Abstract
Objectives:
To provide normative values for isometric strength of 8 different muscle groups among nursing home residents and to investigate their predictive value for the decline of autonomy.
Methods:
This is an analysis of the 1-year follow-up of the SENIOR cohort. At baseline, isometric muscle strength of residents has been assessed for 8 muscle groups using the MicroFET2. The cut-off threshold for low relative isometric muscle strength was defined as the lower quartile. The outcome was the 1-year loss of autonomy (i.e. a decrease of ≥1 point on the ADL scale between baseline and 12-month follow-up). Logistic regressions were carried out to assess the predictive value of isometric muscle strength for the loss of autonomy.
Results:
204 subjects (83.2±8.99 years, 72.5% women) were included. Threshold values of isometric strength were: knee flexors=0.94, knee extensors=1.07, ankle flexors=0.77, ankle extensors=0.88, hip abductors=0.78, hip extensors=0.79, elbow flexors=0.99 and elbow extensors= 0.71 N/kg. After adjustment for age and sex, the cut-off values for knee extensors (p=0.04) and for ankle extensors (p=0.03) were significantly predictive of loss of autonomy.
Conclusions:
The normative values for knee extensors and ankle extensors are independent predictors for loss of autonomy.
Keywords: Isometric Strength, Normative Data, Loss οf Autonomy, Dependence, Nursing Home
Background
Recent clinical and epidemiological studies have shown that muscle strength of middle age and older adults is an important marker of current health, and it is essential to follow people during ageing, injury and rehabilitation[1]. The change in muscle function contributes to the onset and progression of disability and frailty[2,3]. Moreover, muscle strength is associated with motor skills and locomotion[4], one of the five domains (locomotion, sensory, cognition, psychological, vitality) constituting the intrinsic capacity construct defined by the WHO[5]. Muscle strength is an important predictor of dependence[6,7]. Indeed, previous cross-sectional studies have demonstrated that muscle strength is significantly associated with functional limitations such as walking speed[8,9]. Moreover, a minimum level of muscle strength is needed to carry out necessary tasks, such as walking, dressing, etc. Below this minimum threshold level, people are unable to complete these tasks[6].
As the general population increases in age, there is a need for a valid and reliable tool that can quickly and accurately screen for autonomy decline[10]. Autonomy decline is a term used to reflect the loss of an individual’s ability to independently and safely perform activities of daily living (ADL) (usually at home, in the community)[11]. Basic ADL include the fundamental skills typically needed to manage basic physical needs (i.e. everyday tasks), comprised the following areas: bathing, dressing, feeding, continence, transferring, and toileting[12] and instrumental ADL address higher-level tasks, related to independent living in the community (shopping, driving, managing medication and banking)[13]. In this study performed in nursing home setting, we focus on the assessment to independently carry out basic ADL.
A normal part of ageing is the progressive and significant decrease in muscle function (i.e., muscle strength and muscle performance)[4]. Maximum muscle strength occurs between the 20th and 30th years of life[14]. Pronounced changes in the ageing process take place after the 40th year of life[14]. An annual loss of muscle strength of 1.5% is observed up to age 60, and after the sixth decade, the decline amounts to 3% per year[15]. In the literature, muscle function beyond 80 years has not been quantified, specifically in the nursing home setting.
The criterion-referenced assessment of muscle strength involves fixed laboratory-based dynamometers (i.e., isokinetic devices)[16]. A limitation of laboratory-based dynamometers is that they are expensive and not hand-held, which precludes their use as a clinically feasible device in routine patient assessment and in specific conditions (i.e., nursing home settings)[17]. Thus, low-cost and portable dynamometers are an appropriate and convenient method to assess muscle strength in clinical and research practice, if they have been previously validated[16,18]. We previously validated the MicroFET2 hand-held dynamometer (Hoggan Industries, Inc., West Jordan, UT, USA) for obtaining muscle strength measures in a nursing home setting[18]. We concluded that, by using standardized protocol and delivering standardized instructions to patients, the test-retest (intra- and interobserver) of the muscle strength assessed on eight muscle groups with the MicroFET2 dynamometer is highly reliable, excepted for the ankle extensors (intra- and interobserver) and for ankle flexors (intra-observer), making this hand-held dynamometer a potential tool for research in the elderly population. However, these devices still have drawbacks, particularly the absence of large-sample normative data, especially for lower extremity muscle groups and for a specific population such as nursing home residents. The normative data is device dependant[19]. It has been suggested that normative values differ between different equipments and are not interchangeable.
