Abstract
Background:
Zika virus (ZIKV) infection can cause severe birth defects in newborns with no effective currently available treatment. Adoptive transfer of virus-specific T-cells has proven to be safe and effective for the prevention or treatment of many viral infections, and could represent a novel treatment approach for patients with ZIKV infection. However, extending this strategy to the ZIKV setting has been hampered by limited data on immunogenic T-cell antigens within ZIKV. Hence, we have generated ZIKV-specific T-cells and characterized the cellular immune responses against ZIKV antigens.
Method:
T-cell products were generated from peripheral blood of ZIKV-exposed donors, ZIKV-naive adult donors, and umbilical cord blood by stimulation with 15mer overlapping peptide libraries spanning four ZIKV polyproteins (C, M, E, and NS1) using a good manufacturing practice-compliant protocol.
Results:
We successfully generated T-cells targeting ZIKV antigens with clinical relevant numbers. The ex vivo expanded T-cells comprised both CD4+ and CD8+ T-cells that were able to produce Th1 polarized effector cytokines and kill ZIKV-infected HLA matched monocytes, confirming functionality of this unique T-cell product as a potential “off the shelf” therapeutic. Epitope mapping using peptide arrays identified several novel HLA class I and class II-restricted epitopes within NS1 antigen, which is essential for viral replication and immune evasion.
Discussion:
Our findings demonstrate that it is feasible to generate potent ZIKV-specific T-cells from a variety of cell sources including virus naïve donors for future clinical use in an “off the shelf” setting.
Keywords: Cord blood, Epitope mapping, Good manufacturing practice, Immunotherapy, T lymphocytes, Zika virus
INTRODUCTION
Zika virus (ZIKV) is a mosquito-born, enveloped positive-sense single-stranded RNA virus and belongs to the Flaviviridae family, which also includes dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), and Japanese encephalitis virus (JEV) [1,2]. The ZIKV genome encodes three structural proteins: C (capsid), prM/M (premembrane/membrane), and E (envelope); and seven nonstructural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 [3]. While ZIKV is primarily spread through the bite of an infected Aedes species mosquito, cases of sexual, blood-borne, and maternal-fetal transmission have been reported [4,5]. Until recently ZIKV was linked to an endemic, self-limiting, mild symptomatic disease characterized by low-grade fever and rash [6]. Importantly, in the recent outbreaks, ZIKV infection is now known to be associated with microcephaly and other neurodevelopmental birth defects in newborns, through its ability to infect human neural progenitor cells, and Guillain-Barré syndrome in adults, making ZIKV an emerging global public health issue [7–12]. Further, mouse and human studies have shown that ZIKV may also cause male infertility [13–15]. Currently there are no effective treatment or approved vaccination strategies for ZIKV. Hence, there is an urgent need for novel approaches to the prevention and treatment of ZIKV infection.
Adoptive transfer of ex vivo expanded virus-specific T-cells has been shown to be safe and effective for the prevention and treatment for many viral diseases including cytomegalovirus (CMV), Epstein-Barr virus (EBV), adenovirus, and BK virus after hematopoietic stem cell transplantation [16–19]. Moreover, several groups, including ours, have successfully used banked “off the shelf” virus-specific T-cell products, which are feasible for urgent use, from partially HLA-matched healthy seropositive donors to treat viral infections with minimal toxicity [20–23]. This strategy depends upon the knowledge of viral antigens recognized by T-cells. Data on T-cell immunodominant epitopes in human ZIKV infection is limited, but recent studies from mouse models and exposed humans have suggested the importance of both CD4+ and CD8+ T-cell immunity in protection and clearance of ZIKV[24]. CD4+ T-cells have been shown to have an essential role in protection against primary ZIKV infection in an IFNR-deficient murine model [25,26]. Another murine study demonstrated a critical role of CD4+ T-cells on resistance to a lethal ZIKV challenge partly through inducing B-cells to generate neutralizing antibodies [27]. In human donors with a history of ZIKV infection, ZIKV-specific memory CD4+ T-cells producing polyfunctional cytokines have been detected [24,28]. CD8+ T-cells also play a protective role in controlling the ZIKV replication. In mice, depletion of CD8+ T-cells resulted in higher viral loads after infection of ZIKV, but adoptive transfer of memory CD8+ T-cells from ZIKV-infected mice reversed this effect [29,30]. Thus, ZIKV could be a suitable candidate for adoptive immunotherapy, and successful ex vivo expansion of ZIKV-specific T-cell products could provide a new therapeutic strategy against ZIKV infection.
