Abstract
Rationale
Gq signaling in cardiac myocytes is classically considered toxic. Targeting Gq directly to test this is problematic, because cardiac myocytes have many Gq-coupled receptors.
Objective
Test whether Gq coupling is required for the cardioprotective effects of an alpha-1A-adrenergic receptor (AR) agonist.
Methods and Results
In recombinant cells, a mouse alpha-1A-AR with a 6-residue substitution in the third intracellular loop does not couple to Gq signaling. Here we studied a knockin mouse with this alpha-1A-AR mutation. Heart alpha-1A receptor levels and antagonist affinity in the knockin were identical to WT. In WT cardiac myocytes, the selective alpha-1A agonist A61603 stimulated phosphoinositide phospholipase C and myocyte contraction. In myocytes with the alpha-1A knockin, both A61603 effects were absent, indicating that Gq coupling was absent. Surprisingly, A61603 activation of cardioprotective ERK was markedly impaired in the KI mutant myocytes, and A61603 did not protect mutant myocytes from doxorubicin toxicity in vitro. Similarly, mice with the α1A KI mutation had increased mortality after transverse aortic constriction, and A61603 did not rescue cardiac function in mice with the Gq coupling-defective alpha-1A receptor.
Conclusion
Gq coupling is required for cardioprotection by an alpha-1A-AR agonist. Gq signaling can be adaptive.
Keywords: Gq, Gq signaling, cardiac myocytes, alpha-1A-adrenergic receptors, heart failure, cardioprotection, Gproteins, cardioprotection, Cell Signaling, Myocardial Biology, Pathophysiology, Translational Studies
INTRODUCTION
Studies in the late 1990s showed that cardiac overexpression of G alpha q (Gq) can cause death of myocytes and decompensated heart failure.1–3 These results have had a profound effect on thinking, such that current models of pathological hypertrophy, hypertrophy that leads to heart failure, typically point to Gq-coupled receptors as key mediators.4–7
Cardiac myocytes have Gq-coupled receptors for many neurohormones and other ligands, including norepinephrine (α1-adrenergic receptors, ARs), acetylcholine, angiotensin II, apelin, endothelin, histamine, prostaglandin, bradykinin, serotonin, spingosine-1-phosphate, thrombin, urotensin II, and vasopressin.8 However, most Gq-coupled receptors also signal through Gq-independent pathways, so it is very difficult to be sure if Gq signaling by any or all of these receptors is indeed toxic, as models suggest.
α1-ARs are highlighted in most models as mediators of pathological hypertrophy. However, recent studies show that stimulation of one α1-AR subtype, the α1A, with highly selective agonists can prevent cardiotoxicity and heart failure in mouse models.9–12 It is unknown whether these adaptive effects are mediated via the Gq coupling of the α1A-AR, or by a Gq-independent mechanism.
An approach to test these possibilities was provided by the discovery of an α1A mutant receptor that does not couple to Gq, as assayed by absent inositol phosphate (IP) and calcium signaling in HEK293 cells, despite normal ligand binding.13 The α1A mutant involved a 6 amino acid substitution in the 3rd intracellular loop (CCPGCC for DSEQVT).13 This region is analogous to an area of the α1B-AR subtype required for Gq coupling in COS cells;14 and is just C-terminal to 3 amino acids (YVV) of the α1A, deletion of which abolishes calcium and IP responses in PC12 cells.15 None of these mutations have been shown to disrupt Gq binding, and the uncoupling mechanism is not known.
Here we tested a knockin (KI) mouse model with the same 6 amino substitution in the 3rd intracellular loop. We find that α1A ligand binding by this GqKI mutant is normal in heart, but Gq coupling is absent in myocytes by IP signaling and contraction. ERK activation by an α1A agonist is markedly impaired, and cardioprotection is absent in vitro and in vivo. These data show that adaptive effects of the α1A require Gq signaling, and that Gq signaling is not invariably maladaptive.
METHODS
The authors declare that all supporting data are available within the article [and its online supplementary files].
