Abstract
Invariant natural killer T (iNKT) cells represent a specialized subset of innate lymphocytes that recognize lipid and glycolipid antigens presented to them by nonclassical MHC-I CD1d molecules and are able to rapidly secrete copious amounts of a variety of cytokines. iNKT cells possess the ability to modulate innate as well as adaptive immune responses against various pathogens. Intracellular bacteria are one of the most clinically significant human pathogens that effectively evade the immune system and cause a myriad of diseases of public health concern globally. Emerging evidence suggests that iNKT cells can confer immunity to intracellular bacteria but also inflict pathology in certain cases. We summarize the current knowledge on the contribution of iNKT cells in the host defense against intracellular bacterial infections, with a focus on the underlying mechanisms by which these cells induce protective or pathogenic reactions including the pathways of direct action (acting on infected cells) and indirect action (modulating dendritic, NK and T cells). The rational exploitation of iNKT cells for prophylactic and therapeutic purposes awaits a profound understanding of their functional biology.
Key Words: Natural killer T cells, Immune response, T helper cells, Intracellular bacteria
Introduction
Natural killer T (NKT) cells are an unconventional T lymphocyte population that belongs to the family of innate lymphocytes. The term ‘NKT cells’ is nowadays used for CD1d-restricted cells. These cells recognize lipids and glycolipids presented by the nonclassical MHC-I CD1d molecules and share some markers typical of NK cells (such as NK1.1 in mice and CD161 in humans). However, it should be noted that not all NKT cells express NK cell markers, and that not all T cells with NK cell markers are CD1d-restricted NKT cells [1]. NKT cells consist of 2 types: type 1 or classical/invariant NKT (iNKT) and type 2 or nonclassical NKT cells. The iNKT cells are the most widely studied; they express a semi-invariant αβ T cell receptor (TCR) with the use of chain Vα14-Jα18 in mice and Vα24-Jα18 in humans. This unique conformation of αβ TCR chains enables them to specifically recognize lipid and glycolipid antigens presented to them by nonclassical major histocompatibility complex I CD1d molecules [1, 2]. A glycosphingolipid, α-galactosylceramide (α-GalCer), which was originally extracted from a marine poriferan, Agelas mauritianus, is considered to be the prototypic antigen for iNKT cells and has been used to track and target iNKT cells in mice and humans [3, 4, 5, 6, 7]. Recently, some microbial antigens recognized by iNKT cells have been identified, most notably the sphingolipids of Sphingomonas [8, 9, 10, 11, 12]. A hallmark of iNKT cells is the impromptu production of multiple T helper (TH)1, TH2 and TH17 cytokines following their activation [1, 13, 14]. The possession of this characteristic endows iNKT cells with the ability to play a role in diverse conditions, such as tumor rejection, regulation of autoimmune diseases and defense against various pathogens [15, 16]. In contrast to iNKT cells, type 2 NKT cells, although being CD1d-restricted, lack the invariant TCR and do not react with α-GalCer. They express a more diverse αβ TCR repertoire and recognize hydrophobic antigens like sulfatides. Type 2 NKT cells appear to be a heterogeneous population of cells, but their immunobiology is not well understood [16].
Intracellular bacteria possess the ability to gain access to and replicate within the host cells, which allows them to efficiently evade the immune system as well as to successfully continue their life/developmental cycle [17]. Some of these bacteria including Mycobacterium and Chlamydia afflict humans with a wide spectrum of diseases, thus posing a menace to public health across the globe. Accumulating evidence suggests that TH1 responses, characterized by enhanced IFN-γ production, are critical for protective immunity to intracellular bacteria, while TH2 responses may culminate in susceptibility and/or immunopathology [18]. Significant data have been generated from mouse models and human studies to support an important role for iNKT cells in intracellular bacterial infections. While many studies show that iNKT cells elicit protection against bacteria, some have also highlighted a pathogenic effect of these cells on the outcome of infection. Growing evidence further points out that iNKT cells can directly act on infected cells to kill them and/or indirectly impact the quality and quantity of host immune responses via modulation of the function of other immune cells like dendritic cells (DC) [8, 19, 20, 21, 22, 23, 24, 25]. In this article, we review the literature on the role that iNKT cells play in inducing protective immunity and immunopathology against intracellular bacterial infections and the underlying mechanisms that mediate their effector functions. Understanding the functional dynamics of iNKT cells may provide new modes of intervention for prophylactic and therapeutic purposes.
iNKT Cells in Immunity to Intracellular Bacterial Infections in Mouse Models
A great deal of evidence on the role of iNKT cells in intracellular bacterial infections comes from studies using knockout (KO) mice, α-GalCer stimulation and adoptive transfer approaches (table 1). Treatment with α-GalCer has been found to reduce bacterial load and pathology as well as prolong the survival of susceptible mice following Mycobacterium tuberculosis infection, suggesting that the activation of iNKT cells by α-GalCer promotes protective immunity [26]. In combination with isoniazid, an antituberculosis drug, α-GalCer shows a strong synergistic effect in controlling murine pulmonary tuberculosis [27]. Whether the pharmacological ability of α-GalCer to induce protection represents the physiological role played by iNKT cells in vivo is an important question to be addressed. In this context, it is noteworthy that, depending on the administration, α-GalCer can cause anergy or unresponsiveness and a loss of iNKT cells [28, 29]. Following infection with M. tuberculosis, CD1d-KO mice, which lack both iNKT and type 2 NKT cells, showed no significant difference from wild-type (WT) mice in terms of pathology and survival [30, 31, 32, 33]. In line with these findings, studies using Jα18/Jα281-KO mice, which are deficient in iNKT cells, found that there were no appreciable differences in the pulmonary pathology and bacterial burden between KO and WT mice after infection challenge [33, 34]. This indicates that the absence of iNKT cells does not change the outcome of mycobacterial infection, implying that these cells exhibit a state of redundancy and are dispensable for optimal immunity. However, adoptive transfer experiments by Sada-Ovalle et al. [20] recently demonstrated that iNKT cells play a protective role in immune responses against M. tuberculosis infection. Following the adoptive transfer of iNKT cells from naïve mice into irradiated mice infected with M. tuberculosis, the recipient mice showed a reduced bacterial burden in both the lung and spleen [20]. These findings contrast with those from previous studies. The discrepancy could be attributed to the low number of lung-resident iNKT cells that were insufficient to effectively control infection (in the early studies) versus the large number of adoptively transferred iNKT cells (in the study by Sada-Ovalle et al. [20]) that maybe amplified the protective effect. It may be speculated that, in natural infection due to M. tuberculosis, iNKT cells are not activated optimally whereas in a state of enhanced stimulation as observed in the α-GalCer activation model [26], they can exert an active protective response. More studies are needed to address the functional role of iNKT cells and the mechanisms in host immune responses against M. tuberculosis infection.
