Key Points
Question
Is methotrexate or mycophenolate mofetil more effective as first-line immunosuppressive treatment for patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis requiring corticosteroid-sparing therapy?
Findings
In this randomized clinical trial of 216 patients with active noninfectious intermediate uveitis, posterior uveitis, and panuveitis, 66.7% of patients in the methotrexate group achieved corticosteroid-sparing control of inflammation compared with 57.1% in the mycophenolate group, a difference that was not statistically significant.
Meaning
Mycophenolate mofetil was not more effective than methotrexate in treating noninfectious uveitis.
Abstract
Importance
Methotrexate and mycophenolate mofetil are commonly used immunomodulatory therapies for achieving corticosteroid-sparing control of noninfectious uveitis, but there is uncertainty about which drug is more effective.
Objective
To compare the effect of methotrexate and mycophenolate for achieving corticosteroid-sparing control of noninfectious intermediate uveitis, posterior uveitis, and panuveitis.
Design, Setting, and Participants
The First-line Antimetabolites as Steroid-sparing Treatment (FAST) uveitis trial screened 265 adults with noninfectious uveitis requiring corticosteroid-sparing immunosuppressive therapy from 9 referral eye centers in India, the United States, Australia, Saudi Arabia, and Mexico between August 22, 2013, and August 16, 2017. Follow-up ended on August 20, 2018.
Interventions
Patients were randomized to receive oral methotrexate, 25 mg weekly (n = 107), or oral mycophenolate mofetil, 3 g daily (n = 109).
Main Outcomes and Measures
The primary outcome was treatment success at 6 months, which was defined as having control of inflammation in both eyes, no more than 7.5 mg prednisone daily and less than or equal to 2 drops of prednisolone acetate 1%, and no treatment failure due to safety or intolerability. Patients underwent follow-up to 12 months while receiving the same treatment or switched to the other antimetabolite, depending on their 6-month outcome.
Results
Among 216 patients who were randomized (median age, 38 years; 135 (62.5%) women), 194 (89.8%) completed follow-up through 6 months. Treatment success occurred in 64 (66.7%) patients in the methotrexate group vs 56 (57.1%) in the mycophenolate group (difference, 9.5% [95% CI, −5.3% to 21.8%]; odds ratio [OR], 1.50 [95% CI, 0.81 to 2.81]; P = .20). Among patients with posterior uveitis or panuveitis, treatment success was achieved in 58 (74.4%) in the methotrexate group vs 42 (55.3%) in the mycophenolate group (difference, 19.1% [95% CI, 3.6% to 30.6%]; OR, 2.35 [95% CI, 1.16 to 4.90]; P = .02); whereas among patients with intermediate uveitis treatment success occurred in 6 (33.3%) in the methotrexate group vs 14 (63.6%) in the mycophenolate group (difference, −30.3% [95% CI, −51.6% to 1.1%]; OR, 0.29 [95% CI, 0.08 to 1.05]; P = .07; P for interaction = .004). Elevated liver enzymes were the most common nonserious laboratory adverse event, occurring in 14 patients (13.0%) in the methotrexate group and 8 patients (7.4%) in the mycophenolate group.
Conclusions and Relevance
Among adults with noninfectious uveitis, the use of mycophenolate mofetil compared with methotrexate as first-line corticosteroid-sparing treatment did not result in superior control of inflammation. Further research is needed to determine if either drug is more effective based on the anatomical subtype of uveitis.
Trial Registration
ClinicalTrials.gov Identifier: NCT01829295
This randomized trial compares the effects of oral methotrexate, 25 mg weekly vs oral mycophenolate mofetil, 3 g daily, on measures of inflammation in patients with noninfectious uveitis.
Introduction
Uveitis, a group of diseases characterized by sight-threatening intraocular inflammation, is the fifth most common cause of severe vision loss in high-income nations and was estimated to be responsible for 10% to 15% of all blindness cases in the United States in epidemiology studies conducted in the 1990s.1,2 Uveitis can be associated with a systemic disease or be eye limited and affects people of all ages, which may lead to greater years of vision loss than other age-related diseases.3
The standard first-line treatment for noninfectious uveitis is corticosteroids (topical, systemic, injections, and implants). However, corticosteroid therapy has local and systemic adverse effects making its long-term use undesirable.4 Timely management of uveitis is critical to prevent permanent vision loss, and multidisciplinary collaboration is often required given the need for corticosteroid-sparing immunosuppression.
The antimetabolites methotrexate and mycophenolate mofetil are commonly used as initial corticosteroid-sparing treatments for uveitis before progressing to biologic therapies, which are more expensive and have risks for serious adverse effects.5,6,7 Noncomparative retrospective case series generally suggest that mycophenolate mofetil may be more effective and tolerable for patients with uveitis, but there is a lack of evidence comparing the effectiveness of these treatments.5,8,9,10,11,12
The primary objective of this study was to compare the relative effectiveness of methotrexate and mycophenolate mofetil for achieving corticosteroid-sparing control of noninfectious intermediate uveitis, posterior uveitis, and panuveitis.
Methods
Study Design and Eligibility
The First-line Antimetabolites as Steroid-sparing Treatment (FAST) uveitis trial was a National Eye Institute–supported multicenter, randomized, parallel, observer-masked clinical trial at 9 referral eye care centers in India, the United States, Australia, Saudi Arabia, and Mexico. To be eligible for the trial, patients had to be 16 years of age or older, have active noninfectious intermediate uveitis, posterior uveitis, or panuveitis in at least 1 eye, and have a justification for starting corticosteroid-sparing therapy (see eBox in Supplement 1 for complete eligibility criteria). Institutional review board approval was obtained at all centers. All patients provided written informed consent. Race and ethnicity were self-reported by patients based on fixed categories to comply with National Institutes of Health and US Food and Drug Administration guidelines.13,14 All research procedures adhered to the tenets of the Declaration of Helsinki. The study was monitored by an independent data and safety monitoring committee. The trial protocol and statistical analysis plan are available online (Supplement 2 and Supplement 3).
