Ependymoma is the second most frequent malignant central nervous system neoplasm in children and adolescents. Data on frequency, clinical presentation, and outcome of primary metastatic intracranial ependymoma are scarce. This study analyzed the frequency, molecular diagnosis, clinical presentation, and outcome of patients diagnosed with primary metastatic ependymoma and registered in the HIT series.
Keywords: Intracranial ependymoma, Children, Metastases, Therapy, Molecular subgroups
Abstract
Background.
Data on frequency, clinical presentation, and outcome of primary metastatic intracranial ependymoma in children are scarce.
Patients and Methods.
Prospective data on patients younger than 21 years with metastatic intracranial ependymoma at first diagnosis, registered from 2001 to 2014 in the HIT‐2000 trial and the HIT‐2000 Interim Registry, were analyzed.
Results.
Of 453 registered patients with intracranial ependymoma and central neuropathology review, initial staging included spinal magnetic resonance imaging in all patients and lumbar cerebrospinal fluid (CSF) analysis in 402 patients. Ten patients (2.2%) had metastatic disease, including three with microscopic CSF positivity only (M1 metastasis stage, 0.7% of patients with CSF staging). Location of the primary tumor was supratentorial in four patients (all supratentorial RELA‐fused ependymoma [ST‐EPN‐RELA]) and within the posterior fossa in five patients (posterior fossa ependymoma type A [PF‐EPN‐A], n = 4; posterior fossa ependymoma not further classifiable, n = 1), and multifocal in one patient.
All four patients with ST‐EPN‐RELA were alive in first or second complete remission (CR) 7.5–12.3 years after diagnosis. All four patients with macroscopic metastases of posterior fossa or multifocal ependymoma died. Three patients with initial M1 stage (ST‐EPN‐RELA, n = 1; PF‐EPN‐A, n = 2) received chemotherapy and local irradiation and were alive in second or third CR 3.0–9.7 years after diagnosis. Progression‐free and overall survival of the entire cohort at 5 years was 13% (±6%), and 58% (±16%), respectively.
Conclusion.
Primary metastatic disease is rare in children with intracranial ependymoma. Prognosis may depend on molecular subgroup and extent of dissemination, and relevance of CSF analysis for initial staging remains to be clarified.
Implications for Practice.
Childhood ependymoma presenting with metastasis at first diagnosis is very rare with a frequency of 2.4% in this population‐based, well‐characterized cohort. Detection of microscopic metastases in the cerebrospinal fluid was extremely rare, and impact on prognosis and respective treatment decision on irradiation field remains unclear. Initial metastatic presentation occurs in both supratentorial RELA‐fused ependymoma and posterior fossa ependymoma. Prognosis may differ according to extent of metastasis and biological subgroup, with poor prognosis in diffusely spread metastatic posterior fossa ependymoma even after combination therapy with both intensive chemotherapy and craniospinal irradiation, which may help to guide individual therapeutic decisions for future patients.
Introduction
Ependymoma is the second most frequent malignant central nervous system (CNS) neoplasm in children and adolescents after medulloblastoma [1], [2]. Although embryonal CNS tumors frequently show primary leptomeningeal metastases with impact on treatment decisions, the necessity and clinical impact of initial staging of ependymoma is not known. Most patients present with localized disease, where the extent of resection is presently the strongest clinical predictor for progression‐free survival (PFS) and overall survival (OS). PFS rates of 50%–80% at 3–5 years are reported after complete resection [3], [4], [5], [6], [7], [8].
Data are scarce on patients with metastatic ependymoma at presentation. Poor outcome rates have been reported, and no treatment standard exists [9]. Although magnetic resonance imaging (MRI) of the complete CNS is currently considered standard within the diagnostic process and cerebrospinal fluid (CSF) evaluation is widely used, the true frequency of metastatic presentation remains elusive. Furthermore, there are no data on association of molecular‐subgroup‐related metastatic presentation and clinical course.
Molecular evaluations have revealed a high heterogeneity of ependymoma with marked differences according to localization [10], [11], [12], [13], [14]. In most patients, location of the primary tumor is infratentorial, whereas posterior fossa ependymoma type A (PF‐EPN‐A) occurs in younger children and is characterized by an increased DNA methylation, and posterior fossa type B occurs mainly in adolescent and adult patients and frequently shows chromosomal imbalances [11], [13], [15]. An oncogenic fusion of RELA with C11orf95 has been described in the majority of supratentorially located ependymoma, and supratentorial RELA‐fused ependymoma (ST‐EPN‐RELA) was introduced as a separate entity in the 2016 World Health Organization (WHO) classification of CNS tumors [16], [17], [18]. Although preclinical evidence exists on subgroup specific potential drugs, data on the clinical impact of the molecular diagnosis are rare and refer to heterogeneous, retrospective cohorts, with limited information on clinical staging and treatment [19], [20], [21], [22]. Previously described markers for risk association are gain of 1q, which was associated with poor prognosis in several cohorts, whereas histopathological grading could not be confirmed as a clinical prognostic marker [23], [24], [25], [26]. Current treatment standard for localized pediatric intracranial ependymoma consists of maximal safe surgery followed by local radiotherapy, but there is no treatment standard for patients with initial metastatic disease [19].
Within this analysis, frequency, molecular diagnosis, clinical presentation, and outcome were evaluated for patients who were diagnosed with primary metastatic ependymoma and registered in the population‐based prospective HIT‐2000 trial and the HIT‐2000 Interim Registry [27], [28], [29], [30], [31]. (HIT is an abbreviation of the German word Hirntumor, i.e., brain tumor.)
