Abstract
Ribosome assembly cofactors are widely conserved across all domains of life. One such group, the ribosome-associated GTPases (RA-GTPase), act as molecular switches to coordinate ribosome assembly. We previously identified the Staphylococcus aureus RA-GTPase Era as a target for the stringent response alarmone (p)ppGpp, with binding leading to inhibition of GTPase activity. Era is highly conserved throughout the bacterial kingdom and is essential in many species, although the function of Era in ribosome assembly is unclear. Here we show that Era is not essential in S. aureus but is important for 30S ribosomal subunit assembly. Protein interaction studies reveal that Era interacts with the 16S rRNA endonuclease YbeY and the DEAD-box RNA helicase CshA. We determine that both Era and CshA are required for growth at suboptimal temperatures and rRNA processing. Era and CshA also form direct interactions with the (p)ppGpp synthetase RelSau, with RelSau positively impacting the GTPase activity of Era but negatively affecting the helicase activity of CshA. We propose that in its GTP-bound form, Era acts as a hub protein on the ribosome to direct enzymes involved in rRNA processing/degradation and ribosome subunit assembly to their site of action. This activity is impeded by multiple components of the stringent response, contributing to the slowed growth phenotype synonymous with this stress response pathway.
Author summary
The bacterial ribosome is an essential cellular component and as such is the target for a number of currently used antimicrobials. Correct assembly of this complex macromolecule requires a number of accessory enzymes, the functions of which are poorly characterised. Here we examine the function of Era, a GTPase enzyme involved in 30S ribosomal subunit biogenesis in the important human pathogen S. aureus. We uncover that Era is not an essential enzyme in S. aureus, as it is in many other species, but is important for correct ribosome assembly. In a bid to determine a function for this enzyme in ribosomal assembly, we identify a number of protein interaction partners with roles in ribosomal RNA maturation or degradation, supporting the idea that Era acts as a hub protein facilitating ribosomal biogenesis. We also uncover a link between Era and the (p)ppGpp synthetase RelSau, revealing an additional level of control of rRNA processing by the stringent response. With this study we elaborate on the functions of GTPases in ribosomal assembly, processes that are controlled at multiple points by the stringent response.
Introduction
Ribosomes are macromolecular machines responsible for the synthesis of proteins in all living cells. In bacteria these complexes consist of two subunits, with the large 50S subunit containing 33 large proteins (L1-36) and two rRNAs, and the 30S small subunit containing 21 small proteins (S1-21) and the 16S rRNA. As such, assembly of the ribosome is a tightly regulated process, with correct maturation requiring the help of assembly cofactors, one class of which are the P-loop ribosome-associated GTPases (RA-GTPase). These enzymes are widely conserved across all domains of life and act as molecular switches, cycling between inactive GDP-bound, and active, effector-binding GTP-bound states. Within the P-loop GTPase class lies the Era family (Escherichia coli Ras-like protein), which is characterised by the presence of a distinct derivative of a KH domain [1]. Era, the protein after which this family is named, is highly conserved throughout the bacterial kingdom, although is missing in Chlamydia and mycobacterial species [1]. This GTPase is essential in E. coli [2–5], Salmonella Typhimurium [6] and in some strains of Bacillus subtilis, where deletion mutants of B. subtilis strains IS75 and CRK6000 were unobtainable [7, 8], although an era knockout was created in strain BR151 [7]. The reasons behind this difference are not known. The first indication that Era is involved in ribosomal biogenesis came from the ability of the 16S rRNA methyltransferase KsgA to suppress a cold-sensitive phenotype of an Era E200K mutation [9]. While important for ribosomal maturation, additional phenotypic defects associated with depleted cellular levels of Era include cell cycle control and chromosome segregation, as well as carbon and nitrogen metabolism [10–12].
Era is composed of two domains, an N-terminal GTPase domain and a C-terminal RNA-binding K-homology domain [13]. A cryo-electron micrograph structure of Era in complex with the 30S subunit of the ribosome reveals that Era binds into the same pocket as small subunit protein S1 [14]. In the absence of S1, Era interacts with proteins S2, S7, S11 and S18, as well as with a number of helices of the 16S rRNA. In addition, Era interacts with h45 and nearby residues 1530–1539 (GAUCACCUCC) in the 3' minor domain of the 16S rRNA via its CTD region [14, 15]. These residues include the anti-Shine Dalgarno sequence, critical for the formation of the 30S preinitiation complex. Including Era in in vitro reconstitutions of the 30S ribosome promotes the incorporation of several late-stage ribosomal proteins for the RNA [16, 17]. Consequently, it has been proposed that Era functions as a checkpoint protein, and that by binding to the 16S rRNA the formation of the initiation complex is prevented until the appropriate time [14]. In addition to interacting with ribosomal proteins, Era has also been reported to interact with a number of proteins involved in 16S rRNA maturation. One of these, YbeY, is an endonuclease required in E. coli and B. subtilis for the maturation of the 3' end of the 16S rRNA [18, 19]. It is proposed that the binding of YbeY to Era and S11 guide the endonuclease to its site of action [18].
The stringent response is a signalling system used by bacteria to cope with a variety of environmental stresses, the best characterised of which is nutrient deprivation. The opportunistic pathogen Staphylococcus aureus contains three enzymes, RelSau, RelP and RelQ, which upon sensing a stress, synthesise the nucleotides guanosine tetra- and pentaphosphate ((p)ppGpp) [20, 21]. Once produced, this alarmone controls cellular responses to aid survival. Our previous work identified Era, as well as three other GTPase enzymes from S. aureus, as target proteins for (p)ppGpp and demonstrated that the production of (p)ppGpp has a negative impact on mature 70S assembly [22]. Here, we examine the role of Era as an enzyme required for ribosome biogenesis. Unlike in E. coli, this GTPase is not essential for the growth of S. aureus, however mutant cells are defective in 30S subunit maturation. We identify the S. aureus endonuclease YbeY and the DEAD-box RNA helicase CshA as interaction partners for Era, and show that both Era and CshA are crucial for rRNA homeostasis and growth at low temperatures. We additionally demonstrate that both Era and CshA interact with the (p)ppGpp synthetase/hydrolase RelSau, and that cellular rRNA processing is controlled in a stringent response-dependent manner. With this, we propose that Era is a protein that facilitates the interactions between a number of rRNA processing and degrading enzymes and the 30S subunit/16S rRNA, and show that under stress conditions the stringent response is important for this process.
Results
Era is not essential in S. aureus but is required for normal growth and ribosome assembly
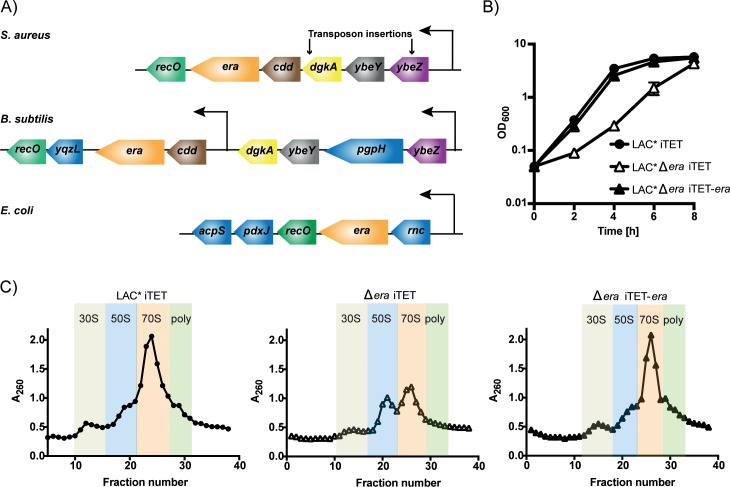
Previous reports show that era is an essential gene in multiple bacterial species [3, 6]. In agreement with this, transposon insertions in this gene have not been identified in a number of published S. aureus transposon libraries [23–25]. Closer inspection of the transposon insertion hits within the era operon obtained in the Nebraska S. aureus mutant library [23] reveals insertions in ybeZ, a gene of unknown function, and at the 3' end of the diacylglycerol kinase dgkA but not within ybeY, cdd, era or recO, suggestive of an additional promoter upstream of era and cdd (Fig 1A). To rule out polar effects and examine whether era in isolation is essential, we replaced the entire coding sequence with the Tet-encoding tetAM open reading frame in the presence of the anhydrotetracycline (Atet)-inducible covering plasmid pCN55iTET-era. Following deletion of the chromosomal copy of era, we attempted to phage transduce this mutation into S. aureus strains containing the empty vector pCN55iTET or the complementing plasmid pCN55iTET-era. Transduction efficiencies were similar with both recipients, albeit smaller colony sizes were observed in the absence of era, indicating that this gene is not essential in S. aureus. We next transduced the era deletion into the community-associated methicillin resistant S. aureus strain LAC* to rule out secondary mutations and used this strain for further studies. Analysis of growth rates revealed that an era mutant strain, while viable, does have a significant growth defect, which can be complemented by the expression of Era from pCN55iTET-era (Fig 1B).
Fig 1. Role of Era in bacterial growth and ribosome assembly.
A) Schematic representation of the era-containing operons from S. aureus, B. subtilis and E. coli. The era operon in S. aureus consists of: ybeZ–encoding for a protein of unknown function; ybeY–a 16S rRNA endoribonuclease; dgkA—a diacylglycerol kinase involved in lipid metabolism; cdd—a cytidine deaminase involved pyrimidine metabolism; era, and recO encoding for a DNA repair protein. Arrows indicate the site of transposon insertions in the S. aureus Nebraska Transposon library. Homologues in B. subtilis and E. coli are coloured similarly, while genes without homologues in this region are in blue. B) Growth of S. aureus strains LAC* iTET, LAC* Δera iTET and LAC* Δera iTET-era. Overnight cultures were diluted to an OD600 of 0.05 and grown in the presence of 100 ng/ml Atet for 8 h. Growth curves were performed three to four times, with averages and standard deviations shown. C) Ribosome profiles from LAC* iTET, LAC* Δera iTET and LAC* Δera iTET-era. Normalised extracts from each strain were layered onto 10–50% sucrose gradients. Gradients were fractionated and analysed for RNA content at an absorbance of 260 nm. 30S, 50S, 70S and polysomes-containing fractions are indicated. Experiments were performed in triplicate (N = 3) with one representative graph shown.
