Abstract
The role of the central nucleus of the amygdala (CeN) in modulating output of noradrenaline in the forebrain was evaluated by recording extracellular, single-unit activity from the noradrenergic nucleus locus ceruleus (LC) during stimulation of the CeN. Short high-frequency trains (200 Hz) delivered at 800 μA in the CeN evoked phasic responses in 90% of the neurons recorded in LC. Single pulses were also effective but less reliably. The responses were complex, multiphasic with an initial latency of 10–20 msec. This early peak was diminished or, in some cases, completely blocked by local or intracerebroventricular application of the corticotrophin releasing factor antagonist α helical CRF (9-41). The later excitatory peak and subsequent inhibition were not effected by the drug treatment. The results underline the reciprocal functional relationship between the amygdaloid complex and the LC and suggest that the LC might be an important “effector” of CeN activation during learning.
Keywords: amygdala central nucleus, locus ceruleus, noradrenaline, CRF, neuromodulation, electrophysiology
Introduction
The central nucleus of the amygdala (CeN) has a well established role in emotional learning (Applegate et al., 1982; Pascoe and Kapp, 1985). More recent evidence suggests that this nucleus plays an essential role in regulation of attention (for review, see Gallagher and Holland, 1994; Holland and Gallagher, 1999). Careful behavioral analyses after lesions led Gallagher and Holland (1994) to conclude that the CeN “regulates the processing of cues when predictive relationships between events are first noticed or altered.” This precisely describes the cognitive context that elicits a robust response of the whole population of cells in the noradrenergic nucleus locus ceruleus (LC).
LC neurons are activated both tonically and phasically during a task requiring sustained attention, suggesting a role in vigilance (Aston-Jones et al., 1991). However, the most striking feature of the response patterns of these neurons is that they show phasic responses to novel stimuli followed by rapid habituation and then renew responding whenever the associated reward contingencies are modified, such as during reversal or extinction (Sara and Segal, 1991;Hervé-Minvielle and Sara, 1995; Vankov et al., 1995). Thus, the LC responds to the predictive value or meaning of the stimulus rather than to its physical properties (Sara et al., 1994). These LC response characteristics observed in the rat were later confirmed in the behaving monkey (Aston-Jones et al., 1997). Thus, LC neurons respond “when predictive relationships between events are first noticed or altered,” in the words of Holland and Gallagher describing the functional role of the CeN.
There is a small but potentially important afferent projection from CeN to LC (Wallace et al., 1989; Luppi et al., 1995). Fibers from CeN terminate in the rostrolateral periceruleus on dendrites identified immunohistochemically as noradrenergic (Van Bockstaele et al., 1996b,1998). Input from CeN to the LC could be important in regulating LC activity during behavior requiring high levels of attention and stimulus processing. LC might, then, participate in mediating the CeN role in attention, through its ubiquitous projections to the forebrain. The postsynaptic facilitory effects of norepinephrine (NE) on stimulus processing in all sensory modalities are well documented (Manunta and Edeline, 1997; Waterhouse et al., 1998; Lecas, 2001; Bouret and Sara, 2002).
The role attributed by Gallagher and Holland to the CeN in attention processes, together with the recent anatomical description of the projections of CeN to LC, encouraged us to examine the functional influence of CeN on LC by recording responses of LC neurons to electrical stimulation of the CeN. The role of corticotropin-releasing factor (CRF) in mediating LC responses was evaluated, because it has been shown that a substantial portion of CeN terminals targeting LC dendrites contain CRF (Van Bockstaele et al. 1996a,b) and CRF has an excitatory effect on LC neurons (Siggins et al., 1985).
Materials and Methods
Animals. Electrophysiological recordings were taken from 41 male Sprague Dawley rats obtained from IFFA Credo (L'Arbresle, France). The rats, weighing 320–420 gm at the time of the recording session, were housed for at least 1 week before the experiment in a temperature-controlled vivarium on a 12 hr light/dark cycle. They were weighed and handled regularly and had access to food and water ad libitum.
Surgery. Rats were anesthetized with urethane, 1.2 gm/kg, which was usually sufficient for the entire recording session, but it was supplemented if there was any sign of discomfort. The rats were mounted in a stereotaxic apparatus with the head positioned so that bregma was 2 mm below lambda, making an angle of approximately −14° from the head level position. Burr holes were drilled over the CeN and LC, the dura was removed, and electrodes were implanted under electrophysiological control. A bipolar stimulating electrode assembly consisted of two tungsten electrodes glued together (0.1–0.5 MΩ) with 500 μm separating the tips. This was aimed at the CeN: −1.8 mm posterior to bregma, 3.8 mm lateral to the midline, and ∼7.6 mm ventral to the surface of the brain. The LC electrode was lowered at −3.9 mm posterior to the lambda suture and 1.15 mm lateral to the midline. LC neurons were usually found at 5.2–5.8 ventral to the surface of the brain, just under the fourth ventricle. They were identified by their broad action potentials, slow firing rate (1.2 Hz), and distinctive excitatory–inhibitory response to contralateral paw pinch.
