Abstract
Little is known about whether the relationship between hypertension and ischemic stroke differs by sex. We examined sex differences in the association between hypertension severity and treatment and ischemic stroke risk. We used a longitudinal cohort study in the continental United States, with oversampling of black individuals and those living in the stroke belt. We included 26,461 participants recruited from 2003–2007 without prevalent stroke at baseline. The main outcome was incident ischemic stroke ascertained by telephone surveillance (with physician adjudication for suspected events). Proportional hazards regression was used to assess the sex-specific association between systolic blood pressure and stroke and between classes of antihypertensive medications and stroke after adjustment for age, race, sex, and age-by-race and sex-by-treatment interaction terms. A priori, p< 0.10 was considered significant for interactions. Among participants (55.4% women, 40.2% black), there were 1084 confirmed ischemic stroke events. In the adjusted model, the risk of stroke per each level of hypertension (referent/ systolic blood pressure <120 mm Hg/ 120–129 mm Hg/ 130–139 mm Hg/ >140 mm Hg) was higher in women (HR 1.25, 95% CI 1.16–1.34) than men (HR 1.14, 95%CI 1.05–1.23) (sex-systolic blood pressure interaction term, p = 0.09). Compared to no medications, with each additional class of medications, stroke risk increased by 23% (HR 1.23, 95%CI 1.14–1.33) for women and 21% (HR 1.21, 95%CI 1.12–1.31) for men (p=0.79). Further work on the biological mechanisms for sex differences in stroke risk associated with hypertension severity and a need for sex-specific clinical guidelines may be warranted.
Keywords: stroke, hypertension, women’s health, primary prevention, sex differences, sex-specific
INTRODUCTION
There are substantial sex differences in age-adjusted stroke incidence,1,2 stroke prevalence,1,2 and in the prevalence and risk associated with cardiometabolic factors including hypertension, atrial fibrillation, diabetes, and smoking.3 An improved understanding of these differences is needed to ensure that stroke prevention strategies are effective for both women and men. Despite higher age-adjusted stroke rates in men in many age categories, women have more strokes and more deaths from stroke than men over the lifetime,1,4–6 thought to be largely due to differences in life expectancy.7 Conflicting data exist in the literature concerning the incidence of stroke in recent years between women and men. While some work suggests that stroke incidence has been decreasing faster in men than women from 1993 to 2010,8 others have shown similar rates of decline in stroke incidence for men and women.9,10 One potential contributor to sex differences in stroke incidence over time and over the lifespan is a sex difference in the way co-morbidities such as hypertension affect stroke risk.3
Hypertension, the most common modifiable stroke risk factor, is known to differ in prevalence, rates of control, and degree of associated stroke risk between women and men. The prevalence of hypertension differs by sex; prevalence is lower in women than men under 60 years of age but higher in women than men after that time point.11 Control of blood pressure (BP) also differs by sex and across the lifespan, as women in older age groups are less likely to have their hypertension controlled compared with men.11,12 Finally, data on the degree of stroke risk associated with hypertension conflict to some extent, with some data showing similar estimates of the association between increasing hypertension and stroke risk13–15 and other showing potentially differing estimates between hypertension and stroke by sex.16 Race and ethnicity play an important role in hypertension prevalence and control as well. The overall age-adjusted prevalence of hypertension is higher in black women than all other race/gender subgroups, and control of hypertension is lower among non-white and Hispanic women compared to white, Non-Hispanic women.11 Previous work in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study found a sex difference in the association of systolic blood pressure (SBP) and use of antihypertensive medications with risk of ischemic stroke in whites but not blacks.17 Finally, though it is well known that hypertension is also a risk factor for hemorrhagic stroke, the focus of the current paper is on ischemic stroke given the significant differences in pathophysiology between ischemic and hemorrhagic strokes as well as low numbers of hemorrhagic strokes in the cohort.
The primary objective of our study was to determine whether there are sex differences in the association between hypertension severity or the treatment of hypertension and risk of ischemic stroke (IS). We also aimed to determine whether sex differences in the association between hypertension severity and stroke risk differ across racial subgroups.
METHODS
Data and Materials Statement
Data and materials can be requested by contacting the REGARDS policies and procedures committee at regardsadmin@uab.edu.
