Abstract
Background:
Autism spectrumdisorder (ASD) commonly presents with co-occurring medical conditions (CoCs). Little is known about patterns in CoCs in a time of rising ASD prevalence.
Aims:
To describe trends in number and type of documented CoCs in 8-year-old children with ASD.
Methods:
We used Autism and Developmental Disabilities Monitoring Network (ADDM) data, a multi-source active surveillance system monitoring ASD prevalence among 8-year-old children across the US. Data from surveillance years 2002, 2006, 2008, and 2010 were used to describe trends in count, categories, and individual CoCs.
Results:
Mean number of CoCs increased from 0.94 CoCs in 2002 to 1.06 CoCs in 2010 (p < 0.001). The percentage of children with ASD with any CoC increased from 44.5% to 56.4% (p < 0.001). CoCs with the greatest increases were in general developmental disability (10.4% to 14.5%), language disorder (18.9% to 23.6%), and motor developmental disability (10.5% to 15.6%). Sex modified the relationship between developmental (P = 0.02) and psychiatric (P < 0.001) CoCs and surveillance year. Race/ethnicity modified the relationship between neurological conditions (P = 0.04) and surveillance year.
Conclusions:
The increase in the percentage of children with ASD and CoCs may suggest the ASD phenotype has changed over time or clinicians are more likely to diagnose CoCs.
Keywords: Autism spectrum disorder, Co-occurring conditions, Trends, Sex, Race/ethnicity
1. Introduction
Autism spectrum disorder (ASD) is characterized by social and communication impairments and repetitive behaviors and restricted interests (American Psychiatric Association, 2000, 2013). Along with these diagnostic features, non-ASD co-occurring conditions (CoCs) are highly prevalent among children with ASD (Baird et al., 2008; Close, Lee, Kaufmann, & Zimmerman, 2012; Lai, Lombardo, & Baron-Cohen, 2014; Levy et al., 2010; Newschaffer et al., 2007). CoCs are associated medical and developmental disorders, distinct from ASD, which may or may not share etiologic origins (Gurney, McPheeters, & Davis, 2006); examples include language disorders, developmental delays (DD), attention deficit hyperactivity disorder (ADHD), intellectual disability (ID), sensory integration disorder, anxiety disorder, and epilepsy (Levy et al., 2010; Simonoff et al., 2008).
Investigating CoCs may provide clues into shared etiologies (Bradley & Isaacs, 2006; Hu, 2012; Ibrahim, Voigt, Katusic, Weaver, & Barbaresi, 2009; Zhang, Xu, Liu, Li, & Xu, 2012), as the neurological mechanisms of CoCs may provide insight in to the mechanisms behind ASD (Maski, Jeste, & Spence, 2011; Sinzig, Walter, & Doepfner, 2009). These conditions complicate ASD presentation and need to be considered when creating interventions specific to a child’s needs. For example, much work has been done in creating interventions adapted for children with ASD and anxiety (Lang, Regester, Lauderdale, Ashbaugh, & Haring, 2010; Reaven, 2009; White, Oswald, Ollendick, & Scahill, 2009) or ADHD (Bradley & Isaacs, 2006; Davis & Kollins, 2012). CoCs are an important consideration in ASD research because they may factor into timing of ASD diagnosis; certain CoCs are associated with later age at ASD diagnosis, including hearing impairment (Mandell, Novak, & Zubritsky, 2005), psychiatric and neurologic conditions (Levy et al., 2010), ADHD (Frenette et al., 2013), and major congenital abnormalities (Frenette et al., 2013). This delay of an ASD diagnosis is associated with increased expenditures to individuals, families and communities (Horlin, Falkmer, Parsons, Albrecht, & Falkmer, 2014). Children with ASD and co-occurring ID are more likely to be diagnosed with ASD at a younger age compared to children without co-occurring ID (Shattuck et al., 2009), which may suggest that clinicians may more easily identify ASD in children with a more severe presentation (Mandell et al., 2005). Once diagnosed with ASD, children with CoCs, specifically those with co-occurring ID or epilepsy have increased service costs (Buescher, Cidav, Knapp, & Mandell, 2014; Lavelle et al., 2014).
It is important to address CoCs in a time when reported ASD prevalence is on the rise. The most recent estimate from the Autism and Developmental Monitoring (ADDM) Network, an active US ASD surveillance system, was 16.8 ASD cases per 1000 8-year-old children in 2014 (Baio et al., 2018) a more than doubling of identified prevalence since the first ADDM Network prevalence report a decade ago (Autism and Developmental Disabilities Monitoring Network 2010 Principal Investigators, 2007). One hypothesis explaining part of this rise is increased awareness of ASD symptoms leading to earlier identification (Koegel, Koegel, Ashbaugh, & Bradshaw, 2014; Warren, Stone, & Humberd, 2009) and/or more ASD diagnoses for children with less severe presentations; this hypothesis is supported by the decrease in proportion of cases with co-occurring ID in ADDM (Autism and Developmental Disabilities Monitoring Network 2010 Principal Investigators, 2014) and other cohorts (Charman et al., 2011; Van Naarden Braun et al., 2015). Another possible explanation is diagnostic accretion or substitution, where a child who was once getting another diagnosis is now either also getting an ASD diagnosis or is reclassified as only having ASD (Coo et al., 2007; Croen, Grether, Hoogstrate, & Selvin, 2002; King & Bearman, 2009). Accretion or substitution would necessitate a child with ASD having at least one other CoC on their health records. By assessing how the proportion of CoCs in children with ASD changes over time, we can evaluate patterns in diagnoses at a population level and temporal changes in extent of CoCs.
The purpose of this study was to describe trends in count and type of CoC in 8-year-old children with ASD, identified by ADDM. We additionally explored whether trends in type and count of CoC differed by sex or race/ethnicity. Findings from the study improve our understanding of how CoCs have changed over time among children with ASD and possible differences in trends by sex and racial/ethnic subgroups
2. Materials and methods
2.1. Study population and case ascertainment
We examined data collected by ADDM for surveillance years (SYs) 2002, 2006, 2008, and 2010. ADDM is a multiple source active surveillance system established by the Centers of Disease Control and Prevention to estimate the biannual prevalence of ASD using a standardized methodology (Autism and Developmental Disabilities Monitoring Network 2010 Principal Investigators, 2014; Baio et al., 2018).
For a child to be identified as potentially having ASD and therefore subject to clinician review to determine ASD surveillance case status, a child had to have been evaluated for developmental concerns and had behaviors consistent with the DSM-IV-TR criteria for autistic disorder, pervasive developmental disorder, or Asperger’s syndrome, as indicated in their health or education records.