Pharmacology. In five experiments, the effect of the CRF antagonist α helical CRF (9-41) (αhCRF) (Sigma, St. Quentin Fallavier, France) was examined. In two experiments, αhCRF was injected into the ventricles (intracerebroventricular injection). A 26 gauge guide cannula was implanted above the lateral ventricle contralateral to the recording site (∼1 mm posterior to bregma and ∼1.5 mm lateral to midline), 1 mm dorsal to the ventricle (3.4 mm below brain surface), and cemented in place with dental cement. Injection was made through a 33 gauge cannula extending 1 mm ventrally from the edge of the guide to reach the ventricle. In three subsequent experiments, a 33 gauge cannula was glued to the recording electrode so that the edge of the cannula was ∼200 μm anterolateral to the tip of the recording electrode. The cannula was attached to flexible tubing into which a 2 μl Hamilton microsyringe was inserted. The electrode–cannula assembly was lowered into the LC as described above.
Two hundred micrograms of αhCRF was dissolved in 190 μl of distilled water and stored as 10 aliquots of 19 μl at −20°C. Just before the injection, the solution was completed with 1 μl of hypertonic saline to make an isotonic solution at a concentration of 1 μg/μl with a neutral pH. For intracerulear injections, 1 μl of this solution was slowly infused into the LC. Three to 4 μl were injected in intracerebroventricular experiments.
Stimulating and recording. The electrophysiological signal was filtered (400–3000 Hz bandpass), amplified (10,000×) (amplifier model # P511; Grass Instruments, West Warwick, RI), and displayed on an oscilloscope and an audio monitor. Wave forms were discriminated online using the Cambridge Electronic Design (CED) (Cambridge, UK) CED1401 digital converter and Spike2 software (CED). Data were stored on a personal computer for additional offline analysis. Stimulation was delivered through an isolation unit in single pulses (200 μsec) or in trains of three pulses at 200 Hz. Stimulation intensities included 200, 500, and 800 μA. Each series consisted of 40–60 stimulations.
Data analysis. Single units were isolated wherever possible, using the Spike2 software. If the spikes were not clearly separable, the file was treated as a multiunit recording. Poststimulus time histograms (PSTHs) and raster displays were generated for neuronal activity 500 msec before and 500 msec after the stimulation, using 2 msec bins. The mean and SD of neuronal firing activity was calculated for the 500 msec prestimulation baseline. A firing rate increase to 2 SDs above the mean of the base line, sustained over at least four bins, was considered an excitatory response. A decrease to 2 SDs below the mean was considered an inhibitory response. Response latencies were thus calculated for each unit or multiunit record.
To quantify baseline and evoked activity, the firing rate was calculated by summing the number of spikes per bin for two 50 msec windows on each PSTH, from 60 to 10 msec before stimulus onset and from 20 to 70 msec after stimulus onset. The mean firing rate in Hertz during these two periods was obtained by multiplying each count by 20 and dividing it by the number of trials (40–60).
To quantify the effect of CRF antagonist injections, spike counts during a baseline and two response windows were computed for successive trials, before and after injection. These windows were 15 msec in duration, starting 15 msec after stimulus onset for the early response window and 30 msec after stimulus onset for the late response window. The 15 msec baseline window ended 10 msec before stimulation. Spike counts obtained before and after αhCRF injection were compared with two-way ANOVA or with a nonparametric multiple comparison (Newman–Keuls analog test) when necessary (when spike counts were very low or not normally distributed). One factor was defined as CeN stimulation with two levels: baseline and response (either early or late). The other factor was αhCRF injection with two levels: before and after injection. A significant interaction between these two factors or a significant effect of the response factor in only one condition (before or after injection) was used as statistical criteria to define an influence of αhCRF on the response to CeN stimulation. For each cell, mean spike count within the defined window, before and after injection, was calculated. The magnitude of early and late phases of the response after αhCRF injection could then be expressed as a difference from the baseline and converted to Hertz. To compare the effect of αhCRF on early and late phases of the response, an average value (across trials) of spike count difference was obtained for each cell for early and late response counts, respectively. A two-way ANOVA was applied to the population of recorded cells, with one factor being defined as response phase (two levels, early and late). The other factor was αhCRF injection with two levels: before and after injection.
Histology. At the end of the stimulation session, the rats were injected with the noradrenergic α2 receptor agonist clonidine (40 μg/kg, i.p.) as a pharmacological control for the recording electrode placement. Clonidine binds to inhibitory autoreceptors in the LC, and this systemically administered dose completely inhibits firing of the noradrenergic neurons with a latency of onset of ∼10 min (Dyon-Laurent et al., 1994). Thirty minutes after injection of clonidine, electrolytic lesions were made in the stimulating and recording sites by passing constant current (9 V) between the tips and the ground for 5 sec. Rats were perfused intracardially with saline and then Formalin (10%). Brains were removed and stored in Formalin. Sixty micrometer sections were cut, stained with cresyl violet, and examined for lesions in the appropriate structures.
