Abstract
To identify novel loci that affect cognitive decline in older adults free of dementia, we conducted genome-wide and gene-based meta-analyses on longitudinal slopes of five cognitive domains (memory, executive function, language, attention/processing speed and visuospatial ability) derived from two population-based cohorts. For decline over time in each cognitive domain, we normalized intra-individual slopes within each cohort, accounting for baseline age, sex and years of education. Normalized slope for each domain was used in cohort-specific genome-wide analyses after including top principal components as covariates followed by genome-wide and gene-based meta-analyses. Both analyses revealed a novel WDFY2 locus at genome-wide (p= 3.37E-08) and gene-wide (p= 7.10E-07) significance levels for the attention/processing speed domain. In the GTEx eQTL analysis, genome-wide significant SNP was associated with RNA expression levels of WDFY2 in several brain regions: cerebellar hemisphere (p= 1.07E-04), cerebellum (p=6.92E-04), hippocampus (p=2.18E-03) and cortex (p= 2.29E-02), and in whole blood (p= 4.41E-05). Our results suggest that WDFY2 genetic variation may affect individual differences in decline over time on tests of attention/processing speed.
Keywords: Cognitive domains, Cognitive decline, genome-wide association, gene-based association
1. Introduction
Cognitive function is an important predictor and determinant of quality of life, especially in old age. General or global cognitive function is derived from multiple theoretical, but moderately correlated cognitive domains (memory, attention, executive function, language, visuospatial skill, processing speed etc.) Interindividual differences in cognitive abilities over the lifespan are likely to have a significant genetic component, as reflected by high heritability estimates (>50%) for both general cognitive ability and major cognitive domains (Harris and Deary, 2011; Polderman et al., 2015; Polmin and Deary 2015).
In order to dissect the genetic component of cognitive function, early studies focused on APOE, an established risk factor for Alzheimer’s disease (AD), and found association of APOE*4 with poor performance on cognitive tests, especially in the memory domain in majority of the studies (Wisdom et al., 2011; Reitz and Mayeux, 2010), albeit not meeting the current standard of genome-wide significance threshold (p<5E-08). Early genome-wide association studies (GWAS) that largely examined general cognitive function as the phenotype also failed to detect genome-wide significant associations despite using large sample sizes (Davies et al., 2011; Lencz et al., 2014; Benyamin et al., 2014). However, recent GWAS on even larger datasets have found multiple genome-wide significant loci for general cognitive function (Davies et al., 2015; Davies et al., 2016; Trampush et al., 2015) and for some specific cognitive domains (Debette et al., 2015; Ibrahim-Verbaas et al., 2016). A recent GWAS meta-analysis on more than 300,000 subjects identified 148 loci for general cognitive function that explained 4.3% of variance in general cognitive function (Davies et al., 2018). Although this number of loci seems high, an equally powered GWAS meta-analysis on more than 250,000 individuals identified 423 loci for human height that explained 16% of the variance in adult height (Wood et al., 2015). This highlights the complexity of cognitive function and challenges resulting from the use of substantially different cognitive tests to construct a general cognitive function phenotype in different studies.
The general or global cognition phenotype may be derived from multiple cognitive domains, where each domain is derived from different cognitive tests. A longitudinal study on the effect of aging on cognitive abilities found that cognitive aging is characterized at all three levels, where 39% of the effect of age was on general cognitive function, 33% at the domains level and 28% at the tests level (Tucker-Drob, 2011). Thus, genetic studies focusing on only a general cognitive function phenotype may not fully characterize the genetic architecture of cognitive function. In the genetics of cognitive decline in aging, investigating specific domains as cognitive endophenotypes may be more informative than collapsing different domains under a unitary construct of global cognitive decline, as different age-associated diseases have various cognitive profiles of impairment and decline (Salmon and Bondi, 2009; Lezak et al., 2012)
In this study, we used longitudinal data on five cognitive domains (memory, executive function, language, attention/processing speed, and visuospatial ability) from two population-based cohorts and conducted GWAS meta-analyses to: a) examine the association of previously reported AD loci with cognitive decline over time, and b) identify novel loci that affect decline in cognitive function across different cognitive domains.
2. Materials and methods
2.1. Sample description
All subjects provided written informed consent and all study procedures were approved by the University of Pittsburgh Institutional Review Boards. Descriptive summary statistics of the two samples are provided in Table 1.
Table 1.
Sample characteristics of the Monongahela-Youghiogheny Health Aging Team (MYHAT) and Monongahela Valley Independent Elders Survey (MoVIES) cohorts
| MYHAT (n=767) | MoVIES (n=378) | |
|---|---|---|
| Age (mean (SD)) | 77.1 (7.3) | 70.1 (4.3) |
| Sex - Female (n(%)) | 464 (60.5) | 254 (67.2) |
| Race - White (n(%)) | 767 (100.0) | 378 (100.0) |
| Years education (mean (SD)) | 13.0 (2.5) | 11.8 (2.3) |
| CDR at baseline | ||
| 0 (normal) | 588 (76.7) | 378 (100.0) |
| 0.5 (mild cognitive impairment) | 179 (23.3) | 0 (0.0) |
2.1.1. Monongahela-Youghiogheny Healthy Aging Team (MYHAT)
MYHAT is an ongoing population-based cohort study based in a region of southwestern Pennsylvania as described previously (Ganguli et al., 2009). From 2006 to 2008, an age-stratified random sample of participants aged 65 years or older was recruited from publicly available voter registration lists. Recruitment criteria included being age 65 or older, living within the selected area, and not living in a long-term care institution. Participants were excluded if they were too ill to participate, had severe hearing or vision impairment, or were decisionally incapacitated. Of 2,036 original participants, 54 were excluded due to substantial baseline cognitive impairment (age-education corrected MMSE of less than 21 out of 30), yielding a sample of 1,982 participants who underwent the full baseline assessment. These participants subsequently underwent annual assessments and had been followed for a maximum of 6 years, or 7 total assessments, at the time of this report. Of the 1982 participants, 906 consented to genotyping, which was done from whole-blood samples. This group did not differ significantly in age, sex, or education from the 204 participants who did not provide DNA. Thirty-one genotyped participants of nonwhite race were excluded from these analyses to prevent confounding by race. A further 8 genotyped participants who had a baseline Clinical Dementia Rating (CDR) of 1.0 or higher, reflecting at least mild dementia, were excluded. Of the remaining participants, 100 were excluded because they lacked any follow-up beyond the baseline assessment, which would be required to quantify cognitive decline. Thus, the final sample size for MYHAT was 767. Mean length of cognitive follow-up for MYHAT was 4.68 years (SD = 1.79, range = 1 to 6 years).
2.1.2. Monongahela Valley Independent Elders Survey (MoVIES)
MoVIES was a population-based cohort study based in an adjoining area of southwestern Pennsylvania (Ganguli et al., 1993). From 1987 to 1989, an age-stratified random sample of participants aged 65 years or older was recruited from publicly available voter registration lists. Recruitment criteria included being age 65 or older, not living in a long-term care institution, fluency in English, not having severe vision or hearing impairment, and at least a sixth-grade education. A total of 1,424 participants were randomly recruited, and an additional 259 volunteers meeting the same inclusion criteria yielded a total MoVIES sample size of 1,683. Participants underwent assessments every two years, on average, and were followed for a maximum of 12 years, or 7 total assessments. Of the original 1,683 participants in 1987–89, we genotyped 887 white individuals who were still alive, participating, and not in nursing homes in 1994, when funding was received for APOE genotyping. Of the specimens, 88% were genotyped using whole blood venipuncture specimens, and 12% using dried blood specimens from finger stick. The 904 MoVIES participants from whom DNA was obtained for APOE genotyping were slightly but significantly younger (mean + SD ages: 71.4 ± 4.9 vs. 74.8 ± 6.5 years), more likely to be female (63.8% vs. 50.7%), and more educated (mean ± SD: 11.3 ± 2.5 vs. 10.8 ± 2.8 years) than the 779 from whom DNA was not obtained (all p < 0.001). Of those who were genotyped for APOE, the 379 participants who provided sufficient DNA for genome-wide genotyping were also slightly but significantly younger (mean age ± SD: 70.1 ± 4.3 vs. 72.3 ± 5.1 years) and better educated (mean ± SD: 11.8 ± 2.3 vs. 10.9 ± 2.5 years) than the 525 whose DNA specimens were insufficient (all p<0.001). For the present study, we had available these 379 MoVIES participants, with CDR=0 throughout the course of the study, whom we had previously genotyped for an AD case-control GWAS (Kamboh et al., 2012). We excluded one genotyped individual because she did not provide neuropsychological data after her baseline assessment. Thus, the final sample size for MoVIES was 378. Mean length of cognitive follow-up for MoVIES was 9.91 years (SD = 2.07, range = 2 to 12 years).
