Abstract
Interferon (IFN)-λs are a new addition to the old IFN family and share many similarities, such as antiviral and antiproliferative characteristics, with type I IFNs. IFN-λs also exhibit unique characteristics in immunomodulation. Accumulating studies have indicated the interactions between IFN-λs and immune cells, which lead to the regulation of the latter. IFN-λs can influence dendritic cells (DCs) and their product, IFN-λs-DCs, can then regulate the function of T cells. On the other hand, IFN-λs can also directly affect T cells through inhibition of the T helper 2 cell (Th2) responses. IFN-λs have varying immunomodulatory functions under different physiological conditions or in different organs and can inhibit tumor growth via regulation of the immune system. Diseases associated with IFN-λs include asthma, allergy, and systemic lupus erythematosus. In this review, we summarize the current knowledge of the biology of IFN-λs and their immunomodulatory function in relevant human diseases.
Key Words: Interferon-λ, Immunomodulation, Allergy, Asthma, Cancer
Introduction
Interferons (IFNs) are defined by their ability to induce host defense to viral infection. There are three types of IFNs, known as types I, II, and III. Type III IFNs, also called IFN-λs, are a new distinct type of IFN initially found in 2003 by two independent groups [1, 2]. Three closely positioned genes on human chromosome 19 were found to encode distinct but paralogous proteins, which were designated IFN-λ1, IFN-λ2, and IFN-λ3 [or interleukin (IL)-29, IL-28A, and IL-28B, respectively] [1]. IFN-λs are also related to the IL-10 family with regard to a classical four-helix bundle structure [3].
IFN-λs and type I IFNs are similar in their expression patterns and biological activities, but research has shown that IFN-λs also have their unique characteristics. Compared to type I IFNs, expression of IFN-λs can be induced in response to a broader spectrum of stimuli, such as diverse viruses and various toll-like receptor (TLR) agonists [4].
Many studies have demonstrated that IFN-λs play a unique role in antiviral defense [1, 5, 6, 7, 8], with their influence on HBV or HCV persistence [9, 10, 11]. However, there is still little known about their function in immunomodulation. This review summarizes the current understanding of the immunomodulatory activities of IFN-λs and their significance in cancer and several immune diseases, such as asthma and SLE. These latest studies suggest that IFN-λs will be a potential therapeutic target in clinical medicine.
Expression Pattern
Similar to type I IFNs, IFN-λ expression is in response to diverse viruses and various TLR agonists [2, 11, 12], and it is induced through transcriptional mechanisms involving IFN regulatory factors (IRFs), NF-ĸB, and activator protein 1 (AP-1) [4, 13]. The similar expression patterns of type I and type III IFN genes are due to the common regulatory elements in their promoters [11, 14]. Nevertheless, the expression of IFN-λs responds to a wider range of stimuli compared with type I IFN [4]. Further studies indicate that the IFN-λ1 gene is regulated by IRF3 and IRF7, thus resembling the IFN-β gene, whereas IFN-λ2/3 gene expression is mainly controlled by IRF7, resembling those of the IFN-α genes [13]. In addition, dendritic cells (DCs), monocytes, mast cells, and epithelial cells are the main IFN-λ-producing cells [15, 16, 17, 18, 19, 20].
Receptor Complex and Signaling
Type III IFNs function through a heterodimeric IFN-λ receptor complex composed of a unique IFN-λR1 chain and the IL-10R2 chain that is also the second subunit of the receptor complexes for IL-10, IL-22, and IL-26 [21, 22]. IFN-λR1 can be alternatively spliced to produce two variant receptors with similar affinity to IFN-λs, and it is possible that these IFN-λ receptor variants are involved in inhibiting ligand binding and/or signal transduction [23].
After IFN-λs bind to IFN-λR, they can activate downstream signaling pathways, such as the Jak-STAT pathway and the mitogen-activated protein kinases (MAPK). Engagement of these pathways by IFN-λs results in recruitment of the IFN-stimulated gene factor 3 (ISGF3) complex to the promoter region of responsive target genes [24, 25, 26]. Additionally, IFN-λs can also induce phosphorylation of protein kinase B (PKB) through the phosphatidylinositol 3-kinase (PI3K) pathway [27]. IFN types III and I induce a similar subset of genes, such as 2′-5′-oligoadenylate synthetase 1 (OAS1) and IFN-stimulated gene 56 (ISG56) [24].
The specificity of the IFN-λ response is regulated through limited receptor expression [8, 24]. Unlike the widespread expression of the receptor of type I IFN, the IFN-λR is only expressed in skin, the respiratory tract, and the gastrointestinal tract. Only epithelial-like cells and, to a lesser extent, some immune cells respond to IFN-λs [28]. Thus, IFN-λs contribute to prevent viral invasion through the skin and mucosal surfaces [29, 30, 31, 32].
Surprisingly, Witte et al. [23] reported that, despite the expression of IFN-λ receptor in immune cells, IFN-λ2 and IFN-λ1 did not activate STAT (signal transducer and activator of transcription) 1 or STAT3 at all in monocytes, T cells, or natural killer (NK) cells, and only minimal activation was observed in B cells, presumably because these cells depend on other pathways for STAT activation.
