Summary
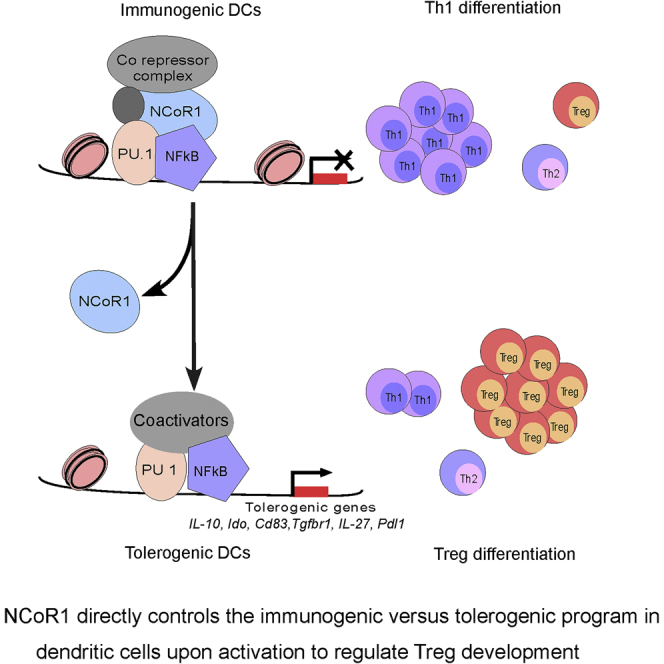
Understanding the mechanisms fine-tuning immunogenic versus tolerogenic balance in dendritic cells (DCs) is of high importance for therapeutic approaches. We found that NCoR1-mediated direct repression of the tolerogenic program in conventional DCs is essential for induction of an optimal immunogenic response. NCoR1 depletion upregulated a wide variety of tolerogenic genes in activated DCs, which consequently resulted in increased frequency of FoxP3+ regulatory T cells. Mechanistically, NCoR1 masks the PU.1-bound super-enhancers on major tolerogenic genes after DC activation that are subsequently bound by nuclear factor-κB. NCoR1 knockdown (KD) reduced RelA nuclear translocation and activity, whereas RelB was unaffected, providing activated DCs a tolerogenic advantage. Moreover, NCoR1DC−/- mice depicted enhanced Tregs in draining lymph nodes with increased disease burden upon bacterial and parasitic infections. Besides, adoptive transfer of activated NCoR1 KD DCs in infected animals showed a similar phenotype. Collectively, our results demonstrated NCoR1 as a promising target to control DC-mediated immune tolerance.
Subject Areas: Molecular Mechanism of Gene Regulation, Immunology, Immune Response, Transcriptomics
Graphical Abstract
Highlights
-
•
NCoR1 directly represses tolerogenic program in mouse cDCs
-
•
Depletion of NCoR1 in cDCs enhanced Treg development ex vivo and in vivo
-
•
NCoR1 masks PU.1-bound super-enhancers on tolerogenic genes in cDCs
-
•
NCoR1DC−/− animals depicted enhanced Treg frequency and infection load
Molecular Mechanism of Gene Regulation; Immunology; Immune Response; Transcriptomics
Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that link innate to adaptive immunity (Steinman, 2006). Upon encounter with pathogens, they get activated resulting in maturation and migration to draining lymph nodes. Primed DCs then polarize naive and memory T helper (Th) cells into various effector subtypes such as Th1, Th2, Th17, or Tregs (Hochweller et al., 2010, Kapsenberg, 2003). The differentiation of these different Th subsets depends on the DC maturation status and the secreted cytokines. DCs are thus considered as major players in fine-tuning immunity versus immune tolerance.
DCs express a range of pattern recognition receptors such as Toll-like receptors (TLRs) to identify a wide plethora of pathogens (Takeuchi and Akira, 2010). Here in this study, we have focused mostly on CD8+ cDC1 DCs. cDC1 DCs are the main antigen cross-presenting DCs for intracellular pathogens detected through TLR3 and TLR9 (Reizis, 2011, Schnorrer et al., 2006). Upon ligation of these receptors, Myd88 and TRIF signaling pathways are stimulated, consequently activating transcription factors (TFs) like nuclear factor (NF)-κB and interferon regulatory factors (IRFs) and a variety of protein kinases (Kawasaki and Kawai, 2014, O'Neill et al., 2013). It has been reported that in DCs canonical NF-κB signaling through IkBα activates both RelA and RelB, whereas in other cells like fibroblasts only non-canonical signaling results in RelB activation (Shih et al., 2012). RelA signaling is important for pro-inflammatory response and cell survival (Shih et al., 2012). On the other side, RelB is reported to be involved in DC maturation and induction of tolerance (Azukizawa et al., 2011, Thomas, 2013). Therefore depending on the stimulus and a fine balance of NF-κB activity, DCs exhibit either immunogenic or regulatory phenotype (Vendelova et al., 2018). The activation stimulus results in signal-specific expression of a large number of DC response genes including cytokines (interleukin [IL]-6, IL-10, IL-12) and co-stimulatory markers (CD40, CD80, and CD86) (Matsushima et al., 2004). Depending on the strength and specificity of these DC responses, naive Th cells differentiate into different effector subtypes (Hochweller et al., 2010, Kaiko et al., 2008). The inflammatory cytokine IL-12 with strong co-stimulatory signals results in Th1, whereas anti-inflammatory signals IL-4, IL-5, and IL-13 induce Th2 development (Liu et al., 2005b) (MacDonald and Maizels, 2008). Similarly, the cytokine IL-10, with strong or weak co-stimulatory signals, respectively, generates Th2 or Tregs (Couper et al., 2008, Saraiva and O'Garra, 2010). Thus, it has been widely proposed that DC responses can be modulated to treat a variety of diseases, such as cancer and autoimmunity (Moreau et al., 2017).
We know that DCs receive multiple stimulatory cues to generate signal-specific responses, and it appears improbable that TFs alone can integrate such a vast number of regulatory signals. One of the well-characterized mechanisms how nuclear receptors (NRs) regulate transcriptional responses is through the recruitment of transcriptional co-regulators (co-repressors or co-activators) (McKenna and O'Malley, 2002, Mouchiroud et al., 2014, Ng et al., 2011). Recent reports show that DC treatment with high-affinity NR ligands such as rosiglitazone and vitamin D modulates expression of co-stimulatory molecules and cytokine genes, which perturbs their functional responses (Agrawal et al., 2016, Farias et al., 2013, Thompson et al., 2007). Analysis of published NCoR1 chromatin sequencing (ChIP-seq) data from macrophages revealed NCoR1 binding on genes accountable for antigen recognition, co-stimulation, and T cell polarization in DCs. This strongly suggested the functional involvement of NR co-repressors like NCoR1 in DCs, as they were identified in complexes with unliganded NRs such as peroxisome proliferator activated receptor gamma (PPARγ) and thyroid receptor (TR) (Cohen et al., 1998, Huang et al., 2009, Li et al., 2013, Mottis et al., 2013, Yu et al., 2005). These co-repressors inhibit gene expression through chromatin compaction by recruiting histone deacetylases (Huang et al., 2009, Perissi et al., 2008). In contrast, we reported NCoR1 enrichment at open chromatin regions marked by H3K27ac/H3K4me1 (Raghav et al., 2012). Despite several reports indicating so, a role of NCoR1 in DC function has not been directly evaluated in a systematic study.
Here, we employed an immunogenomics approach to elucidate the role of NCoR1 in DC function. We demonstrated NCoR1 as a master regulator of the tolerogenic program in cDCs. We found that direct repression of tolerogenic genes by NCoR1 upon DC activation is important to induce an optimal immunogenic response. In addition, we explored the underlying molecular mechanisms and in vivo physiological impact of NCoR1-modulated DC responses in parasite-infected animals.