A potential obstacle for the development of adoptive immunotherapy to treat ZIKV is the limited seroprevalence of ZIKV in non-endemic areas [31]. One approach to overcome this issue is to generate ZIKV-specific T-cell products from ZIKV-seronegative adults and umbilical cord blood. We previously demonstrated that CMV and HIV-specific T-cells can be expanded from virus-naïve donors and T-cell products from these donors can be used for immunotherapeutic application [19,32–35]. Based on the previous success, we sought to generate ZIKV-specific T-cells from not only ZIKV seropositive donors but also naïve donor sources including seronegative healthy adult donors and umbilical cord blood.
Here, we show it is feasible to generate potent ZIKV-specific T-cells from a range of sources including ZIKV-exposed and ZIKV-naïve healthy adult donors, as well as umbilical cord blood using a good manufacturing practice (GMP)-compliant methodology. We also characterized the cellular immune response and identified novel epitopes within ZIKV NS1 antigen, which is essential for viral replication and immune evasion. We anticipate these ZIKV-specific T-cell products potentially represent a novel “off the shelf” therapeutic strategy for patients with ZIKV infection.
METHODS
Donors
Peripheral blood mononuclear cells (PBMCs) from donors who have a history of ZIKV infection (ZIKV-exposed donors) were obtained from ZIKV endemic areas (the University of São Paulo [Brazil]). ZIKV infection was confirmed by a positive real-time reverse-transcriptase PCR assay for ZIKV RNA as previously described [36]. Peripheral blood of ZIKV-naïve healthy adult donors was collected from ZIKV non-endemic areas in the USA (Children’s National Medical Center [Washington, DC] and the National Institutes of Health [Bethesda, MD]). Umbilical cord blood was obtained from MD Anderson Cancer Center (Houston, TX). All human materials were obtained under informed consent approved at each institution in accordance with the Declaration of Helsinki. PBMCs and cord blood mononuclear cells (CBMCs) were isolated by Ficoll gradient centrifugation.
Peptides
For T-cell stimulations, we used ZIKV overlapping peptides of C, M, E, and NS1 (JPT Peptide Technologies, Berlin, Germany). These overlapping ZIKV peptide libraries consisted of 15mer peptides overlapping by 11 amino acids and were selected based on a proprietary algorithm held by JPT that was designed to obtain peptide combinations providing full coverage of ZIKV protein variability. Based on phylogenetic tree, 32, 32, 15, and 30 isolates of ZIKV polyprotein sequences were selected and a total of 48, 49, 146, and 166 peptides were synthesized for C, M, E, and NS1, respectively (Supplementary Table S1).
