Abstract
Astrocyte glutamate release can modulate synaptic activity and participate in brain intercellular signaling. P2X7receptors form large ion channels when activated by ATP or other ligands. Here we show that P2X7 receptors provide a route for excitatory amino acid release from astrocytes. Studies were performed using murine cortical astrocyte cultures. ATP produced an inward current in patch-clamped astrocytes with properties characteristic of P2X7 receptor activation: the current was amplified in low divalent cation medium, blocked by pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), and more potently activated by 3′-O-(4-benzoyl)benzoyl ATP (BzATP) than by ATP itself. Measurement of current reversal potentials showed the relative BzATP-induced permeabilities to different substrates to be Na+, 1 > Cl−, 0.34 >N-methyl-d-glucamine, 0.27 >l-glutamate, 0.15 ≈ d-aspartate, 0.16. Astrocytes exposed to BzATP also became permeable to Lucifer yellow, indicating a large channel opening. Release of l-glutamate and d-aspartate through P2X7 channels was confirmed using radiolabeled tracers. As with the inward current, release of glutamate and d-aspartate was induced by BzATP more potently than ATP, amplified in Ca2+/Mg2+-free medium, and blocked by PPADS or oxidized ATP. Efflux through P2X7channels is a previously unrecognized route of ligand-stimulated, nonvesicular astrocyte glutamate release.
Keywords: d-aspartate, glutamate, P2Z, patch clamp, nonvesicular, purinergic
Introduction
Astrocyte glutamate release can modulate synaptic activity and participate in brain intercellular signaling (Araque et al., 2001; Bezzi et al., 2001; Nedergaard et al., 2002). Astrocyte glutamate release occurs by Ca2+-dependent and Ca2+-independent mechanisms. Ca2+-dependent release is triggered by ligands such as prostaglandins, ATP, and bradykinin as well as by the direct manipulation of astrocyte intracellular free Ca2+ (Parpura et al., 1994; Jeftinija et al., 1996; Bezzi et al., 1998; Sanzgiri et al., 1999; Jeremic et al., 2001). The route by which glutamate is released from astrocytes in response to intracellular Ca2+ elevations is not yet firmly established, but evidence suggests that vesicular release may contribute (Parpura et al., 1995; Araque et al., 2000).
Ca2+-independent astrocyte glutamate release is less well characterized, but studies using brain slices suggest a continuous and rapid release of glutamate from astrocytes by a nonvesicular, Ca2+-independent mechanism (Jabaudon et al., 1999). Ca2+-independent astrocyte glutamate release can occur by reversal of glutamate uptake (Nicholls and Attwell, 1990; Longuemare and Swanson, 1995) and by opening of volume-sensitive anion channels (Kimelberg et al., 1990;Longuemare et al., 1999), but these processes are unlikely to be significant under normal conditions in the brain (Anderson and Swanson, 2000). In this study we investigated the possibility that channel-forming P2X7 receptors could contribute to Ca2+-independent astrocyte glutamate release.
The P2X7 receptor (also termed the P2Z receptor) was originally characterized by its reversible cell-permeabilizing effects (Dahlquist and Diamant, 1974; Steinberg and Silverstein, 1987;Di Virgilio, 1995). P2X7 receptors form homomeric complexes that open large channels when activated by extracellular ATP (North and Surprenant, 2000). In some cell types, these channels are permeable to molecules up to 900 Da in size, whereas in others they are permeable only to smaller molecules or exhibit ion selectivity (Soltoff et al., 1992; Surprenant et al., 1996; Markwardt et al., 1997; Ralevic and Burnstock, 1998). P2X7 receptors in the nervous system have been localized to microglia (Di Virgilio et al., 1999; Ferrari et al., 1999), neuronal processes (Brandle et al., 1998;Deuchars et al., 2001), Müller cells (Pannicke et al., 2000), Schwann cells (Colomar and Amedee, 2001), and astrocytes (Kukley et al., 2001; Panenka et al., 2001). ATP is an important mediator of astrocyte intercellular signaling (Guthrie et al., 1999; Cotrina et al., 2000; Wang et al., 2000), and activation of astrocyte P2X7 receptors has been linked previously to mitogen-activated protein kinase activation, monocyte chemoattractant protein-1 expression, and purine release (Kukley et al., 2001; Panenka et al., 2001).
Mouse cortical astrocyte cultures were used to test the possibility that ATP could induce glutamate release from astrocytes by binding to P2X7 receptors and inducing channel opening. ATP was found to induce an inward current, a permeabilization to Lucifer yellow, and an efflux of excitatory amino acids. Pharmacological characterization of these effects showed a pattern characteristic of P2X7 receptors. These findings suggest a novel mechanism for ligand-mediated release of excitatory amino acids from astrocytes. This work has been published previously in abstract form (Duan et al., 1999a).
Materials and Methods
Radiolabeled glutamate and d-aspartate were obtained from American Radiolabeled Chemicals (St. Louis, MO). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO), except as noted.
Cell cultures. Cultures were prepared from the cortices of 1-d-old mice (Simonsen Laboratories, Gilroy, CA) as described previously (Swanson et al., 1997a) in accordance with a protocol approved by the San Francisco Veterans Affairs Medical Center animal studies committee. Cortices were isolated, freed of meninges, dissociated with papain and trituration, and plated on glass coverslips or 24 well Falcon culture plates. At confluence (day 12–15), most of the cultures were treated for 48 hr with 20 μmcytosine arabinoside to prevent microglial proliferation. This medium was replaced with Eagle's minimal essential medium supplemented with 3% fetal bovine serum (HyClone, Logan, UT), 2 mm glutamine, 100 nm sodium selenate, 200 nm α-tocopherol, and 0.15 mm dibutyryl cAMP to induce the process-bearing morphology (Sensenbrenner et al., 1980; Swanson et al., 1997b). Each study was repeated on cells from at least two different batches of astrocyte cultures at 22–40 d in vitro. For studies measuring reversal potentials, the astrocytes were plated at low density so that single cells could be isolated for whole-cell recording to avoid space clamp problems attributable to gap junctions. In a subset of the cultures, cytosine arabinoside was omitted and the fetal bovine serum supplement was maintained at 10% to promote microglial survival and proliferation.
Reverse transcription-PCR. Total RNA was isolated from cultured mouse cortical astrocytes using the RNAzol B reagent (Tel-Test Inc., Friendswood, TX) and evaluated by denaturing agarose gel electrophoresis. Reverse transcription (RT)-PCR was performed using the ProSTAR Ultra HF RT-PCR system (Stratagene, La Jolla, CA). The 5′ upstream primer was 5′-TCC ACC CTG TCC TAC TTT GG-3′ and the 3′ downstream primer was 5′-CTT GCA GAC TTT TCC CAA GC-3′. PCR used 35 cycles of 30 sec at 95°C, 30 sec at 55°C, 1 min at 72°C, and a final 10 min elongation step at 72°C.