Results
NCoR1 KD cDC Line Showed Enhanced Tolerogenic Responses upon Activation
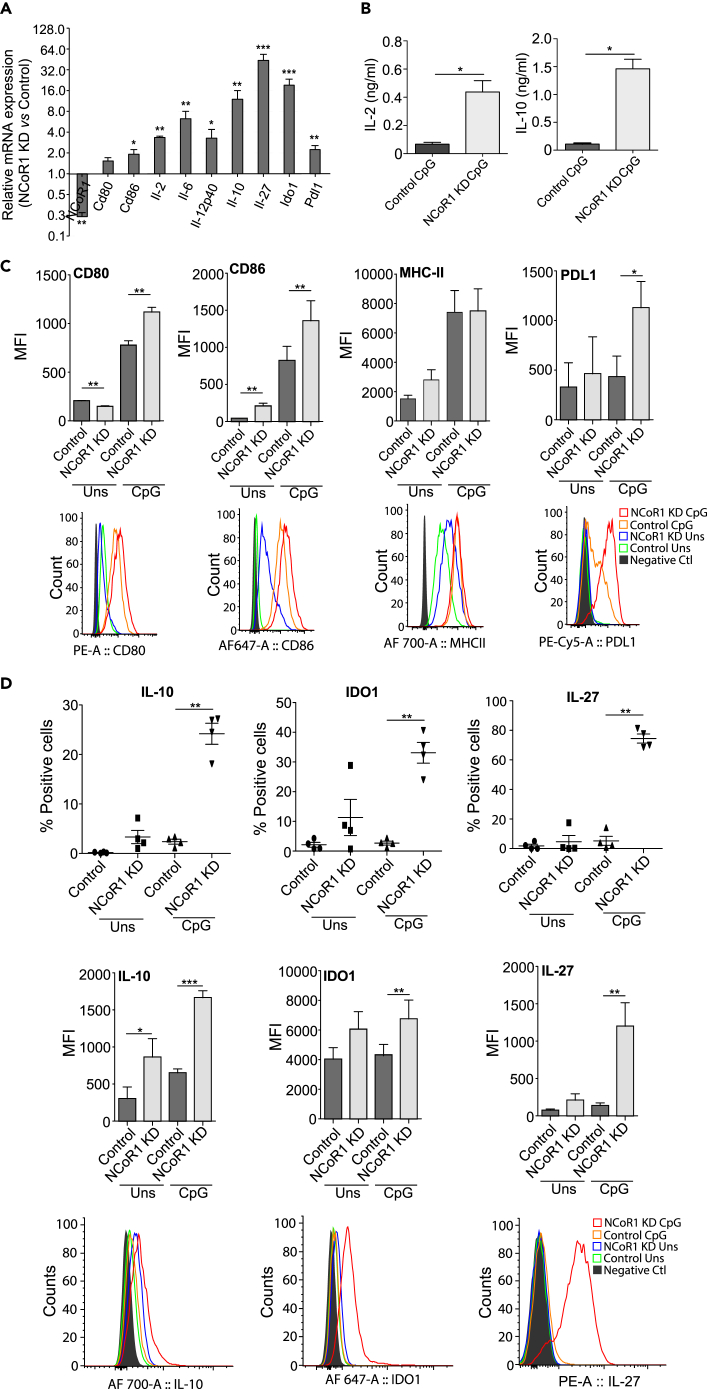
To determine the role of NCoR1 in DCs, we have developed a stable NCoR1 knockdown (KD) and matched empty vector-transduced control cells using lentiviral short hairpin RNA (shRNA) approach in a CD8α+ cDC1 DC line that mimics remarkably the ex vivo isolated cDC1 DCs (Fuertes Marraco et al., 2012, Smita et al., 2018). We found that NCoR1 transcript levels were significantly reduced (≥85%) in NCoR1 shRNA-transduced DCs (Figures 1A and S1A). We first performed qPCR-based immune profiling of control and NCoR1 KD DCs. We found that NCoR1 depletion significantly increased transcripts of several immune-modulatory genes including Il-10, Il-12p40, Il-27, Ido1, and Pdl1 upon 2-, 6-, and 12-h CpG challenge (Figures 1A and S1A). The luminex assay showed significantly increased secretion of IL-2, IL-6, and IL-10 cytokines in the culture supernatants of 6-h CpG-activated NCoR1 KD DCs, whereas the IL-12 cytokine was insignificantly increased (Figures 1B and S1B). The median fluorescence intensity (MFI) shifts showed a significant decrease of CD80 in unstimulated conditions, whereas upon CpG activation CD80, CD86, major histocompatibility complex (MHC)-I, and PDL1 expression were significantly increased (Figures 1C and S1C). CD40 and MHC-II remain unchanged in both the conditions (Figures 1C and S1C). Moreover, the intracellular expression of IL-6, IL-10, IDO1, and IL-27 cytokines showed significantly increased levels in 6-h CpG-challenged NCoR1 KD DCs, whereas IL-12p40 showed an insignificant increase (Figures 1D and S1D). The expression of IL-10 is predominantly dependent on Erk kinase activity (Saraiva and O'Garra, 2010). We found significantly increased pErk+IL-10+pSTAT3- cells in activated NCoR1 KD DCs (Figure S1E).
Figure 1.
NCoR1 KD Enhances Tolerogenic Responses in Conventional DCs upon CpG Challenge
(A) Bar plot depicting the transcript expression of selected immunogenic and tolerogenic response genes in 6 h CpG-stimulated NCoR1 KD cDC1 DCs relative to control cells as quantified by qPCR (n = 3).
(B) Bio-Plex quantitation of the secreted cytokines IL-2 and IL-10 in the culture supernatants of 6 h CpG-challenged NCoR1 KD and control DCs (n = 6).
(C) Graph demonstrating the MFI for DC activation and co-stimulation markers CD80, CD86, MHC-II, and PDL1 in NCoR1 KD and control cDC1 DCs before and after 6 h CpG challenge. Corresponding histogram plot is a representative plot for MFI shifts observed for respective markers (n = 4–8).
(D) Scatterplot showing the percentage positive cells for intracellular expression of IL-10, IDO1, and IL-27 in 0 h and 6 h CpG-stimulated NCoR1 KD and control cDC1 line. Corresponding bar plot and histogram show the MFI shifts observed for the respective genes (n = 4).
p values are calculated using two-tailed paired Student's t test; error bars represent SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. See also Figures S1 and S2.
Moreover, we also developed a stable NCoR1 KD CD11b+ cDC2 DC line (Pigni et al., 2018). Similar to cDC1 DCs, we identified a significantly increased percentage of positive cells for IL-10, IL-27, PDL1, IL-6, and IL-12p40 cytokines after CpG activation in NCoR1-depleted DCs (Figure S1F). Lipopolysaccharide challenge showed similar trends (Figure S1F).
At the same time, to identify if any strong stimulation renders similar responses in NCoR1-depleted cDCs, we challenged NCoR1 KD and matched control cDC1 DCs with pIC (TLR3) and CpG + pIC (TLR3 and TLR9 together) simultaneously. We found that NCoR1 depletion enhanced expression of IL-10, IL-27, IDO-1, and PDL1 along with IL-6 and IL-12p40 in cDC1 DC line with both these stimulations (Figure S2A). Moreover, simultaneous activation showed stronger DC responses (Figure S2A). We also challenged NCoR1 KD and control CD8α+cDC1 DCs with gram-positive (Mycobacterium smegmatis, B. subtilis and Staphylococcus aureus), and gram-negative (Vibrio cholerae, Shigella dysenteriae, and Salmonella typhimurium) bacteria. Interestingly, we found a profound increase of IL-10, IL-27, IDO1, CTLA4, and PDL1 in NCoR1 KD DCs in all these stimulations (Figures S2B and S2C).
Indoleamine 2,3-dioxygenase (IDO) enzyme metabolizes L-tryptophan to L-kynurenine resulting in the inhibition of effector Th cell differentiation (Yan et al., 2010). We found a significantly increased IDO activity in the culture supernatants of NCoR1 KD DCs when compared with controls (Figure S1G). This further substantiated our observation that NCoR1 directly controls the tolerogenic program in cDCs upon activation. Several recent reports showed an enhanced expression of IL-10 along with PDL1, IL-27, and IDO1 in tolerogenic DCs (Kowalczyk et al., 2014, Mellor and Munn, 2004, Tsoumakidou et al., 2014, Yoo and Ha, 2016). Collectively, we demonstrated that NCoR1 depletion induces strong tolerogenic response upon activation in cDCs.
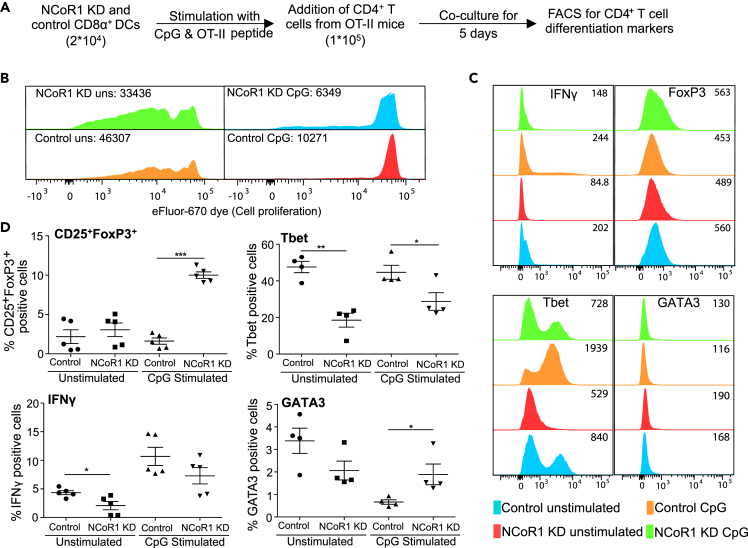
NCoR1 KD cDC1 DCs Increased Frequency of Treg Development Ex Vivo
To access the impact of NCoR1 depletion in cDCs on Th cell differentiation, we co-cultured NCoR1 KD and control cDC1 DCs with naive CD4+ Th cells isolated from OT-II mice (Figure 2A). We found profoundly increased proliferation of Th cells co-cultured with CpG-pulsed NCoR1 KD DCs when compared with control cells (Figure 2B). In addition, the differentiation profile showed a significantly higher number of CD25+FoxP3+ Tregs in NCoR1-depleted conditions (Figure 2C). Tbet and interferon (IFN)-γ-positive cells were significantly reduced, whereas GATA3 showed an increasing trend (Figure 2D) The MFI shifts showed similar trends (Figure 2C). In unstimulated conditions, no changes were observed (Figure 2D). These results confirmed the tolerogenic potential of activated NCoR1 KD cDC1 DCs.
Figure 2.
NCoR1 Depletion Enhances Differentiation of Naive CD4+ Th Cells into Tregs
(A) Experimental outline used to assess the effects of NCoR1 KD on T cell polarization ex vivo.
(B) Representative histograms showing the MFI shifts observed for proliferation of T helper cells co-cultured with unstimulated or CpG-activated NCoR1 KD and control cDC1 DCs (n = 3).
(C) Histogram plots demonstrating the representative MFI shifts observed for FoxP3, Tbet, IFNγ, and GATA3 in helper T cells co-cultured with NCoR1 KD and control cDC1 DCs for 5 days (n = 5).
(D) Scatterplots from flow cytometric analysis showing the percentage positive T helper cells expressing CD25+FoxP3+, Tbet, IFNγ, and GATA3 after 5 days of co-culture with activated NCoR1 KD and control DCs (n = 5).
p values are calculated using two-sample unpaired Student's t test; error bars represent SEM. *p ≤ 0.05, ***p ≤ 0.001.
