Abstract
Neurotrophic factor signaling pathways modulate cellular and behavioral responses to drugs of abuse. In addition, chronic exposure to morphine increases expression of phospholipase Cγ1 (PLCγ1) (a protein involved in neurotrophic signaling) in the ventral tegmental area (VTA), a neural substrate for many drugs of abuse. Using viral-mediated gene transfer to locally alter the activity of PLCγ1, we show that overexpression of PLCγ1 in rostral portions of the VTA (R-VTA) results in increased morphine place preference, whereas PLCγ1 overexpression in the caudal VTA (C-VTA) results in avoidance of morphine-paired compartments. In addition, overexpression of PLCγ1 in R-VTA causes increased preference for sucrose and increased anxiety-like behavior but does not affect responses to stress or nociceptive stimuli. In contrast, overexpression of PLCγ1 in C-VTA decreases preference for sucrose and increases sensitivity to stress and nociceptive stimuli, although there was a tendency for increased anxiety-like behavior as seen for the R-VTA. These results show that levels of PLCγ1 in the VTA regulate responsiveness to drugs of abuse, natural rewards, and aversive stimuli and point to the possibility that distinct topographical regions within the VTA mediate generally positive versus negative responses to emotional stimuli. Moreover, these data also support a role for drug-induced elevations in PLCγ1 expression in the VTA in mediating long-term adaptations to drugs of abuse and aversive stimuli.
Keywords: growth factors, neural plasticity, viral-mediated gene transfer, drug addiction, morphine, stress, depression
Introduction
Neurotrophic factors and the signaling pathways they activate are best characterized for promoting growth, differentiation, and survival of neurons during development. More recently, these factors have been implicated as mediators of neuronal maintenance and plasticity in the adult nervous system (Barde, 1989; Lindsay et al., 1994; Patterson et al., 1996; Lu and Figurov, 1997). Neurotrophic factor levels are altered during aging and in models of neurodegeneration and neuropsychiatric disorders, whereas intracranial infusions of neurotrophic factors can have palliative effects in these models (Gash et al., 1996; Nestler et al., 1996; Duman et al., 1997; Hellweg et al., 1998; Smith et al., 1999).
The mesolimbic dopamine system, which consists of dopamine neurons in the ventral tegmental area (VTA) and their projections to the nucleus accumbens (NAc) (a major efferent region of the VTA) and other limbic regions, is a major substrate believed to regulate motivated behavior and responses to natural reinforcers such as food and sex (Di Chiara and North, 1992; Kelley and Berridge, 2002). Drugs of abuse potently activate this pathway and, after repeated administration, cause long-term adaptations in VTA dopamine neurons and their targets (Wise, 1996; Koob et al., 1998; Nestler, 2001). Among other adaptations, VTA dopamine neurons show increased levels of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis (Beitner-Johnson and Nestler, 1991; Sorg et al., 1993), and they become smaller (Sklair-Tavron et al., 1996) and have diminished levels of neurofilament proteins and axoplasmic transport to the NAc (Beitner-Johnson et al., 1992; Beitner-Johnson and Nestler, 1993).
Some of these biochemical and morphological adaptations of VTA dopamine neurons after chronic drug exposure are similar to changes seen in vitro and in vivo after neuronal injury or reduced neurotrophic support (Nestler et al., 1996). Evidence for this premise comes from studies showing that infusion of certain neurotrophic factors into the VTA opposes the effects of drugs of abuse on these neurons (Berhow et al., 1995, 1996; Sklair-Tavron et al., 1996; Messer et al., 2000). Moreover, chronic morphine exposure alters levels of specific neurotrophic factor-signaling proteins in this brain region (Ortiz et al., 1995; Berhow et al., 1996) for example, phospholipase Cγ1 (PLCγ1) (Wolf et al., 1999). Of the known PLC isoforms, only PLCγ is activated directly by neurotrophic factors (Rhee, 2001) and, of PLCγ isoforms, only PLCγ1 is expressed in brain (Ross et al., 1989). Unlike PLCγ, PLCβ and PLCδ are not regulated by morphine (Wolf et al., 1999).
Despite the evidence that chronic morphine induces PLCγ1in the VTA, the functional consequences of this effect have remained unknown. The present study was designed to address this question by examining the effect of increased PLCγ1 expression in this region, achieved with viral-mediated gene transfer, on behavioral responses to morphine and other emotional stimuli.
Materials and Methods
Animals. Male Sprague Dawley rats (Charles River, Kingston, NY), weighing 350-375 gm at the start of the experiment, were used in this study. All animals were habituated to the animal facility for at least 1 week before experimental manipulation. Rats were double housed in clear polypropylene boxes containing wood shavings in an animal colony maintained at 23-25°C on a 12 hr light/dark cycle in which lights were on between 7:00 A.M. and 7:00 P.M. All animals were provided with food and water ad libitum. Experiments were conducted in accordance with guidelines of the Society for Neuroscience and the institutional animal review committee of The University of Texas Southwestern (Dallas, TX).
Viral vectors. cDNAs for PLCγ1 (obtained from S. G. Rhee, National Institutes of Health, Bethesda, MD) and LacZ were inserted into the herpes simplex virus (HSV) amplicon HSV-PrPUC and packaged into virus using the helper 5dl1.2, as described previously (Neve et al., 1997). The average titer of the recombinant virus stocks was 4.0 × 10 7 infectious units/ml. Titers did not differ by >10% among preparations. All behavioral experiments were commenced on day 3 after viral surgery, a time at which maximal transgene expression caused by these vectors was observed (Carlezon et al., 1998). Expression of the HSV-encoded transgenes is limited to an area of ∼1 mm3 around the injection site, and no expression is seen in either efferent or afferent regions of the injected area. Thus, we found no detectable PLCγ1 or LacZ expression in either the NAc or the dorsal raphe (a major afferent region of the VTA).