Randomization
Patients were randomized (1:1 allocation ratio) to methotrexate or mycophenolate mofetil using permutated blocks of size 4 and 6 and stratified by study site, and allocation was concealed until after enrollment. The principal statistician generated the random allocation sequence using the statistical software R (R Project for Statistical Computing, version 2.12). After investigators confirmed eligibility, the study coordinator obtained the treatment assignment through the online Research Electronic Data Capture (REDCap) system.15
Study Timeline, Masking, and Assessments
All patients completed study visits at baseline, 2 weeks, and every 4 weeks up to 6 months. Scheduling of study visits was based on the enrollment date. The primary outcome was measured at 6 months (Figure 1). Patients with treatment success continued taking their randomized medication for another 6 months. If treatment failed, patients switched to the other antimetabolite plus 6-month follow-up. Personnel responsible for outcome measurements (ophthalmologists, visual acuity examiners, and optical coherence tomography operators) were masked, including after switching medications. Study coordinators and patients were not masked to the medication assignment.
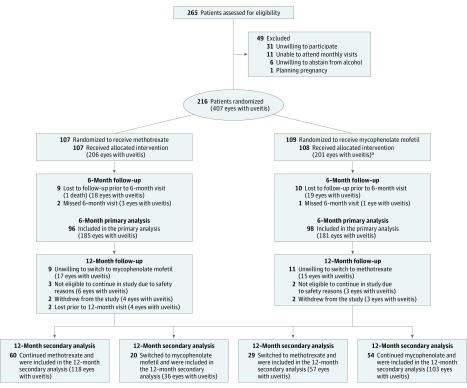
Figure 1. Patient Flow From Screening to 12-Month Analysis.
aOne patient did not receive mycophenolate mofetil due to a medical contraindication discovered postrandomization.
Ophthalmologists, masked to patients’ assigned treatment group, measured anterior chamber cells according to the Standardization of Uveitis Nomenclature (SUN) guidelines. Vitreous haze was assessed by the standardized National Eye Institute scoring system. Retinal and choroidal lesions were assessed by clinical examination and imaging studies. Visual acuity examiners (also masked to patients’ treatment group) measured best spectacle-corrected visual acuity using an ETDRS letter or tumbling “E” logarithm of the minimum angle of resolution chart at 4 m. Macular thickness was assessed using optical coherence tomography. Macular edema thresholds were greater than or equal to 315 μm for Spectralis (Heidelberg Engineering) and greater than or equal to 300 μm for Cirrus (Carl Zeiss Meditec).
Treatment
The University of California San Francisco pharmacy provided study drugs for all sites. Patients were randomized and started treatment with an initial dose for 2 weeks to ensure tolerability (15 mg weekly oral methotrexate or 500 mg twice daily oral mycophenolate mofetil). The dose was increased to a maintenance level for the remainder of the trial: 25 mg weekly for the oral methotrexate group and 1.5 g twice daily for the oral mycophenolate mofetil group. If patients experienced intolerable symptoms or adverse events, dose of the trial drug was reduced (see eTable 3 in Supplement 1 for dosing information) at the discretion of the investigator; masking of study personnel ascertaining outcomes was preserved. Monthly calendars were provided to patients to record any missed doses. Patients were prescribed oral prednisone (1 mg/kg or 60 mg daily [whichever was less]) at enrollment and tapered according to SUN guidelines, with a goal of tapering and holding at 7.5 mg daily prednisone at month 6.16 Following the primary end point, prednisone could be tapered off. If needed, patients took topical corticosteroids but were instructed to taper to 2 drops or less per day of prednisolone acetate 1% or equivalent by month 6. Periocular and intravitreal corticosteroid injections could be administered within the first 90 days after randomization for macular edema if investigators deemed additional treatment was needed; other injections were considered protocol deviations.
Outcomes
The primary outcome was treatment success at the patient level, defined by achieving corticosteroid-sparing control of inflammation in both eyes (or single eye if other eye was unevaluable at baseline) at the month 6 visit, defined by the following:
(1) less than or equal to 0.5+ anterior chamber cells by SUN criteria,16 less than or equal to 0.5+ vitreous haze clinical grading using the NEI scale,16 and no active retinal or choroidal lesions; and (2) no more than 7.5 mg of oral prednisone daily and less than or equal to 2 drops of prednisolone acetate 1% (or equivalent) per day; and (3) no declaration of treatment failure due to intolerability or safety concerns.
Treatment failure due to efficacy was declared if either criterion 1 or 2 (listed in the previous paragraph) was not met at 6 months or declared earlier if a patient had persistent worsening inflammation. Treatment failure could be declared due to safety (eg, if a patient had an abnormal laboratory result meeting the serious adverse event threshold) or intolerability (eg, if a patient was unable or unwilling to continue medication due to adverse effects) at 6 months. The same definition of treatment success was applied to 12-month outcomes.
Prespecified secondary outcomes included treatment success at 6 months by anatomical subtype of uveitis, by enrollment site or country, treatment success at 12 months in patients who continued their randomized antimetabolite and patients who switched to the other antimetabolite, visual acuity, and central subfield macular thickness at 6 months. Additional secondary outcomes not reported in this article were time to control of inflammation; sustained treatment success; treatment success in the Vogt-Koyanagi-Harada subgroup; quality of life by the 36-Item Short Form Survey (SF-36), the National Eye Institute Visual Function Questionnaire (NEI-VFQ-25), and the Indian Vision Function Questionnaire (IND-VFQ); and changes in specific manifestations of uveitis, such as anterior chamber cell inflammation, vitreous haze, retinal or choroidal lesions, and vasculitis.