Subjects, Materials, and Methods
Inclusion Criteria and Diagnostic Evaluation
All pediatric and young adult patients with ependymoma, who were diagnosed in Germany, Austria, or Switzerland between January 1, 2001, and December 31, 2014, were eligible for registration within the population‐based multicenter, prospective HIT‐2000 trial or HIT‐2000 Interim Registry. Only patients with nonmetastatic disease qualified for inclusion within the interventional treatment arms of HIT‐2000, whereas registration was possible irrespective of metastatic status. Eligibility criteria for this analysis were first diagnosis and central neuropathology review of primary intracranial ependymoma (WHO grade II or III), age <21 years at presentation, no previous radio‐ or chemotherapy treatment, registration to the HIT‐2000 trial (NCT00303810) or HIT‐2000 Interim Registry (NCT02238899), and disseminated disease confirmed by cranial and spinal MRI and/or positive CSF cytology. Recruitment period was January 1, 2001, to December 31, 2011, for the HIT‐2000 trial and January 1, 2012, to December 31, 2014, for the HIT‐2000 Interim Registry.
Microscopic disease to CSF (M1 metastasis stage) was defined as at least two single cells with histologically malignant phenotype, or at least one tumor cell cluster detected in CSF, obtained after day 14 postoperatively. Positive CSF results obtained postoperatively but before day 14, and other unclear results, were to be reevaluated later than day 14 postoperatively. Unclear results were not structurally documented. Evaluation of patients with unclear results is not within the scope of this article. Central review of neuropathology was mandatory at the time of inclusion in the trial and was performed according to the respective WHO classification by an experienced neuropathologist. Central neuroradiological review and review of cytospin slides were intended by the protocol but were not performed for all patients. For the patients with metastatic disease, central review of neuropathological diagnosis and neuroradiology was repeated at the time of this analysis, which led to reclassification as anaplastic astrocytoma in one patient. For patients with available tumor material, further characterization was performed by p65 immunohistochemistry as surrogate for RELA fusions, fluorescence in situ hybridization for 1q25, and DNA methylation using Infinium HumanMethylation450 BeadChip (Illumina, San Diego, CA). Classification of DNA methylation results was based on a reference set [13, 32].
The prospective HIT‐2000 trial and the subsequent HIT‐2000 Interim Registry were approved by the ethical committee of the University of Wuerzburg, Germany (ClinicalTrials.gov identifiers, NCT00303810 and NCT02238899). All institutions participating in the study received approval from their institutional review boards, and informed consent was obtained from all patients, parents, or legal guardians.
Data Extraction and Statistical Analysis
All data were prospectively collected within the HIT‐2000 trial or the HIT‐2000 Interim Registry. OS and PFS were estimated using the Kaplan‐Meier method. Results were updated as of January 12, 2017.
Treatment
Because of the expected rarity, there was no specific interventional treatment arm for patients with metastatic ependymoma within the HIT‐2000 trial and HIT‐2000 Interim Registry. Treatment decisions were at the discretion of the treating physician and were based on individual discussion of the case between the physician and the trial coordinating team. Recommendations were based on the respective treatment arms for metastatic medulloblastoma or localized ependymoma with residual tumor and did respect patient age and extent of disease. Primary systemic treatment with chemotherapy was intended for all patients either as HIT‐SKK (cyclophosphamide/vincristine, methotrexate/vincristine, carboplatin/etoposide) or modified HIT‐SKK (mSKK; cyclophosphamide/vincristine, carboplatin/etoposide); mSKK was introduced for incompletely resected ependymoma by the protocol amendment in 2005. (SKK is an abbreviation of the German phrase “Säuglinge und Kleinkinder,” meaning “infants and toddlers.”) For patients <4 years of age, primary intensified chemotherapy treatment with the intention of high‐dose chemotherapy was allowed. For induction treatment either etoposide/carboplatin (until 2005) or modified Head Start treatment (after the 2005 amendment) was used, followed by tandem high‐dose chemotherapy (carboplatin/etoposide, cyclophosphamide/thiotepa) in patients with response to induction treatment [29], [31], [33], [34]. For children older than 4 years at diagnosis, hyperfractionated craniospinal irradiation (CSI) was intended, with 2 × 1 Gy fractions per day and dose prescription of 40 Gy to the craniospinal axis and boost up to 68 Gy to the primary tumor region, or 72 Gy to residual tumor and 50 Gy to metastatic deposits. Dose prescription for children who were not able to receive hyperfractionated radiotherapy was 35.2 Gy to the craniospinal axis, 55 Gy to the tumor region, 59 Gy to the residual tumor, and 49.6 Gy to metastatic deposits, in daily fractions of 1.6 and 1.8 Gy, respectively. For young children (<4 years) with microscopic metastasis to CSF only and clearance of M1 disease after postoperative chemotherapy, application of local irradiation to the tumor bed and avoidance of CSI was recommended.
Depending on the treatment arm and response to initial chemotherapy, maintenance chemotherapy was foreseen after irradiation, with either vincristine/lomustine/cisplatin, mSKK, or temozolomide [31], [33].