Different Functions of IFN-λ Subtypes
IFN-λ2 and IFN-λ3 are virtually identical, sharing 96% identical amino acids, whereas IFN-λ1 has 81% homology to IFN-λ2/3 [33], so many researchers assume that different subtypes of IFN-λs have the same activity. Surprisingly, Dellgren et al. [34] found that IFN-λ3 possesses the highest antiviral activity among all human IFN-λ subtypes, exhibiting a 2-fold higher activity compared to IFN-λ1 and a 16-fold higher activity compared to IFN-λ2. In addition, Liu et al. [35] found that IFN-λ1 had a stronger ability than IFN-λ2/3 to induce IL-12p40, TNF, and IL-10 production in monocyte-derived macrophages in response to R848 stimulation. Further research showed that IFN-λ2 significantly reduced the expression of 89 genes by more than 2-fold in hepatic cells, while no significant downregulation of genes was observed following IFN-λ1 stimulation [36]. Currently, there is still limited data to compare the biological activities of the three different IFN-λs cytokines. It would be useful to figure out whether different subtypes are responsible for different aspects of their functions.
Antiviral and Antiproliferative Function
IFN-λs possess antiviral activity [reviewed in [5, 7, 25]]. Administration of exogenous IFN-λs protects mice from the encephalomyocarditis virus (EMCV), herpes simplex virus-2 (HSV-2), influenza A virus, HCV, and other viruses. However, the IFN-λ-induced antiviral system alone cannot provide full protection against systemic virus infections; the functional type I IFN antiviral system is also required. The latest study shows that mice deficient for both type I and type III IFN receptors cannot efficiently control initial SARS-CoV replication in the lung [37]. In contrast to type I IFNs, antiviral protection of intestinal epithelial cells against rotaviruses mainly relies on the action of the type III IFNs antiviral system [38]. IFN-λ3 single-nucleotide polymorphisms (SNP) can influence IFN-λ expression, which is also associated with mortality risk in HIV-infected patients [39] and prognosis in HCV patients [40, 41, 42, 43, 44, 45].
IFN-λs also elicit antiproliferation responses [46, 47, 48] and induce STAT activation more potently than type I IFNs. IFN-λs lead to apoptosis or G1 phase arrest of cancer cells [49, 50, 51], although not all cell lines have the same susceptibility to IFN-λs [51]. As discussed below, IFN-λs can inhibit tumor growth through modulation of the immune system.
Immunomodulatory Functions of IFN-λs
A study found a discrepancy between the observed antiviral activity in vitro and in vivo, suggesting that type III IFNs do have immunomodulatory properties [52]. The current understanding of the complex role of type III IFNs in overall immunity constitutes an important aspect of IFN-λ biology.
IFN-λs and DCs
IFN-λs have a close relationship with DCs. Although all cells infected by virus can produce IFN-λs, DCs are the main source of IFN-λs after specific stimulation of TLRs [16, 53, 54, 55, 56, 57]. A recent study measuring the expression of IFN subtypes in purified human myeloid DCs (mDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs in response to different TLR agonists revealed that the expression profiles of human IFN-λs subtypes depend on the specific types of TLRs and immune cells [17].
IFN-λs can also impact DCs. There are two kinds of DCs in human periphery blood: pDCs and mDCs. The steady-state expression of IFN-λR1 in pDCs is significantly greater than that of peripheral blood mononuclear cells (PBMCs) or general DCs, suggesting that pDCs are among the most IFN-λ-responsive cell types in the periphery [58, 59]. pDCs treated with IFN-λ1 exhibit enhanced expressions of the homing molecules CCR7 and CD62L, co-culture molecules CD80 and ICOS-L, and reduced production of IL-10, IL-13, and IFN-γ [58].
Human mDCs matured with lipopolysaccharide (LPS) in the presence of IFN-λ1 secrete less IL-10 and more IL-12 than those not exposed to IFN-λ1 [60, 61]. However, Mennechet and Uzé [62] found that IFN-λs induced only a subset of human monocyte-derived DC maturation markers and did not induce IL-12. IFN-λ-treated DCs specifically induce proliferation of a CD4+CD25+Foxp3+ T cell subset with suppressive activity on T cell proliferation [62].
Although further studies are needed at this time, we hypothesize that the initial state of mDCs would influence the function of IFN-λs on themselves. IFN-λ-treated mDCs display a partially matured phenotype and induce regulatory T cells (Tregs) [62], whereas after maturation by LPS stimulation, IFN-λs can promote mDCs to facilitate the T helper 1 (Th1) response and diminish the Th2 response [60, 61].
IFN-λs and CD4+ T Cells
IFN-λ1 can modulate the secretion of Th1 or Th2 cytokines by CD4+ T cells by inhibiting the secretion of IL-4, IL-5, and IL-13 and promoting the secretion of IFN-γ [63]. Furthermore, IFN-λ1 can suppress differentiation towards a Th2 phenotype [53]. Compared to IL-4 and IL-5, IFN-λ1 preferentially inhibits IL-13 production [64] through a decrease in the Th2-restricted transcription factor GATA3 [63]. Th2 cytokines, in turn, also impact IFN-λ production. IL-4-responsive monocytes secrete IL-1 receptor antagonist (IL-Ra), which then acts on pDCs to elevate their IFN-λ1 output [53]. In this way, IL-4 and IFN-λ1 comprise a feedback loop which represents a natural checkpoint for the control of Th2 cytokine production. IFN-λ1 can work against this loss of the CD62LCCR7 population of CD4+ T cells [63] and make memory T cells incapable of entry into the periphery or differentiation.
Intriguingly, an animal experiment showed that neither T cell differentiation nor cytokine production of already differentiated Th0, Th1, or Th2 cells is affected by IFN-λ2 directly [61]. It has been shown that human herpesvirus type 6B (HHV-6B)-induced alterations in the Th1/Th2 balance are mediated mainly through IFN-α instead of IFN-λ1 [57]. This controversy may be caused by different research approaches and models.