In ADDM, a child was eligible if he or she was 8-years-old during the respective SY and had at least one parent or guardian who resided in the surveillance area. Educational and health records were abstracted and reviewed in order to determine ASD case status. Medical, behavioral, psychiatric, and developmental histories, as well as symptoms and diagnoses consistent with DSM-IV-TR, were abstracted for each child, along with all available education records. If more than one record was available for a child, all abstractions were combined into a composite record. Research-trained clinicians reviewed all composite records using a highly structured scoring protocol to determine whether the ASD surveillance case criteria for ASD were met. Clinicians maintained 90% agreement for reliability on final case status and 80–90% agreement for individual variables scored (Rice et al., 2007). All ADDM participating sites functioned as a public health authority under the HIPAA Privacy Rule and met applicable local Institutional Review Board and privacy/confidentiality requirements under 45 CFR 46. The ADDM Network is a public health surveillance system, which does not require participant consent for record review. Further information about ADDM methods is detailed in Rice et al. (2007).
This study includes children identified with ASD in the ADDM network from sites that contributed to four SYs: 2002, 2006, 2008 and 2010. SYs 2000 and 2004 were excluded due to limited site participation and scarcity of data. The eight sites that met entry criteria for this study were located in Alabama, Arizona, Colorado, Georgia, Maryland, Missouri North Carolina, and Wisconsin. All but one site changed their surveillance area boundaries at some point between 2002 and 2010; therefore, a common catchment area was constructed for each site across all four SYs such that temporal comparisons would be appropriate.
2.2. Identifying CoCs
A child was considered to have a CoC if there was a clear statement in the child’s health records by a community professional (e.g. psychologist or developmental pediatrician) that the child met criteria for a specific disorder. Any recorded instance of a CoC from birth (1994 for SY 2002, 1998 for SY 2006, 2000 for SY 2008, and 2002 for SY2010) to time of record abstraction when the child was 8 years old is included. Due to the amount of potential health conditions that could present in children, this paper only presents CoCs that were noted in more than one child in a SY. If the child had a diagnosis that was coded by ADDM clinical reviewers as ‘other non-ASD conditions’ in their composite ADDM record, we examined the accompanying text field for potential CoCs. CoCs may have been noted in this ‘other non-ASD condition’ category because of not being listed as a categorical option in the ADDM abstraction forms, slightly different wordings that prevented accurate categorization (e.g. ADD rather than ADHD), or coding errors. CoCs in the ‘other’ category were thoroughly assessed to exclude rule-out diagnoses or sub-diagnostic level traits, while ensuring proper ascertainment.
Each CoC was placed into one of five broader categories based on groupings of CoCs created by Levy et al. using ADDM data (2010): a) any CoC; b) developmental conditions (ADHD, language disorder, learning disability, intellectual disability, DD general, DD adaptive, DD cognitive, DD motor, DD social personal, DD play, and sensory integration disorder); c) psychiatric conditions (anxiety disorder, conduct disorder, oppositional defiant disorder, obsessive compulsive disorder, bipolar disorder, depression, emotional disorder, mood disorder, mutism, obsessive compulsive disorder, psychosis, reactive attachment disorder, and schizophrenia); d) neurologic diagnoses (encephalopathy, cerebral palsy, seizures or epilepsy, brain injury, vision impairment, hearing loss, Tourette’s syndrome); e) possible causative conditions (tuberous sclerosis, Down syndrome, Fragile X syndrome).
2.3. Data analysis
We modeled trends in the average number for prevalent CoCs using Poisson regression. Since DD sub-categories (general, adaptive, cognitive, motor, social personal, or play) were not exclusive and may have been coded differently by clinicians due to lack of standard definitions for these sub-categories, in instances where a clinician documented more than one DD sub-category we counted DD as a single CoC when deriving our average number of CoCs. The Poisson model was run twice, first controlling for surveillance site, as it is a design variable, and second, additionally controlling for sex and race/ethnicity as potential confounders. Sex and race/ethnicity are associated with different CoCs (Carter et al., 2007; Mayes & Calhoun, 2011; Rubenstein, Wiggins, & Lee, 2015); sex is associated with age at diagnosis (Begeer et al., 2013; Fountain, King, & Bearman, 2011; Shattuck et al., 2009) which has changed over time (Christensen, Bilder et al., 2016; Fountain et al., 2011; Frenette et al., 2013); and race/ethnicity changed in their distribution in ADDM over time (Autism and Developmental Disabilities Monitoring Network 2010 Principal Investigators, 2014). We elected to control for these variables since we were interested in trends for the total sample, not stratified by subgroups. To test for trend, we used a likelihood ratio test (LRT) to examine if the number of prevalent CoCs for any one of SYs 2006, 2008, or 2010 differed from SY 2002 at an alpha level of 0.05.
Additionally, we ran linear-risk regressions to estimate the percentage of children having CoCs in each of the four broad categories, given the category had adequate sample size (developmental, psychiatric, neurological, none) and documented individual CoCs in SY 2002. A linear-risk regression allows us to calculate change in point prevalence over time (as measured in percent), rather than just calculating prevalence ratios (Prentice & Mason, 1986). We present absolute percent change and confidence intervals comparing SYs 2006, 2008, and 2010 to 2002 and CoCs that occurred in at least 5% of our sample for at least one study year (ADHD, DD general, DD adaptive, DD motor, DD cognitive, language disorder, sensory integration disorder, ID and encephalopathy). Our model controlled for site since it is a design variable and we used LRTs to assess trend. Lastly, we addressed sex and race/ethnicity as modifiers by running log-linear regression to calculate risk ratios (RR) between sexes (female compared to male) and between race/ ethnicities (comparing black non-Hispanic, other non-Hispanic, or Hispanic to white non-Hispanic). Models included three covari- ates: site, sex, and race/ethnicity. We tested whether sex or race/ethnicity modified the relationship between CoCs and SY by a running regression model that included interaction terms between sex and SY (three terms) or interaction terms between race/ ethnicity and SY (nine terms). We used LRTs to test whether any of the interaction terms in the given model significantly differed at an alpha = 0.1 level. This test examines whether the trends in risk ratios comparing non-referent groups (females or black non-Hispanic, other non-Hispanic, Hispanic) to the referent (male or white non-Hispanic) do not significantly differ. All analyses were conducted using SAS 9.3 (SAS Institute Inc, 2011).
3. Results
Demographic characteristics are presented in Table 1. Across four SYs, 6379 children from eight participating sites met surveillance case criteria for ASD. The distribution of these 6379 cases across surveillance years was 14.6% from SY2002 (N = 932), 22.4% from SY2006 (N = 1429), 26.1% from SY2008 (N = 1666), and 36.9% from SY2010 (N = 2352). The increase in percentage over SY reflects the increasing prevalence of ASD over the time period in ADDM. Males made up a majority of our sample, with little change over SYs (80.8% male in SY2002, 81.7% male in SY2010). Our sample was largely white non-Hispanic, but percentage white non-Hispanic decreased over SYs (69.3% in 2002, 61.4% in 2010) with an increase in Hispanic ethnicity (2.7% in 2002, 11.6% in 2010).
