Abstract
Energy metabolism and bone homeostasis share several regulatory pathways. The AP1 transcription factor ΔFosB and leptin both regulate energy metabolism and bone, yet whether their pathways intersect is not known. Transgenic mice overexpressing ΔFosB under the control of the Enolase 2 (ENO2) promoter exhibit high bone mass, high energy expenditure, low fat mass and low circulating leptin levels. Since leptin is a regulator of bone and ΔFosB acts on leptin-responsive ventral hypothalamic (VHT) neurons to induce bone anabolism, we hypothesized that regulation of leptin may contribute to the central actions of ΔFosB in the VHT. To address this question, we used Adeno-associated virus (AAV) expression of ΔFosB in the VHT of leptin-deficient ob/ob mice and genetic crossing of ENO2-ΔFosB with ob/ob mice. In both models, leptin deficiency prevented ΔFosB-triggered reduction in body weight, increase in energy expenditure, increase in glucose utilization and reduction in pancreatic islet size. In contrast, leptin deficiency failed to prevent ΔFosB-triggered increase in bone mass. Unlike leptin deficiency, galanin deficiency blocked both the metabolic and the bone ΔFosB-induced effects. Overall, our data demonstrate that, while the catabolic energy metabolism effects of ΔFosB require intact leptin and galanin signaling, the bone mass-accruing effects of ΔFosB require galanin but are independent of leptin.
Keywords: AP1, leptin, galanin, energy, bone, hypothalamus
Introduction
The hypothalamus plays a major role in the systemic regulation of energy metabolism (1–3) and bone (4–10). The past decade of research revealed a high degree of overlap between the neuronal and hormonal/cytokine-dependent mechanisms controlling these processes. One of the most robust such co-regulators is leptin, an adipokine serving as an internal “adipostat”. Leptin is synthesized and secreted by adipocytes and communicates information from the periphery to the brain regarding body’s energy reserves and nutritional status (11). Increased adiposity is associated with increased circulating leptin levels, leading to suppressed feeding and increased energy expenditure. Conversely, a fall in circulating levels signifies energy deficit, promoting feeding and energy conservation (12, 13). In mice, genetic loss of function of either leptin (ob/ob) or the leptin receptor (db/db) genes lead to hyperphagia and major obesity. In humans, both genetic deficiency and leptin insensitivity lead to metabolic disturbances, and can be corrected by exogenous leptin administration (14). Beyond its profound metabolic effects, leptin is reported to act as a regulator of bone and both ob/ob and db/db mice have been reported to display high bone mass phenotypes (7, 15–18), although this question remains controversial (19, 20). ob/ob mice have both increased vertebral bone volume and reduced mineral content and reduced length of the long bones and leptin administration into the third cerebral ventricle usually normalizes the bone phenotypes (16, 20–22). The central site of leptin action appears to differ among the neuronal circuits governing energy metabolism and bone, although in either case it involves the ventral hypothalamic area (VHT), containing the arcuate nucleus (ARC), and ventromedial nucleus (VMH), among others. The ARC harbors two distinct neuronal populations: the anorexigenic, α-melanocyte stimulating hormone derived from proopiomelanocortin (POMC) neurons and the orexigenic, agouti-related peptide (AgRP)-producing neurons, and both express leptin receptors. The VHT also harbors steroidogenic factor1 (SF1)-producing neurons. Leptin’s metabolic effects are believed to be mediated by the aforementioned ARC, VMH, as well as via several extra-hypothalamic nuclei (1, 23), whereas the bone effects appear to follow a different path: Leptin may negatively affect bone mass via action on the serotonergic neurons in the Raphe nuclei of the brainstem (24), projecting to the VMH and activating the sympathetic tone (25). But leptin also affects bone positively via circulating CART, by suppressing osteoclastogenesis (26). Moreover, leptin also exerts direct effects within the bone environment, where it is produced by bone marrow adipocytes (19, 27), making interpretation of the central effects somewhat difficult. Nevertheless, the extent to which leptin-dependent energy metabolism and bone regulating circuits coincide or diverge still remains unclear.
Previously, we demonstrated that AP1 transcription factors are also capable of exerting strong regulation of energy metabolism and bone mass (8, 28–32). AP1 factors form heterodimers between Fos and Jun proteins. ΔFosB, is a naturally occurring splice isoform of FosB, which lacks the C-terminal transactivation domain, and thereby exhibits AP antagonistic properties (33). Mice overexpressing ΔFosB driven by the enolase2 (ENO2) promoter display a lean phenotype with improved glucose metabolism and insulin sensitivity, elevated energy expenditure and high bone mass, due to increased bone formation (29, 31, 32). Moreover, mice in which ΔFosB, or the artificial AP1 antagonist dominant-negative JunD (DNJunD) are stereotactically targeted to the ventral hypothalamus (VHT) phenocopy the ENO2- ΔFosB mice (30, 34). Using a combined anatomical and genetic neuron-specific targeting approach, we recently showed that the high bone mass and boosted energy metabolism are mediated by AP1 antagonism in either AgRP or POMC neurons, and requires the presence in the VHT of the neuromediator galanin (8). Peripheral and central galanin regulates numerous physiological and pathological processes, including neurodegenerative conditions, diabetes and cancer (35), but our work revealed the role of central galanin signaling in bone biology (8).
In contrast to ARC-residing neurons, the expression of AP1 antagonists in VMH-residing SF1 neurons induced high energy, but low bone mass phenotype, showing that the control of energy metabolism and bone can be dissociated. Consequently, peripheral and central cues, such as leptin and galanin, may also affect energy and bone homeostasis processes, together or independently of each other. Since leptin is known to act on ARC neurons, and these neurons express galanin (36) we determined whether any of the effects exerted by ΔFosB on energy expenditure, glucose metabolism and/or bone, were dependent upon the presence of leptin and/or galanin. We used two models to address this question: 1. ΔFosB expression in the VHT of ob/ob or galanin-deficient GALKO mice, using adenoassociated virus (AAV) stereotaxic delivery; and 2. Genetic crossing of ENO2- ΔFosB with the leptin-deficient ob/ob mice. Our results demonstrate that while the catabolic energy metabolism effects of central ΔFosB require intact leptin and intact galanin signaling, the bone mass- inducing effects of ΔFosB require galanin but are independent of leptin. Since ΔFosB-induced energy metabolism and bone effects are dependent on the presence of VHT galanin, this study establishes that central galanin, rather than central leptin, underlies ΔFosB enhancement of bone mass. Taken together, these observations confirm that processes governing whole body energy and bone homeostasis may dissociate at the level of hypothalamic neurons and their regulation.
Materials and methods
Stereotaxic viral transfer of ΔFosB into VHT of mice
Mice were maintained at Harvard animal facilities in compliance with the Institutional Animal Care and Use Committee (IACUC) regulations and housed in a temperature controlled (25°C) environment under a 12-hour light/dark cycle and fed a rodent chow diet (5058, Pico Lab). Leptin null ob/ob mice were purchased from Jackson Laboratories (stock #000632), GALKO mice were generated by David Wynick (37) and were a generous gift from Marina Picciotto (Yale University) and C57BL mice (000664) were purchased from Jackson Laboratories. For all studies, 6–7 week-old males were anesthetized, positioned into stereotaxic frame (David Kopf Instruments), and bilateral holes in the scull were drilled using the following coordinates: AP = 2.0, DV = from −5.7 to 6.0, LAT = 1.2 at 10 degree angle. A viral volume of 0.5 μL ΔFosB-Ires-GFP AVV or Ires-GFP AAV (virus titer 1 × 108 IFU/mL) was delivered on each side, using Hamilton syringe, at a rate of 0.5 μL/min. Injection validation was performed as previously described (34). Animals were allowed to recover and analyzed 8 weeks after-surgery.
