Abstract
Background
Obesity is highly prevalent worldwide. More and more studies have been conducted on the relationship between H. pylori infection and obesity or overweight. But the relationship between them is controversial in the literatures and there is no comprehensive evidence for the correlation.
Aim
To evaluate the prevalence of H. pylori infection in Chinese adult subjects who received routine physical examinations and the relationship between H. pylori and obesity.
Methods
Literatures on H. pylori infection and obesity in Chinese population were searched in online databases. Relevant data were extracted independently by two researchers and meta-analysis was performed by using Review manager 5.3 software.
Results
22 articles were selected with a total sample size of 178033. The pooled prevalence of H. pylori was 42% (95%CI: 37% to 47%) and mean difference of BMI between subjects with and without H. pylori infection was 0.94 (95%CI: -0.04 to 1.91). 9 eligible studies with 27111 subjects were used to calculated pooled OR value because they contained obesity groups. The OR value showed that H. pylori-positive subjects tended to be obese at a risk of 1.20 (95% CI: 1.13 to 1.28).
Conclusion
In China, obesity has association with H. pylori infection. H. pylori infection may be one of the risk factors for obesity.
Introduction
Obesity has become a health problem of global concern and its prevalence is on the rise. The World Health Organization (WHO) classifies people as overweight (25<BMI<30) and obese (BMI>30) according to their body mass index (BMI, kg/m2) which is calculated as one’s body weight (kg) divided by his squared body height (m2) [1]. Another classification for Asian populations was used [overweight: 24<BMI<28, obesity: BMI>28] [2]. A study published in the Lancet reported that the proportion of overweight or obese adults was 37% in men and 38% in women in 2013, and it had increased since 1980 [3]. Meanwhile, the prevalence of obesity in developed country is higher than that in developing country. Although obese people have longer life expectancy than before due to better health care and risk factor management received by them, obesity complications bring them more burden, such as type 2 diabetes, hypertension, chronic kidney disease, fatty liver disease and so on [4–6].
Recently, many studies indicated that Helicobacter pylori (H. pylori, Hp) had relationship with obesity [7, 8]. H. pylori is a gram-negative, bacillus bacterium which colonizes the human stomach and was began to be known to the world since 1984 [9]. H. pylori infected almost half of people worldwide and the number of infected people was 4.4 billion in 2015 [10]. A recent meta-analysis showed that the global prevalence of H. pylori was near 44.3%, and was 42.7% in females while 46.3% in males [10]. The H. pylori infection rate varies in different region which is 50.8% in developing countries while 34.7% in developed country [11]. About one-third of adults are still infected in north European and North American populations, whereas in south and east Europe, South America and Asia, the prevalence of H. pylori is often higher than 50% [12].
This kind of bacterial pathogen is well recognized as one of the main cause of peptic ulcer disease. The organism has also been thought to be a major risk factor for gastric cancer, colon cancer and mucosa-associated lymphoid tissue lymphoma. [13–15] Helicobacter pylori infection usually lasts for life after its first establishing.
Several epidemiological studies have focused on the correlation between H. pylori colonization and BMI and obesity. The results of these studies got contrasting results. Some studies showed that BMI of patients with H. pylori infection was higher than that without [8]. A cohort study in Israel reported H. pylori infection rate was higher in obese subjects than that in normal weight ones [16]. A review showed negative correlation existing between the prevalence of H. pylori and obesity in developed countries [17]. For all we know, the reviews of relationship between H. pylori infection and overweight or obesity among Chinese have not been reported previously. Thus, in the present review, we choose studies reporting Chinese subjects and we aimed: i) to assess the prevalence of H. pylori in China; ii) to examine mean differences in body mass index (BMI) and other factors across groups with or without H. pylori infection and evaluate the risk of H. pylori infection to obesity.
Materials and methods
Search strategy
Six electronic databases including PubMed, EMBASE, Cochrane Library, Web of Science and two Chinese databases, CNKI (China National Knowledge Infrastructure) and WanFang, were searched from their establishment to December 2018 using the following search strategies (Table 1) which included keywords related to H. pylori, obesity and country. The same kind of terms were connected by the Boolean operator “OR” and different kinds of terms were connected by “AND”.
Table 1. Study keywords in search strategy.
| #1 Helicobacter pylori[MeSH Terms]) OR ("Helicobacter pylori"[Title/Abstract] OR "H. pylori"[Title/Abstract] OR "Campylobacter pylori"[Title/Abstract] OR Hp[Title/Abstract] |
| #2 (Obesity[MeSH Terms]) OR (obesity[Title/Abstract] OR obese[Title/Abstract] OR adiposity[Title/Abstract] OR adipose[Title/Abstract] OR overweight[Title/Abstract] OR fatness[Title/Abstract] OR BMI[Title/Abstract] OR "body mass index"[Title/Abstract] OR "weight gain"[Title/Abstract] OR "weight loss"[Title/Abstract] OR "Body weight"[Title/Abstract] OR "body weight changes"[Title/Abstract] OR "over weight"[Title/Abstract]) |
| #3 ((China[MeSH Terms]) OR China[Title/Abstract]) OR Chinese[Title/Abstract] |
| #1 AND #2 AND #3 |
The inclusion criteria were: (1) the subjects of the study were people who underwent physical examinations; (2) the demographic data of the subjects in the literature were complete, and the sample sizes of both the H. pylori-positive group and the H. pylori-negative group, as well as overweight or obesity in each group, or the mean and standard deviation of BMI in each group can be obtained. Studies were excluded if: (1) they were articles such as conferences and reviews; (2) they were about pathological analysis or animal experiments; (3) the samples or contents of them were repeated or very similar to others.