Results
Histology
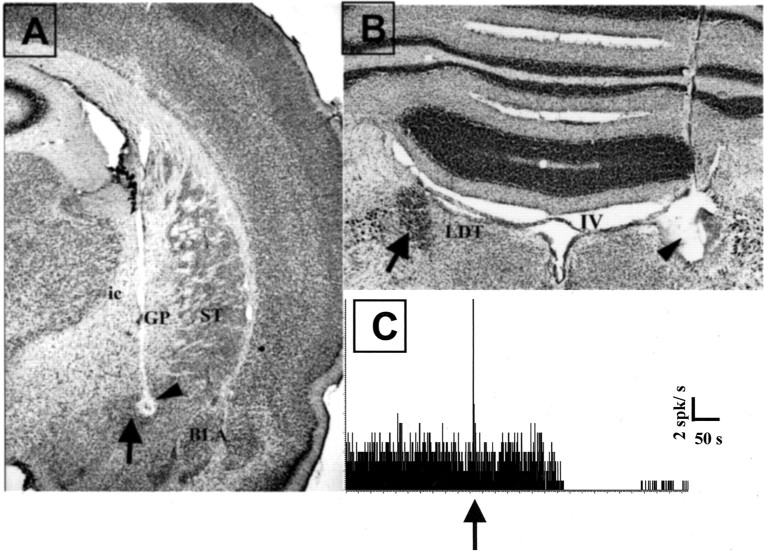
Placement of the stimulating electrode in the CeN is shown in Figure 1A. Placement of the recording electrode in the LC was determined by inspection of the histology as shown in Figure 1B, and the response to clonidine is illustrated in Figure 1C. Thirty-five rats had recording electrodes in the LC, and all of the cells were inhibited after clonidine injection. Of these, 27 also had stimulating electrodes correctly positioned in the CeN. Four stimulating electrodes were situated in the internal capsule, just lateral to CeN, two in the basolateral nucleus of the amygdala (BLA), one in the striatum, and one in the ventral hippocampus.
Fig. 1.
Histological and pharmacological control of electrode placement. A, Histological verification of the recording site in the CeN (arrow). The small lesion (arrowhead) in the CeN was made by passing DC current between the tips of the stimulating electrodes. Note the descending tract of the electrode through the internal capsule (ic) and the globus pallidus (GP). ST, Striatum.B, Histological verification of the recording site in LC. Note on the left the intact LC, the darkly stained nucleus just under the fourth ventricle (IV), as indicated by the arrow. On the contralateral side is the lesioned site of the LC (arrowhead) made by passing DC current between the tip of the recording electrode and the ground. The electrode tract can be seen above the lesion in the cerebellum. C, Example of a pharmacological verification of the recording from a noradrenergic neuron. Note the total inhibition of activity of the unit after systemic injection of clonidine (40 μg/kg) indicated by the arrow. All LC units and multiunit activity responded with total but transient inhibition after clonidine injection. The peak reflects the phasic activation of LC neurons during the injection.
Two recording electrodes were located anterior and medial to LC in the lateral dorsal tegmental nucleus (LDT), three were anterior and dorsolateral to LC in the parabrachial nucleus, and one was in the cerebellum. None of the cells recorded from these sites responded to clonidine. All six of these rats had accurately placed stimulating electrodes in the CeN.
Responses of LC neurons to stimulation of CeN
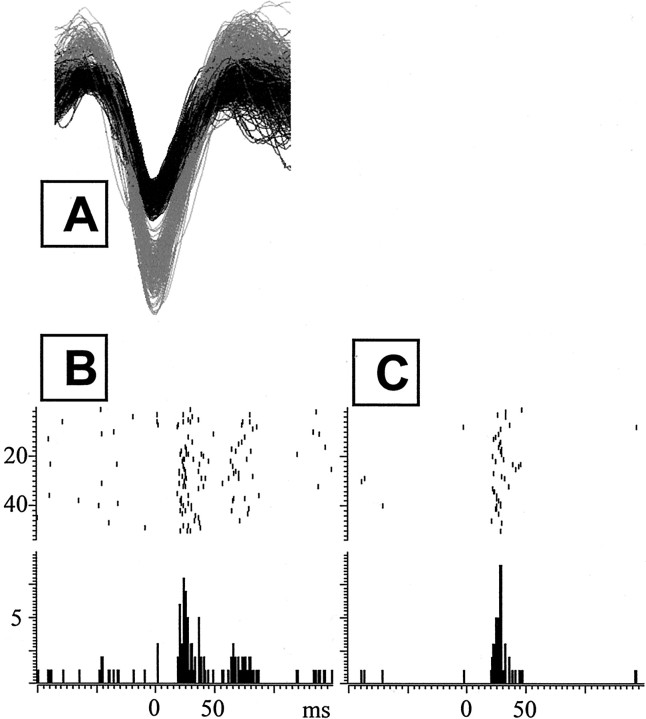
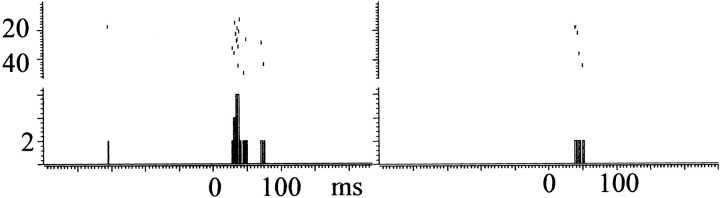
Fifty-three single LC units and 13 multiunit recordings were taken from the 27 rats that had both stimulating electrodes in the CeN and recording electrodes in the LC. Ninety percent (48 of 53) of isolated single units showed a significant response to the CeN stimulation; 44 of these were orthodromically driven. Responses were seen to single pulses at 500 and 800 μA in 20 of 53 single units (38%), with a mean latency of 11.7 ± 0.7 msec, latency being determined as described in Materials and Methods. More reliable responses were elicited by the short train of three pulses delivered at 200 Hz at an intensity of 800 μA. Forty-eight single units responded to the train, with a latency of mean 20.3 ± 0.5 msec from the first pulse. Figure2 shows an example of two single units recorded from the same electrode (Fig. 2A), one response being clearly biphasic, with a latency of 17 msec (Fig.2B), and the other short lasting, monophasic, with a latency of 20 msec (Fig. 2C).
Fig. 2.