Table 1.
Overview of the role of iNKT cells in murine intracellular bacterial infections
| Bacterial species | Route of infection | Primary site of infection | Experimental model/approach for analysis | Effect of iNKT cells on outcome of infection | References |
|---|---|---|---|---|---|
| M. tuberculosis | intranasal | lungs | α-GalCer, CD1d-KO, Jα18-KO, adoptive transfer |
protection, no effect, no effect, protection |
[20, 26, 27, 30, 31, 32, 33, 34] |
| C. trachomatis | intra-articular | knee joint | α-GalCer, CD1d-KO |
protection, protection |
[36] |
| C. muridarum | intranasal | lungs | α-GalCer, CD1d-KO, Jα18-KO |
pathology, pathology, pathology |
[19, 35, 37, 38] |
| intravaginal | genital tract | α-GalCer, CD1d-KO, Jα18-KO |
protection, pathology, pathology |
||
| C. pneumoniae | intranasal | lungs | α-GalCer, Jα18-KO, adoptive transfer |
protection, protection, protection |
[19] |
| L. pneumophila | intranasal | lungs | α-GalCer, Jα-18-KO |
no effect, pathology |
[39] |
| S. typhimurium | oral | spleen and liver | CD1d-KO | no effect | [41] |
| S. choleraesuis | intraperitoneal | spleen and liver | Jα18-KO | pathology | [40] |
| L. monocytogenes | oral | liver | α-GalCer, Jα18-KO, adoptive transfer |
protection, pathology, protection |
[22, 23] |
| E. muris | intraperitoneal | liver | CD1d-KO | protection against mild infection/pathology against lethal infection | [10, 42] |
Using different chlamydial infection models, we and others have extensively investigated the contribution of iNKT cells in the host defense against intracellular bacteria. To delineate the role of iNKT cells, investigators have used various experimental approaches, KO mice (including the CD1d-KO and Jα18-KO strains), and α-GalCer-induced iNKT stimulation. In vivo stimulation with α-GalCer ameliorated the genital-tract Chlamydia muridarum infection, a mouse biovar of Chlamydia trachomatis[35]. Bharhani et al. [36] also reported that the activation of iNKT cells by α-GalCer induced both a protective and a regulatory role in C. trachomatis-induced arthritis. These results were further confirmed by using CD1d-KO mice [36]. In a lung infection model, α-GalCer induced protection against Chlamydophila pneumoniae[19]. Consistent with the finding obtained using the α-GalCer treatment model, when we used Jα18-KO mice, we found that iNKT cells play a critical role in enhancing protective immunity to C. pneumoniae infection through skewing the adaptive T cell responses towards the development of protective type 1 immune responses [19]. Rather surprisingly, we found an increased resistance to C. muridarum infection in CD1d-KO and Jα18-KO mice, which suggested that NKT cells exacerbate the infection [19, 37]. These KO mice exhibited a significantly reduced weight loss, chlamydial in vivo growth and lung pathology compared with WT mice following C. muridarum infection. Further analysis of immune response in the KO mice showed reduced TH2 responses [19, 37]. Consistently, when subjected to a C. muridarum infection of the genital tract, CD1d-KO mice showed reduced pathological changes in the oviduct [38]. The comparison of the iNKT cells in C. pneumoniae and C. muridarum infections has revealed distinct cytokine patterns, i.e. the predominant secretion of IFN-γ in the former and of IL-4 in the latter, which suggests that functionally different iNKT cell subsets (e.g. iNKT-like or type 2-like cells) were generated. The type of iNKT cell response matched the cytokine patterns of CD4 and CD8 T cells and so the type of adaptive immune responses resulted in either a protective or a pathological outcome in these two different infections, although the species are related [19]. The mechanism underlying the generation of differential iNKT cell responses in these two Chlamydia infections remains unclear. It is likely that iNKT cells are activated by certain antigens/epitopes specific for the bacterial species and that the antigenic variability between the C. muridarum and C. pneumoniae species may account for the differential functional roles of these cells. In a broad sense, the above studies suggest that the effect of NKT cell activation or the activation itself is pathogen-specific or even strain-specific and that this may account for the immunological and infectious outcomes. More in-depth studies on the characterization of glycolipid antigens derived from different chlamydial species and the antigenic effects on iNKT cells may provide further insight into the mechanisms underlying the immunological activity exerted by these cells.
