Abstract
Objectives:
Current guidelines recommend delivery of smoking cessation interventions with lung cancer screening (LCS). Unfortunately, there are limited data to guide clinicians and policy-makers in choosing cessation interventions in this setting. Several trials are underway to fill this evidence gap, but results are not expected for several years.
Methods and Materials:
We conducted a systematic review and meta-analysis of current literature on the efficacy of smoking cessation interventions among populations eligible for LCS. We searched PubMed, Medline, and PsycINFO for randomized controlled trials of smoking cessation interventions published from 2010-2017. Trials were eligible for inclusion if they sampled individuals likely to be eligible for LCS based on age and smoking history, had sample sizes >100, follow-up of 6- or 12-months, and were based in North America, Western Europe, Australia, or New Zealand.
Results:
Three investigators independently screened 3,813 abstracts and identified 332 for full-text review. Of these, 85 trials were included and grouped into categories based on the primary intervention: electronic/web-based, in-person counseling, pharmacotherapy, and telephone counseling. At 6-month follow-up, electronic/web-based (odds ratio [OR] 1.14, 95% CI 1.03-1.25), in-person counseling (OR 1.46, 95% CI 1.25-1.70), and pharmacotherapy (OR 1.53, 95% CI 1.33-1.77) interventions significantly increased the odds of abstinence. Telephone counseling increased the odds but did not reach statistical significance (OR 1.21, 95% CI 0.98-1.50). At 12-months, in-person counseling (OR 1.28 95% CI 1.09-1.51) and pharmacotherapy (OR 1.46, 95% CI 1.17-1.84) remained efficacious, although the decrement in efficacy was of similar magnitude across all intervention categories.
Conclusions:
Several categories of cessation interventions are promising for implementation in the LCS setting.
Review Registration (PROSPERO):
CRD42018110322
Keywords: smoking cessation, lung cancer screening, meta-analysis
1. Introduction
The National Lung Screening Trial (NLST) and the NEderlands-Leuvens Longkanker Screenings ONdersoek (NELSON) trial provided evidence that lung cancer screening with low-dose computed tomography (LCS) detected cancers earlier than when clinically symptomatic, and reduced lung cancer mortality by 20%-26% (1, 2). Professional groups, including the U.S. Preventive Services Task Force (USPSTF) and the National Comprehensive Cancer Network (NCCN) recommend LCS for individuals with a high risk of lung cancer based on their age (55-80 and 55-74 years, for the USPSTF and NCCN, respectively), a 30 pack-year smoking history, and other risk factors (3, 4).
The potential benefits of screening may go beyond the early detection of lung cancers. Screening may provide a “teachable moment” for encouraging cessation from smoking for the estimated four million current US smokers eligible for LCS, approximately half of all eligible individuals (5, 6). Smoking cessation, in turn, reduces the risk of several cancer types and cardiopulmonary disease. However, merely undergoing LCS does not influence smoking behaviors (7). Consequently, the Centers for Medicare and Medicaid Services (CMS) mandates that smoking cessation assistance is provided to all current smokers undergoing LCS, but leaves decisions about the type of cessation interventions up to clinicians and screening sites (8).
Presently, there are nine trials in progress in the US that will provide valuable evidence on the efficacy of smoking cessation in the context of LCS (9). However, results are not expected until after 2021. While a number of smoking interventions have been found to be effective in general populations (10), there is a paucity of data on whether these approaches will be effective in older, persistent, heavy smokers eligible for LCS. Only a handful of randomized controlled trials have thus far considered the efficacy of smoking cessation in the screening setting (11-15). Reviews have highlighted the lack of sufficient data needed to make decisions regarding cessation in this setting concluding a need for more data to identify optimal screening strategies for this population (16, 17).
Hence, clinicians and policy-makers now have a mandate to provide cessation to smokers who present for LCS but have limited evidence on the most effective interventions to offer (11-15, 18). To address this lack of information while clinical trials are ongoing, we conducted a systematic review and meta-analysis of recently published clinical trials of smoking cessation that primarily included populations similar to those eligible for LCS. We grouped trials into intervention categories that reflect current clinical guidelines and practice, including electronic/web-based, in-person counseling, pharmacotherapy with drugs currently approved by the Food and Drug Administration, and telephone counseling (9, 10, 19, 20). The results of this analysis are intended to inform current clinical practice at screening sites. As new studies are conducted, the results of this analysis will also support the framework for future research on the expected population effects, costs, and cost-effectiveness of smoking cessation interventions in the LCS setting.
2. Methods
The review follows the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Appendix A.1) (21) and is registered with PROSPERO: http://www.crd.york.ac.uk/PROSPERO/display_record.php?rD=CRD42018110322.
2.1. Data Sources and Searches
Searches were conducted with the help of a health sciences research librarian in PubMed, Medline (Ovid), and PsycINFO for articles published from January 1, 2010, to December 31, 2017. This period was selected to represent current cessation practice. General smoking cessation search terms were used in conjunction with geographic specifications and clinical trial terms (Appendix B.1). The search terms were kept as inclusive as possible to identify all potentially relevant studies. We also searched the bibliographies of selected trials and reviews to identify any articles missed by the database searches. Results of the search were exported into Microsoft Excel 2010 workbooks designed by a health sciences research librarian specifically for screening article eligibility for systematic reviews (22).
2.2. Study Selection
Eligibility criteria were determined a priori. To be eligible, randomized controlled trials published in English tested the efficacy of one or more of four categories of cigarette smoking cessation interventions on 7-day point prevalence at 6- or 12-months post-intervention, had sample sizes >100, were conducted in North America, Western Europe, Australia or New Zealand, so as to reflect populations that could be generalizable to the US; and published between January 1, 2010 to December 31, 2017. The intervention categories included: electronic/web-based, in-person counseling, pharmacotherapy, and telephone counseling. We selected these four categories based on clinical guidelines, expert opinion, and comparability to current ongoing trials in the screening setting (9, 10, 19). Trials that tested multiple interventions or combinations of interventions were deemed eligible for inclusion. Importantly, trials had to include individuals between the ages of 55 and 80 with no signs or symptoms of lung cancer and with indications of heavy smoking (e.g., based on cigarettes per day, or pack-year smoking history) (3, 4, 8). We excluded studies that did not include individuals over 55 (defined as mean age of < two standard deviations below 55 in each study arm or trial-wide), or that focused on light smokers (defined as mean cigarettes per day of <10). Studies could include individuals who were light smokers or below the age of 55. As only a handful of trials reported pack-years, it was not feasible to screen studies using that measure. We excluded trials that focused exclusively on institutionalized populations such as prisoners, long-term care residents, and drug rehabilitation residents, individuals with known cancer or severe COPD, or people with mental illness. Pharmacotherapy interventions needed to use FDA approved drugs to be eligible (i.e., nicotine replacement therapy, bupropion, and varenicline) (20). Interventions that tested a drug not currently approved by the FDA were excluded, as these would not be available in current clinical practice.
Following the deletion of duplicate publications, all trial titles and abstracts were reviewed to determine potential eligibility. If the abstract lacked sufficient evidence to determine eligibility, it was included in the full-text review. Three authors independently screened a sample of papers to measure inter-rater reliability using Cohen's κ where a κ= 0.8 indicates good inter-rater agreement. Disagreements between reviewers regarding eligibility were resolved through discussion to achieve consensus. The remaining titles and abstracts were screened with each abstract screened separately by two authors. The results were reconciled and the final list of studies for full-text review was identified.
The full-text of selected publications was then reviewed to determine final eligibility and identify multiple reports from the same trial. Where multiple reports of the same intervention were found, we used the report with the greatest level of detail regarding the effects of the intervention on smoking cessation at 6 and 12-months. Two reviewers conducted full-text review independently and any uncertainty over inclusion was discussed and resolved among three authors.
2.3. Data Abstraction and Quality Assessment
A data abstraction template was developed in Microsoft Excel 2010. Two reviewers independently abstracted data; disagreements on data elements were resolved by consensus. A random sample was re-abstracted by a third author for quality control; any discrepancies were resolved and the process updated as needed. Each intervention was classified into a category (electronic/web-based, in-person counseling, pharmacotherapy, or telephone counseling). When more than one intervention was included, trials were classified based on their primary focus. In these multimodal trials, primary focus was determined by the trial report. For instance, Burns et al. conducted a two-arm trial in the NY State Quitline where participants in the intervention arm were randomized to receive 4 vs. 8 weeks of NRT. This study would be classified as a pharmacotherapy intervention as both the intervention and control arms received the quitline care, but only the intervention arm received NRT. As another example, Wetter et al. conducted a trial where smokers received and initial group counseling session followed by computer-based treatment vs. no further treatment. The intervention arm in this trial would be considered a multimodality electronic/web-based intervention as the control did not receive the computer-based treatment. Where a primary intervention was not specified we selected the intervention component that had to be fulfilled in order to receive supplemental components. All supplemental intervention types were noted. Trials with multiple intervention arms of the same generic type were combined and compared to the study’s specified control arm (23). When intervention arms were of different intervention types, they were not combined.
Data were abstracted for the self-reported and biochemically verified number of individuals in each arm who were abstinent based on 7-day point prevalence of cessation at 6 and 12-months and the total number in the arm; all data abstraction was based on intention-to-treat. We assumed that participants lost to follow-up were not successful in smoking cessation. Additionally, we abstracted data on sample size, retention rate, the proportion of eligible individuals who enrolled in the trial, age, smoking history (cigarettes per day, years smoking, and pack-years, if available), active vs. minimal/usual care control, motivation to quit, whether or not conflicts of interest were reported by the authors, and funding source. The response rate by intervention arm were not abstracted.
We assessed the methodological quality of trials using an established system (24, 25). Studies were given one point based on having each of the following criteria: 1) a description of the methodology of randomization; 2) randomization resulted in balanced groups; 3) a description of the methods of masking participant allocation; 4) use of double-blinding when feasible; 5) a description of the follow-up rates and reasons for withdrawal; and 6) reported all study outcomes. Trials could receive a total of six points. In this system, a score of two or less was considered poor quality, and scores of three and above were deemed of moderate to high quality (24). The Society for Research on Nicotine and Tobacco recommends that studies with a significant in-person component use biochemical verification (26). Therefore, studies in the in-person counseling groups only received the point for reporting outcomes if they presented biochemically verified results. Additionally, as double-blinding is not always feasible in certain intervention types (electronic/web-based, in-person counseling, or telephone counseling) these interventions were scored out of five points, where two or more points were deemed moderate to high quality (27). Factors such as response rate, or mode of recruitment were not considered as indicators of study quality.
2.4. Data Synthesis and Analysis
The primary analysis was based on self-reported or biochemically verified 7-day abstinence at 6-months in the intervention arm vs. the control arm; 12-month outcomes were a secondary endpoint. When available, biochemically verified cessation rates were used for analysis; otherwise, cessation outcomes were self-reported.
We estimated potential publication bias using contour-enhanced funnel plots where an asymmetric plot suggests the possibility that studies with null intervention effects were less likely to be published than those with significant results (28). Funnel plot asymmetry was assessed using a simple weighted linear regression proposed by Peters, et al. rather than Egger’s test, since the latter does not perform well when examining effects in large numbers of studies with moderate to high heterogeneity (28). A p-value p<0.05 for Peters’ test is considered an indication of possible publication bias.
The DerSimonian and Laird random-effects method was used to determine odds ratios and 95% confidence intervals for each intervention category, where each study effect was weighted by its sample size and variance (29, 30). The random-effects model, which recognizes variance between and within studies, was employed because heterogeneity was expected based on differences in interventions and patient populations. A measure of heterogeneity (I2) was also calculated. I2 values of 50%−75% and ≥75% indicate moderate and high heterogeneity, respectively. For one study, there was no event in the control arm (i.e., no quitters). In this case, 0.5 was added to each cell (intervention and control) to avoid infinite odds (31).
Sensitivity analyses tested the effects on pooled cessation estimates at 6-months for intervention arms that included pharmacotherapy as a supplemental intervention vs. not; single-vs. multi-modality interventions, where the intervention arm included supplementary interventions beyond the primary intervention type (including pharmacotherapy); if the study was able to enroll >50% of eligible patients; and active vs. minimal or no intervention controls. Additionally, we analyzed the impact on effect sizes of omitting trials that were identified as being of poor quality, or were industry-sponsored, on outcomes at 6-months. Finally, we compared the pooled statistics of biochemically-verified-only results with self-report-only results at both 6- and 12-months.
All analyses were conducted in STATA 14.0 (StataCorp. 2015. College Station, TX.).
