Abstract
Plaques containing β-amyloid (Aβ) peptides are one of the pathological features of Alzheimer's disease, and the reduction of Aβ is considered a primary therapeutic target. Amyloid clearance by anti-Aβ antibodies has been reported after immunization, and recent data have shown that the antibodies may act as a peripheral sink for Aβ, thus altering the periphery/brain dynamics. Here we show that peripheral treatment with an agent that has high affinity for Aβ (gelsolin or GM1) but that is unrelated to an antibody or immune modulator reduced the level of Aβ in the brain, most likely because of a peripherally acting effect. We propose that in general, compounds that sequester plasma Aβ could reduce or prevent brain amyloidosis, which would enable the development of new therapeutic agents that are not limited by the need to penetrate the brain or evoke an immune response.
Keywords: Alzheimer's disease, amyloid, Aβ, peripheral sink, sequestration, binding agent
Introduction
β-amyloid (Aβ) is cleaved from the amyloid precursor protein (APP) by sequential proteolytic processing by β- and γ-secretases, which results principally in the generation of Aβ1–40 and Aβ1–42 (Selkoe, 1993). The accumulation of Aβ is thought to be one of the fundamental pathological events in Alzheimer's disease (AD), and its elevation and/or aggregation has been associated with a range of detrimental cellular responses (Small et al., 2001). One therapeutic approach proposed for the treatment of AD has been the reduction of CNS Aβ by antibodies raised against Aβ peptides (Schenk et al., 1999; Janus et al., 2000; Morgan et al., 2000;Weiner et al., 2000; Das et al., 2001) or administered passively (Bard et al., 2000; DeMattos et al., 2001, 2002). One of the proposed mechanisms by which the antibodies may reduce brain Aβ is that anti-Aβ antibodies cross from the periphery to the brain, bind to Aβ in plaques, and stimulate microglial phagocytosis of antibody/Aβ complexes. An alternative proposal based on the observation that anti-Aβ antibodies also significantly influence Aβ transfer between the brain and plasma (DeMattos et al., 2001, 2002) suggests that periphery/brain Aβ dynamics may play a crucial role in the pathogenesis of AD. Because there are several problems associated with immunotherapy, and current clinical trials of actively administered Aβ peptides have been suspended after adverse patient response, we have investigated whether compounds that are unrelated to antibodies, but which have as their salient feature the ability to bind Aβ in the periphery, might be effective in altering the periphery/brain dynamics leading to a reduction of brain Aβ. For these proof-of-concept experiments, we have chosen two compounds with known Aβ binding affinity. Gelsolin is a secretory protein known to bind Aβ via two sites under normal physiological conditions (Chauhan et al., 1999). GM1 is a ganglioside that has a similar affinity for Aβ (Choo-Smith et al., 1997). We propose that derivative drug structures or nonimmunogenic novel structures that specifically bind pathogenic peptides in the periphery may be clinically efficacious, most likely as prophylactic rather than as therapeutic agents.
Materials and Methods
Animals. Mice expressing mutant APPK670N,M671L (mutant APP, Tg2576) (Hsiao et al., 1996) and mutant presenilin (PS)-1M146L(mutant PS-1, line 6.2) (Duff et al., 1996) were crossed to generate PS/APP progeny (Holcomb et al., 1998). Age-matched male and female animals from several litters were equally represented in the vehicle- and drug-treated groups. Two age groups were tested: young mice (at 9–10 weeks of age initially) and mice at 6–7 months of age.
Drug administration and sample preparation. Gelsolin (extracted from bovine plasma; Sigma, St. Louis, MO) or ganglioside GM1 (ammonium salt extracted from bovine brain purchased from Calbiochem, La Jolla, CA) were dissolved in PBS and administered intraperitoneally at a dose of 0.6 and 15 mg/kg body weight, respectively. Gelsolin was injected every 2 d for 3 weeks. GM1 was injected every 2 d for 2 weeks, and the mice were killed after a 1 week washout period. For intracerebroventricular treatment with GM1, an osmotic pump (Alzet, Cupertino, CA) was filled with solution and infused into the lateral ventricle using a brain infusion kit (Alzet) at a dose of 0.15 mg/kg body weight every 2 d for 2 weeks.