Table 1.
Descriptive Characteristics for 8-Year-Old Children with autism spectrum disorder in Autism and Developmental Disabilities Monitoring (ADDM) Network in surveillance years 2002, 2006, 2008, and 2010.
| 2002 | 2006 | 2008 | 2010 | |||||
|---|---|---|---|---|---|---|---|---|
| N = 932 | N = 1429 | N = 1666 | N = 2352 | |||||
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Male | 753 | 80.8 | 1188 | 83.1 | 1368 | 82.1 | 1921 | 81.7 |
| Female | 179 | 19.2 | 241 | 16.9 | 298 | 17.9 | 431 | 18.3 |
| Site | ||||||||
| Alabama | 69 | 7.4 | 144 | 10.8 | 108 | 6.5 | 122 | 5.2 |
| Arizona | 39 | 4.2 | 86 | 6.0 | 141 | 8.5 | 226 | 9.6 |
| Colorado | 28 | 3.0 | 43 | 3.01 | 64 | 3.8 | 367 | 15.6 |
| Georgia | 196 | 21.0 | 294 | 20.6 | 368 | 22.1 | 518 | 22.0 |
| Maryland | 102 | 10.9 | 111 | 7.8 | 171 | 10.3 | 220 | 9.4 |
| Missouri | 205 | 22.0 | 321 | 22.5 | 357 | 21.4 | 359 | 15.3 |
| North Carolina | 112 | 12.0 | 173 | 12.1 | 190 | 11.4 | 210 | 8.9 |
| Wisconsin | 181 | 19.4 | 257 | 18.0 | 267 | 16.0 | 330 | 14.0 |
| Race/ethnicity | ||||||||
| White, non-Hispanic | 621 | 69.3 | 958 | 70.1 | 1060 | 65.9 | 1388 | 61.4 |
| Black, non-Hispanic | 222 | 24.8 | 272 | 19.9 | 347 | 21.6 | 447 | 19.8 |
| Other, non-Hispanic | 29 | 3.2 | 71 | 5.2 | 88 | 5.5 | 162 | 7.2 |
| Hispanic | 24 | 2.7 | 66 | 4.8 | 113 | 7.0 | 263 | 11.6 |
| Missing | 36 | 62 | 58 | 92 | ||||
| Categorized CoCa | ||||||||
| Any CoC | 414 | 44.4 | 749 | 52.4 | 935 | 56.1 | 2352 | 56.0 |
| Developmental | 386 | 41.4 | 707 | 49.5 | 882 | 52.9 | 1262 | 53.7 |
| Neurologic | 60 | 14.6 | 119 | 8.3 | 152 | 9.1 | 204 | 8.7 |
| Psychiatric | 33 | 3.5 | 80 | 5.6 | 126 | 7.6 | 172 | 7.3 |
| Possibly Causative | 3 | 0.3 | 12 | 0.8 | 13 | 0.8 | 19 | 0.8 |
| CoCs count per childb | ||||||||
| Mean (SD) | 0.70 | 1.0 | 0.94 | 1.20 | 1.00 | 1.20 | 1.02 | 1.2 |
| Interquartile interval, median | 1 | 0 | 2 | 1 | 2 | 1 | 2 | 1 |
| Individual CoC | ||||||||
| ADHD | 88 | 9.4 | 191 | 13.4 | 215 | 12.9 | 320 | 13.6 |
| DD general | 101 | 10.8 | 193 | 21.4 | 255 | 15.3 | 351 | 14.9 |
| DD adaptive | 39 | 4.2 | 105 | 7.4 | 141 | 8.5 | 213 | 9.1 |
| DD motor | 85 | 9.1 | 164 | 11.5 | 213 | 12.8 | 369 | 15.7 |
| DD cognitive | 60 | 6.4 | 106 | 7.4 | 113 | 6.8 | 190 | 8.1 |
| DD play | 5 | 0.5 | 8 | 0.6 | 15 | 0.9 | 19 | 0.8 |
| DD personal/social | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 |
| Language disorder | 173 | 18.6 | 283 | 19.8 | 341 | 20.5 | 562 | 23.4 |
| Learning disability | 13 | 1.4 | 29 | 2.0 | 24 | 1.4 | 41 | 1.7 |
| Sensory Integration Disorder | 28 | 3.0 | 88 | 6.2 | 10.6 | 6.4 | 167 | 7.1 |
| ID | 52 | 5.6 | 66 | 4.6 | 69 | 4.1 | 90 | 3.8 |
| Brain injury | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 |
| Cerebral palsy | 12 | 1.3 | 20 | 1.4 | 17 | 1.0 | 29 | 1.2 |
| Hearing loss | 7 | 0.8 | 11 | 0.8 | 10 | 0.6 | 13 | 0.6 |
| Tourette’s syndrome | 1 | 0.1 | 4 | 0.3 | 2 | 0.1 | 0 | 0.0 |
| Vision impairment | 4 | 0.4 | 15 | 1.1 | 6 | 0.4 | 17 | 0.7 |
| Epilepsy/seizure | 24 | 2.6 | 41 | 2.9 | 47 | 2.8 | 60 | 2.6 |
| Encephalopathy | 20 | 2.2 | 50 | 3.5 | 85 | 5.1 | 109 | 4.6 |
| Anxiety | 10 | 1.1 | 27 | 1.9 | 64 | 3.8 | 86 | 3.7 |
| Emotional disturbance | 8 | 0.9 | 20 | 1.4 | 18 | 1.1 | 38 | 1.6 |
| OCD | 1 | 0.1 | 4 | 0.3 | 4 | 0.2 | 9 | 0.4 |
| ODD | 8 | 0.9 | 23 | 1.6 | 22 | 1.3 | 15 | 0.6 |
| Conduct disorder | 1 | 0.1 | 2 | 0.1 | 1 | 0.1 | 1 | 0.0 |
| Bipolar disorder | 2 | 0.2 | 9 | 0.6 | 9 | 0.5 | 6 | 0.3 |
| Depression | 1 | 0.1 | 7 | 0.5 | 2 | 0.1 | 8 | 0.3 |
| Reactive attachment disorder | 2 | 0.2 | 3 | 0.2 | 0 | 0.0 | 1 | 0.0 |
| Behavioral disorder | 0 | 0.0 | 0 | 0.0 | 13 | 0.8 | 30 | 1.3 |
| Mutism | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 0.1 |
| Mood disorder | 5 | 0.5 | 20 | 1.4 | 28 | 1.7 | 47 | 2.0 |
| Down’s syndrome | 3 | 0.3 | 4 | 0.3 | 10 | 0.6 | 16 | 0.7 |
| Fragile X syndrome | 0 | 0.0 | 8 | 0.6 | 3 | 0.2 | 1 | 0.0 |
| Tuberous sclerosis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 |
ADHD: attention deficit hyperactivity disorder.