3. Results
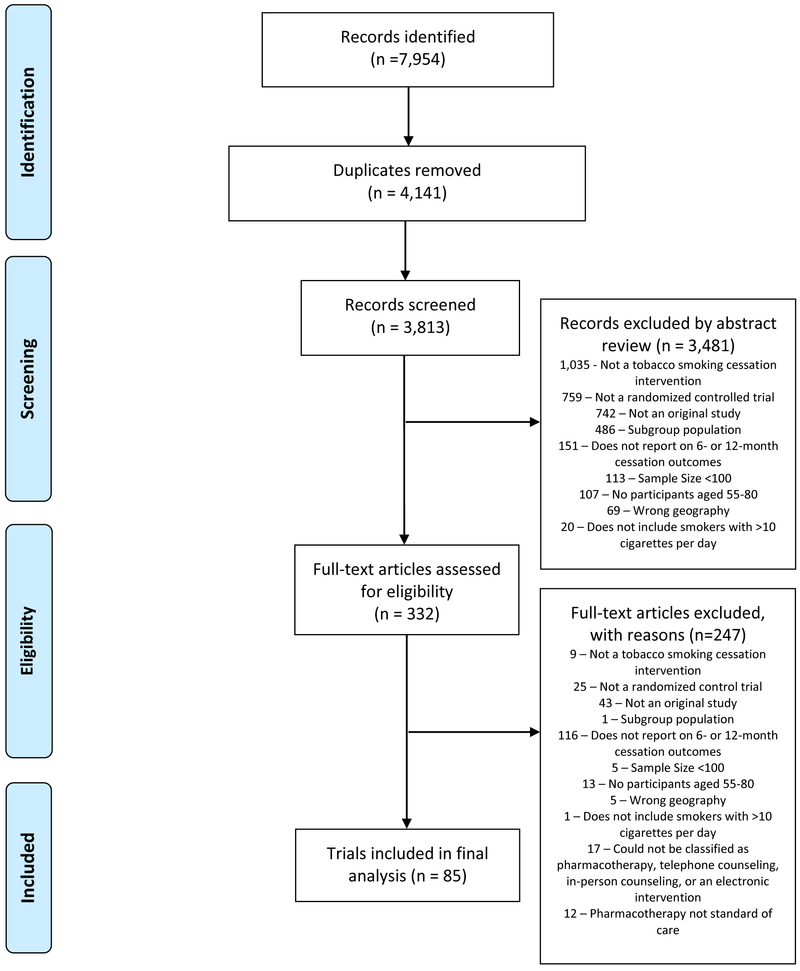
Searches identified 3813 unique articles of potentially eligible trials. The full screening process and reasons for exclusion are outlined in the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) diagram (Figure 1). Through abstract and title review, 332 articles were identified as potentially eligible. Inter-rater reliability was high between the three reviewers (Cohen's κ 0.72-0.84). Following the full-text review, 85 trials were deemed eligible and data were abstracted.
Figure 1: Selection of Trials Published from 2010 to 2018 to Estimate the Efficacy of Smoking Cessation in Lung Screening-eligible Populations.
The PRISMA diagram depicts the flow of studies through the phases of the systematic review from study identification to data analysis. A priori reasons for exclusion are presented at each stage.
The 85 trials in the final analytic sample included 74 that reported 6-month outcomes for 93,827 participants; and 40 that reported 12-month outcomes for 46,844 participants. The trials ranged in size from 103 to 16,430 participants and the majority were conducted in the US (Table 1). We identified 26 publications that used electronic or web-based intervention methods, 25 utilized in-person counseling, 25 utilized pharmacotherapy agents, and 14 utilized telephone counseling. Twenty-seven trials included more than one intervention arm; of these, 22 had intervention arms of similar types that were collapsed into one. Five trials had intervention arms that were categorized into separate primary intervention types (Table 1). Forty-five trials (52.9%) included biochemically verified smoking cessation outcomes. Fourteen trials did not report the number of eligible individuals who declined to participate, of those that did the majority (71.7%) enrolled >50% of eligible individuals. Results for studies with higher (50%+) vs. lower (<50%) participation were similar.
Table 1:
Characteristics of Trials Included in Meta-Analysis of Smoking Cessation Efficacy by Category of Intervention.
| Authors | Ye ar |
Intervention Description |
Multi- Modal Interven tion |
Biochem ical Verificat ion |
Outco me |
Sam ple Size |
Me an Age (SD )* |
Mean Cigarette s per Day (SD)* |
Respo nse Rate at 6- month s |
|---|---|---|---|---|---|---|---|---|---|
| Electronic/Web-Based | |||||||||
| Abroms et al.(43) | 2014 | Text messaging vs. website control | Yes | Yes | 6-months | 503 | 35.7 (10.7) | 17.29 (8.03) | 76% |
| Bock et al.(44) | 2010 | Computer driven individually tailored intervention vs. computer driven individually tailored intervention with NRT | Yes | Yes | 6-months | 300 | ~45.5 (10.8) | ~18.2 (9.1) | 80% |
| Bolman et al.(45) | 2015 | Computer tailored cessation messages with action plan vs. computer tailored cessation messages | No | No | 6-months | 1982 | 38.8 (11.4) | NR | 23% |
| Borland et al.(46) | 2013 | Personalized internet-delivered advice program vs. text messaging program | Yes | No | 6-months | 3530 | 42.1 (NR) | 16.9 (NR) | 86% |
| Bricker et al.(47) | 2017 | Website based behavioural cessation program vs. standard cessation website | No | No | 6- and 12-months | 2637 | 46.2 (13. 4) | NR −33% smoke >20 CPD | 88% |
| Brown et al.(48) | 2014 | Interactive website vs. control website | No | No | 6-months | 4613 | 39.5 (13) | 18.7 (8.9) | 72% |
| Calhoun et al.(49) | 2016 | Internet-based tele-health intervention vs. clinic referral | Yes | No | 12-months | 413 | 42.9 (13. 9) | 15.2 (8.7) | NR |
| Choi et al.(50) | 2014 | Interactive website with nurse counseling by phone vs. state quitline | Yes | No | 6-months | 145 | 42 (9.5) | 20.9 (9.9) | 73% |
| Cobos-Campos et al.(51) | 2017 | Texting intervention + brief counseling vs. brief counseling alone | Yes | Yes | 6-months | 320 | 45 (9.1) | NR −94.6% >5 CPD | 46% |
| Free et al.(52) | 2011 | Text message cessation program vs. non-cessation related text message program | Yes | No | 6-months | 5800 | ~36.8 (11) | NR | 96% |
| Gilbert et al.(53) | 2013 | Computer tailored cessation advice and progress report vs. non-tailored information | Yes | No | 6-months | 6911 | 44.6 (12.2) | 17.8 (9.4) | 75% |
| Houston et al.(54) | 2013 | Cessation website and brief advice vs. usual care | Yes | No | 6-months | 576 | NR −43.2 % aged 45+ | NR | 98% |
| Houston et al.(55) | 2015 | Enhanced cessation website with counselor messenger vs. enhanced cessation website vs. control website | No | No | 6-months | 900 | NR −33 % aged 55+ | NR – 73% smoke >10 CPD | 51% |
| Leykin et al.(56) | 2012 | Four internet-based smoking cessation interventions of increasing intensity | Yes | No | 6- and 12-months | 16430 | ~36.5 (14.5) | −19.5 (10.1) | 25% |
| Loughead et al.(57) | 2016 | Web-based relaxation guide and 8-weeks NRT vs. Web-based relaxation and cognitive conditioning with 8-weeks NRT | Yes | Yes | 6-months | 213 | 43.3 (12.5) | 16.1 (5.7) | 83% |
| Mason et al.(58) | 2012 | Computer tailored cessation advice and progress report vs. non-tailored content | Yes | No | 6-months | 1758 | 37.8 (11.3) | 18.2 (8.7) | NR |
| Moskowitz et al.(59) | 2016 | Internet-based program vs. virtual support and reinforcement | Yes | No | 6-months | 403 | 40.7 (10.6) | 13.1 (6.8) | 50% |
| Reitzel et al.(60) | 2011 | Computer delivered treatment vs. standard treatment; plus pharmacotherapy | Yes | Yes | 6- and 12-months | 303 | 41.4 (10.1) | 22.5 (10.4) | NR |
| Richter et al.(61) | 2015 | 4 computer-based telemedicine sessions in primary care setting vs. 4 sessions of telephone counseling | Yes | No | 6- and 12-months | 566 | 47.4 (12.9) | 19.7 (10.3) | 86% |
| Sherratt et al.(62) | 2018 | Computer-based lung cancer risk projection and brief counseling and personalized pamphlet vs. generic smoking risk pamphlet | Yes | No | 6-months | 302 | ~42 (NR) | ~20 (NR) | 62% |
| Smit et al.(63) | 2012 | Fully automated web-based smoking cessation program vs. no intervention | Yes | No | 6-months | 1129 | ~48.4 (12.2) | 20.6 (12.4) | 26% |
| Stanczyk et al.(64) | 2016 | Text messaging vs. video messaging vs. brief message control | No | No | 6- and 12-months | 2099 | 45.7 (12.8) | 18.8 (8.6) | 58% |
| Westmass et al.(65) | 2018 | Three varying level of intensity of emailed cessation advice | Yes | No | 6-months | 1070 | 40.3 (11.8) | 17.4 (7.9) | 60% |
| Wetter et al.(66) | 2011 | Initial group counseling followed by computer delivered treatment vs. no further treatment | Yes | Yes | 6- and 12-months | 302 | ~44 (11. 2) | ~20.5 (8) | 98% |
| In-person Counseling | |||||||||
| Andrews et al.(67) | 2016 | Community health worker and group support sessions vs. written materials | Yes | Yes | 6- and 12-months | 409 | ~41.1 (14.1) | ~12.6 (7.5) | 93% |
| Bock et al.(68) | 2014 | Motivational enhancement treatment, physician advice, and NRT vs. standard care | Yes | Yes | 6- and 12-months | 846 | 39.6 (11.4) | NR | 50% |
| Brooks et al.(69) | 2017 | Multiple visits by Tobacco Treatment Advocate vs. single visit | Yes | Yes | 12-months | 331 | NR −68 % aged 40+ | NR 43.2% smoke >10 CPD | 76%† |
| Catley et al.(70) | 2016 | 4 sessions of motivational interviewing vs. 4 sessions of health education vs. brief advice | Yes | Yes | 6-months | 255 | 45.8 (10.9) | 17.1 (8.9) | 89% |
| Choi et al.(71) | 2016 | Culturally tailored counseling program vs. untailored counseling program | Yes | Yes | 6-months | 463 | 44.3 (NR) | 15.4 (NR) | 54% |
| Davis et al.(72) | 2014 | Mindfulness counseling program vs. American Lung Association matched program | Yes | Yes | 6-months | 135 | 44.5 (12.7) | 17.7 (8.6) | 44% |
| Garvey et al.(73) | 2012 | Front-loaded counseling vs. weekly counseling | No | No | 6- and 12-months | 278 | 46.9 (11.5) | 17.9 (7.9) | 90% |
| Gifford et al.(74) | 2011 | Bupropion with acceptance and relationship focused behavioural intervention vs. bupropion alone | Yes | Yes | 6-months | 303 | ~45.8 (12.8) | ~24.0 (8.6) | 70% |
| Hooper et al.(55) | 2017 | 8 group sessions of culturally tailored cognitive behavioral therapy vs. 8 standard cognitive behavioural therapy sessions; both with NRT | Yes | Yes | 6- and 12-months | 342 | 49.5 (NR) | 18.0 (10.8) | 87% |
| Kim et al.(75) | 2015 | Culturally tailored counseling vs. standard counseling; plus NRT | Yes | Yes | 12-months | 109 | 49.7 (9.3) | 17.1 (5.8) | 73% |
| Laude et al.(76) | 2017 | In-person cognitive behavioral therapy for 26 weeks vs. 48 weeks | Yes | Yes | 12-months | 219 | ~42.1 (12.1) | ~16.7 (5.9) | 93% |
| Okuyemi et al.(77) | 2013 | NRT and motivational interviewing vs. NRT and brief advice | Yes | Yes | 6-months | 430 | 44.4 (9.9) | 19.3 (13.7) | 75% |
| Pesis-Katz et al.(78) | 2011 | Four sessions with health counselors vs. smoking cessation pamphlets and information on local treatment programs | Yes | Yes | 6-months | 737 | ~45.8 (12) | ~20.2 (10) | 70% |
| Ramos et al.(79) | 2010 | Individual counseling vs. group counseling vs. minimal intervention | Yes | Yes | 12-months | 287 | ~45 (10.9) | ~20 (NR) | 50% |
| Sheffer et al.(80) | 2017 | 6 standard cognitive behavioral therapy sessions vs. 6 socioeconomic status adapted cognitive behavioral therapy session | Yes | Yes | 6-months | 227 | 48.2 (9) | 13.8 (7.4) | 88% |