Responses to a train of 800 μA, of two single units in LC recorded simultaneously from the same electrode.A shows superimposed waveforms of the two units. The unit depicted in B shows biphasic response with the initial phase latency at 17 msec. In the unit depicted inC, the initial latency is 20 msec, and second phase is absent. Top, Raster display of trial-by-trial responses to the stimulation. Bottom, Cumulative PSTH with 2 msec bins.
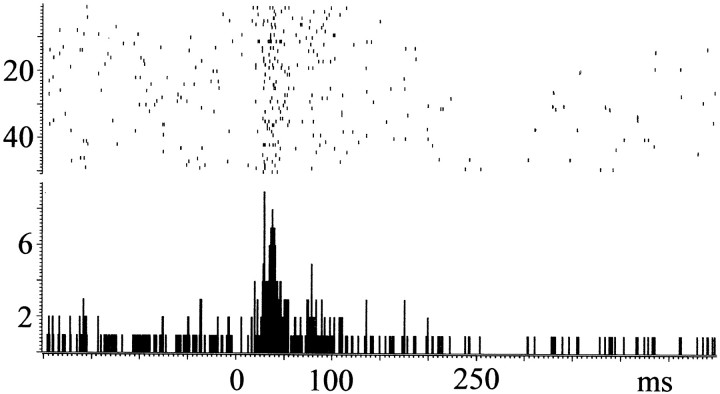
All 13 multiunit recordings taken from LC showed an excitatory response to train, with a latency of 21.3 ± 0.4 msec. An example of a multiunit response is shown in Figure3.
Fig. 3.
Multiunit response LC to a train of 800 μA applied to CeN. Note the relatively low baseline activity, which suggests that only a few neurons are included in this recording. The response consists of a biphasic excitation followed, 200 msec after stimulus, by a long-lasting inhibition. This postexcitation inhibition is probably attributable to an α2 receptor-mediated autoinhibition.
Antidromic responses were seen in four neurons. Response latency ranged from 21 to 24 msec, with a response to every stimulation trial and within cell response latencies being the same on every trial.
One unit responded with a total inhibition lasting for nearly 300 msec; this was the only purely inhibitory response seen in the entire series, and it should be noted that a neighboring unit, recorded from the same electrode, was antidromically driven by the stimulation. Thus, the inhibition seen in the other neuron was probably attributable to release of NE by the antidromically driven cell, which responded to every stimulation.
Responses of LC neurons to stimulation outside of the CeN
Stimulation of the BLA produced excitatory responses in two LC multiunit recordings with latencies of 28 and 32 msec.
In four experiments, the stimulating electrode was located in the internal capsule, and, in two of four multiunit records, there was a response in LC to the 800 μA train with a 28 msec latency. There was no response to the stimulation of the striatum (n = 1) or the ventral hippocampus (n = 1).
Responses to CeN stimulation in non-LC recordings
Verified placements in parabrachial nucleus in three rats (two single units and two multiunit) revealed reliable excitatory responses to CeN stimulation at 500 μA with a variable latency (range of 18–31 msec).
Two multiunit recordings were taken from the LDT, both showing excitatory responses with 25 and 18 msec latencies.
One recording electrode was located in the cerebellum, and there was no response to the CeN stimulation.
Effect of CRF antagonist on LC response to CeN stimulation
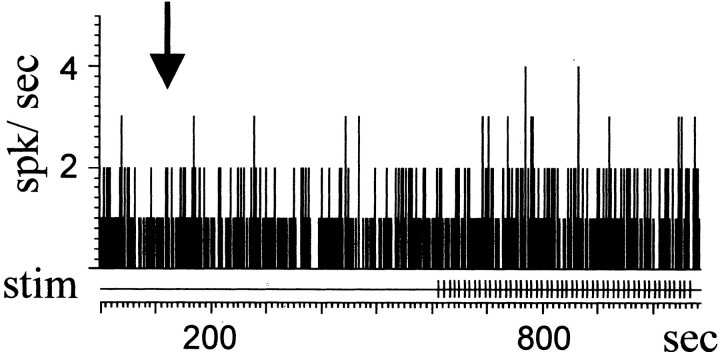
Intraventricular (n = 2) and intracerulear (n = 3) injections of αhCRF were made. Eleven single units were recorded from the five animals. The spontaneous firing rate of LC neurons was not affected by either intracerebroventricular or local injection, (Fig. 4, Table1), enabling a clear comparison of the phasic responses to CeN stimulation before and after αhCRF injection.
Fig. 4.
Local injection does not alter the firing rate of LC neurons. The firing rate of a single unit is shown as a function of time. Neither local αhCRF application (arrow) nor CeN stimulation (lines) modify the baseline activity of the cell.
Table 1.
LC unit firing rate (in Hertz) around CeN stimulation before and after αhCRF injection
| Spontaneous | Evoked | Early | Late | Latency (msec) | Resp Cells | |
|---|---|---|---|---|---|---|
| Control | 1.8 ± 0.4 | 14.7 ± 3.1 | 16 ± 4.5 | 14.2 ± 3.1 | 23.8 ± 1.8 | 11 /11 |
| αhCRF | 1.8 ± 0.6 | 10.3 ± 2.5 | 4.5 ± 2.6* | 13.3 ± 3.4 | 37.4 ± 4.1* | 7 /11 |
Spike count expressed in Hertz, averaged across 11 LC units. Mean ± SEM spontaneous activity (50 msec before stimulus onset). Mean evoked activity (between 20 and 70 msec after stimulus). Early, Mean early response (between 15 and 30 msec after stimulus). Late, Mean net late response (between 30 and 45 msec after stimulus). For these last two variables, spike count during baseline in an equivalent window was subtracted from the evoked spike count. The effect of αhCRF is restricted to the early phase of the evoked activity, which also results in a significant increase in response latency for cells that still showed a response. Resp cells, Number of units responding to the CeN stimulation before and after drug treatment. *p < 0.05.
