ABSTRACT
Consistent asymmetries between the left and right sides of animal bodies are common. For example, the internal organs of vertebrates are left-right (L-R) asymmetric in a stereotyped fashion. Other structures, such as the skeleton and muscles, are largely symmetric. This Review considers how symmetries and asymmetries form alongside each other within the embryo, and how they are then maintained during growth. I describe how asymmetric signals are generated in the embryo. Using the limbs and somites as major examples, I then address mechanisms for protecting symmetrically forming tissues from asymmetrically acting signals. These examples reveal that symmetry should not be considered as an inherent background state, but instead must be actively maintained throughout multiple phases of embryonic patterning and organismal growth.
KEY WORDS: Symmetry, Asymmetry, Nodal, Left-right, Limbs, Somites, Cilia
Summary: Vertebrates are an amalgam of asymmetric and symmetric structures. This Review discusses how symmetries and asymmetries can be generated alongside each other in the embryo and then maintained during growth.
Introduction
The vast majority of animals have body plans based on bilateral symmetry. They possess orthogonal anterior-posterior (A-P) and dorsal-ventral (D-V) axes and therefore have a left and right side. The two sides of a perfectly bilateral animal are mirror images of each other. However, this perfection is rarely realized in the biological world; most animals exhibit left-right (L-R) asymmetries, be they subtle or striking. These asymmetries can be grouped into three categories: (1) fluctuating asymmetries, in which small deviations from L-R symmetry result from developmental noise; (2) anti-symmetries, in which structures are consistently asymmetric but with the direction of asymmetry (i.e. left-handed or right-handed) being random between individuals; and (3) directional asymmetries, in which structures are again consistently asymmetric but in this case the direction of asymmetry is the same in all individuals. Directional asymmetries are common among the bilaterian animals, a major example being the position of the internal organs within vertebrates. The human heart, for example, is offset to the left, as are the stomach and spleen; the gall bladder and liver sit to the right, while the left and right lungs, and the two kidneys, are different sizes and shapes (Fig. 1).
Fig. 1.
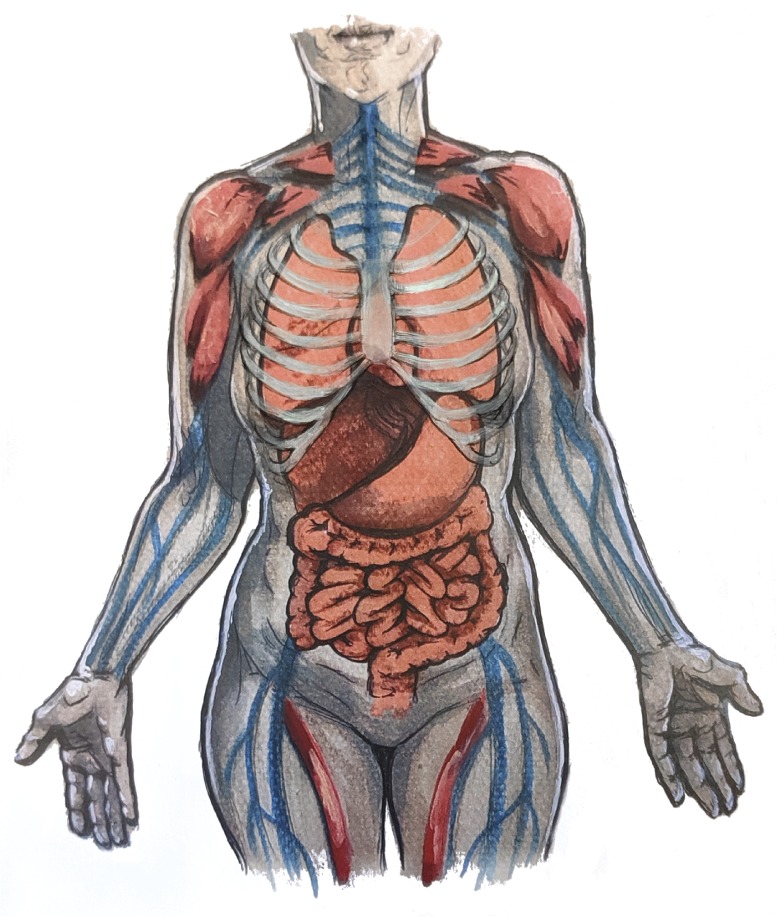
Vertebrates are an amalgam of symmetric and asymmetric structures. Although the muscles and skeleton are largely symmetric between left and right, the majority of the internal organs exhibit asymmetries in their overall position and shape.
Other aspects of embryonic development and growth play out symmetrically. The organs are encased by muscles and skeleton, structures that are close to being L-R symmetric. Appendages that protrude from the body, such as arms and legs, also exhibit L-R symmetry for biomechanical and locomotive reasons (Fig. 1). The organism therefore faces the challenge of controlling symmetries and asymmetries such that they manifest only in the appropriate tissue. For example, an L-R asymmetric pathway involving the secreted TGFβ factor Nodal is present in neurula-stage embryos. This pathway controls many aspects of asymmetric organ morphogenesis (Grimes and Burdine, 2017), but structures such as the somites and limb buds must be protected from its influence. Achieving symmetry is compounded by the fact that symmetric and asymmetric structures form from the same or closely juxtaposed tissues and use many of the same signaling pathways.
After embryogenesis, symmetries are also maintained during growth. Limbs grow seemingly independently for long periods of time, up to 16 years in humans, yet achieve the same size to a precise degree (Wolpert, 2010). The vertebral column is able to maintain L-R symmetry during growth and adulthood, despite the presence of mechanical forces that favor its curvature. The normal development and maintenance of these symmetries is lost in diseases such as Holt-Oram syndrome, where upper limb defects occur with a consistent L-R bias (Newbury-Ecob et al., 1996), and idiopathic scoliosis (see Box 1), where spinal symmetries break down and three-dimensional curves develop during adolescent growth (Cheng et al., 2015).
Box 1. Diseases in which abnormal symmetries and asymmetries manifest.
Heterotaxy, a disorder in which the L-R placement of visceral organs is affected, occurs in around 1 in 10,000 people (Sempou and Khokha, 2019; Sutherland and Ware, 2009). However, when stillbirths are accounted for, heterotaxy is likely much more frequent. Although heterotaxy can impact several organs, the effect on the heart is particularly detrimental owing to its complex L-R construction. Indeed, up to 3% of congenital heart disease (CHD) cases result from heterotaxy (Sutherland and Ware, 2009). A related condition, primary ciliary dyskinesia (PCD), affects around 1 in 10,000-20,000 live births and is caused by abnormal beating of motile cilia (Mitchison and Valente, 2017). This results in defective mucus clearance in the lungs, causing chronic infections, as well as infertility and abnormal organ L-R asymmetry in about half of patients. Other diseases affect tissues that are usually symmetric, such as the spine. Deficits in the bilateral symmetry of somite formation cause congenital scoliosis, a birth defect in which vertebrae are often structurally malformed (Dunwoodie et al., 2002; Li et al., 1997a; Sparrow et al., 2012; Turnpenny et al., 2007). A more common form of scoliosis, termed adolescent idiopathic scoliosis (AIS), occurs in 3-4% of children worldwide. AIS is defined by lateral curves greater than 10° and typically begins during adolescent growth. Spinal bracing or, in extreme cases, surgical intervention are the only available preventative treatments (Cheng et al., 2015). Recent insight into the etiopathogenesis of AIS has emerged. For example, the proprioceptive system (Blecher et al., 2017) as well as cerebrospinal fluid flow (Grimes et al., 2016) have both been implicated in maintaining spinal straightness.
In this Review, I discuss progress in our understanding of how symmetries and asymmetries form alongside one another during development. I first summarize how the vertebrate embryo breaks symmetry to give rise to directional asymmetry of organs. I then discuss models for how the somites and limb buds are able to maintain symmetry in spite of asymmetric molecular cues that influence their development. Finally, I review how the limbs and spine maintain symmetry during long periods of growth. Throughout, I consider diseases in which abnormal symmetries and asymmetries manifest, highlighting the insight that has come from their study.
Breaking symmetry: from asymmetric molecules to asymmetric embryos
To the task of generating an embryo with directional L-R asymmetries, the universe presents a considerable challenge: there is no macroscopic feature of the world that distinguishes left from right. The left and right sides of a bilateral structure can only be defined by referring to a previously agreed upon asymmetric reference. So how can two cells in a bilaterally symmetric embryo that are in equivalent positions but on different sides of the midplane ‘know’ they are on either the left or right side? Directional asymmetries at smaller scales do exist: for example, the violation of symmetry by weak nuclear force (Wu et al., 1957), the use of L but seldom D amino acids by living organisms, as well as the chirality of centrioles (Bornens, 2012) and even entire cells (Taniguchi et al., 2011; Wan et al., 2016). These atomic, molecular and cellular asymmetries are all potential sources of asymmetric patterning information at larger scales, but how they can be transferred to the level of the embryo such that it can differentiate left from right is far from obvious (Brown and Wolpert, 1990).
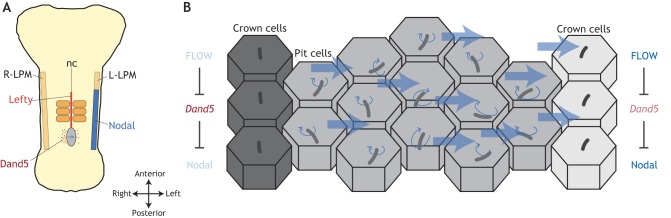
Over the last 20 years, an answer for how vertebrate embryos can tell their left from right has emerged. This model involves midline-located L-R organizers (LROs) – the node in mice, Kupffer's vesicle in zebrafish and the gastroceol roof plate in Xenopus laevis – in which symmetry is broken by a mechanism requiring motile cilia and extracellular fluid flow (Fig. 2) (Essner et al., 2005; Hirokawa et al., 2006; Kramer-Zucker et al., 2005; Nonaka et al., 2002, 1998; Schweickert et al., 2007). Motile cilia are whip-like protrusions of the cell surface built around a microtubule scaffold. They move under the power of dynein motors and drive extracellular fluid flows (Spassky and Meunier, 2017). In LROs, the rotational movement of a field of motile cilia, all in the same direction, generates a fluid flow that is stronger on the left side than the right. This L-R difference is sensed by the embryo and, as a result, L-R asymmetries in gene expression are established around LROs (Fig. 2) (McGrath et al., 2003; Schweickert et al., 2010). Initially, these differences are subtle, but they are then amplified by feedforward loops and the spreading of secreted factors (Nakamura et al., 2006, 2012). This results in broad asymmetrically acting pathways in lateral plate mesoderm (LPM) tissue. Pathways active on one side are prevented from activating on the opposite side by midline barriers (Lenhart et al., 2011; Nakamura et al., 2006). In this way, subcellular asymmetries within cilia are transferred to the scale of the embryo by fluid flow and downstream signaling pathway networks.
Fig. 2.