IFN-λs and CD8+ T Cells
IFN-λs can increase the killing activity of cytotoxic T lymphocytes (CTL). Using DNA vaccination, peripheral CD8+ T cells from animals that were administered with IFN-λ3 show substantially increased cytotoxic responses [65]. IFN-λ3 is able to increase the percentage of splenic CD8+ T cells and reduce Treg cell populations [66], and the CD8+ T cells are more granular and have higher antigen-specific cytolytic degranulation compared to cells taken from the animals that received IL-12 as an adjuvant. NK cells and CTL actually contribute to IFN-λ-induced immune protection of mice with tumor injection [67, 68, 69]. Nevertheless, further experiments showed that IFN-λs cannot directly work on NK cells and CD8+ T cells in vitro [67, 68]. Thus, it remains unclear whether IFN-λs have any efftect on DCs to influence NK cells and CD8+ T cells, or if IFN-λs can change IL-12 and IFN-γ production, which then contributes to the cytolysis of NK cells and CTL.
IFN-λ and Monocytes/Macrophages
IFN-λ1 induces IL-6, IL-8, IL-10, chemokine (C-X-C motif) ligand (CXCL) 9, CXCL10, and CXCL11 in human PBMCs [70, 71]. Examination of purified cell populations isolated from PBMCs demonstrates that monocytes and macrophages are the major IFN-λ1-responsive cellular subsets. IFN-λ1-treated macrophages upregulate the surface expression of the IFN-γR1 chain and therefore are more responsive to IFN-γ [35]. All of this indicates that IFN-λs activate both monocytes and macrophages, and may therefore be important in activating innate immune responses at the site of viral infection. However, the latest research has found that IFN-λ1 sensitized monocytes and macrophages to IL-10 stimulation and seemed to inhibit pro-inflammatory responses [72] (table 1) (fig. 1).
Table 1.
Immunoregulatory functions of IFN-λs
| Subtype | Target cell | Effect and possible mechanism | Overall impression | References |
|---|---|---|---|---|
| λ1 | pDC | increase the expression of the homing molecules CCR7 and CD62L; upregulate co-stimulatory molecules CD80 and ICOS-L, and reduce stimulation of IL-10, IL-13, and IFN-γ production from allogeneic T cells | contribute to pDC activity and prolong pDC survival | 58, 59 |
| λ1, λ2 | mDC (without LPS) | induce partial maturation of DCs; high levels of major MHC class I and MHC class II; express CCR7; low levels of costimulatory molecules, and retained phagocytic ability | induce partial maturation of mDCs | 62 |
| λ1, λ2 | mDC | profoundly inhibit the generation of Th2 and Th17 responses; enhance Th1 polarization, and augment IL-12 secretion | promote mDC maturation | 60, 61 |
| λ1 | CD4+ T cell | alter the Th1/Th2 development of naive human T cells; diminish the development of Th2 cells and lower the secretion of IL-13, and induce CD3+ T cell apoptosis | inhibit Th2, promote Th1, and induce apoptosis | 60, 64, 73 |
| λ1, λ2 | Treg | IL-2-dependent proliferation of CD4+CD25+Foxp3+ T cells | induce proliferation of Treg | 62 |
| λ3 | CD8+ T cell | increase CTL killing activity, and enhance antigen-specific cytolytic degranulation | promote killing activity | 65, 66 |
| λ1, λ2/3 | NK | do not enhance NK cytotoxic activity and chemotaxis directly | no direct influence on NK | 67, 68, 74 |
| λ1 | PBMC | elevate chemokines mRNA levels of CXCL9, CXCL10, and CXCL11, and upregulate IL-6, IL-8, and IL-10 | activate PBMC | 70, 71 |
| λ1 | monocyte | upregulate IL-6, IL-8, and IL-10; change morphology, and more motile | activate monocyte | 71 |
| λ1 | macrophage | upregulate IL-6, IL-8, and IL-10; increase TLR-induced IL-12p40 production, and upregulate IFN-γR1 | activate macrophage | 35, 71 |
| λ2/3 | B cell | enhance TLR7 on B cells | unclear | 75 |
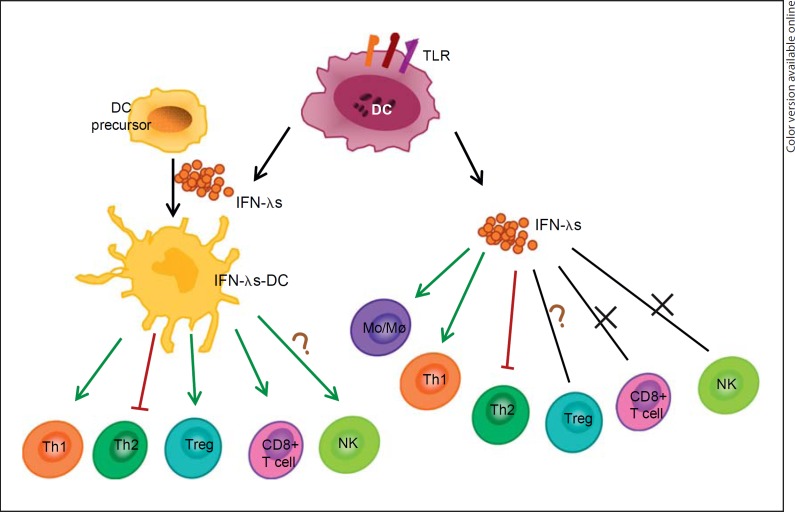
Fig. 1.
Immunomodulatory functions of type III IFN. IFN-λR is expressed in DCs, T cells, and NK cells. DCs can produce IFN-λs in response to the stimulation of TLRs, and IFN-λs can also affect DCs, resulting in the change of MHC, CCR7, and costimulatory molecules. IFN-λs-DCs or IFN-λs themselves can influence the function of T cells, including promotion of Th1, inhibition of Th2, activation of Mo/Mø, and changing the proportion of Treg and the cytotoxicity of CD8+ T cells. Cross marks = IFN-λs could not work directly on this kind of cells; question marks = the pathway was not completely confirmed; green color = promote; red color = inhibit.