During lung infection with Legionella pneumophila, no significant changes in bacterial burden and pathology in the lungs of mice were observed, despite α-GalCer administration; however, following infection, Jα18-KO mice exhibited greater resistance and survival with reduced bacterial loads and less severe lung injury than control mice [39]. Similarly, Jα18-KO mice showed more resistance than the control mice against Listeria monocytogenes infection [23]. However, studies with α-GalCer stimulation and the adoptive transfer of iNKT cells into alymphoid Rag−/− γ−/− mice led to protection against L. monocytogenes infection, with a reduced bacterial burden and systemic production of IFN-γ [22, 23]. Together, these findings show that iNKT cells exert a pathogenic effect in the natural response against L. monocytogenes, but induce protection if they are activated by α-GalCer or adoptively transferred to immune-deficient mice. A distinction in the two subsets, NK1.1+ and NK1.1- iNKT cells, in terms of kinetics and function has been observed in murine listeriosis. Interestingly, the NK1.1+ iNKT cells produce large quantities of IL-4 and exacerbate the disease whereas NK1.1- iNKT cells secreting IFN-γ exert a protective effect [23]. It is becoming increasingly clear that different subsets of iNKT cells could play different roles during microbial infections. In Salmonella choleraesuis infection, reduced hepatic pathology and serum alanine transaminase levels in Jα18-KO mice have suggested a pathogenic role for iNKT cells in the causation of hepatic injury [40]. In another study, although Salmonella typhimurium infection induced rapid activation of iNKT cells, as reflected in their increased numbers, the upregulation of CD69 and the production of IFN-γ, there was no significant difference observed in the bacterial load between the CD1d-KO and control mice [41].
When subjected to a mildly virulent Ehrlichia muris infection, CD1d-KO mice were unable to clear bacteria compared to the WT mice, suggesting that NKT cells play a crucial role in protection against this infection [10]. However, in a lethal model of ehrlichial infection, although NKT cells induced early protection against infection, they promoted Ehrlichia-mediated toxic shock-like syndrome and liver injury [42]. Thus, NKT cells exert both protective as well as pathogenic effects in ehrlichial infection, depending upon the virulence of the bacterial strain used.
Discrepancies in findings from studies using different infection models and experimental approaches regarding the role of iNKT cells in various intracellular bacterial infections underscore careful scrutiny. It is quite possible that the effect of iNKT cell activation on the outcome of infection is influenced by multiple factors, such as type of pathogen/nature of antigens, infective dose and disease stage. Since the host genetic background is a key factor influencing the immune response and outcome of infections, it is of importance to test the effect of iNKT cells in mice with different genetic backgrounds in mouse models of infection. It is also important to use control mice (heterozygous/homozygous WT mice) that are littermates to KO animals. Evidence points out that iNKT cells can play diverse roles in immune responses even in infections by closely related bacterial species, and also in the case of infections due by the same species but at different sites.
iNKT Cells in Immunity to Intracellular Bacterial Infections in Humans
iNKT cell biology in humans with intracellular bacterial infections is still obscure due to the low frequencies of these cells. Emerging evidence in this area is mainly derived from tuberculosis patients. Alteration in the frequencies of iNKT cells has been reported in pulmonary tuberculosis patients. Using antibodies mainly against CD3 and the Vα24 and Vβ11 chains of iNKT cell TCR to identify iNKT cells, some studies have shown a numerical deficiency of iNKT cells in tuberculosis patients [43, 44, 45, 46, 47]. However, other studies contradict these findings and report a steady incline in the number of these cells in the peripheral blood [48, 49, 50, 51]. For example, a flow cytometric analysis of peripheral blood T lymphocytes in patients with pulmonary tuberculosis showed that the number of iNKT cells was much higher in patients than in controls [48], and that these cells secreted enhanced levels of IFN-γ and IL-4 [50]. Another study examined the frequency of iNKT cells in tuberculosis patients with or without diabetes mellitus, showing that these cells are significantly increased in the peripheral blood and bronchoalveolar lavage of the diabetic compared to in the nondiabetic patients [49]. This increase in iNKT cell number may have been due to the higher bacterial burden in the diabetic tuberculosis patients. In humans, iNKT cells can be identified using α-GalCer-loaded CD1d tetramers and T cell markers [52, 53]. By combining the use of CD1d-αGalCer tetramer and anti-Vα24 antibodies, Lee et al. [53] showed that iNKT cells could be stained with more specificity, even at very low frequencies in the blood. iNKT cells can be divided into distinct subsets on the basis of the presence or absence of CD4 and CD8 expression, i.e. CD4+CD8-, CD8+CD4-, CD4+CD8+ and CD4-CD8- iNKT cells. CD4+CD8- iNKT cells have been shown to secrete both TH1 and TH2 cytokines, but CD8+CD4- iNKT cells predominantly produce TH1 cytokines [52, 53]. In active tuberculosis patients, CD4-CD8- iNKT cells are significantly reduced in the blood, while CD4+CD8- iNKT cells increase [54]. In the case of Brucella suis infection, CD4+CD8- iNKT cells have been found to effectively inhibit bacterial growth and/or kill the bacteria through the production of IFN-γ and cytotoxic activities [55]. Recently, Kee et al. [46] conducted a detailed analysis of iNKT cells in patients with active M. tuberculosis infection. They found that the number of these cells was lower in the peripheral blood of both pulmonary and extrapulmonary tuberculosis patients when compared to patients with latent tuberculosis and to healthy controls. The reduction of iNKT cells in the infected patients was significantly correlated to C-reactive protein levels. Furthermore, M. tuberculosis-infected patients show a reduced response to α-GalCer, found to be due to increased iNKT cell apoptosis and reduced CD1d expression. In addition, iNKT cells from tuberculosis patients show higher levels of the inhibitory programmed death-1 (PD-1) receptor, and the blocking of PD-1 signalling improves the response to α-GalCer [46]. Thus, iNKT cell levels and functions appear to be diminished in M. tuberculosis patients and these deficiencies reflect the presence of active tuberculosis. On the other hand, it has been shown that αGalCer-activated human iNKT cells restrict the growth of intracellular M. tuberculosis in vitro in a CD1d-dependent manner [56]. Taken together, these findings suggest a possible role for iNKT cells in host antibacterial resistance to M. tuberculosis infection. From a clinical standpoint, the determination of iNKT cell alterations in the peripheral blood has an important implication for human diseases such as tuberculosis, as analysis of these cells could serve as an immunological adjunct marker for assessing disease activity, albeit with extreme caution. Future studies are needed to examine the biology of these cells in other intracellular bacterial infections. Of note, the method used to analyze iNKT cells appears to be critical for accuracy in flow cytometric analysis because the activated iNKT cells may show downregulation/internalization of some of the surface markers, such as NK1.1 molecules [57]. The use of α-GalCer or its analogue-loaded CD1d tetramers is more reliable for iNKT cell analysis.