Data abstraction
Two investigators reviewed all literature independently and retrieved studies according to inclusion or exclusion criteria. Any disagreement will be determined by discussion participated by a third investigator. Data extraction was carried out from literatures meeting inclusion criteria, mainly including the following contents: authors, publish year, survey year, survey region and methods to diagnose H. pylori, demographic of subjects, prevalence of H. pylori, number of cases with overweight or obesity or the mean and standard deviation of BMI in population with and without H. pylori infection. The eligibility of relevant studies was evaluated using the cross-sectional study quality evaluation criteria recommended by the Agency for Healthcare Research and Quality (AHRQ) [18]. There are 11 items in the evaluation criteria, including the selection of subjects, quality control and data processing, and the answers are "yes", "no" and "unclear". An item would be scored “0” if it was answered “no” or “unclear”; if it was answered “yes”, then the item scored “1”. Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; high quality = 8-11. The process was independently conducted by two researchers at the same time. In case of disagreement, the dispute shall be discussed and decided by a third investigator.
Statistical analysis
The Review Manager 5.3 software was adopted for meta-analysis of prevalence, mean difference and the odds ratio. The results of continuous data were represented by weighted mean difference (WMD) and 95%CI, and the results of classified data were represented by OR and 95%CI. The heterogeneity between studies was determined by I2 test. If I2 > 50%, the random effect model was adopted; if I2 < 50%, the fixed effect model was adopted. To ensure the stability of meta-analysis results, sensitivity analysis was performed, removing one at a time to compare whether there were significant differences in effect values before and after removal. And the publication bias assessment was conducted on the included literature. If the plot was symmetric, it was considered that there was no publication bias.
Results
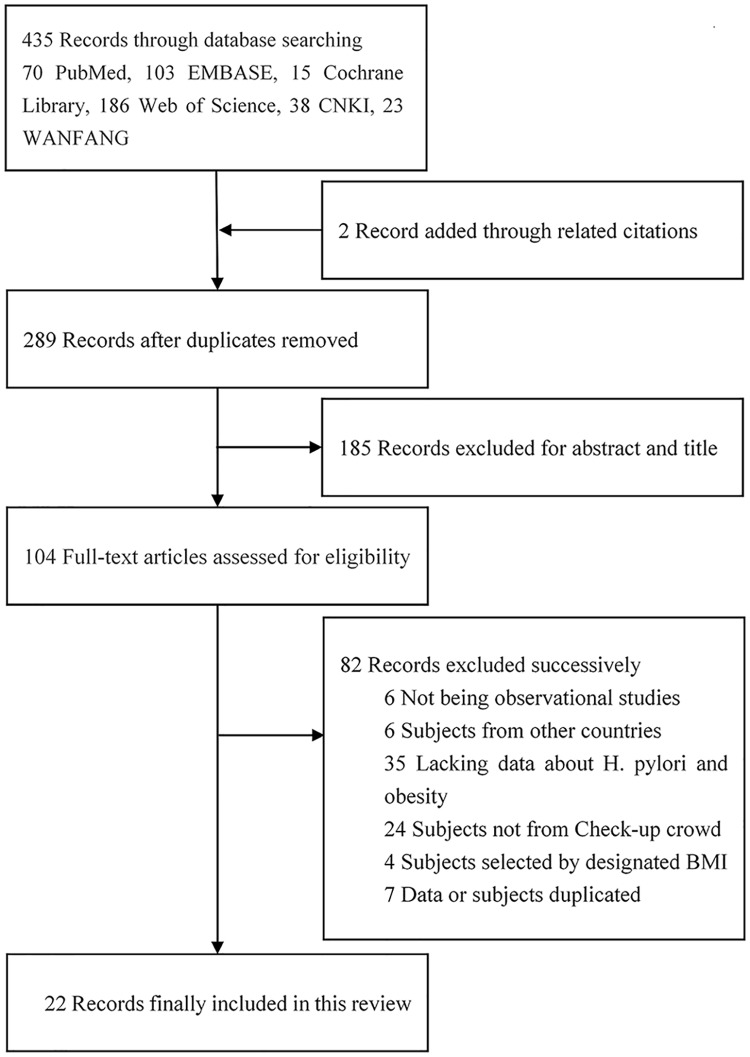
The literature selection process is shown in Fig 1. Of 435 potentially relevant articles (2 articles were added through related citations), 289 relevant articles left after removing duplicates, 185 articles were then excluded due to their titles and abstracts not meeting inclusion criteria. Subsequently, 43 articles were excluded due to the following reasons: not observational studies (n = 6), subjects included did not came from China (n = 6), lacking data about H. pylori and BMI (n = 35), subjects not from Check-up crowd (n = 24), subjects selected by designated BMI (n = 4), data or subjects duplicated (n = 7). Finally, twenty-two observational studies (all cross-sectional studies) met all inclusion criteria. The characteristics and quality assessment of the included cross-sectional studies are presented in Table 2. Totally 178033 subjects involved in this review and 70761 of them infected with H. pylori. Age of studied subjects ranged from 17 years to 91 years or elder age. Test methods of H. pylori infection used in these studies were urea breath test (n = 15), serum diagnosis method through H. pylori-specific IgG antibody (n = 5), rapid urease test (n = 5), biopsy or histology method (n = 1). Stool antigen test was not used in any study.
Fig 1. Literature retrieval flow chart.