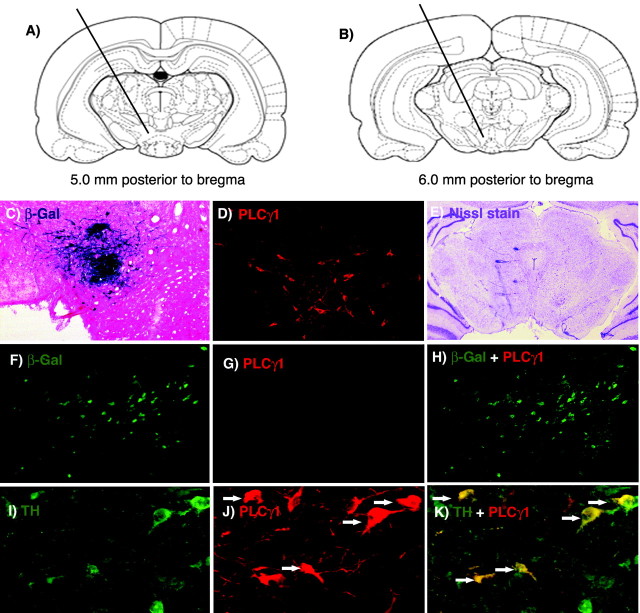
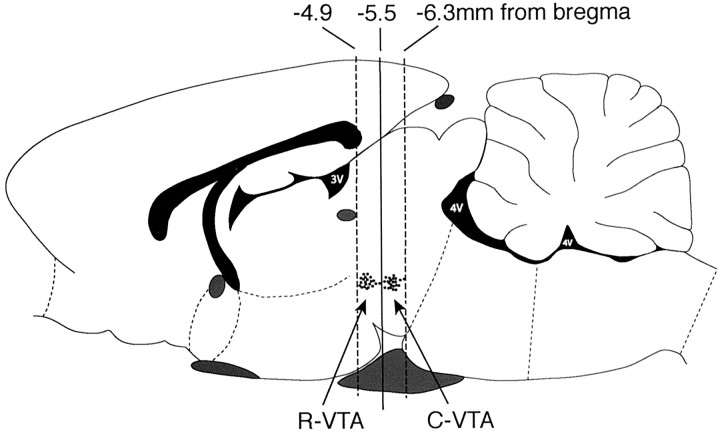
Animal surgery. For viral injections in rats, animals were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and given atropine (0.25 mg/kg, s.c.) to minimize bronchial secretions. Afterward, animals were given unilateral microinjections (2.0 μl over 10 min) of either HSV-PLCγ1 or HSV-LacZ (used as a control) into rostral VTA (R-VTA) (anteroposterior, -4.9; lateral, +2.2; dorsoventral, -7.6 mm below dura) (see Fig. 1 A) or caudal VTA (C-VTA) (anteroposterior, -6.0; lateral, +2.2; dorsoventral, 7.6 mm below dura) (see Fig. 1 B) (Paxinos and Watson, 1997) using a 32 gauge Hamilton syringe angled at 10° from the midline to avoid piercing the sinus system. All needle placements ranging from -4.9 to -5.5 mm from bregma were considered R-VTA, whereas placements ranging from -5.5 to -6.3 mm were considered C-VTA.
Figure 1.
Viral-mediated gene transfer. A, B, Rostral and caudal regions of the VTA to which microinjections of HSV vectors and sham surgery were targeted. C, D, Expression of β-gal (C), revealed by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) assay and PLCγ1 (D), revealed by fluorescence immunohistochemistry (magnification, 20×) 3 d after microinjections of HSV-LacZ or HSV-PLCγ1 into the left VTA. E, Adjacent, Nissl-stained section from the same brain section in D, showing lack of gliosis in the region of transgene expression. F-H, HSV-LacZ microinjection did not alter PLCγ1 levels in the VTA. For example, no colocalization of β-gal (F) and PLCγ1 (G) was observed (merged image in H; magnification, 40×) after a microinjection of HSV-LacZ in the VTA. A comparison of D and G shows the degree of PLCγ1 overexpression achieved with HSV-PLCγ1 microinjections. I-K, Confocal photomicrographs (magnification, 400×) of a representative brain slice from the C-VTA (∼5.8 mm caudal to bregma) double labeled for TH and PLCγ1 to determine the percentage of infected cells that were dopaminergic. I, Cells expressing TH represented by green (Cy2) fluorescence. J, Cells expressing PLCγ1 represented by red (Cy3) fluorescence. K, Merged confocal image of I and J showing that five of the eight brightly labeled PLCγ1 cells (60%) are doubled labeled, represented by yellow fluorescence. Arrows indicate colabeled cells.
Conditioned place preference. Place conditioning was performed exactly as described previously (Carlezon et al., 1997). Briefly, place preference conditioning to morphine sulfate (concentration expressed as base) was performed in a three-compartment apparatus (Med Associates, St. Albans, VT). Before viral injections (day 0), rats were allowed to freely explore the entire apparatus for 30 min to obtain baseline preference to any of the three compartments. Only rats showing no spontaneous preference to either compartment were used (unbiased procedure), which accounted for >80% of all of the animals tested. Rats then received unilateral injections of HSV-PLCγ1, HSV-LacZ, or sham surgery (lowered needle to targeted brain site but no volume injection) into the R-VTA or C-VTA and were allowed to recover for 2 d. After recovery, conditioning trials (two per day) were given on 2 consecutive days (days 3 and 4). On the first conditioning trial, rats received saline (1.0 mg/kg, s.c.) and were confined to one of the large-sized compartments of the apparatus. After 3 hr, rats received morphine (0.125, 0.25, or 0.50 mg/kg, s.c.; National Institute on Drug Abuse, Bethesda, MD) and were confined to the opposite-side compartment. On the final day (day 5), rats were again allowed to freely explore the entire apparatus for 30 min.
Sucrose preference. The sucrose preference test consisted of a two-bottle choice paradigm, performed under red light at the beginning of the dark phase. At the start of the experiment, rats were habituated to drink a 1% sucrose solution for 3 d. On day 4, the sucrose solution was replaced with tap water for an additional 2 d. Two hours (5:00 P.M.) before the test (at the end of day 5), rats were singly housed with access to food. At the start of the dark phase (7:00 P.M.), rats were given access to the two bottles (containing water or 1% sucrose). The position of the sucrose bottle (left or right) was balanced between the experimental groups. Fluid intake was then measured for 30 min. At the end of the testing period, rats were again housed in pairs. The total amount of fluid (water or sucrose) intake was considered baseline preference. Only rats showing a ≤60% preference to sucrose over water were used for the rest of the experiment. Four days after the pretest (i.e., baseline preference), animals received intracranial HSV or sham microinjections into R-VTA or C-VTA. On day 3 after surgery, the sucrose preference test was repeated exactly as performed on day 5 of baseline.