Study Population
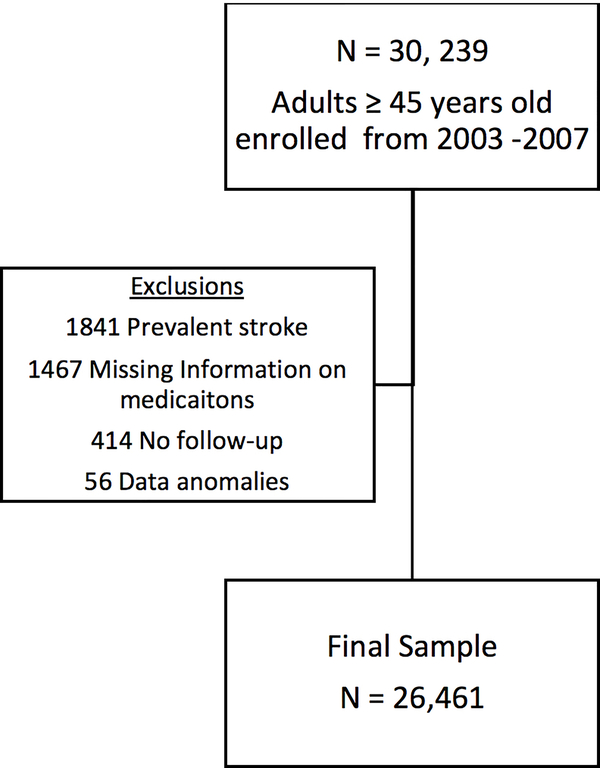
The study population included participants in the REGARDS study, a prospective, national, longitudinal cohort study of 30,239 non-Hispanic black and white adults recruited between January 2003 and October 2007. Adults aged 45 years and older were enrolled from across the continental U.S., with oversampling of black individuals and those living in the stroke belt (North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Arkansas and Louisiana). Further details of REGARDS methodology can be found elsewhere.18 For the current analysis, we included data from 26,461 participants after excluding 1841 due to prevalent stroke at baseline, 1467 due to missing data on medication inventory/ inability to determine number of anti-hypertensive medications, 414 with no follow-up, and 56 because of data anomalies (Figure 1).
Figure 1:
Study Sample
Collection of Exposure and Outcome Data/ Definitions of Exposure and Outcome
Baseline data on patient demographics and use of anti-hypertensive medications were collected through a telephone interview followed by an in-home visit. A physical exam including BP measurements was also completed during the in-home visit. Mean systolic blood pressure (SBP) was calculated from 2 consecutive measurements on the same arm, taken by a trained technician after the patient had been sitting quietly for at least 5 minutes. The protocol for measuring SBP was standardized, and measurements were taken using an aneroid sphygmomanometer that was regularly tested. Most (91%) of the blood pressure measurements were generally taken between 7 AM and 12 noon. Blood pressure measurements were taken in the left arm unless not possible, and a large cuff was used for participants with arm circumference greater than 13 inches. For actual measurements, the cuff was inflated 20 mmHg above the pulse obliteration level and then deflated at a rate of approximately 2mm Hg/second. Throughout the study, blood pressure quality control was monitored centrally for potential digit preference, and study staff were retrained as needed. Surveillance for suspected IS events, the primary outcome in this study, was conducted via computer assisted telephone interviews that occurred every 6 months. Medical records for suspected stroke events were retrieved, and possible events were adjudicated by trained study physicians. Based on review of the medical record including imaging results, physicians first adjudicated suspected events as cases or not cases and then subtyped the cases into ischemic or hemorrhagic. Based on the World Health Organization (WHO), the definition of stroke is “rapidly developing clinical signs of focal, at times global, disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin.” Strokes that didn’t meet the WHO definition but that met REGARDS clinical criteria were also included (symptoms suggestive of stroke with unconfirmed duration or inconclusive imaging; or imaging definitive for stroke without typical symptoms). Those that were not primarily hemorrhagic based on physician review of medical record and imaging results were categorized as ischemic. Only IS were included in this analysis. Additional details of the adjudication process can be found in prior publications.19
Our primary exposure variables were sex, strata of hypertension severity, and number of classes of anti-hypertensive medications that participants reported at the index home visit. Hypertension severity was assessed in two ways, as a continuous variable and as an ordinal variable based on the 2017 American College of Cardiology (ACC)/ American Heart Association (AHA) Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults as follows: normotensive (<120 mm Hg) (reference group)), elevated BP (120–129 mm Hg), Stage 1 hypertension (130–139 mm Hg), and Stage 2 hypertension (>140 mm Hg).20 Medications reported by participants at the baseline visit were classified as anti-hypertensives by trained research staff and categorized into the following classes: angiotensin-converting enzyme inhibitors, aldosterone antagonists, α-blockers, angiotensin II receptor blockers, β-blockers, calcium channel blockers, central agonists, diuretics, or vasodilators. Participants who reported not taking any antihypertensive medications were the reference group, and the other categories were 1, 2, or 3 or more classes of anti-hypertensive medications. The study was approved by the institutional review board of each institution.