Statistical Analyses
Enrollment of 216 patients was estimated to provide 80% power to detect a clinically meaningful absolute difference of approximately 20% in treatment success between the methotrexate group (40%) and the mycophenolate mofetil group (60%), assuming 10% loss to follow-up and a 2-tailed α of 0.05. This risk difference in favor of mycophenolate mofetil was supported by retrospective studies.9,11,17 The prespecified primary outcome analysis was a mixed-effects logistic regression to estimate the association between treatment group as the fixed effect and treatment success at 6 months. Site was included as a random effect. This outcome was evaluated at the patient level. Odds ratios (ORs) and their 95% CIs were calculated from the regression. Equivalent absolute risk differences and 95% CIs are also reported. All patients with a 6-month visit or who were declared as having early treatment failure were analyzed according to their randomization group. Patients missing a primary outcome visit were not included in the primary analysis. As a sensitivity analysis, multiple imputation was used to infer missing primary outcome data. The same logistic model used in the primary outcome analysis was applied at 12 months. A noninferiority analysis with a 2-sided significance level of .05 was prespecified to evaluate whether methotrexate is noninferior to mycophenolate mofetil for achieving treatment success at 6 months, with an absolute margin in risk difference of 10%. The noninferiority margin of 10% was based on investigator consensus and would likely exclude the effect of placebo (Supplement 3).18 Sensitivity analyses were preplanned to examine for heterogeneity in treatment effect across site and country. Because of the potential type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Retrospective studies and clinical trials have demonstrated posterior uveitis and panuveitis to be a more severe and complicated uveitis subtype to treat.19,20,21 We hypothesized that there may be a difference in treatment effect by anatomical uveitis subtype and thus, prespecified a subgroup analysis (intermediate uveitis vs posterior uveitis and panuveitis) of treatment success. The primary outcome logistic regression was used for this subanalysis.
Other secondary outcomes were also examined using the primary outcome logistic regression model. Eye-level outcomes, including change in visual acuity and central subfield macular thickness, were compared between treatment groups with a linear regression model clustering on patient. Only uveitic eyes at enrollment were included in eye-level analyses. Patients with bilateral uveitis had both eyes included.
The frequency and proportion of patients experiencing nonserious and serious ocular, laboratory, and systemic adverse events were calculated for each group. Dose reductions and protocol deviations were compared between treatment groups using a Fisher exact test. Missed doses were compared by group using a Welch t test. Statistical significance was declared at 2-sided P value of less than .05. Permutation CIs were calculated for the primary outcome with permuted P values based on the randomized treatment assignment. Data were analyzed using R version 3.51 (R Project for Statistical Computing).
Results
Two hundred sixteen patients were randomized between August 2013 and August 2017 (107 patients to the methotrexate group and 109 patients to the mycophenolate mofetil group). Six eyes were unevaluable due to phthisis and 19 eyes had no history of uveitis, leaving 206 uveitic eyes in the methotrexate group and 201 uveitic eyes in the mycophenolate mofetil group. Patient follow-up was completed in August 2018, and 194 patients (96 in the methotrexate group and 98 in the mycophenolate mofetil group) had complete information for the primary outcome (Figure 1); 22 patients (10.2%) were lost to follow-up.
Table 1 shows the baseline demographic and clinical characteristics for both groups. Most patients had bilateral uveitis (92.5% in the methotrexate group and 84.4% in the mycophenolate mofetil group). Forty-six patients (21.3%) had intermediate uveitis only or anterior uveitis and intermediate uveitis, and 170 patients (78.7%) had posterior uveitis or panuveitis. In the 180 days prior to enrollment, the highest oral prednisone (or equivalent) dose administered was a median 50 mg (interquartile range [IQR], 40-60 mg) in the methotrexate group and 50 mg (IQR, 40-75 mg) in the mycophenolate mofetil group. The initial corticosteroid dose at enrollment for each group was a median of 50 mg (0.9 mg/kg [IQR, 40-60 mg]). Fifteen patients (6.9%) had previously attempted immunosuppressive therapy more than 12 months prior to enrollment.
Table 1. Baseline Characteristics of Patients by Treatment Assignment.
| Patient-Level Characteristicsa | Methotrexate (n = 107) | Mycophenolate Mofetil (n = 109) |
|---|---|---|
| Age, median (IQR), y | 36 (26-50) | 41 (31-51) |
| Female sex | 75 (70.1) | 60 (55.0) |
| Male sex | 32 (29.9) | 49 (45.0) |
| Hispanic ethnicity | 5 (4.7) | 7 (6.4) |
| Raceb | ||
| Indian subcontinent | 70 (65.4) | 69 (63.3) |
| White | 25 (23.4) | 22 (20.2) |
| Asian | 5 (4.7) | 6 (5.5) |
| Middle Eastern | 5 (4.7) | 5 (4.6) |