Relationship of IFN-λs in Disease
Growing tumors acquire the ability to resist immune recognition and immune-mediated injury [76]. In addition, allergy and systemic lupus erythematosus (SLE) have a hypothetical Th2 cell-cytokine predominance. Since IFN-λs have a special immunomodulatory function, they may play a role in the pathogenesis or therapy of these diseases.
Cancer
Type III IFNs can elicit antitumor activities through both a direct effect on tumor cells themselves and an indirect effect on the antitumor immune responses. The direct antitumor activity of type III IFNs is associated with cell cycle arrest at the G1 phase and apoptosis [51, 77]. For human non-small cell lung cancer (NSCLC), Fujie et al. [78] found that IFN-λ1 significantly inhibited the in vitro growth of a wide range of NSCLC lines in a dose-dependent fashion.
IFN-λs can also work on the immune system to inhibit tumor growth. The proliferation of cancer cells with constitutive expression of IFN-λs is not affected in vitro, but the in vivo tumorigenicity is either suppressed or completely abolished when cells are injected subcutaneously into mice [68, 79], suggesting that IFN-λs engage host mechanisms to inhibit tumor growth. NK cells probably play a critical role in IFN-λ-mediated protection against tumors [50, 67, 69, 80], as IFN-λs sensitize tumor cells to NK cell recognition and activation, and depletion of NK cells inhibits IFN-λ-induced antitumor activity. In addition, Numasaki et al. [68] proposed that polymorphonuclear neutrophils and CD8+ T cells also play equally important roles in IFN-λ2/3-mediated inhibition of MCA205 fibrosarcoma growth, while Sato et al. [69] found that the response of CD8+ T cells is weak in a murine colon26 cancer model. The increased secretion of IL-12 and IFN-γ may also contribute to the immunocytotoxicity induced by IFN-λs [67, 68].
With regard to whether IFN-λ-induced antitumor activity is associated with an antiangiogenic response, research showed that IFN-λs did not affect tumor vascularity in human NSCLC [78], a BNL hepatoma model [67], and esophageal carcinoma [50]. However, Lasfar et al. [79] found that tumors derived from B16.IFN-λ2 cells were less vascular than B16 tumors. Importantly, these experiments were carried out using different subtypes of IFN-λs, leaving the exact relationship between IFN-λs and angiogenesis an open question.
The animal experiments reviewed above indicate that immune cells, cytokines, and antiangiogenesis are possible mechanisms of IFN-λ-mediated protection against tumor. Surprisingly, recent studies showed that IFN-λ1 induced myeloma cell growth and protected cancer cells from dexamethasone-induced cell death [81]. In another study, intratumoral injections of 400 ng IFN-λ1 did not mediate significant suppression of A549 growth in vivo [78]. These inconsistent results may suggest a context-dependent mechanism of action for IFN-λs in different types of tumors.
The potential application of IFN-λs in cancer treatment has been proposed [reviewed in [28, 82]]. Due to the restricted expression of IFN-λ receptors, the adverse side effects are slight and two phase 1 clinical trials have shown good patient tolerance with IFN-λs. Application of IFN-λs will help to modulate the Th1/Th2 balance in cancer patients and break immune tolerance. IFN-λs in combination with IFN-α/β/γ or chemotherapeutic agents can provide a new choice for cancer therapy [51, 74, 78].
Asthma
Researchers have observed deficient induction of IFN-λs by rhinovirus in primary bronchial epithelial cells and alveolar macrophages of patients with asthma exacerbation [83] and human cystic fibrosis [84]. Another discovery is that an SNP rs12979860, which is located 3 kb upstream of IFN-λ3 and influences the production of IFN-λs, is correlated with the immune state in children who develop allergic disease [85], and a relationship between higher levels of a pro-inflammatory cytokine profile at birth with diminished levels of IFN-λs is observed over time in children who carry the SNP.
IFN-λs are thought to inhibit GATA3 expression and suppress Th2-type immune responses [60, 63, 64], which are the hallmarks of allergic diseases [86]. This is further supported by the recent study by Koltsida et al. [61], which shows that IFN-λs can promote Th1 immunity and suppress Th2 responses in the mouse model of allergic asthma [87]. The novel effects of IFN-λ2 on T cell differentiation are not observed in IFN-γ-deficient mice or mice depleted of IL-12p40, indicating that IFN-λ2 induces Th1 effect or function via IL-12 and IFN-γ [87]. IFN-λs are likely the principal IFNs produced during innate responses to respiratory viruses in bronchial epithelial cells [88], and key modulators of the Th2 response [89]. All of this suggests that defective type III IFNs in response to rhinovirus lead to a stronger Th2 response and subsequent allergic diseases. Recent studies have shed further light on the regulation of IFN-λ1 promoter activity in human airway epithelial cells and have shown that BLIMP-1 and ZEB1 may be negative regulators of IFN-λ1 expression [15]. Moriwaki et al. [90] indicated that IL-13, a crucial cytokine responsible for asthma pathogenesis, suppresses dsRNA-induced expression of IFN-λs in airway epithelial cells and alveolar macrophages, and contributes to the impairment of the antiviral defense in asthmatics.
However, Bullens et al. [91] reported that asthma patients have higher mRNA expression of IFN-λ2/3 in sputum than healthy individuals. Moreover, the serum level of IFN-λs is also higher in asthma patients in exacerbation [18]. These studies indicated that IFN-λs are involved in the pathogenesis of allergic inflammation [18, 91]. Thus, the role of IFN-λs in asthma is somewhat controversial, but the mainstream views are that the deficiency of IFN-λs leads to hyperfunction of Th2 cells, and an IFN-λ supplement would alleviate asthmatic symptoms.
