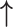Abstract
Background
Adenomyosis commonly occurs with abnormal uterine bleeding (AUB) and is associated with subfertility and a higher miscarriage rate. Recent evidence showed abnormal vascularization in the endometrium in patients with adenomyosis, suggesting a role of angiogenesis in the pathophysiology of AUB and subfertility in adenomyosis and providing a possible treatment target.
Objective and rationale
We hypothesized that the level of abnormal vascularization and expression of angiogenic markers is increased in the ectopic and eutopic endometrium of adenomyosis patients in comparison with the endometrium of control patients. This was investigated through a search of the literature.
Search methods
A systematic search was performed in PubMed and Embase until February 2019. Combinations of terms for angiogenesis and adenomyosis were applied as well as AUB, subfertility or anti-angiogenic therapy. The main search was limited to clinical studies carried out on premenopausal women. Original research articles focusing on markers of angiogenesis in the endometrium of patients with adenomyosis were included. Studies in which no comparison was made to control patients or which were not published in a peer-reviewed journal were excluded. A second search was performed to explore the therapeutic potential of targeting angiogenesis in adenomyosis. This search also included preclinical studies.
Outcomes
A total of 20 articles out of 1669 hits met our selection criteria. The mean vascular density (MVD) was studied by quantification of CD31, CD34, von Willebrand Factor (vWF) or factor-VIII-antibody-stained microvessels in seven studies. All these studies reported a significantly increased MVD in ectopic endometrium, and out of the six articles that took it into account, four studies reported a significantly increased MVD in eutopic endometrium compared with control endometrium. Five articles showed a significantly higher vascular endothelial growth factor expression in ectopic endometrium and three articles in eutopic endometrium compared with control endometrium. The vascular and pro-angiogenic markers α-smooth muscle actin, endoglin, S100A13, vimentin, matrix metalloproteinases (MMPs), nuclear factor (NF)-kB, tissue factor (TF), DJ-1, phosphorylated mammalian target of rapamycin, activin A, folli- and myostatin, CD41, SLIT, roundabout 1 (ROBO1), cyclooxygenase-2, lysophosphatidic acid (LPA) 1,4-5, phospho signal transducer and activator of transcription 3 (pSTAT3), interleukin (IL)-6, IL-22 and transforming growth factor-β1 were increased in ectopic endometrium, and the markers S100A13, MMP-2 and -9, TF, follistatin, myostatin, ROBO1, LPA1 and 4-5, pSTAT3, IL-6 and IL-22 were increased in eutopic endometrium, compared with control endometrium. The anti-angiogenic markers E-cadherin, eukaryotic translation initiation factor 3 subunit and gene associated with retinoic-interferon-induced mortality 19 were decreased in ectopic endometrium and IL-10 in eutopic endometrium, compared with control endometrium. The staining level of vWF and two pro-angiogenic markers (NF-κB nuclear p65 and TF) correlated with AUB in patients with adenomyosis. We found no studies that investigated the possible relationship between markers of angiogenesis and subfertility in adenomyosis patients. Nine articles reported on direct or indirect targeting of angiogenesis in adenomyosis—either by testing hormonal therapy or herbal compounds in clinical studies or by testing angiogenesis inhibitors in preclinical studies. However, there are no clinical studies on the effectiveness of such therapy for adenomyosis-related AUB or subfertility.
Wider implications
The results are in agreement with our hypothesis that increased angiogenesis is present in the endometrium of patients with adenomyosis compared with the endometrium of control patients. It is likely that increased angiogenesis leads to fragile and more permeable vessels resulting in adenomyosis-related AUB and possibly subfertility. While this association has not sufficiently been studied yet, our results encourage future studies to investigate the exact role of angiogenesis in the etiology of adenomyosis and related AUB or subfertility in women with adenomyosis in order to design curative or preventive therapeutic strategies.
Keywords: adenomyosis, angiogenesis, abnormal uterine bleeding, subfertility, anti-angiogenic therapy, endometriosis
Introduction
Adenomyosis is a common gynecological disorder, predominantly occurring in premenopausal women (Bergeron, 2006; Cohen et al., 1995). Adenomyosis may cause abnormal uterine bleeding (AUB) and dysmenorrhea and is associated with a 28% lower clinical pregnancy rate as well as a more than doubled risk of miscarriage in women undergoing IVF with autologous oocytes (Tremellen and Russell, 2011; Martinez-Conejero et al., 2011; Vercellini et al., 2014; Cohen et al., 1995). The main histologic feature of adenomyosis is the presence of endometrial glands and stroma within the myometrium (Bird et al., 1972). The ectopic endometrium is generally associated with smooth muscle hyperplasia (Abbott, 2017). Recently, innovations in ultrasound skill and reporting systems have proven to be of essential value in diagnosing adenomyosis without the need for prior hysterectomy (Van den Bosch et al., 2018; Stoelinga et al., 2018). Therapy options are still scarce and limited to hormonal suppression, hysterectomy, embolization or MRI-guided high-intensity focused ultrasound in experimental settings (de Bruijn et al., 2017; de Bruijn et al., 2017; de Bruijn et al., 2018; Pontis et al., 2016; Dueholm, 2017; Vannuccini et al., 2018; Fan et al., 2012). The pathology of adenomyosis explaining the symptoms of AUB and implantation failure remains inconclusive (Abbott, 2017; Vannuccini et al., 2017). Hypotheses include various biological mechanisms such as alterations in endometrial immunological environment, the increased local estrogen production due to P450 overexpression, alterations in apoptosis and/or increased angiogenesis in the endometrium (Tremellen and Russell, 2012; Brosens et al., 2004; Vannuccini et al., 2017).
Angiogenesis is the process of the outgrowth of new capillary blood vessels from existing blood vessels, which occurs in both physiological and pathological processes (Griffioen and Molema, 2000; Folkman, 1995). It occurs during the proliferative phase of the menstrual cycle when the endometrium is regenerated and is essential for successful embryonic implantation (Burton et al., 2009; Moller et al., 2001). The notion that the ectopic endometrium is surrounded by increased vascularization suggests that the ectopic endometrium releases factors that signal a quiescent vasculature to initiate capillary sprouting. This mechanism was first described in the cancer arena. Tumor cells mutate to start the production of angiogenic factors, which is the initiating event of the formation of new vasculature. This event is referred to as the angiogenic switch (Hanahan and Folkman, 1996) and has motivated the search for both angiogenic factors and angiogenesis inhibitors (Ramjiawan et al., 2017). Estrogen, which is considered a crucial factor in the etiology of adenomyosis, causes mobilization and microvessel incorporation of endothelial progenitor cells resulting in angiogenesis (Chen et al., 2010; Rudzitis-Auth et al., 2016). Abnormal angiogenesis may occur due to modulation of the expression of angiogenesis-regulating genes, such as genes that regulate vascular endothelial growth factor (VEGF). Overexpression of VEGF leads to enhanced vascular sprouting (Hillen and Griffioen, 2007), decreased pericyte coverage and augmented vascular permeability and leakage. Such vasculature is expected to bleed more easily (Weis and Cheresh, 2005).
The role of angiogenesis is well established in endometriosis (Rudzitis-Auth et al., 2016) and has been recognized as a potential treatment target (Laschke and Menger, 2012). Adenomyosis has a strong relationship with endometriosis and can be found in one-third of patients with endometriosis (Larsen et al., 2011). Both endometriosis and adenomyosis are invasive diseases in which the endometrial cells have acquired invasive properties that require angiogenesis to establish at an ectopic site (Kang et al., 2010; Yen et al., 2017). Accordingly, polymorphisms in the angiogenic factor fibroblast growth factor 1 and 2 gene have been found to be associated with the risk of developing both endometriosis and adenomyosis (Kang et al., 2010). In line with endometriosis, where it is known that angiogenesis plays a role in the establishment of ectopic foci outside the uterus, this is also expected to play a role concerning ectopic foci inside the uterus (Groothuis et al., 2005). If angiogenesis exists in the eutopic endometrium, it may be additionally relevant if it is associated with AUB or subfertility. In subfertile patients undergoing surgery for deep endometriosis, pregnancy rates were 68% reduced when patients had coexistent adenomyosis (Vercellini et al., 2014). Although this suggests that adenomyosis negatively impacts the eutopic endometrium, the finding should be interpreted with caution since it was reported in a systematic review including not more than five observational studies. Prior studies have noted the potential role of angiogenesis in the pathophysiology of adenomyosis (Ota and Tanaka, 2003; Ota, 1992; Benagiano et al., 2012). With the knowledge that adenomyosis is found to be a common cause of AUB in women of the perimenopausal age group (Rizvi et al., 2013) and is associated with a higher risk of miscarriage and lower pregnancy rates in women treated with IVF, it is suggested that aberrant angiogenesis plays a role in the pathophysiology of adenomyosis-related AUB and subfertility.
However, very little is known about the role of angiogenesis in adenomyosis. To improve our knowledge of angiogenesis in the endometrium of women with adenomyosis, study its possible relation with alterations in vascular characteristics that may cause AUB and/or subfertility and explore the therapeutic potential of targeting angiogenesis, we performed a literature review to summarize the current knowledge of possible markers related to the angiogenesis in the endometrium of women with adenomyosis. The main objective was to test the hypothesis that abnormal vascularization and the level of angiogenic markers are increased in the ectopic and eutopic endometrium of adenomyosis patients in comparison with the endometrium of control patients. In addition, we review the biology of angiogenesis in adenomyosis and discuss possible therapeutic opportunities.
Methods
This systematic review is conducted in accordance to the Prisma guidelines (Liberati et al., 2009). The methods, inclusion/exclusion criteria, type of studies, study selection, data collection, risk of bias assessment, type of interventions and type of outcome measures were specified in advance and registered in the PROSPERO International prospective register of systematic reviews (CRD42017069832).
Literature search
Two databases, PubMed and Embase, were consulted until February 2019. Combinations of terms for angiogenesis, angiogenic markers and adenomyosis were applied as well as AUB, infertility or anti-angiogenic therapy. References of the studies and related articles were checked for inclusion if not already included in the primary search (see Supplementary Data for full search strategy).
Eligibility criteria
The first search (Search I: angiogenic-related outcome in adenomyosis patients) was limited to laboratory studies carried out on the endometrium of premenopausal women. Original research articles, such as cohort or case control studies, and prospective clinical trials, which investigated the presence of angiogenesis in the endometrium of adenomyotic patients and were published as full papers in peer-reviewed journals, were included. The research needed to focus on markers that indicate the presence of angiogenesis or are known to (among other functions) induce angiogenesis. Papers had to be written in English. Case reports, studies on cells cultured in vitro not taken from women with adenomyosis or studies that did not compare the endometrium of adenomyosis with the endometrium of control patients without adenomyosis or uterine fibroids were excluded. A second search (Search II: anti-angiogenic therapy for adenomyosis) was carried out to evaluate the current status of research into therapeutic options targeting angiogenesis in adenomyosis. For this search, all original research articles, including preclinical studies that evaluated angiogenesis inhibitors in experimental settings, were included.
Data extraction
Data from the included studies were extracted according to a predefined standardized format. The following information was extracted from each of the included articles: first author, year of publication, study design, number of patients included, patient and control characteristics, use of hormones, phase in menstrual cycle, study method, angiogenic-related outcome, type of cells and method of evaluation. All the angiogenic-related markers studied in the articles are summarized by category: vascular outcomes (including mean vascular density: MVD), pro-angiogenic markers and anti-angiogenic markers. We present findings on the differences in tissue expression of the angiogenic-related markers by endometrial type: ectopic (the displaced endometrium to a location within the myometrium, also noted as endometrium with abnormal location (Goteri et al., 2009), adenomyosis (Schindl et al., 2001), adenomyotic lesions (Huang et al., 2014), adenomyotic nodules (Carrarelli et al., 2015), adenomyotic tissue (Li et al., 2013) or adenomyotic foci (Liu et al., 2016) in the articles) or eutopic (the endometrium lining the uterine cavity in adenomyotic patients also noted as endometrium with normal location (Goteri et al., 2009)). Also, we report on the type of cells (endothelial, epithelial or stromal cells) where the angiogenic markers were found and on the tissue expression of angiogenic-related markers during the menstrual phase.
Quality assessment of the included studies
The quality assessment of the included studies was performed by two independent reviewers (MH and CW). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used to assess reporting quality of the included studies (Vandenbroucke et al., 2014), and the Newcastle Ottawa Scale was used to assess methodological quality of all included studies (Stang, 2010). Although the use of quality assessment scales is less common in the field of preclinical medicine, we considered the use of the Gold Standard Publication Checklist (GSPC) to Improve the Quality of Animal Studies, a valid method to enable quality evaluation of the preclinical studies (Hooijmans et al., 2010). The scales will be used as a guide to critically evaluate the quality of the studies, but will not be used to rate articles (da Costa et al., 2011; Sanderson et al., 2007).
Results
Study characteristics
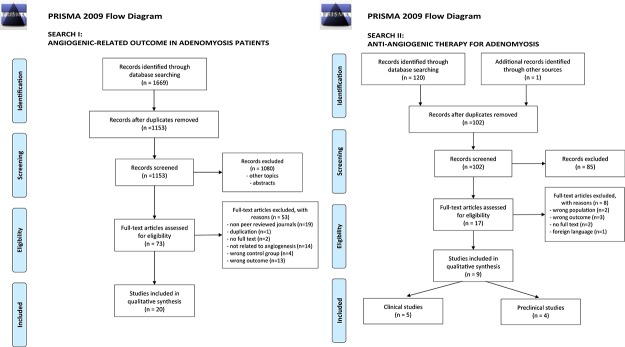
The search using the abovementioned databases resulted in 1669 hits. After removal of duplicates and screening of both the titles and abstracts, 73 articles were retrieved for full-text screening. A total of 20 articles met our selection criteria and were included in our review (Fig. 1a).
Figure 1.
Prisma flow diagrams for the two searches carried out for the systematic review. (a) PRISMA 2009 Flow Diagram Search I: angiogenic-related outcome in adenomyosis patients. The search was limited to laboratory studies carried out on the endometrium of premenopausal women. (b) PRISMA 2009 Flow Diagram Search II: anti-angiogenic therapy for adenomyosis. The search was for all original research articles, including preclinical studies that evaluated angiogenesis inhibitors in experimental settings.
The second search yielded 120 articles, out of which 17 full-text articles were assessed for eligibility and nine were included in this review (Fig. 1b).
The study characteristics of the included articles are shown in Tables I and II. The factors that were identified in the search are not all solely angiogenic factors. Some are usually categorized in the group of inflammatory cytokines, with angiogenesis regulatory functions (Table III). Since the factors were included in this study for their (anti-)angiogenic capacity, they will be referred to as (anti-)angiogenic factors for the remainder of this paper. All articles used immunohistochemical staining to study endometrial samples from premenopausal women. Some articles also used other techniques, such as western blotting, real-time PCR and ELISA. The terms ectopic and eutopic were used by most studies to describe the type of endometrium studied.
Table I.
Study characteristics of the included articles on angiogenic-related outcome.
| Study, year | Study type | N participants | Patient group | Control group | Matching | Menstrual cycle phase | Time of no hormone use | Methods | Angiogenic parameters | Outcome | Association clinical symptom | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eutopic | Ectopic | |||||||||||
| Cai et al., 2018 | Case–control | 80 | 401 | 40 | NA | Proliferative and secretory | 6 months | IHC | eIF3e | - | ↓ | - |
| TGF-β | - |
|
||||||||||
| E-cadherin | - | ↓ | ||||||||||
| Vimentin | - |
|
||||||||||
| Snail | - |
|
||||||||||
| PCNA | - |
|
||||||||||
| Yang et al., 2017 | Case–control | 60 | 10 | 10 | Age | Proliferative | 6 months | IHC and ELISA | LPA1a |