All cells showed a significant response to CeN stimulation (500 μA trains). There were no differences in effect of the CRF antagonist whether it was injected into the ventricle or into LC. Figure5 (left) shows a response of a cell having very little spontaneous activity and a reliable phasic response to the stimulation. The response was significantly decreased after the intracerebroventricular administration of the CRF antagonist (Fig. 5, right).
Fig. 5.
Intracerebroventricular injection of CRF antagonist decreases LC response to CeN stimulation. PSTHs (2 msec bin) and rasters constructed around CeN stimulation (500 μA trains) for an LC unit with very little spontaneous activity. Before αhCRF injection (left), the cell exhibits a clear response to CeN stimulation. After intracerebroventricular injection of αhCRF (right), the response is greatly decreased (nonparametric Newman–Keuls analog test;p < 0.05).
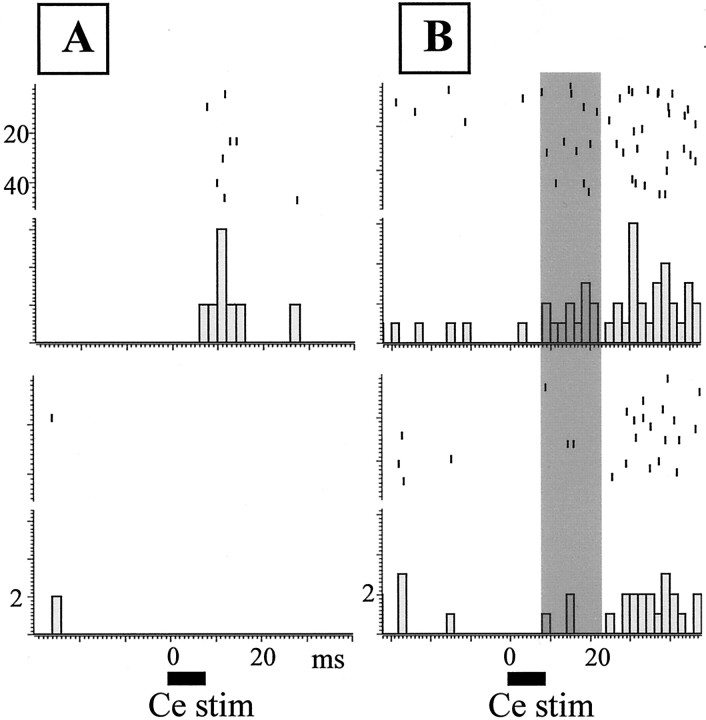
When the whole response window (50 msec) was considered, the injection of αhCRF had no significant effect on response magnitude (Table 1), because the treatment only affected the early phase of the response. For four of the six units that had only presented an early response, this response was completely blocked (Fig.6A). This early response was significantly decreased for the other two units. Five units showed a biphasic response that consisted of an early peak at ∼18 msec and a later peak at ∼30 msec from stimulus onset; an example is seen in Figure 6B. For these cells, clearly only the early phase of the response was altered by αhCRF, the late phase being unaffected.
Fig. 6.
Effects of local injection of CRF antagonist on LC response to CeN stimulation. A, PSTHs and rasters constructed around CeN stimulation (Ce stim) (500 μA trains; horizontal bar) for an LC unit showing a monophasic response (top). This response is completely blocked by the CRF antagonist (bottom; nonparametric Newman–Keuls analog test; p < 0.05). B, Same representation for another single unit showing a biphasic response to CeN stimulation. Before αhCRF injection (top), the cell exhibits a biphasic response with an early (shaded area) and a late phase. After the injection (bottom), the early response is greatly attenuated (30% of its preinjection value; nonparametric Newman–Keuls analog test; p < 0.05), whereas the late phase is spared.
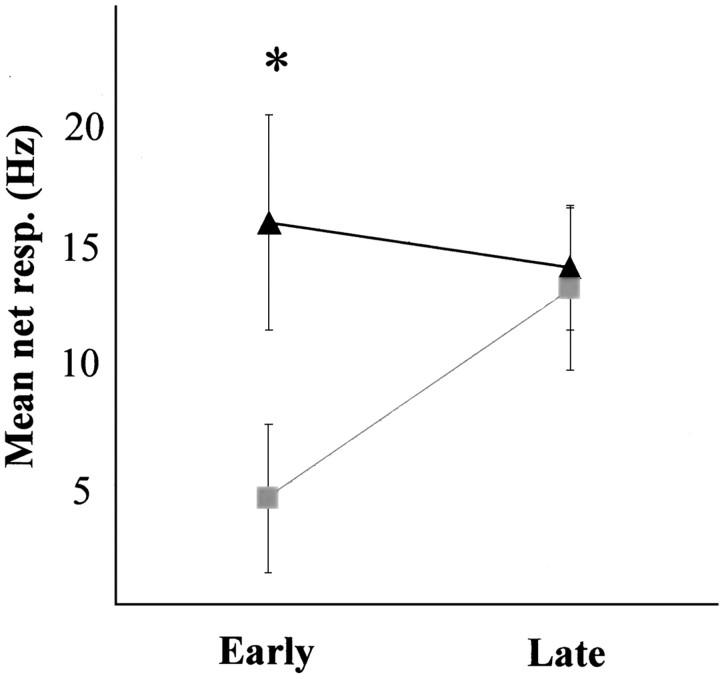
This differential effect of αhCRF on early and late phases of the response is clearly seen when the entire sample is taken into consideration. Figure 7 depicts the mean early and late responses, before and after drug treatments. The drug × phase interaction was significant (F(20,1) = 6.4; p = 0.02), confirming that effect was limited to the early phase of the response.
