Abstract
Nearly one of every five US individuals aged twelve years old or older live with certain types of mental disorders. Men are more likely to use various types of substances, while women tend to be more susceptible to mood disorders, addiction, and eating disorders, all of which are risks associated with suicidal attempts. Fundamental sex differences exist in multiple aspects of functions and activities of neurotransmitter-mediated neural circuits in the central nervous system (CNS). Dysregulation of these neural circuits would lead to various types of mental disorders. The potential mechanisms of sex differences in the CNS neural circuitry regulating mood, reward, and motivation are only beginning to be understood, although they have been largely attributed to the effects of sex hormones on CNS neurotransmission pathways. Understanding this topic is important for developing prevention and treatment of mental disorders that should be tailored differently between men and women. Studies using animal models have provided important insights into pathogenesis, mechanisms, and new therapeutic approaches of human diseases, but some concerns remain to be addressed. The purpose of this chapter is to integrate human and animal studies involving the effects of sex hormone estrogens on CNS neurotransmission, reward processing, and associated mental disorders. We provide an overview of existing evidence for the physiological, behavioral, cellular and molecular actions of estrogens in the context of controlling neurotransmission in the CNS circuits regulating mood, reward and motivation, and discuss related pathology that leads to related mental disorders.
Keywords: Estrogen receptor, Ovariectomy, Dopamine, Serotonin, Glutamate, GABA, Endocannabinoid, Addiction, Reward
1. INTRODUCTION
1.1. Central neurotransmitters regulate mood, reward and motivation
Nearly one of every five individuals aged twelve years old or older live with certain types of mental disorders in the United States 1,2. Mental illnesses, including substance use disorders such as drug addiction and opioid abuse, mood disorders, eating disorders, etc., affect millions of people and are prominent public health threats due to their continuously increased prevalence, difficulty in prevention and treatment, and hundreds of billions of US dollars being spent to care for the patients with mental disorders 3. Additionally, many mental disorders are associated with suicide attempts 4. Therefore, mental disorders take a heavy toll on public health and human livers with enormously high economic costs. We are in drastic need of a better understanding of underlying neurobiological mechanisms behind these disorders.
Several neural circuits within the central nervous system (CNS) regulate mood, reward and motivation, and are modulated by different types of stimuli that individuals respond to and consequently exhibit certain behaviors. These different types of reward stimuli that function as reinforcers to modulate reward circuits include natural rewards (e.g., food and sex) and non-natural rewards (e.g., drug, alcohol, and money). Different types of reward function as stimuli to provide pleasure, enjoyment, and arousal to individuals. While appropriate behavioral responses to reward are beneficial for survival, improper responses to reward, for example, dysregulation of neurotransmitters within the reward circuitry, could be detrimental. Take natural reward stimuli as an example, palatable foods high in calories are a strong reinforcer of neural circuits that control feeding behavior. Rogers and Smit termed compulsive seeking of natural food reward ‘food addiction’ 5, which could lead an individual to develop certain eating disorders and obesity. Similarly, uncontrolled compulsive non-natural reward seeking contributes to destructive substance overuse, potentially leading to substance addiction. Therefore, dysregulation of the CNS reward system serves as a biological factor contributing to the increased prevalence of substance use disorders and related addictions, as well as eating disorders and related obesity.
The reward system includes various brain anatomical regions and pathway structures in the CNS. The reward system is modulated by various neurotransmitters and neuromodulators including dopamine (DA), serotonin (5HT), glutamate, and gamma-aminobutyric acid (GABA); and is influenced by some circulating hormones, including satiety signals, adiposity signals and stress hormones, to alter responses to various types of rewards 6. The neural structures of the reward system are similar across species 6. Animal species from drosophila to humans share a core set of conserved genes 7. Accordingly, neural networks of the reward system also share conserved gene expression profiles across species 8. As animals evolved, different selection pressures likely have acted on distinct brain regions 8. Males and females have dissimilar internal homeostatic states and physiological needs to maintain energy homeostasis 9 and thus would have distinct responses to various types of reward stimuli. Due to specific societal niches held by each sex, selection pressures have had disparate impacts on underlying neural circuitry between the sexes. Albeit the overlapping reward system with similar structures between males and females, functional modulation of shared circuitry by brain chemical messengers are different between the sexes 10 (see Section 2). Additionally, sex hormones interact with these chemical messengers to alter functions between the sexes (see Section 3). Furthermore, there may be more anatomical structural and pathway differences in the CNS reward circuitry between males and females than initially realized. Therefore, it may become cumbersome to define discrete functional differences due to modulations versus true underlying differences between the sexes.
1.2. Dysregulation of neurotransmission leads to mental disorders
The CNS reward system drives the relationship between reward stimuli from the external environment and the internal state of the individuals regulated by homeostatic mechanisms. Motivation to gain reward stimuli from the external environment can change depending on the internal physiological state of the individuals and established associations between external stimuli and reward circuitry from prior contexts. Therefore, the CNS reward system, external stimuli, and internal state of individuals integrate with one another to communicate needs versus costs and evaluate specific reward stimuli, before signals are sent to the regulatory control regions of the brain such as the hypothalamus and the brainstem. Maladjustment of neural circuits of the CNS reward system by either highly palatable foods or drugs of abuse is expected in both eating disorders and substance abuse disorders 11, and is evidenced by their high comorbidity rates 12.
In most of the available literature, areas of the CNS that drive hedonic regulation of food reward by palatable foods 13 and areas that drive homeostatic control of feeding have been studied independently from one another. In actuality, the hedonic circuitry regulating reward responses and the homeostatic circuitry regulating feeding overlap and directly influence one another, depending on each other for proper functioning 14,15. In order to successfully drive appropriate behavior to physiologically maintain whole-body metabolic homeostasis, integration of hedonic reward regions and homeostatic feeding regions with brain areas and neural circuits important for regulating emotion and decision making, along with motor circuits controlling execution of the behaviors occur to change feeding, energy expenditure, and foraging behavior. Such motivational responses due to modified reward circuits are beneficial and necessary for individuals to engage in specific behaviors in order to stay fit and survive. Eating palatable foods, engaging in sexual activity and reproductive behavior, and taking alcohol or other drugs, however, could change neurotransmitter function, neural activity, and modify the reward system 16; while some of these changes could be beneficial, others may be detrimental.
It is noteworthy that cross-sensitization between different types of natural and non-natural reward stimuli take place 17. For example, addiction to a natural reward and overuse of a non-natural reward could strengthen each other, leading to comorbidity between eating disorder and substance use disorders 18. This cross-sensitization could be due to distinctive types of natural and non-natural rewards converging on and activating common neural pathways. Even though distinct rewards may activate similar, overlapping anatomical brain structures, discrete neurochemical modulation may be involved. A better understanding of the reward system at both structural and molecular levels would contribute to our understanding of how different reward stimuli and substances modify reward circuits, leading to mental disorders such as depression, anxiety, and food and substance addictions.
1.3. Sex differences in CNS neurotransmission-related mental disorders
The prevalence of mental disorders differs significantly between men and women. Fundamental sex differences are present in the development of eating disorders, obesity-related metabolic diseases, and substance use disorders. Abundant evidence has established that women are more frequently diagnosed with psychiatric illnesses, in particular eating disorders, anxiety, and depression, compared to men 19,20. Indeed, the prevalence of eating disorders, depression, and anxiety, is about three-fold higher in women than in men 21,22. Although eating disorders, such as anorexia nervosa and bulimia nervosa 23,24 and obesity 25, are more prevalent in women than in men, the incidence of metabolic disorders is more common in men than in women; potentially due to sex differences in fat distribution and energy metabolism 9,26. Contrastingly, while men are more likely to use various types of substances 27, women could become addicted more rapidly from casual drug use, and tend to be more susceptible to some key phases of addiction such as craving and relapse 28–30. Amongst mental disorders, mood disorders are the most strongly associated with suicidal attempts 4. Women have an increased risk of attempting suicide in the general population 31. Published studies in the literature have indicated that while men are at a greater risk of completing suicide than women, the prevalence of attempted suicide is significantly higher in women than men among U. S. population 31. It is noteworthy that, the prevalence of mental disorders is especially high in women during reproductive years following puberty 32, suggesting that elevated and cyclic sex hormone estrogens could predispose women at reproductive ages to develop mental disorders.
It is not suppressing that sex differences exist in almost all aspects of reward- and motivation-related processes. Males and females perceive stimuli and process reward information in different ways and consequently carry out unlike behaviors based on their sex-specific roles. Specifically, males of many species play important roles in hunting and gathering, as well as territorial defense and protection; whereas females of many species play important roles in gestation, lactation and caregiving 9. Therefore, in order to optimize fitness, males and females would need to respond in different ways to metabolic and psychological stressors. Accordingly, decision-making and related behaviors in response to these external and internal pressures would need to be different. Therefore, different selection pressures due to the evolutionary origins of sex-specific reproductive roles and physiological needs of each sex have shaped physiological metabolic responses, psychological decision-making, and other behavioral responses between males and females in addition to neural circuitry. Consequential sex differences seen in susceptibility to psychiatric disorders such as addiction, eating disorders and mood disorders along with metabolic disorders are also apparent.
Importantly, not all sex differences in physiology and behavior are due to differences in socioeconomic status or cultural experience. Sex chromosomes and sex hormones contribute to sex-specific brain differentiation and brain activation during development and adulthood (see Section 2), and distinct sex hormone actions between the sexes play critical roles in the CNS reward system (see Section 3). These sex differences suggest underlying dissimilarities in the brain reward circuitry 26,33. Specifically, dysfunction of reward circuitry is heavily implicated in addiction to food and drugs. The potential mechanisms for sex differences in addiction process and motivational behavior are not well understood. The initiative undertaken by the U.S. National Institutes of Health (NIH) to take sex differences into account in biomedical research is relatively recent 34. Uniform investigations into understanding the sex differences in reward circuitry has yet to take place, particularly for mechanistic molecular studies regarding processes involved in CNS neurotransmission.
1.4. Aim and focus of this chapter
The sex hormones estrogens along with their action are central to physiological regulation and pathological processes during health and diseases in both sexes. This includes behavioral responses involving the CNS reward system, tested using both animal and human models 35. We aim to provide the readers with an overview of current knowledge surrounding sex differences in neural circuits of the reward system involving neurotransmission in the CNS. In this chapter we discuss central estrogenic action in reward-related behavior based on some of the most heavily studied brain regions and neural pathways, involving various neurotransmitters, with some directly but others indirectly activated by estrogens. It is important to note that many other neurotransmitters have been implicated in sex differences, but are beyond the scope of this chapter. Additionally, sex hormones androgens also play vital roles in regulating the reward system but are outside the current scope of focus.
In this chapter, we first introduce the interconnected circuitry including brain structures, neural pathways, and neurotransmitters. We review the studies that have investigated roles of estrogens and their receptors in the regulation of activities and functions of neural pathways involved in reward process. We then discuss current knowledge and questions about estrogenic actions in these pathways, and how these actions are involved in the regulation of reward, focusing on different brain regions and pathways of the reward circuits, involving various neurotransmitters, some directly but others indirectly activated by estrogens.
Although sex dimorphism is known for some circuits, it remains unknown in many other brain regions and pathways. The recent NIH policy promotes studies of animals and cells from both sexes and requests researchers to consider sex as a biological variable 34. Uniform practices in studying sex differences is not always followed, however. Many times researchers may include both males and females in their studies without actually taking sex differences into account. Nevertheless, we can speculate that more sex differences will be reported as more uniform investigations utilizing both males and females in biomedical research take place. Such sex differences could be anatomical in structure and morphology, or functional sex differences due to different modulation of same anatomical structure by sex hormones. This review highlights the gaps in the literature due to lack of examining sex differences and focuses on the effects of sex hormone estrogens on reward-related brain functioning. In light of vulnerability to mental disorders, such as mood disorders, eating disorders, and substance use disorders among females, future studies should try to understand these sex differences.
2. CNS NEUROTRANSMISSION INVOLVED IN REWARD CIRCUITRY
2.1. Structurally interconnected circuitry
Recent studies highlight how brain regions and pathways traditionally studied in terms of discrete functions are currently known as interconnected circuits. For example, connections between metabolic, reward, emotional, and behavioral circuits are discussed with implications for comorbidity witnessed in mood and metabolic disorders 36–41. Similarly, therapeutic implications for a better understanding of eating disorders and associated obesity, either hedonic or homeostatic obesity from neural circuit perspectives, has been observed 36,42–45. Although it is important to understand difference between “hedonic” versus “homeostatic”, definitions are not as discrete as originally implied from the literature; as neural pathways of hedonic and homeostatic regulations are interconnected and depend on one another for normal functioning 42.
While it is clear that scientific community is developing continuous advances in its neurobiological outlook, the one critical component missed is incorporation of how sex factors into the equation. This would be seemingly critical as fundamental sex differences exist in psychiatric disorders such as mood disorders, drug addiction, and eating disorders, as well as associated obesity and metabolic disorders 3,25. It is important to investigate how the CNS reward system is modulated and how reward is shaped by sex differences. One of the critical places to examine sex difference is from the angle of sex hormones, since sex hormones contribute most strikingly to reported sex differences found in physiology, behavior, and pathology 46. Additionally, estrogens play critical roles in neurobiology of feeding and reward regulation in mammals. This is seen in significant increases in food intake and body weight accompanied by behavioral changes of females when endogenous estrogens have been depleted. Sex hormones estrogens and their effects via estrogen receptors (ERs) are discussed in details in Section 3 of this chapter. This line of research that investigates physiological and behavioral events, and cellular and molecular mechanisms of estrogens would aid identifying sex-specific therapeutic targets into psychological and metabolic disorders. The more we understand about underlying sex differences in the CNS neurotransmission and the reward system, the more available sex-specific therapeutics for epidemics such as suicidal attempts and substance abuse will become.
2.2. Human and animal models
Due to advances in imaging technology such as positron-emission tomography (PET) and functional magnetic resonance imaging (fMRI), researchers are able to gain understanding from human studies. An important feature of functional neuroimaging studies is that one can view all changed brain regions simultaneously in human subjects. For example, all activated brain regions can be identified concurrently when testing subjects with hedonic stimuli under different metabolic states. This provides a better understanding of potential interaction among regions as witnessed in the comorbidity observed between mood and metabolic dysregulation. Notably, some human functional neuroimaging studies have demonstrated that, with various types of natural and non-natural reward stimuli, the brain regions involved in reward circuits of men and women are differentially activated 47–49. These studies have begun to provide neural mechanisms supporting sex differences in reward response and related behavior. Specifically, obese subjects typically display greater differential activation in brain reward regions in response to pictures of high versus low energy-dense foods. It is clear that increased hedonic evaluation of foods may contribute to pathophysiology of obesity 50. In addition, pictures of food stimuli elicit greater activation in brain areas related to planning and execution behaviors in men and stronger activation in areas related to cognitive and affective processes in women 51. As mentioned in above Section 1.3 that sex-specific reproductive roles and selection pressures may affect how reward stimuli are perceived and processed between males and females. Activation of brain areas in a sex-specific manner 51 would lead to corresponding behavioral responses such as hunting and defending in men while caregiving in women 9. Other influences such as hormonal milieu, ethnicity, and societal variables need to be taken into account in human imaging studies, offering detailed insights about how much each factor contributes to sex differences in brain circuit activities 47.
There are noteworthy limitations to human studies. First, brain imaging studies indirectly measure neuronal activity based on detection of cerebral glucose metabolism using PET or detection of cerebral blood flow using fMRI. Second, there are many simultaneous factors related to cognition, mood, arousal, memory, experience, etc., that may affect brain responses, which are difficult to control in an experimental design, especially between-subjects and within-subject designs. Third, human imaging studies identify brain regions with changed activity and offer functional understanding, but not mechanistic understanding at the biochemical, cellular or molecular level. For example, changed neural activity or blood flow could be due to either excitation or inhibition, either of which can be caused by different neurotransmitters. Thus, findings from human imaging studies are limited to identifying association, instead of cause-and-effect mechanisms. Fourth, human studies are limited to minor stimuli or low dosages of drugs that may not be comparable to the levels of patients or addicts. Nevertheless, findings from human imaging studies suggest regions with changed activity by reward stimuli, which is different between the sexes.
Due to the similarities of the CNS reward circuitry involving neurotransmitters between humans and animals, most of the current structural and molecular understanding of neural circuits controlling reward and related motivational behavior have come from studies utilizing animal models 6. While dysregulation of neurotransmission of the CNS reward system and related behavior have been shown in a number of animal species in the laboratory setting, they are most commonly investigated in non-human primates and laboratory rodents. For example, human infants, non-human primates, and laboratory rats all share similar hedonic and aversive facial expressions to taste, and the closer the species are to each other phylogenetically, the more similarities can be found 52–54. Specifically, taste reaction to salty sodium chloride solution by infants is less aversive when infants have previously been sodium deficient 55; similar findings are also found in rats 54. Furthermore, “liking” of a stimulus can be enhanced by a physiological state of depletion (i.e., being hungry) or be suppressed by caloric satiety in both rodents and humans 54. In addition, the female estrous cycle with differing levels of estrogens and progesterone are similar in rodents and humans 56. Ovariectomy (OVX) model of removal of the ovaries reduces endogenous circulating estrogens and estrogen signaling, and is commonly used in combination with exogenous estrogen replacement to test organizational versus activational sex differences (see Section 2.4). Thus, animal studies offer benefits to understanding human diseases. Moreover, rodent models may provide us an architype of molecular mechanisms without any influence of gender pressure from society. Indeed, sex differences seen in reward circuit malfunctions such as addiction are similar between humans and rodents. It is noteworthy that fruit fly Drosophila melanogaster is a commonly used model organism that is especially beneficial for understanding genetic components of sex differences without confounding hormonal influence; whereas transgenic mouse models have offered insights on sex chromosome contributions versus activational effects of sex hormones.
2.3. More studies including female subjects and studying sex differences are needed
Sex differences exist in many aspects, including morphological differences of neural structures and activational differences of neurotransmitters 57, which could lead to sex differences in a diverse range of physiology and behavior. While we are discovering more about how sex hormones and different phases of the estrous cycles in females differentially affect reward circuitry 58–61, our current understanding in the reward system at a molecular level is mostly limited to the studies that include male subjects only. Such inadequate knowledge does not answer questions of sex differences, and therapeutic approaches based on these incomplete research findings that have solely used male subjects are likely not relevant or beneficial to females. We did a PubMed search on June 12, 2018 using (reward OR motivation OR addiction OR mood disorder OR eating disorder). The search returned 439,489 hits. When we searched (sex difference OR gender difference) AND (reward OR motivation OR addiction OR mood disorder OR eating disorder), it returned 8,580 hits, which implies that only 1.95% of the publications of current literature has considered sex or gender differences. This leaves one to wonder if the most current understanding of physiological changes and molecular mechanisms underlying the CNS reward system and related disorders is not an incredibly accurate representation, with most of potential mechanisms controlling sex differences seen in physiology and behavior remaining unknown.