For plasma assay, tail blood was collected at predrug, mid-drug, and postdrug treatment times into preweighed tubes containing 10 mm EDTA in PBS. The volume was adjusted to yield a 1:1 ratio of blood/EDTA–PBS. Plasma was separated by centrifugation at 10,000 × g for 5 min.
Mice were perfused with PBS under anesthesia, and brains were dissected into hemispheres. One of each hemisphere was used for ELISA. Brains were extracted either by four-step extraction according to the method of Kawarabayashi et al. (2001) or by two-step extraction according toJanus et al. (2000).
Aβ quantification. Levels of human Aβ40 and Aβ42 in brain extracts and plasma were quantified by ELISA as reported previously (Kawarabayashi et al., 2001) using antibodies supplied by Janssen Pharmaceuticals (Berse, Belgium), as described previously (Refolo et al., 2000). In brief, plates were coated with antibody to either human Aβ40 (JRF/cAβ40/10) or Aβ42 (JRF/cAβ42/26). Freshly thawed samples were diluted and incubated overnight. Signal was detected using a horseradish peroxide-labeled antibody, JRF/Aβtot/17, and an ELISA detection kit (Pierce, Rockford, IL). To confirm that the epitope of ELISA antibodies is not masked, synthetic human Aβ40/Aβ42 (50 fmol/ml) and GM1 (20 μg/ml) or gelsolin (9 μg/ml) were added in mouse plasma and detected as described above.
Statistical analysis. The hypothesis of no difference among treatments was tested using a one-way multivariate ANOVA followed by Fisher's least significant difference post hocpairwise comparisons. All tests contrasted one of the treatments with the vehicle (SPSS, Chicago, IL).
Histochemistry. Hemispheres of brains were fixed in 4% paraformaldehyde overnight and then dehydrated. Two sections at 1.0 mm lateral from the medial line were stained using biotinylated anti-Aβ40/Aβ42 antibody (clone 6E10; Signet, Dedham, MA) and thioflavin S (Sigma). The area covered by staining in the cerebral cortex and hippocampus was measured using microcomputer imaging device software in a blind manner, and an average of two sections was presented as a percentage of total brain area examined. Statistical significance was determined by t test.
GM1 quantification in plasma and blood cells. Plasma and blood cells were separated by centrifugation. Lipid extracts prepared from 10 μl of plasma and 2 μl of blood cell suspension in 0.2% SDS-containing PBS were analyzed on a 96 well plate and on a high-performance thin-layer chromatography plate, respectively, as described by Wu and Ledeen (1988) with slight modification.
Cholesterol assessment. Total cholesterol was measured in 10 μl of plasma from mice at the 2 week time point using a kit (Infinity reagent; Sigma) according to the manufacturer's directions.
Results
Peripheral administration of an Aβ sequestering agent, gelsolin, reduced CNS Aβ in young PS/APP mice
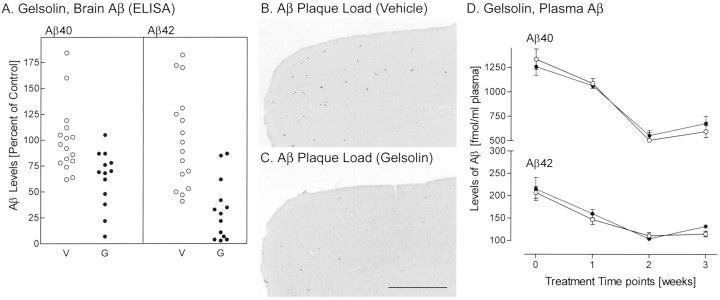