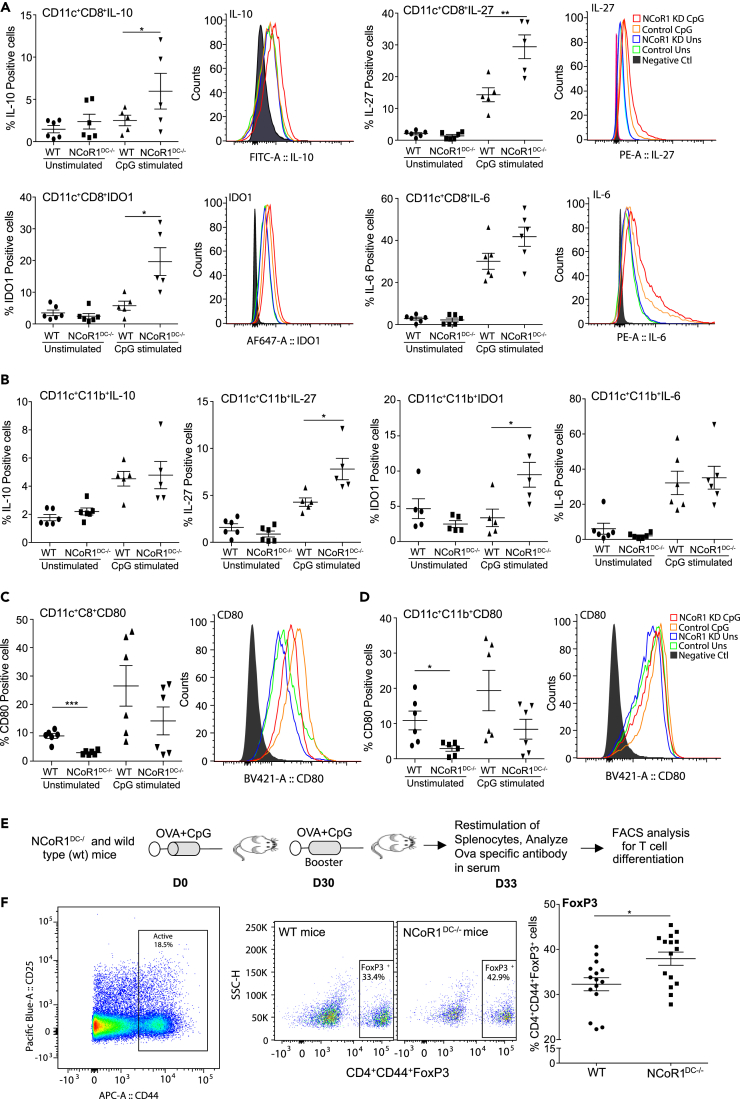
Conventional DCs from NCoR1DC-/- Mice Showed Increased Tolerogenic Responses upon CpG Challenge
To validate the findings in vivo, we developed DC-specific conditional NCoR1 knockout mice by crossing CD11c-Cre strains with floxed NCoR1 mice (Yamamoto et al., 2011). Genotyping PCR confirmed NCoR1 ablation in CD11c+ cDCs (Figure S3A). To obtain sufficient numbers of DCs ex vivo from NCoR1DC−/- and wild-type (WT) mice, we treated them with serum from FLT3L transgenic mice (equivalent to 50 μg FLT3L/mouse/day) for eight consecutive days (Baerenwaldt et al., 2016). First, we profiled DC subtype composition (cDCs and pDCs) after FLT3L treatment. We found that the frequencies of CD8+ (cDC1) and CD11b+ cDCs (cDC2) were profoundly increased in FLT3L-treated mice (Figures S3B and S3C). Then, splenocytes from NCoR1DC−/- and WT mice were stimulated for 6 h with or without CpG and analyzed by fluorescence-activated cell sorting (FACS) to identify the impact of NCoR1 ablation on different subsets of primary CD11c+ cDCs (Figure S3D). We found that NCoR1 loss in CD11chighCD8+ cDC1 DCs significantly enhanced the percentage positive cells of IL-10, IL-27, and IDO1 after activation, whereas IL-6 showed a marginal but insignificant increase (Figure 3A). The MFI shifts showed similar trend (Figure 3A). In addition, CD11b+ cDC2 DCs also showed a significant increase of IL-27 and IDO1 in NCoR1DC−/- when compared with WT mice, whereas IL-6 and IL-10 exhibited an increasing trend (Figures 3B and S3E). Surface expression of CD80 was significantly decreased upon NCoR1 loss of function in both CD8+ and CD11b+ primary cDCs in unstimulated conditions (Figures 3C and 3D). No significant differences were observed for other co-stimulatory genes CD40, CD86, and MHC-II (Figures S3F and S3G). As we found an increased expression of IL-2 cytokine in DC line, we analyzed it in primary DCs using FACS. We found significantly higher IL-2-positive cells in both cDC1 (CD8+) and cDC2 DCs (CD11b+) in CpG-challenged NCoR1-ablated condition (Figure S3I).
Figure 3.
Conventional DCs from NCoR1DC−/- Mice Show Increased Tolerogenic Behavior upon Activation
Splenocytes from NCoR1DC−/- and WT mice were treated with and without CpG for 6 h and the impact of NCoR1 ablation on different DC subsets was analyzed by flow cytometry.
(A) Scatterplots depicting the percentage positive cells for IL-10, IL-27, IDO1, and IL-6 in primary cDC1 DCs from NCoR1DC−/- and WT mice. Corresponding histogram plots depict the MFI shifts (n = 6).
(B) Scatterplots demonstrating the percentage positive cells for IL-10, IL-27, IDO1, and IL-6 in primary cDC2 DCs from NCoR1DC−/- and WT mice (n = 6).
(C) and (D) Scatterplot showing the percentage of CD80-positive cells in primary cDC1 and cDC2 DCs respectively from NCoR1DC−/- and WT mice before and after 6 h CpG activation. Representative histograms depict the MFI shift.
(E) Experimental outline depicting the method employed to identify the in vivo impact of NCoR1 depletion on CD4+ T cell polarization in NCoR1DC−/- and WT mice.
(F) Dot plots showing the percentage of CD4+CD44+FoxP3+-expressing effector T cells from the draining inguinal lymph nodes of NCoR1DC−/- and WT mice vaccinated with CpG and OVA for 1 month (n = 15).
p values are calculated using two-tailed unpaired Student's t test; error bars represent SEM. *p ≤ 0.05, **p ≤ 0.01. See also Figures S3 and S4.
OVA Immunization Enhances Tregs Frequency in NCoR1DC-/- Mice
To access the in vivo impact of NCoR1 deletion in cDCs on Th cell differentiation, we vaccinated NCoR1DC−/- and WT mice with ovalbumin (OVA) and CpG at D0 followed by a booster immunization after 30 days (Figure 3E). Three days after the booster injection, draining lymph nodes were harvested and restimulated with PMA/ionomycin. CD3+CD4+CD44+ effector Th cells were analyzed to assess the differentiation patterns (IFNγ for Th1, IL-13 for Th2, FoxP3 for Tregs, and IL-17 for Th17). We found significantly higher percentages of FoxP3+ cells in NCoR1DC−/- when compared with WT mice (Figure 3F). On the other side, we did not find any change in the IFNγ-, IL-13-, and IL-17-expressing cells in the analyzed effector Th population (Figure S3H). To further check the OVA-specific T cell responses, we also restimulated lymph node cells with OVA-pulsed DCs for 72 h. We found an increased proliferation of effecter Th cells in both WT and NCoR1DC−/- mice when compared with PBS controls (Figure S4A). At the same time, we found a robust and significantly increased FoxP3+ Treg population in CD3+CD4+CD44+ Th cells from NCoR1DC−/- when compared with WT mice (Figures S4A and S4B). These experiments confirmed the development of OVA-specific immune responses in both WT and NCoR1DC−/--immunized animals.
Moreover, we performed ELISA to evaluate OVA-specific antibody titers in immunized NCoR1DC−/- and WT animals to confirm the development of OVA-specific responses. We assayed OVA-specific total IgG and its subtypes IgG1, IgG2a, IgG2b, and IgG3. We found high titers of OVA-specific total IgG in both WT and NCoR1DC−/- animals immunized with OVA + CpG when compared with PBS controls (Figure S4C). We did not observe any significant difference in total IgG levels as well as IgG1, IgG2a, IgG2b, and IgG3 between WT and NCoR1DC−/- mice (Figure S4C).
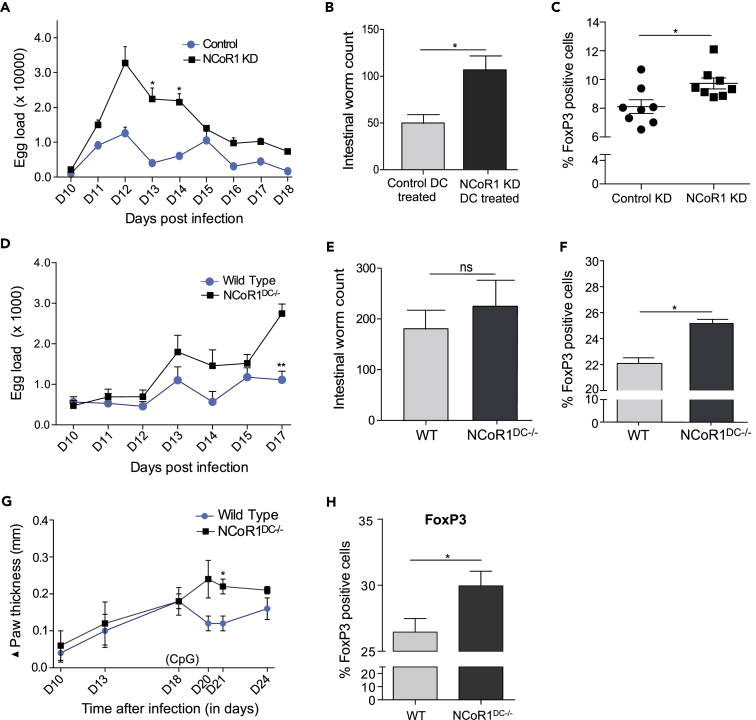
NCoR1 Loss in cDC1 DCs Enhances Treg Development and Parasite Burden
To explore the in vivo implications, we further investigated if NCoR1 KD activated DCs can be adoptively transferred at precise time points to skew T cell polarization into Tregs and the associated disease phenotypes. We employed a well-established helminth infection (Heligmosomoides polygyrus) model in C57BL/6 mice (Filbey et al., 2014). It is reported that the enhanced Treg population in mesenteric lymph nodes (MLNs) increases worm loads in the intestine (Taylor et al., 2012).