Mechanisms of iNKT Cell-Induced Immunity to Intracellular Bacterial Infections
The activation of iNKT cells is an initial but crucial step for their function in immunity, which can be CD1d-dependent and/or CD1d-independent. CD1d-dependent activation of iNKT cells involves the interaction between iNKT TCR and its cognate ligand, whereas in CD1d-independent activation, local cytokines and other immune cells can lead to iNKT-cell activation [24]. Much of our understanding of how iNKT cell activation occurs via iNKT TCR is based on in vitro and in vivo studies using α-GalCer and its analogues, which specifically activate iNKT cells in a CD1d-dependent fashion [3, 4, 5, 6, 7]. While many studies have documented a function for iNKT cells during infection, if microbial antigens are indeed the major activator of iNKT cells has been a contentious issue. Emerging data illustrate that iNKT cells possess antigen sensitivity against glycolipids and lipids derived from some bacterial pathogens, both extracellular and intracellular. The first natural iNKT cell microbial antigen, a tetramannosylated form of phosphatidylinositol, was extracted from Mycobacterium bovis BCG, which activated the iNKT cells, as measured by their IFN-γ production and cytotoxic activities [12]. Recently, we identified the ability of a glycolipid exoantigen purified from C. muridarum (GLXA) to specifically activate iNKT cells. The GLXA activated the cells to express higher levels of the activation marker CD69, and enhanced the production of IFN-γ and IL-4 in vivo [9]. In accordance with these findings, Jiang et al. [38] showed that sonicated C. muridarum activated iNKT and type 2 NKT cells in an antigen-presenting cell-free culture system coated with CD1d molecules and with the use of iNKT or type 2 NKT cell hybridomas. Since the ligands of type 2 NKT cells and iNKT cells are different [58, 59], it is likely that C. muridarum contains different kinds of lipid antigens for these NKT cell types. The nature of antigens specific for activating type 2 NKT cells is not yet clear. Nevertheless, the above findings suggest that the conserved TCR of iNKT cells may be directly involved in mounting the immune responses against intracellular bacterial infections.
It is important to consider that infection induces a very dynamic environment inside the host with changes occurring in the responses by immune cells, their interactions and the release of soluble mediators, such as cytokines and chemokines. Besides antigen-specific activation by direct recognition of microbial glycolipid antigens via their TCR, the activation of iNKT cells can occur through innate or inflammatory stimuli, in conjunction with self-glycolipid antigen recognition [10, 60]. Recently, a study by Brigl et al. [61] investigated the mechanism of iNKT cell activation in response to microbial infection by using a large panel of diverse bacteria, including some intracellular bacterial pathogens like L. monocytogenes and M. tuberculosis. Their findings suggest that innate cytokine-driven activation is the dominant pathway for iNKT cell activation in response to infectious agents that induce the production of IL-12 by antigen-presenting cells like DC after Toll-like receptor-mediated activation. For the function of iNKT cells in the host defense against intracellular bacterial infections, a combination of direct and indirect mechanisms may operate in which the polarization of either mechanism may depend on the type of pathogen, the dose/stage of infection and the genetic background. Once activated, iNKT cells can lead to the generation of immune responses against infection via pathways of direct and/or indirect actions.
Pathway of Direct Action
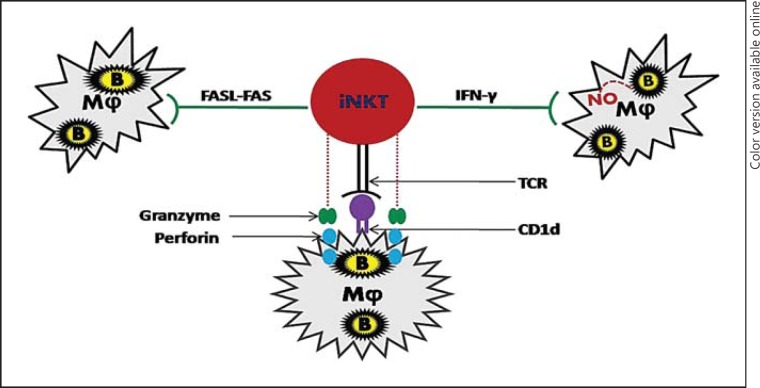
In this pathway, iNKT cells can directly act upon bacteria-infected macrophages and suppress their growth and/or kill them without the assistance of other cell types (fig. 1). Recognition of infected macrophages by iNKT cells is CD1d-dependent and is critical for the inhibition of bacterial replication [20, 21]. Coculture of splenocytes and macrophages infected with M. tuberculosis has shown that IFN-γ production by iNKT cells leads to the production of nitric oxide (NO), which subsequently suppresses the bacterial replication, suggesting that IFN-γ plays a key role in containing bacterial infection [20]. In contrast, although Jα18-KO mice showed increased resistance to L. pneumophila infection compared to WT mice, the level of IFN-γ was not different between the KO and WT mice [39]. Moreover, Selvanantham et al. [62] recently demonstrated that cytosolic peptidoglycan-sensing receptors Nod1 and Nod2 are necessary for optimal IFN-γ production by iNKT cells during S. typhimurium and L. monocytogenes infections. The inclusion of neutralizing anti-IL-12p40 or IL-18 antibodies in the coculture abrogated the production of IFN-γ and NO, resulting in altered control of bacterial growth. This suggests that an iNKT cell-mediated bactericidal effect is dependent on IL-12p40 and IL-18 [20]. Another mechanism used by iNKT cells is Fas-FasL (Fas ligand) signalling, in which the FasL on the iNKT cells interacts with the Fas molecules on the infected macrophages and triggers the death of infected cells. The bactericidal effect of the iNKT cells was severely impaired upon blocking Fas-FasL signalling with anti-Fas antibodies [21]. A recent study revealed that increased FasL expression on iNKT cells is modulated by Toll-like receptor 2 [63]. In addition, iNKT cells have also been shown to release lytic granules such as perforin and granzyme that kill the infected cells [21, 56]. Collectively, an iNKT cell-mediated direct bactericidal effect may result from a single or combination of different mechanisms, such as the production of IFN-γ and NO, the discharge of lytic granules and Fas-FasL interactions.