Generation of Antigen Presenting Cells (APCs)
For dendritic cells (DCs) generation, PBMCs or CBMCs were plated at 1 × 107 cells/well on 6-well plates and incubated for 2 hours in DC media (CellGenix, Freiburg, Germany) supplemented with 2 mmol/l GlutaMax (Gibco, Grand Island, NY). Non-adherent cells were harvested and cryopreserved. Adherent cells were cultured in DC media with IL-4 (1000 U/ml; R&D Systems, Minneapolis, MN) and GM-CSF (800 U/ml, R&D Systems). On day 5, immature DCs were matured using IL-4 (1000 U/ml), GM-CSF (800 U/ml), TNF-α (10 ng/ml), IL-6 (100 ng/ml), IL-1β (10 ng/ml; all R&D Systems), PGE-2 (1 μg/ml; Sigma-Aldrich, St. Louis, MO), and LPS (10 ng/ml, Sigma-Aldrich) and were harvested after 24 hours of maturation to use as APCs. To generate phytohemagglutinin (PHA)-treated lymphoblasts (PHA-blasts), PBMCs or CBMCs were stimulated with PHA (5 μg/ml, Sigma-Aldrich) in the presence of IL-2 (100 U/ml; Prometheus, San Diego, CA) in CTL media consisting of 45% RPMI (GE Healthcare, Logan, UT), 45% Click’s medium (Irvine Scientific, Santa Ana, CA), 10% human AB serum (Gemini BioProducts, West Sacramento, CA) and supplemented with 2 mmol/l GlutaMax. For lymphoblastoid cell lines (LCLs) generation, PBMCs or CBMCs were infected with live B95–8 EBV in complete media consisting of RPMI, 10% FBS (GE Healthcare), and 2 mmol/l GlutaMax containing cyclosporine A (1 μg/ml).
Generation of ZIKV-Specific T-Cell Products from ZIKV-Exposed Donors
PBMCs from ZIKV-exposed donors were pulsed with a mixture of ZIKV overlapping peptides of C, M, E, and NS1 (200 ng/peptide/15 × 106 PBMCs) and plated at 2 × 106 cells/well on 24-well plate with IL-4 (400 IU/ml) and IL-7 (10 ng/ml) in CTL media. After 10 days of stimulation, T-cells were re-stimulated with peptide-pulsed autologous irradiated (30 Gy) PHA-blasts and irradiated (100 Gy) K562 cells that have been genetically modified to express costimulatory molecules, CD80, CD83, CD86, and 4–1BBL (K562-cs; gift from Dr. Cliona Rooney at Baylor College of Medicine) [37]. After 7 days of re-stimulation, T-cell products were harvested and evaluated for antigen specificity and functionality.
Generation of ZIKV-Specific T-Cell Products from ZIKV-Naïve Adult Donors
Matured DCs were harvested from ZIKV-naïve adult donors and pulsed with whole ZIKV overlapping peptides encompassing C, M, E, and NS1 and used as APCs. Non-adherent cells, which contain T-cells, were stimulated with peptide-loaded DCs and cultured in CTL media with a cytokine mixture containing of IL-7 (10 ng/ml), IL-12 (10 ng/ml, R&D Systems), and IL-15 (5 ng/ml, R&D Systems). T-cells were re-stimulated with peptide-pulsed autologous irradiated (30 Gy) PHA-blasts with IL-15 (5 ng/ml) on stimulation 2 (on day 7) and IL-2 (100 U/ml) on stimulation 3 (on day 14). For stimulation 3, irradiated (100 Gy) K562-cs were added. After 7 days of stimulation 3, T-cell products were harvested and evaluated for antigen specificity and functionality.
Generation of ZIKV-Specific T-Cell Products from Umbilical Cord Blood
Matured DCs were harvested from cord blood and pulsed with whole ZIKV overlapping peptides of C, M, E, and NS1 and used as APCs. Non-adherent cells were stimulated peptides-loaded DCs and cultured in CTL media with IL-7 (10 ng/ml), IL-12 (10 ng/ml), and IL-15 (5 ng/ml). T-cells were re-stimulated with peptide-pulsed autologous irradiated (30 Gy) LCLs with IL-15 (5 ng/ml) on stimulation 2 (on day 7) and IL-2 (100 U/ml) on stimulation 3 (on day 14). After 7 days of stimulation 3, T-cell products were harvested and evaluated for antigen specificity and functionality.
IFN-γ Enzyme Linked Immunospot (ELISpot) Assay
Millipore Multi Screen HTS filter plates (MilliporeSigma, Burlington, MA) were coated IFN-γ capture antibody (1-D1K; Mabtech, Nacka Strand, Sweden) at a concentration of 10 μg/ml overnight at 4°C. Plates were blocked for 1 hour and T-cell products were plated at 1 × 105/well in 96-well plate and stimulated with ZIKV overlapping peptides (100 ng/ml). After 24 hours the plates were washed and incubated with biotinylated IFN-γ detection antibody (7-B6–1, Mabtech) at 1 μg/ml for 1 hour at 37°C. After incubation with streptavidin-coupled alkaline phosphatase complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA), plates were developed with AEC substrate (Sigma-Aldrich) solution. IFN-γ spots forming cells were quantified by Zellnet Consulting (Fort Lee, NJ).