Results
Frequency of Metastatic Presentation
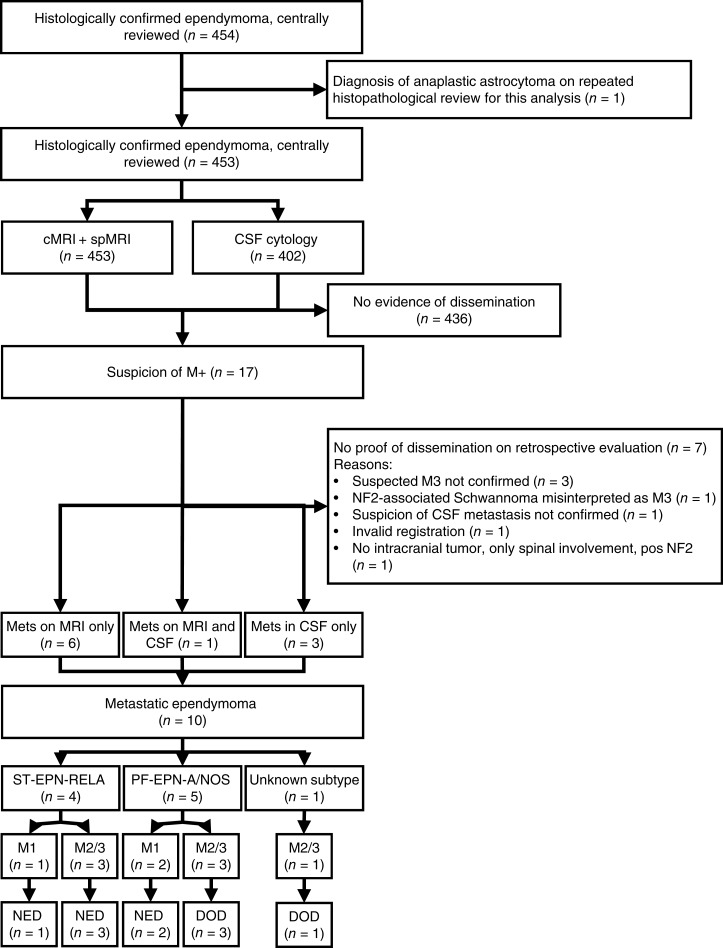
The prospective HIT‐2000 trial and the subsequent HIT‐2000 Interim Registry included 453 patients with intracranial ependymoma; 402 (88.7%) had initial CSF sampling and evaluation, 25 (5.5%) did not undergo initial CSF analysis, and in 26 patients (5.7%) no data concerning initial CSF analysis were available. All patients underwent spinal MRI at diagnosis. In total, 17 patients with newly diagnosed ependymoma were reported with suspected metastasis at the time of primary diagnosis, which could be confirmed by central review in 10 patients. This accounts for 2.2% of all patients with intracranial ependymoma (Fig. 1). Three patients had CSF positivity only (Chang stage M1), comprising 0.7% of patients with intracranial ependymoma, in whom CSF analysis was performed. In one excluded patient, spinal Schwannoma in the context of neurofibromatosis type 2 (NF‐2) were initially misinterpreted as spinal metastases of his posterior fossa ependymoma. At diagnosis, there were no clinical signs of NF‐2. Later during the course of the disease, slowly growing bilateral lesions within the internal auditory meatus raised the suspicion of an underlying NF‐2, which was confirmed genetically and led to the reclassification of the spinal lesions as NF‐2 related Schwannoma (supplemental online Table 1).
Figure 1.
Consort diagram of the patient selection process for this analysis. Supplemental online Table 1 gives details of excluded patients.
Abbreviations: CSF, cerebrospinal fluid; DOD, dead of disease; cMRI, cerebral magnetic resonance imaging; Mets, metastatic spread; MRI, magnetic resonance imaging; NED, no evidence of disease; NF2, neurofibromatosis type 2; PF‐EPN‐A/NOS, posterior fossa ependymoma subgroup A not otherwise specified; spMRI, spinal magnetic resonance imaging; ST‐EPN‐RELA, supratentorial ependymoma with RELA fusion.
Clinical Characteristics of Patients with Metastatic Disease
Centrally confirmed histopathology was anaplastic ependymoma in all 10 patients. Location of the primary tumor was infratentorial in five patients, supratentorial in four patients, and unclear in one patient with a massive tumor that was both supra‐ and infratentorial. All four supratentorially located primary tumors were molecularly classified as ST‐EPN‐RELA. Among the five infratentorially located primary tumors, four belonged to the PF‐EPN‐A subgroup according to methylation profiling, two of them with 1q gain. In one infratentorially located tumor, molecular subgrouping confirmed belonging to the posterior fossa ependymoma group, but further classification was impossible.