A role for Era as a ribosomal subunit assembly cofactor has been reported in both E. coli and B. subtilis, where depletion leads to a decrease in 70S ribosomes and an accumulation of individual 50S and 30S subunits [26, 27]. We reasoned that the growth defect observed in S. aureus may be due to defects in ribosome assembly. To investigate this in the context of a complete era deletion, we analysed the cellular ribosomal content of wildtype, mutant and complemented strains by sucrose density centrifugation. This revealed that the era mutant strain contained fewer polysomes and mature 70S ribosomes, with a concurrent build-up of 50S subunits, a defect that was reversed in the presence of the complementing plasmid (Fig 1C). As cryo-electron microscopy has shown Era from Thermus thermophilus interacting with the 30S subunit [14], an excess of free 50S subunits may be, in and of itself, an indication that there is a defect in small subunit biogenesis, leading to a build-up in free 50S. We reasoned that this defect in ribosome assembly might make the era mutant strain more susceptible to ribosome-targeting antibiotics. In agreement with this, we observed that the minimum inhibitory concentration (MIC) for the Δera strain decreased 2-4-fold when exposed to the 30S-targeting antibiotic spectinomycin (S1A Fig). However, the MIC was unaffected by the 50S targeting antibiotic chloramphenicol (S1B Fig). Together this indicates that while Era is not an essential protein in S. aureus, it is important for optimal growth and ribosome maturation.
Uncovering Era protein interaction dynamics in a native background
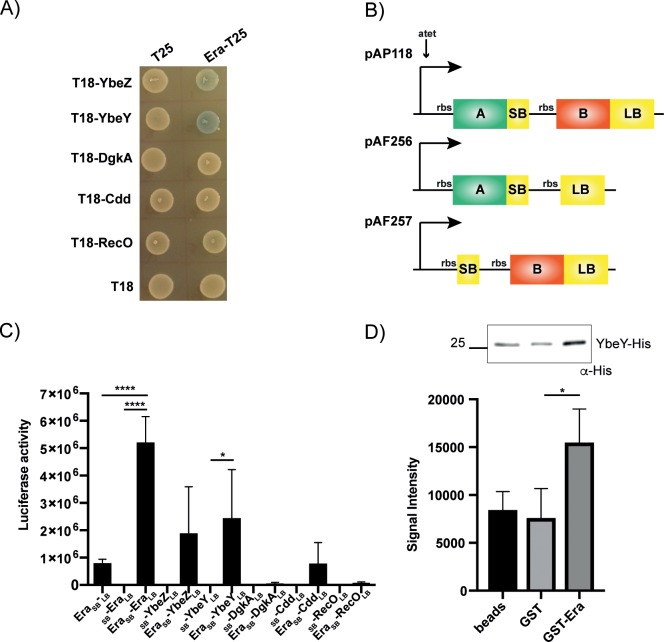
In S. aureus, Era is encoded in an operon with five other genes, one of which encodes YbeY (Fig 1A). YbeY is an endoribonuclease implicated in the maturation of the 3' terminus of 16S rRNA in E. coli, as well as a quality control checkpoint protein that together with RNase R is involved in eliminating defective 70S ribosomes [19, 28]. Era interacts with YbeY in E. coli [18], although these two genes are not encoded in the same operon in that organism (Fig 1A). To determine whether Era from S. aureus interacts with YbeY, and/or any other proteins encoded in the S. aureus era operon, we first used a bacterial two-hybrid approach, heterologously expressing Era in combination with YbeZ, YbeY, DgkA, Cdd or RecO in E. coli. Using this approach, we observed that Era potentially interacts with YbeY and YbeZ (Fig 2A).
Fig 2. Era interacts with other proteins encoded in the same operon.
A) Bacterial two-hybrid showing a positive interaction between Era-T25 and both T18-YbeZ and YbeY. T25 and T18-encoding empty vectors are used as negative controls. N = 3, with one representative image shown. B) Schematic representation of the luciferase vectors used for native protein-protein interaction studies. pAP118 contains an Atet-inducible promoter upstream of two genes–SB encodes for the small bit of the luciferase protein, while LB encodes for the large bit. When genes encoding for interacting proteins (A & B) are translationally fused to SB and LB they associate to produce luciferase. pAF256 and pAF257 are negative control vectors. C) Split luciferase assay demonstrating a significant interaction between Era-Era in S. aureus. The negatives are pAF256-eraSB-LB, which has Era fused to the SB but nothing fused to LB and pAF257-SB-eraLB, which has Era fused to the LB but nothing fused to SB. The average luciferase values and standard deviations of triplicate experiments are plotted (N = 3). Statistical analysis was performed using a one-way ANOVA, followed by Dunnett’s multiple comparisons test (* P < 0.05, **** P < 0.0001). D) Top: affinity pulldown assay using GST or GST-tagged Era coupled to glutathione beads. Beads alone, GST or GST-Era coupled beads were incubated with His-tagged YbeY. After washing, bound YbeY-His was detected using HRP-conjugated anti-His antibodies. N = 3 with one representative image shown above. Below: the mean signal intensities and standard deviations from the 3 repeats are plotted. Statistical analysis was performed using a one-way ANOVA, followed by Dunnett’s multiple comparisons test (* P < 0.05).
In order to look at these interactions natively in an S. aureus background, we adapted a split-luciferase system recently developed to analyse protein-protein interactions in Clostridium difficile for use in S. aureus (Fig 2B) [29]. These vectors, while designed for use in C. difficile, also replicate in S. aureus using an oriV origin of replication. When using this system in S. aureus, we noted an increase in non-specific luciferase activity from the pAF256 control vector over the pAF257 negative control, regardless of the inserted gene. For this reason, only interactions that are statistically increased over both negative controls will be considered positive. The era gene, in combination with itself and each of the operon genes, were cloned into the split-luciferase plasmid pAP118 and introduced into S. aureus strain LAC*. Induction of protein expression with Atet revealed a strong positive interaction for Era with itself (Fig 2C), indicating that Era can form dimers. Dimerisation of Era has previously been observed while solving the crystal structure of the E. coli protein [30] and also by gel filtration chromatography [31], and so this interaction confirms the functionality of the split-luciferase system in S. aureus. In agreement with the bacterial two-hybrid results, no interactions occurred between Era and DgkA, Cdd or RecO. There was a significant interaction between Era and YbeY, but only when compared to the pAF257-ybeY negative control (Fig 2C). This prompted us to investigate the potential interaction between Era-YbeY in vitro. Pulldown assays were performed using glutathione beads coupled to either GST or GST-tagged Era, which were incubated with His-tagged YbeY (Fig 2D). Here we observed low-level cross reactivity of YbeY with the gluthatione beads that could not be reduced despite increased washes. However, His-YbeY was pulled down significantly more in the presence of Era (Fig 2D). Altogether the bacterial two-hybrid, split luciferase and affinity pulldown assays suggest a weak interaction between Era and the endonuclease YbeY from S. aureus, which may form part of a larger complex, as has been suggested for the E. coli protein [18]. With this data we have adapted a luciferase system for confirmation of protein-protein interactions in a native S. aureus background.
Era interacts with the DEAD-box RNA helicase CshA
We wished to shed further light on the role played by Era in the cell and sought to identify unknown interaction partners using a genome-wide bacterial two-hybrid screen. A library of S. aureus genomic DNA fragments cloned into pUT18C was screened against pKNT25-era. Of the 17 hits obtained, 14 contained fragments mapping to cshA, a gene encoding a DEAD-box RNA helicase. Of the remaining 3 plasmids, 2 contained sequences not in-frame with the T18 coding sequence, while 1 contained a sequence that also went blue when co-transformed with the pKNT25 empty vector. CshA functions in mRNA protection and RNA decay in S. aureus [32, 33], while the homologue CsdA from E. coli is involved in the cold shock degradosome [34]. In B. subtilis cells, CshA has also been shown to interact with a number of ribosomal proteins from the 50S subunit, while a deletion strain has a reduction in the amount of mature 70S ribosomes [35].
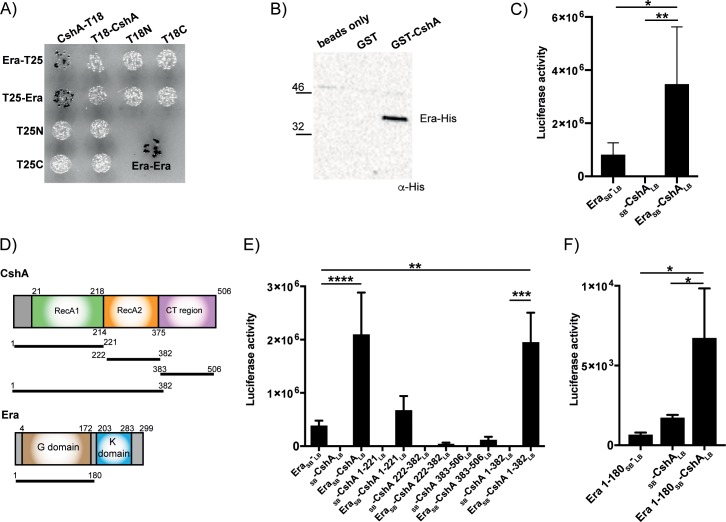
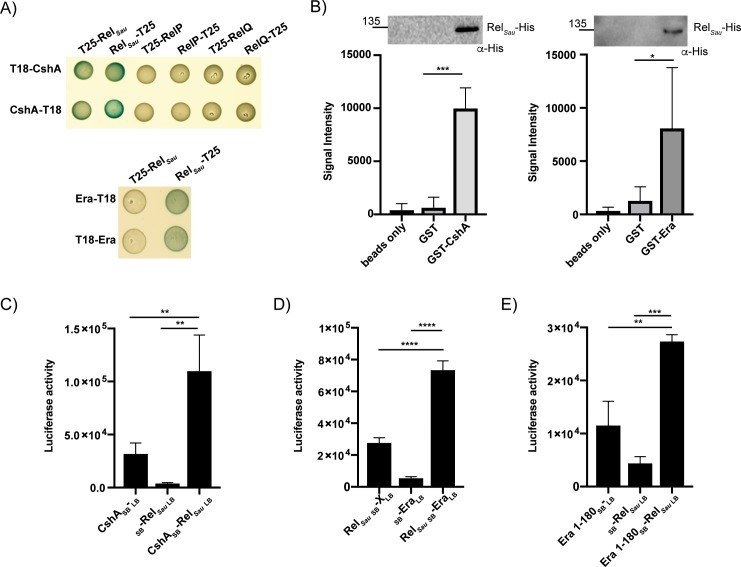
To confirm the interaction between Era and CshA, full-length cshA was cloned into both pUT18 and pUT18C so as to perform a bacterial two-hybrid assay with full-length constructs. Upon co-transformation, interactions occurred between CshA-T18 and both T25-Era and Era-T25 (Fig 3A). To assess this interaction in vitro, we performed pulldown assays using glutathione beads coupled to either GST or GST-tagged CshA and incubated them with His-tagged Era, revealing that while His-Era did not interact with the control GST protein, it was pulled down in the presence of CshA (Fig 3B). Finally, to analyse and confirm this interaction in the context of the native host, we used the split luciferase assay (Fig 2B). Assaying for luciferase activity revealed a 4-fold increase in luciferase activity upon co-expression of Era and CshA (Fig 3C). Together these data confirm the identification of a novel Era interaction partner and suggest that CshA can also interact with proteins that associate with the 30S ribosomal subunit.