We tested the effect of peripherally administered gelsolin on brain Aβ load in two groups of PS/APP mice. No overt detrimental systemic effects (change of body weight or enlarged spleen) were seen, and there was no evidence of CNS inflammation (enhanced glial fibrillary acidic protein immunoreactivity) (data not shown). Both groups of mice received drug treatment under the same protocol, but brain Aβ was extracted by four-step extraction from set 1 mice and by two-step extraction from set 2 mice (Table 1). Four-step extraction yields soluble Aβ in the TBS fraction, membrane-bound Aβ in the Triton X-100 fraction, and insoluble Aβ in the SDS and formic acid (FA) fractions. Two-step extraction yields TBS soluble Aβ, and all other Aβ is extracted in FA. Gelsolin was injected at an age when pathogenesis is first initiated (9–10 weeks), every 2 d for 3 weeks, and brain Aβ load was examined (Table 1). In both sets of mice, brain Aβ was significantly reduced by gelsolin treatment (set 1, p = 0.02, 0.049; set 2,p = 0.01, 0.008 for Aβ40 and Aβ42, respectively). Results for set 1 were lower than for set 2, because a substantial fraction of Aβ had been extracted into the SDS fraction in the four-step extraction. All of the FA-extracted samples from gelsolin-treated mice were analyzed in duplicate, on the same plate, and the results were represented as a percentage of vehicle-treated mice (in total, n = 16 for vehicle andn = 13 for gelsolin; p = 0.003 and 0.00007 for Aβ40 and Aβ42, respectively) (Fig.1A). Amyloid plaque load (n = 5 mice for gelsolin and for vehicle treatment) was assessed by Aβ immunohistochemistry (to detect both diffuse and compact amyloid) and by thioflavin S staining (to detect fibrillar Aβ). Aβ immunoreactive plaque load (percentage of area covered in the cerebral cortex and hippocampus; mean ± SEM) was significantly reduced by gelsolin treatment (vehicle, 0.170 ± 0.013; gelsolin, 0.084 ± 0.019; p = 0.011) (Fig.1B,C). Thioflavin S-positive plaque load showed a trend toward reduction, but the decline did not reach statistical significance (vehicle, 0.028 ± 0.007; gelsolin, 0.013 ± 0.001; p = 0.106). This suggests that diffuse Aβ is the likely target of treatment. Plasma Aβ40 and Aβ42 levels in gelsolin-treated mice (n = 6) and vehicle-treated mice (n = 7) from set 1 were compared before treatment (0 weeks), after 1 week, after 2 weeks, or after 3 weeks of treatment. During the initial stage of Aβ deposition, the levels of Aβ40 and Aβ42 in the plasma of vehicle-treated mice declined (Fig.1D); no difference in plasma Aβ level was detected between vehicle- and gelsolin-treated mice (p > 0.05 at all time points studied).
Table 1.
Brain Aβ40 and Aβ42 in formic acid extracts from drug-treated and vehicle-treated young PS/APP mice
| n | Aβ40 Aβ42 | ||
|---|---|---|---|
| Picomoles of Aβ per gram of tissue | |||
| Gelsolin-treated mice | |||
| Set 11-a | |||
| Vehicle | 7 | 199 ± 29 | 226 ± 46 |
| Gelsolin | 6 | 89 ± 22 | 95 ± 30 |
| (p = 0.02) | (p = 0.049) | ||
| Set 21-b | |||
| Vehicle | 9 | 429 ± 43 | 644 ± 80 |
| Gelsolin | 7 | 190 ± 33 | 314 ± 60 |
| (p = 0.01) | (p = 0.008) | ||
| GM1-treated mice | |||
| Set 11-a | |||
| Vehicle | 7 | 199 ± 29 | 226 ± 46 |
| GM1 | 6 | 112 ± 23 | 104 ± 28 |
| (p = 0.03) | (p = 0.04) | ||
Data are presented as mean ± SEM.
Aβ was extracted by the four-step method (Kawarabayashi et al., 2001).
Aβ was extracted by the two-step formic acid extraction method (Janus et al., 2000).
Fig. 1.
Effect of gelsolin (●, G) or vehicle (○, V) administration on brain (A–C) and plasma (D) Aβ levels. Brain Aβ load was examined by ELISA (A) and Aβ histochemistry. Typical Aβ immunostaining in mice treated with vehicle (B) and gelsolin (C) is shown. Scale bar, 1 mm. Values represent the mean ± SEM.