Asymmetric fluid flow converts cellular chirality to embryo-level asymmetry. (A) Schematic ventral view of a three-somite stage mouse embryo depicting the mouse left-right organizer (LRO) (the node; blue oval) at the posterior tip of the notochord (nc). A leftward fluid flow in the LRO (blue arrow) represses Dand5 on the left, causing derepression and activation of the Nodal pathway in the left LPM (L-LPM). Midline-expressed Lefty inhibits Nodal activity in the right LPM (R-LPM), thereby maintaining unilateral activity. (B) Close-up view of a section of the LRO showing central pit cells harboring posteriorly polarized motile cilia, which rotate clockwise when viewed ventrally. These cilia generate an overall leftward flow in the LRO. This flow is sensed by immotile and unpolarized sensory cilia on the surface of lateral crown cells. In a little-understood sensory pathway, flow signals on the left cause the post-transcriptional repression of Dand5, ultimately promoting left LPM initiation of the Nodal pathway. By contrast, Dand5 remains unrepressed in right-sided crown cells and so Nodal signals do not reach a level compatible with R-LPM Nodal pathway activation.
A second model for the origin of embryonic L-R asymmetry, the ion flux model, argues that asymmetry is established earlier, before cilia are present in the embryo (Vandenberg and Levin, 2013). Indeed, some vertebrates, such as the chick and pig, clearly do not use a cilia/flow system to break embryonic symmetry (Gros et al., 2009), suggesting that other mechanisms must exist in these organisms. In the ion flux model, chiral cytoskeletal elements transport potassium channels and proton pumps to one side of the embryo during cleavage stages (Qiu et al., 2005), ultimately resulting in asymmetries in transmembrane voltage and pH between the left and right halves of the embryo (Adams et al., 2006). Once established, these bioelectric gradients are proposed to then directionally transport charged molecules through gap junctions to one side of the embryo (Fukumoto et al., 2005a,b). Serotonin, for example, is transported to the right side where it represses the expression of the key left-side determinant Nodal (Carneiro et al., 2011).
Both the cilia/flow and ion flux models explain how molecular/cellular chirality can be transferred to the level of the embryo, but distinguishing between the two has proved difficult. It is true that many of the components of the ion flux model also play roles in the cilia/flow mechanism in Xenopus laevis (Beyer et al., 2012a,b; Walentek et al., 2012) but equally some components are used by organisms that do not use the flow mechanism at all (Abe and Kuroda, 2019; Chuang et al., 2007; Davison et al., 2016; Kuroda et al., 2016; Oviedo and Levin, 2007; Tee et al., 2015). These controversies likely reflect a complex evolutionary situation in which several mechanisms for amplifying asymmetry from the level of cytoskeletal chirality exist and potentially intermingle to different extents in different species (Box 2). Which mechanisms are ancestral, and potential reasons for the loss of cilia/flow in some species, have been the subject of excellent reviews (Blum et al., 2014a,b; Vandenberg and Levin, 2013).
Box 2. Bilaterality and asymmetry-generating mechanisms across evolution.
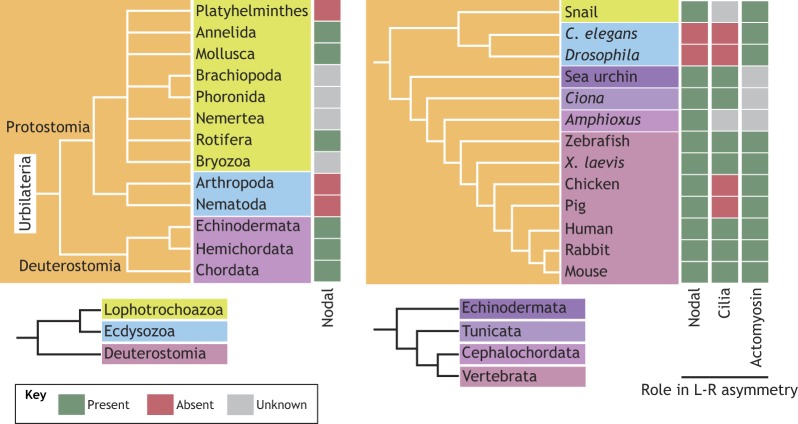
The majority of extant animal species exhibit a bilateral body plan and so are grouped together in the bilateria, the most recent common ancestor of which is called urbilateria (De Robertis and Sasai, 1996). Bilaterality likely evolved, perhaps more than once, before the Cambrian explosion, as it seems to favor locomotion (Genikhovich and Technau, 2017). But once the bilateral body plan appeared, it may have come to dominate the animal kingdom owing to its potential for increased complexity and, thus, its highly evolvable nature. The inter-relationships between the bilateria are shown in the figure (left panel). The presence of Nodal, and its requirement for L-R asymmetry, in both protostomes and deuterostomes suggests it was present in urbilateria but was subsequently lost in some animals such as the ecdysozoa. The panel on the right shows the evolutionary relationships between species that have been well studied with respect to L-R asymmetry. Evolutionary logic suggests that a cilia and flow-based mechanism is a trait shared by the deuterostomes in general, although it has been lost in some species such as the chicken (and likely all birds) and the pig for currently unknown reasons. There is evidence that actomyosin components, such as Myo1D, control L-R asymmetry in many deuterostomes as well as the model ecdysozoans Drosophila and C. elegans, supporting an ancestral role for the cytoskeleton in generating L-R asymmetry within the bilateria (Lebreton et al., 2018; Yuan and Brueckner, 2018). Disruption of Myo1D in the model vertebrates Xenopus laevis and zebrafish results in L-R defects owing to perturbation of left-right organizer flow (Juan et al., 2018; Saydmohammed et al., 2018; Tingler et al., 2018).
Regardless of the upstream mechanisms, the result of embryo-level symmetry breaking is the activation of Nodal in the left but not right LPM (Collignon et al., 1996; Lowe et al., 1996). Lateralized Nodal is conserved across vertebrates as well as some invertebrates (Grande and Patel, 2009) (Box 2). Once activated in the left LPM at the level of the LRO, Nodal spreads throughout the left LPM where it activates the expression of Pitx2, a homeodomain transcription factor that remains asymmetrically expressed into organogenesis (Campione et al., 1999; Piedra et al., 1998; Ryan et al., 1998; Shiratori et al., 2006; Yoshioka et al., 1998). Nodal and Pitx2 asymmetries drive many organ asymmetries during morphogenesis (Grimes and Burdine, 2017). For example, Nodal signaling from the left LPM to the left side of the midline-positioned zebrafish heart results in the leftward migration of cardiac cells. This transforms the initially symmetric heart tube into a leftward-pointing tube, a process termed cardiac jogging (Baker et al., 2008; Grant et al., 2017; Lenhart et al., 2013; Veerkamp et al., 2013). Similarly, Pitx2 expression in the left side of the dorsal mesentery of the gut, a derivative of the LPM, causes asymmetric cell architecture and adhesion changes that drive directional gut tilting, ultimately resulting in stereotyped asymmetric gut coiling in birds and mammals (Davis et al., 2008; Kurpios et al., 2008; Savin et al., 2011; Welsh et al., 2013). The left-sided Nodal-Pitx2 pathway is therefore a major source of L-R asymmetric positional information for cells.
To sum, an embryonic symmetry-breaking event followed by amplification of initially subtle asymmetries by regulatory networks transfers molecular- and cellular-level asymmetries to the scale of the embryo. As a result, the L-R Nodal-Pitx2 pathway is established in the LPM in which Nodal and downstream genes are expressed specifically in the left but not right side. Several other factors, in addition to Nodal and Pitx2, act asymmetrically in embryos (Levin et al., 1995; Meyers and Martin, 1999; Ocaña et al., 2017). I hereafter refer to these embryo-scale asymmetrically acting factors, which usually act in the LPM and its derivatives, as the L-R pathway.
Making symmetry: protecting symmetric structures from asymmetric influences
The asymmetric internal organs are surrounded and supported by symmetric features of the body: muscles, skeleton and limbs. However, the developmental precursors of these, the somites and limb buds, form in an embryo that is patterned asymmetrically by the L-R pathway. The somites form from paraxial mesoderm, a tissue that lies between LROs and LPM, and so may relay asymmetric information, while limb buds outgrow from the molecularly asymmetric LPM itself. How, then, are these precursors able to maintain symmetry during their formation?
A conceptual framework for maintaining symmetry in the face of asymmetric signals
Perhaps the simplest model would be to suppose that the somites and limb buds cannot respond to asymmetric signals. However, we know that both symmetric and asymmetric structures use overlapping molecular cues during their formation, so it is more likely that symmetric structures must be actively protected from asymmetric signals. Indeed, in certain mutant backgrounds, normally symmetric structures become directionally asymmetric, suggesting the existence of active protection mechanisms. How might this be achieved? In Fig. 3, I propose a conceptual framework for the generation of symmetry in the presence of directionally asymmetric signals. This encompasses two models: the balancer model and the wedge model. Both can be visualized as a seesaw with forces acting on either side. A horizontal seesaw implies a symmetric phenotype (Fig. 3Ai), but if the forces result in tilting of the seesaw to the left or the right, then asymmetric left-biased or right-biased phenotypes, respectively, manifest (Fig. 3Aii,iii). Let us suppose that asymmetrically acting L-R influencers can potentially impact the position of the seesaw (Fig. 3Bi). A typical L-R influencer might be the left-sided Nodal pathway, but others also exist, including those that act on the right side. In the balancer model, such L-R influencers are counteracted by an asymmetrically acting contralateral ‘balancer’ (Fig. 3Bi,ii). In the wedge model, by contrast, a symmetrically acting ‘wedge’ holds the seesaw horizontally, preventing the L-R influencer from tilting it (Fig. 3Ci,ii). Both models predict asymmetries in normally symmetric structures when either the balancer (Fig. 3Biv) or the wedge (Fig. 3Civ) is lost. By contrast, symmetry is the most likely outcome upon loss of the L-R influencer (Fig. 3Biii,Ciii), although fluctuating asymmetries may emerge stochastically, especially in the balancer model (Fig. 3Biii). Importantly, balancers do not normally influence the seesaw in the absence of L-R influencers because their own expression is asymmetric and so depends on the L-R pathway. This is not the case for wedges, which are equally active on both sides of the seesaw independently of L-R asymmetric pathways. In the following sub-sections, I consider mechanisms for generating symmetry during limb bud outgrowth and somite formation in light of this conceptual framework.
Fig. 3.
