This article presents the results of a systematic review and network meta‐analysis aiming to evaluate the comparative effectiveness evidence supporting the use of regorafenib and TAS‐102 in patients with refractory metastatic colorectal cancer.
Keywords: TAS‐102, Regorafenib, Refractory metastatic colorectal cancer
Abstract
Background.
Regorafenib at different dosing strategies and TAS‐102 are treatment options for refractory metastatic colorectal cancer (mCRC). We aimed to evaluate the comparative effectiveness evidence supporting these different strategies.
Materials and Methods.
We searched different databases for randomized controlled trials evaluating TAS‐102 or regorafenib in patients with refractory mCRC who failed prior oxaliplatin, irinotecan, and fluoropyrimidine. Outcomes of interest included overall survival (OS) and progression‐free survival (PFS). The overall effect was pooled using the DerSimonian random effects model. We conducted network meta‐analysis based on White's multivariate meta‐regression to pool evidence from direct and indirect comparisons.
Results.
Six trials at low risk of bias (2,445 patients) were included. Direct comparisons showed that Rego 160 and TAS‐102 as monotherapy were superior to best‐supportive care (BSC) in terms of PFS (Rego 160: hazard ratio [HR], 0.4; 95% confidence ratio [CI], 0.26–0.63; TAS‐102: HR, 0.46 CI, 0.40–0.52) and OS (Rego 160: HR, 0.67; CI, 0.48–0.93; TAS‐102: HR, 0.67; CI, 0.57–0.80). Network analysis showed no statistically difference in PFS or OS between Rego 160 and TAS‐102. Rego 80+ was superior to BSC in terms of OS (HR, 0.44; CI, 0.23–0.84) and PFS (HR, 0.37; CI, 0.21–0.66). Rego 80+ was associated with statistically nonsignificant improvement in OS and PFS compared with TAS‐102 and Rego 160.
Conclusion.
Regorafenib 160 and TAS‐102 appear to have similar efficacy. Rego 80+ is shown to be superior to BSC. A trend for improved OS was observed with Rego 80+ versus Rego 160 or TAS 102.
Implications for Practice.
Regorafenib at a dose of 160 mg and TAS‐102 appear to have similar efficacy in patients with refractory metastatic colorectal cancer. Regorafenib with a dose escalation strategy is superior to best‐supportive care. Given its tolerability and the observed trend in survival benefit compared with regorafenib 160, dose escalation strategy of regorafenib (80+) may be the preferred option in this setting.
摘要
背景。不同剂量方案的瑞戈非尼和 TAS‐102 是治疗难治性转移性结直肠癌 (mCRC) 的常见治疗方案。我们旨在对支持这些不同治疗方案的有效性比较证据进行评估。
材料和方法。我们在不同数据库中搜索了评估难治性mCRC患者改用 TAS‐102 或瑞戈非尼是否有效的随机对照试验,这些患者既往用奥沙利铂、伊立替康及氟尿嘧啶治疗失败。所关注的预后包括总生存期 (OS) 和无进展生存期 (PFS)。同时,采用 DerSimonian 随机效应模型对总体效应进行了汇总。我们基于 White 多元荟萃回归模型开展了网络荟萃分析,以便从直接和间接对比中收集证据。
结果。包括六项偏倚风险较低的试验(2 445 名患者)。通过直接对比表明,在PFS方面,瑞戈非尼(剂量为 160 mg,Rego 160)和 TAS‐102 要优于最佳支持疗法 (BSC) [Rego 160:风险比 (HR),0.4;95% 置信比 (CI),0.26–0.63;TAS‐102:HR,0.46;CI,0.40–0.52]及 OS(Rego 160:HR,0.67;CI,0.48–0.93;TAS‐102:HR,0.67;CI,0.57–0.80)。网络分析显示,Rego 160与 TAS‐102 之间的PFS或OS无明显统计学差异。在OS(HR,0.44;CI,0.23–0.84)和PFS(HR,0.37;CI,0.21–0.66)方面,Rego 80+要优于BSC。与 TAS‐102 和Rego 160相比,Rego 80+在OS和PFS方面未带来无明显统计学改善。
结论。瑞戈非尼(剂量为 160 mg)与 TAS‐102 的疗效似乎相似。Rego 80+要优于BSC。对比Rego 80+与Rego 160或 TAS 102 时发现,OS有改善的趋势。