|

|
- |
| LPA2a | ↓ | ↓↓ | ||||||||||
| LPA3a | ↓ | ↓↓ | ||||||||||
| LPA4a |

|

|
||||||||||
| LPA5a |

|

|
||||||||||
| LPA6a | = | = | ||||||||||
| Nie et al., 2016 | Case–control | 68 | 502 | 18 | NR | Proliferative and secretory | 6 months | IHC | vWF |
|
|
Menorrhagia |
| CD68 |
|
|
||||||||||
| Liu et al., 2016 | Cross-sectional case control | 81 | 341 | 206 | Age, parity, menstrual phase | Proliferative and secretory | 6 months | IHC, IF and western blot | TGF-β1a | - |

|
- |
| CD41a | - |

|
||||||||||
| Vimentina | - |

|
||||||||||
| E-cadherina | - | ↓↓↓ | ||||||||||
| VEGFa | - |

|
||||||||||
| CD31a | - |

|
||||||||||
| α-SMAa | - |

|
||||||||||
| Zhihong et al., 2016 | Case–control | 42 | 18 | 24 | NA | Secretory | 1 year1 | IHC, quantitative RT-PCR | IL-6a |
|
- | - |
| IL-10a | ↓ | |||||||||||
| Wang et al., 2016 | Case–control | 40 | 302 | 10 | NR | Proliferative and secretory | 3 months | IHC, in situ TUNEL, western blot | GRIM-19b | ↓/↓↓ | ↓↓/↓↓ | - |
| VEGFb |
 / /
|
 / /
|
||||||||||
| CD34b |
 / /
|
 / /
|
||||||||||
| Guo et al., 2015 | Prospective case–control | 47 | 30 | 17 | NR | Proliferative and secretory | 3 months | IHC and western blot | DJ-1e |
 / /
|
 / /
|
- |
| p-mTORe |
 / /
|
 / /
|
||||||||||
| Carrarelli et al., 2015 | Prospective case–control | 20 | 83 | 12 | NR | Proliferative | 1 month | IHC, RT-PCR | Activin Aa | = |
|
- |
| ActRIIAa |
|

|
||||||||||
| ActRIIBa |

|

|
||||||||||
| Follistatina |

|
↑ | ||||||||||
| Myostatina |

|
↑ | ||||||||||
| Huang et al., 2014 | Prospective case–control | 130 | 100 | 30 | NR | Proliferative, secretory | 3 months | IHC, ELISA, western blot | VEGFc |
 / /
|
 / /
|
- |
| CD31c |
 / /
|
 / /
|
||||||||||
| Wang et al., 2014 | Case–control | 20 | 10 | 10 | NA | NR | NR | IHC | IL-22, IL-22R1, IL-10R2 | ↑ | ↑ | - |
| Li et al., 2013 | Case–control | 31 | 23 | 8 | None | NRb | 3 months | Electrophoretic shift assay, gene, protein expression | NF-kB (p50, p65) | - |

|
Dysmenorrhea |
| COX-2 | - |

|
||||||||||
| VEGF | - |

|
||||||||||
| TF | - |

|
||||||||||
| Nie et al., 2011 | Case–control | 68 | 502 | 18 | NR | Proliferative and secretory | 6 months | IHC | SLITb | = |
|
Dysmenorrhea (SLIT) |
| ROBO1b |
|
|
||||||||||
| CD34b |
|
|
||||||||||
| Liu et al., 2011 | Case–control | 68 | 502 | 18 | NR | Proliferative and secretory | 6 months | IHC | TFb |

|

|
AUB dysmenorrhea |
| Nie et al., 2009 | Retrospective case–control | 68 | 50 | 18 | None | Proliferative and secretory | 6 months | IHC | PR-Ba | ↓↓↓ | ↓ | AUB dysmenorrhea |
| NF-kB (p50, p52, p65)a |
|
|
||||||||||
| Tokyol et al., 2009 | Retrospective case–control | 45 | 25 | 20 | NR | Proliferative and secretory | NR | IHC | MMP-2 | =/= |
 / /
|
- |
| COX-2 | =/= | =/= | ||||||||||
| CD34 | =/= |
 / /
|
||||||||||
| Li et al., 2006 | Prospective case–control | 88 | 68 | 20 | NR | Proliferative and secretory | 3 months | IHC | MMP-2 |
 / /
|
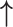 / /
|
- |
| MMP-9 |
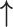 / /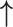
|
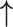 / /
|
||||||||||
| F-VIII Ragb | = |
 / /
|
||||||||||
| VEGFb |
 / /
|
 / /
|
||||||||||
| Hayrabedyan et al., 2005 | Case–control | 50 | 20 | 5 | NR | Secretory | NR | IHC | Endoglinc |
|