Fig. 1.
Pathway of direct action: direct action of iNKT cells upon bacteria-infected macrophages to inhibit bacterial growth. IFN-γ production by iNKT cells stimulates infected macrophages to secrete NO that subsequently inhibits bacterial growth. It can also inhibit the growth of bacteria through enhanced cytotoxic activities, such as the induction of Fas-FasL signalling and the release of perforin and granzyme, which lead to apoptosis of the infected cells. B = Intracellular bacterium; Mφ = macrophage.
Pathway of Indirect Action
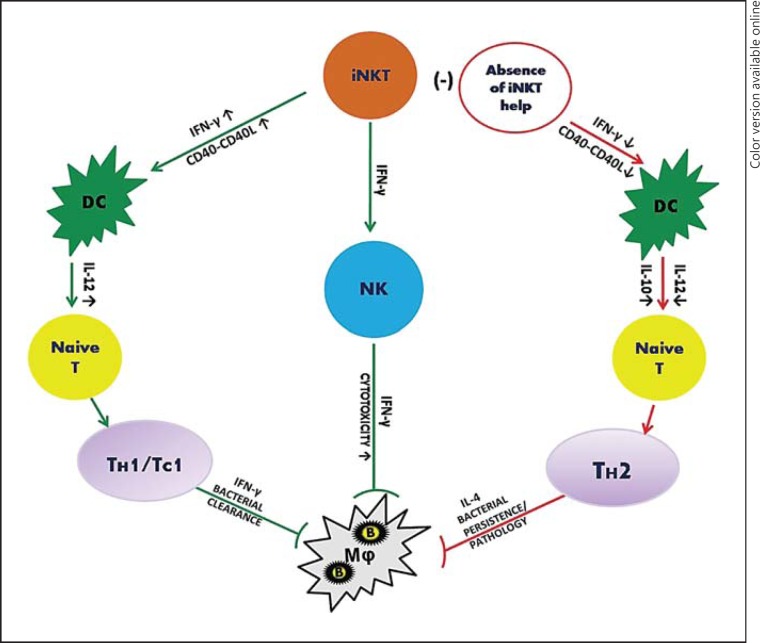
The pathway by which iNKT cells can orchestrate the induction of adaptive immunity by modulating the functions of other immune cells, such as DC and NK cells, is referred to as the pathway of indirect action (fig. 2). Considering the small percentage of iNKT cells compared to effector CD4+ and CD8+ T cells and NK cells, this indirect pathway appears to be more broadly involved in the host defense against infections. Earlier studies using α-GalCer model systems have shown that the activation of iNKT cells induced rapid maturation of DC in vivo and subsequently enhanced T cell immune responses against antigenic challenge [64, 65, 66, 67]. Subsequently, the functional cooperation of iNKT cells with DC in immunity to viral and bacterial infections was also reported [8, 61, 68, 69, 70, 71]. Infection with influenza A virus led to reduced accumulation and maturation of DC in Jα18-KO mice compared to WT mice, but iNKT cells promoted a type 1 IFN response against lymphocytic choriomeningitis virus by plasmacytoid DC interaction, suggesting that iNKT cells can exercise their function through DC [68, 69]. In order to unravel the iNKT cell-mediated mechanisms of protective immunity to intracellular bacterial infections, we recently examined the effect of iNKT cell activation in influencing DC function using Jα18-KO mice, DC-adoptive transfer approaches and a C. pneumoniae lung infection model. Upon adoptive transfer, splenic DC from infected WT mice induced strong TH1 responses whereas those from KO mice induced TH2 responses and increased disease severity following infection challenge, suggesting that iNKT cells modulate DC function to induce the protective T cell responses [70]. Furthermore, splenic DC consist of two subsets, CD8α+ and CD8α- DC, that have different physiological roles. It has been shown that CD8α+ DC induce TH1 dominant responses whereas CD8α- DC induce TH2 responses [72, 73]. In C. pneumoniae infection, iNKT cells preferentially modulated the function of CD8α+ DC in inducing protective TH1 immunity [71]. Similar to splenic DC, iNKT cells transformed the function of lung DC, which reside at the primary site of C. pneumoniae infection, to impact T cell responses [unpubl. observation]. We further examined the molecular basis of iNKT cell-DC crosstalk in this infection model, and found that DC from KO mice showed reduced CD40 expression and IL-12 production whereas enhancing iNKT cell activation using α-GalCer increased CD40 expression and IL-12 production. The coculture of DC with iNKT cells induced a higher IL-12 production by DC in a CD40-CD40L-, IFN-γ- and cell-cell contact-dependent fashion [70]. These findings revealed that the induction of protective type 1 T cell (i.e. both CD4 and CD8 T cells) responses by iNKT cells through DC modulation is largely dependent on IFN-γ and CD40-CD40L signalling.