CoC: documented non-ASD co-occurring condition.
DD: developmental delay.
ID: intellectual disability.
OCD: obsessive-compulsive disorder.
ODD: oppositional defiant disorder.
Not mutually exclusive categories.
CoC count includes only the DD types and co-occurring diagnoses examined in this paper.
Presence of any CoC increased from 44.4% in SY2002 to 56.0% in SY2010. The most common CoC type was developmental, which increased from SY2002 (41.4%) to SY2010 (53.7%). The most common individual CoCs were DD general (10.8% in SY2002, 14.9% in SY2010), language disorder (18.6% in SY2002, 23.4% in SY2010), DD motor (9.1% in SY2002, 15.7% in SY2010) and ADHD (9.4% in SY2002, 13.6% in SY2010).
Fig. 1 presents predicted counts for documented CoCs in each surveillance year, after controlling for site or site, sex, and race/ethnicity. Estimates were similar between the less adjusted and fully adjusted models, with both models showing statistically significant increasing trends (P < 0.001). When only controlling for site, the average number of CoCs increased from 0.92 per child in SY2002 to 1.03 in SY2010. After additional adjustment for sex and race/ ethnicity, the average number increased from 0.94 in SY2002 to 1.06 in SY2010.
Fig. 1.

Mean count and 95% confidence intervals of co-occurring conditions in 8-year-old children with autism spectrum disorder in Autism and Developmental Disability Monitoring (ADDM) Network surveillance years 2002, 2006, 2008, and 2010.
Note: Site model controls for site because it is a design variable.
Note: Full model controls for site, sex, and race/ethnicity.
P for trend with site adjustment is < 0.001.
P for trend with full model adjustment is < 0.001.
Count includes only the DD types and co-occurring diagnoses examined in this paper.
When adjusting for site alone, the percentage of children with any CoC increased significantly (P < 0.001). There were strong increasing trends in categorized developmental CoCs (P < 0.001) and psychiatric CoCs (P < 0.001) (Table 2). Possibly causative CoCs and individual CoCs that occurred in less than 5% of children were excluded from these analyses due to small sample size (See Table 1). Individual CoCs that increased were general developmental disability (4.1% point increase from 2002 to 2010), language disorder (4.7% point increase from 2002 to 2010), and motor developmental disability (5.1% point increase from 2002 to 2010). A significant decrease was seen in intellectual disability (2.5% point decrease from 2002 to 2010).
Table 2.
Change in percentage of 8-year-old children with autism spectrum disorder and given co-occurring conditions in Autism and Developmental Disability Monitoring (ADDM) Network surveillance years 2002, 2006, 2008, and 2010.
| 2002 | 2006 | 2008 | 2010 | Overall Trenda | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | Δb | CI | Δ | CI | Δ | CI | χ2 | P | |
| Categoryc | |||||||||
| Any CoC | 44.5 | 7.8 | 3.7, 11.9 | 11.2 | 7.3, 15.2 | 11.9 | 8.1, 15.7 | 27.1 | < 0.001 |
| Developmental | 42.6 | 7.8 | 3.8, 11.9 | 11.0 | 7.0, 14.9 | 12.5 | 8.7, 16.2 | 41.1 | < 0.001 |
| Neurological | 6.6 | 1.4 | − 0.5, 3.2 | 1.2 | −0.6, 3.1 | 1.5 | − 0.3, 0.3 | 2.6 | 0.1 |
| Psychiatric | 5.1 | 1.6 | 0.3, 3.1 | 2.7 | 1.2, 4.2 | 2.1 | 0.8, 3.4 | 8.2 | 0.004 |
| Individual CoCs | |||||||||
| ADHD | 9.8 | 4.0 | 1.5, 6.4 | 3.3 | 0.1, 5.7 | 3.8 | 1.6, 6.1 | 10.1 | 0.002 |
| DD general | 10.4 | 2.6 | 0.0, 5.2 | 4.8 | 2.2, 7.3 | 4.1 | 1.7, 6.5 | 10.2 | 0.001 |
| DD adaptive | 7.4 | 0.9 | − 0.2, 2.0 | 0.3 | −0.6, 1.2 | 1.4 | 0.1, 2.7 | 6.1 | 0.01 |
| DD motor | 10.5 | 2.0 | − 0.4, 4.0 | 3.0 | 0.6, 5.4 | 5.1 | 2.7, 7.6 | 15.4 | < 0.001 |
| DD cognitive | 7.7 | −0.6 | − 2.2, 1.1 | − 0.7 | −2.2, 0.8 | 0.2 | − 1.5, 1.8 | 0.04 | 0.9 |
| Language disorder | 18.9 | 1.1 | − 2.0, 4.2 | 1.1 | −2.0, 4.1 | 4.7 | 1.7, 7.7 | 8.9 | 0.003 |
| Sensory integration disorder | 3.4 | 3.1 | 1.4, 4.8 | 3.1 | 1.5, 4.7 | 3.9 | 2.3, 5.4 | 20.1 | < 0.001 |
| ID | 5.9 | −1.0 | − 2.7, 0.7 | − 1.9 | −3.5, −0.2 | −2.5 | −4.2, −0.9 | 10.3 | 0.001 |
| Encephalopathy | 4.0 | 1.4 | − 0.8, 5.9 | 1.6 | −0.8, 4.1 | 1.8 | − 0.7, 4.2 | 0 | 1.0 |
Adjusted for study site.
CoC: documented non-ASD co-occurring condition.
ADHD: attention deficit hyperactivity disorder.
DD: developmental delay.
ID: intellectual disability.
Trend test tests whether all of the changes are equal to 0.
Δ is the difference for given study year compared to 2002.
Categories are not mutually exclusive. Bold indicates statistical signficance at an alpha = 0.05 level.