| Smith et al.(81) | 2014 | Culturally tailored in-person counseling for American Indian/Alaska Native vs. non-tailored counseling; plus varenicline | Yes | Yes | 6-months | 103 | 39.8 (13.1) | 14.4 (7.9) | 95% |
| Vidrine et al.(82) | 2016 | Mindfulness-based counseling program vs. cognitive behavioral therapy vs. brief counseling session | Yes | Yes | 6-months | 485 | 48.7 (11.9) | 19.9 (10.1) | 56% |
| Webb et al.(83) | 2010 | Group cognitive behavioral therapy vs. group general health education; plus NRT | Yes | No | 6-months | 154 | 44 (NR) | 13 (NR) | 70% |
| Wewers et al.(84) | 2017 | In-person counselling from community health worker vs. quitline | Yes | Yes | 6- and 12-months | 707 | NR −30.7 % aged 55+ | ~22.3 (11.7) | 85%† |
| Whiteley et al.(85) | 2012 | Cognitive behavioral therapy + exercise vs. cognitive behavioral therapy + contact control | No | Yes | 6- and 12-months | 330 | 43.52 (9.96) | 17.48 (7.16) | 81% |
| Williams et al.(86) | 2016 | In-person counseling 8 session + medication vs. 8 sessions alone vs. 6 sessions | Yes | No | 12-months | 820 | 47.39 (NR) | 18.87 (NR) | 25% |
| Pharmacotherapy | |||||||||
| Anthenelli et al.(87) | 2016 | Varenicline vs. bupropion vs. nrt patch vs. placebo | No | Yes | 6-months | 4028 | ~46.1 (12.8) | ~20.8 (8.2) | 78% |
| Baker et al(88) | 2016 | Varenicline vs. NRT Patch with Lozenge vs. NRT patch | Yes | Yes | 6- and 12-months | 1086 | 48.1 (11.6) | 17.0 (8.3) | 84%† |
| Bullen et al.(89) | 2010 | Pre-cessation NRT in quitline vs. quitline usual care | Yes | Yes | 6-months | 1100 | 39.6 (13.1) | 19 (8.7) | 74% |
| Burns et al.(90) | 2014 | 4 vs. 8 weeks of NRT in state quitline | Yes | No | 6-months | 1495 | NR −15.3% aged 55+ | ~19.8 (NR) | 58% |
| Caldwell and Crane(91) | 2016 | NRT inhaler vs. placebo inhaler both with NRT patch 5 weeks | Yes | No | 6-months | 502 | ~45.2 (11.2) | ~19 (6.7) | 62% |
| Caldwell et al.(92) | 2014 | Nicotine spray vs. placebo both with NRT patch | Yes | Yes | 6- and 12-months | 1423 | ~45.6 (11.4) | ~20 (7.3) | 20% |
| Carpenter et al.(93) | 2011 | NRT sampling and practice quit attempt vs. practice quit attempt alone | Yes | No | 6-months | 849 | ~50.5 (11.8) | ~18.6 (8.8) | 87% |
| Cinciripini et al.(94) | 2013 | 12-weeks of Varenicline, Bupropion, or Placebo plus intensive counseling | Yes | Yes | 6-months | 294 | 44.3 (10.43) | 19.7 (9.36) | 73% |
| Cummings et al.(95) | 2011 | Callers to quitline randomized to 2, 4, or 6 weeks of NRT patch | Yes | No | 6-months | 2806 | NR ~27.9% aged 55+ | NR 67.8% smoke >20 CPD | 60% |
| Ebbert et al.(96) | 2014 | 12 weeks of varenicline/bupro pion combination vs. 12 weeks varenicline/placebo | Yes | Yes | 6- and 12-months | 506 | ~42.2 (12.2) | ~19.5 (7.3) | 60% |
| Gonzales et al.(97) | 2014 | Varenicline vs. Placebo | No | Yes | 6- and 12-months | 498 | 47.5 (NR) | 20 (NR) | 63%† |
| Hughes et al.(98) | 2011 | Varenicline vs. placebo; plus behavioral counseling | Yes | Yes | 6-months | 218 | ~45 (13) | ~19 (9) | 70% |
| Lerman et al.(99) | 2015 | Verenicline + patch vs. patch + placebo vs. Placebo | Yes | Yes | 6- and 12-months | 1246 | 45 (12) | 17.5 (5.9) | 71% |
| Ramon et al.(l00) | 2014 | Varenicline + patch vs. Varenicline + placebo | No | No | 6-months | 341 | ~44.1 (14.8) | ~29.2 (NR) | 71% |
| Rennard et al.(101) | 2012 | Varenicline vs. placebo | Yes | No | 6-months | 650 | ~43.9 (12.5) | ~21.3 (NR) | NR |
| Rose and Behm(102) | 2013 | NRT patch vs. bupropion + NRT patch vs. varenicline | Yes | Yes | 6-months | 335 | ~46.0 (10.8) | ~21.9 (8.8) | 58% |
| Schnoll et al.(103) | 2015 | 8 vs. 24 vs. 54 weeks NRT patch | Yes | Yes | 6- and 12-months | 525 | 46.4 (12.1) | 17.1 (8.4) | 65% |
| Schnoll et al.(104) | 2010 | Nicotine patch vs. nicotine lozenge | Yes | Yes | 6- and 12-months | 568 | ~44.7 (12.7) | ~20.6 (8.9) | 76% |
| Selby et al.(105) | 2014 | NRT, Bupropion, or Varenicline prescription with vs. without payment card | No | Yes | 6-months | 1380 | ~46.5 (12.3) | ~22.2 (9.5) | 65% |
| Stapleton et al.(106) | 2013 | NRT vs. bupropion vs. bupropion plus NRT; plus behavioral support | Yes | Yes | 6-months | 1071 | ~41.2 (12.1) | ~20.3 (9.7) | 62% |
| Tønnesen et al.(107) | 2012 | NRT mouth spray vs. placebo mouth spray | Yes | Yes | 6- and 12-months | 470 | ~47 (10.9) | ~22.7 (8.8) | 50% |
| Tulloch et al.(108) | 2016 | 10 weeks NRT patch vs. 10 weeks patch + gum or inhaler vs. 12 varenicline; all receive counseling | Yes | Yes | 6- and 12-months | 737 | 48.6 (10.8) | 23.2 (10.8) | 69% |
| Walker et al.(109) | 2011 | Quitline with nicotine sampling vs. standard quitline care | Yes | Yes | 6-months | 1410 | ~40.5 (13.4) | ~20 (9.6) | 81% |
| Telephone Counseling | |||||||||
| Bastian et al.(110) | 2013 | Counselor initiated counseling calls vs. tailored self-directed materials | Yes | No | 6- and 12-months | 496 | ~46 (12) | ~20 (11) | 100% |
| Fu et al.(111) | 2015 | Proactive telephone counseling vs. usual care | Yes | No | 12-months | 2406 | NR −46.1% aged 35-64 | 13.6 (9.2) | 74% |
| Klemperer et al.(112) | 2017 | Telephone-based motivational interviewing vs. Telephone based cigarette reduction vs. Brief telephone counseling | Yes | No | 6- and 12-months | 560 | 51 (11) | 20 (8.4) | 63% |
| Klesges et al.(113) | 2015 | Proactive quitline with 8-weeks NRT vs. reactive quitline with 2-weeks NRT | Yes | No | 12-months | 1298 | 39.5 (13.7) | 17.8 (8.5) | 80% |
| Linqvist et al.(114) | 2013 | Motivational interviewing vs. standard treatment in a quitline setting | Yes | No | 12-months | 772 | ~48 (14.2) | NR | 62% |
| Maddison et al.(115) | 2014 | Telephone based exercise program vs. Quitline | Yes | No | 6-months | 906 | 37.5 (12.2) | 19.6 (9.3) | 92% |
| Nohlert et al.(116) | 2014 | Proactive vs. reactive calls in a national quitline. | No | No | 12-months | 586 | NR -56% aged 50+ | NR - 39% smoke >15 CPD | 59%† |
| Sherman et al.(117) | 2018 | Proactive vs. reactive telephones counseling | No | No | 6-months | 2003 | ~53.7 (10.8) | ~17.7 (9.9) | 79% |
| Sumner et al.(118) | 2016 | Directive vs. non-directive telephone counseling | No | No | 6- and 12-months | 518 | ~47 (NR) | ~11 (NR) | 56% |
| Tzelepis et al.(119) | 2010 | Proactive telephone cold-calls vs. mailed written materials | Yes | No | 6- and 12-months | 1562 | ~45.4 (12.7) | ~19.9 (9.6) | 82% |
| Zhu et al.(120) | 2012 | Culturally tailored multilingual telephone counseling vs. self-help materials | Yes | Yes | 6-months | 2277 | NR –52% aged 45+ | NR –54.9% smoke >14 CPD | 90% |
| Zwar et al.(121) | 2015 | Nurse advice + quitline vs. quitline vs. usual care | Yes | No | 12-months | 2390 | ~43.5 (14.3) | ~17.4 (10.7) | 83% |
| Multiple Categories | |||||||||
| Hall et al.(122) | 2011 | Combination of extended behavioural therapy, pharmacotherapy and placebo | Yes | Yes | 6- and 12-months | 406 | 40.7 (9.8) | 19 (7.4) | 95% |
| Levine et al.(123) | 2010 | Weight concern related smoking cessation vs. standard cessation counseling; both with/without placebo or bupropion | Yes | Yes | 6- and 12-months | 349 | 42.0 (10.1) | 20.7 (8.4) | 53% |
| Ramon et al.(124) | 2013 | Individual counselling, combined telephone and individual counselling, or telephone counselling. | Yes | No | 6- and 12-months | 600 | 47.4 (12.1) | ~26.7 (12.9) | 71%† |
| Smit et al.(125) | 2016 | Web-based computer tailoring and nurse counseling vs. computer tailoring alone vs. usual care | Yes | No | 6- and 12-months | 414 | 48.0 (11.9) | NR | 38% |
| Swan et al.(126) | 2010 | Web-based counseling vs. telephone-based counseling vs. combined web and telephone; plus varenicline | Yes | Yes | 6-months | 1202 | 47.3 (NR) | 19.7 (NR) | 74% |
Studies that did not report the information are marked as not reported (NR). Mean age and standard deviation (SD), as well as mean cigarettes per day (CPD) and SD for the entire trial sample, was not reported in all studies. For those studies that only reported mean age and CPD by arm, the values for the intervention arm are provided and marked with a tilde. For those studies that only reported median the median is reported and marked with a tilde and SD is marked as not reported. For those studies that only included age and CPD categories the categories that are most similar to the age and CPD levels of interest for this analysis are reported.
Studies report response rate at 12-months.
There were notable differences in the structure of interventions within the four categories (Table 1). Pharmacotherapy interventions included the use of nicotine replacement for a little as two weeks to up to a year; and included various combinations of NRT and bupropion or varenicline. In-person counseling interventions ranged from short (15 minute) one-time counseling sessions to multiple hour-long individual or group sessions. Both telephone and in-person counseling interventions may have included culturally tailored interventions. Electronic/web-based interventions included website based cessation programs, texting interventions, and email reminders. The majority of trial intervention arms (82.2%) were classified as multimodal interventions, and 53.3% included active controls.
