Abstract
Aim
To present a systematic literature review (SLR) on efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD), aiming to provide a basis for updating the EULAR evidence-based recommendations.
Methods
An SLR was performed according to the standard operating procedures for EULAR-endorsed recommendations. Outcome was determined by efficacy, immunogenicity and safety of vaccination in adult patients with AIIRD, including those receiving immunomodulating therapy. Furthermore, a search was performed on the effect of vaccinating household members of patients with AIIRD on the occurrence of vaccine-preventable infections in patients and their household members (including newborns). The literature search was performed using Medline, Embase and the Cochrane Library (October 2009 to August 2018).
Results
While most investigated vaccines were efficacious and/or immunogenic in patients with AIIRD, some were less efficacious than in healthy control subjects, and/or in patients receiving immunosuppressive agents. Adverse events of vaccination were generally mild and the rates were comparable to those in healthy persons. Vaccination did not seem to lead to an increase in activity of the underlying AIIRD, but insufficient power of most studies precluded arriving at definite conclusions. The number of studies investigating clinical efficacy of vaccination is still limited. No studies on the effect of vaccinating household members of patients with AIIRD were retrieved.
Conclusion
Evidence on efficacy, immunogenicity and safety of vaccination in patients with AIIRD was systematically reviewed to provide a basis for updated recommendations.
Keywords: infections, vaccination, autoimmune diseases
Key messages.
What is already known about this subject?
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk of vaccine-preventable infections and associated complications.
Vaccination may be less efficacious in (subgroups of) patients with AIIRD and could potentially lead to exacerbation of underlying disease.
Evidence-based recommendations of the EULAR for vaccination of adult patients with AIIRD were published in 2011.
What does this study add?
This systematic literature review summarises available evidence on efficacy, immunogenicity and safety of vaccination in AIIRD since October 2009, providing a basis for updated EULAR recommendations.
How might this impact on clinical practice?
The aim of the updated recommendations is to aid health professionals dealing with questions regarding vaccination in patients with AIIRD, whereby reducing infection-related morbidity and mortality.
Introduction
Infectious diseases and associated complications comprise an important cause of morbidity and mortality in patients with autoimmune inflammatory rheumatic diseases (AIIRD). Increased susceptibility to infectious diseases in these patients is most likely due to an immunomodulating effect of the disease itself and/or by use of immunosuppressive medications.1
Vaccination is generally regarded as a safe, efficacious and low-cost method for preventing certain infections. However, vaccination may be less efficacious in (subgroups of) patients with AIIRD, as a result of their immunosuppressed state, and, moreover, could potentially lead to exacerbation of the underlying AIIRD.
In 2011, evidence-based recommendations for vaccination in patients with AIIRD were published. They were formulated by an EULAR task force to aid health professionals dealing with questions regarding vaccination in patients with AIIRD in daily clinical practice, with the aim of reducing infection-related morbidity and mortality in these patients.2 The authors stated that the recommendations needed to be updated on a regular basis as new evidence becomes available.2 Towards this end, the League commissioned another multidisciplinary task force with the purpose of formulating up-to-date recommendations for vaccination in patients with AIIRD.
The current report presents the results of an SLR on efficacy, immunogenicity and safety of vaccination in adult patients with AIIRD, including those using immunomodulating agents. Together with the results of an SLR on incidence and prevalence of VPIs in patients with AIIRD,1 the current SLR provided the task force with a basis for updating the recommendations.3
Methods
The work was performed in accordance with the 2014 EULAR standard operating procedures for EULAR-endorsed recommendations.4
The expert committee first formulated four main research questions (Box 1), based on the 2011 version of the recommendations. The current review reports on the SLR results of three of these four questions, which include the topics of efficacy, immunogenicity and safety of vaccination in adult patients with AIIRD (including those receiving immunosuppressive agents) and the effect of vaccinating their household members on the occurrence of VPIs in both patients and their household members (including newborns). The efficacy of vaccination was defined as the capacity to prevent infections, while the immunogenicity of vaccination refers to the capacity to induce vaccine-specific humoral and/or cellular immune responses. Safety of vaccination in the AIIRD population was determined by the assessment of both the occurrence of adverse effects and the influence on the underlying disease.1
Box 1. Research questions.
What is the incidence or prevalence of vaccine-preventable infections (VPI) in adult patients with AIIRD?*
What is the efficacy, immunogenicity and safety of available vaccines in adult patients with AIIRD?
Are vaccines efficacious and immunogenic in adult patients with AIIRD, treated with immunosuppressive agents and disease-modifying antirheumatic drugs?
What is the effect of vaccinating household contacts of patients with AIIRD on the occurrence of VPI in both patients and household members (including newborns)?
* The systematic literature review covering research question 1 has been submitted for publication separately.1
AIIRD, autoimmune inflammatory rheumatic disease(s).
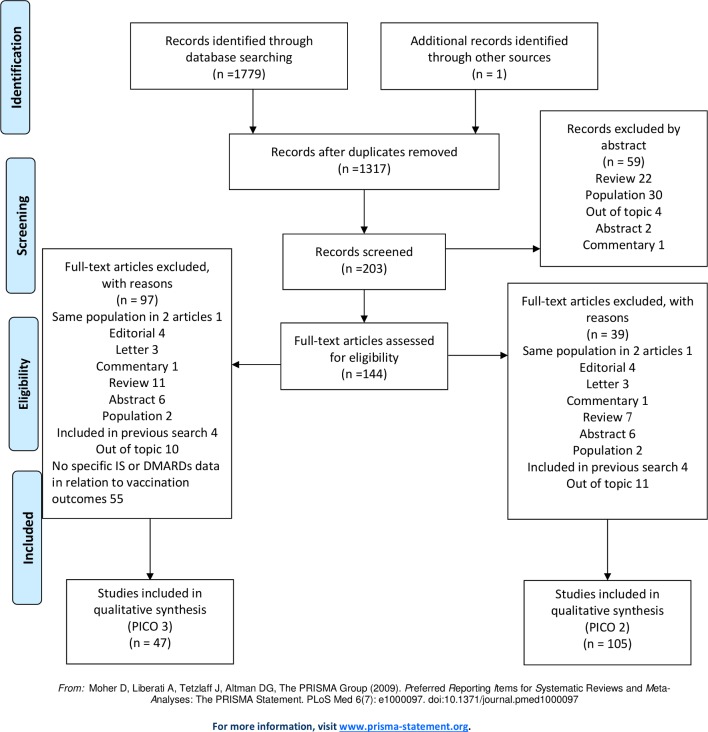
Next, the research questions were adapted according to the PICO-method (population-intervention-comparison-outcome). Population, intervention, comparison and outcome definitions were combined and adapted to be used as search terms (table 1). Medline (via Pubmed), Embase and the Cochrane Library were searched from October 2009 to August 2018. Meta-analyses, randomised trials, cohort studies and case series with at least five participants were eligible. Only English articles on adult patients (≥18 years) were included. Papers with non-original data, case reports, case series with less than five patients, abstracts presented in scientific meetings, and papers included in the previous version of these recommendations were excluded. Papers that were not retrieved in the search, but were relevant in the opinion of the committee, could be added. See figure 1 for the flow chart displaying the search strategy for PICO 2 and 3. For some of the AIIRD, immunomodulating agents and vaccines (diphteria, pertussis, measles, mumps, rubella, Neisseria meningitides, Haemophilus influenzae B and typhoid fever vaccine) that were included in the literature search, no relevant articles were retrieved. No relevant articles were retrieved in the search on the effect of vaccinating household members of patients with AIIRD (research question 4).
Table 1.
Formulation of PICO-questions
| Q2: What is the efficacy, immunogenicity and safety of available vaccines in adult patients with AIIRD? Population: patients with AIIRD* Intervention: immunisation/vaccination with vaccines suitable for adults** Comparison: healthy controls, non-vaccinated patients with AIIRD or none Outcome: efficacy (prevention of vaccine-preventable disease), immunogenicity (laboratory markers for vaccine efficacy, eg, seroprotection/seroconversion) and safety (effect on the underlying autoimmune disease or adverse effects from vaccination) | ||
| Q3: Are vaccines efficacious and immunogenic in adult patients with AIIRD, treated with immunosuppressive agents and disease-modifying antirheumatic drugs (DMARDs)? Population: patients with AIIRD* using immunomodulating agents*** Intervention: immunisation/vaccination with vaccines suitable for adults** Comparison: healthy controls, patients with AIIRD not using analysed agents or none Outcome: efficacy (prevention of vaccine-preventable disease), immunogenicity (laboratory markers for vaccine efficacy, eg, seroprotection/seroconversion) | ||
| Q4: What is the effect of vaccinating household members of patients with AIIRD on the occurrence of VPI in both the patients and household members (including newborns)? Population: patients with AIIRD* Intervention: immunisation/vaccination of household contacts of patients with AIIRD with vaccines suitable for children and adults** Comparison: patients with AIIRD with non-vaccinated household members Outcome: incidence of VPI in patients with AIIRD/safety of household vaccine for patients with AIIRD | ||
| * AIIRD | ** Vaccines | *** Immunomodulating agents |
| Rheumatoid arthritis | Influenza | Glucocorticosteroids |
| Systemic lupus erythematosus | Tetanus toxoid | Methotrexate |
| Antiphospholipid syndrome | Diphtheria | Sulfasalazine |
| Adult Still’s disease | Pertussis | Leflunomide |
| Systemic sclerosis | Measles | Hydroxychloroquine |
| Sjögren syndrome | Mumps | Azathioprine |
| Mixed connective tissue diseases | Rubella | Mycophenolic preparation |
| Relapsing polychondritis | Varicella-zoster virus | Ciclosporine |
| Giant cell arteritis | Human papillomavirus | Tacrolimus |
| Polymyalgia rheumatica | Streptococcus pneumoniae | Cyclophosphamide |
| Takayasu arteritis | Hepatitis A | Rituximab |
| Polyarteritis nodosa | Hepatitis B | Belimumab |
| ANCA-associated vasculitis | Neisseria meningitidis | Abatacept |
| Microscopic polyangiitis | Haemophilus influenzae B | TNFα blocking agents |
| Granulomatosis with polyangiitis | Tickborne encephalitis | Infliximab |
| Eosinophilic granulomatosis with polyangiitis | Typhoid fever | Etanercept |
| Behçet’s disease | Yellow fever | Adalimumab |
| Anti-GBM disease | Certolizumab | |
| Cryoglobulinaemic syndrome | Golimumab | |
| Polymyositis | Anti-IL-6 agents | |
| Dermatomyositis | Tocilizumab | |
| Clinically amyotrophic dermatomyositis | Sarilumab | |
| Inclusion body myositis | Anti-IL-17 agents | |
| Antisynthetase syndrome | Secukinumab | |
| Eosinophilic myositis | Ixekizumab | |
| Eosinophilic fasciitis | Anti-IL-1 agents | |
| Spondyloarthropathies | Canakinumab | |
| Periodic fever syndromes | Anakinra | |
| Familial Mediterranean fever | Rilonacept | |
| TNF-receptor associated syndrome (TRAPS) | Apremilast | |
| Cryopyrin associated periodic syndrome (CAPS) | Tofacitinib | |
| Baricitinib | ||
AIIRD, autoimmune inflammatory rheumatic disease(s); ANCA, antineutrophil cytoplasmic antibodies; GBM, glomerular basement membrane; IL, interleukin; PICO, population-intervention-comparison-outcome; TNF, tumour necrosis factor; VPI, vaccine-preventable infection.
Figure 1.
Flow chart displaying the search strategy for PICO 2 and 3. DMARDs, disease-modifying antirheumatic drugs; IS, immunosuppressives; PICO, population-intervention-comparison-outcome.
Data analysis was performed by CR, VF, MH, SvA and OE. The following information was retrieved from all included articles: name of first author, year of publication, country where the study was performed, years of data inclusion, type of study, vaccine used, addition of adjuvant, type of AIIRD, number of participants, age and sex of participants, disease duration, time of follow-up, medication used and outcome of vaccination (efficacy, immunogenicity and/or safety). The articles were critically assessed (online supplementary file S6—included articles and critical appraisal) by applying tools from the Cochrane Library (online supplementary file S7—critical appraisal criteria) and given a level of evidence based on the Oxford Centre for Evidence-based Medicine approach (table 2). Discrepancies between reviewers were resolved by consensus. The final recommendations were graded according to the level of evidence of the underlying articles (table 3).5
Table 2.
Oxford Centre for Evidence-Based Medicine—levels of evidence
| Level | |
| 1a | Systematic review (with homogeneity) of RCTs |
| 1b | Individual RCT (with narrow CI) |
| 1c | ‘All or none’ |
| 2a | Systematic review (with homogeneity) of cohort studies |
| 2b | Individual cohort study (including low-quality RCT) |
| 2c | ‘Outcomes’ research, ecological studies |
| 3a | Systematic review (with homogeneity) of case-control studies |
| 3b | Individual case-control study |
| 4 | Case series (and poor quality cohort and case-control studies) |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research of ‘first principles’ |
RCT, randomised controlled trial.
Table 3.
Grades of recommendation
| Grade | |
| A | Consistent level 1 studies |
| B | Consistent level 2 or 3 studies or extrapolations from level 1 studies |
| C | Level 4 studies or extrapolations from level 2 or 3 studies |
| D | Level 5 evidence or troublingly inconsistent or inconclusive studies of any level |
rmdopen-2019-001035supp002.pdf (548.6KB, pdf)
rmdopen-2019-001035supp003.pdf (173.1KB, pdf)
Results
Influenza vaccination
Efficacy—immunogenicity—safety
Up to the previous recommendations, one study addressed the issue of efficacy of influenza vaccination in patients with AIIRD.6 Immunogenicity of the vaccine had been evaluated in 26 studies, mainly including patients with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and granulomatosis with polyangiitis (GPA).7–32 Most of these studies demonstrated similar rates of immunogenicity among patients and healthy controls (HC), except for the studies in patients treated with rituximab, whose responses were severely impaired.12 14 33
From the previous recommendations up to August 2018, seven meta-analyses and 50 other studies have been published on efficacy, immunogenicity and safety of influenza vaccination, including the 2009 pandemic H1N1 influenza strain vaccine, in patients with AIIRD (table 4 for seasonal trivalent and table 5 for monovalent pandemic influenza vaccination).
Table 4.