Since the 1980s, several studies were performed with the aim to establish reference values for muscle strength for some of these equipment or methods[20]. Admittedly, it is important to define a clinically standardized cut-off point to identify dynapenic individuals (subjects who have a loss of muscle strength) using isometric strength, which could lead to the efficient identification of patients at risk of autonomy decline[21]. Indeed, early detection of older adults at risk for losing physical independence and better comprehension of the associated risk factors are key determinants for healthy ageing[22]. The use of the cut-off for muscle strength data can help to identify patients who are at a higher risk of loss of autonomy. As strength is highly modifiable, strength tests could be a feasible method for early screening of the population to identify those at risk[6]. In these persons, exercise intervention could improve strength and potentially lower the risk of subsequent loss of autonomy. Thus, this paper aimed to provide normative values for isometric strength, normalized to body weight, of 8 different muscle groups for nursing home residents and to investigate the usefulness of isometric strength as a predictor of autonomy decline among nursing home residents.
Methods
Study design and population
This is an analysis of the 1-year follow-up of the SENIOR cohort (Sample of Elderly Nursing home Individuals: an Observational Research)[23]. The cohort was composed of 662 residents from 28 nursing homes in Liège, Belgium. All the initial data were collected between November 2013 and August 2015 (T0). A second assessment was performed after one year of follow-up (T12). The selection criteria of the SENIOR population were: (1) living in a nursing home in the Province of Liège that was included in this study; (2) being oriented, to provide informed consent and understand the tests; and (3) being able to walk and stand, including with technical assistance. No specific exclusion criterion was defined. All subjects who had both isometric muscle strength assessment at T0 and level of autonomy assessment at T0 and T12 were included in the current analysis. The study was approved by the Ethics Committee of the University Teaching Hospital of Liège under number 2013/178.
Study parameters
Isometric strength of 8 different muscle groups
Maximal isometric muscle strength from 8 different muscle groups (knee extensors and flexors, hip abductors and extensors, ankle flexors and extensors, elbow flexors and extensors) was measured by the investigators (i.e. PhD student, M.Sc student) using a MicroFET2 hand-held dynamometer (Hogan Health Industries, Inc., 8020 South 1300 West, West Jordan, UT, USA). These muscles were chosen because of their strong involvement in the movements of daily living and, therefore, their importance in maintaining autonomy[24-26]. Measurements were performed on the dominant side (writing hand and kicking leg). The protocol was standardized[17]. It consisted of three consecutive maximal contractions for each muscle group, preceded by 3 warm-up trials. Subjects were first shown the movement to be tested and then asked to perform it to confirm their understanding of the movement. Finally, the warm-up trials were performed. The three measurements were taken with 30 seconds intervals between contractions. Subjects were asked to gradually increase their muscle force to a maximum effort which had to be sustained for six seconds. Investigators (i.e. Trained PhD student, M.Sc student) provided standardized encouragement. The highest performance measurement was considered for analysis. The following sequence was employed: knee flexors, knee extensors, ankle flexors, ankle extensors, hip abductors, hip extensors, elbow flexors and elbow extensors. The precise testing positions are described within the literature[17].
Decline of autonomy
The decline of autonomy was defined as a ≥ 1-point decrease on the Katz ADL scale between baseline and 12-month follow-up[12,27]. The Katz ADL scale contains six items (bathing, dressing, toileting, transferring, continence and feeding). A score ranging from 1 to 4 is attributed to each item depending on how independent the individual is when performing the activity. Higher scores indicate higher dependence in ADLs[28].
Demographic characteristics
All subjects were interviewed by a clinical research assistant at baseline to obtain the socio-demographic data. These data included age, gender, weight (to the nearest 0.1 kg) and BMI. Residents were considered having participated in the exercise classes if they attended exercise classes organized by the nursing home in the last two weeks prior to the evaluation. To ensure that residents understood the exercise techniques, cognitive abilities were previously assessed with the Mini-Mental State Examination (MMSE), which consists of a 30-item questionnaire. A maximum score of 30 is attainable for a person without any neuropsychological impairments. Any score greater than or equal to 27 points indicates normal cognition. Below this cut-off, scores can indicate severe (9 points), moderate (10-18 points) or mild (19-24 points) cognitive impairment[29].
Statistical analysis
Quantitative variables that were normally distributed were expressed as a mean ± standard deviation, and quantitative variables that were not normally distributed were reported as the median and percentiles (25th-75th). The Shapiro-Wilk test verified the normal distribution of all parameters. Qualitative variables were reported as absolute and relative frequencies (%). Comparison of isometric strength (i.e., for eight different muscle groups) between subjects with autonomy decline (i.e. ≥1-point decrease on the Katz ADL scale between baseline and 12-month follow-up) and subjects without autonomy decline was assessed by means of the Student’s t-test.