| Black | 3 (2.8) | 6 (5.5) |
| Native American | 2 (1.9) | 1 (0.9) |
| Pacific Islander | 1 (0.9) | 3 (2.8) |
| Uveitis diagnosis | ||
| Vogt-Koyanagi-Harada disease | 49 (45.8) | 44 (40.4) |
| Undifferentiated | 22 (20.6) | 20 (18.3) |
| Retinal vasculitis | 8 (7.5) | 13 (11.9) |
| Sarcoidosis | 7 (6.5) | 8 (7.3) |
| Sympathetic ophthalmia | 3 (2.8) | 8 (7.3) |
| Behçet disease | 5 (4.7) | 3 (2.8) |
| Pars planitis | 2 (1.9) | 5 (4.6) |
| Birdshot chorioretinopathy | 3 (2.8) | 3 (2.8) |
| Other | 8 (7.5) | 5 (4.6) |
| Bilateral uveitis | 99 (92.5) | 92 (84.4) |
| Duration of uveitis, median (IQR), d | 135 (20-550) | 122 (21-783) |
| Anatomic location, based on entire uveitis historyc | ||
| Panuveitis | 60 (56.1) | 61 (56.0) |
| Posterior uveitis | 24 (22.4) | 25 (22.9) |
| Anterior uveitis and intermediate uveitis | 15 (14.0) | 14 (12.8) |
| Intermediate uveitis | 8 (7.5) | 9 (8.3) |
| Systemic corticosteroid use | ||
| Maximum dose of oral prednisone or equivalent in past 180 days, median (IQR), mgd | 50 (40-60) | 50 (40-75) |
| Oral prednisone at baseline, median (IQR), mg | 50 (40-60) | 50 (40-60) |
| Prior treatment with immunosuppressive therapy | 8 (7.5) | 7 (6.4) |
| Eye-Level Characteristics |
Methotrexate (n = 206 eyes) |
Mycophenolate Mofetil (n = 201 eyes) |
| Inflammation at baseline | ||
| Anterior chamber cells | 205 (99.5) | 201 (100.0) |
| 0 | 84 (41.0) | 87 (43.3) |
| 0.5+ | 53 (25.9) | 47 (23.4) |
| 1+ | 46 (22.4) | 47 (23.4) |
| ≥2+ | 22 (10.7) | 20 (10.0) |
| Anterior vitreous cells | 202 (98.1) | 200 (99.5) |
| 0 | 34 (16.8) | 33 (16.5) |
| 0.5+ | 40 (19.8) | 48 (24.0) |
| 1+ | 52 (25.7) | 51 (25.5) |
| ≥2+ | 76 (37.6) | 68 (34.0) |
| Vitreous haze | 202 (98.1) | 200 (99.5) |
| 0 | 85 (42.1) | 97 (48.5) |
| 0.5+ | 30 (14.9) | 34 (17.0) |
| 1+ | 50 (24.8) | 30 (15.0) |
| ≥2+ | 37 (18.3) | 39 (19.5) |
| Active retinal or choroidal lesions, n/N (%) | 125/202 (61.9) | 119/200 (59.5) |
| Macular edemae | 42 (20.4) | 55 (27.3) |
| Central subfield thickness, median (IQR), μmf | 359.0 (331.5-449.5) | 342.0 (316.0-398.5) |
| LogMAR visual acuity, median (IQR)g | 0.28 (0.03-0.52) | 0.30 (0.08-0.60) |
Abbreviations: IQR, interquartile range; logMAR, logarithm of the minimum angle of resolution.
Values are reported as No. (%) unless otherwise indicated.
Seven patients listed being of more than 1 racial heritage.
Anatomic location was assessed given all medical records available.
Corticosteroids in the past 180 days included oral, subcutaneous, and intravenous, and were adjusted to equivalent calculations of oral prednisone.
Macular edema excluded patients with Vogt-Koyanagi-Harada disease who had a serious retinal detachment.
Characteristic was reported in patients with macular edema.
Indicates best-corrected visual acuity of uveitic eyes only.
Six-Month Primary Outcome Analysis
At the 6-month primary end point, 120 patients met the definition of treatment success (Table 2). In the primary analysis, treatment success was achieved in 64 patients in the methotrexate group vs 56 patients in the mycophenolate mofetil group (66.7% vs 57.1%; difference, 9.5% [95% CI, −5.3% to 21.8%]; odds ratio [OR], 1.50 [95% CI, 0.81 to 2.81]; P = .20). Reasons for treatment failure are listed in Table 2.
Table 2. Six-Month Results From a Randomized Clinical Trial Comparing Methotrexate and Mycophenolate Mofetil for Noninfectious Uveitis.
| Patient-Level Characteristics | Methotrexate (n = 96) |
Mycophenolate Mofetil (n = 98) |
Absolute Risk Difference for Treatment Success, % (95% CI) | OR Estimate for Treatment Success (95% CI)a | P Value |
|---|---|---|---|---|---|
| Primary Analysis | |||||
| Treatment success, No. (%)b | 64 (66.7) | 56 (57.1) | 9.5 (−5.3 to 21.8) | 1.50 (0.81 to 2.81) | .20 |
| Treatment failure, No. (%) | 32 (33) | 42 (43) | |||
| Secondary Analyses | |||||
| Reason for treatment failure, no./No. (%) | |||||
| Efficacyc | 26/32 (81) | 38/42 (90) | .55 | ||
| Intolerabilityd | 3/32 (9) | 2/42 (5) | |||
| Safetye | 3/32 (9) | 2/42 (5) | |||
| Treatment success by anatomical location, no./No. (%) | |||||
| Anterior uveitis and intermediate uveitis/intermediate uveitis only | 6/18 (33.3) | 14/22 (63.6) | −30.3 (−51.6 to 1.1) | 0.29 (0.08 to 1.05) | .07f |
| Posterior uveitis/panuveitis | 58/78 (74.4) | 42/76 (55.3) | 19.1 (3.6 to 30.6) | 2.35 (1.16 to 4.90) | .02f |
| Treatment success at 12 mo, no./No. (%) | |||||
| Continued on randomized antimetaboliteg | 48/60 (80) | 40/54 (74) | 5.9 (−12.2 to 17.0) | 1.40 (0.57 to 3.56) | .47 |
| Switched to other antimetaboliteh | 20/29 (69) | 7/20 (35) | 34.2 (6.6 to 52.6) | 4.17 (1.32 to 13.16) | .02 |
| Missed doses, mean (SD), %i | 4.6 (1.0) | 4.3 (0.5) | .87 | ||
| Eye-Level Characteristics |
Methotrexate (n = 185 eyes) |
Mycophenolate Mofetil (n = 181 eyes) |
P Valuej | ||
| Change in logMAR visual acuity, median (IQR)k | −0.10 (−0.32 to 0.00) | −0.12 (−0.31 to 0.00) | .83 | ||
| Reduction in central macular thickness, median (IQR) [No.], μml | −26.00 (−89.00 to 5.00) [42] |
−14.00 (−80.00 to 3.25) [55] |
.95 | ||
Abbreviations: IQR, interquartile range; logMAR, logarithm of the minimum angle of resolution; OR, odds ratio.
Logistic regression with treatment group as a fixed effect and study site as a prespecified random effect.