Data Analysis
Descriptive statistics (frequencies, proportions) were used to compare basic demographics (age, race) by sex. Descriptive statistics (frequencies, proportions, means with standard deviations as appropriate) were also used to determine the prevalence of each stratum of hypertension within sex and race subgroups.
Proportional hazards regression was used to determine both the association of the level of hypertension severity (normal, elevated, stage 1, or stage 2) and the number of classes of anti-hypertensive medications with incident IS. Two product terms were included in the model: Sex-by-SBP category and number of medications-by-SBP category. Models were also adjusted for age, race, and an age-by-race interaction term, which has been shown to be significant in prior REGARDS studies.19 Sex-specific estimates of stroke risk were calculated for each stratum of hypertension severity and number of classes of anti-hypertensive medications. Within each sex, trends in stroke risk across increasing BP severity strata and across increasing number of medication classes were tested for significance. In addition, effect estimates for stroke risk between sexes were compared by testing for heterogeneity. As a sensitivity analysis, models were further adjusted for the remaining Framingham stroke risk factors given that they may be confounders of the association between SBP and stroke: history of diabetes, current atrial fibrillation, current smoking, and prevalent cardiovascular disease. Though it is a Framingham risk factor, left ventricular hypertrophy was not included in the model as it is likely a consequence of SBP and therefore not a confounder.
These analyses were repeated with SBP as a continuous variable to determine the sex-specific increase in stroke risk with 10 mmHg incremental increases in SBP, adjusting for age, race, and an age-by-race interaction term. Finally, proportional regression analyses were repeated following stratification by race to determine whether sex-specific associations between hypertension severity, number of anti-hypertensive medications, and incident IS differed between black and white participants after adjusting for age.
With regard to censoring, 1084 had a stroke endpoint, and 25,377 were censored. Of these, 5505 died of another cause prior to having a stroke event (and were censored at the time of their death), 5831 had withdrawn from the study (and were censored at the last contact where they were known to be stroke-free), 14040 were considered to be in active follow-up (also censored at their last stroke-free contact), and 1 participant had a follow-up status in review.
For all models, adjusted hazard ratios with 95% CI were reported separately for women and men. Chosen a priori, an alpha of 0.05 was used for main effects. As per prior REGARDS analyses, an alpha of 0.10 was chosen a priori for tests of interaction in order to reduce the likelihood of missing a significant interaction term and reporting averaged associations for men and women when the true associations differ by sex. In addition, compared with main effects, detecting group differences using interaction terms require much larger sample sizes; choosing alpha values that are slightly higher may partially compensate for this issue.
RESULTS
Of 26,461 participants included in the analysis, 55.4% (n=14668) were women, and 40.2% (n=10644) were black. Mean age was similar between women and men (64.2 (9.5) versus 65.5 (9.3) years). Over a mean follow-up period of 8.7 +/− 3.6 years, there were 1084 incident IS events. Table 1 shows SBP (as mean (SD) and by categories) along with the number of IS events in each demographic group. Compared with men, there was a higher proportion of women participants in the normotensive group and slightly lower proportions in the Stage 1 and Stage 2 hypertensive groups.