Fig 3. Era interacts with the DEAD-box helicase CshA.
A) Bacterial two-hybrid showing an interaction between CshA-T18 and both Era-T25 and T25-Era. Era is known to form a dimer and an Era-Era interaction is used as a positive control. N = 3 with one representative image shown. B) Affinity pulldown assay using GST or GST-tagged CshA coupled to glutathione beads. Beads were incubated with His-tagged Era and after washing, bound protein was detected using HRP-conjugated anti-His antibodies. N = 3 with one representative image shown. C) Split luciferase assay demonstrating an interaction between Era and CshA in S. aureus. The negative controls have Era fused to the SB but nothing fused to LB, or CshA fused to the LB and nothing to the SB. Cells were grown in the presence of 100 ng/ml Atet at 37°C before being normalised. The average luciferase activity values and standard deviations of triplicate experiments (N = 3) are plotted. D) Schematic representation of CshA and Era. The sizes of each domain construct are indicated by the black lines and numbering. E) Split luciferase assay with Era fused to SB and various truncated domain constructs of CshA fused to LB. F) Split luciferase assay with the G domain of Era (1–180) fused to SB and full-length CshA fused to LB. The average values and standard deviations of three or four experiments are plotted. All statistical analysis was performed using a one-way ANOVA, followed by Dunnett’s multiple comparisons test (* P < 0.05, ** P < 0.005, *** P < 0.0005, **** P < 0.0001).
The N-terminal regions of both CshA and Era are crucial for interactions
CshA is a DEAD-box RNA helicase with an N-terminal helicase core containing two RecA-like domains and a disordered C-terminal (CT) region involved in RNA binding (Fig 3D) [33]. This protein is capable of unwinding both double stranded RNA and RNA-DNA hybrids and is required for both the stabilisation and the degradation of mRNA, the latter of which occurs via interactions of the CT region with components of the RNA degradosome [32, 33].
To determine which regions of both Era and CshA are important for interacting, shorter domain constructs comprising only RecA1 (aa 1–221), RecA2 (aa 222–382), the entire core helicase domain (aa 1–382) or just containing the disordered CT-region (aa 383–506) were cloned, together with Era, into the split luciferase vector pAP118. Interactions with full-length Era were only apparent in the presence of the full core helicase domain (aa 1–382: Fig 3E), indicating that the RNA-binding CT region of CshA is dispensable for this interaction. To determine whether the GTPase domain of Era is required for binding, a shorter construct comprising the N-terminal G domain (aa 1–180) was cloned into pAP118 alongside full-length CshA. This domain is reported to still retain GTPase activity, but can no longer bind to RNA [13, 36]. Luciferase assays reveal that the G domain of Era is sufficient for interacting with CshA (Fig 3F).
Interactions between Era and CshA do not affect enzymatic activity
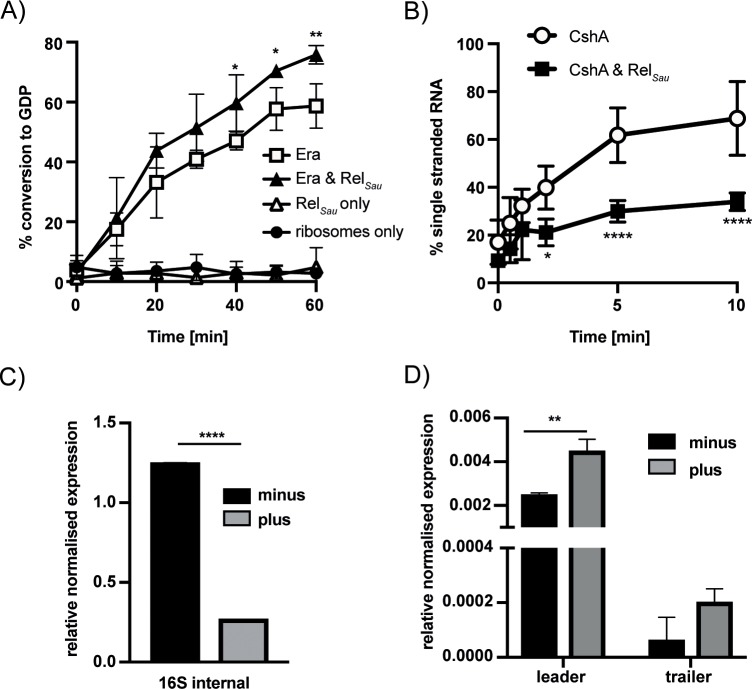
CshA is an RNA helicase, while Era has GTPase activity. To investigate whether the interaction between these two proteins affects the enzymatic activity of either one, we first performed helicase assays. A double stranded RNA oligo was incubated with each protein singly or in combination. While CshA was able to unwind the dsRNA over time, the addition of Era had no effect on its activity (S2A Fig). In addition, the GTPase activity of Era in the presence of 70S ribosomes was unaltered by the addition of either CshA or YbeY (S2B and S2C Fig), indicating that while these proteins do interact, this binding has little effect on their enzymatic functions. As previously proposed, we suggest that these interactions fit with a role for Era as a guide for rRNA/ribosome maturation enzymes to their substrates [14, 18].
CshA and Era are both required for growth at suboptimal temperatures and rRNA homeostasis
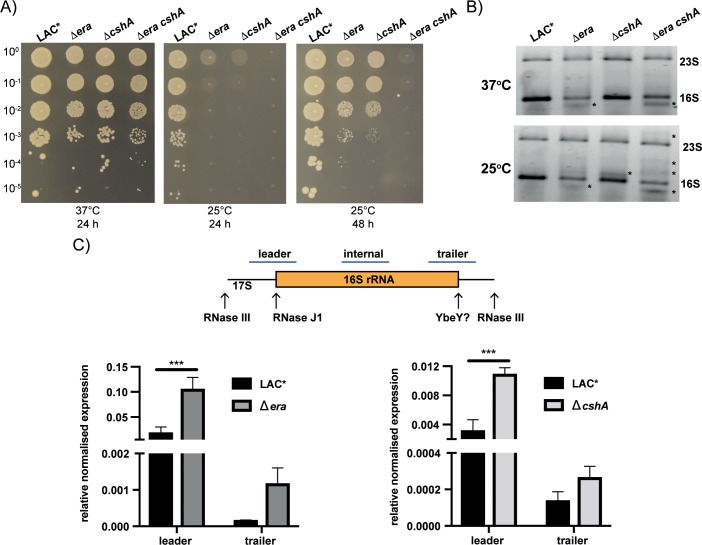
In E. coli, CshA has been linked to survival at low temperatures [37], while strains with depleted levels of Era are sensitive to both cold and heat shock [9, 10]. To examine the importance of these enzymes in S. aureus, we utilised deletion strains in the S. aureus MRSA strain LAC*. While both the S. aureus era and cshA null mutants showed significant growth defects at 37°C (Figs 1B and S3A), the growth of both was severely compromised at 25°C (Figs 4A and S3B). This defect was enhanced in a double Δera cshA mutant, which failed to grow at 25°C even after 48 h (Figs 4A and S3B), highlighting that these two proteins are essential for bacteria to grow at suboptimal temperatures.
Fig 4. Era and CshA are required for cold adaptation and rRNA homeostasis.
A) Serial dilutions of the wildtype LAC*, Δera, ΔcshA and Δera cshA were spotted onto TSA agar plates and incubated at either 37 or 25°C for the times indicated. N = 3 with one representative image shown. B) rRNA profiles from wildtype, era and cshA mutant strains. 500 ng of RNA extracted from LAC*, Δera, ΔcshA and Δera cshA grown to an OD600 of 0.4 at either 37 or 25°C, were run on 0.7% agarose/0.9% synergel gels and stained with Evagreen dye. * highlights the presence of either processing or degradation intermediates. N = 3 with one representative image shown. C) top: schematic of the 17S rRNA, with arrows indicating processing sites that lead to the formation of the mature 16S rRNA. The blue bars indicate the probe regions amplified by RT-PCR. Bottom: RT-PCR of total RNA from the wildtype LAC*, Δera, and ΔcshA strains grown at 25°C using probes as indicated above. rRNA values between strains are normalised to the expression of rho RNA as an internal control before being plotted as mean expression +/- SD relative to the total 16S (internal probe set to a value of 1). The rho transcript was chosen as it has been shown to be highly stable under nutrient-deficient conditions under which the stringent response would be induced [38]. Statistical analysis was performed using a two-way ANOVA, followed by Sidak’s multiple comparisons test (*** P < 0.005). Cycle threshold values were determined for 3 biological repeats in duplicate (N = 3).