2.4. Sex differences in the CNS regulated by sex hormones
2.4.1. Organizational and activational effects of estrogens
Gonadal steroid hormones play important roles in organizing brain structures that control sexually dimorphic neuroendocrine responses and behaviors during critical periods of development. Organizational effects by gonadal steroid hormones are relatively permanent effects 62. Brain circuitry is masculinized or feminized by sex hormones during sexual differentiation that occur during early development stage, which are between week 10 and 20 of pregnancy in humans or from the end of embryonic period through the first postnatal day in rodents, when the surge in androgen secretion by the testes causes early masculinization of male brains 63. Androgens are aromatized to estrogens that masculinize the CNS neural structures and pathways 64 via its organizational effects on some of the components of brain circuitry during the developmental stage. Although it is known that in rodents, masculinization of male brains requires aromatization of androgens to estradiol via aromatase, an enzyme that catalyzes the last step of estrogen synthesis, whether this is the same in humans is unclear. Rodent female developing brains are protected from masculinization by α-fetoprotein that binds to maternal estrogens to form a complex that does not cross the placenta 65, while human female developing brains may be protected by sex hormone-binding globulin 66. In contrast to the permanent, organizational sex-differentiating effects of gonadal hormones that occur early in development, activational effects occur during reproductive life stages. The activational effects modulate activity of brain circuitry and are often reversible. These activational effects work on anatomic structures that have been developed during sexual differentiation in order to differentially modulate brain circuitry between the sexes 62. Sex differences seen in the CNS neurotransmission can be due to organizational and/or activational effects of sex hormones.
2.4.2. Effects of estrogens on tissues and cells
The majority of sex hormones are synthesized in periphery from the gonads. In females, ovarian estrogens are synthesized from the substrate cholesterol via a series of biochemical reactions that are part of the steroidogenic pathways predominantly occurring in the ovaries. In males, relatively small amount of estrogens is produced by Leydig and germ cells of the testis 67. Some sex hormones, termed “neuroestradiol”, are made in the brain from aromatase-expressing neuronal cells that aromatize androgens into estrogens 68. Estrogens affect a wide range of physiological and behavioral functions.
In females, ovarian estrogens play important roles in regulating female secondary sexual characteristics and reproduction 69. Additionally, peripheral estrogens are synthesized at multiple sites throughout the body including the liver, adrenal glands, and adipose tissues 70, where estrogens carry out localized effects to regulate various processes unrelated to reproduction 71. Furthermore, estrogens exert regulatory action in a variety of tissues that do not secrete estrogens, including tissues in the nervous, cardiovascular, and immune systems, as well as at the breast, uterus, and bone 72–76. In the hypothalamus of the CNS, estrogens have been extensively studied for their roles in regulating sexual behavior, release of gonadotropins and prolactin, and regulation of stress response 77. Furthermore, neuroprotective effects of estrogens in neurodegenerative diseases have been demonstrated in cortical and subcortical nuclei within extra-hypothalamic regions 78, working through ERs, to contribute to lower prevalence of Parkinson Disease in females than in males 79. In general, estrogens are critical hormones that have profound effects on physiology and behavior via regulating mood, emotion, mental states, cognition, memory and cognition 80,81.
Widespread distributions of aromatase-expressing neural cell bodies and fibers have been reported in male and female mouse brains 82. Additionally, aromatase-expressing neural cells are found at the median eminence of the hypothalamus in rats 83. Thus, CNS aromatase signaling that converts androgens to neuroestradiol is common in rodents. Neuroestradiol and ovarian estradiol may have complicated interactions. Neonate ovaries are quiescent and testes produce a surge of testosterone, which is converted into estrogens by aromatase-expressing neural cells in the brain 68. Aromatized estrogens are responsible for the masculinization of neuronal pathways, via differentiation of aromatase-expressing neurons and subsequent arborizations in male brains, which leads to male-specific territorial behavior 64. Within the first ten postnatal days of mice, androgens are important for masculinization of brain myelin, as androgens change arborization and synapses 84. Importantly, a greater aromatase signaling has been reported in brains of male mice than female mice 82. Using male and female mice in which enhanced green fluorescent protein (EGFP) is transcribed following physiological activation of cytochrome P450 family 19 A1 gene, a gene that encode aromatase, aromatase expression indicated by EGFP-positive cell bodies is found in many brain regions, with the densest distribution in the bed nucleus of the stria terminalis and medial amygdala, and less dense distribution in the olfactory tubercle, medial amygdaloid nucleus and medial preoptic area 82. There is an apparent sex difference in the distribution of aromatase expression, with the density of EGFP-positive cell bodies and fibers being less in the bed nucleus and medial amygdala of female mice than male mice, implying that autocrine and paracrine effects of estrogens in the brain are more prominent in males than females.
Growing evidence have indicated that local neural origin estrogens influence many brain regions to modulate brain development and behavior. Whether or not neuroestradiol interacts with secretion and activity of neurotransmitters to impact reward circuitry is not well understood, but is highly possible. For example, estrogens are able to increase the release of neurotrophic factors from brain glia cells, which would affect plasticity of neural circuit 85. Another example is that, during puberty, sex hormones continue to stimulate cellular neurogenesis in the anteroventral periventricular nucleus of the hypothalamus and the medial amygdala, along with increasing genesis of astrocytes in the medial amygdala, to maintain sex differences established from perinatal period 86.
It is clear that sex hormones lead to sex differences in brain structures, but this does not mean that structural differences could cause significant functional differences in behaviors between the sexes. De Varies 87 has proposed the dual-function hypothesis that, although it is possible that permanent sex differences in brain structure can develop, functional and behavioral differences may be compensated via modifying levels of sex hormones or gene expressions 87. Therefore, even if behavioral or functional phenotypes do not show an overall sex difference, underlying mechanisms may still differ between the two sexes. Diverse sex-specific signaling pathways may have opposite effects and thus abrogate sex differences, leading to sexual equivalence of overt phenotypes 87. Sex hormones, sex chromosomes, and environmental factors that function as epigenetic factors all contribute to establish sex differences in the brain and behavior 88,89. Many underlying molecular mechanisms controlling sex divergences in physiology and behaviors and sexual differentiation of the brain remain unknown. For example, it is unclear if sex steroid hormones promote neurogenesis, cell differentiation, migration, and death leading to sex differences in the brain and behavior through direct and/or indirect pathways, and the corresponding molecular mechanisms remain unknown 90.
2.4.3. Actions of estrogens via ERs
An organism’s responses to estrogens are the result of a complex interaction between genomic and non-genomic estrogenic signaling (Fig. 1). Human estrogens comprise a group of structurally related steroid molecules, including estradiol, estrone and estriol, which are the most important regulators and probably the best-characterized steroid hormones of female and male reproductive systems. Estrogenic cellular responses are mediated by a number of different subtypes of nuclear and membrane ERs that initiate a complex array of cellular events upon binding to estrogens (Fig. 1). Lipophilic estrogens can pass across cell membrane to enter cells 91 but also can accumulate within cell membrane 92. Thus estrogenic responses are divided into two broad categories. One is a relatively slower, genomic response that is characterized by changes in gene transcription and occurs on a time frame of hours to days after estrogens bind to their classical nuclear ERα and ERβ. The other one induces rapid, non-genomic signaling events via cytosolic pathways involving production of second messengers and activation of intracellular signaling proteins that occur within seconds to minutes of cell stimulation after estrogens bind to non-classical membrane-associated ERs. The genomic mechanisms are relatively well characterized, while the non-genomic estrogenic signaling is less understood and is beginning to be explored.
Figure 1:
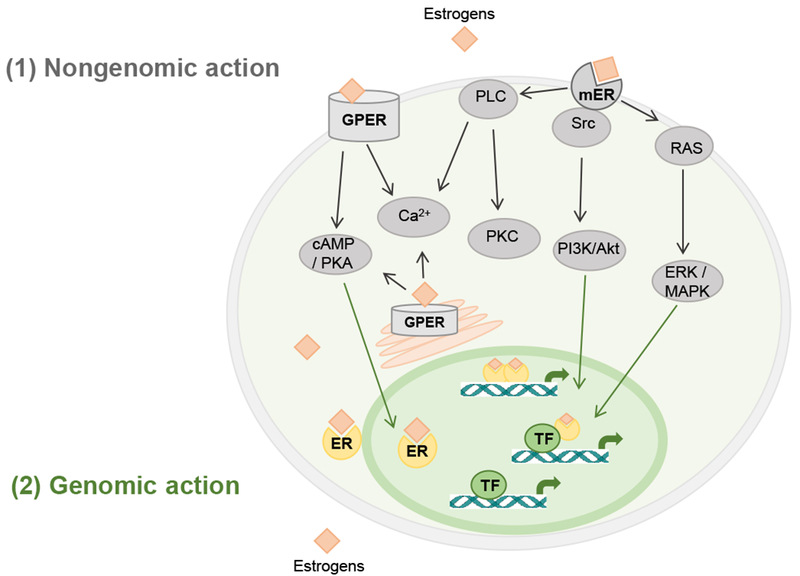
Schematic overview of (1) estrogen-mediated nongenomic signaling pathways via G protein-coupled estrogen receptors and membrane subpopulation of estrogen receptors, and (2) genomic signaling pathways via nuclear estrogen receptors in neural cells.
Akt: protein kinase B; ER: estrogen receptor; ERK: extracellular-regulated kinase; GPER: G protein-coupled estrogen receptor; MAPK: mitogen-activated protein kinase; PI3K: phosphoinositide-3 kinase; PKA: protein kinase A; PKC: protein kinase C; PLC: phospholipase C; RAS: RAS protein; Src: Src kinase; TF: transcription factor.
Estrogen genomic actions via nuclear ERs
Two nuclear ER genes have been identified at distinct chromosomes 93–96. ERα, the first described nuclear ER, has been characterized with specific binding activity using extracts of rat uterus and vagina tissues 97, cloned 96, had its DNA sequenced 95, and its ligand-binding domain crystal structure determined 98. Later ERβ has also been cloned and sequenced 94. Both ERα and ERβ belong to the nuclear hormone receptor family that are presented primarily inside nucleus and are complexed with chaperones, and function as ligand-activated transcription factors. Briefly, binding of estrogens to nuclear ERs in the cytosol and in the nuclei of target cells form estrogen-ER complexes that lead to receptor conformational changes, chaperone dissociation from ER inhibitory protein complexes, and dimerization of the receptors. This is followed by receptor translocation to the nucleus to bind to estrogen responsive elements (ERE) on promoters of hormonally regulated genes, which further recruits co-activators or co-repressors that function as transcription factors, and leads to target gene transcription or alteration in the rate of gene expression 99–101. Ultimately estrogen genomic actions control cellular response, cell growth, cell differentiation, and many other functions 102. Estrogen-ER complex also could modulate gene expression by a non-ERE-mediated mechanism in which ERα and ERβ dimers bind to non-ERE promoter sites of the DNA and interact with other transcription factors through protein-protein interaction, to regulate estrogenic genomic actions. Furthermore, ERs may elicit ligand-independent transcriptional responses and interact with other transcription factors to regulate gene expression 103. In summary, estrogens mediate long-lasting effects via multiple genomic mechanisms in estrogen-regulated tissues and cells.
The most abundant and potent estrogens that binds to nuclear ERs is estradiol. Other natural forms of estrogens such as estrone and estriol 104, along with some environmental and food compounds such as phytoestrogens 105, are also capable of binding nuclear ERs but with much lower affinity than estradiol. Pharmacological reagents are available for investigating estrogenic genomic action following activation of nuclear ERs, including selective ER modulators such as raloxifene and tamoxifen, ERα selective agonist 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT) with 400-fold higher affinity for ERα than ERβ 106, and ERβ selective agonists 2,3-bis(4-Hydroxyphenyl)-propionitrile (DPN) and WAY with 70-fold higher affinity for ERβ than ERα 107.
It is noteworthy that, ERα and ERβ are highly homologous in their DNA- and ligand-binding domains, but lack homology in their transcriptional activation domains 103,108. Additionally, both ERα and ERβ are highly expressed in reproductive tissues and in the CNS, but a greater density of ERα than ERβ is expressed in metabolic tissues such as the kidney, bone, white adipose tissue, and liver, whereas a greater density of ERβ than ERα is expressed in the lung, gastrointestinal tract, bladder, and hematopoietic cells 109,110. Within the CNS, both ERα and ERβ are expressed at the bed nucleus of the stria terminalis, amygdala, medial preoptic nucleus, and locus coeruleus. Differences in ER expression exist among species. For example, in rats, a small number of ERα and no ERβ are expressed in serotonergic neurons at the dorsal raphe nucleus (DRN) in each sex; whereas in mice, both ERα and ERβ are highly expressed in DRN serotonergic neurons in both sexes 111. ERβ expression is less intense than ERα in the suprachiasmatic region, supraoptic nucleus, arcuate nucleus, and amygdala 112. In macaques, ERβ mRNA has been detected using PCR and in situ hybridization in brain regions that lack ERα. Also in macaques, ERβ is highly expressed in the preoptic area, paraventricular nucleus (PVN), and ventromedial nucleus (VMN) of the hypothalamus; the substantia nigra (SN), caudal linear, DRN, and pontine nuclei of the midbrain limbic regions; the dentate gyrus, CA1, CA2, CA3, CA4, and prosubiculum/subiculum areas of the hippocampus; and the temporal lobe 112–114. The presence of ERβ mRNA in some monkey brain regions that lack ERα 112 would help to clarify molecular mechanisms by which estrogens act to regulate physiology, behavior and related disorders, such as hormone secretion, cognition, neuroprotection, reward behavior, and related neurological and mental disorders.
These differences in ERα and ERβ expression suggest differential physiological functions between ERα and ERβ, which is supported by characterizing ERα knockout (KO) and ERβ KO mice 115. Indeed, male and female ERα KO mice manifest metabolic dysregulation with diabetogenic and obese phenotypes 116, whereas ERβ KO mice appear to have improved glucose regulation 117, supporting that both ERα and ERβ mediate important estrogenic action in metabolism but with opposite effects 118. Additionally, dominant expression of ERβ in some brain regions suggests potential involvement of ERβ in regulation of anxiety and stress responses 108. Importantly, studies using ERβ KO mice have demonstrated behavior related to increased levels of depression, anxiety, stress response, and aggression in ERβ KO mice compared to their wildtype counterparts 67,119–123. Therefore, studies using transgenic mouse models have indicated that estrogens have differential effects via acting on respective ERα and ERβ.
Although the focus of this chapter is estrogenic effects, both estrogens and androgens contribute to sex differences in the CNS neurotransmission. Many published studies have demonstrated that differential expression of sex hormone receptors in the brain could lead to sex distinct behavior and biological responses. ERs and androgen receptors (ARs) are expressed in various areas of the brain, including the hypothalamus and the limbic system across species including rodents 111,124, birds 125, domestic species such as ewes and rams 126, nonhuman primates 112, and humans 127. Additionally, different expression between ERs and ARs exists, which could contribute to sex different physiology and behavior. For example, in male mice, ERβ, but not AR, is expressed on DA projections from the VTA to the ventral caudate and to the basolateral amygdala; whereas AR, but not ERβ, is expressed on DA projections from VTA to NAc and the centromedial amygdala 128,129. These studies suggest that estrogens and androgens may have different abilities to carry out differential but coordinated actions on the mesolimbic dopaminergic system in male mice. Furthermore, ER- and AR-positive cells are co-expressed with aromatase-immunoreactive cells in the bed nucleus, lateral septum, medial amygdala and hypothalamus, and often appear to be surrounded by aromatase-positive nerve fibers and terminals 82, suggesting that locally synthesized neuroestradiol in the brain could mediate biological effects by activating ERs and ARs.
Estrogen non-genomic actions via membrane ERs
In addition to estrogen genomic action via nuclear receptors that typically take several hours for the effects to be manifested due to the time needed for gene transcription and protein translation to complete 130, a growing body of evidence supports non-genomic action of estrogens that elicits rapid signal transduction events within minutes 131,132. Such rapid effects cannot be attributed to genomic effects involving transcriptional mechanism and protein biosynthesis that requires a comparably long time from minutes to hours, and thus have been characterized as non-genomic action of membrane receptors that requires only milliseconds to seconds 72,133–137. Estrogen-binding membrane receptors include G-protein coupled ER (GPER) including GPR30 138–142 and Gq-mER 143–146, membrane subpopulation of ERs (mERα/β) 141,147, and ER-X 148,149. Membrane ERs are expressed in various tissues and cells, including reproductive tissues, neurons of the central and peripheral nervous systems, intestinal tissue, pancreatic islets, adipose tissues, skeletal muscle cells, cardiac muscle cells, and inflammatory cells. Different types of membrane ERs have been reviewed in great details previously 35, and we briefly describe the major processes below.
GPERs are highly expressed in many brain regions such as the hypothalamus, pituitary, hippocampus, brainstem, cortex, and striatum 150. Interestingly, sex difference in GPER expression has been reported in the brain 151, with a much higher expression in women than in men 152. Within the CNS, estrogens act on membrane ERs at the striatum to regulate DA release 153. Unlike most of other G protein-coupled receptors, GPER is also localized in the membrane of endoplasmic reticulum. Additionally, membrane ERs could couple to other membrane receptors. For example, estrogens can activate membrane ERs coupled to metabotropic glutamate receptors and activate second messenger signaling at the nucleus accumbens (NAc), a potential mechanism to activate female motivational circuit that is responsible for addiction and substance abuse 154. Estrogens bind to membrane ERs and rapidly mediate multiple intracellular pathways involving various types of second messengers and protein kinases associated with G protein signaling (Fig. 1) 140–142,155. Signaling mechanisms of membrane ERs include rapid activation of phospholipase C, increases in intracellular concentration of Ca2+, and protein kinase C 156, production of cAMP and activation of associated protein kinase A 157,158, activation of Src kinase and subsequent phosphoinositide-3 kinase (PI3K)/Akt, and RAS/mitogen-activated protein kinase (MAPK) activation 102.