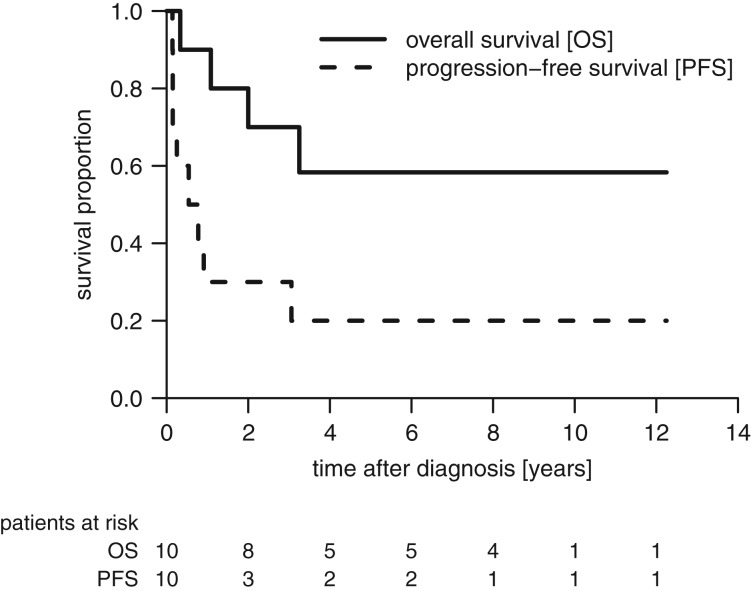
Median age at diagnosis was 3.7 years (range, 0.8–13.4 years). Eight of 10 patients had relapse or progression after a median time of 7.8 months, and four patients died. The six surviving patients had a median follow‐up of 8.6 years (range, 3.0–12.3). The estimated 5‐year PFS and OS (±standard error) for the entire cohort was 13% (±6%) and 58% (±16%), respectively (Fig. 2).
Figure 2.
Kaplan‐Meier estimates for progression‐free survival and overall survival for 10 patients with metastatic ependymoma.
Abbreviations: OS, overall survival; PFS, progression‐free survival.
Patients with Supratentorial Ependymoma and RELA Fusion
Of four patients with ST‐EPN‐RELA, one had CSF dissemination only (M1), and two had single metastatic lesions as single nodular supratentorial lesion (n = 1) or localized leptomeningeal, laminar lesion intraspinal (n = 1). One patient with ST‐EPN‐RELA had extensive, multilocular disease (Table 1). Response to initial postoperative chemotherapy was complete remission (CR) in two patients with single laminar (n = 1) or nodular metastasis (n = 1), with initial resection of the latter. Both patients were older than 4 years at diagnosis and received subsequent CSI and maintenance chemotherapy. Two patients had progression on primary chemotherapy and were considered too young for CSI. After progression, they received individual salvage treatment. One patient with isolated CSF metastasis at diagnosis had local progression with cleared CSF after initial chemotherapy. He received repeated resurgery, local irradiation, and local reirradiation. One patient with initial presentation with extensive multilocular metastases and progression on carboplatin/etoposide induction treatment received salvage chemotherapy with thalidomide and temozolomide based on an individual treatment decision with palliative intention, which led to gradual decrease of disease. The patient received multiple resurgeries and achieved CR without irradiation (Fig. 3). All four patients were alive in first or second CR at last follow‐up 7.5–12.3 years after diagnosis.
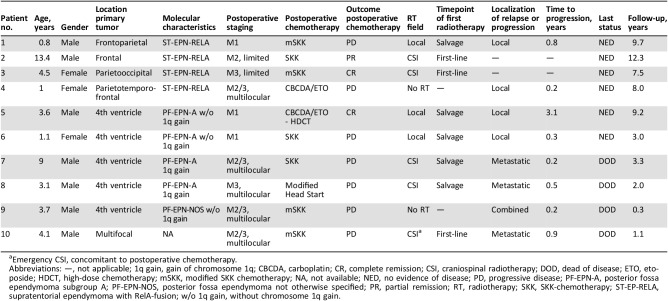
Table 1. Clinical and molecular characteristics and outcome of 10 patients with metastatic ependymoma.
Emergency CSI, concomitant to postoperative chemotherapy.
Abbreviations: —, not applicable; 1q gain, gain of chromosome 1q; CBCDA, carboplatin; CR, complete remission; CSI, craniospinal radiotherapy; DOD, dead of disease; ETO, etoposide; HDCT, high‐dose chemotherapy; mSKK, modified SKK chemotherapy; NA, not available; NED, no evidence of disease; PD, progressive disease; PF‐EPN‐A, posterior fossa ependymoma subgroup A; PF‐EPN‐NOS, posterior fossa ependymoma not otherwise specified; PR, partial remission; RT, radiotherapy; SKK, SKK‐chemotherapy; ST‐EP‐RELA, supratentorial ependymoma with RelA‐fusion; w/o 1q gain, without chromosome 1q gain.
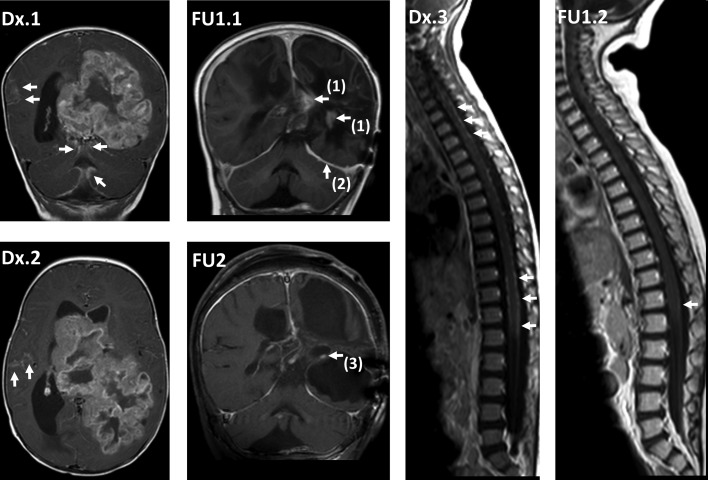
Figure 3.