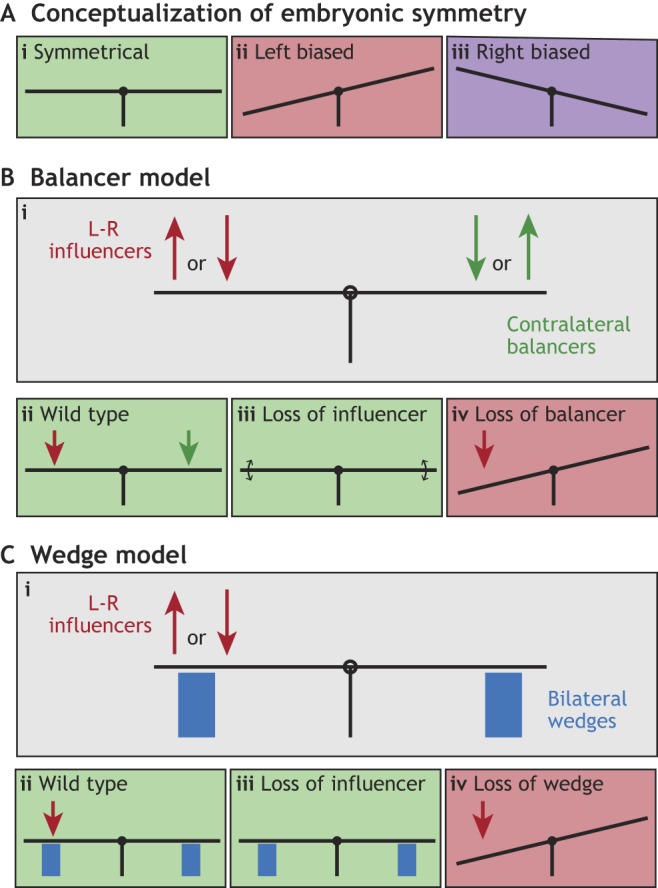
Conceptual framework for maintaining symmetry in the face of asymmetric signals. (A) The embryo is conceptualized as a seesaw that, when horizontal (i), represents a L-R symmetric outcome. Tilting of the seesaw signifies left- or right-biased asymmetries (ii and iii). (B) The balancer model assumes the existence of contralateral balancers that counteract asymmetrically acting L-R influencers (i). In wild-type embryos, the L-R influencer and the balancer perfectly counteract, resulting in symmetry (ii). Symmetry is also the outcome upon loss of the L-R influencer (iii) because balancer activity is also lost as its asymmetric action is downstream of the L-R pathway. However, fluctuating asymmetries might be expected to emerge in such a condition. By contrast, loss of the balancer results in ectopic asymmetries in normally symmetric structures (iv). (C) In the wedge model, bilateral wedges hold the seesaw horizontally, and thereby favor a symmetric outcome, regardless of the action of L-R influencers (i,ii). In this scenario, symmetry is maintained upon loss of the L-R influencer (iii) but asymmetry occurs when the wedge is lost (iv).
Maintaining limb bud symmetry
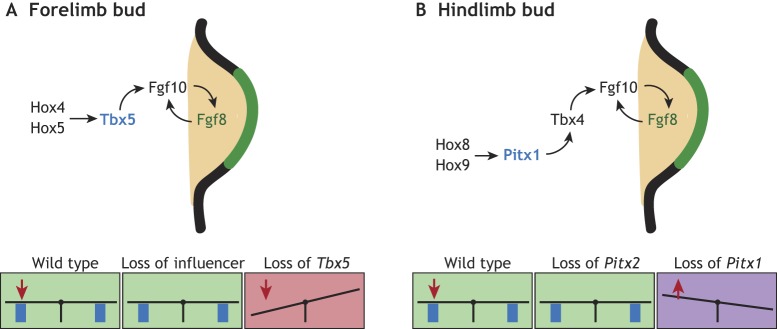
The limbs of vertebrates – arms, legs, wings and fins – begin to form in the embryo when subpopulations of somatic LPM cells are induced to generate a bud. Expression of Fgf10 in the bud-forming LPM induces Fgf8 in the overlying ectoderm; this establishes an Fgf10-Fgf8 positive-feedback loop that promotes limb bud outgrowth (Fig. 4A,B) (Ohuchi et al., 1997; Sekine et al., 1999; Xu et al., 1998). Expression of Fgf10 is initially activated by Tbx transcription factors: Tbx4 in the hindlimb (HL) and Tbx5 in the forelimb (FL) (Naiche and Papaioannou, 2003; Rallis et al., 2003; Rodriguez-Esteban et al., 1999). In the HL, Pitx1 acts upstream of Tbx4, while retinoic acid and Wnt/β-catenin signaling both control Tbx5 expression in the FL (Sheeba and Logan, 2017). The level at which limb bud outgrowth occurs along the anterior-posterior axis is controlled by Hox genes (Fig. 4A,B) (Moreau et al., 2019; Tickle, 2015).
Fig. 4.
The maintenance of limb bud symmetry. (A) Schematic of a mouse forelimb showing the pathway that promotes forelimb outgrowth. Tbx5, which is expressed symmetrically, acts as a wedge to maintain symmetry that buffers an unknown L-R influencer. Loss of Tbx5 causes left-biased forelimb defects. (B) Schematic of a mouse hindlimb showing the pathway that promotes limb bud outgrowth. Pitx2, which is expressed specifically in the left LPM, is an L-R influencer that can disrupt limb symmetry in the absence of Pitx1, which thereby acts as a wedge to buffer against underlying Pitx2 asymmetry.
Evidence, initially from studies of human limb disorders, indicates that active mechanisms are essential for generating limb symmetry. Holt-Oram Syndrome (HOS; OMIM: 142900) is a dominant disorder in which patients exhibit upper limb defects with variable severity but in which the left arm is more highly defective than the right arm (Newbury-Ecob et al., 1996). HOS is caused by haploinsufficiency of the FL factor TBX5 (Basson et al., 1997; Li et al., 1997b). However, modeling this condition in the mouse has proved challenging because mutants in which Tbx5 is lost completely fail to form FLs (Rallis et al., 2003). Recent progress has nevertheless been made with the creation of two mouse models that recapitulate HOS phenotypes (Sulaiman et al., 2016). In one model, here called Tbx5hypomorph, Tbx5 is deleted using a limb-restricted Cre recombinase (Prx1-Cre), causing a loss of FL outgrowth. This defect can be partially rescued by Prx1-specific expression of Tbx5 at hypomorphic levels relative to wild type. In a second model, Tbx5mosaic, a Prx1-Cre line that expresses Cre in a delayed and mosaic fashion, is used to lower Tbx5 levels in the limb-forming region. Both manipulations reduce the effective levels of Tbx5 in the FL in an L-R symmetric manner but, in both cases, left FLs are more severely impacted than right FLs (Sulaiman et al., 2016). This recapitulates the situation in individuals with HOS and provides strong evidence that Tbx5 buffers an underlying FL asymmetry (Fig. 4A).
As Tbx5 is expressed symmetrically, but because lowering its levels induces asymmetry in normally symmetric structures, we can consider Tbx5 as a wedge that counteracts an L-R influencer (Fig. 4A). In wild-type embryos, Tbx5 levels are above a threshold that is high enough to prevent the seesaw from tilting. By contrast, when Tbx5 levels are lowered (e.g. as in Tbx5hypomorph or Tbx5mosaic mutants), the L-R influencer can tilt the seesaw to the left, resulting in left-biased defects. Because the sidedness of FL defects are biased and not random, reversal of embryonic situs should reverse the L-R influencer, and thereby reverse the directionality of the FL defects. Fittingly, reversal of situs at the same time as reduction of Tbx5 levels in inv;Tbx5hypomorph embryos results in right FLs being more severely affected (Sulaiman et al., 2016). However, the identity of the L-R influencer that Tbx5 counteracts is not known.
A second example of how limb symmetry is achieved is provided by the role of Pitx1 in HLs (Fig. 4B). Loss of Pitx1 in the mouse causes severe HL defects owing to reduced chondrogenesis and myogenesis (Szeto et al., 1999). Importantly, HLs are asymmetrically affected, with the right HL being more severely reduced than the left (Lanctot et al., 1999). Similarly, loss-of-function PITX1 mutations cause clubfoot (OMIM: 119800), a condition in which the right leg is often more severely impacted (Gurnett et al., 2008). This asymmetric effect of Pitx1 malfunction is a result of partial compensation by Pitx2 on the left side. Pitx2 is expressed in the left LPM downstream of Nodal signals and, as a result, is present in the early limb-forming field on the left. Experiments in mice in which both Pitx1 and Pitx2 are mutated suggest that the overall dose of Pitx transcription factors is crucial in determining HL outgrowth and identity (Marcil et al., 2003). Thus, in a wild-type embryo, bilaterally high Pitx1 levels ensure that a threshold is overcome for normal HL formation such that L-R differences in Pitx2 do not have an asymmetric effect on the developing limb. Pitx1 could therefore be considered as a wedge that counteracts the L-R influencer Pitx2 (Fig. 4B).
Other molecular factors controlling limb symmetry have been discovered. The transcription factor Sall4 acts upstream of Tbx4 in HL development, yet loss of Sall4 causes unilateral defects with no directional bias (Koshiba-Takeuchi et al., 2006). This observation is currently unexplained but suggests that, in addition to directional asymmetries, stochastic differences between left and right limb buds also exist and must also be buffered to generate a symmetric outcome. Indeed, careful measurements have shown that there can be a high variance in the levels of sonic hedgehog between the left and right limb buds at particular developmental stages (Zhang et al., 2017), yet limb patterning is consistently achieved in a symmetric fashion regardless. Understanding the range of molecular differences that are compatible with a symmetric outcome, as well as how intrinsic noise is buffered, will provide insight into developmental robustness. The symmetric appendages are ideal for such studies as any fluctuating asymmetries between bilaterally paired structures act as readouts of stochastic variation (Debat and Peronnet, 2013).
The symmetry of segmentation
The somites, the embryonic precursors of repetitive body elements, including vertebrae and skeletal muscle, are bilaterally paired structures that form via the anterior to posterior segmentation of the paraxial mesoderm on either side of the notochord (Bénazéraf and Pourquié, 2013). Segmentation of this tissue, termed presomitic mesoderm (PSM), is controlled by the interaction of oscillations of cyclically expressed genes, called the segmentation clock, with opposing gradients of FGF8 (posterior to anterior) and retinoic acid (RA; anterior to posterior) that together set a concentration threshold called the determination front (Fig. 5A). Cells positioned anterior to the determination front can initiate a segmentation program, whereas cells posterior to it cannot, owing to high levels of FGF8 activity (Hubaud and Pourquié, 2014). The number of PSM cells that pass the determination front during one cycle of the segmentation clock thereby sets the size of each somite. The movement of the determination front and the length of the segmentation clock usually occurs synchronously on the left and right sides of the embryo and so somites emerge in a bilaterally symmetric fashion.
Fig. 5.
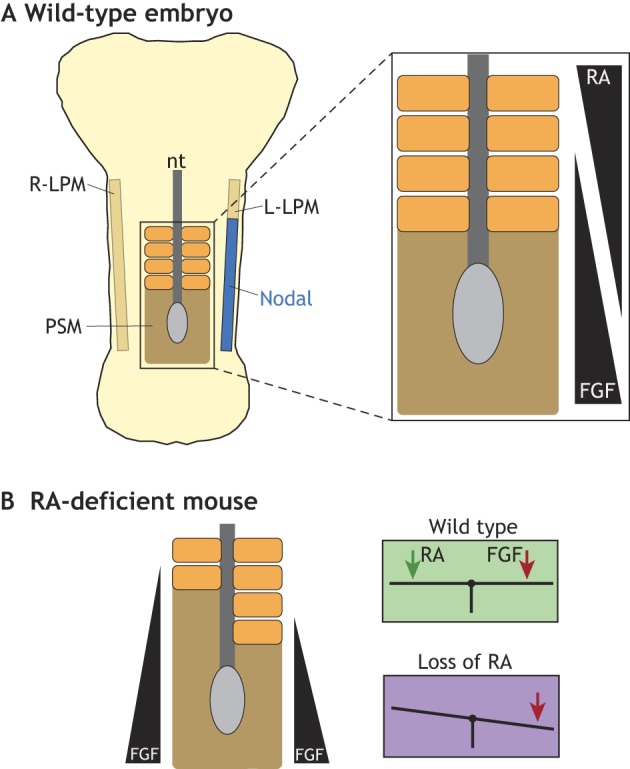
The maintenance of symmetry during segmentation. (A) Schematic of a somite-stage mouse embryo showing the lateral plate mesoderm (LPM) with left-sided L-R pathway activity (blue), the central neural tube (nt) and the paraxial mesoderm (orange), the unsegmented region of which is called the pre-somitic mesoderm (PSM, brown). Opposing gradients of RA and FGF activity determine the position of the determination front, which is the position at which cells initiate the segmentation program. (B) In the absence of RA, mouse embryos exhibit asymmetric somites, with the right side showing a delay owing to right-biased FGF8 activity. This results from the loss of buffering by RA, which acts as a contralateral balancer to the somite desynchronizing activity of FGF8.