Table 2. Characteristics of the different cross-sectional studies.
| Study ID | Survey Year | Method | City | Age | H. pylori prevalence (%) | Related target | Score |
|---|---|---|---|---|---|---|---|
| 01 Wu FJ 2018 [19] | 2016–2017 | UBT | Zhengzhou | 48 | 200/600(0.33) | MS | 7 |
| 02 Wan ZC 2018[20] | 2016 | UBT | Wuhan | 43 | 1687/5168(0.33) | HTN | 9 |
| 03 Wei WZ 2018[21] | 2016 | UBT | Shijiazhuang | 20–91 | 861/1613(0.53) | BL | 8 |
| 04 Wu YH 2017[22] | 2016 | UBT | Qianjiang | ≥18 | 353/823(0.43) | BMI | 8 |
| 05 Gao Y 2018[23] | 2015–2016 | UBT | Wuhan | ≥20 | 2304/6869(0.35) | BL | 8 |
| 06 Chen LW 2018[24] | 2014–2016 | UBT | Taiwan | >30 | 1358/2604(0.52) | obesity | 10 |
| 07 Yang W 2016[25] | 2014 | RUT | Shuozhou | 73.2 | 80/191(0.42) | MS | 9 |
| 08 Li H 2016[26] | 2014 | UBT | Zhejiang | 47 | 3732/8308(0.45) | FLD | 9 |
| 09 Fan N.2018[27] | 2013–2014 | UBT | Shanghai | 48.3 | 17323/28171(0.61) | NAFLD | 9 |
| 10 Kong XL 2017[28] | 2013–2014 | S | Jinan | 48.6 | 4541/22044(0.21) | CKD | 10 |
| 11 Sun Y 2016[29] | 2013–2014 | UBT | Shanghai | 46.1 | 9836/22103(0.45) | BL | 9 |
| 12 Xu CF 2014[30] | 2013 | UBT | Zhejiang | 46 | 3859/8820(0.44) | BMI | 8 |
| 13 Han X 2016[31] | 2013 | UBT | Shiyan | 64 | 15295/30810(0.5) | T2MD | 9 |
| 14 Ma ZH 2013[32] | 2012 | S | Beijing | 48 | 1492/3085(0.48) | BL | 9 |
| 15 Xu MY 2017[33] | 2012–2016 | S | Beijing | 45 | 7804/17791(0.44) | anemia | 9 |
| 16 Song Y 2015[34] | 2011–2013 | UBT | Guiyang | 21–84 | 508/1107(0.46) | BL | 8 |
| 17 Liu A 2013[35] | 2011.1–12 | UBT | Beijing | 47 | 3481/11514(0.3) | HbA1c | 8 |
| 18 Lei YH 2017[36] | 2011 | UBT | Wuhan | 70.5 | 134/427(0.31) | MS | 8 |
| 19 Zhang Y 2015[37] | 2010–2012 | UBT | Wuhan | 52.2 | 839/2050(0.41) | obesity | 9 |
| 20 Yang GH 2014[38] | 2000–2009 | B | Taiwan | ≥60 | 182/324(0.56) | obesity | 10 |
| 21 Ran L 2011[39] | 2009. | S | Chongqing | 21–65 | 651/2188(0.3) | / | 8 |
| 22 Zou D 2011[40] | 2007–2008 | S | Shanghai | 50 | 733/1022(0.72) | GD | 10 |
UBT: urea breath test; RUT: rapid urease test; S: Serology; B: biopsy or histology; BMI: body mass index; FLD: fatty liver disease; NAFLD: Nonalcoholic fatty liver disease; BL: Blood lipid; HTN: hypertension MS: metabolic syndrome HbA1c: glycosylated hemoglobin levels; CKD: chronic kidney disease; GD: gastrointestinal disease.
The prevalence of H. pylori in different subgroups
All included studies were used in the analyses to assess the prevalence of H. pylori. The prevalence of H. pylori among all subjects was 42% (95%CI: 37% to 47%), as shown in Table 3. Subgroup analysis according to test methods and regions was also performed on 22 studies that provided prevalence on adult subjects. The results showed that the pooled detection rate of urea breath test method was higher than that of other methods (serology, biopsy, rapid urease test), and the detection rate of H. pylori in first-tier cities (Beijing, Shanghai) was higher than that in other cities. Stratified analysis was also carried out for subset of those 22 studies which contained stratified information of gender, age and body type. The prevalence of H. pylori in elderly population was higher than it in the middle-aged population and was lowest in the young population. Obese population had highest H. pylori infection rate of 51% (95CI: 42% to 59%) in three body shape groups. After grouping analysis, there was still high heterogeneity, and the data were processed by random effect model. Due to the limited information provided in included literatures, more subgroup analysis were failed to be conducted.
Table 3. Prevalence of H. pylori infection in different subgroups.
| Group | Article N | Total subjects N | Hp(+) N | Prevalence of H. pylori, 95% CI | |
|---|---|---|---|---|---|
| Total | 22 | 178033 | 70761 | 0.42 [0.37, 0.47] | |
| Test method | 22 | 178033 | 70761 | 0.42 [0.37, 0.47] | |
| UBT | 15 | 130987 | 55278 | 0.41 [0.38, 0.45] | |
| Other | 7 | 47046 | 15483 | 0.44 [0.32, 0.56] | |
| Region | 22 | 178033 | 70761 | 0.42 [0.37, 0.47] | |
| First-tier city | 6 | 84087 | 34177 | 0.45 [0.39, 0.51] | |
| Other city | 16 | 93946 | 36584 | 0.41 [0.34, 0.48] | |
| Gender | 18 | 167310 | 66612 | 0.42 [0.38, 0.46] | |
| Male | 18 | 96571 | 38234 | 0.44 [0.38, 0.49] | |
| Female | 18 | 70739 | 28378 | 0.41 [0.35, 0.46] | |
| Age | 5 | 15475 | 5181 | 0.47 [0.38, 0.56] | |
| ≤40 | 3 | 3496 | 1039 | 0.42 [0.24, 0.60] | |
| 40–60 | 3 | 8976 | 3081 | 0.46 [0.24, 0.67] | |
| ≥60 | 5 | 3003 | 1220 | 0.51 [0.32, 0.70] | |
| Body shape | 9 | 42490 | 17354 | 0.47 [0.43, 0.51] | |
| Normal | 8 | 21540 | 8489 | 0.43 [0.37, 0.50] | |
| Overweight | 8 | 15449 | 6497 | 0.47 [0.39, 0.55] | |
| Obese | 9 | 5326 | 2294 | 0.51 [0.42, 0.59] |
Estimated differences in BMI between subjects with and without H. pylori infection
As shown in Table 4, main anthropometric and biochemical characteristics per H. pylori group at baseline were provided in parts of included studies. For original data which presented as median, interquartile, sample mean and standard deviation were estimated from Luo [41], Wan [42]. Mean differences of the physiological and biochemical indexes were estimated, concluded in Table 5. The average HDL-C of the Hp-positive group was lower than that of the H. pylori negative group, but the BMI, age, SBP, DBP, TG, TC and LDL-C of the H. pylori positive group were all higher than that of the negative group. There was still great heterogeneity in the study corresponding to each index.