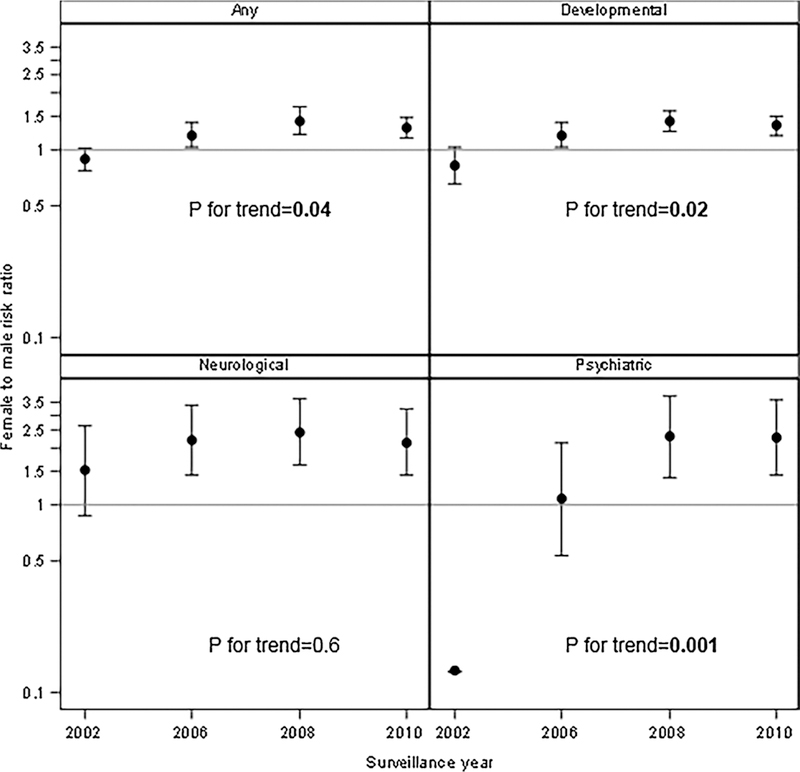
Stratified by sex females had more CoCs in all SY compared to males (1.04 compared to 0.95 in SY 2002, 1.10 to 0.98 in SY2006, 1.23 to 1.06 in SY 2006 and 1.20 to 1.06 in SY 2010), but the change in count over time did not significantly differ between sexes (P = 0.3). RRs comparing females to males and trend for four categorized CoCs are presented in Fig. 2. The trend assess whether SY RRs significantly differ from one another within a CoC category. RRs for any CoC comparing females to males significantly increased over time (P = 0.04), with females initially being less likely to have a CoC in SY2002 (RR = 0.88) to becoming more likely in SY2010 (RR = 1.29). Females were more likely to have developmental CoCs compared to males and this increasing trend in RR over time was statistically significant (P = 0.02). For individuals developmental CoCs with sufficient sample size (DD general, DD adaptive, ID, ADHD, language disability, learning disability, and sensory integration disorder) there were no statistically significant trends. Females were at higher risk of having a neurological CoC in each SY, but these RRs did not change over time (P = 0.6). For psychiatric CoCs, females were at greater risk compared to males and trend significantly increased over time (P < 0.001), although the SY2002 RR had a confidence interval that was very wide (0.013, 0.96).
Fig. 2.
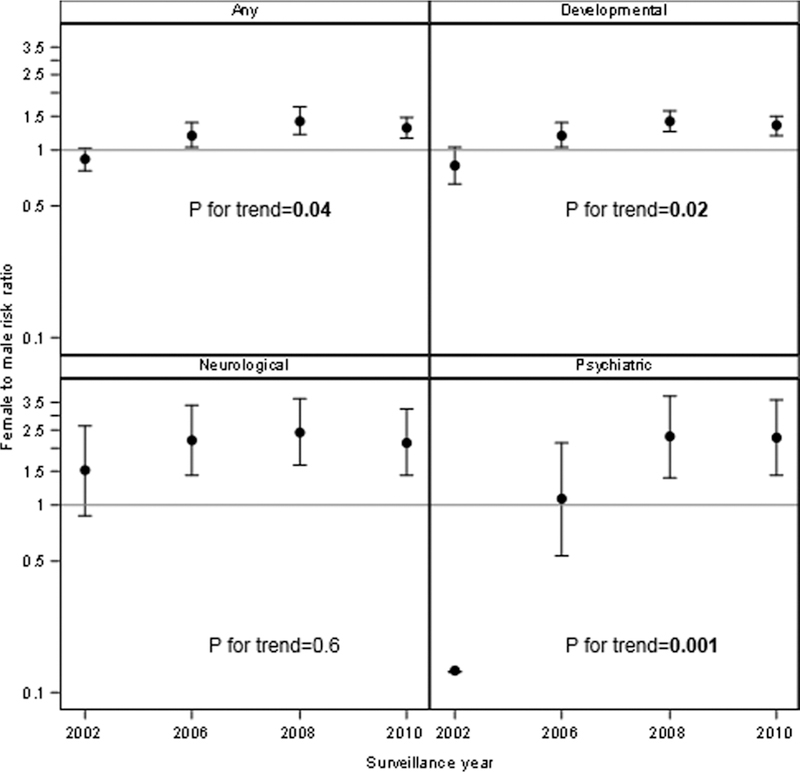
Trends in risk ratios and 95% confidence intervals for categorized co-occurring conditions in 8-year-old children with autism spectrum disorder, comparing females to males in Autism and Developmental Disabilities Monitoring (ADDM) Network surveillance years 2002, 2006, 2008, and 2010.
Note: Confidence limits for 2002 psychiatric are 0.013 to 0.96 (too large to be shown on this graph). Controlling for site and race/ethnicity.
P for trend indicates whether at least one surveillance year RR is statistically different from any of the others.
The average number of CoCs did not differ by race/ethnicity (P = 0.5). Fig. 3 illustrates RRs comparing black non-Hispanic, other non-Hispanic, or Hispanic to white non-Hispanic children in each COC category and the corresponding trend. This trend assessed whether any of the trends in RRs across SYs within a CoC category significantly differed by race. In the psychiatric CoC category, the sample size in the other non-Hispanic group was too small to statistically test in 2002, so 2006 was used as the referent year. The risks for a developmental CoC were slightly higher for black children, children of other race, and Hispanic children compared to white children. When we tested for differences by comparing whether any of the trends over time (RRs comparing white non-Hispanic to black non-Hispanic, other non-Hispanic, or Hispanic over SYs) differed from one another; there was no significant change over time (P = 0.5). White non-Hispanic children were less likely to have any CoCs in all SYs, but there were no changes in CoC count for other race/ethnicities over time (P = 0.3). The only category to meet significance was for neurological CoCs (P = 0.04), which may be driven by a reduction in neurological CoCs among other non-Hispanic children over time. Estimates for neurological CoCs among other non-Hispanic children also became more precise over time. For individual CoCs, those that had sufficient sample size to test had no significant trends (ADHD, DD general, sensory integration disorder, DD language, language disorder and learning disability).
Fig. 3.
Trends in risk ratios and 95% confidence intervals comparing all race/ethnicity groups to the white non-Hispanic referent group for categorized co-occurring conditions in 8-year-old children with autism spectrum disorder in Autism and Developmental Disabilities Monitoring (ADDM) Network surveillance years 2002, 2006, 2008, and 2010.
CoC: documented non-ASD co-occurring condition.
Controlling for site and child sex.
Children with Hispanic ethnicity and psychiatric CoCs were too few in 2002 and a risk ratio could not be estimated.
P for trend evaluates whether trends for race/ethnicity groups statistically differ from one another, within a CoC category.