3.1. Study Outcomes
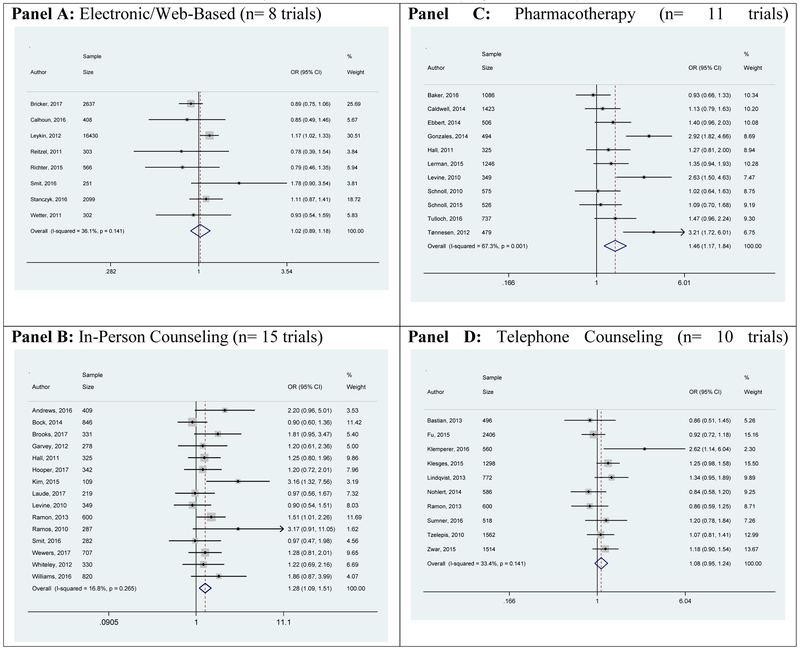
All interventions showed increased odds of quitting smoking (vs control) based on 7-day point prevalence of smoking abstinence at 6-months, but the telephone counseling effect did not reach statistical significance (Figure 2 and Table 2). We found pooled odds ratios and 95% confidence intervals for electronic/web-based of 1.14 (1.03-1.25), in-person counseling 1.46 (1.25-1.70), and pharmacotherapy 1.53 (1.33-1.77) and telephone counseling 1.21 (0.98-1.50).
Figure 2: Odds of Smoking Cessation From Random-Effects Meta-Analysis of Trials with Smokers Potentially Eligible for Lung Screening Based on 7-day Point Prevalence of Abstinence at 6-Months by Primary Intervention Type (n= 74 Trials)*.
Forest plots display weighted odds ratios and 95% confidence intervals of included trials. Trial weights are generated from a random effects analysis. Squares around point estimates indicate study weight relative to the lowest weighted study for each meta-analysis. The vertical dashed line represents the pooled odds ratio with the diamond representing the 95% confidence interval.
* Some trials included more than one intervention of a differing generic type so that the sum of the sample of all intervention types is greater than the total number of trials included.
Table 2:
Odds of Smoking Cessation from Random-Effects Meta-Analysis of Trials with Smokers Potentially Eligible for Lung Screening Based on 7-day Point Prevalence of Abstinence at 6-Months and 12-Months by Primary Intervention Type
| Electronic/We b-Based |
In-Person Counseling |
Pharmacother apy |
Telephone Counseling |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | OR (95% CI) |
I2 (% )* |
n | OR (95% CI) |
I2 (% )* |
n | OR (95% CI) |
I2 (% )* |
n | OR (95% CI) |
I2 (% )* |
|
| Overall at 6-Months | 25 | 1.14 (1.03-1.25) | 57.2 | 20 | 1.46 (1.25-1.70) | 24.7 | 25 | 1.53 (1.33-1.77) | 73.7 | 9 | 1.21 (0.98-1.50) | 74.4 |
| Without Pharmacotherapy | 17 | 1.13 (1.01-1.26) | 53.7 | 3 | 1.16 (0.84-1.62) | 0.0 | NA | NA | NA | 5 | 1.37 (1.02-1.85) | 77.5 |
| With Pharmacotherapy | 8 | 1.13 (0.92-1.40) | 60.3 | 17 | 1.54 (1.29-1.83) | 30.3 | NA | NA | NA | 4 | 1.05 (0.89-1.24) | 0.0 |
| Single Modality | 7 | 1.15 (0.97-1.35) | 55.5 | 2 | 1.22 (0.83-1.78) | 0.0 | 4 | 1.87 (1.47-2.39) | 53.9 | 2 | 1.07 (0.84-1.37) | 16.0 |
| Multi-Modality | 18 | 1.13 (1.00-1.28) | 58.2 | 18 | 1.51 (1.27-1.79) | 29.7 | 21 | 1.47 (1.26-1.71) | 70.1 | 7 | 1.27 (0.98-1.65) | 72.2 |
| Minimal or No Intervention Control | 13 | 1.19 (1.04-1.37) | 43.1 | 8 | 1.57 (1.10-2.25) | 54.2 | 8 | 1.80 (1.28-2.54) | 84.8 | 6 | 1.38 (1.08-1.78) | 69.9 |
| Active Control | 12 | 1.09 (0.95-1.26) | 67.8 | 12 | 1.44 (1.23-1.69) | 0.0 | 17 | 1.42 (1.24-1.62) | 56.9 | 3 | 0.98 (0.78-1.23) | 34.0 |
| Biochemically Verified Results | 7 | 1.31 (0.93-1.83) | 65.6 | 16 | 1.44 (1.20-1.73) | 31.4 | 19 | 1.55 (1.33-1.81) | 69.0 | 2 | 1.52 (0.92-2.49) | 89.6 |
| Self-Reported Results | 20 | 1.16 (1.05-1.29) | 62.3 | 9 | 1.75 (1.40-2.17) | 22.0 | 12 | 1.51 (1.23-1.86) | 78.5 | 7 | 1.10 (0.94-1.30) | 24.9 |
| High Quality | 22 | 1.11 (1.01-1.23) | 57.8 | 16 | 1.44 (1.24-1.67) | 0.0 | 24 | 1.53 (1.32-1.77) | 74.6 | 8 | 1.22 (0.97-1.53) | 77.4 |
| Low Quality | 3 | 1.56 (1.02-2.38) | 34.3 | 4 | 1.68 (0.96-2.96) | 72.0 | 1 | 1.66 (1.20-2.28) | . † | 1 | 1.13 (0.62-2.04) | . † |
| No Conflict of Interest Reported | 22 | 1.14 (1.02-1.28) | 62.0 | 18 | 1.50 (1.27-1.78) | 27.7 | 16 | 1.34 (1.19-1.51) | 46.8 | 9 | 1.21 (0.98-1.50) | 74.4 |
| Conflict of Interest Reported | 3 | 1.17 (1.04-1.32) | 0.0 | 2 | 1.22 (0.82-1.82) | 0.0 | 9 | 2.01 (1.46-2.77) | 82.1 | NA ‡ | NA ‡ | NA ‡ |
| Studies With >50% Enrollment | 14 | 1.16 (1.02-1.31) | 64.3 | 11 | 1.48 (1.19-1.84) | 36.0 | 13 | 1.50 (1.25-1.80) | 70.4 | 8 | 1.22 (0.97-1.53) | 77.4 |
| Studies With <50% Enrollment | 4 | 1.35 (0.96-1.92) | 58.3 | 5 | 1.35 (1.05-1.74) | 0.0 | 8 | 1.43 (1.16-1.77) | 58.7 | 1 | 1.13 (0.62-2.04) | . † |
| Overall at 12-Months | 8 | 1.02 (0.89-1.18) | 36.1 | 15 | 1.28 (1.09-1.51) | 16.8 | 11 | 1.46 (1.17-1.84) | 67.3 | 10 | 1.08 (0.95-1.24) | 33.4 |
| Biochemically Verified Results | 3 | 0.84 (0.60-1.67) | 0.0 | 12 | 1.31 (1.10-1.56) | 24.3 | 11 | 1.46 (1.17-1.84) | 67.3 | 1 | 0.86 (0.59-1.25) | . † |
| Self-Reported Results | 5 | 1.06 (0.90-1.26) | 54.6 | 5 | 1.32 (1.04-1.67) | 0.0 | 1 | 1.32 (0.93-1.86) | . † | 10 | 1.08 (0.95-1.24) | 34.1 |
The I2 statistic is a measure of heterogeneity that describes the percentage of variation across studies not due to chance.
The I2 cannot be calculated for a single intervention in a given group.
No telephone counseling interventions reported a conflict of interest.
At 12 months, overall efficacy was lower across all intervention groups (Table 2 and Appendix B.2) and only pharmacotherapy (OR 1.46 95% CI 1.17-1.84) and in-person counseling (OR 1.28 95% CI 1.09-1.51) remained statistically significant.
3.2. Sensitivity Analyses
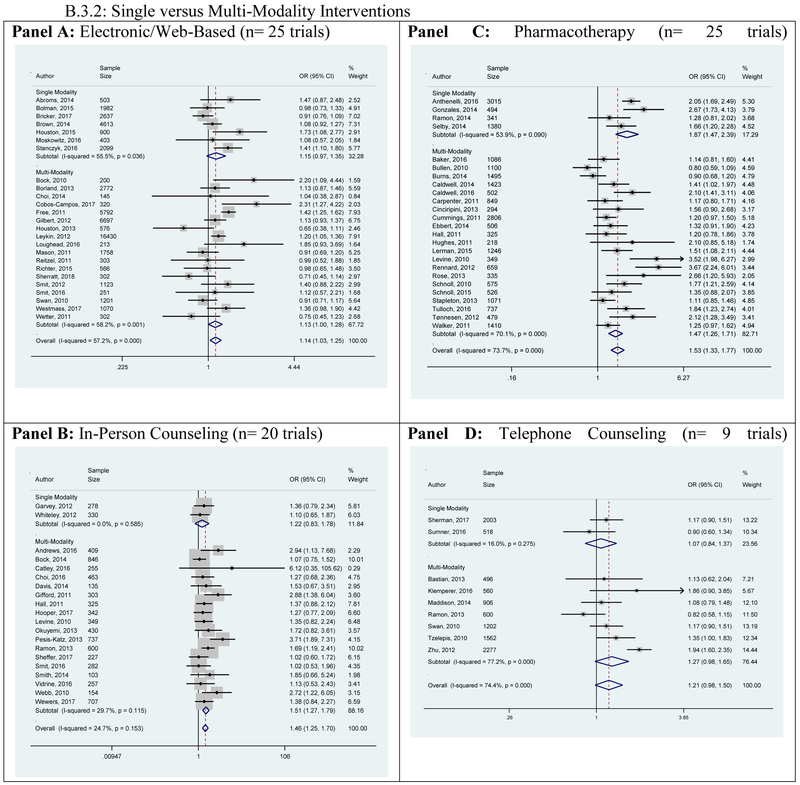
An examination of the role of pharmacotherapy as a supplemental intervention generally increased the odds of cessation, but results were not consistently statistically significant since most interventions included active controls (Table 2 and Appendix B.3.1). Multi-modality approaches had greater efficacy than single modality approaches (Table 2 and Appendix B.3.2), although single-modality pharmacotherapy interventions were more efficacious than other categories of multi-modality interventions; likely due to the use of placebo controls among the single-modality studies. Efficacy was higher for all intervention categories when compared to a minimal or no intervention control vs. an active control arm (Table 2 and Appendix B.3.3).
Results for biochemically verified abstinence did not differ appreciably from self-report at either 6- or 12-month outcomes (Table 2). The removal of poor quality studies, those that were unable to enroll >50% of eligible participants, and of studies that reported a conflict of interest had minimal impact on cessation outcomes at 6-months (Table 2).
3.3. Quality and Bias Assessment
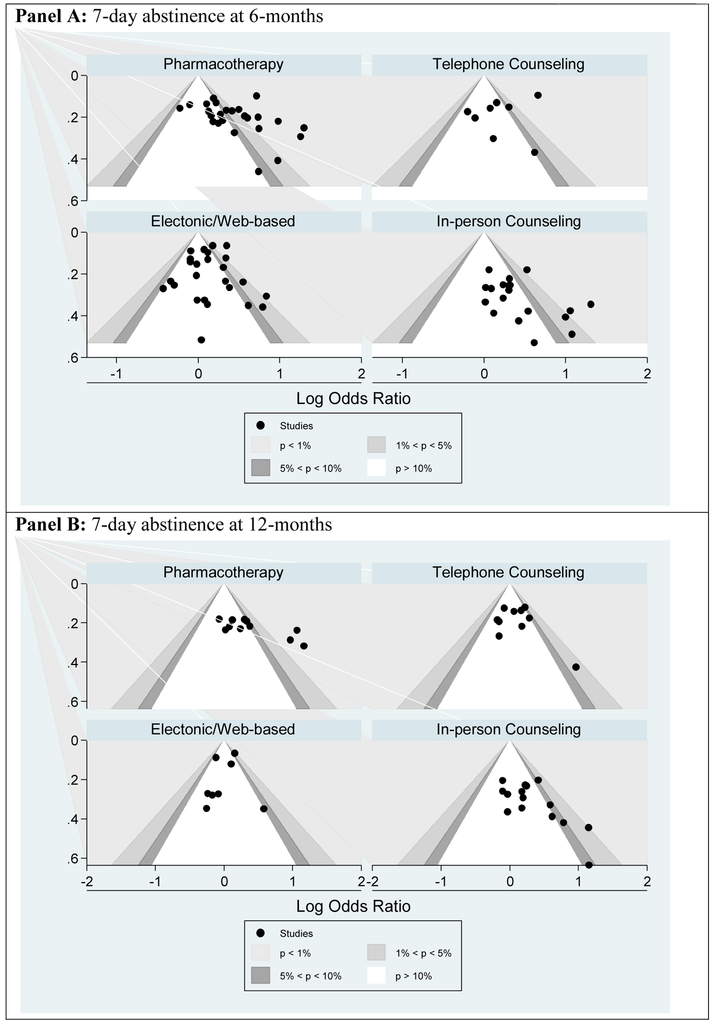
Only nine of the 85 trials included were determined to be low quality (Appendix B.4). The contour-enhanced funnel plots suggest that there may be some publication bias for in-person counseling and pharmacotherapy at 6- and 12-months (Appendix B.5). Peters’ test only found significant evidence of publication bias for the 6-month outcome of telephone counseling (Appendix B.6) suggesting among this group some trials may not have been published due to non-significant results. However, the interpretation of these results must acknowledge the difficulty of regression based measures of publication bias to account for between study heterogeneity (32).