Course of magnetic resonance imaging (MRI) in patient 4, reaching a complete remission without radiotherapy. At diagnosis, cerebral MRI (cMRI) coronal (cor) enhanced T1‐weighted image (T1 CE) (Dx.1) and axial T1 CE (Dx.2) at diagnosis showed large primary tumor in the left hemisphere, crossing the midline and reaching the lateral ventricles, with extensive intracranial leptomeningeal dissemination (arrows), and spinal MRI (spMRI) sagittal (sag) T1 CE (Dx.3) showed extensive spinal leptomeningeal dissemination (arrows). One year after diagnosis, after previous local progression on primary treatment, reoperation, and individual salvage chemotherapy, cMRI cor T1 CE (FU1.1) showed small residual tumor [arrows (1)] and no residual leptomeningeal seeding (partial response). Typical postinterventional subdural enhancement [arrow (2)]. Also 1 year after diagnosis, spMRI sag T1 CE (FU1.2) showed good response with minimal leptomeningeal dissemination [arrow]. Eight years after diagnosis, cMRI cor T1 CE (FU2) showed residual contrast enhancing tissue [arrow (3)]. After initial shrinking, the aspect had been without any change over the last years with regard to size or contrast enhancement and was regarded as gliotic tissue (complete remission). Based on the stability of imaging aspect, histological verification of complete remission was not pursued.
Patients with Infratentorial Ependymoma or Multifocal Presentation
Two of five patients with infratentorial primary tumor had isolated CSF metastasis (M1) only (both PF‐EPN‐A without chromosome 1q gain), and three had extensive metastatic disease (PF‐EPN‐A with chromosome 1q gain [n = 2], posterior fossa ependymoma not otherwise specified without chromosome 1q gain [n = 1]). Response to postoperative chemotherapy was early disease progression in four patients. One patient with isolated CSF dissemination was in CR after postoperative induction and high‐dose chemotherapy and had a local relapse thereafter.
Salvage treatment consisted of resurgery and local radiotherapy for both patients with initially isolated CSF metastasis and local progression or recurrence. Both were in CR at last follow‐up (3.0 and 9.2 years after diagnosis). All three patients with extensive multilocular metastases and progression on chemotherapy died of progressive disease, despite combined salvage treatment with CSI and chemotherapy for two of them.
One patient with extensive multifocal presentation received biopsy only and was treated individually with emergency CSI and concomitant chemotherapy thereafter but was refractory to treatment. Available tumor material was not sufficient for molecular subgroup analysis. See supplemental online Table 2 for detailed description of therapy.
Discussion
Although spinal MRI is clinical standard practice for initial diagnostic evaluation of children and young adults with intracranial ependymoma, and lumbar puncture for CSF evaluation is widely used, only limited data are available on the clinical utility of these staging evaluations and the frequency and clinical course of patients with primarily metastatic presentation. Here, we describe a prospectively documented cohort of pediatric patients with primary metastatic presentation of intracranial ependymoma. These patients were registered within the population‐based multicenter HIT‐2000 trial and HIT‐2000 Interim Registry. Within this series, 453 patients with intracranial ependymoma were registered within a 14‐year recruitment period. Of them, 10 patients had metastatic disease at initial staging, accounting for 2.2% of all patients with newly diagnosed, centrally confirmed ependymoma. Isolated microscopic CSF metastases were very rare (0.7%). The diagnostic standard in this series was high and encompassed mandatory central review of neuropathology, as well as recommendation for central review of MRI and CSF evaluation.
In previous series, frequency of metastatic presentation has been described as 1%–10% and higher [4], [7], [8], [35], [36], [37], [38], [39], [40], [41]. Especially for young patients with ependymoma, the reported frequencies for initial metastatic presentation vary considerably, and in pooled retrospective series, initial metastatic presentation was more frequent in younger patients [9], [42], [43], [44]. The higher rates in some of the earlier series may be based on developing diagnostic standards of neuropathological diagnosis and staging procedures. Misinterpretation of another malignant tumor entity as ependymoma, unspecific CSF findings as early postoperative exfoliated ependymoma cells, ependymal cells or atypical blood mononuclear cells, or unspecific results on MRI imaging as postoperative subdural enhancement may have led to false positive results [32], [38], [45]. Furthermore, other diseases may have mimicked metastases from ependymoma. In our series, one of the excluded patients was retrospectively diagnosed with NF‐2, and the observed intraspinal lesions were retrospectively interpreted as Schwannoma.
Outcome for patients presenting with metastatic ependymoma has been described as poor, with 5‐year event‐free survival and OS of 29% (±7%) and 43% (±8%) in a retrospective series [9]. The outcome rates in our series were comparable, but clinical course was highly heterogeneous and differed with respect to molecular characterization and extent of metastasis.
All four patients with metastatic RELA ependymoma were alive at last follow‐up without evidence of disease 7 to 12 years after diagnosis, with two of them having experienced local progression on primary chemotherapy. Two patients with RELA ependymoma showed response to chemotherapy, including one patient whose tumor and metastases responded to salvage chemotherapy and who is free of disease without having received irradiation.
Although numbers are small, these data support the clinical relevance of the molecular diagnosis. RELA‐fused ependymoma was defined in the 2016 WHO classification on CNS tumors as a separate entity, but clinical data are scarce [13], [16], [17], [18], [46]. Our data show that there are rare cases of metastatic presentation of ST‐EPN‐RELA and that long‐term survival is possible, even after widespread metastatic presentation. Targeting of the nuclear factor‐κB pathway, in which RELA has a crucial role, may be a possible therapeutic target for treatment of these patients in the future. Furthermore, specific chemotherapy treatment may be clinically evaluated based on preclinical models [20].