实践意义:对于难治性转移性结直肠癌患者,瑞戈非尼的剂量为 160 mg时,与 TAS‐102 的疗效相似。瑞戈非尼的剂量递增治疗方案优于最佳支持疗法。鉴于瑞戈非尼的耐受性,以及对比瑞戈非尼(剂量为 160 mg)时所观察到的生存期改善趋势,瑞戈非尼(剂量为 80 mg以上)剂量递增方案可能是此种情况下的首选。
Introduction
The mainstay of treatment for advanced metastatic colorectal cancer (mCRC) includes a combination of chemotherapy and targeted agents aiming to prolong survival while preserving and improving quality of life [1]. First‐ and second‐line treatments usually include a combination of oxaliplatin or irinotecan with a fluoropyrimidine (5‐fluorouracil or capecitabine). In addition, targeted agents are usually added and include monoclonal antibodies that are directed against epidermal growth factor receptor (EGFR) in RAS wild‐type patients, or vascular endothelial growth factor (VEGF) [2]. These combinations have led to a significant improvement in overall survival in mCRC that is now reaching up to a median of 30 months [3], [4]. Beyond the second line, also known as refractory mCRC, treatment options include 2 agents, namely regorafenib and TAS‐102 [5], [6]. Regorafenib is a multikinase inhibitor that blocks the activity of vascular endothelial grown factor receptor (VEGFR) in addition to other kinases involved in tumor angiogenesis [7], whereas TAS‐102 is an oral nucleoside cytotoxic agent that comprised trifluridine and tipiracil hydrochloride at a molar ratio of 1:0.5 [8].
Herein, we present the results of a systematic review and network meta‐analysis that aim to evaluate the comparative effectiveness evidence supporting the use of regorafenib and TAS‐102 in patients with refractory mCRC.
Materials and Methods
The reporting of this systematic review follows the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) statement [9].
Data Sources and Search Methods
A comprehensive literature search was performed for full‐text articles published in print or online from database inception up to August 2018 from electronic databases, MEDLINE, EMBASE, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL). Detailed search strategy is as described in supplemental online Appendix 1. The terms “metastatic colorectal cancer,” “TAS‐102,” “trifluridine/tipiracil,” and “regorafenib” were used to identify the trials of interest. Two individual reviewers (M.B.S. and R.B.) identified and reviewed full‐text articles that were deemed relevant by screening the list of titles. Disagreements between the two reviewers were resolved with consensus.
Study Eligibility and Outcome Measures
We searched for trials that investigated the effect of regorafenib and TAS‐102 in refractory mCRC compared with best‐supportive care (BSC). Only randomized controlled trials (RCTs) were included. Uncontrolled trials were excluded. Outcomes of interest were overall survival (OS), progression‐free survival (PFS), adverse events, and quality of life.
Data Extraction
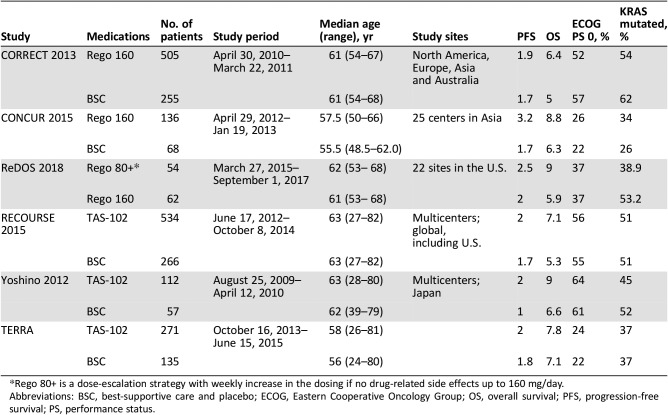
Prespecified data elements were extracted from each trial using a structured data abstraction form, including baseline characteristics, sample size, and interventions used (Table 1). Two reviewers extracted the data from the included trials (M.B.S. and R.B.), and disagreements were resolved by referring to a third reviewer (B.F.). The number of events in each trial was extracted, when available, based on the intention‐to‐treat approach.