Era is an RA-GTPase that, along with a second RA-GTPase RsgA, binds to the 30S ribosomal subunit [14, 39]. In E. coli, overexpression of Era can complement a deletion of rsgA [40]. We first examined whether expression of Era could complement a deletion of rsgA in S. aureus using cross-complementation. This revealed that Era can complement for the loss of RsgA (S4A Fig), although RsgA could not complement for the loss of Era (S4B Fig), suggesting partial overlapping but also distinct functions. We next used cross-complementation of the Δera and ΔcshA mutations to investigate whether Era and CshA act in the same pathway and can functionally complement each other. Cross-complementation of the Δera and ΔcshA mutations did not improve growth at 25°C (S4B and S4C Fig). This indicates that these proteins have separate cellular functions, even though they interact and bacteria lacking both cannot survive cold temperatures. We additionally confirmed that no suppressor mutations had arisen in our Δera cshA double deletion by complementing growth of the double mutant back to ΔcshA levels with pCN55iTET-era (S4E and S4F Fig).
To understand why Era and CshA are so important for growth at cold temperatures, we sought to assess the impact of deleting these genes on the cellular rRNA. We observed that RNA extracted from both the single Δera and double Δera cshA strains grown at 37°C had a processing or degradation intermediate migrating slightly below the 16S band (Fig 4B). At 25°C we also observed a defect for the ΔcshA mutant strain and multiple additional bands were evident in the Δera cshA double mutant (Fig 4B), which could represent either processing intermediates from the 16S, or degradation intermediates from both the 23S and the 16S. An accumulation of degradation products in the cshA mutant could be due to its role in the RNA degradosome [33], or could suggest a role for its helicase activity in rRNA processing. To examine whether an accumulation of unprocessed 17S rRNA could be occurring in these mutant strains when grown at low temperatures, we performed qRT-PCR with RNA extracted from cells grown at 25°C, with probes that either amplified internally to the 16S rRNA sequence (to amplify total 16S rRNA), or flanked either the 5' or 3' processing junctions required to convert 17S into mature 16S rRNA (Fig 4C). When strains were normalised to total 16S rRNA levels, significant increases in unprocessed 5' were evident in both the Δera and ΔcshA strains compared to the wildtype (Fig 4C). Increases were also observed for the processing of the 3' sequence for the Δera mutant, although these did not reach significance (Fig 4C). As Era is known to interact with the 30S ribosomal subunit, these defects could potentially be due to a failure to recruit the endonuclease YbeY, or indeed other processing enzymes, in the absence of Era, while the defects in the ΔcshA strain could be either direct, due to a loss of helicase activity necessary for 16S 5' processing at 25°C, or could reflect the role of CshA in the RNA degradosome, with pre-16S rRNA destined for degradation accumulating in its absence.
RelSau interacts with both Era and CshA
Our previous work demonstrated that the GTPase activity of Era is inhibited by the stringent response alarmone ppGpp [22]. To examine whether ppGpp also interacts with CshA we performed DRaCALA assays, revealing that this nucleotide does not interact with CshA (S5A Fig), nor is the affinity of ppGpp for Era altered in the presence of CshA (Kd of 3.1 ± 0.4 μM in the absence versus 3.2 ± 0.3 μM in the presence of CshA) (S5B Fig).
In E. coli, Era, YbeZ and the (p)ppGpp synthetase/hydrolase enzyme SpoT all interact with YbeY, potentially forming a complex [18]. To investigate whether the stringent response has any additional points of interaction with either Era and CshA in S. aureus, we examined whether one of the (p)ppGpp synthetases might directly interact with either protein via bacterial two-hybrid. This revealed that RelSau, but not RelP or RelQ, interacts with CshA and that RelSau interacts with Era (Fig 5A). To confirm this interaction, we first used affinity pulldown assays, showing that statistically significant interactions occurred between both Era or CshA and RelSau (Fig 5B). Following this, we again used the split luciferase assay, which confirmed an interaction between RelSau and CshA (Fig 5C) or Era (Fig 5D), and also established that the GTPase domain of Era was sufficient for this interaction (Fig 5E).
Fig 5. The stringent response synthetase RelSau interacts with Era and CshA.
A) Top: Bacterial two-hybrid showing an interaction between CshA-T18/T18-CshA and both RelSau-T25 and T25-RelSau. CshA did not interact with the small (p)ppGpp synthetase enzymes RelP or RelQ. Bottom: Bacterial two-hybrid showing an interaction between RelSau-T25 and both Era-T18 and T18-Era. N = 3 with one representative image shown. B) Affinity pulldown assay using GST, GST-tagged CshA and GST-tagged Era coupled to glutathione beads. Beads alone, GST, GST-Era or GST-CshA coupled beads were incubated with His-tagged RelSau. After washing, bound RelSau-His was detected using HRP-conjugated anti-His antibodies. N = 3 or 4 with one representative image shown above. Below, the mean signal intensities and standard deviations from the repeats are plotted. Statistical analysis was performed using a one-way ANOVA, followed by Tukey’s multiple comparisons test (* P < 0.05, *** P < 0.0005). C, D & E) Split luciferase assays demonstrating an interaction between CshA (C), Era (D) or Era 1–180 (E) and RelSau in S. aureus. The negative controls have genes singly fused to either SB or LB. The average values and standard deviations of triplicate experiments (N = 3) are plotted. All statistical analysis was performed using a one-way ANOVA, followed by Tukey’s multiple comparisons test (** P < 0.005, *** P < 0.0005, **** P < 0.0001).
RelSau influences the enzymatic activity of Era and CshA and is important for rRNA processing
We first examined whether the interactions between RelSau and Era/CshA impact the activity of either enzyme. Recombinantly purified full-length RelSau is known to be in the synthetase-off/hydrolase-on conformation in vitro [41]. Using purified RelSau, we determined that Era and CshA have no effect on the pppGpp hydrolase activity of this enzyme (S5C Fig). To look at the enzymatic activities of Era and CshA in the presence of RelSau, we firstly examined the GTPase activity of Era, observing a slight but significant increase in activity in the presence of RelSau (Fig 6A). Following this we performed a helicase assay, revealing a decrease in the unwinding activity of CshA in the presence of RelSau, the opposite effect of binding on the enzymatic activity of Era (Fig 6B).
Fig 6. The stringent response affects Era and CshA enzyme activity and rRNA processing.
A) The GTPase activity of 100 nM Era was measured in the presence of an equal amount of ribosomes, 100 nM RelSau and 1 μM GTP. All reactions contained ribosomes. The RelSau only control reaction contained RelSau and ribosomes but no Era. Hydrolysis of 32P-GTP was monitored by TLC and the percentage GDP formed quantified using ImageJ. Experiments were repeated three times with means and standard deviations shown. Statistical analysis was performed using a two-way ANOVA, followed by Dunnett’s multiple comparisons test (** p < 0.005, * p < 0.05). B) The RNA helicase activity of 0.5 μM CshA with and without RelSau was determined using a Cy3-labelled double stranded RNA oligomer. Reactions were incubated at 25°C for up to 10 min before analysis on a native page gel. Experiments were repeated four times with means and standard deviations shown. Statistical analysis was performed using a two-way ANOVA, followed by Sidak’s multiple comparisons test (* p < 0.05, **** p < 0.0001). C & D) rRNA extracted from LAC*. Strains were grown at 25°C until an OD600 of 0.4. Cells were split and half exposed to 60 μg/ml mupirocin for 1 h (plus), which at 25°C corresponds to one doubling. RT-PCR of total RNA using the probes as indicated in Fig 4C that bind internally to the 16S (C) or to the unprocessed leader and trailer sequences (D) of the 17S rRNA. rRNA values are expressed as mean relative expression +/- SD normalised to the expression of rho RNA as an internal control. For comparisons between two groups (C—LAC* minus v plus) a two-tailed, unpaired Student’s t-test was performed (**** p < 0.0001). For comparisons between more than two groups (D) a two-way ANOVA, followed by Sidak’s multiple comparisons test was performed (** p < 0.005). Cycle threshold values were determined for 3 biological repeats in duplicate (N = 3).
Through RelSau the stringent response appears to interact with RA-GTPases and associated proteins, increasing Era GTPase activity, while decreasing the helicase activity of CshA. To directly analyse the importance of the stringent response for rRNA processing, we grew the wildtype S. aureus LAC* at 25°C, a condition under which we have shown that both Era and CshA are important for viability, and induced the stringent response with mupirocin, an antibiotic that inhibits the isoleucyl-tRNA synthetase mimicking amino acid deprivation [42]. We again performed qRT-PCR with the probes as in Fig 4C that either amplified internally to the 16S rRNA sequence, or either flanked the 5' or 3' processing junctions of the 17S rRNA. For the probe internal to the 16S rRNA we observed a significant decrease in 16S rRNA in the presence of mupirocin (Fig 6C). This is expected, as it is known that rRNA levels decrease upon activation of the stringent response [43]. However, when we compare the amount of unprocessed 5' or 3' sequences relative to the level of 16S in each strain, we observed a significant increase in the amount of unprocessed leader 17S rRNA upon induction of (p)ppGpp (Fig 6D: p = 0.0031). We have previously shown that ppGpp interacts with Era and that mature ribosome formation is inhibited upon induction of the stringent response [22]. Here we propose that the stringent response can also impact RA-GTPase function through direct interactions of RelSau with both Era and CshA. We suggest that Era acts as a hub protein on the 30S ribosome, promoting the association of processing or degradation enzymes like YbeY and CshA. RA-GTPases, including Era, are reported to associate to the ribosome when bound to GTP, but dissociate once hydrolysis has occurred [44]. The increased GTPase activity of Era observed in the presence of RelSau may therefore lead to increased dissociation of Era, and associated enzymes, from the ribosome, stalling maturation. Additionally, the inhibition of the helicase activity of CshA may also delay 17S rRNA processing or degradation, especially at 25°C. The dynamics of RA-GTPase associations to the ribosome are complex, with much further in-depth study required to precisely determine if the Era-YbeY, Era-CshA or RelSau interactions occur on or off the ribosome and the direct impact of these on ribosomal maturation.
Discussion
The synthesis of ribosomes and proteins consumes approximately 40% of the energy within a growing bacterial cell [45]. Ribosomal assembly cofactors are, therefore, an essential group of enzymes for coordinating biogenesis as efficiently as possible. Members of this group include RNA helicases, rRNA and protein modification enzymes, chaperones and RA-GTPases. S. aureus contains over 11 RA-GTPases, each with potentially varying roles in the biogenesis of the 50S and 30S subunits, although the precise functions of each in this process is unclear.