Adoptive transfer of CpG-pulsed NCoR1 KD cDC1 DC line at (Day 10) D10 post helminth infection in mice resulted in significantly increased egg counts at D13 and D14 post-infection (Figure 4A). The excreted egg numbers were found to be consistently higher as measured until D18. In addition, the intestinal worm load was also observed to be significantly higher in NCoR1-depleted cDC1-treated animals (Figures 4B and S5A). Besides, NCoR1 KD cDC-treated mice depicted a profound increase in FoxP3+ effector Th cells in the MLNs when compared with controls (Figures 4C and S5B). No differences were observed in Tbet+ cells, whereas GATA3+ cells were also significantly increased (Figure S5C).
Figure 4.
NCoR1 Loss in cDCs Enhances Treg Frequency In Vivo Leading to Increased Disease Burden in Mice
(A) Line graph demonstrating the egg loads in the feces of H. polygyrus-infected C57BL/6J mice at different days post-helminth infection after treatment with CpG-pulsed NCoR1 KD and control CD8α+ cDC1 DCs. The eggs were counted until D18 post-infection (n = 8).
(B) Bar graph showing the intestinal worm counts from activated NCoR1 KD and control cDC1 DC-treated C57BL/6J mice 15 days post-infection (n = 5).
(C) Scatterplot showing the increased effector CD25+FoxP3+ Treg population in MLN of CpG-pulsed NCoR1 KD DC-treated mice compared with control cell-treated animals.
(D) Line graph demonstrating the egg loads in the feces of H. polygyrus-infected NCoR1DC−/- and WT C57BL/6J mice at different days post-helminth infection after CpG treatment. The eggs were counted until D17 of infection, and then animals were dissected for intestinal worm counting and T cell profiling from MLNs (n = 5).
(E) Bar plot showing the helminth worm counts from the intestines of CpG-treated NCoR1DC−/- and control C57BL/6J mice on D17 post infection (n = 5).
(F) Bar graph depicting the effector CD25+FoxP3+ Treg cells in the MLNs of CpG-treated NCoR1DC−/- and WT mice 17 days post-helminth infection (n = 5).
(G) Line graph demonstrating the paw inflammation in L. major-infected NCoR1DC−/- and WT mice before and after CpG treatment. The CpG treatment was given on D18 after infection. Five animals were used in each group.
(H) Bar plot showing the increase in percentage positive FoxP3 Th cells in draining lymph nodes of NCoR1DC−/- and WT mice at D24 after infection. Five mice were used in each group.
p values are calculated using two-tailed unpaired Student's t test; error bars represent SEM. *p ≤ 0.05, **p ≤ 0.01. See also Figure S5.
To validate our adoptive transfer observations in vivo in NCoR1DC−/- animals, we infected the NCoR1DC−/- and WT mice with helminth larvae and treated the animals with CpG at D10 post-infection. We found a significantly increased helminth egg load at D17 in the feces of NCoR1DC−/- mice compared with WT animals (Figure 4D). The intestinal worm counts were also increased (Figure 4E). In addition, the effector CD44+ Th cell profiling from MLNs of infected animals showed an increased FoxP3+ population in NCoR1-ablated mice (Figure 4F). We did not find any significant differences in the GATA3 and Tbet-expressing Th cells (Figure S5D). We also developed a Leishmania major parasitic infection model. After subcutaneous injection of parasite in the right footpad of NCoR1DC−/- and WT mice, paw thickness was monitored before and after CpG challenge. As cross-presenting DCs are essential for developing immune protection against L. major between 17 and 19 days (Ashok et al., 2014), mice were treated with CpG at day 18 post-infection. We observed a significantly increased (p value ≤ 0.020) foot inflammation at day 21 post infection in NCoR1DC−/- animals when compared with WT mice (Figure 4G). Although the NCoR1DC−/- mice had thicker paws than the WT mice, the differences disappeared 2 weeks after the CpG injection (Figure 4G). Draining lymph nodes from NCoR1DC−/- mice also showed significant increase in FoxP3+ Th cells (Figure 4H). These results indicated a strong potential of NCoR1 in inducing Th cell polarization toward Tregs in vivo and modulating the underlying disease phenotype. Next, to assess the specificity of antigen presentation of DCs, we injected carboxyfluorescein succinimidyl ester (CFSE) labeled CD45.1+ OT-II T cells intravenously in CD45.2+ helminth-infected WT and NCoR1DC−/- mice at D12 of infection. Next day, we injected WT DCs pulsed with or without OVA peptide intravenously, and the animals were kept further for 3 days. Then we harvested the splenocytes to check the OVA-specific proliferation of CD45.1-gated cells. We observed no difference in proliferation of CD45.1+ OT-II T cells analyzed from WT and NCoR1DC−/- mice suggesting there were similar levels of antigen presentation in both WT and NCoR1DC−/- mice (Figure S5E).
Global Profiling of NCoR1 Identifies Its Direct Control on DC Tolerogenicity
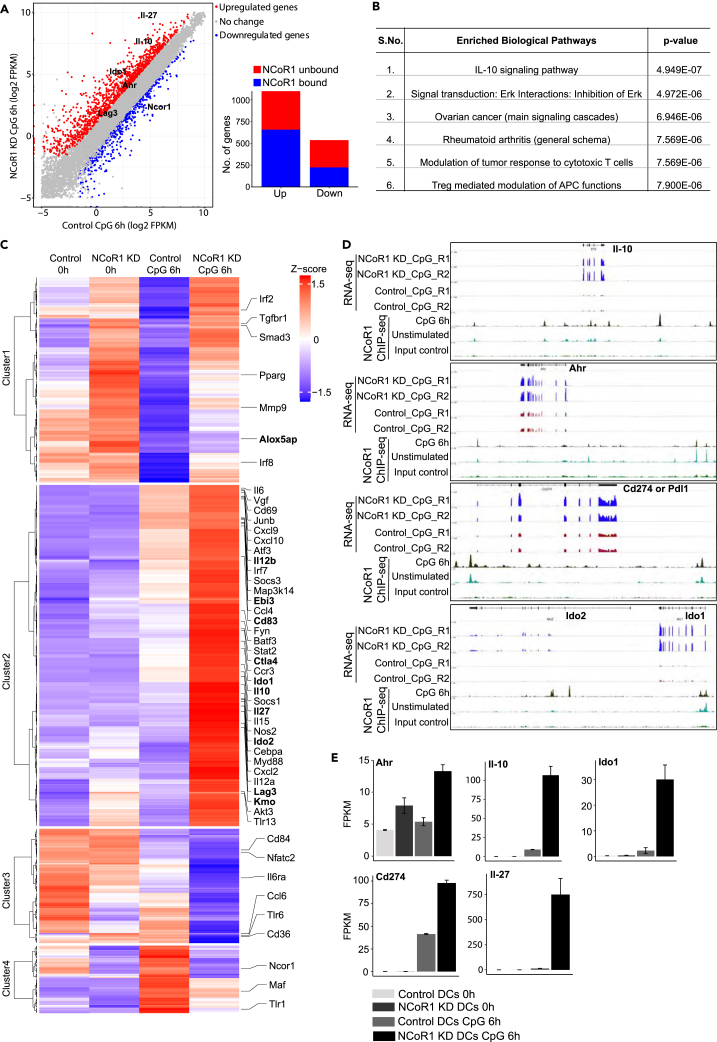
To characterize the molecular mechanisms underlying NCoR1 depletion mediated tolerogenic behavior in cDC1 DCs, we performed RNA sequencing (RNA-seq) analysis of NCoR1 KD and control cDC1 DC line at 0h and 6h after CpG challenge. Besides, we also performed ChIP-seq profiling of NCoR1 in WT cDC1 DC line in similar conditions. We identified ≈13,000 genomic regions bound by NCoR1 at both 0 and 6 h after CpG activation (Table S1). In addition, we performed ChIP-qPCR for 10 randomly selected NCoR1 peaks to validate the ChIP-seq data (Figure S6A, Table S1). Transcriptome profiling of NCoR1 KD cDC1 line identified 1,099 upregulated in contrast to 537 downregulated genes when compared with control DCs (q-value ≤ 0.05 and 2-fold) (Figure 5A, Table S2). On the other side, in unstimulated conditions, only 390 and 360 genes were up- and downregulated, respectively, in NCoR1 KD DCs (Figure 5A, Table S3). Overlap with ChIP-seq data revealed 658 up- and 224 downregulated genes as direct targets of NCoR1 upon CpG activation (Figure 5A, Table S2). The numbers of NCoR1 directly bound and upregulated genes were much higher than the downregulated genes, confirming NCoR1's role as a global transcriptional co-repressor. Pathway enrichment analysis for NCoR1 direct target genes in CpG-activated DCs showed significant enrichment of IL-10 signaling (p value ≤ 10−7) and Tregs mediated modulation of APC functions (p value ≤ 10−6) (Figure 5B, Table S4). In contrast, NCoR1-unbound and NCoR1-regulated genes showed pathway enrichments like “Immune response IFN-alpha/beta signaling via MAPKs” and “Cytoskeleton remodeling TGF and WNT and cytoskeletal remodeling” (Table S5). Next, we examined the expression patterns of NCoR1 direct target genes that are differentially regulated in CpG-activated NCoR1 KD cDC1 DCs. Interestingly, cluster 2 revealed a wide variety of tolerogenic genes such as Lag3, Kmo, Vdr, Cd83, and Tgfbr1 along with Ctla4, Ido1, Ido2, Il-10, Pdl1, and Il-27 (Schinnerling et al., 2015) (Figure 5C, Table S2). integrative genomics viewer (IGV) snapshots and FPKM (fragment counts/kb/million reads) plots from RNA-seq data showed that these genes are highly upregulated after CpG activation in NCoR1-depleted DCs (Figures 5D and 5E). This global analysis further confirmed our in vitro and in vivo observations that NCoR1 KD cDCs develop strong tolerogenic behavior upon activation.