Peripheral administration of the Aβ sequestering agent GM1 also reduced CNS Aβ in young PS/APP mice
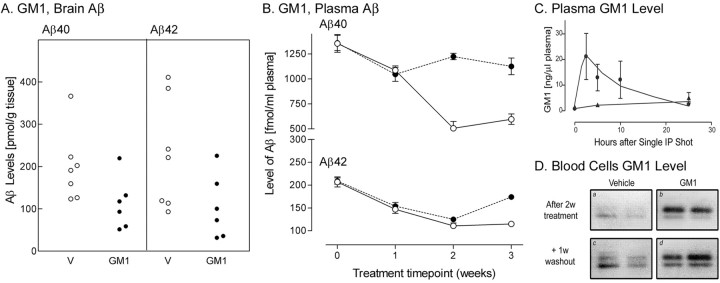
We also tested the hypothesis using a second Aβ sequestering agent, GM1. No overt detrimental systemic effects or evidence of CNS inflammation were seen (data not shown) after 3 weeks of administration. Brain Aβ extracted by the two-step procedure was significantly reduced by treatment with GM1 (p = 0.03, 0.04 for Aβ40 and Aβ42, respectively) (Table 1). In the first week of treatment, the level of plasma Aβ declined in both vehicle-treated animals (n = 6) and GM1-treated animals (n = 7). In the second week, Aβ levels continued to decline in vehicle-treated mice but did not decline in GM1-treated animals; it was therefore effectively elevated relative to vehicle-treated animals (p < 0.001) (Fig.2B). Our data have shown that GM1 is cleared rapidly from the plasma (Fig. 2C) but is maintained in the blood cell fraction for a longer period (Fig.2D). Plasma GM1 has also been shown to be maintained above the endogenous GM1 level for several days after cessation of administration once steady-state levels have been achieved (Rost et al., 1991). We therefore included a 1 week washout period for the GM1 study. After the washout period (3 weeks), the level of Aβ40 was still significantly higher than for vehicle-treated mice (p = 0.004). Aβ42 in GM1-treated mice initially declined similar to vehicle-treated mice, but by the 3 week time point, there was a statistically significant difference between the two groups (p = 0.001). To ensure that the binding of GM1 or gelsolin to Aβ did not reduce the sensitivity of the assay because of epitope masking, ELISA was performed on a known concentration of Aβ with and without the sequestering agents. The epitope of the detection antibody was not masked to any great degree, because ELISA detected 96–105% of added human Aβ.
Fig. 2.
Effect of GM1 (●) or vehicle (○,V) administration on brain (A) and plasma (B) Aβ levels determined by ELISA. The level of GM1 in the plasma (C) was measured after a single administration of drug, over a 24 hr period. GM1 levels were also compared in the blood cell fraction (D) of vehicle-treated and GM1-treated mice 2 weeks after treatment initiation and after a 1 week washout period. The upper, augmented band runs concurrent with administered bovine GM1. Values represent the mean ± SEM.
Because GM1 is known to interact with cholesterol (Kakio et al., 2001), and peripheral cholesterol depletion modulates CNS Aβ levels (Refolo et al., 2001), we measured the level of cholesterol at the 2 week time point in GM1-treated and control mice. Plasma total cholesterol levels were not affected by the dose of GM1 used (0.46 ± 0.031 and 0.45 ± 0.027 mg of total cholesterol per milliliter of plasma after treatment with vehicle and GM1, respectively). Therefore, the effect of GM1 on CNS Aβ levels is not thought to be caused by a reduction of peripheral cholesterol.
We also investigated the relationship between plasma Aβ levels and GM1 kinetics, because GM1 is specifically detectable by cholera toxin B subunit. The level of GM1 was measured in the plasma used for Aβ quantification and also in the cell fraction, because the ganglioside is usually membrane-associated. At the 1 and 2 week time points, 2 d had passed from the last GM1 administration, whereas at the 3 week time point, 8 d had passed. In the blood cell fraction, GM1 was detected as a doublet band by thin-layer chromatography (Fig.2D). Faint lower and upper bands of endogenous GM1 were detected in mice receiving vehicle treatment. In mice receiving GM1 for 2 weeks, the upper band was strongly enhanced, and this was maintained after the washout period. The upper band migrated with standard GM1 extracted from bovine brain, suggesting that most of the GM1 in the blood cell fraction was derived from administered GM1. ELISA measurement of Aβ in the cell fraction from mice at the 2 week time point was uninformative, because the levels were negligible using our preparation protocol. In the plasma fraction, a change in GM1 level was undetectable at any of the time points used for Aβ quantification. However, plasma GM1 was cleared rapidly, because mice treated with a single intraperitoneal injection of the ganglioside showed that GM1 levels in the plasma reached a peak 2.5 hr after the injection and returned to basal levels within 25 hr (Fig. 2C).