A foothold into the molecular basis of bilateral somitogenesis has been revealed by experiments in mouse, chick and zebrafish embryos in which RA signaling is perturbed by inhibition or mutation of the RA biosynthetic enzyme RALDH2 (Kawakami et al., 2005; Sirbu and Duester, 2006; Vermot et al., 2005; Vermot and Pourquié, 2005). This leads to asymmetries in the segmentation clock and the determination front, causing delayed somite formation on one side of the embryo: the right side in mouse and zebrafish; and the left side in chick (Fig. 5B). Biochemical analysis of RA signaling in this context demonstrates that a complex called WHHERE, which consists of WDR5, the histone deacetylases HDAC1 and HDAC2, and the atrophin family protein RERE, positively regulates RA signaling during the control of somite symmetry (Vilhais-Neto et al., 2017, 2010). As such, mutations in Wdr5, Hdac1, Rere and Ehmt2, a gene encoding a histone methyltransferase that can also interact with RERE but more transiently than other members of the WHHERE complex, all cause right-biased somitogenesis delays in the mouse (Vilhais-Neto et al., 2017). Importantly, defective RA signaling does not impact the L-R pathway in the LPM (Niederreither et al., 2001). Moreover, the directional bias of somite asymmetry in Raldh2 and Rere mutants can be reversed when the L-R pathway is reversed (Vermot and Pourquié, 2005). Together, this suggests that RA signals buffer a somite desynchronizing influence from the L-R pathway.
How might this be achieved? Although many of the components of the RA signaling pathway are expressed symmetrically, RA pathway activity itself is asymmetric, being higher in the right PSM than the left in mice (Vilhais-Neto et al., 2010). This occurs because the RERE-interacting protein NR2F2, a nuclear receptor that occupies RA-responsive promoters and positively regulates RA signaling, is expressed asymmetrically within the PSM with a right bias. The molecular identity of the desynchronizing influence is likely FGF8. In mouse embryos, Fgf8 expression is normally restricted to the tail bud, posterior to the node. However, in the absence of RERE, Fgf8 expression expands anteriorly and asymmetrically, more so on the right side (Fig. 5B). As FGF8 represses the program that initiates somite formation, this ectopic encroachment of FGF8 more anteriorly on the right serves to explain the right-sided delay in somite formation in mouse embryos upon loss of RA (Vermot et al., 2005; Vilhais-Neto et al., 2010). Intriguingly, FGF8 has opposite roles in establishing L-R asymmetry in mouse and chick, being a left-side determinant in chick but a right-side determinant in mouse and rabbit (Boettger et al., 1999; Fischer et al., 2002; Meyers and Martin, 1999). Fittingly, NR2F2 is expressed more highly in the left PSM in chick (Vilhais-Neto et al., 2010), a result that coheres with opposite somite formation delays upon reduced RA signaling in chicken and mouse embryos (Vermot et al., 2005; Vermot and Pourquié, 2005). Thus, RA acts as a balancer that counteracts an asymmetric FGF8 signal to maintain somite symmetry (Fig. 5B).
The precise molecular details of this interaction have yet to be resolved but it is worth noting that the expansion of Fgf8 expression in Rere mutants may be a direct effect, as the Fgf8 locus harbors a RA-responsive element at which RERE localizes in the absence of RA, acting as a co-repressor (Kumar and Duester, 2014). In the presence of RA, RERE is released from the Fgf8 locus and replaced by a strong repressive complex containing HDAC1 and PRC2, which deposits repressive chromatin modifications (Kumar and Duester, 2014). Thus, RERE acts as more than a positive regulator of RA signaling, something that is underlined by the fact that Rere/Raldh2 double mutants show a more severe somite symmetry defect than Raldh2 single mutants, although the latter already exhibit a complete loss of RA signaling (Vermot et al., 2005; Vilhais-Neto et al., 2010).
These experiments reveal the importance of controlling the symmetry of the determination front. Defects in the segmentation clock, which is controlled by oscillations of Delta-Notch pathway activity, also result in ectopic somite asymmetry. However, in contrast to RA disruption, treatments that perturb Notch signaling typically result in somite asymmetries without consistent L-R bias (Dunwoodie et al., 2002; Evrard et al., 1998; Holley et al., 2000, 2002; Jiang et al., 2000; Zhang and Gridley, 1998), perhaps partly because the L-R pathway is also randomized in many of these cases owing to the role of Notch signaling in early L-R asymmetric patterning (Boskovski et al., 2013; Kawakami et al., 2005; Matsui et al., 2012; Saude et al., 2005). Some Notch signaling components, such as Suppressor of Hairless, have also been found to control RA biosynthesis (Echeverri and Oates, 2007). Thus, Notch signals are involved in several processes that impinge upon somite symmetry. It is not surprising, therefore, that the majority of genes linked to congenital scoliosis (see Box 1), a disease in which spinal symmetry breaks down due to structural malformations of the vertebrae, play roles in the Notch pathway (Turnpenny et al., 2007).
Some additional factors have been linked to somite symmetry. For example, in zebrafish embryos, loss of the transcription factor DMRT2A causes both abnormal L-R asymmetric patterning and L-R desynchronization of the segmentation clock (Saude et al., 2005). Although mechanistic details are unknown, it is likely that DMRT2A acts in Kupffer's vesicle to prevent asymmetric signals being transferred to the apposed posterior PSM. Precise expression of DMRT2A is controlled by the RNA-binding protein CELF1, which binds to the 3′ untranslated region of dmr2ta and post-transcriptionally dampens its expression (Matsui et al., 2012). As such, CELF1 is also essential for generating symmetric somites (Matsui et al., 2012). Although we do not understand how CELF1 controls symmetry and asymmetry, it is noteworthy that another of its targets is Pkd2, a transient receptor potential (TRP) cation channel that is essential for L-R patterning alongside its protein partner PKD1L1 (Field et al., 2011; Pennekamp et al., 2002). Thus, CELF1 could control L-R patterning, in part, through regulation of PKD2. Interestingly, although DMRT2 is expressed in Hensen's node in chick, it is not present in the mouse node. Moreover, mouse embryos lacking Dmrt2 do not exhibit any L-R defects (Lourenco et al., 2010). Thus, the function of DMRT2 in controlling L-R symmetry and asymmetry seems to have been lost in the lineage leading to the mouse.
Growing symmetrically
Achieving symmetry in size during growth by contralateral communication
A deep and still poorly understood property of biological systems is growth control, a process in which structures achieve their proper size despite intrinsic noise as well as genetic and environmental variation (Debat and Peronnet, 2013; Lander, 2011; Rao et al., 2002; Waddington, 1959). Mechanisms for achieving a certain size in a particular organ span scales, ranging from molecular to organismal levels. For convenience, we can divide mechanisms of size control into two categories: organ intrinsic and organ extrinsic. A typical organ-intrinsic mechanism involves an inhibitor of growth (called a ‘chalone’) being secreted by an organ: when the organ reaches a certain size and the inhibitor crosses a concentration threshold, further growth is repressed (Bullough and Laurence, 1964; McPherron et al., 1997; Plikus et al., 2008). An organ-extrinsic mechanism might instead involve a systemic growth factor secreted distantly that travels to the target organ, or physical constraints imparted on the organ by neighboring tissues. Early examples of extrinsic regulation came from grafting experiments in which limb buds were exchanged between closely related species of different sizes; the size the grafted limb attained was partially influenced by the size of the host body (Ohki-Hamazaki et al., 1997). Organ-intrinsic and organ-extrinsic mechanisms likely collaborate to promote the generation of target organ size robustly (Elgjo and Reichelt, 1994; Ikeda et al., 2009).
The size control of bilaterally paired structures, such as limbs, represents a special case where a third potential mechanism becomes apparent: contralateral regulation, whereby the growth status of a structure on one side of the body could potentially signal to and impinge upon the growth of the equivalent structure on the opposite side. Such communication could be essential in achieving symmetry of size and has recently been found to act in mouse limbs within the context of catch-up growth, a term that refers to the increase in growth rates after a growth-retarding condition such that normal body proportions are ultimately achieved. Specifically, it has been shown that activating the cell cycle repressor p21 in limb chondrocytes in the left side of mouse embryos during late embryogenesis results in decreased bone growth and shorter left limbs (Roselló-Díez et al., 2018). However, the ratio of limb lengths between the two sides is maintained, suggesting that right limb bones respond to the experimentally induced delay in left limb bone growth by reducing their own growth (Roselló-Díez et al., 2018). Indeed, left limb-specific insults result in a systemic growth reduction, mediated by reduced placental efficiency, which is able to maintain body proportions. However, when placental function is re-enhanced, although limb-body proportions become abnormal, limb symmetry is still maintained (Roselló-Díez et al., 2018). This suggests that placental-mediated systemic growth retardation upon left limb growth reduction cannot account for the maintenance of limb symmetry, leaving open the possibility of a more direct limb-limb communication system that maintains limb symmetry upon unilateral growth reduction. The molecular basis of this putative mechanism awaits discovery.
Other studies have also provided evidence of crosstalk between the left and right limbs in the context of injury and regeneration. For example, regeneration studies of the bioelectric properties of Xenopus HL epidermis have revealed that the depolarization that occurs in the amputated HL also occurs in the contralateral HL with the same pattern (Busse et al., 2018). Another example occurs in rats where HL tibial fractures induce growth-promoting BMP expression in both the fractured HL and the contralateral uninjured limb (Fischerauer et al., 2013). These studies reveal that limb crosstalk occurs, but discovering which systems and mechanisms mediate it remains a future challenge. They also warn against the use of contralateral limbs as internal controls.
Achieving and maintaining body straightness
Alongside limb symmetry, the body itself must maintain its overall straight shape (i.e. without lateral or three-dimensional curvatures). During axial elongation, embryonic growth at the posterior must be regulated such that bilateral symmetry is achieved, with defects in this process producing lateral bends in the trunk (Lawton et al., 2013). Elongation occurs when mesodermal progenitor cells undergo an epithelial-to-mesenchymal transition and then migrate into PSM, sorting symmetrically between left and right halves of the embryo. This is possible because the transition increases the disorder of their motion, preventing stable and chiral vortices of migration from forming that would otherwise result in asymmetric sorting between left and right (Das et al., 2017). Thus, increasing disorder at the cellular level promotes the emergence of symmetry of the elongating trunk.