While the RA-GTPase Era has been extensively studied, its precise function in the cell is unknown. Cryo-electron microscopy shows Era interacting with the 30S ribosomal subunit, however this protein has also been implicated in numerous other cellular processes [14]. Era-depleted B. subtilis or E. coli cells are elongated, with defects in septum formation. These cells also contain diffuse nucleoid material, implicating Era in cell division and chromosome segregation [5, 12]. In B. subtilis, Era-depleted cells have defects in spore formation [7], while growth defects in strains containing Era variants with reduced nucleotide binding abilities can be rescued by truncating either rpoN or ptsN [11]. RpoN, which encodes for the alternative sigma factor σ54, is required for nitrogen assimilation and fixation, while PtsN is involved in sugar transport, suggesting that Era is also involved in regulating carbon and nitrogen metabolism. Localisation studies of Era in E. coli have indicated that this protein is present at both the membrane and in the cytoplasm [46], and so it has been suggested that this enzyme cycles between the membrane and the ribosome in response to cellular triggers. In addition to ribosomal biogenesis factors, Era has been reported to interact with MazG, Ndk, Pk and YggG [47–49]. Ndk, a nucleoside diphosphate kinase and Pk, a pyruvate kinase, from Pseudomonas aeruginosa were both shown to form a complex with Era, further implicating Era in energy metabolism [48]. MazG is a nucleoside triphosphate pyrophosphohydrolase, while YggG is a membrane-associated heat shock protein. The significance of these interactions with Era is unknown.
Era has also been implicated in helping cells cope with cold stress. Conditional cold-sensitive mutants of Era have been constructed in E. coli [10] and these mutations could be suppressed by the overexpression of the 16S rRNA methyltransferase KsgA, providing one of the first links between Era and ribosome biogenesis [9]. Era interacts with the universally conserved endonuclease YbeY in E. coli [18]. YbeY is dispensable in E. coli, but is essential in B. subtilis and potentially in S. aureus, given the lack of transposon mutants available [19, 25, 28]. This endonuclease is required for the maturation of the 3' end of the 16S rRNA, and in strains depleted of YbeY the 70S ribosomes are targeted for degradation with the help of RNase R [19, 28]. Era and YbeY are fused in a polypeptide in clostridial species, further highlighting a link between these two proteins. Although not encoded in the same operon as era in E. coli, this prompted us to examine the interactions of all other proteins encoded in the era operon in S. aureus with Era (Fig 2). As in E. coli, YbeY from S. aureus interacts with Era, although quite weakly. YbeZ also interacted weakly by bacterial two-hybrid but not significantly using the quantitative luciferase assay (Fig 2). YbeZ has been shown to interact with YbeY in E. coli, in addition to the (p)ppGpp synthetase/hydrolase enzyme SpoT [18], and ribosomal proteins S7 and L6 [50]. YbeZ has an ATPase domain and an RNA binding motif, and because of its interactions with ribosomal proteins may also bind to the 17S rRNA and in E. coli form a complex with Era, YbeY and SpoT to aid in processing the 17S rRNA to the mature 16S form [18]. So it is possible that YbeY, YbeZ, Era and RelSau in S. aureus all form a complex, and that the interactions between all these proteins may become more apparent upon isolation of the entire complex.
Here we use interaction studies to show that Era also interacts with a protein involved in survival at suboptimal temperatures, the DEAD-box RNA helicase CshA (Fig 3). In B. subtilis, CshA is one of the most abundant RNA helicases produced at low temperatures [35] and in B. subtilis and E. coli it has been implicated in multiple processes, including 50S ribosome biogenesis and interacting with components of the RNA degradosome [33–35, 37]. In S. aureus, CshA has been linked to controlling the turnover of mRNA in the cell [33] [32]. Deletion of cshA results in the stabilisation of some mRNA transcripts, such as the spa mRNA, but CshA also protects a number of other mRNA and sRNA transcripts under stress conditions [32]. Our analysis shows that Era and CshA interact and that the CT disordered region of CshA, which is important for binding to mRNA and the degradosome [33], is dispensable for this interaction (Fig 3). Given that the CshA homologue CsdA in E. coli associates with the 50S ribosomal subunit and is involved in its biogenesis [37], our results showing an interaction of CshA with the 30S binding protein Era, may indicate a wider role for CshA in both 30S and 50S subunit biogenesis, especially at 25°C. This is supported by pulldown experiments performed with CshA from S. aureus, where both 30S and 50S ribosomal proteins were identified as interaction partners for CshA [33]. The precise dynamics of the interactions between CshA, Era and components of the ribosome are a question for future study.
Using cshA and era deletion strains we showed that both single mutants are important for growth at cold temperatures, with a double mutant unable to survive at 25°C (Fig 4). By examining the rRNA of these strains we observe that both mutant strains have increased 17S unprocessed rRNA intermediates (Fig 4C). Additionally, a double Δera cshA mutant has multiple processing and degradation defects (Fig 4B). It has been proposed that Era may act to guide processing enzymes to their site of action [18]. In keeping with this, we have observed that Era and CshA have little effect on the enzymatic activity of each other. Therefore, we support a model whereby Era acts as a hub protein, allowing proteins such as the endonuclease YbeY and the RNA helicase CshA, and potentially others, access to their substrate rRNA. In keeping with this it was observed that over-expressing Era in a ybeY mutant E. coli strain supresses a growth defect and improves 16S rRNA processing in an RNase II, Rnase R and RNase PH-dependent manner, potentially caused by over-recruitment of these exoribonucleases by Era in the absence of YbeY [51].
Cellular levels of rRNA are controlled by the stringent response [43]. Upon activation of the stringent response, transcription from rRNA promoters is reduced (Fig 6C), either by direct binding of ppGpp to the RNA polymerase in Gram negatives, or by tight control of cellular GTP levels in Gram positives [52, 53]. Here we see an additional level of regulation by the stringent response on rRNA. We show a direct interaction between the (p)ppGpp synthetase RelSau and both CshA and Era (Fig 5), and show that RelSau has a relatively small but significant positive effect on the GTPase activity of Era (Fig 6A) and a negative effect on the helicase activity of CshA (Fig 6B). Additionally, we observed that the activation of the stringent response results in increased processing defects in a wildtype strain grown at 25°C (Fig 6D). Era has increased association to the ribosome in the GTP-bound form [44]. Hydrolysis of GTP then acts as a signal to promote dissociation, presumably following a maturation event [54, 55]. The increased GTPase activity of Era in the presence of RelSau could promote premature dissociation of Era from the immature 30S ribosomal subunit, leading to processing defects and contributing to the stalled growth phenotype synonymous with the stringent response. This would be in addition to our previous observation that Era has a higher affinity for ppGpp than GTP [22], which could potentially inhibit the association of the GTPase to the ribosome. All of this would be compounded by the inhibition of helicase activity of CshA, although further research is needed to determine whether any or all of these interactions occur on or off the ribosome.
It is well known that RelSau binds to mature 70S ribosomes and synthesises (p)ppGpp in response to uncharged tRNA docking in the A-site [43]. In addition, a recent paper from Tringuier et al., describes how the accumulation of immature tRNAs with processing defects activates RelBs-dependent production of (p)ppGpp in B. subtilis, leading to the inhibition of 16S rRNA 3' maturation [56]. Here this inhibition is ascribed to decreasing GTP levels upon production of (p)ppGpp, however a direct involvement of (p)ppGpp cannot be ruled out. Our results agree with the reported role for Rel enzymes in rRNA maturation and extend this through the identification of interactions between RelSau and Era/CshA. From this we propose a role for RelSau in controlling immature 30S subunit maturation, in addition to its known function as a sensor of amino acid starvation on mature 70S ribosomes. An interesting area for future study would be to investigate the dynamics of RelSau and Era/CshA interactions in the presence of 30S and 50S subunits, as well as mature 70S ribosomes.
Taken together, we demonstrate a cellular function for Era as a protein important for coordinating 30S ribosomal biogenesis in a stringent response-dependent manner. It is evident that defects in ribosomal assembly lead to altered protein translation, which may be the reason for the plethora of other phenotypes associated with depletion of Era. As Era can complement a defect in RsgA (S4A Fig, [40]), as well as RbfA [26], we propose a broader role for RA-GTPases as hub proteins involved in coordinating biogenesis. It is now of interest to determine what processing events are coordinated by the other RA-GTPases in S. aureus, as well as more broadly in other prokaryotes.
Materials and methods
Bacterial strains and culture conditions
E. coli strains were grown in Luria Bertani broth (LB) and S. aureus strains in tryptic soy broth (TSB) at 37°C or 25°C with aeration. Strains and primers used are listed in S1 and S2 Tables Information on strain and plasmid construction is provided in supplemental S1 protocol.
Luciferase assays
Overnight cultures of S. aureus strains were diluted to an OD600 of 0.05 and grown for 90 min in the presence of 100 ng/ml anhydrotetracycline (Atet). Strains were normalised to an OD600 of 0.1 and luciferase activity measured according to the Nano-Glo Luciferase Assay System Protocol (Promega).
Pull-down experiments and western blotting
Glutathione beads were washed 5 times in 1 x wash solution (25 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100). 1 μM of glutathione-S-transferase (GST), GST-CshA or GST-Era were coupled to the beads by incubating in 1 x wash buffer for 4 h at 4°C. Unbound protein was removed by washing in 1 x wash solution between 5–10 times. Protein-bound beads were incubated with 1 μM His-tagged Era, His-YbeY or His-RelSau in the presence of 1 x binding buffer (25 mM Tris pH 7.5, 150 mM NaCl, 5 mM MgCl2, 0.5% Triton X-100) for 16 h at 4°C. After washing, attached proteins were eluted from the beads with 50 μl elution buffer (25 mM Tris pH 8, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 10 mM reduced glutathione). Samples were mixed 1:1 with 2 x SDS protein sample buffer. Aliquots were separated on 12% SDS-polyacrylamide gels and proteins subsequently transferred to PVDF membranes. Bound His-tagged proteins were detected using HRP-conjugated anti-His antibodies (Sigma) at a 1:500 dilution. Blots were developed by enhanced chemiluminescence and imaged using a ChemiDoc MP imager (Bio-Rad).