Mice were adult males in the C57Bl/6J background. The study was limited to male mice, since female C57BL6J mice are resistant to stresses that cause heart failure in males, and this study was not intended to explore sex differences.9, 16, 17 Horizon Discovery (St. Louis, MO) made the mouse with the knockin that disrupts Gq signaling in recombinant cells (GqKI) in the C57Bl6J background using CRISPR technology. Adult mouse ventricular myocytes (AMVMs) were isolated by perfusion with collagenase and cultured in serum-free medium.
RNA from freshly isolated AMVMs was extracted with RNeasy Mini Kit, and α1 subtype mRNAs normalized to β-actin and GAPDH mRNAs were quantified by RT-qPCR and the ΔΔCq method.
Saturation radioligand binding in total membranes from intact hearts used 3H-prazosin for α1-ARs. The fraction of the α1A subtype was determined using competition binding with the selective antagonist 5-methylurapidil.18
To assess Gq coupling by phosphoinositide phospholipase C (PI-PLC) activation, cultured myocytes were prelabeled with 3H-inositol and stimulated for 1 h with the selective α1A agonist A61603; 3H-inositol phosphates (IPs) were separated by dowex chromatography and quantified. Absolute IP-1 mass was also quantified using the Cisbio IP-One competitive HTRF (Homogeneous Time Resolved Fluorescence) immunoassay (Bedford, MA).
Gq coupling and calcium signaling were also assessed by A61603-stimulated contraction of isolated myocytes, quantifying changes in sarcomere length with an IonOptix system.
ERK1/2 dually phosphorylated on tyrosine and threonine (pERK) was detected in cultured myocytes by immunoblot or immunocytochemistry with a rabbit monoclonal antibody (Ab, Cell Signaling #4370).
A role for Gi was tested using pertussis toxin (PTX), and for β-arrestin, using dephosphorylation of β-arrestin1 detected on immunoblot.
Cultured myocyte survival after incubation with the anthracycline doxorubicin (DOX) was estimated by measuring viable cells with the MTT assay.
Mouse survival and response to chronic A61603 in vivo were tested by severe transverse aortic constriction (TAC, gradient ~110 mmHg). Two weeks after TAC, when function by echo had deteriorated, WT mice were randomized to A61603 or vehicle infusion by osmotic minipump, and GqKI mice were treated with A61603. All operators were blinded, and no mice were excluded. Group sizes were estimated from prior studies in WT mice.
Results are mean ± SE. GraphPad Prism v6.0h was used to test for significant differences (p<0.05) as indicated in the legends.
RESULTS
α1A-AR mRNA levels and binding are normal in the GqKI mutant mouse
The substitution mutation that eliminates Gq coupling in recombinant cells replaces 6 amino acids (228–233) in the 3rd intracellular loop of the α1A (Figure 1A).13 A different close-by α 1A deletion (YVV at residues 208–210 ) also uncouples Gq signaling.15 Gq binding is not reduced.15
FIGURE 1.
Cardiac α1A-AR mRNA and receptor levels are normal in the knockin mouse (GqKI) with a 6-amino acid substitution in the 3rd intracellular loop of the α1A. A. Diagram of the mutation: CCPGCC replaces DSEQVT at amino acid 228. B. α1-AR subtype mRNA levels in AMVMs (AU/HKGs, arbitrary units relative to 2 house keeping genes). C. Saturation binding for total α1-ARs in WT and mutant heart. WT levels are identical to those reported previously (Rokosh et al., 2002; Myagmar et al., 2017).18,34 D. Competition binding to identify the α1A as the site with high 5-methylurapidil affinity, ~20% α1A in both WT and GqKI. Prism was used for nonlinear fitting in C and D. All Ns are hearts.
The mRNA levels of all 3 α1-AR subtypes were similar to wild type (WT) in GqKI adult mouse ventricular myocytes (AMVMs) (Figure 1B). Total α1-AR binding (Figure 1C) and fraction of the α 1A were identical to WT in GqKI hearts (Figure 1D).