Treatment success was defined by achieving corticosteroid-sparing control of inflammation in both eyes at the month 6 visit.
Treatment failure due to efficacy was defined by not achieving the treatment success definition at month 6 or could be declared earlier if patient had persistent worsening inflammation.
Treatment failure due to intolerability was declared if a patient was unable or unwilling to continue medication due to adverse effects.
Treatment failure due to safety was declared if a patient had an abnormal laboratory result that met the serious adverse event threshold.
Interaction between anatomical subtype and treatment group was significant (P = .004).
Of patients who achieved treatment success at 6 months, 60 patients originally randomized to methotrexate and 54 patients originally randomized to mycophenolate mofetil continued on same antimetabolite through 12 months.
Of patients who had treatment failure at 6 months, 29 patients originally randomized to mycophenolate mofetil switched to methotrexate, and 20 patients originally randomized to methotrexate switched to mycophenolate mofetil.
Indicates the number of missed doses over total expected doses throughout a patient’s enrollment in the trial.
P values computed from a linear mixed-effects model.
Decrease indicates gain in visual acuity.
Indicates eyes with macular edema excluding patients who had a serous retinal detachment in the setting of Vogt-Koyanagi-Harada disease.
Prespecified 6-Month Secondary Outcomes
For the noninferiority analysis, the lower bound of the 95% CI of the risk difference derived from the primary analysis was −5.3% and excluded the prespecified 10% noninferiority margin. This provides evidence of methotrexate noninferiority with respect to mycophenolate mofetil.
Treatment success in patients with posterior uveitis and panuveitis was greater in the methotrexate group vs in the mycophenolate mofetil group (74.4% vs 55.3%; difference, 19.1% [95% CI, 3.6% to 30.6%]; OR, 2.35 [95% CI, 1.16 to 4.90]; P = .02; anatomical subtype and treatment interaction, P = .004). Treatment success was not significantly different between groups in patients with intermediate uveitis (33.3% in the methotrexate group vs 63.6% in the mycophenolate mofetil group; difference, −30.3% [95% CI, −51.6% to 1.1%]; OR, 0.29 [95% CI, 0.08 to 1.05]; P = .07).
There was no significant difference in the change in visual acuity between treatment groups (0.2 lines greater in the mycophenolate mofetil group [95% CI, 1.7 lines less to 2.0 lines greater]; P = .83). There was also no significant difference in the change in central subfield macular thickness between treatment groups at 6 months (2.4 μm greater in the methotrexate group [95% CI, −78.0 μm to 82.8 μm]; P = .95). Best spectacle-corrected visual acuity improved by a median of 1.0 line in the methotrexate group (IQR, 0.0 to 3.2 lines), and by a median of 1.2 lines in the mycophenolate mofetil group (IQR, 0.0 to 3.1 lines; Table 2). The central subfield macular thickness decreased by a median of 26 μm in the methotrexate group (IQR, −89 to 5) and 14 μm in the mycophenolate mofetil group (IQR, −80 to 3.25). Seven patients (7 eyes) in the methotrexate group and 5 patients (5 eyes) in the mycophenolate mofetil group were treated per protocol for macular edema with a corticosteroid injection within 90 days of randomization. Four patients (7 eyes) in the methotrexate group and 4 patients (4 eyes) in the mycophenolate mofetil group received a corticosteroid injection that was considered a protocol deviation (>90 days postrandomization).
The mean (SD) proportion of expected doses missed was 4.6% (1.0%) for patients receiving weekly methotrexate and 4.3% (0.5%) for patients receiving daily mycophenolate mofetil (P = .87). A sensitivity analysis of the primary model that included only patients who missed less than 20% of their doses did not substantially change the result. Twenty-one (19.6%) patients in the methotrexate group and 15 (13.8%) patients in the mycophenolate mofetil group reduced their study medication (P = .27), mostly due to intolerability.
Subgroup and Sensitivity Analyses of 6-Month Outcomes
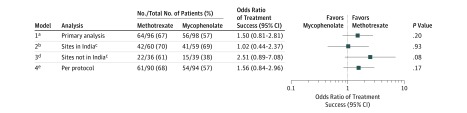
In sensitivity analyses of the primary outcome assessing the effect of clinic location, interaction tests in all models were found not to be statistically significant, indicating a lack of evidence of heterogeneity (India and treatment group, P = .15; country [United States and Mexico combined] and treatment group, P = .45; eTable 7 in Supplement 1). Treatment success was achieved in 22 patients in the methotrexate group vs 15 patients in the mycophenolate mofetil group outside of India (61.1% vs 38.5%; difference, 22.7% [95% CI, −2.6% to 43.1%]; OR, 2.5 [95% CI, 0.9 to 7.1]; P = .08; Figure 2).
Figure 2. Six-Month Primary Outcome and Sensitivity Analyses of the Primary Outcome.
aPrimary model is a logistic regression with treatment group as a fixed effect and study site as a prespecified random effect (n = 194).
bPrimary model with patients from sites in India only (n = 119).
cTreatment group and Indian site interaction term was not significant (P =.15).
dPrimary model with patients from sites not in India only (n = 75).
ePrimary model with patients who missed less than 20% of study medication (n = 184).
A total of 22 of 216 patients (10.2%) missed the 6-month primary outcome visit, 11 patients (50.0%) in the methotrexate group and 11 patients (50.0%) in the mycophenolate mofetil group. Sensitivity analysis of the primary outcome using multiple imputation showed a similar result as the prespecified analysis with treatment success achieved in 66 patients (61.7%) in the methotrexate group vs 59 patients (54.1%) in the mycophenolate mofetil group (difference, 7.6% [95% CI, −5.9% to 19.1%]; OR, 1.4 [95% CI, 0.8 to 2.4]; P = .27).