Figure 5.
Global Profiling of NCoR1 in cDC1 DC Line Depicts Its Direct Control on DC Tolerance
(A) Scatterplot demonstrating the global RNA-seq data of NCoR1 KD and control CD8α+ cDC1 DC line after 6-h CpG challenge. Red and blue dots indicate the significantly up- and downregulated genes, respectively. Genes of interest are marked in bold. The inset bar graph shows the number of genes directly or indirectly controlled by NCoR1 (n = 2), see also Table S2.
(B) Top biological pathways significantly enriched for the list of direct target genes identified from RNA-seq analysis of 6 h CpG-activated NCoR1 KD DCs. See also Table S4.
(C) Heatmap depicting the clusters observed for the list of genes directly regulated by NCoR1 at 0 h and 6 h after CpG challenge. Important DC immune tolerance genes regulated by NCoR1 are marked in bold. See also Tables S2 and S3.
(D) IGV snapshots showing the NCoR1 binding and RNA-seq tag density observed at Il-10, Ahr, Pdl1, and Ido2 tolerogenic genes.
(E) FPKM (fragment counts/kb/million reads) plots demonstrating the levels of transcript expression for important DC tolerogenicgenes identified to be directly regulated by NCoR1 in CD8α+ cDC1 DCs. See also Table S2.
NCoR1 Strongly Represses the PU.1-Bound Enhancers on Tolerogenic Genes
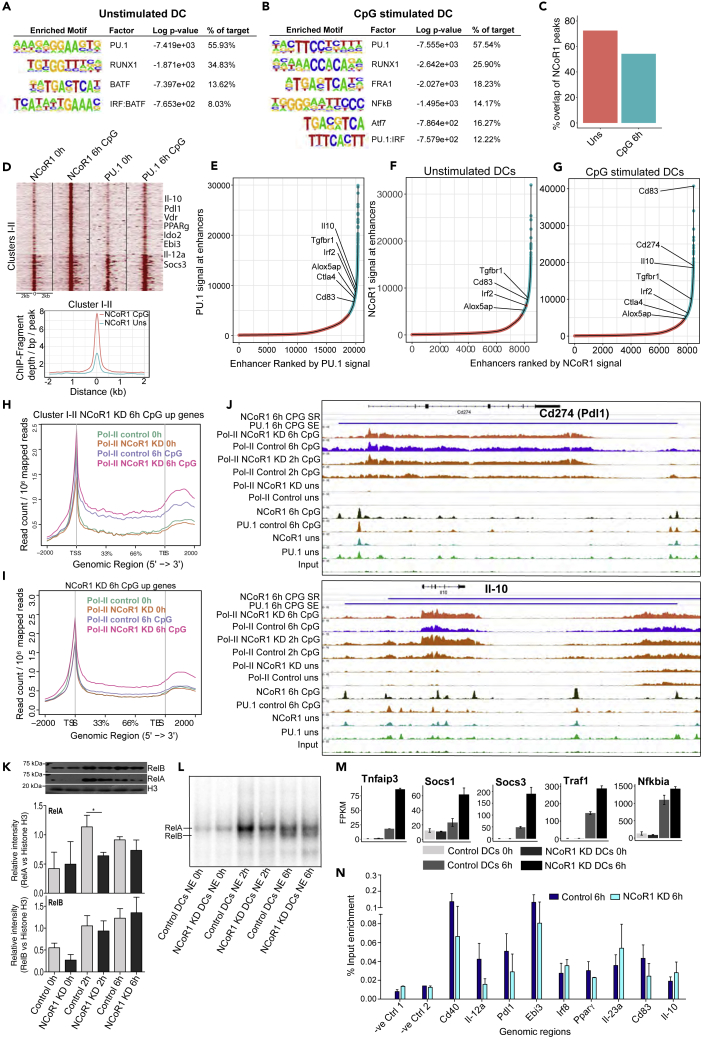
To identify the molecular mechanism underlying NCoR1-mediated DC tolerance, we have performed an integrative genomic analysis. Our de novo motif prediction within NCoR1 ChIP-seq peaks identified PU.1 as highly enriched motifs (55%–60% with p value ≤10−3) in both unstimulated and CpG-challenged conditions (Figures 6A and 6B). Furthermore, RUNX1, BATF, and IRF: BATF motifs were enriched in unstimulated DCs, whereas RUNX1, FRA1, NF-κB, ATF7, and PU.1: IRF motifs were enriched in CpG condition (Figures 6A and 6B). To substantiate our motif predictions, we overlapped NCoR1 binding in cDC1 DC line with publically available TF ChIP-seq data from primary bone marrow-derived dendritic cells (BMDCs) (Garber et al., 2012). We found strong overlap of NCoR1 with PU.1 (50%–55%), NF-–kB (35%–38%), IRF1 (20%–25%), IRF4 (19%–23%), Junb (18%–22%), and the active enhancer mark H3K27ac (60%–65%). Moreover, we performed ChIP-seq and ChIP-qPCR for PU.1 in cDC1 DC line to validate our predictions. We found a strong overlap of PU.1 (≥60%) with NCoR1 peaks, confirming it to be the most putative NCoR1-recruiting factor in DCs (Figures 6C, S6B, and S6C).
Figure 6.
NCoR1 Strongly Represses PU.1-Bound Super-Enhancers Present on Tolerogenic Genes after CpG Activation in CD8α+ cDCs
(A) Top significantly enriched de novo predicted DNA motifs of TFs in NCoR1 ChIP-seq peaks of unstimulated CD8α+ cDC1 DC line.
(B) Top significantly enriched DNA motifs of TFs in NCoR1 ChIP-seq peaks of 6-h CpG-stimulated CD8α+ cDC1 DCs.
(C) Bar graph showing the percentage overlap of NCoR1 with PU.1 ChIP-seq peaks before and after 6 h CpG activation.
(D) SeqMINER clustering showing clusters I-II depicting increased NCoR1 binding after CpG activation in cDC1 DC line when compared with unstimulated cells. Corresponding PU.1 ChIP-seq data lanes show its overlap with NCoR1-bound regions. Corresponding density plot shows the differential enrichment of reads in CpG compared with unstimulated at clusters I-II genomic regions. See also Table S6.
(E) Distribution of PU.1 ChIP-seq signals across the total PU.1-bound regulatory regions in CpG-activated CD8α+ DCs. Bound regions were ranked by the tag counts within the PU.1-enriched peaks to identify the super-enhancer regulatory regions or genes. Tolerogenic genes enriched in the PU.1 super-enhancer regions are marked. See also Table S7.
(F and G) Distribution of NCoR1 ChIP-seq signals across the total NCoR1-bound regulatory regions in unstimulated and CpG-activated CD8α+ DCs. Bound regions were ranked by the tag counts within the NCoR1-enriched peaks to identify the regulatory regions or genes super-repressed by NCoR1. Tolerogenic genes enriched in the NCoR1 super-repressed regions are marked. See also Table S7.
(H) Metagene plot showing the RNA Pol-II enrichment in unstimulated and 6-h CpG-stimulated control and NCoR1 KD DCs within the gene-body regions of the genes that are annotated to NCoR1 bound cluster I-II genomic regions.
(I) Metagene plot depicting the RNA Pol-II enrichment profile in unstimulated and 6 h CpG-stimulated control and NCoR1 KD DCs within the gene-body regions of the genes that are upregulated in 6 h CpG-activated NCoR1 KD DCs in RNA-seq data.
(J) IGV snapshots showing the ChIP-seq enrichments of NCoR1, PU.1, and RNA Pol-II at tolerogenic genes Il-10 and Pdl1 at 0 h and 6 h CpG-stimulated CD8α+ cDC1 DC line. RNA-Pol-II enrichment in CpG-activated control and NCoR1 KD DCs demonstrate the real-time transcription of these representative tolerogenic genes. The horizontal bars above the plot show the PU.1-bound super-enhancer (SE) regions that are strongly repressed (SR) by NCoR1 after CpG activation.
(K) Representative western blot picture for NF-κB subunits RelA and RelB depicting their nuclear translocation at 0 h, 2 h, and 6 h after CpG activation in control and NCoR1 KD CD8α+ cDC1 line. Corresponding densitometric analysis bar plots shows the relative intensity of RelA and RelB from four biological replicates (n = 4).
(L) Electrophoretic mobility shift assay (EMSA) demonstrating the binding activity of RelA and RelB at 0 h, 2 h, and 6 h after CpG activation. RelA and RelB bands are indicated by the marks.
(M) Fragment counts/kb/million read counts plots from control and NCoR1 KD RNA-seq data showing the differential expression of NF-κB negative regulators of NF-κB canonical signaling.