Fig. 7.
Mean ± SEM early and late responses of 11 LC units to CeN stimulation before and after injection of αhCRF. Only the early phase of the response was affected by the treatment. Triangles, Control; squares, αhCRF. An ANOVA reveals a significant interaction between response phase and treatment factors (F(20,1) = 6.4; p = 0.02).
For those cells (n = 7) that still responded to CeN stimulation after αhCRF injection, a significant increase in latency was observed as a consequence of the effect on the early phase of the response (t(6) = −2.6;p = 0.04) (Table 1).
Discussion
Responses of LC units and multiunits to CeN stimulation were consistent and present in the large majority of recorded cells. Although the anatomical projections of CeN to LC have been well described, with some neurochemical definition (Van Bockstaele et al., 1996a,b), this is the first direct demonstration of a functional influence of CeN on LC activity in vivo. The responses elicited were for the most part excitatory, with little variability in initial latency, but most responses were complex, with biphasic excitation (Fig. 2) or excitation followed by inhibition (Fig. 3).
Van Bockstaele et al. (1998) provided strong evidence that a substantial portion of the CeN terminals in the periceruleus region make asymmetric, excitatory synaptic specializations on target cells staining positively for tyrosine hydroxylase. There are abundant synapses on unidentified cells and dendritic processes as well. This region contains a large population of GABAergic neurons that locally regulate LC activity, thus providing an additional mediating mechanism for CeN influence on LC. A substantial number of CeN axon terminals located in the periceruleus region are CRF immunoreactive (Van Bockstaele et al., 1998). Previous studies showed a marked decrease in CRF in LC region after lesions of the CeN, suggesting that this nucleus is a major source of CRF in LC (Koegler-Muly et al., 1993). Moreover, recent studies using a combination of cellular and molecular techniques clearly confirm the existence of CRF receptors in LC neurons (Fox et al., 2002). Electrophysiological studies, in vivo andin vitro, have indicated that CRF has an excitatory effect on LC neurons (Siggins et al., 1985; Curtis et al., 1995), so CRF should be a strong candidate for mediating at least part of the LC response to CeN stimulation. The present results confirm this by showing that the short latency early part of the response is significantly attenuated or completely blocked after local application of the CRF antagonist in the LC. The presence of vesicles in these terminals that were not CRF immunoreactive suggests that other peptides and neurotransmitters are likely to be colocalized with CRF in CeN terminals (Van Bockstaele et al., 1998). Indeed, immunohistochemical studies revealed large populations of neurotensin, enkephalin, somatostatin, substance P, cholecystokinin, and vasoactive peptide containing neurons within the CeN (Cassell et al., 1986). The residual early response sometimes seen after the CRF antagonist could be mediated by any of these. The late part of the response, spared by the injection, is most likely polysynaptic.
The majority of extrinsic projections from CeN are GABAergic (Swanson and Petrovich, 1998); their short-latency inhibitory influence might delay expression of the excitatory response in LC. It should be noted that the stimulus artifact associated with the train stimulation had a duration of 13 msec, so it could be masking an early inhibitory response.
Specificity of CeN stimulation
Stimulating electrodes located in BLA and the internal capsule evoked responses in LC. The BLA does not project to LC but does have a small direct projection to the CeN and another via the lateral amygdala (LA) (Pitkanen et al., 1997). Thus, the polysynaptic influence would account for the longer-latency response of LC cells to this stimulation. Responses in LC were also elicited, less reliably, from the internal capsule. Data from our laboratory suggest that fibers originating in the FR2 region of frontal cortex and projecting to the region of the LC pass via the internal capsule. Electrical stimulation of the FR2 produces an excitatory response in LC (Briois and Sara, 1997; Jodo et al., 1998) that is abolished by cutting this fiber system (L. Briois and S. J. Sara, unpublished observations). It is important to note that stimulation of other electrode placements, near but outside of the CeN, namely in the ventral hippocampus or the ventral striatum, did not evoke responses in LC.
It is possible that there is current spread to other structures in the vicinity that could mediate the effect of the CeN stimulation. The most likely candidate is the bed nucleus of the stria terminalis (BNST), just adjacent to the CeN and a major output pathway from the amygdala. This nucleus does send a large projection to the periceruleus region. Recent studies by Van Bockstaele et al. (1996a) have shown that, like CeN projections, BNST terminals synapse on both noradrenergic and non-noradrenergic dendrites in the region. The major difference is that the BNST synapses are mostly symmetrical, i.e., of the inhibitory type. Thus, current spread to the BNST could be contributing to the response in LC, but it most likely does not account for the consistent excitatory responses observed. In fact, current spread to the BNST might account for the puzzling observation that LC responses to short trains had significantly longer latencies than responses to single pulses of the same intensity. According to the ultrastructural studies of Van Bockstaele, activation of the BNST, more likely during a train than during a brief pulse, would provide a competing inhibitory influence to LC, delaying the appearance of the excitatory response.