Table 1:
Systolic Blood Pressure and Stroke Outcomes, by Sex and Race Categories
| Characteristic | Women (n=14,668) | Men (n=11,793) | ||||
|---|---|---|---|---|---|---|
| All (n=14668) N (%) | Black (n=6674) N (%) | White (n=7994) N (%) | All (n=11793) N (%) | Black (n=3970) N (%) | White (n=7823) N (%) | |
| Systolic blood pressure (mm Hg) (mean, SD) | 126.0 (16.8) | 129.5 (17.2) | 123.0 (15.8) | 128.5 (15.9) | 131.5 (16.7) | 126.9 (15.3) |
| Systolic Blood Pressure (mm Hg) | ||||||
| Normotensive (<120) | 5121 (34.9) | 1793 (26.9) | 3328 (41.6) | 3328 (28.2) | 860 (21.7) | 2468 (31.5) |
| Elevated (120–129) | 3985 (27.2) | 1774 (26.6) | 2211 (27.6) | 3277 (27.8) | 1025 (25.8) | 2252 (28.8) |
| Stage 1 (130–139) | 2834 (19.3) | 1492 (22.3) | 1342 (16.8) | 2653 (22.5) | 985 (24.8) | 1668 (21.3) |
| Stage 2 (>140) | 2728 (18.6) | 1615 (24.2) | 1113 (13.9) | 2535 (21.5) | 1100 (27.7) | 1435 (18.3) |
| Ischemic stroke events | 542 | 269 | 273 | 542 | 179 | 363 |
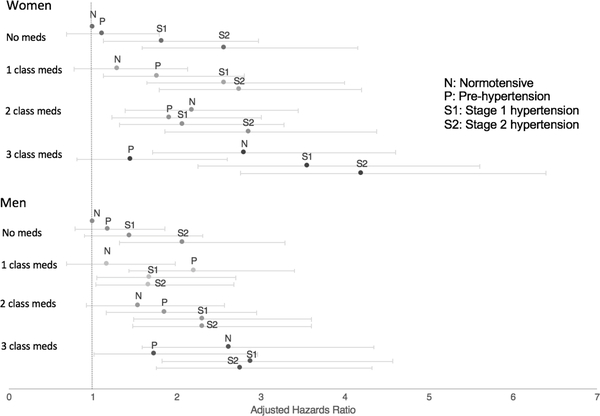
Table 2 displays sex-specific estimates of the risk of IS associated with increasing SBP by category, stratified by number of classes of antihypertensive medications; information is displayed graphically in Figure 2. For both sexes, in all strata of number of anti-hypertensive medications, the IS risk increased with increasing SBP. Effect sizes, however, were often greater for women than men, most clearly demonstrated by higher risk estimates in 3 of 4 strata of number of classes of antihypertensives. For example, for those participants on 3 classes of medications, the average increase in IS risk per BP level was 1.26 (95% CI 1.07–1.47) for women and 1.07 (95% CI 0.91–1.26) for men (p=0.18). Average risk increases were not significantly different by sex in individual strata. The overall pooled effect estimate for women vs. men (1.25 (95% CI 1.16–1.34) vs. 1.14 (95%CI 1.05–1.23)), was significant at p=0.09 (an alpha of 0.10 was chosen a priori for tests of interaction). When SBP was treated as a continuous variable, women had higher risk of stroke per 10 mm Hg increase in SBP as indicated by larger effect estimates (1.15, 95%CI 1.10–1.20, vs. 1.08, 95%CI 1.03–1.14, P=0.09). The data in Table 2 also show increasing stroke risk with increasing numbers of anti-hypertensive medications in all strata of SBP. Pooled effect estimates of the average increase in stroke risk per increase in number of medications were similar by sex (1.23, 95%CI 1.14–1.33 for women, 1.21 95%CI 1.12–1.31 for men, p=0.79).
