Abstract
The Developing Countries Vaccine Manufacturers Network (DCVMN) convened vaccine manufacturing experts and leaders from local and global public health organizations for its 19th Annual General Meeting. Lectures and panel discussions centered on international cooperation for better access to vaccines, and partnerships in areas ranging from vaccine research and process development, to clinical studies, regulatory, supply chain and emergency preparedness and response.
Global vaccine market trends and changes that will impact vaccine financing and procurement methods were discussed as well as capital sources, including funding, for the development of new or improved vaccines.
DCVMN members presented their progress in developing novel Hexavalent, Meningitis, Pneumococcal Conjugate Vaccine, Shigella, Mumps, Rotavirus, Yellow Fever, Polio, Hepatitis E and Dengue vaccines, and a novel monoclonal antibody cocktail for post-bite prophylaxis against rabies infections.
Access to and availability of vaccines is enhanced through sharing of best practices for vaccine quality control, reducing redundant testing and promoting development of harmonized common standards. Eligible stakeholders were encouraged to join the WHO-National Control Laboratory Network for Biologicals which serves as a platform for collaboration and technical exchange in this area.
Increasing regulatory convergence at the regional and global levels through mechanisms such as joint dossier review and the WHO Collaborative Registration Procedure can help to accelerate vaccine access globally. Additionally, four proposals for streamlining procedures and alignment of dossiers were discussed.
Successful partnerships between a broad range of stakeholders, including international organizations, manufacturers, academic research institutes and regulators have provided support for, and in some cases accelerated, vaccine innovation, clinical trials and registration, WHO prequalification, vaccine introduction and access. Strong partnerships, based on experience and trust, help leverage opportunities and are critically important to advancing the shared goal of providing quality vaccines for all people.
Keywords: Public-private partnerships, Immunization, Vaccine market trends, Technology innovation, Developing countries
1. Introduction
The 19th Annual General Meeting of the Developing Countries Vaccine Manufacturers Network (DCVMN) in Kunming, China, gathered around 315 professionals - 30% of which were female - from 34 countries, 41 corporate manufacturers, 14 corporate partners, and local and global health organizations. The meeting aimed to tighten collaborations, deepen understanding, and enhance partnerships, contributing to future vaccine development and manufacturing. This report provides a summary of major points discussed throughout the meeting.
DCVMN President, M. Datla, thanked the Institute of Medical Biology, Chinese Academy of Medical Sciences, for hosting the event and welcomed representatives from WHO, UNICEF, PAHO, PATH, CEPI, CHAI, Gavi, IVI, GHIF, AVAREF, NIBSC, Intravacc, Imperial College London, and BMGF 1. In 2018, three new companies joined the Network, bringing the total to 54 corporate members; six vaccines from five member companies received WHO prequalification (WHO PQ); and members endorsed a five-year strategy to strengthen capabilities to produce a sustainable supply of vaccines.
C. Lou (Science and Technology Department), Y. Xu (Yunnan Health Commission) and J. Ju (Medical Product Administration of Yunnan Province) encouraged experts and entrepreneurs to advance industrial technology systems, promote international certification of vaccines and establish a production and supply base that meets international standards. Working together, international organizations, national health administrations and vaccine manufacturers can strengthen partnerships for health.
M. Simao (WHO) noted that life expectancy was extended by 25 years in the last decade; however, health inequities persist within and between countries, and challenges have expanded to industrialized countries. WHO’s new strategy 2019–2023 [1] aims to address inequities to ensure higher healthcare coverage, prevent emergencies and promote better health for a further three billion people, at all ages. As of October 2018, there were Ebola, Cholera, Yellow Fever (YF) and Meningitis outbreaks in nine countries [2]. Vaccines can address health emergencies; however, only 30% of national regulatory authorities (NRAs) globally can perform core regulatory functions to provide efficient oversight of registration, including the assessment, quality control and post-marketing surveillance of vaccines. This can lead to delayed, access to prevention in emergency situations, thus the need to rely on international mechanisms for product regulation, including the WHO Collaborative Registration Procedure (CRP) [3].
2. Public and private partnerships to enhance access to vaccines
A. Oswald (BMGF) interviewed Bill Gates (by recorded video), reflecting on achievements of the first Decade of Vaccines. Since the 1990s, annual child mortality decreased from over 11 million to around 5 million currently 2. “In saving children’s lives there is nothing as phenomenal as vaccines”, said Mr. Gates. In this context, ensuring simple procurement practices and guaranteed supply, avoiding fragmentation, working on policies and technical advice for countries, as well as stockpiles, should help drive global and regional vaccine uptake, increasing access to vaccines for everyone. In general, some redundancy in manufacturing is needed to ensure economies of scale and sustainable global supply capacity if one factory goes offline. He commended DCVMN companies for exploring state-of-the-art manufacturing technologies and stated that BMGF is committed to a strong dialogue and willing to share risks that make a difference to global health. Mr. Gates concluded: “we are looking forward to the partnerships we can have over the next decade”.
S. Davis (PATH) explained how PATH’s extensive vaccine innovation and access capabilities can support manufacturers and other partners through end-to-end partnerships, from lead identification and preclinical studies, to process development, clinical trials and registration, to WHO prequalification, introduction and global access efforts. Examples included partnerships with Serum Institute of India (SII) on MenAfriVac, Bharat Biotech on Rotavirus, CDIBP 3 on Japanese encephalitis (JE) and Beijing Biotech Institute on Oral Polio Vaccine (OPV) to achieve WHO PQ and supply global markets. PATH relies on its partnerships with industry, research institutes, foundations, and regulators, and success is based on experience and trust.
E. Kadilli (UNICEF) highlighted significant progress on reducing child mortality over the past 30 years, with immunizations as key contributor. Achievements have been facilitated through strong partnerships, including with vaccine manufacturers, across four streams of work: procurement and market shaping; supply planning and coordination; country support and sustainability; and strengthening country ownership and long-term immunization sustainability through funding e.g. Gavi co-financing. However, achieving the Sustainable Development Goal targets for child mortality by 2030 [4] requires innovations, including programmatic intelligence, understanding the barriers and deepening insights to leverage partnerships with the private sector to increase immunization rates (Fig. 1 ).
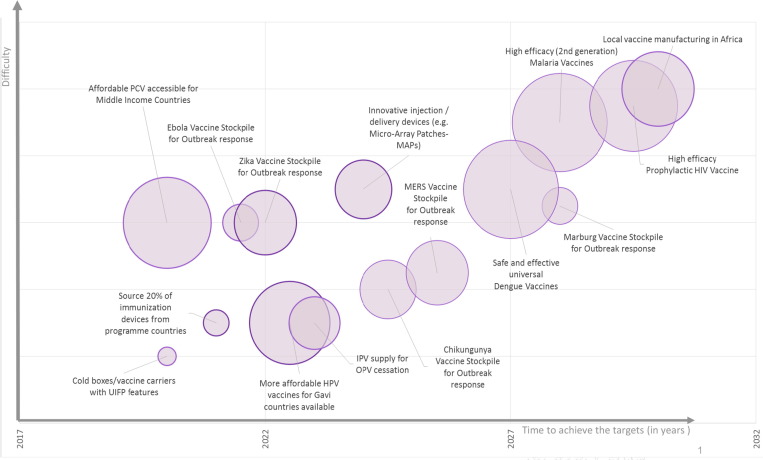
Fig. 1.
Illustration of 15 global immunization targets set by UNICEF and global stakeholders to further reduce child mortality, to be achieved from 2020 to 2030. Based on these examples of desirable outcomes, UNICEF is shifting its approach to also engage pre-licensure, including with the private sector and at the executive level, advocating for required investments to meet the future needs of children and adolescents, and to consider the entire ecosystem. The relative level of difficulty to achieve these targets is reflected on the Y axis and time to achievement is shown on the X axis. The size of bubbles denotes the potential impact should goals/targets be achieved. Access to affordable PCV for middle income countries may be the next high impact achievement. Size of bubbles denotes the potential impact should goals/targets be achieved. All information subjectively estimated. Abbreviations: PCV: Pneumococcal Conjugate Vaccine; UIFP: user-independent freeze prevention; HPV: Human Papillomavirus; IPV: Inactivated Polio Vaccine; OPV: Oral Polio Vaccine; MERS: Middle East Respiratory Syndrome. (Figure courtesy of E. Kadilli).