Generation of ENO2-ΔFosB-ob/ob mice
To generate ΔFosB transgenic Lepob/ob mice (ENO2-ΔFosB-ob/ob), a modified tetracycline-regulated system was used (28, 29, 31). We first crossed heterozygous ob/+ mice to the mice carrying the tetracycline transactivator (tTA) under the control of the ENO2 promoter (ENO2-tTA) and to the mice carrying the ΔFosB transgene under the control of the tetracycline-responsive promoter, TetOp (TetOp-ΔFosB) to obtain ENO2-tTATg/0/Lepob/+ mice and TetOp-ΔFosBTg/0/Lepob/+ mice, respectively. Then, ENO2-tTATg/0/Lepob/+ mice and TetOp-ΔFosBTg/0/Lepob/+ mice were crossed to obtain ENO2-tTATg/0/TetOp-ΔFosBTg/0/Lepob/ob mice (ENO2-ΔFosB-ob/ob). The mice with the following genotypes were used as the littermate controls: ENO2-tTATg/0/TetOp-ΔFosB0/0/Lepob/ob, ENO2-tTA0/0/TetOp-ΔFosBTg/0/Lepob/ob or ENO2-tTA0/0/TetOp-ΔFosB0/0/Lepob/ob (C-ob/ob), ENO2-tTATg/0/TetOp-ΔFosB0/0/Lep+/+, ENO2-tTA0/0/TetOp-ΔFosBTg/0/Lep+/+ or ENO2-tTA0/0/TetOp-ΔFosB0/0/Lep+/+ (C), and ENO2-tTATg/0/TetOp-ΔFosBTg/0/Lep+/+ (ENO2-ΔFosB). For genotyping, primers used were as follows: 5’-GTCCTCATCCATCACTGCTTCCA-3’ (forward) and 5’-CTACCAGCTATGTCTGTAGAGACA-3’ (reverse) for ENO2-tTA, 5’-CTCAGTACCTGTCTTCGGTGG-3’ (forward) and 5’-GATCTCCGACTCCAGCTCTG-3’ (reverse) for TetOp-ΔFosB. For Lepob genotyping, DdeI restriction digestion was performed following PCR using 5’-TGTCCAAGATGGACCAGACTC-3’ (forward) and 5’-ACTGGTCTGAGGCAGGGAGCA-3’ (reverse) primers. Male mice were analyzed and sacrificed at 35–40 weeks of age.
Calorimetric analysis of energy expenditure
Metabolic parameters were assessed using a paramagnetic oxygen sensor equipped Comprehensive Lab Animal Monitoring System CLAMS (Columbus Instruments) for the measurement of energy expenditure and locomotion, allowing ad libitum access to food and water, with simultaneous monitoring of up to 8 animals. Mice were placed in cages and allowed to acclimatize for 24 hours. Subsequently, measurements were taken every 18 minutes, for a period of 48 hours. RER is the ratio of CO2 produced relative to O2 consumed. Oxidation of carbohydrates results in RER of 1 and oxidation of fatty acids results in 0.7. Energy expenditure is a measure of heat [(3.815 + 1.232 x RER) x O2 consumed] normalized to body weight. Locomotion is indicated a total count of beam-breaking on x and z axis.
Glucose tolerance test and insulin tolerance test
Glucose tolerance test (GTT) was performed by administrating glucose (2.0 mg/g BW, Sigma) i.p. after a 16-hr fast. Blood glucose levels were monitored using glucose test strips and a glucometer (OneTouch ultra, LifeScan) at indicated times. Blood was also collected from tails using EDTA-treated microcapillaries and plasma insulin levels were measured using an EIA kit (ALPCO). For insulin tolerance test (ITT), mice were fasted for 4 hrs and injected insulin (1.0 mU/g BW, Lilly) intraperitoneally, and blood glucose levels were measured at indicated times. ITT data are presented as percentage of initial blood glucose concentration. The area under the curve (AUC) was calculated by the linear trapezoidal method. The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was calculated according to the formula; HOMA-IR = fasted insulin (μU/mL) x fasted glucose (mg/dL) / 405.
Insulin pancreatic β-cell immunohistochemistry
Pancreases were collected, fixed overnight in 4% PFA, embedded in paraffin and sectioned at 5 μm. Sections were immunostained using rabbit anti-insulin polyclonal antibody (4590, Cell Signaling) and counterstained with hematoxylin. Quantitative histomorphometric analysis of islet area and number was performed using Image J software (National Institute of Health).
Evaluation of bone volume by micro-CT, histomorphometry and serum P1NP and CTX
Upon completion of metabolic assessment, to determine the bone formation rate, mice were injected with fluorescent compounds prior to sample collection, as follows: 20 mg/kg calcein (day 0) and 40 mg/kg demeclocycline (day 4) and sacrificed on day 6 after the first injection. For micro-CT, femurs were removed and placed in 70% ethanol until scanning by SCANCO μ-CT35 (outsourced to Harvard School of Dental Medicine). Scans were conducted with an isotropic voxel size of 7 μm, x-ray tube potential of 70 kVP, an x-ray intensity of 0.145 mA, and an integration time of 600 ms per tomographic projection. From the scans, a region starting 280 μm proximal to the distal femoral growth plate and extending trabecular bone 2.1 mm was selected for trabecular bone analysis. A Gaussian filter using a fixed threshold at 26% of maximal grayscale value was applied to the femur images. Three-dimensional images were reconstructed from the 2-dimensional images from the contoured regions. For bone density, tibiae were removed, fixed in 4% formaldehyde, then infiltrated with a mixture of 90% methyl methacrylate (Sigma-Aldrich), 10% dibutyl phthalate (Sigma-Aldrich), and 0.15% benzoyl peroxide (Polyscience) at 4°C. Tibiae were then embedded in a mixture composed of 85% methyl methacrylate (Sigma-Aldrich), 15% dibutyl phthalate (Sigma-Aldrich), and 0.05% benzoyl peroxide (Polyscience). Polymerization was performed at 37°C. Standard undecalcified sections (4 μm) were prepared using a Reichert-Jung microtome (Cambridge Scientific) and were stained with von Kossa. Quantitative bone histomorphometric measurements were performed according to standardized protocols using the OsteoMeasure system (OsteoMetrics). Serum P1NP and CTX were measured using commercial kits according to the manufacturer’s instructions (Immunodiagnostic Systems).
Western blot evaluation of galanin expression
Hypothalami were isolated and lysed using SBN buffer: 1 mM EDTA, 50 mM Tris-HCl, 150 mM NaCl, 10% Glycerol, 1% NP40, pH 7.5 containing protease inhibitor cocktail (Sigma-Aldrich). Following 15 minutes incubation on ice, cell lysates were collected, centrifuged at 12,000 g for 15 min and supernatants were stored at −20ºC. Total protein quantification was performed using BCA protein assay kit (Thermo Scientific). Equal amounts of protein (50 μg) were heated in 1X loading buffer containing 1% DTT at 100ºC for 5 min, subjected to 10–15% SDS-PAGE, and transferred to nitrocellulose membrane using semi-dry TransBlot Turbo (BioRad). Primary rabbit anti-galanin (LSBio) antibodies were used in 1:1000 dilution in 5% bovine serum albumin in PBS blocking solution. Respective secondary anti-rabbit antibodies (CellSignaling) were used at 1:10,000 in 5% dry milk in PBS. Signal was developed with Western Lightning Plus ECL (PerkinElmer).