Selective pharmacological reagents, such as GPER agonists including G1 159,160 and antagonists including G15 161 with high affinity and high selectivity for GPERs, are available for elucidating estrogenic non-genomic action following binding of membrane ERs. It is noteworthy that tamoxifen and raloxifene can bind to and activate both nuclear ERs and GPERs 107,141,162,163. Besides selective agonists and antagonists, a few genetic mouse models lacking GPER gene have been used to advance our understanding in the physiological roles of GPERs 164–167.
The research field of estrogenic non-genomic action via membrane ERs has received increasing attention during the recent decades. A PubMed search on June 8, 2018 with the keywords “estrogen” and “non-genomic” yielded 799 published papers since 1979, with 708 (88.61%) of these papers being published since 2000 in the current millennium. This focused area of understanding estrogen signaling has seen a surge of interest and represents one of the fastest emerging areas in the field of estrogen research.
3. EFFECTS OF ESTROGENS ON CNS NEURTRANSMISSION
3.1. Overview of effects of estrogens on neurotransmission-mediated CNS circuitry
Traditional neurotransmitters such as DA, glutamate, GABA, and 5HT; and non-traditional neurotransmitter such as endocannabinoids, along with their receptors and transporters, are expressed in different brain regions that are interconnected parts of the reward circuits (Fig. 2), to regulate mood and reward-related behavior. The same neurotransmitters can be used in multiple pathways of the reward system. For example, DA can be used in mesolimbic pathway and mesocortical pathway (see Section 3.2). Additionally, one reward pathway can be activated by different types of reward stimuli (e.g., DA and 5HT neurotransmission are activated by palatable food reward and drug reward), while multiple pathways involving in different neurotransmitters can be activated by one same type of reward stimulus (i.e. cross-sensitization; Section 1.2). For example, estrogens modulate responses to reward stimuli via regulation of multiple aspects and components of DA and 5HT systems in the mesolimbic nuclei and the hypothalamus in response to drug reward stimuli 168. The mechanism by which estrogens influence neurotransmission could be via nuclear and membrane-associated ERs. For example, estradiol treatment in OVX rats decreases mRNA levels of ERα in the amygdala and the hypothalamus and decreases mRNA levels of ERβ in amygdala; increases mRNA levels of DA receptors D1 in the hypothalamus, D2 in the midbrain, and D3 in the ventral tegmental area (VTA), while decreases D3 receptor mRNA levels in the midbrain; and increases 5HT2C receptor mRNA levels in the midbrain and the hypothalamus 168. Therefore, estrogens regulate expression of genes for various specific subtypes of DA receptors and 5HT receptors in a region-specific manner, to contribute to behavioral responses to changes of internal and external environment. In this section, we discuss sex differences and focus on the action of estrogens in interconnected CNS reward circuits regulated by traditional and nontraditional neurotransmitters.
Figure 2:
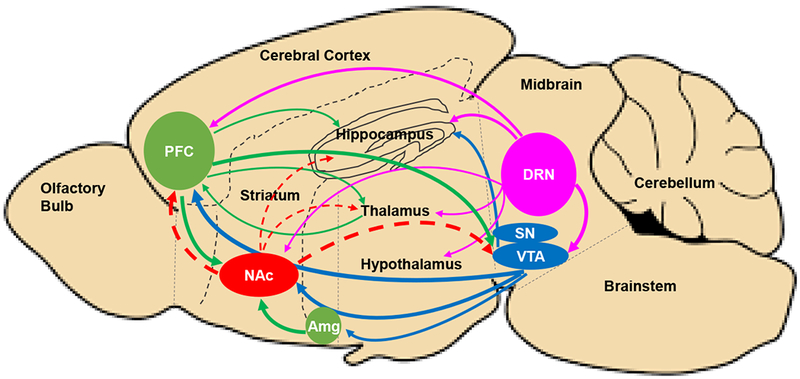
Schematic image of a sagittal rodent brain section illustrating common, interconnected networks of neural circuits among species, involving classic neurotransmitters, including dopamine (blue), serotonin (pink), glutamate (green), and GABA (red), in reward, addiction, and motivation. Pathways with greater activity in females or enhanced by estrogens are indicated using solid lines. Pathways with lower activity in females or suppressed by estrogens are indicated using dashed lines. For clarity only major projections of most prominent neurotransmitter represented within each brain region are shown.
Amg: Amygdala; DRN: dorsal raphe nucleus; NAc: nucleus accumbens; PFC: prefrontal cortex; SN: substantia nigra; VTA: ventral tegmental area.
3.2. Sex differences and modulation of dopamine pathway by estrogens
3.2.1. Dopaminergic pathway
DA is a critical neurotransmitter implicated in reward and motivation. A large amount of human and animal studies support that many rewarding stimuli, including palatable foods and various types of substance, regardless of their dissimilar action mechanisms, converge on a shared dopaminergic pathway. Specifically, dopaminergic cell bodies originate in the VTA of the midbrain, and dopaminergic axons project either directly or indirectly to various brain regions, predominantly terminating at the NAc in the ventral striatum, termed the mesolimbic VTA-NAc pathway, and less predominantly projecting to amygdala, bed nucleus of stria terminalis, hippocampus, lateral hypothalamus, and the lateral septal area 169. DA activity in the mesolimbic projections from the VTA to NAc, a main dopaminergic pathway, is implicated in decision making, reward, motivation, cognition, prediction, validation and drug addiction 169. While the mesolimbic dopaminergic pathway is the major one involved in the reward pathway, other neurotransmitters including 5HT, norepinephrine, endogenous opioids, GABA, and glutamate transmission could also play critical roles 169. Activation of dopaminergic input from the VTA to NAc enhances DA signaling and neurotransmission 170. Specifically, presentation of a reward induces DA release from the VTA into the NAc and increases DA receptor binding affinity, both of which induce associated learning processes and multiple aspects of motivational behavior to obtain rewards 171.
DA signaling is modulated by sex hormones. Estrogens, the most important hormones affecting dopamine neurotransmission, account for many sex differences in the reward system and related behavior, and have been heavily studied in both animals and humans. DA has been tested in women as well as female gonadally intact rats and OVX rats with or without estrogen treatment. Below human and animal studies have indicated that estrogens lead to functional variation of the VTA-NAc dopaminergic pathway between the sexes 128, contributing to the sex differences in reward and motivational behavior such as addiction 172.
3.2.2. Sex differences and modulation of dopamine pathway by estrogens - human studies
Advance in imaging technology such as fMRI or PET combined with DA-specific binding has provided a better understanding of the reward circuits in humans. It has been reported that palatable foods simulate more brain activation than low-calorie foods in all groups in human brain imaging studies. Greater activation of DA signaling, especially in regions related to VTA-NAc dopaminergic pathway, has been reported in obese individuals than normal weight individuals 173, implying that greater activation of dopaminergic reward pathway could be a potential mechanism leading to elevated motivational and reward-associated behavior for consuming palatable foods in obese individuals.
Feeding is regulated by an interaction between physiological, homeostatic state and reward value of food. Striatum and orbitofrontal cortex (OFC), a prefrontal cortex (PFC) region in the frontal lobes, are involved in control of food intake that is associated with monetary and food rewards and interacts with circulating satiety signals 44,174. Specifically, ventral striatum is activated by “wanting” while OFC is activated by “liking” of food reward. Physiological hunger increases “wanting” of food, but does not increase “liking” of food. Thus metabolic needs and related satiety signals could change the activity of the CNS reward system to regulate hedonic evaluation of food reward and related motivational behavior. In studies that measure fMRI of adult women with a normal BMI range while performing monetary- or food-related reward during fasted or satiety state, activities of OFC and ventral striatum increase due to receiving money reward and/or food reward 44,174. Such activation of OFC and striatum is influenced by metabolic state, with fasted women having greater activation than satiated women 44,174. Therefore, physiological hunger state sensitizes the CNS reward system to stimulate feeding in healthy women. Contradictory to the activated reward system by fasting in healthy women, the reward systems of women with anorexia nervosa are not activated by physiological hunger 174. The diminished sensitivity of reward response during hunger underpins potential neural mechanisms for why patients with anorexia nervosa are not motivated to eat when fasted.
Activation of the reward circuit to food cues in women depends on phase of the estrous cycle 175, as the activity and sensitivity of neurotransmitters may fluctuate over the course of the female estrous cycles. Van Vugt and Reid have attested that female estrous cycle naturally represents different gonadal hormone environment that changes DA signaling for food and drug reward 175. Even though sex hormone-induced sex differences in reward pathways is widely accepted knowledge, only a few neuroimaging studies on food reward response have actually evaluated reward responses to food stimuli during different phases of the female estrous cycles 58–61. Therefore, there is a need to account for estrous cycle phase as a variable when include women in human imaging studies. Some human studies that have analyzed data based on different phases of the estrous cycles report that, women during luteal phase have less stimulation by amphetamine and cocaine than men; whereas during follicular phase when levels of estrogens are naturally higher than luteal phase, women experience greater stimulation by amphetamine and cocaine than men do 176–178. Thus, this finding suggests that reward stimulation by drugs are related to levels of estrogens.
To summarize, DA neurotransmission sensitivity may fluctuate over the course of the estrous cycle. The potential augmentation of modulatory effects of dopaminergic system by food and drug reward stimuli by estrogens suggest that DA responsiveness would be at its highest during the estradiol-dominated follicular and periovulatory phases of the estrous cycle in women. While results of many human imaging studies may be inconsistent due to factors such as the women estrous cycle not being accounted for, human imaging studies generally indicate greater sensitivity in DA response to reward in women than in men. Women show greater sensitivity to gamble win and loss than men do 179. The mesolimbic responses are more sensitive to reward in women than men, which is dependent on estrogens during various reward stimuli, including monetary 60, appetitive 175, and amphetamine and cocaine 176–178.
3.2.3. Sex differences and modulation of dopamine pathway by estrogens - animal studies
Food and drug reward stimuli have been tested in animal studies. Female rats are more susceptible to palatable food than male rats, with greater expression of neural activation marker Fos in mesocorticolimbic regions of the reward circuits, whereas no sex difference is found in regions of the hypothalamus or amygdala 180, suggesting that increased sensitivity of female rats to palatable food is through “hedonic” mechanism, rather than “homeostatic” mechanisms which is regulated by the regions in the hypothalamus. This study provides initial evidence that palatable foods may be more rewarding to females than to males, possibly due to heightened responsiveness of neural substrates that mediate hedonic and motivational responses to palatable food, which in part, may underlie sex differences in binge eating proneness 180.
It is interesting that estrogens have opposite effects on feeding via hedonic and homeostatic mechanisms. The effects of estrogens on caloric intake is regulated by homeostatic regulation, while the effects of estrogens on macronutrient selection are dependent on activation of the reward pathways. In terms of homeostatic regulation, estrogens suppress caloric intake 181 via activating anorexigenic neurons of the hypothalamus 182. Indeed, in many species caloric intake varies across the female estrous cycles, eating least during periovulatory estrous phase when estrogen levels are high and eating most during diestrus when estrogen levels are low 183. The decrease in feeding during estrus is due to smaller meal sizes and concurrently increases in meal frequency 183. Both meal size and meal frequency are two parameters of spontaneous feeding regulated separately in a homeostatic manner. In terms of hedonic regulation, estrogens are known for enhancing the sensitivity to highly palatable foods and for increasing DA responses of brain reward regions 184,185.
It is noteworthy that selection of macronutrients with various palatability also varies during the estrous cycles. Inconsistent findings in macronutrient selection, however, have been reported in rats, as one study reported increased carbohydrate intake but decreased fat intake during estrus 186, while other studies reported increased fat intake but decreased carbohydrate intake during estrus 187,188, comparing with other phases of the estrous cycle. These inconsistent findings in macronutrient selection observed among different studies could be due to different forms of macronutrients and food properties being tested. It is possible that different sweet and fatty tastes could also contribute to macronutrient selection.
Besides food reward stimuli, females are also more sensitive to drug reward than males. Many drugs such as cocaine and amphetamine produce sex-specific effects on neural activity at various brain regions. Adult female rats are more sensitive to cocaine than adult male rats. For example, there is a greater increase in striatal DA in response to cocaine 189 and amphetamine 190 administration in females than in males. Female rats require more self-administration of cannabinoid 191, cocaine 168,192, and amphetamine 190 than male rats. OVX with reduced endogenous levels of estrogens reduces self-administration rates, and conversely estrogen treatment enhances the hyperactivity induced by cocaine in OVX rats 168, implying the stimulating regulation of drug reward by estrogens.
It is noteworthy that female rats generally show increased neural activity with single cocaine exposure but reduced activity with repeated exposure, while male rats generally show a trend with opposite effects 193. Repeated exposure of drugs could affect various brain circuits involving a number of neurotransmitters modulating reward, learning, memory, emotion, visual process and locomotion in hippocampal, amygdala and midbrain areas.
3.2.4. Underlying mechanisms of modulation of dopamine pathway by estrogens
Effects of estrogens on sexual differentiation
Anatomical differences due to sex differentiation of the brain have been reported in some brain regions of the reward system, such as NAc and medial amygdala, leading to sex-distinct motivational behavior and susceptibility of related disorders. Such sex differentiation is at least partially attributed to brain masculinization by fetal sex hormones testosterone and estradiol. Estrogens that are converted from neonatal testosterone surge may be the cause of down-regulating excitatory synaptic dopaminergic input into the striatal NAc core in adult males comparing to females 194. Specifically, increased synaptic excitability in female rats exists before puberty, which is abolished following neonatoal testosterone and estradiol treatment 194, implying critical roles of sex hormones in sex differentiation of excitatory synaptic input to the NAc core during neonatal period. Long-term increases in tyrosine hydroxylase (TH) in SN and VTA have been reported in neonatal male rats receiving injections of testosterone that is aromatized to estrogens 195. It is noteworthy that sexual dimorphism in VTA-NAc dopaminergic pathway reported in polygynous rodent species may be missing in monogamous species. For example, Campi et al. 196 used immunohistochemical labeling of TH to compare number of dopaminergic neurons in the VTA and used tract tracing to accurately delineate boundaries of the VTA in male and female California mice, a monogamous species. They reported that no sex difference in either volume or number of TH-immunoreactive neurons in the VTA 196.
Compared to adult male rats, adult proestrous female rats have larger spine heads for the spines next to TH-immunoreactive neurons, greater spine density, and greater excitatory input onto medium spiny neurons of striatal region of the NAc core 185,197, suggesting more profound synaptic connectivity, glutamatergic input, and dopaminergic modulation in females than in males. Sex differentiation in the volume and functional connectivity in the medial amygdala has been reported in male and female prepubertal rats at 25-29 days of age 198 and adult rats 199. Male prepubertal rats had about 80% more excitatory synapses than females, implying that sex difference in organization exists in the medial amygdala 198. Adult male rats have larger neuron size than adult female rats that is accounted for by circulating androgen 199, whereas adult males have a greater number of neurons than females that cannot be explained by circulating androgen 200. These findings suggest that while the greater number of neurons in the medial amygdala is an organized sex difference occurring during perinatal period, the larger neuron size and volume of the medial amygdala in males are maintained during adulthood by male sex hormones 200.
Effects of estrogens on DA neurotransmission
DA neurotransmission of the VTA-NAc pathway associated with motivation is modulated by estrogens, which leads to functional differences of the mesolimbic dopaminergic pathway, accounting for many sex differences in the reward process and related behavior reported in human and animal studies 128. In general, elevated circulating levels of estrogens in rodents, either naturally during their estrous cycles or exogenously by estrogen treatment, contribute to elevated dopaminergic signaling. Estrogens affect multiple aspects of dopaminergic neurotransmission both presynaptically and postsynaptically, including (1) DA synthesis, release, and degradation; (2) presynaptic and postsynaptic receptors; and (3) DA transporters that uptake DA from synapse to terminate DA neurotransmission. The mechanisms by which estrogens influence dopaminergic system could be via nuclear and membrane-associated ERs. Pertinent to the effects of estrogens on the CNS reward system, ERs are distributed in dopaminergic pathways involved in reward 129,201. In male mice, dopaminergic projections from the VTA to the ventral caudate express ERβ, while dopaminergic projections to the dorsal caudate do not express ERβ. Dopaminergic projections to the basolateral amygdala also express ERβ 129. Estrogens also act rapidly via membrane-associated ERs on dopaminergic cells in the striatum to affect DA release 153,202.
First, estrogens increase activity of TH and thus DA synthesis in the NAc 203 via acting on nuclear ERs 204 and membrane ER 205,206; induces presynaptic DA release 207 in the striatum 208,209; and decrease DA turnover in the NAc and reduce clearance and degradation of DA so that DA remains at synapse for a longer period 210. Consistent with changes by estrogen treatment, OVX reduces TH immunoreactivity in neurons of the SN and VTA 211, which is restored by estrogen replacement 211. OVX also reduces DA content in the VTA 212, and estrogen replacement increases DA release in NAc as measured by microdialysis in adult female OVX rats 184. The majority of studies that explore sex differences have used rodents. One study shows that dopaminergic neuron densities in the SN are much greater in gonadally intact female nonhuman primates African green monkeys than male and OVX female monkeys 213.
Second, estrogens and testosterone regulate DA receptor density and function. DA receptor density can be measured using receptor autoradiography. Testosterone decreases D1 and D2 receptors in the NAc 214, while estrogens upregulate the density of D1 receptor in the striatum 215. Additionally, naturally elevated estrogen level during luteal phase across the female estrous cycle upregulates D2 receptor at caudate nucleus and putamen 216 and at striatum 217; whereas OVX reduces D2 receptor densities at striatum 217. Therefore, estrogens upregulate D1 and D2 receptors.
D1 receptors are a crucial determinant of risk-taking behavior in probability discounting, defined as decrease in subjective value of a reward as the likelihood of receiving this reward decreases 218,219. Treatment with D1 antagonists, either systemically 220 or locally in NAc or PFC decreases risk-taking in probability discounting 219,221. In contrast, treatment with a D1 agonist increases risk taking in this task 219,221. Adult male rats have a higher density of D1 in the striatum than females, but this difference does not appear until puberty 222. The dependence of striatal D1 density on hormonal environment may explain increased basal level of risk taking in testosterone-treated males. The sex difference and effects of estrogens in D1 receptor density may underlie the tendency for increased risk taking under influence of substances exhibited in males.