4. Discussion
This meta-analysis is the only large synthesis of data on the efficacy of multiple categories of current smoking cessation interventions with populations similar to those eligible for LCS. We found that most classes of smoking cessation interventions were effective in increasing abstinence at 6 months among patients eligible for LCS. Among these, the most efficacious were pharmacological interventions, followed by in-person counseling and web-based approaches. Telephone counseling did not reach statistical significance at the 95% level, although the direction of the association is promising. This non-significant result may, in part, be due to the smaller number of telephone counseling studies (n=9 at 6-months). Multimodal interventions appeared to be more efficacious than a single modality. Finally, the odds of 6-month cessation appear to persist to 12-months among pharmacotherapy and in-person counseling interventions suggesting that LCS sites should consider the implementation of these interventions.
Our study is unique in its focus on cessation in older age groups and those with a heavier smoking history, making the results relevant to the LCS setting. Our results are similar to those of preliminary reports of studies in the field that suggest a range of smoking cessation interventions will be effective for individuals eligible for LCS (11-18). The results of four prior reviews of smoking cessation interventions for older adults suggested, like our meta-analysis, that most currently recommended approaches to cessation might be effective among older smokers (17, 19, 33, 34). However, three reviews did not pool study effectiveness. Two of these reviews considered only studies conducted in the screening setting, but were limited by small numbers of observational and randomized controlled trials and a dearth of substantial high quality data inappropriate for meta-analyzing (16, 17). We found small effects, and our summary odds ratios had lower point estimates (but overlapping confidence intervals) than previous meta-analyses of smoking cessation in general populations (10, 35-38). In contrast to studies in the general population, telephone counseling was not statistically significantly associated at the 95% level with cessation in our sample of trials that included older and heavier smokers, although the point estimate suggests a positive association and the number of trials was the smallest among the intervention categories (10, 36).
Our finding of lower intervention efficacy in LCS populations compared to use of the same interventions when applied in the general populations could be due to a greater difficulty to quit among long-term, heavy smokers compared to other smokers. Alternatively, our estimates may be lower because we included trials with both active and minimal care control groups, whereas the previous reviews compared intervention groups to minimal intervention controls (10, 35, 36). Our sensitivity analysis removing studies with an active control resulted in more comparable, albeit still lower point estimates of efficacy, across all intervention categories (10, 27, 35, 36).
Our findings show that multimodal interventions are likely to be more efficacious than single-modality interventions, although the results were inconclusive due to smaller samples in sub-group analyses. The greater efficacy in the single-modality pharmacotherapy arms is likely due to the four studies in the single-modality sub-group being placebo-controlled trials compared to predominantly active control trials in the multi-modality group (Table 1). This will be an important area for future investigation, since, if effective, single modality approaches are likely to be less costly than multi-faceted interventions. Our results, like those of others (10, 36), suggest that supplemental pharmacotherapy will be beneficial as part of multi-modality approaches to improving the odds of cessation in the LCS setting. It is encouraging that our results support cessation at 12-months among pharmacotherapy and in-person counseling interventions. Since long-term abstinence is necessary for the realization of screening benefits on mortality, it will be critical to re-evaluate the long-term maintenance of abstinence as new research studies become available. The ongoing NCI-funded Smoking Cessation at Lung Examination (SCALE) Collaboration trials were designed to address these gaps and the results are expected after 2021 (9).
The results of our study must be considered in the current context of LCS. To date, fewer than 5% of eligible individuals have presented for lung cancer screening (39, 40). Individuals who present for LCS are likely different from those who are eligible and not referred or those who are referred, but do not attend. The characteristics of these individuals will likely impact their willingness to accept cessation and their ultimate success in quitting smoking. We are unaware of research that looks at the different characteristics of those who do and don’t present for screening. However, it is possible that due to the healthy adherer bias (41), individuals who present for lung cancer screening are more likely to quit on their own. This would likely reduce the efficacy of an intervention tested in this setting, as participants in both the intervention and control arms would be more likely to quit on their own. Additionally, studies examining smoking cessation in lung screening trials found cessation to be associated with screen-detected abnormalities which could further bias results towards the null (42). It is hoped that future research by the SCALE Trials will provide some insight into these interactions. This meta-analysis has several strengths, including the large pooled sample size, the rigor of the methods, quality of included studies, and focus on trials that included smokers eligible for LCS. There are also several limitations that should be noted in considering our results. Our subgroup analysis by enrollment rate suggests that the results of these studies are likely to be generalizable to the target populations of the studies. However, none of the studies in this meta-analysis solely included individuals eligible for LCS. All trials included some individuals that were younger and with a lighter smoking history than necessary to qualify for lung cancer screening, potentially over-estimating effects that may be seen among smokers eligible for LCS. The effects seen in cessation trials in the LCS setting could also vary based on implementation difficulties, measurement differences, or differences in settings and populations. More attention should be focused on smoking cessation interventions for the LCS population, given the opportunity that screening provides for bringing smokers into cessation services and the mandate from the Centers for Medicaid and Medicare Services to include cessation as part of effective screening programs (8). Second, the pooling of studies into generic categories limits the ability to look more in depth at individual interventions or combinations of individual interventions, including the types or intensity of counseling, pharmacotherapy, and electronic interventions. Due to the limited number of studies in this setting, we were unable to compare results by specific types of pharmacotherapy or intervention intensity. Determining the most effective and feasible regimens in LCS is an important priority for future research.
5. Conclusion
The results of our meta-analysis provide important information to guide LCS sites, clinical practices, and health systems that are faced with having to make decisions about integrating smoking cessation interventions in their LCS practices ahead of definitive studies about cessation specific to screening populations. We found that multiple categories of cessation interventions are likely to be efficacious in a population similar to those undergoing LCS, but that screening sites looking for the most efficacious intervention could consider pharmacotherapy or in-person counseling since electronic/web-based and telephone counseling interventions either has non-significant effects at 6 months and/or failed to show effects on cessation at 12-months. With a wide range of possible effective interventions for screening sites to choose from, implementation will depend on feasibility, scalability, acceptability, cost, and specific characteristics of each environment and patient population. Results from ongoing clinical trials are expected to address several dimensions of implementation, efficacy, and cost (9). Until then, our results provide a useful framework for estimating the impact of different models of care for the integration of smoking cessation into the LCS setting.
Supplementary Material
Highlights:
Many efficacious interventions exist that could be implemented by screening sites.
Cessation estimates are lower than the general population.
Multi-modality interventions appear to be most efficacious.
Cessation persists at 12-months in two intervention categories.
Acknowledgements
This research was funded by the National Institutes of Health at the National Cancer Institute Grants U01CA199284, U01CA199218, and R01CA207228. The content of this article reflects the views of the authors. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
We would like to acknowledge the Smoking Cessation in Lung Examination (SCALE) Collaboration for their input and support for this work. We would also like to acknowledge the work of research librarian Helena VonVille who assisted in the development and conduct of the literature search, and shared invaluable article screening templates.
This research was funded by the National Institutes of Health at the National Cancer Institute Grants U01CA199284, U01CA199218, and R01CA207228. The content of this article reflects the views of the authors. The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Abbreviations:
- CMS
Centers for Medicare and Medicaid Services
- FDA
Food and Drug Administration
- LCS
lung cancer screening with low-dose computed tomography
- NCCN
National Comprehensive Cancer Network
- NELSON
Nederlands-Leuvens Longkanker Screenings ONdersoek trial
- NLST
National Lung Screening Trial
- OR
Odds Ratio
- SCALE
Smoking Cessation at Lung Examination trials
- USPSTF
United States Preventive Services Task Force
- 95% CI
95% Confidence Interval
Appendix
Appendix B.1:
Search Terms*
| Medline Search Strategy | |
|---|---|
| 1 | smoking/ or pipe smoking/ or tobacco smoking/ or cigar smoking/ or cigarette smoking/ or vaping/ |
| 2 | (cigar* or ecigarette* or smoking or tobacco or vaping).ti,ab,kw. |
| 3 | 1 or 2 |
| 4 | (abstinence or cessation or quit or quits or quitting or "stop smoking" or "stopped smoking").ti,ab,kw. |
| 5 | 3 and 4 |
| 6 | smoking cessation/ or smoking reduction/ or "tobacco use cessation"/ or "Tobacco Use Cessation Products"/ |
| 7 | 5 or 6 |
| 8 | limit 7 to (english language and yr="2010 - 2017") |
| 9 | (8 and (adult/ or aged/ or middle aged/)) or (8 not (adolescent/ or young adult/ or child/ or infant/)) |
| 10 | (9 and (north america/ or exp united states/ or exp australia/ or exp canada/ or exp europe/)) or (9 not (exp africa/ or exp asia/ or exp south america/)) |
| 11 | ("clinical trial" or "clinical trial, phase i" or "clinical trial, phase ii" or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or "multicenter study" or "randomized controlled trial").pt. or double-blind method/ or clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ or controlled clinical trials as topic/ or randomized controlled trials as topic/ or early termination of clinical trials as topic/ or multicenter studies as topic/ or (randomi?ed adj7 (studies or study or trial or trials)).ti,ab,kw. or (controlled adj3 trial*).ti,ab,kw. or (clinical adj2 trial*).ti,ab,kw. or ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab,kw. or ("4 arm" or "four arm").ti,ab,kw. |
| 12 | 10 and 11 |
| PsycINFO Search Strategy | |
| 1 | tobacco smoking/ |
| 2 | (cigar* or smoking or tobacco).ti,ab,id. |
| 3 | 1 or 2 |