On the other hand, three of five patients with metastasized posterior fossa ependymoma died of their disease 0.3 to 3.3 years after diagnosis despite intensive combined radiotherapy and chemotherapy treatment.
Gain of 1q25 has repeatedly been shown as a negative prognostic marker for infratentorial ependymoma and is present in less than 10%–20% of posterior fossa ependymoma [23], [24], [25], [47]. In our series, we detected gain of 1q in two of three patients with molecularly characterized posterior fossa ependymoma and extensive metastatic presentation.
The clinical relevance of lumbar CSF evaluation for initial staging of ependymoma has been discussed before, with differing estimates on frequency of microscopic CSF dissemination. In total, only very few patients with ependymoma and microscopic CSF metastasis have been described [9], [36], [37], [44], [48]. Available reports are based on limited retrospective series and are difficult to compare because of heterogeneous sampling standards. In our prospective series, sampling was based on postoperative lumbar puncture, with eventual repuncture in case of questionable or positive result before day 14 after surgery. Central review of CSF cytospins was recommended and widely used, leading to a high diagnostic standard. All three patients (one with ST‐EPN‐RELA, two with PF‐EPN‐A) with microscopic metastasis only were initially treated with postoperative chemotherapy and received local irradiation after progression or recurrence and resurgery. Interestingly, all three patients were alive at last follow‐up, and despite omission of craniospinal irradiation, none had experienced metastatic relapse. The favorable outcome of isolated M1 metastasis together with the very low rate of 0.7% of patients with isolated positive CSF cytology in our series supports further questioning of the clinical utility of routine lumbar CSF sampling in ependymoma [36]. Because of the low numbers, verification of this result is warranted before omitting CSF sampling for routine diagnosis.
Our data point out the importance of initial staging by full spinal MRI and lumbar CSF sampling within clinical trials. Verification of diagnostic results by central review may enhance validity and comparability of results and should at least be performed before proceeding to more toxic therapy, including craniospinal irradiation, based on a positive diagnostic imaging or CSF result. However, the low incidence of metastasis limits the sample size of our population‐based prospective series, and the heterogeneity of the described patients precludes uniform treatment recommendations. Based on the described heterogeneity of clinical presentation and response to therapy, inclusion of metastatic patients in future subgroup‐specific innovative treatment trials seems more reasonable as the intent to define and evaluate any uniform treatment strategy for metastasized ependymoma without subgroup discrimination. However, based on our data, it can be concluded that conventional chemotherapy was not effective for postoperative disease control in the majority of patients.
In the meantime, until more innovative treatments are available, treatment decisions may be made on an individual basis considering staging results, molecular characteristics of the tumor, and the limitations to radiotherapy given by the age of the patient.
Conclusion
Besides the rarity of metastatic presentation in intracranial ependymoma of childhood and adolescence, our series clearly shows the heterogeneity of this group and the clinical relevance of molecular diagnosis and thorough diagnostic workup. Development of specific, innovative treatment options is needed and will be based on the biological subgroup.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank Susanne Becker, Christine Lindow, Antje Stiegmann, and Wiebke Treulieb for excellent data management and Katja Petrasch and Renate Schmid for their meticulous preview of all centrally reviewed cerebrospinal fluid cytospin samples. We thank Ahrend Koch, Kathy Keyvani, Wolfgang Brück, Christian Mawrin, Christian Hartmann, and Andreas Ebersdobler for the submission of tumor samples for molecular characterization. K.v.H., M.M., B.O.J., S.R., M.W.M., B.B., T.P., S.T., G.F., C.K., and R.D.K. receive support from the German Childhood Cancer Foundation ‐ Deutsche Kinderkrebsstiftung; K.v.H., M.M., B.O.J., and S.R. receive additional support from the Damp Foundation (Damp‐Stiftung); M.B. receives support from the Styrian Childhood Cancer Foundation ‐ Steirische Kinderkrebshilfe. K.P. is currently affiliated with the Department of Radiation Oncology, Klinikum Chemnitz, Germany. This study was presented in part at the 17th International Symposium on Pediatric Neuro‐Oncology, Liverpool, U.K.
Contributed equally.
Contributor Information
Martin Benesch, Email: martin.benesch@klinikum-graz.at.
Katja von Hoff, Email: katja.von-hoff@charite.de.