2.2. Neuropsychological assessments
Neuropsychological assessment tests were grouped into five cognitive domains on a theoretical basis, including attention/processing speed, executive function, language, memory, and visuospatial ability, as shown in Table 2.
Table 2.
Neuropsychological Tests done in Monongahela-Youghiogheny Health Aging Team (MYHAT) and Monongahela Valley Independent Elders Survey (MoVIES) cohorts
| Neuropsychological Tests | ||
|---|---|---|
| Cognitive domain | MYHAT | MoVIES |
| Attention/processing speed | Trailmaking Test A | Trailmaking Test A |
| Digit Span Forward | ||
| Executive function | Trailmaking Test B | Trailmaking Test B |
| Initial Letter Fluency | Initial Letter Fluency | |
| Clock Drawing Test | Clock Drawing Test | |
| Language | Boston Naming Test | Boston Naming Test |
| Animal Fluency | Animal Fluency | |
| Token Test | ||
| Memory | Story Immediate Recall | Story Immediate Recall |
| Story Delayed Recall | Story Delayed Recall | |
| Visual Reproduction Immediate Recall | Word List Learning | |
| Visual Reproduction Delayed Recall | Word List Delayed Recall | |
| Fuld Object Memory Evaluation | ||
| Visuospatial skill | Block Design | Constructional Praxis |
2.3. Cognitive slopes normalization
Cognitive Domain Composites: In each cognitive domain, z-scores were created by first standardizing each test score according to the sample baseline mean and standard deviation, and then averaging the standardized test scores within each domain for participants with at least one non-missing test score in that domain. Global z-scores were created by averaging all of the standardized test scores for participants who were not missing more than one test score.
2.3.1. Cognitive Decline Slopes
To create the cognitive decline phenotypes used in GWAS, we extracted age, sex, and education-adjusted person-specific slopes of cognitive domain z-scores, using a procedure similar to that reported in De Jager et al (2012). For MYHAT and MoVIES samples separately, a longitudinal linear mixed model was fit for each of the cognitive domains and for the global score. Age, sex, years of education were included as covariates, both as main effects and in interactions with time. A random intercept and random slope were included in the model. Since the estimated person-specific slope distributions were left-skewed due to a few participants who showed more rapid cognitive decline, we rescaled the slopes so they conformed to a normal distribution (Peng et al., 2007). We first ranked the slope values, then scaled the ranks to the interval [0.1, 0.99], and finally transformed the scaled ranks to a standard normal distribution using the inverse standard normal cumulative distribution function (qnorm in R).
2.4. Genotyping, imputation and quality control
Genome-wide genotyping was carried out using the Omni1-Quad chip in the MoVIES sample (Kamboh et al., 2012) and Illumina Omni2.5 chip in the MYHAT sample. APOE genotyping was performed as described previously (Kamboh et al., 2012). Imputation of non-genotyped single-nucleotide polymorphisms (SNPs) was performed with IMPUTE2 (Howie et al., 2009) using the 1000 Genomes Project Phase III (May 2013 release) data as the reference panel. As part of the quality control, SNPs with imputation info score <0.5, minor allele frequency (MAF) <0.01, P<1E-06 in the Hardy Weinberg equilibrium test and the missing rate >5% were removed along with insertions and deletions. After quality control measures, 5.6 million genotyped and imputed SNPs were included in the GWAS analysis. Genetic association analyses were conducted on normalized slope for each domain after including first four principal components (PCs) calculated using smartPCA (Price et al.,2006).
2.5. Meta-analysis
METAL (Willer et al., 2010) software was used to perform meta-analysis on the two GWAS of normalized slopes for each domain. The summary effect size was calculated by averaging the study-specific effect sizes, with weights reflecting the standard errors from the study-specific effect sizes. The standard threshold of p<5E-08 statistical significance for genome-wide analyses was used.
2.6. Gene-based analysis
Gene-based analysis was conducted using MAGMA (de Leeuw et al., 2015) by inputting the SNP data from the meta-analysis. Input SNPs were mapped to 18,440 protein coding genes. A gene-wide significance threshold for gene-based association was used as p=2.71E-06 (0.05/18,440).
2.7. Functional annotations
We performed the following analyses in order to evaluate the biological significance of statistically significant signals.
Differentially expressed genes: We searched for differentially expressed genes using gene expression data from AlzBase (http://alz.big.ac.cn/alzBase/) that includes transcription data from brain and blood from participants without dementia, and with mild cognitive impairment, early stage AD, and late stage AD subjects.
2.7.1. Human brain gene expression
We evaluated the expression level of top genes in human brain tissues from the Barres Human and Mouse Brain RNA-Seq Resource (http://www.brainrnaseq.org/).
2.7.2. Expression quantitative trait loci (eQTL) analysis
We first identified variants in linkage disequilibrium (LD) (R2 ≥ 0.8) with the genome-wide significant SNPs listed in Table 3. The SNiPA website (https://snipa.helmholtz-muenchen.de/snipa3/) was used to search for variants in LD, using the 1000 Genomes, Phase 3v5 variant set for the European population. We then searched the list of variants for genes functionally linked via eQTLs to our expanded list of variants. Finally, we searched the Genotype-Tissue Expression (GTEx) database (https://gtexportal.org/home/) for eQTL associations in various brain tissues and whole blood.
Table 3:
Genome-wide significant SNPs (p<5E-08) form the meta-analysis in the attention/processing speed domain
| SNP | CHR | BP | A1 | A2 | Region | GENE | MAF MYHAT | MAF MoVIES | MAF Meta | LD** | BETA Meta | P-value Meta | BETA MYHAT | p-value MYHAT | BETA MoVIES | p-value MoVIES |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs17532412 | 13 | 52308103 | A | C | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 3.37E-08 | 0.21 | 7.57E-04 | 0.49 | 1.47E-06 |
| rs9535744 | 13 | 52311481 | C | G | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 3.37E-08 | 0.21 | 7.57E-04 | 0.49 | 1.47E-06 |
| rs9535749 | 13 | 52328844 | A | T | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 3.37E-08 | 0.21 | 7.57E-04 | 0.49 | 1.47E-06 |
| *rs9535753 | 13 | 52335201 | C | T | UTR3 | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 3.37E-08 | 0.21 | 7.57E-04 | 0.49 | 1.47E-06 |
| rs17532524 | 13 | 52316599 | A | G | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 0.99 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs17532936 | 13 | 52332745 | G | A | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| *rs2296029 | 13 | 52335765 | T | C | UTR3 | WDFY2 | 0.18 | 0.15 | 0.17 | 0.99 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs73199746 | 13 | 52323602 | A | T | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9526793 | 13 | 52327020 | A | G | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 0.99 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9526794 | 13 | 52330268 | C | T | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9535743 | 13 | 52309076 | T | C | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9535745 | 13 | 52312297 | G | A | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 0.99 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9535747 | 13 | 52322184 | G | C | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 0.99 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9535750 | 13 | 52330240 | C | G | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9535751 | 13 | 52333057 | T | C | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9535752 | 13 | 52333283 | A | C | intronic | WDFY2 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
| rs9535756 | 13 | 52339750 | G | C | intergenic | WDFY2, DHRS12 | 0.18 | 0.15 | 0.17 | 1.00 | 0.28 | 4.78E-08 | 0.21 | 6.66E-04 | 0.47 | 3.21E-06 |
Bold font indicates genotyped SNPs
Linkage disequilibrium (LD) with rs9535753
MAF: minor allele frequency which is denoted by A1
3. Results
3.1. Sample characteristics
The main characteristics of the two study populations are shown in Table 1 and neuropsychological assessment tests performed within each domain are listed in Table 2. Pearson correlation between the five cognitive domains was low to moderate (range r= 0.03 to 0.66) in both the MYHAT and MoVIES samples (Supplementary Figure S1).