4. Discussion
To our knowledge, this is the first study to examine temporal trends of CoCs in ASD. Overall, the frequency of CoCs increased slightly, specifically for developmental conditions like ADHD and DD subtypes. This may be the result of relaxation of earlier diagnostic practice that discouraged dual diagnoses of ASD and ADHD (Hanson et al., 2013). The percentage of children with ASD with any CoC increased significantly over the study period. These trends suggest that children with ASD are either presenting with more conditions, having more conditions identified or documented, or clinicians are more likely to give an additional non-ASD diagnosis. Increased utilization of early screening (Radecki, Sand-Loud, O’Connor, Sharp, & Olson, 2011) may impact this observed rise, as children screened early have more time to gain diagnoses before the age of eight.
Although slight (0.08% point increase from 2002 to 2010), the change in average number and type of CoCs may be a sign of changing ASD phenotype. This may be due to a wider diagnostic net and greater awareness of less severe ASD presentations; ADDM has consistently seen a decrease in percentage of children with co-occurring ID (Autism and Developmental Disabilities Monitoring Network 2010 Principal Investigators, 2007, 2014; Autism and Developmental Disabilities Monitoring Network Year 2012 Principal Investigators, 2012; Christensen, Baio et al., 2016; Baio et al., 2018). Additionally, the increase in early intervention programs (Eldevik et al., 2009; Fava & Strauss, 2014; Reichow, Barton, Boyd, & Hume, 2012) might prevent or reduce the development of CoCs like anxiety (Dawson & Burner, 2011; Koegel et al., 2014), language disorders (Brian, Smith, Zwaigenbaum, Roberts, & Bryson, 2016; Dawson & Burner, 2011; Koegel et al., 2014), and DDs (Fava & Strauss, 2014; Gengoux et al., 2015); however, we observed increases for each of these CoCs.
Understanding CoCs may help elucidate etiology of ASD. ADHD and Tourette’s syndrome phenotypes are similar to the ASD phenotype, especially among those children with ASD (Hanson et al., 2013; Kern et al., 2015; Sinzig et al., 2009; Ullebo, Posserud, Heiervang, Obel, & Gillberg, 2012). These CoCs have similar neurological connectivity with long-range under-connectivity and short- range over-connectivity (Kern et al., 2015) and share certain copy number variants that could indicate etiologic origin (Martin et al., 2014). Being that they present differently when paired with ASD, examining the etiologic pathways of these CoCs may provide insight into ASD etiology. Psychiatric CoCs may share similar heritable origins of ASD (Duvekot, van der Ende, Constantino, Verhulst, & Greaves-Lord, 2016; Piven & Palmer, 1999). By focusing on disorders that have this similar etiology, more phenotypically homogenous ASD subgroups can be created (Lai, Lombardo, Chakrabarti, & Baron-Cohen, 2013; Veatch, Veenstra-Vanderweele, Potter, Pericak-Vance, & Haines, 2014) and can be used to better understand ASD presentation. If the increase in ASD prevalence we see over time is associated with a different CoC pattern, it would then be important to study ASD etiology incorporating the biology of the CoCs and craft intervention for these groups.
Females were at higher risk compared to males of having one of the categorized CoCs, with significant increasing trends in developmental and psychiatric conditions. This may be an indication that females with ASD are more likely than males to be identified when presentation is more severe, meaning more CoCs like ID (Carter et al., 2007; Dworzynski, Ronald, Bolton, & Happe, 2012; Hiller, Young, & Weber, 2015; Kirkovski, Enticott, & Fitzgerald, 2013; Rubenstein et al., 2015) or that females are getting evaluated for ASD later than males and clinicians are giving non-ASD diagnoses to try and explain symptoms (Begeer et al., 2013; Giarelli et al., 2010). There is increased effort to better understand the female ASD phenotype (Halladay et al., 2015; Lai, Lombardo, Auyeung, Chakrabarti, & Baron-Cohen, 2015), which could lead to more recognition, diagnosis, and increase in services for females.
We found few differences in CoCs when comparing race/ethnicities, with the only significant difference being a slight elevation in prevalence of for neurological conditions for other non-Hispanic children compared to white non-Hispanic children. Our results suggest that there may not be racial/ethnic disparity in how CoCs are documented in children with who have ASD. Past studies have shown that non-white children with ASD were less likely to receive specialty care (gastrointestinal clinic, neurology, psychology/psychiatry) (Broder-Fingert, Shui, Pulcini, Kurowski, & Perrin, 2013) and that there are racial/ethnic disparities in ASD identification. An earlier study analyzed ADDM SY2002 data and reported that black, Hispanic, and children of other race were less likely to have a formal documentation of ASD diagnosis in their school or medical records by age 8 compared to white non-Hispanic children (Mandell et al., 2009). The study found that minority children who met the diagnostic criteria for ASD were less likely to have been previously diagnosed than white children; and that those with ID (IQ < 70) were more likely to have been diagnosed with ASD. Black children were less likely to have been diagnosed with ASD whether or not they had ID. It will be important to continue to monitor these trends to evaluate the extent of racial/ethnic disparity and how it is associated with diagnostic practice and CoCs.
This study had some limitations. We used cross-sectional surveillance data, which prevent us from examining how children received diagnoses longitudinally. CoCs may be recorded better in more recent years, and that children from earlier SYs may be more likely to have incomplete records. Additionally, we extracted CoC data from abstracted records, which prevents us from identifying undocumented conditions. We included most CoCs that were documented but excluded CoCs that only were present in one child, which may lead to a slight underestimation in average CoC count. Further, we assumed that any CoCs truly co-occurred with ASD and were not conditions that were incorrectly diagnosed, possibly to explain ASD traits. We believe that this is a valid assumption since many conditions may be used to explain ASD traits prior to ASD diagnosis are conditions that often co-occur with ASD (for example, ID, ADHD) (American Psychiatric Association, 2013; Daniels & Mandell, 2014). This study was limited to eight sites that consistently had relevant data and is not a nationally representative sample. Although each site’s data are population-based, results may not be generalizable to the entire U.S. population. In this study we did not assess temporality of CoCs, that is, whether they occurred before or after ASD diagnosis. This may have some implication for how CoCs affect the diagnostic process. This question was outside the scope of this work and will need to be explored in future studies.
This study has several strengths. We were able to utilize a large population-based set of 8-year-old children with ASD to identify trends in categorized and individual CoCs. All data included in this study were collected by ADDM where all participating sites implement a standardized data collection protocol in the same communities over four surveillance years, allowing us to perform a tread analysis with less confounding.
5. Conclusion
This study examined trends in CoCs in a sample of 8-year-old children with ASD. We found that average number of conditions have increased slightly, mainly for developmental conditions. The percentage of children with any CoC has increased, suggesting that either the ASD phenotype has changed or clinicians are more apt to give diagnoses of CoCs. We found that females were more likely to have developmental or psychiatric CoCs as compared to males, and that the female-male risk ratios for these CoCs increased over time. It is important to monitor diagnostic trends of CoCs to ensure timely and correct diagnosis for children with ASD. Future research should assess the relationship between the timing of receiving an ASD diagnosis and a CoC.