|
- |
| S100A13c | ↑ | - | ||||||||||
| Schindl et al., 2001 | Retrospective case–control | 70 | 53 | 17 | NR | Proliferative and secretory | 4 months | IHC | CD34b | = |
 / /
|
- |
| Ota et al., 2001 | Case LISTNUM –control | 83 | 334 | 50 | NR | Proliferative and secretory | None | IHC | COX-2b | =/= | =/= | - |
| Ota et al., 1998 | Case–control | 71 | 425 | 29 | NR | Proliferative and secretory | None | IHC | vWFb |
 / /
|
- | - |
Superscripts: 1focal and diffuse, 2all diffuse, 3all focal adenomyosis, 4group includes nine patients with ovarian cyst, 5includes nine patients with leiomyoma less than one-third of uteri corpus uteri, 6includes patients with carcinoma in situ; ano comparison performed between the phases, bno (significant) differences observed between the phases, cno P-value known for difference between the phases
Symbols: - not included in the study, = no difference observed compared with control endometrium; ↑ increase, ↓ decrease;  or ↓ significant difference compared with control endometrium with P < 0.05;
or ↓ significant difference compared with control endometrium with P < 0.05;  or ↓↓: significant difference compared with control endometrium with P ≤ 0.01;
or ↓↓: significant difference compared with control endometrium with P ≤ 0.01;  or ↓↓↓ significant difference compared with control endometrium with P ≤ 0.001
or ↓↓↓ significant difference compared with control endometrium with P ≤ 0.001
/: difference between the menstrual phases indicating: proliferative phase / secretory phase
NR, not reported; IHC, immunohistochemistry; IF, immunohistofluorescence; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; p-mTOR, phosphorylated mammalian target of rapamycin; COX-2, cyclooxygenase-2; LPA, Lysophosphatidic acid; pSTAT3, phospho signal transducer and activator of transcription 3; IL-, interleukin-; α-SMA, alpha smooth muscle actin; TGF-β, transforming growth factor beta; GRIM-19, gene associated with retinoic-interferon-induced mortality 19; eIF3e, eukaryotic translation initiation factor 3 subunit; PCNA, proliferating cell nuclear antigen; vWF, von Willebrand factor; TF, tissue factor; ROBO1, roundabout 1; AUB, abnormal uterine bleeding
Table II.
Potential anti-angiogenic strategies for treatment of adenomyosis.
| Substance group | Mechanism | Anti-angiogenic agent | Studies | Effect on endo- or myometrium | Effect on clinical symptoms | |
|---|---|---|---|---|---|---|
| GnRH agonist | Reduction of estrogen and progesterone concentrations | Leuprolide acetate | (Khan et al., 2010) | Decreased staining of vWF (MVD) and angiogenesis in endo- and myometrium. No difference in MVD in adenomyotic lesions | - | |
| Progestogens | Reduction of estrogen concentration and progesterone receptors | Levonorgestrel (LNg-IUS) | (Laoag-Fernandez et al., 2003) | Decrease in VEGF expression in endometrial epithelium and stroma | No correlation between number of bleeding days and expression levels of VEGF | |
| Growth factor inhibitors | Alleviating expression of IL-6, IL-8, TGF-β, EGF, VEGF, MMP-2 | Berberine | (Liu et al., 2018)(Liu et al., 2017) | Inhibit proliferation and viability of eutopic and ectopic endometrial stromal cells | - | |
NF- B inhibitor B inhibitor |
Andrographolide (Andrographis paniculata) | (Li et al., 2013) | Decrease in mRNA and protein levels of COX-2, VEGF and TF | - | ||
| Preclinical studies | Mechanism | Anti-angiogenic agent | Studies | Model | Effect on endo- or myometrium | Effect on clinical symptoms |
| Anti-platelet therapy | Thromboxane A2 (TXA2) synthesis inhibitor or platelet depletion | Ozagrel; rat anti-mouse GPIbα polyclonal IgG antibody | (Zhu et al., 2016) | ICR mice with neonatal dosing of tamoxifen | Both ozagrel and platelet depletion reduced the depth of myometrial invasion, platelet aggregation and staining level of COX-2 and NF- B p65 B p65 |
Diminished hyperalgesia (improvement in hot plate latency) |
| Growth factor inhibitors | Anti-VEGF antibody | Bevacizumab | (Huang et al., 2014) | Xenotransplantation of human adenomyosis in NODSCID mice | Reduce blood vessel formation | - |
| Annexin A2 inhibition (ANXA2) | ANXA2 knockout via siRNA | (Zhou et al., 2012) | Nude mouse model | Inhibition of endometrial tissue growth | Diminished hyperalgesia (improvement in hot plate latency) | |
| Fumagillin analogues | Reduction mean surface area blood vessels | TNP-470 | (Zhou et al., 2003) | Virgin female SHN mice implanted with pituitary gland | Inhibition of endothelial cell migration and proliferation | - |
MVD, mean vascular density; LNG-IUS, levonorgestrel-releasing intrauterine system; EGF, epidermal growth factor; NODSCID, non-obese diabetic severe combined immunodeficient,
Table III.
Outcome of vascular characteristics and angiogenic markers.
= No difference; ↑/↓ increase/decrease compared with control endometrium;  / ↓ significant increase/decrease compared with control endometrium;
/ ↓ significant increase/decrease compared with control endometrium;  /↓↓ significant increase/decrease compared with eutopic endometrium;
/↓↓ significant increase/decrease compared with eutopic endometrium;  /↓↓↓ significant increase/decrease compared with control and eutopic endometrium. *Type of cells not reported, **Inflammatory cytokines with angiogenesis regulatory functions.
/↓↓↓ significant increase/decrease compared with control and eutopic endometrium. *Type of cells not reported, **Inflammatory cytokines with angiogenesis regulatory functions.
Assessment of risk of bias
The methodological quality was assessed using the STROBE checklist for the quality of reporting (Table IV), the Newcastle Ottawa Scale for possible sources of bias (Table V) and the GSPC for the preclinical studies (Table VI) to assess the reporting as well as the methodological quality of the included studies. Only 12 studies reported specifications of the location and inclusion dates of the conducted study. The lack of information poses risk of selection bias of study participants. Only a limited number of studies controlled for possible confounders, such as comorbidity, menstrual phase, age or parity (Liu et al., 2016; Yang et al., 2017; Cai et al., 2018; Liu et al., 2018). The preclinical animal studies on anti-angiogenic therapy often lacked clear reasoning why a specific animal model was chosen, a clear description of how the disease was defined and full disclosure on the type of anesthesia/euthanasia, description of general well-being and relevant physiological parameters of the animals. The discussion of the findings and clinical relevance, on the other hand, was accurate in all included studies.
Table IV.
STROBE: Strengthening the Reporting of Observational Studies in Epidemiology.
| Cai et al., 2018 | Liu, 2018 | Liu, 2017 | Yang et al., 2017 | Liu, 2016 | Zhihong, 2016 | Wang, 2016 | Guo, 2015 | Carrarelli, 2015 | Huang, 2014 | Wang, 2014 | Li et al., 2013 | Nie, 2011 | Liu, 2011 | Goteri, 2009 | Khan, 2010 | Nie, 2009 | Tokyol, 2009 | Li, 2006 | Hayrabedyan, 2005 | Laoag-Fernandez, 2003 | Schindl, 2001 | Ota, 2001 | Ota, 1998 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item 5: setting, locations, dates | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Item 6: eligibility criteria, selection of participants | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Item 6b: matching criteria | 0 | NA | NA | 1 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Item 7: define variables | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Item 8: data sources and methods of assessment | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Item 9: sources of bias | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Item 10: study size | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Item 12: statistical methods | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Item 13: participant results | NA | NA | NA | 1 | NA | 1 | NA | NA | NA | NA | NA | 1 | NA | NA | NA | NA | 1 | NA | NA | NA | 1 | NA | NA | NA |
| Item 14: descriptive results | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Item 16: unadjusted estimates results | NA | 1 | 1 | NA | 0 | NA | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | NA | 1 | NA | NA | 0 | 1 | NA | NA | NA |
Assessment of risk of bias according to the STROBE checklist for observational studies: each item is classified as adequate (1), inadequate (0) or not applicable ‘NA’ in the evaluation of a paper
Table V.
Newcastle Ottawa Scale for case control studies.
| Cai et al., 2018 | Liu, 2018 | Liu, 2017 | Yang et al., 2017 | Nie, 2016 | Liu, 2016 | Zhihong, 2016 | Wang, 2016 | Guo, 2015 | Carrrelli, 2015 | Huang, 2014 | Wang, 2014 | Li et al., 2013 | Nie, 2011 | Liu X. 2011 | Khan. 2010 | Nie, 2009 | Tokyol, 2009 | Li, 2006 | Hayrabedyan, 2005 | Laoag-Fernandez, 2003 | Schindl, 2001 | Ota, 2001 | Ota, 1998 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection: | ||||||||||||||||||||||||
| 1. Is the case definition adequate? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2. Representativeness of the cases | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 3. Selection of controls | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | NA | 0 | 0 | 0 |
| 4. Definition of controls | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | NA | 0 | 1 | 1 |
| Comparability: | ||||||||||||||||||||||||
| 1a. Study controls for important factor | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1b. Study controls for additional factor | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Exposure | ||||||||||||||||||||||||
| Ascertainment of exposure | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Same method of ascertainment | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
Assessment of risk of bias according to the Newcastle Ottawa Scale for case control studies: each item is classified as adequate (1), inadequate (0) or not applicable ‘NA’ in the evaluation of a paper
Table VI.
Gold standard publication checklist.
| Zhu, 2016 | Huang, 2014 | Zhou, 2012 | Zhou, 2003 | |
|---|---|---|---|---|
| Introduction | ||||
| Background information (3p) | 3 | 2 | 2 | 2 |
| Research question or hypothesis (2p) | 1 | 0 | 1 | 1 |
| Clinical relevance (2p) | 1 | 0 | 1 | 2 |
| Methods | ||||
| Experimental design (1p) | 1 | 1 | 1 | 1 |
| Experimental groups and controls (19p) | 14 | 6 | 13 | 13 |
| Regulations and ethics (2p) | 2 | 1 | 1 | 1 |
| The intervention (12p) | 12 | 4 | 7 | 11 |
| Outcome (3p) | 2 | 1 | 2 | 2 |
| Results (7p) | 6 | 4 | 3 | 3 |
| Discussion (3p) | 3 | 3 | 3 | 3 |
| Total score out of 54 | 45 | 22 | 34 | 39 |
Assessment of risk of bias: each topic contains a list of items which ought to be addressed in every research article on animal experiments (Hooijmans et al., 2010). The total of items per topic (1 point per item) is stated behind each topic
Microvascular density and vascular characteristics
In nine articles, vascular characteristics or the MVD in endometrium of patients with adenomyosis was studied by quantification of microvessels through either CD31, CD34, von Willebrand Factor (vWF) or factor VIII (FVIII) antibody staining (Liu et al., 2016; Wang et al., 2016; Nie et al., 2011; Tokyol et al., 2009; Huang et al., 2014; Li et al., 2006; Schindl et al., 2001; Nie and Liu, 2016; Ota et al., 1998). Staining of these markers exclusively occurs in endothelial cells of blood vessels (Table III). In all articles that assessed the ectopic endometrium, the MVD was significantly increased compared with the control endometrium. Six studies found a significantly increased MVD in the eutopic endometrium from adenomyosis patients compared with the control endometrium (Liu et al., 2016; Huang et al., 2014; Wang et al., 2016; Nie et al., 2011; Ota et al., 1998; Nie and Liu, 2016), while two studies reported no significant difference (Schindl et al., 2001; Tokyol et al., 2009), and one did not study the difference between eutopic endometrium and control endometrium (Liu et al., 2016).
One article assessed the characteristics of capillaries in the endometrium through staining vWF (Ota et al., 1998). Compared with the control endometrium, the eutopic endometrium displayed an increase in mean and total surface area of capillaries, irrespective of the menstrual cycle phase. The ectopic endometrium was not investigated in this study. The highest increase was reported for the total surface area of capillaries; this was 11.6 times higher in the eutopic endometrium in adenomyosis patients than in the endometrium of controls during the proliferative phase. One group studied endoglin (Eng), a transmembrane homodimer glycoprotein that is known as an angiogenic marker because it is overexpressed in activated endothelial cells (Hayrabedyan et al., 2005). Eng staining was not observed in the endothelial cells of microvessels of the control endometrium, while it was expressed in all microvessels of the eutopic and ectopic endometrium, with stronger expression in eutopic than in ectopic endometrium.
Pro-angiogenic and vascular markers
A total of 22 markers were studied for their angiogenic characteristics in the 20 articles retrieved by our search (Table III). Five articles compared VEGF expression in the endometrium of adenomyosis patients with controls (Li et al., 2006; Wang et al., 2016; Huang et al., 2014; Liu et al., 2016; Li et al., 2013). All studies reported a significantly higher VEGF expression in the ectopic endometrium compared with control endometrium. In three articles, the eutopic endometrium was compared with the control endometrium (Wang et al., 2016; Huang et al., 2014; Li et al., 2006), where VEGF expression was reported to be increased in the eutopic endometrium compared with control endometrium. Immunoreactivity of cyclo-oxygenase 2 (COX-2) was studied in three studies (Tokyol et al., 2009; Ota et al., 2001; Li et al., 2013). One study reported significantly increased protein expression levels of COX-2 in adenomyotic tissue compared with control tissue (Li et al., 2013). Both other studies reported no significant difference in COX-2 expression between the groups.