Estrogens also upregulate D2 receptor, which is associated with sex differences seen in reward-related behaviors in humans 216,223. Downregulation of D2 receptor is associated with obesity in humans. Human neuroimaging studies using PET and D2 receptor radioligand [C-11]raclopride that assess and compare D2 receptor availability between normal weight and obese individuals have reported reduced striatal D2 receptor availability in obese people 224. Additionally neuroimaging studies have revealed exaggerated responses in motivation and reward neural circuits and emotion regions in response to food images in obese individuals, with greater activations in PFC and limbic regions comparing to healthy weight individuals 225. In rodent studies, comparing to lean rats, obese rats that binge daily on sucrose show greater consumption of palatable sucrose that is resistant to disruption by compulsive-like feeding behavior, an aversive conditioned stimulus, continuously increased release of DA in the NAc 226 and downregulation of striatal D2 receptors 227. Additionally, lentivirus-mediated knockdown of striatal D2 receptors rapidly accelerates the development of addiction-like reward deficits and the onset of compulsive-like food seeking in rats with extended access to palatable high-fat foods 227. Therefore, downregulation of D2 receptors due to elevated DA release is associated with compulsive-like feeding behavior and obesity in both humans and rodents.
Comparing to food reward stimuli, effects of estrogens on DA receptors following drug reward stimuli are more complicated. Febo et al. has reported that, one week of estrogen treatment increases D2/D3 receptor-induced G-protein activation in cingulate cortex, lowers D2/D3 receptor-induced G-protein activation in the VTA of cocaine-sensitized OVX rats, and no difference in striatum NAc between OVX rats with and without estrogen treatment after cocaine administration 228. Thus, cocaine-induced changes in D2/D3 receptor activation and function are regulated by estrogens in a region-specific manner, which could be an underlying mechanism by which estrogens regulate behavioral sensitization to cocaine.
It is notable that, although estrogens have an overall facilitating effect on dopaminergic neurotransmission, both stimulating 207,229 and inhibiting 230,231 effects of estrogens on dopaminergic neurotransmission can be found in the literature. For example, chronic treatment of estradiol at a supraphysiological dose (1 μg twice a day for 2 weeks) reduces DA content in NAc and VTA 232. Such chronic 2-week of estrogen treatment has little effect on D1 and D3 receptor expression in VTA or NAc, but downregulates D2 receptor in dorsal and ventral striatum 233, while 3-week estrogen treatment in OVX rats increases D3 receptor in VTA 168.
Third, another critical player involved in DA neurotransmission is DA transporters. There are some discrepancy regarding to effects of estrogens on DA transporters. For example, estrogens reduce DA transporters in NAc shell to delay the termination of DA neurotransmission 234; while a different study has reported reduced DA transporter expression in NAc in OVX rats 235. DA transporter in the NAc has been reported to be upregulated 236, decreased 231,234, or not changed 237 during proestrus or by estrogen treatment. The discrepancies of estrogenic effects on DA system is not surprising considering inconsistent assays utilized for assessing DA transporters, and could be explained by dissimilar methods of estrogen treatment used in different studies, such as administration mode, dose, duration, and testing time following treatment.
It has been recently shown, in a model of relapse to cocaine, that estrogens have significant effects on extracellular DA levels induced by cocaine challenge in dorsolateral striatum of female rats 238. These results represent a new research line for the role of estrogens in compulsive drug seeking. At the behavioral level, it has been shown that amphetamine administration to OVX rats does not produce place preference behavior 239, a behavioral paradigm widely used in neurobiological studies of addiction to drugs of abuse. In this work, replacement with estradiol or ERβ-selective agonist DPN restores the effect of amphetamine in the place preference behavior test 239. In other behavioral paradigms, it has been observed that administration of estradiol increases locomotor activity and behavioral sensitization induced by cocaine in OVX rats 240. Lastly, in an animal model of cocaine self-administration, one of the most important model for evaluating all stages in addiction, it has been observed that female rats have a higher number of lever responses at low and high doses of cocaine as compared to males 241. In contrast, OVX rats have a lower lever responding than intact females 241. Indeed, OVX induces depressive-like behaviors, such as decrease in sucrose preference and decrease in escape-related behaviors when animals are exposed to drugs of abuse 242, similar as the animals with inhibition of VTA dopaminergic neurons.
To summarize, although some of the literature may be contradictory, most studies have indicated that estrogens enhance DA neurotransmission and are particularly potent in activating DA function in the reward system, which accounts for the sex differences seen in reward-related behaviors.
3.3. Sex differences and modulation of serotonergic pathway by estrogens
3.3.1. Serotonergic pathway
The central 5HT pathway originates from 5HT-producing neurons in the midbrain and hindbrain, including the DRN and medial raphe nuclei of the midbrain. The serotonergic neurons of the DRN project to the PFC and the hippocampus that regulate integrative cognition and memory processes from higher order functions, to the limbic system for arousal control and balancing mood, and to the diencephalic thalamus and the hypothalamus that regulate pituitary hormone secretion, energy homeostasis, controlling eating behavior and mediating satiety, stress, and sexual behavior 243. The serotonergic neurons of the caudal nuclei project to the spinal cord and interact with numerous autonomic and sensory systems. Dysregulation of 5HT neurotransmission would impact cognition and memory, resulting in mood disorders such as depression and anxiety 244, and would disturb emotions related to eating and shaping the hedonic response to food 245,246. 5HT has been identified as a key signal mediating physiological and behavioral functions linked to stress response and feeling of satiety.
The 5HT system is complex. At least seven major families and 15 different subtypes of 5HT receptors, including G-protein coupled 5HT receptors and ligand-gated ion channels, have been characterized for intracellular signal transduction 247. 5HT and its agonists have been used to activate serotonergic pathways in human studies and animal studies that study 5HT neurotransmission as one of the mechanisms linking mood disorder and eating disorders such as anorexia nervosa and bulimia nervosa and 248.
There may be species differences in the effect of estrogens on 5HT neural function. In general, findings from human and animal studies suggest that females have an overall higher level of 5HT in the CNS than males 249 and estrogen treatment increases 5HT levels in the CNS. Both ERα and ERβ are expressed in serotonergic neurons in the hypothalamus, while ERβ but not ERα is expressed in serotonergic neurons at the DRN in guinea pigs 250 and in nonhuman primates 251,252, suggesting that estrogens act via ERβ at the serotonergic neurons in the DRN to regulate gene expression. Changes in the expression of multiple genes by estrogens could be associated with an increase in 5HT neurotransmission.
Serotonergic neurotransmission is dependent on a multitude of processes, including (1) 5HT synthesis regulated by tryptophan hydroxylase (TPH), the rate-limiting enzyme in 5HT biosynthesis; (2) 5HT signaling via 5HT2A and 2C receptors 253; (3) 5HT release from the firing neurons whose activity is inhibited by 5HT1A autoreceptor; receptor expression and binding; (4) serotonin transporter (SERT) for 5HT reuptake, predominantly located presynaptically at nerve terminals and also present on cell bodies and dendrites 254. SERT moves 5HT from the synaptic cleft into the presynaptic serotonergic neuron to be degraded by monoamine oxidase (MAO). Thus SERT reduces 5HT concentration at synaptic cleft and terminates 5HT neurotransmission 255. Dysfunction of SERT-mediated 5HT uptake has been implicated in depression and anxiety disorders. SERT is the site of action of widely used antidepressants known as selective serotonin reuptake inhibitors (SSRIs). By altering the expression or activity of SERT, estrogens could alter serotonergic neurotransmission. SSRIs block SERT for 5HT reuptake to increase 5HT levels in synaptic cleft, usually ameliorate depressive symptoms in humans, suggesting that serotonergic system is critical in psychiatric illnesses such as depression. Additionally, 5HT projection of GABA neurons could either stimulate or inhibit release of GABA in different brain regions. Thus unravelling sex differences in serotonergic action and how it is influenced by estrogens could be complex.
3.3.2. Sex differences and modulation of 5HT pathway by estrogens - human studies
While the prevalence of depression in women is much higher than in men, this sex difference is manifested in women of reproductive ages 256. Mood disorders and eating disorders associated with reproduction in women are of a varied nature, as precipitated depressive symptoms could be due to elevated cyclic levels of sex hormones in some women but due to loss of sex hormones in others 252. The roles of CNS 5HT in the severity of depression and in the loss of emotional wellbeing are examined using a combined PET and 5HT transporter radiotracer with fMRI in men and women 257. Men present a strengthened connectivity to the OFC, while women present a strengthened projection to the ventral striatum. Both brain regions are involved in mediating emotional response to reward stimuli such as palatable food. This study suggests that 5HT connections to other brain regions of the reward circuitry is different between men and women. In women, severity of depression is positively correlated with BMI and with the activity in ventral striatum 257, suggesting that increased body mass may convey to other mood aspects in women.
3.3.3. Sex differences and modulation of 5HT pathway by estrogens - animal studies
Besides acting on reward pathway, serotonergic neurotransmission regulates feeding in animals 258, but sex differences in serotonergic control of feeding is not conclusive due to inconsistent literature. In one study, eating-inhibitory effect of a SSRI fenfluramine is more evident in intact, cycling rats during estrus than during diestrus 259, and more evident in female rats than in male rats, indicating that estrogens upregulate 5HT-mediated feeding inhibition. In another study, no difference in eating responses to 5HT agonists and an SSRI fluoxetine between female and male rats 260. Furthermore, one study has showed that estradiol treatment in OVX rats increases effects of fenfluramine in feeding suppression 261, but another study does not show any difference in the effect of chronic fenfluramine treatment between OVX rats with and without estradiol treatment 262. Such discrepancy could be due to different duration and dosages of estrogen treatments, and different aspects of 5HT neurotransmission targeted by different chemicals.
3.3.4. Underlying mechanisms of modulation of 5HT pathway by estrogens
First, estrogens upregulate expression and activity of TPH to increase 5HT biosynthesis. Estrogen administration has been found to increase TPH mRNA level 263. In OVX guinea pigs, estrogen treatment alone increases the protein expression of TPH at the DRN 250. Similarly, in spayed nonhuman primate macaques, estrogen treatment increases TPH mRNA level 263 and protein level 264 compared to control-treated and progesterone-treated spayed macaques, suggesting that estrogens induce expression of TPH. Interestingly, while estrogen treatment alone increases levels of TPH mRNA and TPH protein, it does not affect 5HT content; rather, combined treatment of estrogen and progesterone increases 5HT levels at the DRN and at the projected medial basal hypothalamus in guinea pigs 250. Thus there is a discrepancy in the sex hormone regulation of TPH mRNA and TPH protein levels versus 5HT production in estrogen-treated animals, possibly due to activity of TPH that is regulated by phosphorylation by protein kinase A 265. Only phosphorylated TPH has catalytic activity 266. It is possible that progesterone treatment increases protein kinase A expression and activity of TPH. Based on these information, we can speculate that postmenopausal women would have a lower level of 5HT synthesis due to lower levels of estrogens and progesterone, and consequently lower levels of TPH mRNA and TPH protein. However, not all postmenopausal women are depressed 256. It is possible that after loss of ovarian function, there may be an adjustment in the 5HT neurons so that TPH protein levels recover in many women. This also suggests that mechanism related to lower TPH expression is a potential point of vulnerability in pre- and postmenopausal women who experience mental disorders such as depression related to decreased level of 5HT.
Second, estrogens regulate 5HT receptors 5HT2A and 2C, which are G protein-linked protein molecules and exhibit classic features of G protein-coupled receptors.
5HT2A receptor has been implicated in suicide and depression, and its mRNA are found in the brain areas relevant for controlling mood, mental state, and cognition 267. In humans, 5HT2A receptor mRNA is localized in cortex and hippocampus, but not in DRN, striatum, SN, or cerebellum 268. Estrogens increase 5HT2A receptor mRNA and binding site densities in male rat brain. There is a general agreement between rat and monkey regarding the localization of 5HT2A mRNA in the hypothalamus. In macaque hypothalamus 5HT2A receptor mRNA is expressed in the periventricular nuclei, supraoptic nucleus, mammillary bodies, subthalamic capsule, and moderately expressed in the thalamus as determined using in situ hybridization 269. Healthy men have significantly higher levels of 5HT2A receptor binding capacity than healthy women in the frontal and cingulate cortices as determined with PET and radiotracer 18F-labeled altanserin 270. Findings from human and rat studies suggest that estrogens increase 5HT2A binding in higher forebrain regions although little regulation has been observed at the mRNA level in the macaque hypothalamus.
The NAc receives major inputs from the amygdala and projects to the cortex and hypothalamus. These regions are essential for cognition, emotion, mental state, mood, and neuroendocrine control. Thus, estrogen-stimulated increase in 5HT2A receptor densities in these stated regions to control behavior and mood. A single injection of estradiol in OVX rats induces a significant increase in 5HT2A receptor labeling in the NAc, the amygdala, DRN, anterior frontal, anterior cingulate and primary olfactory cortex 271,272. Moreover, at the time of spontaneous estrogen-induced LH surge, 5HT2A receptor densities increase compared to diestrous females or males in the frontal and cingulate cortex, olfactory tubercle and NAc 273. OVX reduces 5HT2A receptor mRNA and protein levels, and long-term estrogen replacement reverses this effect in frontal cortex 274,275.
5HT2C, a key contributor of many psychiatric and neurological disorders 253, is the most prominent 5HT receptor subtype in rat brain 276. 5HT2C mRNA and protein are found in discrete regions of rat brain such as the choroid plexus, olfactory bulb, NAc, amygdala, SN, and hypothalamus 277–279. In the primate hypothalamus, using in situ hybridization, dense populations of 5HT2C mRNA-labeled cells are found in the anterior hypothalamus, periventricular nuclei, VMN, dorsal hypothalamic area, lateral hypothalamus, arcuate nucleus, and infundibular nucleus 269. Estradiol treatment increases 5HT2C receptor content in the dorsal part of the caudal brainstem, but not in the hypothalamus 280, suggesting that estradiol increases 5HT signaling by increasing the numbers of 5HT2C receptors in the caudal brainstem. Interestingly, estrogen treatment in spayed female macaques decreases 5HT2C receptor mRNA in the VMN, dorsal and posterior hypothalamus, but not other hypothalamic areas 269. VMN, but not dorsal or posterior hypothalamus, contains neurons that express ERs. The decrease in 5HT2C receptor mRNA in the VMN could be due to a direct action of estrogens through ERs. Downregulation of 5HT2C receptor gene in the dorsal and posterior hypothalamus that devoid of ERs could be due to changes of other processes of 5HT neurotransmission.
Third, estrogens regulate 5HT autoinhibition via 5HT1A autoreceptor. 5HT1A autoreceptor suppresses 5HT synthesis via inhibiting firing of serotonergic neurons at DRN and median raphe 281 and inhibiting 5HT release in the hippocampus 282,283. Therefore, blocking 5HT1A receptors has antidepressant-like activity 284. Estrogen treatment reduces 5HT1A mRNA level in the dentate gyrus, CA2 region of the hippocampus, and DRN of OVX rats 285 and in the DRN and median raphe of spayed rhesus monkeys 286 measured by in situ hybridization. The 5HT1A autoreceptor is linked to an inhibitory G protein of Gi/o/z family 287. Estrogens decrease basal and activated GTP binding in the DRN 288. In summary, the majority of evidence suggests that, in rodents and nonhuman primates, estrogens downregulate the activity and expression of 5HT1A autoreceptor, as well as decreases the availability of GTP binding, to enhance 5HT effects.
Fourth, estrogen treatment reduces 5HT uptake to presynaptic cells, via decreasing gene expression, translation, protein phosphorylation, trafficking, and stability of SERT 254. Estrogens reduce SERT mRNA signal in the DRN of estrogen-treated spayed rhesus monkeys compared to the spayed control group 289, and decrease [3H]paroxetine binding, a selective indicator of 5HT reuptake sites, in the hippocampus of estrogen-treated rats 290. Acute estrogen administration decreases SERT mRNA levels 289 and 5HT1A mRNA levels and binding 291. Thus, we can speculate that estrogen replacement therapy in postmenopausal women would decrease expression of SERT gene and SERT protein, thus 5HT may remain in the extracellular space for a longer period of time to continue neurotransmission. However, such reasoning does not explain the observations that humans with depression having lower levels of 5HT reuptake sites than healthy humans 292,293. It is possible that there is blunted 5HT release and reduced levels of 5HT in humans with depression to begin with, there is also less 5HT reuptake.
Fifth, estrogens decrease 5HT metabolism via degradation by MAO after 5HT is taken up into the presynaptic neurons. A decrease in the activity of MAO-A or -B would be reflected by a relative increase in availability of active 5HT and a decrease in its metabolites, 5-hydroxyindoleacetic acid (5HIAA) and homovanillic acid (HVA). In support, MAO inhibitors increase the concentration of 5HT and decrease the concentration of 5HIAA in rat brain and in human plasma 294. MAO-A and MAO-B mRNA are found in the DRN and similar nuclei of the hypothalamus known to contain ERs of the nonhuman primate macaque 295. Additionally, treatment of ovarian hormones, estrogen and progesterone, decrease gene transcription of MAO-A at DRN and hypothalamic PVN, LH and VMN; and decrease gene transcription of MAO-B at hypothalamic POA, LH and VMN, but not at DRN, of spayed macaques 295. Similarly, estrogen treatment in OVX rats reduces MAO-A activity in the hypothalamus and amygdala 296–298. If this change in gene expression is reflected by a change in protein, then ovarian hormones can increase extracellular concentrations of 5HT, by decreasing their metabolic oxidation.
To summarize, estrogens enhance 5HT signaling via increasing 5HT biosynthesis, upregulating expression and binding of receptors 5HT2A and 5HT2c, downregulating activity and expression of 5HT1A autoreceptor, decreasing expression of SERT gene and SERT protein and allowing 5HT remain in the extracellular space for a longer period of time to continue neurotransmission, and decreasing 5HT metabolism.
3.4. Sex differences and modulation of glutamatergic and GABAergic pathways by estrogens
3.4.1. Glutamatergic and GABAergic pathways
Glutamate is the main excitatory neurotransmitter whereas GABA is the most abundant and widely distributed inhibitory neurotransmitter in the CNS. There are multiple glutamatergic pathways, including (1) the descending cortical brainstem pathway that projects from cortical pyramidal neurons in the PFC to brainstorm neurotransmitter centers at VTA/SN to regulate DA release; (2) the descending pathway that projects from the PFC to the striatum NAc; (3) the ascending thalamocortical pathways that project from the thalamus to pyramidal neurons in the PFC; (4) the descending pathway that projects from the PFC to the thalamus; and (5) projections among cortical pyramidal neurons 299. Glutamate acts mostly via its N-methyl-D-aspartate (NMDA) receptor in a few brain regions that underlie cognitive functions, including the hippocampus, amygdala, and PFC with glutamatergic projection to VTA and NAc, especially potentiates the rewarding effects of use of substances 300. Gluatmatergic transmission onto medium spiny neurons in the NAc core, a ventral striatum region where abnormal functioning is implicated in patients with eating disorders 174,301,302.