Table 1. Baseline characteristics of the included trials.
Rego 80+ is a dose‐escalation strategy with weekly increase in the dosing if no drug‐related side effects up to 160 mg/day.
Abbreviations: BSC, best‐supportive care and placebo; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; PFS, progression‐free survival; PS, performance status.
Risk of Bias Assessment and Quality of Evidence
The methodologic quality was assessed using the Cochrane risk‐of‐bias tool [10]. Two reviewers independently assessed trial quality by examining several components: generation of allocation, concealment of allocation, blinding of participants, caregivers, data collectors, and outcome assessors, incomplete outcome data, and any other potential source of bias. The quality of evidence (i.e., certainty in the estimates) was evaluated using the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) [11].
Statistical Analysis
We extracted hazard risk (HR) from the included trials. Logarithm‐transformed HR was pooled using the DerSimonian and Laird random effects model. We conducted network meta‐analysis based on White's multivariate meta‐regression to pool evidence from direct and indirect comparisons. We used the I2 statistic to assess for heterogeneity across the included trials. Two sided p value <.05 was deemed statistically significant.
Results
Included Studies
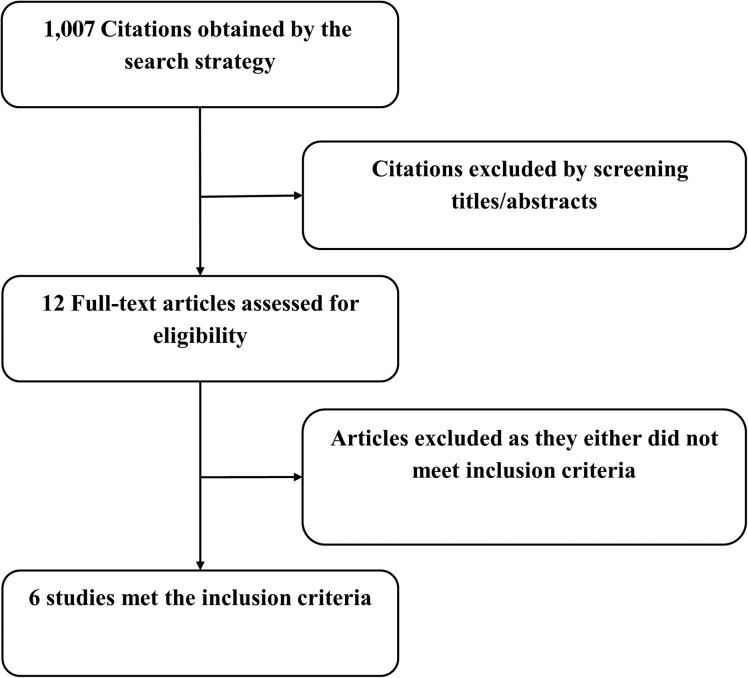
A total of 1,007 titles and abstracts were identified by the screening electronic search strategy, of which 12 full‐text articles met the eligibility for assessment (Fig. 1). Six trials met the inclusion criteria [7], [12], [13], [14], [15], [16]. (Fig. 1; Table 1).
Figure 1.
Flow chart showing the search and screening process.