Forced swim test. The forced swim test is a 2 d procedure in which rats are forced to swim under conditions in which they cannot escape. On the first day, rats are forced to swim. Initially, they engage in a variety of escape-like behaviors, but they eventually adopt a posture of immobility in which they make only the movements necessary to maintain their head above water. When retested 24 hr later, rats become immobile more quickly. However, antidepressant treatment between the forced swim exposures can significantly increase their escape-like behaviors, an effect that has been correlated with antidepressant activity in humans (Porsolt et al., 1977; Cryan et al., 2002). Rats received HSV-PLCγ1, HSV-LacZ, or sham surgery injections into R-VTA or C-VTA as described above. On day 3 after surgery, rats were placed in plastic cylinders (30 × 45 cm) filled to 30 cm depth (so that the paws and tail do not touch the bottom) with 25°C water and forced to swim for 15 min. At the end of this period, rats were removed from the water, dried with towels, and kept in a warm enclosure for 30 min. All cylinders were emptied and cleaned between rats. Twenty-four hours after the forced swim, rats were retested for 5 min under identical conditions, and sessions were videotaped by a camera attached to the ceiling of the testing room. Raters unaware of the treatment conditions scored the videotapes. In this study, the latency to become immobile was the dependent variable. Latency to immobility was defined as the time at which the rat first initiated a stationary posture that did not reflect attempts to escape from the water (Lucki, 1997; Pliakas et al., 2001). To qualify as immobility, this posture had to be clearly visible and maintained for ≥2.0 sec.
Locomotor activity. A separate group of rats was used to examine whether gene transfer treatments affected general locomotor activity 24 hr after day 1 of forced swimming. Rats received HSV microinjections or sham surgery into R-VTA or C-VTA and were placed for 1 hr in automated (75 cm diameter × 15 cm wide, four photocell beams) circular activity chambers (Med Associates).
Response to nociceptive stimuli. In this test, we exposed rats to an electric foot-shock session in an apparatus consisting of a computerized box with a grid floor (Barrot et al., 2002). The threshold of foot-shock intensity required to induce a behavioral response was determined. After 2 min of habituation to the testing chamber, rats received a foot shock every 30 sec starting at 0.05 mA, with a 0.05 mA increment between each shock (to a maximum of 1.0 mA). The first appearance of a flinch, an audible vocalization, and a jump were recorded. The test session was terminated after all three behavioral responses were observed in each animal.
Elevated-plus maze. Rats receiving HSV microinjections or sham surgery into R-VTA or C-VTA were tested for 5 min on the elevated-plus maze, a behavioral test of anxiety-like behavior. The maze was made of gray plastic and consisted of two perpendicular, intersecting runways (12 cm wide × 100 cm long) (Barrot et al., 2002). One runway had tall walls (40-cm-high “closed arms”), whereas the other one had no walls (“open arms”). The arms were connected by a central area, and the maze was elevated 1 m from the floor. Testing was conducted between 9:00 A.M. and 1:00 P.M. under controlled light conditions (∼90 lux). At the beginning of the 5 min observation, animals were placed in the central area facing one of the open arms, and the cumulative time spent in the open arms was videotaped by a camera placed on the ceiling of the testing room. Raters unaware of the treatment conditions scored the videotapes.
Histology. At the end of the behavioral experiments, rats were anesthetized with an overdose of chloral hydrate and were perfused transcardially with 0.9% saline, followed by cold 4% paraformaldehyde. The brains were removed, postfixed overnight in 4% paraformaldehyde, and stored in 20% glycerol solution. Coronal sections (45 μm) through the midbrain were taken on a microtome and stored in 0.1 m sodium phosphate buffer with 0.05% azide. Sections were processed for verification of injection placements or transgene expression using immunohistochemistry as described below. Data obtained from rats with placements outside the intended brain regions (<10% of all experimental animals) were not included in the analyses.
Transgene detection. Immunohistochemical staining was used to examine the ability of the HSV constructs to drive expression of PLCγ1 and LacZ [β-galactosidase (β-gal)] within the VTA. Midbrain free-floating coronal sections were processed for immunohistochemistry using the following antibodies: PLCγ1 (1:2000 mixed mouse monoclonal; Upstate Biotechnology, Lake Placid, NY); β-galactosidase (1:5000 goat polyclonal; Biogenesis, Poole, UK); or TH (1:5000, rabbit polyclonal; Chemicon, Temecula, CA). Adjacent sections were blocked in 3% normal donkey serum (NDS) and incubated overnight in one of the primary antibodies mentioned above, along with 0.3% Triton X-100 (Fisher Scientific, Pittsburgh, PA) and 1% NDS. Sections were incubated with the appropriate biotinylated anti-goat, anti-mouse, or anti-rabbit secondary antibody (1:200; Jackson ImmunoResearch, West Grove, PA) for 2 hr at room temperature. Stained sections were then slide mounted (Fisher Scientific), dehydrated in ethanol and citrosolv, and coverslipped with clear DPX adhesive (Sigma, St. Louis, MO). Slides were then visualized and photographed using a fluorescence microscope and a digital camera.
Statistical analysis. Significance was measured using one-way and two-way ANOVAs. When appropriate, Student's t test and F test were used to determine statistical significance of preplanned comparisons involving two groups. Data are expressed as the mean ± SEM. Statistical significance was defined as p < 0.05.
Results
Viral-mediated gene transfer in the VTA
Figure 1, A and B, shows the R-VTA and C-VTA to which microinjections of HSV vectors (HSV-LacZ or HSV-PLCγ1) were aimed, and Figure 2 shows the range of injected rostral and caudal regions targeted in a typical experiment. We separately targeted rostral and caudal subregions of the VTA on the basis of previous evidence that manipulation of the two regions can differentially regulate morphine reward (Carlezon et al., 2000b). As reported previously for HSV-LacZ and several other HSV vectors (Carlezon et al., 1997, 1998, 2000b; Barrot et al., 2002), we found that expression of LacZ (Fig. 1C) and PLCγ1 (Fig. 1D) was maximal between days 3 and 4 after virus injection, and it significantly declined thereafter as a result of the transient nature of transgene expression (Neve et al., 1997). Viral-mediated expression was restricted to an area of the VTA of ∼1 mm in diameter and was accompanied by minimal damage (Fig. 1E) that was indistinguishable from that caused by microinjection of vehicle alone (10% sucrose). No change in PLCγ1 immunoreactivity was present in rats given HSV-LacZ microinjections, confirming that increased PLCγ1 expression in HSV-PLCγ1-treated animals is not a nonspecific reaction to surgery or viral infection (Fig. 1F-H). Confocal microscopy (Fig. 1 I-K) revealed that 52% of the neurons overexpressing PLCγ1 in R-VTA were dopaminergic (i.e., TH positive), whereas in C-VTA, 65% of the PLCγ1-infected neurons were double labeled. This difference, which is similar to previous findings (Carlezon et al., 2000b), did not reach statistical significance (p > 0.1). As found in previous studies using HSV vectors, we found no detectable expression of the viral-encoded transgenes in glial cells (data not shown).