α-Smooth muscle actin (SMA), S100A13, vimentin, matrix metalloproteinases (MMPs) 2 and 9, nuclear factor (NF)-kB (subunits p50/p65), tissue factor (TF), DJ-1, phosphorylated mammalian target of rapamycin (p-mTOR), activin A, folli- and myostatin, platelet count through CD41, SLIT, roundabout 1 (ROBO1), lysophosphatidic acid (LPA) 1,4-5, phospho signal transducer and activator of transcription 3 (pSTAT3), interleukin (IL)-6, IL-22 and transforming growth factor (TGF)-β1 are markers that were identified in this search as being involved in angiogenesis and vascular establishment. They are studied in one or two articles per factor (Table III). The immunohistochemical staining and protein expression levels of all markers were significantly increased in the ectopic endometrium compared with the endometrium of control patients. For the markers S100A13, MMP-2 and MMP-9, TF, follistatin, myostatin, ROBO1, LPA1 and 4-5, pSTAT3, IL-6 and IL-22, an increase in expression was also observed in eutopic compared with control endometrium.
Anti-angiogenic markers
Four articles that reported on markers with an anti-angiogenic capacity were identified (Tables I and III). Two articles reported decreased immunostaining levels of E-cadherin in the ectopic endometrium compared with control endometrium (Cai et al., 2018; Liu et al., 2016). IL-10 was investigated in endometrial samples obtained during the implantation window after ovarian stimulation and was lower in the eutopic endometrium of adenomyosis patients than in endometrium samples of control patients (Zhihong et al., 2016). The immunohistochemical staining of gene associated with retinoic-interferon-induced mortality (GRIM)-19 was less positive in the ectopic endometrium compared with the eutopic endometrium, which in turn was less positive than the control endometrium (ectopic<eutopic<control) (Wang et al., 2016). Eukaryotic translation initiation factor 3 subunit (eIF3e) immunoreactivity was significantly decreased in the ectopic endometrium compared with the control endometrium. eIF3e staining levels correlated positively with those of E-cadherin and negatively with those of TGF-β1 and vimentin (Cai et al., 2018).
Interferon (IFN)-α and tumor necrosis factor (TNF)-α are other known (pro- and anti-, respectively) angiogenic markers that have been reported to play an important role in several angiogenesis-related pathologies (von Marschall et al., 2003; Jin et al., 2019). We found no studies that investigated the expression of these markers in the endometrium of adenomyosis patients versus control patients. Sotnikova et al. (2002) studied cytokine expression in supernatants of mononuclear cells from the eutopic and ectopic endometrium from adenomyosis patients in comparison with control patients and found increased levels of both IFN-α and TNF-α in supernatants derived from the eutopic and ectopic endometrium compared with the control endometrium. However, no association with angiogenesis was considered. Li et al. (2013) studied the effect of TNF-α on the expression of NF-κB DNA-binding activity and protein expression of NF-κB-mediated genes COX-2, VEGF and TF in stromal cells of the ectopic endometrium of adenomyosis patients and the endometrium of control patients. The NF-κB-binding activity, assessed by electrophoretic mobility shift assay, and its protein (p50 and p65) expression levels were significantly higher in the ectopic endometrium than in the control endometrium (P < 0.01 and P < 0.001 respectively). Also, the protein levels of COX-2 (P < 0.0017), VEGF (P < 0.0006) and TF (P < 0.0013) were significantly higher in the ectopic endometrium than in controls.
Type of cells expressing (anti-)angiogenic markers
All markers for MVD and vascular characteristics were specific for endothelial cells in order to quantify and characterize blood vessels in the studied samples. Immunoreactivity of S100A13 (Hayrabedyan et al., 2005), MMP-2 and ROBO1, a receptor for SLIT, was also observed on the surface and within vascular endothelial cells (Nie et al., 2011; Tokyol et al., 2009). Glandular epithelial cells (Wang et al., 2016; Li et al., 2006; Goteri et al., 2009; Liu et al., 2016; Lai et al., 2016; Orazov et al., 2016), stromal cells (Li et al., 2006; Goteri et al., 2009; Li et al., 2013; Orazov et al., 2016) and endothelial cells (Goteri et al., 2009; Orazov et al., 2016) in the endometrium were stained positive for VEGF in women with adenomyosis whereas the staining in the control endometrium was either very low or not reported (Wang et al., 2016; Li et al., 2006; Goteri et al., 2009; Liu et al., 2016; Li et al., 2013). The expression of COX-2 was observed not only in the endothelial, glandular (Ota et al., 2001; Tokyol et al., 2009) and stromal (Li et al., 2013) cells but also in plasma cells (Ota et al., 2001). S100A13 (Hayrabedyan et al., 2005) staining was present in glandular epithelial cells, stromal cells and vascular smooth muscle cells as well as in the perivascular space. For the markers activin A and its receptors ActRIIA and ActRIIB, the type of cell was not studied.
Immunoreactivity of α-SMA, TGF-β1 and vimentin (only ectopic) (Liu et al., 2016; Cai et al., 2018), MMP 2–9 (Li et al., 2006; Tokyol et al., 2009), TF (Liu et al., 2011; Li et al., 2013), DJ-1/p-mTOR (Guo et al., 2015), follistatin, myostatin (Carrarelli et al., 2015), LPA1,4–5 (Yang et al., 2017), pSTAT3 (Wang et al., 2016), IL-6 and IL-10 (only eutopic) (Zhihong et al., 2016) and IL-22 (Wang et al., 2014) was reported in both glandular epithelial cells and stromal cells. Tissue expression of E-cadherin, eIF3e (Liu et al., 2016; Cai et al., 2018), SLIT (Nie et al., 2011) and GRIM-19 (Wang et al., 2016) was only reported in epithelial cells, while NF-κB (p50, p65) (Li et al., 2013; Nie et al., 2009) and CD41 (Liu et al., 2016) expression was only reported in the stroma. ROBO1 (Nie et al., 2011) and MMP-2 were reported in both endothelial and epithelial cells.
Tissue expression of angiogenic markers during the menstrual cycle
Eighteen studies mentioned the phase of the menstrual cycle in which the samples were collected (Table I). Two of the six articles on MVD in which the difference between the menstrual phases was taken into account reported an increased MVD in the secretory phase compared with the early proliferative phase (Huang et al., 2014; Tokyol et al., 2009), whereas the other studies found no difference in MVD between the secretory and proliferative phases (Nie et al., 2011; Li et al., 2006; Schindl et al., 2001; Wang et al., 2016; Nie and Liu, 2016). All Eng samples were taken in the secretory phase (Hayrabedyan et al., 2005). The levels of staining for vWF were increased in all adenomyotic samples, with no difference between the menstrual phases (Ota et al., 1998). One study reported that the staining of VEGF had a greater density in the secretory phase compared with the proliferative phase (Huang et al., 2014), while two studies found no difference between the phases (Li et al., 2006; Wang et al., 2016) and two others did not compare menstrual phases (Li et al., 2013; Liu et al., 2016).
In the secretory phase, there was an increased expression reported of the pro-angiogenic parameters COX-2, MMP-2 and MMP-9 (only for eutopic endometrium) (Tokyol et al., 2009; Li et al., 2006). However, Ota et al. (2001) reported no difference in COX-2 expression between the phases. No difference in expression between the phases was reported for SLIT and ROBO1 (Nie et al., 2011), TF (Liu et al., 2011) and GRIM-19 or pSTAT3 (Wang et al., 2016). DJ-1 and p-mTOR were the only parameters with a higher expression reported in the proliferative phase than in the secretory phase (Guo et al., 2015). Differences between the two menstrual phases were not studied for α-SMA, TGF-β1, platelet aggregation (CD41), vimentin, E-cadherin, eIF3e (Liu et al., 2016; Cai et al., 2018), NF-κB (Nie et al., 2009; Li et al., 2013) and IL-22 (Wang et al., 2014). The samples for LPA (Yang et al., 2017), activin A, follistatin and myostatin (Carrarelli et al., 2015) were all collected in the proliferative phase, while the samples for IL-6 and IL-10 (Zhihong et al., 2016) and Eng were only taken in the secretory phase (Hayrabedyan et al., 2005).
Angiogenesis in relation to AUB and subfertility
Most studies included in this review only described indirect effects of the MVD or (anti-)angiogenic markers on adenomyosis-related symptoms of AUB or subfertility (Fig. 2). One study found that the MVD in both eutopic and ectopic endometrium was significantly higher in women who reported heavy menses than those who reported light/moderate menses (Nie and Liu, 2016). Two studies assessed the relation between an angiogenic marker and the presence of AUB. One study (Nie et al., 2009) reported NF-κB nuclear p65 immunoreactivity to be associated with heavier menstrual bleeding and increased p65 in the ectopic endometrium to be significantly associated with the severity of dysmenorrhea in adenomyosis patients. Another study reported an increased level of TF staining in patients that reported symptoms of heavy menstrual bleeding compared with patients with light/moderate menstrual bleeding (Liu et al., 2011).
Figure 2.
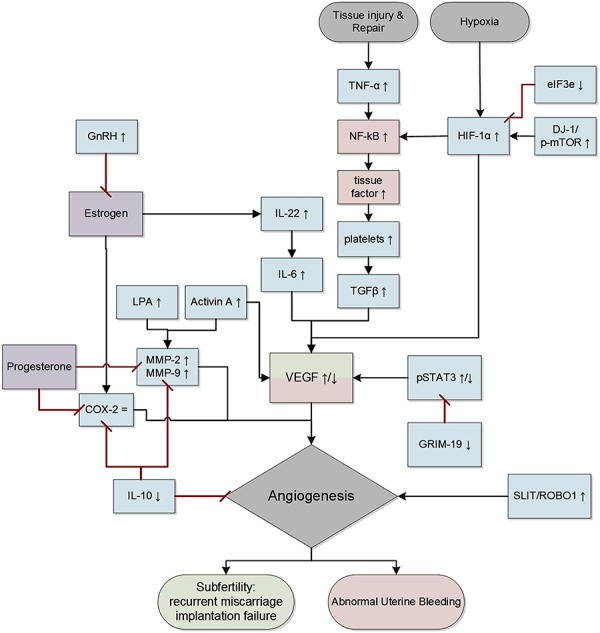
Overview of interactions between the (anti-)angiogenic markers reported in this review. Red: association with abnormal uterine bleeding; green: association with subfertility. ↑ increase, = no change, ↓ decrease. TNF-α, tumor necrosis factor alpha; NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells; HIF-1α, hypoxia-inducible factor 1 alpha; eIF3e, eukaryotic translation initiation factor 3 subunit; DJ-1; p-mTOR, phosphorylated mammalian target of rapamycin; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor; IL-, interleukin-; LPA, lysophosphatidic acid; MMP, matrix metalloproteinase; COX-2, cyclooxygenase-2; pSTAT3, phospho signal transducer and activator of transcription 3; GRIM-19, gene associated with retinoic-interferon-induced mortality 19; SLIT; ROBO1, roundabout 1. Note: adjusted figure based on information described in the literature. However, these interactions do not necessarily represent the actual or complete biological pathways. Diagram made with Microsoft Visio 2010.
Anti-angiogenic therapy for adenomyosis
The search on anti-angiogenic therapy in adenomyosis identified nine articles reporting on targeting angiogenesis in (pre)clinical studies (Table II). GnRH agonists (GnRHa) may act indirectly, through estrogen or estrogen’s effect on the expression of VEGF, on angiogenesis in the endometrium (Khan et al., 2010). As GnRHa induces a hypo-estrogenic state, they lower VEGF and COX-2, which in turn may result in a downregulation of the abnormal endometrial vascularity (Khan et al., 2010). The vWF-positive MVD in biopsy specimens obtained after reduction surgery and hysterectomy for adenomyosis in women with GnRHa therapy for 3–6 months was significantly decreased compared with the non-treated group (Khan et al., 2010). The levonorgestrel-releasing intra-uterine system (LNg-IUS) is a common treatment modality for menorrhagia, and the first line of treatment for adenomyosis (Vannuccini et al., 2018). One study demonstrated that after 3 months of LNg-IUS use, the level of expression of VEGF was decreased in the eutopic endometrium of patients with adenomyosis, while the effect on the ectopic endometrium was not studied (Laoag-Fernandez et al., 2003). On the contrary, the level of VEGF expression was not correlated to the number of bleeding days. A number of herbal compounds have been studied for their anti-angiogenic potential. Andrographolide has been reported to disrupt NF- B activation (Li et al., 2013). Treating adenomyosis patients with andrographolide resulted in reduced expression levels of COX-2, VEGF and TF (Li et al., 2013; Zheng et al., 2018). Another study investigated the growth inhibitory effect of berberine and reported a decrease in the expression levels of IL-6, IL-8, TGF-β, EGF, VEGF and MMP-2, thus inhibiting the growth of ectopic endometrial stromal cells (Liu et al., 2017; Liu et al., 2018). This effect was also partly exerted through the inhibition of nuclear NF-
B activation (Li et al., 2013). Treating adenomyosis patients with andrographolide resulted in reduced expression levels of COX-2, VEGF and TF (Li et al., 2013; Zheng et al., 2018). Another study investigated the growth inhibitory effect of berberine and reported a decrease in the expression levels of IL-6, IL-8, TGF-β, EGF, VEGF and MMP-2, thus inhibiting the growth of ectopic endometrial stromal cells (Liu et al., 2017; Liu et al., 2018). This effect was also partly exerted through the inhibition of nuclear NF-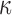 B/p65 translocation.
B/p65 translocation.
Four studies were identified that targeted angiogenesis in a preclinical study (Table II). In ovariectomized mice xenografted with human adenomyosis lesions and treated with bevacizumab (anti-VEGF monoclonal antibody), the MVD decreased and the expression of VEGF was reduced (Huang et al., 2014). Another study targeted angiogenesis in mice that developed adenomyosis after pituitary gland implantation using TNP-470, a potent inhibitor of angiogenesis known from arthritis research (Zhou et al., 2003). The mean surface area of blood vessels in the endometrium of the TNP-470-treated group reduced to 60.5% of that in the control group, and the TNP-470-treated group did not develop signs of uterine adenomyosis as opposed to 80% in the control group. Zhou et al. (2012) identified the angiogenic potential of overexpressed annexin A2 (ANXA2), an estrogen-responsive protein, through hypoxia inducible factor (HIF)-1α/VEGF-A activation, in the ectopic endometrium compared with the eutopic endometrium of adenomyosis patients. Expression of ANXA2 was associated with the epithelial-to-mesenchymal transition (EMT), as well as to the severity of dysmenorrhea in adenomyosis patients. In an in vivo nude mouse model, they demonstrated that ANXA2 knockdown reduced angiogenesis, suggesting its potential as therapeutic target.