During body elongation, the axis also straightens from a curled position into a straight head-to-tail axis. This is readily observed in the zebrafish embryo, which is initially curled ventrally around the yolk sphere. Over the first 1.5 days of development, the embryo extends and the trunk moves dorsally, thereby straightening the body axis (Fig. 6A). Recent evidence suggests that the Reissner fiber, a proteinacous thread extending along the inside of the central canal of the spinal cord in vertebrates, is essential for zebrafish body straightening (Fig. 6B) (Cantaut-Belarif et al., 2018). Loss of the Reissner fiber causes ventral curves, a phenotype that is also present in zebrafish mutants that lack cilia motility (Hjeij et al., 2014; Jaffe et al., 2016). Fittingly, motile cilia were found to be essential for formation of the Reissner fiber (Cantaut-Belarif et al., 2018). Although the Reissner fiber is unlikely to mechanically straighten the body, it could signal the status of curvature of the embryo. For example, in a straight embryo, the Reissner fiber may be positioned centrally in the spinal canal but, in ventrally curved embryos, it could touch mechano-responsive cells on the wall of the central canal, and thereby relay information about body curvature (Driever, 2018). Another potential model involves the Reissner fiber functioning as a transport conduit that allows chemical signals secreted in the brain to reach the spinal canal (Grimes, 2019; Zhang et al., 2018). For example, adrenaline secreted in the brain signals, directly or indirectly, to sensory cells in the spinal canal, termed cerebrospinal fluid-contacting neurons (CSF-cNs), to upregulate the expression of urotensin neuropeptides (Zhang et al., 2018). These neuropeptides then signal to muscles specifically on the dorsal side, causing their contraction and thereby the dorsal movements of the body during its straightening (Zhang et al., 2018). Adrenaline signals may be transported from their origin in the brain down the spinal canal by cilia-driven CSF flow and/or via interactions with the Reissner fiber. Future work is needed to explain how the status of curvature is sensed; CSF-cNs, which are both chemo- and mechano-responsive cells, are attractive candidates for this role.
Fig. 6.
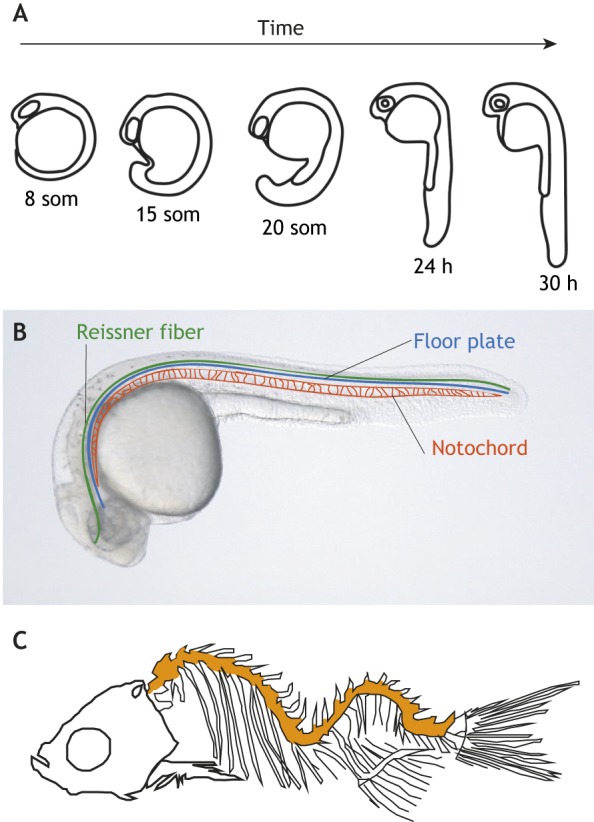
Zebrafish body symmetry depends on motile cilia-dependent mechanisms. (A) Schematic of the first 30 h of zebrafish development. Initially, during somite (som) stages, zebrafish embryos are curved around the yolk ball. As the body axis extends, the trunk moves dorsally to straighten this initial curve. (B) Image of a 30 h zebrafish embryo schematically showing the position of the Reissner fiber, the floor plate of the neural tube and the underlying notochord. (C) Schematic skeleton of a mutant zebrafish in which cilia motility is inactivated. Mutants exhibit three-dimensional spinal curves (orange) that resemble aspects of idiopathic scoliosis.
Motile cilia are also required for the maintenance of a straight spine during adolescent growth in zebrafish (Grimes et al., 2016). Making use of a temperature-sensitive mutation in the cilia motility gene cfap298 to inactivate cilia-driven CSF flow only after embryonic periods allowed researchers to demonstrate that CSF flow is essential during growth phases to prevent the formation of spinal curves in zebrafish (Grimes et al., 2016). The curves that result (Fig. 6C) exhibit hallmarks of idiopathic scoliosis, a prevalent disease characterized by three-dimensional spinal curves (see Box 1) (Cheng et al., 2015). Precisely how CSF flow promotes spinal straightness is unknown, but the sensory CSF-cNs have again been implicated (Sternberg et al., 2018). Zebrafish research is now poised to make important strides in understanding the maintenance of spinal straightness and diseases of aberrant spinal curvature such as idiopathic scoliosis (Boswell and Ciruna, 2017).
Conclusions
Given that we are roughly bilaterally symmetric animals, and so too are most of the animals we see and study, it is easy to think of symmetry as a natural state for vertebrates and their embryos. But, as the examples in this Review attest, this is not the case. Instead, symmetries must be actively generated and maintained, with asymmetries from various sources being buffered against. Considering vertebrate evolutionary history, perhaps this is not so surprising. Both limb buds and somites evolved in an asymmetric context, in embryos that already possessed embryo-scale L-R asymmetric pathways. Indeed, the somites of amphioxus, an animal that sits at the base of the chordates (Box 2), develop in a consistently asymmetric way (Brend and Holley, 2009; Conklin, 1932; Minguill; and Garcia-Fernandez, 2002; Schubert et al., 2001). Not surprisingly, amphioxus embryos contain a functional L-R asymmetric pathway (Li et al., 2017; Yu et al., 2002).
Going back further, there is the question of the origin of bilaterality itself. This is currently a complex and little-understood issue, and several plausible scenarios are debated (Genikhovich and Technau, 2017). More-detailed phylogenies combined with the functional study of non-traditional model organisms will help address these issues in the future. A related question is how an overall bilateral shape can develop from an egg given that many of the molecular and cellular structures that make up eggs and early embryos are chiral. These asymmetries must be somehow compensated for during embryogenesis. Fruitful avenues of research into these issues might come from the study of planarian flatworms. These animals are highly symmetric, although subtle cryptic asymmetries do exist (Nogi et al., 2005), and may resemble early bilaterians. Planarian research is therefore well placed to inform us about how bilateral body plans can be built from asymmetric molecular and cellular components.
Although this Review is largely focused on directional asymmetries governed by the L-R pathway, it should be noted that other sources of asymmetry also exist. The investigation of these promises to teach us much about developmental mechanisms. For example, the study of fluctuating asymmetries, small deviations between left and right, may shed light on the basis of developmental stability in the face of intrinsic noise (Debat and Peronnet, 2013). Additionally, many organisms exhibit conspicuous L-R asymmetries, such as the claws of the fiddler crab, the beaks of crossbills and wrybills, and the tusk of the narwhal. These asymmetries emerge through the interaction of complex genetics and the environment. One particularly tractable example is the facial asymmetries of blind Mexican cavefish (Powers et al., 2017; Yamamoto et al., 2003). As cavefish are less inbred than traditional models, this represents an exciting case for understanding how complex genetic variation and environmental factors can impinge upon biological traits. Last, the L-R pathway is not the only source of directional asymmetries in the embryo; these can also emerge in an organ-intrinsic fashion. For example, the dextral looping of the zebrafish heart is controlled by a heart-intrinsic cytoskeleton-dependent mechanism (Desgrange et al., 2018; Noël et al., 2013). Thus, asymmetric information is transferred from smaller to larger scales multiple times independently within the same embryo (Chin et al., 2018; Ray et al., 2018), rather than emerging in a single symmetry-breaking event.
Overall, maintaining symmetry on a canvas of molecular-, cellular- and embryo-level asymmetry requires active processes that act at the level of individual organs through to the entire organism during both embryonic patterning and growth. To understand how symmetries are achieved, we must understand the forces that promote asymmetries, whether those be intrinsic noise or directional signals, and how those are antagonized. This will require an integrated holistic view of development that considers several interacting tissues as well as systemic signals that act at a distance.
Acknowledgements
I thank members of the Grimes lab for discussion, Sarah Stednitz for artwork in Fig. 1 and Elizabeth Robertson for support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
D.T.G. is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases K99/R00 award mechanism, by the Donald E. and Delia B. Baxter Foundation and by the University of Oregon. Deposited in PMC for release after 12 months.