Western blots and immunostaining. Homogenates were prepared from mouse brain cortices and from cortical mouse astrocyte cultures. Thirty micrograms of protein was run on 10% SDS polyacrylamide gels, electrophoretically transferred onto polyvinylidene fluoride membranes, and placed in a blocking solution containing affinity-purified polyclonal anti-P2X7 receptor antiserum (Chemicon, Temecula, CA) at a 1:500 dilution for 12–18 hr at 4°C. In some cases the antiserum was preadsorbed with a fivefold molar excess of the peptide antigen. Antibody binding was visualized with a peroxidase-labeled anti-rabbit IgG and the 3–3′-diaminobenzidene method. Immunostaining of the astrocyte cultures was performed after fixation for 20 min in 4% paraformaldehyde. Cultures on glass coverslips were washed with a blocking buffer containing 10% goat serum and 0.1% Triton X-100 and then incubated with primary antibodies for 18 hr at 4°C. The primary antibodies were rabbit anti-P2X7 (Chemicon) diluted 1:2000 along with either mouse anti-glial fibrillary acidic protein (GFAP; Chemicon) diluted 1:2000 to identify astrocytes or rat anti-mouse F4/80 (Serotec, Oxford, UK) diluted 1:500 to identify microglia. After washing to remove excess primary antibody, the cultures were incubated for 90 min at room temperature with fluorescence-tagged mouse monoclonal secondary antibodies (Molecular Probes, Eugene, OR): Alexa-fluor 488 anti-rabbit IgG for the P2X7 antibody, Alexa-fluor 594 anti-rat IgG for the F4/80 antibody, and Alexa-fluor anti-mouse IgG for the GFAP antibody. Excess antibody was removed, the coverslips were mounted on glass slides, and cells were imaged using a Leica (Nussloch, Germany) TCS confocal microscope with 2 μm optical sections.
Whole-cell patch-clamp recording. Except as specified, the pipette solution contained (in mm): 100N-methyl-d-glucamine chloride (NMDG-Cl), 3 Mg-ATP, 5 EGTA, and 10 HEPES. The external solution contained (in mm): 100 NaCl, 1 CaCl2, 1.2 MgCl2, 5 HEPES, and 10 glucose. For studies in low Ca2+/Mg2+external solutions, CaCl2 was decreased to 0.3 mm and MgCl2 to 0.1 mm. For both pipette and external solutions, the pH was adjusted to 7.2 with NMDG and the osmolarity was adjusted to 290 mOsm with sucrose. Recordings for the reversal potential (Vrev) determinations began at least 15 min after establishing whole-cell patch configurations to allow a full exchange between cytoplasm and pipette solutions. Connection of the perfusion chamber to ground was established via a 3m KCl agarose bridge. Liquid junction potentials (VL values) were calculated with Clampex 7 software (Axon Instruments, Foster City, CA) and corrected by the equation Vm =Vp −VL, whereVp is the command voltage (holding potential) and Vm is the actual potential difference across the membrane. Voltage pulse generation, data digitization, and data analysis were performed with a DigiData 1200 analog-to-digital/digital-to-analog interface with pClamp 7 software (Axon Instruments, Union City, CA). The “U-tube” solution change method (Duan and Cooke, 1999), which can change the solution surrounding a cell completely within 100–200 msec, was used to apply drugs. All drugs were added from concentrated, iso-osmolar, buffered (pH 7.2) stock solutions.
Glutamate and d-aspartate release.Studies were performed at 37°C in a balanced salt solution (BSS) as described previously (Longuemare and Swanson, 1995). The standard BSS contained (in mm): 135 NaCl, 3.1 KCl, 1.2 CaCl2, 1.2 MgSO4, 0.5 KH2PO4, 2 glucose, and 5.0 1,4-piperazinediethanesulfonic acid, adjusted to a final pH of 7.2 and to 280–320 mOsm. Ca2+ and Mg2+ were in some cases replaced by molar equivalents of Na+. All drugs were added from concentrated, iso-osmolar, buffered (pH 7.2) stock solutions. Astrocytes were loaded with 0.17 μCi/ml [l-14C(U)] glutamate (0.8 nmol/ml) or 0.34 μCi/ml [d-2,3-3H]-aspartate (0.03 pmol/ml) for 60 min and then transferred to BSS. For studies using 14C-glutamate, the cultures were preincubated for 30 min with 1 mm amino-oxyacetic acid and 0.5 mm methionine sulfoximine before adding 14C-glutamate to inhibit the metabolism of glutamate to other radiolabeled metabolites (Farinelli and Nicklas, 1992). In some studies, the cultures were incubated with agents to assess other potential routes of excitatory amino acid efflux. l-Transpyrrolidine-2,4-dicarboxylic acid (PDC), used to block glutamate transporter reversal, was loaded into the cells by incubating the cultures in 50 μmPDC for 60 min after the preincubation withd-aspartate (Longuemare and Swanson, 1995). Thapsigargin and EGTA, used to assess the calcium dependency ofd-aspartate release, were incubated with the cultures for 40 min in Ca2+-free medium after the preincubation with d-aspartate. The calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) was added to the cultures as its acetoxy-methyl ester (AM) during the terminal 30 min of the d-aspartate preincubation.
The studies used 6 min incubations with purinergic receptor agonists. Incubations were terminated by the removal of the medium and lysis of the cells with 0.1N NaOH. Media and cell lysates were taken for scintillation counting to determine the percentage of radiolabeled substrate (14C glutamate or3H d-aspartate) released in each culture well during the 6 min incubation period. Consistent with previous experience (Longuemare and Swanson, 1995), the total radiolabel loading (14C or3H per milligram of protein) varied by <30% between studies, including studies using PDC preloading.
Lucifer yellow uptake. One-half of the cultures were preincubated with a 0.3 mm concentration of the irreversible P2X7 antagonist oxidized ATP (Ox-ATP) (Murgia et al., 1993; Evans et al., 1995; North and Surprenant, 2000) for 2 hr in standard culture medium. The astrocytes on coverslips were then washed and transferred either to standard BSS or Ca2+/Mg2+-free BSS at 37°C. The cells were incubated for 5 min with 0.5 mg/ml Lucifer yellow, with or without 300 μm3′-O-(4-benzoyl)benzoyl ATP (BzATP). Incubations were terminated by washing into standard BSS, and fluorescence photomicrographs were obtained within 10 min using a laser scanning microscope (LSM 510; Zeiss, Oberkochen, Germany) using 458 nm excitation. Five fields were randomly selected from each coverslip (four corner and one center) for image capture and measurement of whole-field fluorescence intensities. Whole-field fluorescence intensities were normalized to the mean intensity of the cultures treated with no BzATP in Ca2+/Mg2+-free BSS.
Cell survival. Rupture of astrocyte membranes was assessed by measuring the loss of lactate dehydrogenase (LDH) from the cultures. At designated time points the medium was removed and cells were solubilized in a solution of 3% Triton X-100, 0.02% bovine serum albumin, and 5 mm potassium phosphate buffer, pH 7.5. LDH activity in BzATP-treated culture wells was measured by the method of Koh and Choi (1987) and expressed as percentage of activity in control wells from the same 24 well plate.