Statistical analysis
All data are expressed either as line graphs, showing mean ± SD, or as box whisker graphs, showing distribution of data into quartiles, highlighting the mean and outliers. These “whisker” lines indicate variability outside the upper and lower quartiles, and any point outside those lines or whiskers is considered an outlier. The significance of differences between groups was determined using JMP 8.0 Statistical Discovery Software (SAS Institute 2000) by 2-way ANOVA, followed by Tukey-Kramer honest significant difference (HSD) test. Differences were considered significant at P < 0.05. Groups marked by different letters significantly differ from each other (e.g., a is significantly different from b, but a is not significantly different from ab).
Results
ΔFosB expression in VHT does not affect body weight and energy expenditure in the absence of leptin
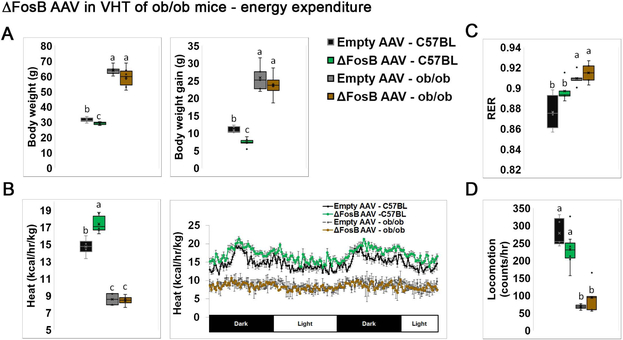
We have previously shown that ENO2-ΔFosB mice display almost two-fold lower circulating leptin levels, yet prolonged restoration of serum leptin to normal physiological levels, using osmotic pump infusion, failed to reduce the high bone mass phenotype (38). This suggested that ΔFosB-induced anabolic skeletal effects are not due to decreased blood leptin and still occur in the presence of normal levels of leptin. However, the possibility remained that the complete absence of leptin, rather than leptin sufficiency, would affect the ΔFosB-induced energy and/or bone phenotype, i.e. that leptin is required for ΔFosB to increase bone formation and bone mass. To address this question, we first stereotaxically delivered ΔFosB or GFP AVV (control) to the VHT of leptin-null ob/ob or wild-type C57BL (control) mice. Metabolic analysis revealed that in C57BL, centrally targeted ΔFosB decreases body weight, body weight gain (Figure 1A), and increases energy expenditure (Figure 1B), but not the respiratory exchange ratio (RER) or locomotion (Figure 1C,D) confirming our earlier observations that central AP1 antagonism shifts energy metabolism to a catabolic state (8, 28–32). On the other hand, ΔFosB expression in the VHT of ob/ob mice failed to trigger changes in either body weight or energy expenditure (Figure 1A, B). As expected, ob/ob mice were markedly more obese with suppressed energy expenditure compared to wild type control. Overall, these observations suggest that leptin is required to mediate ΔFosB whole body energy catabolic effects.
Figure 1. ΔFosB in VHT expression does not affect body weight and energy expenditure in leptin deficient ob/ob mice.
The VHTs of male 6- to 7-week old ob/ob or C57BL (control) mice were stereotactically injected with ΔFosB AAV or Empty AAV (control), and metabolic profiles were assessed 8 weeks after surgery. A. Body weight and body weight gain. B. Calorimetric analysis of energy expenditure. C. Respiratory exchange ratio (RER). D. Locomotion. Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, P < 0.05, comparing 4 groups (n = 7–8). Groups marked by different letters significantly differ from each other (e.g., a is significantly different from b, but a is not significantly different from ab).
ΔFosB expression in the VHT does not affect glucose clearance in the absence of leptin
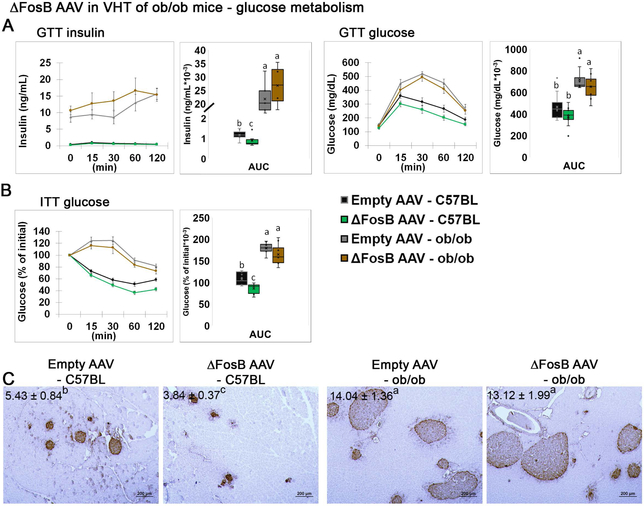
Glucose homeostasis is tightly linked to adiposity and energy regulation. Previously, we showed that mice expressing AP1 antagonists, ΔFosB or DNJunD in the VHT exhibit improved glucose tolerance and increased insulin sensitivity in target organs, with suppressed insulin secretion from pancreatic islets, via activation of the sympathetic nervous system (SNS) (34). These effects persisted in aged animals, suggesting that central AP1 antagonism confers protection against age-related deterioration in glucose metabolism (34). Here we tested whether the beneficial effects of ΔFosB on glucose metabolism require the presence of leptin. While ΔFosB expression in the VHT reduced insulin levels in glucose tolerance tests (GTT) (Figure 2A) and glucose levels in the insulin tolerance test (ITT) (Figure 2B) in wild-type mice, no changes were observed in ob/ob mice. Similarly, insulin immunohistochemistry showed markedly reduced islet size in wild-type mice injected with AAV ΔFosB, and no changes in ob/ob mice, which displayed enlarged islets regardless the presence of ΔFosB in the VHT, previously associated with insulin resistance (39, 40). Overall, these observations suggest that, similar to the increase in energy expenditure, leptin is required to mediate the effects of ΔFosB on glucose metabolism.
Figure 2. ΔFosB in VHT expression does not affect glucose metabolism in leptin deficient ob/ob mice.
The VHTs of male 6- to 7-week old ob/ob or C57BL (control) mice were stereotactically injected with ΔFosB AAV or Empty AAV (control), and glucose metabolism profiles were assessed 8 weeks after surgery. A. Glucose tolerance test, showing insulin and glucose levels and areas under the curve (AUC). B. Insulin tolerance test and AUC. C. Insulin immunohistochemistry, counterstained with hematoxylin. The numbers indicate average area occupied by islets (% β-cell area/pancreas area). Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, P < 0.05, comparing 4 groups (n = 7–8).