Figure 2.
HSV injection sites in R-VTA and C-VTA. The figure shows the injection sites targeting R-VTA and C-VTA in a typical experiment, such as that shown in Figure 3A.
PLCγ1 regulation of morphine-conditioned place preference
Conditioned place preference has been widely used to assess the rewarding or aversive properties of drugs. In this behavioral assay, animals learn to prefer environments associated previously with rewarding drug effects, while they avoid environments associated with aversive drug effects (Hoffman, 1989). As seen in Figure 3A, time spent in the morphine-paired compartment varied as a function of viral vector treatment and VTA region (viral treatment × region interaction, F(2,107) = 9.2; p < 0.0002). Animals receiving HSV-PLCγ1 injections into the R-VTA spent significantly more time in environments paired with threshold doses of morphine [0.25 (p = 0.06) and 0.50 (p = 0.04) mg/kg], whereas rats with PLCγ1 microinjections into the C-VTA did not consistently approach the morphine-paired environments when compared with their HSV-LacZ or sham controls. In fact, microinjecting HSV-PLCγ1 into the C-VTA resulted in avoidance of the morphine-paired compartments [0.25 (p = 0.001) and 0.50 (p = 0.0001)]. Both the increased reward seen with PLCγ1 overexpression in R-VTA and the aversion seen with PLCγ1 overexpression in C-VTA showed a clear dose response. In contrast, microinjections of HSV-PLCγ1 into the nearby substantia nigra did not make these doses of morphine rewarding or aversive (data not shown).
Figure 3.
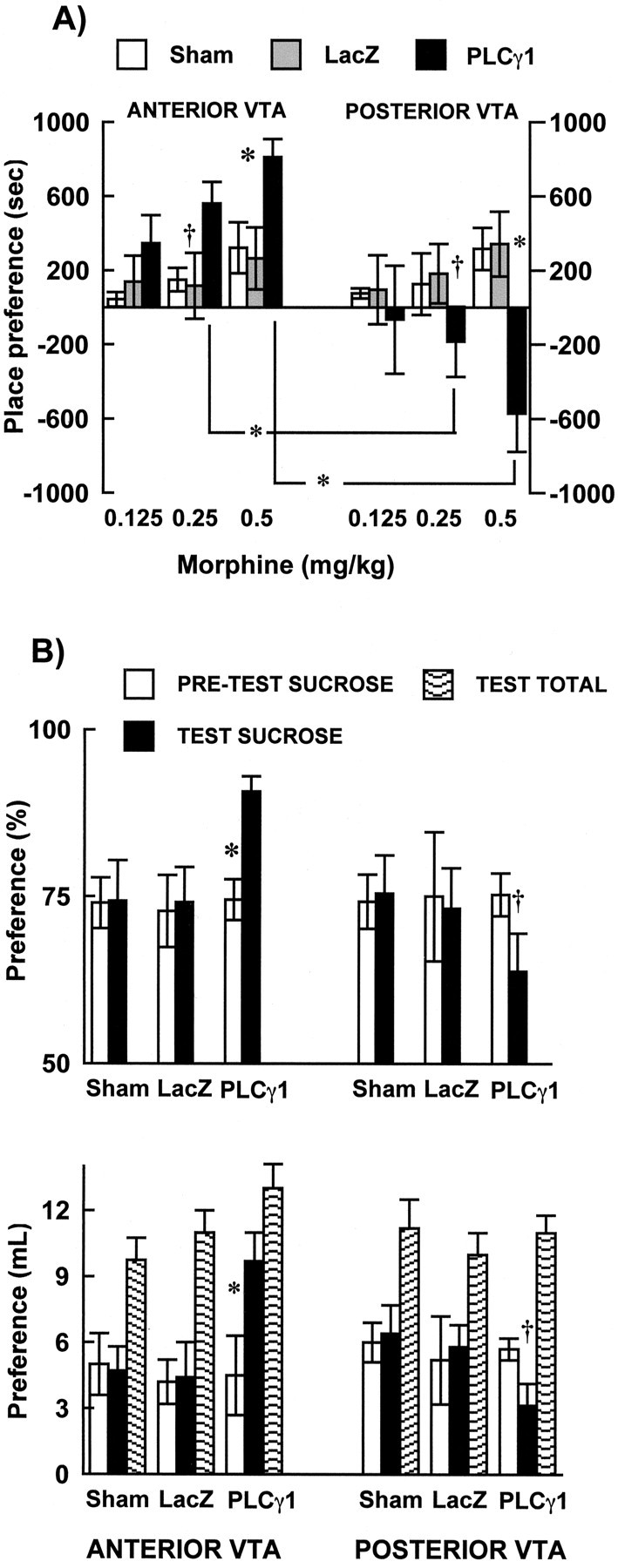
PLCγ1 regulates responses to rewarding stimuli. A, Morphine (0.125, 0.25, and 0.50 mg/kg, s.c.) place conditioning. PLCγ1 overexpression in R-VTA enhanced sensitivity to threshold doses of morphine, whereas overexpression of this protein in C-VTA resulted in place aversion. Sham surgery and β-gal expression (via HSV-LacZ) had no effect on place conditioning. *p < 0.05; †p = 0.06. B, Sucrose preference. Sham surgery and β-gal expression did not affect sucrose preference, regardless of VTA region. PLCγ1 overexpression in R-VTA significantly increased sucrose preference, whereas PLCγ1 in C-VTA decreased sucrose preference, respectively. Data are presented as percentage difference of total liquid intake between pretest and posttest (top) or as difference in liquid intake between sucrose and water bottle (bottom). PLCγ1 overexpression did not affect to tal fluid (sucrose plus water) intake (bottom). *p < 0.05; † p = 0.065. Error bars indicate SEM.