Statistical analysis. Each study was performed using astrocytes from at least two different culture preparation dates.n denotes the number of individual cells assessed in the patch-clamp studies or the number of culture wells assessed in the other studies. Statistical differences were determined by Student'st test for two-group comparisons or by one-way ANOVA with the Tukey test for multiple group comparisons.
Results
The murine cortical astrocyte cultures used for these studies form a confluent layer of process-bearing cells, of which >95% express the astrocyte-specific marker GFAP (Swanson et al., 1997a; Duan et al., 1999b). As shown in Figure 1, RT-PCR and Western blots confirm expression of the P2X7receptor in these cultures. Localization of P2X7receptors on the astrocyte cell membranes was confirmed by P2X7 immunostaining in cultures that were costained for GFAP. A positive control for the P2X7 immunostaining was prepared by promoting microglial proliferation in the cortical cultures (Di Virgilio et al., 1999; Colomar and Amedee, 2001). Microglia were identified by their characteristic morphology and by F4/80 epitope immunoreactivity (Perry et al., 1985) (Fig. 1C). P2X7immunoreactivity on both astrocytes and microglia was eliminated by omission of the anti-P2X7 antibody or by adding excess P2X7 target epitope peptide to the cultures with the antibody (data not shown).
Fig. 1.

Astrocytes express P2X7 receptors.A, RT-PCR showing P2X7 mRNA expression in mouse astrocyte cultures. The expected product was 253 bp.+RT and −RT denote laneswith and without reverse transcriptase, respectively. The left lane shows molecular weight markers. B, Western blot showing P2X7 mRNA expression in mouse astrocyte cultures and in mouse cortex. The expected P2X7 band is at 72 kDa. Immunostaining is blocked by the coincubation of antibody with P2X7 peptide epitope. C, Immunostaining of astrocyte cultures showed P2X7 immunoreactivity over astrocyte surfaces surrounding GFAP intermediate filaments.D, Immunostaining of microglia (as a positive control) showed colocalization of P2X7 with the mouse microglia surface marker F4/80. The magnification in D is sixfold greater than inC. P2X7 immunostaining in both astrocytes and microglia was blocked by coincubation of antibody with the P2X7 peptide epitope (data not shown).
ATP induces an inward current in astrocytes that is mediated by P2X7 receptors
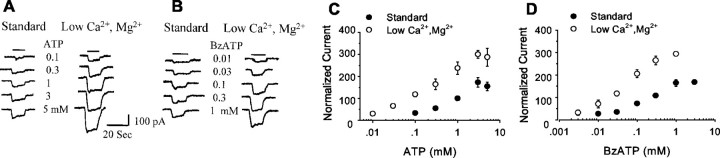
ATP-induced currents were studied in mouse astrocyte cultures by the whole-cell patch-clamp technique. The application of ATP at concentrations of ≥0.1 mm induced an inward current in 33 of 48 cells recorded. The current developed quickly and showed small desensitization (Fig. 2). The P2Y agonists UTP and 2-chloro ATP produced no detectable currents, suggesting that the effect of ATP was mediated by a P2X receptor. BzATP produced a current similar to that produced by ATP, but BzATP was a more potent agonist (Fig. 2). The EC50 for BzATP was ∼100 μm, whereas the EC50 for ATP was ∼300 μm. This difference suggests that a component of the ATP-induced inward current was carried either by P2X1 or P2X7 receptors, because these are the only receptor types known to be more potently activated by BzATP than by ATP (Bianchi et al., 1999; North and Surprenant, 2000). One way that P2X7 receptors can be distinguished from P2X1 receptors is by the amplified response of P2X7 receptors in low divalent cation solutions (Di Virgilio, 1995; Ralevic and Burnstock, 1998; North and Surprenant, 2000). As shown in Figure 2, the inward currents induced by both ATP and BzATP were amplified in low Ca2+, low Mg2+ solution. Under these conditions BzATP was again more potent than ATP, with the approximate EC50 values being 50 and 300 μm, respectively.
Fig. 2.
Whole-cell patch-clamp recordings showed inward currents during exposure to ATP or to the P2X7receptor-selective agonist BzATP. Representative recordings from single astrocytes are shown in A and B. Dose–response curves from averaged data (means ± SE) are plotted in C and D and expressed as the percentage of the 1 mm ATP-induced responses in normal divalent cation (standard) medium (n = 3–10 for each data point). Currents recorded in low Ca2+, low Mg2+ medium were significantly greater than in normal medium at all ATP and BzATP concentrations tested (p < 0.05). External solution, 100 mm NaCl; internal solution, 100 mm NMDG-Cl; holding potential, −40 mV.
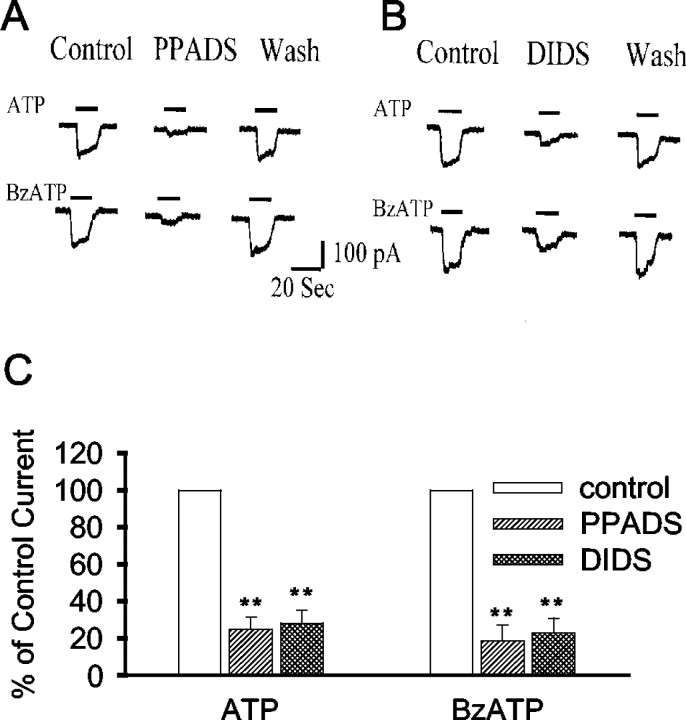
The effects of receptor antagonists also suggested that the ATP response was mediated by P2X7 receptors. Suramin, which is a potent P2X1 antagonist with only weak activity at P2X7 receptors (Chessell et al., 1997; Ralevic and Burnstock, 1998; Bianchi et al., 1999; North and Surprenant, 2000), reduced inward currents only at concentrations of >1 mm (data not shown). The nonspecific P2 receptor antagonist pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (Chessell et al., 1997) inhibited both ATP- and BzATP-induced currents (Fig. 3), providing a positive control for this response. Inhibition of the ATP- and BzATP-induced currents with 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) (Fig. 3) is also consistent with, although not specific for, flux through an P2X7-activated ion channel (Soltoff et al., 1993).
Fig. 3.