Zhou et al., 2016 investigated the effect of high- and low-dose anti-platelet therapy using a thromboxane A2 synthesis inhibitor (ozagrel) or platelet depletion therapy using the rat anti-mouse GPIbα polyclonal IgG antibody in an adenomyosis mouse model. Platelet activation is related to angiogenesis since its co-occurrence with TGF-β1 release leads to EMT and fibroblast-to-myofibroblast transdifferentiation. Both ozagrel and platelet depletion therapy reduced depth of myometrial invasion, platelet aggregation and staining level of COX-2 and NF-κB p65. There were no adverse effects observed after ozagrel treatment. However, in the high-dose platelet depletion group, one mouse died and two appeared lethargic, while there were no apparent side effects in the low-dose depletion therapy group. Both treatment strategies resulted in significantly reduced platelet counts, depth of myometrial infiltration and staining level of COX-2 and NF-κB p65.
Discussion
Main findings
This study gives an overview of the angiogenesis-related markers in the endometrium of women with adenomyosis. Out of all outcomes of the included articles, MVD and VEGF were the most often studied parameters. The majority of articles that included these two markers reported an increase in levels in both ectopic and eutopic endometrium in adenomyosis patients compared with control patients. Increased VEGF leads to more angiogenesis, which in turn results in an increased MVD. The markers studied in the included 20 articles either directly or indirectly influence the expression of VEGF, MVD or capillary characteristics, suggesting their impact on angiogenesis in both ectopic and eutopic located endometrium (Table III and Fig. 2). This is in agreement with our hypothesis that adenomyosis patients have an increased MVD and display more angiogenic markers in the eutopic and ectopic endometrium than healthy individuals. Direct correlation with AUB or fertility outcomes has not been studied extensively, and so far only the staining levels of vWF, NF-κB nuclear p65 and TF had a direct correlation with AUB.
The staining of pro- and anti-angiogenic markers was reported in all types of endometrium cells: these include endothelial cells, epithelial cells and stromal cells (Table III). The endometrium consists of endothelial cells lining the blood vessels, glandular columnar epithelial cells lining the cavity of the uterus and the glands and stromal cells that make up the layer of connective tissue underlying the layer of epithelial cells. The expression of angiogenic markers in all cell types and in both eutopic and ectopic endometrium suggests that angiogenesis stimulates the invasiveness of eutopic endometrial tissue and the maintenance of the ectopic endometrium (Zhou et al., 2012). The formation of new and fragile blood vessels in the eutopic endometrium might lead to AUB and impair embryo receptivity.
There was no strong evidence for a difference in expression of angiogenic markers between the proliferative or secretory phases of the menstrual cycle. Continuous regeneration and degeneration of the endometrium during the menstrual cycle under the influence of estradiol (E2) has been associated with VEGF expression in endometrial epithelial cells (Moller et al., 2001), causing a higher expression of VEGF in the secretory phase than in the early (Day 6–10) proliferative phase (Chen et al., 2010; Huang et al., 2014). This is surprising, since we expected an increased level of angiogenesis in the proliferative phase after the menses to rebuild the endometrial layer under the influence of increased E2 levels. The inconsistency with our findings may be explained by different expression of VEGF within the early, mid and late proliferative phases (Sugino et al., 2002), which were not taken into account in the included studies.
Interpretation of the findings
The potential role of angiogenesis in the pathophysiology of adenomyosis and its related symptoms is complex. It is a process that is suggested to be triggered by tissue injury and repair (TIAR) causing reactive EMT in response to hypoxic and hormonal stimuli (Leyendecker et al., 2009; Ibrahim et al., 2017). An interpretation of the findings of our review and their potential interaction is depicted in Fig. 2.
The underlying pathogenesis relies on the theory that endometrial cells invade the myometrium at sites in the junctional zone that are weakened, either from genetic predisposition or from uterine auto-traumatization and induced hypoxia (Benagiano et al., 2012). In this theory, hyper and non-synchronized peristalses of the uterus following physiological processes such as menstruation or sperm transport cause chronic damage to the junctional zone, where the endometrium lies adjacent to the myometrium (Leyendecker, 2015). When the endometrium invades these disruptions in the junctional zone, ectopic foci of endometrium establish and cause local inflammation and hypoxia. Exposure to ovarian estrogens, but also local estrogen production due to local estrogen sulfatase and aromatase activity in the adenomyotic tissue, may play an additional role (Bulun, 2009; Yamamoto et al., 1993). These events are directly related to the increased angiogenesis in these tissues since they lead to the production of VEGF, thus causing angiogenesis (Goteri et al., 2009). Hypoxia is suggested to be a driving factor for physiological angiogenesis to induce endometrial repair during menstruation (Maybin et al., 2018). A vicious cycle is established when estrogen increases uterine peristalsis again and more auto-traumatization follows (Vannuccini et al., 2017).
Hereby, this TIAR theory provides insight into the role of the immune system and inflammation in adenomyosis. The reciprocal relationship between angiogenesis and the immune system has long been recognized (Griffioen and Molema, 2000). On the one hand, it is known that angiogenesis can be regulated by immune cells (Dirkx et al., 2006; Sica et al., 2008; Dings et al., 2011) and cytokines produced by immune cells (Castermans et al., 2008; Yao et al., 1999). On the other hand, immunity is heavily regulated by angiogenesis (Griffioen et al., 1996; Griffioen et al., 1996; Dirkx et al., 2003; Dirkx et al., 2006). This makes the separation between angiogenesis and immunity difficult, also reflected by the cytokines in Table III, and the correlation with increased macrophage number in adenomyotic tissue (Nie and Liu, 2016). Most leukocytes produce a series of angiogenic factors, including VEGF, TGF-β and ILs (Carmeliet, 2003; Ramjiawan et al., 2017), and neutrophils and natural killer cells were recognized as initiators of abnormal angiogenesis in endometriosis (Li et al., 2001).
VEGF and angiogenesis appear to play a key role in the development of adenomyosis, which is represented by the fact that polymorphisms in the VEGF gene seem to be related to the risk of developing adenomyosis (Kang et al., 2009). VEGF is a specific and important angiogenic parameter given its stimulating effect on the proliferation of endothelial cells (Hillen and Griffioen, 2007). The increased VEGF activity in the eutopic and ectopic endometrium in adenomyosis compared with control endometrium suggests increased vascular sprouting and number of fragile blood vessels that bleed more easily (Fig. 3) (Hillen and Griffioen, 2007; Griffioen and Molema, 2000). Moreover, increased VEGF has been reported to be associated with increased neurogenesis, possibly also explaining symptoms of dysmenorrhea (Orazov et al., 2016). During hypoxia, the expression of HIF-1α may lead to upregulation of the VEGF gene through activation of the NF-κB pathway (Groenman et al., 2007; Semenza et al., 1991; Tong et al., 2006; van Uden et al., 2011; Chen et al., 2015b; de Rooij et al., 2004). This can take place via DJ-1 (Vasseur et al., 2009; Slomovitz and Coleman, 2012), a multifunctional protein overexpressed in a variety of endometrial tumors and various stages of endometriosis that is associated with cell proliferation, migration and invasion (Rai and Shivaji, 2011). Enhanced staining of DJ-1 and HIF-1α in adenomyosis patients implies increased VEGF, which is known to be associated with more permeable and leaky vessels (Zhang and Salamonsen, 2002). Also, platelet aggregation was increased in adenomyotic lesions (Liu et al., 2016; Cai et al., 2018). Platelets are considered an important player in angiogenesis, as they contain over 80% of the total circulating VEGF (Peterson et al., 2010).
Figure 3.
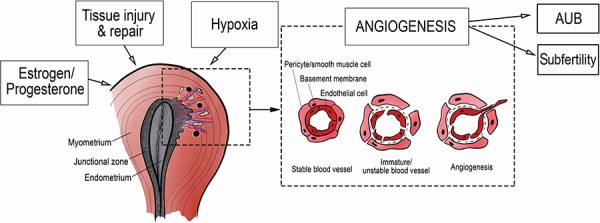
Hypothesis on angiogenesis and abnormal uterine bleeding. AUB, abnormal uterine bleeding.
Estrogen and progesterone are known angiogenic factors in the uterus, implicating the hormonal role for angiogenesis in adenomyosis patients (Hyder et al., 2000). Estrogen is known to induce EMT—the transition of endometrial epithelial cells (associated with a decrease in E-cadherin) to mesenchymal cells, accompanied by an increase in vimentin (Chen et al., 2010; Ribatti, 2017). This process is linked to angiogenesis, and the same factors that are involved in EMT may drive endothelial cells toward a pro-angiogenic state in adenomyosis (Chen et al., 2010; Khan et al., 2015; An et al., 2017). The presence of EMT was evident by the increased expression of TGF-beta and vimentin and decreased E-cadherin staining in the epithelial cells of the ectopic endometrium in adenomyosis patients (Liu et al. 2016; Chen et al., 2010; Cai et al. 2018). TGF-beta derived from platelets is known to activate transcription markers involved in EMT (Zhang et al., 2016), and its presence was previously reported in adenomyotic mice (Ribatti, 2017; Shen et al., 2016). eIF3e, a eukaryotic initiation factor that seems to have an anti-angiogenic effect [silencing of eIF3e has previously been associated with increased angiogenesis through HIF-1α activation (Chen et al. 2010)] was studied for its supposed role in EMT in adenomyosis. Indeed, decreased expression of eIF3e was related to increased TGF-beta and vimentin levels in the ectopic endometrium (Cai et al. 2018). Staining of vimentin appeared in the cytoplasm of stromal cells in control and adenomyosis groups. It was stained positive in the cytoplasm of epithelial cells in the ectopic adenomyotic endometrium but negative in the control endometrium (Liu et al., 2016; Cai et al., 2018). Thus, vimentin may serve as a marker for adenomyotic tissue. α-SMA is a molecular marker for pericytes (used to determine the maturity of vessels) and predominates within vascular smooth muscle cells (Morikawa et al., 2002). Decreased levels of α-SMA expression were reported in women with menorrhagia compared with controls (Abberton et al., 1999), suggesting an increase in immature leaky vessels. Our expectation was contradicted in this review since increased α-SMA expression was reported in adenomyosis patients. However, the increase in TGF-β1 and α-SMA was reported only in the endometrial epithelium and stromal cells (Liu et al., 2016) and only provides evidence of fibrogenesis, which might have evolved secondary to angiogenesis in the myometrium (Liu et al., 2016).
Estrogen is also a potent stimulus for COX-2, which in turn leads to increased levels of prostaglandins in the uterus (Bulun et al., 2000; Chen et al., 2010). Symptoms of AUB and dysmenorrhea are associated with increased prostaglandin levels (Maia et al., 2005), while COX-2 was associated with angiogenic markers in vitro (Leung et al., 2003). However, this could not be confirmed in vivo, since COX-2 expression was not different between the ectopic and control endometrium (Tokyol et al., 2009).
The other angiogenic markers identified in this review, such as IL-6, IL-22, LPA (1-6), SLIT/ROBO, activin A, follistatin, myostatin and pSTAT3, stimulate endometrial stromal cells to produce VEGF (Carrarelli et al., 2015; Rocha et al., 2012; Wang et al., 2014; Wang et al., 2016) and induce autocrine activity, attraction, migration and proliferation of vascular endothelial cells (Wu et al., 2005; Kozian et al., 1997; Dickinson and Duncan, 2010; Wang et al., 2003; Wang et al., 2008; Yang et al., 2017). However, previous studies reported no positive correlation of SLIT/ROBO with MVD (Wang et al., 2003), and when LPA was added to a culture of endometrial stromal cells, there were no differences in the release of VEGF between cases and controls (Yang et al., 2017). The presence of activin A was previously linked to the development of AUB and dysmenorrhea since it increased VEGF in endometriosis patients (Rocha et al., 2012). Activin A has been linked to physiological processes, such as embryo implantation, and pathologies including endometrical carcinoma, where it acts by activating MMP-2 and -9 (Jones et al., 2006). MMPs are known for their great impact on tissue degradation during menstruation (Salamonsen and Woolley, 1996), and a link with increased angiogenesis in tumors has been reported (Bergers et al., 2000; Fang et al., 2000; Tang et al., 2005). MMP-2 and -9 were increased in adenomyosis patients, and the positive correlation with VEGF indicates increased angiogenesis (Li et al., 2006). STAT3 is reported to upregulate VEGF expression and stimulate growth of tumors through angiogenesis (Niu et al., 2002). By inhibiting STAT3, GRIM-19 can downregulate the VEGF expression. IL-10 is a known anti-angiogenic and anti-inflammatory factor that is produced by uterine natural killer cells and macrophages. IL-10 inhibits angiogenesis through downregulation of COX-2 and MMP-2 and -9 (Moore et al., 2001; Zhihong et al., 2016). A significantly lower expression of IL-10 reported in the adenomyosis endometrium compared with the control endometrium indicates an upregulation of angiogenesis.
Pathology of angiogenesis in adenomyosis in relation to AUB