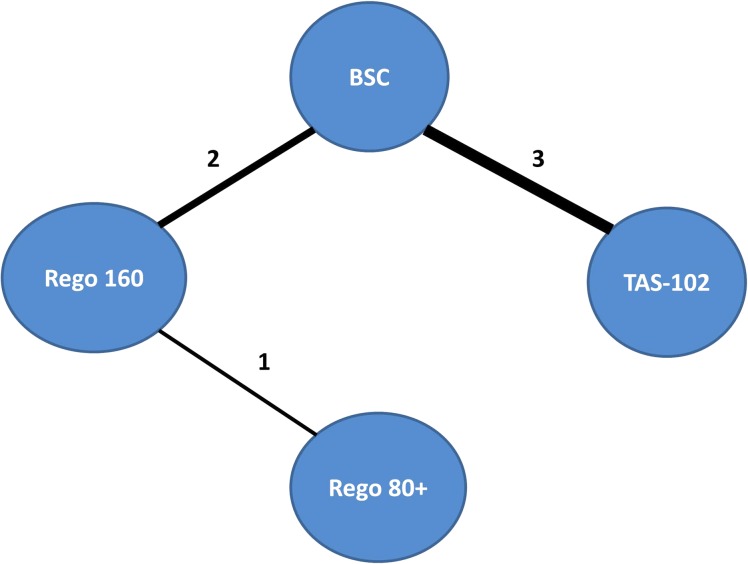
These six included trials encompassing a total of 2,445 patients. Three trials evaluated TAS‐102 versus BSC [14], [15], [16]. Two trials compared regorafenib 160 mg with BSC, and one trial compared two different dosing strategies of regorafenib (ReDOS trial): Rego 160 (regorafenib at 160 mg once daily for the first 3 weeks of each 4 week cycle) versus a dose escalation strategy, Rego 80+ (regorafenib at 80 mg per day, weekly dose escalation if no significant drug‐related toxicities, up to 160 mg per day) [7], [12], [13]. Median age in these trials ranged from 24–81 years. All the trials included patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1 except Yoshino 2012, which also included patients with ECOG PS of 2 (Table 1; Fig. 2).
Figure 2.
Evidence network of the different studied interventions that are included in the network meta‐analysis. The numbers refer to the number of trials, and the thickness of the connecting line corresponds to the number of trials between comparators.
Abbreviation: BCC, best‐supportive care.
TAS‐102 Versus BSC
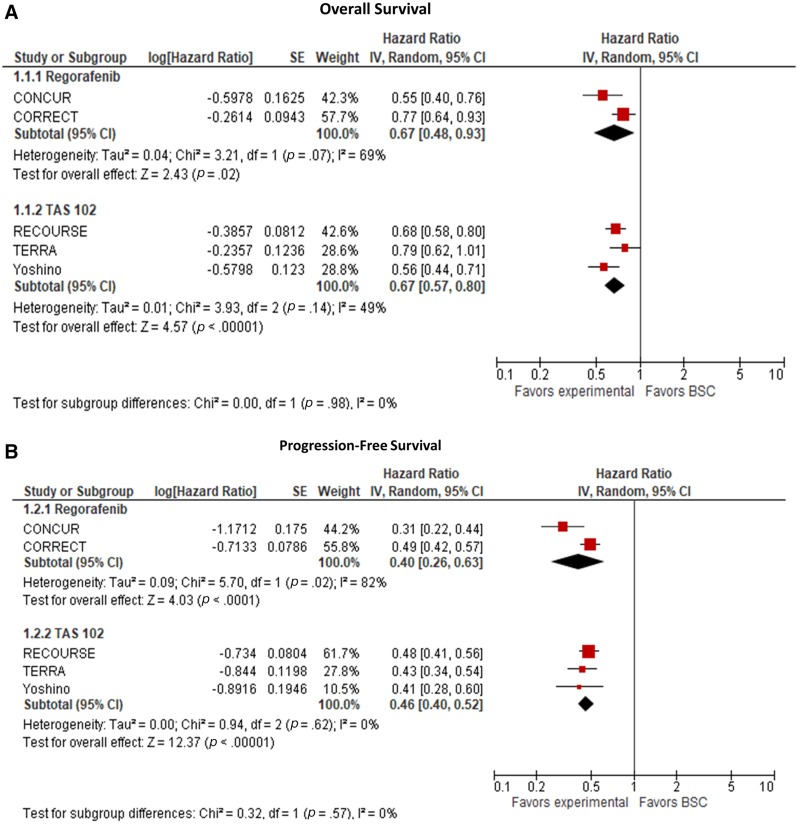
Three RCTs evaluated the efficacy of TAS‐102 versus BSC in patients with refractory advanced mCRC (Table 1). The first trial was a multicenter phase II trial in Japan [16]. The OS benefit seen in this trial was confirmed in two other phase III trials: TERRA, which was conducted in China, Korea, and Thailand, and RECOURSE, a global phase III study that led to the U.S. Food and Drug Administration approval of TAS‐102 in the U.S. [14], [15]. Pooling the results of the three trials that evaluated the efficacy of TAS‐102 versus BSC demonstrated an improvement in both PFS (HR, 0.46; 95% confidence interval [CI], 0.40–0.52) and OS (HR, 0.67; 95% CI, 0.57–0.80; Fig. 3).