ΔFosB expression in the VHT increases bone mass despite the absence of leptin
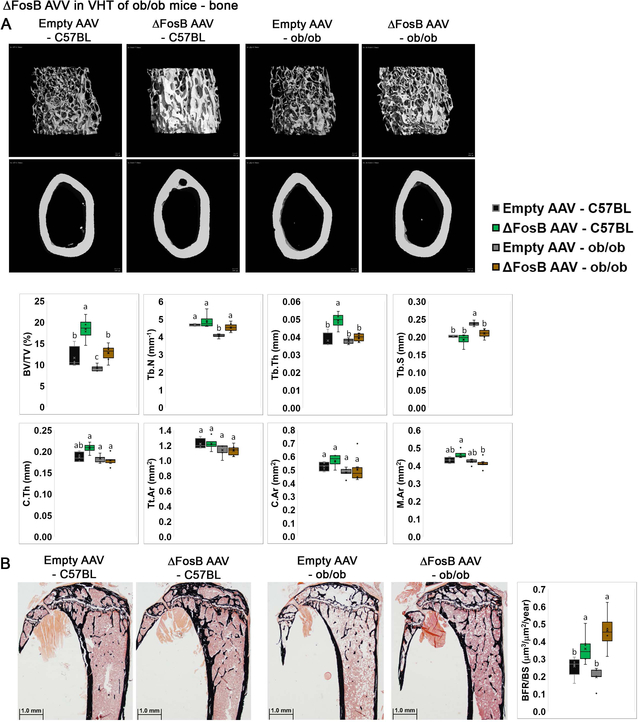
Leptin plays a major role in the neuroendocrine regulation of skeletal homeostasis, exerting both direct and indirect effects on bone (4, 7, 27, 41, 42). Previously, we showed that AP1 antagonism increases bone formation in transgenic ENO2-ΔFosB mice (28, 29, 31, 32, 38), when targeted to the VHT (30), or selectively targeted to either POMC or AgRP neurons (8). Since leptin receptors are abundantly present on ARC and VMH neurons, we determined whether the bone effects of central AP1 antagonism require leptin signaling. Interestingly, ΔFosB expression in the VHT increased bone volume/total volume (BV/TV) in both wild-type and ob/ob mice, as evident from micro-CT analysis, by 58.7% and 38.3% respectively (Figure 3A). ΔFosB expression in the VHT also increased trabecular number (Tb. N.) in ob/ob mice (Figure 3A). Moreover, and explaining the increase in bone mass, dynamic histomorphometric analysis revealed an increased bone formation rate (BFR/BS) following ΔFosB expression in the VHT in both wild-type and ob/ob mice (Figure 3B). These observations demonstrated that while ΔFosB whole body energy catabolic effects require intact leptin signaling, the bone mass effects circumvent the leptin pathways.
Figure 3. ΔFosB in VHT expression increases bone mass in wild-type or leptin deficient ob/ob mice.
The VHTs of male 6- to 7-week old ob/ob or C57BL (control) mice were stereotactically injected with ΔFosB AAV or Empty AAV (control), and bone profiles were assessed 8 weeks after surgery by micro-CT in femurs. A. Micro-CT analysis of femurs. B. Von Kossa staining and histomorphometric analysis of tibiae. Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, P < 0.05, comparing 4 groups (n = 7–8).
ENO-2 transgenic ΔFosB expression does not affect body weight, energy expenditure, or glucose metabolism but increases bone mass despite leptin deficiency
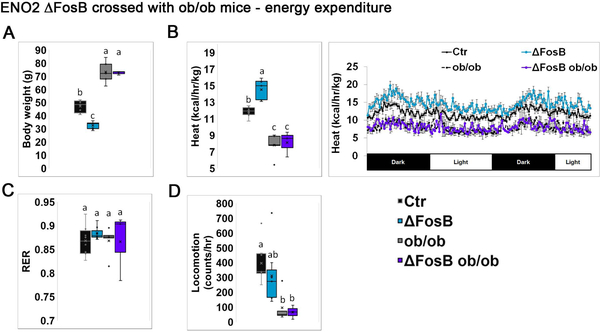
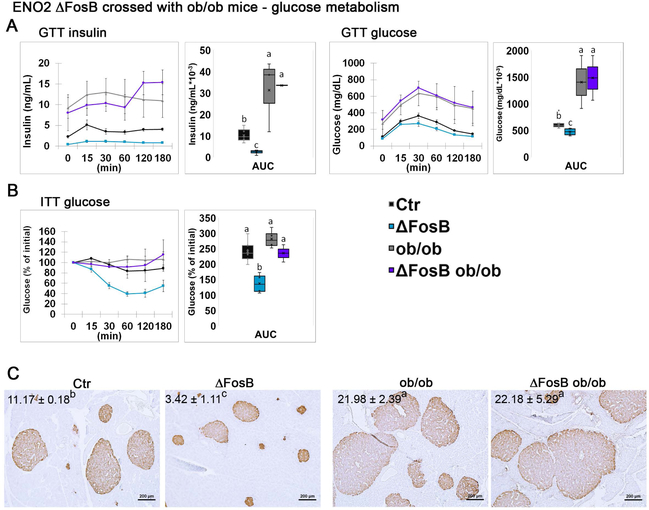
In the studies described above, we used the viral mediated gene transfer model (using AAVs), which is characterized by both temporal and anatomical restrictions, i.e. ΔFosB expression is targeted solely to the VHT and for a period of 8 weeks. To avoid these limitations we then explored the role of leptin in a transgenic mouse model expressing ΔFosB from conception on the background of leptin deficiency. For this purpose we crossed ENO2-ΔFosB with ob/ob mice and subjected aged mice (35–40 weeks old) to metabolic and bone assessment. Calorimetric energy analysis revealed that aged ENO2-ΔFosB mice have an enhanced metabolic profile, manifested by low body weight (Figure 4A), high energy expenditure (Figure 4B), as well as suppressed GTT insulin and glucose levels (Figure 5A), suppressed ITT glucose levels (Figure 5B) and smaller pancreatic islet size (Figure 5C) compared to wild-type control, confirming our earlier observations (Sato et al., 2017). In contrast, ENO2-ΔFosB-ob/ob mice were obese (Figure 4A), had suppressed energy expenditure (Figure 4B), elevated GTT insulin and glucose levels (Figure 5A) and enlarged pancreatic islets (Figure 5C), to an extent similar to ob/ob mice. These observations show that long term transgenic expression of ΔFosB on the background of total leptin deficiency fails to correct the metabolic phenotype of ob/ob mice. Overall, these data confirm our AAV-derived observations that leptin is required to mediate ΔFosB whole body energy and glucose metabolism beneficial effects.
Figure 4. Broad genetic ΔFosB expression does not affect body weight and energy expenditure in leptin deficient ob/ob mice.
ENO2-ΔFosB were crossed with ob/ob to generate ENO2-ΔFosB-ob/ob mice. Analysis was performed on 35–40 weeks old animals. Respective background strains were used as controls (see materials and methods, Generation of ENO2-ΔFosB-ob/ob mice). A. Body weight. B. Calorimetric analysis of energy expenditure. C. Respiratory exchange ratio (RER). D. Locomotion. Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, P < 0.05, comparing 4 groups (n = 4–5).
Figure 5. Broad genetic ΔFosB expression does not affect glucose metabolism in leptin deficient ob/ob mice.
ENO2-ΔFosB were crossed with ob/ob to generate ENO2-ΔFosB-ob/ob mice. Analysis was performed on 35–40 weeks old animals. Respective strains were used as controls (see materials and methods, Generation of ENO2-ΔFosB-ob/ob mice). A. Glucose tolerance test, showing insulin and glucose levels and areas under the curve (AUC). B. Insulin tolerance test and AUC. C. Insulin immunohistochemistry, counterstained with hematoxylin. The numbers indicate average area occupied by islets (% β-cell area/pancreas area). Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, P < 0.05, comparing 4 groups (n = 4–5).