Table 2:
Sex-specific Hazard Ratios Demonstrating Association between Increasing Systolic Blood Pressure, Number of Classes of Blood Pressure Medications, and Risk of Ischemic Stroke
| Systolic blood pressure | No Medications | 1 class of medications | 2 classes of medications | 3 classes of medications | Change per class, and p-value for effect modification by sex | Pooled effect of medications,* and p-value for effect modification by sex | |
|---|---|---|---|---|---|---|---|
| Normal | W | 1.00 (ref) | 1.29 (0.78 – 2.13) | 2.18 (1.38 – 3.44) | 2.80 (1.71 – 4.60) | 1.43 (1.23 – 1.67) | W: 1.23 (1.14 – 1.33) M: 1.21 (1.12 – 1.31) P = 0.79 |
| M | 1.00 (ref) | 1.17 (0.68 – 1.98) | 1.54 (0.92 – 2.56) | 2.62 (1.59 – 4.34) | 1.36 (1.15 – 1.60) | ||
| P = 0.64 | |||||||
| Elevated | W | 1.11 (0.69 – 1.79) | 1.77 (1.12 – 2.80) | 1.91 (1.22 – 3.00) | 1.45 (0.81 – 2.60) | 1.13 (0.95 – 1.34) | |
| M | 1.18 (0.75 – 1.85) | 2.20 (1.43 – 3.40) | 1.85 (1.16 – 2.95) | 1.73 (1.01 – 2.96) | 1.14 (0.98 – 1.32) | ||
| P = 0.96 | |||||||
| Stage 1 | W | 1.82 (1.12 – 2.97) | 2.56 (1.64 – 3.99) | 2.07 (1.31 – 3.27) | 3.55 (2.25 – 5.60) | 1.19 (1.01 – 1.40) | |
| M | 1.44 (0.90 – 2.31) | 1.68 (1.05 – 2.70) | 2.31 (1.48 – 3.60) | 2.88 (1.82 – 4.57) | 1.27 (1.10 – 1.48) | ||
| P = 0.54 | |||||||
| Stage 2 | W | 2.56 (1.58 – 4.15) | 2.74 (1.79 – 4.19) | 2.86 (1.86 – 4.38) | 4.20 (2.76 – 6.39) | 1.18 (1.02 – 1.37) | |
| M | 2.07 (1.31 – 3.28) | 1.66 (1.03 – 2.68) | 2.30 (1.47 – 3.60) | 2.75 (1.75 – 4.32) | 1.13 (0.97 – 1.31) | ||
| P = 0.68 | |||||||
| Change per class | W | 1.38 (1.18 – 1.62) | 1.29 (1.11 – 1.49) | 1.10 (0.95 – 1.28) | 1.26 (1.07 – 1.47) | ||
| P-value for effect modification by sex | M | 1.27 (1.09 – 1.47) | 1.06 (0.90 – 1.23) | 1.14 (0.99 – 1.33) | 1.07 (0.91 – 1.26) | ||
| P = 0.44 | P = 0.069 | P = 0.72 | P = 0.18 | ||||
| Pooled effect of BP† | W | 1.25 (1.16 – 1.34) | |||||
| M | 1.14 (1.05 – 1.23) | ||||||
| P = 0.091 | |||||||
W: Women; M: men; BP: blood pressure; Effect estimates are hazard ratios adjusted by age, race, age-by-race interaction term.
Across strata of BP, 3-degree freedom test for differences within sex, p=0.15 (women), p=0.28 (men)
Across strata of medication classes, 3-degree freedom test for differences within sex p = 0.22 (women), P=0.31 (men)
Figure 2:
Sex-specific Associations between Increasing Systolic Blood Pressure by Category, Number of Classes of Blood Pressure Medications, and Risk of Ischemic Stroke. Within each grouping of the number of medications, the four data points correspond to normotensive (<120 mmHg), pre-hypertension (120–129 mmHg), stage 1 (130–139 mm Hg) and stage 2 (>140 mm Hg) blood pressure strata. (Hazard ratios adjusted by age, race, and age-by-race interaction term)
Supplemental tables S1 and S2 (online supplement) show the sex-specific effects of increasing hypertension severity by strata of hypertension across strata of number of anti-hypertensives in blacks and white participants separately. Among black participants, the pooled effect estimate of increased stroke risk per increasing level of hypertension severity was 1.26 for women (95%CI 1.13–1.41) and 1.19 for male participants (95%CI 1.03–1.36, p=0.51) (Table S1). Among white participants, the pooled effect estimate of increased stroke risk per increasing level of hypertension severity was 1.24 for women (95%CI 1.11–1.38) and 1.12 for male participants (95%CI 1.02–1.23, p=0.17) (Table S2). Similar results were obtained when hypertension severity was treated as a continuous variable. Among black participants, women and men had similar increases in IS risk per 10 mmHg increase in SBP, respectively (1.15, 95%CI 1.08–1.23 vs. 1.13, 95%CI 1.04–1.24, p=0.79). Among white participants, per 10 mmHg increase in SBP, the risk of IS differed by sex (women: 1.14, 95%CI 1.07–1.22 vs. men 1.06, 95%CI 0.99–1.13, p=0.093).