The molecular weight of GM1 is 1564, and ∼1% of peripherally administered GM1 has been shown to cross the blood–brain barrier (BBB) (Saulino and Schengrund, 1994). To assess whether Aβ sequestration in the brain rather than the plasma could account for our results, we infused GM1 directly into the brains of mice for ∼3 weeks and measured Aβ levels (n = 7 for vehicle andn = 4 for GM1 treatment). The amount of GM1 infused into the right lateral ventricle (0.15 mg/kg body weight every 2 d) was calculated to be approximately equivalent to 1% of that administered peripherally (15 mg/kg body weight every 2 d). Aβ levels were unchanged in the plasma of mice during or after intracerebroventricular GM1 treatment (data not shown). Aβ40 and Aβ42 levels were significantly increased in the soluble Aβ fraction after 3 weeks of treatment (p = 0.04, 0.001 for Aβ40 and Aβ42, respectively). Levels in the Triton X-100, SDS, or FA fractions were unchanged (Table 2). Therefore, administration of GM1 into the brain ventricles directly results in accumulation of Aβ in the soluble fraction but does not lead to a change in the amount of insoluble/aggregated Aβ.
Table 2.
Brain Aβ40 and Aβ42 in TBS and formic acid extracts after intracerebroventricular infusion of GM1 or vehicle
| n | Aβ40 Aβ42 | ||
|---|---|---|---|
| Picomoles of Aβ per gram of tissue | |||
| TBS-soluble Aβ | |||
| Vehicle infusion | 7 | 1.1 ± 0.1 | 0.7 ± 0.1 |
| GM1 infusion | 4 | 5.8 ± 1.6 | 2.1 ± 0.2 |
| (p = 0.04) | (p = 0.001) | ||
| FA-soluble Aβ | |||
| Vehicle infusion | 7 | 179 ± 43 | 210 ± 37 |
| GM1 infusion | 4 | 212 ± 26 | 231 ± 15 |
| (p = 0.29) | (p = 0.98) | ||
Aβ was extracted by the four-step method (Kawarabayashi et al., 2001). Data are presented as mean ± SEM.
GM1 administration was not effective in reducing CNS Aβ in mice with severe amyloid burden
To assess whether GM1 would be effective in reducing Aβ levels in mice with more severe amyloid pathology, we administered GM1 to mice at 6–7 months of age every 2 d for 2 weeks, followed by the 1 week washout period (n = 5 for vehicle or GM1 treatment). Using this protocol, brain and plasma levels of Aβ were unaffected (data not shown). Although it is possible that a longer administration period of GM1 would impact plasma and CNS Aβ levels to a greater extent, it is also possible that the amyloid load is too great in these mice (or the plaques are too stable) to be impacted by treatment at this stage in disease progression. Interestingly, published vaccine-based therapeutic approaches also failed to reduce CNS Aβ in older mice with significant amyloid load (Morgan et al., 2000; Das et al., 2001), although cognitive impairment was ameliorated (Morgan et al., 2000). Cognitive status in GM1- or gelsolin-treated mice has not yet been assessed.
Discussion
The recent observations of elevated levels of Aβ in the plasma of another transgenic mouse line (PDAPP) mice after passive immunization with anti-Aβ antibodies (DeMattos et al., 2001, 2002) and in PS/APP mice after active Aβ immunization (Lemere et al., 2002) suggest that alteration of peripheral/brain Aβ dynamics may be a possible therapeutic target, and that this could be achieved simply through peripheral sequestration of Aβ using compounds that have a high binding affinity for Aβ. We have shown that this is indeed the case, using two systemically administered compounds that bind Aβ peptides but that do not cross the BBB to any great degree. Our data support recent findings that the Fab fragment of an anti-Aβ antibody, which lacks immunomodulative effects and acts simply as an Aβ binding agent, reduced brain Aβ levels significantly (Bacskai et al., 2002).