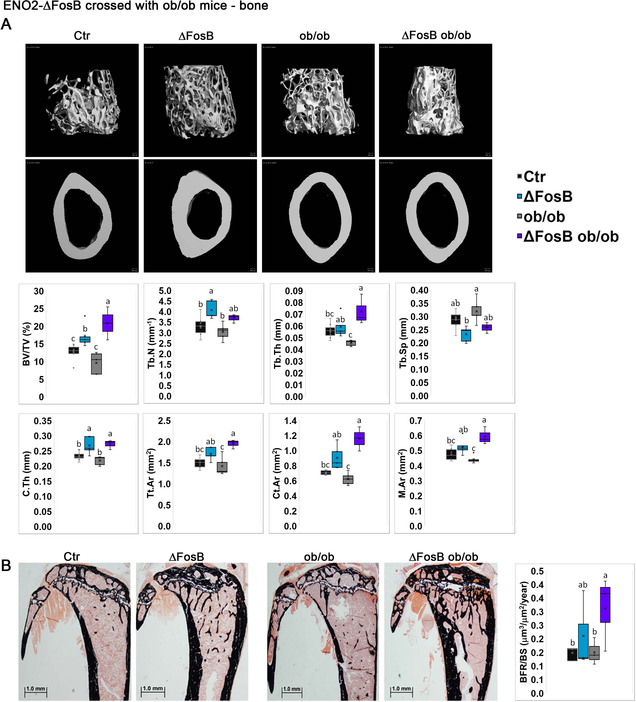
Next, we explored the effect of leptin deficiency on bone parameters in ENO2-ΔFosB-ob/ob mice. Of note, and in contrast with the reports of Karsenty et. al., (16, 24, 25) but in agreement with Hamrick et. al., (2004) and Turner et. al. (2013), we found ob/ob bone density to be significantly lower than control mice in long bones (Figure 3B) at 6–7 weeks of age. Nevertheless, and comparably to the elevated bone mass observed following ΔFosB stereotaxically targeted to the VHT of ob/ob mice (Figure 3), transgenic ΔFosB expression markedly increased trabecular BV/TV, and even more so in ob/ob mice, by 31.5% and 116.0% in ENO2-ΔFosB and ENO2-ΔFosB-ob/ob mice respectively, compared to their respective controls (Figure 6A,B). Increase in Tb.N and increase in Tb.Th. seem to account for an increase in bone mass in each mouse model, respectively. Most interestingly, ENO2-ΔFosB-ob/ob mice reached a significantly higher bone mass than ΔFosB mice (Figure 6A), showing that the effects of ΔFosB are more pronounced in the absence of leptin. This suggests that leptin may actually limit the effects of ΔFosB on bone in wild-type animals. Consequently, ENO2-ΔFosB-ob/ob mice also displayed 25.0% higher cortical thickness (C.Th.) than ob/ob mice (Figure 6A). Taken together, these data demonstrate that unlike the effects on metabolism and glucose, ΔFosB increases bone mass in a leptin independent manner.
Figure 6. Broad genetic ΔFosB expression increases bone mass in leptin deficient ob/ob mice.
ENO2-ΔFosB were crossed with ob/ob to generate ENO2-ΔFosB-ob/ob mice. Bone analysis was performed on 35–40 weeks old animals, by micro-CT in femurs. Respective strains were used as controls (see materials and methods, Generation of ENO2-ΔFosB-ob/ob mice). A. Micro-CT analysis of femurs. B. Von Kossa staining and histomorphometric analysis of tibiae. Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, P < 0.05, comparing 4 groups (n = 4–5).
ΔFosB expression in the VHT fails to affect energy, glucose metabolism or bone mass in the absence of galanin
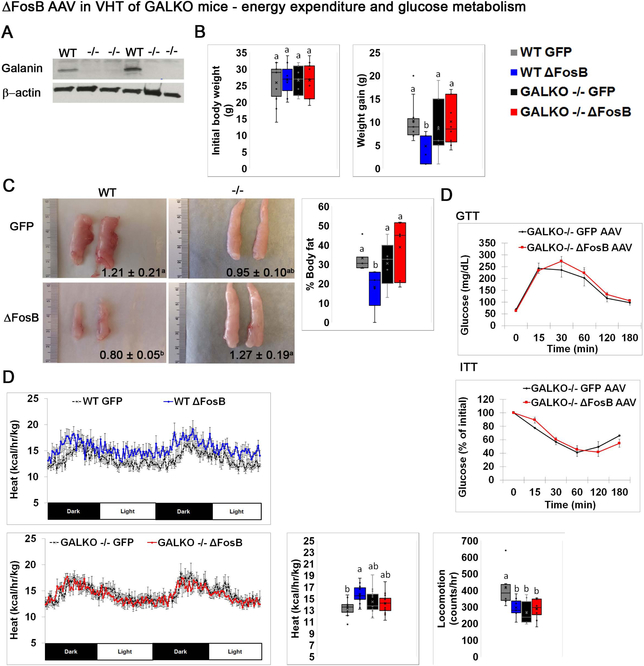
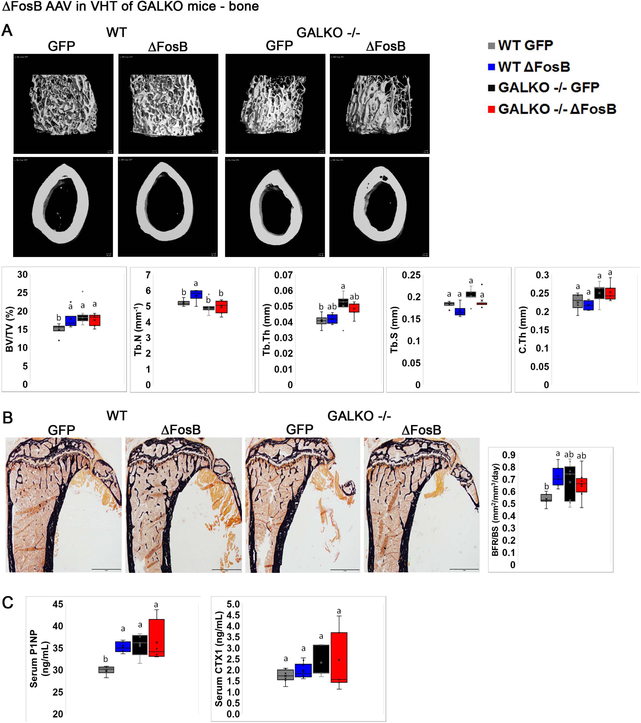
Recently, we demonstrated that ΔFosB or DNJunD AP1 antagonism in AgRP- and POMC-expressing neurons in the ARC increases energy, glucose metabolism and bone formation by upregulating the expression of the neuromediator galanin in the VHT (8). Our data above suggest that leptin is required for the metabolic boost, but not for the induction of high bone mass by ΔFosB. Since leptin is known to signal in the galanin-expressing VHT neurons and increase galanin mRNA transcription (36, 43), we hypothesized that galanin could reside downstream of leptin and its deficiency may prevent ΔFosB bone and/or energy metabolism effects. To address this question, we stereotaxically delivered ΔFosB or GFP AVV (control) to the VHT of galanin-null GALKO (37) or wild-type (control) mice. Galanin deletion was validated by western blot (Figure 7A). Confirming our expectations, metabolic analysis revealed that absence of galanin prevents the reduction in body weight (Figure 7B), body adiposity and fat pad size (Figure 7C), as well as the improved glucose utilization (Figure 7D) and higher energy expenditure (Figure 7E), otherwise seen following ΔFosB expression in the VHT of wild-type mice. Moreover, galanin deficiency, unlike leptin deficiency, also prevented the induction of the high bone mass phenotype, as GALKO-ΔFosB and GALKO-GFP groups display the same % BV/TV (Figure 8A), BFR/BS (Figure 8B) and bone formation P1NP serum marker levels (Figure 8C). Overall, these data demonstrate that, in contrast with leptin, galanin is required to mediate ΔFosB energy catabolic and bone anabolic effects via the VHT.
Figure 7. Global deletion of galanin prevents ΔFosB-induced increases in glucose and metabolism.