Systemic Lupus Erythematosus
IFN-λ1 mRNA expression and serum protein levels in patients with SLE are higher compared to normal controls, suggesting that IFN-λ1 is probably involved in the renal disorder and arthritis progression of SLE and associated with disease progression. IFN-λ1 stimulates the production of CXCL10 (IP-10), CXCL9 (MIG), and IL-8 by PBMCs from SLE patients [92]. These chemokines play an important role in the inflammation process of SLE by recruiting leukocytes to inflammatory sites and promoting disease aggravation. Recently, the expression of IFN-λ2/3 was found to be high in activated CD4+ T cells of SLE patients [93]. Significantly, enhanced IFN-λ1 could also be measured in the serum of cutaneous lupus erythematosus patients with active skin lesions. Functional analyses revealed that human keratinocytes are able to produce high levels of IFN-λ1 but only low amounts of IFN-α/β/γ in response to immunostimulatory nucleic acids [94].
In SLE patients, IFN-λ secretion is enhanced and leads to upregulation of several inflammatory proteins, and cytokine imbalances contribute to immune dysfunction, trigger inflammation, and induce organ damage. Therefore, there is a potential to use anti-IFN-λ monoclonal antibodies to neutralize excess IFN-λs in SLE patients.
Food Allergy
He et al. [73] found that IFN-λs are involved in the development and maintenance of oral tolerance in the intestines of mice. Interaction between IFN-λs and their receptor induces apoptosis of T cells and their subsequent phagocytosis by DCs, which leads to the generation of tolerogenic DCs and Tregs in vitro and in vivo. On the other hand, IFN-λ-treated DCs retain their phagocytic ability and induce Treg proliferation [62]. Thus, IFN-λs are functional in the generation of tolerogenic DCs and Tregs, keeping the immune activation in control and helping to restore immune homeostasis. Surprisingly, He et al. [95] also reported that eosinophils express IFN-λs that can induce intestinal epithelial barrier dysfunction and promote the initiation of aberrant Th2 polarization in the intestine. So it remains to be further investigated whether IFN-λs are involved in the development of oral tolerance or food allergy (table 2).
Table 2.
IFN-λs and relevant diseases
| Subtype | Experimental system | Effect and possible mechanism | References |
|---|---|---|---|
| λ1, λ2/3 | antitumor function in mice | regulate innate and adaptive immune responses of NK, T cells, and DCs, and antiangiogenesis | 50, 67–69, 79, 80 |
| λ1, λ2/3 | asthma | modulate lung DC function to promote Th1 immune skewing and suppress allergic airway disease, and decreased IFN-λ production correlating with severity of rhinovirus-induced asthma exacerbation and virus load | 18, 19, 61, 83, 84, 90 |
| λ2 | Con A-induced hepatitis | induce Th1 cytokine production and T cell-mediated liver injury | 96 |
| λ1, λ2/3 | SLE | significantly enhanced in patients with SLE, and stimulate the production of CXCL10 (IP-10), CXCL9 (MIG), and IL-8 | 92–94 |
| λ | food allergy | controversial: induced apoptosis of T cells or intestinal epithelial cells, and suppressed or induced antigen-specific Th2 cell-mediated inflammation | 73, 95 |
Concluding Remarks
It has been widely accepted that type I IFNs play an exclusive role as early mediators of the innate response to viruses, as well as regulators of the subsequent responses from components of the adaptive immune system [97]. IFN-α-DCs can play a role in the generation of antitumor T cell immunity and in the pathogenesis of some autoimmune disorders [98]. IFN-λs are new members of the IFN family of cytokines, and many research studies have shown that type III IFNs and type I IFNs share lots of biological similarities. The therapeutic efficacy could be augmented and the side effects could be reduced when both IFN types are used in combination [78, 99].
We believe IFN-λs not only assist type I IFNs but also modulate the immune response independently. IFN-λs can function via DCs and also directly work on T cells. Under physiological conditions, IFN-λs promote differentiation of immune cells and activate the immune system. However, under pathological conditions, their abnormal secretion is associated with the pathogenesis of immunological diseases, such as cancer, SLE, asthma, and food allergy. In consideration of its immunomodulatory function, IFN-λs have potential as a new target of treatment in these diseases. Potential applications include inhibition of the activity of IFN-λ to ameliorate symptoms of SLE patients, or supplementation of IFN-λs to modulate the imbalance of T helper cells in cancer patients.
In summary, the newly discovered IFN-λs and their special functions have attracted great attention to the old IFN family. It has been realized that the immunomodulatory activities of IFN-λs are complex and intriguing. We hypothesize that IFN-λs possibly have dual characteristics, functioning diversely in different circumstances. Although further studies to elucidate the mechanism of the function of IFN-λs are needed, the current evidence suggests that IFN-λs have great therapeutic potential, and can provide novel strategies for the clinical treatment of many diseases.