3.2. Association of APOE and other known AD loci
As APOE*4 is an established risk factor for AD and is associated with poor performance on cognitive testing in older subjects, especially in the memory domain, we first examined its association (Supplementary Table S1) along with other known risk loci for AD with each domain. As expected, APOE*4 showed the most significant association in meta-analysis with memory decline (p= 7.18E-06; β= −0.29), followed by language (p= 1.65E–04; β= −0.24) and executive function (p= 1.11E-03; β= −0.21) decline. However, no association of APOE*4 was observed with decline in visuospatial function or attention domains.
Since the causative genes in other AD loci are unknown, we examined regional association around the top IGAP (International Genomic Alzheimer Project) significant SNP within each region (Efthymiou et al., 2017) and the results are presented in Supplementary Tables S2.1–S2.6. The top SNP within each region in a given domain with nominal p<0.001 is highlighted. Two loci showed associations with more than one domain, including EPHA1 with visuospatial (p= 1.24E-05) and memory (p= 5E-04), and ABCA7 with executive function (p= 3E-04), language (p= 4E-04), attention (p= 5E-04) and visuospatial (p= 7E-04).
3.3. Genome-wide analysis
Next we examined the entire GWAS data, along with APOE and other AD loci in order to identify novel signals for cognitive domains. Quantile-quantile (QQ) plots and lambda values for the meta-analysis for each domain showed that the combined results from meta-analysis were not inflated in their test statistics (Supplementary Figure 2). Genome-wide p-values for each domain are shown in Manhattan plots in Supplementary Figures S3.1–S3.6. Overall, meta p-values for the top SNPs in a specific domain were more significant than the corresponding meta p-values in the global cognitive domain (Supplementary Tables 3.1–3.5).
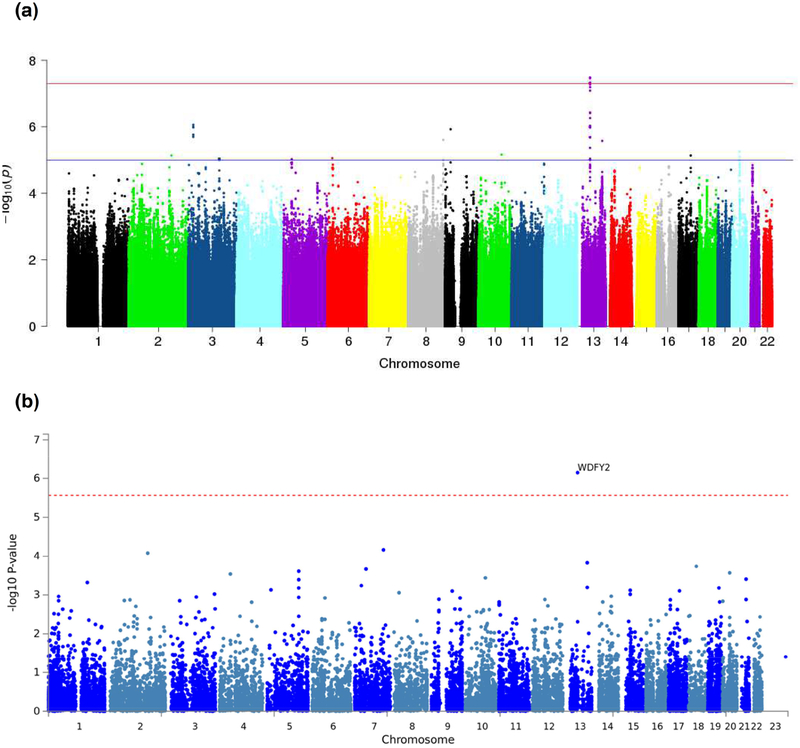
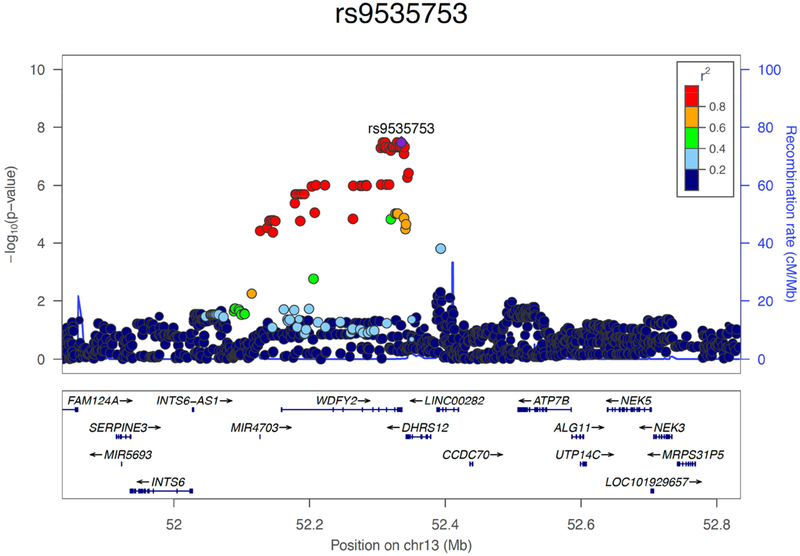
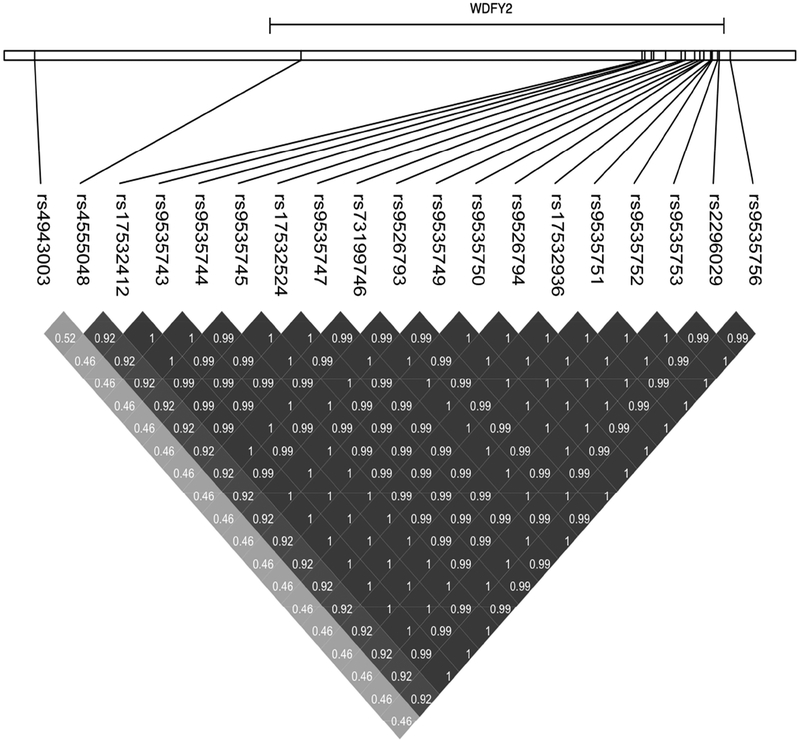
A genome-wide significant association was observed for decline in the attention domain on chromosome 13 in the WDFY2 gene (Figure 1a) where multiple SNPs showed identical p-values passing the genome-wide significant threshold of p<5E-08 (Table 3; Figure 2). Among the top 4 SNPs having identical p= 3.37E-08, one located in 3’UTR of WDFY2 (rs9535753T/C) was genotyped and the remaining were imputed. Of the next 13 SNPs with identical p= 4.78E-08, only one was genotyped and it was also located in 3’UTR (Table 3). Although almost complete LD between these SNPs (Figure 3) makes it difficult to ascertain which one is driving the association, for our discussion purposes here we have denoted rs9535753 as the sentinel SNP because it was genotyped, has a potential functional significance given that it is located in 3’UTR, and it was among those with the lowest p-value.