PLCγ1 regulation of sucrose preference
To generalize the place-conditioning effects of PLCγ1 in the VTA to a natural reward, and to a behavioral paradigm free of associative memory, we studied sucrose preference. Overall analysis indicated that HSV microinjections did not significantly affect the total fluid intake of the rats (water plus sucrose) (Fig. 3B) during the testing day. However, similar to the effects seen with morphine place conditioning, sucrose preference varied as a function of VTA region, viral treatment, and testing (viral treatment × region × test day interaction, F(2,106) = 3.7; p < 0.02). PLCγ1 overexpression in R-VTA increased sucrose preference when compared with pretesting scores (p < 0.003) and with the LacZ (p < 0.05) and sham (p < 0.05) control groups during the test day (Fig. 3B). Conversely, PLCγ1 overexpression in C-VTA showed a notable, although marginally significant (p = 0.065), decrease in sucrose preference when compared with their sucrose scores at pretest and a significant difference when compared with preference scores for the HSV-LacZ (p < 0.05) or sham (p < 0.05) groups. As an additional control, we found that HSV-PLCγ1 microinjections into the substantia nigra did not affect sucrose preference (data not shown).
PLCγ1 regulation of forced swimming
Given the increasing evidence that the mesolimbic dopamine system regulates responses to aversive stimuli as well as rewarding ones (Barrot et al., 2002), it was of interest to study the effect of PLCγ1 overexpression in behavioral tests of aversion. We first used the forced swim test to study animal responses to stressful conditions (Pliakas et al., 2001; Cryan et al., 2002). In this test, animals initially struggle trying to escape, but within 1 or 2 min, they become immobile. The amount of time rats engaged in escape-directed behaviors (i.e., latency to immobility) in the forced swim test was dependent on viral treatment. Animals receiving HSV-PLCγ1 into the C-VTA had significantly shorter times to become immobile (treatment main effect, F(2,13) = 5.2; p < 0.02) than rats receiving sham (p < 0.014) or HSV-LacZ (p < 0.017) microinjections into the C-VTA (Fig. 4A). In contrast, no difference in the latency to immobility was apparent in animals receiving HSV-PLCγ1 into the R-VTA. To assess whether the effects observed in the forced swim test could be confounded by changes in general locomotor activity after viral-mediated gene transfer, separate groups of HSV-PLCγ1, HSV-LacZ, or sham surgery animals were analyzed for locomotor behavior. As seen in Figure 4B, no significant differences were apparent during PLCγ1 overexpression in either the C-VTA or R-VTA (F(2,24) = 1.6; p > 0.2), when locomotor activity was assessed 24 hr after day 1 of forced swimming (day 4 after HSV microinjections). HSV-PLCγ1 microinjections into the substantia nigra did not alter the responses of the animals in the forced swim test (data not shown).
Figure 4.
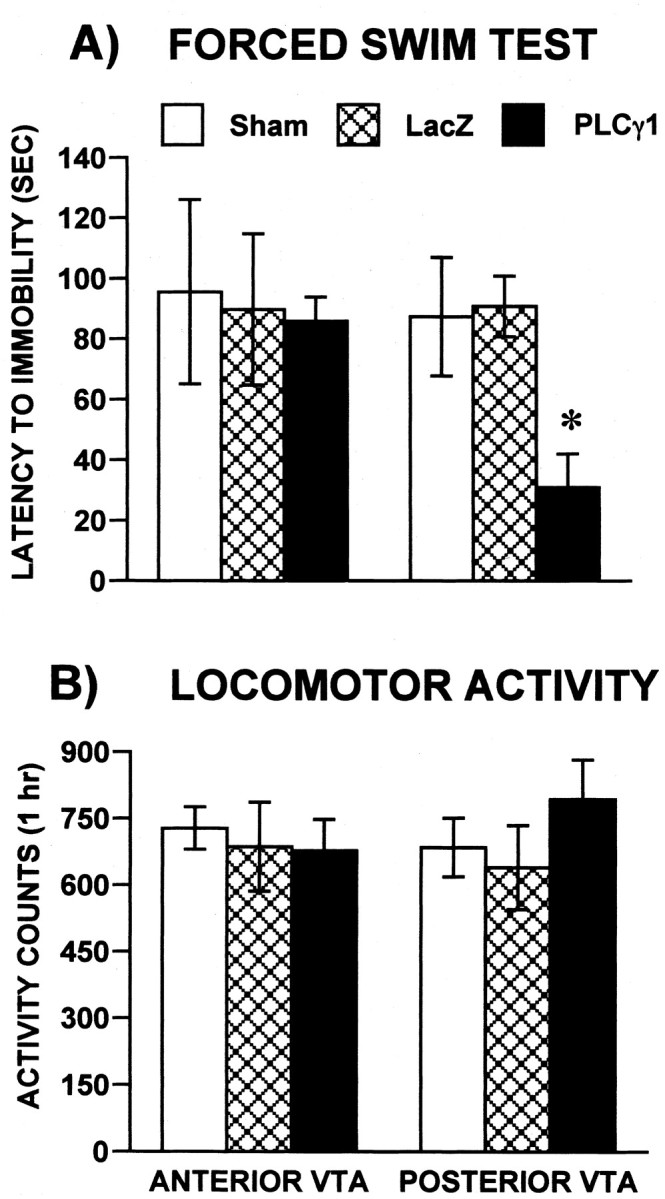
PLCγ1 regulates responses to swim stress. A, Latencies to become immobile varied as a function of viral vector treatment and VTA region. Latencies were significantly decreased in rats treated with HSV-PLCγ1 in C-VTA. HSV-LacZ or sham injections had no effect. Data are presented as latencies (mean ± SEM, in seconds) during the 5 min test on day 4 after HSV microinjections. B, There were no group differences when activity, rather than swimming, was quantified during testing day. *p < 0.002. Error bars indicate SEM.
PLCγ1 regulation of responses to nociceptive stimuli
Our findings thus far show that PLCγ1 overexpression in the R-VTA and C-VTA can differentially modulate behavioral responses to rewarding and stressful stimuli. We next assessed the influence of PLCγ1 overexpression in the VTA on unconditioned behavioral responses to nociceptive stimuli. When compared with control groups, overexpression of PLCγ1 in the C-VTA decreased the threshold foot-shock intensities required to elicit vocalization (F(1,42) = 6.37; p < 0.05) or jumping (F(1,42) = 8.43; p < 0.001) without significantly affecting the threshold intensity eliciting a flinch reaction (Fig. 5A). These data suggest that animals receiving HSV-PLCγ1 into the C-VTA are more sensitive to this mild nociceptive stimulus. In contrast, animals receiving HSV-PLCγ1 into the R-VTA showed no difference in nociceptive responses (Fig. 5A).
Figure 5.