The cut-off value of isometric strength of each muscle was calculated using the lowest quartile point. Then, the association between isometric muscle strength cut-off value and the decline of autonomy was assessed, in univariate analysis, using the Chi-squared test. Logistic regression analyses were used to test this association, in multivariate analysis (i.e., adjustment on age and sex). Finally, sensibility, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the muscle strength were calculated[30], and the usefulness of the cut-off values for isometric strength to predict the decline of autonomy was assessed by means of the area under the ROC curve (AUC). An AUC value of 0.5 indicates no discriminative value. ROC curves with an AUC ≤0.75 are not clinically useful and an AUC of 0.97 has a very high clinical value[31]. By means of a Chi-squared test, post hoc analyses were also conducted to compare the risk of autonomy decline between the weakest 25% and the strongest 25% subjects.
The data analyses were performed using Statistica12 software (TIBCO Statistica, Palo Alto, CA). The results were considered statistically significant when the 2-tailed P values were less than.05.
Results
Sample
Out of the 662 residents from the SENIOR cohort, 204 were included in the current analysis. The excluded residents did not have complete assessments (i.e., isometric strength assessment at T0, as well as assessment of the level of autonomy at T0 and T12). The mean age of the sample was 83.7±8.52 years, with 71.5% of the sample being female. The average weight of the population was 65.7±14.8 kg and the mean BMI was 26.3±5.78 kg/m2. The mean MMSE score was 24.8±4.28 points. About a quarter of the population, 23.8% took part in exercise class proposed in nursing home. Within the studied sample, 66 (32.4%) subjects had a loss of autonomy whereas 138 (67.6%) subjects did not lose autonomy over the 12 months of follow-up. Note that both groups were comparable in terms of age (p=0.13) and gender (p=0.29).
The development of isometric muscle strength reference thresholds
[Table 1] shows the mean isometric strength normalized to body weight in the sample, for residents with loss of autonomy and those without loss of autonomy.
Table 1.
Isometric strength normalized to body weight for each muscle group, according to autonomy decline.
| Isometric strength (N/kg) | Total population (n=204) | Autonomy decline (n=66) | No autonomy decline (n=138) | p-value |
|---|---|---|---|---|
| Knee flexors | 1.29 ± 0.51 | 1.25 ± 0.47 | 1.33 ± 0.48 | 0.26 |
| Knee extensors | 1.58 ± 0.86 | 1.45 ± 0.62 | 1.75 ± 0.95 | 0.04 |
| Ankle flexors | 1.16 ± 0.60 | 1.04 ± 0.52 | 1.34 ± 0.72 | 0.09 |
| Ankle extensors | 1.37 ± 0.70 | 1.27 ± 0.58 | 1.46 ± 0.58 | 0.03 |
| Hip abductors | 1.18 ± 0.51 | 1.09 ± 0.46 | 1.23 ± 0.54 | 0.09 |
| Hip extensors | 1.27 ± 0.62 | 1.16 ± 0.52 | 1.40 ± 0.69 | 0.02 |
| Elbow flexors | 1.36 ± 0.53 | 1.37 ± 0.54 | 1.45 ± 0.54 | 0.33 |
| Elbow extensors | 0.96 ± 0.38 | 0.94 ± 0.37 | 1.01 ± 0.36 | 0.27 |
Subjects with decline of autonomy had lower isometric muscle strength for knee extensors (p=0.04), ankle extensors (p=0.03) and hip extensors (p=0.02), compared to the subjects without decline of autonomy.
Note that no significant difference was observed for isometric muscle strength normalized to body weight between men and women (p-values= 0.05; 0.40; 0.09; 0.08; 0.84; 0.74 for knee extensors, ankle flexors and extensors, hip extensors, elbow flexors and extensors, respectively), with the exception of knee flexors (p=0.04) and hip abductors (p=0.03). Therefore, the analyses will not be separated by gender in this article.
To create isometric muscle strength reference thresholds for nursing home residents, the cut-off value of the isometric strength of each muscle was calculated using the lowest quartile point (lowest 25%) (Table 2).
Table 2.