Fig. 2.
Pathway of indirect action: modulation of DC, NK and T cells by iNKT cells in order to influence the outcome of infection. iNKT cells induce IL-12 secretion by DC, which influences naïve T cells to get transformed into TH1/TC1 (cytotoxic T cells) cells and confer protective CD4/CD8 T cell responses against infection. iNKT cell-mediated modulation of DC function is largely dependent upon CD40-CD40 ligand interaction and IFN-γ production. In certain bacterial infections, in the absence of assistance from iNKT cells, DC function is modulated and the DC direct T cells towards a TH2 response, with increased IL-4 secretion, resulting in susceptibility and/or immunopathology. In addition, iNKT cells can also influence NK cells to modulate the immune response. iNKT cells secrete IFN-γ that induces NK cells to enhance their cytotoxic activity and kill the bacteria inside the macrophages. B = Intracellular bacterium; CD40L = CD40 ligand; Mφ = macrophage; (-) = absence; ↑ = higher; ↓ = lower.
The interaction of iNKT cells with other immune cells is, however, not confined to DC alone. iNKT cells can also communicate with NK cells to modulate the immune responses against chlamydial infection, which is in agreement with the well-documented findings in α-GalCer-stimulated models. During C. muridarum infection, a deficiency of iNKT cells leads to the reduced expansion of NK cells with differential changes in IFN-γ production and the degranulation of NK cells. This enhances IFN-γ production but, at the same time, inhibits cytolytic function [74].
Conclusions
The role of iNKT cells in the host defense against intracellular bacteria is in the process of being elucidated. These cells not only induce innate immunity, but also steer the direction and magnitude of subsequent adaptive immunity via various mechanisms, including the modulation of functions of other cell types of the immune system such as DC, NK cells and T cells, thus serving as a bridge between innate and adaptive immunity. Although iNKT cells are mostly involved in protection against these bacterial infections, some studies point towards a pathogenic role of iNKT cells where they exert a deleterious effect on the outcome of infection. Thus, these cells can be either beneficial or detrimental to the host antibacterial defense, depending upon the type of pathogen, site of infection and host background. Since most data concerning the role of iNKT cells in host defense to infections have been generated from studies in mouse models, it still has to be ascertained if the knowledge gained can be extrapolated to humans. How to rationally harness the therapeutic potential of iNKT cells to combat infections is a pertinent question to be addressed in time to come. Caution should be taken, however, while attempting to develop iNKT cell-based therapies, as this may lead to pathological responses in some situations if it is not carefully designed. Future modalities should be developed on the basis of extensive studies and the functional characterization and dynamics of iNKT cell responses with respect to specific intracellular bacterial infections.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research, the Manitoba Health Research Council (MHRC) and the Manitoba Institute of Child Health (MICH) to X.Y., who was the Canada Research Chair in Infection and Immunity. S.S. is a recipient of an MHRC/MICH graduate scholarship. Ms. Shipra Shekhar deserves special thanks for her help in drawing the figures.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 3.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 4.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda JL, Gapin L, Fazilleau N, Warren K, Naidenko OV, Kronenberg M. Natural killer T cells reactive to a single glycolipid exhibit a highly diverse T cell receptor beta repertoire and small clone size. Proc Natl Acad Sci USA. 2001;98:12636–12641. doi: 10.1073/pnas.221445298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinjo Y, Ueno K. iNKT cells in microbial immunity: recognition of microbial glycolipids. Microbiol Immunol. 2011;55:472–482. doi: 10.1111/j.1348-0421.2011.00338.x. [DOI] [PubMed] [Google Scholar]
- 9.Peng Y, Zhao L, Shekhar S, Liu L, Wang H, Chen Q, Gao X, Yang X, Zhao W. The glycolipid exoantigen derived from Chlamydia muridarum activates invariant natural killer T cells. Cell Mol Immunol. 2012;9:361–366. doi: 10.1038/cmi.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 11.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 12.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SH, Schaible UE. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci USA. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-gamma upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 14.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a Th2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 15.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann SH. Intracellular pathogens: living in an extreme environment. Immunol Rev. 2011;240:5–10. doi: 10.1111/j.1600-065X.2010.01001.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 19.Joyee AG, Qiu H, Wang S, Fan Y, Bilenki L, Yang X. Distinct NKT cell subsets are induced by different Chlamydia species leading to differential adaptive immunity and host resistance to the infections. J Immunol. 2007;178:1048–1058. doi: 10.4049/jimmunol.178.2.1048. [DOI] [PubMed] [Google Scholar]