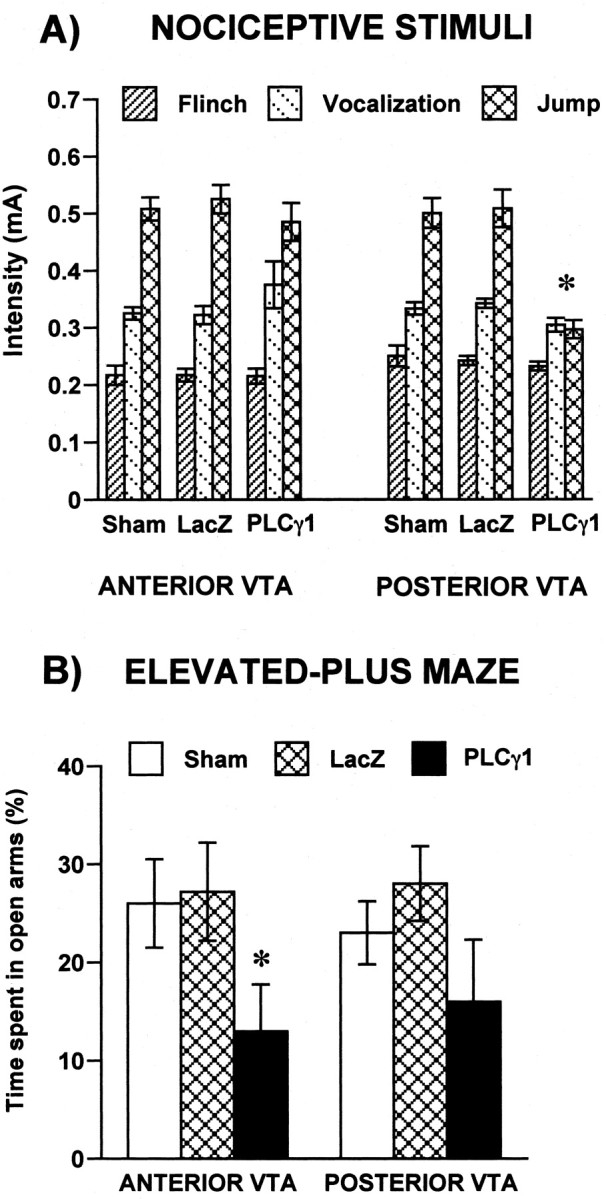
PLCγ1 regulates responses to nociceptive and anxiogenic stimuli. A, Rats with HSV-PLCγ1 overexpression in C-VTA vocalized and jumped in response to lower foot-shock intensities than rats overexpressing PLCγ1 in R-VTA. HSV-LacZ and sham groups did not differ in their responses to foot shock. B, Animals overexpressing PLCγ1 in R-VTA spent significantly less time in the open arms of the elevated-plus maze than the LacZ and sham controls. There was a trend, although not statistically significant, for decrease in time spent in the open arms in rats overexpressing PLCγ1 in C-VTA compared with their respective LacZ and sham controls. *p < 0.05. Error bars indicate SEM.
PLCγ1 regulation of elevated-plus maze behavior
We also studied the effect of PLCγ1 overexpression in the R-VTA and C-VTA on anxiety-like behavior using the elevated-plus maze. Time spent in the open arms of the plus maze (a measure of anxiety-like behavior) was affected by viral treatment (condition main effect, F(2,36) = 8.51; p < 0.0009), but it did not vary as a function of VTA region (Fig. 5B). Animals receiving HSV-PLCγ1 in R-VTA spent significantly less time in the open arms of the maze than the HSV-LacZ (p = 0.01) and sham (p = 0.005) controls, an indication of increased anxiety-like behavior. However, there was a trend for a similar decrease in time spent on the open arms in rats microinjected with HSV-PLCγ1 in C-VTA compared with the HSV-LacZ or sham controls (p = 0.065 in each case).
Discussion
Previous reports have implicated neurotrophic factors in the cellular and behavioral adaptations occurring in the VTA after prolonged exposure to drugs of abuse (see Introduction). Moreover, chronic exposure to drugs of abuse has been shown to alter several components of neurotrophic factor-signaling cascades within this brain region. One example was our demonstration that repeated exposure to morphine increases levels of PLCγ1 in the VTA (Wolf et al., 1999). Although PLCγ1 has been implicated in mediating several neurobiological processes (Kamat and Carpenter, 1997; Rhee, 2001), the functional role this signaling protein plays in mediating responses to drugs of abuse has remained unknown. Thus, in the present study, we mimicked the biological response of PLCγ1 induction observed after chronic morphine by using viral-mediated gene transfer to locally increase PLCγ1 levels in the VTA. We showed that increased expression of PLCγ1 in the VTA modulates behavioral responses to morphine and to sucrose (a natural reward), as well as several aversive stimuli. In addition, we showed that regulation of these behavioral responses elicited by emotional stimuli is dependent on the subregion of the VTA in which PLCγ1 is overexpressed.
Our findings describe two distinct behavioral phenotypes caused by PLCγ1 overexpression in the rostral versus caudal aspects of the VTA. In the R-VTA, increased levels of PLCγ1 increase the sensitivity of an animal to the rewarding effects of morphine as well as the sucrose preference of an animal, while causing little change in its responses to aversive stimuli. In contrast, in the C-VTA, increased levels of PLCγ1 cause the opposite effects on reward, with reduced responses to morphine and sucrose observed, but also induce greater sensitivity to several types of aversive stimuli, including swim stress and nociceptive and anxiogenic challenges. These data thereby suggest two distinct functional loops mediated by drug-induced upregulation of PLCγ1 expression in rostral and caudal subregions of the VTA. In the R-VTA, upregulation of PLCγ1 would appear to mediate a state of sensitized responses to drug and natural rewards. In contrast, in the C-VTA, upregulation of PLCγ1 would appear to mediate a depressed emotional state characterized by reduced sensitivity to reward and enhanced sensitivity to negative emotional stimuli.