In contrast to the excitatory effects carried out by glutamate, GABAergic neurotransmission is known to inhibit signaling and function of other neurotransmitters, such as DA 303,304 and 5HT 305,306, through GABA receptors GABAA and GABAB that are highly expressed in the cortex, hippocampus, thalamus, basal ganglia and cerebellar brain areas. A sequence of neurons consists of a glutamate-GABA-DA neurocircuit loop that starts from glutamatergic neurons in the PFC, fires on GABA interneurons to release GABA, and leads to dopaminergic neurons in the mesolimbic VTA/SN regions. Dopaminergic system contains GABAergic projection that inhibits DA release. This loop allows an accurate amount of DA and dopaminergic activity to occur to maintain appropriate, non-psychotic states. Removal of this GABAergic inhibition on VTA/SN DA neurons, such as during substance use, would increase DA neuron firing rates and activity, and thus induce reward-related bursts 307. Among a number of structures, NAc is a major source of GABAergic input to the VTA inhibits the activity of dopaminergic neurons at the VTA 308. Additionally, a recent study has demonstrated that D1 dopamine receptor expressing cells in specific sub-regions of the NAc project to VTA in mice to regulate motivational behavior 309. Therefore, CNS glutamate and GABA neurotransmission mediate multiple brain areas to respond to reward stimuli via modulating neurotransmission of other neurotransmitters.
A body of literature has indicated that estrogens enhance glutamatergic synaptic transmission in the hippocampus 310 while reduce GABA-mediated signaling in hippocampal, amygdala, and midbrain areas in vivo 304,311. Additionally, estrogens suppress GABAergic input in cultured rat hippocampal neurons in vitro 312. Consequently, due to hippocampal modulation by the interaction between estrogens and glutamatergic and GABAergic pathways, estrogens affect memory, long-term potentiation, and associated responses to reward stimuli.
3.4.2. Sex differences and modulation of glutamatergic and GABAergic pathways by estrogens - cell culture and animal studies
There is a scarcity of human studies on sex differences in or effects of estrogens on glutamate or GABA neurotransmission. The impact of sex hormones on the glutamatergic and GABAergic systems has been mostly studied in vitro using cultured hippocampal cells 313, in ex vivo brain slices 306,310, and in vivo animal models 303,304,314.
Estrogens facilitate glutamatergic neurotransmission in vitro 313. In vivo functional glutamatergic neurotransmission and glutamate receptor expression in the PFC following repeated stress paradigm are seen in female rats and estrogen-treated male rats, whereas glutamatergic neurotransmission and glutamate receptor expression are reduced in stressed males 314. Additionally, endogenous estrogens of female rats and exogenous estrogen treatment in male rats increase resilience to stress and preserve hippocampal functioning in rats 314,315. Thus, function of the hippocampal-amygdala-PFC glutamatergic pathway is dependent on functional estrogen signaling to provide protection against repeated stress. Furthermore, blocking of aromatase, the enzyme of estrogen synthesis, results in stress-induced glutamatergic deficits and memory impairment in female rats 314. Therefore, female rodents have an endogenously synthesized estrogen source, termed neuroestradiol (see Section 2.4) to counter insults such as stress and maintain normal function. In vivo studies using rats 304 and baboons 303 also have demonstrated that estrogens reduce GABAergic activity to increase DA content in the mesolimbic VTA-NAc pathway. Overall, findings support that estrogens enhance glutamatergic pathways and suppress GABAergic pathways.
3.4.3. Underlying mechanisms of modulation of glutamatergic and GABAergic pathways by estrogens
Estrogens facilitate glutamatergic signaling, via upregulating the expression 316 and increasing the distribution 317 of NMDA glutamate receptor in neurons, and via potentiating neuronal sensitivity to synaptic input mediated by NMDA receptor 318. In contrast, blockade of NMDA receptors with antagonists attenuates the impact of estrogens on neural cells that correlate of memory, such as long-term potentiation 319. It is noteworthy that estrogens act on both neural cells and astrocytes to mediate neuroprotective effects in the hippocampus of estradiol-treated female rats 320. Specifically, estradiol treatment improves density of neural cells, upregulates glutamine synthetase activity, and increases astrocyte glutamine transporter expression in the hippocampus. Therefore, neuroprotective properties of estrogens are reasonably linked to astrocytic activity 320.
Estrogens act on various subtypes of ERs, including nuclear ERα and ERβ and membrane ERs, to potentiate glutamatergic pre- and post- synaptic transmission in CA1 pyramidal neurons of the hippocampus of male and female rats 310. Selective ER agonists have been used to investigate sex differences in the mechanisms underlying estrogen-induced potentiation. Interestingly, presynaptic effects of estradiol is similarly initiated by a selective ERα agonist PPT in males, but by a selective ERβ agonist WAY in females 310. Additionally, the effects of estradiol of increasing postsynaptic transmission activity are mimicked by a selective ERβ agonist WAY in males, but by a selective GPER agonist G1 in females, 310. Therefore, although estrogens potentiate glutamatergic transmission at hippocampus in both sexes, sex-specific mechanisms with activation of distinct subtypes of ERs are involved in presynaptic and postsynaptic events. Besides acting on various subtypes of ERs, estrogens also activate membrane ERs that are coupled to metabotropic glutamate receptors to activate second messenger signaling at the NAc, known as ER/mGluR signaling 154, a potential mechanism to activate female motivational circuit that is responsible for addiction and substance abuse. Furthermore, a selective ERβ agonist DPN can regulate growth factor / trophic factor signaling to modulate glutamatergic and cholinergic synapse pathways, as well as retrograde endocannabinoid signaling, to provide neurogenesis, neuromodulation and neuroprotection in the hippocampal formation of OVX rats 321.
To summarize, sex differences in molecular regulation of excitatory synapses in the hippocampus exist, suggesting that different therapeutics that target distinct ERs would affect sex-specific excitatory hippocampal activity in males and females.
3.5. Sex differences and modulation of endocannabinoid pathway by estrogens
3.5.1. Endocannabinoid pathway
The CNS endocannabinoid system is a neuromodulatory system composed of endocannabinoids, cannabinoid receptors CB1 and CB2, along with many intracellular proteins involving endocannabinoid signal transduction. The endocannabinoid system regulates diverse biological, physiological and behavioral actions such as pain processing, inflammation, energy metabolism and sexual behavior engaging the CNS, various peripheral organs and tissues (e.g., gut, liver, pancreas, and adipose tissue), and circulating hormones including gonadal hormones. The endocannabinoid system has implication in eating disorders and addiction 322.
There are over 60 cannabinoid compounds including Δ9-tetrahydrocannabinol (THC), the primary psychotropic constituent. Endocannabinoids, such as anandamide and 2-arachidonoyl glycerol (2AG), are two major endogenous cannabinoids, produced from fatty acid metabolism, and function as lipid-based retrograde neurotransmitters 323. Endocannabinoids bind to two G protein-coupled cannabinoid receptors CB1 and CB2 324,325, with CB1 mostly presented in the CNS cortex, PVN and VMN of the hypothalamus, hippocampus, brainstem, and mesocorticolimbic brain regions including amygdala, NAc, and SN to increase appetite 326,327; and CB2 mostly presented in immune cells and in peripheral organs and tissues, including the intestine, liver, and adipose tissue, to regulate lipid and glucose metabolism 328.
CNS endocannabinoids are responsible for psychological effects of caloric intake via modulating feedback loop involved in hypothalamic appetite regulation by acting on its endocannabinoid receptors. Peripheral orexigenic ghrelin increases the levels of hypothalamic endocannabinoids anandamide and 2AG 329, while anorexigenic adiposity hormone leptin decreases anandamide and 2AG 330. Endocannabinoids regulate appetite mostly via their action on CB1 receptor. The administration of CB1 receptor agonists into hypothalamic nuclei such as the PVN 331 and VMN 332 increases energy intake. Besides CB1 and CB2, G-protein-coupled receptor 55 (GPR55) also has binding affinity for endocannabinoids. Dysfunction of endocannabinoid system appears to be a risk factor for anorexia nervosa. Loss of GPR55, as seen in some patients diagnosed with anorexia nervosa, induces less phosphorylated ERK when cells are treated with anandamide. Thus, low-functioning of GPR55 increases vulnerability to the development of eating disorders, such as anorexia nervosa 333. In the NAc, activation of CB1 inhibits effects of GABA, glutamate, and acetylcholine transmission 334 and increases DA release 335; while antagonists of CB1 decrease alcohol, cocaine, and opiate consumption 336,337. These studies suggest that activation of endocannabinoid system increases motivation to consume alcohol and other drugs 336,337.
Sex differences and effects of estrogens in cannabinoid system have been documented in both humans and animal models. The striking sex differences in the regulation of endocannabinoid pathway regulating motivation and energy homeostasis are pervasive and far-reaching based on available literature, including endocannabinoid-mediated neonatal development of the amygdala 338 and hippocampal neurogenesis 339, learning and memory during adolescence and adulthood 340, energy metabolism 341,342, and drug addiction 343.
3.5.2. Sex differences and modulation of endocannabinoid pathway by estrogens - human studies
Population-based surveys of adolescents found a sex difference in the prevalence rate of marijuana smoking, with males being highly frequent cannabis users comparing to females, identified in several clinical studies and in anecdotal observations 344. It is possible that cannabis smoking is associated with different emotional or mental states in men versus women. Moreover, some possible bias in the results coming from epidemiological studies may occur because women appear to receive more health care information than men, possibly due to women’s superior communication skills in general. Another survey has also revealed sex differences in correlation of frequent and heavy cannabis use. In particular, comparing with adolescent males, adolescent females appear to be less influenced by cannabis use of their peers and by the social environment established in school 345. Additionally, adolescent females reporting relatively poor mental health are at greater risk for frequent and heavy cannabis use than adolescent males 345, suggesting that mental health status is correlated with female’s, but not male’s, cannabis use.
Differences in a variety of cannabinoid effects exist between the two sexes. In women, the circulating levels of anandamide are higher during follicular phase and highest during ovulation when levels of estrogens are increasing and peak, and lower during the luteal phase when levels of estrogens are decreasing 346,347. Physiological and behavioral effects of endocannabinoids are different between the sexes, which could be related to sex differences in body fat distribution and related drug disposition. High concentrations of lipophilic cannabinoids are sequestered in adipose tissues. Women have a higher percentage of body fat than men do 25, which could retain more endocannabinoids and their metabolites at adipose tissues and reduce their circulating levels. Consequently women would experience weaker effects of cannabinoids than men do.
Sex differences exist in the endocannabinoid regulation of appetite. Most studies using cannabinoids or cannabinoid receptor agonists to increase energy intake and to ameliorate lack of appetite and body wasting in the treatment of cancer- and HIV/AIDS-related cachexia include only male participants 348–350. One study with similar percentages of male and female participants, however, failed to show increase in appetite by THC or cannabis extract 351, suggesting that estrogens attenuate endocannabinoid regulation of appetite. The RIO-North American clinical trial with approximately 80% women participants, in contrast, has demonstrated anti-obesity effects of Rimonabant, a CB1 receptor antagonist, such as effective reduction in body weight, adiposity, and waist circumference 352. It is noteworthy that more than 40% of women in the RIO-North American clinical trial are either peri- or post-menopausal women. Thus, it is possible that estrogen-induced attenuation of endocannabinoid signaling is not manifested due to decreasing levels of estrogens in these woman participants.
The potential attenuation of modulatory effects of cannabinoid system on appetite by estrogens suggests that cannabinoid responsiveness is at its lowest during estradiol-dominated follicular and periovulatory phases of the estrous cycle in women. Under hypoestrogenic states such as during primary amenorrhea (as seen in anorexia nervosa) or secondary amenorrhea (as seen in menopause), cannabinoid responsiveness would proceed to its full extent. Indeed, a man-made cannabinoid dronabinol that contains THC significantly increases weight gain in women with anorexia nervosa 353.
3.5.3. Sex differences and modulation of endocannabinoid pathway by estrogens - animal studies
Sex differences in cannabinoid-induced behavior have been found to attribute to activational effects of sex hormones. Research findings have suggested that estrogens alter endocannabinoid signaling via modulating expression of various proteins in the endocannabinoid system.
First, the levels of endocannabinoids are different between males and females 354, which could be altered by estrogens. Female rats have higher content of a major endocannabinoid anandamide than males in the hypothalamus and the anterior pituitary gland 355. The content of anandamide in the hypothalamus and the anterior pituitary gland also fluctuates across various phases of female estrous cycle, with peak values during the estrus and the nadir values during diestrus in the anterior pituitary gland, and an opposite tendency in the hypothalamus 355. In contrast, the content of the other major endocannabinoid 2AG is not different between males and females, and does not change during the female estrous cycle 343,355.
Second, expressions of genes and proteins of endocannabinoid-associated proteins including CB1 receptor are different between sexes, which could also be regulated by estrogens 355. For example, male rats have higher levels of CB1 receptor mRNA transcripts in the anterior pituitary gland than normal cycling female rats at the different stages of the estrous cycle 355. Additionally, CB1 receptor mRNA transcripts fluctuate during the female estrous cycle, reaching maximum magnitude in diestrus when estrogen levels are low and reaching lowermost value in estrus immediately after estrogen’s peak 355. Furthermore, CB1 receptor mRNA levels are lower in estradiol-treated OVX rats than OVX rats without estrogen replacement 355. Therefore, estrogens suppress the expression of CB1.
Third, behavioral responses to cannabinoids are sex-specific and are regulated by estrogens. One example is that the endocannabinoid system may be differentially sensitive in its modulation of appetitive behavior in females versus males. Sex differences in the cannabinoid regulation of caloric intake has been demonstrated in rodents. Administration of a cannabinoid CP 55,940 into the fourth ventricle stimulates consumption of sweetened condensed milk in male rats at a much lower dose than that observed in female rats 356, suggesting that male rats are more sensitive to cannabinoid’s effects. Similarly, male guinea pigs are more sensitive to hyperphagic and hypophagic effects of CB1 receptor agonist WIN 55,212-2 and antagonist AM251, respectively, than female guinea pigs 357. Specifically, CB1 agonist WIN 55,212-2 has larger increases in caloric intake, meal size and meal duration in males than in females, and CB1 antagonist AM251 has larger decreases in caloric intake and meal frequency in males than in females 357. Such sex differences persist even in the absence of sex hormones. CB1 agonist WIN 55,212-2 stimulates caloric intake to a greater extent in orchidectomized males than in OVX females 357, suggesting organizational effects of sex hormones on cannabinoid system. Interestingly, estradiol replacement in OVX females reduces energy intake, and rapidly and markedly attenuates the increase in energy intake caused by a CB1 receptor agonist WIN 55,212-2 342, suggesting activational effects by estrogens on cannabinoid system. Therefore, estrogens could have both organizational and activational effects on cannabinoid signaling to regulate appetite behavior. Another example is that the endocannabinoid system is more sensitive in its modulation of reward behavior in females versus males. Intravenous self-administration of the CB1 receptor agonist WIN 55,212-2 in female Long Evans and Lister Hooded rats is more rapidly acquired, more robustly maintained, and more slowly extinguished than in their male counterparts 191. Moreover, after both drug and cue priming, gonadally intact female rats reinstate responding for the cannabinoid at higher level than males and OVX females 343. Perinatal exposure to THC, the primary psychoactive ingredient in marijuana, decreases endogenous opioid polypeptide hormone proenkephalin gene expression in the caudate-putamen of female but not male rats 358; while female, but not male, rats that have been perinatally exposed to THC self-administer more morphine once they are adults 359. In general, cycling females respond more sensibly to THC-induced effects when tested in estrous with relatively higher levels of estrogens than in diestrous 360. Furthermore, endocannabinoids may be differentially metabolized to active and inactive metabolites in male and female rats 361. Levels of THC metabolites in brain tissues are higher in females than in males, likely contributing to the greater behavioral effects of THC in female compared to male rats 362.
3.5.4. Underlying mechanisms of modulation of endocannabinoid pathway by estrogens
In general, estrogens modulate endocannabinoid signaling in a CNS region-specific manner via multiple mechanisms such as regulating CB receptor expression, density, and affinity, to regulate responses to cannabinoids.
First, estrogens suppress CB1 receptor expression. As mentioned in above section 3.5.3 that male rats have greater gene expression levels of CB1 receptor in the anterior pituitary gland than cycling female rats 355. In addition, CB1 receptor gene expression in the anterior pituitary fluctuates throughout the female estrous cycle, increasing to peak value when estrogen levels decrease in diestrus while decreasing to nadir following estrogen peak 355. Furthermore, estrogen replacement in OVX rats reduces CB1 receptor mRNA levels in the anterior pituitary 355. Similarly, high levels of estrogens, as seen in cycling females and estrogen-treated OVX females, downregulate hypothalamic CB1 receptor gene expression and lower CB1 binding relative to low estrogen levels as seen in male rats and OVX female rats respectively 363.
Second, estrogens regulate density of CB receptors in the medial basal hypothalamus of female rats, with the highest density of CB receptors during diestrus and the lowest during estrus 364. Additionally, OVX reduces CB1 receptor density in the limbic forebrain 364, hippocampus and the amygdala 363, but the opposite is seen in the hypothalamus 363, all of which can be reversed by estradiol treatment 363,364.
Third, estrogens reduce binding affinity of CB receptors to cannabinoids. The binding affinity of CB receptors to cannabinoids is higher when level of estrogens is lower in the limbic forebrain, hypothalamus, and hippocampus, but opposite in the amygdala. Specifically, binding affinity of receptors to cannabinoids is the highest during diestrus and the lowest during estrus in the limbic forebrain, mesencephalon, and striatum 364; is higher in male rats and lower in cycling females 364; increases following OVX, which is normalized by estrogen treatment in the limbic forebrain, mesencephalon, and striatum 364 and is upregulated by OVX in the hypothalamus and the hippocampus 363. In agreement with the reduced CB1 receptor expression and affinity in the hypothalamus and related CNS feeding circuits induced by estrogen treatment in OVX rats, estrogen replacement in OVX females attenuates the ability of CB1 receptor agonist WIN 55,212-2 to increase energy intake 342,343. In contrast, males and OVX females have lower magnitude of cannabinoid receptor binding in the amygdala relative to cycling females and estrogen-treated OVX females 363.