| 4 | (abstinence or cessation or quit or quits or quitting or "stop smoking" or "stopped smoking").ti,ab,id. |
| 5 | 3 and 4 |
| 6 | smoking cessation/ |
| 7 | 5 or 6 |
| 8 | clinical trials/ or "treatment outcome clinical trial".md. or ((randomi?ed adj7 trial*) or ((single or doubl* or tripl* or treb*) and (blind* or mask*)) or (controlled adj3 trial*) or (clinical adj2 trial*)).ti,ab,id. |
| 9 | 7 and 8 |
| 10 | (aged 65 yrs older or middle age 40 64 yrs).ag. |
| 11 | (adolescence 13 17 yrs or school age 6 12 yrs or thirties 30 39 yrs).ag. |
| 12 | (9 and 10) or (9 not 11) |
| 13 | limit 12 to (all journals and english language and yr="2010 - 2017") |
| PubMed Search Strategy | |
| 1 | smoking[mesh:noexp] OR pipe smoking[mesh:noexp] OR tobacco smoking[mesh:noexp] OR cigar smoking[mesh:noexp] OR cigarette smoking[mesh:noexp] OR vaping[mesh:noexp] |
| 2 | (cigar*[tiab] OR ecigarette*[tiab] OR smoking [tiab] OR tobacco [tiab] OR vaping [tiab]) |
| 3 | #1 OR #2 |
| 4 | (abstinence[tiab] OR cessation[tiab] OR quit[tiab] OR quits[tiab] OR quitting[tiab] OR "stop smoking"[tiab] OR "stopped smoking"[tiab]) |
| 5 | #3 AND #4 |
| 6 | smoking cessation[mesh:noexp] OR smoking reduction[mesh:noexp] OR "tobacco use cessation"[mesh:noexp] OR "Tobacco Use Cessation Products"[mesh:noexp] |
| 7 | #5 OR #6 |
| 8 | #7 AND english[1a] AND 2010:2017[dp] |
| 9 | (#8 AND (adult[mesh:noexp] OR aged[mesh:noexp] OR middle aged[mesh:noexp])) OR (#8 NOT (adolescent[mesh:noexp] OR young adult[mesh:noexp] OR child[mesh:noexp] OR infant[mesh:noexp])) |
| 10 | (#9 AND (north america[mesh:noexp] OR united states[mesh] OR australia[mesh] OR canada[mesh] OR europe[mesh])) OR (#9 NOT (africa[mesh] OR asia[mesh] OR south america[mesh])) |
| 11 | Clinical Trial [PT:NoExp] OR "clinical trial, phase i"[pt] OR "clinical trial, phase ii"[pt] OR "clinical trial, phase iii" [pt] OR "clinical trial, phase iv"[pt] OR "controlled clinical trial" [pt] OR "multicenter study" [pt] OR "randomized controlled trial" [pt] OR "Clinical Trials as Topic"[mesh:noexp] OR "clinical trials, phase i as topic"[MeSH Terms:noexp] OR "clinical trials, phase ii as topic"[MeSH Terms:noexp] OR "clinical trials, phase iii as topic"[MeSH Terms:noexp] OR "clinical trials, phase iv as topic"[MeSH Terms:noexp] OR "controlled clinical trials as topic"[MeSH Terms:noexp] OR "randomized controlled trials as topic"[MeSH Terms:noexp] OR "early termination of clinical trials" [MeSH Terms:noexp] OR "multicenter studies as topic" [MeSH Terms:noexp] OR “Double-Blind Method”[Mesh] OR ((randomised[TIAB] OR randomized[TIAB]) AND (trial[TIAB] OR trials[tiab] OR study[tiab] OR studies[tiab])) OR ((single[TIAB] OR double[TIAB] OR doubled[TIAB] OR triple[TIAB] OR tripled[TIAB] OR treble[TIAB] OR treble[TIAB]) AND (blind* [TIAB] OR mask* [TIAB])) OR ("4 arm"[tiab] OR "four arm"[tiab]) |
| 12 | #10 AND #11 |
| PubMed Search Strategy | |
| 1 | smoking[mesh:noexp] OR pipe smoking [mesh:noexp] OR tobacco smoking[mesh:noexp] OR cigar smoking[mesh:noexp] OR cigarette smoking[mesh:noexp] OR vaping[mesh:noexp] |
| 2 | (cigar* [tiab] OR ecigarette*[tiab] OR smoking[tiab] OR tobacco[tiab] OR vaping[tiab]) |
| 3 | #1 OR #2 |
| 4 | (abstinence[tiab] OR cessation [tiab] OR quit[tiab] OR quits[tiab] OR quitting [tiab] OR "stop smoking"[tiab] OR "stopped smoking"[tiab]) |
| 5 | #3 AND #4 |
| 6 | smoking cessation[mesh:noexp] OR smoking reduction[mesh:noexp] OR "tobacco use cessation" [mesh:noexp] OR "Tobacco Use Cessation Products"[mesh:noexp] |
| 7 | #5 OR #6 |
| 8 | #7 AND english[1a] AND 2010:2017[dp] |
| 9 | (#8 AND (adult[mesh:noexp] OR aged[mesh:noexp] OR middle aged[mesh:noexp])) OR (#8 NOT (adolescent[mesh:noexp] OR young adult[mesh:noexp] OR child[mesh:noexp] OR infant[mesh:noexp])) |
| 10 | (#9 AND (north america[mesh:noexp] OR united states[mesh] OR australia[mesh] OR canada[mesh] OR europe[mesh])) OR (#9 NOT (africa[mesh] OR asia[mesh] OR south america[mesh])) |
| 11 | Clinical Trial [PT:NoExp] OR "clinical trial, phase i" [pt] OR "clinical trial, phase ii" [pt] OR "clinical trial, phase iii"[pt] OR "clinical trial, phase iv"[pt] OR "controlled clinical trial"[pt] OR "multicenter study" [pt] OR "randomized controlled trial" [pt] OR "Clinical Trials as Topic"[mesh:noexp] OR "clinical trials, phase i as topic"[MeSH Terms:noexp] OR "clinical trials, phase ii as topic"[MeSH Terms:noexp] OR "clinical trials, phase iii as topic"[MeSH Terms:noexp] OR "clinical trials, phase iv as topic"[MeSH Terms:noexp] OR "controlled clinical trials as topic"[MeSH Terms:noexp] OR "randomized controlled trials as topic"[MeSH Terms:noexp] OR "early termination of clinical trials" [MeSH Terms:noexp] OR "multicenter studies as topic” [MeSH Terms:noexp] OR “Double-Blind Method”[Mesh] OR ((randomised[TIAB] OR randomized[TIAB]) AND (trial[TIAB] OR trials[tiab] OR study[tiab] OR studies[tiab])) OR ((single[TIAB] OR double[TIAB] OR doubled[TIAB] OR triple[TIAB] OR tripled[TIAB] OR treble[TIAB] OR treble[TIAB]) AND (blind*[TIAB] OR mask*[TIAB])) OR ("4 arm"[tiab] OR "four arm"[tiab]) |
| 12 | #10 AND #11 |
On the advice of a research librarian, search terms were kept as broad as possible to find all potentially relevant smoking cessation studies. Studies were then eliminated during abstract and full-text review based on the a priori inclusion criteria (Figure 1).
Appendix B.2: Odds of Smoking Cessation From Random-Effects Meta-Analysis of Trials with Smokers Potentially Eligible for Lung Screening Based on 7-day Point Prevalence of Abstinence at 12-Months by Primary Intervention Type (n= 40 Trials)*.
Forest plots display weighted odds ratios and 95% confidence intervals of included trials. Trial weights are generated from a random effects analysis. Squares around point estimates indicate study weight relative to the lowest weighted study for each meta-analysis. The vertical dashed line represents the pooled odds ratio with the diamond representing the 95% confidence interval.
* Some trials included more than one intervention of a differing generic type so that the sum of the sample of all intervention types is greater than the total number of trials included.
Appendix B.3: Sensitivity Analyses Examining Change in Odds of Smoking Cessation From Random-Effects Meta-Analysis of Trials with Smokers Potentially Eligible for Lung Screening Based on 7-day Point Prevalence of Abstinence at 6-Months by Primary Intervention Type (n= 74 Trials)*.
Forest plots display weighted odds ratios and 95% confidence intervals of included trials. Trial weights are generated from a random effects analysis. Squares around point estimates indicate study weight relative to the lowest weighted study for each meta-analysis. The vertical dashed line represents the pooled odds ratio with the diamond representing the 95% confidence interval.
* Some trials included more than one intervention of a differing generic type so that the sum of the sample of all intervention types is greater than the total number of trials included.
Appendix B.4:
Study Quality Assessment
| Author, Year | Randomized (1/0) |
Similar Patient Characteristics (1/0) |
Double Blind (1/0) |
Masking (1/0) |
Withdrawal (1/0) |
Selective Reporting (1/0) |
Total |
|---|---|---|---|---|---|---|---|
| Electronic/Web-Based | |||||||
| Abroms, 2014 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Bock, 2010 | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Bolman, 2015 | 1 | 1 | NA | 1 | 1 | 1 | 5 |
| Borland, 2013 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Bricker, 2017 | 1 | 1 | NA | 1 | 1 | 1 | 5 |
| Brown, 2014 | 1 | 1 | NA | 1 | 1 | 1 | 5 |
| Calhoun, 2016 | 1 | 1 | NA | 0 | 1 | 0 | 3 |
| Choi, 2014 | 0 | 0 | NA | 1 | 1 | 1 | 3 |
| Cobos-Campos, 2017 | 1 | 0 | NA | 0 | 1 | 1 | 3 |
| Free, 2011 | 1 | 1 | NA | 1 | 1 | 1 | 5 |
| Gilbert, 2013 | 1 | 0 | NA | 0 | 1 | 1 | 3 |
| Houston, 2013 | 1 | 0 | NA | 1 | 0 | 1 | 3 |
| Houston, 2015 | 1 | 1 | NA | 1 | 0 | 1 | 4 |
| Leykin, 2012 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Loughead, 2016 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Mason, 2012 | 1 | 1 | NA | 1 | 1 | 1 | 5 |
| Moskowitz, 2016 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Reitzel, 2011 | 0 | 1 | NA | 0 | 0 | 1 | 2 |
| Richter, 2015 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Sheratt, 2018 | 1 | 0 | NA | 1 | 0 | 1 | 3 |
| Smit, 2012 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Stanczyk, 2016 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Westmass, 2018 | 1 | 1 | NA | 0 | 0 | 1 | 3 |
| Wetter, 2011 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| In-Person Counseling | |||||||
| Andrews, 2016 | 0 | 1 | NA | 1 | 1 | 1 | 4 |
| Bock, 2014 | 1 | 0 | NA | 0 | 1 | 0 | 2 |
| Brooks, 2017 | 1 | 0 | NA | 0 | 1 | 1 | 3 |
| Catley, 2016 | 1 | 1 | NA | 1 | 1 | 1 | 5 |
| Choi, 2016 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Davis, 2014 | 0 | 0 | NA | 1 | 1 | 1 | 3 |
| Garvey, 2012 | 1 | 1 | NA | 0 | 0 | 0 | 2 |
| Gifford, 2011 | 1 | 1 | NA | 0 | 1 | 0 | 3 |
| Hooper, 2017 | 0 | 1 | NA | 0 | 1 | 1 | 3 |
| Kim, 2015 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Laude, 2017 | 1 | 0 | NA | 0 | 1 | 1 | 3 |
| Okuyemi, 2013 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Pesis-Katz, 2011 | 0 | 1 | NA | 0 | 0 | 0 | 1 |
| Ramos, 2010 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Sheffer, 2017 | 1 | 0 | NA | 1 | 0 | 1 | 3 |
| Smith, 2014 | 0 | 0 | NA | 0 | 1 | 1 | 2 |
| Vidrine, 2016 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Webb, 2010 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Wewers, 2017 | 0 | 1 | 1 | 1 | 1 | 1 | 5 |
| Whiteley, 2012 | 0 | 1 | NA | 1 | 1 | 1 | 4 |
| Williams, 2016 | 0 | 0 | NA | 0 | 0 | 0 | 0 |
| Pharmacotherapy | |||||||
| Anthenelli, 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Baker, 2016 | 1 | 0 | 0 | 0 | 1 | 1 | 3 |
| Bullen, 2010 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Burns, 2014 | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| Caldwell, 2016 | 1 | 1 | 1 | 1 | 0 | 0 | 4 |
| Caldwell, 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Carpenter, 2011 | 1 | 1 | NA | 0 | 0 | 1 | 3 |
| Cinciripini, 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Cummings, 2011 | 0 | 1 | NA | 1 | 1 | 1 | 4 |
| Ebbert, 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Gonzales, 2014 | 1 | 1 | 1 | 1 | 0 | 1 | 5 |
| Hughes, 2011 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Lerman, 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Ramon, 2014 | 1 | 1 | 0 | 1 | 1 | 1 | 5 |
| Rennard, 2012 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Rose, 2013 | 0 | 1 | 1 | 1 | 0 | 1 | 4 |
| Schnoll, 2015 | 1 | 1 | 0 | 0 | 1 | 1 | 4 |
| Schnoll, 2010 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| Selby, 2014 | 1 | 0 | NA | 0 | 0 | 1 | 2 |
| Stapleton, 2013 | 1 | 0 | 0 | 0 | 1 | 1 | 3 |
| Tønnesen, 2012 | 1 | 1 | 1 | 1 | 0 | 1 | 5 |
| Tulloch, 2016 | 1 | 1 | 0 | 0 | 1 | 1 | 4 |
| Walker, 2011 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Telephone Counseling | |||||||
| Bastian, 2013 | 0 | 1 | NA | 0 | 1 | 0 | 2 |
| Fu, 2015 | 0 | 1 | NA | 1 | 1 | 1 | 4 |
| Klemperer, 2017 | 1 | 1 | NA | 1 | 1 | 0 | 4 |
| Klesges, 2015 | 1 | 1 | NA | 1 | 1 | 1 | 5 |
| Lindqvist, 2013 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Maddison, 2014 | 0 | 1 | NA | 1 | 1 | 1 | 4 |
| Nohlert, 2014 | 0 | 0 | NA | 0 | 1 | 1 | 2 |
| Sherman, 2018 | 1 | 0 | NA | 0 | 1 | 1 | 3 |
| Sumner, 2016 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Tzelepis, 2010 | 1 | 0 | NA | 1 | 1 | 1 | 4 |
| Zhu, 2012 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Zwar, 2015 | 0 | 1 | NA | 1 | 1 | 1 | 4 |
| Multiple Interventions | |||||||
| Hall, 2011 | 1 | 1 | 0 | 1 | 1 | 1 | 5 |
| Levine, 2010 | 0 | 1 | 1 | 0 | 1 | 1 | 4 |
| Ramon, 2013 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Smit, 2016 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
| Swan, 2010 | 1 | 1 | NA | 0 | 1 | 1 | 4 |
Appendix B.5: Contour-Enhanced Funnel Plots to Assess Publication Bias of Smoking Cessation Interventions from a Random Effects Meta-Analysis by Intervention Type.