Author Contributions
Conception/design: Martin Benesch, Martin Mynarek, Stefan Rutkowski, Katja von Hoff
Provision of study material or patients: Martin Mynarek, Klaus Pietschmann, Björn‐Ole Juhnke, Irene Schmid, Christof M. Kramm, Peter Vorwerk, Andreas Beilken, Carl Friedrich Classen, Pablo Hernáiz Driever, Gabriele Kropshofer, Thomas Imschweiler, Andreas Lemmer, Rolf‐Dieter Kortmann, Stefan Rutkowski, Katja von Hoff
Collection and/or assembly of data: Martin Benesch, Martin Mynarek, Hendrik Witt, Monika Warmuth‐Metz, Torsten Pietsch, Brigitte Bison, Stefan M. Pfister, Kristian W. Pajtler, Marcel Kool, Ulrich Schüller, Klaus Pietschmann, Björn‐Ole Juhnke, Stephan Tippelt, Gudrun Fleischhack, Rolf‐Dieter Kortmann, Stefan Rutkowski, Katja von Hoff
Data analysis and Interpretation: Martin Benesch, Martin Mynarek, Stefan Rutkowski, Katja von Hoff
Manuscript writing: Martin Benesch, Martin Mynarek, Stefan Rutkowski, Katja von Hoff
Final approval of manuscript: Martin Benesch, Martin Mynarek, Hendrik Witt, Monika Warmuth‐Metz, Torsten Pietsch, Brigitte Bison, Stefan M. Pfister, Kristian W. Pajtler, Marcel Kool, Ulrich Schüller, Klaus Pietschmann, Björn‐Ole Juhnke, Stephan Tippelt, Gudrun Fleischhack, Irene Schmid, Christof M. Kramm, Peter Vorwerk, Andreas Beilken, Carl Friedrich Classen, Pablo Hernáiz Driever, Gabriele Kropshofer, Thomas Imschweiler, Andreas Lemmer, Rolf‐Dieter Kortmann, Stefan Rutkowski, Katja von Hoff
Disclosures
The authors indicated no financial relationships.
References
- 1.Peris‐Bonet R, Martinez‐Garcia C, Lacour B et al. Childhood central nervous system tumours‐incidence and survival in Europe (1978‐1997): Report from Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2064–2080. [DOI] [PubMed] [Google Scholar]
- 2.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: A surveillance, epidemiology, and end results study. J Neurosurg 2009;110:725–729. [DOI] [PubMed] [Google Scholar]
- 3.Rousseau P, Habrand JL, Sarrazin D et al. Treatment of intracranial ependymomas of children: Review of a 15‐year experience. Int J Radiat Oncol Biol Phys 1994;28:381–386. [DOI] [PubMed] [Google Scholar]
- 4.Timmermann B, Kortmann RD, Kühl J et al. Combined postoperative irradiation and chemotherapy for anaplastic ependymomas in childhood: Results of the German prospective trials HIT 88/89 and HIT 91. Int J Radiat Oncol Biol Phys 2000;46:287–295. [DOI] [PubMed] [Google Scholar]
- 5.Merchant TE, Li C, Xiong X et al. Conformal radiotherapy after surgery for paediatric ependymoma: A prospective study. Lancet Oncol 2009;10:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conter C, Carrie C, Bernier V et al. Intracranial ependymomas in children: Society of Pediatric Oncology experience with postoperative hyperfractionated local radiotherapy. Int J Radiat Oncol Biol Phys 2009;74:1536–1542. [DOI] [PubMed] [Google Scholar]
- 7.Massimino M, Gandola L, Giangaspero F et al. Hyperfractionated radiotherapy and chemotherapy for childhood ependymoma: Final results of the first prospective AIEOP (Associazione Italiana di Ematologia‐Oncologia Pediatrica) study. Int J Radiat Oncol Biol Phys 2004;58:1336–1345. [DOI] [PubMed] [Google Scholar]
- 8.Massimino M, Miceli R, Giangaspero F et al. Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro Oncol 2016;18:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacharoulis S, Ji L, Pollack IF et al. Metastatic ependymoma: A multi‐institutional retrospective analysis of prognostic factors. Pediatr Blood Cancer 2008;50:231–235. [DOI] [PubMed] [Google Scholar]
- 10.Taylor MD, Poppleton H, Fuller C et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 2005;8:323–335. [DOI] [PubMed] [Google Scholar]
- 11.Mack SC, Witt H, Piro RM, et al. Epigenomic alterations define lethal CIMP‐positive ependymomas of infancy. Nature 2014;506:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer S, Prebble E, Davison V et al. Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am J Pathol 2002;161:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pajtler KW, Witt H, Sill M et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 2015;27:728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korshunov A, Witt H, Hielscher T et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol 2010;28:3182–3190. [DOI] [PubMed] [Google Scholar]
- 15.Witt H, Mack SC, Ryzhova M et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 2011;20:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker M, Mohankumar KM, Punchihewa C et al. C11orf95‐RELA fusions drive oncogenic NF‐κB signalling in ependymoma. Nature 2014;506:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietsch T, Wohlers I, Goschzik T et al. Supratentorial ependymomas of childhood carry C11orf95‐RELA fusions leading to pathological activation of the NF‐κB signaling pathway. Acta Neuropathol 2014;127:609–611. [DOI] [PubMed] [Google Scholar]
- 18.Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol 2016;131:803–820. [DOI] [PubMed] [Google Scholar]
- 19.Ramaswamy V Hielscher T Mack SC et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: A retrospective multicohort analysis. J Clin Oncol 2016;34:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzaridis T, Milde T, Pajtler KW et al. Low‐dose actinomycin‐D treatment re‐establishes the tumoursuppressive function of P53 in RELA‐positive ependymoma. Oncotarget 2016;7:61860–61873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack SC, Pajtler KW, Chavez L et al. Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature 2018;553:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witt DA, Donson AM, Amani V et al. Specific expression of PD‐L1 in RELA‐fusion supratentorial ependymoma: Implications for PD‐1‐targeted therapy. Pediatr Blood Cancer 2018;65:e26960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendrzyk F, Korshunov A, Benner A et al. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res 2006;12:2070–2079. [DOI] [PubMed] [Google Scholar]