Currents induced by 1 mm ATP or 0.1 mm BzATP were reversibly inhibited by 20 μmPPADS, a P2 receptor antagonist, and by 1 mm DIDS.Traces in A and B are representative recordings from single patched astrocytes.C shows pooled data from five cells under each condition (means ± SE).**p < 0.01. Recordings were made with 100 mm NaCl in the external solution and 100 mm NMDG-Cl in the pipette. Holding potential, −40 mV.
An additional distinguishing characteristic of P2X7 receptors is that they gate channels permeable to molecules up to 900 Da in size. Lucifer yellow is a 457 Da fluorescent dye that has been shown previously to enter cells via P2X7 receptors (Ballerini et al., 1996;Coutinho-Silva et al., 1999). As shown in Figure4, astrocytes treated with 300 μm BzATP in Ca2+/Mg2+-free medium exhibited a rapid and large increase in Lucifer yellow permeability. This effect was diminished in standard medium and was blocked by preincubating the cultures with Ox-ATP (Fig.4B). Ox-ATP (ATP dialdehyde) is a selective P2X7 antagonist when used as an irreversible inhibitor (Murgia et al., 1993; Evans et al., 1995; North and Surprenant, 2000). The profile of BzATP > ATP ≫ UTP agonist potency, response amplification in low divalent cation medium, weak inhibition by suramin, large channel formation in response to BzATP, and irreversible blockade by Ox-ATP is unique to P2X7 receptors.
Fig. 4.
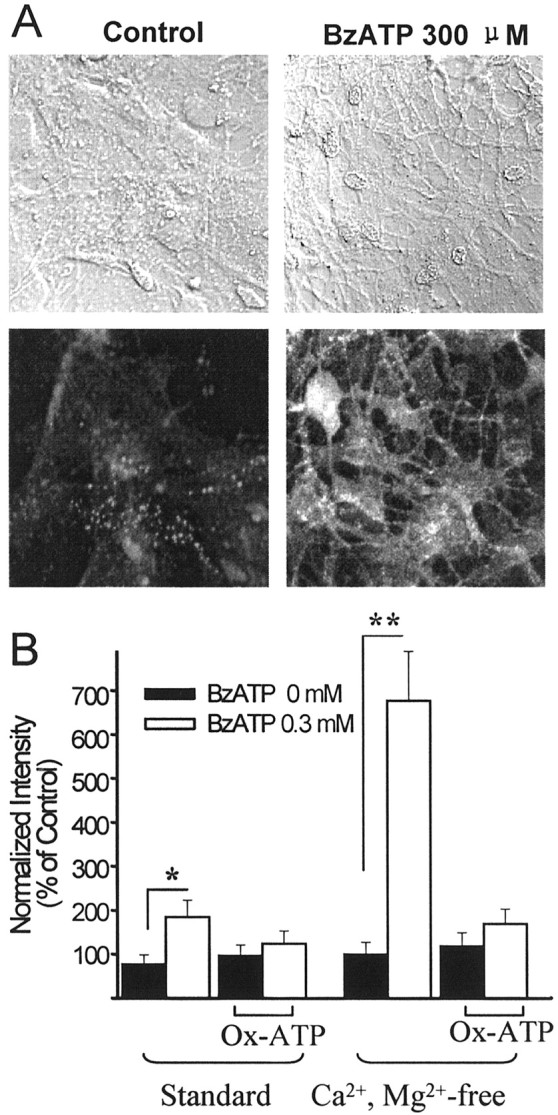
BzATP permeabilizes astrocytes to Lucifer yellow.A,top, Differential interference contrast images corresponding to the Lucifer yellow fluorescence images at the bottom. Cells were incubated for 5 min in Lucifer yellow in Ca2+/Mg2+-free BSS, with or without 300 μm BzATP. B, BzATP (0.3 mm) increased the Lucifer yellow fluorescence intensity in astrocytes; this effect was amplified in the Ca2+/Mg2+-free BSS. Pretreatment with the P2X7 receptor antagonist Ox-ATP (0.3 mm) for 2 hr inhibited the effect of BzATP in both standard and Ca2+/Mg2+-free BSS. Data were normalized to the averaged intensity measured in cultures in the Ca2+/Mg2+-free solution. *p < 0.05; **p < 0.01;n = 15 for each group. Error bars indicate SE.
The astrocyte P2X7 channel is permeable to glutamate
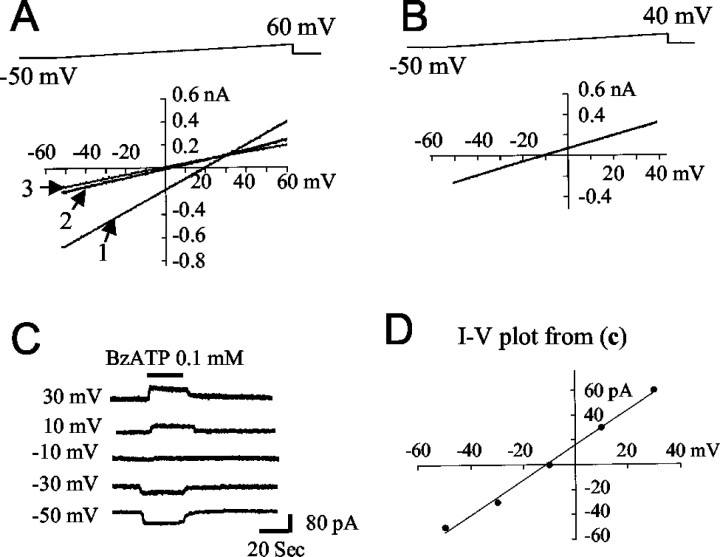
Permeability of the P2X7-gated channel to glutamate and other ions was determined by constructing current–voltage curves in voltage-clamp configuration as illustrated in Figure 5; the reversal potentials of these currents are provided in Table 1. The relative BzATP-induced permeabilities to the different substrates, as calculated by the Goldman–Hodgkin–Katz equation, were Na+, 1 > Cl−, 0.34 > NMDG, 0.27 >l-glutamate, 0.15 ≈ d-aspartate, 0.16. Although the permeability to glutamate is less than that of Cl−, the steep intracellular/extracellular gradient for glutamate provides a strong outward driving force for this anion (Erecinska and Silver, 1990;Anderson and Swanson, 2000). In the following experiments we directly measured ATP- and BzATP-induced release of glutamate from the astrocyte cultures.
Fig. 5.
The reversal potentials of BzATP-induced currents under different external and internal ion conditions were determined by two methods. In A and B, 2 sec voltage ramp pulses were applied (top) and current–voltage curves obtained in the presence of 0.1 mm BzATP were subtracted by values obtained in the absence of BzATP. InA, the pipette contained 100 mm NMDG-Cl and the bath contained 100 mm NaCl (1), 100 mm NMDG-Cl (2), or 50 mm NMDG-Cl (3). In B, the pipette contained 100 mm NMDG-glutamate and the bath contained 100 mm NMDG-Cl. C, BzATP-induced currents were recorded under different holding potentials and plotted in D. The pipette contained 100 mmNMDG-glutamate and the bath contained 100 mm NMDG-Cl. Values obtained by these methods were in close agreement and are presented in Table 1.