References
- 1.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 3.Zdanov A. Structural analysis of cytokines comprising the IL-10 family. Cytokine Growth Factor Rev. 2010;21:325–330. doi: 10.1016/j.cytogfr.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iversen MB, Paludan SR. Mechanisms of type III interferon expression. J Interferon Cytokine Res. 2010;30:573–578. doi: 10.1089/jir.2010.0063. [DOI] [PubMed] [Google Scholar]
- 5.Ank N, West H, Paludan SR. IFN-lambda: novel antiviral cytokines. J Interferon Cytokine Res. 2006;26:373–379. doi: 10.1089/jir.2006.26.373. [DOI] [PubMed] [Google Scholar]
- 6.Kempuraj D, Donelan J, Frydas S, Iezzi T, Conti F, Boucher W, et al. Interleukin-28 and 29 (IL-28 and IL-29): new cytokines with anti-viral activities. Int J Immunopathol Pharmacol. 2004;17:103–106. doi: 10.1177/039463200401700201. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Liu X, Zhou Y, Su SB. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. J Leukoc Biol. 2009;86:23–32. doi: 10.1189/jlb.1208761. [DOI] [PubMed] [Google Scholar]
- 8.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, et al. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 9.Pagliaccetti NE, Robek MD. Interferon-lambda in the immune response to hepatitis B virus and hepatitis C virus. J Interferon Cytokine Res. 2010;30:585–590. doi: 10.1089/jir.2010.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly C, Klenerman P, Barnes E. Interferon lambdas: the next cytokine storm. Gut. 2011;60:1284–1293. doi: 10.1136/gut.2010.222976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, et al. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- 14.Witte K, Witte E, Sabat R, Wolk K. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev. 2010;21:237–251. doi: 10.1016/j.cytogfr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R, Eskdale J, Gallagher G. Regulation of IFN-lambda1 promoter activity (IFN-lambda1/IL-29) in human airway epithelial cells. J Immunol. 2011;187:5636–5644. doi: 10.4049/jimmunol.1003988. [DOI] [PubMed] [Google Scholar]
- 16.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, et al. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 17.Hillyer P, Mane VP, Schramm LM, Puig M, Verthelyi D, Chen A, et al. Expression profiles of human interferon-alpha and interferon-lambda subtypes are ligand- and cell-dependent. Immunol Cell Biol. 2012;90:774–783. doi: 10.1038/icb.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He S, Li T, Chen H, Ma W, Yao Q, Yang H, et al. CD14+ cell-derived IL-29 modulates proinflammatory cytokine production in patients with allergic airway inflammation. Allergy. 2011;66:238–246. doi: 10.1111/j.1398-9995.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 19.He S, Zhang H, Chen H, Yang H, Huang T, Chen Y, et al. Expression and release of IL-29 by mast cells and modulation of mast cell behavior by IL-29. Allergy. 2010;65:1234–1241. doi: 10.1111/j.1398-9995.2010.02349.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, et al. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol. 2009;182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotenko SV, Langer JA. Full house: 12 receptors for 27 cytokines. Int Immunopharmacol. 2004;4:593–608. doi: 10.1016/j.intimp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76:314–321. doi: 10.1189/jlb.0204117. [DOI] [PubMed] [Google Scholar]
- 23.Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, et al. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009;10:702–714. doi: 10.1038/gene.2009.72. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotenko SV. IFN-lambdas. Curr Opin Immunol. 2011;23:583–590. doi: 10.1016/j.coi.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 27.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, et al. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G960–G968. doi: 10.1152/ajpgi.00126.2005. [DOI] [PubMed] [Google Scholar]
- 28.Lasfar A, Abushahba W, Balan M, Cohen-Solal KA. Interferon lambda: a new sword in cancer immunotherapy. Clin Dev Immunol. 2011;2011:349575. doi: 10.1155/2011/349575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulverer JE, Rand U, Lienenklaus S, Kugel D, Zietara N, Kochs G, et al. Temporal and spatial resolution of type I and III interferon responses in vivo. J Virol. 2010;84:8626–8638. doi: 10.1128/JVI.00303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 34.Dellgren C, Gad HH, Hamming OJ, Melchjorsen J, Hartmann R. Human interferon-lambda3 is a potent member of the type III interferon family. Genes Immun. 2009;10:125–131. doi: 10.1038/gene.2008.87. [DOI] [PubMed] [Google Scholar]
- 35.Liu BS, Janssen HL, Boonstra A. IL-29 and IFNalpha differ in their ability to modulate IL-12 production by TLR-activated human macrophages and exhibit differential regulation of the IFNgamma receptor expression. Blood. 2011;117:2385–2395. doi: 10.1182/blood-2010-07-298976. [DOI] [PubMed] [Google Scholar]
- 36.Diegelmann J, Beigel F, Zitzmann K, Kaul A, Goke B, Auernhammer CJ, et al. Comparative analysis of the lambda-interferons IL-28A and IL-29 regarding their transcriptome and their antiviral properties against hepatitis C virus. PLoS One. 2010;5:e15200. doi: 10.1371/journal.pone.0015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahlakoiv T, Ritz D, Mordstein M, Dediego ML, Enjuanes L, Muller MA, et al. Combined action of type I and type III interferon restricts initial replication of SARS-Coronavirus in the lung but fails to inhibit systemic virus spread. J Gen Virol. 2012 doi: 10.1099/vir.0.046284-0. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parczewski M, Bander D, Leszczyszyn-Pynka M, Urbanska A, Socha L, Boron-Kaczmarska A. IL28B CC genotype is associated with higher all-cause mortality in antiretroviral-treated HIV-infected patients. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/AID.2011.0354. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Langhans B, Kupfer B, Braunschweiger I, Arndt S, Schulte W, Nischalke HD, et al. Interferon-lambda serum levels in hepatitis C. J Hepatol. 