Abstract
Objective:
The primary aim of this investigation was to evaluate substance-specific and nonspecific associations between parental and sibling histories of alcohol, cannabis, amphetamine, and hallucinogen use disorders with proband risk for these conditions. A second aim was to evaluate whether the specificity of substance use disorder (SUD) risk to probands varied by family member (i.e., father, mother, and any sibling).
Method:
Lifetime SUD diagnostic data for this family-based investigation were derived from semistructured interviews of community residents. Participants were an age-based cohort (probands), selected at random during adolescence and followed longitudinally until age 30, and their first-degree family members (n = 803 probands and families).
Results:
Findings generally supported substance-specific and nonspecific forms of familial risk related to a particular type of SUD in probands. Family-based alcohol use disorder (AUD) demonstrated the greatest degree of risk specificity of any substance category, in that no other family SUD category predicted proband AUD. Family-based AUD, however, was also the most consistent nonspecific predictor of nonalcohol forms of SUD among probands. Among family members, the most consistent unique effects associated with a substance-specific risk to probands were observed for siblings.
Conclusions:
Findings support both the generality and specificity of risk associated with the abuse of or dependence on specific substances within families and highlight the impact of siblings on SUD risk to other siblings. Study findings underscore the need for a better understanding of malleable family-based factors that promote and reduce SUD risk among members.
In the united states, past-year prevalence rates of alcohol use disorder (AUD) and drug use disorders (inclusive of cannabis, amphetamines, club drugs, cocaine, hallucinogens, opiates, sedatives, and inhalants) have been estimated to be approximately 14% and 4%, respectively, in the general population (Grant et al., 2015, 2016). Worldwide, substance use disorders (SUDs) account for 20% of all disability-adjusted life years and 81% of all premature deaths that result from any psychiatric condition (Whiteford et al., 2013). SUDs are, as a consequence, common and serious public health concerns.
Although there are likely multiple developmental pathways that culminate in SUDs, it is increasingly clear that important mechanisms underlying SUD risk include those that are family based (Chassin et al., 2016; Kendler et al., 2012, 2013; Merikangas & McClair, 2012). Parental SUDs, for example, are known risk factors for SUDs in offspring (e.g., Chassin et al., 1999; Kendler et al., 2012; Kosty et al., 2015; Lieb et al., 2002; Merikangas et al., 2009; Sher et al., 1991), as are SUDs among siblings (Kendler et al., 2013). An understudied topic within the family SUD literature pertains to whether SUD risk shared among family members is substance specific or nonspecific with respect to a particular drug category. Family-based studies (Bierut et al., 1998; Kendler et al., 1997; Meller et al., 1988; Merikangas et al., 1998) have typically found evidence of a specific risk related to a particular substance type or drug category (e.g., the effect of family AUD histories on proband risk for AUD), as well as a general or nonspecific risk for any form of SUD (e.g., the effect of family AUD histories on proband risk for cannabis, amphetamine, and hallucinogen use disorders). Similarly, twin studies often report evidence of genetic and environmental factors that signify a nonspecific vulnerability to psychoactive substance abuse across drug categories, as well as a specific vulnerability for the abuse of certain drug classes that is independent from nonspecific vulnerability factors (Kendler et al., 1999, 2000, 2003; McGue et al., 2000; True et al., 1999; Tsuang et al., 1998; Young et al., 2006). Nonspecific heritable factors appear to be more influential as the level of drug involvement increases (e.g., from occasional use to dependence; Young et al., 2006) and may change with increasing age when the scope of misused substances typically narrows (Kendler et al., 2008; Vrieze et al., 2012).
Research into the family aggregation of SUDs has also sought to identify the primary sources of risk within families. In a twin study, Hicks et al. (2004) found that drug-specific resemblance, when distinguished at two levels (i.e., alcohol dependence vs. other drug dependence), was more pronounced among sibling pairs than between parent–offspring pairs (see also Bornovalova et al., 2010; Hicks et al., 2011, 2013). Other research found that offspring substance-related histories have robust associations with substance use or SUD histories among other siblings (Kendler et al., 2013), sometimes more so than with parents’ substance-related histories (Whiteman et al., 2013). Among parents, findings are equivocal as to whether fathers’ substance use or SUD histories are more predictive of offspring substance use or SUD than mothers’ histories or whether risk to offspring depends on which parent had an SUD (Chassin et al., 1991; Ohannessian et al., 2005; Kendler et al., 1997; Sørensen et al., 2011).
This family-based study from the Oregon Adolescent Depression Project investigated whether familial sources of SUD risk are limited to the corresponding substance or drug class (i.e., substance specific), are nonspecific with respect to substance type (i.e., substance general), or are some combination of these. Studies reviewed above have generally used unrepresentative samples, relied on offspring reports of parental or sibling drug use, or restricted analyses to AUD versus an all-inclusive drug category (e.g., drug use disorder). The present research sought to overcome these limitations in an investigation with two primary research questions:
(a) to what extent are there substance-specific versus nonspecific effects of family-based AUDs, cannabis use disorders (CUDs), amphetamine use disorders (AMPs), and hallucinogen use disorders (HUDs) on proband risk for these disorders, and
(b) what are the primary sources of familial influence (i.e., mother, father, any sibling) for substance-specific effects on proband risk for AUDs, CUDs, AMPs, and HUDs?
We hypothesized that findings would generally support drug specificity in risk as revealed by significant effects of each family SUD variable (e.g., family AUD) to the corresponding proband SUD variable (proband AUD). We also hypothesized some degree of nonspecificity in the familial aggregation of SUDs, as indicated by significant effects of specific family SUD variables (e.g., family AUD) on a range of different proband SUD categories (proband CUD, AMP, and HUD). In analyses involving sources of familial influence for substance-specific effects, our hypotheses are based on emerging research that suggests that siblings will generally demonstrate more robust associations with proband SUD risk than either parent.
Method
Participants
Probands.
The proband sample was initially selected at random from nine high schools in five Oregon communities. The Time 1 (T1; around age 16) sample of 1,709 adolescents was demographically similar to corresponding regional census data (Lewinsohn et al., 1993). T2 was initiated 1 year after T1, and 1,507 (88%) participants were reassessed. When continuing and noncontinuing participants were compared, attrition was higher for youth with disruptive behavior disorders (17% vs. 11%) and males with any form of SUD (26% vs. 14%). No other statistically significant differences in specific Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R; American Psychiatric Association, 1987), Axis I disorder rates were noted.
At T3 (around age 24), a stratified sampling procedure was implemented whereby eligible participants included all persons with a positive history of a psychiatric diagnosis by T2 (n = 644) and a randomly selected subset of never mentally ill participants (n = 457 of 863 persons). Of these 1,101 eligible persons, 941 (85%) completed T3. Diagnostic status assessed at T2 was not significantly related to attrition by T3 among eligible participants.