Table 1.
Reversal potentials of BzATP-induced inward currents
| External solution1-a | Pipette solution1-b | Reversal potential |
|---|---|---|
| 100 mm NaCl | 100 mmNMDG-Cl | 19.9 ± 0.5 |
| 100 mmNMDG-Cl | 100 mm NMDG-Cl | 0 ± 0.4 |
| 50 mm NMDG-Cl | 100 mm NMDG-Cl | 1.7 ± 0.5 |
| 100 mm NMDG-Cl | 100 mmNMDG-l-glutamate1-c | −9.3 ± 0.7 |
| 100 mm NMDG-Cl | 100 mmNMDG-d-aspartate1-c | −8.8 ± 0.3 |
Reversal potentials under each condition were determined using both methods described in Figure 3. Values are means ± SE;n = 6–16 cells. Osmolarity of both external and pipette solutions was adjusted to 290 mOsm with sucrose when needed.
Also contained 5 mmHEPES.
Also contained 0.3 mmCaCl2, 0.1 mm MgCl2, 5 mm HEPES, and 10 mm glucose.
Also contained 2 mmNMDG-Cl.
P2X7 receptor activation causes excitatory amino acid release from astrocyte cultures
Radiotracer loading of astrocytes was achieved by transporter-mediated uptake of 14Cl-glutamate or 3Hd-aspartate from the medium. Release of radiolabeled glutamate or d-aspartate under control conditions was typically 10–20% of the total radiolabel loaded when the cultures were placed in standard BSS and 25–35% when the cells were placed in Ca2+/Mg2+-free BSS. Astrocyte glutamate release was significantly increased by exposure to ATP or BzATP (Fig.6A). As observed in the whole-cell patch-clamp studies, BzATP was a more potent agonist than ATP, and the responses to both agonists were markedly amplified in Ca2+/Mg2+-free BSS. Similar results were observed usingd-aspartate as a tracer for glutamate flux (Fig.6B). Under Ca2+/Mg2+-free conditions, which accentuate P2X7-mediated responses, the EC50 for BzATP was ∼10 μm, whereas the EC50 for ATP was ∼25 μm.d-Aspartate is a glutamate analog that is taken up by plasma membrane glutamate transporters and has a permeability similar to glutamate at P2X7 receptors (from Table 1), but that differs from l-glutamate in that it is not rapidly metabolized and not packaged by vesicular transporters (Naito and Ueda, 1985; Anderson and Swanson, 2000). The intracellular concentration of d-aspartate after loading was ∼10 nm (C. Anderson, unpublished observations), which is negligible compared with intracellular aspartate and glutamate concentrations (Erecinska and Silver, 1990). Because studies using glutamate and d-aspartate showed similar results, most subsequent experiments were performed using 3H d-aspartate to avoid potential confounding effects of the agents required to prevent rapid glutamate metabolism.
Fig. 6.
ATP and BzATP induce an efflux of preloaded14C glutamate and 3H d-aspartate.A, 14C glutamate release induced by 6 min incubations in standard BSS (left) or Ca2+/Mg2+-free BSS (right). BzATP produced a greater response than ATP under both conditions (p < 0.05), and the effects of both agonists were amplified in the Ca2+/Mg2+-free BSS. Cultures used for 14C glutamate studies were pretreated with methionine sulfoximine and amino-oxyacetic acid to inhibit the metabolism of glutamate to other 14C-labeled compounds (see Materials and Methods). B, 3H d-aspartate release induced by 6 min incubations in standard BSS (left) or Ca2+/Mg2+-free BSS (right). The dose–response curves indicate that BzATP is a more potent agonist than ATP and that the effects of both agonists are amplified in Ca2+/Mg2+-free medium. C, The effect of ATP on 3Hd-aspartate release was amplified by the removal of both Mg2+ and Ca2+ but not by the removal of either cation alone. Values in each panel are means ± SE; n ≥ 6. +, With; −, without. **p < 0.01.
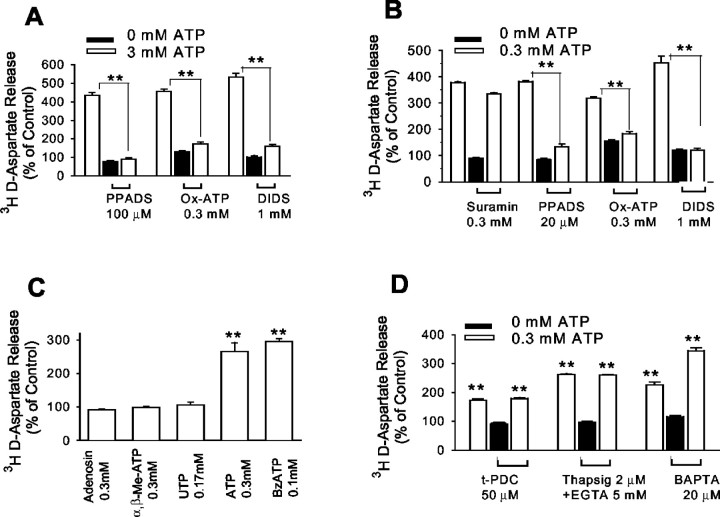
To determine whether the response amplification observed in Ca2+/Mg2+ free medium was attributable specifically to the lack of Ca2+, experiments were performed using BSS prepared with Ca2+ and Mg2+ removed independently. As shown in Figure 6C, the effect of 0.3 mm ATP ond-aspartate release was substantially reduced by the presence of either 1 mmMg2+ or 1 mmCa2+, consistent with the properties of P2X7 receptors (Chessell et al., 1997; Ralevic and Burnstock, 1998; Bianchi et al., 1999; North and Surprenant, 2000). Studies with other purinergic receptor agonists and antagonists also suggest that ATP-induced excitatory amino acid release occurs through astrocyte P2X7 channels. As shown in Figure7A, ATP-inducedd-aspartate release was blocked by the nonselective P2 receptor antagonist PPADS, by the anion channel blocker DIDS, and by a 30 min preincubation with the irreversible, P2X7-selective antagonist Ox-ATP. These agents were equally effective in Ca2+/Mg2+-free medium, whereas the P2X1-selective antagonist suramin was ineffective (Fig. 7B). ATP-induced release was not mimicked by other purinergic ligands, including the P1 agonist adenosine, the P2Y agonist UTP, and the potent P2X1 agonist α,β-methylene ATP (Fig.7C).
Fig. 7.