Figure 3.
Meta‐analyses of the included trials. (A): Overall survival analysis. (B): Progression‐free survival analysis.
Abbreviation CI, confidence interval.
Rego 160 Versus BSC
Two RCTs evaluated the efficacy of Rego 160 versus BSC in patients with refractory advanced mCRC; one was conducted globally (CORRECT), and the other was in Asia (CONCUR) [7], [13]. Both trials showed that regorafenib 160 mg improved PFS and OS over placebo in patients with refractory mCRC (Table 1). A meta‐analysis of these two trials showed that compared with BSC, Rego 160 demonstrated an improvement in both PFS (HR, 0.40; 95% CI, 0.26–0.63) and OS (HR, 0.67; 95% CI, 0.48–0.93; Fig. 3).
Network Meta‐Analysis
Six trials were included in the network meta‐analysis (Table 1; Fig. 2). Both Rego 160 and TAS‐102 appear to have similar efficacy in refractory mCRC in terms of PFS (HR, 0.93; 95% CI, 0.66–1.32) and OS (HR, 1.00; 95% CI, 0.72–1.41; Table 2). The dose escalation strategy of regorafenib (Rego 80+) was found to be superior to BSC in terms of OS (HR, 0.44; 95% CI, 0.23–0.84) and PFS (HR, 0.37; 95% CI, 0.21–0.66). In contrast, neither TAS‐102 nor Rego 160 was found to have superior OS or PFS compared with Rego 80+.
Table 2. Network meta‐analysis of the included studies.
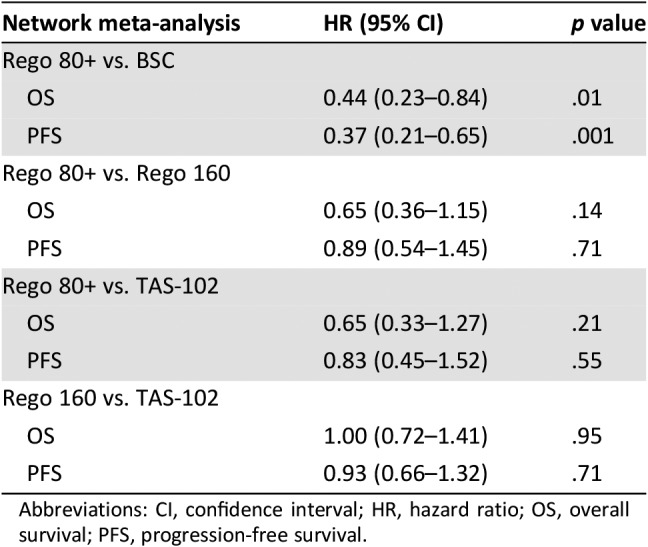
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival.
Adverse Events and Quality of Life
TAS‐102 and regorafenib are known to have different toxicity profiles. Therefore, only descriptive data are reported here, with no statistical analysis comparing the two. Pertinent adverse events were collected and summarized in supplemental online Table 1. TAS‐102 is associated with more cytopenias compared with regorafenib, which is more associated with hand‐foot skin reaction (HFSR) and hypertension (supplemental online Table 1). Quality of life data were reported in the trials evaluating the effectiveness of regorafenib 160 versus BSC (CONCUR and CORRECT) [7], [13]. These trials showed a similar deterioration in quality of life in both the regorafenib and placebo groups. In contrast, unlike the regorafenib 160, the dose escalation strategy did not appear to compromise quality of life at the second week of therapy initiation in the ReDOS trial [12]. None of the TAS‐102 trials reported quality of life results. However, it was noted in the RECOURSE trial that the median time to the change of ECOG PS to 2 or worse in TAS‐102 group was longer (5.7 months) than the placebo group (4 months) [14].
Risk of Bias
A qualitative assessment was performed by assessing various indicators for each individual study using the aforementioned tools for risk of bias and quality assessment. Overall, the trials were deemed to be at low risk for bias (supplemental online Figs. 1 and 2).