References
- Abe M. and Kuroda R. (2019). The development of CRISPR for a mollusc establishes the formin Lsdia1 as the long-sought gene for snail dextral/sinistral coiling. Development 146, dev175976 10.1242/dev.175976 [DOI] [PubMed] [Google Scholar]
- Adams D. S., Robinson K. R., Fukumoto T., Yuan S., Albertson R. C., Yelick P., Kuo L., McSweeney M. and Levin M. (2006). Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133, 1657-1671. 10.1242/dev.02341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K., Holtzman N. G. and Burdine R. D. (2008). Direct and indirect roles for Nodal signaling in two axis conversions during asymmetric morphogenesis of the zebrafish heart. Proc. Natl. Acad. Sci. USA 105, 13924-13929. 10.1073/pnas.0802159105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson C. T., Bachinsky D. R., Lin R. C., Levi T., Elkins J. A., Soults J., Grayzel D., Kroumpouzou E., Traill T. A., Leblanc-Straceski J. et al. (1997). Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat. Genet. 15, 30-35. 10.1038/ng0197-30 [DOI] [PubMed] [Google Scholar]
- Bénazéraf B. and Pourquié O. (2013). Formation and segmentation of the vertebrate body axis. Annu. Rev. Cell Dev. Biol. 29, 1-26. 10.1146/annurev-cellbio-101011-155703 [DOI] [PubMed] [Google Scholar]
- Beyer T., Danilchik M., Thumberger T., Vick P., Tisler M., Schneider I., Bogusch S., Andre P., Ulmer B., Walentek P. et al. (2012a). Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 22, 33-39. 10.1016/j.cub.2011.11.027 [DOI] [PubMed] [Google Scholar]
- Beyer T., Thumberger T., Schweickert A. and Blum M. (2012b). Connexin26-mediated transfer of laterality cues in Xenopus. Biol. Open 1, 473-481. 10.1242/bio.2012760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher R., Krief S., Galili T., Biton I. E., Stern T., Assaraf E., Levanon D., Appel E., Anekstein Y., Agar G. et al. (2017). The proprioceptive system masterminds spinal alignment: insight into the mechanism of scoliosis. Dev. Cell 42, 388-399.e383. 10.1016/j.devcel.2017.07.022 [DOI] [PubMed] [Google Scholar]
- Blum M., Feistel K., Thumberger T. and Schweickert A. (2014a). The evolution and conservation of left-right patterning mechanisms. Development 141, 1603-1613. 10.1242/dev.100560 [DOI] [PubMed] [Google Scholar]
- Blum M., Schweickert A., Vick P., Wright C. V. E. and Danilchik M. V. (2014b). Symmetry breakage in the vertebrate embryo: when does it happen and how does it work? Dev. Biol. 393, 109-123. 10.1016/j.ydbio.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T., Wittler L. and Kessel M. (1999). FGF8 functions in the specification of the right body side of the chick. Curr. Biol. 9, 277-280. 10.1016/S0960-9822(99)80119-5 [DOI] [PubMed] [Google Scholar]
- Bornens M. (2012). The centrosome in cells and organisms. Science 335, 422-426. 10.1126/science.1209037 [DOI] [PubMed] [Google Scholar]
- Boskovski M. T., Yuan S., Pedersen N. B., Goth C. K., Makova S., Clausen H., Brueckner M. and Khokha M. K. (2013). The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality. Nature 504, 456-459. 10.1038/nature12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell C. W. and Ciruna B. (2017). Understanding idiopathic scoliosis: a new zebrafish school of thought. Trends Genet. 33, 183-196. 10.1016/j.tig.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Brend T. and Holley S. A. (2009). Balancing segmentation and laterality during vertebrate development. Semin. Cell Dev. Biol. 20, 472-478. 10.1016/j.semcdb.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Brown N. A. and Wolpert L. (1990). The development of handedness in left/right asymmetry. Development 109, 1-9. [DOI] [PubMed] [Google Scholar]
- Bullough W. S. and Laurence E. B. (1964). Mitotic control by internal secretion: the role of the chalone-adrenalin complex. Exp. Cell Res. 33, 176-194. 10.1016/S0014-4827(64)81025-9 [DOI] [PubMed] [Google Scholar]
- Busse S. M., McMillen P. T. and Levin M. (2018). Cross-limb communication during Xenopus hindlimb regenerative response: non-local bioelectric injury signals. Development 145, dev164210 10.1242/dev.164210 [DOI] [PubMed] [Google Scholar]
- Campione M., Steinbeisser H., Schweickert A., Deissler K., van Bebber F., Lowe L. A., Nowotschin S., Viebahn C., Haffter P., Kuehn M. R. et al. (1999). The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development 126, 1225-1234. [DOI] [PubMed] [Google Scholar]
- Cantaut-Belarif Y., Sternberg J. R., Thouvenin O., Wyart C. and Bardet P. L. (2018). The reissner fiber in the cerebrospinal fluid controls morphogenesis of the body axis. Curr. Biol. 28, 2479-2486.e2474. 10.1016/j.cub.2018.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro K., Donnet C., Rejtar T., Karger B. L., Barisone G. A., Diaz E., Kortagere S., Lemire J. M. and Levin M. (2011). Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev. Biol. 11, 29 10.1186/1471-213X-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. C., Castelein R. M., Chu W. C., Danielsson A. J., Dobbs M. B., Grivas T. B., Gurnett C. A., Luk K. D., Moreau A., Newton P. O. et al. (2015). Adolescent idiopathic scoliosis. Nat. Rev. Dis. Primers 1, 15030 10.1038/nrdp.2015.30 [DOI] [PubMed] [Google Scholar]
- Chin A. S., Worley K. E., Ray P., Kaur G., Fan J. and Wan L. Q. (2018). Epithelial cell chirality revealed by three-dimensional spontaneous rotation. Proc. Natl. Acad. Sci. USA 115, 12188-12193. 10.1073/pnas.1805932115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C.-F., Vanhoven M. K., Fetter R. D., Verselis V. K. and Bargmann C. I. (2007). An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell 129, 787-799. 10.1016/j.cell.2007.02.052 [DOI] [PubMed] [Google Scholar]
- Collignon J., Varlet I. and Robertson E. J. (1996). Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 381, 155-158. 10.1038/381155a0 [DOI] [PubMed] [Google Scholar]
- Conklin E. G. (1932). The embryology of amphioxus. J. Morphol. 54, 69-151. 10.1002/jmor.1050540103 [DOI] [Google Scholar]
- Das D., Chatti V., Emonet T. and Holley S. A. (2017). Patterned disordered cell motion ensures vertebral column symmetry. Dev. Cell 42, 170-180.e175. 10.1016/j.devcel.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. M., Kurpios N. A., Sun X., Gros J., Martin J. F. and Tabin C. J. (2008). The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev. Cell 15, 134-145. 10.1016/j.devcel.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A., McDowell G. S., Holden J. M., Johnson H. F., Koutsovoulos G. D., Liu M. M., Hulpiau P., Van Roy F., Wade C. M., Banerjee R. et al. (2016). Formin is associated with left-right asymmetry in the pond snail and the frog. Curr. Biol. 26, 654-660. 10.1016/j.cub.2015.12.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debat V. and Peronnet F. (2013). Asymmetric flies: the control of developmental noise in Drosophila. Fly (Austin) 7, 70-77. 10.4161/fly.23558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M. and Sasai Y. (1996). A common plan for dorsoventral patterning in Bilateria. Nature 380, 37-40. 10.1038/380037a0 [DOI] [PubMed] [Google Scholar]
- Desgrange A., Le Garrec J. F. and Meilhac S. M. (2018). Left-right asymmetry in heart development and disease: forming the right loop. Development 145, dev162776 10.1242/dev.162776 [DOI] [PubMed] [Google Scholar]
- Driever W. (2018). Developmental biology: Reissner's fiber and straightening of the body axis. Curr. Biol. 28, R833-R835. 10.1016/j.cub.2018.05.080 [DOI] [PubMed] [Google Scholar]
- Dunwoodie S. L., Clements M., Sparrow D. B., Sa X., Conlon R. A. and Beddington R. S. (2002). Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy gene Dll3 are associated with disruption of the segmentation clock within the presomitic mesoderm. Development 129, 1795-1806. [DOI] [PubMed] [Google Scholar]
- Echeverri K. and Oates A. C. (2007). Coordination of symmetric cyclic gene expression during somitogenesis by Suppressor of Hairless involves regulation of retinoic acid catabolism. Dev. Biol. 301, 388-403. 10.1016/j.ydbio.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Elgjo K. and Reichelt K. L. (1994). Beta-receptor blockade by propranolol modifies the effect of the inhibitory, endogenous epidermal pentapeptide on epidermal cell flux at the G2-M transition but not at the G1-S transition. Epithelial Cell Biol. 3, 32-37. [PubMed] [Google Scholar]
- Essner J. J., Amack J. D., Nyholm M. K., Harris E. B. and Yost H. J. (2005). Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132, 1247-1260. 10.1242/dev.01663 [DOI] [PubMed] [Google Scholar]
- Evrard Y. A., Lun Y., Aulehla A., Gan L. and Johnson R. L. (1998). lunatic fringe is an essential mediator of somite segmentation and patterning. Nature 394, 377-381. 10.1038/28632 [DOI] [PubMed] [Google Scholar]
- Field S., Riley K.-L., Grimes D. T., Hilton H., Simon M., Powles-Glover N., Siggers P., Bogani D., Greenfield A. and Norris D. P. (2011). Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development 138, 1131-1142. 10.1242/dev.058149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Viebahn C. and Blum M. (2002). FGF8 acts as a right determinant during establishment of the left-right axis in the rabbit. Curr. Biol. 12, 1807-1816. 10.1016/S0960-9822(02)01222-8 [DOI] [PubMed] [Google Scholar]
- Fischerauer E. E., Manninger M., Seles M., Janezic G., Pichler K., Ebner B. and Weinberg A. M. (2013). BMP-6 and BMPR-1a are up-regulated in the growth plate of the fractured tibia. J. Orthop. Res. 31, 357-363. 10.1002/jor.22238 [DOI] [PubMed] [Google Scholar]
- Fukumoto T., Blakely R. and Levin M. (2005a). Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev. Neurosci. 27, 349-363. 10.1159/000088451 [DOI] [PubMed] [Google Scholar]
- Fukumoto T., Kema I. P. and Levin M. (2005b). Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 15, 794-803. 10.1016/j.cub.2005.03.044 [DOI] [PubMed] [Google Scholar]
- Genikhovich G. and Technau U. (2017). On the evolution of bilaterality. Development 144, 3392-3404. 10.1242/dev.141507 [DOI] [PubMed] [Google Scholar]
- Grande C. and Patel N. H. (2009). Nodal signalling is involved in left-right asymmetry in snails. Nature 457, 1007-1011. 10.1038/nature07603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. G., Patterson V. L., Grimes D. T. and Burdine R. D. (2017). Modeling syndromic congenital heart defects in zebrafish. Curr. Top. Dev. Biol. 124, 1-40. 10.1016/bs.ctdb.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Grimes D. T. (2019). Developmental biology: go with the flow to keep the body straight. Curr. Biol. 29, R101-R103. 10.1016/j.cub.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Grimes D. T. and Burdine R. D. (2017). Left-right patterning: breaking symmetry to asymmetric morphogenesis. Trends Genet. 33, 616-628. 10.1016/j.tig.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. T., Boswell C. W., Morante N. F. C., Henkelman R. M., Burdine R. D. and Ciruna B. (2016). Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 352, 1341-1344. 10.1126/science.aaf6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J., Feistel K., Viebahn C., Blum M. and Tabin C. J. (2009). Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science 324, 941-944. 10.1126/science.1172478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett C. A., Alaee F., Kruse L. M., Desruisseau D. M., Hecht J. T., Wise C. A., Bowcock A. M. and Dobbs M. B. (2008). Asymmetric lower-limb malformations in individuals with homeobox PITX1 gene mutation. Am. J. Hum. Genet. 83, 616-622. 10.1016/j.ajhg.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Tanaka Y., Okada Y. and Takeda S. (2006). Nodal flow and the generation of left-right asymmetry. Cell 125, 33-45. 10.1016/j.cell.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Hjeij R., Onoufriadis A., Watson C. M., Slagle C. E., Klena N. T., Dougherty G. W., Kurkowiak M., Loges N. T., Diggle C. P., Morante N. F. et al. (2014). CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am. J. Hum. Genet. 95, 257-274. 10.1016/j.ajhg.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley S. A., Geisler R. and Nusslein-Volhard C. (2000). Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev. 14, 1678-1690. [PMC free article] [PubMed] [Google Scholar]