(N) RelA ChIP-qPCR depicting the enrichment of RelA at 10 randomly selected genomic regions when compared with negative control genomic regions.
p values are calculated using two-tailed paired Student's t test; fold change error is depicted as SEM. *p ≤ 0.05. See also Figures S6 and S7.
To identify the mechanistic control of gene regulation by NCoR1, we did SeqMINER (Ye et al., 2011) clustering to identify the regulatory regions differentially bound by NCoR1 in CpG-activated cDCs compared with unstimulated conditions (Figure S6B). Two of these genomic clusters (clusters I-II) showed increased NCoR1 binding, whereas clusters III-XI showed similar enrichment after activation (Figures 6D and S7A). Interestingly, these regions were annotated to 214 genes that were significantly upregulated in NCoR1 KD DCs upon CpG activation, which includes major tolerogenic genes Il-10, Pdl1, Ido2, Vdr, and Cd83 (Table S6). The cluster I-II genomic regions were also enriched for TFs PU.1, IRF1, IRF4, Junb, and H3K27ac marks, whereas RelA and RelB were enriched only after activation (Figure S6B). Thus, these regulatory regions appear to be the hotspots where several of these activating TFs appear to form complexes.
Moreover, to identify if enhancers at tolerogenic genes are strongly repressed by NCoR1 after CpG challenge, we ran the super-enhancer discovery program to rank PU.1- and NCoR1-bound signals (Table S7). We found that PU.1 super-enhancers on tolerance-inducing genes like Il-10, Pdl1, Cd83, Ctla4, and Tgfβr1 were strongly repressed by NCoR1 after CpG activation (Figures 6E–6G). Our ChIP-qPCR analysis further confirmed the enrichment of PU.1 at these NCoR1-bound genomic regions (Figure S7B). This suggested that NCoR1 masks the PU.1-bound enhancers after DC activation to prevent induction of the major tolerogenic genes. We performed RNA Pol-II ChIP-seq and ChIP-qPCR in control and NCoR1 KD cDC line before and after CpG activation to estimate the changes in transcription rate of differentially regulated genes (Figure S7C). We observed profoundly increased gene body RNA Pol-II tag counts for the cluster I-II annotated genes that are upregulated in NCoR1-depleted cDCs (Figures 6H–6J). The downregulated genes showed a decreased Pol-II tag density (Figures S7C and S7D). Representative IGV snapshots depicted the normalized ChIP-seq and RNA-seq tag counts at major tolerogenic genes Il-10 and Pdl1 (Figure 6J).
Collectively, these results demonstrated that NCoR1 directly represses the tolerogenic enhancers after DC activation. Hence upon NCoR1 depletion, these genes are derepressed leading to their enhanced transcription and concomitant mRNA expression after DC activation. This NCoR1-mediated repression of the tolerogenic program appears to be a very important event to prevent the cDCs from going into tolerogenic modality under the strong inflammatory stimulus.
NCoR1 Depletion Perturbs NF-κB Activity on Tolerogenic Genes
We found a significant enrichment of NF-κB DNA motif in NCoR1-bound regions, and a fine balance of NF-κB activity controlling the immunogenic versus regulatory phenotype in DCs has been widely reported (Dohler et al., 2017, Thomas, 2013, Vendelova et al., 2018). Therefore, two mechanisms could be suggested: (1) NCoR1 directly represses PU.1-regulated tolerogenic genes and (2) it directly represses inhibitors of RelA (NF-κB), which provides for immunogenic functions. Therefore, we probed NF-κB nuclear translocation (RelA and RelB) and binding activity in NCoR1 KD and control cDC1 DCs before and after CpG activation. We found significantly inhibited RelA translocation and activity in NCoR1-depleted DCs at 2 h after activation, whereas RelB activity was unaffected (Figures 6K and 6L). We found increased RelA nuclear localization and binding at 2 h when compared with 6 h, whereas RelB comes up at 6 h after CpG activation. Interestingly, NCoR1 had little, if any, impact on RelB activity, which is known for immune tolerance in DCs. In addition, we also observed significantly increased expression of negative regulators of NF-κB signaling, i.e., Tnfaip3, Nfkbia, and Traf1 along with cytokine-signaling regulators Socs1 and Socs3 in NCoR1 KD DCs (Figure 6M). RelA ChIP-qPCR further confirmed an overall decreased RelA enrichment on selected tolerogenic/immunogenic genes except Il-10 and Il-23a in activated NCoR1-depleted DCs (Figure 6N). This indicated toward a role of NCoR1 in perturbing NF-κB activity to fine-tune DCs immunogenic versus tolerogenic state.
Discussion
DCs link innate to adaptive immunity and regulate a fine balance of inflammatory and tolerogenic responses to prevent immune pathology. Although the signaling pathways underlying immunogenic and tolerogenic program are explored, the mechanisms underlying fine control of this balance in DCs is of paramount interest for therapeutic approaches and are largely unknown. Using in vitro, ex vivo, and in vivo models, we revealed that NCoR1 is a master repressor of the tolerogenic program in cDC1 DCs and its depletion renders DCs tolerogenic, irrespective of activation by any strong TLR ligand or microbe. NCoR1-depleted DCs have strong potential to polarize Th cells into Tregs ex vivo and in vivo with a concomitant increase in disease phenotype. Moreover, we demonstrated using integrative genomic analyses that NCoR1 strongly represses tolerogenic genes and its KD modulates NF-κB activity in activated DCs that could shift immunogenic program toward tolerance.
A number of reports suggested that treatment of DCs with a variety of strong NR ligands such as vitamin-D and dexamethasone induces DC tolerance by suppressing their activation along with an increased IL-10 production (Anderson et al., 2017). NRs are well known to control their target genes by switching from co-repressor to co-activator complexes upon stimulation (McKenna and O'Malley, 2002, Mouchiroud et al., 2014). Recently, NCoR1 co-repressor has been reported to interact with non-NR TFs such as NF-κB and AP1 that are crucial for DC function (Barish et al., 2012, Huang et al., 2009, Li et al., 2013). Activation signals remove NCoR1 from co-repressor complex through recruitment of activating TFs like NF-κB or through complex destabilization by kinases such as p38 and pErk (Ghisletti et al., 2009). In DCs CpG stimulation activates canonical NF-κB signaling along with phosphorylation of p38 and Erk kinases (O'Neill et al., 2013, Yu et al., 2004). Activation of Erk kinase is predominantly reported to induce IL-10 expression, whereas p38 largely induces inflammatory cytokines like IL-12 (Saraiva and O'Garra, 2010, Yu et al., 2004). We found a profoundly increased pErk activity and concomitantly increased IL-10 levels in activated NCoR1 KD DCs. At the same time PDL1, IL-27, and IDO1 genes were upregulated, which are also reported to induce strong tolerogenic responses (Raker et al., 2015, Shiokawa et al., 2009, Sumpter and Thomson, 2011, Yoo and Ha, 2016). The IL-10 cytokine further induces Pdl1 through STAT3 signaling (Wolfle et al., 2011). The expression of major tolerogenic genes like Ido1, Pdl1, and Il-10 are also induced by IL-27 cytokine, an IL-12 family member cytokine through STAT1 and STAT3 signaling (Carbotti et al., 2015, Murugaiyan et al., 2009). Both these STATs were increased in NCoR1 KD DCs (Carbotti et al., 2015), which are known to induce regulatory Tr1 cells by inhibiting Th17 cell development (Kushwah and Hu, 2011). The increased IL-2 could also enhance the secretion of IL-27-induced IL-10 (Murugaiyan et al., 2009). Studies suggest that STAT3 is also phosphorylated via both IL-6 and IL-10 signaling in immune cells (Niemand et al., 2003). It is unlikely that increased IL-6 is responsible for this STAT3 activation in NCoR1 KD cells as the IL-6rα expression was significantly decreased after CpG stimulation. There is a concomitant increase in Socs3 as well, which strongly inhibits STAT3 phosphorylation leaving open the IL-10 signaling pathway (Ahmed and Ivashkiv, 2000). Socs2 and Socs3 are also reported to suppress uncontrolled inflammatory cytokine expression and were highly elevated in tolerogenic-state mature DCs (Connor et al., 2017, Yoshimura et al., 2005).
Contrary to the reported suppression of IL-12 by enhanced IL-10 signaling in DCs (Ma et al., 2015), we noticed a marginal increase of IL-12 in NCoR1 KD DCs when compared with the robust increase of IL-10 production. Last but not the least, IL-12p40 homo-dimers are inhibitory and IL-12p70 is not significantly upregulated in NCoR1 KD DCs. Type-I IFN signaling is also suggested to stimulate IL-10 and suppress IL-12 and IL-23 cytokines in DCs (Yen et al., 2015). Interestingly, we found Ifnβ1 and its signaling intermediate Stat2 significantly increased in CpG-stimulated NCoR1 KD DCs. IFNβ1 is also known to represses IL-12 expression (Zhao et al., 2015).
Some of the surface markers and secreted factors that showed a marginal increase after NCoR1 KD are often considered inflammatory. Nevertheless, co-stimulation and antigen presentation by MHC class-II are required for Treg generation (Price et al., 2015). Therefore, an increase of co-stimulatory molecules can also contribute to Treg differentiation. As Tregs express CD25 (IL-2Rα), increased IL-2 secreted by NCoR1 KD DCs could induce Treg proliferation and clonal expansion and further contribute to tolerogenicity. In RNA-seq, we find upregulation of IL-12p35 and this molecule has inflammatory (IL-12p70) as well as anti-inflammatory/tolerogenic properties (IL-35 composed of IL-12p35 and EBI3) (Collison et al., 2010).