Male wild type and homozygote GALKO mice were stereotaxically injected with ΔFosB AAV or GFP AAV as control. Energy metabolism was analyzed 6 weeks post-surgery. A. Western blot of galanin expression in the hypothalamus of control (wild-type) and GALKO mice (n=3). B. Initial weights pre-operatively and after 6 weeks. C. Representative images of eWAT with average pad weight (grams). D. GTT and ITT. E. Calorimetric analysis of energy expenditure and locomotion. Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, comparing 4 groups (n=6–8), p<0.05.
Figure 8. Global deletion of galanin prevents ΔFosB-induced increases in bone mass.
Male 6- to 7-week old wild type and homozygote GALKO mice were stereotaxically injected with ΔFosB AAV or GFP AAV as control. Bone was analyzed 7 weeks post-surgery. A. Micro-CT analysis of femurs. B. Von Kossa staining and histomorphometric analysis of tibiae. C. Serum P1NP and CTX. Statistical analysis included 2-way ANOVA followed by Tukey-Kramer HSD test, comparing 4 groups (n=6–8), p<0.05.
Discussion
This study focused on understanding whether leptin mediates some or all of the effects of the AP1 antagonist ΔFosB on energy expenditure, glucose metabolism and/or bone density, and whether the regulation of these functions is interrelated. We used two animal models where ΔFosB is expressed either in a restricted area of the brain and for a limited period of time (ΔFosB AAV gene mediated transfer to VHT) or constitutively (ENO2-ΔFosB) mostly in the brain but also in other tissues. Both of these models were then tested on the background of leptin deficiency by utilizing the leptin-deficient ob/ob mouse. Both animal models established clearly that if leptin is required to mediate the VHT effects of ΔFosB on metabolism, leading to body weight reduction, increased energy expenditure and improved glucose clearance, the central ΔFosB-induced high bone density effect is independent of leptin. In fact, our results show that leptin may actually limit the influence of ΔFosB on bone homeostasis. In contrast to leptin, we found, using galanin deficient mice, that the neuromediator galanin is required to mediate ΔFosB effects in the VHT not only on energy metabolism and glucose but also on bone mass.
The concept of energy homeostasis refers to the combined processes that manage fuel intake (feeding), storage (adiposity) and usage (energy expenditure, encompassing basal metabolism, exercise, and thermogenesis). The regulation of glucose is tightly linked to energy metabolism, as these systems coordinate to ensure adequate nutrient supply to the tissues (44). Historically, energy homeostasis was thought to be regulated by the central nervous system (CNS), whereas glucose homeostasis was viewed predominantly as a peripheral process, driven by pancreatic insulin production and glucose surge into the liver and skeletal muscle. Recent findings made this concept evolve by showing that the same ARC neurons responding to “anorexigenic” leptin signaling to control whole body adiposity, are also crucial for the regulation of glucose. The direct action of leptin in the ARC, via targeted expression of leptin receptors, was shown to be sufficient to restore normoglycemia in otherwise hyperglycemic db/db mice (45). Deletion of leptin receptors in POMC neurons leads to mild-obesity (46, 47) whereas mice lacking both leptin and insulin receptors in POMC neurons display insulin resistance (47). Re-expression of leptin receptors in POMC neurons normalizes blood glucose and improves hepatic insulin resistance, hyperglucagonemia, and dyslipidemia in the otherwise hyperglycemic leptin receptor-null mice (48). It was reported that leptin action depolarizes (activates) POMC neurons (49), while simultaneously hyperpolarizes (inactivates) AgRP neurons (50). ~30% of POMC neurons and ~10–20% of AgRP neurons are believed to be leptin responsive, respectively (51). AgRP neurons, which co-exist side by side with POMC neurons, were also demonstrated to be critical in the leptin regulation of energy and glucose homeostasis. Ablation of AgRP neurons in ob/ob mice showed reduced feeding and improved glucose tolerance (52), while overexpression of leptin receptor in AgRP neurons of ob/ob mice normalized blood glucose (53).
Collectively, the aforementioned studies, deciphering the action of leptin in the ARC, formed the basis of the “hunger-satiety” model, where long-term energy balance was suggested to depend on the circulating levels of the “anorexigenic” leptin and other neuroendocrine molecules, such as “orexigenic” ghrelin. Recent studies, measuring the AgRP and POMC neurons dynamics, however, revealed that a mere presentation of palatable food inhibits AgRP neuronal firing, (54, 55), activating POMC neurons (56). Therefore, it appears that homeostatic regulation of energy balance, relying on hormonal deviation from a physiological set-point, such as fluctuating leptin levels, is distinct from the sensory regulation (food “anticipation”) (57). In this context, our present work demonstrates that central or broader transgenic expression of ΔFosB fails to trigger favorable effects on glucose utilization in the absence of leptin, as shown by GTT and ITT tests and unchanged pancreatic islet size in ob/ob mice. These results establish that leptin is positioned downstream of ΔFosB in a signaling cascade that regulates glucose metabolism. Although, we did not target specific neuronal populations in this report, we have recently shown that AP1 antagonism in either AgRP- or POMC-expressing neurons produces a multifaceted energy catabolism effect, characterized by increased energy expenditure, reduced adiposity, and glucose utilization, without effects on feeding (34). Based on this literature, it is plausible to assume that leptin signaling mediates the ΔFosB-induced long-term homeostatic energy balance, rather than short-term sensory balance.
Information from the leptin sensitive 1st order VHT neurons is translated to changes in cellular composition, activity and connectivity with 2nd order intra- and extra-hypothalamic neurons (1), which trigger changes in the pituitary hormone secretion and/or autonomic nervous system, SNS and PNS, function to maintain energy homeostasis (9, 58). Here we show that in addition to leptin, galanin is required to mediate ΔFosB effects on energy balance, as GALKO mice fail to respond to ΔFosB VHT expression. Recent reports using mice with conditional leptin receptor deletion from galanin neurons, demonstrated that galanin is an important mediator of leptin’s anorexigenic and nutrient rewarding action in lateral hypothalamic area (LHA) (36, 43). Although, LHA is anatomically distinct from the ARC, it is plausible to assume that the metabolic action of leptin may be mediated by the 1st order AgRP, POMC, and galanin-expressing neurons, as well as by heterogenic populations (expressing one or more neuropeptides), altering the SNS tone, that we recently showed to be important for the central ΔFosB effects on energy and glucose homeostasis (34).