Quality of Evidence
The quality of the evidence for the comparisons of both regorafenib versus BSC and TAS‐102 versus BSC was high (for the outcome of OS). However, because of imprecision, the quality was low for the indirect comparison (regorafenib vs. TAS‐102; supplemental online Table 2).
Discussion
In this systematic review and meta‐analysis of patients with refractory mCRC, we found that regorafenib 160 and TAS‐102 have comparable efficacy and are superior to placebo. Similarly, the data demonstrate that the dose escalation strategy of regorafenib 80+ is superior to placebo.
In our analysis, neither TAS‐102 nor Rego 160 was found to have superior OS or PFS compared with dose escalation strategy of regorafenib (Rego 80+). In contrast, a trend toward the hazard ratio for OS favored Rego 80+ over TAS‐102 and Rego 160.
In addition to extending survival, one of the main goals of palliative therapy is to preserve or improve quality of life compared with placebo. This could be best achieved when the treatment controls the disease without side effects that significantly deteriorate quality of life. TAS‐102 and regorafenib have different side effect profiles with more hematological toxicities associated with TAS‐102 compared with HFSR, and hypertension with regorafenib [7], [12], [13], [14], [15], [16]. Both the CONCUR and CORRECT trials showed similar deterioration in quality of life measures in patients who received regorafenib versus placebo [7], [13]. Interestingly, patients who received the dose escalation strategy of regorafenib did not appear to have a decrease in quality of life at the second week of therapy initiation compared with full‐dose regorafenib [12]. This is related to the lower rates of adverse events reported with the Rego 80+ compared with Rego 160.
The sequencing of treatment in refractory mCRC is a key component in enabling patients to receive all available agents which will likely translate to improved patient outcomes [17]. There are no major randomized trials comparing agents that are used in the refractory setting. Recently, the REVERCE study (randomized phase II study of regorafenib followed by cetuximab vs. the reverse sequence in patients with mCRC) showed that early introduction of regorafenib prior to cetuximab was associated with longer OS (17.4 vs. 11.6 months; HR, 0.61l; 95% CI, 0.39–0.96) and trended to an improvement in PFS and PFS2 [18]. In addition, we learn from the RECOURSE trial that patients with prior regorafenib exposure appeared to maintain benefit from TAS‐102 compared with those who are regorafenib naive [14]. It is therefore possible that regorafenib may provide improved benefit when allowed to be used as indicated but earlier in the treatment paradigm [17]. Clinically, multiple factors should be taken into consideration when choosing between the two agents, including comorbidities, toxicity profile, and patient preferences.
Of note, Abrahao et al. previously reported a network meta‐analysis of only three (CORRECT, CONCUR, and RECOURSE) of the 6 included trials here comparing regorafenib 160 with TAS‐102 and found similar efficacy between the two [19]. Our study expands on this by including three additional landmark trials and provides simultaneous assessment of the relative efficacy of the different dosing strategies for regorafenib. Limitations of our study are related to both the network analysis and individual trials. Given the limited number of direct comparative effectiveness trials, estimates based purely on indirect treatment comparisons typically warrant lower confidence compared with those based on combined direct‐ and indirect‐treatment comparisons. In addition, our analysis was performed with study‐level data rather than individual patient data, which would limit the power of our analysis.
Conclusion
In patients with refractory mCRC, regorafenib 160 (once daily for the first 3 weeks of each 4 week cycle), and TAS‐102 appear to have similar efficacy and are superior to BSC. In addition, the dose escalation strategy of regorafenib 80+ (80 mg per day, weekly dose escalation if no significant drug‐related toxicities, up to 160 mg per day) is superior to BSC. Given its tolerability and the observed trend in OS benefit compared with regorafenib 160, dose escalation strategy of regorafenib (80+) may be the preferred option in this setting.
See http://www.TheOncologist.com for supplemental material available online.
Footnotes
For Further Reading: Toshikazu Moriwaki, Shota Fukuoka, Hiroya Taniguchi et al. Propensity Score Analysis of Regorafenib Versus Trifluridine/Tipiracil in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapy (REGOTAS): A Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study. The Oncologist 2018;23:7–15.