These data indicate that the mutant receptor is expressed and binds normally in heart and myocytes, as seen previously in recombinant cells.13
This mutation will not alter α1A localization. The α1A is expressed constitutively in the cardiac myocyte nucleus,19–24 with a nuclear localization signal in the C-terminal tail, quite distant from the KI mutation made in the proximal 3rd intracellular loop.22
The GqKI mutant α1A does not couple to Gq in myocytes
We tested Gq coupling in cultured AMVMs using the selective α1A agonist A61603 to activate PI-PLC, the prototypical Gq response. A61603 activated PI-PLC robustly in WT myocytes, measured by stimulated accumulation both of 3H-IP1 (Figure 2A) and IP1 mass (Figure 2B). Activation was absent in GqKI cells (Figure 2AB).
FIGURE 2.
The GqKI mutant α1A does not couple to Gq in myocytes. TOP: The mutant receptor does not mediate activation of phosphoinositide-phospholipase C (PI-PLC) by the α1A agonist A61603 (60 min) in adult mouse ventricular myocytes (AMVMs), measured by A. labeling with 3H-inositol, or B. inositol phosphate mass assay. Sigmoidal dose-response curves were fit in Prism; p values are from unpaired t test of the calculated Emax; N = cultures from different hearts. BOTTOM: C. The mutant receptor does not mediate an inotropic response to A61603 (A6,100 nM, 5–10 min at peak response), shown as percent basal length for individual cells from 4 hearts (left) and for hearts from which the cells were isolated (right). Both a WT and a GqKI mouse were studied on each experimental day; thus for GqKI cells that did not respond to A61603, WT cells on the same day did respond. P values by paired t test for cells, and by unpaired t test for hearts.
We also tested Gq coupling by assaying contraction stimulated by A61603, which is a calcium-dependent process.25 A61603 stimulated shortening in WT cells but had no effect on contraction in GqKI cells, whether displayed on a per cell or per heart basis (Figure 2C).
These data confirm in normal adult cardiac ventricular myocytes that the mutant receptor does not couple to Gq signaling.
No role for β-arrestin or Gi
We considered whether the KI that eliminated Gq signaling also disrupted signaling via β-arrestin or Gi. There is no evidence in cardiac myocytes that the α1A couples to β-arrestin, and even in recombinant cells, where α1A levels are an average 700-fold higher than in myocytes, the receptor does not recruit β-arrestiin.26–30 To explore β-arrestin involvement in myocytes, we used an approach that did not involve knockout or overexpression, but rather tested if α1A agonist stimulation caused dephosphorylation of β-arrestin, a first step in β-arrestin activation.31, 32 As shown in Online Figure I A, total β-arrestin1 and β-arrestin1 phosphorylated at S412, the major regulated site, were detected readily in AMVMs. However, the selective α1A agonist A61603 did not cause β-arrestin dephosphorylation. Myocyte fractionation to cytosol and membrane components also did not reveal β-arrestin dephosphorylation (Online Figure I B). Interestingly, the β-AR agonist isoproterenol did not dephosphorylate β-arrestin1 (Online Figure I AB). These results support those in recombinant cells that the α1A does not couple to β-arrestin.26–30
Similarly, we showed previously that Gi is not involved in α1-receptor activation of PI-PLC, using pertussis toxin (PTX) in neonatal rat ventricular myocytes.33 Here we used PTX to test α1A coupling to ERK activation via Gi. Online Figure II shows that PTX has no effect on A61603 activation of ERK.