What this paper adds.
We found that average number of co-occurring medical conditions (CoCs) have increased slightly over time in a surveillance system of children aged 8 years old with autism spectrum disorder (ASD). The increase was mainly driven by more developmental condition diagnoses. The percentage of children with any CoC has increased, suggesting that either the ASD phenotype has changed or clinicians are more apt to give diagnoses of CoCs. We found that females were more likely to have developmental or psychiatric CoCs as compared to males, and that the female-male risk ratios for these CoCs increased over time. It is important to monitor diagnostic trends of CoCs to ensure timely and correct diagnosis for children with ASD while reducing sex or race related disparity.
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network 2010 Principal Investigators (2007). Prevalence of autism spectrum disorders-autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR: Surveillance Summaries, 56, 1–11. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network 2010 Principal Investigators (2014). Prevalence of autism spectrum disorder among children aged 8 years — Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveillance Summaries, 63, 1–22. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Year 2012 Principal Investigators (2012). Prevalence of autism spectrum disorders: Autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Morbidity and Mortality Weekly Report. Surveillance Summaries, 61(3), 1–19. [PubMed] [Google Scholar]
- Baird G, Charman T, Pickles A, Chandler S, Loucas T, Meldrum D, et al. (2008). Regression, developmental trajectory and associated problems in disorders in the autism spectrum: The SNAP study. Journal of Autism and Developmental Disorders, 38, 1827–1836. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins LD, Christensen DL, et al. (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morb. Mortal. Wkfy. Rep. Surveill Summ. 67(6), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, et al. (2013). Sex differences in the timing of identification among children and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43, 1151–1156. [DOI] [PubMed] [Google Scholar]
- Bradley EA, & Isaacs BJ (2006). Inattention, hyperactivity, and impulsivity in teenagers with intellectual disabilities, with and without autism. The Canadian Journal of Psychiatry, 51, 598–606. [DOI] [PubMed] [Google Scholar]
- Brian JA, Smith IM, Zwaigenbaum L, Roberts W, & Bryson SE (2016). The Social ABCs caregiver-mediated intervention for toddlers with autism spectrum disorder: Feasibility, acceptability, and evidence of promise from a multisite study. Autism Research, 9, 899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder-Fingert S, Shui A, Pulcini CD, Kurowski D, & Perrin JM (2013). Racial and ethnic differences in subspecialty service use by children with autism. Pediatrics, 132, 94–100. [DOI] [PubMed] [Google Scholar]
- Buescher AVS, Cidav Z, Knapp M, & Mandell D (2014). Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatrics, 168, 721–728. [DOI] [PubMed] [Google Scholar]
- Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, & Tager-Flusberg H (2007). Sex differences in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37, 86–97. [DOI] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, & Baird G (2011). IQ in children with autism spectrum disorders: Data from the Special Needs and Autism Project (SNAP). Psychological Medicine, 41, 619–627. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, et al. (2016). Prevalence and characteristics of autism spectrum disorder among children aged 8 years-Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR: Surveillance Summaries, 65, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Bilder DA, Zahorodny W, Pettygrove S, Durkin MS, Fitzgerald RT, et al. (2016). Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the autism and developmental disabilities monitoring network. Journal of Developmental and Behavioral Pediatrics, 37, 1–8. [DOI] [PubMed] [Google Scholar]
- Close HA, Lee LC, Kaufmann CN, & Zimmerman AW (2012). Co-occurring conditions and change in diagnosis in autism spectrum disorders. Pediatrics, 129, e305–e316. [DOI] [PubMed] [Google Scholar]
- Coo H, Ouellette-Kuntz H, Lloyd JEV, Kasmara L, Holden JJA, & Lewis MES (2007). Trends in autism prevalence: Diagnostic substitution revisited. Journal of Autism and Developmental Disorders, 38, 1036–1046. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Hoogstrate J, & Selvin S (2002). The changing prevalence of autism in California. Journal of Autism and Developmental Disorders, 32, 207–215. [DOI] [PubMed] [Google Scholar]
- Daniels AM, & Mandell DS (2014). Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism, 18, 583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NO, & Kollins SH (2012). Treatment for co-occurring attention deficit/hyperactivity disorder and autism spectrum disorder. Neurotherapeutics, 9, 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, & Burner K (2011). Behavioral interventions in children and adolescents with autism spectrum disorder: A review of recent findings. Current Opinion in Pediatrics, 23, 616–620. [DOI] [PubMed] [Google Scholar]
- Duvekot J, van der Ende J, Constantino JN, Verhulst FC, & Greaves-Lord K (2016). Symptoms of autism spectrum disorder and anxiety: Shared familial transmission and cross-assortative mating. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57, 759–769. [DOI] [PubMed] [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, & Happe F (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? Journal of the American Academy of Child and Adolescent Psychiatry, 51, 788–797. [DOI] [PubMed] [Google Scholar]
- Eldevik S, Hastings RP, Hughes JC, Jahr E, Eikeseth S, & Cross S (2009). Meta-analysis of Early Intensive Behavioral Intervention for children with autism. Journal of Clinical Child and Adolescent Psychology, 38, 439–450. [DOI] [PubMed] [Google Scholar]
- Fava L, & Strauss K (2014). Response to Early Intensive Behavioral Intervention for autism—An umbrella approach to issues critical to treatment individualization. International Journal of Developmental Neuroscience, 39, 49–58. [DOI] [PubMed] [Google Scholar]
- Fountain C, King MD, & Bearman PS (2011). Age of diagnosis for autism: Individual and community factors across 10 birth cohorts. Journal of Epidemiology and Community Health, 65, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette P, Dodds L, MacPherson K, Flowerdew G, Hennen B, & Bryson S (2013). Factors affecting the age at diagnosis of autism spectrum disorders in Nova Scotia, Canada. Autism, 17, 184–195. [DOI] [PubMed] [Google Scholar]
- Gengoux GW, Berquist KL, Salzman E, Schapp S, Phillips JM, Frazier TW, et al. (2015). Pivotal response treatment parent training for autism: Findings from a 3-month follow-up evaluation. Journal of Autism and Developmental Disorders, 45, 2889–2898. [DOI] [PubMed] [Google Scholar]
- Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, et al. (2010). Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disability and Health Journal, 3, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney JG, McPheeters ML, & Davis MM (2006). Parental report of health conditions and health care use among children with and without autism. Archives of Pediatrics and Adolescent Medicine, 160, 825. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. (2015). Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Molecular Autism, 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, Cerban BM, Slater CM, Caccamo LM, Bacic J, & Chan E (2013). Brief report: Prevalence of attention deficit/hyperactivity disorder among individuals with an autism spectrum disorder. Journal of Autism and Developmental Disorders, 43, 1459–1464. [DOI] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2015). Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism, 20, 75–84. [DOI] [PubMed] [Google Scholar]