Implications for Practice: Previous studies of patients with metastatic colorectal cancer refractory to standard chemotherapy had demonstrated that both regorafenib and trifluridine/tipiracil could result in increased overall survival compared with placebo, but there are no head‐to‐head trials. This large, multicenter, observational study retrospectively compared the efficacy of regorafenib and trifluridine/tipiracil in 550 patients with metastatic colorectal cancer refractory to standard chemotherapy who had access to both drugs. Although no difference in overall survival was found between the two drugs in adjusted analysis using propensity score, regorafenib showed favorable survival in patients aged <65 years, whereas trifluridine/tipiracil was favored in patients aged ≥65 years in the subgroup analysis.
Disclosures
Joleen M. Hubbard: Bayer (SAB), Bayer, Taiho (RF); Daniel H. Ahn: Astellas, Eisai, Cardinal Health, Lexicon, Exelixis (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Van Cutsem E, Oliveira J; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow‐up. Ann Oncol 2009;20(suppl 4):61–63. [DOI] [PubMed] [Google Scholar]
- 2.Hochster HS, Grothey A, Hart L et al. Improved time to treatment failure with an intermittent oxaliplatin strategy: Results of concept. Ann Oncol 2014;25:1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel A, Hofheinz RD, Kubicka S et al. Treatment decisions in metastatic colorectal cancer ‐ Beyond first and second line combination therapies. Cancer Treat Rev 2017;59:54–60. [DOI] [PubMed] [Google Scholar]
- 4.Yoshino T, Arnold D, Taniguchi H et al. Pan‐Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO‐ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018;29:44–70. [DOI] [PubMed] [Google Scholar]
- 5.Food U.S. and Administration Drug. Prescribing information for Stivarga (regorafenib). Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/203085s007lbl.pdf. 2017. [Google Scholar]
- 6.Food U.S. and Adminsitration Drug. Prescribing information for Lonsurf (trifluridine and tipiracil). Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207981s000lbl.pdf. 2015. [Google Scholar]
- 7.Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 8.Peters GJ. Therapeutic potential of TAS‐102 in the treatment of gastrointestinal malignancies. Ther Adv Med Oncol 2015;7:340–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 2009;62:e1000100. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gotzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brignardello‐Petersen R, Bonner A, Alexander PE et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta‐analysis. J Clin Epidemiol 2018;93:36–44. [DOI] [PubMed] [Google Scholar]
- 12.Bekaii‐Saab TS, Ou FS, Anderson DM et al. Regorafenib dose optimization study (reDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC)–An accru network study. J Clin Oncol 2018;36:611a. [Google Scholar]
- 13.Li J, Qin S, Xu R et al. Regorafenib plus best supportive care versus placebo plus best supportive care in asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2015;16:619–629. [DOI] [PubMed] [Google Scholar]
- 14.Mayer RJ, Van Cutsem E, Falcone A et al. Randomized trial of TAS‐102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–1919. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Kim TW, Shen L et al. Results of a randomized, double‐blind, placebo‐controlled, phase III trial of trifluridine/tipiracil (TAS‐102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: The TERRA study. J Clin Oncol 2018;36:350–358. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino T, Mizunuma N, Yamazaki K et al. TAS‐102 monotherapy for pretreated metastatic colorectal cancer: A double‐blind, randomised, placebo‐controlled phase 2 trial. Lancet Oncol 2012;13:993–1001. [DOI] [PubMed] [Google Scholar]
- 17.Bekaii‐Saab T, Kim R, Kim TW et al. Third‐ or later‐line therapy for metastatic colorectal cancer: Reviewing best practice. Clin Colorectal Cancer 2019;18:e117–e129. [DOI] [PubMed] [Google Scholar]
- 18.Shitara K, Yamanaka T, Denda T et al. REVERCE: Randomized phase II study of regorafenib followed by cetuximab versus the reverse sequence for metastatic colorectal cancer patients previously treated with fluoropyrimidine, oxaliplatin, and irinotecan. J Clin Oncol 2018;36(suppl):557a. [DOI] [PubMed] [Google Scholar]
- 19.Abrahao ABK, Ko YJ, Berry S et al. A comparison of regorafenib and TAS‐102 for metastatic colorectal cancer: A systematic review and network meta‐analysis. Clin Colorectal Cancer 2018;17:113–120. [DOI] [PubMed] [Google Scholar]