Funnel plots compare the effect estimate of a study to some measure of its precision. Larger more powerful studies are placed at the top and smaller less powerful studies are at the bottom. Contour-enhanced funnel plots add areas of statistical significance to aid in the identification of areas of significance or non-significance from which studies appear to be missing.
Appendix B.6:
Results of Peters et al.’s Regression Test for Publication Bias of Smoking Cessation Interventions from a Random Effects Meta-Analysis by Intervention Type at 6- and 12-Months
| Intervention Category | 6-Month p-Value | 12-Month p-Value |
|---|---|---|
| Electronic/Web-Based | 0.871 | 0.552 |
| In-Person Counseling | 0.866 | 0.325 |
| Pharmacotherapy | 0.212 | 0.122 |
| Telephone Counseling | 0.048 | 0.992 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
None declared.
Presented in part at the 2019 Society for Research on Nicotine and Tobacco Annual Meeting, San Francisco, California, 20-23 February 2019; and at the 2019 American Society of Preventive Oncology Annual Meeting, Tampa, Florida, 10-12 March 2019.
This systematic review is registered with PROSPERO and is available at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018110322.
References
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Koning HJ, Van der Aalst CM, ten Haaf K, Oudkerk M. Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. . IASLC WCLC 2018. Toronto; 2018. [Google Scholar]
- 3.U.S.Preventive Services Task Force. Final Recommendation Statement: Lung Cancer: Screening. October 2014. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening Accessed December 18, 2017.
- 4.National Comprehensive Cancer Network. NCCN Guidelines for Patients: Lung Cancer Screening. 2017. https://www.nccn.org/patients/guidelines/lung_screening/files/assets/basic-html/page-1.html Accessed December 18, 2017.
- 5.Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56(1):125–34. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381–5. [DOI] [PubMed] [Google Scholar]
- 7.Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc. 2014;11(4):619–27. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services (CMS). Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). 2015. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 Accessed on December 18, 2017. [Google Scholar]
- 9.Joseph AM, Rothman AJ, Almirall D, Begnaud A, Chiles C, Cinciripini PM, et al. Lung Cancer Screening and Smoking Cessation Clinical Trials: SCALE Collaboration. Am J Respir Crit Care Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf Accessed December 18, 2017; 2008. [Google Scholar]
- 11.Taylor KL, Hagerman CJ, Luta G, Bellini PG, Stanton C, Abrams DB, et al. Preliminary evaluation of a telephone-based smoking cessation intervention in the lung cancer screening setting: A randomized clinical trial. Lung Cancer. 2017;108:242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark MM, Cox LS, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer. 2004;44(1):13–21. [DOI] [PubMed] [Google Scholar]
- 13.Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer. 2012;76(2):211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Aalst CM, de Koning HJ, van den Bergh KA, Willemsen MC, van Klaveren RJ. The effectiveness of a computer-tailored smoking cessation intervention for participants in lung cancer screening: a randomised controlled trial. Lung Cancer. 2012;76(2):204–10. [DOI] [PubMed] [Google Scholar]
- 15.Marshall HM, Courtney DA, Passmore LH, McCaul EM, Yang IA, Bowman RV, et al. Brief Tailored Smoking Cessation Counseling in a Lung Cancer Screening Population is Feasible: A Pilot Randomized Controlled Trial. Nicotine Tob Res. 2016;18(7):1665–9. [DOI] [PubMed] [Google Scholar]
- 16.Iaccarino JM, Duran C, Slatore CG, Wiener RS, Kathuria H. Combining smoking cessation interventions with LDCT lung cancer screening: A systematic review. Prev Med. 2019;121:24–32. [DOI] [PubMed] [Google Scholar]
- 17.Pineiro B, Simmons VN, Palmer AM, Correa JB, Brandon TH. Smoking cessation interventions within the context of Low-Dose Computed Tomography lung cancer screening: A systematic review. Lung Cancer. 2016;98:91–8. [DOI] [PubMed] [Google Scholar]
- 18.Bade M, Bahr V, Brandt U, Eigentopf A, Bruchert T, Gross ML, et al. Effect of smoking cessation counseling within a randomised study on early detection of lung cancer in Germany. J Cancer Res Clin Oncol. 2016;142(5):959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields PG, Herbst RS, Arenberg D, Benowitz NL, Bierut L, Luckart JB, et al. Smoking Cessation, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(11):1430–68. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Clinical Excellence, ed. Smoking cessation services in primary care, pharmacies, local authorities and workplaces, particularly for manual working groups, pregnant women and hard to reach communities NICE Public Health Guidance 10. London: National Institute for Health and Clinical Excellence, 2008. [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, w64. [DOI] [PubMed] [Google Scholar]
- 22.VonVille HM: Excel workbooks for systematic reviews. http://libguides.sph.uth.tmc.edu/excel_workbook_home.
- 23.Rucker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res Synth Methods. 2017;8(4):392–403. [DOI] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 25.Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed: The Cochrane Collaboration; 2011. [Google Scholar]
- 26.Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–59. [DOI] [PubMed] [Google Scholar]
- 27.Myung SK, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM. Effects of Web- and computer-based smoking cessation programs: meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(10):929–37. [DOI] [PubMed] [Google Scholar]
- 28.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 31.Catley D, Goggin K, Harris KJ, Richter KP, Williams K, Patten C, et al. A Randomized Trial of Motivational Interviewing: Cessation Induction Among Smokers With Low Desire to Quit. Am J Prev Med. 2016;50(5):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L, Moreno SG. Assessing publication bias in meta-analyses in the presence of between-study heterogeneity. Journal of the Royal Statistical Society: Series A (Statistics in Society). 2010;173(3):575–91. [Google Scholar]
- 33.Chen D, Wu LT. Smoking cessation interventions for adults aged 50 or older: A systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zbikowski SM, Magnusson B, Pockey JR, Tindle HA, Weaver KE. A review of smoking cessation interventions for smokers aged 50 and older. Maturitas. 2012;71(2):131–41. [DOI] [PubMed] [Google Scholar]
- 35.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2017(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013(8):Cd002850. [DOI] [PubMed] [Google Scholar]
- 37.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013(5):Cd009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. Cmaj. 2008;179(2):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charkhchi P, Kolenic GE, Carlos RC. Access to Lung Cancer Screening Services: Preliminary Analysis of Geographic Service Distribution Using the ACR Lung Cancer Screening Registry. J Am Coll Radiol. 2017;14(11):1388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huo J, Hong YR, Bian J, Guo Y, Wilkie DJ, Mainous AG 3rd. Low Rates of Patient-Reported Physician-Patient Discussion about Lung Cancer Screening among Current Smokers: Data from Health Information National Trends Survey. Cancer Epidemiol Biomarkers Prev. 2019;28(5):963–73. [DOI] [PubMed] [Google Scholar]
- 41.Ladova K, Vlcek J, Vytrisalova M, Maly J. Healthy adherer effect - the pitfall in the interpretation of the effect of medication adherence on health outcomes. J Eval Clin Pract. 2014;20(2):111–6. [DOI] [PubMed] [Google Scholar]
- 42.Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl. Cancer Inst. 2014;106(6):dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: a text messaging program for smoking cessation. American Journal of Preventive Medicine. 2014;47(3):242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bock BC, Hudmon KS, Christian J, Graham AL, Bock FR. A tailored intervention to support pharmacy-based counseling for smoking cessation. Nicotine & Tobacco Research. 2010;12(3):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolman C, Eggers SM, van Osch L, Te Poel F, Candel M, de Vries H. Is Action Planning Helpful for Smoking Cessation? Assessing the Effects of Action Planning in a Web-Based Computer-Tailored Intervention. Substance use & misuse. 2015;50(10):1249–60. [DOI] [PubMed] [Google Scholar]
- 46.Borland R, Balmford J, Benda P. Population-level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction. 2013;108(3):618–28. [DOI] [PubMed] [Google Scholar]
- 47.Bricker JB, Mull KE, McClure JB, Watson NL, Heffner JL. Improving quit rates of web-delivered interventions for smoking cessation: full-scale randomized trial of WebQuit.org versus Smokefree.gov . Addiction (Abingdon, England). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown J, Michie S, Geraghty AWA, Yardley L, Gardner B, Shahab L, et al. Internet-based intervention for smoking cessation (StopAdvisor) in people with low and high socioeconomic status: a randomised controlled trial. The Lancet Respiratory Medicine. 2014;2(12):997–1006. [DOI] [PubMed] [Google Scholar]
- 49.Calhoun PS, Datta S, Olsen M, Smith VA, Moore SD, Hair LP, et al. Comparative Effectiveness of an Internet-Based Smoking Cessation Intervention Versus Clinic-Based Specialty Care for Veterans. Journal of substance abuse treatment. 2016;69:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi SH, Waltje AH, Ronis DL, Noonan D, Hong O, Richardson CR, et al. Web-enhanced tobacco tactics with telephone support versus 1-800-QUIT-NOW telephone line intervention for operating engineers: randomized controlled trial. Journal of Medical Internet Research. 2014;16(11):e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cobos-Campos R, Apinaniz Fernandez de Larrinoa A, Saez de Lafuente Morinigo A, Parraza Diez N, Aizpuru Barandiaran F. Effectiveness of Text Messaging as an Adjuvant to Health Advice in Smoking Cessation Programs in Primary Care. A Randomized Clinical Trial. Nicotine & Tobacco Research. 2017;19(8):901–7. [DOI] [PubMed] [Google Scholar]
- 52.Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378(9785):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilbert HM, Leurent B, Sutton S, Alexis-Garsee C, Morris RW, Nazareth I. ESCAPE: a randomised controlled trial of computer-tailored smoking cessation advice in primary care. Addiction. 2013;108(4):811–9. [DOI] [PubMed] [Google Scholar]
- 54.Houston TK, Delaughter KL, Ray MN, Gilbert GH, Allison JJ, Kiefe CI, et al. Cluster-randomized trial of a web-assisted tobacco quality improvement intervention of subsequent patient tobacco product use: a National Dental PBRN study. BMC Oral Health. 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houston TK, Sadasivam RS, Allison JJ, Ash AS, Ray MN, English TM, et al. Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study. Implementation Science. 2015;10:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leykin Y, Aguilera A, Torres LD, Perez-Stable EJ, Munoz RF. Interpreting the outcomes of automated internet-based randomized trials: example of an International Smoking Cessation Study. Journal of Medical Internet Research. 2012;14(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loughead J, Falcone M, Wileyto EP, Albelda B, Audrain-McGovern J, Cao W, et al. Can brain games help smokers quit?: Results of a randomized clinical trial. Drug & Alcohol Dependence. 2016;168:112–8. [DOI] [PubMed] [Google Scholar]
- 58.Mason D, Gilbert H, Sutton S. Effectiveness of web-based tailored smoking cessation advice reports (iQuit): a randomized trial. Addiction. 2012;107(12):2183–90. [DOI] [PubMed] [Google Scholar]
- 59.Moskowitz JM, McDonnell DD, Kazinets G, Lee H. Online smoking cessation program for Korean Americans: Randomized trial to test effects of incentives for program completion and interim surveys. Preventative Medicine: An International Journal Devoted to Practice & Theory. 2016;86:70–6. [DOI] [PubMed] [Google Scholar]
- 60.Reitzel LR, McClure JB, Cofta-Woerpel L, Mazas CA, Cao Y, Cinciripini PM, et al. The efficacy of computer-delivered treatment for smoking cessation. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richter KP, Shireman TI, Ellerbeck EF, Cupertino AP, Catley D, Cox LS, et al. Comparative and Cost Effectiveness of Telemedicine Versus Telephone Counseling for Smoking Cessation. Journal of Medical Internet Research. 2015;17(5):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherratt FC, Marcus MW, Robinson J, Field JK. Utilizing Lung Cancer Risk Prediction Models to Promote Smoking Cessation: Two Randomized Controlled Trials. American Journal of Health promotion : AJHP. 2016. [DOI] [PubMed] [Google Scholar]
- 63.Smit ES, de Vries H, Hoving C. Effectiveness of a Web-based multiple tailored smoking cessation program: a randomized controlled trial among Dutch adult smokers. Journal of Medical Internet Research. 2012;14(3):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanczyk NE, de Vries H, Candel MJJM, Muris JWM, Bolman CAW. Effectiveness of video- versus text-based computer-tailored smoking cessation interventions among smokers after one year. Preventive medicine. 2016;82:42–50. [DOI] [PubMed] [Google Scholar]