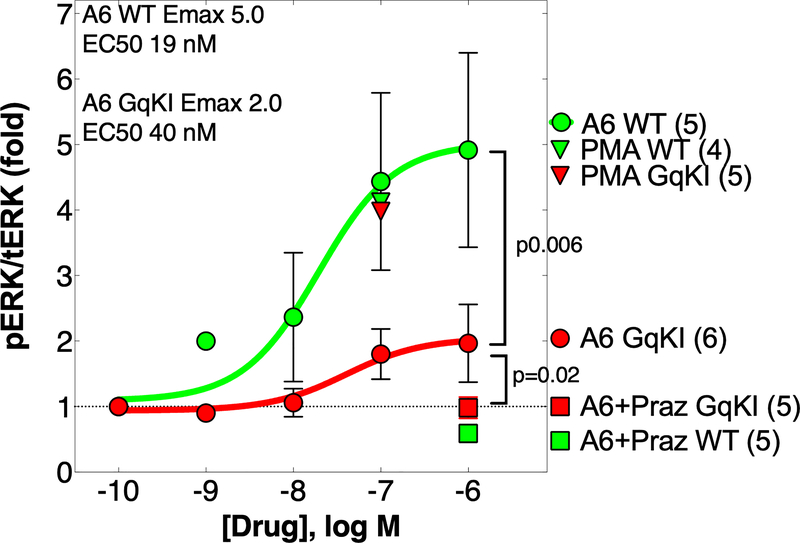
Gq coupling is required for full ERK activation by an α1A agonist in AMVMs
Previous studies established that ERK is required for cardioprotection by the α1A.10, 24 In WT cultured AMVMs, the α1A agonist A61603 activated ERK by 5-fold (Figure 3). In GqKI myocytes, A61603 activated ERK by only 2-fold, a significant 60% reduction vs. WT (Figure 3). As a positive control, phorbol myristate acetate (PMA) increased pERK by a maximum 5-fold equally in WT and GqKI cells, and as a negative control, the α1-AR antagonist prazosin blocked the A61603 effect completely in both WT and GqKI myocytes (Figure 3).
FIGURE 3.
Gq coupling is required for full ERK activation by an α1A agonist in AMVMs. AMVMs cultured 24 h in serum-free medium were treated 5 min with the drugs indicated, and phospho-ERK and total-ERK were quantified by immunoblot. A6=A61603; GqKI=α1A with Gq knockin. PMA=phorbol myristate acetate was a positive control for ERK activation in the GqKI. Praz=prazosin, an α1-AR antagonist, blocks A61603 activation of ERK in both WT and GqKI, confirming an α1 effect. Sigmoidal dose-response curves were fit in Prism; p values are from unpaired t test of the calculated Emax of A6 WT and A6 GqKI; N = cultures from different hearts.
Thus Gq coupling is required for full ERK activation by an α1A agonist in myocytes. Residual ERK activation can be detected in GqKI myocytes when α1A-mediated ERK activation is robust (Figure 3), but not when activation is less strong (Online Figure II).
Previously, we observed that stimulation of the α1A increased pERK only in the myocyte cytoplasm, not in the nucleus,34 as noted by others.19 Online Figures III A and III B show that pERK is limited to the cytoplasm after α1A agonist stimulation of WT myocytes and myocytes with the KI mutant α1A. PMA did not increase nuclear pERK (Online Figure III B).
Gq coupling is required for cardioprotection by the α1A in vitro and in vivo
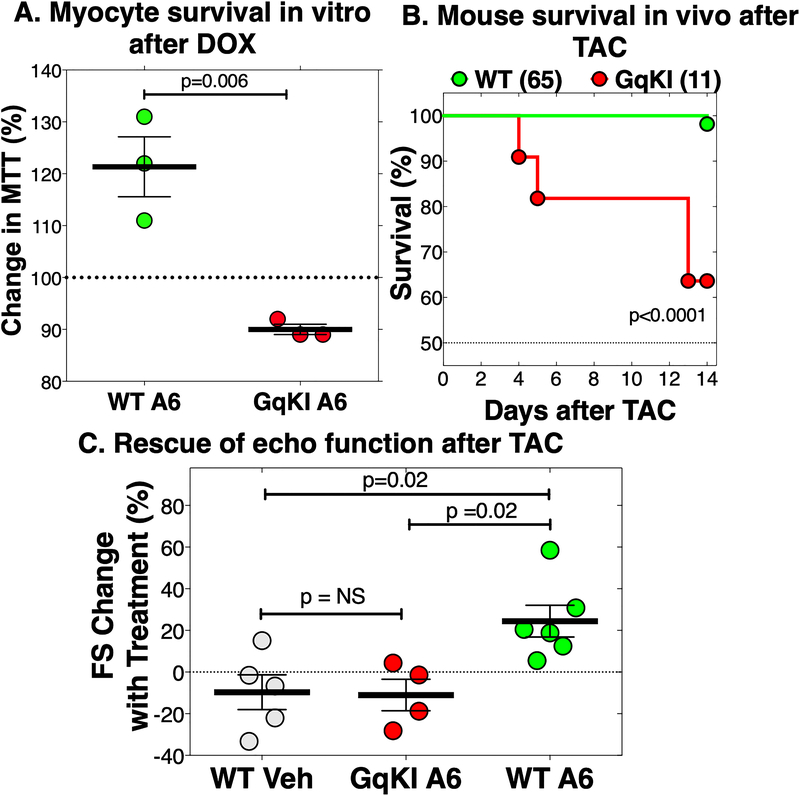
We used the cardiotoxic anthracycline DOX to test cardioprotection by the α1A in vitro, because the α1A is required for AMVM protection from DOX.9 Co-incubation of A61603 with DOX 5 μM for 24 h protected WT myocytes, as quantified by the MTT assay for viable mitochondria (Figure 4A). No protection was seen in GqKI myocytes (Figure 4A).