The opposite effects of PLCγ1 overexpression in R-VTA versus C-VTA on measures of drug reward are in agreement with several previous studies that have demonstrated that topographical differences within the VTA mediate the rewarding and aversive properties of drugs (Ikemoto et al., 1997, 1998; Carlezon et al., 2000b; Olson et al., 2001), and we now extend these previous findings to a natural reward, namely sucrose. Although the mechanism(s) underlying these topographical differences remain unknown, two explanations have been offered. The first speculates that distinct populations of dopamine neurons within the VTA might mediate the divergent behavioral effects observed between R-VTA and C-VTA. Neuroanatomical studies indicate that dopamine neurons from more rostral portions of the VTA innervate primarily, but not exclusively, the NAc shell, whereas dopamine neurons from C-VTA project predominantly, but not exclusively, to cortical areas (Emson and Koob, 1978; Brog et al., 1993). Moreover, these projections show differential regulation by morphine: morphine increases extracellular dopamine levels in the NAc shell but has no effect in prefrontal cortical areas (Bassareo et al., 1996), whereas morphine withdrawal is associated with decreased extracellular dopamine levels in the NAc shell but increased levels in prefrontal cortex (Acquas et al., 1991; Pothos et al., 1991; Bassareo et al., 1995). Thus, within this framework, it is conceivable that increased PLCγ1 activity in R-VTA dopamine neurons enhances reward to morphine and sucrose while resulting in opposite effects when PLCγ1 activity is enhanced in C-VTA dopamine neurons.
The second explanation focuses on distinct populations of nondopaminergic, most likely GABAergic, neurons in the R-VTA versus C-VTA, which differentially regulate drug and sucrose reward. GABAergic neurons in the VTA have long been known to regulate the activity of VTA dopamine neurons (Di Chiara and North, 1992; Johnson and North, 1992) and, more recently, have been shown to project directly to the NAc (Van Bockstaele and Pickel, 1995; Steffensen et al., 1998), thereby providing two mechanisms by which these neurons control activity of the mesolimbic reward pathway. GABAergic activity in the VTA has been shown to play an increasingly important role in modulating the behavioral and cellular responses to rewarding (Roberts and Brebner, 2000; Laviolette and van der Kooy, 2001; Steffensen et al., 2001) and aversive (Bonci and Williams, 1997; Chieng and Williams, 1998) stimuli. The HSV vectors used in this study are not selective for a particular type of neuron (Neve et al., 1997; Carlezon et al., 2000a), and appear to infect all neuronal types within a given brain region with approximately equal efficiency. However, because the density of dopaminergic neurons in the VTA decreases from caudal to rostral subregions, it is possible that PLC-γ1 overexpression may occur to a greater extent in nondopaminergic cells in the R-VTA compared with the C-VTA. Indeed, whereas a clear majority of HSV-infected cells in the C-VTA are dopaminergic (i.e., TH positive), a somewhat smaller percentage of infected cells in the R-VTA are dopaminergic. However, additional work is needed to determine whether this difference in PLC-γ1 overexpression in dopaminergic versus nondopaminergic cells between R-VTA and C-VTA can explain the differential behavioral effects observed. In addition, it will be important in future investigations to determine whether the morphine-induced upregulation of PLCγ1 occurs predominantly in dopaminergic, nondopaminergic, or both cell types in the VTA. GABAergic neurons are a likely candidate for the nondopaminergic cells involved in this phenomenon. Unfortunately, it has not been possible to directly identify cell bodies of these neurons in the VTA because of limitations in available antibodies.
Our findings that PLCγ1 overexpression in the C-VTA alters the sensitivity of an animal to aversive stimuli lends additional support to the notion that the mesolimbic reward pathway may play a role in the symptoms of depression and other stress-related syndromes (Kapur and Mann, 1992; Naranjo et al., 2001; Pliakas et al., 2001; Yadid et al., 2001; Nestler et al., 2002). Thus, animals that received HSV-PLCγ1 injections in the C-VTA exhibited shorter latency to immobility in the forced swim test, an effect opposite to that of antidepressant treatments (Cryan et al., 2002). In contrast, microinjections of HSV-PLCγ1 into R-VTA had no effect in this assay. The decreased latency to immobility obtained with HSV-PLCγ1 injections in the C-VTA was not caused by changes in general motor activity, which was unaffected by PLCγ1 overexpression. Additionally, sham treatment or injection of HSV-LacZ into the C-VTA did not affect latency to immobility, indicating that surgery or viral infection per se does not affect forced swimming. Additional studies with foot-shock stress and the elevated-plus maze found that PLCγ1 overexpression in the C-VTA increased the responses of the animals to nociceptive and anxiogenic stimuli. Thus, the induction of PLCγ1 in C-VTA, by decreasing responses to rewarding stimuli while increasing responses to aversive stimuli, could contribute to a similar constellation of symptoms, which are seen in many drug addicts, particularly during early phases of drug withdrawal (Gawin and Kleber, 1986; Gawin et al., 1989; Barr et al., 2002). It also would be interesting to investigate the possible involvement of PLCγ1 in the C-VTA now in mediating these and certain other symptoms of depression (American Psychiatric Association, 1994).
To summarize, results of the present study establish the functional importance of morphine-induced upregulation of PLCγ1 expression in the VTA. Our findings define two distinct feedback loops whereby PLCγ1 induction in R-VTA versus C-VTA mediate distinct behavioral adaptations to chronic morphine exposure. Additional understanding of the mechanisms underlying this PLCγ1-induced behavioral plasticity will lead to a better understanding of the neural and molecular basis of drug addiction.
Footnotes
This work was supported by grants (E.J.N.) and a National Research Service Award (C.A.B.) from the National Institute on Drug Abuse and the National Institute of Mental Health (E.J.N.).
Correspondence should be addressed to Dr. Eric J. Nestler, Department of Psychiatry, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390-9070. E-mail: eric.nestler@utsouthwestern.edu.
M. Barrot's present address: Unité Mixte de Recherche 7519, Centre National de la Recherche Scientifique, University Louis Pasteur, 67084 Strasbourg Cedex, France.
Copyright © 2003 Society for Neuroscience 0270-6474/03/237569-08$15.00/0
References
- Acquas E, Carboni E, Di Chiara G ( 1991) Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol 193: 133-134. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association ( 1994) Diagnostic and statistical manual of psychiatric disorders, Ed 4. Washington, DC: American Psychiatric Association.