Efficacy, immunogenicity and safety of trivalent influenza vaccination in patients with AIIRD (October 2009–August 2018)
| First author +ref. | Year | Study design | No. cases | Efficacy | Immunogenicity | Safety | Influence of IS on eff./imm. | LoE | ||
| Eff. | Imm. | Saf. | ||||||||
| Subesinghe75 | 2018 | Meta-analysis | 7 studies in RA | – | See column influence of IS | – | MTX and anti-TNF not associated with reduced immunogenicity | – | 2a | – |
| Huang42 | 2017 | Meta-analysis | 13 studies in RA (also including pts <18 years) | – | Reduced immunogenicity RA compared with HCs for H1N1 strain, not for H3N2 and B Respective SP: 60%, 68%, 61% Lower response with non-adjuvanted vaccine |
Disease activity not influenced by vaccination AE significantly more frequent in RA (RR 1.77; 95% CI 1.02 to 3.08) |
GC: No influence Anti-TNF, RTX: Lower SP rate for H1N1, but not for SC or other strains Other biologics: Lower SP and SC for H1N1 |
– | 2a | 2a |
| Burmester201 | 2017 | Meta-analysis | Total in analysis: 171 RA-anti-TNF vacc. 382 RA-anti-TNF- non-vacc. All using adalimumab |
Influenza-related AE occurred in 5% of vaccinated pts versus 14% of non-vacc. | – | – | – | 2a | – | – |
| Hua77 | 2014 | Meta-analysis | 7 studies in RA | – | See column influence of IS | – | RTX: reduced immunogenicity Anti-TNF: no influence For MTX, results differed depending on method of analysis |
– | 2a | – |
| Park79 | 2018 | RCT | 2 RA groups:
|
– | Better response for all strains in patients who hold MTX 2 weeks after vaccination (SP difference H1N1 11% (95% CI 2% to 19%), H3N2 16% (6% to 26%), B 14.7% (5% to 25%) | No SAE eight flares (5%) in MTX-cont. and 17 (11%) in MTX-hold group (p=0.07) |
– | – | 1b-2b | 2b |
| Park78 | 2017 | RCT | 4 RA groups on MTX:
|
– | Adequate response Better results in pts who stopped MTX 2 weeks before and after vaccination |
No SAE Flares tended to be more common in groups 2 and 3 (not significant) |
– | – | 1b-2b | 2b |
| Kivitz202 | 2014 | RCT | 107 RA-CZP 109 RA-PCB |
– | No difference | No difference in AE: 62.3% in PCB versus 63.6% in CZP, mostly mild/moderate Disease activity NR |
Reduced on MTX | – | 1b-2b | 4 |
| Chen34 | 2018 | Cohort (retrospective database) | 3748 RA-vacc 3748 RA non-vacc |
Reduced risk of morbidity and mortality in vaccinated pts | – | – | – | 2b | – | – |
| Jain39 | 2017 | Cohort | 51 RA-MTX 51 RA-naïve 45 HCs |
– | No difference | No influence on disease activity No difference in AE |
See column immunogenicity | – | 2b | 4 |
| Winthrop84 Part A |
2016 | Cohort | 102 RA-TFC 98 RA-PCB |
– | Similar proportions of satisfactory response | – | Reduced in TFC/MTX | – | 2b | – |
| Winthrop84 Part B |
2016 | Cohort | 92 RA-TFC cont. 91 RA-TFC stop | No difference | No | – | 2b | – | ||
| Alten83 | 2016 | Cohort | 184 RA ABT+MTX | – | Adequate response | – | See column immunogenicity | – | 2b | – |
| Luque Ramos203 | 2016 | Cohort (retrospective database) | 111482 RA 555410 HCs |
Trend towards higher hospital admittance rates for pneumonia in areas with lower influenza and pneumococcal vaccine uptake | – | – | – | 5 | – | – |
| Kogure74 | 2014 | Cohort | 57 RA: 9 biologics 34 MTX 8 TAC 10 GC 14 SASP |
– | Seroprotection: H1N1 63%, H3N2 81%, influenza B 26% |
No change in disease activity, no AE. | Reduced on biologics | – | 2b | 4 |
| Milanetti41 Both seasonal and pandemic |
2014 | Cohort | 30 RA 13 HCs |
– | No difference | Milder AE in patients. No changes in disease activity |
No effect of anti-TNF or ABT | – | 2b | 4 |
| Kobashigawa36 | 2013 | Cohort (prospective) | 17735 RA in 4 seasons (12.2%–38.7% vacc) |
Vaccination associated with reduced self-reported risk of influenza | – | – | No | 2b | – | – |
| Milanovic37 | 2013 | Cohort | 19 SLE–vacc. 11 SLE 15 RA-vacc. 22 RA 13 SjS-vacc. 19 SjS |
Lower incidence of influenza and bact. Complications among vaccinated pts | Sign. difference in GMT between vacc./unvacc. SLE, but not in RA and SjS. |
No changes in disease activity | No | 4 | 2b | 4 |
| Tsuru81 | 2013 | Cohort | 38 TCZ (28 RA/10 CD) 39 RA anti-TNF/DMARD |
– | No difference | – | No | – | 2b | 4 |
| Mori73 | 2012 | Cohort | 62 RA-TCZ 65 RA-MTX 49 RA-TCZ +MTX 18 RA-DC |
Adequate immune response, but lower on MTX | No systemic AE No flares |
Reduced on MTX | – | 2b | 4 | |
| Kogure72 | 2012 | Cohort | RA treated with Japanese Kampo medicine: 24 RA+MTX 16 RA-DC |
– | No difference Low response in general |
No AE No influence on disease activity |
No influence of MTX | – | 4 | 4 |
| Arad38 | 2011 | Cohort | 29 RA-RTX (16<5 mo, 13>5 mo) 17 RA-DC 16 HCs |
– | Humoral immunity: reduced in RA-RTX Similar percentage of influenza-specific IFN-γ producing CD4+ cells in RA groups |
No change in disease activity | Humoral immunity: Reduced on RTX Cellular immunity: No |
– | 2b | 4 |
| Kobie40 | 2011 | Cohort | 61 RA-anti-TNF 70 RA-MTX 33 RA-DC 97 HCs |
– | Reduced in RA-anti-TNF | – | Reduced on anti-TNF | – | 2b | – |
| Rehnberg107 | 2010 | Cohort | 11 RA 6 mo post-RTX 8 RA 6 d pre-RTX 10 RA-DC |
– | Lower frequency influenza-specific B cells in peripheral blood in post-RTX group 6 d after vacc. Lower humoral response 21 d after vacc. in post-RTX group |
– | Reduced on RTX | – | 4 | – |
| Salemi71 | 2010 | Cohort | 22 RA-anti-TNF 10 HCs |
– | Lower in RA | No SAE No difference in AE No change in disease activity ANA appearance/increase similar RA and HCs |
– | – | 2b | 4 |
| Huang47 | 2016 | Meta-analysis | 15 studies in SLE (also including pts<18 years) | – | Reduced immunogenicity SLE compared with HCs for H1N1 and B, but not for H3N2 Respective SP: 66%, 64%, 60% Lower response with non-adjuvanted vaccine |
Disease activity not influenced by vaccination No difference in AE between SLE and HCs |
GC, AZA or IS in general: reduced immunogenicity HCsQ: No difference |
– | 2a | 2a |
| Pugès45 | 2016 | Meta-analysis | 17 studies in SLE | – | Immunogenicity depends on viral strains: reduced against A and preserved for B | No influence on disease activity | – | – | 2a | 2a |
| Liao46 | 2016 | Meta-analysis | 18 studies in SLE | – | Reduced in SLE for H1N1 and H3N2, but not for B Respective SP: 68%, 76%, 66% |
All side effects mild and transient Similar rate of AE in SLE and HCs 2 severe flares |
– | – | 2a | 2a |
| Chang35 | 2016 | Cohort (retrospective database) | 1765 SLE-vacc. 8360 SLE non-vacc. |
Reduction of complications of influenza in vaccinated patients | – | – | – | 2b | – | – |
| Launay204 | 2013 | Cohort | 27 SLE | Percentages of responders at day 30 are 55.5%, 18.5% and 55.5%, for H1N1, H3N2 and influenza B, respectively | Increase in rheumatoid factor levels, after vacc. No flares. |
4 | 4 | |||
| Vista205 | 2012 | Cohort | 101 SLE 101 HCs |
– | – | Similar proportion new onset anticardiolipin antibodies | – | – | – | 4 |
| Crowe44 | 2011 | Cohort | 72 SLE 72 HCs |
– | No difference. More high responses in African-American subjects. |
19.4%/26.4% flare 6/12 weeks postvacc. More low responders with flare at 6 weeks. |
Reduced on steroids | – | 4 | 4 |
| Wallin43 | 2009 | Cohort | 47 SLE:
27 HCs |
– | No difference in seroprotection | Overall stable disease | Reduced on steroids | – | 2b | 4 |
| Jaeger53 | 2017 | Cohort | 107 injections influenza vaccine in 55 CAPS |
– | – | AE in 7% of injections Fever in 2% No SAE |
– | – | – | 4 |
| Caso51 | 2016 | Cohort | 25 PsA-vacc. 25-PsA DC |
– | – | Higher tender joint count and ESR after 1 month, more episodes mild symptoms in PsA- vacc. |
– | – | – | 4 |
| Jeffs48 | 2015 | Cohort | 24 AAV-vacc. 67 AAV-non vacc. 53 HCs |
– | Adequate, but lower response in AAV | No SAE Significant increase in local AE following vaccination only in HCs No change in disease activity |
– | – | 2b | 2b |
| Polachek50 | 2015 | Cohort | 63 PsA 4 Pso 30 HCs |
– | No difference | Increased CRP in patients 4–6 weeks postvacc. |
No | – | 2b | 4 |
| Litinsky49 | 2012 | Cohort | 26 SSc 16 HCs |
– | Increased in SSc for H1N1 No difference for H3N2 and influenza B |
Overall stable disease | Increased on combination iloprost and calcium channel blockers for H1N1 and influenza B | – | 2b | 4 |
| Kostianovsky52 Both seasonal and pandemic |
2012 | Cohort | 74 systemic vasculitis 32 SSc 29 SLE 23 SjS 28 other AIIRD |
– | No difference | 19 flares | No | – | 4 | 4 |
The table is structured as follows: First studies in RA, then SLE followed by other autoimmune inflammatory rheumatic diseases (AIIRD). Within this organisation, articles are clustered in study design (meta-analyses, RCT, cohort studies, case series) and presented in order of publication year.
AAV, ANCA-associated vasculitis; ABT, abatacept; ANA, antinuclear antibodies; AZA, azathioprine; bact., bacterial; CAPS, cryopyrin associated periodic syndrome; CD, Castleman’s disease; CD, cluster of differentiation; cont., continued; CRP, C reactive protein; CZP, certolizumab pegol; d, days; DC, disease control; DMARD, disease-modifying antirheumatic drug; eff., efficacy; ESR, erythrocyte sedimentation rate; GC, glucocorticoids; GMT, geometrical mean titre; HC, healthy controls; HCQ, hydroxychloroquine; IFN, interferon; imm, immunogenicity; IS, immunosuppressives; LoE, level of evidence; mo., months; MTX, methotrexate; No., number; NR, not reported; PCB, placebo; PsA, psoriatic arthritis; Pso, psoriasis; pts, patients; RA, rheumatoid arthritis; RCT, randomised controlled trial; ref., reference; RR, relative risk; RTX, rituximab; (S)AE, (serious) adverse event(s); saf., safety; SASP, salazosulfapyridine; SC, seroconversion; sign, significant; SjS, Sjögren’s syndrome; SLE, systemic lupus erythematosus; SP, seroprotection; SSc, systemic sclerosis; TAC, tacrolimus; TCZ, tocilizumab; TFC, tofacitinib; TNF, tumor necrosis factor; vacc., vaccinated; yrs, years.
Table 5.
Efficacy, immunogenicity and safety of monovalent (H1N1) pandemic influenza vaccination in patients with AIIRD (October 2009–August 2018)
| First author +ref. | Year | Study design | No. cases | Efficacy | Immunogenicity | Safety | Influence of IS on eff./ imm. | LoE | ||
| Eff. | Imm. | Saf. | ||||||||
| Milanetti41 Both seasonal and pandemic, adj. (MF59) |
2014 | Cohort | 30 RA 13 HC |
– | No difference | More mild AE in patients | No effect of anti-TNF or ABA | – | 2b | 4 |
| Kapetanovic62 Adj. (AS03) |
2014 | Cohort | 50 RA-MTX 38 RA-anti-TNF 53 RA-anti-TNF+MTX 5 RA-ABA- 10 RA-RTX 2 RA TCZ 41 SpA-anti-TNF 51 SpA-anti-TNF+MTX Two doses in 58% |
– | Reduced in RA-RTX Increased in SpA-anti-TNF Increased after two doses, except for RA-MTX and RA- RTX |
One pneumonia 8.2% of patients reported that vaccination influenced their rheumatic disease |
Reduced on RTX and ABA (only five pts) | – | 2b | 4 |
| Ribeiro82 Non-adj. Subanalysis of58 |
2013 | Cohort | 11 RA-ABA 33 RA-MTX DC 55 HC |
– | Reduced in RA-ABA | No difference AE. | Reduced on ABA | – | 2b | 4 |
| Adler59 AS03 adjuvanted |
2012 | Cohort | 47 RA 59 SpA 15 vasculitis 28 CTD 40 HC |
– | Reduced in patients (but not in SpA and CTD) |
No difference in AE. Increase disease activity in 32 patients | Reduced on ABA, RTX (n=8) and MTX 2 responders in RTX group: 1 and 3 mo after RTX |
– | 2b | 4 |
| França60 Non-adj. |
2012 | Cohort | 41 RA-anti-TNF 79 SpA-anti-TNF 41 RA-DC 75 SpA-DC 117 HC |
– | Reduced in SpA-anti-TNF but not for etanercept | More mild systemic AE in patients on anti- TNF |
Reduced on MTX (RA). Reduced on anti-TNF (SpA) (except etanercept) |
– | 2b | 4 |
| Iwamoto61 Mostly non-adj. |
2012 | Cohort | 89 RA 14 HC |
– | Reduced (non-significant) in RA Seroprotection 55.1% |
1 facial palsy | Lower (non-significant) on biologics | – | 2b | 5 |
| Saad54 Non-adj. |
2011 | Cohort | 1668 AIIRD* 234 HC |
– | Reduced in AIIRD versus HC Reduced in SLE and RA |
Overall stable disease | No | – | 2b | 4 |
| Gabay55 Adj. (AS03) |
2011 | Cohort | 82 RA 45 SpA 46 other AIIRD 138 HC |
– | Reduced in patients No difference after two doses in patients (seroprotection after 1 and 2 doses 75% and 85%, respectively) |
Overall stable disease | Reduced on DMARDs and within 3 mo. after B cell depletion | – | 2b | 4 |
| Miraglia56 Non-adj. |
2011 | Cohort | 1152 Immunocompromised†: 260 RA 83 JIA |
– | Seroprotection in 61.5% of patients with RA and in 85.5% of patients with JIA |
Mild systemic AE in more than 20% of RA and JIA |
– | – | 2b | 4 |
| Elkayam57 Adj. (MF59) |
2011 | Cohort | 41 RA 21 SLE 17 PsA 15 AS 25 HC |
– | Reduced in patients with RA/PsA Seroprotection in 60%–76% of patients |
Overall stable disease | Reduced on leflunomide and infliximab | – | 2b | 4 |
| Ribeiro58 Non-adj. |
2011 | Cohort | 340 RA 234 HC |
– | Reduced in RA No influence of disease activity |
More local AE in HC. More mild systemic AE in RA | Reduced on MTX | – | 2b | 2b |
| Müller206 Adj. (AS03) |
2013 | Case series | 16 RA+SjS | – | SC in B cell depleted: 22%, non-depleted: 57% | More influenza-like symptoms in B cell depleted patients | Low response with RTX | – | 4 | 5 |
| Borba64 Non-adj. |
2012 | Cohort | 555 SLE 170 HC |
– | Reduced in SLE with therapy (except for antimalarials) No difference in HC and SLE without therapy |
Overall stable disease | Reduced for steroids and IS Restored when using concomitant antimalarials |
– | 2b | 2b |
| Kostianovsky52 Both seasonal and pandemic, non-adj. |
2012 | Cohort | 74 systemic vasculitis 32 SSc 29 SLE 23 SjS 28 other AIIRD |
– | No difference | 19 flares | No | – | 4 | 4 |
| Lu63 Non-adj. |
2011 | Cohort | 21 SLE 15 HC |
– | No difference | Changes in autoantibody levels Overall stable clinical disease activity one flare |
No | – | 2b | 4 |
| Urowitz207 Both adj. and non-adj. |
2011 | Cohort | 103 SLE: 51 adj. 52 non-adj. |
– | – | No difference Overall stable disease |
– | – | – | 2b |
| Mathian69 Non-adj. |
2011 | Cohort | 111 SLE | – | Increased after booster vaccination (seroprotection after 1 and 2 doses 67% and 80%, respectively) | No severe AE Overall stable disease |
Reduced on IS | – | 2b | 4 |
| Brauner67 | 2017 | Cohort | 14 SjS 18 HC |
Higher levels of influenza-specific IgG in patients, and higher avidity | Antibody titres to non-influenza (incl autoantigens Ro/SSA and La/SSB) antigen increased in patients, but not in HC. | – | – | 4 | 4 | |
| Sampaio-Barros68 | 2017 | Cohort | 92 SSc 92 HC |
– | Higher GMT SSc Comparable SP and SC |
No difference in AE No SAE |
No | – | 2b | 4 |
| De Medeiros208 Non-adj. |
2014 | Cohort | 45 PAPS 33 HC |
– | – | No change in overall frequencies of autoantibodies | – | – | – | 2b |
| Miossi66 Non-adj. |
2013 | Cohort | 69 MCTD 69 HC |
– | No difference | Overall stable disease | No | – | 2b | 4 |
| Shinjo65 Non-adj. |
2012 | Cohort | 37 DM +21 PM 116 HC |
– | No difference | No difference Overall stable disease |
No | – | 2b | 4 |
The table is structured as follows: First studies in RA, then systemic lupus erythematosus (SLE) followed by other autoimmune inflammatory rheumatic diseases (AIIRD). Within this organisation, presented in order of publication year.
*Group consisted of patients with SLE (n=572), RA (n=343), psoriatic arthritis (n=101), ankylosing spondylitis (n=152), Behçet’s disease (n=85), dermatomyositis (n=45), systemic sclerosis (n=127), mixed connective tissue disease (n=69), primary antiphospholipid syndrome (n=54), primary Sjögren’s syndrome (n=36), Takayasu’s arteritis (n=30), polymyositis (n=28), granulomatosis with polyangiitis (n=26).