To summarize, estrogen fluctuation along the estrous cycle as well as changes of estrogens after OVX and estrogen replacement modulate endocannabinoid signal transduction via changing CB receptor expression, density, and affinity. Such responses occur in a brain region-dependent and sex-specific manner. These findings corroborate sex hormone-dependent differences in the sensitivity of certain neuronal processes to cannabinoid treatment, which are different between sexes, fluctuate along the estrous cycle, and are changed by OVX and estrogen treatment. Additionally, these findings provide putative endocannabinoid signaling-related molecular and biochemical mechanisms mediating behavioral and physiological effects of estrogens on energy metabolism, mood, memory, motivation, and many other CNS functions 365 by modulating the endocannabinoid system in the brain.
4. CONCLUSIONS
The culmination of human and animal studies from recent decades has revealed extensive sex differences in CNS neurotransmission and neural circuits in different species, many of which are regulated by estrogens. Although these studies have elucidated considerable mechanistic insights underpinnings essential differences in central neural circuits involving various neurotransmitters to regulate physiology and behavior between males and females, they also urge more demands to be accomplished, especially regarding cellular and molecular events regulated by estrogens learned from animal studies to be effectively translated to the human conditions and to be appropriately tailored into therapeutic strategies in men and women, thus to be leveraged to better serve human health and to combat and cure mental disorders.
Sex as a biological factor has been receiving more attention in biomedical research. Although some neural circuits have known sex dimorphism, sex difference remains unknown for many others in the literature. Differential effects of male and female sex hormones substantially influence many aspects of physiology, behavior, metabolism and related diseases. The knowledge of sex differences in CNS neurotransmission that impact the reward system and associated motivational behavior, along with how neurotransmission-mediated CNS circuitry is influenced by sex hormones is critically important for continued function and vitality in women, as they are linked to risks of developing mental disorders with greater prevalence in women than in men, especially nowadays women live long past menopause. Experimental intervention in humans is frequently difficult to interpret due to problems in symptom variability and inconsistency in diagnosis, categorization, inclusion and exclusion criteria of human subjects in clinical trials. Appropriately, animal studies incorporating both male and female subjects in scientific premise such as physiological and behavioral responses to various types of stimulants, stressors, metabolic challenges, pharmacological treatments, genetic manipulations, etc. are on the rise. Up till now, however, many of available animal studies either use only male subjects, or use both male and female subjects but data are not analyzed separately at the disregard of confounding sex-related variables. Studies that including female subjects or examining sex differences at cellular and molecular levels are lacking. Thus more research incorporating both females and males in all aspects of neuroscience and behavioral research is needed.
A few concerns need to be addressed in order to drive research forward. First, it is open to debate whether evaluating different phases of the female estrous cycle would be a poor or wise choice to accurately represent sex differences seen in everyday life. Some researchers do not include phase as a factor because they believe that this would be an inaccurate representation of sex differences that is witnessed in real life. Other researchers do not include phase as a factor simply because a large number of female subjects are needed for comparison among different phases. In some published studies, sex differences are not established until phases of estrous cycle are analyzed separately 366,367. In order to advance our understanding, different phases are recommended to be analyzed as a factor to reveal sex differences that may not be seen when various phases are analyzed together. Second, while the combination treatment of OVX with hormone replacement provides us an experimental model of manipulating hormones to study the effects of sex hormones on neurotransmission in neural circuits, and to differentiate potential organizational versus activational effects of hormones, this model does not represent natural variations in hormonal milieu seen in menopausal women. It is challenging to find an appropriate animal model for human menopause. OVX is an accepted animal model that simulates human menopause. OVX however induces prompt menopause and skips perimenopause period of irregular estrous cycles, thus does not satisfactorily mimic hormonal changes during perimeopausal period. Third, young-adult and middle-aged female subjects, with drastic differences in neural circuitry activities and functions from aging or aged female animals, are usually included in OVX and hormone treatment studies. Thus, better animal models with similarities in endocrine, neural, and reproductive attributes as menopausal women are needed.
In conclusion, although some important insights into the neuroendocrine bases of sex differences in neurotransmission of brain circuits and related mental disorders have been achieved, investigation of these topics is still at an early stage. In recent decades preclinical and clinical researches have paid attention to include female subjects, although females are still underrepresented in many lines of investigation. A considerable part of clinical studies is based on preclinical research that are female predominant. Due to high prevalence of anxiety-related disorders and eating disorders in women 19,20,21,22, preclinical studies that exclude female subjects appear inevitably incomplete and biased. With regard to preventing and treating mental disorders in men and women, there is an urgent need to study both sexes. As investigators are asked to consider sex as a biological factor to ensure that women get the same benefits from medical research as men 34, there is no longer a justification for limiting research to only one sex. We can speculate that more sex differences would be reported in future. If both preclinical animal studies and human studies routinely included subjects of both sexes, greater progress in the field would be reached in a shorter time.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (DK090823) and the American Heart Association (16GRNT31110008) to HS, and doctoral fellowship from the Department of Biology to KNK and QZ.
Abbreviations
- 2AG
2-arachidonoyl glycerol
- 5HT
serotonin
- CNS
central nervous system
- ER
estrogen receptor
- DA
dopamine
- fMRI
functional magnetic resonance imaging
- GABA
gamma-aminobutyric acid
- MAO
monoamine oxidase
- NAc
Nucleus accumbens
- NMDA
N-methyl-D-aspartate receptor
- OFC
orbitofrontal cortex
- OVX
ovariectomy
- PET
positron-emission tomography
- PFC
prefrontal cortex
- PVN
paraventricular hypothalamic nucleus
- SERT
serotonin transporter
- SN
substantia nigra
- SSRI
selective serotonin reuptake inhibitor
- TH
tyrosine hydroxylase
- TPH
tryptophan hydroxylase
- VMN
ventromedial hypothalamic nucleus
- VTA
ventral tegmental area
References
- 1.Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2017. (Accessed June 12, 2018, 2018, at https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm.)
- 2.Reeves WC, Strine TW, Pratt LA, et al. Mental illness surveillance among adults in the United States. MMWR Suppl 2011;60:1–29. [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality (CBHSQ) 2016 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, Rockville, MD: 2017. [Google Scholar]
- 4.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the national comorbidity survey. Arch Gen Psychiatry 1999;56:617–26. [DOI] [PubMed] [Google Scholar]
- 5.Rogers PJ, Smit HJ. Food craving and food “addiction”: a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav 2000;66:3–14. [DOI] [PubMed] [Google Scholar]
- 6.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology 2008;199:457–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey UB, Nichols CD. Human disease models in drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev 2011;63:411–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science 2012;336:1154–7. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 2009;30:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowrey WR, Portman DS. Sex differences in behavioral decision-making and the modulation of shared neural circuits. Biol Sex Differ 2012;3:8-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction In: Carter C, Dalley J, eds. Brain Imaging in Behavioral Neuroscience Current Topics in Behavioral Neurosciences. Current Topics in Behavioral Neurosciences: Springer, Berlin, Heidelberg; 2011. [DOI] [PubMed] [Google Scholar]
- 12.Sansone RA, Sansone LA. Obesity and substance misuse: is there a relationship? Innov Clin Neurosci 2013;10:30–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav 2009;97:537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science 1962;135:374–5. [DOI] [PubMed] [Google Scholar]
- 15.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science 1962;135:375–7. [DOI] [PubMed] [Google Scholar]
- 16.Becker JB, Taylor JR. Sex differences in motivation In: Becker JB, Berkley K, Geary N, Hampson E, Herman JP, Young EA, eds. Sex Differences in the Brain: from Genes to Behavior. Oxford, UK: Oxford University Press; 2008:177–99. [Google Scholar]
- 17.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience 2003;122:17–20. [DOI] [PubMed] [Google Scholar]
- 18.Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci 2012;13:279. [DOI] [PubMed] [Google Scholar]
- 19.Gender and women’s mental health. 2016. (Accessed June 12, 2018, 2018, at http://www.who.int/mental_health/prevention/genderwomen/en/.)
- 20.Grant BF, Weissman MM. Gender and the prevalence of psychiatric disorders In: Narrow WE, First MB, Sirovatka PJ, Regier DA, eds. Age and gender considerations in psychiatric diagnosis: A research agenda for DSM-V. Arlington, VA, US: American Psychiatric Publishing, Inc.; 2007:31–45. [Google Scholar]
- 21.Klein LC, Corwin EJ. Seeing the unexpected: how sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Curr Psychiatry Rep 2002;4:441–8. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC. Epidemiology of women and depression. J Affect Disord 2003;74:5–13. [DOI] [PubMed] [Google Scholar]
- 23.Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry 2006;19:389–94. [DOI] [PubMed] [Google Scholar]
- 24.Striegel-Moore RH, Rosselli F, Perrin N, et al. Gender difference in the prevalence of eating disorder symptoms. Int J Eat Disord 2009;42:471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedrich MJ. Global obesity epidemic worsening. JAMA 2017;318:603-. [DOI] [PubMed] [Google Scholar]
- 26.Lovejoy JC, Sainsbury A, the Stock Conference Working G. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 2009;10:154–67. [DOI] [PubMed] [Google Scholar]
- 27.Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlates of drug use and dependence in the united states: Results from the national comorbidity survey. Arch Gen Psychiatry 1995;52:219–29. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy AP, Epstein DH, Phillips KA, Preston KL. Sex differences in cocaine/heroin users: Drug-use triggers and craving in daily life. Drug Alcohol Depend 2013;132:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hitschfeld MJ, Schneekloth TD, Ebbert JO, et al. Female smokers have the highest alcohol craving in a residential alcoholism treatment cohort. Drug Alcohol Depend 2015;150:179–82. [DOI] [PubMed] [Google Scholar]
- 30.Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology 2014;39:1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Results from the 2013 National Survey on Drug Use and Health: Mental Health Findings. HHS Publication No. (SMA) 14–4887, 2014. (Accessed June 12, 2018, 2018, at http://www.samhsa.gov/data/sites/default/files/NSDUHmhfr2013/NSDUHmhfr2013.pdf.)
- 32.Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? J Psychiatry Neurosci 2008;33:331–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Fattore L Reward processing and drug addiction: does sex matter? Front Neurosci 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H, Senthil Kumar SPD, Liu X. G protein-coupled estrogen receptor in energy homeostasis and obesity pathogenesis. In: Tao Y-X, ed. Prog Mol Biol Transl Sci: Academic Press; 2013:193–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jauch-Chara K, Oltmanns KM. Obesity - a neuropsychological disease? Systematic review and neuropsychological model. Prog Neurobiol 2014;114:84–101. [DOI] [PubMed] [Google Scholar]
- 37.Kanoski SE, Grill HJ. Hippocampus contributions to food Intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry 2017;81:748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso-Alonso M, Woods SC, Pelchat M, et al. Food reward system: current perspectives and future research needs. Nutr Rev 2015;73:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sweeney P, Yang Y. Neural circuit mechanisms underlying emotional regulation of homeostatic feeding. Trends Endocrinol Metab 2017;28:437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C, Lee S, Elmquist Joel K. Circuits controlling energy balance and mood: inherently intertwined or just complicated intersections? Cell Metab 2014;19:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrario CR, Labouèbe G, Liu S, et al. Homeostasis meets motivation in the battle to control food intake. J Neurosci 2016;36:11469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab 2018;27:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CM, Kanoski SE. Homeostatic and non-homeostatic controls of feeding behavior: Distinct vs. common neural systems. Physiol Behav 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon JJ, Wetzel A, Sinno MH, et al. Integration of homeostatic signaling and food reward processing in the human brain. JCI Insight 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu YH, Vasselli JR, Zhang Y, Mechanick JI, Korner J, Peterli R. Metabolic vs. hedonic obesity: a conceptual distinction and its clinical implications. Obes Rev 2015;16:234–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci 2012;32:2241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tunç B, Solmaz B, Parker D, et al. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos Trans R Soc Lond B Biol Sci 2016;371:20150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite 2011;57:421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict Behav 2014;39:789–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev 2012;13:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res 2013;243:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev 2001;25:53–74. [DOI] [PubMed] [Google Scholar]
- 53.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 1978;143:263–79. [DOI] [PubMed] [Google Scholar]
- 54.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 2000;24:173–98. [DOI] [PubMed] [Google Scholar]
- 55.Crystal SR, Bernstein IL. Infant salt preference and mother’s morning sickness. Appetite 1998;30:297–307. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen SE, Herrera AY. 1.14 - Sex steroids, learning and memory In: Pfaff DW, Joëls M, eds. Hormones, Brain and Behavior (Third Edition). Oxford: Academic Press; 2017:399–422. [Google Scholar]
- 57.Feis D-L, Brodersen KH, von Cramon DY, Luders E, Tittgemeyer M. Decoding gender dimorphism of the human brain using multimodal anatomical and diffusion MRI data. NeuroImage 2013;70:250–7. [DOI] [PubMed] [Google Scholar]
- 58.McVay MA, Copeland AL, Newman HS, Geiselman PJ. Food cravings and food cue responding across the menstrual cycle in a non-eating disordered sample. Appetite 2012;59:591–600. [DOI] [PubMed] [Google Scholar]
- 59.Frank S, Laharnar N, Kullmann S, et al. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Res 2010;1350:159–66. [DOI] [PubMed] [Google Scholar]
- 60.Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A 2007;104:2465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alonso-Alonso M, Ziemke F, Magkos F, et al. Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: relation to fasting and feeding. Am J Clin Nutr 2011;94:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold AP. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 2009;55:570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 2009;30:883–925. [DOI] [PubMed] [Google Scholar]
- 64.Wu MV, Manoli DS, Fraser EJ, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 2009;139:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bakker J, De Mees C, Douhard Q, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 2006;9:220. [DOI] [PubMed] [Google Scholar]
- 66.Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod 2011;85:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nomura M, Andersson S, Korach KS, Gustafsson J-Å, Pfaff DW, Ogawa S. Estrogen receptor-β gene disruption potentiates estrogen-inducible aggression but not sexual behaviour in male mice. Eur J Neurosci 2006;23:1860–8. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy MM, Schwarz JM, Wright CL, Dean SL. Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol 2008;20:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology 1998;139:5070–81. [DOI] [PubMed] [Google Scholar]
- 70.Hess R Estrogen in the adult male reproductive tract: A review. Reprod Biol Endocrinol 2003;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baquedano MS, Saraco N, Berensztein E, et al. Identification and developmental changes of aromatase and estrogen receptor expression in prepubertal and pubertal human adrenal tissues. J Clin Endocrinol Metab 2007;92:2215–22. [DOI] [PubMed] [Google Scholar]
- 72.Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen signaling in the hypothalamus. In: Gerald L, ed. Vitam Horm: Academic Press; 2005:123–45. [DOI] [PubMed] [Google Scholar]
- 73.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999;20:279–307. [DOI] [PubMed] [Google Scholar]
- 74.Teede HJ. Sex hormones and the cardiovascular system: effects on arterial function in women. Clin Exp Pharmacol Physiol 2007;34:672–6. [DOI] [PubMed] [Google Scholar]
- 75.Turner RT, Riggs BL, Spelsberg TC. Skeletal effects of estrogen. Endocr Rev 1994;15:275–300. [DOI] [PubMed] [Google Scholar]
- 76.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med 2002;346:340–52. [DOI] [PubMed] [Google Scholar]
- 77.Bohler HCL Jr, Thomas Zoeller R, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res 1990;8:259–62. [DOI] [PubMed] [Google Scholar]
- 78.Ramon S-Z, Gonzalo C, Georgina MR, Pedro E, Victor DR. Sex hormones and brain dopamine functions. Cent Nerv Syst Agents Med Chem 2014;14:62–71. [DOI] [PubMed] [Google Scholar]
- 79.Al Sweidi S, Sánchez MG, Bourque M, Morissette M, Dluzen D, Di Paolo T. Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson’s disease. J Neuroendocrinol 2012;24:48–61. [DOI] [PubMed] [Google Scholar]
- 80.Luine VN. Sex steroids and cognitive function. J Neuroendocrinol 2008;20:866–72. [DOI] [PubMed] [Google Scholar]
- 81.Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol 1998;25:764–75. [DOI] [PubMed] [Google Scholar]
- 82.Stanić D, Dubois S, Chua HK, et al. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One 2014;9:e90451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kenealy BP, Kapoor A, Guerriero KA, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci 2013;33:19051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abi Ghanem C, Degerny C, Hussain R, et al. Long-lasting masculinizing effects of postnatal androgens on myelin governed by the brain androgen receptor. PLoS Genet 2017;13:e1007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carrer HF, Cambiasso MJ. Sexual differentiation of the brain: genes, estrogen, and neurotrophic factors. Cell Mol Neurobiol 2002;22:479–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci 2008;11:995–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 2004;145:1063–8. [DOI] [PubMed] [Google Scholar]
- 88.Yang T, Shah NM. Molecular and neural control of sexually dimorphic social behaviors. Curr Opin Neurobiol 2016;38:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Forger NG. Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philos Trans R Soc Lond B Biol Sci 2016;371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forger NG, Strahan JA, Castillo-Ruiz A. Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol 2016;40:67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muller RE, Johnston TC, Traish AM, Wotiz HH. Studies on the mechanism of estradiol uptake by rat uterine cells and on estradiol binding to uterine plasma membranes. Adv Exp Med Biol 1979;117:401–21. [DOI] [PubMed] [Google Scholar]