2011;54:859–865. doi: 10.1016/j.jhep.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Li JH, Lao XQ, Tillmann HL, Rowell J, Patel K, Thompson A, et al. Interferon-lambda genotype and low serum low-density lipoprotein cholesterol levels in patients with chronic hepatitis C infection. Hepatology. 2010;51:1904–1911. doi: 10.1002/hep.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, Cronin KD, et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology. 2010;52:1888–1896. doi: 10.1002/hep.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selvarajah S, Tobler LH, Simmons G, Busch MP. Host genetic basis for hepatitis C virus clearance: a role for blood collection centers. Curr Opin Hematol. 2010;17:550–557. doi: 10.1097/MOH.0b013e32833e7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 45.Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–1345.e1. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 46.Numasaki M, Tagawa M, Iwata F, Suzuki T, Nakamura A, Okada M, et al. IL-28 elicits antitumor responses against murine fibrosarcoma. J Immunol. 2007;178:5086–5098. doi: 10.4049/jimmunol.178.8.5086. [DOI] [PubMed] [Google Scholar]
- 47.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 48.Zitzmann K, Brand S, Baehs S, Goke B, Meinecke J, Spottl G, et al. Novel interferon-lambdas induce antiproliferative effects in neuroendocrine tumor cells. Biochem Biophys Res Commun. 2006;344:1334–1341. doi: 10.1016/j.bbrc.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 49.Maher SG, Sheikh F, Scarzello AJ, Romero-Weaver AL, Baker DP, Donnelly RP, et al. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther. 2008;7:1109–1115. doi: 10.4161/cbt.7.7.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Kawamura K, Okamoto S, Fujie H, Numasaki M, Namba M, et al. Adenoviruses-mediated transduction of human oesophageal carcinoma cells with the interferon-lambda genes produced anti-tumour effects. Br J Cancer. 2011;105:1302–1312. doi: 10.1038/bjc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q, Kawamura K, Ma G, Iwata F, Numasaki M, Suzuki N, et al. Interferon-lambda induces G1 phase arrest or apoptosis in oesophageal carcinoma cells and produces anti-tumour effects in combination with anti-cancer agents. Eur J Cancer. 2010;46:180–190. doi: 10.1016/j.ejca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Megjugorac NJ, Gallagher GE, Gallagher G. IL-4 enhances IFN-lambda1 (IL-29) production by plasmacytoid DCs via monocyte secretion of IL-1Ra. Blood. 2010;115:4185–4190. doi: 10.1182/blood-2009-09-246157. [DOI] [PubMed] [Google Scholar]
- 54.Wolk K, Witte K, Witte E, Proesch S, Schulze-Tanzil G, Nasilowska K, et al. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc Biol. 2008;83:1181–1193. doi: 10.1189/jlb.0807525. [DOI] [PubMed] [Google Scholar]
- 55.Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iversen MB, Ank N, Melchjorsen J, Paludan SR. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J Virol. 2010;84:4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nordstrom I, Eriksson K. HHV-6B induces IFN-lambda1 responses in cord plasmacytoid dendritic cells through TLR9. PLoS One. 2012;7:e38683. doi: 10.1371/journal.pone.0038683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29) J Leukoc Biol. 2009;86:1359–1363. doi: 10.1189/jlb.0509347. [DOI] [PubMed] [Google Scholar]
- 59.Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, et al. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol. 2012;189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jordan WJ, Eskdale J, Srinivas S, Pekarek V, Kelner D, Rodia M, et al. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun. 2007;8:254–261. doi: 10.1038/sj.gene.6364382. [DOI] [PubMed] [Google Scholar]
- 61.Koltsida O, Hausding M, Stavropoulos A, Koch S, Tzelepis G, Ubel C, et al. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med. 2011;3:348–361. doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 63.Dai J, Megjugorac NJ, Gallagher GE, Yu RY, Gallagher G. IFN-lambda1 (IL-29) inhibits GATA3 expression and suppresses Th2 responses in human naive and memory T cells. Blood. 2009;113:5829–5838. doi: 10.1182/blood-2008-09-179507. [DOI] [PubMed] [Google Scholar]
- 64.Srinivas S, Dai J, Eskdale J, Gallagher GE, Megjugorac NJ, Gallagher G. Interferon-lambda1 (interleukin-29) preferentially down-regulates interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology. 2008;125:492–502. doi: 10.1111/j.1365-2567.2008.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrow MP, Yan J, Pankhong P, Shedlock DJ, Lewis MG, Talbott K, et al. IL-28B/IFN-lambda 3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol Ther. 2010;18:1714–1723. doi: 10.1038/mt.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, et al. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood. 2009;113:5868–5877. doi: 10.1182/blood-2008-11-190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, et al. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother. 2010;59:1059–1071. doi: 10.1007/s00262-010-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Numasaki M, Tagawa M, Iwata F, Suzuki T, Nakamura A, Okada M, et al. IL-28 elicits antitumor responses against murine fibrosarcoma. J Immunol. 2007;178:5086–5098. doi: 10.4049/jimmunol.178.8.5086. [DOI] [PubMed] [Google Scholar]
- 69.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 70.Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon lambda-1 (IFN-lambda1/IL-29) induces ELR(-) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-gamma-independent manner. Genes Immun. 2007;8:177–180. doi: 10.1038/sj.gene.6364372. [DOI] [PubMed] [Google Scholar]
- 71.Jordan WJ, Eskdale J, Boniotto M, Rodia M, Kellner D, Gallagher G. Modulation of the human cytokine response by interferon lambda-1 (IFN-lambda1/IL-29) Genes Immun. 2007;8:13–20. doi: 10.1038/sj.gene.6364348. [DOI] [PubMed] [Google Scholar]