Prespecified 12-Month Outcomes
Of the 120 patients who were declared a 6-month treatment success, 114 were followed up for an additional 6 months. Forty-eight of 60 (80.0%) patients in the methotrexate group and 40 of 54 (74.1%) patients in the mycophenolate mofetil group remained a treatment success at 12 months, with the majority (50.0% for methotrexate and 55.0% for mycophenolate mofetil) discontinuing prednisone. Forty-nine of the 68 eligible patients in whom treatment failed in the first 6 months and who did not have a serious laboratory adverse event switched to the other antimetabolite: 20 of the 32 (62.5%) patients originally randomized to methotrexate and 29 of the 42 (69.0%) originally randomized to mycophenolate mofetil. There was greater treatment success at 12 months in the methotrexate group (69.0%) in patients for whom mycophenolate mofetil had previously failed vs patients in the mycophenolate mofetil group (35.0%) in whom methotrexate had failed (difference, 34.2% [95% CI, 6.6% to 52.6%]; OR, 4.2 [95% CI, 1.3 to 13.2]; P = .02).
Adverse Events
Table 3 shows the frequency and proportion of patients experiencing nonserious or serious ocular, laboratory, and systemic adverse events in each treatment group at 6 months. Nonserious liver function test abnormalities occurred more frequently in patients receiving methotrexate (14 [13.0%]) vs mycophenolate mofetil (8 [7.4%]). Fatigue and headache were the most common nonserious systemic adverse events (62 patients [57.9%] and 55 patients [51.4%]) in the methotrexate group and also in the mycophenolate mofetil group (59 patients [54.6%] and 45 patients [41.7%]). Fourteen serious adverse events occurred through the primary outcome with 5 deemed drug-related by the masked medical monitor: 3 (2.8%) in the methotrexate group and 2 (1.9%) in the mycophenolate mofetil group. All drug-related serious adverse events were elevated liver function tests. Twelve-month adverse events are reported in eTables 8 and 9 in Supplement 1.
Table 3. Six-Month Adverse Events From a Randomized Clinical Trial Comparing Methotrexate and Mycophenolate Mofetil for Noninfectious Uveitis.
| Event Typea | No. (%) of Patients Reporting ≥1 Adverse Event | |
|---|---|---|
| Methotrexate (n = 107) |
Mycophenolate Mofetil (n = 109)b |
|
| Nonserious ocular | ||
| Ocular hypertension >24 mm Hg | 10 (9.3) | 13 (12.0) |
| Peripheral and/or central vitreous hemorrhage | 3 (2.8) | 3 (2.8) |
| Suspect or confirmed glaucoma diagnosis | 2 (1.9) | 2 (1.9) |
| Intraocular pressure <5 mm Hg without structural damage | 0 | 1 (0.9) |
| Visually significant cataract, surgery indicated | 5 (4.7) | 2 (1.9) |
| Decrease in vision or defective vision (self-reported) | 9 (8.4) | 19 (17.6) |
| Eye pain (self-reported) | 9 (8.4) | 4 (3.7) |
| Serious ocular | ||
| Glaucoma | 0 | 1 (0.9) |
| Retinal detachment | 1 (0.9) | 0 |
| Nonserious laboratory | ||
| Elevated ALT or AST (2 to 5 times upper limit of normal <28 d) | 14 (13.0) | 8 (7.4) |
| Low hemoglobin (>6.5 to <9 g/dL lasting <28 d) | 2 (1.9) | 3 (2.8) |
| Low leukocyte count (>1000 and <2500/μL lasting <28 d) | 3 (2.8) | 1 (0.9) |
| Elevated creatinine (>1.5 to <2 mg/dL lasting <28 d) | 1 (0.9) | 0 |
| Serious laboratory | ||
| Extremely elevated ALT or AST (>5 times the upper limit of normal or 2 to 5 times the upper limit of normal ≥28 d)c | 3 (2.8) | 2 (1.9) |
| Nonserious systemic | ||
| Fatigue | 62 (57.9) | 59 (54.6) |
| Headache | 55 (51.4) | 45 (41.7) |
| Mood changes (self-reported, not requiring therapy) | 33 (30.8) | 26 (24.1) |
| Hair loss | 6 (5.6) | 2 (1.9) |
| Nausea | 42 (39.3) | 30 (27.8) |
| Muscle weakness, no decrease in function | 32 (29.9) | 26 (24.1) |
| Numbness or tingling | 25 (23.4) | 18 (16.7) |
| Diarrhea | 25 (23.4) | 24 (22.2) |
| Dyspnea | 21 (19.6) | 22 (20.4) |
| Vomiting | 26 (24.3) | 24 (22.2) |
| Allergic reaction | 14 (13.1) | 11 (10.2) |
| Fever <39°C for 12 h | 11 (10.3) | 18 (16.7) |
| Mild congestive heart failure or arrhythmia not requiring therapy | 4 (3.7) | 2 (1.9) |
| Systemic infection | 25 (23.4) | 27 (25.0) |
| Other systemic (no treatment required) | 65 (60.7) | 63 (58.3) |
| Serious systemic | ||
| Death | 1 (0.9) | 0 |
| Diarrhea | 0 | 1 (0.9) |
| Disability or permanent damage | 1 (0.9) | 0 |
| Hospitalization | 2 (1.9) | 2 (1.9) |
| Serious systemic infection | 0 | 1 (0.9) |
| Vomiting | 1 (0.9) | 0 |
| Other serious eventd | 3 (2.8) | 1 (0.9) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
SI conversion: for creatinine to μmol/L, multiply by 88.4.
Criteria for defining serious and nonserious adverse events are included in eTable 1 in Supplement 1.
One patient in the mycophenolate mofetil group never received mycophenolate mofetil due to medical contraindication discovered postrandomization.
All drug-related serious adverse events were due to extremely elevated ALT, AST, or both in some patients.
Indicates stroke, injury to finger, and lower limb pain within the methotrexate group and extreme flank pain within the mycophenolate mofetil group.
Discussion
Among adults with noninfectious uveitis, the use of mycophenolate mofetil compared with methotrexate as first-line corticosteroid-sparing treatment did not result in superior control of inflammation.