Of the 941 T3 probands, 816 (87%) participated in T4 (approximately age 30). Discontinuation from T3 to T4 was more common among participants with an SUD history (17% vs. 11%) but not for any specific form of SUD. No other significant diagnostic differences between continuers and noncontinuers were observed for DSM-IV (American Psychiatric Association, 1994) Axis I disorders. Because proband attrition between T3 and T4 was related to a history of an SUD diagnosis, probands who completed T1 through T3 but not T4 and their families were included in this research, in addition to probands who completed T1 through T4 and their family members.1 The 941 participants (57% female, 89% White, 53% married) who, at minimum, completed the T1 through T3 evaluations constituted the eligible proband sample for this research.
First-degree family members.
At about proband age 24, first-degree family members were interviewed. For the 941 probands who completed T1–T3 evaluations, interview data (described below) were available for 2,565 first-degree relatives: 801 biological mothers, 786 biological fathers, and 978 siblings 18 years of age or older. Complete data were obtained for all first-degree family members for 531 of 941 families (56%). Of the remaining families, 272 (29%) had data available for some family members but were missing data for one or more other members (mean proportion of first-degree relatives with data = 61%). No family data were available for 138 probands (15%). These probands and their family members were excluded from this research. Data from 803 probands and their first-degree family members were used for this investigation.
Diagnostic assessments
Probands.
During T1, T2, and T3, participants were interviewed with the Epidemiologic and Present Episode versions of the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Chambers et al., 1985; Orvaschel et al., 1982). Follow-up diagnostic evaluations at T2 and T3 also included the Longitudinal Interval Follow-Up Evaluation (LIFE; Keller et al., 1987) that, in combination with the K-SADS, provided detailed information related to the presence and course of disorders since the previous interview.
The T4 assessment included the administration of the LIFE and the Structured Clinical Interview for DSM-IV Axis I Disorders–Non-Patient Edition (SCID-NP; First et al., 1994). Diagnostic categories were evaluated in accordance with DSM-III-R criteria (American Psychiatric Association, 1987) at T1 and T2 and DSM-IV criteria (American Psychiatric Association, 1994) at T3 and T4. There was, however, sufficient additional symptom information collected during T1 and T2 to permit DSM-IV–based evaluations of individual SUD categories (see Rohde et al., 2007). As a consequence, all SUD diagnoses in this research were based on DSM-IV diagnostic criteria.
Audiotapes of randomly selected diagnostic interviews were evaluated for interrater reliability by a second rater (see Rohde et al., 2007, for details). Because the kappa statistic can become inflated, deflated, or unreliable when few positive cases are observed, interrater reliability was computed only for diagnostic categories coded as present on at least 10 occasions summed over two raters. AUD, CUD, and HUD were diagnosed with sufficient frequency among raters during at least one of the four assessment waves, and the mean κ statistics for those categories were .73, .79, and .77, respectively. AMP was insufficiently prevalent among reliability assessments to permit kappa calculations. Proband diagnostic data used in this research were based on judgments made by raters who directly interviewed participants.
First-degree family members.
Lifetime diagnostic assessments of parents and adult siblings of probands were performed with the SCID-NP (First et al., 1994). Information on family members was primarily obtained through direct interviews and supplemented with reports from another family member. When direct interviews were not possible (38% of all first-degree relatives), we attempted to interview at least two first-degree relatives about the psychiatric history of the target relative based on the family history method of assessment (Mannuzza & Fyer, 1990), a procedure with demonstrated validity (Rice et al., 1995). Final diagnostic decisions were based on DSM-IV criteria and the best-estimate method (Leckman et al., 1982). Two of four senior diagnosticians blind to the proband’s diagnosis independently derived diagnoses for each relative based on all available data, with disagreements resolved by consensus. Interdiagnostician agreement was excellent for individual SUD categories evaluated for fathers, mothers, and siblings (all κs > .75).
Data analyses
Specific SUD categories selected for evaluation exceeded minimal thresholds of lifetime prevalence (>1%) for, separately, fathers, mothers, siblings, and probands in the sample of 803 families. Two separate sets of primary analyses were performed based on different sample compositions. In “complete sample” analyses, data were analyzed for families with diagnostic data available for at least one family member (n = 803 families). In families without siblings, or when data were missing for a specific family member, individual SUD categories for that member were coded as “diagnosis absent” or unaffected. In “restricted sample” analyses, the sample was limited to families in which the proband had at least one sibling and for whom data about lifetime SUD history were available (n = 633 families). In this sample, missing data for individual family members were also coded as absent for each SUD category. Path analysis models with categorical outcomes were conducted separately for complete and restricted samples with Mplus (Muthén & Muthén, 1998–2017) and robust maximum likelihood estimation.
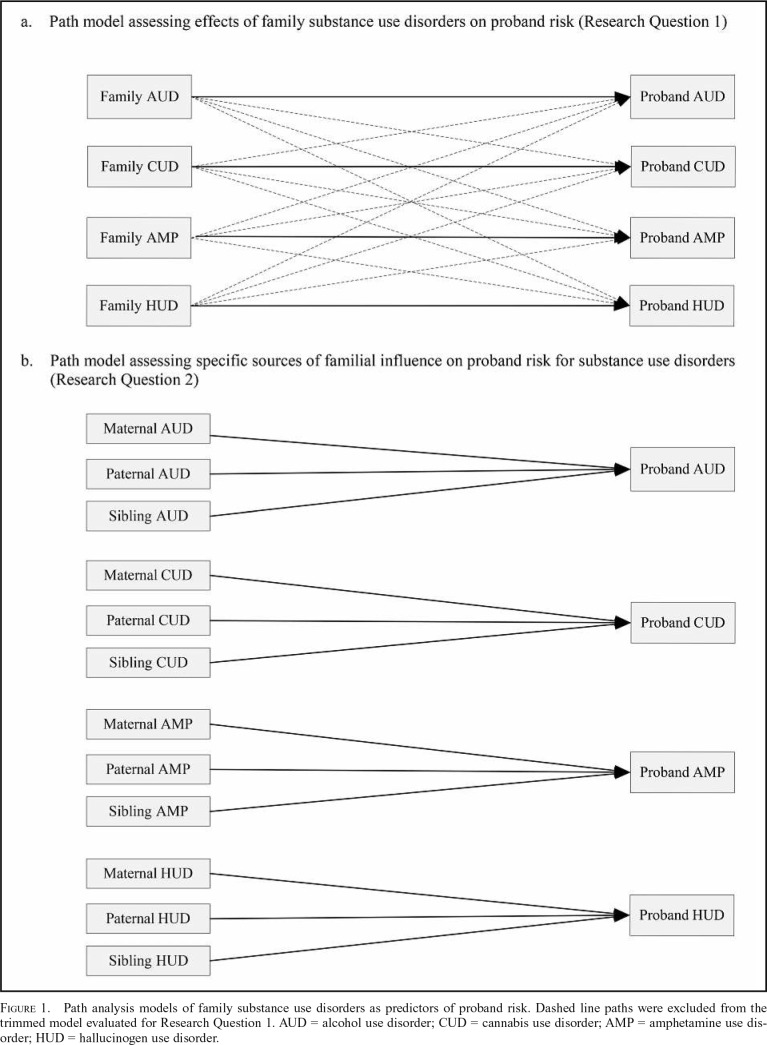
For the first research question, predictor variables included the number of family members with lifetime AUDs, CUDs, AMPs, and HUDs, computed as the sum of within-substance diagnoses across family member categories (i.e., mother, father, and any siblings; minimum = 0, maximum = 3). Criterion variables included binary representation of probands’ lifetime history of these same SUD categories (absent = 0, present = 1). We examined two competing path models, illustrated in Figure 1a. First, we estimated a trimmed model that included only paths from individual family SUD categories to the corresponding proband variable (e.g., family AUD to proband AUD). Second, we examined a full model that included all paths from each family variable to each proband variable (e.g., family AUD to proband AUD, CUD, AMP, and HUD).