Supplemental table S3 displays the prevalence of additional Framingham stroke risk factors by sex and systolic blood pressure category, and supplemental table S4 displays sex-specific estimates of the risk of IS associated with increasing SBP by category, stratified by number of classes of antihypertensive medications and adjusted by the four additional Framingham risk factors: diabetes, atrial fibrillation, current smoking, and prevalent cardiovascular disease. In this model, the overall pooled hazard of stroke with increasing SBP was 1.24 (95%CI 1.15–1.35) for women vs. 1.13 (95%CI 1.04–1.23) for men and was significant at p=0.08.
DISCUSSION
In this large, national, prospective cohort study, the association between increasing hypertension severity and incident IS was almost twice as large in women compared with men. This sex difference remained after adjustment for other conventional stroke risk factors. While further research is needed to confirm our findings in other study populations and to investigate potential pathophysiologic differences in the link between hypertension and cerebrovascular disease, our findings suggest that a sex-specific approach to BP control and risk factor modification should be considered.
Few prior studies have been designed to detect sex differences in the association between hypertension severity and stroke risk either in the participant sampling or data analysis phases, and those that have reported sex-specific associations between stroke risk and BP have shown conflicting results.13,16,17,21 Our study adds to the current literature by evaluating this question in a large prospective biracial cohort, by taking into account the number of medications used to treat the hypertension, and by using the 2017 ACC/AHA High BP Guideline with 120 mmHg as the threshold for a diagnosis of hypertension.20 Another recent study from REGARDS also demonstrated a larger association between factors such as hypertension, diabetes, heart disease and stroke events in women compared with men, though these differences were only present among whites.17 A previous large study of older adults demonstrated a stronger association between hypertension and stroke in women compared with men, but the severity of hypertension was not assessed.16 A secondary analysis of SPRINT data that assessed sex-specific effects of intensive BP lowering on the composite outcome of CVD events also found sex differences. The effect estimate included 1.0 for women but not for men, suggesting a statistically significant benefit for men but only a trend towards a significant benefit for women. The p-value for the interaction was non-significant.21 Women, however, were underrepresented in SPRINT, comprising only 35% of participants. A large meta-analysis of studies that reported sex-specific associations between SBP and stroke risk found similar risks for women and men, but did not account for possible treatment differences.13 These studies, in conjunction with our current findings of a stronger association between hypertension severity and IS in women compared with men, suggest the need for future well designed studies powered to better understand possible sex specific effects of hypertension on stroke risk.
Possible explanations for our finding that hypertension severity carries a greater stroke risk for women than men are numerous and require further investigation. First, biologic and/or hormonal mechanisms that link hypertension to vascular dysfunction and disease may well differ between the sexes. For example, there is evidence that beta blockers are less effective in reducing sympathetic nerve activity and peripheral vascular resistance in postmenopausal women compared with men.22 It is also plausible that there are sex specific synergistic effects between hypertension and other risk factors such as diabetes that contribute to vascular dysfunction and thus increase stroke risk.16 In a large study of Taiwanese adults over age 65, the combined effect of having hypertension and diabetes on stroke risk was greater for women than men.16 Third, a difference in hypertension treatment and adherence leading to increased stroke risk for women is also a possibility. We were able to adjust for numbers of classes of anti-hypertensive medications but were not able to evaluate factors such as doses of antihypertensive medications and changes in medications over time in response to participants’ BP measurements.
It is unclear why there might be a sex difference in stroke risk associated with increasing hypertension severity among white participants but not black participants. There are differences in the distribution of hypertension severity by race that must be considered. In our dataset, overall there were fewer black men than all other subgroups. Compared to white women and men, black women and men had more severe hypertension and were less likely to be in the normotensive category. Treatment disparities could also be a factor; one could speculate that if BP over time is treated more aggressively in white men compared with other race/sex subgroups, a sex difference would be seen between white men and women but not between black men and women. Some existing data, however, do not support such a difference in treatment aggressiveness between blacks and whites.23 Finally, there may be confounding variables (i.e. socioeconomic status and health insurance) that affect the association between BP and stroke risk in black and white participants for which we did not account in our study.