The relatively sparse number of antidromically driven responses was initially unexpected, because the CeN receives a substantial noradrenergic input. A recent report has shown, however, that the majority of the noradrenergic fibers in the CeN originate, not in LC as originally suggested by Fallon et al. (1978), but in the medulla oblongata catecholamine cell groups (Asan, 1998). This corroborates a previous study using double labeling to show that only ∼5% of LC neurons were retrogradely labeled from the CeN (Petrov et al., 1993).
Parabrachial and LDT responses to CeN stimulation
Responses to CeN stimulation in the parabrachial nucleus and in the LDT are not surprising, because the CeN projects directly to both of these regions (Hopkins and Holstege, 1978); the responses had slightly longer latencies than those in LC, but there were not enough data to evaluate the reliability of this difference.
Functional significance
There has been much discussion concerning the relative roles of the various nuclei of the amygdala in learning and memory processes. There is a general consensus that the amygdala plays an important role in pavlovian fear conditioning and inhibitory avoidance conditioning (Kapp et al., 1979; Pascoe and Kapp, 1985; Davis, 1994; McGaugh et al., 1996; LeDoux, 2000) and that the LA is involved in processing information concerning the conditioned stimulus (CS), whereas the CeN governs reactive responses (Amorapanth et al., 2000). There is, however, disagreement as to whether the amygdala is the site of plasticity underlying the associative fear learning or whether the role of the amygdala is to modulate memory formation in other brain regions (Fanselow and LeDoux, 1999; Cahill et al., 1999; Vazdarjanova and McGaugh, 1999; Wilensky et al., 2000). Consideration of the functional consequences of the CeN influence on LC might put the question in a new light. Activation of CeN during conditioning will cause an increase in firing of LC cells, which in turn will release NE in the BLA and LA, because there is a projection from LC to both of these regions (Fallon et al., 1978). As we know from the extensive work of McGaugh et al. (1996), this increase in NE in the amygdala will enhance memory consolidation by interaction with hormones and neurotransmitters. The CeN excitation of LC will also release NE in forebrain regions. This should result in increased vigilance and attention (Berridge and Foote, 1991) and enhanced sensory processing (Manunta and Edeline, 1997; Waterhouse et al., 1998; Lecas, 2001;Bouret and Sara, 2002), thereby facilitating learning.
Several studies have shown that stimulation of the amygdala enhances synaptic transmission, plasticity, and long-term potentiation in the dentate gyrus (DG) of the hippocampus (Ikegaya et al., 1995, 1996;Akirav and Richter-Levin, 1999). The activation of LC by stimulation of CeN directly or via BLA should result in a massive release of NE from LC terminals, found profusely in the DG. There is a large literature showing noradrenergic-induced enhancement of cellular excitability (Harley and Sara, 1992), synaptic transmission, (Sara and Bergis, 1991;Kitchigina et al., 1997), and synaptic plasticity (Neumann and Harley, 1983) in vivo in the rat DG. If activation of the amygdala occurs during learning, then its influence on LC should promote synaptic plasticity in the hippocampus, thought to be necessary for memory formation.
There are surprisingly few electrophysiological studies of the involvement of CeN in learning to lend support to the view of CeN function put forth by Gallagher and Holland (see Introduction). The extensive work of Kapp et al. (1994) has, however, clearly shown that this nucleus is activated by CSs in pavlovian aversive conditioning (Kapp et al., 1994). It is evident that CeN activity during learning can influence forebrain structures through several different pathways.Gallagher and Holland (1994) have, indeed, provided evidence from lesion and pharmacological studies that the cholinergic system is involved in mediating the CeN role in attention. In those studies, however, the conditioned orientating response, suppressed after CeN lesion, was spared by lesions of the cholinergic system (Holland and Gallagher, 1999), suggesting the involvement of other outputs. Our electrophysiological studies showing that responses are evoked in LC cells under similar cognitive conditions as for CeN (see Introduction) together with the present results showing the strong excitatory influence of CeN on LC makes a strong case for considering LC to be another effector of CeN activity. The widespread LC projections on both sensory and limbic areas, particularly the BLA and the hippocampus, should facilitate neuronal processing across these areas, as well as the formation of a distributed memory trace.
Footnotes
This work was supported by Centre National de la Recherche Scientifique (CNRS) Unité Mixte de Recherche 7102. S.B. was supported by a predoctoral grant from the French Ministère de l'Education Nationale, Recherche et Technologie. A bilateral grant from the CNRS–Beckman Institute to S.J.S and M. Gabriel supported A.D. We recognize the essential contribution of Y. Moricard in providing excellent histology. Prof. E. Thomas and Dr. E. Yadin participated in some of the preliminary experiments.
Correspondence should be addressed to Dr. Susan J. Sara, Neuromodulation and Memory Processes, Unité Mixte de Recherche 7102, Université Pierre & Marie Curie, 9 Quai St. Bernard, 75005 Paris, France. E-mail: sjsara@ccr.jussieu.fr.