J. Chu (CHAI) led a discussion on enhancing partnerships to accelerate vaccine innovation, by illustrating that lives could be saved through improved success in the development of innovative vaccines (Fig. 2 ).
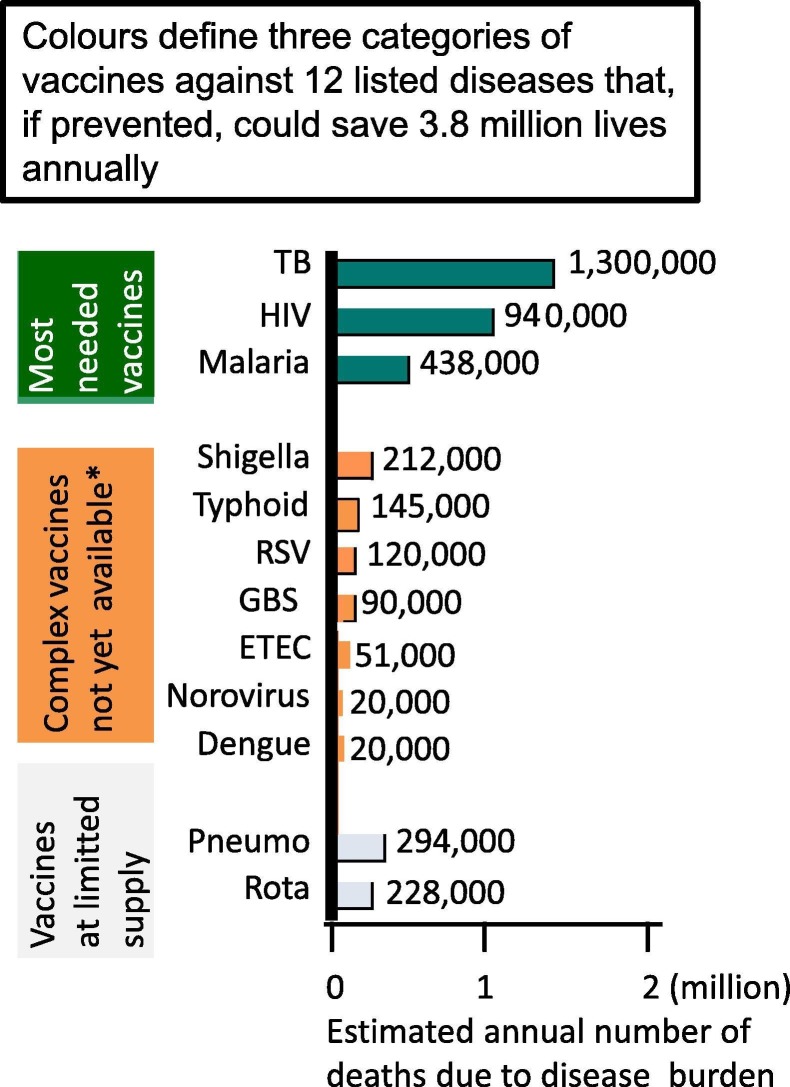
Fig. 2.
Graphic illustration of lives that could be saved annually through the development of innovative vaccines and improved global availability of vaccines. Bar chart illustrates the mortality of global disease burden due to 12 diseases that could be prevented through innovations in vaccines against TB, HIV, Malaria, Shigella, Typhoid, RSV, GBS, ETEC, Norovirus and Dengue vaccines to save 3.3 million lives. Higher availability of Pneumococcal and new generation Rotavirus vaccines could save additional lives, bringing the total estimated lives saved to 3.8 million annually. Bar sizes are only subjectively estimated, indicative of the number of deaths reported in 132 countries. Coloured areas divide the vaccines into three main categories: green = most needed vaccines; orange = not yet available vaccines; grey = vaccines available at limited supply. Improving vaccines manufacturing, supply capacity and availability would save 3.8 million lives. All rates reported here are age-standardised and derived from WHO and Lancet disease burden estimates, represented in a simple illustrative manner, as to data published in 2017 (at the time of the meeting). The purpose of GBD 2017 is to serve as a global public good, freely available for policy makers and the public seeking to improve human health. A detailed description of Data and statistical modelling tools are available at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32203-7/fulltext. (*) Note that new typhoid vaccines became globally available after 2018. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
M. Zuma (BioManguinhos) described three success factors for technology transfer initiatives to introduce new vaccines: innovation throughout the transfer process when establishing manufacturing in a new location; mutual trust between partners to share development information, production capacity, industry expertise and capabilities to innovate; and a supportive environment, including large public markets and government purchasing power.
S. Davis (PATH) added that innovation also includes packaging, distribution tools and addressing next-generation technology transfer and business models. He stressed that manufacturers need incentives to innovate, even with open-source intellectual property approaches and tiered pricing models.
M. Simao (WHO) commented that collaborations to achieve WHO PQ are partnerships that help encourage innovation.
P. Tippoo shared Biovac’s engagements with WHO, PATH, and BMGF towards building vaccine development and manufacturing capacity in South Africa. He highlighted that building partnerships takes time, explaining that it took more than three years before launching the development of a novel Group B Streptococcus vaccine. Access to training and experts allowed the project to ramp up quickly.
H. Iyer (BMGF) highlighted that a key challenge for manufacturers is to assess the potential of new vaccines. Future challenges include mRNA vaccines, biomarkers, adaptive clinical trial design and human challenge models for infection.
Panelists encouraged manufacturers to leverage their innovation capabilities through partnerships.
3. Procurement and globalization of vaccines
E. Baker (Gavi) discussed major global vaccine market trends and estimated changes that will impact vaccine financing and procurement methods (Fig. 3A, Fig. 3B ). It will become increasingly challenging for global actors to operate in this environment, not only due to changes in source of financing and increasing costs of immunization programs, but also due to uncertainties around procurement channels (see also Fig. 3A, Fig. 3B legend).
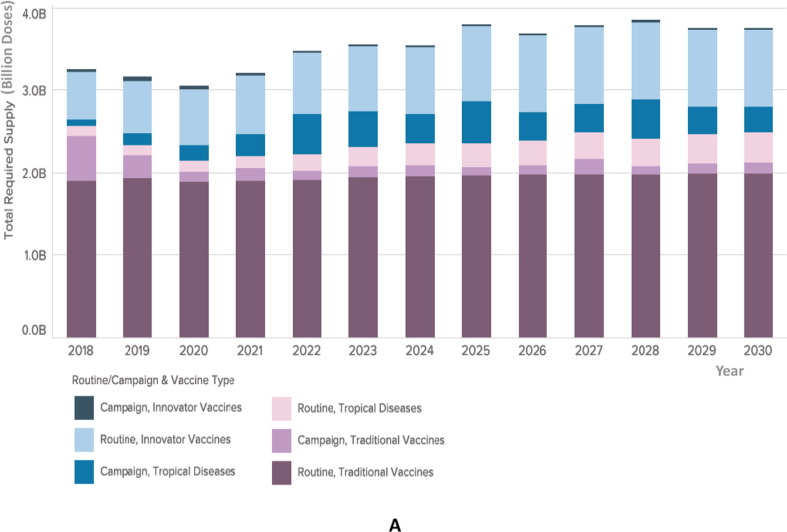
Fig. 3A.