Increased angiogenesis resulting in an increased amount of leaky fragile vessels may be the cause of AUB in women with adenomyosis (Leyendecker et al., 2009) (Fig. 3). The correlation between AUB and the MVD through staining of vWF, and increased levels of TF and NF-κB was the only evidence found to directly support this theory. TF is a glycoprotein in the cell membrane that is involved in the clotting cascade and platelet activation (Liu et al. 2011; Krikun et al., 2008). Increased levels of TF with the co-occurrence of enlarged and widened blood vessels in bleeding sites of the endometrium were linked to angiogenesis in the endometrium (Krikun et al., 2009; Krikun et al., 2008; Liu et al., 2011). The expression of TF was positively correlated with AUB and dysmenorrhea (Liu et al., 2011).
The NF-κB heterodimer belongs to a family of nuclear transcription markers and is known to be involved in the degradation of basement membrane and the remodeling of the extracellular matrix, which is one of the earliest processes in angiogenesis. Also, it has been suggested to play a pivotal role in endometriosis (Guo 2007). However, the exact role of NF-κB in angiogenesis seems to differ among cell types and is inconclusive (Tabruyn and Griffioen 2008; Tabruyn et al. 2009). Thus, suggesting that NF-κB’s role in angiogenesis explains its association with dysmenorrhea and the severity of AUB in adenomyosis patients is disputable (Nie et al., 2010; Li et al., 2013). TNF-α, a known pro-angiogenic factor, can activate NF-κB, which translocates and binds to the nucleus of its target genes (Gilmore, 2006) and leads to higher protein levels of COX-2, VEGF and TF. This binding activity was associated with the severity of dysmenorrhea in adenomyosis patients (Li et al., 2013).
Pathology of angiogenesis in adenomyosis in relation to subfertility
VEGF and angiogenesis are crucial for embryonic implantation, decidual vascularization and the early stages of development (Burton et al., 2009). Any disruption in this process might lead to fertility problems. Unfortunately, a direct relationship between angiogenic markers and subfertility has not been studied in adenomyosis patients and could therefore not be established in this review. According to our hypothesis, there is indirect evidence to support the relationship between the increase in angiogenic markers in the endometrium of adenomyosis patients summarized in this review and the observed lower probability of clinical pregnancy and a 2-fold risk of miscarriage in this population undergoing IVF (Campo et al., 2012; Vercellini et al., 2014). One study reported increased levels of the angiogenic factor IL-6 and decreased IL-10 during the window of implantation in adenomyosis patients versus control patients after ovarian stimulation with GnRHa, indicating that increased angiogenesis might be involved in the observed compromised endometrium receptivity (Zhihong et al., 2016). No pregnancy outcomes were available to support this hypothesis.
Other studies have explored the relation between VEGF and abortions or miscarriages. Compared with control patients, there was an increased VEGF-A expression in women with spontaneous miscarriages. In women with recurrent miscarriages, decreased VEGF-A and VEGF-C with increased levels of VEGF-R1 and -R2, TGF-β1 and α-SMA staining and reduced levels of IL-10 were reported (Lash et al., 2012; Plevyak et al., 2002). Another study reported a decreased GRIM-19, resulting in an increased STAT3 expression in villous samples of patients with miscarriages compared with controls (Chen et al., 2015). They reported a lower VEGF expression in women with miscarriages in the same study (Chen et al., 2015). Suppression of adenomyosis by long-term downregulation with GnRHa, which might indirectly reduce VEGF expression, resulted in improvement in IVF outcomes with no difference in clinical pregnancy rates and early pregnancy loss in comparison to controls (Mijatovic et al., 2010; Costello et al., 2011). These results suggest that abnormal levels of VEGF (subtypes) may lead to a disbalance in the angiogenesis process that may play a role in a higher miscarriage rate. According to Lash et al. (2012), women with recurrent miscarriage have more matured vessels in the endometrium compared with controls, which contradicted our hypothesis of less matured vessels being the cause of infertility. This contradiction might be explained by the following: for an ongoing pregnancy, a certain balance of vessel maturity should be achieved. Possibly both overly matured vessels and less matured vessels can lead to miscarriages. In case of more matured vessels, less production of blood vessels or less adaptation of blood flow to the implanted fetus might be the cause of a higher risk for miscarriage. However, to the best of our knowledge there is no literature to further explore this theory.
Strengths and limitations
This is the first review of available literature describing possible angiogenic-related markers in the adenomyotic endometrium (both ectopic and eutopic) and relating the pathophysiology of angiogenesis to clinical symptoms of adenomyosis, such as AUB and fertility problems. Strengths of this review include the systematic approach in accordance with the Prisma guidelines using two databases (PubMed and Embase), its registration in the PROSPERO International prospective register of systematic reviews and the use of quality checklists to assess the included articles for risk of bias. Another strength of the review is the stratified presentation of the results concerning the outcomes of vascular characteristics (MVD), (anti-)angiogenic markers, type of cells and menstrual phase. However, not all articles reported the menstrual phase, age or parity of the participants or adjusted the results accordingly. Neither were other potential confounders, such as medication use or comorbidity, most importantly endometriosis, taken into account in most articles. The lack of information on these factors seriously limits the interpretability of the observed differences in the angiogenic profile of the endometrium and prevents further insight into the possible correlation of these individual factors with angiogenesis in the endometrium. Despite the limitation of the unknown effect of concomitant endometriosis, the presence of angiogenesis in the eutopic endometrium in adenomyosis seems most relevant for the association with AUB or subfertility (Vercellini et al., 2014).
Other limitations of the articles include the relatively small sample size and the heterogeneity of the study population and of the angiogenic markers. Most of the studied markers were only studied and reported in a few articles, hampering the ability to draw solid conclusions on all individual factors in relation to angiogenesis in adenomyosis. Some possible relevant angiogenic markers, such as IFN-alpha and TNF-alpha, were not studied in the endometrium of adenomyosis patients. Additionally, some articles included other endometrial abnormalities in their control group, and these abnormalities may share pathological pathways with adenomyosis (Tokyol et al. 2009). Also, many articles lacked a pre-reported definition of adenomyosis or a description of the location of the biopsies taken. Differences in depth of the endometrial invasion might lead to different angiogenic results. The additional search on potential anti-angiogenic therapy for adenomyosis further strengthens this review and stresses the clinical relevance of the topic. However, the heterogeneity of the anti-angiogenic agents and (animal) models used severely limits the interpretability of these results. Moreover, the animal studies lacked a complete description of the applied methods and the results. For example, incomplete reporting of physiological parameters of the animals limits the understanding of the side-effect profile of the applied therapy.
Conclusion
The increased expression of angiogenic markers and decrease in anti-angiogenic markers, as well as an increase in MVD and capillary characteristics in the ectopic and eutopic endometrium in comparison with the endometrium in control patients without adenomyosis, support our hypothesis that increased angiogenesis plays a role in the pathogenesis of adenomyosis.
Future perspectives
The implicated role of these markers in AUB and subfertility outcomes, such as lowered pregnancy rates and increased miscarriage rates during IVF, is only based on indirect evidence. Direct evidence requires future studies focusing on the relation between angiogenesis and these clinical outcomes in adenomyosis patients. Confirmation of this hypothesized relation might give future tools for the development of targeted selective therapies. When a positive correlation between ongoing angiogenesis and the occurrence of adenomyosis can be confirmed, it is tempting to speculate on the use of angiostatic drugs for treatment of adenomyosis. Similar to endometriosis, where the benefit of angiostatic therapy has been validated (Laschke and Menger, 2012; Zheng et al., 2018), it is suggested that adenomyosis can be efficiently treated with inhibitors of angiogenesis as well. To date, studies of anti-angiogenic treatment regimens for adenomyosis are still lacking. Available literature on the treatment of adenomyosis report on hormonal therapies, some of which (in)directly influence angiogenesis, on preclinical studies or on the effects of herbal compounds which appear to inhibit vascular growth factors (Table II) (Streuli et al., 2014; Benagiano et al., 2009). Since endometriosis has been recognized as an angiogenic-dependent disease for a long time, anti-angiogenic compounds have been studied in endometriosis to a greater extent and a number of in vitro and in vivo studies show promising results (Nap et al., 2005; Nap et al., 2004; Korbel et al., 2018; Hu et al., 2017). However, there are no clinical studies on the effectiveness of such therapies for adenomyosis-related AUB or subfertility. Treatment that is targeted at attenuating angiogenesis has been studied most extensively in the field of cancer research (Wentink et al., 2015; Hurwitz et al., 2004; Dings et al., 2006; Wu et al., 2019). Angiostatic approaches in the field of cancer, however, have only limited effects on patient survival. This is due to the fact that cancer cells have the capacity to become resistant to angiogenesis inhibitors, e.g. through drifting towards dependency to alternative growth factors (van Beijnum et al., 2015; Bani et al., 2017; Huijbers et al., 2016). Inhibition of angiogenesis may be extremely attractive for non-malignant diseases in which cells are not genetically unstable, as was observed in ophthalmology applications. Treatment of age-related macula degeneration, for example, appears to have resulted in a renaissance of therapeutic intervention (Rosenfeld et al., 2006). Therefore, such treatment also seems attractive for reversing or inhibiting angiogenesis in adenomyosis. Nonetheless, the clinical applicability of these compounds in the population of adenomyosis patients is much more restricted than in the patients with cancer, whose compounds were originally developed for: in the latter population, a life-threatening disease needs to be treated, and for that reason, the safety requirements differ completely from the requirements for a drug that treats a benign disease in a population that wants to preserve fertility and accepts fewer side effects (Laschke and Menger, 2012). Since angiogenesis in the reproductive tract is one of the only physiologically occurring angiogenesis mechanisms in the human body, this function would need to be preserved.
Local application of a therapeutic agent would ideally reduce angiogenesis and adenomyosis-related AUB and fertility difficulties without disrupting physiological angiogenesis in the reproductive organs.
Supplementary Material
Authors’ roles
M.H.: conceptualization, data analysis and writing. C.W.: conceptualization and data analysis. F.G.: supervision, review and editing. V.M.: review and editing. W.H.: review and editing. J.H. and A.W.G: supervision, review and editing. All authors also contributed to the critical revision of the intellectual content and approved the final version of the paper.
Funding
No external funding was received.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Abberton KM, Healy DL, Rogers PA. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod 1999;14:3095–3100. [DOI] [PubMed] [Google Scholar]
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)-pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol 2017;40:68–81. [DOI] [PubMed] [Google Scholar]
- An M, Li D, Yuan M, Li Q, Zhang L, Wang G. Different macrophages equally induce EMT in endometria of adenomyosis and normal. Reproduction 2017;154:79–92. [DOI] [PubMed] [Google Scholar]
- Bani M, Decio A, Giavazzi R, Ghilardi C. Contribution of tumor endothelial cells to drug resistance: anti-angiogenic tyrosine kinase inhibitors act as p-glycoprotein antagonists. Angiogenesis 2017;20:233–241. [DOI] [PubMed] [Google Scholar]
- Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev 2015;67:441–461. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Brosens I, Carrara S. Adenomyosis: new knowledge is generating new treatment strategies. Womens Health (Lond) 2009;5:297–311. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98:572–579. [DOI] [PubMed] [Google Scholar]
- Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20:511–521. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z et al. . Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000;2:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus--revisited. Am J Obstet Gynecol 1972;112:583–593. [DOI] [PubMed] [Google Scholar]
- Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, Hagglund L, Mardesic T, Varila E, Zech J et al. . High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod 2004;19:352–356. [DOI] [PubMed] [Google Scholar]