- Horlin CC, Falkmer M, Parsons R, Albrecht MA, & Falkmer T (2014). The cost of autism spectrum disorders. PLoS One, 9, e106552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VW (2012). Subphenotype-dependent disease markers for diagnosis and personalized treatment of autism spectrum disorders. Disease Markers, 33, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, & Barbaresi WJ (2009). Incidence of gastrointestinal symptoms in children with autism: A population-based study. Pediatrics, 124, 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Geier DA, King PG, Sykes LK, Mehta JA, & Geier MR (2015). Shared brain connectivity issues, symptoms, and comorbidities in autism spectrum disorder, attention deficit/hyperactivity disorder, and Tourette syndrome. Brain Connectivity, 5, 321–335. [DOI] [PubMed] [Google Scholar]
- King M, & Bearman P (2009). Diagnostic change and the increased prevalence of autism. International Journal of Epidemiology, 38, 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkovski M, Enticott PG, & Fitzgerald PB (2013). A review of the role of female gender in autism spectrum disorders. Journal of Autism and Developmental Disorders, 43, 2584–2603. [DOI] [PubMed] [Google Scholar]
- Koegel LK, Koegel RL, Ashbaugh K, & Bradshaw J (2014). The importance of early identification and intervention for children with or at risk for autism spectrum disorders. International Journal of Speech-Language Pathology, 16, 50–56. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, & Baron-Cohen S (2015). Sex/gender differences and autism: Setting the scene for future research. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, & Baron-Cohen S (2014). Autism. Lancet, 383, 896–910. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Chakrabarti B, & Baron-Cohen S (2013). Subgrouping the autism “spectrum”: Reflections on DSM-5. PLoS Biology, 11, e1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Regester A, Lauderdale S, Ashbaugh K, & Haring A (2010). Treatment of anxiety in autism spectrum disorders using cognitive behaviour therapy: A systematic review. Developmental Neurorehabilitation, 13, 53–63. [DOI] [PubMed] [Google Scholar]
- Lavelle TA, Weinstein MC, Newhouse JP, Munir K, Kuhlthau KA, & Prosser LA (2014). Economic burden of childhood autism spectrum disorders. Pediatrics, 133, e520–e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SE, Giarelli E, Lee LC, Schieve LA, Kirby RS, Cunniff C, et al. (2010). Autism spectrum disorder and co-occurring developmental, psychiatric, and medical conditions among children in multiple populations of the United States. Journal of Developmental and Behavioral Pediatrics, 31, 267–275. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Novak MM, & Zubritsky CD (2005). Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics, 116, 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Wiggins LD, Carpenter LA, Daniels J, DiGuiseppi C, Durkin MS, et al. (2009). Racial/ethnic disparities in the identification of children with autism spectrum disorders. American Journal of Public Health, 99, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Cooper M, Hamshere ML, Pocklington A, Scherer SW, Kent L, et al. (2014). Biological overlap of attention-deficit/hyperactivity disorder and autism spectrum disorder: Evidence from copy number variants. Journal of the American Academy of Child and Adolescent Psychiatry, 53, 761–770 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maski KP, Jeste SS, & Spence SJ (2011). Common neurological co-morbidities in autism spectrum disorders. Current Opinion in Pediatrics, 23, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, & Calhoun SL (2011). Impact of IQ, age, SES, gender, and race on autistic symptoms. Research in Autism Spectrum Disorders, 5, 749–757. [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. (2007). The epidemiology of autism spectrum disorders. Annual Review of Public Health, 28, 235–258. [DOI] [PubMed] [Google Scholar]
- Piven J, & Palmer P (1999). Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. American Journal of Psychiatry, 156, 557–563. [DOI] [PubMed] [Google Scholar]
- Prentice RL, & Mason MW (1986). On the application of linear relative risk regression models. Biometrics, 42, 109–120. [PubMed] [Google Scholar]
- Radecki L, Sand-Loud N, O’Connor KG, Sharp S, & Olson LM (2011). Trends in the use of standardized tools for developmental screening in early childhood: 2002–2009. Pediatrics, 128, 14–19. [DOI] [PubMed] [Google Scholar]
- Reaven JA (2009). Children with high-functioning autism spectrum disorders and co-occurring anxiety symptoms: Implications for assessment and treatment. Journal for Specialists in Pediatric Nursing, 14, 192–199. [DOI] [PubMed] [Google Scholar]
- Reichow B, Barton EE, Boyd BA, & Hume K (2012). Early intensive behavioral intervention (EIBI) foryoung children with autism spectrum disorders (ASD). The Cochrane Database of Systematic Reviews, 10, CD009260. [DOI] [PubMed] [Google Scholar]
- Rice CE, Baio J, Van Naarden Braun K, Doernberg N, Meaney FJ, Kirby RS, et al. (2007). A public health collaboration for the surveillance of autism spectrum disorders. Paediatric and Perinatal Epidemiology, 21, 179–190. [DOI] [PubMed] [Google Scholar]
- Rubenstein E, Wiggins LD, & Lee LC (2015). A review of the differences in developmental, psychiatric, and medical endophenotypes between males and females with autism spectrum disorder. Journal of Developmental and Physical Disabilities, 27, 119–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc (2011). SAS/STAT Software, version 9.3.
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, et al. (2009). Timing of identification among children with an autism spectrum disorder: Findings from a population-based surveillance study. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Walter D, & Doepfner M (2009). Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome? Journal of Attention Disorders, 13, 117–126. [DOI] [PubMed] [Google Scholar]
- Ullebo AK, Posserud MB, Heiervang E, Obel C, & Gillberg C (2012). Prevalence of the ADHD phenotype in 7- to 9-year-old children: Effects of informant, gender and non-participation. Social Psychiatry and Psychiatric Epidemiology, 47, 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Naarden Braun K, Christensen D, Doernberg N, Schieve L, Rice C, Wiggins L, et al. (2015). Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PloS One, 10, e0124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch OJ, Veenstra-Vanderweele J, Potter M, Pericak-Vance MA, & Haines JL (2014). Genetically meaningful phenotypic subgroups in autism spectrum disorders. Genes, Brain, and Behavior, 13, 276–285. [DOI] [PubMed] [Google Scholar]
- Warren Z, Stone W, & Humberd Q (2009). A training model for the diagnosis of autism in community pediatric practice. Journal of Developmental and Behavioral Pediatrics, 30, 442–446. [DOI] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, & Scahill L (2009). Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review, 29, 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu Q, Liu J, Li SC, & Xu X (2012). Risk factors for autistic regression: Results of an ambispective cohort study. Journal of Child Neurology, 27, 975–981. [DOI] [PubMed] [Google Scholar]