- 20.Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessoles S, Dudal S, Besra GS, Sanchez F, Lafont V. Human CD4+ invariant NKT cells are involved in antibacterial immunity against Brucella suis through CD1d-dependent but CD4-independent mechanisms. Eur J Immunol. 2009;39:1025–1035. doi: 10.1002/eji.200838929. [DOI] [PubMed] [Google Scholar]
- 22.Ranson T, Bregenholt S, Lehuen A, Gaillot O, Leite-de-Moraes MC, Herbelin A, Berche P, Di Santo., JP Invariant V alpha 14+ NKT cells participate in the early response to enteric Listeria monocytogenes infection. J Immunol. 2005;175:1137–1144. doi: 10.4049/jimmunol.175.2.1137. [DOI] [PubMed] [Google Scholar]
- 23.Emoto M, Yoshizawa I, Emoto Y, Miamoto M, Hurwitz R, Kaufmann SH. Rapid development of a gamma interferon-secreting glycolipid/CD1d-specific Valpha14+ NK1.1- T-cell subset after bacterial infection. Infect Immun. 2006;74:5903–5913. doi: 10.1128/IAI.00311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 25.Behar SM, Porcelli SA. CD1-restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215–250. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- 26.Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immun. 2002;70:6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sada-Ovalle I, Skold M, Tian T, Besra GS, Behar SM. Alpha-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2010;182:841–847. doi: 10.1164/rccm.200912-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayakawa Y, Berzins SP, Crowe NY, Godfrey DI, Smyth MJ. Antigen-induced tolerance by intrathymic modulation of self-recognizing inhibitory receptors. Nat Immunol. 2004;5:590–596. doi: 10.1038/ni1069. [DOI] [PubMed] [Google Scholar]
- 30.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1d or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Souza CD, Cooper AM, Frank AA, Ehlers S, Turner J, Bendelac A, Orme IM. A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am J Respir Cell Mol Biol. 2000;23:188–193. doi: 10.1165/ajrcmb.23.2.4063. [DOI] [PubMed] [Google Scholar]
- 32.Sousa AO, Mazzaccaro RJ, Russell RG, Lee FK, Turner OC, Hong S, Van Kaer L, Bloom BR. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci USA. 2000;97:4204–4208. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, Nakayama T, Taniguchi M, Saito A. Minimal contribution of Valpha14 natural killer T cells to Th1 response and host resistance against mycobacterial infection in mice. Microbiol Immunol. 2002;46:207–210. doi: 10.1111/j.1348-0421.2002.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara I, Yamada H, Mizuno S, Li CY, Nakayama T, Taniguchi M. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis (Edinb) 2002;82:97–104. doi: 10.1054/tube.2002.0331. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Zhao L, Peng Y, Liu J, Qi M, Chen Q, Yang X, Zhao W. Protective role of α-galactosylceramide-stimulated natural killer T cells in genital tract infection with Chlamydia muridarum. FEMS Immunol Med Microbiol. 2012;65:43–54. doi: 10.1111/j.1574-695X.2012.00939.x. [DOI] [PubMed] [Google Scholar]
- 36.Bharhani MS, Chiu B, Na KS, Inman RD. Activation of invariant NKT cells confers protection against Chlamydia trachomatis-induced arthritis. Int Immunol. 2009;21:859–870. doi: 10.1093/intimm/dxp052. [DOI] [PubMed] [Google Scholar]
- 37.Bilenki L, Wang S, Yang J, Fan Y, Joyee AG, Yang X. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J Immunol. 2005;175:3197–3206. doi: 10.4049/jimmunol.175.5.3197. [DOI] [PubMed] [Google Scholar]
- 38.Jiang J, Karimi O, Ouburg S, Champion CI, Khurana A, Liu G, Freed A, Pleijster J, Rozengurt N, Land JA, Surcel HM, Tiitinen A, Paavonen J, Kronenberg M, Morre SA, Kelly KA. Interruption of CXCL13-CXCR5 axis increases upper genital tract pathology and activation of NKT cells following chlamydial genital infection. PLoS One. 2012;7:e47487. doi: 10.1371/journal.pone.0047487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayakawa K, Tateda K, Fuse ET, Matsumoto T, Akasaka Y, Ishii T, Nakayama T, Taniguchi M, Kaku M, Standiford TJ, Yamaguchi K. Paradoxically high resistance of natural killer T (NKT) cell-deficient mice to Legionella pneumophila: another aspect of NKT cells for modulation of host responses. J Med Microbiol. 2008;57:1340–1348. doi: 10.1099/jmm.0.47747-0. [DOI] [PubMed] [Google Scholar]
- 40.Ishigami M, Nishimura H, Naiki Y, Yoshioka K, Kawano T, Tanaka Y, Taniguchi M, Kakumu S, Yoshikai Y. The roles of intrahepatic Valpha14(+) NK1.1(+) T cells for liver injury induced by Salmonella infection in mice. Hepatology. 1999;29:1799–1808. doi: 10.1002/hep.510290605. [DOI] [PubMed] [Google Scholar]
- 41.Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immunol. 2005;35:2100–2109. doi: 10.1002/eji.200425846. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson HL, Crossley EC, Thirumalapura N, Walker DH, Ismail N. Regulatory roles of CD1d-restricted NKT cells in the induction of toxic shock-like syndrome in an animal model of fatal ehrlichiosis. Infect Immun. 2008;76:1434–1444. doi: 10.1128/IAI.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montoya CJ, Catano JC, Ramirez Z, Rugeles MT, Wilson SB, Landay AL. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin Immunol. 2008;127:1–6. doi: 10.1016/j.clim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Snyder-Cappione JE, Nixon DF, Loo CP, Chapman JM, Meiklejohn DA, Melo FF, Costa PR, Sandberg JK, Rodrigues DS, Kallas EG. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. J Infect Dis. 2007;195:1361–1364. doi: 10.1086/513567. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland JS, Jeffries DJ, Donkor S, Walther B, Hill PC, Adetifa IM, Adegbola RA, Ota MO. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis (Edinb) 2009;89:398–404. doi: 10.1016/j.tube.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Kee SJ, Kwon YS, Park YW, Cho YN, Lee SJ, Kim TJ, Lee SS, Jang HC, Shin MG, Shin JH, Suh SP, Ryang DW. Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect Immun. 2012;80:2100–2108. doi: 10.1128/IAI.06018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barcelos W, Martins-Filho OA, Guimaraes TM, Oliveira MH, Spindola-de-Miranda S, Carvalho BN, Toledo Vde., P Peripheral blood mononuclear cells immunophenotyping in pulmonary tuberculosis patients before and after treatment. Microbiol Immunol. 2006;50:597–605. doi: 10.1111/j.1348-0421.2006.tb03834.x. [DOI] [PubMed] [Google Scholar]