Isometric muscle strength reference thresholds for 8 different muscle groups.
| Muscle groups | Isometric strength (N/kg) | ||
|---|---|---|---|
| P25 valu | |||
| Total population | Women | Men | |
| Knee flexors | 0.94 | 0.89 | 1.06 |
| Knee extensors | 1.07 | 1.01 | 1.26 |
| Ankle flexors | 0.77 | 0.72 | 0.85 |
| Ankle extensors | 0.88 | 0.84 | 0.97 |
| Hip abductors | 0.78 | 0.72 | 0.93 |
| Hip extensors | 0.79 | 0.74 | 0.95 |
| Elbow flexors | 0.99 | 0.90 | 1.27 |
| Elbow extensors | 0.71 | 0.65 | 0.93 |
These threshold values ranged between 0.71 (elbow extensors) to 1.07 N/kg (knee extensors) for the total population. As expected, the values were higher for men than women. The difference according to gender is more marked for some muscle groups (e.g., elbow flexors and extensors).
Association between isometric muscle strength reference thresholds and the decline of autonomy
The thresholds values for isometric strength (P25) were significantly associated with the decline of autonomy for knee extensors, ankle flexors and ankle extensors (p=0.03, 0.04 and 0.0009, respectively) in univariate analyses (Table 3). Indeed, subjects with muscle strength lower than P25 are at higher risk for loss of autonomy.
Table 3.
Association between isometric muscle strength reference thresholds and the decline of autonomy.
| Muscle groups | Loss of autonomy OR (95% CI) | p-value | p-value* | AUC (95% CI) | Sensibility (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Knee flexors | 0.44 (0.76-2.75) | 0.55 | 0.56 | 0.56 (0.48-0.65) | 38.5 | 75.4 | 71.6 | 43.1 |
| Knee extensors | 1.63 (1.03-2.59) | 0.03 | 0.04 | 0.60 (0.52-0.58) | 50 | 73 | 77.9 | 40.7 |
| Ankle flexors | 1.94 (1.03-3.67) | 0.04 | 0.09 | 0.61 (0.53-0.70) | 47.7 | 74.6 | 74.2 | 47.7 |
| Ankle extensors | 1.77 (1.02-3.05) | 0.009 | 0.03 | 0.59 (0.50-0.67) | 62 | 82 | 74.3 | 42 |
| Hip abductors | 1.69 (0.90-3.19) | 0.19 | 0.13 | 0.57 (0.48-0.65) | 29.5 | 99.9 | 85.7 | 38.6 |
| Hip extensors | 1.87 (0.80-3.24) | 0.55 | 0.30 | 0.60 (0.51-0.68) | 59.5 | 64 | 77.4 | 47.7 |
| Elbow flexors | 1.32 (0.75-2.32) | 0.53 | 0.57 | 0.56 (0.47-0.64) | 55.4 | 59.3 | 73.4 | 42.9 |
| Elbow extensors | 1.60 (0.69-3.69) | 0.79 | 0.44 | 0.56 (0.47-0.64) | 38.5 | 72.6 | 71 | 40.3 |
p-value adjusted for age and sex. Legend: AUC= Area Under the Curve, PPV= Positive Predictive Value, NPV= Negative Predictive Value.
After adjustment for age and sex, the reference threshold for isometric strength of knee extensors was independently associated with autonomy decline (OR=1.63, 95% CI=1.03-2.59, p=0.04). Subjects with low isometric strength of the knee extensors are 1.63 times more likely to have a loss of autonomy over a period of one year.
The reference threshold for isometric strength of ankle extensors was independently associated with autonomy decline, after adjustment for age and sex (OR=1.77, 95% CI=1.02-3.05, p=0.03). Subjects with low isometric strength of the ankle extensors are almost twice as likely to have a loss of autonomy over a period of one year.
The AUC value for the ankle extensors is 0.57 (sensibility= 62% and specificity= 82%; VPP= 74.3%; VPN= 42%). From a public health point of view, if we want to rely upon the specificity of the measurement (specificity of 90%) or the true negative rate, the cut-off value for isometric strength of the ankle extensors is 0.74 kg/m2. For this threshold value, the specificity of the measurement is low (19.5%), the VPP is 78.8% and the VPN is 35.2%. In this circumstance, the isometric strength test could be used for screening the population. More robust follow-up tests should then be used for the diagnostic (Figure 1).
Figure 1.
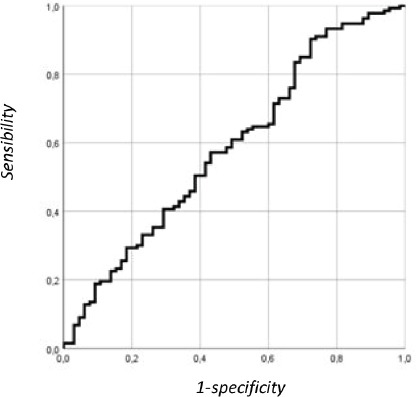
ROC curve illustrating the discriminatory power of ankle extensor strength to assess loss of autonomy.