Illustration of forecasted global demand of vaccines analyzed by routine or campaign use and vaccine type. The columns chart illustrates the estimated total required supply of vaccines, in billions of doses, displayed in the Y axis. The years included in the forecast are reflected in the X axis (2018–2030). Total global demand for vaccines is expected to grow from 3 billion doses annually presently, to 4 billion doses annually in 2030. Vaccines are color-coded according to the routine or campaign use and vaccine type. Traditional vaccines represent the largest number of doses and are depicted in dark purple and light purple colors, on the bottom of the columns. Innovative or novel vaccines are depicted in dark blue and light blue, represented on the top of each column. Vaccines targeting regional tropical diseases (Cholera, Japanese Encephalitis, Typhoid fever, Dengue fever) are depicted in pink and blue areas. Demand for traditional vaccines (e.g. Pentavalent) may grow the least, while newer vaccines (PCV, Rotavirus, HPV) and vaccines targeting regional tropical diseases are likely to grow more. Note that the number of doses of traditional vaccines used in catchup campaigns, such as measles, are likely to decrease over time, as routine vaccine coverage becomes more stable. Note: volumes exclude OPV demand. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
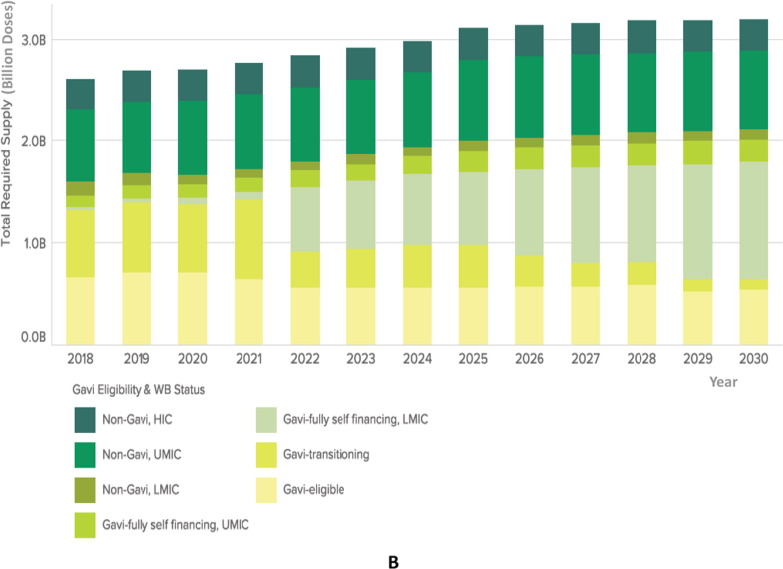
Fig. 3B.
Illustration of forecasted global routine demand by country income group and Gavi eligibility. The total required supply of vaccines, in billions of doses, is displayed in the Y axis. Vaccines are color coded according to Gavi eligibility and World Bank status. Gavi countries (fully eligible, transitioning, self-financing) are depicted in yellow and light green shading, while non-Gavi High Income Countries (HICs) are depicted in dark green colors, on the top of the columns. The years included in the forecast are reflected in the X axis (2018–2030). Demand by income group may change considerably over the next decade as many Gavi-eligible countries transition to become self-financing. As a result, non-Gavi self-financing Middle Income Countries (MICs) will comprise the majority proportion of global demand by 2028. Supply requirements are unlikely to change in non-Gavi MICs and HICs. Abbreviations: WB: World Bank; HIC: high income countries; UMIC: upper-middle income countries; LMIC: lower-middle income countries. Source: Global Vaccine Market Model, owned by Linksbridge, July 2018. (Figures courtesy of E. Baker). Note: volumes include routine vaccine program demand only. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
B. Giersing (WHO) remarked that Gavi financing played a big role in access to traditional and newer vaccines. Financing will be crucially important for vaccines lacking a dual market (public and private markets or developing and industrialized markets). However, as Gavi countries develop self-financing capacity, a lower rate of vaccine uptake was observed in the past [5] and can be addressed through new procurement mechanisms.
A. Ottosen (UNICEF) mentioned innovative procurement approaches such as shifting to multi-year tenders, 10-year contracts for pneumococcal conjugate vaccines (PCV) and multi-phased tenders, all implemented following consultations with industry, aiming to achieve vaccine security, including a diverse supplier base. UNICEF procurement considers price, but also other factors that contribute to ensure sustainability of markets and access to vaccines for immunization programmes.
J. Fitzsimmons (PAHO Revolving Fund) commented on collaborative approaches for shared demand forecasting of vaccines against regional epidemics, illustrated by WHO’s Eliminate Yellow Fever Epidemics (EYE) 4 Initiative and the International Coordinating Group on Vaccine Provision 5. In 2017, this mechanism facilitated access to additional supply of YF vaccines for procurement during a constrained global supply situation.
D. Hein (Gavi) emphasized that the global vaccine market value doubled in the last decade because of innovative vaccines and attractive prices in high income countries (HICs). However, the value of traditional vaccines stagnated and some outbreak vaccines lack markets in HICs. Manufacturers operating in these segments face volatility and unpredictability, besides regulatory fragmentation. Gavi’s approach is aggregation and long-term visibility for financing.
R. Iqbal (BMGF) reflected on supply factors that shape globalization. BMGF invests in vaccines to ensure an adequate supply base, aligning with Gavi principles for healthy markets, minimizing shortage risks and increasing market sharing among manufacturers, fostering multiple supply options. BMGF also invests in manufacturing technology platforms that reduce production costs.
4. Funding landscape for vaccines
G. Rockman (GHIF) introduced examples of capital sources for the development of new vaccines, noting the difference between funding, that doesn’t need repayment, and financing, that seeks a financial return. He outlined the profile of CEPI, BMGF-SIF and GHIF funding (Table 1 ) before opening the discussion, noting that GHIF will scale up its efforts to finance global health technologies with new sources of capital in 2019, and will rebrand itself as Adjuvant.
Table 1.
Comparative overview of CEPI, BMGF-SIF and GHIF funding assessments across six aspects (listed in the first column), including funding nature, targeted products, focus areas, typical value range of funds, funding structures and early/late phases of development funded. (Table courtesy of G. Rockman).
| Coalition Epidemic Preparedness Innovations (CEPI) | Bill & Melinda Gates Foundation Strategic Investment Fund (BMGF SIF) | Adjuvant The Global Health Investment Fund | |
|---|---|---|---|
| Profile | Primarily a Grant Funder to Support R&D for Interventions Against Epidemic Threats. Has the flexibility to develop other investment tools going forward. | BMGF SIF uses a variety of financing tools to stimulate private-sector innovation, encourage market-driven efficiencies and attract external capital to initiatives that support BMGF’s charitable mission | Adjuvant is an impact investment fund that uses venture capital and private equity strategies to support global health R&D projects with commercial financial return prospects |
| Interventions they Fund | Primarily Vaccines | Vaccines, Therapeutics, Diagnostics, and Other Technologies | Vaccines, Therapeutics, Diagnostics, and Other Technologies |
| Areas of Focus | WHO R&D Blueprint Priority Pathogens; e.g. Ebola, Lassa, MERS, Nipah, “Disease X” | HIV, TB, Malaria, and Other Neglected Infectious Diseases, Maternal and Child Health Challenges | HIV, TB, Malaria, and Other Neglected Infectious Diseases, Maternal and Child Health Challenges |
| Typical R&D Funding Amounts per Project | $10–50 Million | $5–100 Million | $5–50 Million |
| Funding Structure(s) Available | Milestone-Based Grants. Flexibility to develop other investment tools. Also entering into “Development partnerships” with aligned non-profit organizations. Also putting in place tools for surge funding to expedite R&D during outbreaks. | Loans, Equity Investments, Project Financing, Volume Guarantees, and Other Innovative Finance Mechanisms (note that BMGF’s Global Health Program makes traditional grants as well; SIF capital is used when an investment structure is more suitable) | Loans, Equity Investments, Project Financing |
| Phases of Development Funded | Early Stage (Preclinical, Phase I, and Phase II) | Early Stage, Late Stage, Commercialization/Scale-Up | Late Stage (Validating Phase II Data or Later Required), Commercialization/Scale-Up |
F. Kristensen (CEPI) provided an overview of the coalition’s development of vaccines against priority pathogens – the Middle East Respiratory Syndrome (MERS), Lassa and Nipah viruses, and platforms for rapid vaccine development against any new threat. CEPI’s mission is to enable equitable access to these vaccines for affected populations during outbreaks through being both a funder and a facilitator for improved preparedness, response and sustainability.