- Bruijn AM, Lohle PN, Huirne JA, Vries J, Twisk M, Group QU-T, Hehenkamp WJ. Uterine artery embolization versus hysterectomy in the treatment of symptomatic adenomyosis: protocol for the randomized QUESTA Trial. JMIR Res Protoc 2018;7:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn AM, Smink M, Hehenkamp WJK, Nijenhuis RJ, Smeets AJ, Boekkooi F, Reuwer P, Van Rooij WJ, Lohle PNM. Uterine artery embolization for symptomatic adenomyosis: 7-year clinical follow-up using UFS-Qol questionnaire. Cardiovasc Intervent Radiol 2017a;40:1344–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn AM, Smink M, Lohle PNM, Huirne JAF, Twisk JWR, Wong C, Schoonmade L, Hehenkamp WJK. Uterine artery embolization for the treatment of adenomyosis: a systematic review and meta-analysis. J Vasc Interv Radiol 2017b;28:1629–1642 e1621. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Zeitoun KM, Takayama K, Sasano H. Molecular basis for treating endometriosis with aromatase inhibitors. Hum Reprod Update 2000;6:413–418. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction 2009;138:895–902. [DOI] [PubMed] [Google Scholar]
- Cai X, Shen M, Liu X, Nie J. The possible role of eukaryotic translation initiation factor 3 subunit e (eIF3e) in the epithelial-mesenchymal transition in adenomyosis. Reprod Sci 2018;1933719118773490. [DOI] [PubMed] [Google Scholar]
- Campo S, Campo V, Benagiano G. Infertility and adenomyosis. Obstet Gynecol Int 2012;2012:786132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med 2003;9:653–660. [DOI] [PubMed] [Google Scholar]
- Carrarelli P, Yen CF, Arcuri F, Funghi L, Tosti C, Wang TH, Huang JS, Petraglia F. Myostatin, follistatin and activin type II receptors are highly expressed in adenomyosis. Fertil Steril 2015;104:744–752.e741. [DOI] [PubMed] [Google Scholar]
- Castermans K, Tabruyn SP, Zeng R, Beijnum JR, Eppolito C, Leonard WJ, Shrikant PA, Griffioen AW. Angiostatic activity of the antitumor cytokine interleukin-21. Blood 2008;112:4940–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Deng X, Yang Y, Shen Y, Chao L, Wen Y, Sun Y. Expression of GRIM-19 in missed abortion and possible pathogenesis. Fertil Steril 2015a;103:138–146.e133. [DOI] [PubMed] [Google Scholar]
- Chen L, Endler A, Uchida K, Horiguchi S, Morizane Y, Iijima O, Toi M, Shibasaki F. Int6/eIF3e silencing promotes functional blood vessel outgrowth and enhances wound healing by upregulating hypoxia-induced factor 2alpha expression. Circulation 2010a;122:910–919. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu J, He B, Li Y, Liu S, Wu B, Wang S, Zhang S, Xu X, Wang J. Vascular endothelial growth factor (VEGF) regulation by hypoxia inducible factor-1 alpha (HIF1A) starts and peaks during endometrial breakdown, not repair, in a mouse menstrual-like model. Hum Reprod 2015b;30:2160–2170. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Li HY, Chang YL, Yuan CC, Tai LK, Lu KH, Chang CM, Chiou SH. Suppression of migratory/invasive ability and induction of apoptosis in adenomyosis-derived mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil Steril 2010b;94:1972–1979, 1979 e1971-1974. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Li HY, Huang CH, Twu NF, Yen MS, Wang PH, Chou TY, Liu YN, Chao KC, Yang MH. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol 2010c;222:261–270. [DOI] [PubMed] [Google Scholar]
- Cohen I, Beyth Y, Tepper R, Figer A, Shapira J, Cordoba M, Yigael D, Altaras MM. Adenomyosis in postmenopausal breast cancer patients treated with tamoxifen: a new entity? Gynecol Oncol 1995;58:86–91. [DOI] [PubMed] [Google Scholar]
- Costa BR, Cevallos M, Altman DG, Rutjes AW, Egger M. Uses and misuses of the STROBE statement: bibliographic study. BMJ Open 2011;1:e000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MF, Lindsay K, McNally G. The effect of adenomyosis on in vitro fertilisation and intra-cytoplasmic sperm injection treatment outcome. Eur J Obstet Gynecol Reprod Biol 2011;158:229–234. [DOI] [PubMed] [Google Scholar]
- Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction 2010;139:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dings RP, Chen X, Hellebrekers DM, Eijk LI, Zhang Y, Hoye TR, Griffioen AW, Mayo KH. Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J Natl Cancer Inst 2006;98:932–936. [DOI] [PubMed] [Google Scholar]
- Dings RP, Vang KB, Castermans K, Popescu F, Zhang Y, Oude Egbrink MG, Mescher MF, Farrar MA, Griffioen AW, Mayo KH. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res 2011;17:3134–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkx AE, oude Egbrink MG, Castermans K, Schaft DW, Thijssen VL, Dings RP, Kwee L, Mayo KH, Wagstaff J, Bouma-ter Steege JC et al. . Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J 2006a;20:621–630. [DOI] [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Kuijpers MJ, Niet ST, Heijnen VV, Bouma-ter Steege JC, Wagstaff J, Griffioen AW. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res 2003;63:2322–2329. [PubMed] [Google Scholar]
- Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol 2006b;80:1183–1196. [DOI] [PubMed] [Google Scholar]
- Dueholm M. Uterine adenomyosis and infertility, review of reproductive outcome after in vitro fertilization and surgery. Acta Obstet Gynecol Scand 2017;96:715–726. [DOI] [PubMed] [Google Scholar]
- Fan TY, Zhang L, Chen W, Liu Y, He M, Huang X, Orsi F, Wang Z. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol 2012;81:3624–3630. [DOI] [PubMed] [Google Scholar]
- Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci USA 2000;97:3884–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 2006;25:6680–6684. [DOI] [PubMed] [Google Scholar]
- Goteri G, Lucarini G, Montik N, Zizzi A, Stramazzotti D, Fabris G, Tranquilli AL, Ciavattini A. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int J Gynecol Pathol 2009;28:157–163. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 1996a;88:667–673. [PubMed] [Google Scholar]
- Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996b;56:1111–1117. [PubMed] [Google Scholar]
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 2000;52:237–268. [PubMed] [Google Scholar]
- Groenman FA, Rutter M, Wang J, Caniggia I, Tibboel D, Post M. Effect of chemical stabilizers of hypoxia-inducible factors on early lung development. Am J Physiol Lung Cell Mol Physiol 2007;293:L557–L567. [DOI] [PubMed] [Google Scholar]
- Groothuis PG, Nap AW, Winterhager E, Grummer R. Vascular development in endometriosis. Angiogenesis 2005;8:147–156. [DOI] [PubMed] [Google Scholar]
- Guo J, Gao J, Yu X, Luo H, Xiong X, Huang O. Expression of DJ-1 and mTOR in eutopic and ectopic endometria of patients with endometriosis and adenomyosis. Gynecol Obstet Invest 2015;79:195–200. [DOI] [PubMed] [Google Scholar]
- Guo SW. Nuclear factor-kappab (NF-kappaB): an unsuspected major culprit in the pathogenesis of endometriosis that is still at large? Gynecol Obstet Invest 2007;63:71–97. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353–364. [DOI] [PubMed] [Google Scholar]
- Hayrabedyan S, Kyurkchiev S, Kehayov I. Endoglin (cd105) and S100A13 as markers of active angiogenesis in endometriosis. Reprod Biol 2005;5:51–67. [PubMed] [Google Scholar]
- Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev 2007;26:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Leenaars M, Ritskes-Hoitinga M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern Lab Anim 2010;38:167–182. [DOI] [PubMed] [Google Scholar]
- Hu Z, Cheng J, Xu J, Ruf W, Lockwood CJ. Tissue factor is an angiogenic-specific receptor for factor VII-targeted immunotherapy and photodynamic therapy. Angiogenesis 2017;20:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Chen YJ, Chou TY, Chen CY, Li HY, Huang BS, Tsai HW, Lan HY, Chang CH, Twu NF et al. . Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med 2014;18:1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers EJ, Beijnum JR, Thijssen VL, Sabrkhany S, Nowak-Sliwinska P, Griffioen AW. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist Updat 2016;25:26–37. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E et al. . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, Stancel GM. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect 2000;108:785–790. [DOI] [PubMed] [Google Scholar]
- Ibrahim MG, Sillem M, Plendl J, Chiantera V, Sehouli J, Mechsner S. Myofibroblasts are evidence of chronic tissue microtrauma at the endometrial-myometrial junctional zone in uteri with adenomyosis. Reprod Sci 2017;24:1410–1418. [DOI] [PubMed] [Google Scholar]
- Jin F, Zheng X, Yang Y, Yao G, Ye L, Doeppner TR, Hermann DM, Wang H, Dai Y. Impairment of hypoxia-induced angiogenesis by LDL involves a HIF-centered signaling network linking inflammatory TNFalpha and angiogenic VEGF. Aging (Albany NY) 2019;11:328–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Findlay JK, Farnworth PG, Robertson DM, Wallace E, Salamonsen LA. Activin A and inhibin A differentially regulate human uterine matrix metalloproteinases: potential interactions during decidualization and trophoblast invasion. Endocrinology 2006;147:724–732. [DOI] [PubMed] [Google Scholar]
- Kang S, Li SZ, Wang N, Zhou RM, Wang T, Wang DJ, Li XF, Bui J, Li Y. Association between genetic polymorphisms in fibroblast growth factor (FGF)1 and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum Reprod 2010;25:1806–1811. [DOI] [PubMed] [Google Scholar]
- Kang S, Zhao J, Liu Q, Zhou R, Wang N, Li Y. Vascular endothelial growth factor gene polymorphisms are associated with the risk of developing adenomyosis. Environ Mol Mutagen 2009;50:361–366. [DOI] [PubMed] [Google Scholar]
- Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Masuzaki H. Involvement of hepatocyte growth factor-induced epithelial-mesenchymal transition in human adenomyosis. Biol Reprod 2015;92:35. [DOI] [PubMed] [Google Scholar]
- Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod 2010;25:642–653. [DOI] [PubMed] [Google Scholar]
- Korbel C, Gerstner MD, Menger MD, Laschke MW. Notch signaling controls sprouting angiogenesis of endometriotic lesions. Angiogenesis 2018;21:37–46. [DOI] [PubMed] [Google Scholar]
- Kozian DH, Ziche M, Augustin HG. The activin-binding protein follistatin regulates autocrine endothelial cell activity and induces angiogenesis. Lab Invest 1997;76:267–276. [PubMed] [Google Scholar]
- Krikun G, Lockwood CJ, Paidas MJ. Tissue factor and the endometrium: from physiology to pathology. Thromb Res 2009;124:393–396. [DOI] [PubMed] [Google Scholar]
- Krikun G, Schatz F, Taylor H, Lockwood CJ. Endometriosis and tissue factor. Ann N Y Acad Sci 2008;1127:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TH, Wu PH, Wu WB. Involvement of NADPH oxidase and NF-kappaB activation in CXCL1 induction by vascular endothelial growth factor in human endometrial epithelial cells of patients with adenomyosis. J Reprod Immunol 2016;118:61–69. [DOI] [PubMed] [Google Scholar]
- Laoag-Fernandez JB, Maruo T, Pakarinen P, Spitz IM, Johansson E. Effects of levonorgestrel-releasing intra-uterine system on the expression of vascular endothelial growth factor and adrenomedullin in the endometrium in adenomyosis. Hum Reprod 2003;18:694–699. [DOI] [PubMed] [Google Scholar]
- Larsen SB, Lundorf E, Forman A, Dueholm M. Adenomyosis and junctional zone changes in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol 2011;157:206–211. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Menger MD. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update 2012;18:682–702. [DOI] [PubMed] [Google Scholar]
- Lash GE, Innes BA, Drury JA, Robson SC, Quenby S, Bulmer JN. Localization of angiogenic growth factors and their receptors in the human endometrium throughout the menstrual cycle and in recurrent miscarriage. Hum Reprod 2012;27:183–195. [DOI] [PubMed] [Google Scholar]
- Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol 2003;23:1317–1322. [PubMed] [Google Scholar]
- Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet 2009;280:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, Bottcher B, Wildt L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch Gynecol Obstet 2015;291:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen M, Liu X, Guo SW. Constitutive and tumor necrosis factor-alpha-induced activation of nuclear factor-kappaB in adenomyosis and its inhibition by andrographolide. Fertil Steril 2013a;100:568–577. [DOI] [PubMed] [Google Scholar]
- Li B, Chen M, Liu X, Guo SW. Constitutive and tumor necrosis factor-α-induced activation of nuclear factor-κB in adenomyosis and its inhibition by andrographolide. Fertil Steril 2013b;100:568–577. [DOI] [PubMed] [Google Scholar]
- Li T, Li YG, Pu DM. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol Obstet Invest 2006;62:229–235. [DOI] [PubMed] [Google Scholar]
- Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A et al. . Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab 2001;86:1823–1834. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen L, Jiang C, Guo J, Xie Y, Kang L, Cheng Z. Berberine inhibits the LPS-induced proliferation and inflammatory response of stromal cells of adenomyosis tissues mediated by the LPS/TLR4 signaling pathway. Exp Ther Med 2017;14:6125–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Luo N, Guo J, Xie Y, Chen L, Cheng Z. Berberine inhibits growth and inflammatory invasive phenotypes of ectopic stromal cells: Imply the possible treatment of adenomyosis. J Pharmacol Sci 2018a;137:5–11. [DOI] [PubMed] [Google Scholar]
- Liu L, Luo N, Guo J, Xie Y, Chen L, Cheng Z. Berberine inhibits growth and inflammatory invasive phenotypes of ectopic stromal cells: Imply the possible treatment of adenomyosis. J Pharmacol Sci 2018b;137:5–11. [DOI] [PubMed] [Google Scholar]
- Liu X, Nie J, Guo SW. Elevated immunoreactivity to tissue factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Hum Reprod 2011;26:337–345. [DOI] [PubMed] [Google Scholar]
- Liu X, Shen M, Qi Q, Zhang H, Guo SW. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum Reprod 2016;31:734–749. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Bilgicyildirim A, Inacker M, Stalf T, Huppert P, Mall G, Bottcher B, Wildt L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study.. Arch Gynecol Obstet 2015;291:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia H Jr, Maltez A, Studard E, Zausner B, Athayde C, Coutinho E. Effect of the menstrual cycle and oral contraceptives on cyclooxygenase-2 expression in the endometrium. Gynecol Endocrinol 2005;21:57–61. [DOI] [PubMed] [Google Scholar]