- 65.Westmaas JL, Bontemps-Jones J, Hendricks PS, Kim J, Abroms LC. Randomised controlled trial of stand-alone tailored emails for smoking cessation. Tobacco control. 2018;27(2):136–46. [DOI] [PubMed] [Google Scholar]
- 66.Wetter DW, McClure JB, Cofta-Woerpel L, Costello TJ, Reitzel LR, Businelle MS, et al. A randomized clinical trial of a palmtop computer-delivered treatment for smoking relapse prevention among women. Psychology of Addictive Behaviors. 2011;25(2):365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrews JO, Mueller M, Dooley M, Newman SD, Magwood GS, Tingen MS. Effect of a smoking cessation intervention for women in subsidized neighborhoods: A randomized controlled trial. Preventive medicine. 2016;90:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bock BC, Papandonatos GD, de Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low-income smokers: motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research. 2014;16(4):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brooks DR, Burtner JL, Borrelli B, Heeren TC, Evans T, Davine JA, et al. Twelve-Month Outcomes of a Group-Randomized Community Health Advocate-Led Smoking Cessation Intervention in Public Housing. Nicotine Tob Res. 2018;20(12):1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Catley D, Goggin K, Harris KJ, Richter KP, Williams K, Patten C, et al. A Randomized Trial of Motivational Interviewing: Cessation Induction Among Smokers With Low Desire to Quit. American Journal of Preventive Medicine. 2016;50(5):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi WS, Beebe LA, Nazir N, Kaur B, Hopkins M, Talawyma M, et al. All Nations Breath of Life: A Randomized Trial of Smoking Cessation for American Indians. American Journal of Preventive Medicine. 2016;51(5):743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis JM, Manley AR, Goldberg SB, Smith SS, Jorenby DE. Randomized trial comparing mindfulness training for smokers to a matched control. Journal of substance abuse treatment. 2014;47(3):213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garvey AJ, Kalman D, Hoskinson RA Jr., Kinnunen T, Wadler BM, Thomson CC, et al. Front-loaded versus weekly counseling for treatment of tobacco addiction. Nicotine & Tobacco Research. 2012;14(5):578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy. 2011;42(4):700–15. [DOI] [PubMed] [Google Scholar]
- 75.Kim SS, Kim S-H, Fang H, Kwon S, Shelley D, Ziedonis D. A Culturally Adapted Smoking Cessation Intervention for Korean Americans: A Mediating Effect of Perceived Family Norm Toward Quitting. Journal of Immigrant & Minority Health. 2015;17(4):1120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laude JR, Bailey SR, Crew E, Varady A, Lembke A, McFall D, et al. Extended treatment for cigarette smoking cessation: a randomized control trial. Addiction. 2017;112(8):1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okuyemi KS, Goldade K, Whembolua G-L, Thomas JL, Eischen S, Sewali B, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction. 2013;108(6):1136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pesis-Katz I, Williams GC, Niemiec CP, Fiscella K. Cost-effectiveness of intensive tobacco dependence intervention based on self-determination theory. American Journal of Managed Care. 2011;17(10):e393–8. [PMC free article] [PubMed] [Google Scholar]
- 79.Ramos M, Ripoll J, Estrades T, Socias I, Fe A, Duro R, et al. Effectiveness of intensive group and individual interventions for smoking cessation in primary health care settings: a randomized trial. BMC Public Health. 2010;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheffer CE, Bickel WK, Franck CT, Panissidi L, Pittman JC, Stayna H, et al. Improving tobacco dependence treatment outcomes for smokers of lower socioeconomic status: A randomized clinical trial. Drug & Alcohol Dependence. 2017;181:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith SS, Rouse LM, Caskey M, Fossum J, Strickland R, Culhane JK, et al. Culturally-Tailored Smoking Cessation for Adult American Indian Smokers: A Clinical Trial. The Counseling psychologist. 2014;42(6):852–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, et al. Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. J Consult Clin Psychol. 2016;84(9):824–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Webb MS, de Ybarra DR, Baker EA, Reis IM, Carey MP. Cognitive-behavioral therapy to promote smoking cessation among African American smokers: a randomized clinical trial. Journal of Consulting & Clinical Psychology. 2010;78(1):24–33. [DOI] [PubMed] [Google Scholar]
- 84.Wewers ME, Shoben A, Conroy S, Curry E, Ferketich AK, Murray DM, et al. Effectiveness of Two Community Health Worker Models of Tobacco Dependence Treatment Among Community Residents of Ohio Appalachia. Nicotine & Tobacco Research. 2017;19(12):1499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whiteley JA, Williams DM, Dunsiger S, Jennings EG, Ciccolo JT, Bock BC, et al. YMCA commit to quit: randomized trial outcomes. American Journal of Preventive Medicine. 2012;43(3):256–62. [DOI] [PubMed] [Google Scholar]
- 86.Williams GC, Niemiec CP, Patrick H, Ryan RM, Deci EL. Outcomes of the Smoker's Health Project: a pragmatic comparative effectiveness trial of tobacco-dependence interventions based on self-determination theory. Health Educ Res. 2016;31(6):749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–20. [DOI] [PubMed] [Google Scholar]
- 88.Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, et al. Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: A Randomized Clinical Trial. JAMA. 2016;315(4):371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bullen C, Howe C, Lin R-B, Grigg M, Laugesen M, McRobbie H, et al. Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction. 2010;105(8):1474–83. [DOI] [PubMed] [Google Scholar]
- 90.Burns EK, Hood NE, Goforth E, Levinson AH. Randomised trial of two nicotine patch protocols distributed through a state quitline. Tobacco control. 2016;25(2):218–23. [DOI] [PubMed] [Google Scholar]
- 91.Caldwell BO, Crane J. Combination Nicotine Metered Dose Inhaler and Nicotine Patch for Smoking Cessation: A Randomized Controlled Trial. Nicotine & Tobacco Research. 2016;18(10):1944–51. [DOI] [PubMed] [Google Scholar]
- 92.Caldwell BO, Adamson SJ, Crane J. Combination rapid-acting nicotine mouth spray and nicotine patch therapy in smoking cessation. Nicotine & Tobacco Research. 2014;16(10):1356–64. [DOI] [PubMed] [Google Scholar]
- 93.Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Archives of Internal Medicine. 2011;171(21):1901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70(5):522–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cummings KM, Hyland A, Carlin-Menter S, Mahoney MC, Willett J, Juster HR. Costs of giving out free nicotine patches through a telephone quit line. Journal of Public Health Management & Practice. 2011;17(3):E16–23. [DOI] [PubMed] [Google Scholar]
- 96.Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzales D, Hajek P, Pliamm L, Nackaerts K, Tseng LJ, McRae TD, et al. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: a randomized, placebo-controlled trial. Clinical Pharmacology & Therapeutics. 2014;96(3):390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine & Tobacco Research. 2011;13(10):955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lerman C, Schnoll RA, Hawk LW Jr., Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. The Lancet Respiratory Medicine. 2015;3(2):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Medicine. 2014;12:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine & Tobacco Research. 2012;14(3):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. American Journal of Psychiatry. 2013;170(8):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Internal Medicine. 2015;175(4):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Annals of Internal Medicine. 2010;152(3):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Selby P, Brosky G, Oh P, Raymond V, Arteaga C, Ranger S. A pragmatic, randomized, controlled study evaluating the impact of access to smoking cessation pharmacotherapy coverage on the proportion of successful quitters in a Canadian population of smokers motivated to quit (ACCESSATION). BMC Public Health. 2014;14:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stapleton J, West R, Hajek P, Wheeler J, Vangeli E, Abdi Z, et al. Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: effectiveness in clinical practice. Addiction. 2013;108(12):2193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. European Respiratory Journal. 2012;40(3):548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Medicine. 2016;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, et al. Does improved access and greater choice of nicotine replacement therapy affect smoking cessation success? Findings from a randomized controlled trial. Addiction. 2011;106(6):1176–85. [DOI] [PubMed] [Google Scholar]
- 110.Bastian LA, Fish LJ, Peterson BL, Biddle AK, Garst J, Lyna P, et al. Assessment of the impact of adjunctive proactive telephone counseling to promote smoking cessation among lung cancer patients' social networks. American Journal of Health Promotion. 2013;27(3):181–90. [DOI] [PubMed] [Google Scholar]
- 111.Fu SS, van Ryn M, Nelson D, Burgess DJ, Thomas JL, Saul J, et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial. Thorax. 2016;71(5):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR. Motivational, reduction and usual care interventions for smokers who are not ready to quit: a randomized controlled trial. Addiction. 2017;112(1):146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klesges RC, Ebbert JO, Talcott GW, Thomas F, Richey PA, Womack C, et al. Efficacy of a Tobacco Quitline in Active Duty Military and TRICARE Beneficiaries: A Randomized Trial. Military medicine. 2015;180(8):917–25. [DOI] [PubMed] [Google Scholar]
- 114.Lindqvist H, Forsberg LG, Forsberg L, Rosendahl I, Enebrink P, Helgason AR. Motivational interviewing in an ordinary clinical setting: a controlled clinical trial at the Swedish National Tobacco Quitline. Addictive Behaviors. 2013;38(7):2321–4. [DOI] [PubMed] [Google Scholar]
- 115.Maddison R, Roberts V, McRobbie H, Bullen C, Prapavessis H, Glover M, et al. Exercise counseling to enhance smoking cessation outcomes: the Fit2Quit randomized controlled trial. Annals of Behavioral Medicine. 2014;48(2):194–204. [DOI] [PubMed] [Google Scholar]
- 116.Nohlert E, Ohrvik J, Helgason AR. Effectiveness of proactive and reactive services at the Swedish National Tobacco Quitline in a randomized trial. Tobacco Induced Diseases. 2014;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sherman SE, Krebs P, York LS, Cummins SE, Kuschner W, Guvenc-Tuncturk S, et al. Telephone care co-ordination for tobacco cessation: randomised trials testing proactive versus reactive models. Tobacco control. 2018;27(1):78–82. [DOI] [PubMed] [Google Scholar]
- 118.Sumner W 2nd, Walker MS, Highstein GR, Fischer I, Yan Y, McQueen A, et al. A randomized controlled trial of directive and nondirective smoking cessation coaching through an employee quitline. BMC Public Health. 2016;16:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tzelepis F, Paul CL, Wiggers J, Walsh RA, Knight J, Duncan SL, et al. A randomised controlled trial of proactive telephone counselling on cold-called smokers' cessation rates. Tobacco control. 2011;20(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu S-H, Cummins SE, Wong S, Gamst AC, Tedeschi GJ, Reyes-Nocon J. The effects of a multilingual telephone quitline for Asian smokers: a randomized controlled trial. Journal of the National Cancer Institute. 2012;104(4):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zwar N, Richmond R, Halcomb E, Furler J, Smith J, Hermiz O, et al. Quit in general practice: a cluster randomised trial of enhanced in-practice support for smoking cessation. BMC Family Practice. 2010;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hall SM, Humfleet GL, Munoz RF, Reus VI, Prochaska JJ, Robbins JA. Using extended cognitive behavioral treatment and medication to treat dependent smokers. American Journal of Public Health. 2011;101(12):2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, et al. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Archives of Internal Medicine. 2010;170(6):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramon JM, Nerin I, Comino A, Pinet C, Abella F, Carreras JM, et al. A multicentre randomized trial of combined individual and telephone counselling for smoking cessation. Preventive medicine. 2013;57(3):183–8. [DOI] [PubMed] [Google Scholar]
- 125.Smit ES, Candel MJJM, Hoving C, de Vries H. Results of the PAS Study: A Randomized Controlled Trial Evaluating the Effectiveness of a Web-Based Multiple Tailored Smoking Cessation Program Combined With Tailored Counseling by Practice Nurses. Health communication. 2016;31(9):1165–73. [DOI] [PubMed] [Google Scholar]
- 126.Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine. 2010;38(5):482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.