Pharmacology of ATP-inducedd-aspartate release indicates action at P2X7receptors. A, Standard BSS. B–D, Ca2+/Mg2+-free BSS.A, ATP-induced d-aspartate release is blocked by DIDS, by the P2 receptor antagonist PPADS, and by a 2 hr preincubation with the irreversible P2X7-selective inhibitor Ox-ATP. B, ATP was ∼10-fold more potent in Ca2+/Mg2+-free medium than in standard medium (compare with A). The P2X7receptor inhibitors also blocked ATP-induced d-aspartate release in Ca2+/Mg2+-free medium, whereas the P2X1 antagonist suramin was ineffective.C, d-Aspartate release was induced by BzATP and ATP but not by agonists of other purinergic receptor subtypes.Adenosin, Adenosine; α,β-Me-ATP, α,β-methylene ATP. D, ATP-induced release was not attenuated by preloading with PDC to block glutamate uptake reversal, by preloading the astrocytes with the calcium chelator BAPTA, or by depleting cell calcium with preincubation in Ca2+-free medium containing EGTA and thapsigargin (Thapsig). Preincubations with PDC, BAPTA-AM, and EGTA/thapsigargin were performed at 37°C for 40 min and followed by exchange with Ca2+/Mg2+-free BSS before thed-aspartate release assay. Values in eachpanel are means ± SE; n ≥ 6. **p < 0.01.
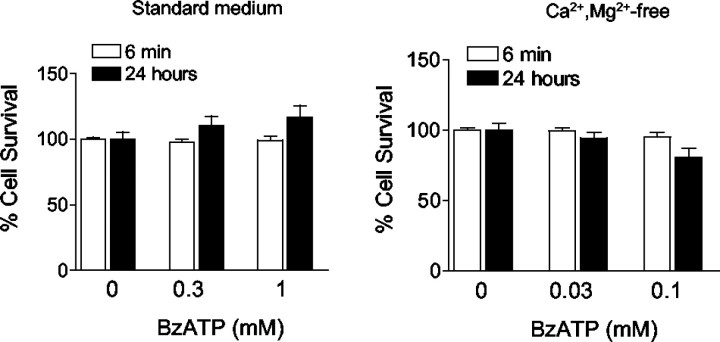
Evidence for ATP-induced glutamate or aspartate release by routes other than the P2X7 channel was not observed. Release by Ca2+-dependent processes was rendered unlikely by the amplified response observed in Ca2+/Mg2+-free medium. In addition, ATP-induced release was not inhibited in astrocytes preincubated for 40 min in Ca2+-free medium containing the calcium chelator EGTA plus the calcium pump inhibitor thapsigargin (Fig.7D). Similarly, preincubation for 40 min with a 20 μm concentration of the calcium chelator BAPTA-AM did not attenuate ATP-inducedd-aspartate release (Fig. 7D). Inhibition of uptake reversal by preloading cultures with PDC (Longuemare and Swanson, 1995) also had no effect on ATP-induced release. Because extended activation of P2X7receptors causes cell lysis in some cell types, we also tested the possibility that excitatory amino acids were released because of the rupture of cell membranes by using intracellular LDH as an index of cell membrane integrity (Koh and Choi, 1987). LDH activity in the astrocyte cultures was not reduced at the end of the 6 min BzATP incubation period used for the radiotracer efflux studies in either standard BSS or Ca2+/Mg2+-free BSS at any BzATP concentration tested (Fig.8). Intracellular LDH activity was also unchanged 24 hr after incubation with BzATP, excluding the possibility of delayed cell lysis or delayed leakage of LDH.
Fig. 8.
BzATP does not cause astrocyte cell lysis. Astrocytes were incubated with BzATP for 6 min in standard BSS (left) or Ca2+/Mg2+-free BSS (right). Cell survival was assessed by measuring intracellular LDH content in the cultures either immediately or 24 hr after the 6 min incubations. There was no significant change in LDH content under any of these conditions. Error bars indicate SE;n = 8.
Discussion
Both glutamate and ATP serve as signaling molecules between astrocytes and neurons. The present findings suggest a mechanism by which glutamate can be released from astrocytes in response to extracellular ATP binding to P2X7 receptors. Although the channel opened by P2X7 ligand binding is not highly selective for glutamate, the strong driving force for glutamate release compared with other anions favors a significant glutamate efflux through the activated channel. Efflux through astrocyte P2X7 channels is a previously unrecognized route of ligand-induced, nonvesicular astrocyte glutamate release.
ATP is a ligand for several purinergic receptor subtypes. A feature characteristic of P2X7 receptors is that BzATP is a more potent and effective agonist than ATP (Ralevic and Burnstock, 1998; Bianchi et al., 1999; North and Surprenant, 2000). However, BzATP is also an agonist at several other P2X receptor subtypes, especially P2X1, in which, as with P2X7 receptors, it is significantly more potent than ATP itself. P2X1 receptors can be pharmacologically distinguished from P2X7receptors by the fact that α,β-methylene ATP is a potent agonist, and suramin is a potent antagonist at the P2X1but not at the P2X7 subtype (Bianchi et al., 1999; North and Surprenant, 2000). In addition, Ox-ATP (ATP dialdehyde) is an irreversible P2X7 antagonist that does not irreversibly block P2X1 or any other recognized P2X receptor subtype (Evans et al., 1995; North and Surprenant, 2000). The general P2X antagonist PPADS was initially thought to be relatively ineffective at P2X7 receptors (Surprenant et al., 1996), but subsequent studies showed this to be an unreliable discriminator because of species differences (Chessell et al., 1998).
Another characteristic feature of P2X7 receptors is the capacity to form large channels permeable to propidium and other dyes. However, P2X2 and P2X4 receptors can also form large pores with extended agonist exposure (Virginio et al., 1999), and P2X7 channels do not have uniform permeability characteristics across all cell types (Steinberg and Silverstein, 1987;Nuttle and Dubyak, 1994). A more consistent feature of P2X7 receptors is response amplification in low divalent cation medium; P2X7 receptors are the only known P2X receptor subtype with this property (Ralevic and Burnstock, 1998; Bianchi et al., 1999; North and Surprenant, 2000).
In the present study, several lines of evidence were developed to establish that the ATP- and BzATP-induced d-aspartate release from astrocytes resulted from the activation of P2X7 receptors: (1) BzATP and ATP produced an inward current that was amplified in low divalent cation medium, with BzATP being the more potent agonist. (2) BzATP-induced inward currents were carried by glutamate and d-aspartate. (3) BzATP rapidly permeabilized astrocytes to the large anionic dye Lucifer yellow. (4) Permeability to Lucifer yellow was strongly amplified in Ca2+/Mg2+-free medium and irreversibly blocked by the selective P2X7 antagonist Ox-ATP. (5) BzATP induced a net efflux of glutamate and d-aspartate, with BzATP being the more potent agonist. (6) BzATP- and ATP-induced release ofd-aspartate was amplified in low divalent cation medium. (7) BzATP- and ATP-induced d-aspartate efflux was irreversibly inhibited by Ox-ATP and not inhibited by 0.3 mm suramin. (8) Inward currents and d-aspartate efflux were not induced by α,β-methylene ATP, adenosine, or UTP. These data are all consistent with action at a P2X7 receptor and are inconsistent with action at any other known receptor type.