†Group consisted of patients with cancer (n=319), RA (n=260), HIV infection (n=256), kidney transplant recipients (n=85), juvenile idiopathic arthritis (n=83) and elderly persons (n=149).
ABA, abatacept; adj., adjuvanted; AS, ankylosing spondylitis; AS, adjuvant system; DC, disease control; DM, dermatomyositis; DMARD, disease-modifying antirheumatic drug; eff., efficacy; GMT, geometrical mean titre; HC, healthy controls; imm, immunogenicity; IS, immunosuppressives; JIA, juvenile idiopathic arthritis; LoE, level of evidence; (M)CTD, (mixed) connective tissue disease; mo., months; MTX, methotrexate; No., number; PAPS, primary antiphospholipid syndrome; PM, polymyositis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; ref., reference; RTX, rituximab; (S)AE, (serious) adverse event(s); saf., safety; SC, seroconversion; SjS, Sjögren’s syndrome; SP, seroprotection; SpA, spondyloarthropathy; SSc, systemic sclerosis; TCZ, tocilizumab; TNF, tumor necrosis factor.
Five studies addressed the efficacy of influenza vaccination. Retrospective database analysis studies reported a reduced all-cause mortality rate and risk of hospitalisation for influenza-related complications in patients with RA34 and SLE35 who received trivalent seasonal subunit influenza vaccine. A prospective Japanese study following a total of 17 735 patients with RA during four influenza seasons, found that trivalent subunit influenza vaccination was associated with a lower self-reported rate of influenza infections (RR 0.83, 95% CI 0.71 to 0.95).36 These findings are supported by two prospective cohort studies.6 37
Most studies on influenza vaccination in patients with AIIRD, however, address immunogenicity, mainly by assessing the development of a protective level of antibodies (titre value ≥40, as measured by the haemagglutination inhibition assay). For RA, most of these studies report similar responses in patients and HCs.7 9–15 20 38–41 A meta-analysis including a total of 886 patients with RA and 685 controls concluded that 60%, 68% and 61% of patients with RA reached seroprotective antibody levels following influenza vaccination for the H1N1, H3N2 and B strain, respectively. Only for the H1N1 influenza strain, the strain for which most data were available, responses were significantly lower in patients than in HCs.42
For SLE most studies report similar, adequate immune responses using trivalent seasonal subunit influenza vaccine,17 22–25 43 44 although modestly lower responses compared with HCs were also reported.21 26–29 Two meta-analyses reported an adequate but lower response against influenza A strains (H1N1 and H3N2) but not against influenza B in patients with SLE as compared with HCs,45 46 while another meta-analysis reported a reduced immunogenicity in SLE for H1N1 and B strains, but not for H3N2.47 Reported pooled seroprotection rates in patients with SLE are 66%–68%, 64%–76% and 60%–66% against H1N1, H3N2 and B strains, respectively.46 47
Likewise, in other AIIRD, including patients with spondyloarthropathies, antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis and primary systemic sclerosis (pSS), adequate serological responses to influenza vaccination were found.30–32 48–53
Regarding the pandemic monovalent subunit influenza vaccine, most larger studies report reduced immunogenicity in patients with AIIRD (mostly RA and SLE), although protective antibody levels were reached in the majority of patients.41 52 54–68 A second booster dose of vaccine, given 3–4 weeks after the first, improved immunogenicity, resulting in seroprotection levels comparable to those of HCs.55 62 69 This phenomenon has also been shown in patients with SLE who received seasonal influenza vaccine for the first time.70 High disease activity levels did not preclude reaching seroprotection in a study that included 340 patients with RA, of which 14.5% had a DAS 28 (Disease Activity Score in 28 joints) value above 5.1.58
Influenza vaccination did not influence activity of the underlying AIIRD in patients with RA,7 8 12 14 15 38 39 42 54 55 57 71–73 SLE,6 19 21 26 28 37 43 45–47 ANCA-associated vasculitis30 31 48 or systemic sclerosis.32 49 Adverse events of influenza vaccination in patients with AIIRD were comparable to those in HCs in most studies,7 19 21 23 30 59 65 including a meta-analysis in patients with SLE,46 In contrast, a meta-analysis including 13 studies in patients with RA concluded that local, mild adverse events occurred significantly more frequently in patients with RA.42
Influence of immunomodulating agents
The influence of immunomodulating agents on influenza vaccine efficacy and immunogenicity is summarised in table 6. No influence of methotrexate (MTX) on influenza immunogenicity was found in most studies39 40 43 62 72 74 including one meta-analysis in patients with RA.75 In some, a modest reduction in immunogenicity was observed.58–60 73 76 In another meta-analysis, results on the influence of MTX differed depending on whether response rates per influenza strain, or for at least two of the three strains, were analysed. In case of the latter approach, the negative impact of MTX was significant.77 Interestingly, temporary discontinuation of MTX was shown to significantly improve immunogenicity of seasonal influenza vaccination in patients with RA in two studies by Park et al.78 79 Discontinuation of MTX for 2 weeks after influenza vaccination led to a 11%–16% (depending on influenza strain) higher seroprotection rate compared with patients with RA who continued the use of MTX. Flare rates tended to be higher in patients with RA who temporarily halted MTX use, but the increase in disease activity was transient.78 79
Table 6.
Influence of disease-modifying antirheumatic drugs on influenza and pneumococcal vaccine efficacy and immunogenicity
| Efficacy | Immunogenicity | LoE Immunogenicity | ||
| Influenza | Pneumococcal | |||
| MTX | No data | Adequate for influenza/reduced for pneumococcal | 2a | 2b |
| Other cs-DMARD | No data | Only for HCQ Adequate |
4 | 4 |
| Anti-TNFα | No data | Adequate | 2a | 2b |
| B cell depletion | No data | Reduced | 2a | 2b |
| Belimumab | No data | Pneumococcal: preserved | – | 2b |
| Tocilizumab | No data | Preserved | 2b | 2b |
| Abatacept | No data | Controversial Probably mildly reduced |
4 | 4 |
| Tofacitinib | No data | Adequate for influenza, reduced for pneumococcal | 2b | 2b |
| Glucocorticoids (±other IS) | No data | Adequate for influenza, mildly reduced in high doses GC for pneumococcal | 4 | 2b |
cs-DMARD, conventional synthetic disease-modifying antirheumatic drugs; GC, glucocorticoids; HCQ, hydroxychloroquine;IS, immunosuppressives; LoE, level of evidence; MTX, methotrexate; TNF, tumour necrosis factor.
Hydroxychloroquine does not influence the development of an adequate immune response to influenza vaccination.47 64 66 The same holds true for the use of TNFα-blocking agents in the majority of studies,11 13 20 41 80 including two meta-analyses in RA.75 77 Another meta-analysis reported a lower seroprotection, but not seroconversion rate in patients with RA on anti-TNF α, only for the H1N1 influenza strain.42 Four studies did report a modestly reduced response to influenza vaccination in patients using anti-TNFα.10 16 40 60
B cell depleting therapy has been associated with hampered antibody responses following influenza vaccination in multiple studies. A negative influence of B cell depleting therapy was observed in two meta-analyses that pooled data from cohort studies. Patient numbers in analyses were low however, and CIs were wide.42 77 The interval between administration of rituximab and vaccination differed between studies. A study that included both patients with RA vaccinated 4–8 weeks (n=11) and 6–10 months after (n=12) the administration of rituximab demonstrated no response to influenza vaccination in the first, early group and a modestly restored response in the late group.14 The use of rituximab did not seem to affect cell-mediated immune responses to influenza vaccination in a study with a limited number of patients.38
Two studies demonstrated that patients with RA treated with tocilizumab, an IL-6 receptor blocking agent, were able to mount a satisfactory antibody response following influenza vaccination.73 81
Controversial data have been published on the effect of abatacept on influenza vaccine immunogenicity. Most of the studies were small in patient numbers, but they reported a substantial negative effect.59 62 82 One relatively large but uncontrolled study, including 184 patients with RA, reported an adequate humoral response to influenza vaccination.83
Only one study investigated the influence of tofacitinib on influenza vaccine immunogenicity in AIIRD. Tocafitinib alone did not seem to affect the immune response to the vaccine, but a combination of tofacitinib and methotrexate was associated with a lower response.84
The effect of glucocorticoids on the immune response to influenza vaccine has mainly been studied in combination with other immunosuppressive agents. The antibody response is generally adequate in patients who were on glucocorticoids at the time of influenza vaccination,21–23 42 47 although some studies did find a mildly reduced response.17 43 44 64 69
Summary and clinical implications
Seasonal trivalent influenza vaccination is associated with a reduced incidence of bacterial complications, hospital admissions and mortality in patients with RA and SLE. It has also been proven to be immunogenic in the majority of studies in patients with AIIRD, even when treated with immunosuppressive agents, with the exclusion of B cell depletion. Although studies that are sufficiently powered with regard to safety are lacking, in the majority of studies disease activity remained stable and only mild adverse events were reported, comparable with HCs. Therefore, the updated EULAR recommendation on influenza states that influenza vaccination should be strongly considered for the majority of patients with AIIRD.3
Pneumococcal vaccination
Efficacy—immunogenicity—safety
To date, 91 pneumococcal serotypes have been identified, 30 of them being responsible for up to 90% of all infections.85 Although the pneumococcal polysaccharide vaccine that includes 23 serotypes (PPSV23) was found to prevent invasive pneumococcal infections in the general population, it did not generate immunity in children younger than 2 years of age and had a limited efficacy in reducing non-bacteraemic pneumonia.86 Therefore, in 2000 a pneumococcal conjugate vaccine comprising seven antigens (PCV7) was developed and expanded to 13 serotypes (PCV13) which was licensed in 2010 based on immunogenicity outcome studies.87 88 In 2015, a randomised controlled study performed in the older healthy population demonstrated the capacity of PCV13 to prevent vaccine-type pneumococcal, bacteraemic and non-bacteraemic community-acquired pneumonia as well as vaccine-type invasive disease.89
Up to the previous recommendations, 15 studies addressed the issue of immunogenicity and safety of PCV13 and PPSV23 in patients with AIIRD: 7 studies in RA,8 90–95 8 in SLE,95–102 2 in patients with spondyloarthropathy (SpA)91 103 and 1 in pSS.104 Adequate as well as reduced immunogenic responses compared with controls were reported in these studies. Treatment with rituximab, TNFα blockers and MTX seemed to impair the humoral response to the pneumococcal vaccine.16 90 92 93
From the previous recommendations and up to August 2018, 34 studies53 76 81 83 84 105–133 and two meta-analyses45 77 have been published on the efficacy, immunogenicity and safety of PPSV23 and the conjugated vaccines PCV7 and PCV13, including evaluation of a combined strategy (tables 7 and 8).106 128 133
Table 7.
Efficacy, immunogenicity and safety of 23-valent pneumococcal polysaccharide vaccine (PPSV23) in patients with AIIRD (October 2009–August 2018)
| First author +ref. | Year | Study design | No. cases | No. ST |
Efficacy | Immunogenicity | Safety | LoE | ||
| Eff. | Imm. | Saf. | ||||||||
| Izumi120 | 2017 | RCT | 464 RA–vaccinated 436 RA–placebo |
NA | Similar efficacy in vaccinated versus placebo | – | No safety issue | 1b-2b | – | – |
| Kivitz76 | 2014 | RCT | 110 RA-Certolizumab (68%+MTX) 114 RA-Placebo (68%+MTX) |
6 | – | No difference between certolizumab and placebo | – | – | 1b-2b | – |
| Hesselstrand131 | 2018 | Cohort | 44 SSc:
49 HC |
2 | – | Lower response in patients treated with DMARDs | No safety issue | – | 2b | 4 |
| Jaeger53 | 2017 | Cohort | 16 patients with CAPS | NA | – | – | Significant side effects | – | 4 | – |
| Chatham125 | 2017 | Cohort | 34 SLE PPSV23 4 weeks before, and 45 SLE 24 weeks after belimumab |
23 | – | Adequate response, not affected by belimumab | No safety issue | – | 2b | 4 |
| Broyde130 | 2016 | Cohort (retrospective) | 88 RA and SpA vaccinated 42 RA and SpA non-vaccinated |
NA | – | Preserved immunogenicity after 7 years | – | – | 2b | – |
| Winthrop84 Part A |
2016 | Cohort | 102 RA-Tofacitinib 98 RA-Placebo |
12 | – | Reduced response in tofacitinib-treated patients | – | – | 2b | – |
| Winthrop84 Part B |
2016 | Cohort | 92 RA-Cont Tofacitinib 91 RA-Stop Tofacitinib |
12 | – | No difference between groups | – | – | 2b | – |
| Alten83 | 2016 | Cohort | 125 RA ABA+MTX | 5 | – | Adequate response | No safety issue | 2b | 4 | |
| Rezende116 | 2016 | Cohort | 54 SLE | 7 | – | Poor immunogenicity | – | 2b | – | |
| Migita109 | 2015 | Cohort | 35 RA-DMARDs 55 RA-MTX 21 RA-ABA +MTX |
2 | – | Reduced response in abatacept-treated patients | No safety issue | 2b | 4 | |
| Migita124 | 2015 | Cohort | 35 RA-DMARDs 55 RA-MTX 24 RA-Golimumab +MTX |
2 | – | Reduced response in golimumab-treated patients | No safety issue | 2b | 4 | |
| Migita119 | 2015 | Cohort | 35 RA-DMARDs 55 RA-MTX 29 RA-Tacrolimus 14 RA-Tacrolimus +MTX |
2 | – | Higher response in tacrolimus-treated patients | No safety issue | 2b | 4 | |
| Bingham112 | 2015 | Cohort | 27 RA-MTX 54 RA-MTX +TCZ |
12 | – | Similar response in patients treated with MTX or MTX +TCZ | No safety issue | 2b | 4 | |
| Fischer115 | 2015 | Cohort | 57 vaccinated/122 non-vaccinated RA, SpA, vasc., CTD | NR | – | Adequate response | – | 4 | – | |
| Tsuru81 | 2014 | Cohort | 21 RA-TCZ | 12 | – | All TCZ-treated patients responded | – | 2b | – | |
| Mori113 | 2013 | Cohort | 62 RA-MTX 54 RA-MTX+TCZ 50 RA-TCZ 24 RA-DMARDs |
2 | – | Better response in patients treated with TCZ | No safety issue | 2b | 4 | |
| Coulson121 | 2011 | Cohort (retrospective) | 124 RA vaccinated 28 RA non-vaccinated |
NA | Reduced rate of pneumonia in vaccinated | Preserved immunogenicity after 7 years | – | 4 | 2b | |
| Rehnberg107 | 2010 | Cohort | 11 RA-RTX 36 weeks 8 RA-Pre-RTX 1 week 10 RA-DC |
NR | – | Reduced in patients treated with RTX | – | 4 | – | |
ABA, abatacept; CAPS, cryopyrin-associated periodic syndrome; cont., continued; CTD, connective tissue disease; DC, disease control; DMARD, disease-modifying antirheumatic drug; eff., efficacy; HC, healthy controls; imm., immunogenicity; LoE, level of evidence; MTX, methotrexate; No., number; NR, not reported; PCV, pneumococcal conjugate vaccine; PPSV, pneumococcal polysaccharide vaccine; RA, rheumatoid arthritis; RCT, randomised controlled trial; Ref., reference; RTX, rituximab; saf., safety; SLE, systemic lupus erythematosus; SpA, spondyloarthropathy; SSc, systemic sclerosis; ST, serotypes; TCZ, tocilizumab; vasc., vasculitis.
Table 8.