- Marschall Z, Scholz A, Cramer T, Schafer G, Schirner M, Oberg K, Wiedenmann B, Hocker M, Rosewicz S. Effects of interferon alpha on vascular endothelial growth factor gene transcription and tumor angiogenesis. J Natl Cancer Inst 2003;95:437–448. [DOI] [PubMed] [Google Scholar]
- Martinez-Conejero JA, Morgan M, Montesinos M, Fortuno S, Meseguer M, Simon C, Horcajadas JA, Pellicer A. Adenomyosis does not affect implantation, but is associated with miscarriage in patients undergoing oocyte donation. Fertil Steril 2011;96:943–950. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Murray AA, Saunders PTK, Hirani N, Carmeliet P, Critchley HOD. Hypoxia and hypoxia inducible factor-1alpha are required for normal endometrial repair during menstruation. Nat Commun 2018;9:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic V, Florijn E, Halim N, Schats R, Hompes P. Adenomyosis has no adverse effects on IVF/ICSI outcomes in women with endometriosis treated with long-term pituitary down-regulation before IVF/ICSI. Eur J Obstet Gynecol Reprod Biol 2010;151:62–65. [DOI] [PubMed] [Google Scholar]
- Moller B, Rasmussen C, Lindblom B, Olovsson M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reprod 2001;7:65–72. [DOI] [PubMed] [Google Scholar]
- Moore KW, Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 2002;160:985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nap AW, Dunselman GA, Griffioen AW, Mayo KH, Evers JL, Groothuis PG. Angiostatic agents prevent the development of endometriosis-like lesions in the chicken chorioallantoic membrane. Fertil Steril 2005;83:793–795. [DOI] [PubMed] [Google Scholar]
- Nap AW, Griffioen AW, Dunselman GA, Bouma-Ter Steege JC, Thijssen VL, Evers JL, Groothuis PG. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab 2004;89:1089–1095. [DOI] [PubMed] [Google Scholar]
- Nie J, Liu X. Immunoreactivity of CD68, granulocyte-macrophage colony-stimulating factors receptor and vonwillebrand factor and its association with dysmenorrhea severity and the amount of menses in adenomyosis. Int J Clin Exp Med 2016;9:20856–20865. [Google Scholar]
- Nie J, Liu X, Guo SW. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am J Obstet Gynecol 2010;202:346e341–346e348. [DOI] [PubMed] [Google Scholar]
- Nie J, Liu X, Zheng Y, Geng JG, Guo SW. Increased immunoreactivity to SLIT/ROBO1 and its correlation with severity of dysmenorrhea in adenomyosis. Fertil Steril 2011;95:1164–1167. [DOI] [PubMed] [Google Scholar]
- Nie J, Lu Y, Liu X, Guo SW. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil Steril 2009;92:886–889. [DOI] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D et al. . Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002;21:2000–2008. [DOI] [PubMed] [Google Scholar]
- Orazov MR, Nosenko EN, Radzinsky VE, Khamoshina MB, Lebedeva MG, Sounov MA. Proangiogenic features in chronic pelvic pain caused by adenomyosis. Gynecol Endocrinol 2016;32:7–10. [DOI] [PubMed] [Google Scholar]
- Ota H. Evaluation of hysteroscopy in the diagnosis of adenomyosis. Jpn J Fertil Steril 1992;37:49–55. [Google Scholar]
- Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod 2001;16:561–566. [DOI] [PubMed] [Google Scholar]
- Ota H, Igarashi S, Tanaka T. Morphometric evaluation of stromal vascularization in the endometrium in adenomyosis. Hum Reprod 1998;13:715–719. [DOI] [PubMed] [Google Scholar]
- Ota H, Tanaka T. Stromal vascularization in the endometrium during adenomyosis. Microsc Res Tech 2003;60:445–449. [DOI] [PubMed] [Google Scholar]
- Peterson JE, Zurakowski D, Italiano JE Jr, Michel LV, Fox L, Klement GL, Folkman J. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol 2010;85:487–493. [DOI] [PubMed] [Google Scholar]
- Plevyak M, Hanna N, Mayer S, Murphy S, Pinar H, Fast L, Ekerfelt C, Ernerudh J, Berg G, Matthiesen L et al. . Deficiency of decidual IL-10 in first trimester missed abortion: a lack of correlation with the decidual immune cell profile. Am J Reprod Immunol 2002;47:242–250. [DOI] [PubMed] [Google Scholar]
- Pontis A, D'Alterio MN, Pirarba S, Angelis C, Tinelli R, Angioni S. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol 2016;32:696–700. [DOI] [PubMed] [Google Scholar]
- Rai P, Shivaji S. The role of DJ-1 in the pathogenesis of endometriosis. PLoS One 2011;6:e18074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017;20:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. Epithelial-mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp Cell Res 2017;353:1–5. [DOI] [PubMed] [Google Scholar]
- Rizvi G, Pandey H, Pant H, Chufal SS, Pant P. Histopathological correlation of adenomyosis and leiomyoma in hysterectomy specimens as the cause of abnormal uterine bleeding in women in different age groups in the Kumaon region: a retroprospective study. J Midlife Health 2013;4:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AL, Carrarelli P, Novembri R, Pascalis F, Luisi S, Reis FM, Petraglia F. Activin A stimulates interleukin 8 and vascular endothelial growth factor release from cultured human endometrial stromal cells: possible implications for the pathogenesis of endometriosis. Reprod Sci 2012;19:832–838. [DOI] [PubMed] [Google Scholar]
- Rooij JD, Hosgor M, Ijzendoorn Y, Rottier R, Groenman FA, Tibboel D, Krijger RR. Expression of angiogenesis-related factors in lungs of patients with congenital diaphragmatic hernia and pulmonary hypoplasia of other causes. Pediatr Dev Pathol 2004;7:468–477. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431. [DOI] [PubMed] [Google Scholar]
- Rudzitis-Auth J, Nenicu A, Nickels RM, Menger MD, Laschke MW. Estrogen stimulates homing of endothelial progenitor cells to endometriotic lesions. Am J Pathol 2016;186:2129–2142. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Woolley DE. Matrix metalloproteinases in normal menstruation. Hum Reprod 1996;11:124–133. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007;36:666–676. [DOI] [PubMed] [Google Scholar]
- Schindl M, Birner P, Obermair A, Kiesel L, Wenzl R. Increased microvessel density in adenomyosis uteri. Fertil Steril 2001;75:131–135. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA 1991;88:5680–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod 2016;31:355–369. [DOI] [PubMed] [Google Scholar]
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol 2008;18:349–355. [DOI] [PubMed] [Google Scholar]
- Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res 2012;18:5856–5864. [DOI] [PubMed] [Google Scholar]
- Sotnikova N, Antsiferova I, Malyshkina A. Cytokine network of eutopic and ectopic endometrium in women with adenomyosis. Am J Reprod Immunol 2002;47:251–255. [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- Stoelinga B, Hehenkamp WJK, Nieuwenhuis LL, Conijn MMA, Waesberghe J, Brolmann HAM, Huirne JAF. Accuracy and reproducibility of sonoelastography for the assessment of fibroids and adenomyosis, with magnetic resonance imaging as reference standard. Ultrasound Med Biol 2018;44:1654–1663. [DOI] [PubMed] [Google Scholar]
- Streuli I, Dubuisson J, Santulli P, Ziegler D, Batteux F, Chapron C. An update on the pharmacological management of adenomyosis. Expert Opin Pharmacother 2014;15:2347–2360. [DOI] [PubMed] [Google Scholar]
- Sugino N, Kashida S, Karube-Harada A, Takiguchi S, Kato H. Expression of vascular endothelial growth factor (VEGF) and its receptors in human endometrium throughout the menstrual cycle and in early pregnancy. Reproduction 2002;123:379–387. [DOI] [PubMed] [Google Scholar]
- Tabruyn SP, Griffioen AW. NF-kappa B: a new player in angiostatic therapy. Angiogenesis 2008;11:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabruyn SP, Memet S, Ave P, Verhaeghe C, Mayo KH, Struman I, Martial JA, Griffioen AW. NF-kappaB activation in endothelial cells is critical for the activity of angiostatic agents. Mol Cancer Ther 2009;8:2645–2654. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 2005;65:3193–3199. [DOI] [PubMed] [Google Scholar]
- Tokyol C, Aktepe F, Dilek FH, Sahin O, Arioz DT. Expression of cyclooxygenase-2 and matrix metalloproteinase-2 in adenomyosis and endometrial polyps and its correlation with angiogenesis. Int J Gynecol Pathol 2009;28:148–156. [DOI] [PubMed] [Google Scholar]
- Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Li D. VEGF is upregulated by hypoxia-induced mitogenic factor via the PI-3K/Akt-NF-kappaB signaling pathway. Respir Res 2006;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremellen K, Russell P. Adenomyosis is a potential cause of recurrent implantation failure during IVF treatment. Aust N Z J Obstet Gynaecol 2011;51:280–283. [DOI] [PubMed] [Google Scholar]
- Tremellen KP, Russell P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: adenomyosis and macrophages. J Reprod Immunol 2012;93:58–63. [DOI] [PubMed] [Google Scholar]
- Uden P, Kenneth NS, Webster R, Muller HA, Mudie S, Rocha S. Evolutionary conserved regulation of HIF-1beta by NF-kappaB. PLoS Genet 2011;7:e1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bosch T, Bruijn AM, Leeuw RA, Dueholm M, Exacoustos C, Valentin L, Bourne T, Timmerman D, JAF H. A sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol 2018. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke JP, Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, Initiative S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500–1524. [DOI] [PubMed] [Google Scholar]
- Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril 2018;109:398–405. [DOI] [PubMed] [Google Scholar]
- Vannuccini S, Tosti C, Carmona F, Huang SJ, Chapron C, Guo SW, Petraglia F. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online 2017;35:592–601. [DOI] [PubMed] [Google Scholar]
- Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci USA 2009;106:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellini P, Consonni D, Barbara G, Buggio L, Frattaruolo MP, Somigliana E. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online 2014a;28:704–713. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod 2014b;29:964–977. [DOI] [PubMed] [Google Scholar]
- Wang B, Xiao Y, Ding BB, Zhang N, Yuan X, Gui L, Qian KX, Duan S, Chen Z, Rao Y et al. . Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003;4:19–29. [DOI] [PubMed] [Google Scholar]
- Wang J, Deng X, Yang Y, Yang X, Kong B, Chao L. Expression of GRIM-19 in adenomyosis and its possible role in pathogenesis. Fertil Steril 2016;105:1093–1101. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Zhao Y, Han B, Ma YG, Zhang J, Yang DM, Mao JW, Tang FT, Li WD, Yang Y et al. . Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci 2008;99:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang L, Shao J, Wang Y, Jin LP, Li DJ, Li MQ. L-22 enhan ces the invasiveness of endometrial stromal cells of adenomyosis in an autocrine manner. Int J Clin Exp Pathol 2014;7:5762–5771. [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005;437:497–504. [DOI] [PubMed] [Google Scholar]
- Wentink MQ, Huijbers EJ, Gruijl TD, Verheul HM, Olsson AK, Griffioen AW. Vaccination approach to anti-angiogenic treatment of cancer. Biochim Biophys Acta 2015;1855:155–171. [DOI] [PubMed] [Google Scholar]
- Wu WT, Chen CN, Lin CI, Chen JH, Lee H. Lysophospholipids enhance matrix metalloproteinase-2 expression in human endothelial cells. Endocrinology 2005;146:3387–3400. [DOI] [PubMed] [Google Scholar]
- Wu XG, Zhou CF, Zhang YM, Yan RM, Wei WF, Chen XJ, Yi HY, Liang LJ, Fan LS, Liang L et al. . Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 2019. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Noguchi T, Tamura T, Kitawaki J, Okada H. Evidence for estrogen synthesis in adenomyotic tissues. Am J Obstet Gynecol 1993;169:734–738. [DOI] [PubMed] [Google Scholar]
- Yang B, Wang L, Wan X, Li Y, Yu X, Qin Y, Luo Y, Wang F, Huang O. Elevated plasma levels of lysophosphatidic acid and aberrant expression of lysophosphatidic acid receptors in adenomyosis. BMC Womens Health 2017;17:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Sgadari C, Furuke K, Bloom ET, Teruya-Feldstein J, Tosato G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood 1999;93:1612–1621. [PubMed] [Google Scholar]
- Yen CF, Huang SJ, Lee CL, Wang HS, Liao SK. Molecular characteristics of the endometrium in uterine adenomyosis and its biochemical microenvironment. Reprod Sci 2017;24:1346–1361. [DOI] [PubMed] [Google Scholar]
- Zhang J, Salamonsen LA. Expression of hypoxia-inducible factors in human endometrium and suppression of matrix metalloproteinases under hypoxic conditions do not support a major role for hypoxia in regulating tissue breakdown at menstruation. Hum Reprod 2002;17:265–274. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol 2016;428:1–16. [DOI] [PubMed] [Google Scholar]
- Zheng W, Cao L, Xu Z, Ma Y, Liang X. Anti-angiogenic alternative and complementary medicines for the treatment of endometriosis: a review of potential molecular mechanisms. Evid Based Complement Alternat Med 2018;2018:4128984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhihong N, Yun F, Pinggui Z, Sulian Z, Zhang A. Cytokine profiling in the eutopic endometrium of adenomyosis during the implantation window after ovarian stimulation. Reprod Sci 2016;23:124–133. [DOI] [PubMed] [Google Scholar]
- Zhou S, Yi T, Liu R, Bian C, Qi X, He X, Wang K, Li J, Zhao X, Huang C et al. . Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics 2012;11:M112.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YF, Mori T, Kudo H, Asakai R, Sassa S, Sakamoto S. Effects of angiogenesis inhibitor TNP-470 on the development of uterine adenomyosis in mice. Fertil Steril 2003;80:788–794. [DOI] [PubMed] [Google Scholar]
- Zhu B, Chen Y, Shen X, Liu X, Guo SW. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence.. Reprod Biol Endocrinol 2016;14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.