Table 4. Main anthropometric and biochemical characteristics per H. pylori group at baseline provided in part of included studies.
| Study ID | Age | BMI (kg/m2) |
SBP (mmHg) |
DBP (mmHg) |
TG (mmol/L) |
TC (mmom/L) |
HDL-c (mmom/L) |
LDL-c (mmom/L) |
|
|---|---|---|---|---|---|---|---|---|---|
| 01 | Hp(+) | 50±12.6 | 26.2±2.73 | 138±11 | 86±11 | 2.1±1.31 | 5.1±1.11 | 1.3±0.22 | 3.6±1.12 |
| Hp(-) | 48±13.6 | 23.3±2.21 | 125±12 | 75±10 | 1.1±0.76 | 4.9±1.22 | 1.6±0.21 | 3.1±0.88 | |
| 02 | Hp(+) | 44±11.9 | 24.2±3.34 | 124±18 | 76±12 | 1.5±1.1 | 4.6±0.87 | 1.2±0.3 | 2.8±0.74 |
| Hp(-) | 42±12.1 | 23.6±3.38 | 122±16 | 75±11 | 1.5±1.17 | 4.6±0.87 | 1.2±0.3 | 2.7±0.75 | |
| 03 | Hp(+) | NA | 25.3±4.3 | NA | NA | 1.37±1.1 | 4.83±1.25 | 1.39±0.46 | 2.69±1.05 |
| Hp(-) | NA | 25.4±4.4 | NA | NA | 1.33±1.03 | 4.92±1.21 | 1.43±0.47 | 2.76±0.99 | |
| 07 | Hp(+) | 75±10.8 | 24.3±2.7 | 132±14 | 76±10 | 1.2±0.52 | 4.4±0.88 | NA | NA |
| Hp(-) | 72±11.1 | 23.1±2.74 | 133±13 | 74±8 | 1.3±0.81 | 4.2±1.15 | NA | NA | |
| 08 | Hp(+) | 47±10.8 | 24.1±3.2 | 127±18 | 78±11 | 1.4±0.73 | 4.9±0.92 | 1.1±0.28 | 2.6±0.64 |
| Hp(-) | 47±11.6 | 23.8±3.19 | 127±18 | 77±12 | 1.4±0.79 | 4.8±0.93 | 1.1±0.29 | 2.6±0.63 | |
| 09 | Hp(+) | 48±14.9 | 23.9±3.3 | 128±20 | 74±12 | 1.5±1.2 | 4.8±0.9 | 1.4±0.4 | 3±0.8 |
| Hp(-) | 48±15.1 | 23.5±3.2 | 126±19 | 73±11 | 1.4±1.2 | 4.8±0.9 | 1.5±0.4 | 2.9±0.8 | |
| 10 | Hp(+) | 52±14 | 25.8±3.5 | 133±20 | 80±12 | 1.5±1.15 | 5±0.94 | 1.4±0.25 | 3±0.73 |
| Hp(-) | 48±14.3 | 25.3±3.6 | 130±19 | 78±12 | 1.5±1.25 | 5±0.97 | 1.4±0.25 | 3±0.37 | |
| 11 | Hp(+) | NA | NA | NA | NA | 1.70±1.43 | 5.08±0.975 | 1.31±0.32 | 2.99±0.80 |
| Hp(-) | NA | NA | NA | NA | 1.61±1.32 | 0.06±0.98 | 1.35±0.38 | 2.96±0.79 | |
| 12. | Hp(+) | 46±9.6 | 24±3.3 | 126±17 | 78±11 | 1.4±0.73 | 4.8±0.88 | 1.1±0.27 | 2.6±0.61 |
| Hp(-) | 46±11.1 | 23.7±3.18 | 126±18 | 77±12 | 1.3±0.78 | 4.8±0.88 | 1.1±0.28 | 2.5±0.59 | |
| 13 | Hp(+) | 64±8.6 | 24.3±3.36 | 140±23 | 80±13 | 1.5±1.07 | 4.8±1.12 | 1.5±0.44 | 2.7±0.87 |
| Hp(-) | 65±8.3 | 24.3±3.36 | 139±22 | 79±12 | 1.5±1.06 | 4.7±1.14 | 1.5±0.46 | 2.7±0.89 | |
| 14 | Hp(+) | 50±18.9 | 24.5±3.27 | NA | NA | 5±0.92 | 1.6±1.26 | 1±0.21 | 2.8±0.69 |
| Hp(-) | 47±19.2 | 24.3±3.35 | NA | NA | 4.9±0.92 | 1.5±1.3 | 1±0.22 | 2.7±0.73 | |
| 18 | Hp(+) | 71±7.9 | 24.6±2.87 | 131±16 | 78±10 | 1.9±1.51 | 4.9±0.85 | 1.4±0.37 | 2.7±0.77 |
| Hp(-) | 70±7.4 | 24.1±3.2 | 132±16 | 79±9 | 1.4±0.72 | 4.9±0.85 | 1.6±0.47 | 2.6±0.72 | |
| 19 | Hp(+) | 52±11.3 | 25.3±3.36 | 125±13 | 77±10 | 1.5±0.91 | 4.8±0.88 | 1.2±0.3 | 2.9±0.83 |
| Hp(-) | 53±11.3 | 25±3.04 | 124±13 | 78±11 | 1.5±0.84 | 4.7±0.91 | 1.2±0.29 | 2.8±0.76 | |
| 20 | Hp(+) | 67±5.3 | 25.1±3.6 | NA | NA | NA | NA | NA | NA |
| Hp(-) | 68±5.8 | 24.4±3.3 | NA | NA | NA | NA | NA | NA |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Hp, Helicobacter pylori; N, number. Data are presented as numbers or mean ± standard deviation.