Immunogenicity and safety of 7-valent and 13-valent pneumococcal conjugate vaccine (PCV7 and PCV13), including prime boosting with 23-valent pneumococcal polysaccharide vaccine (PPSV23), in patients with AIIRD (October 2009–August 2018)
| First author +ref. | Year | Study design | No. cases | Strategy | No. ST | Immunogenicity | Safety | LoE | |
| Imm. | Saf. | ||||||||
| PCV7 | |||||||||
| Grabar128 | 2017 | RCT | 46 SLE: 27 placebo +PPSV23 19 PCV7 +PPSV23 |
NA | 7 | Adequate immunogenicity No differences between groups |
No safety issue | 1b | 4 |
| David Morgan105 | 2016 | Cohort | 92 AAV | NA | 7 | Preserved immunogenicity in patients on remission | – | 2b | – |
| Nagel123 | 2015 | Cohort | 248 RA 249 SpA |
NA | 2 | Good correlation between levels of immunogenicity and incidence of pneumonia | – | 2b | – |
| Kapetanovic108 | 2013 | Cohort | 173 RA (TCZ, RTX, ABA, MTX) 86 SpA controls |
NA | 2 | Reduced response in patients treated with ABA and RTX | No safety issue | 2b | 4 |
| Kapetanovic127 | 2013 | Cohort | 163 RA 139 SpA |
NA | 2 | Reduced immunogenicity after 1.5 y | – | 2b | – |
| Kapetanovic110 | 2011 | Cohort | 253 RA 252 SpA (MTX, anti-TNF) |
NA | 2 | Reduced response in pts treated with MTX | No safety issue | 2b | 4 |
| Kapetanovic111 | 2011 | Cohort | 201 RA (PCV7) 201 RA (PPSV23) |
NA | 2 | Similar immunogenicity for PCV7 and PPSV23 | No safety issue | 2b | 4 |
| PCV13, including prime boosting with PPSV23 | |||||||||
| Nguyen106 | 2017 | RCT | 98 RA 63 bDMARD 35 csDMARD-DC |
PCV 13+PPSV23 PCV13 +PPSV23 PCV13 +PCV13+PPSV23 |
12 | Adequate and similar response in the three arms | No safety issue | 2b | 4 |
| Bahuaud133 | 2018 | Cohort | 23 RA | PCV13 +PPSV23 | 10 | Adequate short-term response Functional antibodies decreased after 2 years |
– | 2b | – |
| Kapetanovic118 | 2017 | Cohort | 10 RA-MTX 10 RA-DC |
PCV13 | 2 | Reduced response in MTX-treated patients | – | 4 | – |
| Nived117 | 2017 | Cohort | 49 vasculitis 49 HC |
PCV13 | 2 | Adequate response, similar in both groups | No safety issue | 2b | 4 |
| Nagel114 | 2017 | Cohort | 47 SLE 21 HC |
PCV13 | 12 | Decreased response in IS-treated patients with SLE, preserved under HCQ and belimumab | No safety issue | 2b | 4 |
| Rakoczi129 | 2016 | Cohort | 22 RA 24 OA |
PCV13 | NR | Adequate immunogenicity, but lower in RA | No safety issue | 2b | 4 |
| Groh132 | 2017 | Case series | 19 AAV
|
PCV13/PCV 7±PPSV23 | 7 | Decreased response on induction, preserved on maintenance therapy | – | 4 | – |
AAV, ANCA-associated vasculitis; ABA, abatacept; ANCA, antineutrophil cytoplasmic antibodies; bDMARD, biological disease-modifying antirheumatic drug; (cs)DMARD, (conventional synthetic) DMARD; DC, disease control; HC, healthy controls; HCQ, hydroxychloroquine; imm., immunogenicity; IS, immunosuppressives; LoE, level of evidence; MTX, methotrexate; No., number; NR, not reported; OA, osteoarthritis; pts, patients; RA, rheumatoid arthritis; RCT, randomised controlled trial; ref., reference; RTX, rituximab; saf., safety; SLE, systemic lupus erythematosus; SpA, spondyloarthropathy; ST, serotypes; TCZ, tocilizumab; TNF, tumor necrosis factor; y, years.
Regarding efficacy of pneumococcal vaccination in AIIRD, a randomised double-blind trial on the clinical efficacy of PPSV23 in preventing pneumonia in patients with RA did not demonstrate an increased efficacy of the vaccine over placebo, emphasising the need for a more efficacious vaccine.120 In contrast, a retrospective study on the long-term effect of PPSV23 in 180 patients with RA treated with MTX showed a relative risk of 9.7 to develop pneumonia among non-vaccinated patients.121 Vaccination with PCV7 tended to reduce the risk of pneumococcal infections in patients with RA and SpA.122 In this cohort, a direct correlation was shown between the postvaccination levels of antipneumococcal antibodies and the risk of pneumococcal infections: more robust antibody responses after vaccination with PCV7 were associated with lower risk of serious pneumococcal infections.123 The humoral immunogenicity and safety of PPSV23 were demonstrated in RA,76 81 109 112 113 119 124 SLE,116 125 134 and, to a limited extent, in SpA and other rheumatic diseases.115 The long-term immunogenicity of PPSV23 was evaluated in two studies in patients with RA, treated with MTX121 and biologics.130 Both have shown a long-term duration of protective antibodies, up to 7 years.
Humoral immunogenicity of PCV7 is similar to that of PPSV23,111 but was shown to decrease after 1.5 years.127 A randomised controlled study in patients with SLE aiming at evaluating the immunogenicity of the combination of PCV7 and PPSV23 in comparison with PPSV23, showed an adequate and similar response in the two groups.128 The immunogenicity of PCV7 is preserved in patients with ANCA-associated vasculitis on remission.105
The immunogenicity of PCV13 has been evaluated in small groups of patients with RA,118 129 SLE114 and pSS.131 It induced an adequate humoral response.
Three studies evaluated the prime-boost strategy. In SLE, the combination of PCV7 and PPSV23 was not more immunogenic than PPSV23 alone.128 Another randomised controlled study evaluated the serological response to PCV13 followed by PPSV23 after 16–24 weeks in patients with RA, with one of the arms including two doses of PCV13. This study demonstrated an adequate response in patients with RA (87% and 94% on biological disease-modifying antirheumatic drugs (DMARDs) and conventional synthetic DMARDs, respectively), without additional effect of two PCV13 injections.106 An additional study has questioned the long-term effect of the prime boosting strategy using PCV13 and PPSV23, showing reduced levels of functional antibodies 2 years after vaccination.133
No safety issues following pneumococcal vaccination in most of the AIIRDs were reported, independent of vaccine type (see tables 7 and 8). In contrast, data from the b-CONFIDENT Study in patients with cryopyrin associated periodic syndrome (CAPS) and from a case series of seven patients with CAPS showed that PPSV23 might induce severe local reactions and systemic reactions in these patients (fever, headache, meningismus, nausea), necessitating hospitalisation.126 All symptoms resolved within a period of 3–17 days.
Influence of immunomodulating agents
Humoral immunogenicity of PPSV23 has been shown to be reduced by MTX,119 abatacept,109 golimumab,124 tofacitinib84 and rituximab,90 but not to be affected by certolizumab76 and belimumab.125 Immunogenicity following PCV7 vaccination is reduced by the use of MTX,110 abatacept and rituximab,108 but not by TNFα blockers.110 Additionally, the humoral response of PCV13 is reduced under MTX.118 A randomised controlled study in patients with RA that evaluated the serological response to PCV13 followed by PPSV23 after 16–24 weeks, showed a significantly decreased response in patients treated with rituximab. The prime-boost strategy with PCV13 did not improve the response106 (see table 6 for summary).
Summary and clinical implications
Stepwise pneumococcal vaccination, according to the prime-boost strategy (PCV13 followed by PPSV23, with an interval of at least 8 weeks between the two vaccinations) is currently recommended by the Centers for Disease Control and Prevention (CDC) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) for young children, adults above 65 years of age and patients at risk for pneumococcal disease.135 This is mainly based on expert opinion, although studies conducted in the general population136 137 and in patients with HIV138 did show an augmented immunogenic response following combined vaccination.
Pneumococcal vaccination should be strongly considered for the majority of patients with AIIRD for the following considerations: (1) The increased risk of non-invasive and invasive pneumococcal disease in patients with AIIRD.1 (2) Good efficacy, immunogenicity and a favourable safety profile of pneumococcal vaccines (with the exception of patients with CAPS). (3) In line with the present recommendations of the CDC87 139 and the ESCMID.135 Given the insufficient evidence for the efficacy of the combination of PCV13 and PPSC23, the choice and sequence of pneumococcal vaccination should be in concordance with local guidelines.
Hepatitis A vaccination
Efficacy—immunogenicity—safety
Hepatitis A virus (HAV) vaccine, an inactivated vaccine, is very efficacious in preventing hepatitis A.140 141 There are however no studies on the efficacy of HAV vaccination in patients with AIIRD. All three studies on immunogenicity HAV vaccination in patients with AIIRD have been published after the 2011 version EULAR recommendations2 and SLR142 (table 9).
Table 9.
Efficacy, immunogenicity and safety of hepatitis A vaccination in patients with AIIRD
| First author +ref. | Year | Study design | No. cases | Efficacy | Immunogenicity | Safety | Influence of IS on eff./imm. | LoE | ||
| Eff. | Imm. | Saf. | ||||||||
| Rosdahl146 | 2018 | Cohort | 69 RA 58 HC |
– | Reduced in RA | No SAE Mild systemic AE in 17% (HC and RA combined) |
– | – | 2b | 4 |
| Askling145 | 2014 | Cohort | 53 RA: 15 anti-TNF 21 anti-TNF +MTX 17 MTX |
– | Seroprotection in 10% after 1 month, 83% at month 7 (1 month after second dose) | One meningoencephalitis 2.5 weeks after second dose Mild AE in 2 |
Possibly reduced on MTX | – | 2b | 4 |
| Van den Bijllaardt147 | 2013 | Cohort (retrospective) |
173 IS-treated: 31 anti-TNF 123 DMARD 19 Other |
– | – | Reduced imm. on anti-TNF in multivariate regression analysis | – | 2b | – | |
AIIRD, autoimmune inflammatory rheumatic disease; DMARD, disease-modifying antirheumatic drug; eff., efficacy; HC, healthy control; imm., immunogenicity; IS, immunosuppressives; LoE, Level of evidence; MTX, methotrexate; No., number; RA, rheumatoid arthritis; ref., reference; (S)AE, (serious) adverse event; Saf., safety; TNF, tumor necrosis factor.
In healthy persons, the HAV vaccine is highly immunogenic, resulting in seroprotection in ≥95% only 1 month after the first vaccine dose.143 144 In patients with RA, it has been shown to be less immunogenic. The percentage of seroprotected patients with RA after 1 month varied between 10%145 and 60%–68%146 in two studies that used different methods. A three-dose schedule (0, 1 and 6 months or 0 (double dose) and 6 months) resulted in 99% seroconversion in patients with RA after 12 months.146 A double dose of vaccine at baseline did not result in an improved seroconversion rate after 1 month, compared with the usual dose (68% vs 60%).146
In terms of safety, there are no data on the influence of vaccination on activity of the underlying AIIRD. Adverse events were generally mild, and reported in up to 17% of patients.145 146 Askling et al reported one case of meningoencephalitis which occurred in a patient with an RA 2.5 weeks after the second dose of HAV vaccine.145
Influence of immunomodulating agents
Using a cut-off for seroprotection of anti-HAV ≥10 mIU/mL instead of 20 mIU/ml, significantly more patients with RA using only an anti-TNFα agent (73%, n=15) reached seroprotection than those using a combination of anti-TNF and MTX (15%, n=21) or MTX alone (6%, n=17).145 In a study of 173 immunosuppressive-treated patients (31 anti-TNF, 123 classic DMARD and 19 other), the use of anti-TNF was associated with lower seroprotection rates in a multivariate logistic regression analysis (see table 9).147
Summary and clinical implications
Since a single dose of HAV vaccine does not seem to afford sufficient protection in a substantial percentage of patients with AIIRD, it is recommended to administer a second dose of vaccine 6 months after the first and to determine postvaccination antibody titres. If this is not possible, as in the case of a last-minute traveller, it should be borne in mind that a patient with AIIRD may not be protected after a single dose of HAV vaccine. Passive immunisation for the specific journey may be considered.
Hepatitis B vaccination
Efficacy—immunogenicity—safety
The incidence of hepatitis B virus (HBV) infections has markedly decreased in countries where HBV vaccination is routinely implemented.148 Although no antibody level gives complete protection against transient infection, there is a clear association between antibody level and risk of HBV infection.149 In general, a level of antihepatitis B surface antigen ≥10 mIU/ml is considered protective.
Up to the previous version of recommendations, a total of four studies reported on the immunogenicity of HBV vaccination in patients with RA,150 SLE,151 AS152 and Behçet’s disease.153 One additional study in patients with RA had been published since then (online supplementary table S1).154
rmdopen-2019-001035supp001.pdf (116.3KB, pdf)
This recent study, including 46 patients with RA and 9 HCs, reported a significantly lower percentage of patients versus HCs reaching seroprotective antibody levels (64% in patients vs 100% in HCs).154 Another controlled study from 2005, with 13 patients with Behçet’s disease and 15 HCs reported no difference in immunogenicity of the HBV vaccine.153 A response to the vaccine was demonstrated in all remaining studies on HBV vaccination in patients with AIIRD that did not include a control group150–152 (online supplementary table S1).
The HBV vaccine did not lead to changes in overall disease activity in patients with RA and Behçet’s disease.150 153 154
Influence of immunomodulating agents
A severely hampered antibody response to HBV vaccination was noted in patients with AS treated with TNF-blocking agents.152
Summary and clinical implications
HBV vaccine should be administered to patients with AIIRD at risk of infection, for example, medical personnel, patients having an infected family member, intravenous drug users, men who have sex with men, and patients travelling to or residents from endemic countries. It is advised to determine vaccination response. For non-responders several strategies are available to try to reach seroprotection. A booster vaccination or passive immunisation should be considered for an unvaccinated patient or a patient with insufficient response exposed to HBV. See recommendations of the CDC via https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hepb.pdf.
Tetanus toxoid vaccination
Efficacy—immunogenicity—safety
The efficacy of tetanus toxoid vaccination in the prevention of tetanus has never been studied in a vaccine trial. The incidence of tetanus has been shown to decrease dramatically in vaccinated populations,155 156 although this was not specified for the AIIRD population. The protective antibody level for tetanus is generally considered to be ≥0.1 IU/mL. Tetanus is extremely rare in fully immunised adults who received their last dose of vaccine within the preceding 10 years.
Reports on immunogenicity of tetanus toxoid vaccination in patients with RA showed satisfactory antibody responses.9 112 157 Most studies in patients with SLE reported adequate response rates.102 157–159 One small study from 1980 including nine patients with SLE and nine HCs showed a diminished response in the patients with SLE, with a blunted response in three of them.160
Most studies did not report on safety of tetanus toxoid vaccination. One randomised controlled trial (RCT) showed a higher incidence of mild/moderate adverse events after combined tetanus toxoid and pneumococcal vaccination in patients with RA on MTX who recently started the use of tocilizumab, compared with patients with RA on MTX only (online supplementary table S2).112
Influence of immunomodulating agents
Rituximab administered 24 weeks before vaccination did not affect response to the tetanus toxoid vaccine in patients with RA.90 An RCT in 54 patients with RA on MTX who started tociluzimab 3 weeks before tetanus toxoid vaccination, and 27 RA MTX disease controls, showed no difference in immunogenicity of the tetanus toxoid vaccine between groups. However, there were only three patients in the tocilizumab + MTX group who did not have a seroprotective antibody level at baseline. Two out of these three patients reached a protective level 5 weeks after vaccination.112
An observational study on immunogenicity of pneumococcal, tetanus toxoid and H. influenzae type B vaccine in 73 patients with SLE reported a trend towards a lower response in patients on glucocorticoids and azathioprine, which was not specified for tetanus toxoid vaccination.102
Summary and clinical implications
As satisfactory immune responses were observed in patients with AIIRD following tetanus toxoid vaccination, mostly similar to the response in HCs, and no serious adverse events have been reported, the updated EULAR recommendations conclude that patients with AIIRD should receive tetanus toxoid vaccination according to national recommendations for the general population. Since no data are available on efficacy or immunogenicity of tetanus toxoid vaccination in patients who received B cell depleting therapy within the preceding 6 months, passive immunisation with tetanus immunoglobulins should be considered in these patients in case of an event with high risk of acquiring tetanus, when the vaccine would otherwise be indicated, according to expert opinion.
Herpes zoster vaccination
Efficacy—immunogenicity—safety
Up to the previous recommendations, no data were available on herpes zoster vaccination in patients with AIIRD. Since 2010 seven relevant studies have been published (table 10).
Table 10.