Young PS/APP mice administered peripherally with gelsolin or GM1 show a substantial decrease in aggregated Aβ40 and Aβ42 in the brain. Because of its high molecular weight (86,000 according to SDS-PAGE), gelsolin is very unlikely to enter the brain when administered peripherally. A small amount of GM1 would be expected to enter the brain, but this is unlikely to be the major cause of reduced CNS Aβ, because administration of GM1 into the brain ventricles directly resulted in an increase in soluble Aβ, but insoluble Aβ levels were unchanged. Although we do not have direct evidence that peripheral sequestration of Aβ is the mechanism underlying the reduction of CNS Aβ, our data are supported by previous studies that have strongly suggested that sequestration of Aβ in the periphery by antibodies affects the dynamics of Aβ efflux and/or influx, which is associated with a concomitant reduction of Aβ peptides in the brain (DeMattos et al., 2001, 2002; Lemere et al., 2002). Although gelsolin and GM1 administration led to a significant reduction in brain Aβ levels, plasma Aβ was affected differently in the two experiments. Vehicle-treated mice undergoing the initial stages of amyloidogenesis show a decline in plasma Aβ, a situation similar to that seen in APP Tg2576 mice in the initial stages of amyloid deposition (Kawarabayashi et al., 2001). The profile for gelsolin-treated animals was very similar to that seen after vehicle treatment, and no change of plasma Aβ was seen between the two groups. Aβ levels in GM1-treated mice were maintained at a higher level relative to vehicle-treated mice, and the profile for Aβ40 and Aβ42 was different, with Aβ40 being affected earlier. It is unknown whether this reflects the initially higher proportion of Aβ40 relative to Aβ42 in the plasma, faster clearance of the GM1/Aβ42 complex relative to GM1/Aβ40 in the periphery, or slower efflux of Aβ42 from the brain; we also do not know whether the Aβ species sequestered preferentially is Aβ40.
The effect of peripheral sequestration with GM1 or gelsolin on plasma Aβ was minor compared with the elevation in plasma Aβ seen with passive immunization, but the effects on CNS Aβ levels were similar (Lemere et al., 2002). The lower levels of plasma Aβ in the current study may reflect the lower affinity of gelsolin and GM1 for Aβ (low micromolar range) (Choo-Smith et al., 1997) compared with anti-Aβ antibodies (low picomolar range) (DeMattos et al., 2001), or they may reflect faster clearance of the GM1–Aβ or gelsolin–Aβ complex compared with an IgG–Aβ complex. Lower plasma Aβ levels are unlikely to be an artifact of epitope masking however, because the ELISA recognizes Aβ in plasma with or without the sequestering agent equally well. At this time, it is difficult to interpret the significance of plasma Aβ levels relative to brain levels, because the clearance and metabolism of the drugs tested are not clearly defined in this system. Additional investigation using a compound with well established pharmacodynamics will be more meaningful.
The relevant contribution of plasma versus CNS Aβ to the plaques is unclear. Two studies have shown that Aβ can be cleared to the plasma from the brain (DeMattos et al., 2001, 2002); other studies have shown that radiolabeled peripheral Aβ passes into the brain, where it contributes to the plaque (Ghilardi et al., 1996), and a third study has shown that CNS-derived Aβ can contribute to plaques even in the absence of peripheral Aβ (Calhoun et al., 1999). At this stage it is unknown whether peripheral sequestration of Aβ prevents the influx or enhances the efflux of Aβ between the brain and the plasma, but this study shows that the mechanism is effective for a range of different compounds that have as their common feature the ability to bind Aβ.
Although our results show GM1 and gelsolin to be at least as effective as immunomodulation-based methods for lowering CNS Aβ levels in the PS/APP mice, the use of these compounds as systemic Aβ sequestering agents is not proposed as a treatment for AD, but rather as a proof-of-concept for a prophylactic approach that may be more flexible, more reliable, and less likely to cause side effects in long-term administration paradigms than immunization-based therapies. There are significant implications for novel drug design not only for AD but also for vascular amyloidosis and other amyloidoses, such as the British, Finnish, and Danish dementias. In addition, it is possible that the peripheral prion protein might be a target, because recent studies have shown that scrapie in transgenic mice can be ameliorated by administration of anti-prion antibodies (Peretz et al., 2001). Given the caveats and limitations associated with antibody-based therapeutics, new drug therapies would be a welcome addition to our pharmacopedic arsenal.