Figure 1.
Path analysis models of family substance use disorders as predictors of proband risk. Dashed line paths were excluded from the trimmed model evaluated for Research Question 1. AUD = alcohol use disorder; CUD = cannabis use disorder; AMP = amphetamine use disorder; HUD = hallucinogen use disorder.
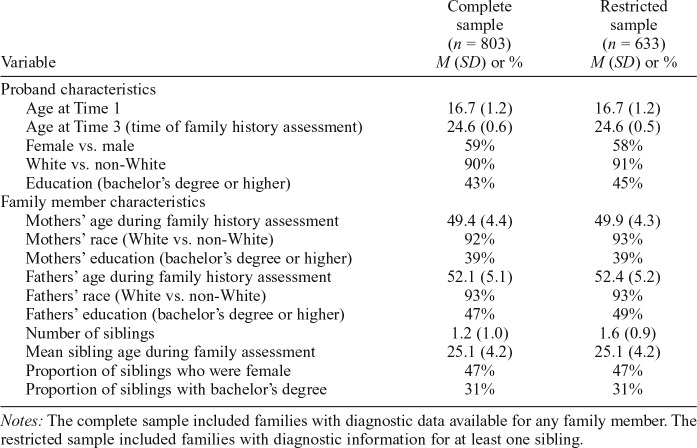
For the second research question, predictor variables included binary indicators denoting lifetime history of AUDs, CUDs, AMPs, and HUDs among fathers, mothers, and any sibling. Criterion variables included binary indicators of probands’ lifetime history of these same SUD categories. To avoid the evaluation of more parameters than the data can reasonably permit (i.e., overfitting), analyses involving sources of familial risk evaluated only substance-specific effects (Figure 1b). Significance tests and odds ratios (ORs) were used to evaluate which familial sources had unique associations with proband risk for individual SUD categories.
Results
Descriptive data
Tables 1 and 2, respectively, present demographic characteristics and lifetime rates of SUD categories for probands, mothers, fathers, and any siblings in the complete and restricted samples.
Table 1.
Demographic characteristics of probands and family members
| Complete sample (n = 803) M (SD) or % | Restricted sample (n = 633) M (SD) or % | |
| Variable | ||
| Proband characteristics | ||
| Age at Time 1 | 16.7 (1.2) | 16.7 (1.2) |
| Age at Time 3 (time of family history assessment) | 24.6 (0.6) | 24.6 (0.5) |
| Female vs. male | 59% | 58% |
| White vs. non-White | 90% | 91% |
| Education (bachelor’s degree or higher) | 43% | 45% |
| Family member characteristics | ||
| Mothers’ age during family history assessment | 49.4 (4.4) | 49.9 (4.3) |
| Mothers’ race (White vs. non-White) | 92% | 93% |
| Mothers’ education (bachelor’s degree or higher) | 39% | 39% |
| Fathers’ age during family history assessment | 52.1 (5.1) | 52.4 (5.2) |
| Fathers’ race (White vs. non-White) | 93% | 93% |
| Fathers’ education (bachelor’s degree or higher) | 47% | 49% |
| Number of siblings | 1.2 (1.0) | 1.6 (0.9) |
| Mean sibling age during family assessment | 25.1 (4.2) | 25.1 (4.2) |
| Proportion of siblings who were female | 47% | 47% |
| Proportion of siblings with bachelor’s degree | 31% | 31% |
Notes: The complete sample included families with diagnostic data available for any family member. The restricted sample included families with diagnostic information for at least one sibling.
Table 2.