Construction of bacterial two-hybrid library
Genomic DNA of S. aureus was extracted and partially digested by incubation with Sau3AI at 37°C for 20 min. Digested DNA was run on a 0.8% agarose gel and fragments of 500 to 1000 bp and 1000 to 3000 bp gel extracted and purified. This was repeated five times from separate genomic preps and DNA fragments pooled. The vector pUT18C was digested overnight at 37°C with BamHI and dephosphorylated with antarctic phosphatase for 90 min. Genomic DNA fragments were ligated into the pUT18C linearized vector using T4 ligase, transformed into E. coli DH5α competent cells (New England Biolabs) and plated onto LB agar with carbenicillin. Plates were scraped and transformant plasmids isolated using the GeneJET plasmid purification kit (Thermo scientific).
Bacterial two-hybrid
Bacterial two hybrid plasmids containing genes of interest were co-transformed into E. coli BTH101 cells, plated on LB agar containing 150 μg/ml carbenicillin and 30 μg/ml kanamycin and incubated at 30°C overnight. Colonies were isolated, grown overnight at 30°C in LB broth containing 0.5 mM IPTG and 5 μl spotted onto LB agar containing 0.5 mM IPTG and 40 μg/ml X-gal. Plates were incubated at 30°C for up to 48 h. In some cases, BTH101 transformants were incubated overnight with 0.5 mM IPTG and spotted directly onto LB agar containing IPTG, X-gal and appropriate antibiotics.
RNA extraction
Strains of S. aureus were grown overnight at 37°C and diluted to an OD600 of 0.05. Cultures were grown to an OD600 of 0.4 at either 37 or 25°C and harvested. RNA was extracted using the RiboPure RNA Purification Kit (Invitrogen) as per guidelines. RNA was visualised using a modified agarose gel containing 0.7% agarose and 0.9% Synergel (Diversified Biotech) in 0.5 X TBE (44.5 mM Tris, 1 mM EDTA pH 8, 44.5 mM boric acid) as per Wachi et al. 1999 [57]. RNA was visualised using EvaGreen fluorescent nucleic acid dye (Biotium) on a ChemiDoc MP imager (Bio-Rad).
RT-PCR
RNA was extracted as described above. Complementary DNA was synthesised from 1.5 μg RNA with transcriptor reverse transcriptase (Sigma) and random primers. RT-PCR was performed on 100 ng of cDNA in triplicate using SYBR Green Jumpstart Taq readymix (Sigma). Primers RMC379/380 (16S internal), RMC570/571 (leader) and RMC572/548 (trailer) were designed to amplify 200–220 bp target regions in the 17S rRNA. The housekeeping gene rho was amplified with primers RMC573/574. The rho transcript was chosen as it has been shown to be highly stable under nutrient-deficient conditions under which the stringent response would be induced [38]. Cycle threshold values were determined for 3 biological repeats in duplicate. For each reaction, the ratio to rho transcript number was calculated as follows: 2-(Ct target–Ct rho).
Growth curves
S. aureus strains were grown overnight in TSB medium containing the appropriate antibiotics. Overnight cultures were diluted to a starting OD600 of 0.05 in the presence of 100 ng/ml Atet where appropriate, incubated at 25°C or 37°C with aeration and OD600 values determined at 2 h intervals. Growth curves were performed three to four times, and averages and standard deviations are plotted.
Minimum Inhibitory Concentrations
Overnight cultures of wildtype LAC* and Δera strains were adjusted to an OD600 of 0.05 in Mueller-Hinton broth and 100 μl incubated in 96 well plates with 2-fold dilutions of various antimicrobials at the following starting concentrations: spectinomycin 250 μg/ml and chloramphenicol 64 μg/ml. Plates were incubated at 37°C overnight with shaking. Assays were performed four times, and averages and standard deviations are plotted.
Protein purifications
Proteins were purified from 1–2 L E. coli cultures. Cultures were grown to an OD600 0.5–0.7, expression induced with 1 mM IPTG and incubated for 3 h at 37°C. Protein purifications were performed by either nickel or glutathione affinity chromatography. For nickel purifications, cell pellets were resuspended in 5 ml Buffer A (50 mM Tris pH 7.5, 150 mM NaCl, 5% glycerol, 10 mM imidazole), lysed with 100 μg lysozyme and sonication and the filtered cell lysate loaded onto a 1 ml HisTrap HP Ni2+ column (GE Healthcare) before elution using a gradient of Buffer B (50 mM Tris pH 7.5, 150 mM NaCl, 5% glycerol, 500 mM imidazole). GST-tagged proteins were resuspended in 5 ml PBS, lysed and the cell lysate was loaded onto a 1 ml GSTrap HP column (GE Healthcare) before elution using 50 mM Tris pH 8.0, 10 mM reduced glutathione. Protein containing fractions were dialysed in 50 mM Tris pH 7.5, 200 mM NaCl, 5% glycerol before storage at -80°C. Protein concentrations were determined by A280 readings.
Synthesis of (p)ppGpp and differential radial capillary action of ligand assay (DRaCALA)
The synthesis of (p)ppGpp and DRaCALA binding assays were performed as described previously [22].
Ribosomal profiles from S. aureus cell extracts
Crude isolations of ribosomes from S. aureus cell extracts were achieved as described by Chin Loh et al. with some modifications [27]. Briefly, 100 ml cultures of the different S. aureus strains were grown to an OD600 of 0.4 in TSB 100 ng/ml Atet. 100 μg/ml chloramphenicol was added to each culture and incubated for 3 min, before being cooled to 4°C to produce runoff ribosomes. Pelleted cells were suspended in association buffer (20 mM Tris-HCl pH 7.6, 8 mM MgCl2, 30 mM NH4Cl and 2 mM β-mercaptoethanol), normalized to an OD600 of 15, lysed by the addition of 0.2 μg/ml lysostaphin and 75 ng/ml DNase and incubated for 60 min at 37°C. The extracts were centrifuged at 17,000 x g for 10 min and subsequently 250 μl were layered onto 10–50% sucrose density gradients made in association buffer. Gradients were centrifuged for 7 h at 192,100 x g. Gradients were fractionated by upwards displacement of 250 μl aliquots, which were analysed for RNA content at an absorbance of 260 nm.
Helicase assay
Reactions were set up as described [58]. Briefly, 250 nM of Cy3-labelled duplex RNA (Cy3-GCUUUACGGUGCUA, AACAAAACAAAAUAGCACCGUAAAGC) purchased from IDT, was incubated with 0.5 μM recombinant protein in reaction buffer (20 mM Tris pH 7.5, 50 mM potassium acetate, 5 mM magnesium acetate, 10 mM DTT, 0.1 mg/ml BSA, 10 U RNasin). The 0.5 μM recombinant protein used is at a concentration similar to that used in the affinity pulldown assays (1 μM) that show an Era-CshA interaction. The reaction was initiated with the addition of 1 mM ATP and incubated at 25°C for 10 min. Reactions were terminated by removing an aliquot at the indicated timepoints mixing with an equal volume of stop buffer (1% SDS, 0.5 mM EDTA, 20% glycerol) and loaded onto 20% native polyacrylamide gels before being electrophoresed in 0.5 x TBE. Bands were imaged using a ChemiDoc MP imager (Bio-Rad).
Nucleotide hydrolysis assays
The ability of proteins to hydrolyse GTP was determined by incubating 100 nM recombinant Era with equimolar YbeY/CshA or RelSau, 100 nM S. aureus ribosomes, 1 μM GTP and 2.78 nM α-32P-GTP in 40 mM Tris pH 7.5, 100 mM NaCl, 10 mM MgCl2 at 37°C for the indicated times. All reactions were set up in the absence of Era as controls. pppGpp hydrolysis assays were performed by incubating 100 nM recombinant RelSau in the presence and absence of equimolar GST, GST-Era or GST-CshA, 1 μM pppGpp and 2.78 nM α-32P-pppGpp in 50 mM Tris pH 8.5, 0.1 mM MnCl2, 20 mM KCl at 37°C for the indicated times. All reactions were set up in the absence of RelSau as controls. Hydrolysis reactions were inactivated with the addition of formic acid to a final concentration of 1.2 M. Precipitated proteins were pelleted by centrifugation at 17,000 x g for 10 min. Reaction products were then visualized by spotting 1 μl on PEI-cellulose thin layer chromatography (TLC) plates (Merck Millipore) and separated using 1 M KH2PO4, pH 3.6 buffer. The radioactive spots were visualised using an LA 7000 Typhoon PhosphorImager and images quantified using ImageJ.
Data provision
All raw data for the above methods are provided in the supplemental raw data file.
Supporting information
The susceptibility of LAC* and Δera to A) spectinomycin and B) chloramphenicol was measured by growing the strains in 96 well plates with the indicated concentration of each antibiotic. OD600 readings were determined after 24 h of growth and plotted as % survival compared to growth without antibiotic. Experiments were repeated four times and mean and standard deviation plotted.
(TIF)
A) The RNA helicase activity of 0.5 μM CshA with and without an equal concentration of Era were determined using a Cy3-labelled double stranded RNA oligomer. Reactions were incubated at 25°C for up to 10 min before analysis on a native page gel. Experiments were repeated three-four times with means and standard deviations shown. Statistical analysis was performed using a two-way ANOVA, followed by Sidak’s multiple comparisons test. B & C) The GTPase activity of 100 nM Era was measured in the presence of an equal amount of ribosomes and 1 μM GTP, plus and minus 100 nM CshA (B) or 100 nM YbeY (C). All reactions contained ribosomes. Reactions lacking Era were included as controls. Hydrolysis of 32P-GTP was monitored by TLC and the percentage GDP formed quantified using ImageJ. Experiments were repeated five times with means and standard deviations shown. Statistical analysis was performed using a two-way ANOVA, followed by Dunnett’s multiple comparisons test.
(TIF)
Growth of S. aureus strains LAC*, LAC* Δera, LAC* ΔcshA and LAC* Δera cshA at A) 37°C and B) 25°C. Overnight cultures were diluted to an OD600 of 0.05 and grown for 24 h. Growth curves were performed in triplicate, with averages and standard deviations shown.