Efficacy, immunogenicity and safety of live-attenuated herpes zoster vaccination in patients with AIIRD
| First author +ref. | Year | Study design | No. cases | Efficacy | Immunogenicity | Safety | Influence IS on eff./imm. | LoE | ||
| Eff. | Imm. | Saf. | ||||||||
| Winthrop170 | 2017 | RCT | 55 RA MTX+TFC* 57 RA MTX+PCB* |
– | Similar CMI response. Trend towards higher humoral response MTX+TFC | 3 SAE in MTX-TFC group versus 0 in MTX+PCB: one cholangitis, one bronchitis and one disseminated primary varicella in seronegative patient. Mild AE no difference. |
See column immunogenicity. | – | 2b | 4 |
| Russell173 | 2015 | RCT | 206 GC vacc. (25% PMR) 100 GC PCB-vacc (31% PMR) Mostly no AIIRD. >10–20 mg: n=39 |
– | Higher postvacc. humoral response in vacc. | More injection-site AE and headache in vacc. Other systemic and serious AE: no difference. | No influence of limited daily GC dose | – | 2b | 4 |
| Koh169 | 2018 | Cohort | 41 RA 28 OA |
– | Lower CMI response in RA. Similar humoral response. | No SAE. Mild systemic AE in 11.6% of all participants. 6 RA flares during 12 weeks postvacc. Median Disease Activity Index unchanged. |
– | – | 2b | 4 |
| Zhang167 and Yun168 |
2012 2017 |
Cohort (retrospective database) | Total: 463 541 Vacc: 18 683 (4.0%) Vacc on biologics: 633 7780 vaccinated patients in analysis |
Lower incidence of HZ in vacc. patients. Rapid decline difference incidence rate vacc. and unvacc. 6 years postvacc: no longer significant. |
– | <42 of vacc: HZ incidence decreased, no cases of hospitalised meningitis or encephalitis, no HZ in patients using biologics | Lower HZ incidence in vacc. patients using biologics, DMARDs or GC alone | 2b | – | 4 |
| Guthridge171 | 2013 | Cohort | 10 SLE 10 HC |
– | Similar proportion of subjects with 50% increase in CMI measures postvacc. | No difference. No flares. |
– | – | 2b | 4 |
*Tofacitinib or placebo was started 2–3 weeks postvaccination.
†Patients with rheumatoid arthritis (n=292 169), psoriasis (n=89 565), psoriatic arthritis (n=11 030), ankylosing spondylitis (n=4026) and/or inflammatory bowel disease (n=66 751).
AIIRD, auto-immune inflammatory rheumatic disease; CMI, cell-mediated immunity; DMARD, disease-modifying antirheumatic drug; eff., efficacy; GC, glucocorticoids; HC, healthy controls; HZ, herpes zoster; imm., immunogenicity; IS, immunosuppressives; LoE, Level of evidence; MTX, methotrexate; No., number; OA, osteoarthritis; PCB, placebo; PMR, polymyalgiarheumatica; RA, rheumatoid arthritis; RCT, randomised controlled trial; ref., reference; (S)AE, serious adverse event(s); Saf., safety; SLE, systemic lupus erythematosus; TFC, tofacitinib; vacc., vaccinated.
Currently, two different vaccines are available for the prevention of herpes zoster in varicella-zoster virus (VZV)-seropositive healthy adults above the age of 50 years: one is a live-attenuated vaccine and the other is an adjuvanted subunit (non-live) vaccine. All studies on zoster vaccination in patients with AIIRD have been performed using the live-attenuated zoster vaccine. This vaccine has been shown to decrease the risk of herpes zoster in adults above the age of 50 years by 38%–70%,161 162 with lowest efficacy in those above 70 years.161 Of note, the AS01 adjuvanted subunit (non-live) vaccine has recently been shown to be safe and more efficacious than the live-attenuated vaccine in healthy adults above the age of 50 and 70 years.161 163 164 Vaccine efficacy ranged between 91% and 98% and did not significantly differ between age groups.163 164 Of note, safety of the adjuvant system AS01, which contributes to the generation of a particularly strong cellular immune response,165 166 has not yet been determined in patients with AIIRD.
Vaccination with the live-attenuated zoster vaccine was associated with a reduced incidence of herpes zoster in patients with immune-mediated diseases (RA, psoriatic arthritis, ankylosing spondylitis, psoriasis and inflammatory bowel diseases) over 60 years of age, as reported in a large retrospective database study by Zhang et al. In total, 7780 vaccinated and over 800 000 unvaccinated patients were included in their analysis.167 A rapid decline in vaccine efficacy was observed in the same study population. Six years after vaccination, the difference in herpes zoster incidence between groups of vaccinated and unvaccinated subjects was no longer significant.168
Immunogenicity of the live-attenuated zoster vaccine has been investigated in patients with RA169 170 and SLE.171 Both patient groups were able to mount cell-mediated immune responses to the vaccine,169–171 which is crucial for the protection against herpes zoster.172 However, live-attenuated zoster vaccination resulted in lower cell-mediated immunity in patients with RA (n=41) than in controls (n=28).169 Because no correlate of protection for herpes zoster infection is known, and methods for assessing herpes zoster immunity are not uniform, interpretation and comparison of studies is difficult.
Disease activity of the underlying AIIRD did not seem to be affected by live zoster vaccination, but numbers of analysed patients are low.169–171 In a study in patients with RA, who were randomised to receive tofacitinib (n=55) or placebo (n=57) 2–3 weeks following vaccination, three serious adverse events were observed in the tofacitinib group versus none in the placebo group. Of note, one case of disseminated primary varicella occurred in a patient who was VZV-seronegative at baseline.170 In the retrospective database study by Zhang et al, the zoster vaccine did not seem to induce infection within 42 days after vaccination. On the contrary: a reduced incidence of herpes zoster was seen in the vaccinated patients. No cases of hospitalised meningitis or encephalitis were identified in this period.167
Influence of immunomodulating agents
The favourable effect of vaccination on herpes zoster incidence in patients with autoimmune diseases is present regardless of medication use, including biologics (used by 633 vaccinated patients), as reported in the same large database study by Zhang et al.167 A daily dose of 5–20 mg of corticosteroids in a heterogeneous patient group (206 patients received zoster vaccination, of whom 25% were patients with polymyalgia rheumatica) did not seem to affect humoral immune response to live-attenuated zoster vaccination. Unfortunately, effect on cell-mediated immune response was not reported.173 Zoster vaccination resulted in a similar cell-mediated response in patients with RA who started the use of tofacitinib or placebo 2–3 weeks after vaccination (see table 10).170
Summary and clinical implications
Although large prospective trials that are sufficiently powered for assessing safety are lacking, the safety and efficacy profile of the live-attenuated zoster vaccine seem to be favourable for VZV-seropositive patients with AIIRD. However, the vaccine contains live-attenuated virus and, therefore, should still be considered with caution in the immunocompromised patient. Before administering the zoster vaccine, it is advisable to affirm the VZV-seropositive status of the patient. In case of a VZV-seronegative patient, a less potent VZV vaccine approved for preventing primary varicella in children may be considered. Based on expert opinion, the zoster vaccine is preferably administered 4 weeks prior to initiation and not during treatment with biologics and targeted synthetic DMARDs.
As noted earlier, the novel non-live AS01 adjuvanted subunit vaccine has been shown to be more efficacious than the live-attenuated vaccine in healthy adults above the age of 50–70 years. Whether this also holds true for the AIIRD population and whether the adjuvant system AS01 is safe in this patient group, is most interesting and warrants further investigation.
Yellow fever vaccination
Efficacy—immunogenicity—safety
The yellow fever vaccine is a live-attenuated vaccine. Several cases of visceral dissemination of yellow fever of the vaccine type have been reported, with clinical features similar to wild type yellow fever, including high mortality.174–176 The vaccine is therefore generally contraindicated in immunocompromised patients177–180 (online supplementary table S3).
Only limited observational studies have been published on yellow fever vaccination in patients with AIIRD, mostly concerning revaccination (see online supplementary table S3 for details). The reported immunogenicity results are mainly adequate, and similar as in HC.177–180
A study of 34 glucocorticoid-treated patients (among whom 9 were patients with RA and 14 with chronic inflammatory conditions, revaccination in 44%) and 68 HC, reported more moderate and severe local reactions in patients (12% vs 2%).179 No serious systemic adverse events of yellow fever vaccination were reported in the previously mentioned studies in patients with AIIRD. As the vaccine has been contraindicated in immunocompromised patients for years, numbers of studies and included patients are however very low and, as stated, most patients were revaccinated. Of note, lethal outcomes of fellow fever vaccination have been reported in immunocompromised patients, including a female patient with RA and SLE, who was possibly treated with glucocorticoids and MTX.181
Influence of immunomodulating agents
Due to the heterogeneous populations of the small studies on yellow fever vaccination in patients with AIIRD, it is difficult to discern the separate influences of different immunosuppressive agents. In a study of corticosteroid-treated patients, of whom 44% were vaccinated for yellow fever earlier in life, seroprotection against yellow fever was reached in all 20 analysed patients. A trend towards a lower yellow fever antibody response was observed in anti-TNFα-treated patients with RA (n=17) compared with HC (n=15).180
Summary and clinical implications
Reported immunogenicity results of yellow fever revaccination among patients with AIIRD are mainly adequate and similar as in HC. However, the available data on the safety of yellow fever vaccination in this group are very limited, and potential sequelae are serious (including death). Patients with AIIRD under immunosuppression should, therefore, avoid yellow fever vaccination in general. Temporarily withholding immunosuppressive therapy may be considered for patients with AIIRD travelling to endemic countries.
Human papillomavirus vaccination
Efficacy—immunogenicity—safety
Data on the efficacy of human papillomavirus (HPV) vaccination originate from vaccine trials in HCs that investigate the capacity of HPV vaccination to prevent premalignant lesions. A vaccine efficacy of 66% to prevent cervical intraepithelial neoplasia grade III was reported in a bivalent (HPV types 16 and 18) HPV vaccine trial after 4.5–10 years of follow-up.182
The correlation between the level of anti-HPV antibodies and protection against the development of cervical carcinoma is not known, since there are no reports on cervical carcinoma in vaccinated subjects. The level of seroprotection is based on comparison with naturally infected subjects.183
To date, no studies on the efficacy of HPV vaccination to prevent malignancies or premalignant lesions in the AIIRD population have been published, but there are studies on HPV vaccine immunogenicity in patients with AIIRD, all published after 2010 (online supplementary table S4). All but one study, performed in patients with juvenile idiopathic arthritis (JIA),184 include patients with SLE and used a quadrivalent (HPV types 6, 11, 16 and 18) vaccine.185–189 Seroconversion rates in patients were high and usually similar to those of healthy subjects, although two studies reported a lower geometrical mean titre for HPV 16 in patients with SLE after 5 years of follow-up,185 and also for patients with JIA,184 compared with HCs.
Rates of vaccine adverse events similar to HCs were reported for both patients with SLE188 and patients with JIA.184 Disease activity after vaccination was stable.184 186 188 189
Influence of immunomodulating agents
Mok et al reported that anti-HPV titres were lower in vaccinated patients with SLE using immunsosuppressive agents, especially in those receiving a combination of mycophenolate mofetil (MMF) and glucocorticoids.188 After 5 years of follow-up it was noted that patients with SLE that no longer were anti-HPV seropositive received significantly longer and higher doses of glucocorticoids and MMF than those with persistent seropositivity.185
Summary and clinical implications
Immunogenicity of HPV vaccination in the AIIRD population is high and, in the majority of studies, similar to that in HCs. The vaccine appears to be safe in patients with AIIRD. Therefore, the vaccine is recommended for patients with AIIRD, in accordance with recommendations for the general population. Patients with SLE, in particular, are advised to receive HPV vaccination, since they were shown to be at high risk of contracting a genital HPV infection, including the serotypes that are considered to be high risk for developing cervical dysplasia.190–194
Tickborne encephalitis vaccination
Efficacy—immunogenicity—safety
The available tickborne encephalitis (TBE) vaccines are inactivated vaccines. The vaccine is highly immunogenic in the general adult population.195 Excellent effectiveness of TBE vaccination in prevention of TBE was demonstrated in an Austrian study.196 Nonetheless, cases of vaccine failure have been reported, which occurred mainly in older or immunocompromised persons197.
To date, only one study on vaccination against TBE in patients with AIIRD has been published (online supplementary table S5). This Swedish study by Hertzell et al enrolled 65 patients with RA and 1 patient with AS on anti-TNF (n=16), a combination of MTX and anti-TNF (n=36) or MTX alone (n=14). Four doses of vaccine instead of three were offered to patients and HCs ≥60 years of age. One month after the last dose of vaccine, 39% of the patients and 79% of the HCs had developed protective levels of neutralising TBE antibodies. The difference was statistically significant. An extra dose of vaccine in patients with an undetectable antibody level at month 13 resulted in seroprotection in 4 out of 10 patients, all of whom were older than 60 years. No serious adverse events occurred.198
Influence of immunomodulating agents
In the study by Hertzell et al, regarding only patients ≥60 years of age, it seemed that a lower percentage of those treated with a combination of MTX and anti-TNF reached seroprotective anti-TBE levels than those treated with MTX or anti-TNF alone (25% vs 38%–40%).198
Summary and clinical implications
Although the updated recommendations do not specifically provide recommendations regarding TBE vaccination, an overarching principle states that non-live vaccines can be administered to patients with AIIRD, including during their use of glucocorticoids and DMARDs. The only available study on TBE vaccination in the AIIRD patient population showed a diminished immunogenicity response in these patients. As such, determining TBE-antibody levels after the last vaccine dose and, if necessary, giving an extra dose of vaccine may be considered.
Discussion
This SLR summarises the available data on efficacy, immunogenicity and safety of vaccination in patients with AIIRD, including patients with AIIRD using immunomodulating agents. Since the first version of EULAR recommendations and accompanying SLR on vaccination of adult patients with AIIRD were published in 2011,2 142 there has been a large expansion in the amount of available evidence on this topic, necessitating an update. By defining four research questions, we were able to address incidence/prevalence of VPIs, efficacy, immunogenicity and safety of vaccination, as well as the influence of immunomodulating agents on vaccine efficacy/immunogenicity in patients with AIIRD. To enable the presentation of a clear overview of the large amount of available data, the evidence on the incidence and prevalence of VPI diseases in patients with AIIRD was presented in a separate SLR, which was submitted for publication simultaneously with this report. The literature search for the fourth research question, dealing with the effect of vaccinating household members of patients with AIIRD on the occurrence of VPI in these patients and household members (including newborns), did not result in finding any relevant studies. Therefore, the recommendation encouraging household members of patients with AIIRD to receive vaccines according to national guidelines with the exception of oral polio vaccine, as formulated in the updated recommendations, is based on expert opinion. This recommendation follows guidelines of international societies such as the Infectious Diseases Society of America.199 Also the recommendation of avoiding live-attenuated vaccines during the first 6 months of life in newborns of mothers treated with biologics during the second half of pregnancy is based on expert opinion. This recommendation is in line with the EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation.200
One strength of our current approach, compared with the 2011 SLR, is the clear separation between efficacy and immunogenicity of vaccination, allowing a just interpretation of the data by readers. When assessing the different vaccines, evidence on efficacy, defined as the capacity of a vaccine to prevent infection, was considered to be of higher quality than evidence on immunogenicity, which refers to the capacity of vaccines to induce humoral and/or cellular immune responses. Unfortunately, because of the necessarily large sample size, using a clinical end point was essentially infeasible for some groups of AIIRD and/or vaccines. Consequently, only a small number of studies used a clinical end point.
It is important to note that although a large number of articles was included, for certain AIIRDs, vaccines and immunomodulating treatments the number of publications was still very limited. The majority of studies was performed in patients with RA and SLE. Results in these patient groups cannot uncontestedly be extrapolated one on one to patients with other AIIRDs.
Regarding safety of vaccination in the AIIRD population, both the occurrence of adverse events and influence on underlying disease of vaccination were assessed. Although since 2011 the amount of available evidence has considerably grown, studying safety of vaccination in patients with AIIRD remains a challenge. While vaccination did not lead to significant harms in the large majority of the included studies, these studies were too small or not properly designed to be able to detect the occurrence of rare adverse events.
In conclusion, evidence on efficacy, immunogenicity and safety of vaccination in patients with AIIRD (including those using immunomodulation agents), from October 2009 to August 2018, was systematically reviewed to provide a basis for updated evidence-based recommendations for vaccination in patients with AIIRD,3 in order to aid physicians, nurses and other health professionals dealing with questions regarding vaccination in patients with AIIRD in daily clinical practice.
Footnotes
CR and VF contributed equally.