The AUC value for the knee extensors is 0.60 (0.52-0.58) (sensibility= 50% and specificity= 73%; VPP= 77.9%; VPN= 40.7%). From a public health point of view, if we want to rely upon the specificity of the measurement (specificity of 90%) or true negative rate, the cut-off value for isometric strength of the knee extensors is 0.73 kg/m2. For this threshold value, the specificity of the measurement is low (24.6%), the VPP is 82.5% and the VPN is 36.5%. In this circumstance, the isometric strength test could be used for screening the population. More robust follow-up tests should then be used for the diagnostic of the population (Figure 2).
Figure 2.
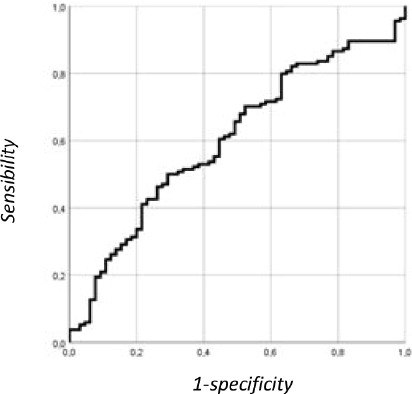
ROC curve illustrating the discriminatory power of knee extensor strength to assess loss of autonomy.
Discussion
Increasingly compelling evidence has highlighted the potential role of muscular strength capacity as a protective factor for health across populations[32]. Clinicians often have access to muscle strength measurement tools but are unable to interpret the results (i.e., due to the lack of reference values and the difficulty in choosing “key muscles”). To aid clinicians in the assessment of muscular strength, we determined, by means of this prospective study, the reference values for isometric strength of 8 different muscle groups among physically active nursing home residents. Based on these reference values, the current results showed that, after adjustment for age and sex, poor maximal isometric strength of the knee extensors or ankle extensors was associated with an increased risk of loss of autonomy among nursing home residents. These two muscle groups can, therefore, be considered “key muscles” to evaluate in clinical practice. Hence, the establishment of these normative data is essential not only from an epidemiological point of view to predict adverse health outcomes but also from a clinical point of view to individualize patient care. The assessment of muscle strength, using the normative data for MicroFET2, would make it possible to evaluate one of the 5 domains of intrinsic capacities, namely, locomotion.
As there is substantial covariance between strength capacity and body mass, and, moreover, that the link between muscle strength and both physical function and chronic health is directly mediated by the proportion of strength relative to body mass, these new reference values have been normalized to body weight[32,33]. The significant correlation between body weight and muscle strength is not surprising because joints of the lower limbs must support body weight and muscle strength plays a role in joint stability[34]. In addition, relative strength is more discriminative than absolute strength because two subjects with differing body weight and equal muscle strength do not present with the same functional outcome. This is consistent with other recent efforts to identify normative values or t-scores in non-U.S. cohorts and samples[35-38]. Note that in our cohort, the results vary greatly if strength is expressed in absolute value (i.e., no association between absolute strength and loss of autonomy) or in relative value.
To our knowledge, the predictive value of isometric muscle strength in the loss of autonomy has been poorly investigated, specifically in the nursing home setting. Moreover, previous studies have only focussed on specific muscle groups (i.e., grip strength and knee extensors). Thus, the present study could serve to fill the gap in the current literature. This study explored more comprehensively the relationship between the muscle strength of 8 different lower limb and upper limb muscle groups and the loss of autonomy among elderly nursing home residents. The investigated muscles are all strongly involved in the movements of daily life. Our results are in line with a previous finding that muscle strength is a predictor of ADL dependence[6]. In a prospective study performed among community-dwelling people aged 75 years, Rantanen et al. showed that those performing in the lowest third for grip strength, arm flexion strength, knee extension strength, trunk extension strength and trunk flexion strength had the greatest risk of ADL-dependence after 5 years. It is intuitive that the strength of a muscle group directly involved in a specific task is the strongest predictor of disability in that task[6]. The two muscle groups we identified as predictors of loss of autonomy are muscles of the lower limbs (ankle and knee extensors) that play an important role in supporting body weight against gravity and are the most involved in mobility and locomotion[25,39]. Moreover, our study is the first to provide a threshold value for ankle extensor strength in the prediction of loss of autonomy.