Figure 1:
(a) Manhattan plot showing the p-values of SNPs form genome-wide meta-analysis for the attention/processing speed domain. The red line represents the genome-wide significance threshold (p= 5E-08), and the blue line represents the suggestive significance threshold (p= 1E-05). (b) Manhattan plot showing the p-values of gene-based meta-analysis for the attention/processing speed domain. The red dotted line represents the gene-wide significance threshold (p= 2.71E-06).
Figure 2:
Regional plot of the WDFY2 region on chromosome 13 in the meta-analysis of the attention/processing speed domain. The relative location of genes and the direction of transcription are shown in the lower portion of the figure, and the chromosomal position is shown on the x -axis. The light blue line shows the recombination rate across the region (right y -axis), and the left y axis shows the significance of the associations. The purple diamond shows the p-value for rs9535753 (p= 3.37E-08) that is among the most significant SNPs in the meta-analysis. The circles show the p-values for all other SNPs and are color coded according to the level of LD with rs9535753 in the 1000 Genome Project EUR population.
Figure 3:
Linkage disequilibrium (LD) pattern of WDFY2 genome-wide significant SNPs (p<5E-08) for attention/processing speed as shown in Table 3, along with two SNPs (rs49rs4555048 and rs4943000 at far left) that are eQTLs for WDFY2 in AlzBase database.
The effect (minor) C allele of rs9535753 was associated with slower decline in attention over time (β=0.28) as compared to the common T allele. In addition to its genome-wide significance with attention, rs9535753 (and those in complete LD with this) also showed association with decline in executive function (p= 1.94E-05; β= 0.22), but not with other domains. (Supplementary Table S4).
3.4. Gene-based analysis
Gene-based analysis on SNPs derived from meta-analysis was performed using MAGMA that uses a multiple regression approach to properly incorporate LD between markers and to detect multi-marker effects. A gene-wide significance signal was seen for the attention domain, also implicating the WDFY2 gene (p= 7.10E-07) on chromosome 13 (Figure 1b, Table 4). WDFY2 was also the top gene for executive function (p= 2.12E-05; Table 4).
Table 4:
Result of gene-based analysis showing the top genes (p<1E-04) for each domain
| Domain | ENSG # | Gene | CHR | START | END | # of SNPs | *p -value |
|---|---|---|---|---|---|---|---|
| Attention/ processing speed | |||||||
| ENSG00000139668 | WDFY2 | 13 | 52158644 | 52336171 | 151 | 7.10E-07 | |
| ENSG00000106348 | IMPDH1 | 7 | 1.28E+08 | 1.28E+08 | 38 | 6.79E-05 | |
| Executive Function | |||||||
| ENSG00000139668 | WDFY2 | 13 | 52158644 | 52336171 | 151 | 2.12E-05 | |
| ENSG00000198837 | DENND4B | 1 | 1.54E+08 | 1.54E+08 | 15 | 2.23E-05 | |
| ENSG00000143614 | GATAD2B | 1 | 1.54E+08 | 1.54E+08 | 142 | 3.37E-05 | |
| ENSG00000160741 | CRTC2 | 1 | 1.54E+08 | 1.54E+08 | 7 | 4.34E-05 | |
| ENSG00000143570 | SLC39A1 | 1 | 1.54E+08 | 1.54E+08 | 9 | 8.08E-05 | |
| ENSG00000101166 | SLMO2 | 20 | 57608200 | 57617964 | 9 | 9.49E-05 | |
| ENSG00000143578 | CREB3L4 | 1 | 1.54E+08 | 1.54E+08 | 9 | 9.68E-05 | |
| Language | |||||||
| ENSG00000196466 | ZNF799 | 19 | 12500830 | 12512085 | 10 | 2.15E-05 | |
| ENSG00000138758 | SEPT11 | 4 | 77870856 | 77961537 | 150 | 4.32E-05 | |
| Memory | |||||||
| ENSG00000072415 | MPP5 | 14 | 67707826 | 67802536 | 43 | 2.14E-05 | |
| ENSG00000130203 | APOE | 19 | 45409011 | 45412650 | 5 | 4.29E-05 | |
| ENSG00000162782 | TDRD5 | 1 | 1.8E+08 | 1.8E+08 | 256 | 4.46E-05 | |
| ENSG00000170054 | SERPINA9 | 14 | 94929054 | 94946026 | 64 | 6.58E-05 | |
| ENSG00000172717 | FAM71D | 14 | 67656110 | 67695267 | 35 | 7.34E-05 | |
| ENSG00000072401 | UBE2D1 | 10 | 60094735 | 60130513 | 24 | 7.42E-05 | |
| ENSG00000100554 | ATP6V1D | 14 | 67761088 | 67826982 | 39 | 7.84E-05 | |
| ENSG00000134001 | EIF2S1 | 14 | 67826714 | 67853233 | 19 | 8.15E-05 | |
| Visuospatial Function | |||||||
| ENSG00000183873 | SCN5A | 3 | 38589548 | 38691164 | 223 | 2.30E-05 | |
| ENSG00000166825 | ANPEP | 15 | 90328120 | 90358633 | 98 | 2.82E-05 | |
| ENSG00000118507 | AKAP7 | 6 | 1.31E+08 | 1.32E+08 | 264 | 5.96E-05 | |
| ENSG00000158717 | RNF166 | 16 | 88762903 | 88772829 | 39 | 6.12E-05 | |
| ENSG00000160613 | PCSK7 | 11 | 1.17E+08 | 1.17E+08 | 73 | 7.76E-05 | |
| ENSG00000148842 | CNNM2 | 10 | 1.05E+08 | 1.05E+08 | 336 | 9.66E-05 |
Input SNPs were mapped to 18,440 protein coding genes. Gene-wide significance was defined as p= 2.71E-06 (0.05/18,440).
3.5. Functional bioinformatics analyses
In the GTEx expression data, WDFY2 is expressed in multiple tissues, including in different human brain regions (Supplementary Figure 4). Furthermore, RNA-Seq of cell types isolated from mouse and human brain show its expression in astrocytes, neurons, microglial and oligodendrocytes (http://www.brainrnaseq.org/ Supplementary Figure 5).
We evaluated the potential biological significance of WDFY2 genome-wide significant SNPs in affecting gene expression in blood and brain tissues In AlzBase database WDFY2 was shown to be downregulated in the listed two transcriptome studies of AD.
In the GTEx eQTL analysis (Supplementary Table S5), the effect C allele of the sentinel SNP (rs9535753 and those in LD with this) was associated with higher RNA expression levels of WDFY2 in several brain regions: cerebellar hemisphere (p=1.07E-04; β=0.34), cerebellum (p=6.92E-04; β=0.35), hippocampus (p= 2.16E-03; β=0.30) and cortex (p= 2.29E-02; β=0.20) as well as in whole blood (p= 3.34E-05 β= 0.20). We also looked at the eQTL data for WDFY2 in AlzBase that lists two SNPs to be cis eQTL for WDFY2 in two brain regions: rs4555048 (an intronic WDFY2 variant located at position 52185855 bp) in visual cortex (p=6.74E-07) and rs4943003 (located upstream of MIR 4703 at position 52090383 bp) in prefrontal cortex (p=3.50E-07). While rs4555048 was in high LD with all genome-wide significant WDFY2 SNPs (r2=0.92; Figure 3) and also showed significant association with attention/speed processing (p=2.07E-06), rs4943003 is in moderate LD with genome-wide significant SNPs (r2=0.46; Figure 3) and with rs4555048 (r2=0.52; Figure 3) and showed a modest association with attention/speed processing (p=0.018).