Contributors: CR, in collaboration with SvA, reviewed articles on efficacy, immunogenicity and safety of vaccination for influenza, hepatitis A, hepatitis B, tetanus toxoid, herpes zoster, yellow fever and tickborne encephalitis in patients with AIIRD, and drafted the first version of the manuscript. VF, in collaboration with OE, performed the literature search and reviewed articles on vaccination in patients with AIIRD on the various immunomodulating therapies. MH reviewed articles on efficacy, immunogenicity and safety of vaccination of human papillomavirus vaccination in patients with AIIRD. OE initiated the project, reviewed articles on efficacy, immunogenicity and safety of vaccination for pneumococcal disease in patients with AIIRD, and wrote the paragraphs on pneumococcal vaccination. All authors collaborated in discussions during two meetings, and read and approved the final manuscript.
Funding: This project was supported by EULAR.
Competing interests: KW reports personal fees from Pfizer, during the conduct of the study; grants and personal fees from Biotest, CSL Behring, personal fees from LFB, Grifols, Baxter, Roche, Octapharma, grants from BMS, outside the submitted work. AM reports grants and personal fees from ABBVIE, Pfizer, BMS, UCB, MERCK, during the conduct of the study. MD reports grants and personal fees from PFIZER, ABBVIE, UCB, NOVARTIS, LILLY, MERCK, ROCHE, grants from BMS, outside the submitted work. OE reports grants and personal fees from Pfizer, Abbvie, Roche, Janssen, personal fees from Novartis and Lilly. All other authors have no conflicts of interests to declare.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Furer V, Rondaan C, Heijstek M, et al. Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review Informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD. RMD Open 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011;70:414–22. 10.1136/ard.2010.137216 [DOI] [PubMed] [Google Scholar]
- 3.Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2019. 10.1136/annrheumdis-2019-215882. [Epub ahead of print: 14 Aug 2019]. [DOI] [PubMed] [Google Scholar]
- 4.van der Heijde D, Aletaha D, Carmona L, et al. 2014 update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis 2015;74:8–13. 10.1136/annrheumdis-2014-206350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips B, Ball C, Sackett D, et al. Last update by Howick J. Oxford centre for evidence-based medicine – levels of evidence (March 2009), 2018. [Google Scholar]
- 6.Stojanovich L. Influenza vaccination of patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (rA). Clin Dev Immunol 2006;13:373–5. 10.1080/17402520600800820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers A, Scheifele D, Patterson C, et al. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J Rheumatol 1994;21:1203–6. [PubMed] [Google Scholar]
- 8.Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol 2007;34:272–9. [PubMed] [Google Scholar]
- 9.Denman EJ, Denman AM, Greenwood BM, et al. Failure of cytotoxic drugs to suppress immune responses of patients with rheumatoid arthritis. Ann Rheum Dis 1970;29:220–31. 10.1136/ard.29.3.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapetanovic MC, Saxne T, Nilsson J-A, et al. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology 2007;46:608–11. 10.1093/rheumatology/kel366 [DOI] [PubMed] [Google Scholar]
- 11.Kubota T, Nii T, Nanki T, et al. Anti-Tumor necrosis factor therapy does not diminish the immune response to influenza vaccine in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2007;17:531–3. 10.3109/s10165-007-0632-5 [DOI] [PubMed] [Google Scholar]
- 12.Oren S, Mandelboim M, Braun-Moscovici Y, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis 2008;67:937–41. 10.1136/ard.2007.077461 [DOI] [PubMed] [Google Scholar]
- 13.Nii T, Kubota T, Nanki T, et al. Reevaluation of antibody titers 1 year after influenza vaccination in patients with rheumatoid arthritis receiving TNF blockers. Mod Rheumatol 2009;19:216–8. 10.3109/s10165-008-0135-z [DOI] [PubMed] [Google Scholar]
- 14.van Assen S, Holvast A, Benne CA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 2010;62:75–81. 10.1002/art.25033 [DOI] [PubMed] [Google Scholar]
- 15.Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006;65:191–4. 10.1136/ard.2005.036434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelinck LBS, van der Bijl AE, Beyer WEP, et al. The effect of anti-tumour necrosis factor alpha treatment on the antibody response to influenza vaccination. Ann Rheum Dis 2008;67:713–6. 10.1136/ard.2007.077552 [DOI] [PubMed] [Google Scholar]
- 17.Herron A, Dettleff G, Hixon B, et al. Influenza vaccination in patients with rheumatic diseases. safety and efficacy. JAMA 1979;242:53–6. [PubMed] [Google Scholar]
- 18.Turner-Stokes L, Cambridge G, Corcoran T, et al. In vitro response to influenza immunisation by peripheral blood mononuclear cells from patients with systemic lupus erythematosus and other autoimmune diseases. Ann Rheum Dis 1988;47:532–5. 10.1136/ard.47.7.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Porto F, Laganà B, Biselli R, et al. Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. safety and immunogenicity. Vaccine 2006;24:3217–23. 10.1016/j.vaccine.2006.01.028 [DOI] [PubMed] [Google Scholar]
- 20.Elkayam O, Bashkin A, Mandelboim M, et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum 2010;39:442–7. 10.1016/j.semarthrit.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 21.Williams GW, Steinberg AD, Reinertsen JL, et al. Influenza immunization in systemic lupus eruthematosus. A double-blind trial. Ann Intern Med 1978;88:729–34. 10.7326/0003-4819-88-6-729 [DOI] [PubMed] [Google Scholar]
- 22.Ristow SC, Douglas RG, Condemi JJ. Influenza vaccination of patients with systemic lupus erythematosus. Ann Intern Med 1978;88:786–9. 10.7326/0003-4819-88-6-786 [DOI] [PubMed] [Google Scholar]
- 23.Brodman R, Gilfillan R, Glass D, et al. Influenzal vaccine response in systemic lupus erythematosus. Ann Intern Med 1978;88:735–40. 10.7326/0003-4819-88-6-735 [DOI] [PubMed] [Google Scholar]
- 24.Louie JS, Nies KM, Shoji KT, et al. Clinical and antibody responses after influenza immunization in systemic lupus erythematosus. Ann Intern Med 1978;88:790–2. 10.7326/0003-4819-88-6-790 [DOI] [PubMed] [Google Scholar]
- 25.Pons VG, Reinertsen JL, Steinberg AD, et al. Decreased cell-mediated cytotoxicity against virus-infected cells in systemic lupus erythematosus. J Med Virol 1979;4:15–23. 10.1002/jmv.1890040103 [DOI] [PubMed] [Google Scholar]
- 26.Abu-Shakra M, Press J, Varsano N, et al. Specific antibody response after influenza immunization in systemic lupus erythematosus. J Rheumatol 2002;29:2555–7. [PubMed] [Google Scholar]
- 27.Mercado U, Acosta H, Avendaño L. Influenza vaccination of patients with systemic lupus erythematosus. Rev Invest Clin 2004;56:16–20. [PubMed] [Google Scholar]
- 28.Holvast A, Huckriede A, Wilschut J, et al. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann Rheum Dis 2006;65:913–8. 10.1136/ard.2005.043943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Więsik-Szewczyk E, Romanowska M, Mielnik P, et al. Anti-Influenza vaccination in systemic lupus erythematosus patients: an analysis of specific humoral response and vaccination safety. Clin Rheumatol 2010;29:605–13. 10.1007/s10067-010-1373-y [DOI] [PubMed] [Google Scholar]
- 30.Holvast A, Stegeman CA, Benne CA, et al. Wegener's granulomatosis patients show an adequate antibody response to influenza vaccination. Ann Rheum Dis 2009;68:873–8. 10.1136/ard.2008.092924 [DOI] [PubMed] [Google Scholar]
- 31.Zycinska K, Romanowska M, Nowak I, et al. Antibody response to inactivated subunit influenza vaccine in patients with Wegener's granulomatosis. J Physiol Pharmacol 2007;58(Pt 2):819–28. [PubMed] [Google Scholar]
- 32.Setti M, Fenoglio D, Ansaldi F, et al. Flu vaccination with a virosomal vaccine does not affect clinical course and immunological parameters in scleroderma patients. Vaccine 2009;27:3367–72. 10.1016/j.vaccine.2009.01.078 [DOI] [PubMed] [Google Scholar]
- 33.Gelinck LBS, Teng YKO, Rimmelzwaan GF, et al. Poor serological responses upon influenza vaccination in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 2007;66:1402–3. 10.1136/ard.2007.071878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C-M, Chen H-J, Chen W-S, et al. Clinical effectiveness of influenza vaccination in patients with rheumatoid arthritis. Int J Rheum Dis 2018;21:1246–53. 10.1111/1756-185X.13322 [DOI] [PubMed] [Google Scholar]
- 35.Chang C-C, Chang Y-S, Chen W-S, et al. Effects of annual influenza vaccination on morbidity and mortality in patients with systemic lupus erythematosus: a nationwide cohort study. Sci Rep 2016;6:37817 10.1038/srep37817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobashigawa T, Nakajima A, Taniguchi A, et al. Vaccination against seasonal influenza is effective in Japanese patients with rheumatoid arthritis enrolled in a large observational cohort. Scand J Rheumatol 2013;42:445–50. 10.3109/03009742.2013.788733 [DOI] [PubMed] [Google Scholar]
- 37.Milanovic M, Stojanovich L, Djokovic A, et al. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med 2013;229:29–34. 10.1620/tjem.229.29 [DOI] [PubMed] [Google Scholar]
- 38.Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine 2011;29:1643–8. 10.1016/j.vaccine.2010.12.072 [DOI] [PubMed] [Google Scholar]
- 39.Jain VK, Bhashini N, Balajee LK, et al. Effect of disease-modifying antirheumatic drug therapy on immune response to trivalent influenza vaccine in rheumatoid arthritis. Indian J Med Res 2017;145:464–70. 10.4103/ijmr.IJMR_920_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobie JJ, Zheng B, Bryk P, et al. Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis Res Ther 2011;13 10.1186/ar3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milanetti F, Germano V, Nisini R, et al. Safety and immunogenicity of co-administered MF59-adjuvanted 2009 pandemic and plain 2009-10 seasonal influenza vaccines in rheumatoid arthritis patients on biologicals. Clin Exp Immunol 2014;177:287–94. 10.1111/cei.12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Wang H, Tam WWS. Is rheumatoid arthritis associated with reduced immunogenicity of the influenza vaccination? A systematic review and meta-analysis. Curr Med Res Opin 2017;33:1901–8. 10.1080/03007995.2017.1329140 [DOI] [PubMed] [Google Scholar]
- 43.Wallin L, Quintilio W, Locatelli F, et al. Safety and efficiency of influenza vaccination in systemic lupus erythematosus patients. Acta Reumatol Port 2009;34:498–502. [PubMed] [Google Scholar]
- 44.Crowe SR, Merrill JT, Vista ES, et al. Influenza vaccination responses in human systemic lupus erythematosus: impact of clinical and demographic features. Arthritis Rheum 2011;63:2396–406. 10.1002/art.30388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pugès M, Biscay P, Barnetche T, et al. Immunogenicity and impact on disease activity of influenza and pneumococcal vaccines in systemic lupus erythematosus: a systematic literature review and meta-analysis. Rheumatology 2016;55:1664–72. 10.1093/rheumatology/kew211 [DOI] [PubMed] [Google Scholar]
- 46.Liao Z, Tang H, Xu X, et al. Immunogenicity and safety of influenza vaccination in systemic lupus erythematosus patients compared with healthy controls: a meta-analysis. PLoS One 2016;11:e0147856 10.1371/journal.pone.0147856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Wang H, Wan L, et al. Is systemic lupus erythematosus associated with a declined immunogenicity and poor safety of influenza vaccination?: a systematic review and meta-analysis. Medicine 2016;95:e3637 10.1097/MD.0000000000003637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeffs LS, Peh CA, Jose MD, et al. Randomized trial investigating the safety and efficacy of influenza vaccination in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology 2015;20:343–51. 10.1111/nep.12416 [DOI] [PubMed] [Google Scholar]
- 49.Litinsky I, Balbir A, Zisman D, et al. Vaccination against influenza in patients with systemic sclerosis. Clin Exp Rheumatol 2012;30(2 Suppl 71):S7–11. [PubMed] [Google Scholar]
- 50.Polachek A, Korobko U, Mader-Balakirski N, et al. Immunogenicity and safety of vaccination against seasonal 2012 influenza virus among patients with psoriatic arthritis and psoriasis. Clin Exp Rheumatol 2015;33:181–6. [PubMed] [Google Scholar]
- 51.Caso F, Ramonda R, Del Puente A, et al. Influenza vaccine with adjuvant on disease activity in psoriatic arthritis patients under anti-TNF-α therapy. Clin Exp Rheumatol 2016;34:507–12. [PubMed] [Google Scholar]
- 52.Kostianovsky A, Charles P, Alves J-F, et al. Immunogenicity and safety of seasonal and 2009 pandemic A/H1N1 influenza vaccines for patients with autoimmune diseases: a prospective, monocentre trial on 199 patients. Clin Exp Rheumatol 2012;30(1 Suppl 70):S83–9. [PubMed] [Google Scholar]
- 53.Jaeger VK, Hoffman HM, van der Poll T, et al. Safety of vaccinations in patients with cryopyrin-associated periodic syndromes: a prospective registry based study. Rheumatology 2017;56:1484–91. 10.1093/rheumatology/kex185 [DOI] [PubMed] [Google Scholar]
- 54.Saad CGS, Borba EF, Aikawa NE, et al. Immunogenicity and safety of the 2009 non-adjuvanted influenza A/H1N1 vaccine in a large cohort of autoimmune rheumatic diseases. Ann Rheum Dis 2011;70:1068–73. 10.1136/ard.2011.150250 [DOI] [PubMed] [Google Scholar]
- 55.Gabay C, Bel M, Combescure C, et al. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum 2011;63:1486–96. 10.1002/art.30325 [DOI] [PubMed] [Google Scholar]
- 56.Miraglia JL, Abdala E, Hoff PM, et al. Immunogenicity and reactogenicity of 2009 influenza A (H1N1) inactivated monovalent non-adjuvanted vaccine in elderly and immunocompromised patients. PLoS One 2011;6:e27214 10.1371/journal.pone.0027214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elkayam O, Amir S, Mendelson E, et al. Efficacy and safety of vaccination against pandemic 2009 influenza A (H1N1) virus among patients with rheumatic diseases. Arthritis Care Res 2011;63:1062–7. 10.1002/acr.20465 [DOI] [PubMed] [Google Scholar]
- 58.Ribeiro ACM, Guedes LKN, Moraes JCB, et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice. Ann Rheum Dis 2011;70:2144–7. 10.1136/ard.2011.152983 [DOI] [PubMed] [Google Scholar]
- 59.Adler S, Krivine A, Weix J, et al. Protective effect of A/H1N1 vaccination in immune-mediated disease--a prospectively controlled vaccination study. Rheumatology 2012;51:695–700. 10.1093/rheumatology/ker389 [DOI] [PubMed] [Google Scholar]
- 60.França ILA, Ribeiro ACM, Aikawa NE, et al. Tnf blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology 2012;51:2091–8. 10.1093/rheumatology/kes202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwamoto M, Homma S, Onishi S, et al. Low level of seroconversion after a novel influenza A/H1N1/2009 vaccination in Japanese patients with rheumatoid arthritis in the 2009 season. Rheumatol Int 2012;32:3691–4. 10.1007/s00296-011-2118-1 [DOI] [PubMed] [Google Scholar]
- 62.Kapetanovic MC, Kristensen L-E, Saxne T, et al. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res Ther 2014;16 10.1186/ar4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu C-C, Wang Y-C, Lai J-H, et al. A/H1N1 influenza vaccination in patients with systemic lupus erythematosus: safety and immunity. Vaccine 2011;29:444–50. 10.1016/j.vaccine.2010.10.081 [DOI] [PubMed] [Google Scholar]
- 64.Borba EF, Saad CGS, Pasoto SG, et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology 2012;51:1061–9. 10.1093/rheumatology/ker427 [DOI] [PubMed] [Google Scholar]
- 65.Shinjo SK, de Moraes JCB, Levy-Neto M, et al. Pandemic unadjuvanted influenza A (H1N1) vaccine in dermatomyositis and polymyositis: immunogenicity independent of therapy and NO harmful effect in disease. Vaccine 2012;31:202–6. 10.1016/j.vaccine.2012.10.063 [DOI] [PubMed] [Google Scholar]