- Holley S. A., Julich D., Rauch G. J., Geisler R. and Nusslein-Volhard C. (2002). her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development 129, 1175-1183. [DOI] [PubMed] [Google Scholar]
- Hubaud A. and Pourquié O. (2014). Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 15, 709-721. 10.1038/nrm3891 [DOI] [PubMed] [Google Scholar]
- Ikeda O., Ozaki M., Murata S., Matsuo R., Nakano Y., Watanabe M., Hisakura K., Myronovych A., Kawasaki T., Kohno K. et al. (2009). Autonomic regulation of liver regeneration after partial hepatectomy in mice. J. Surg. Res. 152, 218-223. 10.1016/j.jss.2008.02.059 [DOI] [PubMed] [Google Scholar]
- Jaffe K. M., Grimes D. T., Schottenfeld-Roames J., Werner M. E., Ku T. S., Kim S. K., Pelliccia J. L., Morante N. F., Mitchell B. J. and Burdine R. D. (2016). c21orf59/kurly controls both cilia motility and polarization. Cell Rep. 14, 1841-1849. 10.1016/j.celrep.2016.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.-J., Aerne B. L., Smithers L., Haddon C., Ish-Horowicz D. and Lewis J. (2000). Notch signalling and the synchronization of the somite segmentation clock. Nature 408, 475-479. 10.1038/35044091 [DOI] [PubMed] [Google Scholar]
- Juan T., Géminard C., Coutelis J.-B., Cerezo D., Polès S., Noselli S. and Fürthauer M. (2018). Myosin1D is an evolutionarily conserved regulator of animal left-right asymmetry. Nat. Commun. 9, 1942 10.1038/s41467-018-04284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Raya A., Raya R. M., Rodríguez-Esteban C. and Izpisua Belmonte J. C. (2005). Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature 435, 165-171. 10.1038/nature03512 [DOI] [PubMed] [Google Scholar]
- Koshiba-Takeuchi K., Takeuchi J. K., Arruda E. P., Kathiriya I. S., Mo R., Hui C.-C., Srivastava D. and Bruneau B. G. (2006). Cooperative and antagonistic interactions between Sall4 and Tbx5 pattern the mouse limb and heart. Nat. Genet. 38, 175-183. 10.1038/ng1707 [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker A. G., Olale F., Haycraft C. J., Yoder B. K., Schier A. F. and Drummond I. A. (2005). Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 132, 1907-1921. 10.1242/dev.01772 [DOI] [PubMed] [Google Scholar]
- Kumar S. and Duester G. (2014). Retinoic acid controls body axis extension by directly repressing Fgf8 transcription. Development 141, 2972-2977. 10.1242/dev.112367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda R., Fujikura K., Abe M., Hosoiri Y., Asakawa S., Shimizu M., Umeda S., Ichikawa F. and Takahashi H. (2016). Diaphanous gene mutation affects spiral cleavage and chirality in snails. Sci. Rep. 6, 34809 10.1038/srep34809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpios N. A., Ibanes M., Davis N. M., Lui W., Katz T., Martin J. F., Belmonte J. C. I. and Tabin C. J. (2008). The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc. Natl. Acad. Sci. USA 105, 8499-8506. 10.1073/pnas.0803578105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot C., Moreau A., Chamberland M., Tremblay M. L. and Drouin J. (1999). Hindlimb patterning and mandible development require the Ptx1 gene. Development 126, 1805-1810. [DOI] [PubMed] [Google Scholar]
- Lander A. D. (2011). Pattern, growth, and control. Cell 144, 955-969. 10.1016/j.cell.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. K., Nandi A., Stulberg M. J., Dray N., Sneddon M. W., Pontius W., Emonet T. and Holley S. A. (2013). Regulated tissue fluidity steers zebrafish body elongation. Development 140, 573-582. 10.1242/dev.090381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton G., Géminard C., Lapraz F., Pyrpassopoulos S., Cerezo D., Spéder P., Ostap E. M. and Noselli S. (2018). Molecular to organismal chirality is induced by the conserved myosin 1D. Science 362, 949-952. 10.1126/science.aat8642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart K. F., Lin S.-Y., Titus T. A., Postlethwait J. H. and Burdine R. D. (2011). Two additional midline barriers function with midline lefty1 expression to maintain asymmetric Nodal signaling during left-right axis specification in zebrafish. Development 138, 4405-4410. 10.1242/dev.071092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart K. F., Holtzman N. G., Williams J. R. and Burdine R. D. (2013). Integration of nodal and BMP signals in the heart requires FoxH1 to create left-right differences in cell migration rates that direct cardiac asymmetry. PLoS Genet. 9, e1003109 10.1371/journal.pgen.1003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Johnson R. L., Stern C. D., Kuehn M. and Tabin C. (1995). A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82, 803-814. 10.1016/0092-8674(95)90477-8 [DOI] [PubMed] [Google Scholar]
- Li G., Liu X., Xing C., Zhang H., Shimeld S. M. and Wang Y. (2017). Cerberus-Nodal-Lefty-Pitx signaling cascade controls left-right asymmetry in amphioxus. Proc. Natl. Acad. Sci. USA 114, 3684-3689. 10.1073/pnas.1620519114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Krantz I. D., Deng Y., Genin A., Banta A. B., Collins C. C., Qi M., Trask B. J., Kuo W. L., Cochran J. et al. (1997a). Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet. 16, 243-251. 10.1038/ng0797-243 [DOI] [PubMed] [Google Scholar]
- Li Q. Y., Newbury-Ecob R. A., Terrett J. A., Wilson D. I., Curtis A. R., Yi C. H., Gebuhr T., Bullen P. J., Robson S. C., Strachan T. et al. (1997b). Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat. Genet. 15, 21-29. 10.1038/ng0197-21 [DOI] [PubMed] [Google Scholar]
- Lourenco R., Lopes S. S. and Saúde L. (2010). Left-right function of dmrt2 genes is not conserved between zebrafish and mouse. PLoS ONE 5, e14438 10.1371/journal.pone.0014438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe L. A., Supp D. M., Sampath K., Yokoyama T., Wright C. V. E., Potter S. S., Overbeek P. and Kuehn M. R. (1996). Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature 381, 158-161. 10.1038/381158a0 [DOI] [PubMed] [Google Scholar]
- Marcil A., Dumontier E., Chamberland M., Camper S. A. and Drouin J. (2003). Pitx1 and Pitx2 are required for development of hindlimb buds. Development 130, 45-55. 10.1242/dev.00192 [DOI] [PubMed] [Google Scholar]
- Matsui T., Sasaki A., Akazawa N., Otani H. and Bessho Y. (2012). Celf1 regulation of dmrt2a is required for somite symmetry and left-right patterning during zebrafish development. Development 139, 3553-3560. 10.1242/dev.077263 [DOI] [PubMed] [Google Scholar]
- McGrath J., Somlo S., Makova S., Tian X. and Brueckner M. (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114, 61-73. 10.1016/S0092-8674(03)00511-7 [DOI] [PubMed] [Google Scholar]
- McPherron A. C., Lawler A. M. and Lee S.-J. (1997). Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83-90. 10.1038/387083a0 [DOI] [PubMed] [Google Scholar]
- Meyers E. N. and Martin G. R. (1999). Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science 285, 403-406. 10.1126/science.285.5426.403 [DOI] [PubMed] [Google Scholar]
- Minguillón C. and Garcia-Fernàndez J. (2002). The single amphioxus Mox gene: insights into the functional evolution of Mox genes, somites, and the asymmetry of amphioxus somitogenesis. Dev. Biol. 246, 455-465. 10.1006/dbio.2002.0660 [DOI] [PubMed] [Google Scholar]
- Mitchison H. M. and Valente E. M. (2017). Motile and non-motile cilia in human pathology: from function to phenotypes. J. Pathol. 241, 294-309. 10.1002/path.4843 [DOI] [PubMed] [Google Scholar]
- Moreau C., Caldarelli P., Rocancourt D., Roussel J., Denans N., Pourquié O. and Gros J. (2019). Timed collinear activation of hox genes during gastrulation controls the avian forelimb position. Curr. Biol. 29, 35-50.e34. 10.1016/j.cub.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche L. A. and Papaioannou V. E. (2003). Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development 130, 2681-2693. 10.1242/dev.00504 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Mine N., Nakaguchi E., Mochizuki A., Yamamoto M., Yashiro K., Meno C. and Hamada H. (2006). Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev. Cell 11, 495-504. 10.1016/j.devcel.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Saito D., Kawasumi A., Shinohara K., Asai Y., Takaoka K., Dong F., Takamatsu A., Belo J. A., Mochizuki A. et al. (2012). Fluid flow and interlinked feedback loops establish left-right asymmetric decay of Cerl2 mRNA. Nat. Commun. 3, 1322 10.1038/ncomms2319 [DOI] [PubMed] [Google Scholar]
- Newbury-Ecob R. A., Leanage R., Raeburn J. A. and Young I. D. (1996). Holt-Oram syndrome: a clinical genetic study. J. Med. Genet. 33, 300-307. 10.1136/jmg.33.4.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K., Vermot J., Messaddeq N., Schuhbaur B., Chambon P. and Dolle P. (2001). Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128, 1019-1031. [DOI] [PubMed] [Google Scholar]
- Noël E. S., Verhoeven M., Lagendijk A. K., Tessadori F., Smith K., Choorapoikayil S., den Hertog J. and Bakkers J. (2013). A Nodal-independent and tissue-intrinsic mechanism controls heart-looping chirality. Nat. Commun. 4, 2754 10.1038/ncomms3754 [DOI] [PubMed] [Google Scholar]
- Nogi T., Yuan Y. E., Sorocco D., Perez-Tomas R. and Levin M. (2005). Eye regeneration assay reveals an invariant functional left-right asymmetry in the early bilaterian, Dugesia japonica. Laterality 10, 193-205. 10.1080/1357650054200001440 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Shiratori H., Saijoh Y. and Hamada H. (2002). Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96-99. 10.1038/nature00849 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M. and Hirokawa N. (1998). Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829-837. 10.1016/S0092-8674(00)81705-5 [DOI] [PubMed] [Google Scholar]
- Ocaña O. H., Coskun H., Minguillón C., Murawala P., Tanaka E. M., Galcerán J., Muñoz-Chápuli R. and Nieto M. A. (2017). A right-handed signalling pathway drives heart looping in vertebrates. Nature 549, 86-90. 10.1038/nature23454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki-Hamazaki H., Katsumata T., Tsukamoto Y., Wada N. and Kimura I. (1997). Control of the limb bud outgrowth in quail-chick chimera. Dev. Dyn. 208, 85-91. [DOI] [PubMed] [Google Scholar]
- Ohuchi H., Nakagawa T., Yamamoto A., Araga A., Ohata T., Ishimaru Y., Yoshioka H., Kuwana T., Nohno T., Yamasaki M. et al. (1997). The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development 124, 2235-2244. [DOI] [PubMed] [Google Scholar]
- Oviedo N. J. and Levin M. (2007). Gap junctions provide new links in left-right patterning. Cell 129, 645-647. 10.1016/j.cell.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Pennekamp P., Karcher C., Fischer A., Schweickert A., Skryabin B., Horst J., Blum M. and Dworniczak B. (2002). The ion channel polycystin-2 is required for left-right axis determination in mice. Curr. Biol. 12, 938-943. 10.1016/S0960-9822(02)00869-2 [DOI] [PubMed] [Google Scholar]
- Piedra M. E., Icardo J. M., Albajar M., Rodriguez-Rey J. C. and Ros M. A. (1998). Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell 94, 319-324. 10.1016/S0092-8674(00)81475-0 [DOI] [PubMed] [Google Scholar]
- Plikus M. V., Mayer J. A., de la Cruz D., Baker R. E., Maini P. K., Maxson R. and Chuong C.-M. (2008). Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340-344. 10.1038/nature06457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A. K., Davis E. M., Kaplan S. A. and Gross J. B. (2017). Cranial asymmetry arises later in the life history of the blind Mexican cavefish, Astyanax mexicanus. PLoS ONE 12, e0177419 10.1371/journal.pone.0177419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D., Cheng S.-M., Wozniak L., McSweeney M., Perrone E. and Levin M. (2005). Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev. Dyn. 234, 176-189. 10.1002/dvdy.20509 [DOI] [PubMed] [Google Scholar]