Lifetime rates of individual substance use disorder categories among family members
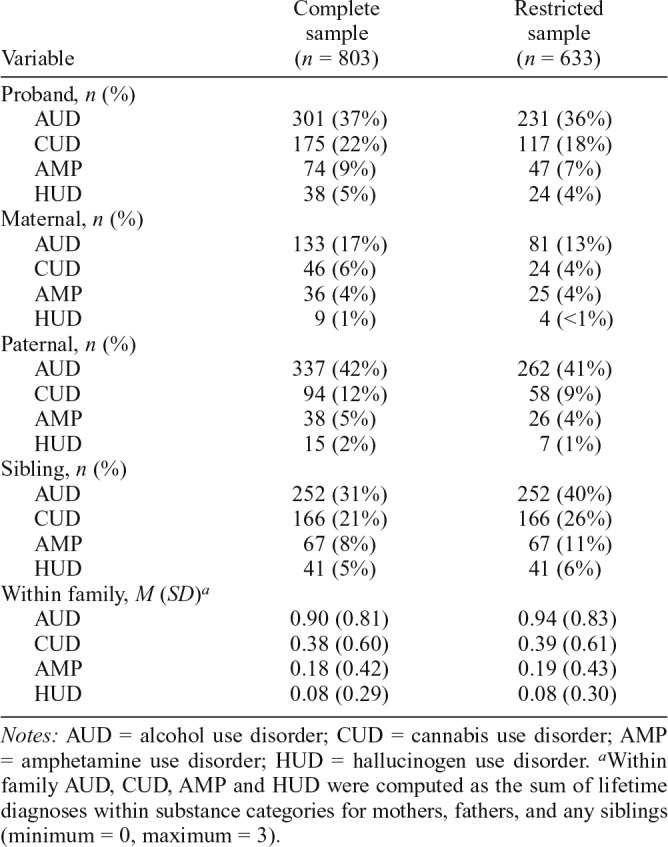
| Variable | Complete sample (n = 803) | Restricted sample (n = 633) |
| Proband, n (%) | ||
| AUD | 301 (37%) | 231 (36%) |
| CUD | 175 (22%) | 117 (18%) |
| AMP | 74 (9%) | 47 (7%) |
| HUD | 38 (5%) | 24 (4%) |
| Maternal, n (%) | ||
| AUD | 133 (17%) | 81 (13%) |
| CUD | 46 (6%) | 24 (4%) |
| AMP | 36 (4%) | 25 (4%) |
| HUD | 9 (1%) | 4 (<1%) |
| Paternal, n (%) | ||
| AUD | 337 (42%) | 262 (41%) |
| CUD | 94 (12%) | 58 (9%) |
| AMP | 38 (5%) | 26 (4%) |
| HUD | 15 (2%) | 7 (1%) |
| Sibling, n (%) | ||
| AUD | 252 (31%) | 252 (40%) |
| CUD | 166 (21%) | 166 (26%) |
| AMP | 67 (8%) | 67 (11%) |
| HUD | 41 (5%) | 41 (6%) |
| Within family, M (SD)a | ||
| AUD | 0.90 (0.81) | 0.94 (0.83) |
| CUD | 0.38 (0.60) | 0.39 (0.61) |
| AMP | 0.18 (0.42) | 0.19 (0.43) |
| HUD | 0.08 (0.29) | 0.08 (0.30) |
Notes: AUD = alcohol use disorder; CUD = cannabis use disorder; AMP = amphetamine use disorder; HUD = hallucinogen use disorder.
Within family AUD, CUD, AMP and HUD were computed as the sum of lifetime diagnoses within substance categories for mothers, fathers, and any siblings (minimum = 0, maximum = 3).
Effects of family SUDs on proband risk
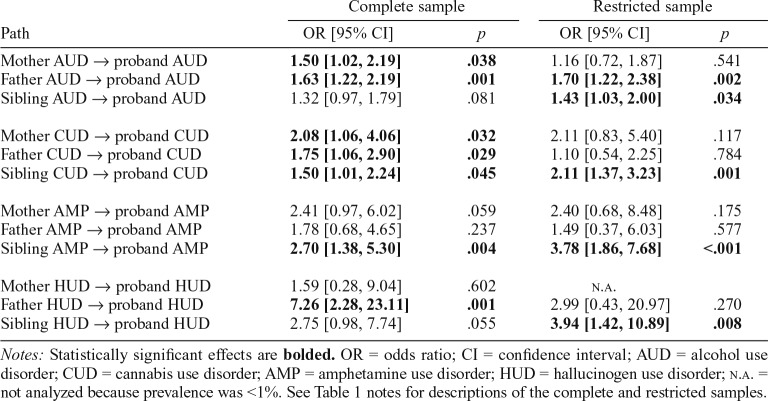
The first research question evaluated substance-specific versus nonspecific effects of family AUDs, CUDs, AMPs, and HUDs on proband risk for these disorders. Results of the trimmed and full path models based on complete and restricted samples are summarized in Table 3.
Table 3.
Effects of family substance use disorders on proband risk: Path analysis model results
| Path | Complete sample |
Restricted sample |
||||
| OR [95% CI] |
p | OR [95% CI] |
p | |||
| Trimmed path model result | ||||||
| Family AUD → proband AUD | 1.49 [1.24, 1.78] | <.001 | 1.47 [1.21, 1.79] | <.001 | ||
| Family CUD → proband CUD | 1.70 [1.32, 2.20] | <.001 | 1.73 [1.28, 2.32] | <.001 | ||
| Family AMP → proband AMP | 2.33 [1.51, 3.59] | <.001 | 2.70 [1.66, 4.40] | <.001 | ||
| Family HUD → proband HUD | 3.38 [1.72, 6.67] | <.001 | 3.06 [1.32, 7.11] | .009 | ||
| Full path model results | ||||||
| Family AUD → proband AUD | 1.45 [1.19, 1.75] | <.001 | 1.46 [1.18, 1.81] | .001 | ||
| Family CUD → proband AUD | 1.18 [0.89, 1.55] | .245 | 1.21 [0.89, 1.65] | .232 | ||
| Family AMP → proband AUD | 0.94 [0.63, 1.41] | .776 | 0.84 [0.54, 1.30] | .425 | ||
| Family HUD → proband AUD | 0.94 [0.55, 1.60] | .820 | 0.88 [0.49, 1.60] | .682 | ||
| Family AUD → proband CUD | 1.18 [0.95, 1.47] | .138 | 1.44 [1.12, 1.85] | .004 | ||
| Family CUD → proband CUD | 1.47 [1.08, 1.99] | .013 | 1.42 [1.00, 2.03] | .052 | ||
| Family AMP → proband CUD | 0.97 [0.64, 1.48] | .897 | 0.95 [0.59, 1.52] | .832 | ||
| Family HUD → proband CUD | 1.69 [0.97, 2.92] | .062 | 1.44 [0.76, 2.72] | .258 | ||
| Family AUD → proband AMP | 1.36 [1.02, 1.83] | .037 | 1.65 [1.17, 2.34] | .005 | ||
| Family CUD → proband AMP | 1.01 [0.66, 1.54] | .975 | 0.88 [0.54, 1.43] | .600 | ||
| Family AMP → proband AMP | 1.62 [0.97, 2.70] | .064 | 1.84 [1.03, 3.27] | .038 | ||
| Family HUD → proband AMP | 2.20 [1.13, 4.29] | .020 | 2.20 [0.95, 5.10] | .065 | ||
| Family AUD → proband HUD | 1.55 [1.04, 2.32] | .033 | 1.65 [1.01, 2.70] | .045 | ||
| Family CUD → proband HUD | 0.96 [0.49, 1.86] | .899 | 1.04 [0.44, 2.41] | .935 | ||
| Family AMP → proband HUD | 0.76 [0.31, 1.83] | .534 | 0.92 [0.33, 2.53] | .866 | ||
| Family HUD → proband HUD |
3.34 [1.32, 8.42] |
.011 |
2.58 [0.81, 8.20] |
.108 |
||
| Model comparison test | χ2 difference |
df |
p |
χ2 difference |
df |
p |
| Trimmed vs. full model | 21.40 | 12 | .045 | 24.50 | 12 | .017 |
Notes: Statistically significant effects (p < .05) are bolded. OR = odds ratio; CI = confidence interval; AUD = alcohol use disorder; CUD = cannabis use disorder; AMP = amphetamine use disorder; HUD = hallucinogen use disorder. See Table 1 for notes on the definitions of the complete and restricted samples.