J. Yip (BMGF) shared the features of SIF in guarantees, loans, fund investments, and equity investments in addition to core grants and contracts with manufacturers for R&D and other product improvements. SIF investments focus on infectious diseases affecting disadvantaged populations. Since 2009, SIF has invested US$1.9 billion in over 70 companies and academic laboratories, fostering product development and delivery.
M. Datla (Biological E) commented on R&D funding and financing changes over the last decade in India. Previously, there was no venture capital for vaccines, so grants were critical. Grant funding is mission-oriented, restrictive, with financial objectives, usually leading to access agreements to lower prices for a specific volume or time window. Private equity requires 3–5-year cycles and is therefore not ideal for vaccines.
S. Prasad (Bharat Biotech) stated that one significant grant helped to advance manufacturing of Rotavirus vaccines. Investments are based on predictability; however, it is often difficult to measure financial success parameters, leading to unpredictable rate of return on investments on vaccines without long-term supply contracts.
5. Vaccine research and development
V. Pavliak (IVI) invited experts to comment on innovations to foster vaccine affordability.
D. Robinson (BMGF) commented that innovative simplified processes can translate into capital savings and product costs in the developing world. Further, the recently founded Gates Medical Research Institute (GMRI) will take a small biotech approach to innovation for drugs, diagnostics and vaccines, to be transferred to manufacturers, focusing on diseases affecting the developing world, prioritizing TB, HIV, and malaria.
R. Shattock (Imperial College London) noted that industry typically finalizes the manufacturing process before conducting clinical trials, which are expensive and time consuming. Trials that do not require fixed manufacturing processes, applying adaptive clinical trial design, offer a promising alternative.
D. Dat added that Vabiotech transferred Oral Cholera Vaccine (OCV) technology to IVI, to develop a high-quality vaccine through clinical trials in various populations. The optimized manufacturing technology expanded the manufacturing capacity and was transferred to other interested manufacturers.
A. Tomar (Cadila Biologicals) explained that efficient and low-cost manufacturing must ensure enough production capacity to achieve economies of scale. Procurement systems influence the manufacturing process, thus it is critical to engage with the appropriate stakeholders to increase awareness and ensure a market for new products. Cadila will license a new Rabies vaccine thanks to successful partnerships.
R. Suri (Panacea) added that affordable vaccines require a longer-term approach that considers sustainable manufacturing costs. An innovative example is a process change for Inactivated Polio Vaccine (IPV) which reduces the cost by tenfold.
Panelists concluded that manufacturers can add value to future vaccines.
6. Future vaccines
D. Robinson (BMGF) illustrated innovation through supported proof-of-concept studies of Univercells’ modular manufacturing platforms for vaccines, antibodies or proteins [6]. The low capital costs of the platforms facilitate engagement of regional and local manufacturers. GMRI will also address proof-of-concept studies to develop new diagnostics, drugs, and bioproducts. Furthermore, a well-trained workforce will ensure that high quality is top priority for manufacturers.
R. Suri (Panacea) summarized the ten-year effort to bring a fully liquid wP 6-IPV-based Hexavalent vaccine to the market. After proving safety and tolerability, an open-label, randomized, multicenter study showed non-inferiority seroprotection in 6–10-week-old infants, achieving broad and long-term protection compared to licensed combination vaccines [7]. The vaccine was approved by the Indian NRA.
R. Dhere (SII) mentioned that thermostable ACYWX Meningitis vaccines are most suitable to prevent future outbreaks. Despite a significant drop of Meningitis type A cases through vaccination since 2010, increased cases related to serogroups C-Y-W-X were observed in Africa [8]. Thus, polysaccharide conjugation [9] and serogroup-specific cell banking were developed for uniform high-yield growth kinetics. Safety and immunogenicity of the ACYWX vaccine was confirmed [10]. Efficacy studies will start in 2019, aiming for WHO PQ in 2020. Preliminary studies to add serotype B have started.
S. Yang (Walvax) revealed the immunogenicity and safety profile of a 13-valent PCV for infants in a randomized, double-blind controlled clinical study, demonstrating non-inferiority compared to Prevnar 7, the only licensed vaccine in China in 2016. The vaccine is likely to be licensed by 2019. A lot-to-lot consistency and non-inferiority study of the PCV-13, will compare it to Prevnar 13, licensed in China last year.
L. Du (Zhifei) described the development of a vaccine against Shigella, the second leading cause of diarrhea worldwide, causing 1.31 million deaths annually [11]. A bivalent Shigella conjugate vaccine candidate is being developed, using the O-polysaccharide from LPS conjugated to carrier protein with two strains: Shigella flexneri 2a and Plesiomonas shigelloides. Early studies in healthy subjects to identify dosage and immunization schedule are underway.
Y. Che (IMBCAMS) presented a new live attenuated Mumps vaccine to address re-emerging outbreaks related to F genotype SP viral strain. Virus shedding studies indicated a favorable safety and immunogenicity profile. In 2017, a Phase II trial demonstrated non-inferiority of seroconversion of neutralizing and hemagglutination-inhibiting antibodies, and significant increase in cellular immunity. Efficacy studies will follow.
C. Kirkwood (BMGF) acknowledged the Rotavirus vaccines-driven improvement in child health and highlighted efforts towards improving vaccine performance and delivery. Despite the good safety profile of four WHO PQ live attenuated oral rotavirus vaccines, 90 million children worldwide still lack access [11], and intussusception and lower efficacy remain a concern in some countries. Biofarma is developing a G3P [6] strain oral vaccine with 3-dose schedule, showing excellent protection against rotavirus gastroenteritis [12]. Injectable non-replicating vaccines may improve the safety and efficacy and could be used in combination vaccines. Two candidates are being developed: a trivalent non-replicating rotavirus vaccine expressed in E. coli, developed by PATH - SK Bioscience as partner- showed good safety and immunogenicity (e.g. neutralizing antibodies) in human clinical studies [13], clinical protection will be evaluated. The inactivated whole-virus particle Rotavirus vaccine developed by the U.S. CDC - SII as partner - showed protective immunity in animals.
S. Kumar (Zydus Cadila) introduced a novel monoclonal antibody (mAb) cocktail for post-bite prophylaxis against rabies infections. The rabies virus progresses from peripheral tissues to the spinal cord, causing brain inflammation and fatal paralysis. Immediate wound wash followed by rabies vaccine and anti-rabies-immunoglobulin (RIG) neutralize the virus and protect against the disease. RabiMabs is a cocktail of two serum-free, anti-G protein, monoclonal antibodies, IgG1 and IgG2b, neutralizing viruses isolated from many countries and animals. Clinical data from a randomized, multicentre, open label comparator-controlled study demonstrated safety, tolerability and efficacy when co-administered with rabies vaccine, VaxiRab N, indicating non-inferiority to Imogam [14].
S. Missailidis (BioManguinhos) described a new YF vaccine production process aimed at doubling its capacity, and the development of a purified YF vaccine made from 17DD virus strain in Vero cell culture, inactivated with β-propiolactone. Production capacity of the latter was increased through a scale-up process optimized by GE Healthcare technology, and antibiotic-free formulation improved its quality. Furthermore, BioManguinhos initiated three parallel Zika vaccine projects to allow comparative clinical trials: a Vero-cells inactivated virus vaccine for pregnant women during epidemics, an attenuated vaccine and a recombinant chimeric virus of the YF 17DD strain expressing Zika virus proteins. Immunogenicity and neurovirulence in non-human primates were studied.