Footnotes
This work was supported by grants from the National Institutes of Health to K.D. and Y.M. and from the Alzheimer Association to Y.M. We thank Dr. Marc Mercken (Johnson & Johnson Pharmaceutical Research and Development, Beerse, Belgium) for the gift of anti-Aβ antibodies for ELISA.
Correspondence should be addressed to Dr. Karen Duff or Dr. Yasuji Matsuoka, The Center for Dementia Research, Nathan Kline Institute/New York University, 140 Old Orangeburg Road, Orangeburg, NY 10962. E-mail:duff@nki.rfmh.org or matsuoka@nki.rfmh.org.
References
- 1.Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc Natl Acad Sci USA. 1999;96:14088–14093. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauhan VP, Ray I, Chauhan A, Wisniewski HM. Binding of gelsolin, a secretory protein, to amyloid β-protein. Biochem Biophys Res Commun. 1999;258:241–246. doi: 10.1006/bbrc.1999.0623. [DOI] [PubMed] [Google Scholar]
- 5.Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Aβ-(1–40) peptide to anglioside-containing membrane vesicles. J Biol Chem. 1997;272:22987–22990. doi: 10.1074/jbc.272.37.22987. [DOI] [PubMed] [Google Scholar]
- 6.Das P, Murphy MP, Younkin LH, Younkin SG, Golde TE. Reduced effectiveness of Aβ1–42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001;22:721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- 7.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 9.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 10.Ghilardi JR, Catton M, Stimson ER, Rogers S, Walker LC, Maggio JE, Mantyh PW. Intra-arterial infusion of [125I]Aβ 1–40 labels amyloid deposits in the aged primate brain in vivo. NeuroReport. 1996;7:2607–2611. doi: 10.1097/00001756-199611040-00040. [DOI] [PubMed] [Google Scholar]
- 11.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 13.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, George-Hyslop P, Westaway D. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 14.Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem. 2001;276:24985–24990. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- 15.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemere CA, Spooner ET, LaFrancois J, Malester B, Mori C, Leverone JF, Clements JT, Selkoe DJ, Duff KE. Aβ immunization of PS/APP mice leads to decreased cerebral Aβ and a corresponding increase in serum Aβ. Soc Neurosci Abstr. 2002;27:687.10. [Google Scholar]
- 17.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 18.Peretz D, Williamson RA, Kaneko K, Vergara J, Leclerc E, Schmitt-Ulms G, Mehlhorn IR, Legname G, Wormald MR, Rudd PM, Dwek RA, Burton DR, Prusiner SB. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature. 2001;412:739–743. doi: 10.1038/35089090. [DOI] [PubMed] [Google Scholar]
- 19.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 20.Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- 21.Rost KL, Brockmoller J, Weber W, Roots I. Multiple-dose pharmacokinetics of ganglioside GM1 after intravenous and intramuscular administration to healthy volunteers. Clin Pharmacol Ther. 1991;50:141–149. doi: 10.1038/clpt.1991.118. [DOI] [PubMed] [Google Scholar]
- 22.Saulino MF, Schengrund CL. Differential accumulation of gangliosides by the brains of MPTP-lesioned mice. J Neurosci Res. 1994;37:384–391. doi: 10.1002/jnr.490370310. [DOI] [PubMed] [Google Scholar]
- 23.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 24.Selkoe DJ. Physiological production of the β-amyloid protein and the mechanism of Alzheimer's disease. Trend Neurosci. 1993;16:403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- 25.Small DH, Mok SS, Bornstein JC. Alzheimer's disease and Aβ toxicity: from top to bottom. Nat Rev Neurosci. 2001;2:595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 26.Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazadeh S, Hancock WW, Selkoe DJ. Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 27.Wu GS, Ledeen R. Quantification of gangliotetraose gangliosides with cholera toxin. Anal Biochem. 1988;173:368–375. doi: 10.1016/0003-2697(88)90201-1. [DOI] [PubMed] [Google Scholar]