Phenotyping
ZIKV-specific T-cell products were stained with fluorophore-conjugated antibodies against CD3, CD4, CD8, CD14, CD16, CD19, CD25, CD45RO, CD62L, CD127, CCR7, PD-1, LAG-3, TIM-3, and CTLA-4 (Miltenyi Biotec, Bergisch Gladbach, Germany; BioLegend, San Diego, CA). All samples were acquired on a CytoFLEX cytometer (Beckman Coulter, Brea, CA) and the data was analyzed with FlowJo X (FlowJo LLC, Ashland, OR).
Multiplex Cytokine Assay
ZIKV-specific T-cell products were plated on 1 × 105/well in 96-well plate, stimulated with ZIKV overlapping peptides (100 ng/ml) or control, and incubated overnight. Supernatants were harvested and the cytokine profile analysis was performed using the Bio-plex Pro Human 17-plex Cytokine Assay kit (Bio-Rad, Hercules, CA), and read on a Bio-plex MAGPIX instrument (Luminex, Austin, TX).
Intracellular Cytokine Staining
1 × 106 ZIKV-specific T-cell products were stimulated with ZIKV overlapping peptides (100 ng/ml), media alone, actin (negative control), or staphylococcal enterotoxin B (SEB, positive control) in the presence of brefeldin A and CD28/CD49d (BD Biosciences, San Jose, CA) for 6 hours. T-cells were fixed, permeabilized with Cytofix/Cytoperm solution (BD Biosciences) and stained with IFN-γ and TNF-α antibodies (Miltenyi Biotec).
Coculture Experiments
CD14+ monocytes were isolated from PBMCs using CD14 Microbeads (Miltenyi Biotec) and infected with ZIKV strain PRVABC59 (ATCC, Manassas, VA) at a multiplicity of infection of 10 for 1 hour in DC media. Monocytes were harvested after 48 hours of infection and used as targets. ZIKV infected monocytes were mixed with ZIKV-specific T-cell products or control PHA-blasts from the same donor at a monocyte to T-cell ratio of 1:2 in CTL media. After 24 hours of coculture, cells were collected and stained with monoclonal antibodies to detect T-cells (CD3+) and monocytes (CD14+). Cells were fixed, permeabilized and stained with a pan-flavivirus anti-E antibody (clone 4G2, ATCC). Samples were acquired on a FACS Calibur (BD Biosciences) and the data was analyzed with FlowJo X.
51Chromium (51Cr) Release Assay
Cytotoxicity was measured by standard 51Cr release assay. Briefly, ZIKV peptide-pulsed or unpulsed autologous PHA-blasts (negative control) were labeled with 51Cr (Perkin Elmer, Waltham, MA) for 1 hour, washed, and resuspended with ZIKV-specific T-cell products at multiple effector to target ratios, and incubated for 4 hours. Supernatants were transferred to a Luma plate (Perkin Elmer), and 51Cr release was measured on a MicroBeta2 counter (Perkin Elmer). Maximum release was determined by addition of 1% Triton X-100 to target cells. Specific lysis was determined as follows: (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100.
Epitope Mapping
ZIKV-specific T-cell products were tested for specificity to NS1 individual peptides in IFN-γ ELISpot. The NS1 15mer peptides were pooled according to the matrix as shown in Supplementary Figure S1. Cross-reactive pools were analyzed and individual peptides were tested to confirm epitope specificity. The HLA-restriction of antigen recognition was tested using blocking antibodies against HLA class I and II. 1 × 105/well cells were treated with monoclonal anti-HLA A/B/C or DR/DP/DQ antibody (Dako Agilent, Santa Clara, CA) at 10 μg/ml for 1 hour. After incubation cells were plated with actin (negative control), ZIKV single peptides, and SEB (positive control). ELISpot plates were incubated and developed as described earlier.