Our finding of a stronger association between stroke risk and increasing hypertension severity in women vs. men suggests that, pending future confirmation of such sex differences, sex-specific hypertension guidelines for stroke prevention may be warranted. The SPRINT trial demonstrated a statistically non-significant benefit of controlling SBP to < 120 mm Hg compared with the prior target of < 140 mm Hg in women. Due to issues with statistical power, however, based on these data, it is unknown whether the threshold for treating hypertension should be equivalent in men and women. Future trials should be designed with sufficient power to adequately assess treatment effects in each sex. Additionally, we need more data from both pre-clinical and clinical research to assess how risk factors like diabetes and hypertension act synergistically to increase cardiovascular disease and stroke risk in women vs. men. Further studies of the role that endogenous sex hormones may play in the association between hypertension and stroke in women should also be considered.
We found that, in general, at a fixed level of BP, the risk of IS increased with the number of classes of anti-hypertensive medications reported by participants, even among normotensive individuals. Our data do not suggest that stroke risk increases with use of larger numbers of antihypertensive medications, but rather that stroke risk is lower if a person requires a fewer number of medications to maintain a specific BP level. This is consistent with our prior data,24 but in the present study, our findings additionally demonstrate no sex differences in the impact of the number of antihypertensive medications on stroke risk. Our findings of increased IS risk with an increasing number of medications, even among those in the normotensive category, suggest that controlling BP with medication does not fully mitigate the biologic and/or physiologic mechanisms that contribute to stroke risk among those diagnosed with hypertension. Such factors are important targets for future interventions. Our findings further suggest that most efficient approach for BP control is the primordial prevention of hypertension. At least 5 approaches with class 1-A evidence have been shown to prevent or delay the onset of hypertension.25,26
Our study has several limitations. First, we were unable to investigate the effect of changing hypertension severity over time, as our BP measurements were taken at the time of study enrollment only. Our study can be used to assess the sex-specific association between SBP at a given time point and subsequent stroke risk, which is of importance when considering strategies to reduce stroke risk across populations. Another limitation of our study is that, though we adjusted for the effect of age on the relationship between hypertension severity and stroke risk, we did not report risk estimates in specific age groups because of the small numbers of participants in the various age, sex, and race-specific strata. We could also not adjust for age at menopause. In addition, though we investigated the effect of increasing numbers of anti-hypertensive medications on stroke risk, this study was not designed to address adherence with medications or adjustment of medications over time, which could vary by sex and race. Studying provider and patient behaviors around the treatment of hypertension over time is an important future research direction. Despite these limitations, the design of REGARDS as a longitudinal cohort study of almost 30,000 individuals, the oversampling of black individuals, and the availability of adjudicated stroke events make REGARDS an ideal study to evaluate the association between hypertension severity and stroke. Finally, the focus of our manuscript was the association between SBP and ischemic stroke, elevation of which is known to be associated with increased risk of cardiovascular disease and stroke. There may also be sex differences in the way in which diastolic blood pressure and pulse pressure affect stroke risk,27–28 a topic for future research. Hemorrhagic strokes were not included in our study given the clear pathophysiologic differences between IS and hemorrhagic stroke though should also be evaluated in a separate study with sufficient number of strokes to evaluate this topic.
PERSPECTIVES
In summary, the relationship between severity of hypertension and risk of IS was greater in women than men. Treatment with additional classes of antihypertensive medications was associated with increased IS risk in both women and men. Further work on the biological mechanisms of sex differences in severity of HTN, and its CVD complications, including stroke, and the potential need for sex-specific clinical guidelines for hypertension prevention and treatment is warranted.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
Using a large, longitudinal prospective cohort study of black and white individuals, we investigated whether the risk of ischemic stroke associated with hypertension severity and anti-hypertensive medication use increases similarly for women compared with men. We also evaluated the association between incident stroke and hypertension severity in sex/race subgroups.
What is Relevant?
Among 26,461 participants in the REGARDS study, after adjusting for age, race, an age-by-race interaction term, sex, number of anti-hypertensive medications (treatment) and a sex-by-treatment interaction term, the risk of ischemic stroke per each level of hypertension severity was greater in women than men (HR 1.25 in women (95%CI 1.16–1.34) vs. 1.14 (95%CI 1.05–1.23) in men). Trends in our findings suggest these sex differences are larger among white individuals compared with black individuals.