References
- 1.Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J Neurosci. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 3.Applegate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Res. 1982;238:457–462. doi: 10.1016/0006-8993(82)90123-8. [DOI] [PubMed] [Google Scholar]
- 4.Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- 6.Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- 7.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouret S, Sara SJ (2002) Locus coeruleus activation modulates firing rate and temporal organization of odor-induced single cell responses in rat piriform cortex. Eur J Neurosci 2371–2382. [DOI] [PubMed]
- 9.Briois L, Sara SJ. Locus ceruleus responses to frontal cortex stimulation after local injection of GABA antagonists or lesion of the nucleus paragigantocellularis. Soc Neurosci Abstr. 1997;23:930.2. [Google Scholar]
- 10.Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- 11.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- 12.Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- 13.Davis M. The role of the amygdala in emotional learning. Int Rev Neurobiol. 1994;36:225–266. doi: 10.1016/s0074-7742(08)60305-0. [DOI] [PubMed] [Google Scholar]
- 14.Dyon-Laurent C, Herve A, Sara SJ. Noradrenergic hyperactivity in hippocampus after partial denervation: pharmacological, behavioral, and electrophysiological studies. Exp Brain Res. 1994;99:259–266. doi: 10.1007/BF00239592. [DOI] [PubMed] [Google Scholar]
- 15.Fallon J, Koziell D, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and autorhinal cortex. J Comp Neurol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- 16.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 17.Fox K, Wolff I, Curtis A, Pernar L, van Bockstaele E, Valentino R. Multiple lines of evidence for the existence of corticotropin releasing factor receptors on locus coeruleus neurons. Soc Neurosci Abstr. 2002;28:637.9. [Google Scholar]
- 18.Gallagher M, Holland PC. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci USA. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harley CW, Sara SJ. Locus coeruleus bursts induced by glutamate trigger delayed perforant path spike amplitude potentiation in the dentate gyrus. Exp Brain Res. 1992;89:581–587. doi: 10.1007/BF00229883. [DOI] [PubMed] [Google Scholar]
- 20.Hervé-Minvielle A, Sara SJ. Rapid habituation of auditory responses of locus coeruleus cells in anaesthetized and awake rats. NeuroReport. 1995;6:1363–1368. doi: 10.1097/00001756-199507100-00001. [DOI] [PubMed] [Google Scholar]
- 21.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- 23.Ikegaya Y, Abe K, Saito H, Nishiyama N. Medial amygdala enhances synaptic transmission and synaptic plasticity in the dentate gyrus of rats in vivo. J Neurophysiol. 1995;74:2201–2203. doi: 10.1152/jn.1995.74.5.2201. [DOI] [PubMed] [Google Scholar]
- 24.Ikegaya Y, Saito H, Abe K. The basomedial and basolateral amygdaloid nuclei contribute to the induction of long-term potentiation in the dentate gyrus in vivo. Eur J Neurosci. 1996;8:1833–1839. doi: 10.1111/j.1460-9568.1996.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 25.Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- 26.Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- 27.Kapp BS, Supple WF, Jr, Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behav Neurosci. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 29.Koegler-Muly SM, Owens MJ, Ervin GN, Kilts CD, Nemeroff CB. Potential corticotropin-releasing factor pathways in the rat brain as determined by bilateral electrolytic lesions of the central amygdaloid nucleus and the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1993;5:95–98. doi: 10.1111/j.1365-2826.1993.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 30.Lecas JC. Noradrenergic modulation of tactile responses in rat cortex. Current source-density and unit analyses. C R Acad Sci III. 2001;324:33–44. doi: 10.1016/s0764-4469(00)01276-2. [DOI] [PubMed] [Google Scholar]
- 31.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Luppi P-H, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- 33.Manunta Y, Edeline JM. Effects of noradrenaline on frequency tuning of rat auditory cortex neurons. Eur J Neurosci. 1997;9:833–847. doi: 10.1111/j.1460-9568.1997.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 34.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann R, Harley C. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res. 1983;273:162–165. doi: 10.1016/0006-8993(83)91106-x. [DOI] [PubMed] [Google Scholar]
- 36.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons in the awake rabbit. Brain Res Bull. 1985;14:331–338. doi: 10.1016/0361-9230(85)90194-7. [DOI] [PubMed] [Google Scholar]
- 37.Petrov T, Krukoff TL, Jhamandas JH. Branching projections of catecholaminergic brainstem neurons to the paraventricular hypothalamic nucleus and the central nucleus of the amygdala in the rat. Brain Res. 1993;609:81–92. doi: 10.1016/0006-8993(93)90858-k. [DOI] [PubMed] [Google Scholar]
- 38.Pitkanen A, Savander V, LeDoux J. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 39.Sara SJ, Bergis O. Enhancement of excitability and inhibitory processes in hippocampal dentate gyrus by noradrenaline: a pharmacological study in awake, freely moving rats. Neurosci Lett. 1991;126:1–5. doi: 10.1016/0304-3940(91)90356-x. [DOI] [PubMed] [Google Scholar]
- 40.Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- 41.Sara SJ, Vankov A, Hervé A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 42.Siggins GR, Gruol D, Aldenhoff J, Pittman Q. Electrophysiological actions of corticotropin-releasing factor in the central nervous system. Fed Proc. 1985;44:237–242. [PubMed] [Google Scholar]
- 43.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 44.Van Bockstaele EJ, Colago EEO, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996a;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Van Bockstaele EJ, Chan J, Pickel VM. Input from central nucleus of the amygdalaefferents to pericoeruleardendrites, some of which contain tyrosine hydroxylase immunoreactivity. J Neurosci Res. 1996b;45:289–302. doi: 10.1002/(SICI)1097-4547(19960801)45:3<289::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 47.Vankov A, Hervé-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 48.Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallace DM, Magnuson DJ, Gray TS. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci Lett. 1989;97:252–258. doi: 10.1016/0304-3940(89)90606-x. [DOI] [PubMed] [Google Scholar]
- 50.Waterhouse BD, Moises HC, Woodward DJ. Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res. 1998;790:33–44. doi: 10.1016/s0006-8993(98)00117-6. [DOI] [PubMed] [Google Scholar]
- 51.Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]