Beyond the metabolic effects, and often considered as a secondary effect of the metabolic changes, leptin is known to act as a regulator of bone (9, 15, 24, 27, 59–61). Interestingly, the central effect of leptin on bone appears to utilize neuronal pathways other than within the ARC. Several lines of evidence showed that peripheral leptin acts on the brainstem, where it inhibits serotonin production and release by the Raphe nuclei. These serotonergic neurons then project to the VMH, where, via its action on serotonin 2C receptors (Htr2c) receptors, increase SNS tone and inhibit bone accrual (24, 62), supporting earlier observations that intact VMH is required for leptin to regulate bone mass (25). Opposing this notion, another study showed that leptin does not directly affect brain serotonin neurons (63). They observed that while leptin hyperpolarizes some non-serotonin producing Raphe neurons, it does not alter the activity of serotonergic ones. Thus, further research is needed to resolve the leptin - serotonin link in the control of bone. Of interest, the brainstem Raphe nucleus, via which the leptin pathway was suggested to take its course, was recently shown to contain GABAergic and glutamatergic neurons which exert reciprocal and bidirectional regulation of feeding and body weight, independently of leptin (64). Transcriptional analysis of these neurons revealed that glutamatergic, but not GABAergic Raphe nucleus terminals also express markers of serotonin synthesis. These findings raise the possibility that these excitatory and inhibitory brainstem neurons may also be involved in the regulation of bone biology independently of leptin (9). Moreover, leptin being produced by the adipocytes, it is also present in the bone marrow and can affect bone cells peripherally, in a paracrine fashion, as suggested by several studies (65–67). Strong evidence for leptin’s role in the bone microenvironment came from a study where bone marrow from db/db mice was transplanted into lethally irradiated wild-type mice, resulting in markedly suppressed BFR in the femur and lumbar vertebrae, to values indistinguishable from db/db mice (19). Our present work demonstrates that central or transgenic expression of ΔFosB induces a robust increase in bone density, as seen by micro-CT, regardless of leptin’s absence. In fact, the absence of leptin may actually enhance the bone response as indicated by the fact that the proportional increase in bone density is much higher in the ob/ob mice than in the control mice, reaching significantly higher values in the absence than in the presence of leptin (Figure 6A). This finding is consistent with the concept that leptin exerts mostly a negative influence on bone homeostasis (16, 19, 25) and also the hypothesis that leptin mediates is effects on bone mass via routes other than the ARC, possibly the brainstem. Moreover, this finding is consistent with our recent report showing that AP1 antagonism in either ARC neuron type – AgRP or POMC, promotes bone formation and density, via a galanin-dependent mechanism (8). Therefore, it is plausible to assume that leptin acts on hypothalamic galanin-expressing neurons, blocking the effect of galanin. It is likely that leptin acts on galanin-expressing neurons, other than AgRP or POMC, since leptin receptor deletion from these neurons, previously shown to increase body weight and adiposity (68), did not affect bone (69). Interestingly, although the bone-accrual response to AP1 antagonism is enhanced in the absence of leptin, the femoral % BV/TV of ob/ob mice is lower at 15 weeks of age (Figure 3A) and unchanged at 40 weeks of age (Figure 6A), suggesting that the effects of leptin are complex and may vary with age.
The limitations of this study include possible variability in the stereotaxic injection site, due to human error, although the accuracy of injection was validated in each mouse brain and animals with off-target virus delivery were excluded from the analysis. Other limitations are unknown identity of targeted neurons, lack of information on the electro-chemical neuronal activity, as well as age difference between two experimental models. Physiologically, ΔFosB expression is regulated in the brain by several circumstances, such during drug addiction and c-Fos is a known marker of neuronal activation (70) and we speculate that ΔFosB overexpression influences neuronal firing and future studies may shed light on the link between neuronal activation/suppression and bone biology.
Given that we observe no effects of ΔFosB on energy and glucose metabolism while its effects on bone are maintained in the absence of leptin, our results establish again, as we have recently shown (Idelevich et al, 2018), that the changes in bone are independent of the changes in energy and glucose metabolism, and vice versa. Indeed, a collection of published data gave impetus to the idea that changes in energy regulation, and consequently glucose metabolism, can influence bone homeostasis (25, 61, 71) and reciprocally, that bone remodeling can affect energy and glucose metabolism (72, 73). Our findings, as well as our earlier report on SF1 neurons (Idelevich et al., 2018) show instead that, whereas they certainly can influence each other, the energy, glucose and bone phenotypes are not ineluctably linked, whether centrally or at the periphery. In other words, in our models the changes in bone remodeling and homeostasis are neither the cause nor the consequence of the changes in energy and/or glucose metabolism and vice-versa. In conclusion, our study establishes that ΔFosB in the VHT can regulate energy expenditure, insulin sensitivity and bone density but demonstrates that, in this model, while the metabolic effects are leptin- and galanin-dependent, the bone effects are not only leptin- independent but also galanin-dependent and independent of changes in energy expenditure and/or glucose metabolism.
Acknowledgements
This work was funded by a grant (AG-040222) from the National Institute of Aging, NIH to R.B.; A.I. was the recipient of a Dean Scholarship from Harvard School of Dental Medicine. The authors are very grateful to Dr. Eric Nestler (Mount Sinai Icahn School of Medicine) for his advice and help with stereotaxic experiments and to Dr. Marina Picciotti (Yale University School of Medicine) for providing us with the GALKO mice.
References
- 1.Waterson MJJ, Horvath TLL. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. [Internet]. Cell Metab. 2015;22(6):962–70. [DOI] [PubMed] [Google Scholar]
- 2.Richard D. Cognitive and autonomic determinants of energy homeostasis in obesity [Internet]. Nat. Rev. Endocrinol 2015;11(8):489–501. [DOI] [PubMed] [Google Scholar]
- 3.Kim K-S, Seeley RJ, Sandoval DA. Signalling from the periphery to the brain that regulates energy homeostasis [Internet]. Nat. Rev. Neurosci 2018;19(4):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houweling P, Kulkarni RN, Baldock PA. Neuronal control of bone and muscle. [Internet]. Bone 2015;80:95–100. [DOI] [PubMed] [Google Scholar]
- 5.Quiros-Gonzalez I, Yadav VK. Central genes, pathways and modules that regulate bone mass. [Internet]. Arch. Biochem. Biophys 2014;561:130–6. [DOI] [PubMed] [Google Scholar]
- 6.Dimitri P, Rosen C. The Central Nervous System and Bone Metabolism: An Evolving Story [Internet]. Calcif. Tissue Int 2017;100(5):476–485. [DOI] [PubMed] [Google Scholar]
- 7.Karsenty G Convergence between bone and energy homeostases: leptin regulation of bone mass. [Internet]. Cell Metab. 2006;4(5):341–8. [DOI] [PubMed] [Google Scholar]
- 8.Idelevich A et al. Neuronal hypothalamic regulation of body metabolism and bone density is galanin-dependent [Internet]. J. Clin. Invest [published online ahead of print: March 29, 2018]; doi: 10.1172/JCI99350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idelevich A, Baron R. Brain to bone: What is the contribution of the brain to skeletal homeostasis? [Internet]. Bone [published online ahead of print: May 16, 2018]; doi: 10.1016/j.bone.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elefteriou F Impact of the Autonomic Nervous System on the Skeleton [Internet]. Physiol. Rev 2018;98(3):1083–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain–adipose crosstalks [Internet]. Nat. Rev. Neurosci 2018;19(3):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan WW, Myers MG. Leptin and the maintenance of elevated body weight [Internet]. Nat. Rev. Neurosci 2018;19(2):95–105. [DOI] [PubMed] [Google Scholar]
- 13.Cui H, López M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. [Internet]. Nat. Rev. Endocrinol 2017;13(6):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. [Internet]. Dis. Model. Mech 2017;10(6):679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. [Internet]. Nature 2012;481(7381):314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducy P et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. [Internet]. Cell 2000;100(2):197–207. [DOI] [PubMed] [Google Scholar]
- 17.Hamrick M, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine [Internet]. Bone 2004;34(3):376–383. [DOI] [PubMed] [Google Scholar]
- 18.Baldock PA et al. Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. [Internet]. J. Bone Miner. Res 2005;20(10):1851–7. [DOI] [PubMed] [Google Scholar]