The cut-off point for isometric strength of the knee extensors (Percentile 25) in our study is 1.07 N/kg, which is higher than the value observed in the study by Rantanen et al. (363 kg=37.02 N= ±0.56 N/kg)[6]. This difference can be explained by the protocol of muscle strength assessment, which is different between the study by Rantanen and our study (e.g., 1st tertile in the study by Rantanen and 1st quartile in our study; angle of 60° in the study by Rantanen and 90° in our study; the mean of three measurements was taken for the analysis in the study by Rantanen, whereas the best value was used in our study; the dynamometer used varies between studies). Moreover, the study by Rantanen was conducted in northern countries (i.e., Denmark, Sweden, and Finland) and our study was conducted in Belgium. We can hypothesize that the health status of nursing home populations is different between these countries (i.e., the definition of nursing home is country specific). As muscle strength measurements show a great deal of variation across geographical regions and national contexts, it is important to have region-specific reference values[40]. Compared to the population studied by Rantanen, our population was older (87.7 vs. 75 years old). Moreover, our population had a lower mean body weight compared to the population studied by Rantanen (65.7±14.8 vs. 75.8±10.5 kg). It is also important to note that our sample was predominantly female; this ratio is comparable to the sex ratio that is generally present in nursing homes due to the longer life expectancy for women compared to men.
For screening purposes, the threshold values for isometric knee extensor and ankle extensor strength could be identified as 0.73 and 0.74 kg/m2, respectively. With these values, the specificity of the measurement to predict the loss of autonomy is 90%; however, the sensibility is very low. Given this circumstance, the isometric strength tests could be used as a first line of testing, for screening, but more robust tests should follow for diagnostic. Previous studies had suggested that the MicroFET2 may be acceptable to recommend its use at the individual level (i.e., for screening purposes)[41] and could be used for research purposes at the population level[17].
Identifying interventions to prevent or delay the loss of autonomy is currently a public health priority in the successful management of the ageing population[42]. From a public health point of view, a preventive strategy should be implemented in nursing homes to improve the muscle strength of knee extensors and ankle extensors. In addition, from a clinical point of view, individualized strategies should be developed to manage muscle weakness. A systematic measurement of muscle strength is required for this individualized process.
Our study has certain limitations. First, due to the selection criteria of the study population, our results cannot be extrapolated to all nursing home residents, but they are applicable for physically active residents. Second, we used P25 to define the cut-off value of isometric strength; the results could be different with another defined cut-off point. Potential confounding factors related to loss of autonomy were not considered (e.g., the presence of multiple diseases, genetic factors). Nevertheless, according to Rantanen, adjustment for chronic diseases (such as diabetes, stroke, arthritis, chronic obstructive pulmonary disease and coronary heart disease) did not materially decrease the predictive power of muscle strength on loss of autonomy[6]. This could be explained because the effect of disease on disability is mediated through decline in muscle in muscle strength[43]. Third, the brief period of one year between the first and second assessments might have influenced the lack of association between isometric strength of certain muscles and loss of autonomy. It would be interesting to confirm our results after a longer-term period before follow-up. Finally, about a quarter of our population participated in exercise class proposed in nursing home, which may have led to an increase in mean strength. Consequently, the latter may be overestimated compared to the general population in a nursing home.It has been established that muscle strength can increase substantially through physical exercise and strength training at all ages, even in the frailest subset of older people[44,45]. Therefore, the threshold defined in the present study offers an interesting perspective from a public health perspective, to consider if weak people who improve their muscle strength also improve their autonomy.
In conclusion, this study presented reference values for the isometric strength of 8 different muscle groups for people in the nursing home setting, derived from the SENIOR population. Of these threshold values, two are independent predictors for a loss of autonomy (i.e., knee extensors and ankle extensors). From a public health point of view, the isometric strength test, which is acceptable and suitable for an elderly population, could be used at the population level in research settings and at the individual level in clinical practice for screening purposes. The prediction of loss of autonomy, achieved through a relatively simple measure of isometric strength, could be useful for assessing locomotion, a key domain of intrinsic capacities.