4. Discussion
The process and measurement of cognitive aging is multifaceted and is characterized by changes in different cognitive variables attributable to declines in general (global) cognitive function, domain-specific, and test-specific aspects of cognition (Tucker-Drob, 2011; Harris et al., 2011). Different cognitive domains reflect functioning of different brain regions and circuits which are differentially impaired in different disorders and thus may be considered as cognitive endophenotypes that are potentially informative for genetic studies. Full genetic contribution to age-related cognitive decline can ideally be captured by focusing on both general and domain-specific cognitive skills. However, previous GWAS have largely focused on a general cognitive function phenotype and may therefore have missed domain-specific genes/loci as well as loci that are specific to cognitive decline rather than baseline cognitive function. In this study, we followed two longitudinal cohorts free of dementia at baseline, and calculated intraindividual slopes of linear decline over time in five cognitive domains (memory, executive function, language, attention/ processing speed, and visuospatial ability). We performed genome-wide and gene-based meta-analyses to capture genetic variation associated with decline in each individual domain, and compared results with global cognitive decline as constructed from the five domains
Among the known AD loci, APOE*4 showed the strongest association with memory change/decline, as predicted and providing validation to our cognitive assessment and statistical methods. Two other known AD loci showed associations with more than one domain at p<1E-03, including EPHA1 with visuospatial ability and memory and ABCA7 with executive function, language, attention and visuospatial ability. However, none of these AD genes were the top genes in their respective domains.
Our GWAS meta-analysis identified a novel WDFY2 locus (p= 3.37E-08) for the attention domain, which we would have missed had we used the global cognitive decline as a unitary phenotype. The attention domain comprised two tasks, reflecting verbal working memory storage capacity and psychomotor speed/visual search. Interestingly, these functions and tasks are not typical impaired in early AD-type neurodegeneration. More broadly, attention is a set of cognitive functions supporting all other higher cognitive functions, by allowing the appropriate selection of stimuli and maintenance of concentration (i.e., vigilance). As a multidimensional function with complex anatomic and neurochemical underpinnings, including sub-cortical networks, attention may be disturbed in a variety of medical conditions across the lifespan. There are shared processes and networks with executive functions, as well (Gitelman, 2003). The credence to shared processes and networks between attention and executive functions is provided by our genetic data where genome-wide significant WDFY2 SNPs also showed association with decline in executive function (p= 1.94E-05; β= 0.22), but not with other domains, and WDFY2 was the top gene for both attention (p= 7.10E-07) and executive function (p= 2.12E-05) in the gene-based analysis.
Although the sample sizes in our two cohorts were relatively small, both showed consistent and directional association for the top GWAS attention signal that provides support for a genuine association. Additional support to the genome-wide analysis (based on single SNP test in a genome) is provided by the gene-based analysis (based on multiple SNPs test within a gene) that also identified the WDFY2 gene as being gene-wide significant (p= 7.10E-07). The two WDFY2 genotyped SNPs that showed genome-wide significance (rs9535753; p= 3.37E-08 and rs2296029; p= 4.78E-08) are located in 3’UTR of WDFY2. 3’UTRs of mRNAs are known to be involved in the regulation of mRNA stability, translation, and mRNA localization. In addition, the formation of 3’UTR-mediated protein-protein interactions can also enable the transmit of genetic information stored in 3’UTRs to proteins (Mayr, 2018). In view of the wide range of functions associated with 3’UTRs, it is plausible that the identified SNPs in 3’UTR of WDFY2 are functional. However, we cannot rule out the possibility that other SNPs with genome-wide significance that were in tight LD with these two SNPs, or yet to be discovered SNPs in this region are involved in driving the association at this locus. Indeed, our all genome-wide significant SNPs were associated with WDFY2 expression in different brain regions, at least at nominal significance.
WDFY2 [WD (tryptophan-aspartic acid dipeptide) repeat and FYVE domain containing 2] is a phosphatidylinositol 3-phosphate binding-protein that is localized to early endosomes necessary for endocytosis (Hayakawa et al., 2006). WDFY2-enriched endosomes also serve as a scaffold that enables specificity of insulin signaling through protein kinase Akt (Walz et al. 2010). WDFY2 has also been identified as a tumor suppression gene via inactivation of the Akt pathway (Wang et al., 2017). WDFY2 is widely expressed in multiple tissues, including the brain. A network analysis of bipolar disorder (BD) GWAS data has identified WDFY2 as one of the four hub genes that might indirectly affect the risk of BD by interacting with genes directly related to BD (Xie et al., 2017). To our knowledge, WDFY2 has not been previously implicated in GWAS of cognitive function or AD. However, WDFY2 has been identified as a differentially co-expressed gene along with TAX1BP3 or SLC35E1, where their co-expression was decreased in prefrontal cortex of AD cases relative to controls (Narayanan et al., 2014). Similarly, AlzBase database shows lower expression of WDFY2 in AD, indicating its potential role in AD or AD-related dementia. In our study, the effect (minor) allele of the top WDFY2 SNP (rs9535753 and other SNPs in LD; all located in WDFY2) was associated with slower decline in attention over time, and was also associated with higher expression of WDFY2 in different brain regions in the GTEx data. The underlying mechanism of this association is not clear at present. Another member of the WDFY family, WDFY4, which is predominantly expressed in immune tissues has been found to be genetically associated with lupus and rheumatoid arthritis (Yang et al., 2010; Zhang et al., 2014). It is likely that the observed association of WFDY2 with a cognitive endophenotype and its differential expression in AD brains is also due to an immune-related mechanism, similar to that observed with some AD-associated genes (Pimenova et al., 2018), since WFDY2 is also expressed in microglial/macrophage cells in the brain (Supplementary Figure 5).
Strengths of this study include our focus on the genetics of cognitive decline in older adults from two well-characterized population-based cohorts. Participants were free of dementia at baseline and had measured trajectories of cognitive endophenotypes over time in multiple cognitive domains. Identical findings from genome-wide and gene-wide tests provide credence to our observed novel genetic association with the attention domain that is further supplemented by functional analyses, including gene expression data in relevant brain cells and regions. The main study limitation is the absence of replication cohorts. This was largely beyond our control as most of the published studies have used general cognition in GWAS as compared to our focus on domain-specific cognitive endophenotypes. This limitation is somewhat alleviated by the fact that the association and the direction of our top attention signal was similar in our both cohorts and that our genome-wide analysis finding was confirmed in the gene-based analysis. While our finding of the association WDFY2 with decline over time in attention appears to be novel, it will need to be replicated before its role is more thoroughly investigated.
In conclusion, we report a novel locus for decline in attention that also showed suggestive association with decline in executive function. Future larger studies focusing on domain-specific cognitive endophenotypes may help us to broaden our understanding about the complex genetic architect of cognitive change in aging.
Supplementary Material
Highlights.