Exploratory hypothesis-generating secondary analyses assessed treatment success by anatomical subgroup of uveitis and at 12 months. Methotrexate was found to be more effective in patients with posterior uveitis or panuveitis—the most severe type of uveitis and the largest subgroup in this trial. Although the results favored mycophenolate mofetil, no significant difference was found in treatment success with the less-severe subgroup, intermediate uveitis. Ultimately, treatment success by anatomical location needs to be explored further. In both treatment groups, most patients who achieved treatment success at 6 months remained a success through 12 months. Treatment success in the 12-month analysis of patients switching treatment after not achieving control was superior in patients switching to methotrexate after mycophenolate mofetil.
Overall, treatment failures due to intolerability and safety were low. Abnormalities in liver function tests were more common with methotrexate treatment as expected.
Although the biologic adalimumab is US Food and Drug Administration–approved for uveitis, antimetabolites are still commonly used as initial corticosteroid-sparing therapy because of their lower costs and fewer associated risks. Retrospective noncomparative case series suggest that mycophenolate mofetil may be more effective than methotrexate as a corticosteroid-sparing therapy for uveitis, but the heterogeneity in definitions of treatment success, patient populations, and medication dosing in these reports make it difficult to compare the effectiveness of methotrexate and mycophenolate mofetil.5,11,12,17,21,22,23,24,25,26 Additionally, low doses of methotrexate (≤15 mg) were routinely used in these previous case series.5,19,20 A pilot trial, conducted as a precursor to this trial, with 25 mg of weekly oral methotrexate but a lower dose of daily mycophenolate mofetil (2 g daily) found consistent results to the current finding. In this trial, the standard maximum doses for methotrexate and mycophenolate mofetil were compared. Even with these high doses, few patients’ treatment failed due to intolerability. The higher dose of methotrexate used in this trial compared with previous retrospective studies on uveitis may have contributed to the finding that mycophenolate mofetil was not superior. It is possible that using 25 mg of methotrexate subcutaneously may result in even greater uveitis control given the increased bioavailability with subcutaneous administration.27
In rheumatology, disease-specific treatment guidelines exist. For example, methotrexate is the preferred initial treatment for rheumatoid arthritis and mycophenolate mofetil for lupus nephritis.28,29,30 Comparative trials are important to evaluate the relative efficacy of available treatment options, and this has been lacking for uveitis. The findings of this trial have implications for clinical practice because they provide scientific justification that mycophenolate mofetil is not more effective than methotrexate as a corticosteroid-sparing immunosuppressive therapy for uveitis. Patients with posterior uveitis and panuveitis have more vision complications than other types of uveitis and often have chronic inflammation requiring long-term immunosuppressive therapy.20,21 Methotrexate may be an effective, reasonable, first-line choice for these patients.
The strengths of this study include its randomized design, a large and geographically diverse patient population that increases generalizability, standardized treatment, and the masking of graders to reduce bias. The definition of treatment success in this trial was a clinically relevant composite outcome of assessing inflammation in all parts of both eyes and incorporating tolerability and safety. Many uveitis trials have focused on only 1 aspect of inflammation, yet in clinical practice, treatment is not adequate if it does not control inflammation in all parts of the eye and corticosteroids cannot be tapered.
Limitations
This study has several limitations. First, patients were not masked to their medication. However, study personnel responsible for measuring outcomes were masked through 12 months, including when patients switched medication. The masking of graders, along with using highly reproducible grading scales, mitigated the limitation of subjective inflammation grading for the definition of treatment success.31 Second, patients with a heterogeneous group of uveitis etiologies were included to facilitate enrollment. A difference in treatment effect between intermediate uveitis vs posterior uveitis and panuveitis was found, but there was not sufficient statistical power to compare between uveitis diagnoses. Third, most patients were enrolled in India, but those results did not drive the primary outcome.
Conclusions
Among adults with noninfectious uveitis, the use of mycophenolate mofetil compared with methotrexate as first-line corticosteroid-sparing treatment did not result in superior control of inflammation. Further research is needed to determine if either drug is more effective based on the anatomical subtype of uveitis.
eBox. All Eligibility Criteria for Enrollment in the FAST Uveitis Randomized Clinical Trial
eTable 1. Definitions of Serious and Nonserious Adverse Events
eTable 2. Enrollment Totals by Center
eTable 3. Dose Reduction Guidelines for Study Medication
eTable 4. Six-Month Patient-Reported Other Ocular Adverse Events
eTable 5. Six-Month Patient-Reported Mood-Related Adverse Events
eTable 6. Six-Month Patient-Reported Other Systemic Adverse Events
eTable 7. Tests of Homogeneity of Effect for the 6-Month Primary Outcome
eTable 8. Six- to 12-Month Adverse Events from a RCT Comparing Methotrexate and Mycophenolate Mofetil for Noninfectious Uveitis in Patients Continuing on Treatment after Treatment Success
eTable 9. Six- to 12-Month Adverse Events from a RCT Comparing Methotrexate and Mycophenolate Mofetil for Noninfectious Uveitis in Patients Switching to the Other Antimetabolite
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491-500. doi: 10.1016/j.ophtha.2003.06.014 [DOI] [PubMed] [Google Scholar]
- 2.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80(9):844-848. doi: 10.1136/bjo.80.9.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbaum JT, Dick AD. The eyes have it: a rheumatologist’s view of uveitis. Arthritis Rheumatol. 2018;70(10):1533-1543. doi: 10.1002/art.40568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492-513. doi: 10.1016/S0002-9394(00)00659-0 [DOI] [PubMed] [Google Scholar]
- 5.Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115(10):1826-1832. doi: 10.1016/j.ophtha.2008.04.026 [DOI] [PubMed] [Google Scholar]
- 6.Jaffe GJ, Dick AD, Brézin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932-943. doi: 10.1056/NEJMoa1509852 [DOI] [PubMed] [Google Scholar]
- 7.Dick AD, Rosenbaum JT, Al-Dhibi HA, et al. ; Fundamentals of Care for Uveitis International Consensus Group . Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: Fundamentals Of Care for Uveitis (FOCUS) initiative. Ophthalmology. 2018;125(5):757-773. doi: 10.1016/j.ophtha.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 8.Teoh SC, Hogan AC, Dick AD, Lee RWJ. Mycophenolate mofetil for the treatment of uveitis. Am J Ophthalmol. 2008;146(5):752-760, 760.e1-760.e3. doi: 10.1016/j.ajo.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 9.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112(8):1472-1477. doi: 10.1016/j.ophtha.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 10.Baltatzis S, Tufail F, Yu EN, Vredeveld CM, Foster CS. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology. 2003;110(5):1061-1065. doi: 10.1016/S0161-6420(03)00092-7 [DOI] [PubMed] [Google Scholar]
- 11.Gangaputra S, Newcomb CW, Liesegang TL, et al. ; Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study . Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116(11):2188-98.e1. doi: 10.1016/j.ophtha.2009.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobrin L, Christen W, Foster CS. Mycophenolate mofetil after methotrexate failure or intolerance in the treatment of scleritis and uveitis. Ophthalmology. 2008;115(8):1416-1421, 1421.e1. doi: 10.1016/j.ophtha.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm. Accessed June 24, 2019.