Because P2X7 channels in several cell types are permeable to large anionic dyes such as Lucifer yellow and fura-2 (Steinberg and Silverstein, 1987; Nuttle and Dubyak, 1994) it is not surprising that they are permeable to other anions as well. However, anion permeability of P2X7 channels has not been extensively studied, and the literature suggests that permeability to anions, like cations, varies with cell type. In cultured mouse Schwann cells, a K+-dependent Cl− conductance has been observed with the activation of P2X7 receptors (Colomar and Amedee, 2001). Coutinho-Silva and Persechini (1997) showed that P2X7 pores in macrophages and J774 cell are permeable both to large cations (NMDG) and to anions (glutamate), whereas P2X7 receptors in human B lymphocytes were reported to have negligible anion permeability (Bretschneider et al., 1995; Markwardt et al., 1997). The relative permeability of the P2X7 channel to Na+, NMDG+, Cl−, and glutamate estimated in the present study (Table 1) suggests that P2X7 channels in astrocytes, although showing relative selectivity for Na+ among tested ions, discriminate poorly between NMDG+and Cl−. However, it remains possible that activation of P2X7 receptors could simultaneously open both cation channels permeable to large cations such as NMDG and anion channels permeable to organic anions such asl-glutamate and d-aspartate.
Although the permeability of astrocyte P2X7channels to l-glutamate and d-aspartate was lower than that of Cl− or Na+ (relative permeabilities 0.15, 0.16, 0.34, and 1.0, respectively), radiotracer studies confirmed that a significant net efflux of l-glutamate andd-aspartate resulted from the activation of P2X7 channels. Ion flux is determined not only by channel conductance but also by the ion concentrations and transmembrane ion gradient. Astrocyte intracellular glutamate is normally in the millimolar range, and astrocyte Na+-dependent transporters maintain an intracellular/extracellular glutamate ratio of ∼10,000:1 (Erecinska and Silver, 1990; Anderson and Swanson, 2000), which greatly exceeds the transmembrane gradient of the other common anions and cations. The fraction of total ion flux attributable to glutamate is difficult to calculate, because the individual ion fluxes may not be independent of one another (Levitan and Garber, 1998). However, the radiotracer measurements indicate that full activation of astrocyte P2X7 receptors could lead to a substantial glutamate efflux. The approximately threefold increase over basal glutamate release produced by maximally stimulating BzATP or ATP concentrations equates to the release of >30% of the intracellular glutamate pool within the 6 min observation interval.
Sustained activation of P2X7 receptors leads to cell lysis in some cell types (Surprenant et al., 1996). The LDH release studies performed here showed that cell lysis is not the cause of excitatory amino acid efflux from astrocytes during P2X7 receptor stimulation. The reason that P2X7 receptor stimulation leads to cell lysis in some cell types but not others has not been established, but it may be related to the density of receptor expression. It may also be significant that P2X7 receptors in the brain are exclusively monomeric, whereas P2X7 receptors expressed elsewhere form homomeric multimers (Kim et al., 2001).
Previous reports have described a Ca2+-dependent mechanism for ligand-induced glutamate release from astrocytes and Schwann cells (Parpura et al., 1994; Araque et al., 1998; Bezzi et al., 1998;Jeftinija and Jeftinija, 1998), including Ca2+-dependent glutamate release induced by low micromolar ATP and blocked by suramin (Jeremic et al., 2001). The ATP-induced release described here was not blocked by suramin and was independent of Ca2+, because it was not attenuated in cultures preincubated with the Ca2+-pump inhibitor thapsigargin in Ca2+-free medium or in cultures loaded with the Ca2+-binding agent BAPTA. Quite the opposite, d-aspartate release was amplified severalfold in Ca2+/Mg2+-free medium. These findings may be reconciled by postulating that ligand-induced astrocyte release can occur by more than one mechanism. Alternatively, Ca2+-dependent processes could act upstream of P2X7 receptor activation. Because ATP functions as an autocrine and paracrine messenger (Wang et al., 1996; Cotrina et al., 1998; Guthrie et al., 1999; Queiroz et al., 1999), factors that induce astrocyte ATP release in one cell, such as elevated intracellular Ca2+ (Guthrie et al., 1999; Queiroz et al., 1999), could induce glutamate release by stimulating P2X7 receptors on the same or nearby cells. In this way, ligands that cause an increase in intracellular calcium could indirectly lead to glutamate release through a process involving Ca2+-dependent ATP release and subsequent, Ca2+-independent activation of P2X7 receptors.
Astrocyte P2X7 receptors are expressed in brain astrocytes (Kukley et al., 2001; Panenka et al., 2001), and ATP is an important mediator of astrocyte intercellular signaling (Guthrie et al., 1999; Cotrina et al., 2000; Wang et al., 2000). Moreover, because glutamate can in turn induce ATP release from astrocytes (Queiroz et al., 1999), these reciprocal effects could contribute to a propagating signal. However, it should be noted that ATP is a weak P2X7 agonist in the presence of normal extracellular Ca2+ and Mg2+ concentrations. This weak response to ATP is common to all P2X7-mediated effects and all cell types on which P2X7 receptors are expressed (North and Surprenant, 2000). Nevertheless, gene-deletion studies have demonstrated that P2X7 receptors on macrophages are activated in vivo (Labasi et al., 2002). It is possible that P2X7 receptor activation is achieved by transient, high ATP concentrations in confined spaces. Alternatively, the potency of ATP may be markedly increased in the presence of a coagonist or a priming stimulus (Le Feuvre et al., 2002). Given these considerations, the present findings indicate that astrocyte P2X7 receptors have the potential to conduct rapid nonvesicular glutamate release, but the physiological conditions in which astrocyte P2X7receptors are activated remain to be established.
Footnotes
This work was supported by the National Institutes of Health (R.A.S.), the Department of Veterans Affairs (R.A.S., E.C.K.), and by a joint fellowship of the Heart and Stroke Foundation of Canada and the Canadian Institutes of Health Research (C.M.A.).
Correspondence should be addressed to Dr. Raymond A. Swanson, Neurology (127), Veterans Affairs Medical Center, 4150 Clement Street, San Francisco, CA 94121. E-mail: ray@itsa.ucsf.edu.
S. Duan's and Y. Chen's present address: Institute of Neuroscience, Chinese Academy of Sciences, 320 Yue-yang Road, Shanghai 200031, China.