- 48.Al Majid FM, Abba AA. Immunophenotypic characterisation of peripheral T lymphocytes in pulmonary tuberculosis. J Postgrad Med. 2008;54:7–11. doi: 10.4103/0022-3859.39182. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, Xiao HP, Cui HY, Sugawara I. Significant increase in natural-killer T cells in patients with tuberculosis complicated by type 2 diabetes mellitus. J Int Med Res. 2011;39:105–111. doi: 10.1177/147323001103900113. [DOI] [PubMed] [Google Scholar]
- 50.Kulpraneet M, Sukwit S, Sumransurp K, Chuenchitra T, Santiwatanakul S, Srisurapanon S. Cytokine production in NK and NKT cells from Mycobacterium tuberculosis infected patients. Southeast Asian J Trop Med Public Health. 2007;38:370–375. [PubMed] [Google Scholar]
- 51.Zahran WA, Ghonaim MM, Koura BA, El-Banna H, Ali SM, El-Sheikh N. Human natural killer T cells (NKT), NK and T cells in pulmonary tuberculosis: potential indicators for disease activity and prognosis. Egypt J Immunol. 2006;13:67–78. [PubMed] [Google Scholar]
- 52.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Im JS, Kang TJ, Lee SB, Kim CH, Lee SH, Venkataswamy MM, Serfass ER, Chen B, Illarionov PA, Besra GS, Jacobs WR, Jr, Chae GT, Porcelli SA. Alteration of the relative levels of iNKT cell subsets is associated with chronic mycobacterial infections. Clin Immunol. 2008;127:214–224. doi: 10.1016/j.clim.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bessoles S, Dudal S, Besra GS, Sanchez F, Lafont V. Human CD4+ invariant NKT cells are involved in antibacterial immunity against Brucella suis through CD1d-dependent but CD4-independent mechanisms. Eur J Immunol. 2009;39:1025–1035. doi: 10.1002/eji.200838929. [DOI] [PubMed] [Google Scholar]
- 56.Gansert JL, Kiessler V, Engele M, Wittke F, Rollinghoff M, Krensky AM, Porcelli SA, Modlin RL, Stenger S. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170:3154–3161. doi: 10.4049/jimmunol.170.6.3154. [DOI] [PubMed] [Google Scholar]
- 57.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to suphatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godfrey DI, Rossjohn J. New ways to turn on NKT cells. J Exp Med. 2011;208:1121–1125. doi: 10.1084/jem.20110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selvanantham T, Escalante NK, Cruz Tleugabulova M, Fiévé S, Girardin SE, Philpott DJ, Mallevaey T. Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection. J Immunol. 2013;191:5646–5654. doi: 10.4049/jimmunol.1301412. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu H, Matsuguchi T, Fukuda Y, Nakano I, Hayakawa T, Takeuchi O, Akira S, Umemura M, Suda T, Yoshikai Y. Toll-like receptor 2 contributes to liver injury by Salmonella infection through Fas ligand expression on NKT cells in mice. Gastroenterology. 2002;123:1265–1277. doi: 10.1053/gast.2002.36006. [DOI] [PubMed] [Google Scholar]
- 64.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 67.Hermans IF, Silk JD, Gileadi U, Masri SH, Shepherd D, Farrand KJ, Salio M, Cerundolo V. Dendritic cell function can be modulated through cooperative actions of TLR ligands and invariant NKT cells. J Immunol. 2007;178:2721–2729. doi: 10.4049/jimmunol.178.5.2721. [DOI] [PubMed] [Google Scholar]
- 68.Paget C, Ivanov S, Fontaine J, Blanc F, Pichavant M, Renneson J, Bialecki E, Pothlichet J, Vendeville C, Barba-Spaeth G, Huerre MR, Faveeuw C, Si-Tahar M, Trottein F. Potential role of inavariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J Immunol. 2011;186:5590–5602. doi: 10.4049/jimmunol.1002348. [DOI] [PubMed] [Google Scholar]
- 69.Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron AS, Tomkiewicz C, Herbelin A, Barouki R, von Herrath M, Dalod M, Lehuen A. NKT cell-plasmacytoid dendritic cell cooperation via Ox40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 70.Joyee AG, Qiu H, Fan Y, Wang S, Yang X. Natural killer T cells are critical for dendritic cells to induce immunity in Chlamydial pneumonia. Am J Respir Crit Care Med. 2008;178:745–756. doi: 10.1164/rccm.200804-517OC. [DOI] [PubMed] [Google Scholar]
- 71.Joyee AG, Uzonna J, Yang X. Invariant NKT cells preferentially modulate the function of CD8 alpha+ dendritic cell subset in inducing type 1 immunity against infection. J Immunol. 2010;184:2095–2106. doi: 10.4049/jimmunol.0901348. [DOI] [PubMed] [Google Scholar]
- 72.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao L, Gao X, Peng Y, Joyee AG, Bai H, Wang S, Yang J, Zhao W, Yang X. Differential modulating effect of natural killer (NK) T cells on interferon-γ production and cytotoxic function of NK cells and its relationship with NK subsets in Chlamydia muridarum infection. Immunology. 2011;134:172–184. doi: 10.1111/j.1365-2567.2011.03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]