- 19.Turner RT et al. Peripheral leptin regulates bone formation [Internet]. J. Bone Miner. Res 2013;28(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid IR, Baldock PA, Cornish J. Effects of Leptin on the Skeleton. [Internet]. Endocr. Rev 2018;39(6):938–959. [DOI] [PubMed] [Google Scholar]
- 21.Philbrick KA, Wong CP, Branscum AJ, Turner RT, Iwaniec UT. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. [Internet]. J. Endocrinol 2017;232(3):461–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCabe IC et al. Novel leptin receptor signaling mutants identify location and sex-dependent modulation of bone density, adiposity, and growth. [Internet]. J. Cell. Biochem [published online ahead of print: September 30, 2018]; doi: 10.1002/jcb.27726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. [Internet]. EMBO Rep. 2012;13(12):1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav VK et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. [Internet]. Cell 2009;138(5):976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda S et al. Leptin regulates bone formation via the sympathetic nervous system. [Internet]. Cell 2002;111(3):305–17. [DOI] [PubMed] [Google Scholar]
- 26.Singh MK, Elefteriou F, Karsenty G. Cocaine and amphetamine-regulated transcript may regulate bone remodeling as a circulating molecule. [Internet]. Endocrinology 2008;149(8):3933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idelevich A, Sato K, Baron R. What are the effects of leptin on bone and where are they exerted? [Internet]. J. Bone Miner. Res 2013;28(1):18–21. [DOI] [PubMed] [Google Scholar]
- 28.Sabatakos G et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. [Internet]. Nat. Med 2000;6(9):985–90. [DOI] [PubMed] [Google Scholar]
- 29.Kveiborg M et al. DeltaFosB induces osteosclerosis and decreases adipogenesis by two independent cell-autonomous mechanisms. [Internet]. Mol. Cell. Biol 2004;24(7):2820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe GC et al. Energy expenditure and bone formation share a common sensitivity to AP-1 transcription in the hypothalamus. [Internet]. J. Bone Miner. Res 2012;27(8):1649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe GC et al. Increased energy expenditure and insulin sensitivity in the high bone mass DeltaFosB transgenic mice. [Internet]. Endocrinology 2009;150(1):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabatakos G et al. Doubly truncated FosB isoform (Delta2DeltaFosB) induces osteosclerosis in transgenic mice and modulates expression and phosphorylation of Smads in osteoblasts independent of intrinsic AP-1 activity. [Internet]. J. Bone Miner. Res 2008;23(5):584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. [Internet]. Nat. Neurosci 2003;6(11):1208–15. [DOI] [PubMed] [Google Scholar]
- 34.Sato K et al. Hypothalamic ΔFosB prevents age-related metabolic decline and functions via SNS [Internet]. Aging (Albany. NY) [published online ahead of print: January 20, 2017]; doi: 10.18632/aging.101157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang R et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. [Internet]. Pharmacol. Rev 2015;67(1):118–75. [DOI] [PubMed] [Google Scholar]
- 36.Laque A et al. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons [Internet]. Mol. Metab 2015;4(10):706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynick D et al. Galanin regulates prolactin release and lactotroph proliferation. [Internet]. Proc. Natl. Acad. Sci. U. S. A 1998;95(21):12671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kveiborg M et al. The Increased Bone Mass in ΔFosB Transgenic Mice Is Independent of Circulating Leptin Levels [Internet]. Endocrinology 2002;143(11):4304–4309. [DOI] [PubMed] [Google Scholar]
- 39.Tomita T, Doull V, Pollock HG, Krizsan D. Pancreatic islets of obese hyperglycemic mice (ob/ob). [Internet]. Pancreas 1992;7(3):367–75. [DOI] [PubMed] [Google Scholar]
- 40.Kim J-Y et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. [Internet]. J. Clin. Invest 2007;117(9):2621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. [Internet]. Metabolism. 2015;64(1):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karsenty G, Olson EN. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. [Internet]. Cell 2016;164(6):1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laque A et al. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action [Internet]. Am. J. Physiol. Metab 2013;304(9):E999–E1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grayson BE, Seeley RJ, Sandoval DA. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis [Internet]. Nat. Rev. Neurosci 2013;14(1):24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coppari R et al. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. [Internet]. Cell Metab. 2005;1(1):63–72. [DOI] [PubMed] [Google Scholar]
- 46.Balthasar N et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. [Internet]. Neuron 2004;42(6):983–91. [DOI] [PubMed] [Google Scholar]
- 47.Hill JW et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. [Internet]. Cell Metab. 2010;11(4):286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berglund ED et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. [Internet]. J. Clin. Invest 2012;122(3):1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cowley MA et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. [Internet]. Nature 2001;411(6836):480–4. [DOI] [PubMed] [Google Scholar]
- 50.Pinto S et al. Rapid Rewiring of Arcuate Nucleus Feeding Circuits by Leptin [Internet]. Science (80-. ). 2004;304(5667):110–115. [DOI] [PubMed] [Google Scholar]
- 51.Fujikawa T, Coppari R. Living without insulin: the role of leptin signaling in the hypothalamus. [Internet]. Front. Neurosci. 2015;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility [Internet]. Proc. Natl. Acad. Sci 2012;109(8):3155–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonçalves GHM, Li W, Garcia AVC-G, Figueiredo MS, Bjørbæk C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. [Internet]. Cell Rep. 2014;7(4):1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Lin Y-C, Kuo T-W, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. [Internet]. Cell 2015;160(5):829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Betley JN et al. Neurons for hunger and thirst transmit a negative-valence teaching signal [Internet]. Nature 2015;521(7551):180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandelblat-Cerf Y et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. [Internet]. Elife 2015;4. doi: 10.7554/eLife.07122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Knight ZA. Making sense of the sensory regulation of hunger neurons. [Internet]. Bioessays 2016;38(4):316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. [Internet]. Trends Neurosci. 2013;36(2):65–73. [DOI] [PubMed] [Google Scholar]
- 59.Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases [Internet]. J. Bone Miner. Metab 2015;33(5):474–485. [DOI] [PubMed] [Google Scholar]
- 60.Froguel P et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. [Internet]. Nature 1998;392(6674):398–401. [DOI] [PubMed] [Google Scholar]
- 61.Elefteriou F et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. [Internet]. Nature 2005;434(7032):514–20. [DOI] [PubMed] [Google Scholar]
- 62.Oury F et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. [Internet]. Genes Dev. 2010;24(20):2330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam DD et al. Leptin Does Not Directly Affect CNS Serotonin Neurons to Influence Appetite [Internet]. Cell Metab. 2011;13(5):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nectow AR et al. Identification of a Brainstem Circuit Controlling Feeding [Internet]. Cell 2017;170(3):429–442.e11. [DOI] [PubMed] [Google Scholar]
- 65.Burguera B et al. Leptin increases proliferation of human steosarcoma cells through activation of PI(3)-K and MAPK pathways. [Internet]. Med. Sci. Monit 2006;12(11):BR341–9. [PubMed] [Google Scholar]
- 66.Hess R, Pino AM, Ríos S, Fernández M, Rodríguez JP. High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. [Internet]. J. Cell. Biochem 2005;94(1):50–7. [DOI] [PubMed] [Google Scholar]
- 67.Scheller EL et al. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. [Internet]. Stem Cells 2010;28(6):1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van de Wall E et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. [Internet]. Endocrinology 2008;149(4):1773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JG et al. AgRP Neurons Regulate Bone Mass [Internet]. Cell Rep. 2015;13(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joo J-Y, Schaukowitch K, Farbiak L, Kilaru G, Kim T-K. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. [Internet]. Nat. Neurosci [published online ahead of print: November 23, 2015]; doi: 10.1038/nn.4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fulzele K et al. Insulin Receptor Signaling in Osteoblasts Regulates Postnatal Bone Acquisition and Body Composition [Internet]. Cell 2010;142(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee NK et al. Endocrine Regulation of Energy Metabolism by the Skeleton [Internet]. Cell 2007;130(3):456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferron M et al. Insulin Signaling in Osteoblasts Integrates Bone Remodeling and Energy Metabolism [Internet]. Cell 2010;142(2):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]