Table 5. Summary of mean differences on anthropometric and biochemical characteristics between Hp (+) and Hp (-) groups.
| Variable | Number of study | Mean difference (95% CI), % | Q Statistics | I2 (%) |
|---|---|---|---|---|
| BMI | 12 | 0.94 [-0.04, 1.91]** | 220 | 95 |
| Age | 12 | 0.60 [0.38, 0.81] | 367 | 97 |
| SBP | 10 | 2.12 [0.83, 3.41] ** | 225 | 96 |
| DBP | 10 | 1.42 [0.70, 2.14] ** | 150 | 94 |
| TG | 11 | 0.10 [0.05, 0.16] ** | 167 | 94 |
| TC | 11 | 0.04 [0.01, 0.07] ** | 33 | 70 |
| HDLC | 10 | -0.06 [-0.09, -0.04] ** | 450 | 98 |
| LDLC | 10 | 0.06 [0.03, 0.09] ** | 75 | 88 |
** p < 0.01 between Hp(+) group and Hp(-) group
Meta-analysis of the impact of H. pylori on obesity
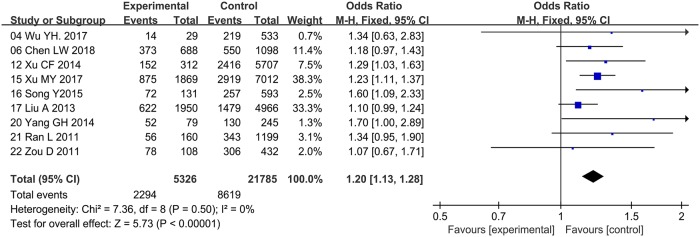
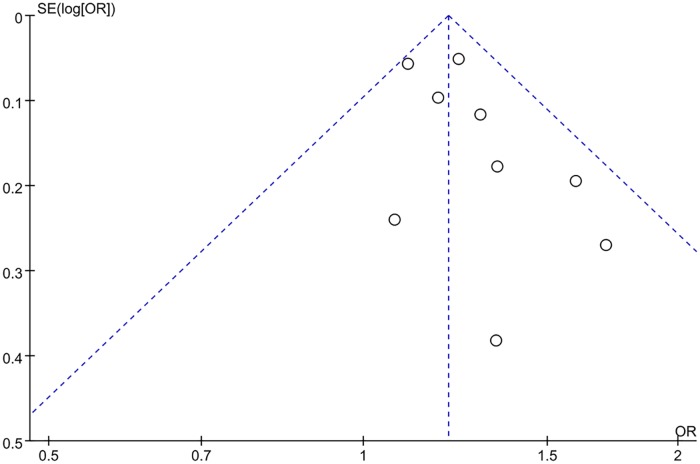
Nine references were included for this meta-analysis, because only in these studies, we could get case number of patients with H. pylori in both obesity and normal weight groups. There was no statistical heterogeneity being found (I2 = 0.0%), so we selected a fixed-effect model for this analysis (Fig 2). The population size, demographic data, and the H. pylori infection rate for this subset can be queried by their name in Table 2. The results showed that the H. pylori infection rate in the obese patients group was lower than that in the normal-weighted control group (OR = 1.20, 95% CI: 1.13 to 1.28). It shows that there is no significant publication bias in this study (see Fig 3 for funnel plot).
Fig 2. Forest plot of the odds ratio with corresponding 95% confidence intervals for H. pylori infection on obese subjects versus normal weight subjects.
Size of squares reflect the statistical weight of each study. Pooled OR value is indicated by an unshaded diamond.
Fig 3. Funnel plot of the association between H. pylori infection and obesity.
Discussion
This study is the first systematic review and meta-analysis about the relationship of H. pylori infection and obesity in China. The estimated prevalence of H. pylori in Chinese adult subjects receiving routine physical examination was 42% (95%CI: 37% to 47%). One comprehensive survey of H. pylori infection from 2002 to 2004 in China reported that the average total infection rate of H. pylori was 56.22% [43]. Among the 22 studies we included, 21 were surveyed after 2007, indicating a decline in H. pylori infection rates in China. Another review also showed a decline in H. pylori infection rates in China up to 19 January 2015. [44] On the other hand, our results also suggests that H. pylori may be one of risk factors for obesity with pooled OR of 1.15 (95% CI: 1.09 to 1.22). Besides, the mean difference of H. pylori-positive BMI was higher than that of negative, and the H. pylori infection rate of obese people was higher than that of normal people, indicating that there was a positive correlation between H. pylori infection and obesity. Compared with H. pylori-negative group, the BMI, systolic blood pressure, diastolic blood pressure, triglycerides, total cholesterol and serum LDL cholesterol of the H. pylori-positive group were all enhanced, and the average HDL cholesterol decreased, which testify to a worse energy metabolism.