W. Meng (Sinovac) shared the clinical development of Sabin IPV (sIPV), facilitated by WHO. Two products were designed: pure sIPV and alum-adjuvanted sIPV for multi-dose vials. Phase 2 clinical studies showed >95% seroconversion rate and high GMT values proportional to antigen dosage. Non-inferiority phase 3 studies comparing Sabin IPV to Salk IPV are underway, aiming for WHO PQ and approval in 2021.
J. Shih (Innovax) discussed Hepatitis E and cervical cancer vaccines. Hepatitis E outbreaks in Africa and Southeast Asia showed serious risks for pregnant women [15]. The vaccine, produced in E. coli from genotype 1, showed cross protection against genotype 4, and is approved in China and Pakistan. A Phase I trial is planned in the United States. A bivalent HPV16/18 vaccine targeting 9–45-year-old females, also produced in E. coli, showed safety and efficacy in clinical studies compared to similar bivalent and quadrivalent HPV vaccines. Studies in adolescent girls, with 2-dose schedule, showed comparable immunogenicity to 3 doses. A 9-valent vaccine is planned.
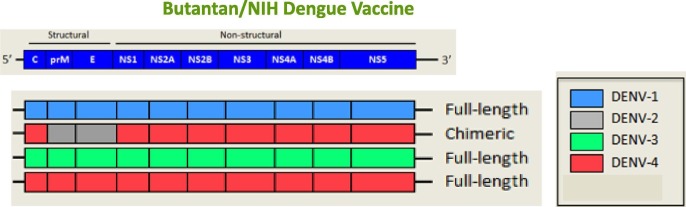
A. Precioso (Butantan) reported on a second-generation recombinant Dengue vaccine. Dengue is caused by antigenically distinct serotypes 1, 2, 3 and 4, with most endemic countries reporting circulation of all four serotypes. Infection with one serotype confers lifelong immunity to that serotype (homotypic protection) while cross immunity to other serotypes (heterotypic protection) persists for one or two years. Most severe dengue cases are observed with secondary heterotypic infection and antibody-dependent enhancement [16]. The live attenuated lyophilized vaccine (Fig. 4 ) was safe, immunogenic and well tolerated and is undergoing a randomized, multicentre, double-blind, placebo-controlled Phase III trial in Brazil, with one dose given subcutaneously to subjects 2–59 years old. [17] This vaccine has 32 antigens and is expected to confer at least 80% protection against symptomatic disease. Previous dengue exposure was not associated with adverse reactions.
Fig. 4.
Butantan/NIH recombinant attenuated vaccine strategy for dengue. The upper diagram shows schematically the strategy used by the Butantan, in cooperation with the US National Institutes of Health (NIH), to develop a second-generation recombinant Dengue vaccine. The Dengue virus genome is about 11,000 bases of positive-sense, single stranded RNA (ssRNA) that codes for three structural proteins (capsid protein C, membrane protein M, envelope protein E) and seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, NS5). It also includes short non-coding regions on both the 5′ and 3′ ends (black lines). The vaccine constructs comprise three full-length dengue viruses types 1, 3, 4 (depicted in blue, green and red in the lower diagram), attenuated by one or more 30-nucleotide deletions in the 3′ untranslated region (NS1 to NS5). The non-structural proteins are derived from type-1, type-3, and type-4 vaccines. The type-2 component is a chimeric virus, carrying virus type 2 structural M and E genes and capsid and non-structural genes of virus type 4 genome. Abbreviations: DENV-1: Dengue virus type 1; DENV-2: Dengue virus type 2; DENV-3: Dengue virus type 3; DENV-4: Dengue virus type 4. NS = non structural genes (Figure courtesy of A. Precioso). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
7. Future quality Control (QC) assays and international standards: Technology transfer initiatives
U. Rosskopf (WHO) introduced the Global WHO-National Control Laboratory (NCL) Network for Biologicals, established in 2016. Responsible NRAs/NCLs provide rigorous oversight of vaccines by testing thousands of vaccine lots against approved specifications. The Network promotes exchange of technical information, predominantly on testing of WHO PQ vaccines; efficient use of resources; and mutual recognition of lot release, thereby reducing costs, minimizing risk of inaccurate results and fostering 3R principles 7 [18], [19], [20]. In 2017, a shared electronic platform was created for Network members to exchange quality and technical information in a confidential setting. The Network comprises NCLs of vaccine-producing countries, WHO test laboratories, NRAs/NCLs of countries that receive UN-procured vaccines (and also non-prequalified vaccines), UN agencies, manufacturer associations and other stakeholders.
I. Feavers (NIBSC) discussed vaccine testing using new methods developed during the vaccine life cycle, exemplified by the NIBSC Meningitis Group working with a manufacturer to assure vaccine batch quality by introducing an in vitro test to measure the presence of pyrogens, thereby reducing the use of animals. Another example is the histamine sensitization test for acellular pertussis vaccines that evaluated the CHO cell intoxication clustering assay in a collaborative study [21]. A third example is the evaluation of deep sequencing (DS) as an alternative for MAPREC 8 [22] in polio vaccines manufacturing. An international collaborative study with participating NCLs and vaccine manufacturers assessed consistency of OPV. Sabin poliovirus type 3 showed good correlation between MAPREC and DS. The study will build a database of mutational composition of seed viruses and vaccine batches from different manufacturers. The new approach could be applied to other licensed vaccines.
D. Boyle and N. Agarwal (PATH) expressed the need for high-quality antibodies to test and characterize PCV during R&D and manufacturing processes. In collaboration with a manufacturer, hybridomas were generated and screened for quality (e.g. absence of cross-reactivity, and binding affinity). In 2019, a set of 12 affordable mAbs will be commercially available. A second set of mAbs will follow. The solution presented here is a globally accessible, commercially sustainable repository of high-quality affordable mAbs against 24 of the most common pneumococcal serotypes.
G. Kersten (Intravacc) proposed a testing scheme for DTP 9 based-vaccines, to reduce the use of animals in manufacturing by serological alternatives, such as cell culture and immune-physico-chemical methods. Potency tests for toxoid vaccines are based on a lethal challenge in animals although new methods for Tetanus [23] and Diphtheria [24] have received regulatory acceptance. An alternative serological potency test for Pertussis vaccines is proposed for use in conjunction with a T-helper cell responses (qualitative) assay. Alternatively, an ELISA 10 to quantify key antigens in wP vaccines could be used next to the serology assay. Such a consistency approach will support regulatory acceptance. A study outline is under consideration by stakeholders.
S. Boyle (BMGF) and K. Mahmood (PATH) jointly presented the establishment of international reference reagents for sIPV. In 2014, experts and vaccine manufacturers discussed assays to measure the D-antigen content of sIPV products and harmonization of potency tests. NIBSC assessed the suitability of WHO International Standard (IS) 12/104 for conventional IPV (cIPV) to measure sIPV products through a collaborative study including products from several manufacturers. Despite good performance of cIPV IS, it was considered unsuitable for sIPV. Assay validation and inter-laboratory variability for in-house methods improved when using a sIPV sample as reference. A second collaborative study confirmed that D-antigen measurements of sIPV improved when using sIPV samples as reference. Based on characterization and stability data, the first WHO IS for sIPV was endorsed by the WHO Expert Committee on Biological Standardization in October 2018 [25], and is available from NIBSC for use in potency testing.
8. Fostering regulatory convergence
E. Cooke (WHO) introduced WHO regulatory activities, focusing on the CRP for accelerating registration in emerging countries. The CRP is based on principles of cooperation, reliance, harmonization and voluntary information sharing to avoid duplication of efforts, enabling faster and efficient access to quality vaccines while respecting sovereignty and national decision-making. Thus far, 34 countries have accepted the CRP. Since 2013, the CRP has enabled registration of 350 medicines within an average of 90 days, and implementation of its principles contributed to achieving 26 vaccine registrations. In 2017, a Pentavalent was registered in Ethiopia within 6 months, and cholera vaccines were registered in Nigeria and Caribbean countries in 3–5 months. Still, there is a need to optimize the CRP for vaccines by better defining priority vaccines and priority countries.