References
- 1.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 3.Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 5. Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D'Alimonte I, Trubiani O, Caciagli F, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. NeuroReport 7 1996. 2533 2537 arsid4682859 [DOI] [PubMed] [Google Scholar]
- 6. Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391 1998. 281 285 arsid4682859 [DOI] [PubMed] [Google Scholar]
- 7.Bezzi P, Domercq M, Vesce S, Volterra A. Neuron-astrocyte cross-talk during synaptic transmission: physiological and neuropathological implications. Prog Brain Res. 2001;132:255–265. doi: 10.1016/S0079-6123(01)32081-2. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 9.Brandle U, Kohler K, Wheeler-Schilling TH. Expression of the P2X7-receptor subunit in neurons of the rat retina. Brain Res Mol Brain Res. 1998;62:106–109. doi: 10.1016/s0169-328x(98)00254-x. [DOI] [PubMed] [Google Scholar]
- 10.Bretschneider F, Klapperstuck M, Lohn M, Markwardt F. Nonselective cationic currents elicited by extracellular ATP in human B-lymphocytes. Pflügers Arch. 1995;429:691–698. doi: 10.1007/BF00373990. [DOI] [PubMed] [Google Scholar]
- 11.Chessell IP, Michel AD, Humphrey PP. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br J Pharmacol. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chessell IP, Simon J, Hibell AD, Michel AD, Barnard EA, Humphrey PP. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- 13.Colomar A, Amedee T. ATP stimulation of P2X7 receptors activates three different ionic conductances on cultured mouse Schwann cells. Eur J Neurosci. 2001;14:927–936. doi: 10.1046/j.0953-816x.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- 14.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutinho-Silva R, Persechini PM. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. AM J Physiol. 1997;273:C1793–C1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, Kanellopoulos JM, Motta-Ly I, Dautry-Varsat A, Ojcius DM. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276:C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- 18.Dahlquist R, Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 1974;34:368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- 19.Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation,and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 21.Di Virgilio F, Sanz JM, Chiozzi P, Falzoni S. The P2Z/P2X7 receptor of microglial cells: a novel immunomodulatory receptor. Prog Brain Res. 1999;120:355–368. doi: 10.1016/s0079-6123(08)63569-4. [DOI] [PubMed] [Google Scholar]
- 22.Duan S, Cooke IM. Selective inhibition of transient K+ current by La3+ in crab peptide-secretory neurons. J Neurophysiol. 1999;81:1848–1855. doi: 10.1152/jn.1999.81.4.1848. [DOI] [PubMed] [Google Scholar]
- 23. Duan S, Keung EC, Chen YR, Swanson RA. ATP-activated glutamate permeable non-selective channels in cultured mouse astrocytes: involvement of P2Z/P2X7 like receptors. Soc Neurosci Abstr 25 1999a. 890.10 arsid5071066 [Google Scholar]
- 24.Duan S, Farrell K, Guenza JK, Stein BA, Swanson RA. Glutamate induces a rapid upregulation of astrocyte glutamate transport and redistribution of GLAST. J Neurosci. 1999b;19:10193–10200. doi: 10.1523/JNEUROSCI.19-23-10193.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 26.Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2Xpurinoceptors). Mol Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- 27.Farinelli SE, Nicklas WJ. Glutamate metabolism in rat cortical astrocyte cultures. J Neurochem. 1992;58:1905–1915. doi: 10.1111/j.1471-4159.1992.tb10068.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- 29.Guthrie PB, Knappenberger J, Segal M, Bennett MVL, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci USA. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeftinija SD, Jeftinija KV. ATP stimulates release of excitatory amino acids from cultured Schwann cells. Neuroscience. 1998;82:927–934. doi: 10.1016/s0306-4522(97)00310-2. [DOI] [PubMed] [Google Scholar]
- 32.Jeftinija SD, Jeftinija KV, Stefanovic G, Liu F. Neuroligand-evoked calcium-dependent release of excitatory amino acids from cultured astrocytes. J Neurochem. 1996;66:676–684. doi: 10.1046/j.1471-4159.1996.66020676.x. [DOI] [PubMed] [Google Scholar]
- 33.Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Spelta V, Sim J, North RA, Surprenant A. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem. 2001;276:23262–23267. doi: 10.1074/jbc.M102253200. [DOI] [PubMed] [Google Scholar]
- 35.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 37.Kukley M, Barden JA, Steinhauser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia. 2001;36:11–21. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- 38.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 39.Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem. 2002;277:3210–3218. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- 40.Levitan I, Garber SS. Anion competition for a volume-regulated current. Biophys J. 1998;75:226–235. doi: 10.1016/S0006-3495(98)77509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longuemare MC, Swanson RA. Excitatory amino acid release from astrocytes during energy failure by reversal of sodium-dependent uptake. J Neurosci Res. 1995;40:379–386. doi: 10.1002/jnr.490400312. [DOI] [PubMed] [Google Scholar]
- 42.Longuemare MC, Rose CR, Farrell K, Ransom BR, Waxman SG, Swanson RA. K+-induced reversal of astrocyte glutamate uptake is limited by compensatory changes in intracellular Na+. Neuroscience. 1999;93:285–292. doi: 10.1016/s0306-4522(99)00152-9. [DOI] [PubMed] [Google Scholar]
- 43.Markwardt F, Lohn M, Bohm T, Klapperstuck M. Purinoceptor-operated cationic channels in human B lymphocytes. J Physiol (Lond) 1997;498:143–151. doi: 10.1113/jphysiol.1997.sp021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- 45.Naito S, Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985;44:99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- 46.Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- 47.Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 48.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 49.Nuttle LC, Dubyak GR. Differential activation of cation channels and non-selective pores by macrophage P2Z purinergic receptors expressed in Xenopus oocytes. J Biol Chem. 1994;269:13988–13996. [PubMed] [Google Scholar]
- 50.Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pannicke T, Fischer W, Biedermann B, Schadlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A. P2X7 receptors in Muller glial cells from the human retina. J Neurosci. 2000;20:5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 53.Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin,and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- 54.Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 55.Queiroz G, Meyer DK, Meyer A, Starke K, von Kugelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- 56.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 57.Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J Neurobiol. 1999;41:221–229. [PubMed] [Google Scholar]
- 58.Sensenbrenner M, Devilliers G, Bock E, Porte A. Biochemical and ultrastructural studies of cultured rat astroglial cells: effect of brain extract and dibutyryl cyclic AMP on glial fibrillary acidic protein and glial filaments. Differentiation. 1980;17:51–61. doi: 10.1111/j.1432-0436.1980.tb01081.x. [DOI] [PubMed] [Google Scholar]
- 59.Soltoff SP, McMillian MK, Talamo BR. ATP activates a cation-permeable pathway in rat parotid acinar cells. Am J Physiol. 1992;262:C934–C940. doi: 10.1152/ajpcell.1992.262.4.C934. [DOI] [PubMed] [Google Scholar]
- 60.Soltoff SP, McMillian MK, Talamo BR, Cantley LC. Blockade of ATP binding site of P2 purinoceptors in rat parotid acinar cells by isothiocyanate compounds. Biochem Pharmacol. 1993;45:1936–1940. doi: 10.1016/0006-2952(93)90455-6. [DOI] [PubMed] [Google Scholar]
- 61.Steinberg TH, Silverstein SC. Extracellular ATP4 promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987;262:3118–3122. [PubMed] [Google Scholar]
- 62.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 63.Swanson RA, Farrell K, Stein BA. Astrocyte energetics, function, and death under conditions of incomplete ischemia: a mechanism of glial death in the penumbra. Glia. 1997a;21:142–153. doi: 10.1002/(sici)1098-1136(199709)21:1<142::aid-glia16>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 64.Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997b;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Haydon PG, Yeung ES. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem. 2000;72:2001–2007. doi: 10.1021/ac9912146. [DOI] [PubMed] [Google Scholar]