(TIF)
A) Growth of LAC* iTET, LAC* ΔrsgA iTET, LAC* ΔrsgA iTET-rsgA and LAC* ΔrsgA iTET-era at 37°C. B) Growth of LAC* iTET, LAC* Δera iTET and LAC* Δera iTET-rsgA at 37°C. C) Growth of LAC* iTET, LAC* Δera iTET, LAC* Δera iTET-era and LAC* Δera iTET-cshA at 25°C. D) Growth of LAC* iTET, LAC* ΔcshA iTET, LAC* ΔcshA iTET-cshA and LAC* ΔcshA iTET-era at 25°C. E) Growth of LAC* iTET, LAC* ΔcshA iTET, LAC* Δera iTET, LAC* ΔcshA era iTET and LAC* ΔcshA era iTET-era at 37°C. F) Growth of LAC* iTET, LAC* ΔcshA iTET, LAC* Δera iTET, LAC* ΔcshA era iTET and LAC* ΔcshA era iTET-era at 25°C. Overnight cultures were diluted to an OD600 of 0.05 and grown in the presence of 100 ng/ml Atet for 8 h to 24 h at either 37°C or 25°C. Growth curves were performed three to four times, with averages and standard deviations shown.
(TIF)
A) DRaCALA binding assays with recombinant GST, GST-CshA and Era-His and 32P-labelled GTP and ppGpp. Quantification was carried out using ImageJ. The average values and standard deviations of triplicate experiments are plotted. B) Binding curves and Kd determination for 32P-ppGpp and Era in the absence and presence of CshA. C) Hydrolysis activity of RelSau on 32P-pppGpp in the absence and presence of Era and CshA. 100 nM of each protein were incubated with 1 μM pppGpp over the course of 5 min at 37°C before reactions were quenched. Reactions lacking RelSau, or including just GST in place of GST-Era/CshA were included as controls. Experiments were repeated two to four times with means and standard deviations plotted. Statistical analysis was performed using a two-way ANOVA, followed by Dunnett’s multiple comparisons test.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The work was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society, https://wellcome.ac.uk:104110/Z/14/Z to RMC; as well as the University of Sheffield’s 2022 Futures and the MRC DiMeN programs to RMC. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of manuscript.
References
- 1.Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. 10.1006/jmbi.2001.5378 [DOI] [PubMed] [Google Scholar]
- 2.March PE, Lerner CG, Ahnn J, Cui X, Inouye M. The Escherichia coli Ras-like protein (Era) has GTPase activity and is essential for cell growth. Oncogene. 1988;2:539–544. [PubMed] [Google Scholar]
- 3.Inada T, Kawakami K, Chen SM, Takiff HE, Court DL, Nakamura Y. Temperature-sensitive lethal mutant of era, a G protein in Escherichia coli. J Bacteriol. 1989;171:5017–5024. 10.1128/jb.171.9.5017-5024.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takiff HE, Chen SM, Court DL. Genetic analysis of the rnc operon of Escherichia coli. J Bacteriol. 1989;171:2581–2590. 10.1128/jb.171.5.2581-2590.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gollop N, March PE. A GTP-binding protein (Era) has an essential role in growth rate and cell cycle control in Escherichia coli. J Bacteriol. 1991;173:2265–2270. 10.1128/jb.173.7.2265-2270.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson PE, Matsunaga J, Simons EL, Simons RW. Structure and regulation of the Salmonella Typhimurium rnc-era-recO operon. Biochimie. 1996;78:1025–1034. 10.1016/s0300-9084(97)86726-0 [DOI] [PubMed] [Google Scholar]
- 7.Minkovsky N, Zarimani A, Chary VK, Johnstone BH, Powell BS, Torrance PD, et al. Bex, the Bacillus subtilis homolog of the essential Escherichia coli GTPase Era, is required for normal cell division and spore formation. J Bacteriol. 2002;184:6389–6394. 10.1128/JB.184.22.6389-6394.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto T, Loh PC, Hirai T, Asai K, Kobayashi K, Moriya S, et al. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology. 2002;148:3539–3552. 10.1099/00221287-148-11-3539 [DOI] [PubMed] [Google Scholar]
- 9.Lu Q, Inouye M. The gene for 16S rRNA methyltransferase (ksgA) functions as a multicopy suppressor for a cold-sensitive mutant of era, an essential RAS-like GTP-binding protein in Escherichia coli. J Bacteriol. 1998;180:5243–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner CG, Inouye M. Pleiotropic changes resulting from depletion of Era, an essential GTP-binding protein in Escherichia coli. Mol Microbiol. 1991;5:951–957. 10.1111/j.1365-2958.1991.tb00770.x [DOI] [PubMed] [Google Scholar]
- 11.Powell BS, Court DL, Inada T, Nakamura Y, Michotey V, Cui X, et al. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. Enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J Biol Chem. 1995;270:4822–4839. 10.1074/jbc.270.9.4822 [DOI] [PubMed] [Google Scholar]
- 12.Britton RA, Powell BS, Dasgupta S, Sun Q, Margolin W, Lupski JR, et al. Cell cycle arrest in Era GTPase mutants: a potential growth rate-regulated checkpoint in Escherichia coli. Mol Microbiol. 1998;27:739–750. 10.1046/j.1365-2958.1998.00719.x [DOI] [PubMed] [Google Scholar]
- 13.Johnstone BH, Handler AA, Chao DK, Nguyen V, Smith M, Ryu SY, et al. The widely conserved Era G-protein contains an RNA-binding domain required for Era function in vivo. Mol Microbiol. 1999;33:1118–1131. 10.1046/j.1365-2958.1999.01553.x [DOI] [PubMed] [Google Scholar]
- 14.Sharma MR, Barat C, Wilson DN, Booth TM, Kawazoe M, Hori-Takemoto C, et al. Interaction of Era with the 30S ribosomal subunit implications for 30S subunit assembly. Mol Cell. 2005;18:319–329. 10.1016/j.molcel.2005.03.028 [DOI] [PubMed] [Google Scholar]
- 15.Tu C, Zhou X, Tarasov SG, Tropea JE, Austin BP, Waugh DS, et al. The Era GTPase recognizes the GAUCACCUCC sequence and binds helix 45 near the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 2011;108:10156–10161. 10.1073/pnas.1017679108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamaru D, Amikura K, Shimizu Y, Nierhaus KH, Ueda T. Reconstitution of 30S ribosomal subunits in vitro using ribosome biogenesis factors. RNA. 2018;24:1512–1519. 10.1261/rna.065615.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunner AE, Nord S, Wikstrom PM, Williamson JR. The effect of ribosome assembly cofactors on in vitro 30S subunit reconstitution. J Mol Biol. 2010;398:1–7. 10.1016/j.jmb.2010.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercruysse M, Kohrer C, Shen Y, Proulx S, Ghosal A, Davies BW, et al. Identification of YbeY-Protein Interactions Involved in 16S rRNA Maturation and Stress Regulation in Escherichia coli. MBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgardt K, Gilet L, Figaro S, Condon C. The essential nature of YqfG, a YbeY homologue required for 3' maturation of Bacillus subtilis 16S ribosomal RNA is suppressed by deletion of RNase R. Nucleic Acids Res. 2018;46:8605–8615. 10.1093/nar/gky488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemos JA, Lin VK, Nascimento MM, Abranches J, Burne RA. Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol Microbiol. 2007;65:1568–1581. 10.1111/j.1365-2958.2007.05897.x [DOI] [PubMed] [Google Scholar]
- 21.Cassels R, Oliva B, Knowles D. Occurrence of the regulatory nucleotides ppGpp and pppGpp following induction of the stringent response in staphylococci. J Bacteriol. 1995;177:5161–5165. 10.1128/jb.177.17.5161-5165.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corrigan RM, Bellows LE, Wood A, Grundling A. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc Natl Acad Sci U S A. 2016;113:E1710–1719. 10.1073/pnas.1522179113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4:e00537–00512. 10.1128/mBio.00537-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhuri RR, Allen AG, Owen PJ, Shalom G, Stone K, Harrison M, et al. Comprehensive identification of essential Staphylococcus aureus genes using Transposon-Mediated Differential Hybridisation (TMDH). BMC Genomics. 2009;10:291 10.1186/1471-2164-10-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santiago M, Matano LM, Moussa SH, Gilmore MS, Walker S, Meredith TC. A new platform for ultra-high density Staphylococcus aureus transposon libraries. BMC Genomics. 2015;16:252 10.1186/s12864-015-1361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue K, Alsina J, Chen J, Inouye M. Suppression of defective ribosome assembly in a rbfA deletion mutant by overexpression of Era, an essential GTPase in Escherichia coli. Mol Microbiol. 2003;48:1005–1016. 10.1046/j.1365-2958.2003.03475.x [DOI] [PubMed] [Google Scholar]
- 27.Loh PC, Morimoto T, Matsuo Y, Oshima T, Ogasawara N. The GTP-binding protein YqeH participates in biogenesis of the 30S ribosome subunit in Bacillus subtilis. Genes Genet Syst. 2007;82:281–289. [DOI] [PubMed] [Google Scholar]
- 28.Jacob AI, Kohrer C, Davies BW, RajBhandary UL, Walker GC. Conserved bacterial RNase YbeY plays key roles in 70S ribosome quality control and 16S rRNA maturation. Mol Cell. 2013;49:427–438. 10.1016/j.molcel.2012.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira Paiva AM, Friggen AH, Qin L, Douwes R, Dame RT, Smits WK. The Bacterial Chromatin Protein HupA Can Remodel DNA and Associates with the Nucleoid in Clostridium difficile. J Mol Biol. 2019. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Court DL, Ji X. Crystal structure of ERA: a GTPase-dependent cell cycle regulator containing an RNA binding motif. Proc Natl Acad Sci U S A. 1999;96:8396–8401. 10.1073/pnas.96.15.8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier TI, Peery RB, McAllister KA, Zhao G. Era GTPase of Escherichia coli: binding to 16S rRNA and modulation of GTPase activity by RNA and carbohydrates. Microbiology. 2000;146 (Pt 5):1071–1083. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Corvaglia AR, Leo S, Cheung A, Francois P. Characterization of RNA Helicase CshA and Its Role in Protecting mRNAs and Small RNAs of Staphylococcus aureus Strain Newman. Infect Immun. 2016;84:833–844. 10.1128/IAI.01042-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giraud C, Hausmann S, Lemeille S, Prados J, Redder P, Linder P. The C-terminal region of the RNA helicase CshA is required for the interaction with the degradosome and turnover of bulk RNA in the opportunistic pathogen Staphylococcus aureus. RNA Biol. 2015;12:658–674. 10.1080/15476286.2015.1035505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prud'homme-Genereux A, Beran RK, Iost I, Ramey CS, Mackie GA, Simons RW. Physical and functional interactions among RNase E, polynucleotide phosphorylase and the cold-shock protein, CsdA: evidence for a 'cold shock degradosome'. Mol Microbiol. 2004;54:1409–1421. 10.1111/j.1365-2958.2004.04360.x [DOI] [PubMed] [Google Scholar]