Furthermore, we characterized the direct and indirect target genes of NCoR1 for mechanistic insight. We found a strong overlap of PU.1 binding with NCoR1, suggesting it as the most putative TF-tethering NCoR1 in DCs. Integrative genomics demonstrated that NCoR1 masks the PU.1-bound super-enhancers at the major tolerogenic genes including Il-10, Cd83, Ctla4, and Pdl1. Therefore, upon NCoR1 depletion these genes are derepressed leading to enhanced transcription as evident by increased gene body RNA Pol-II counts after activation. We also observed a significant enrichment of NF-κB at NCoR1 peaks in CpG-activated condition, which suggested involvement of NF-κB TF in NCoR1-mediated effects. Recent reports also indicated that perturbations in the activity of different NF-κB subunits like RelA and RelB modulate DCs' inflammatory versus tolerogenic phenotype (Azukizawa et al., 2011). We identified that RelA-binding activity was profoundly reduced after activation, whereas RelB was unaffected. In addition, NCoR1 directly represses negative regulators of canonical NF-κB signaling such as Tnfaip3 (A20), Traf1, and Pias1, which are well reported to repress inflammatory responses by inhibiting RelA activity (Kool et al., 2011, Liu et al., 2005a, Sen and Smale, 2010, Vereecke et al., 2009). We also noticed increased RelB translocation and activity from 2 to 6 h after CpG activation in CD8α+ cDCs, whereas the RelA activity was reduced. It would be further interesting to characterize how this switch of NF-κB activity regulates DC responses. In addition, we showed that increased NCoR1 biding after CpG activation overlaps with binding of other activating TFs. Therefore, it is highly probable that other activating TFs may also bind at these tolerogenic enhancers after NCoR1 depletion. Treatment with live bacteria showed even more pronounced tolerogenic effects compared with CpG challenge, which suggests that multiple strong stimulations further enhance tolerogenicity in NCoR1 KD DCs. Altogether, our integrative genomic analysis identified NCoR1 as a master regulator of the tolerogenic program in DCs.
Limitations of the Study
In this study we have majorly used cDC1 mutuDC line and validated the findings ex vivo and in vivo using primary DCs. It is important to mention that from a single WT C57BL/6 mice one can get nearly 100,000 cDC1 DCs in good condition from splenocytes, using magnetic bead-based kits. Therefore, using primary DCs is a major limitation for high-throughput genomic analysis such as ChIP-seq and bulk RNA-seq that we reported here.
Acknowledgments
We thank Johan Auwerx for providing the NCoR1fl/fl mice, Genotypic technology for NGS, Fabienne Tacchini-Cottier, Ivo Regli, and Marianna Koga for helping in the parasite experiments and Matteo Pigni for providing the CD11b cDC line. Nicola Harris lab at EPFL, Lausanne, provided the helminth larvae. We thank Christine Lavanchy, Viplov K. Biswas and Vanessa Mack for their technical help. Ton Rolink provided the FLT3L transgenic mice. We thank Bart Deplancke, Marjan Biocanin, and Punit Prasad for constructive comments to edit the manuscript and Girdhari Lal of NCCS Pune, India for suggestions during revision. A.A. is supported by DBT-JRF. S.S. is supported by ILS fellowship. G.P.M. is supported by DBT BINC fellowship and DST-SNSF grant. Grant funds from DST-SNSF (DST/INT/SWISS/SNSF/P-47/2015), DBT-India Ramalingaswami fellowship, SERB-India (EMR/2016/000717), DBT-India (BT/PR15908/MED/12/725/2016), (HAO_SNF-Switzerland 310030_132492) supported this study; ILS provided intramural support and infrastructure.
Author Contributions
Conceptualization, A.A., M.S., S.S., S.K.R., and H.A-O.; Methodology, A.A., S.S., M.S., D.G., U.A.S., and B.G.; Investigation, A.A., M.S., S.S., S.B., H.A-O., and S.K.R.; Writing – Original Draft, A.A., M.S., H.A-O., B.G., and S.K.R.; Data Curation, G.P.M. and S.K.R.; Formal Analysis, G.P.M. and S.M.W.; Supervision, S.K.R. and H.A-O.; Visualization, A.A., S.S., G.P.M., and M.S.; Funding Acquisition, S.K.R. and H.A-O.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.024.
Contributor Information
Hans Acha-Orbea, Email: hans.acha-orbea@unil.ch.
Sunil Kumar Raghav, Email: sunilraghav@ils.res.in.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Supplemental Information
GeneGo metacore software was used for the analysis.
GeneGo Metacore software was used for the analysis
References
- Agrawal S., Ganguly S., Tran A., Sundaram P., Agrawal A. Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging (Albany NY) 2016;8:1223–1235. doi: 10.18632/aging.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.T., Ivashkiv L.B. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J. Immunol. 2000;165:5227–5237. doi: 10.4049/jimmunol.165.9.5227. [DOI] [PubMed] [Google Scholar]
- Anderson A.E., Swan D.J., Wong O.Y., Buck M., Eltherington O., Harry R.A., Patterson A.M., Pratt A.G., Reynolds G., Doran J.P. Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-beta1. Clin. Exp. Immunol. 2017;187:113–123. doi: 10.1111/cei.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok D., Schuster S., Ronet C., Rosa M., Mack V., Lavanchy C., Marraco S.F., Fasel N., Murphy K.M., Tacchini-Cottier F. Cross-presenting dendritic cells are required for control of Leishmania major infection. Eur. J. Immunol. 2014;44:1422–1432. doi: 10.1002/eji.201344242. [DOI] [PubMed] [Google Scholar]
- Azukizawa H., Dohler A., Kanazawa N., Nayak A., Lipp M., Malissen B., Autenrieth I., Katayama I., Riemann M., Weih F. Steady state migratory RelB+ langerin+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells. Eur. J. Immunol. 2011;41:1420–1434. doi: 10.1002/eji.201040930. [DOI] [PubMed] [Google Scholar]
- Baerenwaldt A., von Burg N., Kreuzaler M., Sitte S., Horvath E., Peter A., Voehringer D., Rolink A.G., Finke D. Flt3 ligand regulates the development of innate lymphoid cells in fetal and adult mice. J. Immunol. 2016;196:2561–2571. doi: 10.4049/jimmunol.1501380. [DOI] [PubMed] [Google Scholar]
- Barish G.D., Yu R.T., Karunasiri M.S., Becerra D., Kim J., Tseng T.W., Tai L.J., Leblanc M., Diehl C., Cerchietti L. The Bcl6-SMRT/NCoR cistrome represses inflammation to attenuate atherosclerosis. Cell Metab. 2012;15:554–562. doi: 10.1016/j.cmet.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbotti G., Barisione G., Airoldi I., Mezzanzanica D., Bagnoli M., Ferrero S., Petretto A., Fabbi M., Ferrini S. IL-27 induces the expression of IDO and PD-L1 in human cancer cells. Oncotarget. 2015;6:43267–43280. doi: 10.18632/oncotarget.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.N., Wondisford F.E., Hollenberg A.N. Two separate NCoR (nuclear receptor corepressor) interaction domains mediate corepressor action on thyroid hormone response elements. Mol. Endocrinol. 1998;12:1567–1581. doi: 10.1210/mend.12.10.0188. [DOI] [PubMed] [Google Scholar]
- Collison L.W., Chaturvedi V., Henderson A.L., Giacomin P.R., Guy C., Bankoti J., Finkelstein D., Forbes K., Workman C.J., Brown S.A. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor L.M., Tang S.C., Cognard E., Ochiai S., Hilligan K.L., Old S.I., Pellefigues C., White R.F., Patel D., Smith A.A. Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles. J. Exp. Med. 2017;214:125–142. doi: 10.1084/jem.20160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., Riley E.M. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Dohler A., Schneider T., Eckert I., Ribechini E., Andreas N., Riemann M., Reizis B., Weih F., Lutz M.B. RelB(+) steady-state migratory dendritic cells control the peripheral pool of the natural Foxp3(+) regulatory T cells. Front Immunol. 2017;8:726. doi: 10.3389/fimmu.2017.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias A.S., Spagnol G.S., Bordeaux-Rego P., Oliveira C.O., Fontana A.G., de Paula R.F., Santos M.P., Pradella F., Moraes A.S., Oliveira E.C. Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS Neurosci. Ther. 2013;19:269–277. doi: 10.1111/cns.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey K.J., Grainger J.R., Smith K.A., Boon L., van Rooijen N., Harcus Y., Jenkins S., Hewitson J.P., Maizels R.M. Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunol. Cell Biol. 2014;92:436–448. doi: 10.1038/icb.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes Marraco S.A., Grosjean F., Duval A., Rosa M., Lavanchy C., Ashok D., Haller S., Otten L.A., Steiner Q.G., Descombes P. Novel murine dendritic cell lines: a powerful auxiliary tool for dendritic cell research. Front Immunol. 2012;3:331. doi: 10.3389/fimmu.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M., Yosef N., Goren A., Raychowdhury R., Thielke A., Guttman M., Robinson J., Minie B., Chevrier N., Itzhaki Z. A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol. Cell. 2012;47:810–822. doi: 10.1016/j.molcel.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S., Huang W., Jepsen K., Benner C., Hardiman G., Rosenfeld M.G., Glass C.K. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochweller K., Wabnitz G.H., Samstag Y., Suffner J., Hammerling G.J., Garbi N. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc. Natl. Acad. Sci. U S A. 2010;107:5931–5936. doi: 10.1073/pnas.0911877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ghisletti S., Perissi V., Rosenfeld M.G., Glass C.K. Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol. Cell. 2009;35:48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko G.E., Horvat J.C., Beagley K.W., Hansbro P.M. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M., van Loo G., Waelput W., De Prijck S., Muskens F., Sze M., van Praet J., Branco-Madeira F., Janssens S., Reizis B. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Kowalczyk A., D'Souza C.A., Zhang L. Cell-extrinsic CTLA4-mediated regulation of dendritic cell maturation depends on STAT3. Eur. J. Immunol. 2014;44:1143–1155. doi: 10.1002/eji.201343601. [DOI] [PubMed] [Google Scholar]