- 66.Miossi R, Fuller R, Moraes JCB, et al. Immunogenicity of influenza H1N1 vaccination in mixed connective tissue disease: effect of disease and therapy. Clinics 2013;68:129–33. 10.6061/clinics/2013(02)OA02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brauner S, Folkersen L, Kvarnström M, et al. H1N1 vaccination in Sjögren's syndrome triggers polyclonal B cell activation and promotes autoantibody production. Ann Rheum Dis 2017;76:1755–63. 10.1136/annrheumdis-2016-210509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sampaio-Barros PD, Andrade DCO, Seguro LCP, et al. Pandemic non-adjuvanted influenza A H1N1 vaccine in a cohort of patients with systemic sclerosis. Rheumatology 2018;57:1721–5. 10.1093/rheumatology/kex330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathian A, Devilliers H, Krivine A, et al. Factors influencing the efficacy of two injections of a pandemic 2009 influenza A (H1N1) nonadjuvanted vaccine in systemic lupus erythematosus. Arthritis Rheum 2011;63:3502–11. 10.1002/art.30576 [DOI] [PubMed] [Google Scholar]
- 70.Holvast A, van Assen S, de Haan A, et al. Effect of a second, booster, influenza vaccination on antibody responses in quiescent systemic lupus erythematosus: an open, prospective, controlled study. Rheumatology 2009;48:1294–9. 10.1093/rheumatology/kep200 [DOI] [PubMed] [Google Scholar]
- 71.Salemi S, Picchianti-Diamanti A, Germano V, et al. Influenza vaccine administration in rheumatoid arthritis patients under treatment with TNFalpha blockers: safety and immunogenicity. Clin Immunol 2010;134:113–20. 10.1016/j.clim.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 72.Kogure T, Harada N, Oku Y, et al. The observation of humoral responses after influenza vaccination in patients with rheumatoid arthritis treated with Japanese Oriental (Kampo) medicine: an observational study. Evidence-Based Complementary and Alternative Medicine 2012;2012:1–6. 10.1155/2012/320542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mori S, Ueki Y, Hirakata N, et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann Rheum Dis 2012;71:2006–10. 10.1136/annrheumdis-2012-201950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kogure T, Harada N, Tatsumi T, et al. Investigation of clinical characteristics as predictive factors for the humoral immune response to the influenza vaccine in patients with rheumatoid arthritis. Clin Rheumatol 2014;33:323–8. 10.1007/s10067-013-2483-0 [DOI] [PubMed] [Google Scholar]
- 75.Subesinghe S, Bechman K, Rutherford AI, et al. A systematic review and Metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J Rheumatol 2018;45:733–44. 10.3899/jrheum.170710 [DOI] [PubMed] [Google Scholar]
- 76.Kivitz AJ, Schechtman J, Texter M, et al. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase IV trial. J Rheumatol 2014;41:648–57. 10.3899/jrheum.130945 [DOI] [PubMed] [Google Scholar]
- 77.Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014;66:1016–26. 10.1002/acr.22246 [DOI] [PubMed] [Google Scholar]
- 78.Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2017;76:1559–65. 10.1136/annrheumdis-2017-211128 [DOI] [PubMed] [Google Scholar]
- 79.Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018;77:annrheumdis-2018-213222–904. 10.1136/annrheumdis-2018-213222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kapetanovic MC. Further evidence for influenza and pneumococcal vaccination in patients treated with disease modifying antirheumatic drugs and anti-tumor necrosis factor agents. J Rheumatol 2014;41:626–8. 10.3899/jrheum.140063 [DOI] [PubMed] [Google Scholar]
- 81.Tsuru T, Terao K, Murakami M, et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod Rheumatol 2014;24:511–6. 10.3109/14397595.2013.843743 [DOI] [PubMed] [Google Scholar]
- 82.Ribeiro AC, Laurindo IM, Guedes LK, et al. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res 2013;65:476–80. 10.1002/acr.21838 [DOI] [PubMed] [Google Scholar]
- 83.Alten R, Bingham CO, Cohen SB, et al. Antibody response to pneumococcal and influenza vaccination in patients with rheumatoid arthritis receiving abatacept. BMC Musculoskelet Disord 2016;17:231 10.1186/s12891-016-1082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. 10.1136/annrheumdis-2014-207191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fedson DS, Nicolas-Spony L, Klemets P, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines 2011;10:1143–67. 10.1586/erv.11.99 [DOI] [PubMed] [Google Scholar]
- 86.Mirsaeidi M, Schraufnagel DE. Pneumococcal vaccines: understanding centers for disease control and prevention recommendations. Ann Am Thorac Soc 2014;11:980–5. 10.1513/AnnalsATS.201401-042CME [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention (CDC) Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep 2012;61:394–5. [PubMed] [Google Scholar]
- 88.Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013;31:3577–84. 10.1016/j.vaccine.2013.04.085 [DOI] [PubMed] [Google Scholar]
- 89.Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015;372:1114–25. 10.1056/NEJMoa1408544 [DOI] [PubMed] [Google Scholar]
- 90.Bingham CO, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010;62:64–74. 10.1002/art.25034 [DOI] [PubMed] [Google Scholar]
- 91.Elkayam O, Caspi D, Reitblatt T, et al. The effect of tumor necrosis factor blockade on the response to pneumococcal vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum 2004;33:283–8. 10.1053/j.semarthrit.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 92.Visvanathan S, Keenan GF, Baker DG, et al. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J Rheumatol 2007;34:952–7. [PubMed] [Google Scholar]
- 93.Kapetanovic MC, Saxne T, Sjöholm A, et al. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology 2006;45:106–11. 10.1093/rheumatology/kei193 [DOI] [PubMed] [Google Scholar]
- 94.Gelinck LBS, van der Bijl AE, Visser LG, et al. Synergistic immunosuppressive effect of anti-TNF combined with methotrexate on antibody responses to the 23 valent pneumococcal polysaccharide vaccine. Vaccine 2008;26:3528–33. 10.1016/j.vaccine.2008.04.028 [DOI] [PubMed] [Google Scholar]
- 95.Elkayam O, Paran D, Caspi D, et al. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis 2002;34:147–53. 10.1086/338043 [DOI] [PubMed] [Google Scholar]
- 96.Klippel JH, Karsh J, Stahl NI, et al. A controlled study of pneumococcal polysaccharide vaccine in systemic lupus erythematosus. Arthritis Rheum 1979;22:1321–5. 10.1002/art.1780221201 [DOI] [PubMed] [Google Scholar]
- 97.Croft SM, Schiffman G, Snyder E, et al. Specific antibody response after in vivo antigenic stimulation in systemic lupus erythematosus. J Rheumatol 1984;11:141–6. [PubMed] [Google Scholar]
- 98.Lipnick RN, Karsh J, Stahl NI, et al. Pneumococcal immunization in patients with systemic lupus erythematosus treated with immunosuppressives. J Rheumatol 1985;12:1118–21. [PubMed] [Google Scholar]
- 99.Jarrett MP, Schiffman G, Barland P, et al. Impaired response to pneumococcal vaccine in systemic lupus erythematosus. Arthritis Rheum 1980;23:1287–93. 10.1002/art.1780231110 [DOI] [PubMed] [Google Scholar]
- 100.McDonald E, Jarrett MP, Schiffman G, et al. Persistence of pneumococcal antibodies after immunization in patients with systemic lupus erythematosus. J Rheumatol 1984;11:306–8. [PubMed] [Google Scholar]
- 101.Tarján P, Sipka S, Maródi L, et al. No short-term immunological effects of pneumococcus vaccination in patients with systemic lupus erythematosus. Scand J Rheumatol 2002;31:211–5. 10.1080/030097402320318396 [DOI] [PubMed] [Google Scholar]
- 102.Battafarano DF, Battafarano NJ, Larsen L, et al. Antigen-Specific antibody responses in lupus patients following immunization. Arthritis Rheum 1998;41:1828–34. [DOI] [PubMed] [Google Scholar]
- 103.Mease PJ, Ritchlin CT, Martin RW, et al. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J Rheumatol 2004;31:1356–61. [PubMed] [Google Scholar]
- 104.Mercado U, Acosta H, Diaz-Molina R. Antibody response to pneumococcal polysaccharide vaccine in systemic sclerosis. J Rheumatol 2009;36:1549–50. 10.3899/jrheum.081227 [DOI] [PubMed] [Google Scholar]
- 105.David Morgan M, Richter A, Al-Ali S, et al. Association of low B cell count and IgG levels with infection, and poor vaccine response with all-cause mortality in an immunosuppressed vasculitis population. Arthritis Care Res 2016;68:853–60. 10.1002/acr.22757 [DOI] [PubMed] [Google Scholar]
- 106.Nguyen MTT, Lindegaard H, Hendricks O, et al. Initial serological response after prime-boost pneumococcal vaccination in rheumatoid arthritis patients: results of a randomized controlled trial. J Rheumatol 2017;44:1794–803. 10.3899/jrheum.161407 [DOI] [PubMed] [Google Scholar]
- 107.Rehnberg M, Brisslert M, Amu S, et al. Vaccination response to protein and carbohydrate antigens in patients with rheumatoid arthritis after rituximab treatment. Arthritis Res Ther 2010;12 10.1186/ar3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crnkic Kapetanovic M, Saxne T, Jönsson G, et al. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther 2013;15 10.1186/ar4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Migita K, Akeda Y, Akazawa M, et al. Effect of abatacept on the immunogenicity of 23-valent pneumococcal polysaccharide vaccination (PPSV23) in rheumatoid arthritis patients. Arthritis Res Ther 2015;17 10.1186/s13075-015-0863-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kapetanovic MC, Roseman C, Jönsson G, et al. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum 2011;63:3723–32. 10.1002/art.30580 [DOI] [PubMed] [Google Scholar]
- 111.Kapetanovic MC, Roseman C, Jönsson G, et al. Heptavalent pneumococcal conjugate vaccine elicits similar antibody response as standard 23-valent polysaccharide vaccine in adult patients with RA treated with immunomodulating drugs. Clin Rheumatol 2011;30:1555–61. 10.1007/s10067-011-1856-5 [DOI] [PubMed] [Google Scholar]
- 112.Bingham CO, Rizzo W, Kivitz A, et al. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Ann Rheum Dis 2015;74:818–22. 10.1136/annrheumdis-2013-204427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mori S, Ueki Y, Akeda Y, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann Rheum Dis 2013;72:1362–6. 10.1136/annrheumdis-2012-202658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nagel J, Saxne T, Geborek P, et al. Treatment with belimumab in systemic lupus erythematosus does not impair antibody response to 13-valent pneumococcal conjugate vaccine. Lupus 2017;26:1072–81. 10.1177/0961203317695465 [DOI] [PubMed] [Google Scholar]
- 115.Fischer L, Gerstel PF, Poncet A, et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases – a longitudinal study. Arthritis Res Ther 2015;17 10.1186/s13075-015-0663-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rezende RPV, Ribeiro FM, Albuquerque EMN, et al. Immunogenicity of pneumococcal polysaccharide vaccine in adult systemic lupus erythematosus patients undergoing immunosuppressive treatment. Lupus 2016;25:1254–9. 10.1177/0961203316636472 [DOI] [PubMed] [Google Scholar]
- 117.Nived P, Nagel J, Saxne T, et al. Immune response to pneumococcal conjugate vaccine in patients with systemic vasculitis receiving standard of care therapy. Vaccine 2017;35:3639–46. 10.1016/j.vaccine.2017.05.044 [DOI] [PubMed] [Google Scholar]
- 118.Kapetanovic MC, Nagel J, Nordström I, et al. Methotrexate reduces vaccine-specific immunoglobulin levels but not numbers of circulating antibody-producing B cells in rheumatoid arthritis after vaccination with a conjugate pneumococcal vaccine. Vaccine 2017;35:903–8. 10.1016/j.vaccine.2016.12.068 [DOI] [PubMed] [Google Scholar]
- 119.Migita K, Akeda Y, Akazawa M, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tacrolimus. Arthritis Res Ther 2015;17 10.1186/s13075-015-0662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Izumi Y, Akazawa M, Akeda Y, et al. The 23-valent pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis: a double-blinded, randomized, placebo-controlled trial. Arthritis Res Ther 2017;19 10.1186/s13075-016-1207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coulson E, Saravanan V, Hamilton J, et al. Pneumococcal antibody levels after pneumovax in patients with rheumatoid arthritis on methotrexate. Ann Rheum Dis 2011;70:1289–91. 10.1136/ard.2010.144451 [DOI] [PubMed] [Google Scholar]
- 122.Nagel J, Geborek P, Saxne T, et al. The risk of pneumococcal infections after immunization with pneumococcal conjugate vaccine compared to non-vaccinated inflammatory arthritis patients. Scand J Rheumatol 2015;44:271–9. 10.3109/03009742.2014.984754 [DOI] [PubMed] [Google Scholar]
- 123.Nagel J, Geborek P, Saxne T, et al. The association between antibody levels before and after 7-valent pneumococcal conjugate vaccine immunization and subsequent pneumococcal infection in chronic arthritis patients. Arthritis Res Ther 2015;17 10.1186/s13075-015-0636-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Migita K, Akeda Y, Akazawa M, et al. Opsonic and antibody responses to pneumococcal polysaccharide in rheumatoid arthritis patients receiving golimumab plus methotrexate. Medicine 2015;94:e2184 10.1097/MD.0000000000002184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chatham W, Chadha A, Fettiplace J, et al. A randomized, open-label study to investigate the effect of belimumab on pneumococcal vaccination in patients with active, autoantibody-positive systemic lupus erythematosus. Lupus 2017;26:1483–90. 10.1177/0961203317703495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Walker UA, Hoffman HM, Williams R, et al. Brief Report: Severe Inflammation Following Vaccination Against Streptococcus pneumoniae in Patients With Cryopyrin-Associated Periodic Syndromes. Arthritis Rheumatol 2016;68:516–20. 10.1002/art.39482 [DOI] [PubMed] [Google Scholar]
- 127.Crnkic Kapetanovic M, Saxne T, Truedsson L, et al. Persistence of antibody response 1.5 years after vaccination using 7-valent pneumococcal conjugate vaccine in patients with arthritis treated with different antirheumatic drugs. Arthritis Res Ther 2013;15 10.1186/ar4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grabar S, Groh M, Bahuaud M, et al. Pneumococcal vaccination in patients with systemic lupus erythematosus: a multicenter placebo-controlled randomized double-blind study. Vaccine 2017;35:4877–85. 10.1016/j.vaccine.2017.07.094 [DOI] [PubMed] [Google Scholar]
- 129.Rákóczi Éva, Perge B, Végh E, et al. Evaluation of the immunogenicity of the 13-valent conjugated pneumococcal vaccine in rheumatoid arthritis patients treated with etanercept. Joint Bone Spine 2016;83:675–9. 10.1016/j.jbspin.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 130.Broyde A, Arad U, Madar-Balakirski N, et al. Longterm efficacy of an antipneumococcal polysaccharide vaccine among patients with autoimmune inflammatory rheumatic diseases. J Rheumatol 2016;43:267–72. 10.3899/jrheum.150397 [DOI] [PubMed] [Google Scholar]
- 131.Hesselstrand R, Nagel J, Saxne T, et al. Immunogenicity and safety of pneumococcal vaccination in patients with systemic sclerosis. Rheumatology 2018;57:625–30. 10.1093/rheumatology/kex471 [DOI] [PubMed] [Google Scholar]
- 132.Groh M, Puéchal X, Terrier B, et al. Failure of pneumococcal immunization during remission induction treatment of ANCA-associated vasculitis: the Pneumovas pilot 1 study. Joint Bone Spine 2017;84:643–4. 10.1016/j.jbspin.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 133.Bahuaud M, Beaudouin-Bazire C, Husson M, et al. Immunogenicity and persistence of a prime-boost re-vaccination strategy for pneumococcal vaccines in patients with rheumatoid arthritis. Hum Vaccin Immunother 2018;14:1464–70. 10.1080/21645515.2018.1438091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alyasin S, Adab M, Hosseinpour A, et al. Immunogenicity of 23-valent pneumococcal vaccine in children with systemic lupus erythematosus. Iran J Immunol 2016;13:204–19. doi:IJIv13i3A6 [PubMed] [Google Scholar]
- 135.Esposito S, Bonanni P, Maggi S, et al. Recommended immunization schedules for adults: clinical practice guidelines by the ESCMID vaccine Study Group (EVASG), European geriatric medicine Society (EUGMS) and the world association for infectious diseases and immunological disorders (WAidid). Hum Vaccin Immunother 2016;12:1–18. 10.1080/21645515.2016.1150396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine–naïve adults 60–64 years of age. Vaccine 2014;32:2364–74. 10.1016/j.vaccine.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 137.Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013;31:3594–602. 10.1016/j.vaccine.2013.04.084 [DOI] [PubMed] [Google Scholar]