Acknowledgements
The authors would like to thank all the participants who participated in this study. We also thank directory and healthcare staff from the nursing homes for their collaboration in the study.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Turusheva A, Frolova E, Degryse JM. Age-related normative values for handgrip strength and grip strength's usefulness as a predictor of mortality and both cognitive and physical decline in older adults in northwest Russia. Journal of musculoskeletal & neuronal interactions. 2017;17(1):417–432. [PMC free article] [PubMed] [Google Scholar]
- 2.Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. The journal of nutrition, health & aging. 2008;12(7):493–8. doi: 10.1007/BF02982711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckinx F, Rolland Y, Reginster J-Y, Ricour C, Petermans J, Bruyère O. Burden of frailty in the elderly population:perspectives for a public health challenge. Archives of Public Health. 2015;73:19. doi: 10.1186/s13690-015-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckinx F, Croisier JL, Reginster JY, Petermans J, Goffart E, Bruyere O. Relationship between Isometric Strength of Six Lower Limb Muscle Groups and Motor Skills among Nursing Home Residents. The Journal of frailty & aging. 2015;4(4):184–7. doi: 10.14283/jfa.2015.70. [DOI] [PubMed] [Google Scholar]
- 5.Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Cooper C, Martin FC, Reginster JY, Vellas B, Beard JR. Evidence for The Domains Supporting The Construct of Intrinsic Capacity. J Gerontol A Biol Sci Med Sci. 2018 Feb 2; doi: 10.1093/gerona/gly011. [DOI] [PubMed] [Google Scholar]
- 6.Rantanen T, Avlund K, Suominen H, Schroll M, Frandin K, Pertti E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res. 2002;14(3 Suppl):10–5. [PubMed] [Google Scholar]
- 7.Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging clinical and experimental research. 2004;16(6):481–6. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Guralnik JM, Buchner D, Kasper J, Lamb SE, Simonsick EM, Corti MC, Bandeen-Roche K, Fried LP. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities:the Women's Health and Aging Study. The journals of gerontology Series A, Biological sciences and medical sciences. 1997;52(5):M275–85. doi: 10.1093/gerona/52a.5.m275. [DOI] [PubMed] [Google Scholar]
- 9.Rantanen T, Guralnik JM, Izmirlian G, Williamson JD, Simonsick EM, Ferrucci L, Fried LP. Association of muscle strength with maximum walking speed in disabled older women. American journal of physical medicine & rehabilitation. 1998;77(4):299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Beaton K, Grimmer K. Tools that assess functional decline:systematic literature review update. Clinical interventions in aging. 2013;8:485–94. doi: 10.2147/CIA.S42528. doi:10.2147/cia.s42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. Journal of the American Geriatrics Society. 2002;50(6):1045–50. doi: 10.1046/j.1532-5415.2002.50259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz S. Assessing self-maintenance:activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–7. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 13.Graf C. The Lawton Instrumental Activities of Daily Living (IADL) Scale. Medsurg nursing:official journal of the Academy of Medical-Surgical Nurses. 2009;18(5):315–6. [PubMed] [Google Scholar]
- 14.Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles, ligaments and tendons journal. 2013;3(4):346–50. [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 16.Mentiplay BF, Perraton LG, Bower KJ, Adair B, Pua YH, Williams GP, McGaw R, Clark RA. Assessment of Lower Limb Muscle Strength and Power Using Hand-Held and Fixed Dynamometry:A Reliability and Validity Study. PLoS ONE. 2015;10(10):e0140822. doi: 10.1371/journal.pone.0140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckinx F, Croisier JL, Reginster JY, Dardenne N, Beaudart C, Slomian J, Leonard S, Bruyere O. Reliability of muscle strength measures obtained with a hand-held dynamometer in an elderly population. Clinical physiology and functional imaging. 2017;37(3):332–340. doi: 10.1111/cpf.12300. [DOI] [PubMed] [Google Scholar]
- 18.Buckinx F, Croisier JL, Reginster JY, Dardenne N, Beaudart C, Slomian J, Leonard S, Bruyere O. Reliability of muscle strength measures obtained with a hand-held dynamometer in an elderly population. Clin Physiol Funct Imaging. 2015 doi: 10.1111/cpf.12300. [DOI] [PubMed] [Google Scholar]
- 19.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–59. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 20.Benfica PDA, Aguiar LT, Brito SAF, Bernardino LHN, Teixeira-Salmela LF, Faria C. Reference values for muscle strength:a systematic review with a descriptive meta-analysis. Brazilian journal of physical therapy. 2018;22(5):355–369. doi: 10.1016/j.bjpt.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulac M, Boutros GE, Pion C, Barbat-Artigas S, Gouspillou G, Aubertin-Leheudre M. Is handgrip strength normalized to body weight a useful tool to identify dynapenia and functional incapacity in post-menopausal women? Brazilian journal of physical therapy. 2016;20(6):510–516. doi: 10.1590/bjpt-rbf.2014.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical independence in older adults:the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle. 2017;8(2):245–250. doi: 10.1002/jcsm.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckinx F, Reginster JY, Petermans J, Croisier JL, Beaudart C, Brunois T, Bruyere O. Relationship between frailty, physical performance and quality of life among nursing home residents:the SENIOR cohort. Aging Clin Exp Res. 2016 doi: 10.1007/s40520-016-0616-4. [DOI] [PubMed] [Google Scholar]