Trimmed path model.
For the trimmed model, each path from a specific family SUD category to the corresponding proband SUD category was statistically significant in the complete and restricted sample analyses (all ps < .01). These findings indicate that, for each substance category, there was a significant corresponding risk to the proband based on the lifetime histories of that specific form of SUD among first-degree family members. Chi-square difference tests that comparatively evaluated the data fit of nonspecific SUD aggregation (full path model) to the trimmed path model, however, indicated that the full path model had significantly better fit in the complete (p = .045) and restricted samples (p = .017).
Full path model.
The findings from the full path model are generally consistent with both specificity and nonspecificity perspectives on the familial aggregation of SUDs, although the patterns of findings varied according to the substance category. Specificity of risk was most clearly evident with respect to AUD, whereby family AUD was the only significant predictor of proband AUD in the complete and restricted samples (ORs = 1.45 and 1.46, respectively, ps ≤ .001). Statistically significant evidence of specificity was also apparent for the family aggregation of CUD in the complete sample (OR = 1.47, p = .013) and AMP in the restricted sample (OR = 1.84, p = .038). Effects that fell just above the threshold for statistical significance for family CUD in the restricted sample and family AMP in the complete sample were consistent with the direction and pattern of statistically significant findings for these SUD categories in the complete and restricted samples, respectively (Table 3). Evidence for HUD specificity was observed only for the complete sample (OR = 3.34, p = .011).
As indicated in Table 3, statistically significant effects for nonspecific family aggregation of SUD were found for the prediction of proband CUD in the restricted sample, for proband AMP in the complete and restricted samples, and for proband HUD in the complete and restricted samples. Other related effects that fell just above the threshold for statistical significance are directionally consistent with and supplement the pattern of statistically significant nonspecific risk findings (Table 3).
Sources of familial risk
We also sought to isolate unique sources of family risk (i.e., mother, father, and sibling effects) associated with probands’ SUD risk. Findings based on complete and restricted samples are summarized in Table 4.
Table 4.
Sources of familial influence on proband risk for specific substance use disorders: Path analysis model results
| Complete sample |
Restricted sample |
|||
| Path | OR [95% CI] | p | OR [95% CI] | p |
| Mother AUD →proband AUD | 1.50 [1.02, 2.19] | .038 | 1.16 [0.72, 1.87] | .541 |
| Father AUD →proband AUD | 1.63 [1.22, 2.19] | .001 | 1.70 [1.22, 2.38] | .002 |
| Sibling AUD →proband AUD | 1.32 [0.97, 1.79] | .081 | 1.43 [1.03, 2.00] | .034 |
| Mother CUD →proband CUD | 2.08 [1.06, 4.06] | .032 | 2.11 [0.83, 5.40] | .117 |
| Father CUD →proband CUD | 1.75 [1.06, 2.90] | .029 | 1.10 [0.54, 2.25] | .784 |
| Sibling CUD →proband CUD | 1.50 [1.01, 2.24] | .045 | 2.11 [1.37, 3.23] | .001 |
| Mother AMP →proband AMP | 2.41 [0.97, 6.02] | .059 | 2.40 [0.68, 8.48] | .175 |
| Father AMP →proband AMP | 1.78 [0.68, 4.65] | .237 | 1.49 [0.37, 6.03] | .577 |
| Sibling AMP →proband AMP | 2.70 [1.38, 5.30] | .004 | 3.78 [1.86, 7.68] | <.001 |
| Mother HUD →proband HUD | 1.59 [0.28, 9.04] | .602 | n.a. | |
| Father HUD →proband HUD | 7.26 [2.28, 23.11] | .001 | 2.99 [0.43, 20.97] | .270 |
| Sibling HUD →proband HUD | 2.75 [0.98, 7.74] | .055 | 3.94 [1.42, 10.89] | .008 |
Notes: Statistically significant effects are bolded. OR = odds ratio; CI = confidence interval; AUD = alcohol use disorder; CUD = cannabis use disorder; AMP = amphetamine use disorder; HUD = hallucinogen use disorder; n.a. = not analyzed because prevalence was <1%. See Table 1 notes for descriptions of the complete and restricted samples.
In the complete sample, statistically significant maternal effects after we controlled for other family members were found in the prediction of proband AUD (OR = 1.50, p = .038) and CUD (OR = 2.08, p = .032), with the effect for AMP falling just above the threshold significance level (OR = 2.41, p = .059). Fathers’ histories of AUD were a significant unique predictor of proband AUD after we controlled for other family members in the complete (OR = 1.63, p = .001) and restricted (OR = 1.70, p = .002) sample analyses. For other SUD categories, significant unique paternal effects in the complete sample were observed for CUD (OR = 1.75, p = .029) and HUD (OR = 7.26, p = .001). Statistically significant sibling-related effects after we controlled for other family members were generally robust across complete and restricted sample analyses (Table 4). Other effects that fell just above the threshold for statistical significance for siblings were directionally consistent with the pattern of statistically significant findings.
Discussion
There were two primary aims of this study. First, we evaluated whether proband risk for SUD was associated with a general or nonspecific family liability for SUDs versus a disorder-specific vulnerability related to a particular substance or drug category. Second, we evaluated whether specificity of risk varied according to the familial relation to the proband (i.e., mother, father, and any sibling). With respect to the first aim, findings supported both specific and nonspecific forms of familial risk related to the emergence of a particular type of SUD in probands. Whereas trimmed path model findings provided robust support for the intrafamily specificity of SUD risk, the full path model produced a better fit to the data and provided evidence of both specific and nonspecific family SUD effects on proband risk for particular forms of SUD. AUD demonstrated the greatest degree of specificity, as evidenced by the observation that no other family-based SUD predicted proband AUD. Depending on the sample composition (complete or restricted), evidence of specificity of family SUD effects was also observed for probands’ risk for CUD, AMP, and HUD.
Nonspecific family SUD effects were observed for proband risk for CUD, AMP, and HUD but not AUD. Family-based AUD was the most robust nonspecific risk factor for nonalcohol SUDs in probands. Among individuals, alcohol use and misuse often (Kandel et al., 1992) but not always (Robins, 1993; Tarter et al., 2006) precede the use and misuse of illicit substances. Nonspecific effects of family-based AUD on proband SUD risk may reflect the commonality of AUD in the sample and a typical developmental sequence for multiple drug involvement. Because alcohol was the only licit substance investigated,2 it was also likely to be the most readily available substance within households. Altogether, findings suggest an especially pernicious overall effect of family-based AUD in relation to an increased risk for multiple forms of SUD among probands. Findings also imply that SUD risk within families may be substantially reduced if preventive efforts explicitly target alcohol misuse.