- 92.Jackson V, Chalkley R. The binding of estradiol-17 beta to the bovine endometrial nuclear membrane. J Biol Chem 1974;249:1615–26. [PubMed] [Google Scholar]
- 93.Walter P, Green S, Greene G, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A 1985;82:7889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A 1996;93:5925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greene G, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science 1986;231:1150–4. [DOI] [PubMed] [Google Scholar]
- 96.Green S, Walter P, Kumar V, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 1986;320:134–9. [DOI] [PubMed] [Google Scholar]
- 97.Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science 1973;182:126–34. [DOI] [PubMed] [Google Scholar]
- 98.Brzozowski AM, Pike ACW, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997;389:753–8. [DOI] [PubMed] [Google Scholar]
- 99.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev 2007;28:726–41. [DOI] [PubMed] [Google Scholar]
- 100.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 2001;276:36869–72. [DOI] [PubMed] [Google Scholar]
- 101.Nilsson S, Mäkelä S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev 2001;81:1535–65. [DOI] [PubMed] [Google Scholar]
- 102.Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol 2009;308:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas C, Gustafsson J-Å. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 2011;11:597–608. [DOI] [PubMed] [Google Scholar]
- 104.Pipil S, Kumar V, Rawat VS, Sharma L, Sehgal N. In silico and in vivo analysis of binding affinity of estrogens with estrogen receptor alpha in Channa punctatus (Bloch). Fish Physiol Biochem 2015;41:31–40. [DOI] [PubMed] [Google Scholar]
- 105.McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol 2010;10:620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem 2000;43:4934–47. [DOI] [PubMed] [Google Scholar]
- 107.Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology 2010;86:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for oestrogen receptor β in adult brain function. J Neuroendocrinol 2012;24:160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuiper GGJM, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997;138:863–70. [DOI] [PubMed] [Google Scholar]
- 110.Matthews J, Gustafsson J-Å. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv 2003;3:281–92. [DOI] [PubMed] [Google Scholar]
- 111.Sheng Z, Kawano J, Yanai A, et al. Expression of estrogen receptors (α, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res 2004;49:185–96. [DOI] [PubMed] [Google Scholar]
- 112.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERβ) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res 2000;76:191–204. [DOI] [PubMed] [Google Scholar]
- 113.Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-alpha knockout mous. Endocrinology 1997;138:5649–52. [DOI] [PubMed] [Google Scholar]
- 114.Mitra SW, Hoskin E, Yudkovitz J, et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor α. Endocrinology 2003;144:2055–67. [DOI] [PubMed] [Google Scholar]
- 115.Hewitt S, Korach K. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction 2003;125:143–9. [DOI] [PubMed] [Google Scholar]
- 116.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci U S A 2000;97:12729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nilsson S, Gustafsson JA. Estrogen receptors: therapies targeted to receptor subtypes. Clin Pharmacol Ther 2011;89:44–55. [DOI] [PubMed] [Google Scholar]
- 118.Liang YQ, Akishita M, Kim S, et al. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord 2002;26:1103–9. [DOI] [PubMed] [Google Scholar]
- 119.Krȩżel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. Proc Natl Acad Sci U S A 2001;98:12278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsuda MC, Yamaguchi N, Nakata M, Ogawa S. Modification of female and male social behaviors in estrogen receptor beta knockout mice by neonatal maternal separation. Front Neurosci 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imwalle DB, Gustafsson J-Å, Rissman EF. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav 2005;84:157–63. [DOI] [PubMed] [Google Scholar]
- 122.Tomihara K, Soga T, Nomura M, et al. Effect of ER-β gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav 2009;96:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ogawa S, Chan J, Chester AE, Gustafsson J-Å, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci U S A 1999;96:12887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vito CC, DeBold JF, Fox TO. Androgen and estrogen receptors in adult hamster brain. Brain Res 1983;264:132–7. [DOI] [PubMed] [Google Scholar]
- 125.Voigt C, Ball GF, Balthazart J. Sex differences in the expression of sex steroid receptor mRNA in the quail brain. J Neuroendocrinol 2009;21:1045–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scott CJ, Tilbrook AJ, Simmons DM, et al. The distribution of cells containing estrogen receptor-alpha (ERalpha) and ERbeta messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: comparison of males and females. Endocrinology 2000;141:2951–62. [DOI] [PubMed] [Google Scholar]
- 127.Swaab DF, Chung WCJ, Kruijver FPM, Hofman MA, Hestiantoro A. Sex differences in the hypothalamus in the different stages of human life. Neurobiol Aging 2003;24:S1–S16. [DOI] [PubMed] [Google Scholar]
- 128.Greenberg GD, Trainor BC. Sex differences in the social behavior network and mesolimbic dopamine system In: Shansky RM, ed. Sex differences in the central nervous system. San Diego: Academic Press; 2016:77–106. [Google Scholar]
- 129.Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol 2004;476:348–62. [DOI] [PubMed] [Google Scholar]
- 130.O’Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol 2004;18:1859–75. [DOI] [PubMed] [Google Scholar]
- 131.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 2005;67:335–76. [DOI] [PubMed] [Google Scholar]
- 132.Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev 2003;83:965–1016. [DOI] [PubMed] [Google Scholar]
- 133.Brann DW, Hendry LB, Mahesh VB. Emerging diversities in the mechanism of action of steroid hormones. J Steroid Biochem Mol Biol 1995;52:113–33. [DOI] [PubMed] [Google Scholar]
- 134.Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: Two sides of the coin. J Cell Physiol 2006;207:594–604. [DOI] [PubMed] [Google Scholar]
- 135.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology 2006;147:5557–63. [DOI] [PubMed] [Google Scholar]
- 136.Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: Role of estrogen receptor α and estrogen receptor β in neurons and glia. Neuroscience 2006;138:851–8. [DOI] [PubMed] [Google Scholar]
- 137.Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med 2007;25:139,53. [DOI] [PubMed] [Google Scholar]
- 138.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997;45:607–17. [DOI] [PubMed] [Google Scholar]
- 139.Filardo EJ, Quinn JA, Frackelton AR, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 2002;16:70–84. [DOI] [PubMed] [Google Scholar]
- 140.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 2008;70:165–90. [DOI] [PubMed] [Google Scholar]
- 141.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005;307:1625–30. [DOI] [PubMed] [Google Scholar]
- 142.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005;146:624–32. [DOI] [PubMed] [Google Scholar]
- 143.Qiu J, Bosch MA, Tobias SC, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 2003;23:9529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiu J, Bosch MA, Tobias SC, et al. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 2006;26:5649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology 2008;149:6113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roepke TA, Bosch MA, Rick EA, et al. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 2010;151:4926–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci U S A 2000;97:11603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Toran-Allerand CD, Guan X, MacLusky NJ, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci 2002;22:8391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Toran-Allerand CD. Estrogen and the brain: beyond ER-α, ER-β, and 17β-estradiol. Ann N Y Acad Sci 2005;1052:136–44. [DOI] [PubMed] [Google Scholar]
- 150.Brailoiu E, Dun SL, Brailoiu GC, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 2007;193:311–21. [DOI] [PubMed] [Google Scholar]
- 151.Canonaco M, Giusi G, Madeo A, et al. A sexually dimorphic distribution pattern of the novel estrogen receptor G-protein-coupled receptor 30 in some brain areas of the hamster. J Endocrinol 2008;196:131–8. [DOI] [PubMed] [Google Scholar]
- 152.Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol 2010;320:16–24. [DOI] [PubMed] [Google Scholar]
- 153.Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Front Neuroendocrinol 1996;17:402–39. [DOI] [PubMed] [Google Scholar]
- 154.Tonn Eisinger KR, Larson EB, Boulware MI, Thomas MJ, Mermelstein PG. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 2000;14:1649–60. [DOI] [PubMed] [Google Scholar]
- 156.Pietras R, Nemere I, Szego C. Steroid hormone receptors in target cell membranes. Endocrine 2001;14:417–27. [DOI] [PubMed] [Google Scholar]
- 157.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A 1994;91:8517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Szego CM, Davis JS. Adenosine 3’,5’-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A 1967;58:1711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2006;2:207–12. [DOI] [PubMed] [Google Scholar]
- 160.Broughton BRS, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol 2010;298:H1055–H61. [DOI] [PubMed] [Google Scholar]
- 161.Dennis MK, Burai R, Ramesh C, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol 2009;5:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chow RWY, Handelsman DJ, Ng MKC. Minireview: rapid actions of sex steroids in the endothelium. Endocrinology 2010;151:2411–22. [DOI] [PubMed] [Google Scholar]
- 163.Lin BC, Suzawa M, Blind RD, et al. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res 2009;69:5415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang C, Dehghani B, Magrisso IJ, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 2008;22:636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Isensee J, Meoli L, Zazzu V, et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 2009;150:1722–30. [DOI] [PubMed] [Google Scholar]
- 166.Mårtensson UEA, Salehi SA, Windahl S, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009;150:687–98. [DOI] [PubMed] [Google Scholar]
- 167.Otto C, Fuchs I, Kauselmann G, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 2009;80:34–41. [DOI] [PubMed] [Google Scholar]
- 168.Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res 2002;100:75–83. [DOI] [PubMed] [Google Scholar]
- 169.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord 2012;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 2017;18:73. [DOI] [PubMed] [Google Scholar]
- 171.Volkow ND, Wise RA, Baler R. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 2017;18:741. [DOI] [PubMed] [Google Scholar]
- 172.Becker JB. Sex differences in addiction. Dialogues Clin Neurosci 2016;18:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage 2008;41:636–47. [DOI] [PubMed] [Google Scholar]
- 174.Wierenga CE, Bischoff-Grethe A, Melrose AJ, et al. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol Psychiatry 2015;77:642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Van Vugt DA, Reid RL. Neuroimaging menstrual cycle associated changes in appetite. In: Hollins-Martin C, van den Akker O, Martin C, Preedy VR, eds. Handbook of diet and nutrition in the menstrual cycle, periconception and fertility; 2014:169–88. [Google Scholar]
- 176.White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 2002;73:729–41. [DOI] [PubMed] [Google Scholar]
- 177.Justice AJH, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav 2000;66:509–15. [DOI] [PubMed] [Google Scholar]
- 178.Munro CA, McCaul ME, Wong DF, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 2006;59:966–74. [DOI] [PubMed] [Google Scholar]
- 179.Kamarajan C, Rangaswamy M, Chorlian DB, et al. Theta oscillations during the processing of monetary loss and gain: A perspective on gender and impulsivity. Brain Res 2008;1235:45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sinclair EB, Hildebrandt BA, Culbert KM, Klump KL, Sisk CL. Preliminary evidence of sex differences in behavioral and neural responses to palatable food reward in rats. Physiol Behav 2017;176:165–73. [DOI] [PubMed] [Google Scholar]
- 181.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav 2009;97:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 2007;13:89–94. [DOI] [PubMed] [Google Scholar]
- 183.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 2013;305:R1215–R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm Behav 2009;56:11–23. [DOI] [PubMed] [Google Scholar]
- 185.Wissman AM, May RM, Woolley CS. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct 2012;217:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Geiselman PJ, Martin JR, Vanderweele DA, Novin D. Dietary self-selection in cycling and neonatally ovariectomized rats. Appetite 1981;2:87–101. [DOI] [PubMed] [Google Scholar]
- 187.Bartness TJ, Waldbillig RJ. Dietary self-selection in intact, ovariectomized, and estradiol-treated female rats. Behav Neurosci 1984;98:125–37. [DOI] [PubMed] [Google Scholar]
- 188.Wurtman JJ, Baum MJ. Estrogen reduces total food and carbohydrate intake, but not protein intake, in female rats. Physiol Behav 1980;24:823–7. [DOI] [PubMed] [Google Scholar]
- 189.Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology 2005;31:1193–202. [DOI] [PubMed] [Google Scholar]
- 190.Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 1993;610:127–34. [DOI] [PubMed] [Google Scholar]
- 191.Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 2007;152:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology 2000;152:132–9. [DOI] [PubMed] [Google Scholar]
- 193.Perez PD, Hall G, Zubcevic J, Febo M. Cocaine differentially affects synaptic activity in memory and midbrain areas of female and male rats: an in vivo MEMRI study. Brain Imaging Behav 2018;12:201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cao J, Dorris DM, Meitzen J. Neonatal masculinization blocks increased excitatory synaptic input in female rat nucleus accumbens core. Endocrinology 2016;157:3181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Espinosa P, Silva RA, Sanguinetti NK, et al. Programming of dopaminergic neurons by neonatal sex hormone exposure: effects on dopamine content and tyrosine hydroxylase expression in adult male rats. Neural Plast 2016;2016:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Campi KL, Jameson CE, Trainor BC. Sexual dimorphism in the brain of the monogamous California mouse (Peromyscus californicus). Brain Behav Evol 2013;81:236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Forlano PM, Woolley CS. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 2010;518:1330–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci 2005;25:10759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A 1999;96:7538–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol 2008;506:851–9. [DOI] [PubMed] [Google Scholar]
- 201.Becker JB. Sexual differentiation of motivation: a novel mechanism? Horm Behav 2009;55:646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chiodo LA, Caggiula AR. Alterations in basal firing rate and autoreceptor sensitivity of dopamine neurons in the substantia nigra following acute and extended exposure to estrogen. Eur J Pharmacol 1980;67:165–6. [DOI] [PubMed] [Google Scholar]
- 203.Hernández ML, Fernández-Ruiz JJ, de Miguel R, Ramos JA. Time-dependent effects of ovarian steroids on tyrosine hydroxylase activity in the limbic forebrain of female rats. J Neural Transm Gen Sect 1991;83:77–84. [DOI] [PubMed] [Google Scholar]
- 204.Maharjan S, Serova L, Sabban EL. Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors α and β and interactions with cyclic AMP. J Neurochem 2005;93:1502–14. [DOI] [PubMed] [Google Scholar]
- 205.Maharjan S, Serova LI, Sabban EL. Membrane-initiated estradiol signaling increases tyrosine hydroxylase promoter activity with ERα in PC12 cells. J Neurochem 2010;112:42–55. [DOI] [PubMed] [Google Scholar]
- 206.Yanagihara N, Liu M, Toyohira Y, et al. Stimulation of catecholamine synthesis through unique estrogen receptors in the bovine adrenomedullary plasma membrane by 17β-estradiol. Biochem Biophys Res Commun 2006;339:548–53. [DOI] [PubMed] [Google Scholar]
- 207.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 1990;118:169–71. [DOI] [PubMed] [Google Scholar]
- 208.Becker J, Ramirez VD. Dynamics of endogenous catecholamine release from brain fragments of male and female rats. Neuroendocrinology 1980;31:18–25. [DOI] [PubMed] [Google Scholar]
- 209.Becker JB, Ramirez VD. Experimental studies on the development of sex differences in the release of dopamine from striatal tissue fragments in vitro. Neuroendocrinology 1981;32:168–73. [DOI] [PubMed] [Google Scholar]
- 210.Shimizu H, Bray GA. Effects of castration, estrogen replacement and estrus cycle on monoamine metabolism in the nucleus accumbens, measured by microdialysis. Brain Res 1993;621:200–6. [DOI] [PubMed] [Google Scholar]
- 211.Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol 2010;22:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Russo SJ, Festa ED, Fabian SJ, et al. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience 2003;120:523–33. [DOI] [PubMed] [Google Scholar]
- 213.Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci 2000;20:8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wallin-Miller KG, Chesley J, Castrillon J, Wood RI. Sex differences and hormonal modulation of ethanol-enhanced risk taking in rats. Drug Alcohol Depend 2017;174:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hruska RE, Nowak MW. Estrogen treatment increases the density of D1 dopamine receptors in the rat striatum. Brain Res 1988;442:349–50. [DOI] [PubMed] [Google Scholar]
- 216.Czoty PW, Riddick NV, Gage HD, et al. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 2008;34:548. [DOI] [PubMed] [Google Scholar]
- 217.Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res 1994;637:163–72. [DOI] [PubMed] [Google Scholar]
- 218.Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev 2015;58:147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stopper CM, Khayambashi S, Floresco SB. Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology 2012;38:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology 2008;34:681. [DOI] [PubMed] [Google Scholar]
- 221.St. Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci 2011;31:8625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yoest KE, Cummings JA, Becker JB. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem 2014;14:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Volkow ND, Fowler JS, Wang G-J, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993;14:169–77. [DOI] [PubMed] [Google Scholar]
- 224.Wang G-J, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet 2001;357:354–7. [DOI] [PubMed] [Google Scholar]
- 225.Martin LE, Holsen LM, Chambers RJ, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 2010;18:254–60. [DOI] [PubMed] [Google Scholar]
- 226.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005;134:737–44. [DOI] [PubMed] [Google Scholar]
- 227.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 2010;13:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Febo M, González-Rodríguez LA, Capó-Ramos DE, González-Segarra NY, Segarra AC. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem 2003;86:405–12. [DOI] [PubMed] [Google Scholar]
- 229.Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem 1994;62:1750–6. [DOI] [PubMed] [Google Scholar]
- 230.Morel GR, Carón RW, Cónsole GM, et al. Estrogen inhibits tuberoinfundibular dopaminergic neurons but does not cause irreversible damage. Brain Res Bull 2009;80:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17β-estradiol. Eur J Pharmacol 1998;345:207–11. [DOI] [PubMed] [Google Scholar]
- 232.Dupont A, Di Paolo T, Gagné B, Barden N. Effects of chronic estrogen treatment on dopamine concentrations and turnover in discrete brain nuclei of ovariectomized rats. Neurosci Lett 1981;22:69–74. [DOI] [PubMed] [Google Scholar]
- 233.Lammers C-H, D’Souza U, Qin Z-H, Lee S-H, Yajima S, Mouradian MM. Regulation of striatal dopamine receptors by estrogen. Synapse 1999;34:222–7. [DOI] [PubMed] [Google Scholar]
- 234.Rehavi M, Goldin M, Roz N, Weizman A. Regulation of rat brain vesicular monoamine transporter by chronic treatment with ovarian hormones. Brain Res Mol Brain Res 1998;57:31–7. [DOI] [PubMed] [Google Scholar]
- 235.Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res 2010;1321:51–9. [DOI] [PubMed] [Google Scholar]
- 236.Bossé R, Rivest R, Di Paolo T. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Brain Res Mol Brain Res 1997;46:343–6. [DOI] [PubMed] [Google Scholar]