We followed two longitudinal cohorts free of dementia at baseline, and calculated intraindividual slopes of linear decline over time in five cognitive domains
We performed genome-wide and gene-based meta-analyses to capture genetic variation associated with decline in each individual domain
Both analyses revealed a novel WDFY2 locus at genome-wide and gene-wide significance levels for the attention/processing speed domain
Acknowledgments
This study was supported by NIH grants AG023651, AG07562, AG030653 and AG041718.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no actual or potential conflicts of interest
Appendix. Supplementary material
CDR: Clinical Dementia Rating
References
- Benyamin B, Pourcain B, Davis OS, Davies G, Hansell NK, Brion MJ, Kirkpatrick RM, Cents RA, Franic S, Miller MB, Haworth CM, Meaburn E, Price TS, Evans DM, Timpson N, Kemp J, Ring S, McArdle W, Medland SE, Yang J, Harris SE, Liewald DC, Scheet P, Xiao X, Hudziak JJ, de Geus EJ, Jaddoe VW, Starr JM, Verhulst FC, Pennell C, Tiemeier H, Iacono WG, Palmer LJ, Montgomery GW, Martin NG, Boomsma DI, Posthuma D, McGue M, Wright MJ, Davey Smith G, Deary IJ, Plomin R, Visscher PM 2014. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol Psychiatry 19(2), 253–258. doi: 10.1038/mp.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Armstrong N, Bis JC, Bressler J, Chouraki V, Giddaluru S, Hofer E, Ibrahim-Verbaas CA, Kirin M, Lahti J, van der Lee SJ, Le Hellard S, Liu T, Marioni RE, Oldmeadow C, Postmus I, Smith AV, Smith JA, Thalamuthu A, Thomson R, Vitart V, Wang J, Yu L, Zgaga L, Zhao W, Boxall R, Harris SE, Hill WD, Liewald DC, Luciano M, Adams H, Ames D, Amin N, Amouyel P, Assareh AA, Au R, Becker JT, Beiser A, Berr C, Bertram L, Boerwinkle E, Buckley BM, Campbell H, Corley J, De Jager PL, Dufouil C, Eriksson JG, Espeseth T, Faul JD, Ford I, Gottesman RF, Griswold ME, Gudnason V, Harris TB, Heiss G, Hofman A, Holliday EG, Huffman J, Kardia SL, Kochan N, Knopman DS, Kwok JB, Lambert JC, Lee T, Li G, Li SC, Loitfelder M, Lopez OL, Lundervold AJ, Lundqvist A, Mather KA, Mirza SS, Nyberg L, Oostra BA, Palotie A, Papenberg G, Pattie A, Petrovic K, Polasek O, Psaty BM, Redmond P, Reppermund S, Rotter JI, Schmidt H, Schuur M, Schofield PW, Scott RJ, Steen VM, Stott DJ, van Swieten JC, Taylor KD, Trollor J, Trompet S, Uitterlinden AG, Weinstein G, Widen E, Windham BG, Jukema JW, Wright AF, Wright MJ, Yang Q, Amieva H, Attia JR, Bennett DA, Brodaty H, de Craen AJ, Hayward C, Ikram MA, Lindenberger U, Nilsson LG, Porteous DJ, Raikkonen K, Reinvang I, Rudan I, Sachdev PS, Schmidt R, Schofield PR, Srikanth V, Starr JM, Turner ST, Weir DR, Wilson JF, van Duijn C, Launer L, Fitzpatrick AL, Seshadri S, Mosley TH Jr., Deary IJ 2015. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N=53949). Mol Psychiatry 20(2), 183–192. doi: 10.1038/mp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, Hagenaars SP, Ritchie SJ, Marioni RE, Fawns-Ritchie C, Liewald DCM, Okely JA, Ahola-Olli AV, Barnes CLK, Bertram L, Bis JC, Burdick KE, Christoforou A, DeRosse P, Djurovic S, Espeseth T, Giakoumaki S, Giddaluru S, Gustavson DE, Hayward C, Hofer E, Ikram MA, Karlsson R, Knowles E, Lahti J, Leber M, Li S, Mather KA, Melle I, Morris D, Oldmeadow C, Palviainen T, Payton A, Pazoki R, Petrovic K, Reynolds CA, Sargurupremraj M, Scholz M, Smith JA, Smith AV, Terzikhan N, Thalamuthu A, Trompet S, van der Lee SJ, Ware EB, Windham BG, Wright MJ, Yang J, Yu J, Ames D, Amin N, Amouyel P, Andreassen OA, Armstrong NJ, Assareh AA, Attia JR, Attix D, Avramopoulos D, Bennett DA, Bohmer AC, Boyle PA, Brodaty H, Campbell H, Cannon TD, Cirulli ET, Congdon E, Conley ED, Corley J, Cox SR, Dale AM, Dehghan A, Dick D, Dickinson D, Eriksson JG, Evangelou E, Faul JD, Ford I, Freimer NA, Gao H, Giegling I, Gillespie NA, Gordon SD, Gottesman RF, Griswold ME, Gudnason V, Harris TB, Hartmann AM, Hatzimanolis A, Heiss G, Holliday EG, Joshi PK, Kahonen M, Kardia SLR, Karlsson I, Kleineidam L, Knopman DS, Kochan NA, Konte B, Kwok JB, Le Hellard S, Lee T, Lehtimaki T, Li SC, Liu T, Koini M, London E, Longstreth WT Jr., Lopez OL, Loukola A, Luck T, Lundervold AJ, Lundquist A, Lyytikainen LP, Martin NG, Montgomery GW, Murray AD, Need AC, Noordam R, Nyberg L, Ollier W, Papenberg G, Pattie A, Polasek O, Poldrack RA, Psaty BM, Reppermund S, Riedel-Heller SG, Rose RJ, Rotter JI, Roussos P, Rovio SP, Saba Y, Sabb FW, Sachdev PS, Satizabal CL, Schmid M, Scott RJ, Scult MA, Simino J, Slagboom PE, Smyrnis N, Soumare A, Stefanis NC, Stott DJ, Straub RE, Sundet K, Taylor AM, Taylor KD, Tzoulaki I, Tzourio C, Uitterlinden A, Vitart V, Voineskos AN, Kaprio J, Wagner M, Wagner H, Weinhold L, Wen KH, Widen E, Yang Q, Zhao W, Adams HHH, Arking DE, Bilder RM, Bitsios P, Boerwinkle E, Chiba-Falek O, Corvin A, De Jager PL, Debette S, Donohoe G, Elliott P, Fitzpatrick AL, Gill M, Glahn DC, Hagg S, Hansell NK, Hariri AR, Ikram MK, Jukema JW, Vuoksimaa E, Keller MC, Kremen WS, Launer L, Lindenberger U, Palotie A, Pedersen NL, Pendleton N, Porteous DJ, Raikkonen K, Raitakari OT, Ramirez A, Reinvang I, Rudan I, Dan R, Schmidt R, Schmidt H, Schofield PW, Schofield PR, Starr JM, Steen VM, Trollor JN, Turner ST, Van Duijn CM, Villringer A, Weinberger DR, Weir DR, Wilson JF, Malhotra A, McIntosh AM, Gale CR, Seshadri S, Mosley TH Jr., Bressler J, Lencz T, Deary IJ 2018. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun 9(1), 2018. doi: 10.1038/s41467-018-04362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE, Ritchie SJ, Luciano M, Fawns-Ritchie C, Lyall D, Cullen B, Cox SR, Hayward C, Porteous DJ, Evans J, McIntosh AM, Gallacher J, Craddock N, Pell JP, Smith DJ, Gale CR, Deary IJ 2016. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N=112 151). Mol Psychiatry 21(6), 758–767. doi: 10.1038/mp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Tenesa A, Payton A, Yang J, Harris SE, Liewald D, Ke X, Le Hellard S, Christoforou A, Luciano M, McGhee K, Lopez L, Gow AJ, Corley J, Redmond P, Fox HC, Haggarty P, Whalley LJ, McNeill G, Goddard ME, Espeseth T, Lundervold AJ, Reinvang I, Pickles A, Steen VM, Ollier W, Porteous DJ, Horan M, Starr JM, Pendleton N, Visscher PM, Deary IJ 2011. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol Psychiatry 16(10), 996–1005. doi: 10.1038/mp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Shulman JM, Chibnik LB, Keenan BT, Raj T, Wilson RS, Yu L, Leurgans SE, Tran D, Aubin C, Anderson CD, Biffi A, Corneveaux JJ, Huentelman MJ, Rosand J, Daly MJ, Myers AJ, Reiman EM, Bennett DA, Evans DA 2012. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol Aging 33(5), 1017 e1011–1015. doi: 10.1016/j.neurobiolaging.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, Posthuma D 2015. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol 11(4), e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Ibrahim Verbaas CA, Bressler J, Schuur M, Smith A, Bis JC, Davies G, Wolf C, Gudnason V, Chibnik LB, Yang Q, deStefano AL, de Quervain DJ, Srikanth V, Lahti J, Grabe HJ, Smith JA, Priebe L, Yu L, Karbalai N, Hayward C, Wilson JF, Campbell H, Petrovic K, Fornage M, Chauhan G, Yeo R, Boxall R, Becker J, Stegle O, Mather KA, Chouraki V, Sun Q, Rose LM, Resnick S, Oldmeadow C, Kirin M, Wright AF, Jonsdottir MK, Au R, Becker A, Amin N, Nalls MA, Turner ST, Kardia SL, Oostra B, Windham G, Coker LH, Zhao W, Knopman DS, Heiss G, Griswold ME, Gottesman RF, Vitart V, Hastie ND, Zgaga L, Rudan I, Polasek O, Holliday EG, Schofield P, Choi SH, Tanaka T, An Y, Perry RT, Kennedy RE, Sale MM, Wang J, Wadley VG, Liewald DC, Ridker PM, Gow AJ, Pattie A, Starr JM, Porteous D, Liu X, Thomson R, Armstrong NJ, Eiriksdottir G, Assareh AA, Kochan NA, Widen E, Palotie A, Hsieh YC, Eriksson JG, Vogler C, van Swieten JC, Shulman JM, Beiser A, Rotter J, Schmidt CO, Hoffmann W, Nothen MM, Ferrucci L, Attia J, Uitterlinden AG, Amouyel P, Dartigues JF, Amieva H, Raikkonen K, Garcia M, Wolf PA, Hofman A, Longstreth WT Jr., Psaty BM, Boerwinkle E, DeJager PL, Sachdev PS, Schmidt R, Breteler MM, Teumer A, Lopez OL, Cichon S, Chasman DI, Grodstein F, Muller-Myhsok B, Tzourio C, Papassotiropoulos A, Bennett DA, Ikram MA, Deary IJ, van Duijn CM, Launer L, Fitzpatrick AL, Seshadri S, Mosley TH Jr. 2015. Genome-wide studies of verbal declarative memory in nondemented older people: the Cohorts for Heart and Aging Research in enomic Epidemiology consortium. Biol Psychiatry 77(8), 749–763. doi: 10.1016/j.biopsych.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthymiou AG, Goate AM 2017. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol Neurodegener 12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Belle S, Ratcliff G, Seaberg E, Huff FJ, von der Porten K, Kuller LH 1993. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol 48(4), M152–161. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Snitz B, Vander Bilt J, Chang CC 2009. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry 24(11), 1277–1284. doi: 10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR 2003. Attention and its disorders. Br Med Bull 65, 21–34. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ 2011. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends Cogn Sci 15(9), 388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Hayakawa A, Leonard D, Murphy S, Hayes S, Soto M, Fogarty K, Standley C, Bellve K, Lambright D, Mello C, Corvera S 2006. The WD40 and FYVE domain containing protein 2 defines a class of early endosomes necessary for endocytosis. Proc Natl Acad Sci U S A 103(32), 11928–11933. doi: 10.1073/pnas.0508832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J 2009. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5(6), e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim-Verbaas CA, Bressler J, Debette S, Schuur M, Smith AV, Bis JC, Davies G, Trompet S, Smith JA, Wolf C, Chibnik LB, Liu Y, Vitart V, Kirin M, Petrovic K, Polasek O, Zgaga L, Fawns-Ritchie C, Hoffmann P, Karjalainen J, Lahti J, Llewellyn DJ, Schmidt CO, Mather KA, Chouraki V, Sun Q, Resnick SM, Rose LM, Oldmeadow C, Stewart M, Smith BH, Gudnason V, Yang Q, Mirza SS, Jukema JW, deJager PL, Harris TB, Liewald DC, Amin N, Coker LH, Stegle O, Lopez OL, Schmidt R, Teumer A, Ford I, Karbalai N, Becker JT, Jonsdottir MK, Au R, Fehrmann R, Herms S, Nalls M, Zhao W, Turner ST, Yaffe K, Lohman K, van Swieten JC, Kardia S, Knopman DS, Meeks WM, Heiss G, Holliday EG, Schofield PW, Tanaka T, Stott DJ, Wang J, Ridker P, Gow AJ, Pattie A, Starr JM, Hocking LJ, Armstrong NJ, McLachlan S, Shulman JM, Pilling LC, Eiriksdottir G, Scott RJ, Kochan NA, Palotie A, Hsieh YC, Eriksson JG, Penman A, Gottesman RF, Oostra BA, Yu L, DeStefano AL, Beiser A, Garcia M, Rotter JI, Nothen MM, Hofman A, Slagboom PE, Westendorp R, Buckley BM, Wolf PA, Uitterlinden AG, Psaty BM, Grabe HJ, Bandinelli S, Chasman DI, Grodstein F, Raikkonen K, Lambert JC, Porteous DJ, Price JF, Sachdev PS, Ferrucci L, Attia JR, Rudan I, Hayward C, Wright AF, Wilson JF, Cichon S, Franke L, Schmidt H, Ding J, de Craen A, Fornage M, Bennett DA, Deary IJ, Ikram MA, Launer LJ, Fitzpatrick AL, Seshadri S, van Duijn CM, Mosley TH 2016. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol Psychiatry 21(2), 189–197. doi: 10.1038/mp.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh MI, Demirci FY, Wang X, Minster RL, Carrasquillo MM, Pankratz VS, Younkin SG, Saykin AJ, Jun G, Baldwin C, Logue MW, Buros J, Farrer L, Pericak-Vance MA, Haines JL, Sweet RA, Ganguli M, Feingold E, Dekosky ST, Lopez OL, Barmada MM 2012. Genome-wide association study of Alzheimer’s disease. Transl Psychiatry 2, e117. doi: 10.1038/tp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM, Djurovic S, Melle I, Sundet K, Christoforou A, Reinvang I, Mukherjee S, DeRosse P, Lundervold A, Steen VM, John M, Espeseth T, Raikkonen K, Widen E, Palotie A, Eriksson JG, Giegling I, Konte B, Ikeda M, Roussos P, Giakoumaki S, Burdick KE, Payton A, Ollier W, Horan M, Donohoe G, Morris D, Corvin A, Gill M, Pendleton N, Iwata N, Darvasi A, Bitsios P, Rujescu D, Lahti J, Hellard SL, Keller MC, Andreassen OA, Deary IJ, Glahn DC, Malhotra AK 2014. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consortium (COGENT). Mol Psychiatry 19(2), 168–174. doi: 10.1038/mp.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D 2012. Neuropsychological assessment (5th ed.). New York, NY, US: Oxford University Press. [Google Scholar]
- Walz HA, Shi X, Chouinard M, Bue CA, Navaroli DM, Hayakawa A, Zhou QL, Nadler J, Leonard DM, Corvera S 2010. Isoform-specific regulation of Akt signaling by the endosomal protein WDFY2. J Biol Chem. 285,14101–14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C 2018. What Are 3’ UTRs Doing? Cold Spring Harb Perspect Biol. doi: 10.1101/cshperspect.a034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan M, Huynh JL, Wang K, Yang X, Yoo S, McElwee J, Zhang B, Zhang C, Lamb JR, Xie T, Suver C, Molony C, Melquist S, Johnson AD, Fan G, Stone DJ, Schadt EE, Casaccia P, Emilsson V, Zhu J 2014. Common dysregulation network in the human prefrontal cortex underlies two neurodegenerative diseases. Mol Syst Biol 10, 743. doi: 10.15252/msb.20145304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Yu RK, Dehoff KL, Amos CI 2007. Normalizing a large number of quantitative traits using empirical normal quantile transformation. BMC Proc 1 Suppl 1, S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenova AA, Raj T, Goate AM 2018. Untangling Genetic Risk for Alzheimer’s Disease. Biol Psychiatry 83(4), 300–310. doi: 10.1016/j.biopsych.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Deary IJ 2015. Genetics and intelligence differences: five special findings. Mol Psychiatry 20(1), 98–108. doi: 10.1038/mp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, Posthuma D 2015. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 47(7), 702–709. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38(8), 904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Reitz C, Mayeux R 2010. Use of genetic variation as biomarkers for mild cognitive impairment and progression of mild cognitive impairment to dementia. J Alzheimers Dis 19(1), 229–251. doi: 10.3233/jad-2010-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW 2009. Neuropsychological assessment of dementia. Annu Rev Psychol 60, 257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampush JW, Lencz T, Knowles E, Davies G, Guha S, Pe’er I, Liewald DC, Starr JM, Djurovic S, Melle I, Sundet K, Christoforou A, Reinvang I, Mukherjee S, DeRosse P, Lundervold A, Steen VM, John M, Espeseth T, Raikkonen K, Widen E, Palotie A, Eriksson JG, Giegling I, Konte B, Ikeda M, Roussos P, Giakoumaki S, Burdick KE, Payton A, Ollier W, Horan M, Scult M, Dickinson D, Straub RE, Donohoe G, Morris D, Corvin A, Gill M, Hariri A, Weinberger DR, Pendleton N, Iwata N, Darvasi A, Bitsios P, Rujescu D, Lahti J, Le Hellard S, Keller MC, Andreassen OA, Deary IJ, Glahn DC, Malhotra AK 2015. Independent evidence for an association between general cognitive ability and a genetic locus for educational attainment. Am J Med Genet B Neuropsychiatr Genet 168B(5), 363–373. doi: 10.1002/ajmg.b.32319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM 2011. Global and domain-specific changes in cognition throughout adulthood. Dev Psychol 47(2), 331–343. doi: 10.1037/a0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen X, Tong S, Zhou H, Sun J, Gou Y, Wu F, Hu J, Xu J, Ding G 2017. Overexpression of WDFY2 inhibits prostate cancer cell growth and migration via inactivation of Akt pathway. Tumour Biol 39(6), 1010428317704821. doi: 10.1177/1010428317704821. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR 2010. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26(17), 2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA 2011. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 32(1), 63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, Chu AY, Estrada K, Luan J, Kutalik Z, Amin N, Buchkovich ML, Croteau-Chonka DC, Day FR, Duan Y, Fall T, Fehrmann R, Ferreira T, Jackson AU, Karjalainen J, Lo KS, Locke AE, Magi R, Mihailov E, Porcu E, Randall JC, Scherag A, Vinkhuyzen AA, Westra HJ, Winkler TW, Workalemahu T, Zhao JH, Absher D, Albrecht E, Anderson D, Baron J, Beekman M, Demirkan A, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Fraser RM, Goel A, Gong J, Justice AE, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Lui JC, Mangino M, Mateo Leach I, Medina-Gomez C, Nalls MA, Nyholt DR, Palmer CD, Pasko D, Pechlivanis S, Prokopenko I, Ried JS, Ripke S, Shungin D, Stancakova A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Afzal U, Arnlov J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Bluher M, Bolton JL, Bottcher Y, Boyd HA, Bruinenberg M, Buckley BM, Buyske S, Caspersen IH, Chines PS, Clarke R, Claudi-Boehm S, Cooper M, Daw EW, De Jong PA, Deelen J, Delgado G, Denny JC, Dhonukshe-Rutten R, Dimitriou M, Doney AS, Dorr M, Eklund N, Eury E, Folkersen L, Garcia ME, Geller F, Giedraitis V, Go AS, Grallert H, Grammer TB, Grassler J, Gronberg H, de Groot LC, Groves CJ, Haessler J, Hall P, Haller T, Hallmans G, Hannemann A, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hemani G, Henders AK, Hillege HL, Hlatky MA, Hoffmann W, Hoffmann P, Holmen O, Houwing-Duistermaat JJ, Illig T, Isaacs A, James AL, Jeff J, Johansen B, Johansson A, Jolley J, Juliusdottir T, Junttila J, Kho AN, Kinnunen L, Klopp N, Kocher T, Kratzer W, Lichtner P, Lind L, Lindstrom J, Lobbens S, Lorentzon M, Lu Y, Lyssenko V, Magnusson PK, Mahajan A, Maillard M, McArdle WL, McKenzie CA, McLachlan S, McLaren PJ, Menni C, Merger S, Milani L, Moayyeri A, Monda KL, Morken MA, Muller G, Muller-Nurasyid M, Musk AW, Narisu N, Nauck M, Nolte IM, Nothen MM, Oozageer L, Pilz S, Rayner NW, Renstrom F, Robertson NR, Rose LM, Roussel R, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Schunkert H, Scott RA, Sehmi J, Seufferlein T, Shi J, Silventoinen K, Smit JH, Smith AV, Smolonska J, Stanton AV, Stirrups K, Stott DJ, Stringham HM, Sundstrom J, Swertz MA, Syvanen AC, Tayo BO, Thorleifsson G, Tyrer JP, van Dijk S, van Schoor NM, van der Velde N, van Heemst D, van Oort FV, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Waldenberger M, Wennauer R, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Bergmann S, Biffar R, Blangero J, Boomsma DI, Bornstein SR, Bovet P, Brambilla P, Brown MJ, Campbell H, Caulfield MJ, Chakravarti A, Collins R, Collins FS, Crawford DC, Cupples LA, Danesh J, de Faire U, den Ruijter HM, Erbel R, Erdmann J, Eriksson JG, Farrall M, Ferrannini E, Ferrieres J, Ford I, Forouhi NG, Forrester T, Gansevoort RT, Gejman PV, Gieger C, Golay A, Gottesman O, Gudnason V, Gyllensten U, Haas DW, Hall AS, Harris TB, Hattersley AT, Heath AC, Hengstenberg C, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Jacobs KB, Jarvelin MR, Jousilahti P, Jula AM, Kaprio J, Kastelein JJ, Kayser M, Kee F, Keinanen-Kiukaanniemi SM, Kiemeney LA, Kooner JS, Kooperberg C, Koskinen S, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimaki T, Lupoli S, Madden PA, Mannisto S, Manunta P, Marette A, Matise TC, McKnight B, Meitinger T, Moll FL, Montgomery GW, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Ouwehand WH, Pasterkamp G, Peters A, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ritchie M, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PE, Sebert S, Sever P, Shuldiner AR, Sinisalo J, Steinthorsdottir V, Stolk RP, Tardif JC, Tonjes A, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PI, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hayes MG, Hui J, Hunter DJ, Hveem K, Jukema JW, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, Marz W, Melbye M, Moebus S, Munroe PB, Njolstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Perusse L, Peters U, Powell JE, Power C, Quertermous T, Rauramaa R, Reinmaa E, Ridker PM, Rivadeneira F, Rotter JI, Saaristo TE, Saleheen D, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Strauch K, Stumvoll M, Tuomilehto J, Uusitupa M, van der Harst P, Volzke H, Walker M, Wareham NJ, Watkins H, Wichmann HE, Wilson JF, Zanen P, Deloukas P, Heid IM, Lindgren CM, Mohlke KL, Speliotes EK, Thorsteinsdottir U, Barroso I, Fox CS, North KE, Strachan DP, Beckmann JS, Berndt SI, Boehnke M, Borecki IB, McCarthy MI, Metspalu A, Stefansson K, Uitterlinden AG, van Duijn CM, Franke L, Willer CJ, Price AL, Lettre G, Loos RJ, Weedon MN, Ingelsson E, O’Connell JR, Abecasis GR, Chasman DI, Goddard ME, Visscher PM, Hirschhorn JN, Frayling TM 2014. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 46(11), 1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Yang X, Deng X, Ma M, Shu K 2017. A Genome-Wide Association Study and Complex Network Identify Four Core Hub Genes in Bipolar Disorder. Int J Mol Sci 18(12). doi: 10.3390/ijms18122763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Shen N, Ye DQ, Liu Q, Zhang Y, Qian XX, Hirankarn N, Ying D, Pan HF, Mok CC, Chan TM, Wong RW, Lee KW, Mok MY, Wong SN, Leung AM, Li XP, Avihingsanon Y, Wong CM, Lee TL, Ho MH, Lee PP, Chang YK, Li PH, Li RJ, Zhang L, Wong WH, Ng IO, Lau CS, Sham PC, Lau YL 2010. Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS Genet 6(2), e1000841. doi: 10.1371/journal.pgen.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bo L, Zhang H, Zhuang C, Liu R 2014. E26 transformation-specific-1 (ETS1) and WDFY family member 4 (WDFY4) polymorphisms in Chinese patients with rheumatoid arthritis. Int J Mol Sci 15(2), 2712–2721. doi: 10.3390/ijms15022712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.