- 138.Lesprit P, Pédrono G, Molina J-M, et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS 2007;21:2425–34. 10.1097/QAD.0b013e3282887e91 [DOI] [PubMed] [Google Scholar]
- 139.Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep 2015;64:944–7. 10.15585/mmwr.mm6434a4 [DOI] [PubMed] [Google Scholar]
- 140.Werzberger A, Mensch B, Kuter B, et al. A controlled trial of a formalin-inactivated hepatitis a vaccine in healthy children. N Engl J Med 1992;327:453–7. 10.1056/NEJM199208133270702 [DOI] [PubMed] [Google Scholar]
- 141.Innis BL, et al. Protection against hepatitis A by an inactivated vaccine. JAMA 1994;271:1328–34. 10.1001/jama.1994.03510410040030 [DOI] [PubMed] [Google Scholar]
- 142.van Assen S, Elkayam O, Agmon-Levin N, et al. Vaccination in adult patients with auto-immune inflammatory rheumatic diseases: a systematic literature review for the European League against rheumatism evidence-based recommendations for vaccination in adult patients with auto-immune inflammatory rheumatic diseases. Autoimmun Rev 2011;10:341–52. 10.1016/j.autrev.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 143.Orr N, Klement E, Gillis D, et al. Long-Term immunity in young adults after a single dose of inactivated hepatitis A vaccines. Vaccine 2006;24:4328–32. 10.1016/j.vaccine.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 144.Schmidtke P, Habermehl P, Knuf M, et al. Cell mediated and antibody immune response to inactivated hepatitis a vaccine. Vaccine 2005;23:5127–32. 10.1016/j.vaccine.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 145.Askling HH, Rombo L, van Vollenhoven R, et al. Hepatitis a vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Travel Med Infect Dis 2014;12:134–42. 10.1016/j.tmaid.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 146.Rosdahl A, Herzog C, Frösner G, et al. An extra priming dose of hepatitis A vaccine to adult patients with rheumatoid arthritis and drug induced immunosuppression - A prospective, open-label, multi-center study. Travel Med Infect Dis 2018;21:43–50. 10.1016/j.tmaid.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 147.van den Bijllaardt W, Siers HM, Timmerman‐Kok C, et al. Seroprotection after hepatitis A vaccination in patients with Drug‐Induced immunosuppression. J Travel Med 2013;20:278–82. 10.1111/jtm.12050 [DOI] [PubMed] [Google Scholar]
- 148.Poland GA, Jacobson RM. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med 2004;351:2832–8. 10.1056/NEJMcp041507 [DOI] [PubMed] [Google Scholar]
- 149.Jack AD, Hall AJ, Maine N, et al. What level of hepatitis B antibody is protective? J Infect Dis 1999;179:489–92. 10.1086/314578 [DOI] [PubMed] [Google Scholar]
- 150.Elkayam O, Yaron M, Caspi D. Safety and efficacy of vaccination against hepatitis B in patients with rheumatoid arthritis. Ann Rheum Dis 2002;61:623–5. 10.1136/ard.61.7.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuruma KAM, Borba EF, Lopes MH, et al. Safety and efficacy of hepatitis B vaccine in systemic lupus erythematosus. Lupus 2007;16:350–4. 10.1177/0961203307078225 [DOI] [PubMed] [Google Scholar]
- 152.Franco Salinas G, de Rycke L, Cantaert T, et al. Tnf blockade impairs T cell dependent antibody responses. Ann Rheum Dis 2009;68(suppl 3). [Google Scholar]
- 153.Erkek E, Ayaslioglu E, Erkek AB, et al. Response to vaccination against hepatitis B in patients with Behcet's disease. J Gastroenterol Hepatol 2005;20:1508–11. 10.1111/j.1440-1746.2005.03903.x [DOI] [PubMed] [Google Scholar]
- 154.Intongkam S, Samakarnthai P, Pakchotanon R, et al. Efficacy and safety of hepatitis B vaccination in rheumatoid arthritis patients receiving disease-modifying antirheumatic drugs and/or biologics therapy. JCR: Journal of Clinical Rheumatology 2018. 10.1097/RHU.0000000000000877 [DOI] [PubMed] [Google Scholar]
- 155.Scheibel I. The uses and results of active tetanus immunization. Bull World Health Organ 1955;13:381–94. [PMC free article] [PubMed] [Google Scholar]
- 156.Roush SW, Murphy TV, Vaccine-Preventable Disease Table Working Group . Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 2007;298:2155–63. 10.1001/jama.298.18.2155 [DOI] [PubMed] [Google Scholar]
- 157.Devey ME, Bleasdale K, Isenberg DA. Antibody affinity and IgG subclass of responses to tetanus toxoid in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol 1987;68:562–9. [PMC free article] [PubMed] [Google Scholar]
- 158.Abe T, Homma M. Immunological reactivity in patients with systemic lupus erythematosus: humoral antibody and cellular immune responses. Acta Rheumatol Scand 1971;17:35–46. 10.3109/rhe1.1971.17.issue-1-4.06 [DOI] [PubMed] [Google Scholar]
- 159.Kashef S, Ghazizadeh F, Derakhshan A, et al. Antigen-Specific antibody response in juvenile-onset SLE patients following routine immunization with tetanus toxoid. Iran J Immunol 2008;5:181–4. doi:IJIv5i3A7 [DOI] [PubMed] [Google Scholar]
- 160.Nies K, Boyer R, Stevens R, et al. Anti-Tetanus toxoid antibody synthesis after booster immunization in systemic lupus erythematosus. Comparison of the in vitro and in vivo responses. Arthritis Rheum 1980;23:1343–50. 10.1002/art.1780231203 [DOI] [PubMed] [Google Scholar]
- 161.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med Overseas Ed 2005;352:2271–84. 10.1056/NEJMoa051016 [DOI] [PubMed] [Google Scholar]
- 162.Schmader KE, Levin MJ, Gnann JW, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clinical Infectious Diseases 2012;54:922–8. 10.1093/cid/cir970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96. 10.1056/NEJMoa1501184 [DOI] [PubMed] [Google Scholar]
- 164.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016;375:1019–32. 10.1056/NEJMoa1603800 [DOI] [PubMed] [Google Scholar]
- 165.Didierlaurent AM, Collignon C, Bourguignon P, et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J.i. 2014;193:1920–30. 10.4049/jimmunol.1400948 [DOI] [PubMed] [Google Scholar]
- 166.Fochesato M, Dendouga N, Boxus M. Comparative preclinical evaluation of AS01 versus other adjuvant systems in a candidate herpes zoster glycoprotein E subunit vaccine. Hum Vaccin Immunother 2016;12:2092–5. 10.1080/21645515.2016.1154247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012;308:43–9. 10.1001/jama.2012.7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yun H, Xie F, Baddley JW, et al. Longterm effectiveness of herpes zoster vaccine among patients with autoimmune and inflammatory diseases. J Rheumatol 2017;44:1083–7. 10.3899/jrheum.160685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koh JH, Lee J, Kim SH, et al. Safety, and humoral and cell-mediated immune responses to herpes zoster vaccine in patients with rheumatoid arthritis. J Rheumatol 2018;45:465–9. 10.3899/jrheum.170936 [DOI] [PubMed] [Google Scholar]
- 170.Winthrop KL, Wouters AG, Choy EH, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol 2017;69:1969–77. 10.1002/art.40187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guthridge JM, Cogman A, Merrill JT, et al. Herpes zoster vaccination in SLE: a pilot study of immunogenicity. J Rheumatol 2013;40:1875–80. 10.3899/jrheum.130170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers 2015;1 10.1038/nrdp.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Russell AF, Parrino J, Fisher CL, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine 2015;33:3129–34. 10.1016/j.vaccine.2015.04.090 [DOI] [PubMed] [Google Scholar]
- 174.de Menezes Martins R, Fernandes Leal MdaL, Homma A. Serious adverse events associated with yellow fever vaccine. Hum Vaccin Immunother 2015;11:2183–7. 10.1080/21645515.2015.1022700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martin M, Tsai TF, Cropp B, et al. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. The Lancet 2001;358:98–104. 10.1016/S0140-6736(01)05327-2 [DOI] [PubMed] [Google Scholar]
- 176.Vasconcelos PFC, Luna EJ, Galler R, et al. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. The Lancet 2001;358:91–7. 10.1016/S0140-6736(01)05326-0 [DOI] [PubMed] [Google Scholar]
- 177.Wieten RW, Goorhuis A, Jonker EFF, et al. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and immune-compromised patients. J Infect 2016;72:713–22. 10.1016/j.jinf.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 178.Oliveira ACV, Mota LMH, Santos-Neto LL, et al. Seroconversion in patients with rheumatic diseases treated with immunomodulators or immunosuppressants, who were inadvertently revaccinated against yellow fever. Arthritis Rheumatol 2015;67:582–3. 10.1002/art.38960 [DOI] [PubMed] [Google Scholar]
- 179.Kernéis S, Launay O, Ancelle T, et al. Safety and immunogenicity of yellow fever 17D vaccine in adults receiving systemic corticosteroid therapy: an observational cohort study. Arthritis Care Res 2013;65:1522–8. 10.1002/acr.22021 [DOI] [PubMed] [Google Scholar]
- 180.Scheinberg M, Guedes-Barbosa LS, Mangueira C, et al. Yellow fever revaccination during infliximab therapy. Arthritis Care Res 2010;62:896–8. 10.1002/acr.20045 [DOI] [PubMed] [Google Scholar]
- 181.Whittembury A, Ramirez G, Hernández H, et al. Viscerotropic disease following yellow fever vaccination in Peru. Vaccine 2009;27:5974–81. 10.1016/j.vaccine.2009.07.082 [DOI] [PubMed] [Google Scholar]
- 182.Lehtinen M, Lagheden C, Luostarinen T, et al. Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials. BMJ Open 2017;7:e015867 10.1136/bmjopen-2017-015867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dauner JG, Pan Y, Hildesheim A, et al. Characterization of the HPV-specific memory B cell and systemic antibody responses in women receiving an unadjuvanted HPV16 L1 VLP vaccine. Vaccine 2010;28:5407–13. 10.1016/j.vaccine.2010.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Esposito S, Corona F, Barzon L, et al. Immunogenicity, safety and tolerability of a bivalent human papillomavirus vaccine in adolescents with juvenile idiopathic arthritis. Expert Rev Vaccines 2014;13:1387–93. 10.1586/14760584.2014.943195 [DOI] [PubMed] [Google Scholar]
- 185.Mok CC, Ho LY, To CH. Long-Term immunogenicity of a quadrivalent human papillomavirus vaccine in systemic lupus erythematosus. Vaccine 2018;36:3301–7. 10.1016/j.vaccine.2018.04.056 [DOI] [PubMed] [Google Scholar]
- 186.Dhar JP, Essenmacher L, Dhar R, et al. The safety and immunogenicity of quadrivalent HPV (qHPV) vaccine in systemic lupus erythematosus. Vaccine 2017;35:2642–6. 10.1016/j.vaccine.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 187.Dhar JP, Essenmacher L, Dhar R, et al. The effect of history of abnormal Pap smear or preceding HPV infection on the humoral immune response to quadrivalent human papilloma virus (qHPV) vaccine in women with systemic lupus erythematosus. Hum Vaccin Immunother 2018;14:2318–22. 10.1080/21645515.2018.1469592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mok CC, Ho LY, Fong LS, et al. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case–control study. Ann Rheum Dis 2013;72:659–64. 10.1136/annrheumdis-2012-201393 [DOI] [PubMed] [Google Scholar]
- 189.Soybilgic A, Onel KB, Utset T, et al. Safety and immunogenicity of the quadrivalent HPV vaccine in female Systemic Lupus Erythematosus patients aged 12 to 26 years. Pediatric Rheumatology 2013;11 10.1186/1546-0096-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Klumb EM, Pinto AC, Jesus GR, et al. Are women with lupus at higher risk of HPV infection? Lupus 2010;19:1485–91. 10.1177/0961203310372952 [DOI] [PubMed] [Google Scholar]
- 191.Lee Y-H, Choe J-Y, Park S-H, et al. Prevalence of human papilloma virus infections and cervical cytological abnormalities among Korean women with systemic lupus erythematosus. J Korean Med Sci 2010;25:1431–7. 10.3346/jkms.2010.25.10.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lyrio LDC, Grassi MFR, Santana IU, et al. Prevalence of cervical human papillomavirus infection in women with systemic lupus erythematosus. Rheumatol Int 2013;33:335–40. 10.1007/s00296-012-2426-0 [DOI] [PubMed] [Google Scholar]
- 193.Mendoza-Pinto C, García-Carrasco M, Vallejo-Ruiz V, et al. Incidence of cervical human papillomavirus infection in systemic lupus erythematosus women. Lupus 2017;26:944–51. 10.1177/0961203316686708 [DOI] [PubMed] [Google Scholar]
- 194.Méndez-Martínez S, García-Carrasco M, Jiménez-Herrera EA, et al. Factors of the epidemiological triad that influence the persistence of human papilloma virus infection in women with systemic lupus erythematosus. Lupus 2018;27:1542–6. 10.1177/0961203318773176 [DOI] [PubMed] [Google Scholar]
- 195.Kollaritsch H, Paulke-Korinek M, Holzmann H, et al. Vaccines and vaccination against tick-borne encephalitis. Expert Rev Vaccines 2012;11:1103–19. 10.1586/erv.12.86 [DOI] [PubMed] [Google Scholar]
- 196.Heinz FX, Holzmann H, Essl A, et al. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 2007;25:7559–67. 10.1016/j.vaccine.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 197.Andersson CR, Vene S, Insulander M, et al. Vaccine failures after active immunisation against tick-borne encephalitis. Vaccine 2010;28:2827–31. 10.1016/j.vaccine.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 198.Hertzell KB, Pauksens K, Rombo L, et al. Tick-Borne encephalitis (TBE) vaccine to medically immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Vaccine 2016;34:650–5. 10.1016/j.vaccine.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 199.Rubin LG, Levin MJ, Ljungman P, et al. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2013;2014:309–18. [DOI] [PubMed] [Google Scholar]
- 200.Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. 10.1136/annrheumdis-2015-208840 [DOI] [PubMed] [Google Scholar]
- 201.Burmester GR, Landewé R, Genovese MC, et al. Adalimumab long-term safety: infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:414–7. 10.1136/annrheumdis-2016-209322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kivitz AJ, Schechtman J, Texter M, et al. Vaccine responses in patients with rheumatoid arthritis treated with Certolizumab pegol: results from a single-blind randomized phase IV trial. J Rheumatol 2014;41:648–57. 10.3899/jrheum.130945 [DOI] [PubMed] [Google Scholar]
- 203.Luque Ramos A, Hoffmann F, Callhoff J, et al. Influenza and pneumococcal vaccination in patients with rheumatoid arthritis in comparison with age- and sex-matched controls: results of a claims data analysis. Rheumatol Int 2016;36:1255–63. 10.1007/s00296-016-3516-1 [DOI] [PubMed] [Google Scholar]
- 204.Launay O, Paul S, Servettaz A, et al. Control of humoral immunity and auto-immunity by the CXCR4/CXCL12 axis in lupus patients following influenza vaccine. Vaccine 2013;31:3492–501. 10.1016/j.vaccine.2013.05.095 [DOI] [PubMed] [Google Scholar]
- 205.Vista ES, Crowe SR, Thompson LF, et al. Influenza vaccination can induce new-onset anticardiolipins but not β2-glycoprotein-I antibodies among patients with systemic lupus erythematosus. Lupus 2012;21:168–74. 10.1177/0961203311429554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Muller RB, Maier R, Hoschler K, et al. Efficient boosting of the antiviral T cell response in B cell-depleted patients with autoimmune rheumatic diseases following influenza vaccination. Clin Exp Rheumatol 2013;31:723–30. [PubMed] [Google Scholar]
- 207.Urowitz MB, Anton A, Ibanez D, et al. Autoantibody response to adjuvant and nonadjuvant H1N1 vaccination in systemic lupus erythematosus. Arthritis Care Res 2011;63:1517–20. 10.1002/acr.20599 [DOI] [PubMed] [Google Scholar]
- 208.de Medeiros DM, Silva CA, Bueno C, et al. Pandemic influenza immunization in primary antiphospholipid syndrome (PAPS): a trigger to thrombosis and autoantibody production? Lupus 2014;23:1412–6. 10.1177/0961203314540351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2019-001035supp002.pdf (548.6KB, pdf)
rmdopen-2019-001035supp003.pdf (173.1KB, pdf)
rmdopen-2019-001035supp001.pdf (116.3KB, pdf)