The specificity of risk conveyance observed in this study likely reflects, in part, distinct etiologic influences that increase risk for particular types of SUDs. Investigations into potential sources that influence the intrafamily specificity of substance use or SUD risk are limited but include macroenvironmental contextual factors such as community-based controls or laws (McGue et al., 2000), the setting within which one resides (e.g., rural or urban; Legrand et al., 2008), exposure to substance-specific media content (Tucker et al., 2013), and drug availability (Robins, 1993); subcultural affiliations such as pub patronage, rave culture, and cannabis social clubs (Baldwin et al., 2014; Decorte, 2015; Reynolds, 1999); family-shared environmental factors, such as parental modeling and attitudes about drug use (Ryan et al., 2010) and sibling drug use (Kendler et al., 2013; Whiteman et al., 2013); and biological processes such as neural adaptations arising from repeated exposures to particular substances (Koob & Le Moal, 2008), subjective response profiles to specific substances (Haberstick et al., 2011), and substance-specific genetic factors such as those related to the metabolism of certain drugs (Beirut, 2011; Tsuang et al., 1998).
The second study aim was to identify the greatest familial sources of risk. Comparisons of findings between the complete and restricted samples in Table 4 revealed both similarities and differences. The distinguishing feature differentiating these samples was the requirement for the restricted sample that families include at least one sibling with data. As a consequence, when compared with findings from the complete sample, sibling effects in the restricted sample were generally stronger and maternal and paternal effects were generally weaker. Overall, comparisons between the complete and restricted samples in relation to the sources of family influence suggest that sibling effects often superseded those associated with either parent in families in which siblings are present. In samples that include a mix of families with and without siblings, however, both parental and sibling SUD histories often emerged as unique factors associated with proband risk. Although there is prospective evidence that older siblings are more likely to influence younger siblings’ substance use than contrariwise (Chassin et al., 2016), there are also indications that reciprocal influences are possible, particularly when siblings are of the same sex and close in age (McGue et al., 1996; Whiteman et al., 2013). Data for this study, however, are unable to clarify the directional or reciprocal nature of sibling influence with respect to birth order.
In the complete sample and depending on the type of substance, mothers’ and fathers’ SUD histories also afforded a unique risk to probands independent of that of siblings. As noted by Chassin et al. (2016), lower prevalence rates of individual SUD categories among mothers compared with fathers often make the detection of significant effects owing to maternal factors more difficult to reliably observe. Our findings are consistent with this suggestion, as effect sizes associated with maternal SUD categories were generally consistent with, and sometimes exceeded, those associated with paternal SUD categories.
Although this study has strengths, it also has limitations. First, because analyses were based on lifetime SUD histories, we were unable to evaluate whether proband risk was primarily influenced by SUD episodes among family members occurring before or during the probands’ lifetime. In addition, for those family members whose episodes occurred during the probands’ lifetime, we were unable to consistently ascertain whether the proband was residing in the same household as affected family members. We are, as a consequence, unable to determine if the findings reported here would be similar depending on whether probands had direct exposure to substance-abusing family members.
Second, because we included probands who dropped out after T3 for whom family data were available, the resulting SUD history data for these probands (9% of all probands) ceased at age 24 as compared with the remaining proband sample, which extended to age 30. Although rates of first incidence episodes for AUD and CUD after age 24 were relatively low in the Oregon Adolescent Depression Project proband sample compared with peak developmental periods (Farmer et al., 2015; Seeley et al., 2019), it is likely that some proportion of T3 dropouts had additional SUD episodes following study attrition. The potential impact of these unavailable data on study findings is unclear, although findings based solely on probands who completed all four assessments were highly similar to those presented in this report (see Footnote 1).
Third, this study did not evaluate mechanisms underlying risk transmission. The observation that sibling concordance with proband substance use or abuse is often greater than that of either parent (Whiteman et al., 2013) highlights the potential importance of environmental influences in accounting for the aggregation of SUDs within families. Living in a household with a sibling who uses a particular drug may enhance the specificity of the proband’s risk for use of that same substance (McGue et al., 2000).
Fourth, we did not examine the role of SUD comorbidity among family members on proband risk for specific SUD categories or comorbid SUD in probands. Fifth, power was limited to detect significant effects of family SUD categories with low prevalence rates (e.g., family member HUD), and findings based on these variables should be interpreted with caution. Sixth, because of limited power, we did not evaluate biological sex or race and ethnicity as potential moderators of observed effects.
Observations from this research contribute to understandings about SUD etiology within families. Findings related to nonspecific effects support the notion that common causal pathways underlie the risk for multiple forms of SUD. Evidence of specificity of SUD risk also highlights the potential importance of unique environmental and biological factors that influence more restricted forms of substance use or drug preferences. Overall, study findings indicate a need for a better understanding of malleable aspects of the family environment that increase and mitigate SUD risk, particularly those related to the conveyance of AUD risk within families and SUD risk transmission between siblings.
Acknowledgment
The authors thank Peter M. Lewinsohn for making study data available for this investigation.
Footnotes
This research was supported by National Institutes of Health Grants MH40501, MH50522, and DA12951 to Peter M. Lewinsohn and R01AA023842 to Richard F. Farmer and John R. Seeley. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The findings reported in Tables 3 and 4 are highly similar to findings obtained when the sample was restricted to probands who completed all four assessments (T1 through T4) and their families. These data are available from the first author on request.