The precise mechanism underlying these findings is not well established. There is a number of potential factors which may be involved. (1) Ghrelin and leptin, gastrointestinal hormones, are both involved in metabolic control and energy balance. Ghrelin is produced in the stomach and can stimulate food intake. Leptin has an opposite effect. It was mainly synthesized in adipose tissue [45] and also produced by P cells of the gastric epithelium [46]. Studies reported lower serum leptin levels [47] and lower serum Ghrelin levels in H. pylori-positive patients [47, 48, 49]. Leptin can inhibit eating, and its reduction may be involved in excessive eating and obesity. While the decrease of plasma Ghrelin concentration represented a physiological adaptation to the positive energy balance associated with obesity [50]. (2) Insulin resistance is an important risk factor in lots of common metabolic disorders [51, 52]. H. pylori infection was found to have a potential role in promoting insulin resistance as observed in a Japanese study [53]. So, people with H. pylori infection may be more likely to get obesity. (3) Obesity may interact with H. pylori infection. Recently, growing evidence has implicated the intestinal immune system as an important contributor to metabolic disease including obesity [54]. It has been reported that the ability of monocytes to convert into macrophages was decreased in morbid obesity patients [55], which indicate that immune environment of obese people is more powerful for H. pylori survival. On the other hand, pre-adipocytes could develop phagocytic activity toward microorganisms as macrophage-like cells until they stop proliferating and differentiate into adipocytes [56], which suggests that H. pylori infection may stimulate the growth of adipose tissue to participate in the immune process.
Our result supports a positive relationship between H. pylori infection and obesity. But, studies included in this review are all the cross-sectional studies so this study cannot establish a causal relationship between them. In addition, this study has several limitations. First, studies included are unable to distinguish the H. pylori genotypes. H. pylori type I strains which expressing cagAand vacA are more virulent than type II strains and may bring more effect on metabolism. Studies have shown that the variation of the 3′ Region of the cagA Gene in H. pylori is closely related to the pathological changes and clinical outcomes caused by the infection of the strain [57]. But how these gene differences affect obesity remains unclear. Second, subjects in our study were people who took health examination from urban area, so we may underestimate the prevalence of H. pylori in China. More researches on H. pylori infection and obesity in rural areas are needed. Last, obesity is a chronic disease that is also affected by heredity and lifestyle. There are existing more than one major gene influencing BMI in Chinese sample [58]. In Chinese population, Genetic variation in the FTO gene is strongly associated with obesity and BMI, and its effect size on BMI is comparable with that in the European population [59]. NOC Gene, which is one of circadian clock genes, is also associated with obesity and BMI [60]. Besides, the AC3 genetic polymorphisms are associated with obesity in adults but not in children [61]. So, further studies are needed to control the confounders and to verify or strengthen the association. It would be important to note that the evaluation of the H. pylori infection rate in several subgroups and the calculation of pooled values were not determined by all studies but some subsets. Because only these subsets contains the data needed for the calculation. Notice that there was significant heterogeneity between studies when we compared the mean difference of biochemical characteristics, but hardly any heterogeneity when evaluated the risk of obesity for H. pylori-positive subjects, we thought this was due to the intrinsic properties of the object being analyzed. For the former’s heterogeneities, we considered difference in survey regions, H. pylori test methods, socioeconomic status of subjects, and other factors as sources of them.
H. pylori, a pathogenic bacteria of gastrointestinal diseases, has relationship with many extra-intestinal diseases. However, H. pylori is not on the adverse effects of all diseases. It presents a protective factor in the onset of certain diseases, which may inspire new diagnosis and therapeutic methods for obesity and other diseases.
Supporting information
(DOC)
Acknowledgments
We are grateful to the authors of the original research. We also appreciate the language editing of Yujing Lu, an English professor in Lanzhou University.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study is supported by Lanzhou Innovation and Entrepreneurship Technology Project from Lanzhou Science and Technology Bureau (award number: 2016-RC-24). Grant Recipient is Guowei Yu.
References
- 1.World Health Organization. Body mass index classification [Internet]. [cited 2019 Oct 13]. https://www.who.int/gho/ncd/risk_factors/bmi_text/en/.
- 2.Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. Asia Pacific Journal of Clinical Nutrition 2003. January; 11(s8):S732–S737. 10.1046/j.1440-6047.11.s8.19.x [DOI] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384(9945):766–781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaboration, Asia Pacific Cohort Studies. The burden of overweight and obesity in the Asia–Pacific region. Obesity Reviews An Official Journal of the International Association for the Study of Obesity 2007; 8:191–196. 10.1111/j.1467-789X.2006.00292.x . [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A, Snehalatha C. Rising Burden of Obesity in Asia. Journal of Obesity 2010. August; 2010:1–8. 10.1155/2010/868573 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368(9548):1681–1688. 10.1016/S0140-6736(06)69703-1 . [DOI] [PubMed] [Google Scholar]
- 7.DiBaise JK, Zhang H, Crowell MD, Rosa K, Krajmalnik BR, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clinic proceedings 2008. April; 83(4):460–469. 10.4065/83.4.460 [DOI] [PubMed] [Google Scholar]