D. Maïga (WHO/AFRO) explained the impact of joint scientific and ethics reviews of clinical trial applications in Africa, where growing public health needs require faster access to quality-assured medical products. AVAREF facilitated the development and use of several vaccines in Africa, particularly MenAfriVac and Ebola, serving as a Pan-African ethics and regulatory harmonization platform. In a joint review, experts from NRAs and/or Ethics Committees (ECs) of various countries review a common dossier and collectively prepare questions to discuss with the applicant. Fostering NRA convergence and harmonization of procedures between countries requires the agreement of the manufacturer and/or sponsor, as well as agreement of the target countries (NRAs and ECs) following WHO/AVAREF guidance [26].
H. Langar (WHO/EMRO) described the CRP-facilitated registration of polio vaccines through joint dossier review in Eastern Mediterranean Region (EMR) countries. In 2015, WHO recommended the introduction of IPV, and to ensure at least two available brands of IPV were registered in any of 21 EMR countries: two countries lacked registered IPV and five countries had only one registered IPV brand. The joint registration procedure was legally accepted by the countries for issuance of a marketing authorization (MA), with commitment to no further testing or site inspections before granting the MA. A joint evaluation meeting, focused on IPV vaccine dossiers as submitted to the NRAs, was conducted in October 2014. [27] Participating NRAs reported to their respective registration committee for final decision, and five countries registered IPV in 2015. Notably, participation of manufacturers in the meeting accelerated the process, addressing questions and concerns expressed by NRAs.
N. Dellepiane (DCVMN) discussed possible improvements in vaccine registration procedures in emerging countries, addressing previously identified challenges. [28] The joint industry Regulatory Experts Working Group developed four proposals for streamlining procedures and alignment of dossiers. First, registration procedures could rely on WHO PQ certificates or apply the CRP. Dossier evaluation could rely on batch release data of tests and inspections already conducted by WHO. Registration procedures for non-PQ vaccines could rely on dossiers and data provided by the manufacturing country’s NRA, through mutual recognition agreements, or based on release tests conducted by WHO-contracted laboratories (see U. Rosskopf, above). Second, alignment of dossier numbering systems across countries and regions could be improved by following the ICH CTD 11 (EU Notice to Applicants) [29], also recognized by WHO. Third, use an application form template (proposed in tabular format), maintaining the three key sections and main subheadings. Fourth, consider pre-submission meetings between the applicant, regulators and post-marketing pharmacovigilance systems to improve registration procedures. Dr. Dellepiane concluded that more regulatory convergence practices can improve registrations and access to vaccines globally.
9. Novel initiatives to improve vaccine coverage and equity
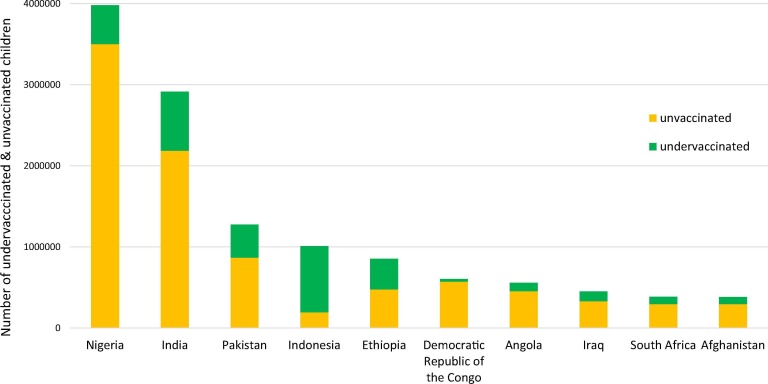
B. Giersing (WHO) introduced two new multi-stakeholder initiatives to improve vaccine delivery. Total Systems Effectiveness (TSE) is a framework that assists decision-makers to identify and select vaccine products according to attributes that best meet their needs. Vaccine Innovation Prioritisation Strategy (VIPS) aims to prioritize delivery technologies with identified product attributes, to clarify to manufacturers and other stakeholders regarding investment decisions. Both TSE and VIPS assume that differentiated delivery approaches are needed, given that most unvaccinated populations live in clustered areas, located in key countries (Fig. 5 ), focusing on poor and vulnerable populations.
Fig. 5.
Top 10 countries with most under- and un-vaccinated children for DTP3, in 2017. Graphical bar chart representation of countries with the largest numbers of undervaccinated and unvaccinated children for the third dose of DTP, under one year old (0–12 months). The number of undervacccinated and unvaccinated children is reflected in the Y axis and the countries are listed along the X axis. Globally, 19.9 million infants are unimmunized, with the greatest numbers of unimmunized children under one year old concentrated in Nigeria and India. Source: WHO/UNICEF coverage estimates 2017 revision, July 2018. Immunization Vaccines and Biologicals (IVB), World Health Organization. 194 WHO Member States. Date of slide: 18 July 2018. (Figure courtesy of B. Giersing).
Examples of such innovations include heat-stable/freeze-stable formulations, labelling to track temperature exposures, jet-injectors, powder-inhaler or nebulizer devices, compact pre-filled auto-disable devices/syringes, blow-fill-seal containers, integrated reconstitution devices, microarray patches and controlled temperature chain use of vaccines.
D. Kristensen (PATH) moderated a discussion on innovative technologies, TSE, and VIPS to help countries enhance coverage and equity through suitable vaccines for specific needs.
R. Park (EuBiologics) mentioned that a change in OCV vial presentation, from glass to plastic, reduced the storage volume/cold chain footprint by 50%, and cost of goods by 30%, and also facilitated vaccine administration by field workers, thereby increasing OCV coverage and achieving set goals.
V. Hsu (BMGF) explained that BMGF sees community needs as the priority, and investment decisions in supporting OCV product innovations were driven by policies to expand the OCV stockpile from 200,000 doses in 2013 to 12 million doses in 2018, saving many lives.
R. Kapoor (National University of Singapore) noted that the TSE framework guides LMICs 12 to identify criteria for product selection. In MICs 13, TSE brings different groups together to better inform the decision-making process and improve transparency, enabling comparison between different vaccines for the same disease, e.g. Rotarix and Rotateq, besides comparison of vaccines across diseases. The framework informs countries on cost, feasibility, packaging, schedule, etc., and helps manufacturers understand the basis for decision-making and needs.
D. Hein (Gavi) confirmed that VIPS and TSE help Gavi drive vaccine innovation to meet country needs and support immunization coverage and equity. The process provides an aligned perspective and greater clarity to manufacturers to make informed investment decisions.
S. Jadhav (SII) stated that it may be costly for manufacturers to develop/produce many product variations driven by TSE and VIPS, therefore some uniformity based on the preferred product profiles defined by WHO, is needed. He added that introduction of new technologies should coexist with already established technologies for some time.
Global partners and DCVMN suppliers agreed that a stable vaccine supply is needed and that industry’s understanding of demand and market evolution can help guide investment decisions.