Oregon’s medical cannabis law was passed 4 years before the T4 assessment and only fully implemented 2 years after legislation approval. By the end of the T4 assessment period, only about 0.3% of Oregon’s population applied for, but did not necessarily receive, a medical cannabis card. Legislation approving the use of cannabis for recreational purposes in Oregon occurred after all Oregon Adolescent Depression Project assessments had been completed.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (3rd ed., rev.) Washington, DC: Author; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed.) Washington, DC: Author; 1994. [Google Scholar]
- Baldwin J. M., Stogner J. M., Miller B. L. It’s five o’clock somewhere: An examination of the association between happy hour drinking and negative consequences. Substance Abuse Treatment, Prevention, and Policy. 2014;9:17. doi: 10.1186/1747-597X-9-17. doi:10.1186/1747-597X-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut L. J. Genetic vulnerability and susceptibility to substance dependence. Neuron. 2011;69:618–627. doi: 10.1016/j.neuron.2011.02.015. doi:10.1016/j.neuron.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut L. J., Dinwiddie S. H., Begleiter H., Crowe R. R., Hesselbrock V., Nurnberger J. I., Jr., Reich T. Familial transmission of substance dependence: Alcohol, marijuana, cocaine, and habitual smoking: A report from the Collaborative Study on the Genetics of Alcoholism. Archives of General Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. doi:10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Bornovalova M. A., Hicks B. M., Iacono W. G., McGue M. Familial transmission and heritability of childhood disruptive disorders. American Journal of Psychiatry. 2010;167:1066–1074. doi: 10.1176/appi.ajp.2010.09091272. doi:10.1176/appi.ajp.2010.09091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers W. J., Puig-Antich J., Hirsch M., Paez P., Ambrosini P. J., Tabrizi M. A., Davies M. The assessment of affective disorders in children and adolescents by semistructured interview: Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. doi:10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chassin L., Haller M., Lee M. R., Handley E., Bountress K., Beltran I. Familial factors influencing offspring substance use and dependence. In: Sher K. J., editor. Oxford handbook of substance use and substance use disorders (Vol. 1, pp. 449–482) NewYork, NY: Oxford University Press; 2016. [Google Scholar]
- Chassin L., Pitts S. C., DeLucia C., Todd M. A longitudinal study of children of alcoholics: Predicting young adult substance use disorders, anxiety, and depression. Journal of Abnormal Psychology. 1999;108:106–119. doi: 10.1037//0021-843x.108.1.106. doi:10.1037/0021-843X.108.1.106. [DOI] [PubMed] [Google Scholar]
- Chassin L., Rogosch F., Barrera M. Substance use and symptomatology among adolescent children of alcoholics. Journal of Abnormal Psychology. 1991;100:449–463. doi: 10.1037//0021-843x.100.4.449. doi:10.1037/0021-843X.100.4.449. [DOI] [PubMed] [Google Scholar]
- Decorte T. Cannabis social clubs in Belgium: Organizational strengths and weaknesses, and threats to the model. International Journal on Drug Policy. 2015;26:122–130. doi: 10.1016/j.drugpo.2014.07.016. doi:10.1016/j.drugpo.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Farmer R. F., Kosty D. B., Seeley J. R., Duncan S. C., Lynskey M. T., Rohde P., Lewinsohn P. M. Natural course of cannabis use disorders. Psychological Medicine. 2015;45:63–72. doi: 10.1017/S003329171400107X. doi:10.1017/S003329171400107X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. Structured clinical interview for Axis I DSM-IV disorders, non-patient edition. NewYork, NY: NewYork State Psychiatric Institute; 1994. [Google Scholar]
- Grant B. F., Goldstein R. B., Saha T. D., Chou S. P., Jung J., Zhang H., Hasin D. S. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. doi:10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Saha T. D., Ruan W. J., Goldstein R. B., Chou S. P., Jung J., Hasin D. S. Epidemiology of DSM-5 drug use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. doi:10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick B. C., Zeiger J. S., Corley R. P., Hopfer C. J., Stallings M. C., Rhee S. H., Hewitt J. K. Common and drug-specific genetic influences on subjective effects to alcohol, tobacco and marijuana use. Addiction. 2011;106:215–224. doi: 10.1111/j.1360-0443.2010.03129.x. doi:10.1111/j.1360-0443.2010.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks B. M., Foster K. T., Iacono W. G., McGue M. Genetic and environmental influences on the familial transmission of externalizing disorders in adoptive and twin offspring. JAMA Psychiatry. 2013;70:1076–1083. doi: 10.1001/jamapsychiatry.2013.258. doi:10.1001/jamapsychiatry.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks B. M., Krueger R. F., Iacono W. G., McGue M., Patrick C. J. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. doi:10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hicks B. M., Schalet B. D., Malone S. M., Iacono W. G., McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior Genetics. 2011;41:459–475. doi: 10.1007/s10519-010-9417-2. doi:10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D. B., Yamaguchi K., Chen K. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. Journal of Studies on Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. doi:10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Keller M. B., Lavori P. W., Friedman B., Nielsen E., Endicott J., McDonald-Scott P., Andreasen N. C. The Longitudinal Interval Follow-up Evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. doi:10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Davis C. G., Kessler R. C. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: A family history study. British Journal of Psychiatry. 1997;170:541–548. doi: 10.1192/bjp.170.6.541. doi:10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Karkowski L. M., Neale M. C., Prescott C. A. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. doi:10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Karkowski L., Prescott C. A. Hallucinogen, opiate, sedative and stimulant use and abuse in a population-based sample of female twins. Acta Psychiatrica Scandinavica. 1999;99:368–376. doi: 10.1111/j.1600-0447.1999.tb07243.x. doi:10.1111/j.1600-0447.1999.tb07243.x. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Ohlsson H., Sundquist K., Sundquist J. Within-family environmental transmission of drug abuse: A Swedish national study. JAMA Psychiatry. 2013;70:235–242. doi: 10.1001/jamapsychiatry.2013.276. doi:10.1001/jamapsychiatry.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Prescott C. A., Myers J., Neale M. C. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. doi:10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Schmitt E., Aggen S. H., Prescott C. A. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. doi:10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Sundquist K., Ohlsson H., Palmér K., Maes H., Winkleby M. A., Sundquist J. Genetic and familial environmental influences on the risk for drug abuse: A national Swedish adoption study. Archives of General Psychiatry. 2012;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. doi:10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. doi:10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kosty D. B., Farmer R. F., Seeley J. R., Gau J. M., Duncan S. C., Lewinsohn P. M. Parental transmission of risk for cannabis use disorders to offspring. Addiction. 2015;110:1110–1117. doi: 10.1111/add.12914. doi:10.1111/add.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman J. F., Sholomskas D., Thompson W. D., Belanger A., Weissman M. M. Best estimate of lifetime psychiatric diagnosis: A methodological study. Archives of General Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. doi:10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Legrand L. N., Keyes M., McGue M., Iacono W. G., Krueger R. F. Rural environments reduce the genetic influence on adolescent substance use and rule-breaking behavior. Psychological Medicine. 2008;38:1341–1350. doi: 10.1017/S0033291707001596. doi:10.1017/S0033291707001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P. M., Hops H., Roberts R. E., Seeley J. R., Andrews J. A. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. Journal of Abnormal Psychology. 1993;102:133–144. doi: 10.1037//0021-843x.102.1.133. doi:10.1037/0021-843X.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lieb R., Merikangas K. R., Höfler M., Pfister H., Isensee B., Wittchen H.-U. Parental alcohol use disorders and alcohol use and disorders in offspring: A community study. Psychological Medicine. 2002;32:63–78. doi: 10.1017/s0033291701004883. doi:10.1017/S0033291701004883. [DOI] [PubMed] [Google Scholar]