- 24.Kilday JP, Mitra B, Domerg C, et al. Copy number gain of 1q25 predicts poor progression‐free survival for pediatric intracranial ependymomas and enables patient risk stratification: A prospective European clinical trial cohort analysis on behalf of the Children's Cancer Leukaemia Group (CCLG), Societe Francaise d'Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP). Clin Cancer Res 2012;18:2001–2011. [DOI] [PubMed] [Google Scholar]
- 25.Godfraind C, Kaczmarska JM, Kocak M et al. Distinct disease‐risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol 2012;124:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison DW, Kocak M, Figarella‐Branger D et al. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerber NU, von Hoff K, Resch A et al. Treatment of children with central nervous system primitive neuroectodermal tumors/pinealoblastomas in the prospective multicentric trial HIT 2000 using hyperfractionated radiation therapy followed by maintenance chemotherapy. Int J Radiat Oncol Biol Phys 2014;89:863–871. [DOI] [PubMed] [Google Scholar]
- 28.Friedrich C, von Bueren AO, von Hoff K et al. Treatment of young children with CNS‐primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy. Neuro Oncol 2013;15:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Bueren AO, von Hoff K, Pietsch T et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: Results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol 2011;13:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lannering B, Rutkowski S, Doz F et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard‐risk medulloblastoma: Results from the randomized multicenter HIT‐SIOP PNET 4 trial. J Clin Oncol 2012;30:3187–3193. [DOI] [PubMed] [Google Scholar]
- 31.von Hoff K, Kortmann RD, Gerber NU et al. Risk‐adapted treatment for non‐metastatic ependymoma: Preliminary results of the non‐randomized prospective phase II clinical trial HIT 2000. Neuro Oncol 2014;16(suppl 1):EM‐028A. [Google Scholar]
- 32.Capper D, Jones DTW, Sill M et al. DNA methylation‐based classification of central nervous system tumours. Nature 2018;555:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Bueren AO, Kortmann RD, von Hoff K et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol 2016;34:4151–4160. [DOI] [PubMed] [Google Scholar]
- 34.Mynarek M, von Hoff K, Mueller K et al. Intensification of induction chemotherapy improves outcomes in patients with metastatic medulloblastoma below 4 years of age: Results of a prospective, non‐randomized phase II clinical trial. Neuro Oncol 2014;16(suppl 1):MB‐071A. [Google Scholar]
- 35.Massimino M, Gandola L, Barra S et al. Infant ependymoma in a 10‐year AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) experience with omitted or deferred radiotherapy. Int J Radiat Oncol Biol Phys 2011;80:807–814. [DOI] [PubMed] [Google Scholar]
- 36.Poltinnikov IM, Merchant TE. CSF cytology has limited value in the evaluation of patients with ependymoma who have MRI evidence of metastasis. Pediatr Blood Cancer 2006;47:169–173. [DOI] [PubMed] [Google Scholar]
- 37.Fangusaro J, Van Den Berghe C, Tomita T et al. Evaluating the incidence and utility of microscopic metastatic dissemination as diagnosed by lumbar cerebro‐spinal fluid (CSF) samples in children with newly diagnosed intracranial ependymoma. J Neurooncol 2011;103:693–698. [DOI] [PubMed] [Google Scholar]
- 38.Bouffet E, Capra M, Bartels U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst 2009;25:1293–1301. [DOI] [PubMed] [Google Scholar]
- 39.Pollack IF, Gerszten PC, Martinez AJ et al. Intracranial ependymomas of childhood: Long‐term outcome and prognostic factors. Neurosurgery 1995;37:655–666; discussion 666–657. [DOI] [PubMed] [Google Scholar]
- 40.Duffner PK, Krischer JP, Sanford RA et al. Prognostic factors in infants and very young children with intracranial ependymomas. Pediatr Neurosurg 1998;28:215–222. [DOI] [PubMed] [Google Scholar]
- 41.Timmermann B, Kortmann RD, Kuhl J et al. Role of radiotherapy in anaplastic ependymoma in children under age of 3 years: Results of the prospective German brain tumor trials HIT‐SKK 87 and 92. Radiother Oncol 2005;77:278–285. [DOI] [PubMed] [Google Scholar]
- 42.Geyer JR, Sposto R, Jennings M et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: A report from the Children's Cancer Group. J Clin Oncol 2005;23:7621–7631. [DOI] [PubMed] [Google Scholar]
- 43.Grill J, Le Deley MC, Gambarelli D et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: A multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol 2001;19:1288–1296. [DOI] [PubMed] [Google Scholar]
- 44.Moreno L, Pollack IF, Duffner PK et al. Utility of cerebrospinal fluid cytology in newly diagnosed childhood ependymoma. J Pediatr Hematol Oncol 2010;32:515–518. [DOI] [PubMed] [Google Scholar]
- 45.Warmuth‐Metz M, Kuhl J, Krauss J et al. Subdural enhancement on postoperative spinal MRI after resection of posterior cranial fossa tumours. Neuroradiology 2004;46:219–223. [DOI] [PubMed] [Google Scholar]
- 46.Pajtler KW, Mack SC, Ramaswamy V et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol 2017;133:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pajtler KW, Wen J, Sill M et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol 2018;136:211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian X, Goumnerova LC, De Girolami U et al. Cerebrospinal fluid cytology in patients with ependymoma: A bi‐institutional retrospective study. Cancer 2008;114:307–314. [DOI] [PubMed] [Google Scholar]