- 8.Arslan Erol, Halil Atılgan İrfan Yavaşoğlu. The prevalence of Helicobacter pylori in obese subjects. European Journal of Internal Medicine 2009. November; 20(7):695–697. 10.1016/j.ejim.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Mentis A, Lehours P. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 2015; 20(S1):1–7. 10.1111/hel.12250 [DOI] [PubMed] [Google Scholar]
- 10.Michael MYS, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-analysis. Gastroenterology 2017; 153:420–429. 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 11.Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, et al. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics 2018. April; 47(7):868–876. 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]
- 12.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter 2015; 19(s1):1–5. 10.1111/hel.12165 [DOI] [PubMed] [Google Scholar]
- 13.Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Alimentary Pharmacology & Therapeutics 2010; 11(S1):71–88. 10.1046/j.1365-2036.11.s1.5.x [DOI] [PubMed] [Google Scholar]
- 14.Sonnenberg A, Genta RM. Helicobacter pylori is a Risk Factor for Colonic Neoplasms. The American Journal of Gastroenterology 2013; 108(2):208–215. 10.1038/ajg.2012.407 [DOI] [PubMed] [Google Scholar]
- 15.Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Letters 2011; 305(2):228–238. 10.1016/j.canlet.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamad S, Yaara LW, Doran B, David I, Tsachi TP, Doron C, et al. Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders: a cohort study. European Journal of Gastroenterology & Hepatology; 2017:1–3. 10.1097/meg.0000000000001014 [DOI] [PubMed] [Google Scholar]
- 17.Lender N, Tally NJ, Enck P, Haag S, Zipfel S, Morrison M, et al. Review article: Associations between Helicobacter pylori and obesity—an ecological study. Alimentary Pharmacology & Therapeutics 2014. July; 40(1):24–31. 10.1111/apt.12790 [DOI] [PubMed] [Google Scholar]
- 18.Rostom A, Dube C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US) 2004 Sep; (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. http://www.ncbi.nlm.nih.gov/books/NBK35156
- 19.Wu FJ, Fang LF. [Study of the Correlation Between Metabolic Syndrome and Helicobacter Hylori Infection]. Journal of New Medicine. 2018. March; 28(3):277–279. [Google Scholar]
- 20.Wan ZC, Hu L, Hu M, Lei XM, Huang YC, Lv YM. Helicobacter pylori infection and prevalence of high blood pressure among Chinese adults. Journal of Human Hypertension. 2018. February; 32(2):158–164. 10.1038/s41371-017-0028-8 . [DOI] [PubMed] [Google Scholar]
- 21.Wei WZ, Liu YR, Wei XH, Zhang JH, Gao M, Tian JL. [Relationship between Helicobacter Pylori Infection with Hyperhomocysteinemia and Related Indexes of Lipid Metabolism in Healthy People Taking Medical Examinations]. Medical and Pharmaceutical Journal of Chinese People’s Liberation Army 2018. February; 30(2):31–33. 10.3969/j.issn.2095-140x.2018.02.009 [DOI] [Google Scholar]
- 22.Wu YH, Qi G. [Correlation between helicobacter pylori infection and body mass index in rural communities in Qianjiang, Hubei province.] Journal of Yangtze University (Natural Science Edition). 2017. 14(20):50–51. [Google Scholar]
- 23.Gao Y, Ye B, Me D. [Correlation between helicobacter pylori infection and Blood lipid metabolism]. Medical Journal of Wuhan University. 2018. March; 39(2):291–295. [Google Scholar]
- 24.Chen LW, Kuo SF, Chen C, Chien C, Lin CL, Chien RN. A community-based study on the association between Helicobacter pylori Infection and obesity. Scientific Reports. 2018. July 16; 8(1):10746 10.1038/s41598-018-28792-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Xuan CF. Influence of Helicobacter pylori Infection on Metabolic Syndrome in Old Chinese People. Gastroenterology Research and Practice. 2016. June; 2016:6951264 10.1155/2016/6951264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li HZ, Ma L, Chen J, Zen WL, Jin LM, Xu XH et al. ZJU index is associated with prevalence of Helicobacter pylori infection in a Chinese population. International Journal of Clinical and Experimental Medicine. 2016; 9(11):22324–22330. [Google Scholar]
- 27.Fan N, Peng L, Xia Z, Zhang L, Wang Y, Peng Y. Helicobacter pylori Infection Is Not Associated with Non-alcoholic Fatty Liver Disease: A Cross-Sectional Study in China. Frontiers in Microbiology 2018. January; 9:73 10.3389/fmicb.2018.00073 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong X, Xu D, Li F, Ma X, Su H, Xu D. Association of H. pylori infection with chronic kidney disease among Chinese adults. International Urology and Nephrology. 2017. May;49(5):845–850. 10.1007/s11255-016-1498-2 . [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Fu D, Wang YK, Liu M, Liu XD. Prevalence of Helicobacter pylori infection and its association with lipid profiles. Bratisl Lek Listy. 2016. 117(9):521–524. . [DOI] [PubMed] [Google Scholar]
- 30.Xu CF, Yan M, Sun Y, Joo J, Wan X, Yu C, et al. Prevalence of Helicobacter pylori Infection and its Relation with Body Mass Index in a Chinese Population. Helicobacter. 2014. December; 19(6):437–42. 10.1111/hel.12153 . [DOI] [PubMed] [Google Scholar]
- 31.Han X, Li YR, Wang J, Liu B, Hu H, Li X, et al. Helicobacter pylori infection is associated with type 2 diabetes among a middle- and old-age Chinese population. Diabetes-Metabolism Research and Reviews. 2016. January; 32(1):95–101. 10.1002/dmrr.2677 . [DOI] [PubMed] [Google Scholar]
- 32.Ma ZH, Guo J, Yuan BS, Liu L. [Study on the correlation between helicobacter pylori infection and serum lipid level]. Chin J Clinicians (Electronic Edition), 2013. October; 7(19):8958–8959. [Google Scholar]
- 33.Xu MY, Cao B, Yuan BS, Yin J, Liu L, Lu QB, et al. Association of anaemia with Helicobacter pylori infection: a retrospective study. Scientific Reports. 2017. October; 7:13434 10.1038/s41598-017-13955-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y, Ran LM, Liu YP, Hua YS, Mo L, Wu CW, et al. [Association of Helicobacter pylori infection with lipids levels]. Guizhou Medical Journal, 2015. November; 39(11):973:975. 10.3969/j.issn.1000-744x.2015.11.005 [DOI] [Google Scholar]