- 237.Morissette M, Paolo TD. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem 1993;60:1876–83. [DOI] [PubMed] [Google Scholar]
- 238.Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: Neurochemistry and behavior. Drug Alcohol Depend 2014;135:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Silverman JL, Koenig JI. Evidence for the involvement of ERβ and RGS9-2 in 17-β estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav 2007;52:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Segarra AC, Agosto-Rivera JL, Febo M, et al. Estradiol: A key biological substrate mediating the response to cocaine in female rats. Horm Behav 2010;58:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology 2012;37:2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tye KM, Mirzabekov JJ, Warden MR, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2012;493:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 1992;72:165–229. [DOI] [PubMed] [Google Scholar]
- 244.Coppen AJ, Doogan DP. Serotonin and its place in the pathogenesis of depression. J Clin Psychiatry 1988;49 Suppl:4–11. [PubMed] [Google Scholar]
- 245.Haahr ME, Rasmussen PM, Madsen K, et al. Obesity is associated with high serotonin 4 receptor availability in the brain reward circuitry. NeuroImage 2012;61:884–8. [DOI] [PubMed] [Google Scholar]
- 246.Pollak Dorocic I, Fürth D, Xuan Y, et al. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 2014;83:663–78. [DOI] [PubMed] [Google Scholar]
- 247.Murphy DL, Andrews AM, Wichems CH, Li Q, Tohda M, Greenberg B. Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry 1998;59:4–12. [PubMed] [Google Scholar]
- 248.Blundell JE, Lawton CL, Halford JCG. Serotonin, eating behavior, and fat intake. Obes Res 1995;3:471S–6S. [DOI] [PubMed] [Google Scholar]
- 249.Carlsson M, Carlsson A. A regional study of sex differences in rat brain serotonin. Prog Neuropsychopharmacol Biol Psychiatry 1988;12:53–61. [DOI] [PubMed] [Google Scholar]
- 250.Lu NZ, Shlaes TA, Gundlah C, Dziennis SE, Lyle RE, Bethea CL. Ovarian steroid action on tryptophan hydroxylase protein and serotonin compared to localization of ovarian steroid receptors in midbrain of guinea pigs. Endocrine 1999;11:257–67. [DOI] [PubMed] [Google Scholar]
- 251.Bethea CL, Brown NA, Kohama SG. Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology 1996;137:4372–83. [DOI] [PubMed] [Google Scholar]
- 252.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 2002;23:41–100. [DOI] [PubMed] [Google Scholar]
- 253.Palacios JM, Pazos A, Hoyer D. A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology 2017;234:1395–418. [DOI] [PubMed] [Google Scholar]
- 254.Hoffman BJ, Hansson SR, Mezey É, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol 1998;19:187–231. [DOI] [PubMed] [Google Scholar]
- 255.Rubinow DR, Schmidt PJ, Roca CA. Estrogen–serotonin interactions: implications for affective regulation. Biol Psychiatry 1998;44:839–50. [DOI] [PubMed] [Google Scholar]
- 256.Weissman M, Olfson M. Depression in women: implications for health care research. Science 1995;269:799–801. [DOI] [PubMed] [Google Scholar]
- 257.Melasch J, Rullmann M, Hilbert A, et al. Sex differences in serotonin–hypothalamic connections underpin a diminished sense of emotional well-being with increasing body weight. Int J Obes 2016;40:1268. [DOI] [PubMed] [Google Scholar]
- 258.Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: a review. Neurosci Biobehav Rev 2011;35:1419–49. [DOI] [PubMed] [Google Scholar]
- 259.Eckel LA, Rivera HM, Atchley DPD. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am J Physiol Regul Integr Comp Physiol 2005;288:R1486–R91. [DOI] [PubMed] [Google Scholar]
- 260.Currie PJ, Braver M, Mirza A, Sricharoon K. Sex differences in the reversal of fluoxetine-induced anorexia following raphe injections of 8-OH-DPAT. Psychopharmacology 2004;172:359–64. [DOI] [PubMed] [Google Scholar]
- 261.Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav 2005;86:331–7. [DOI] [PubMed] [Google Scholar]
- 262.Souquet AM, Rowland NE. Dexfenfluramine: action with estradiol on food intake and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 1990;258:R211–R5. [DOI] [PubMed] [Google Scholar]
- 263.Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci 1996;16:7021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol Psychiatry 2000;47:562–76. [DOI] [PubMed] [Google Scholar]
- 265.Kuhn DM, Arthur R, States JC. Phosphorylation and activation of brain tryptophan hydroxylase: identification of serine-58 as a substrate site for protein kinase A. J Neurochem 1997;68:2220–3. [DOI] [PubMed] [Google Scholar]
- 266.Banik U, Wang G-A, Wagner PD, Kaufman S. Interaction of phosphorylated tryptophan hydroxylase with 14-3-3 proteins. J Biol Chem 1997;272:26219–25. [DOI] [PubMed] [Google Scholar]
- 267.Sumner BEH, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res 1998;59:205–14. [DOI] [PubMed] [Google Scholar]
- 268.Burnet PWJ, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res 1995;676:157–68. [DOI] [PubMed] [Google Scholar]
- 269.Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res 1999;63:325–39. [DOI] [PubMed] [Google Scholar]
- 270.Biver F, Lotstra F, Monclus M, et al. Sex difference in 5HT2 receptor in the living human brain. Neurosci Lett 1996;204:25–8. [DOI] [PubMed] [Google Scholar]
- 271.Sumner BEH, Fink G. Effects of acute estradiol on 5-hydroxytryptamine and dopamine receptor subtype mRNA expression in female rat brain. Mol Cell Neurosci 1993;4:83–92. [DOI] [PubMed] [Google Scholar]
- 272.Sumner BEH, Fink G. Estrogen increases the density of 5-Hydroxytryptamine2A receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol 1995;54:15–20. [DOI] [PubMed] [Google Scholar]
- 273.Sumner BEH, Fink G. The density of 5-hydoxytryptamine2A receptors in forebrain is increased at pro-oestrus in intact female rats. Neurosci Lett 1997;234:7–10. [DOI] [PubMed] [Google Scholar]
- 274.Biegon A, Reches A, Snyder L, McEwen BS. Serotonergic and noradrenergic receptors in the rat brain: modulation by chronic exposure to ovarian hormones. Life Sci 1983;32:2015–21. [DOI] [PubMed] [Google Scholar]
- 275.Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience 1998;83:829–36. [DOI] [PubMed] [Google Scholar]
- 276.Molineaux SM, Jessell TM, Axel R, Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci U S A 1989;86:6793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hoffman BJ, Mezey E. Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain. FEBS Lett 1989;247:453–62. [DOI] [PubMed] [Google Scholar]
- 278.Wolf WA, Schutz LJ. The serotonin 5-HT2C receptor is a prominent serotonin receptor in basal ganglia: evidence from functional studies on serotonin-mediated phosphoinositide hydrolysis. J Neurochem 1997;69:1449–58. [DOI] [PubMed] [Google Scholar]
- 279.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol 1995;351:357–73. [DOI] [PubMed] [Google Scholar]
- 280.Rivera HM, Santollo J, Nikonova LV, Eckel LA. Estradiol increases the anorexia associated with increased 5-HT2C receptor activation in ovariectomized rats. Physiol Behav 2012;105:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sprouse JS, Aghajanian GK. (−)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol 1986;128:295–8. [DOI] [PubMed] [Google Scholar]
- 282.Sharp T, McQuade R, Bramwell S, Hjorth S. Effect of acute and repeated administration of 5-HT1A receptor agonists on 5-HT release in rat brain in vivo. Naunyn Schmiedebergs Arch Pharmacol 1993;348:339–46. [DOI] [PubMed] [Google Scholar]
- 283.Bohmaker K, Eison AS, Yocca FD, Meller E. Comparative effects of chronic 8-OH-DPAT, gepirone and ipsapirone treatment on the sensitivity of somatodendritic 5-HT1A autoreceptors. Neuropharmacology 1993;32:527–34. [DOI] [PubMed] [Google Scholar]
- 284.Singh A, Lucki I. Antidepressant-like activity of compounds with varying efficacy at 5-HT1A receptors. Neuropharmacology 1993;32:331–40. [DOI] [PubMed] [Google Scholar]
- 285.Birzniece V, Johansson IM, Wang MD, Seckl JR, Bäckström T, Olsson T. Serotonin 5-HT(1A) receptor mRNA expression in dorsal hippocampus and raphe nuclei after gonadal hormone manipulation in female rats. Neuroendocrinology 2001;74:135–42. [DOI] [PubMed] [Google Scholar]
- 286.Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience 1999;89:267–77. [DOI] [PubMed] [Google Scholar]
- 287.Raymond JR, Mukhin YV, Gettys TW, Garnovskaya MN. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br J Pharmacol 1999;127:1751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology 2002;27:12. [DOI] [PubMed] [Google Scholar]
- 289.Pecins-Thompson M A Brown N, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res 1998;53:120–9. [DOI] [PubMed] [Google Scholar]
- 290.Mendelson SD, McKittrick CR, McEwen BS. Autoradiographic analyses of the effects of estradiol benzoate on [3H]paroxetine binding in the cerebral cortex and dorsal hippocampus of gonadectomized male and female rats. Brain Res 1993;601:299–302. [DOI] [PubMed] [Google Scholar]
- 291.Osterlund MK, Halldin C, Hurd YL. Effects of chronic 17beta-estradiol treatment on the serotonin 5-HT(1A) receptor mRNA and binding levels in the rat brain. Synapse 2000;35:39–44. [DOI] [PubMed] [Google Scholar]
- 292.Gross-Isseroff R, Biegon A, Voet H, Weizman A. The suicide brain: a review of postmortem receptor/transporter binding studies. Neurosci Biobehav Rev 1998;22:653–61. [DOI] [PubMed] [Google Scholar]
- 293.Malison RT, Price LH, Berman R, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998;44:1090–8. [DOI] [PubMed] [Google Scholar]
- 294.Celada P, Pérez J, Alvarez E, Artigas F. Monoamine oxidase inhibitors phenelzine and brofaromine increase plasma serotonin and decrease 5-hydroxyindoleacetic acid in patients with major depression: relationship to clinical improvement. J Clin Psychopharmacol 1992;12:309–15. [PubMed] [Google Scholar]
- 295.Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology 2002;160:271–82. [DOI] [PubMed] [Google Scholar]
- 296.Holschneider DP, Kumazawa T, Chen K, Shih JC. Tissue-specific effects of estrogen on monoamine oxidase A and B in the rat. Life Sci 1998;63:155–60. [DOI] [PubMed] [Google Scholar]
- 297.Luine VN, Rhodes JC. Gonadal hormone regulation of MAO and other enzymes in hypothalamic areas. Neuroendocrinology 1983;36:235–41. [DOI] [PubMed] [Google Scholar]
- 298.Ortega-Corona BG, Valencia-Sánchez A, Kubli-Garfias C, et al. Hypothalamic monoamine oxidase activity in ovariectomized rats after sexual behavior restoration. Arch Med Res 1994;25:337–40. [PubMed] [Google Scholar]
- 299.Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4 ed: Cambridge University Press; 2013. [Google Scholar]
- 300.D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci 2015;9:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zastrow A, Kaiser S, Stippich C, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry 2009;166:608–16. [DOI] [PubMed] [Google Scholar]
- 302.Keating C, Tilbrook AJ, Rossell SL, Enticott PG, Fitzgerald PB. Reward processing in anorexia nervosa. Neuropsychologia 2012;50:567–75. [DOI] [PubMed] [Google Scholar]
- 303.Dewey S, Smith G, Logan J, et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci 1992;12:3773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.McGinnis MY, Gordon JH, Gorski RA. Influence of γ-aminobutyric acid on lordosis behavior and dopamine activity in estrogen primed spayed female rats. Brain Res 1980;184:179–91. [DOI] [PubMed] [Google Scholar]
- 305.Andrade R, Malenka R, Nicoll R. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science 1986;234:1261–5. [DOI] [PubMed] [Google Scholar]
- 306.Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience 1997;77:155–66. [DOI] [PubMed] [Google Scholar]
- 307.Paladini CA, Roeper J. Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience 2014;282:109–21. [DOI] [PubMed] [Google Scholar]
- 308.Beier Kevin T, Steinberg Elizabeth E, DeLoach Katherine E, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 2015;162:622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yang H, de Jong JW, Tak Y, Peck J, Bateup HS, Lammel S. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 2018;97:434–49. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Oberlander JG, Woolley CS. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci 2016;36:2677–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Febo M, Segarra AC. Cocaine alters GABAB-mediated G-protein activation in the ventral tegmental area of female rats: modulation by estrogen. Synapse 2004;54:30–6. [DOI] [PubMed] [Google Scholar]
- 312.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci 1998;18:2550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Yokomaku D, Numakawa T, Numakawa Y, et al. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol 2003;17:831–44. [DOI] [PubMed] [Google Scholar]
- 314.Wei J, Yuen EY, Liu W, et al. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry 2013;19:588. [DOI] [PubMed] [Google Scholar]
- 315.Bredemann TM, McMahon LL. 17β Estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology 2014;42:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci 1996;16:6830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Adams MM, Fink SE, Janssen WGM, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol 2004;474:419–26. [DOI] [PubMed] [Google Scholar]
- 318.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci 1997;17:1848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol 1999;81:925–9. [DOI] [PubMed] [Google Scholar]
- 320.Sarfi M, Elahdadi Salmani M, Goudarzi I, Lashkar Boluki T, Abrari K. Evaluating the role of astrocytes on β-estradiol effect on seizures of Pilocarpine epileptic model. Eur J Pharmacol 2017;797:32–8. [DOI] [PubMed] [Google Scholar]
- 321.Sárvári M, Kalló I, Hrabovszky E, Solymosi N, Rodolosse A, Liposits Z. Long-term estrogen receptor beta agonist treatment modifies the hippocampal transcriptome in middle-aged ovariectomized rats. Front Cell Neurosci 2016;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Viveros M-P, Bermúdez-Silva F-J, Lopez-Rodriguez A-B, Wagner EJ. The endocannabinoid system as a pharmacological target derived from its CNS role in energy homeostasis and reward: applications in eating disorders and addiction. Pharmaceuticals 2011;4:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci 2012;367:3216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Van Sickle MD, Duncan M, Kingsley PJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005;310:329–32. [DOI] [PubMed] [Google Scholar]
- 325.Gong J-P, Onaivi ES, Ishiguro H, et al. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res 2006;1071:10–23. [DOI] [PubMed] [Google Scholar]
- 326.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998;83:393–411. [DOI] [PubMed] [Google Scholar]
- 327.Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 1990;87:1932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Jourdan T, Djaouti L, Demizieux L, Gresti J, Vergès B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 2010;59:926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One 2008;3:e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001;410:822. [DOI] [PubMed] [Google Scholar]
- 331.Verty ANA, McGregor IS, Mallet PE. Paraventricular hypothalamic CB1 cannabinoid receptors are involved in the feeding stimulatory effects of Δ9-tetrahydrocannabinol. Neuropharmacology 2005;49:1101–9. [DOI] [PubMed] [Google Scholar]
- 332.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 2001;134:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ishiguro H, Onaivi E, Horiuchi Y, et al. Functional polymorphism in the GPR55 gene is associated with anorexia nervosa. Synapse 2011;65:103–8. [DOI] [PubMed] [Google Scholar]
- 334.Schoffelmeer ANM, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and μ opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology 2006;51:773–81. [DOI] [PubMed] [Google Scholar]
- 335.Sperlágh B, Windisch K, Andó RD, Sylvester Vizi E. Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem Int 2009;54:452–7. [DOI] [PubMed] [Google Scholar]
- 336.Zhou Y, Huang T, Lee F, Kreek MJ. Involvement of endocannabinoids in alcohol “binge” drinking: studies of mice with human fatty acid amide hydrolase genetic variation and after CB1 receptor antagonists. Alcohol Clin Exp Res 2016;40:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Moreira FA, Jupp B, Belin D, Dalley JW. Endocannabinoids and striatal function: implications for addiction-related behaviours. Behav Pharmacol 2015;26:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci USA 2010;107:20535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LAM. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol 2009;30:343–57. [DOI] [PubMed] [Google Scholar]
- 340.Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol 2007;18:563–9. [DOI] [PubMed] [Google Scholar]
- 341.Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 2007;18:27–37. [DOI] [PubMed] [Google Scholar]
- 342.Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol 2009;622:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol 2010;160:724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kohn L, Kittel F, Piette D. Peer, family integration and other determinants of cannabis use among teenagers. ijamh 2004;16:359. [DOI] [PubMed] [Google Scholar]
- 345.Tu AW, Ratner PA, Johnson JL. Gender differences in the correlates of adolescents’ cannabis use. Subst Use Misuse 2008;43:1438–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril 2010;93:1989–96. [DOI] [PubMed] [Google Scholar]
- 347.Habayeb OMH, Taylor AH, Evans MD, et al. Plasma levels of the endocannabinoid anandamide in women--a potential role in pregnancy maintenance and labor? J Clin Endocrinol Metab 2004;89:5482–7. [DOI] [PubMed] [Google Scholar]
- 348.Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage 2005;29:358–67. [DOI] [PubMed] [Google Scholar]
- 349.Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995;10:89–97. [DOI] [PubMed] [Google Scholar]
- 350.Nelson K, Walsh D, Deeter P, Sheehan F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J Palliat Care 1994;10:14–8. [PubMed] [Google Scholar]
- 351.Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the cannabis-study-group. J Clin Oncol 2006;24:3394–400. [DOI] [PubMed] [Google Scholar]
- 352.Pi-Sunyer F, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, f RI-NASG. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: Rio-north america: a randomized controlled trial. JAMA 2006;295:761–75. [DOI] [PubMed] [Google Scholar]
- 353.Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int J Eat Disord 2014;47:18–23. [DOI] [PubMed] [Google Scholar]
- 354.Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol 2010;160:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.González S, Bisogno T, Wenger T, et al. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun 2000;270:260–6. [DOI] [PubMed] [Google Scholar]
- 356.Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav 2004;80:611–6. [DOI] [PubMed] [Google Scholar]
- 357.Diaz S, Farhang B, Hoien J, et al. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology 2009;89:424–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Corchero J, García-Gil L, Manzanares J, Fernández-Ruiz JJ, Fuentes JA, Ramos JA. Perinatal Δ9-tetrahydrocannabinol exposure reduces proenkephalin gene expression in the caudate-putamen of adult female rats. Life Sci 1998;63:843–50. [DOI] [PubMed] [Google Scholar]
- 359.Vela G, Martín S, García-Gil La, et al. Maternal exposure to Δ9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central μ opioid receptors in adult offspring female rats. Brain Res 1998;807:101–9. [DOI] [PubMed] [Google Scholar]
- 360.Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Δ9-tetrahydrocannabinol in male and female rats. Eur J Pharmacol 2008;578:37–42. [DOI] [PubMed] [Google Scholar]
- 361.Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of Δ9-tetrahydrocannabinol in the rat. Biochem Pharmacol 1991;41:1187–94. [DOI] [PubMed] [Google Scholar]
- 362.Tseng AH, Craft RM. CB1 receptor mediation of cannabinoid behavioral effects in male and female rats. Psychopharmacology 2004;172:25–30. [DOI] [PubMed] [Google Scholar]
- 363.Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 2010;35:1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.de Fonseca FR, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci 1994;54:159–70. [DOI] [PubMed] [Google Scholar]
- 365.Mize AL, Alper RH. Acute and long-term effects of 17β-estradiol on Gi/o coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPγS binding. Brain Res 2000;859:326–33. [DOI] [PubMed] [Google Scholar]
- 366.Henricks AM, Berger AL, Lugo JM, et al. Sex differences in alcohol consumption and alterations in nucleus accumbens endocannabinoid mRNA in alcohol-dependent rats. Neuroscience 2016;335:195–206. [DOI] [PubMed] [Google Scholar]
- 367.Zhu Z, Liu X, Senthil Kumar SPD, Zhang J, Shi H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm Behav 2013;63:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