10. Progress toward securing future vaccine supply
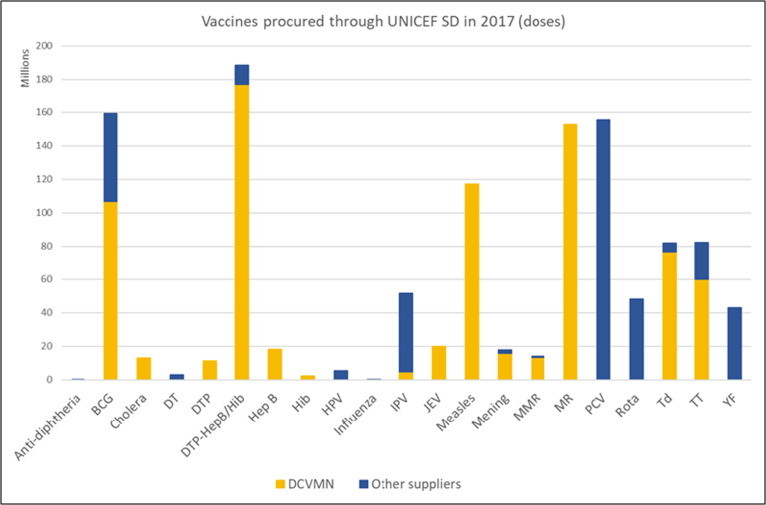
Y. Momeni (UNICEF) provided an update for 2017 (Fig. 6 ), where around 100 countries were supplied 2.4 billion doses of vaccine through UNICEF, at a value of US$1.3 billion, reaching 45% of children under 5 years of age. [30] Eleven DCVMN companies supplied 1.3 billion doses (54%) of traditional vaccines at a value of US$400 million. The supply crises in early 2000, where UNICEF experienced severe vaccine shortages due to mergers, acquisitions and exits from the UNICEF market, highlighted the importance of longer-term forecasting for manufacturing planning to secure access to supply. Currently, UNICEF is experiencing insufficient supply of Rotavirus, IPV and HPV, and is relying on a limited supplier base for PCV, Typhoid and JE vaccines. UNICEF is in the process of implementing sustainable procurement approaches requiring manufacturing policies and encouraging innovations that reduce carbon footprint and waste disposal.
Fig. 6.
Graphical representation of vaccines procured through the UNICEF Supply Division in 2017 (doses). The number of vaccine doses procured is reflected in the Y access (in millions of doses) while the 21 types of vaccines procured are listed in the X axis. The figure illustrates vaccines supplied by DCVMN companies (in yellow) and vaccines supplied by other vaccine companies (in blue). DCVMN companies supplied 1.3 billion doses (54%) of the 2.4 billion doses procured by UNICEF. Abbreviations: BCG: Bacille Calmette Guérin; DT: Diphtheria and Tetanus; DTP: Diphtheria, Tetanus and Pertussis; DTP Hep B/Hib: Diphtheria, Tetanus, Pertussis, Hepatitis B, Haemophilus influenzae type b; Hep B: Hepatitis B; Hib: Haemophilus influenzae type b; HPV: Human Papillomavirus; IPV: Inactivated Polio Vaccine; JEV: Japanese Encephalitis Virus; Mening: Meningitis; MMR: Measles, Mumps and Rubella; MR: Measles and Rubella; PCV: Pneumococcal Conjugate Vaccine; Rota: Rotavirus; Td: Tetanus and Diphtheria; TT: Tetanus Toxoid; YF: Yellow Fever. (Figure courtesy of Y. Momeni).
N. Steensma (CHAI) offered support to companies to help develop their business strategy including building a business case for entering a market and gathering market intelligence. In light of Gavi transition and more countries becoming Middle Income Countries (MICs), and in response to requests from DCVM manufacturers, CHAI will increase its support to provide market intelligence on MICs.
R. Suri (Panacea) highlighted trust, transparency and fairness as key characteristics through dealing with UNICEF for many years, requesting better demand visibility, including changes related to vaccination campaigns. Improvement in multi-phased tenders, which led to market exits in Pentavalent, could be considered. There is consensus among manufacturers that it is very challenging to negotiate contractual terms which are outside of UNICEF’s standard contractual arrangements.
L. Yang (CNBG) appreciated the collaboration with UNICEF on processes and forecasting, including proactive problem-solving leading up to, and after, becoming a supplier. She requested aggregate forecasts across UNICEF and self-procuring countries, and support to work with governments to even out demand spikes. She highlighted the great potential of Chinese suppliers, with 48 registered vaccine manufacturers.
L. Shi (Walvax) said that the company is committed to achieving WHO PQ, ensuring products meet international standards. Walvax highlighted their concerns regarding country product preferences, or lack of preference.
The panel agreed that WHO prequalification is an important international label for quality assurance and compliance, irrespective of country of production. Participants also discussed the challenges surrounding country product preferences, registration requirements and conflicting priorities, especially for products newly prequalified by WHO, where country programs drive for earlier access despite increased complexity in legislation and regulatory requirements.
11. Closing lecture and concluding remarks
C. Nannei and S. Goldin (WHO) delivered the closing lecture focused on the progress and challenges for sustainable influenza vaccine production to ensure pandemic preparedness. A holistic approach to preparedness should include surveillance, evidence-based policies, strategies to deploy influenza vaccines, and sufficient vaccine supply. Considerations for rapid deployment of vaccines in an emergency include regulatory capacity, distribution systems, monitoring systems, healthcare workers’ familiarity with influenza immunization, vaccination policies for target groups and communication strategies. From 2006 to 2016, global seasonal influenza vaccine manufacturing capacity tripled from 500 million doses to 1.5 billion doses. During the same period, the estimated global pandemic influenza production capacity grew from 1.46 billion to 6.37 billion potential doses. WHO is developing a new Global Influenza Strategy for 2019–2030 to be launched in early 2019, highlighting the need for sustainable local production of influenza vaccines. DCVMN is a key partner in global influenza preparedness and response activities as an advocate for sustainable vaccine production capacity in developing countries.
Presentations and discussions throughout the meeting demonstrated progress and achievements made possible by international cooperation and highlighted the importance of partnerships in fostering innovations for public health, particularly in developing countries. Over the years, the support of the Bill and Melinda Gates Foundation, through PATH and other partners, unlocked economies of scale and sustainable vaccine supply capacity for developing countries, as exemplified by partnerships on MenAfriVac, Rotavirus, Japanese encephalitis and new Polio vaccines to achieve WHO PQ and supply global markets. Pooled procurement/financing mechanisms utilized by PAHO Revolving Fund, UNICEF and GAVI, in partnership with countries, help to protect people against known and emerging infectious diseases. Partnerships with WHO enhanced influenza vaccine manufacturing capacity, thereby increasing pandemic influenza preparedness. Enduring partnerships are the basis to innovation and strengthening industry in developing countries over the coming decades. Manufacturers and partners can take pride in their collective work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to all speakers and moderators whose contributions made the meeting possible and contributed to its success. We are particularly thankful to the experts who graciously served as session rapporteurs: W. Bakker (Intravacc), K. Balaji (PATH), Clarke Cole (CHAI), J. Li (IMBCAMS) and A. Ottonsen (UNICEF). We thank Ms. S. Ramirez for editorial assistance. We thank corporate partners for supporting DCVMN with unrestricted educational grants: Alfa Wasserman, Applikon Biotechnology, Bausch & Stroebel, Bioengineering, Biozeen, Bosch, GEA, GE Healthcare, Gihon, IQVIA, Merck Group, Munters, OMPI (Stevanato Group), Rommelag, Temptime Corporation and Univercells. This meeting was partly supported by a grant from the Bill & Melinda Gates Foundation, Grant no. OPP1153279.
Footnotes
Report on the 19th annual general meeting of the Developing Countries Vaccine Manufacturers Network, 29–31st October 2018, Kunming, China.
IMPORTANT NOTE: This report summarizes the views of an international group of experts as presented at a scientific conference in a given time and context and does not necessarily represent the decisions or the stated policy of any institution or corporation.
Acronyms: WHO: World Health Organization; UNICEF: United Nations International Children’s Fund; PAHO: Pan American Health Organization; PATH: Program for Appropriate Technology in Health; CEPI: Coalition for Epidemic Preparedness Innovations; CHAI: Clinton Health Access Initiative; Gavi: Gavi, the Vaccine Alliance; IVI: International Vaccine Institute; GHIF: Global Health Investment Fund; AVAREF: African Vaccine Regulatory Forum; NIBSC: National Institute for Biological Standards and Control; BMGF: Bill & Melinda Gates Foundation.