- Kushwah R., Hu J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci. 2011;1:20. doi: 10.1186/2045-3701-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Spann N.J., Kaikkonen M.U., Lu M., Oh D.Y., Fox J.N., Bandyopadhyay G., Talukdar S., Xu J., Lagakos W.S. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell. 2013;155:200–214. doi: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Yang R., Wong K.A., Getman C., Stein N., Teitell M.A., Cheng G., Wu H., Shuai K. Negative regulation of NF-kappaB signaling by PIAS1. Mol. Cell. Biol. 2005;25:1113–1123. doi: 10.1128/MCB.25.3.1113-1123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao S., Kim S., Chung E.Y., Homma Y., Guan X., Jimenez V., Ma X. Interleukin-12: an update on its immunological activities, signaling and regulation of gene expression. Curr. Immunol. Rev. 2005;1:119–137. doi: 10.2174/1573395054065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Yan W., Zheng H., Du Q., Zhang L., Ban Y., Li N., Wei F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res. 2015;4:1465. doi: 10.12688/f1000research.7010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A.S., Maizels R.M. Alarming dendritic cells for Th2 induction. J. Exp. Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima H., Yamada N., Matsue H., Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J. Immunol. 2004;173:531–541. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- McKenna N.J., O'Malley B.W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Moreau A., Alliot-Licht B., Cuturi M.C., Blancho G. Tolerogenic dendritic cell therapy in organ transplantation. Transpl. Int. 2017;30:754–764. doi: 10.1111/tri.12889. [DOI] [PubMed] [Google Scholar]
- Mottis A., Mouchiroud L., Auwerx J. Emerging roles of the corepressors NCoR1 and SMRT in homeostasis. Genes Dev. 2013;27:819–835. doi: 10.1101/gad.214023.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L., Eichner L.J., Shaw R.J., Auwerx J. Transcriptional coregulators: fine-tuning metabolism. Cell Metab. 2014;20:26–40. doi: 10.1016/j.cmet.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G., Mittal A., Lopez-Diego R., Maier L.M., Anderson D.E., Weiner H.L. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J. Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.S., Chang T.H., Tailor P., Ozato K., Kino T. Virus-induced differential expression of nuclear receptors and coregulators in dendritic cells: implication to interferon production. FEBS Lett. 2011;585:1331–1337. doi: 10.1016/j.febslet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemand C., Nimmesgern A., Haan S., Fischer P., Schaper F., Rossaint R., Heinrich P.C., Muller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J. Immunol. 2003;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- O'Neill L.A., Golenbock D., Bowie A.G. The history of Toll-like receptors - redefining innate immunity. Nat. Rev. Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- Perissi V., Scafoglio C., Zhang J., Ohgi K.A., Rose D.W., Glass C.K., Rosenfeld M.G. TBL1 and TBLR1 phosphorylation on regulated gene promoters overcomes dual CtBP and NCoR/SMRT transcriptional repression checkpoints. Mol. Cell. 2008;29:755–766. doi: 10.1016/j.molcel.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigni M., Ashok D., Stevanin M., Acha-Orbea H. Establishment and characterization of a functionally competent type 2 conventional dendritic cell line. Front Immunol. 2018;9:1912. doi: 10.3389/fimmu.2018.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.G., Idoyaga J., Salmon H., Hogstad B., Bigarella C.L., Ghaffari S., Leboeuf M., Merad M. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat. Immunol. 2015;16:1060–1068. doi: 10.1038/ni.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghav S.K., Waszak S.M., Krier I., Gubelmann C., Isakova A., Mikkelsen T.S., Deplancke B. Integrative genomics identifies the corepressor SMRT as a gatekeeper of adipogenesis through the transcription factors C/EBPbeta and KAISO. Mol. Cell. 2012;46:335–350. doi: 10.1016/j.molcel.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Raker V.K., Domogalla M.P., Steinbrink K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol. 2015;6:569. doi: 10.3389/fimmu.2015.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B. Intracellular pathogens and CD8(+) dendritic cells: dangerous liaisons. Immunity. 2011;35:153–155. doi: 10.1016/j.immuni.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Schinnerling K., Garcia-Gonzalez P., Aguillon J.C. Gene expression profiling of human monocyte-derived dendritic cells - searching for molecular regulators of tolerogenicity. Front Immunol. 2015;6:528. doi: 10.3389/fimmu.2015.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer P., Behrens G.M., Wilson N.S., Pooley J.L., Smith C.M., El-Sukkari D., Davey G., Kupresanin F., Li M., Maraskovsky E. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. U S A. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Smale S.T. Selectivity of the NF-{kappa}B response. Cold Spring Harb Perspect. Biol. 2010;2:a000257. doi: 10.1101/cshperspect.a000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih V.F., Davis-Turak J., Macal M., Huang J.Q., Ponomarenko J., Kearns J.D., Yu T., Fagerlund R., Asagiri M., Zuniga E.I. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat. Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiokawa A., Tanabe K., Tsuji N.M., Sato R., Hachimura S. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol. Lett. 2009;125:7–14. doi: 10.1016/j.imlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Smita S., Ahad A., Ghosh A., Biswas V.K., Koga M.M., Gupta B., Acha-Orbea H., Raghav S.K. Importance of EMT factor ZEB1 in cDC1 "MutuDC line" mediated induction of Th1 immune response. Front Immunol. 2018;9:2604. doi: 10.3389/fimmu.2018.02604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M. Linking innate to adaptive immunity through dendritic cells. Novartis Found. Symp. 2006;279:101–109. discussion 109-113, 216-109. [PubMed] [Google Scholar]
- Sumpter T.L., Thomson A.W. The STATus of PD-L1 (B7-H1) on tolerogenic APCs. Eur. J. Immunol. 2011;41:286–290. doi: 10.1002/eji.201041353. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Taylor M.D., van der Werf N., Maizels R.M. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33:181–189. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Thomas R. RelB and the aryl hydrocarbon receptor: dendritic cell tolerance at the epithelial interface. Immunol. Cell Biol. 2013;91:543–544. doi: 10.1038/icb.2013.51. [DOI] [PubMed] [Google Scholar]
- Thompson P.W., Bayliffe A.I., Warren A.P., Lamb J.R. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4+ T cells. Cytokine. 2007;39:184–191. doi: 10.1016/j.cyto.2007.07.191. [DOI] [PubMed] [Google Scholar]
- Tsoumakidou M., Tousa S., Semitekolou M., Panagiotou P., Panagiotou A., Morianos I., Litsiou E., Trochoutsou A.I., Konstantinou M., Potaris K. Tolerogenic signaling by pulmonary CD1c+ dendritic cells induces regulatory T cells in patients with chronic obstructive pulmonary disease by IL-27/IL-10/inducible costimulator ligand. J. Allergy Clin. Immunol. 2014;134:944–954.e8. doi: 10.1016/j.jaci.2014.05.045. [DOI] [PubMed] [Google Scholar]
- Vendelova E., Ashour D., Blank P., Erhard F., Saliba A.E., Kalinke U., Lutz M.B. Tolerogenic transcriptional signatures of steady-state and pathogen-induced dendritic cells. Front Immunol. 2018;9:333. doi: 10.3389/fimmu.2018.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke L., Beyaert R., van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Wolfle S.J., Strebovsky J., Bartz H., Sahr A., Arnold C., Kaiser C., Dalpke A.H., Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur. J. Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Williams E.G., Mouchiroud L., Canto C., Fan W., Downes M., Heligon C., Barish G.D., Desvergne B., Evans R.M. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147:827–839. doi: 10.1016/j.cell.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Zhang G.X., Gran B., Fallarino F., Yu S., Li H., Cullimore M.L., Rostami A., Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T., Krebs A.R., Choukrallah M.A., Keime C., Plewniak F., Davidson I., Tora L. seqMINER: an integrated ChIP-seq data interpretation platform. Nucleic Acids Res. 2011;39:e35. doi: 10.1093/nar/gkq1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J.H., Kong W., Hooper K.M., Emig F., Rahbari K.M., Kuo P.C., Scofield B.A., Ganea D. Differential effects of IFN-beta on IL-12, IL-23, and IL-10 expression in TLR-stimulated dendritic cells. J. Leukoc. Biol. 2015;98:689–702. doi: 10.1189/jlb.3HI0914-453R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S., Ha S.J. Generation of tolerogenic dendritic cells and their therapeutic applications. Immune Netw. 2016;16:52–60. doi: 10.4110/in.2016.16.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Nishinakamura H., Matsumura Y., Hanada T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res. Ther. 2005;7:100–110. doi: 10.1186/ar1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Markan K., Temple K.A., Deplewski D., Brady M.J., Cohen R.N. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J. Biol. Chem. 2005;280:13600–13605. doi: 10.1074/jbc.M409468200. [DOI] [PubMed] [Google Scholar]
- Yu Q., Kovacs C., Yue F.Y., Ostrowski M.A. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J. Immunol. 2004;172:6047–6056. doi: 10.4049/jimmunol.172.10.6047. [DOI] [PubMed] [Google Scholar]
- Zhao G.N., Jiang D.S., Li H. Interferon regulatory factors: at the crossroads of immunity, metabolism, and disease. Biochim. Biophys. Acta. 2015;1852:365–378. doi: 10.1016/j.bbadis.2014.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GeneGo metacore software was used for the analysis.
GeneGo Metacore software was used for the analysis