FIGURE 4.
Gq coupling is required for cardioprotection by an α1A agonist in vitro with doxorubicin, and in vivo with transverse aortic constriction (TAC). A: Cultured WT or GqKI AMVMs were treated 24 h with doxorubicin (DOX, 5 μM), in the presence of A61603 (A6, 100 nM) or Vehicle. Cell survival was quantified by the MTT assay for viable mitochondria. Values are percent increase in MTT with A6 vs. vehicle; each point is a culture from a different heart, with 2–3 35mm dishes for each group in each culture, with MTT read in triplicate; p by unpaired t test. B: Adult male mice had TAC to create a gradient ~110 mmHg; survival over 2 weeks was 98% in WT and 64% in GqKI; p value by Gehan-Beslow-Wilcoxon test. C. At 2 weeks after TAC, when fractional shortening (FS) by echo had dropped from baseline 62±1% to an average 40±1% (n=17), WT mice and surviving GqKI mice were treated with A6 10 ng/kg/d by osmotic minipump. In WT mice, A6 for 2 more weeks increased FS by 24%, to 50±2%, or 82% of baseline. In GqKI mice treated with A61603, FS fell further (−11%), the same as WT mice treated with vehicle (−10%). Values are percent change in FS for the same mouse with treatment between 2 weeks and 4 weeks; p by ordinary one-way ANOVA with Tukey’s multiple comparisons test. Complete echo data are in Online Table I and Online Figure IV
In vivo, in a model of severe pressure overload (gradient ~110 mmHg) by transverse aortic constriction (TAC),35 only 1 of 65 WT mice died over 2 weeks, whereas survival was significantly reduced in GqKI animals (Figure 4B). A61603 treatment by osmotic minipump at 10 ng/kg/d, a dose with no effect on blood pressure,9 was started at 2 weeks after TAC, when fractional shortening (FS) by echo had dropped by one-third. This model might simulate disease treatment in patients. In WT mice, A61603 for 2 additional weeks improved FS quantified over time in individual mice by 24%, or to 82% of baseline pre-TAC (Figure 4C). In GqKI mice treated 2 weeks with A61603, FS was not improved, but decreased a further −11±8%, the same as WT mice treated with vehicle (−10±8%) (Figures 4C and Online Figure IV, Online Table I).
The degree of overall hypertrophy was the same in all 3 TAC groups (WT treated with vehicle, WT treated with A61603, and GqKI treated with A61603), as was the degree of atrial enlargement (Online Table II). Body weight and liver and lung weights were the same as Sham in all 3 TAC groups (Online Table II), suggesting no overt volume overload.
These data show that Gq coupling is required for cardioprotection by the α1A in vitro and in vivo.
DISCUSSION
Our major new finding is that Gq signaling is required for cardioprotection by the α1A-AR. A 6-residue substitution in the 3rd intracellular loop eliminated Gq signaling in knockin AMVMs, as measured by two PI-PLC assays and by contraction. Other mutations in the same 3rd loop of the α1A and the α1B also disrupt Gq signaling but not Gq binding in recombinant cells.13–15 Absence of Gq signaling caused a marked reduction in maximum α1A-AR agonist activation of myocyte ERK, and eliminated α1A-AR agonist ability to protect myocytes from DOX in vitro and to rescue cardiac function after TAC in vivo. The Gq-mutant receptor was expressed and bound ligands normally and was able to mediate some ERK activation, but did not confer protection.
A potential confounder would be if the α1A couples to β-arrestin, and if the 6-base knockin that eliminated Gq coupling also disrupted β-arrestin association. However, studies in recombinant cells with very high α1A levels show that the α1A does not recruit β-arrestin,26–30 plus we found no evidence for α1A activation of β−arrestin1 (Online Figure I). Also, we found no evidence for α1A activation of ERK via Gi (Online Figure II).
The groundbreaking idea that Gq signaling is maladaptive in heart came from overexpression in cardiac transgenic mice.1–3 In contrast with this idea that Gq signaling is maladaptive, our current data show clearly that Gq signaling can be adaptive in myocytes and heart, at least for the α1A receptor. Myocytes have many Gq-coupled receptors (Introduction), and these might signal to Gq differently, or have more Gq-independent actions. Gq-coupled receptor function can differ as a function of subcellular localization.20 Therefore, targeting Gq directly might not be the best way to uncover myocyte biology of the diverse Gq-coupled receptors.
Consistent with our prior finding that ERK activation is required for myocyte protection by the α1A,10, 24 ERK activation was reduced by 60–100% with the mutant α1A that did not protect. ERK activation by the mutant α1A receptor could be detected when overall ERK activation was sufficiently robust, but activation was insufficient in quantity or quality to protect myocytes from death. Less myocyte death seems to explain the most significant α1A adaptive effect, i.e. protection of cardiac function in vivo,9, 10, 12 as seen in this study. In turn, reduced oxidative stress, rescued mitochondria, and more ATP could explain less myocyte death.10, 12 Cardiac protection by α1A agonist in WT mice was not due to a change in the overall extent of hypertrophy (Online Table II).
In summary, we show that Gq signaling is required for α1A cardioprotection, and infer that Gq signaling can be adaptive, not just maladaptive as thought now.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
GPCR signaling through Gq is considered toxic in heart failure.
The α1A adrenergic receptor is coupled to Gq, but is cardioprotective.
Resolving these contradictions is important.
What New Information Does This Article Contribute?
A new mouse model has a mutant α1A receptor that does not couple to Gq.
In mutant mouse cardiac myocytes in vitro, an α1A agonist does not activate cardioprotective signaling or protect myocytes from death with a toxic stimulus.
In vivo in wild type mice, an α1A agonist rescues depressed cardiac function after pressure overload, without changing the overall degree of hypertrophy.
In vivo in mice with the mutant α1A that does not couple to Gq, all adaptive effects of α1A agonist are lost.
These data show for the first time that Gq signaling can be adaptive, not maladaptive as generally believed. This conclusion comes from a new mouse model with an α1A adrenergic receptor 6-base knockin substitution mutation in the proximal 3rd intracellular loop. This mutation disrupts Gq signaling. Loss of Gq signaling eliminates α1A-mediated survival signaling in vitro and rescue of cardiac function in heart failure in vivo. Rescue in vivo does not alter the overall degree of myocardial hypertrophy. Current models of hypertrophy and heart failure that place Gq signaling from GPCRs at the center of maladaptive remodeling need to be revised. Coupling to Gq should not bias against a potential new drug to treat heart failure.
ACKNOWLEDGEMENTS
We thank Horizon Discovery (St. Louis, MO) for constructing the Gq mutant mouse.
SOURCES OF FUNDING
Support was from Janssen Research & Development, the NIH (PCS), the Department of Veterans Affairs (PCS, AJB), and the Western States Affiliate of the American Heart Association (AJB, B-EM). The Northern California Institute for Research and Education administered grants, and the Veterans Affairs Medical Center, San Francisco, California, provided resources.
Nonstandard Abbreviations and Acronyms
- A6
A61603 (N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl]methanesulfonamide hydrobromide), an α1A-AR selective agonist
- α1-ARs
alpha-1-adrenergic receptors
- Ab
antibody
- AMVM
adult mouse ventricular myocyte
- AR
adrenergic receptor
- Arr
arrestin
- DOX
doxorubicin
- ERK
extracellular signal regulated kinase
- GqKI
knock-in mouse with α1A-adrenergic receptor with impaired G alpha q coupling
- HTRF
Homogeneous Time Resolved Fluorescence
- IP
inositol phosphate
- pERK
phosphorylated-ERK
- PI-PLC
phosphoinositide-phospholipase C
- PMA
phorbol myristate acetate
- PTX
pertussis toxin
- RT-qPCR
reverse transcription quantitative real time polymerase chain reaction
- TAC
transverse aortic constriction
- WT
wild type
Footnotes
DISCLOSURES
UCSF owns a patent with PCS as inventor for use of A61603 in heart failure, and PCS is involved in a company to pursue this.
REFERENCES
- 1.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB and Dorn GW 2nd. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH and Dorn GW 2nd. Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. 1998;95:10140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakata Y, Hoit BD, Liggett SB, Walsh RA and Dorn GW 2nd. Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation. 1998;97:1488–95. [DOI] [PubMed] [Google Scholar]
- 4.Dorn GW 2nd. The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49:962–70. [DOI] [PubMed] [Google Scholar]
- 5.Hill JA and Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. [DOI] [PubMed] [Google Scholar]
- 6.Bernardo BC, Weeks KL, Pretorius L and McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M and Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Gareri C and Rockman HA. G-Protein-Coupled Receptors in Heart Disease. Circ Res. 2018;123:716–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery MD, Chan T, Swigart PM, Myagmar BE, Dash R and Simpson PC. An alpha-1A adrenergic receptor agonist prevents acute doxorubicin cardiomyopathy in male mice. PLoS One. 2017;12:e0168409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beak J, Huang W, Parker JS, Hicks ST, Patterson C, Simpson PC, Ma A, Jin J and Jensen BC. An oral selective alpha-1A adrenergic receptor agonist prevents doxorubicin cardiotoxicity. JACC Basic Transl Sci. 2017;2:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowley PM, Wang G, Joshi S, Swigart PM, Lovett DH, Simpson PC and Baker AJ. alpha1A-Subtype adrenergic agonist therapy for the failing right ventricle. Am J Physiol Heart Circ Physiol. 2017;313:H1109–H1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowley PM, Wang G, Swigart PM, Raghunathan A, Reddy N, Dulam P, Lovett DH, Simpson PC and Baker AJ. Reversal of right ventricular failure by chronic alpha1A-subtype adrenergic agonist therapy. Am J Physiol Heart Circ Physiol. 2019;316:H224–H232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copik AJ, Ma C, Kosaka A, Sahdeo S, Trane A, Ho H, Dietrich PS, Yu H, Ford AP, Button D and Milla ME. Facilitatory interplay in alpha 1a and beta 2 adrenoceptor function reveals a non-Gq signaling mode: implications for diversification of intracellular signal transduction. Mol Pharmacol. 2009;75:713–28. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Jiang H and Simon MI. Different alpha 1-adrenergic receptor sequences required for activating different G alpha subunits of Gq class of G proteins. J Biol Chem. 1995;270:9828–32. [DOI] [PubMed] [Google Scholar]
- 15.Lee D, Robeva A, Chen Z and Minneman KP. Mutational uncoupling of alpha1A-adrenergic receptors from G proteins also uncouples mitogenic and transcriptional responses in PC12 cells. J Pharmacol Exp Ther. 2003;306:471–7. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E and Simpson PC. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003;111:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W and Simpson PC. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rokosh DG and Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci U S A. 2002;99:9474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahl EF, Wu SC, Healy CL, Perry J and O’Connell TD. ERK mediated survival signaling is dependent on the Gq-G-protein coupled receptor type and subcellular localization in adult cardiac myocytes. J Mol Cell Cardiol. 2019;127:67–73. [DOI] [PubMed] [Google Scholar]
- 20.Dahl EF, Wu SC, Healy CL, Harsch BA, Shearer GC and O’Connell TD. Subcellular compartmentalization of proximal Galphaq-receptor signaling produces unique hypertrophic phenotypes in adult cardiac myocytes. J Biol Chem. 2018;293:8734–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu SC, Dahl EF, Wright CD, Cypher AL, Healy CL and O’Connell TD. Nuclear localization of a1A-adrenergic receptors is required for signaling in cardiac myocytes: an “inside-out” a1-AR signaling pathway. J Am Heart Assoc. 2014;3:e000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright CD, Wu SC, Dahl EF, Sazama AJ and O’Connell TD. Nuclear localization drives alpha1-adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal. 2012;24:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright CD, Chen Q, Baye NL, Huang Y, Healy CL, Kasinathan S and O’Connell TD. Nuclear alpha1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ Res. 2008;103:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC and O’Connell TD. An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–72. [DOI] [PubMed] [Google Scholar]
- 25.Cowley PM, Wang G, Chang AN, Makwana O, Swigart PM, Lovett DH, Stull JT, Simpson PC and Baker AJ. The alpha1A-adrenergic receptor subtype mediates increased contraction of failing right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2015;309:H888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copik AJ, Baldys A, Nguyen K, Sahdeo S, Ho H, Kosaka A, Dietrich PJ, Fitch B, Raymond JR, Ford AP, Button D and Milla ME. Isoproterenol acts as a biased agonist of the alpha-1A-adrenoceptor that selectively activates the MAPK/ERK pathway. PLoS One. 2015;10:e0115701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oganesian A, Yarov-Yarovoy V, Parks WC and Schwinn DA. Constitutive coupling of a naturally occurring human alpha1a-adrenergic receptor genetic variant to EGFR transactivation pathway. Proc Natl Acad Sci U S A. 2011;108:19796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustafa S, See HB, Seeber RM, Armstrong SP, White CW, Ventura S, Ayoub MA and Pfleger KD. Identification and profiling of novel alpha1A-adrenoceptor-CXC chemokine receptor 2 heteromer. J Biol Chem. 2012;287:12952–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akinaga J, Lima V, Kiguti LR, Hebeler-Barbosa F, Alcantara-Hernandez R, Garcia-Sainz JA and Pupo AS. Differential phosphorylation, desensitization, and internalization of alpha1A-adrenoceptors activated by norepinephrine and oxymetazoline. Mol Pharmacol. 2013;83:870–81. [DOI] [PubMed] [Google Scholar]
- 30.Stanasila L, Abuin L, Dey J and Cotecchia S. Different internalization properties of the alpha1a- and alpha1b-adrenergic receptor subtypes: the potential role of receptor interaction with beta-arrestins and AP50. Mol Pharmacol. 2008;74:562–73. [DOI] [PubMed] [Google Scholar]
- 31.Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA and Lefkowitz RJ. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272:31051–7. [DOI] [PubMed] [Google Scholar]
- 32.Meng D, Lynch MJ, Huston E, Beyermann M, Eichhorst J, Adams DR, Klussmann E, Houslay MD and Baillie GS. MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J Biol Chem. 2009;284:11425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karliner JS, Kagiya T and Simpson PC. Effects of pertussis toxin on alpha 1-agonist-mediated phosphatidylinositide turnover and myocardial cell hypertrophy in neonatal rat ventricular myocytes. Experientia. 1990;46:81–4. [DOI] [PubMed] [Google Scholar]
- 34.Myagmar BE, Flynn JM, Cowley PM, Swigart PM, Montgomery MD, Thai K, Nair D, Gupta R, Deng DX, Hosoda C, Melov S, Baker AJ and Simpson PC. Adrenergic receptors in individual ventricular myocytes: the beta-1 and alpha-1B are in all cells, the alpha-1A Is in a subpopulation, and the beta-2 and beta-3 are mostly absent. Circ Res. 2017;120:1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr. and Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.