- Mannuzza S., Fyer A. J. Family informant schedule and criteria (FISC), July 1990 revision. NewYork, NY: Anxiety Disorders Clinic, NewYork State Psychiatric Institute; 1990. [Google Scholar]
- McGue M, Elkins I, Iacono W. G. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. doi:10.1002/1096-8628(20001009)96:5<671::AID-AJMG14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- McGue M., Sharma A., Benson P. Parent and sibling influences on adolescent alcohol use and misuse: Evidence from a U.S. adoption cohort. Journal of Studies on Alcohol. 1996;57:8–18. doi: 10.15288/jsa.1996.57.8. doi:10.15288/jsa.1996.57.8. [DOI] [PubMed] [Google Scholar]
- Meller W. H., Rinehart R., Cadoret R. J., Trough Ton E. Specific familial transmission in substance abuse. International Journal of the Addictions. 1988;23:1029–1039. doi: 10.3109/10826088809056183. doi:10.3109/10826088809056183. [DOI] [PubMed] [Google Scholar]
- Merikangas K. R., Li J. J., Stipelman B., Yu K., Fucito L., Swendsen J., Zhang H. The familial aggregation of cannabis use disorders. Addiction. 2009;104:622–629. doi: 10.1111/j.1360-0443.2008.02468.x. doi:10.1111/j.1360-0443.2008.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K. R., McClair V. L. Epidemiology of substance use disorders. Human Genetics. 2012;131:779–789. doi: 10.1007/s00439-012-1168-0. doi:10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K. R., Stolar M., Stevens D. E., Goulet J., Preisig M. A., Fenton B., Rounsaville B. J. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. doi:10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. Mplus user’s guide (8th ed.) Los Angeles: Authors; 1998–2017. [Google Scholar]
- Ohannessian C. M., Hesselbrock V. M., Kramer J., Kuperman S., Bucholz K. K., Schuckit M. A., Nurnberger J. I. The relationship between parental psychopathology and adolescent psychopathology: An examination of gender patterns. Journal of Emotional and Behavioral Disorders. 2005;13:67–76. doi:10.1177/10634266050130020101. [Google Scholar]
- Orvaschel H., Puig-Antich J., Chambers W., Tabrizi M. A., Johnson R. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-e. Journal of the American Academy of Child Psychiatry. 1982;21:392–397. doi: 10.1016/s0002-7138(09)60944-4. doi:10.1016/S0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- Reynolds S. Generation ecstasy: Into the world of techno and rave culture. NewYork, NY: Routledge; 1999. [Google Scholar]
- Rice J. P., Reich T., Bucholz K. K., Neuman R. J., Fishman R., Rochberg N., Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. doi:10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins L. N. Vietnam veterans’ rapid recovery from heroin addiction: A fluke or normal expectation? Addiction. 1993;88:1041–1054. doi: 10.1111/j.1360-0443.1993.tb02123.x. doi:10.1111/j.1360-0443.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Rohde P., Lewinsohn P. M., Seeley J. R., Klein D. N., Andrews J. A., Small J. W. Psychosocial functioning of adults who experienced substance use disorders as adolescents. Psychology of Addictive Behaviors. 2007;21:155–164. doi: 10.1037/0893-164X.21.2.155. doi:10.1037/0893-164X.21.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S. M., Jorm A. F., Lubman D. I. Parenting factors associated with reduced adolescent alcohol use: A systematic review of longitudinal studies. Australian and New Zealand Journal of Psychiatry. 2010;44:774–783. doi: 10.1080/00048674.2010.501759. doi:10.1080/00048674.2010.501759. [DOI] [PubMed] [Google Scholar]
- Seeley J. R., Farmer R. F., Kosty D. B., Gau J. M. Prevalence, incidence, recovery, and recurrence of alcohol use disorders from childhood to age 30. Drug and Alcohol Dependence. 2019;194:45–50. doi: 10.1016/j.drugalcdep.2018.09.012. doi:10.1016/j.drugalcdep.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher K. J., Walitzer K. S., Wood P. K., Brent E. E. Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. doi:10.1037/0021-843X.100.4.427. [DOI] [PubMed] [Google Scholar]
- Sørensen H. J., Manzardo A. M., Knop J., Penick E. C., Madarasz W., Nickel E. J., Mortensen E. L. The contribution of parental alcohol use disorders and other psychiatric illness to the risk of alcohol use disorders in the offspring. Alcoholism: Clinical and Experimental Research. 2011;35:1315–1320. doi: 10.1111/j.1530-0277.2011.01467.x. doi:10.1111/j.1530-0277.2011.01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter R. E., Vanyukov M., Kirisci L., Reynolds M., Clark D. B. Predictors of marijuana use in adolescents before and after licit drug use: Examination of the gateway hypothesis. American Journal of Psychiatry. 2006;163:2134–2140. doi: 10.1176/ajp.2006.163.12.2134. doi:10.1176/ajp.2006.163.12.2134. [DOI] [PubMed] [Google Scholar]
- True W. R., Xian H., Scherrer J. F., Madden P. A., Bucholz K. K., Heath A. C., Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Archives of General Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. doi:10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tsuang M. T., Lyons M. J., Meyer J. M., Doyle T., Eisen S. A., Goldberg J., Eaves L. Co-occurrence of abuse of different drugs in men: The role of drug-specific and shared vulnerabilities. Archives of General Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. doi:10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Tucker J. S., Miles J. N., D’Amico E. J. Cross-lagged associations between substance use-related media exposure and alcohol use during middle school. Journal of Adolescent Health. 2013;53:460–464. doi: 10.1016/j.jadohealth.2013.05.005. doi:10.1016/j.jadohealth.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze S. I., Hicks B. M., Iacono W. G., McGue M. Decline in genetic influence on the co-occurrence of alcohol, marijuana, and nicotine dependence symptoms from age 14 to 29. American Journal of Psychiatry. 2012;169:1073–1081. doi: 10.1176/appi.ajp.2012.11081268. doi:10.1176/appi.ajp.2012.11081268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford H. A., Degenhardt L., Rehm J., Baxter A. J., Ferrari A. J., Erskine H. E., Vos T. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. doi:10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Whiteman S. D., Jensen A. C., Maggs J. L. Similarities in adolescent siblings’ substance use: Testing competing pathways of influence. Journal of Studies on Alcohol and Drugs. 2013;74:104–113. doi: 10.15288/jsad.2013.74.104. doi:10.15288/jsad.2013.74.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. E., Rhee S. H., Stallings M. C., Corley R. P., Hewitt J. K. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: General or specific? Behavior Genetics. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. doi:10.1007/s10519-006-9066-7 [DOI] [PubMed] [Google Scholar]