Data based on United Nations Sustainable Development Goals report 2017, page 21, available at https://unstats.un.org/sdgs/report/2017/.
CDIBP: Cheng-Du Institute of Biological Products.
wP = whole cell Pertussis.
The principles of the 3Rs (Replacement, Refinement and reduction) of animals in research is a European-led initiative that provides an internationally recognized framework for more ethical animal use in testing. The 3Rs principles encourage alternatives to animal testing and, where the use of animals cannot be avoided, aim to reducing their use and improve animal welfare. For more information see https://www.nc3rs.org.uk/the-3rs.
MAPREC: Mutant Analysis by PCR and Restriction Enzyme Cleavage.
DTP : Diphtheria-Tetanus-Pertussis.
ELISA: enzyme-linked immunosorbent assay.
ICH CTD: The International Council for Harmonisation (ICH) Common Technical Document (CTD).
LMIC: low and middle-income countries.
MIC: middle income countries.
Contributor Information
Sonia Pagliusi, Email: s.pagliusi@dcvmn.net.
Yanchun Che, Email: cheyanchun@imbcams.com.cn.
Shaozhong Dong, Email: dsz@imbcams.com.cn.
References
- 1.WHO Thirteenth general programme of work 2019−2023. Available at https://apps.who.int/iris/bitstream/handle/10665/324775/WHO-PRP-18.1-eng.pdf.
- 2.WHO Emergencies. Availableat http://www.who.int/emergencies/crises/en/
- 3.WHO Collaborative procedure between the World Health Organization (WHO) Prequalification Team and national regulatory authorities in the assessment and accelerated national registration of WHO-prequalified pharmaceutical products and vaccines. TRS, No. 996, 2016, Annex 8 https://www.who.int/medicines/publications/pharmprep/WHO_TRS_996_web.pdf.
- 4.UN Sustainable Development Goal 3: Ensure healthy lives and promote well-being for all at all ages. Available from: https://www.un.org/sustainabledevelopment/health/.
- 5.Cernuschi T., Gaglione S., Bozzanic F. Challenges to sustainable immunization systems in Gavi transitioning countries. Vaccine. 2018 Oct 29;36(45):6858–6866. doi: 10.1016/j.vaccine.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Univercells’ modular manufacturing platforms for vaccines, antibodies or proteins. Available from: https://www.univercells.com/our-technology/.
- 7.Mohanty L. A randomized, open label trial to evaluate and compare the immunogenicity and safety of a novel liquid hexavalent DTwP-Hib/Hep B-IPV (EasySix) to licensed combination vaccines in healthy infants. Vaccine. 2018;36(17):2378–2384. doi: 10.1016/j.vaccine.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Mustapha M.M., Harrison L.H. Vaccine prevention of meningococcal disease in Africa: Major advances, remaining challenges. Hum Vaccines Immunother. 2018;14(5):1107–1115. doi: 10.1080/21645515.2017.1412020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chilukuri S.R. Process development and immunogenicity studies on a serogroup 'X' Meningococcal polysaccharide conjugate vaccine. Biologicals. 2014;42(3):160–168. doi: 10.1016/j.biologicals.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen W.H. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect Dis. 2018;18(10):1088–1096. doi: 10.1016/S1473-3099(18)30400-6. [DOI] [PubMed] [Google Scholar]
- 11.Collaborators G.B.D.D.D. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018;18(11):p. 1211–28. [DOI] [PMC free article] [PubMed]
- 12.Bines J.E. Human neonatal rotavirus vaccine (RV3-BB) to Target Rotavirus from Birth. N Engl J Med. 2018;378(8):719–730. doi: 10.1056/NEJMoa1706804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groome M. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2017;17(8):843–853. doi: 10.1016/S1473-3099(17)30242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanofi Pasteur. Product monograph: IMOGAM® Rabies Pasteurized - Rabies Immune Globulin, Pasteurized (Human) Solution for Injection. Available from: https://www.vaccineshoppecanada.com/document.cfm?file=imogam_rabies_e.pdf
- 15.Cooper B.S., White L.J., Siddiqui R. Reactive and pre-emptive vaccination strategies to control hepatitis E infection in emergency and refugee settings: a modelling study. PLoS Negl Trop Dis. 2018;12(9):e0006807. doi: 10.1371/journal.pntd.0006807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzelnick LC, Harris E, and Participants in the Summit on Dengue Immune Correlates of, Immune correlates of protection for dengue: state of the art and research agenda. Vaccine 2017;35(36):p. 4659–69. [DOI] [PMC free article] [PubMed]
- 17.Precioso A.R. Clinical evaluation strategies for a live attenuated tetravalent dengue vaccine. Vaccine. 2015;33(50):7121–7125. doi: 10.1016/j.vaccine.2015.09.105. [DOI] [PubMed] [Google Scholar]
- 18.WHO WHO-National Control Laboratory Network for Biologicals (WHO-NNB). Available from: https://www.who.int/immunization_standards/vaccine_quality/who_nnb/en/.
- 19.WHO Collaboration – Global network of national vaccine control laboratories. WHO Drug Information, 2017;31(1):p. 3–10.
- 20.WHO Regulatory networks – Update on the WHO-National Control Laboratory Network for Biologicals. WHO Drug Information 2018;32(2):p. 189–93.
- 21.Isbrucker R. Transferability study of CHO cell clustering assays for monitoring of pertussis toxin activity in acellular pertussis vaccines. Pharmeur Bio Sci Notes. 2016;2015:97–114. [PubMed] [Google Scholar]
- 22.WHO Standard Operating Procedure: Mutant Analysis by PCR And Restriction Enzyme Cleavage (MAPREC) for Oral Poliovirus (Sabin) Vaccine Types 1, 2 or 3 Version 5 (2012). Available from: https://www.who.int/biologicals/vaccines/MAPREC_SOP_Final_09112012.pdf
- 23.WHO Recommendations to assure the quality, safety and efficacy of tetanus vaccines (adsorbed) Replacement of Annex 2 of WHO Technical Report Series, No. 800, and Annex 5 of WHO Technical Report Series, No. 927. WHO Technical Report Series No. 980, 2014 Annex 5.
- 24.WHO Recommendations to assure the quality, safety and efficacy of diphtheria vaccines (adsorbed) Replacement of Annex 2 of WHO Technical Report Series, No. 800, and Annex 5 of WHO Technical Report Series, No. 927. WHO Technical Report Series No. 980, 2014 Annex 4.
- 25.NIBSC & WHO International Standard - Sabin Inactivated Polio Vaccine (sIPV). Instructions for use - Version 2.0; Available from: https://www.nibsc.org/documents/ifu/17-160.pdf.
- 26.AVAREF Publications: Guideline for Joint and Assisted Reviews of Clinical Trial Applications. AVAREF 2017-JRCTA; Available from: http://afro.who.int/about-us/leadership/avaref.
- 27.Langar H., Dehaghi R.O.A., Dellepiane N. Joint evaluation of marketing authorization files of inactivated polio vaccines in countries of the Eastern Mediterranean Region. East Mediterr Health J. 2018;24(6):588–594. doi: 10.26719/2018.24.6.588. [DOI] [PubMed] [Google Scholar]
- 28.Dellepiane N, Pagliusi S, and Registration Experts Working Group., Challenges for the registration of vaccines in emerging countries: differences in dossier requirements, application and evaluation processes. Vaccine 2018;36(24):p. 3389–96. [DOI] [PMC free article] [PubMed]
- 29.European Commission. EU Volume 2 B Notice to applicants. Medicinal products for human use. Presentation and format of the dossier. Common Technical Document (CTD). Available from: https://ec.europa.eu/health/documents/eudralex/vol-2_en.
- 30.UNICEF Supply Annual Report 2017. Available from: https://www.unicef.org/supply/files/Unicef_External_Annual_Report_2017.pdf.