- Rallis C., Bruneau B. G., Del Buono J., Seidman C. E., Seidman J. G., Nissim S., Tabin C. J. and Logan M. P. (2003). Tbx5 is required for forelimb bud formation and continued outgrowth. Development 130, 2741-2751. 10.1242/dev.00473 [DOI] [PubMed] [Google Scholar]
- Rao C. V., Wolf D. M. and Arkin A. P. (2002). Control, exploitation and tolerance of intracellular noise. Nature 420, 231-237. 10.1038/nature01258 [DOI] [PubMed] [Google Scholar]
- Ray P., Chin A. S., Worley K. E., Fan J., Kaur G., Wu M. and Wan L. Q. (2018). Intrinsic cellular chirality regulates left-right symmetry breaking during cardiac looping. Proc. Natl. Acad. Sci. USA 115, E11568-E11577. 10.1073/pnas.1808052115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Esteban C., Tsukui T., Yonei S., Magallon J., Tamura K. and Izpisua Belmonte J. C. (1999). The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature 398, 814-818. 10.1038/19769 [DOI] [PubMed] [Google Scholar]
- Roselló-Díez A., Madisen L., Bastide S., Zeng H. and Joyner A. L. (2018). Cell-nonautonomous local and systemic responses to cell arrest enable long-bone catch-up growth in developing mice. PLoS Biol. 16, e2005086 10.1371/journal.pbio.2005086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A. K., Blumberg B., Rodriguez-Esteban C., Yonei-Tamura S., Tamura K., Tsukui T., de la Peña J., Sabbagh W., Greenwald J., Choe S. et al. (1998). Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature 394, 545-551. 10.1038/29004 [DOI] [PubMed] [Google Scholar]
- Saude L., Lourenco R., Goncalves A. and Palmeirim I. (2005). terra is a left-right asymmetry gene required for left-right synchronization of the segmentation clock. Nat. Cell Biol. 7, 918-920. 10.1038/ncb1294 [DOI] [PubMed] [Google Scholar]
- Savin T., Kurpios N. A., Shyer A. E., Florescu P., Liang H., Mahadevan L. and Tabin C. J. (2011). On the growth and form of the gut. Nature 476, 57-62. 10.1038/nature10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydmohammed M., Yagi H., Calderon M., Clark M. J., Feinstein T., Sun M., Stolz D. B., Watkins S. C., Amack J. D., Lo C. W. et al. (2018). Vertebrate myosin 1d regulates left-right organizer morphogenesis and laterality. Nat. Commun. 9, 3381 10.1038/s41467-018-05866-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Holland L. Z., Stokes M. D. and Holland N. D. (2001). Three amphioxus Wnt genes (AmphiWnt3, AmphiWnt5, and AmphiWnt6) associated with the tail bud: the evolution of somitogenesis in chordates. Dev. Biol. 240, 262-273. 10.1006/dbio.2001.0460 [DOI] [PubMed] [Google Scholar]
- Schweickert A., Weber T., Beyer T., Vick P., Bogusch S., Feistel K. and Blum M. (2007). Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 17, 60-66. 10.1016/j.cub.2006.10.067 [DOI] [PubMed] [Google Scholar]
- Schweickert A., Vick P., Getwan M., Weber T., Schneider I., Eberhardt M., Beyer T., Pachur A. and Blum M. (2010). The nodal inhibitor Coco is a critical target of leftward flow in Xenopus. Curr. Biol. 20, 738-743. 10.1016/j.cub.2010.02.061 [DOI] [PubMed] [Google Scholar]
- Sekine K., Ohuchi H., Fujiwara M., Yamasaki M., Yoshizawa T., Sato T., Yagishita N., Matsui D., Koga Y., Itoh N. et al. (1999). Fgf10 is essential for limb and lung formation. Nat. Genet. 21, 138-141. 10.1038/5096 [DOI] [PubMed] [Google Scholar]
- Sempou E. and Khokha M. K. (2019). Genes and mechanisms of heterotaxy: patients drive the search. Curr. Opin. Genet. Dev. 56, 34-40. 10.1016/j.gde.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Sheeba C. J. and Logan M. P. (2017). the roles of T-box genes in vertebrate limb development. Curr. Top. Dev. Biol. 122, 355-381. 10.1016/bs.ctdb.2016.08.009 [DOI] [PubMed] [Google Scholar]
- Shiratori H., Yashiro K., Shen M. M. and Hamada H. (2006). Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development 133, 3015-3025. 10.1242/dev.02470 [DOI] [PubMed] [Google Scholar]
- Sirbu I. O. and Duester G. (2006). Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nat. Cell Biol. 8, 271-277. 10.1038/ncb1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow D. B., Chapman G., Smith A. J., Mattar M. Z., Major J. A., O'Reilly V. C., Saga Y., Zackai E. H., Dormans J. P., Alman B. A. et al. (2012). A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell 149, 295-306. 10.1016/j.cell.2012.02.054 [DOI] [PubMed] [Google Scholar]
- Spassky N. and Meunier A. (2017). The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423-436. 10.1038/nrm.2017.21 [DOI] [PubMed] [Google Scholar]
- Sternberg J. R., Prendergast A. E., Brosse L., Cantaut-Belarif Y., Thouvenin O., Orts-Del'Immagine A., Castillo L., Djenoune L., Kurisu S., McDearmid J. R. et al. (2018). Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat. Commun. 9, 3804 10.1038/s41467-018-06225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman F. A., Nishimoto S., Murphy G. R., Kucharska A., Butterfield N. C., Newbury-Ecob R. and Logan M. P. (2016). Tbx5 buffers inherent left/right asymmetry ensuring symmetric forelimb formation. PLoS Genet. 12, e1006521 10.1371/journal.pgen.1006521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M. J. and Ware S. M. (2009). Disorders of left-right asymmetry: heterotaxy and situs inversus. Am. J. Med. Genet. C Semin. Med. Genet. 151C, 307-317. 10.1002/ajmg.c.30228 [DOI] [PubMed] [Google Scholar]
- Szeto D. P., Rodriguez-Esteban C., Ryan A. K., O'Connell S. M., Liu F., Kioussi C., Gleiberman A. S., Izpisua-Belmonte J. C. and Rosenfeld M. G. (1999). Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 13, 484-494. 10.1101/gad.13.4.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Maeda R., Ando T., Okumura T., Nakazawa N., Hatori R., Nakamura M., Hozumi S., Fujiwara H. and Matsuno K. (2011). Chirality in planar cell shape contributes to left-right asymmetric epithelial morphogenesis. Science 333, 339-341. 10.1126/science.1200940 [DOI] [PubMed] [Google Scholar]
- Tee Y. H., Shemesh T., Thiagarajan V., Hariadi R. F., Anderson K. L., Page C., Volkmann N., Hanein D., Sivaramakrishnan S., Kozlov M. M. et al. (2015). Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat. Cell Biol. 17, 445-457. 10.1038/ncb3137 [DOI] [PubMed] [Google Scholar]
- Tickle C. (2015). How the embryo makes a limb: determination, polarity and identity. J. Anat. 227, 418-430. 10.1111/joa.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingler M., Kurz S., Maerker M., Ott T., Fuhl F., Schweickert A., LeBlanc-Straceski J. M., Noselli S. and Blum M. (2018). A conserved role of the unconventional myosin 1d in laterality determination. Curr. Biol. 28, 810-816.e813. 10.1016/j.cub.2018.01.075 [DOI] [PubMed] [Google Scholar]
- Turnpenny P. D., Alman B., Cornier A. S., Giampietro P. F., Offiah A., Tassy O., Pourquié O., Kusumi K. and Dunwoodie S. (2007). Abnormal vertebral segmentation and the notch signaling pathway in man. Dev. Dyn. 236, 1456-1474. 10.1002/dvdy.21182 [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N. and Levin M. (2013). A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev. Biol. 379, 1-15. 10.1016/j.ydbio.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerkamp J., Rudolph F., Cseresnyes Z., Priller F., Otten C., Renz M., Schaefer L. and Abdelilah-Seyfried S. (2013). Unilateral dampening of Bmp activity by nodal generates cardiac left-right asymmetry. Dev. Cell 24, 660-667. 10.1016/j.devcel.2013.01.026 [DOI] [PubMed] [Google Scholar]
- Vermot J. and Pourquié O. (2005). Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature 435, 215-220. 10.1038/nature03488 [DOI] [PubMed] [Google Scholar]
- Vermot J., Gallego Llamas J., Fraulob V., Niederreither K., Chambon P. and Dolle P. (2005). Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science 308, 563-566. 10.1126/science.1108363 [DOI] [PubMed] [Google Scholar]
- Vilhais-Neto G. C., Maruhashi M., Smith K. T., Vasseur-Cognet M., Peterson A. S., Workman J. L. and Pourquié O. (2010). Rere controls retinoic acid signalling and somite bilateral symmetry. Nature 463, 953-957. 10.1038/nature08763 [DOI] [PubMed] [Google Scholar]
- Vilhais-Neto G. C., Fournier M., Plassat J.-L., Sardiu M. E., Saraf A., Garnier J.-M., Maruhashi M., Florens L., Washburn M. P. and Pourquié O. (2017). The WHHERE coactivator complex is required for retinoic acid-dependent regulation of embryonic symmetry. Nat. Commun. 8, 728 10.1038/s41467-017-00593-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington C. H. (1959). Canalization of development and genetic assimilation of acquired characters. Nature 183, 1654-1655. 10.1038/1831654a0 [DOI] [PubMed] [Google Scholar]
- Walentek P., Beyer T., Thumberger T., Schweickert A. and Blum M. (2012). ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development. Cell Rep. 1, 516-527. 10.1016/j.celrep.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Wan L. Q., Chin A. S., Worley K. E. and Ray P. (2016). Cell chirality: emergence of asymmetry from cell culture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150413 10.1098/rstb.2015.0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh I. C., Thomsen M., Gludish D. W., Alfonso-Parra C., Bai Y., Martin J. F. and Kurpios N. A. (2013). Integration of left-right Pitx2 transcription and Wnt signaling drives asymmetric gut morphogenesis via Daam2. Dev. Cell 26, 629-644. 10.1016/j.devcel.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. (2010). Arms and the man: the problem of symmetric growth. PLoS Biol. 8, e1000477 10.1371/journal.pbio.1000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. S., Ambler E., Hayward R. W., Hoppes D. D. and Hydson R. P. (1957). Experimental test of parity conservation in beta decay. Phys. Rev. 105, 1413 10.1103/PhysRev.105.1413 [DOI] [Google Scholar]
- Xu X., Weinstein M., Li C., Naski M., Cohen R. I., Ornitz D. M., Leder P. and Deng C. (1998). Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125, 753-765. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Espinasa L., Stock D. W. and Jeffery W. R. (2003). Development and evolution of craniofacial patterning is mediated by eye-dependent and -independent processes in the cavefish Astyanax. Evol. Dev. 5, 435-446. 10.1046/j.1525-142X.2003.03050.x [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Meno C., Koshiba K., Sugihara M., Itoh H., Ishimaru Y., Inoue T., Ohuchi H., Semina E. V., Murray J. C. et al. (1998). Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell 94, 299-305. 10.1016/S0092-8674(00)81473-7 [DOI] [PubMed] [Google Scholar]
- Yu J. K., Holland L. Z. and Holland N. D. (2002). An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left-right axis formation. Evol. Dev. 4, 418-425. 10.1046/j.1525-142X.2002.02030.x [DOI] [PubMed] [Google Scholar]
- Yuan S. and Brueckner M. (2018). Left-right asymmetry: myosin 1D at the center. Curr. Biol. 28, R567-R569. 10.1016/j.cub.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Zhang N. and Gridley T. (1998). Defects in somite formation in lunatic fringe-deficient mice. Nature 394, 374-377. 10.1038/28625 [DOI] [PubMed] [Google Scholar]
- Zhang R., Lee C., Lawson L. Y., Svete L. J., McIntyre L. M. and Harfe B. D. (2017). SHH protein variance in the limb bud is constrained by feedback regulation and correlates with altered digit patterning. G3 (Bethesda) 7, 851-858. 10.1534/g3.116.033019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jia S., Chen Z., Chong Y. L., Xie H., Feng D., Wu X., Song D. Z., Roy S. and Zhao C. (2018). Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat. Genet. 50, 1666-1673. 10.1038/s41588-018-0260-3 [DOI] [PubMed] [Google Scholar]