- 72.Liu BS, Janssen HL, Boonstra A. Type I and III interferons enhance IL-10 receptor expression on human monocytes and macrophages, resulting in IL-10-mediated suppression of TLR-induced IL-12. Eur J Immunol. 2012;42:2431–2440. doi: 10.1002/eji.201142360. [DOI] [PubMed] [Google Scholar]
- 73.He SH, Chen X, Song CH, Liu ZQ, Zhou LF, Ma WJ, et al. Interferon-lambda mediates oral tolerance and inhibits antigen-specific, T-helper 2 cell-mediated inflammation in mouse intestine. Gastroenterology. 2011;141:249–258. doi: 10.1053/j.gastro.2011.04.006. 258.e1-e2. [DOI] [PubMed] [Google Scholar]
- 74.Guenterberg KD, Grignol VP, Raig ET, Zimmerer JM, Chan AN, Blaskovits FM, et al. Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis. Mol Cancer Ther. 2010;9:510–520. doi: 10.1158/1535-7163.MCT-09-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinha S, Guo Y, Thet S, Yuan D. IFN type I and type II independent enhancement of B cell TLR7 expression by natural killer cells. J Leukoc Biol. 2012;92:713–722. doi: 10.1189/jlb.0212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 77.Hui X, Chen H, Zhang S, Ma X, Wang X, Huang B. Antitumor activities of recombinant human interferon (IFN)-lambda1 in vitro and in xenograft models in vivo for colon cancer. Cancer Lett. 2011;311:141–151. doi: 10.1016/j.canlet.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Fujie H, Tanaka T, Tagawa M, Kaijun N, Watanabe M, Suzuki T, et al. Antitumor activity of type III interferon alone or in combination with type I interferon against human non-small cell lung cancer. Cancer Sci. 2011;102:1977–1990. doi: 10.1111/j.1349-7006.2011.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, et al. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- 80.Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Pulido J, et al. Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer. Cancer Res. 2010;70:4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novak AJ, Grote DM, Ziesmer SC, Rajkumar V, Doyle SE, Ansell SM. A role for IFN-lambda1 in multiple myeloma B cell growth. Leukemia. 2008;22:2240–2246. doi: 10.1038/leu.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steen HC, Gamero AM. Interferon-lambda as a potential therapeutic agent in cancer treatment. J Interferon Cytokine Res. 2010;30:597–602. doi: 10.1089/jir.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 84.Vareille M, Kieninger E, Alves MP, Kopf BS, Moller A, Geiser T, et al. Impaired type I and type III interferon induction and rhinovirus control in human cystic fibrosis airway epithelial cells. Thorax. 2012;67:517–525. doi: 10.1136/thoraxjnl-2011-200405. [DOI] [PubMed] [Google Scholar]
- 85.Gaudieri S, Lucas M, Lucas A, McKinnon E, Albloushi H, Rauch A, et al. Genetic variations in IL28B and allergic disease in children. PLoS One. 2012;7:e30607. doi: 10.1371/journal.pone.0030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 87.Edwards MR, Johnston SL. Interferon-lambda as a new approach for treatment of allergic asthma? EMBO Mol Med. 2011;3:306–308. doi: 10.1002/emmm.201100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, et al. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 89.Gallagher G, Megjugorac NJ, Yu RY, Eskdale J, Gallagher G, Siegel R, et al. The lambda interferons: guardians of the immune-epithelial interface and the T-helper 2 response. J Interferon Cytokine Res. 2010;30:603–615. doi: 10.1089/jir.2010.0081. [DOI] [PubMed] [Google Scholar]
- 90.Moriwaki A, Matsumoto K, Matsunaga Y, Fukuyama S, Matsumoto T, Kan-o K, et al. IL-13 suppresses double-stranded RNA-induced IFN-lambda production in lung cells. Biochem Biophys Res Commun. 2011;404:922–927. doi: 10.1016/j.bbrc.2010.12.082. [DOI] [PubMed] [Google Scholar]
- 91.Bullens DM, Decraene A, Dilissen E, Meyts I, De Boeck K, Dupont LJ, et al. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clin Exp Allergy. 2008;38:1459–1467. doi: 10.1111/j.1365-2222.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- 92.Wu Q, Yang Q, Lourenco E, Sun H, Zhang Y. Interferon-lambda1 induces peripheral blood mononuclear cell-derived chemokines secretion in patients with systemic lupus erythematosus: its correlation with disease activity. Arthritis Res Ther. 2011;13:R88. doi: 10.1186/ar3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin SC, Kuo CC, Tsao JT, Lin LJ. Profiling the expression of interleukin (IL)-28 and IL-28 receptor alpha in systemic lupus erythematosus patients. Eur J Clin Invest. 2012;42:61–69. doi: 10.1111/j.1365-2362.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 94.Zahn S, Rehkamper C, Kummerer BM, Ferring-Schmidt S, Bieber T, Tuting T, et al. Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNlambda) in cutaneous lupus erythematosus. J Invest Dermatol. 2011;131:133–140. doi: 10.1038/jid.2010.244. [DOI] [PubMed] [Google Scholar]
- 95.He SH, Song CH, Liu Z, Zhang H, Ma W, Zhou LF, et al. Eosinophil-derived interferon-lambda contributes to initiation of allergen-related inflammation in the intestine. Cytokine. 2012;58:186–192. doi: 10.1016/j.cyto.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Siebler J, Wirtz S, Weigmann B, Atreya I, Schmitt E, Kreft A, et al. IL-28A is a key regulator of T-cell-mediated liver injury via the T-box transcription factor T-bet. Gastroenterology. 2007;132:358–371. doi: 10.1053/j.gastro.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 97.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–208. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 98.Rizza P, Moretti F, Belardelli F. Recent advances on the immunomodulatory effects of IFN-alpha: implications for cancer immunotherapy and autoimmunity. Autoimmunity. 2010;43:204–209. doi: 10.3109/08916930903510880. [DOI] [PubMed] [Google Scholar]
- 99.Pagliaccetti NE, Robek MD. Interferon-lambda in HCV infection and therapy. Viruses. 2010;2:1589–1602. doi: 10.3390/v2081589. [DOI] [PMC free article] [PubMed] [Google Scholar]