Summary
In the future, sex specific guidelines for control of hypertension may be warranted to improve stroke prevention strategies in both women and men.
Acknowledgments
SOURCES OF FUNDING
The REGARDS research project is supported by a cooperative agreement (U01 NS041588) from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, the Department of Health and Human Service. TEM is funded by a K23 from the NHLBI [1K23HL140081-01A1].
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
None of the authors have conflicts to disclose.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madsen TE, Howard VJ, Jiménez M, et al. Impact of Conventional Stroke Risk Factors on Stroke in Women. Stroke.2018;49:536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leading Causes of Death in Males, CDC [Internet]. Available from: https://www.cdc.gov/men/lcod/. Accessed November 1, 2018.
- 5.Leading Causes of Death in Females, CDC. [Internet]. Available from: https://www.cdc.gov/women/lcod/. Accessed November 1, 2018.
- 6.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation. 2016;133:e38–48. [DOI] [PubMed] [Google Scholar]
- 7.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen TE, Khoury J, Alwell K, et al. Sex-specific stroke incidence over time in the Greater Cincinnati/Northern Kentucky Stroke Study. Neurology. 2017;89:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giroud MM, Delpont B, Daubail B, et al. Temporal Trends in Sex Differences with Regard to Stroke Incidence: The Dijon Stroke Registry (1987–2012). Stroke. 2017;48:846–9. [DOI] [PubMed] [Google Scholar]
- 10.Koton S, Schneider ALC, Rosamond WD, et al. STroke incidence and mortality trends in us communities, 1987 to 2011. JAMA. 2014;312:259–68. [DOI] [PubMed] [Google Scholar]
- 11.Yoon Sung, Margaret et al. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief. 2015;220:1–8. [PubMed] [Google Scholar]
- 12.Daugherty SL, Masoudi FA, Ellis JL, et al. Age-dependent gender differences in hypertension management. J Hypertens. 2011;29:1005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters SA, Huxley R, Woodward M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: A systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke. 2013;44:2394–401. [DOI] [PubMed] [Google Scholar]
- 14.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383:1899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turin TC, Okamura T, Afzal AR, et al. Hypertension and lifetime risk of stroke. J Hypertens. 2016;34:116–22. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y-J, Chen H-C, Chou P. Gender Difference in the Interaction Effects of Diabetes and Hypertension on Stroke among the Elderly in the Shih-Pai Study, Taiwan. PLoS One. 2015;10:e0136634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard VJ, Madsen TE, Kleindorfer D. Sex and race differences in incident ischemic stroke and risk factors. JAMA Neurol. 2018; 2018 December 10 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology. 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 19.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Circulation. 2018;138:e426–83. [DOI] [PubMed] [Google Scholar]
- 21.Foy C, Lovato L, Vitolins M. Gender, blood pressure, and cardiovascular and renal outcomes in adults with hypertension from the Systolic Blood Pressure Intervention Trial. J Hypertens. 2018;36:904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: Myths and reality. Curr Hypertens Rep. 2013;15:321–30. [DOI] [PubMed] [Google Scholar]
- 23.Plescia M, Huston S, Bostrom S, Yow A. Racial Disparities in Blood Pressure Control and Treatment Differences in a Medicaid Population, North Carolina, 2005–2006. Prev Chronic Dis. 2011;8:A55. [PMC free article] [PubMed] [Google Scholar]
- 24.Howard G, Banach M, Cushman M, et al. Is Blood Pressure Control for Stroke Prevention the Correct Goal?: The Lost Opportunity of Preventing Hypertension. Stroke. 2015;46:1595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: Clinical and Public Health Advisory from the National Blood Pressure Education Program. JAMA. 2002;288:1882–7. [DOI] [PubMed] [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Ovbiagele B. Post-stroke diastolic blood pressure and risk of recurrent vascular events. Eur J Neurol. 2017;24(11):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence JD. Systolic blood pressure targets, diastolic J curve and cuff artefact in blood pressure measurement: a note of caution. Eur J Neurol. 2017;24(11):1323–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.