- 24.Tikkanen O, Haakana P, Pesola AJ, Hakkinen K, Rantalainen T, Havu M, Pullinen T, Finni T. Muscle activity and inactivity periods during normal daily life. PLoS ONE. 2013;8(1):e52228. doi: 10.1371/journal.pone.0052228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of biomechanics. 2001;34(11):1387–98. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 26.Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56(10):B443–8. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- 27.van Abbema D, van Vuuren A, van den Berkmortel F, van den Akker M, Deckx L, Buntinx F, van Kampen R, Lambooij E, de Boer M, de Vos-Geelen J, Tjan-Heijnen VC. Functional status decline in older patients with breast and colorectal cancer after cancer treatment:A prospective cohort study. Journal of geriatric oncology. 2017;8(3):176–184. doi: 10.1016/j.jgo.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of adl:a standardized measure of biological and psychosocial function. Jama. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Tombaugh TN, McIntyre NJ. The mini-mental state examination:a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 30.Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value:the case of tests with continuous results. Biochemia medica. 2016;26(3):297–307. doi: 10.11613/BM.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. Cjem. 2006;8(1):19–20. doi: 10.1017/s1481803500013336. [DOI] [PubMed] [Google Scholar]
- 32.Peterson MD, Krishnan C. Growth Charts for Muscular Strength Capacity With Quantile Regression. Am J Prev Med. 2015;49(6):935–8. doi: 10.1016/j.amepre.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zvijac JE, Toriscelli TA, Merrick WS, Papp DF, Kiebzak GM. Isokinetic concentric quadriceps and hamstring normative data for elite collegiate American football players participating in the NFL Scouting Combine. J Strength Cond Res. 2014;28(4):875–83. doi: 10.1519/JSC.0b013e3182a20f19. [DOI] [PubMed] [Google Scholar]
- 34.Li PY, Yin QS, Hang HY, Li JY, Shen HY, Wang ZJ, Wang Q. [Impact of muscle strength on knee joint stability in static loading] Nan fang yi ke da xue xue bao =Journal of Southern Medical University. 2010;30(12):2625–8. [PubMed] [Google Scholar]
- 35.Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM. Normative values of cognitive and physical function in older adults:findings from the Irish Longitudinal Study on Ageing. J Am Geriatr Soc. 2013;61(Suppl 2):S279–90. doi: 10.1111/jgs.12195. [DOI] [PubMed] [Google Scholar]
- 36.Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, Kirkwood TB, Lawlor DA, Robinson SM, Starr JM, Steptoe A, Tilling K, Kuh D, Cooper C, Sayer AA. Grip strength across the life course:normative data from twelve British studies. PloS one. 2014;9(12):e113637. doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohannon RW, Magasi S. Identification of dynapenia in older adults through the use of grip strength t-scores. Muscle & nerve. 2015;51(1):102–5. doi: 10.1002/mus.24264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spruit MA, Sillen MJ, Groenen MT, Wouters EF, Franssen FM. New normative values for handgrip strength:results from the UK Biobank. J Am Med Dir Assoc. 2013;14(10):775.e5–11. doi: 10.1016/j.jamda.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Freddolini M, Placella G, Gervasi GL, Morello S, Cerulli G. Quadriceps muscles activity during gait:comparison between PFPS subjects and healthy control. Musculoskeletal surgery. 2017;101(2):181–187. doi: 10.1007/s12306-017-0469-9. [DOI] [PubMed] [Google Scholar]
- 40.Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength:a systematic review and meta-analysis of normative data. Age Ageing. 2016;45(2):209–16. doi: 10.1093/ageing/afv192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hars M, Herrmann FR, Trombetti A. Reliability and minimal detectable change of gait variables in community-dwelling and hospitalized older fallers. Gait Posture. 2013;38:1010–4. doi: 10.1016/j.gaitpost.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Romera L, Orfila F, Segura JM, Ramirez A, Moller M, Fabra ML, Lancho S, Bastida N, Foz G, Fabregat MA, Marti N, Cullell M, Martinez D, Gine M, Bistuer A, Cendros P, Perez E. Effectiveness of a primary care based multifactorial intervention to improve frailty parameters in the elderly:a randomised clinical trial:rationale and study design. BMC geriatr. 2014;14:125. doi: 10.1186/1471-2318-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbrugge LM, Jette AM. The disablement process. Social science & medicine (1982) 1994 Jan. 38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 44.Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults:a meta-analysis. Ageing research reviews. 2010;9(3):226–37. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ralston GW, Kilgore L, Wyatt FB, Baker JS. The Effect of Weekly Set Volume on Strength Gain:A Meta-Analysis. Sports Med. 2017;47(12):2585–2601. doi: 10.1007/s40279-017-0762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