- 35.Lehnik-Habrink M, Rempeters L, Kovacs AT, Wrede C, Baierlein C, Krebber H, et al. DEAD-Box RNA helicases in Bacillus subtilis have multiple functions and act independently from each other. J Bacteriol. 2013;195:534–544. 10.1128/JB.01475-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao G, Meier TI, Peery RB, Matsushima P, Skatrud PL. Biochemical and molecular analyses of the C-terminal domain of Era GTPase from Streptococcus pneumoniae. Microbiology. 1999;145 (Pt 4):791–800. [DOI] [PubMed] [Google Scholar]
- 37.Charollais J, Dreyfus M, Iost I. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res. 2004;32:2751–2759. 10.1093/nar/gkh603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sihto HM, Tasara T, Stephan R, Johler S. Validation of reference genes for normalization of qPCR mRNA expression levels in Staphylococcus aureus exposed to osmotic and lactic acid stress conditions encountered during food production and preservation. FEMS Microbiol Lett. 2014;356:134–140. 10.1111/1574-6968.12491 [DOI] [PubMed] [Google Scholar]
- 39.Daigle DM, Brown ED. Studies of the interaction of Escherichia coli YjeQ with the ribosome in vitro. J Bacteriol. 2004;186:1381–1387. 10.1128/JB.186.5.1381-1387.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell TL, Brown ED. Genetic interaction screens with ordered overexpression and deletion clone sets implicate the Escherichia coli GTPase YjeQ in late ribosome biogenesis. J Bacteriol. 2008;190:2537–2545. 10.1128/JB.01744-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gratani FL, Horvatek P, Geiger T, Borisova M, Mayer C, Grin I, et al. Regulation of the opposing (p)ppGpp synthetase and hydrolase activities in a bifunctional RelA/SpoT homologue from Staphylococcus aureus. PLoS Genet. 2018;14:e1007514 10.1371/journal.pgen.1007514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiss S, Pane-Farre J, Fuchs S, Francois P, Liebeke M, Schrenzel J, et al. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob Agents Chemother. 2012;56:787–804. 10.1128/AAC.05363-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cashel M, Gentry D.R., Hernandez V.J., and Vinella D. The stringent response In E. coli and Salmonella: Washington DC: ASM press; 1996. [Google Scholar]
- 44.Sayed A, Matsuyama S, Inouye M. Era, an essential Escherichia coli small G-protein, binds to the 30S ribosomal subunit. Biochem Biophys Res Commun. 1999;264:51–54. 10.1006/bbrc.1999.1471 [DOI] [PubMed] [Google Scholar]
- 45.Wilson DN, Nierhaus KH. The weird and wonderful world of bacterial ribosome regulation. Crit Rev Biochem Mol Biol. 2007;42:187–219. 10.1080/10409230701360843 [DOI] [PubMed] [Google Scholar]
- 46.Gollop N, March PE. Localization of the membrane binding sites of Era in Escherichia coli. Res Microbiol. 1991;142:301–307. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Inouye M. MazG, a nucleoside triphosphate pyrophosphohydrolase, interacts with Era, an essential GTPase in Escherichia coli. J Bacteriol. 2002;184:5323–5329. 10.1128/JB.184.19.5323-5329.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chopade BA, Shankar S, Sundin GW, Mukhopadhyay S, Chakrabarty AM. Characterization of membrane-associated Pseudomonas aeruginosa Ras-like protein Pra, a GTP-binding protein that forms complexes with truncated nucleoside diphosphate kinase and pyruvate kinase to modulate GTP synthesis. J Bacteriol. 1997;179:2181–2188. 10.1128/jb.179.7.2181-2188.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y, Zhang B, Dong K, Zhang X, Hou L, Wang T, et al. Up-regulation of yggG promotes the survival of Escherichia coli cells containing Era-1 mutant protein. FEMS Microbiol Lett. 2007;275:8–15. 10.1111/j.1574-6968.2007.00860.x [DOI] [PubMed] [Google Scholar]
- 50.Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, Canadien V, et al. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature. 2005;433:531–537. 10.1038/nature03239 [DOI] [PubMed] [Google Scholar]
- 51.Ghosal A, Babu VMP, Walker GC. Elevated Levels of Era GTPase Improve Growth, 16S rRNA Processing, and 70S Ribosome Assembly of Escherichia coli Lacking Highly Conserved Multifunctional YbeY Endoribonuclease. J Bacteriol. 2018;200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kriel A, Bittner AN, Kim SH, Liu K, Tehranchi AK, Zou WY, et al. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol Cell. 2012;48:231–241. 10.1016/j.molcel.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross W, Sanchez-Vazquez P, Chen AY, Lee JH, Burgos HL, Gourse RL. ppGpp Binding to a Site at the RNAP-DksA Interface Accounts for Its Dramatic Effects on Transcription Initiation during the Stringent Response. Mol Cell. 2016;62:811–823. 10.1016/j.molcel.2016.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Himeno H, Hanawa-Suetsugu K, Kimura T, Takagi K, Sugiyama W, Shirata S, et al. A novel GTPase activated by the small subunit of ribosome. Nucleic Acids Res. 2004;32:5303–5309. 10.1093/nar/gkh861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Achila D, Gulati M, Jain N, Britton RA. Biochemical characterization of ribosome assembly GTPase RbgA in Bacillus subtilis. J Biol Chem. 2012;287:8417–8423. 10.1074/jbc.M111.331322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trinquier A, Ulmer JE, Gilet L, Figaro S, Hammann P, Kuhn L, et al. tRNA Maturation Defects Lead to Inhibition of rRNA Processing via Synthesis of pppGpp. Mol Cell. 2019;74:1227–1238 10.1016/j.molcel.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 57.Wachi M, Umitsuki G, Shimizu M, Takada A, Nagai K. Escherichia coli cafA gene encodes a novel RNase, designated as RNase G, involved in processing of the 5' end of 16S rRNA. Biochem Biophys Res Commun. 1999;259:483–488. 10.1006/bbrc.1999.0806 [DOI] [PubMed] [Google Scholar]
- 58.Xu L, Wang L, Peng J, Li F, Wu L, Zhang B, et al. Insights into the Structure of Dimeric RNA Helicase CsdA and Indispensable Role of Its C-Terminal Regions. Structure. 2017;25:1795–1808 e1795. 10.1016/j.str.2017.09.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The susceptibility of LAC* and Δera to A) spectinomycin and B) chloramphenicol was measured by growing the strains in 96 well plates with the indicated concentration of each antibiotic. OD600 readings were determined after 24 h of growth and plotted as % survival compared to growth without antibiotic. Experiments were repeated four times and mean and standard deviation plotted.
(TIF)
A) The RNA helicase activity of 0.5 μM CshA with and without an equal concentration of Era were determined using a Cy3-labelled double stranded RNA oligomer. Reactions were incubated at 25°C for up to 10 min before analysis on a native page gel. Experiments were repeated three-four times with means and standard deviations shown. Statistical analysis was performed using a two-way ANOVA, followed by Sidak’s multiple comparisons test. B & C) The GTPase activity of 100 nM Era was measured in the presence of an equal amount of ribosomes and 1 μM GTP, plus and minus 100 nM CshA (B) or 100 nM YbeY (C). All reactions contained ribosomes. Reactions lacking Era were included as controls. Hydrolysis of 32P-GTP was monitored by TLC and the percentage GDP formed quantified using ImageJ. Experiments were repeated five times with means and standard deviations shown. Statistical analysis was performed using a two-way ANOVA, followed by Dunnett’s multiple comparisons test.
(TIF)
Growth of S. aureus strains LAC*, LAC* Δera, LAC* ΔcshA and LAC* Δera cshA at A) 37°C and B) 25°C. Overnight cultures were diluted to an OD600 of 0.05 and grown for 24 h. Growth curves were performed in triplicate, with averages and standard deviations shown.
(TIF)
A) Growth of LAC* iTET, LAC* ΔrsgA iTET, LAC* ΔrsgA iTET-rsgA and LAC* ΔrsgA iTET-era at 37°C. B) Growth of LAC* iTET, LAC* Δera iTET and LAC* Δera iTET-rsgA at 37°C. C) Growth of LAC* iTET, LAC* Δera iTET, LAC* Δera iTET-era and LAC* Δera iTET-cshA at 25°C. D) Growth of LAC* iTET, LAC* ΔcshA iTET, LAC* ΔcshA iTET-cshA and LAC* ΔcshA iTET-era at 25°C. E) Growth of LAC* iTET, LAC* ΔcshA iTET, LAC* Δera iTET, LAC* ΔcshA era iTET and LAC* ΔcshA era iTET-era at 37°C. F) Growth of LAC* iTET, LAC* ΔcshA iTET, LAC* Δera iTET, LAC* ΔcshA era iTET and LAC* ΔcshA era iTET-era at 25°C. Overnight cultures were diluted to an OD600 of 0.05 and grown in the presence of 100 ng/ml Atet for 8 h to 24 h at either 37°C or 25°C. Growth curves were performed three to four times, with averages and standard deviations shown.
(TIF)
A) DRaCALA binding assays with recombinant GST, GST-CshA and Era-His and 32P-labelled GTP and ppGpp. Quantification was carried out using ImageJ. The average values and standard deviations of triplicate experiments are plotted. B) Binding curves and Kd determination for 32P-ppGpp and Era in the absence and presence of CshA. C) Hydrolysis activity of RelSau on 32P-pppGpp in the absence and presence of Era and CshA. 100 nM of each protein were incubated with 1 μM pppGpp over the course of 5 min at 37°C before reactions were quenched. Reactions lacking RelSau, or including just GST in place of GST-Era/CshA were included as controls. Experiments were repeated two to four times with means and standard deviations plotted. Statistical analysis was performed using a two-way ANOVA, followed by Dunnett’s multiple comparisons test.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.