- 35.Liu A, Zhu L. [Association between Helicobacter pylori infection and glycosylated hemoglobin levels]. Chinese Journal of General Practitioners. 2013. August; 12(8):643–645. 10.3760/cma.j.issn.1671-7368.2013.08.021 [DOI] [Google Scholar]
- 36.Lei YH, Chen H, Liu J, Zhao SX, Wang F. [Correlation between metabolic syndrome and helicobacter pylori infection in elderly patients]. Chinese Journal of Geriatrics 2017. December; 31(12):1161–1163. [Google Scholar]
- 37.Zhang Y, Du T, Chen X, Yu X, Tu L, Zhang C, et al. Association between Helicobacter pylori infection and overwe ight or obesity in a Chinese population. The Journal of Infection in Developing Countries. 2015. September 27; 9(9):945–953. 10.3855/jidc.6035 . [DOI] [PubMed] [Google Scholar]
- 38.Yang GH, Wu JS, Yang YC, Huang YH, Lu FH, Chang CJ. Obesity Associated with Increased Risk of Gastric Helicobacter Pylori Infection in an Elderly Chinese Population. Journal of the American Geriatrics Society. 2014. January; 62(1):190–192. 10.1111/jgs.12618 . [DOI] [PubMed] [Google Scholar]
- 39.Ran L, Deng XJ, Qu XY, You JR, Wang YH, Luo R. [A Study on the Infection of Helicobacter Pylori among Occupational Population in Chongqing]. Chinese General Practice. 2011. February; 14(2C):589–591. 10.3969/j.issn.1007-9572.2011.06.005 [DOI] [Google Scholar]
- 40.Zou DW, He J, Ma XQ, Liu WB, Chen J, Shi X, et al. Helicobacter pylori infection and gastritis: The Systematic Investigation of gastrointestinal diseases in China (SILC). Journal of Gastroenterology and Hepatology. 2011. May; 26(5):908–915. 10.1111/j.1440-1746.2010.06608.x . [DOI] [PubMed] [Google Scholar]
- 41.Wan X, Wan W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014. December 19; 14:135 10.1186/1471-2288-14-135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo DH, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range and/or mid-quartile range. Statistical Methods in Medical Research 2018. June; 27(6):1785–1805. 10.1177/0962280216669183 . [DOI] [PubMed] [Google Scholar]
- 43.Zhang WD, Hu FL, Xiao SD, Xu ZM. [Prevalence of Helicobacter pylori infection in China]. The Team of Collaboration of Helicobacter pylori research in China] 2010; 15(5):265–270. 10.3969/j.issn.1672-2159.2010.05.001 [DOI] [Google Scholar]
- 44.Peter N, Johansson S, Michael MB. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathogens 2016; 8:8 10.1186/s13099-016-0091-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahima RS, Flier JS. Leptin. Annual Review of Physiology 2000; 62:413–37. 10.1146/annurev.physiol.62.1.413 . [DOI] [PubMed] [Google Scholar]
- 46.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature 1998. August 20; 394(6695):790–3. 10.1038/29547 . [DOI] [PubMed] [Google Scholar]
- 47.Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ, et al. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. Journal of Clinical Endocrinology & Metabolism 2008. June; 93(6):2350–7. 10.1210/jc.2007-2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, et al. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. Journal of Clinical Endocrinology & Metabolism 2005. January; 90(1):10–6. 10.1210/jc.2004-1330 . [DOI] [PubMed] [Google Scholar]
- 49.Hajime I, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, et al. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. American Journal of Gastroenterology 2005; 100(8):1711–1720. [DOI] [PubMed] [Google Scholar]
- 50.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating Ghrelin Levels Are Decreased in Human Obesity. Diabetes 2001; 50(4):707–709. 10.2337/diabetes.50.4.707 [DOI] [PubMed] [Google Scholar]
- 51.Reaven GM. Role of insulin resistance in human disease. Diabetes 1988; 32(12):1595–1606. [DOI] [PubMed] [Google Scholar]
- 52.Defronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Netherlands Journal of Medicine 1997. May; 50(5):191–197. 10.1016/s0300-2977(97)00012-0 . [DOI] [PubMed] [Google Scholar]
- 53.Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, et al. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter 2009. October; 14(5):144–50. 10.1111/j.1523-5378.2009.00705.x . [DOI] [PubMed] [Google Scholar]
- 54.Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metabolism 2016. March 8; 23(3):413–26. 10.1016/j.cmet.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 55.Arslan E, Atilgan H, Yavaşoğlu I. The prevalence of Helicobacter pylori in obese subjects. Eur J Intern Med. 2009; 20:695–697. 10.1016/j.ejim.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 56.Cousin B, Munoz O, Andre M, Fontanilles Am, Dani C, Cousin JL, et al. A role for preadipocytes as macrophage-like cells. The FASEB Journal 1999. February; 13(2):305–12. 10.1096/fasebj.13.2.305 . [DOI] [PubMed] [Google Scholar]
- 57.Azuma T, Yamakawa A, Yamazaki S, Fukuta K, Ohtani M, Ito Y, et al. Correlation between Variation of the 3′Region of the cagA Gene in Helicobacter pylori and Disease Outcome in Japan. Journal of Infectious Diseases, 2002. December; 186(11):1621–1630. 10.1086/345374 [DOI] [PubMed] [Google Scholar]
- 58.Liu PY, Li YM, Li MX, Malkin I, Qin YJ, Chen XD, et al. Lack of Evidence for a Major Gene in the Mendelian Transmission of BMI in Chinese. Obesity research, 2004. December; 12(12):1967–1973. 10.1038/oby.2004.247 [DOI] [PubMed] [Google Scholar]
- 59.Chang YC, Liu PH, Lee WJ, Chang TJ, Jiang YD, Li HY, et al. Common Variation in the Fat Mass and Obesity-Associated (FTO) Gene Confers Risk of Obesity and Modulates BMI in the Chinese Population. Diabetes, 2008. August; 57(8):2245–2252. 10.2337/db08-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang YC, Chiu YF, Liu PH, Hee SW, Chang TJ, Jiang YD, et al. Genetic Variation in the NOC Gene Is Associated with Body Mass Index in Chinese Subjects. PLoS One, 2013. July; 8(7):e69622 10.1371/journal.pone.0069622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Wu M, Zhu W, Shen J, Shi X, Yang J, et al. Evaluation of the association between the AC3 genetic polymorphisms and obesity in a Chinese Han population. PLoS One. 2010. November; 5(11):e13851 10.1371/journal.pone.0013851 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.