- Barde Y ( 1989) Trophic factors and neuronal survival. Neuron 2: 1525-1534. [DOI] [PubMed] [Google Scholar]
- Barr A, Markou A, Phillips A ( 2002) A “crash” course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci 23: 475-482. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ ( 2002) CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA 99: 11435-11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Tanda G, Di Chiara G ( 1995) Increase of extracellular dopamine in the medial prefrontal cortex during spontaneous and naloxone-precipitated opiate abstinence. Psychopharmacology (Berl) 122: 202-205. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Tanda G, Petromilli P, Giua C, Di Chiara G ( 1996) Nonpsychostimulant drugs of abuse and anxiogenic drugs activate with differential selectivity dopamine transmission in the nucleus accumbens and in the medial prefrontal cortex of the rat. Psychopharmacology (Berl) 124: 293-299. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Nestler EJ ( 1991) Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem 57: 344-347. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Nestler EJ ( 1993) Chronic morphine impairs axoplasmic transport in the mesolimbic dopamine system of the rat brain. NeuroReport 5: 57-60. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ ( 1992) Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J Neurosci 12: 2165-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, Nestler EJ ( 1995) Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience 68: 969-979. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ ( 1996) Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci 16: 4707-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Williams JT ( 1997) Increased probability of GABA release during withdrawal from morphine. J Neurosci 17: 796-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS ( 1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338: 255-278. [DOI] [PubMed] [Google Scholar]
- Carlezon Jr WA, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ ( 1997) Sensitization to morphine induced by viral-mediated gene transfer. Science 277: 812-814. [DOI] [PubMed] [Google Scholar]
- Carlezon Jr WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ ( 1998) Regulation of cocaine reward by CREB. Science 282: 2272-2275. [DOI] [PubMed] [Google Scholar]
- Carlezon Jr WA, Nestler EJ, Neve RL ( 2000a) Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol 14: 47-67. [DOI] [PubMed] [Google Scholar]
- Carlezon Jr WA, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ ( 2000b) Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci 20: RC62(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Williams JT ( 1998) Increased opioid inhibition of GABA release in nucleus accumbens during morphine withdrawal. J Neurosci 18: 7033-7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I ( 2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23: 238-245. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, North RA ( 1992) Neurobiology of opiate abuse. Trends Pharmacol Sci 13: 185-193. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ ( 1997) A molecular and cellular theory of depression. Arch Gen Psychiatry 54: 597-606. [DOI] [PubMed] [Google Scholar]
- Emson PC, Koob GF ( 1978) The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res 142: 249-267. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak P, Collins F, Hoffer B, Gerhardt GA ( 1996) Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380: 252-255. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD ( 1986) Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 43: 107-113. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD, Byck R, Rounsaville BJ, Kosten TR, Jatlow PI, Morgan C ( 1989) Desipramine facilitation of initial cocaine abstinence. Arch Gen Psychiatry 46: 117-121. [DOI] [PubMed] [Google Scholar]
- Hellweg R, von Richthofen S, Anders D, Baethge C, Ropke S, Hartung HD, Gericke CA ( 1998) The time course of nerve growth factor content in different neuropsychiatric diseases—a unifying hypothesis. J Neural Transm 105: 871-903. [DOI] [PubMed] [Google Scholar]
- Hoffman DC ( 1989) The use of place conditioning in studying the neuropharmacology of drug reinforcement. Brain Res Bull 23: 373-387. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ ( 1997) Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci 111: 369-380. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ ( 1998) Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav 61: 87-92. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA ( 1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12: 483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Carpenter G ( 1997) Phospholipase C-gamma1: regulation of enzyme function and role in growth factor-dependent signal transduction. Cytokine Growth Factor Rev 8: 109-117. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mann JJ ( 1992) Role of the dopaminergic system in depression. Biol Psychiatry 32: 1-17. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC ( 2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22: 3306-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE ( 1998) Neuroscience of addiction. Neuron 21: 467-476. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D ( 2001) GABA(A) receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci 13: 1009-1015. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS ( 1994) Neurotrophic factors: from molecule to man. Trends Neuroscience 17: 182-190. [DOI] [PubMed] [Google Scholar]
- Lu B, Figurov A ( 1997) Role of neurotrophins in synapse development and plasticity. Rev Neurosci 8: 1-12. [DOI] [PubMed] [Google Scholar]
- Lucki I ( 1997) The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol 8: 523-532. [DOI] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon Jr WA, Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ ( 2000) Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron 26: 247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo CA, Tremblay LK, Busto UE ( 2001) The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry 25: 781-823. [DOI] [PubMed] [Google Scholar]
- Nestler EJ ( 2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2: 119-128. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Berhow MT, Brodkin ES ( 1996) Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol Psychiatry 1: 190-199. [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM ( 2002) Neurobiology of depression. Neuron 34: 13-25. [DOI] [PubMed] [Google Scholar]
- Neve RL, Howe JR, Hong S, Kalb RG ( 1997) Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience 79: 435-447. [DOI] [PubMed] [Google Scholar]
- Olson VG, Wolf DH, Russell D, Hughes T, Neve RL, Nestler EJ ( 2001) Regulation of drug reward by CREB in ventral midbrain. Soc Neurosci Abstr 27: 977.17. [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ ( 1995) Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci 15: 1285-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER ( 1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16: 1137-1145. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C ( 1997) The rat brain in stereotaxic coordinates, Ed 3. San Diego: Academic. [DOI] [PubMed]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon Jr WA ( 2001) Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M ( 1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730-732. [DOI] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG ( 1991) Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res 566: 348-350. [DOI] [PubMed] [Google Scholar]
- Rhee SG ( 2001) Regulation of phosphoinositide-specific phopholipase C. Annu Rev Biochem 70: 281-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Brebner K ( 2000) GABA modulation of cocaine self-administration. Ann NY Acad Sci 909: 145-158. [DOI] [PubMed] [Google Scholar]
- Ross CA, MacCumber MW, Glatt CE, Snyder SH ( 1989) Brain phospholipase C isozymes: differential mRNA localizations by in situ hybridization. Proc Natl Acad Sci USA 86: 2923-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi W-X, Lane SB, Harris HW, Bunney BS, Nestler EJ ( 1996) Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA 93: 11202-11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH ( 1999) Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci USA 96: 10893-10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Chen SY, Kalivas PW ( 1993) Time course of tyrosine hydroxylase expression after behavioral sensitization to cocaine. J Pharmacol Exp Ther 266: 424-430. [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ ( 1998) Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci 18: 8003-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Lee RS, Stobbs SH, Henriksen SJ ( 2001) Responses of ventral tegmental area GABA neurons to brain stimulation reward. Brain Res 906: 190-197. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM ( 1995) GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res 682: 215-221. [DOI] [PubMed] [Google Scholar]
- Wise RA ( 1996) Neurobiology of addiction. Curr Opin Neurobiol 6: 243-251. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Numan S, Nestler EJ, Russell DS ( 1999) Regulation of phospholipase Cgamma in the mesolimbic dopamine system by chronic morphine administration. J Neurochem 73: 1520-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Overstreet DH, Zangen A ( 2001) Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res 896: 43-47. [DOI] [PubMed] [Google Scholar]