- 14.US Food and Drug Administration (FDA) Collection of race and ethnicity data in clinical trials. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials?source=govdelivery&utm_medium=email&utm_source=govdelivery. Published 2016. Accessed June 24, 2019.
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group . Standardization of uveitis nomenclature for reporting clinical data: results of the first international workshop. Am J Ophthalmol. 2005;140(3):509-516. doi: 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel E, Thorne JE, Newcomb CW, et al. Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol. 2010;149(3):423-32.e1, 2. doi: 10.1016/j.ajo.2009.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleiss JL, Levin B, Paik MC. 6.9 Standard errors for measures of association In: Balding DJ, Cressie NAC, Fisher NI, et al. , eds. Statistical Methods for Rates and Proportions. 3rd ed Hoboken, NJ: John Wiley & Sons; 2003. [Google Scholar]
- 19.Rathinam SR, Babu M, Thundikandy R, et al. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology. 2014;121(10):1863-1870. doi: 10.1016/j.ophtha.2014.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempen JH, Van Natta ML, Altaweel MM, et al. ; Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group . Factors predicting visual acuity outcome in intermediate, posterior, and panuveitis: the Multicenter Uveitis Steroid Treatment (MUST) trial. Am J Ophthalmol. 2015;160(6):1133-1141.e9. doi: 10.1016/j.ajo.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabs DA. Immunosuppression for the uveitides. Ophthalmology. 2018;125(2):193-202. doi: 10.1016/j.ophtha.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32(2):362-365. [PubMed] [Google Scholar]
- 23.Bom S, Zamiri P, Lightman S. Use of methotrexate in the management of sight-threatening uveitis. Ocul Immunol Inflamm. 2001;9(1):35-40. doi: 10.1076/ocii.9.1.35.3983 [DOI] [PubMed] [Google Scholar]
- 24.Dev S, McCallum RM, Jaffe GJ. Methotrexate treatment for sarcoid-associated panuveitis. Ophthalmology. 1999;106(1):111-118. doi: 10.1016/S0161-6420(99)90011-8 [DOI] [PubMed] [Google Scholar]
- 25.Holz FG, Krastel H, Breitbart A, Schwarz-Eywill M, Pezzutto A, Völcker HE. Low-dose methotrexate treatment in noninfectious uveitis resistant to corticosteroids. Ger J Ophthalmol. 1992;1(3-4):142-144. [PubMed] [Google Scholar]
- 26.Shah SS, Lowder CY, Schmitt MA, Wilke WS, Kosmorsky GS, Meisler DM. Low-dose methotrexate therapy for ocular inflammatory disease. Ophthalmology. 1992;99(9):1419-1423. doi: 10.1016/S0161-6420(92)31790-7 [DOI] [PubMed] [Google Scholar]
- 27.Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ≥15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73(8):1549-1551. doi: 10.1136/annrheumdis-2014-205228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dooley MA, Jayne D, Ginzler EM, et al. ; ALMS Group . Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365(20):1886-1895. doi: 10.1056/NEJMoa1014460 [DOI] [PubMed] [Google Scholar]
- 29.Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960-977. doi: 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 30.Singh JA, Saag KG, Bridges SL Jr, et al. ; American College of Rheumatology . 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1-25. doi: 10.1002/acr.22783 [DOI] [PubMed] [Google Scholar]
- 31.Kempen JH, Ganesh SK, Sangwan VS, Rathinam SR. Interobserver agreement in grading activity and site of inflammation in eyes of patients with uveitis. Am J Ophthalmol. 2008;146(6):813-8.e1. doi: 10.1016/j.ajo.2008.06.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eBox. All Eligibility Criteria for Enrollment in the FAST Uveitis Randomized Clinical Trial
eTable 1. Definitions of Serious and Nonserious Adverse Events
eTable 2. Enrollment Totals by Center
eTable 3. Dose Reduction Guidelines for Study Medication
eTable 4. Six-Month Patient-Reported Other Ocular Adverse Events
eTable 5. Six-Month Patient-Reported Mood-Related Adverse Events
eTable 6. Six-Month Patient-Reported Other Systemic Adverse Events
eTable 7. Tests of Homogeneity of Effect for the 6-Month Primary Outcome
eTable 8. Six- to 12-Month Adverse Events from a RCT Comparing Methotrexate and Mycophenolate Mofetil for Noninfectious Uveitis in Patients Continuing on Treatment after Treatment Success
eTable 9. Six- to 12-Month Adverse Events from a RCT Comparing Methotrexate and Mycophenolate Mofetil for Noninfectious Uveitis in Patients Switching to the Other Antimetabolite
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement