ABSTRACT
Traditional pairwise meta-analysis (PMA) is a very useful method that pools evidence from one study design type if appropriate; its widespread use in nutrition research is an important phenomenon. Recently, a promising method for more advanced evidence-synthesis, called network meta-analysis (NMA), was introduced. NMA is an extension of PMA that enables simultaneous comparison of multiple interventions. NMA combines direct evidence (i.e., trials comparing 2 interventions directly) and indirect evidence (i.e., from a connected route via ≥1 comparators, e.g. placebo) in a network of studies. NMAs have the potential to advance knowledge in the field of nutrition as they provide insights that cannot be obtained by individual 2-arm randomized controlled trials or PMA. Thus, in this perspective paper, we aim to summarize the current (methodologic) status of published NMAs in nutrition research and emphasize advances and strengths in comparison with traditional PMA through specific examples, and highlight potential pitfalls and limitations. NMA is an emerging methodology in the field of nutrition research. A PubMed search identified only 23 nutrition research-related NMAs published since the inception of journals up to January 8, 2019 (61% of them published since 2017), compared with >5000 published PMAs. Moreover, we aim to highlight the scientific concepts and standards through the use of the following NMA example: “Which type of oils/solid fats offers the greatest impact on blood lipids?” In this regard, we discuss intervention definitions, transitivity/similarity, statistical methods, description and visualization of results, inconsistency, ranking, dissemination bias, assessing the certainty of evidence by Grading of Recommendations Assessment, Development and Evaluation, and reporting guidelines. We expect that rigorously conducted NMAs based on high-quality systematic reviews will become the new evidence synthesis benchmark in nutrition research. However, caution is warranted because abuse and misinterpretations of PMA and NMA findings could hamper the scientific field and possibly decision-making regarding public policy.
Keywords: network meta-analysis, nutrition, evidence synthesis, diet, ranking
Introduction
During the last few decades, systematic reviews (SRs) have increased remarkably in number and continue to replace the narrative reviews previously used to summarize findings from multiple studies. Narrative reviews are often viewed critically for their lack of transparency in selection of evidence and therefore their inherent subjectivity (1); however, their key contribution is to deepen understanding of, for example, mechanisms (2). With the tremendous increase in scientific publications (3), the methodologic approach of narrative reviews is becoming less useful for the description of the consequences of an intervention or exposure, and systematic approaches to summarizing the scientific literature should become the preferred option (4). SRs aim to provide a comprehensive and objective summary of all relevant research evidence addressing specific questions according to prespecified eligibility criteria (5). Many SRs contain meta-analyses (MAs), a statistical method used to quantitatively summarize data from independent studies. In all fields of health sciences, including nutritional sciences (6), SRs and MAs have become an important tool for estimating the effects and associations based on intervention and observational studies. SRs are used to inform clinical practice guidelines and to contribute to health technology assessment reports, thereby supporting the transfer of research into evidence-based health care practice (5).
Pairwise meta-analysis (PMA) is a very useful method that pools effect estimates of randomized controlled trials (RCTs), observational studies such as prospective cohort studies, or other study design types (5) that compare 2 interventions/exposures directly (so-called direct evidence), i.e. intervention/exposure compared with control, or intervention/exposure compared with intervention/exposure.
The widespread implementation of PMAs is an important phenomenon, but the reporting and methodologic quality of SRs have to date often been inconsistent and flawed (7–10). The misuse of MAs in nutrition research has recently been criticized. By combining heterogeneous studies, including highly diverse participant demographics and study methods, the variability in findings that can reduce statistical power may increase, making “true” effects more difficult to identify (11). Although high-quality SRs and MAs may help to merge and ultimately enhance evidence-based dietary guidelines (12–17), some concerns have been expressed regarding their validity and application in nutrition research (18, 19). For example, RCTs in nutrition research are often prone to inherent methodologic constraints: sometimes they cannot be controlled with “true” placebos but rather by a limitation of certain aspects of nutrient compositions, food groups, or dietary patterns; other limitations include the lack of double blinding, poor compliance and adherence, crossover bias, and high drop-out rates (20). When conducting high-quality SRs and MAs, these study limitations need to be considered, assessed, and their impact on the findings of the SR/MA evaluated.
A promising, more recently introduced evidence-synthesis method is network meta-analysis (NMA) (also called multiple-treatment MA or mixed-treatment comparisons), which is an extension of PMA that enables a simultaneous comparison of multiple interventions. NMA combines direct (i.e., from trials directly comparing 2 interventions) and indirect (i.e., from a connected route via ≥1 intermediate comparators) evidence in a network of trials or studies. In this way, it enables inferences about every possible comparison between pairs of interventions in the network, even when some comparisons have never been evaluated directly in a trial or study. NMA offers the opportunity to synthesize large amounts of data relating to clinical outcomes and to rank interventions in terms of their relative efficacy, and might improve the precision of the effect estimates (21).
NMA has been applied widely in many medical fields, especially in psychiatry, focusing on the comparison of different pharmacologic treatments (22). In other medical fields, for example endocrinology, NMA methodology has also been applied successfully, e.g. to investigate the safety and effectiveness of long-acting compared with intermediate-acting insulin to treat patients with type 1 diabetes (23), and the resulting evidence has been implemented in the most recent “standards of medical care in diabetes” published yearly by the American Diabetes Association evaluating pharmacologic approaches to glycemic treatment (24). Findings from other NMAs (25, 26) investigating the health impact of different training modalities in patients with type 2 diabetes (T2D) have been included in the 2019 position paper of the European Association of Preventive Cardiology (27). In cases where multiple treatment options are available, guideline developers will increasingly consider NMA for establishing timely recommendations (28).
Therefore, high-quality NMAs have the potential to also give an insight into long-standing questions in nutrition research that have not yet been answered by individual trials or by PMA (29). Thus, in this perspective article, we aim to summarize the current (methodologic) status of published NMAs in nutrition research. In addition, we emphasize advances and strengths in comparison with traditional PMA through specific examples, highlighting also the pitfalls and limitations of NMA. Moreover, we will describe the scientific concepts of NMA and emphasize recent and future developments and the implications of this advanced evidence-synthesis method for nutrition research.
Current Status
Published NMAs in nutrition research
NMA is an emerging methodology in the field of nutrition research. A systematic search of PubMed was conducted, from the inception of journals up to January 8, 2019, with the following search-terms: (network meta-analysis[tiab] OR multiple treatments meta-analysis[tiab] OR mixed-treatment comparison[tiab] OR multiple treatments comparison[tiab]) AND (diet*[tiab] OR nutrition[tiab] OR food*[tiab] OR nutrient*[tiab]). This search yielded 92 references, whereas for traditional MA >5400 references were identified. Out of these 92 hits, only 23 NMAs (25%) dealt with a nutrition-related topic (30–52). An overview of the identified nutrition-related NMAs is given in Table 1. The identified NMAs were published between 2011 and 2018, 14 (61%) of them since 2017, and often in general medical journals. The number of included studies varied between 8 and 80 RCTs, and the number of participants between 840 and >17,000. The number of interventions (including placebo intervention and control/standard intervention) within an NMA varied between 3 and 20, and the number of outcomes ranged between 1 and 11. Five NMAs (22%) compared the effects of various dietary approaches (e.g. Mediterranean, low fat). Only 10 (43%) of the NMAs were based an a priori study protocol and 13 (57%) used frequentist methodology. Interestingly, it seems that the frequentist approach is used increasingly in more recently published NMAs, probably due to the availability of new software packages for NMA (e.g. network Stata, “netmeta” for R). The NMA methodologic approaches applied are summarized in Table 2 (53, 54).
TABLE 1.
Nutrition-related NMAs identified in PubMed1
| First author (reference) | Year | Journal | Studies included, n | Number and disease status of included participants | Number and type of included interventions/placebo/control (usual care) | Number and type of included outcomes | A priori protocol | NMA statistical approach |
|---|---|---|---|---|---|---|---|---|
| Schwingshackl (30) | 2018 | Am J Clin Nutr | 66 | 3595 participants | 10 food groups | 10 LDL-C, TG, TC, HDL-C, FG, HbA1c, HOMA-IR, SBP, DBP, CRP | Y | Frequentist |
| Pan (31) | 2018 | J Evid Based Med | 10 | 921 patients with T2D | 5 dietary approaches | 9 HbA1c, FG, TC, HDL-C, LDL-C, TG, BW, BMI, WC | Y | Frequentist |
| Schwingshackl (32) | 2018 | J Lipid Res | 54 | 2065 participants | 13 oils | 4 TC, HDL-C, LDL-C, TG | Y | Frequentist |
| Liang (33) | 2018 | Medicine | 17 | 1931 patients with AD or MCI | 5 exercise, music therapy, nutrition therapy, computerized cognitive training, control | 2 MMSE, NPI | N | Bayesian |
| Schwingshackl (34) | 2018 | Crit Rev Food Sci Nutr | 67 | 17,230 patients with pre- and hypertension | 13 dietary approaches, control | 2 SBP, DBP | Y | Frequentist |
| Zou (35) | 2018 | Eur J Gastroenterol Hepatol | 19 | 846 patients with NAFLD | 6 diet, exercise, control | 4 ALT, AST, BMI, HOMA-IR | U | Frequentist |
| Schwingshackl (36) | 2018 | Eur J Epidemiol | 56 | 4937 patients with T2D | 9 dietary approaches, control | 2 HbA1c, FG | Y | Frequentist |
| Gutiérrez-Castrellón (37) | 2017 | Medicine | 32 | 2242 children with Infantile colic | 9 drug, diet, acupuncture, herbal, massage, reassurance/education, manipulative, L. retueri DSM17938, control | 1 infantile colic | U | Frequentist |
| Muñoz Fernández (38) | 2017 | J Am Med Dir Assoc | 21 | 2329 patients with AD | 7 single antioxidants, complex antioxidants, polymeric formula, polypeptide, ω-3 fatty acid, B-vitamins, placebo | 1 cognitive outcome | N | Bayesian |
| Ha (39) | 2017 | PLoS One | 21 | 1865 pregnant women | 7 dietary approaches | 4 HbA1c, FG, HOMA-IR, FI | Y | Bayesian |
| Yu (40) | 2017 | Medicine | 27 | 4649 preterm infants | 6 food additives, placebo | 5 ACM, NEC-incidence, NEC- related mortality, sepsis, hospitalization days | N | Bayesian |
| Song (41) | 2017 | Oncotarget | 11 | 840 patients with gastric cancer | 5 immunonutrition | 3 IC, NIC, LOHS | N | Frequentist |
| Sekercioglu (52) | 2017 | PLoS One | 29 | 8335 patients with chronic kidney disease | 8 diet, phosphate binder, placebo | 3 serum phosphate, calcium, PTH | Y | Frequentist |
| Iftikhar (42) | 2017 | Sleep Med | 80 | 7882 patients with obstructive sleep apnea | 5 continuous positive airway pressure, mandibular advancement devices, diet, exercise, control | 5 AHI, ESS, ODI, sleep efficiency, O2 nadir | Y | Frequentist |
| Sekercioglu (43) | 2016 | PLoS One | 28 | 8335 patients with chronic kidney disease | 7 diet, phosphate binder, placebo | 3 ACM, CVM, hospitalization | Y | Frequentist |
| Lehert (44) | 2015 | Climacteric | 24 | NA | 11 dietary approaches, dietary supplements, social engagement, exercise | 3 memory, general intelligence, screening cognition | N | Frequentist |
| Song (45) | 2015 | Medicine | 27 | NA patients with gastric cancer | 4 immunonutrition, enteral nutrition | 3 PIC, PNIC, PH | N | Frequentist |
| Stevens (46) | 2015 | Diabetes Res Clin Pract | 30 | NA patients with high risk of T2D | 20 drugs, diet, exercise, usual care | 1 progression of T2D | N | Bayesian |
| Mazaki (47) | 2015 | Ann Surg | 74 | 7572 patients with gastrointestinal surgery | 4 immunonutrition, enteral nutrition | 9 AI, OC, ACM, WI, Pneumonia, IAA, AL, sepsis, UTI | N | Bayesian |
| Schwingshackl (48) | 2014 | Syst Rev | 22 | 3521 patients with overweight or obesity | 3 diet, exercise | 11 BW, WC, FM, WHR, TC, LDL-C, HDL-C, TG, DBP, SBP, VO2 max | Y | Bayesian |
| Carter (49) | 2014 | J Hum Nutr Diet | 8 | NA patients with diabetes and non-diabetes | 7 dietary approaches, usual care | 3 HbA1c, FG, HOMA-IR | U | Bayesian |
| Dunkley (50) | 2012 | Diabetes Obes Metab | 13 | 3907 patients with metabolic syndrome | 12 exercise, diet, drug, usual care | 2 T2D incidence, CVD, MS reversal | N | Bayesian |
| Wiebe (51) | 2011 | BMC Med | 53 | 1126 patients with diabetes and non-diabetes | 6 sweeteners | 9 BW, EI, HbA1c, HOMA-IR, TC, HDL-C, TG | N | Bayesian |
1ACM, all-cause mortality; AD, Alzheimer's disease; AHI, apnea hypopnea index; AI, any infection; AL, anastomotic leak; ALT, alanine transaminase; AST, aspartate transaminase; BW, body weight; CVD, cardiovascular disease; CVM, cardiovascular mortality; DBP, diastolic blood pressure; EI, energy intake; ESS, Epworth Sleepiness Scores; FI, fasting insulin; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; FG, fasting glucose; FM, fat mass, IAA, intra-abdominal abscess; IC, infectious complications; LDL-C, low-density lipoprotein cholesterol; LOHS, length of hospital stay; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MS, metabolic syndrome; N, no; NA, not applicable; NAFLD, nonalcoholic fatty liver disease patients; NEC, necrotizing enterocolitis; NIC, noninfectious complications; NMA, network meta-analysis; NPI, neuropsychiatric inventory; OC, overall complications; ODI, oxygen desaturation index; PH, postoperative hospitalization; PIC, postoperative infectious complications; PNIC, postoperative noninfectious complications; PTH, parathyroid hormone; SBP, systolic blood pressure; T2D, type 2 diabetes; TC, total cholesterol; TG, triacylglycerols; U, unclear; UTI, urinary tract infection; VO2max, maximum oxygen uptake; WC, waist circumference; WHR, waist-to-hip ratio; WI, wound infection; Y, yes.
TABLE 2.
Methodological features applied across the included NMAs1
| NMA methodology | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ranking | |||||||||||
| First author (reference) | Risk of bias2 | Network plot | Similarity/ transitivity | Contribution matrix | SUCRA or P score | Other ranking | Inconsistency testing4 | Subgroup or sensitivity analyses | Funnel plot5 | Certainty of the evidence | PRISMA NMA checklist cited |
| Schwingshackl (30) | Y | Y | Y | Y | Y | N | Y | Y | Y | Y (CINeMA) | Y |
| Pan (31) | Y | Y | N | N | Y | N | Y | Y | N | N | Y |
| Schwingshackl (32) | Y | Y | Y | Y | Y | N | Y | Y | Y | Y (CINeMA) | Y |
| Liang (33) | Y | Y | N | N | Y | N | N | N | N | N | N |
| Schwingshackl (34) | Y | Y | Y | Y | Y | N | Y | Y | Y | Y (CINeMA) | Y |
| Zou (35) | Y | Y | N | N | Y | N | N | N | N | N | Y |
| Schwingshackl (36) | Y | Y | Y | Y | Y | N | Y | Y | Y | Y (CINeMA) | Y |
| Gutiérrez-Castrellón (37) | Y | Y | N | Y | Y | N | N | N | Y | N | Y |
| Muñoz Fernández (38) | Y | Y | N | N | N | Y | Y | N | N | N | N |
| Ha (39) | Y | Y | Y | N | Y | N | Y | N | N | Y (GRADE) | Y |
| Yu (40) | Y | Y | N | N | Y | N | N | Y | Y | Y (GRADE) | N |
| Song (41) | N3 | Y | N | N | Y | N | N | N | Y | N | N |
| Sekercioglu (52) | Y | Y | Y | N | Y | N | Y | Y | Y | Y (GRADE) | Y |
| Iftikhar (42) | Y | Y | N | N | Y | N | Y | N | Y | N | Y |
| Sekercioglu (43) | Y | Y | Y | Y | N | N | Y | Y | Y | Y (GRADE) | N |
| Lehert (44) | N | N | N | N | N | N | Y | N | N | N | NA |
| Song (45) | Y | Y | N | N | Y | N | Y | N | Y | N | NA |
| Stevens (46) | N3 | Y | N | N | N | Y | Y | Y | N | N | NA |
| Mazaki (47) | Y | Y | N | N | Y | N | Y | Y | Y | N | NA |
| Schwingshackl (48) | Y | N | Y | N | N | Y | Y | Y | N | N | NA |
| Carter (49) | N3 | N | N | N | N | N | Y | Y | N | N | NA |
| Dunkley (50) | Y | Y | N | N | N | Y | N | N | NA | N | NA |
| Wiebe (51) | N3 | Y | N | N | N | Y | Y | Y | NA | N | NA |
| % Y | 78% | 87% | 35% | 26% | 87% | 74% | 57% | 57% | 35% | 67% | |
1NA, not applicable (methodology not yet published); CINeMA, Confidence In Network Meta-Analysis; GRADE, Grading of Recommendations Assessment, Development and Evaluation for NMA; N, no; NMA, network meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (54); SUCRA, surface under the cumulative ranking curves; Y, yes.
2Risk of bias assessed by the tool of the Cochrane collaboration (55).
3Study quality assessed with Jadad scale (53).
4Inconsistency: loop-specific approach (56), side-splitting approach (57), design-by-treatment approach (58).
5Application of the comparison-adjusted funnel plot (59).
Examples of PMAs compared with NMAs
Dietary approaches in the management of T2D
The most recent nutrition recommendation position paper of the American Diabetes Association concluded that evidence suggests that there is not an ideal percentage of calories from carbohydrate, protein, and fat for all people with diabetes (60). This position paper included PMAs (61, 62) that dealt with the question of which dietary approach (low fat, Mediterranean, vegetarian, high protein, moderate carbohydrate, low carbohydrate, low glycemic index/load) offers the greatest benefits in the management of T2D (63). However, because no RCTs have ever been conducted comparing, for example, the Mediterranean diet with a vegetarian diet, or a high-protein diet with a low-carbohydrate diet, no conclusions could be drawn from the use of the PMA methodology. This important question has recently been addressed through the use of NMA methodology: for reducing HbA1c, the low-carbohydrate diet was ranked as the most effective dietary approach, and for reducing fasting glucose, the Mediterranean diet was ranked best. The NMA also revealed that all included dietary approaches significantly reduced HbA1c (−0.82% to −0.47% reduction) and fasting glucose (−1.61 to −1.00 mmol/L reduction) compared with a control diet (no intervention) (36). However, for most of the comparisons the certainty of evidence was rated low, limiting our confidence in the effect estimates.
Dietary approaches in the management of elevated blood pressure
A recent PMA compared several dietary approaches (e.g., Dietary Approaches to Stop Hypertension [DASH] diet, Mediterranean, low sodium) and a control diet (usual diet). The authors concluded that some dietary patterns might be more effective than others, without ranking the dietary approaches based on blood pressure–lowering impact (64). However, one of the most important questions that remains to be answered is which dietary approach offers the greatest effect in the management of elevated blood pressure. In a recent NMA of 67 RCTs (>17,000 patients with prehypertension and hypertension), the effects of 13 different dietary approaches (e.g. low carbohydrate, DASH, low fat) were compared. For systolic blood pressure and diastolic blood pressure the DASH diet was ranked the most effective dietary approach. Compared with a control diet, the DASH, Mediterranean, low-carbohydrate, paleolithic, high-protein, low-glycemic index, low-sodium, and low-fat dietary approaches were more effective in reducing systolic blood pressure (−8.73 to −2.32 mm Hg) and diastolic blood pressure (−4.85 to −1.27 mm Hg) (34). However, the findings were limited by very low to moderate certainty of evidence, with the exception of the DASH compared with the low-fat dietary approach, for which the certainty of evidence was rated high (34).
Impact of oils and solid fats on blood lipids
A traditional PMA showed that ω-3 (n–3) and ω-6 fatty acid–rich plant oils showed more pronounced LDL cholesterol– and total cholesterol (TC)–reducing effects compared with olive oil (65), whereas palm oil showed negative effects on LDL cholesterol compared with vegetable oils low in saturated fats (66). An important question that still remained to be answered was: which type of oil/solid fat offers the greatest effect on blood lipids combining direct and indirect evidence? This question has recently been addressed by investigating the effects of 13 oils and solid fats (safflower, sunflower, canola, hempseed, flaxseed, corn, olive, soybean, palm, and coconut oil, and beef fat, lard, and butter) on blood lipids (32) (Table 3). Safflower oil was ranked as the most effective oil for reducing LDL cholesterol and TC, followed by canola oil and sunflower oil; for reducing triacylglycerols soybean oil was ranked highest, followed by corn oil and palm oil; lard and butter were ranked lowest for improving LDL cholesterol and TC; for increasing HDL cholesterol, coconut oil was ranked highest, followed by palm oil and beef fat (32). Compared with butter, all vegetable oils were more effective in reducing LDL cholesterol (−0.42 to −0.23 mmol/L). The certainty of evidence for LDL cholesterol was rated mostly low or moderate where both direct and indirect evidence was available; whereas for comparisons based only on indirect evidence, the certainty of evidence was mostly rated low or very low.
TABLE 3.
Example of application of the PICOS criteria regarding the research question: which oils/solid fats offer the greatest improvements in blood lipids?1
| Criteria | Description |
|---|---|
| Participants | Participants aged ≥18 y |
| Interventions (comparator) | Eligible types of intervention/comparison of ≥2 of the following oils/solid fats: • safflower oil • sunflower oil • canola oil • hempseed oil • flaxseed oil • olive oil • corn oil • soybean oil • palm oil • coconut oil • lard • beef fat • butter |
| Outcomes | Primary outcome: LDL cholesterol; Secondary outcomes: TC, HDL cholesterol, triacylglycerols |
| Study design | Randomized parallel or crossover intervention trials with minimum intervention period of 21 d |
1PICOS, participants, interventions, comparisons, outcomes, and study design; TC, total cholesterol.
Future nutrition-relevant NMA topics
With the emerging methodology of NMA other pertinent questions in nutrition research could hopefully be answered in the future, such as the following:
Which type of dietary sugar (glucose, fructose, sucrose, or starch) is the most harmful for cardiometabolic risk factors?
Which dietary approach offers the greatest benefits in the management of obesity?
The findings of high-quality NMAs are important for deriving future dietary guidelines by answering pertinent questions that could otherwise not be answered with traditional MAs. For example, the USDA Dietary Guidelines for Americans 2015 answered the following question: what is the relation between dietary patterns and measures of body weight or obesity? (67) Findings from a future NMA investigating the effects of dietary patterns on measures of body weight or obesity would be of importance to provide additional indirect evidence answering this question.
Scientific Concepts
Essential steps before conducting an MA
Before conducting an MA, a high-quality SR needs to be undertaken, providing the most comprehensive qualitative synthesis of evidence. SRs are a form of observational research, and the methods for the SR should be agreed upon before the review commences. An essential part of good scientific practice for conducting an SR is the availability of a detailed protocol of each SR explicitly defining the participants (P), interventions (I) or exposures (E), comparisons (C), outcomes (O), and study design (S) (PICOS or PECOS criteria; see Table 3) and prespecifying analytic plans including subgroup and sensitivity analyses (68). High-quality SRs result in reduced bias and random error through transparent, explicit, reproducible, comprehensive, and rigorous processes that examine all of the available evidence for a specific research question (5). A comprehensively conducted systematic search for all available studies is a key element of each high-quality SR, followed by aspects of study selection and data extraction, which should be independently conducted by ≥2 people (5). Another key element is the risk-of-bias (RoB) assessment of each individual primary study by applying suggested assessment tools (55, 69).
Authors should comply with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist guidelines that ensure high-quality results when evidence-synthesis methods are used (70). Transparent reporting of the PRISMA checklist includes, for example, the presentation of the full electronic search strategy for ≥1 database, presentation of the study selection process and the data extraction process, as well as evaluation of the RoB.
A meta-analysis should probably NOT be conducted when...
According to the Cochrane Handbook, 3 major reasons exist where meta-analyzing primary studies could be more of a hindrance than a help (5).
Clinical or epidemiologic primary studies that are too diverse: the most important type of diversity is in relation to the comparisons/exposures within the primary studies. Sometimes, it might not make sense to combine all identified primary studies in a PMA. It might be more appropriate to consider them, for example, in separate subgroups. NMA can actually serve to explain heterogeneity by refining the definition of treatments or exposures: exposures that seem to be too heterogeneous (for example different doses) can be regarded as different nodes in the network. Decisions about whether or not an MA should be conducted are inevitably subjective and are not amenable to statistical solutions, but require profound discussion and clinical or epidemiologic knowledge, or a combination, and judgment. In some cases, consensus may be hard to reach.
High RoB in primary studies. If bias is present in the majority of the primary studies, an MA will simply compound the errors and generate an “invalid” estimate that may be mistakenly interpreted as having more certainty.
Evidence of serious publication or reporting bias. Hence, meta-analyses are at high risk of producing invalid summary estimates.
In the following paragraphs we will briefly describe the scientific concepts of NMA by using as an example the aforementioned NMA research question (i.e., impact of oils and solid fat on blood lipids [32]).
How to define the interventions
One of the first steps when planning an NMA is the consideration of which interventions should form the nodes of the network and how to define them. There is a broad spectrum between “splitting,” i.e., making very fine distinctions between interventions, and “lumping” interventions together that are roughly similar (71–73). Taking the example provided in Table 3, one possibility is to make a very fine distinction between oils/solid fats (comparison of all 13 oils/solid fats as shown in Figure 1), or to lump these oils/solid fats into 4 major categories (sources rich in saturated fat, monounsaturated fat, or ω-6 or ω-3 fatty acids).
FIGURE 1.
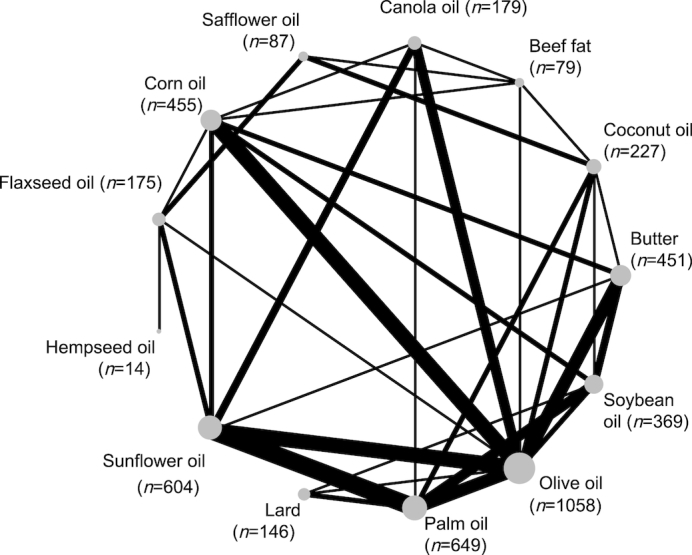
Network diagram taking into account 13 different oils and solid fats. The size of the nodes is proportional to the total number of participants allocated to the oils or solid fats, and the thickness of the lines is proportional to the number of studies evaluating each direct comparison. The line width is proportional to the number of studies that provide direct evidence for the respective comparison. The size of the blue points corresponds to the number of observations receiving the respective treatment and is also printed next to the treatment label. The treatment sequence on the circle was determined automatically such that a graph with a small number of line crossings is generated.
Transitivity, similarity
An important assumption of the validity of an NMA, often called the transitivity assumption, is that trials comparing different sets of interventions are similar in terms of important characteristics that may influence the outcome of interest (21, 74, 75). To evaluate transitivity/similarity, the distribution of potential effect modifiers across the available trials should be compared (for example, in Figure 1: if large differences in energy intake across trials exist, the transitivity assumption may be violated). However, only 8 out of the identified 23 NMAs (35%) evaluated transitivity/similarity (Table 2).
Statistical methods and software
Recent years have brought an expansion of statistical methods (76). Whereas in the first years Bayesian methodologic approaches dominated the field (77, 78), more recently standard regression methods based on a frequentist approach have also become widespread. This has been supported by the emergence of specialized software modules in popular statistical packages such as R (79) and Stata (80). Although Bayesian methods are in general more flexible, frequentist methods are often easier to apply for nonstatisticians (81, 82). They are also computationally less time consuming. Nevertheless, an expert should be consulted to make sure the complex assumptions are not violated and methods are appropriately used.
WinBUGS is the main resource for Bayesian network meta-analysis and is a comprehensive, albeit rather technical, implementation (83–85). The R package gemtc (86) also uses BUGS or alternatively JAGS for statistical analyses and provides a graphic front end for the user. Frequentist methods are available in Stata (80) and several R packages including netmeta (87) and metafor (88). An overview of R packages for network meta-analysis is given by Neupane et al. (89).
Description and visualization of results
The network structure is typically visualized by a network plot showing treatments as nodes and existing pairwise comparisons as edges between corresponding treatment nodes. Typically, a circular presentation of nodes is used. However, other presentation types are sometimes preferable (90). The network plot in Figure 1, which was generated with the R function netgraph of the R package netmeta, shows the common presentation with treatments on a circle. The graph shows a highly connected network where olive oil was the main comparator for the other interventions. It was recently shown that such well-connected network structures lead to a greater gain of precision when indirect evidence is added (91).
A forest plot can be used to summarize the results of the network meta-analysis if a common comparator exists, e.g., a placebo group. The forest plot in Figure 2 shows all treatment comparisons with lard as a reference. The comparisons are sorted by decreasing P score which is described below in the subsection on ranking of treatments. The forest plot shows that only safflower (mean difference: −0.95 mmol/L; 95% CI: −1.81, −0.08 mmol/L) and sunflower oil (mean difference: −0.81 mmol/L; 95% CI: −1.49, −0.13 mmol/L) are more effective in reducing TC compared with lard. All other oils/solid fats, with the exception of butter (which slightly increases TC), slightly reduce TC.
FIGURE 2.
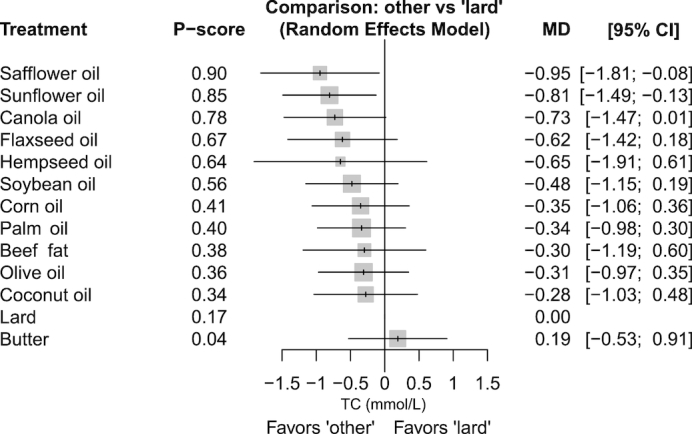
Summary effect estimates for the comparison of different oils and solid fats on TC (mmol/L). Lard is defined as the reference treatment. P scores are defined such that they are between 0 and 1, where 0 means that a treatment is always worst, and 1 means that a treatment is always best compared with the other treatments in the network. Safflower oil (P score: 0.90) was ranked best to improve TC, followed by sunflower oil (0.85) and canola oil (0.78). MD, mean difference. TC, total cholesterol.
Another common approach for presentation of NMA results is a league table, which shows all pairwise network comparisons in a square matrix. Typically, different information is provided in the lower and upper triangles. As shown in Table 4, all network estimates and corresponding 95% CIs are shown in the lower triangle and available estimates from direct treatment comparisons are shown in the upper triangle. For example, in the comparison of beef fat with canola oil (in the top left corner), estimates are rather similar when taking the width of the 95% CI into account: the network estimate is 0.43 mmol/L (95% CI: −0.27, 1.13 mmol/L) and the estimate from the direct pairwise comparison is 0.61 mmol/L (95% CI: −0.41, 1.62 mmol/L).
TABLE 4.
League table with network and direct treatment estimates (95% CI) for TC (in mmol/L)1
| Beef fat | 0.61 (−0.41, 1.62) | −0.34 (−1.26, 0.58) | 0.61 (−0.41, 1.62) | — | — | — | — | 0.32 (−0.69, 1.33) | — | 0.36 (−0.56, 1.28) | — | — |
| 0.43 (−0.27, 1.13) | Canola oil | — | 0.00 (−0.97, 0.97) | — | — | — | — | −0.34 (−0.85, 0.18) | −0.10 (−1.07, 0.87) | — | — | −0.20 (−0.82, 0.41) |
| −0.02 (−0.67, 0.63) | −0.45 (−1.01, 0.11) | Coconut oil | — | −0.20 (−1.23, 0.83) | — | — | — | 0.20 (−0.49, 0.90) | 0.05 (−0.65, 0.74) | 0.60 (−0.08, 1.28) | −0.29 (−1.34, 0.76) | — |
| 0.06 (−0.61, 0.72) | −0.38 (−0.84, 0.09) | 0.07 (−0.43, 0.58) | Corn oil | −0.90 (−1.58, −0.21) | 0.38 (−0.67, 1.43) | — | — | −0.16 (−0.58, 0.25) | — | — | 0.15 (−0.51, 0.82) | 1.34 (0.63, 2.05) |
| −0.49 (−1.19, 0.21) | −0.92 (−1.42, −0.41) | −0.47 (−0.98, 0.04) | −0.54 (−0.96, −0.13) | Butter | — | — | — | 0.35 (−0.08, 0.79) | — | — | 0.81 (0.23, 1.39) | 1.04 (0.04, 2.04) |
| 0.32 (−0.41, 1.05) | −0.11 (−0.70, 0.48) | 0.34 (−0.24, 0.92) | 0.26 (−0.26, 0.79) | 0.81 (0.23, 1.38) | Flaxseed oil | 0.03 (−0.95, 1.01) | — | −0.11 (−1.06, 0.84) | — | 0.55 (−0.21, 1.30) | — | −0.10 (−0.87, 0.66) |
| 0.35 (−0.87, 1.57) | −0.08 (−1.22, 1.06) | 0.37 (−0.77, 1.51) | 0.29 (−0.81, 1.40) | 0.84 (−0.29, 1.97) | 0.03 (−0.95, 1.01) | Hempseed oil | — | — | — | — | — | — |
| −0.30 (−1.19, 0.60) | −0.73 (−1.47, 0.01) | −0.28 (−1.03, 0.48) | −0.35 (−1.06, 0.36) | 0.19 (−0.53, 0.91) | −0.62 (−1.42, 0.18) | −0.65 (−1.91, 0.61) | Lard | 0.24 (−0.77, 1.25) | 0.31 (−0.40, 1.03) | — | 0.63 (−0.40, 1.66) | — |
| 0.01 (−0.63, 0.65) | −0.42 (−0.81, −0.03) | 0.03 (−0.41, 0.47) | −0.04 (−0.37, 0.28) | 0.50 (0.15, 0.85) | −0.31 (−0.80, 0.18) | −0.34 (−1.43, 0.75) | 0.31 (−0.35, 0.97) | Olive oil | −0.13 (−0.57, 0.32) | — | 0.25 (−0.45, 0.94) | 0.67 (0.25, 1.09) |
| 0.04 (−0.62, 0.71) | −0.39 (−0.82, 0.04) | 0.06 (−0.39, 0.51) | −0.01 (−0.40, 0.37) | 0.53 (0.12, 0.94) | −0.28 (−0.80, 0.25) | −0.31 (−1.41, 0.80) | 0.34 (−0.30, 0.98) | 0.03 (−0.26, 0.32) | Palm oil | — | 0.11 (−0.37, 0.58) | 0.42 (0.03, 0.81) |
| 0.65 (−0.06, 1.36) | 0.22 (−0.46, 0.90) | 0.67 (0.10, 1.24) | 0.59 (−0.04, 1.23) | 1.14 (0.48, 1.80) | 0.33 (−0.26, 0.91) | 0.30 (−0.84, 1.44) | 0.95 (0.08, 1.81) | 0.64 (0.04, 1.23) | 0.61 (−0.01, 1.22) | Safflower oil | — | — |
| 0.18 (−0.50, 0.87) | −0.25 (−0.73, 0.24) | 0.20 (−0.28, 0.69) | 0.13 (−0.26, 0.52) | 0.67 (0.27, 1.07) | −0.14 (−0.70, 0.42) | −0.17 (−1.29, 0.96) | 0.48 (−0.19, 1.15) | 0.17 (−0.17, 0.51) | 0.14 (−0.20, 0.48) | −0.47 (−1.11, 0.18) | Soybean oil | — |
| 0.51 (−0.15, 1.18) | 0.08 (−0.33, 0.49) | 0.53 (0.05, 1.01) | 0.46 (0.08, 0.83) | 1.00 (0.59, 1.41) | 0.19 (−0.31, 0.69) | 0.16 (−0.93, 1.25) | 0.81 (0.13, 1.49) | 0.50 (0.21, 0.78) | 0.47 (0.18, 0.76) | −0.14 (−0.75, 0.48) | 0.33 (−0.06, 0.71) | Sunflower oil |
1Network estimates are shown in the lower triangle comparing the treatment in the column with that in the row. The upper triangle shows available direct pairwise comparisons (treatment in the row compared with the column). The effect estimates presented in this table are based on our recently published NMA (32). Whereas the effect estimates in the original NMA (32) were reported as a 10% isocaloric exchange (effect estimates of each primary study have been standardized) between the different types of oils and solid fats, in this table no standardization has been conducted for total cholesterol. Instead, postintervention values with the corresponding SD (see Supplementary Table S2 of the corresponding NMA [32]) from each primary study were used to calculate effect estimates. NMA, network meta-analysis; TC, total cholesterol.
Inconsistency
To evaluate the presence of local statistical inconsistency (i.e., disagreement between the direct and indirect evidence), the loop-specific approach (detecting loops of evidence that might present important inconsistency) (56), as well as the side-splitting approach (57), should be applied. In the loop-specific approach, the inconsistency is tested for each loop. Loops are closed connections of direct evidence (56). For example, a closed triangular network is illustrated in Figure 1 for the comparison of palm oil with olive oil, palm oil with soybean oil, and olive oil with soybean oil. The side-splitting approach (side = separating indirect and direct evidence) tests for discrepancies between direct evidence (PMA) and indirect evidence for each individual comparison available in the overall network. In the side-splitting approach, one direct comparison at a time will be excluded (i.e. olive oil compared with canola oil in Figure 1) and the NMA will obtain the indirect relative effect for olive oil compared with canola oil.
Global methods, such as the design-by-treatment interaction model, investigate the presence of inconsistency jointly from all possible sources of evidence in the entire network simultaneously (58). Different relative effects of interventions in studies can therefore often be plausibly assumed depending on the design. For example, the effects of a very-low-calorie diet and a low-calorie diet in a 3-arm study (including only patients with obesity) that also investigates intermittent fasting may differ from those in a 2-arm comparison (including only patients with normal weight). The design-by-treatment interaction model aims to explain inconsistency by allowing effects to be different in studies with different designs.
Sources of inconsistency may be explored by subgroup (e.g., age) or sensitivity analyses (e.g., excluding high RoB studies) or meta-regression. High-quality NMAs draw conclusions only from analyses that are prespecified before inspecting the study findings, but even these findings should be interpreted cautiously (92).
Ranking
By conducting NMA, it is possible to derive a relative ranking of the different interventions for the given outcome through the use of the distribution of the ranking probabilities and the surface under the cumulative ranking curves (SUCRA) (93). SUCRA values are a concept developed in the context of Bayesian statistics. Their value is between 0 and 1, where 0 means that a treatment is always worst and 1 means that a treatment is always best compared with the other treatments in the network. In practice, the researcher has to specify whether small values (such as for HbA1c) or large values (HDL cholesterol) are desired for the specific clinical outcome. SUCRA values can also be obtained in a frequentist framework, either through the use of resampling methods (80) or an analytic approach (P score), with corresponding interpretation and identical rankings (94). Taking our example from Figure 2, safflower oil (P score: 0.90) was ranked best to improve TC, followed by sunflower oil (0.85) and canola oil (0.78).
Dissemination bias
In order to evaluate dissemination bias, a funnel plot can principally be created for each direct pairwise comparison. However, a disadvantage of this approach is that funnel plots are often uninformative as only a small number of studies are available in each pairwise comparison. Instead, Chaimani et al. (95) introduced a comparison-adjusted funnel plot which shows all pairwise comparisons in a single plot, which typically contains the recommended number of at least ten estimates for the evaluation of funnel plot asymmetry (96, 97). Advanced methods to adjust for publication bias and related biases in NMA were also suggested by Chaimani and Salanti (59). The crucial assumption of comparison-adjusted funnel plots, however, is that treatments have been ordered in a “meaningful way” (95). For instance, treatments can be ordered from oldest to newest treatment if newer treatments are expected to be favored in smaller trials, i.e., if small studies showing a favorable effect for the newer treatment have a larger probability of being published than small studies showing the opposite effect.
Grading the certainty of evidence
Evaluating the certainty of evidence in an NMA is a very important part of every type of MA. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach considers the following items: study limitations, imprecision, inconsistency, indirectness, and publication bias; the approach has been expanded for NMAs to assess the certainty of evidence (98–101). The Confidence In Network Meta-Analysis (CINeMA) framework, which is an improvement of a previously suggested approach (102), can facilitate judgments about the outcome-specific certainty of evidence in an NMA. CINeMA is an adaptation of the GRADE approach and considers the following items: RoB, indirectness, imprecision, heterogeneity, publication bias, and inconsistency (102). CINeMA has been recently implemented in a web application available at http://cinema.ispm.ch. Grading the certainty of evidence according to GRADE or CINeMA leads to judgments based on 4 levels of evidence certainty: high, moderate, low, and very low.
Reporting guidelines
Authors of NMAs of health care interventions are encouraged to follow the PRISMA NMA statement (103). In congruence with the standard PRISMA guidelines for PMAs (104), authors of NMAs should state explicitly the study questions being addressed with reference to PICOS or PECOS criteria. The inclusion criteria for the PICOS or PECOS criteria should be defined in the light of transitivity/similarity with respect to an NMA. One main difference between PMA and NMA is that the number of interventions is likely to be larger and the distinction between intervention and comparison is often not obvious (in Figure 1 and Table 3, for example, olive oil might be used as the experimental treatment in one trial and as the control comparison in another trial). Table 3 shows an example of our previously published NMA that used PICOS criteria regarding the research question (32): which oil/solid fat is most effective in improving blood lipids?
Compared with the standard PRISMA statement for PMAs (70), study protocols require extensions for NMAs, i.e., potential effect modifiers need to be defined to evaluate transitivity (92). Moreover, a network plot should be provided and characteristics of the network plot should be described (Figure 1). Regarding summary measures, treatment rankings and SUCRA values should be described and reported. For the assessment of inconsistency, statistical methods used to detect disagreement between direct and indirect evidence should be described, and inconsistency should be explored with various models.
It is recommended that authors of NMAs report and compare the findings of both NMA and PMA and be aware of the potential impact of including treatments mainly based on indirect evidence in a network (i.e., star networks) (91).
Future Directions
Component NMA
Treatments in NMA can be complex—for example, combinations of ≥2 treatments or of common components. Whereas a standard NMA handles all included treatments (single or combination therapy) as different nodes in the network, component NMA models assume that the effect of a combined treatment is the sum of the effects of its components (such that equal components cancel each other out, e.g., olive oil [25 g/d] + canola oil [25 g/d] compared with olive oil [50 g/d] = canola oil [50 g/d]). These assumptions are inevitably much more problematic in nutrition research than in other medical fields such as psychiatry, because the example of combining olive oil with canola oil treatments could result in added calories (i.e., if the dose of olive oil or canola oil is ignored) or restricting other nutrients (very difficult to assess in nutrition research), and thus the credibility of an additive model as conferring benefit is limited. At best, some interactions could be added to an additive model in a synergistic or antagonistic sense if they are biologically plausible (105).
Component NMA models allow the effects of treatment components to be estimated in the context of multicomponent interventions, borrowing strength from studies with common components and comparing the estimates to the standard NMA. These models even allow estimates of effects across disconnected networks if the parts of the network have enough common components (106).
Living NMA
Updating SRs is generally more efficient than starting again from the beginning when new evidence emerges (107). Prospectively planned living MA, i.e., NMA that is continuously updated as soon as new evidence becomes available, can facilitate timely recommendations (108) and contribute to reducing research waste by providing strong evidence against the null hypothesis earlier than living PMA (109). Cochrane hosts and encourages living SRs (https://community.cochrane.org/review-production/production-resources/living-systematic-reviews). A recent study that considered 77 NMAs showed that performing living NMA seems to be feasible. Most NMAs (∼75%) had <4 new trials included per year (110). In a specific example, Crequit et al. (111) spent ∼2 mo on a 1-y NMA update, which corresponds to 11% of the initial workload (18 mo).
Use of NMA for design of future studies
The design of new studies should ideally be based on all existing evidence about the underlying clinical or health-related research question. If there are several alternative interventions, an up-to-date NMA is the optimal basis for planning a new study. NMA has therefore been proposed as a tool to optimally plan the design and estimate the required sample size of a new trial (112–115). As shown in the example in Figure 1, future intervention trials may compare the effects of hempseed oil with those of coconut oil or butter, or may also compare the effects of canola or flaxseed oil with those of butter. By taking into account Figure 1, we can additionally show that the sample size for several treatment groups was very low (<100 participants per intervention arm for hempseed oil, safflower oil, and beef fat) which should be considered when planning future trials.
Salanti et al. (112) recently introduced a 4-step framework:
Perform NMA on a research question of interest.
Define targeted comparison or comparisons between the treatments of interest (i.e., olive oil),
Decide whether NMA answers the research question (i.e., hempseed oil compared with olive oil).
Estimate the features of a future trial that will update the network to answer the research question (calculation of sample size within a conditional framework considering available evidence).
This framework aims to identify the optimal design for a new trial that will both update the existing evidence and minimize the required sample size.
NMA of observational studies
If studies are suitable, it is technically possible to conduct NMAs of observational studies. Overall, NMAs of observational studies have been only rarely applied across scientific disciplines. To date, NMAs of observational studies have not been directly applied in the field of nutrition research, but in the adjacent field of obesity research. In a recent NMA of 19 observational studies Wiebe et al. (116) investigated whether vitamin B-12 concentrations are lower in people with higher BMI. The authors observed no association between serum or plasma B-12 concentrations and BMI. In another NMA the different definitions of metabolic health and risk of T2D were investigated (117). It was shown that metabolically unhealthy patients have a 2- to 4-fold higher risk of T2D compared with patients classified as healthy across all BMI categories.
In summary, we highlight in this perspective article that NMA is a highly attractive evidence-synthesis method that has recently reached the field of nutrition research. NMAs have the potential to advance knowledge in the field of nutrition because they provide insights that cannot be obtained by individual trials or PMA, and provide an important basis for the design of novel trials. We expect that rigorously conducted NMAs based on high-quality SRs will become the new evidence-synthesis benchmark in nutrition research. However, caution is warranted since abuse and misinterpretations of both PMA and NMA findings would hamper the scientific field and possibly decision-making in public policy.
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—LS, GS, GR, and JJM: contributed to the conception and design of the paper; LS, GS, and GR: were involved in the acquisition and analysis of the data; LS, GS, GR, and JJM: interpreted the results; LS, GS, GR, and JJM: drafted the manuscript; LS, GS, GR, and JJM: provided critical revisions; and all authors: have read and approved the final manuscript.
Notes
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
The authors reported no funding received for this study. GR is funded by DFG (German Research Foundation), grant RU1747/1-2.
Author disclosures: LS, GS, GR, and JJM, no conflicts of interest.
Abbreviations used: CINeMA, Confidence In Network Meta-Analysis; DASH, Dietary Approaches to Stop Hypertension; GRADE, Grading of Recommendations Assessment, Development and Evaluation; MA, meta-analysis; NMA, network meta-analysis; PECOS, participants, exposures, comparisons, outcomes, and study design; PICOS, participants, interventions, comparisons, outcomes, and study design; PMA, pairwise meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; RoB, risk of bias; SR, systematic review; TC, total cholesterol; T2D, type 2 diabetes.
References
- 1. Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376–80. [DOI] [PubMed] [Google Scholar]
- 2. Greenhalgh T, Thorne S, Malterud K. Time to challenge the spurious hierarchy of systematic over narrative reviews?. Eur J Clin Invest. 2018;48(6):e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larsen PO, von Ins M. The rate of growth in scientific publication and the decline in coverage provided by Science Citation Index. Scientometrics. 2010;84(3):575–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faggion CM Jr., Bakas NP, Wasiak J. A survey of prevalence of narrative and systematic reviews in five major medical journals. BMC Med Res Methodol. 2017;17(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins J, Green S.. Cochrane handbook for systematic reviews of interventions version 5.1.0. [updated March 2011] The Cochrane Collaboration; 2011. [Google Scholar]
- 6. Schwingshackl L, Schlesinger S, Devleesschauwer B, Hoffmann G, Bechthold A, Schwedhelm C, Iqbal K, Knuppel S, Boeing H. Generating the evidence for risk reduction: a contribution to the future of food-based dietary guidelines. Proc Nutr Soc. 2018;77(4):432–44. [DOI] [PubMed] [Google Scholar]
- 7. Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94(3):485–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Page MJ, Moher D.. Mass production of systematic reviews and meta-analyses: an exercise in mega-silliness?. Milbank Q. 2016;94(3):515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pussegoda K, Turner L, Garritty C, Mayhew A, Skidmore B, Stevens A, Boutron I, Sarkis-Onofre R, Bjerre LM, Hrobjartsson A et al.. Systematic review adherence to methodological or reporting quality. Syst Rev. 2017;6(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chalmers I, Glasziou P.. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374(9683):86–9. [DOI] [PubMed] [Google Scholar]
- 11. Barnard ND, Willett WC, Ding EL. The misuse of meta-analysis in nutrition research. JAMA. 2017;318(15):1435–6. [DOI] [PubMed] [Google Scholar]
- 12. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, Henauw SD, Michels N, Devleesschauwer B, Schlesinger S et al.. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2017:1–20.. 10.1080/10408398.2017.1392288. [DOI] [PubMed] [Google Scholar]
- 13. Schwingshackl L, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Laure Preterre A, Iqbal K, Bechthold A, De Henauw S, Michels N, Devleesschauwer B et al.. Food groups and risk of colorectal cancer. Int J Cancer. 2018;142(9):1748–58. [DOI] [PubMed] [Google Scholar]
- 15. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 16. Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, Andriolo V, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlesinger S, Neuenschwander M, Schwedhelm C, Hoffmann G, Bechthold A, Boeing H, Schwingshackl L. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2019;10(2):205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brannon PM, Taylor CL, Coates PM. Use and applications of systematic reviews in public health nutrition. Annu Rev Nutr. 2014;34:401–19. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr. 2008;138(12):2297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwingshackl L, Knuppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopantelis E, Iqbal K et al.. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. [DOI] [PubMed] [Google Scholar]
- 22. Petropoulou M, Nikolakopoulou A, Veroniki A-A, Rios P, Vafaei A, Zarin W, Giannatsi M, Sullivan S, Tricco AC, Chaimani A et al.. Bibliographic study showed improving statistical methodology of network meta-analyses published between 1999 and 2015. J Clin Epidemiol. 2017;82:20–8. [DOI] [PubMed] [Google Scholar]
- 23. Tricco AC, Ashoor HM, Antony J, Beyene J, Veroniki AA, Isaranuwatchai W, Harrington A, Wilson C, Tsouros S, Soobiah C et al.. Safety, effectiveness, and cost effectiveness of long acting versus intermediate acting insulin for patients with type 1 diabetes: systematic review and network meta-analysis. BMJ. 2014;349:g5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–-2019. Diabetes Care2019;42(Suppl 1):S90–S102. [DOI] [PubMed] [Google Scholar]
- 25. Pan B, Ge L, Xun YQ, Chen YJ, Gao CY, Han X, Zuo LQ, Shan HQ, Yang KH, Ding GW et al.. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. 2018;15(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwingshackl L, Missbach B, Dias S, Konig J, Hoffmann G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia. 2014;57(9):1789–97. [DOI] [PubMed] [Google Scholar]
- 27. Kemps H, Krankel N, Dorr M, Moholdt T, Wilhelm M, Paneni F, Serratosa L, Ekker Solberg E, Hansen D, Halle M et al.. Exercise training for patients with type 2 diabetes and cardiovascular disease: what to pursue and how to do it. A position paper of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol. 2019. 10.1177/2047487318820420. [DOI] [PubMed] [Google Scholar]
- 28. Kanters S, Ford F, Druyts E, Thorlund K, Mills E, Bansback N. Use of network meta-analysis in clinical guidelines. Bull World Health Organ. 2016;94:782–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwingshackl L, Buyken A, Chaimani A. Network meta-analysis reaches nutrition research. Eur J Nutr. 2019;58(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwingshackl L, Hoffmann G, Iqbal K, Schwedhelm C, Boeing H. Food groups and intermediate disease markers: a systematic review and network meta-analysis of randomized trials. Am J Clin Nutr. 2018;108(3):576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan B, Wu Y, Yang Q, Ge L, Gao C, Xun Y, Tian J, Ding G. The impact of major dietary patterns on glycemic control, cardiovascular risk factors, and weight loss in patients with type 2 diabetes: a network meta-analysis. J Evid Based Med. 2019;12(1):29–39. [DOI] [PubMed] [Google Scholar]
- 32. Schwingshackl L, Bogensberger B, Bencic A, Knuppel S, Boeing H, Hoffmann G. Effects of oils and solid fats on blood lipids: a systematic review and network meta-analysis. J Lipid Res. 2018;59(9):1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang JH, Xu Y, Lin L, Jia RX, Zhang HB, Hang L. Comparison of multiple interventions for older adults with Alzheimer disease or mild cognitive impairment: a PRISMA-compliant network meta-analysis. Medicine (Baltimore). 2018;97(20):e10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwingshackl L, Chaimani A, Schwedhelm C, Toledo E, Punsch M, Hoffmann G, Boeing H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: a systematic review and network meta-analysis. Crit Rev Food Sci Nutr. 2018;1–14.. 10.1080/10408398.2018.1463967. [DOI] [PubMed] [Google Scholar]
- 35. Zou TT, Zhang C, Zhou YF, Han YJ, Xiong JJ, Wu XX, Chen YP, Zheng MH. Lifestyle interventions for patients with nonalcoholic fatty liver disease: a network meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(7):747–55. [DOI] [PubMed] [Google Scholar]
- 36. Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. 2018;33:157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gutierrez-Castrellon P, Indrio F, Bolio-Galvis A, Jimenez-Gutierrez C, Jimenez-Escobar I, Lopez-Velazquez G. Efficacy of Lactobacillus reuteri DSM 17938 for infantile colic: systematic review with network meta-analysis. Medicine (Baltimore). 2017;96(51):e9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munoz Fernandez SS, Ivanauskas T, Lima Ribeiro SM. Nutritional strategies in the management of Alzheimer disease: systematic review with network meta-analysis. J Am Med Dir Assoc. 2017;18(10):897.e13–.e30. [DOI] [PubMed] [Google Scholar]
- 39. Ha V, Bonner AJ, Jadoo JK, Beyene J, Anand SS, de Souza RJ. The effects of various diets on glycemic outcomes during pregnancy: a systematic review and network meta-analysis. PLoS One. 2017;12(8):e0182095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu W, Sui W, Mu L, Yi W, Li H, Wei L, Yin W. Preventing necrotizing enterocolitis by food additives in neonates: a network meta-analysis revealing the efficacy and safety. Medicine (Baltimore). 2017;96(21):e6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song GM, Liu XL, Bian W, Wu J, Deng YH, Zhang H, Tian X. Systematic review with network meta-analysis: comparative efficacy of different enteral immunonutrition formulas in patients underwent gastrectomy. Oncotarget. 2017;8(14):23376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iftikhar IH, Bittencourt L, Youngstedt SD, Ayas N, Cistulli P, Schwab R, Durkin MW, Magalang UJ. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med. 2017;30:7–14. [DOI] [PubMed] [Google Scholar]
- 43. Sekercioglu N, Thabane L, Diaz Martinez JP, Nesrallah G, Longo CJ, Busse JW, Akhtar-Danesh N, Agarwal A, Al-Khalifah R, Iorio A et al.. Comparative effectiveness of phosphate binders in patients with chronic kidney disease: a systematic review and network meta-analysis. PLoS One. 2016;11(6):e0156891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lehert P, Villaseca P, Hogervorst E, Maki PM, Henderson VW. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric. 2015;18(5):678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Song GM, Tian X, Zhang L, Ou YX, Yi LJ, Shuai T, Zhou JG, Zeng Z, Yang HL. Immunonutrition support for patients undergoing surgery for gastrointestinal malignancy: preoperative, postoperative, or perioperative? A Bayesian network meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94(29):e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Woods HB, Davies M, Goyder E. Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pract. 2015;107(3):320–31. [DOI] [PubMed] [Google Scholar]
- 47. Mazaki T, Ishii Y, Murai I. Immunoenhancing enteral and parenteral nutrition for gastrointestinal surgery: a multiple-treatments meta-analysis. Ann Surg. 2015;261(4):662–9. [DOI] [PubMed] [Google Scholar]
- 48. Schwingshackl L, Dias S, Hoffmann G. Impact of long-term lifestyle programmes on weight loss and cardiovascular risk factors in overweight/obese participants: a systematic review and network meta-analysis. Syst Rev. 2014;3:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carter P, Achana F, Troughton J, Gray LJ, Khunti K, Davies MJ. A Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: a network meta-analysis. J Hum Nutr Diet. 2014;27(3):280–97. [DOI] [PubMed] [Google Scholar]
- 50. Dunkley AJ, Charles K, Gray LJ, Camosso-Stefinovic J, Davies MJ, Khunti K. Effectiveness of interventions for reducing diabetes and cardiovascular disease risk in people with metabolic syndrome: systematic review and mixed treatment comparison meta-analysis. Diabetes Obes Metab. 2012;14(7):616–25. [DOI] [PubMed] [Google Scholar]
- 51. Wiebe N, Padwal R, Field C, Marks S, Jacobs R, Tonelli M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med. 2011;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sekercioglu N, Angeliki Veroniki A, Thabane L, Busse JW, Akhtar-Danesh N, Iorio A, Cruz Lopes L, Guyatt GH. Effects of different phosphate lowering strategies in patients with CKD on laboratory outcomes: a systematic review and NMA. PLoS One. 2017;12(3):e0171028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 54. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 55. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91. [DOI] [PubMed] [Google Scholar]
- 57. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932–44. [DOI] [PubMed] [Google Scholar]
- 58. Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014;33(21):3639–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chaimani A, Salanti G.. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods. 2012;3(2):161–76. [DOI] [PubMed] [Google Scholar]
- 60. Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(Suppl 1):S120–43. [DOI] [PubMed] [Google Scholar]
- 61. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Sato M, Sugawara A, Totsuka K, Shimano H, Ohashi Y et al.. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32(5):959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kirk JK, Graves DE, Craven TE, Lipkin EW, Austin M, Margolis KL. Restricted-carbohydrate diets in patients with type 2 diabetes: a meta-analysis. J Am Diet Assoc. 2008;108(1):91–100. [DOI] [PubMed] [Google Scholar]
- 63. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–16. [DOI] [PubMed] [Google Scholar]
- 64. Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016;67(4):733–9. [DOI] [PubMed] [Google Scholar]
- 65. Ghobadi S, Hassanzadeh-Rostami Z, Mohammadian F, Nikfetrat A, Ghasemifard N, Raeisi Dehkordi H, Faghih S. Comparison of blood lipid-lowering effects of olive oil and other plant oils: a systematic review and meta-analysis of 27 randomized placebo-controlled clinical trials. Crit Rev Food Sci Nutr. 2018;1–15.. 10.1080/10408398.2018.1438349. [DOI] [PubMed] [Google Scholar]
- 66. Sun Y, Neelakantan N, Wu Y, Lote-Oke R, Pan A, van Dam RM. Palm oil consumption increases LDL cholesterol compared with vegetable oils low in saturated fat in a meta-analysis of clinical trials. J Nutr. 2015;145(7):1549–58. [DOI] [PubMed] [Google Scholar]
- 67. United States Department of Agriculture Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015. [Google Scholar]
- 68. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group.Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I et al.. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sturtz S, Bender R.. Unsolved issues of mixed treatment comparison meta-analysis: network size and inconsistency. Res Synth Methods. 2012;3(4):300–11. [DOI] [PubMed] [Google Scholar]
- 72. Caldwell DM, Welton NJ.. Approaches for synthesising complex mental health interventions in meta-analysis. Evid Based Ment Health. 2016;19(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. James A, Yavchitz A, Ravaud P, Boutron I. Node-making process in network meta-analysis of nonpharmacological treatment are poorly reported. J Clin Epidemiol. 2018;97:95–102. [DOI] [PubMed] [Google Scholar]
- 74. Leucht S, Chaimani A, Cipriani AS, Davis JM, Furukawa TA, Salanti G. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci. 2016;266(6):477–80. [DOI] [PubMed] [Google Scholar]
- 75. Mavridis D, Giannatsi M, Cipriani A, Salanti G. A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health. 2015;18(2):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Efthimiou O, Debray TP, van Valkenhoef G, Trelle S, Panayidou K, Moons KG, Reitsma JB, Shang A, Salanti G. GetReal in network meta-analysis: a review of the methodology. Res Synth Methods. 2016;7(3):236–63. [DOI] [PubMed] [Google Scholar]
- 77. Lu G, Ades AE.. Assessing evidence inconsistency in mixed treatment comparisons. J Am Statist Assoc. 2006;101(474):447–59. [Google Scholar]
- 78. Lu G, Ades A.. Modeling between-trial variance structure in mixed treatment comparisons. Biostatistics. 2009;10(4):792–805. [DOI] [PubMed] [Google Scholar]
- 79. R Core Team. R: a language and environment for statistical computing. [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org/. [Google Scholar]
- 80. White IR. Network meta-analysis. Stata J. 2015;15(4):951–85. [Google Scholar]
- 81. Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. [DOI] [PubMed] [Google Scholar]
- 82. Rucker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3(4):312–24. [DOI] [PubMed] [Google Scholar]
- 83. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity—subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33(5):618–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33(5):641–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–99. [DOI] [PubMed] [Google Scholar]
- 87. Rücker G, Krahn U, König J, Efthimiou O, Schwarzer G.. netmeta: network meta-analysis using frequentist methods. [Internet]. 2019. Available from: https://github.com/guido-s/netmeta; http://meta-analysis-with-r.org. [Google Scholar]
- 88. Viechtbauer W. metafor: meta-analysis package for R. [Internet]. R package version 2.0-0 2018. Available from: https://CRAN.R-project.org/package = metafor. [Google Scholar]
- 89. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. 2014;9(12):e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rucker G, Schwarzer G.. Automated drawing of network plots in network meta-analysis. Res Synth Methods. 2016;7(1):94–107. [DOI] [PubMed] [Google Scholar]
- 91. Lin L, Xing A, Kofler MJ, Murad MH. Borrowing of strength from indirect evidence in 40 network meta-analyses. J Clin Epidemiol. 2019;106:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J Clin Epidemiol. 2017;83:65–74. [DOI] [PubMed] [Google Scholar]
- 93. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. [DOI] [PubMed] [Google Scholar]
- 94. Rucker G, Schwarzer G.. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Method. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH et al.. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 98. Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clin Res Ed). 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 99. Brignardello-Petersen R, Murad MH, Walter SD, McLeod S, Carrasco-Labra A, Rochwerg B, Schunemann HJ, Tomlinson G, Guyatt GH. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60–7. [DOI] [PubMed] [Google Scholar]
- 100. Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, Hazlewood GS, Alhazzani W, Mustafa RA, Murad MH et al.. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44. [DOI] [PubMed] [Google Scholar]
- 101. Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC, Murad MH, Agoritsas T, Izcovich A, Schünemann HJ, Guyatt GH. GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol. 2019;108:77–85. [DOI] [PubMed] [Google Scholar]
- 102. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP et al.. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 104. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9., W64. [DOI] [PubMed] [Google Scholar]
- 105. Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol. 2009;169(9):1158–65. [DOI] [PubMed] [Google Scholar]
- 106. Madan J, Chen Y-F, Aveyard P, Wang D, Yahaya I, Munafo M, Bauld L, Welton N. Synthesis of evidence on heterogeneous interventions with multiple outcomes recorded over multiple follow-up times reported inconsistently: a smoking cessation case-study. J R Stat Soc Series A Stat Soc. 2014;177(1):295–314. [Google Scholar]
- 107. Garner P, Hopewell S, Chandler J, MacLehose H, Schunemann HJ, Akl EA, Beyene J, Chang S, Churchill R, Dearness K et al.. When and how to update systematic reviews: consensus and checklist. BMJ. 2016;354:i3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Akl EA, Meerpohl JJ, Elliott J, Kahale LA, Schunemann HJ. Living systematic reviews: 4. Living guideline recommendations. J Clin Epidemiol. 2017;91:47–53. [DOI] [PubMed] [Google Scholar]
- 109. Nikolakopoulou A, Mavridis D, Furukawa TA, Cipriani A, Tricco AC, Straus SE, Siontis GCM, Egger M, Salanti G. Living network meta-analysis compared with pairwise meta-analysis in comparative effectiveness research: empirical study. BMJ. 2018;360:k585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Crequit P, Martin-Montoya T, Attiche N, Trinquart L, Vivot A, Ravaud P. Living network meta-analysis was feasible when considering the pace of evidence generation. J Clin Epidemiol. 2019;108:10–16.. 10.1016/j.jclinepi.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 111. Crequit P, Trinquart L, Yavchitz A, Ravaud P. Wasted research when systematic reviews fail to provide a complete and up-to-date evidence synthesis: the example of lung cancer. BMC Med. 2016;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Salanti G, Nikolakopoulou A, Sutton AJ, Reichenbach S, Trelle S, Naci H, Egger M. Planning a future randomized clinical trial based on a network of relevant past trials. Trials. 2018;19(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nikolakopoulou A, Mavridis D, Salanti G. Using conditional power of network meta-analysis (NMA) to inform the design of future clinical trials. Biom J. 2014;56(6):973–90. [DOI] [PubMed] [Google Scholar]
- 114. Nikolakopoulou A, Mavridis D, Salanti G. Planning future studies based on the precision of network meta-analysis results. Stat Med. 2016;35(7):978–1000. [DOI] [PubMed] [Google Scholar]
- 115. Nikolakopoulou A, Mavridis D, Egger M, Salanti G. Continuously updated network meta-analysis and statistical monitoring for timely decision-making. Stat Methods Med Res. 2018;27(5):1312–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wiebe N, Field CJ, Tonelli M. A systematic review of the vitamin B12, folate and homocysteine triad across body mass index. Obes Rev. 2018;19(11):1608–18. [DOI] [PubMed] [Google Scholar]
- 117. Lotta LA, Abbasi A, Sharp SJ, Sahlqvist AS, Waterworth D, Brosnan JM, Scott RA, Langenberg C, Wareham NJ. Definitions of metabolic health and risk of future type 2 diabetes in BMI categories: a systematic review and network meta-analysis. Diabetes Care. 2015;38(11):2177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