Data analysis
Results were evaluated using descriptive statistics (means, SEM, and ranges). Two-tailed Student’s t-test was used to compare the 2 groups and 1-way ANOVA with Tukey’s post hoc test was used to compare 3 or more groups. Data analysis was performed in Graphpad Prism (GraphPad Software, La Jolla, CA).
RESULTS
ZIKV-Specific T-Cells Can Be Generated from ZIKV-Exposed Donors, ZIKV-Naïve Adult Donors, and Umbilical Cord Blood
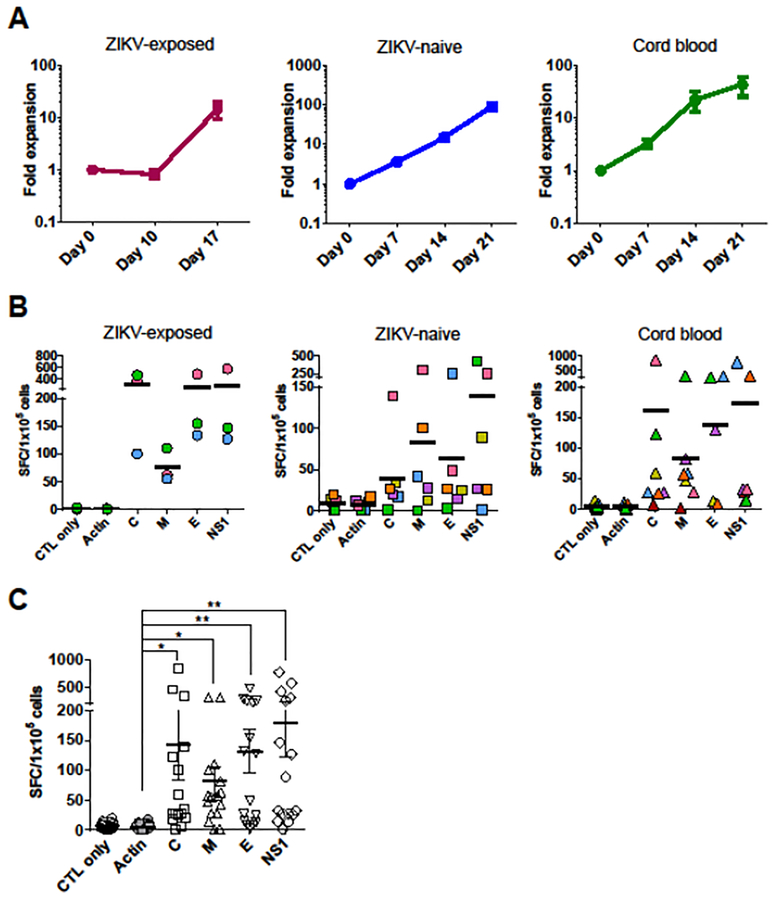
To evaluate whether ZIKV-specific T-cells can be expanded from seropositive donors by using the similar methodology as CMV-specific T cells [17], PBMCs from ZIKV-exposed donors were stimulated with ZIKV overlapping peptide libraries. Because little is known about T cell immunodominant epitopes in human ZIKV infection, we chose C, M, E, and NS1 proteins for T-cell stimulation according to previous studies [28,29,38,39]. PBMCs were pulsed with C, M, E, and NS1 overlapping peptides in the presence of IL-4 and IL-7 and expanded with peptide-pulsed PHA-blasts and costimulatory molecule expressing K562-cs. Resultant T-cell products showed a mean expansion of 14.5 ± 5.1-fold (range, 6.0–23.8) in total cell numbers after 17 days of stimulation (Figure 1A). We determined the specificity of the expanded T-cell products by IFN-γ ELISpot. T-cells specifically released IFN-γ against ZIKV antigens C (mean ± SEM, 306.2 ± 107.8 spot-forming cells [SFCs]/1 × 105 cells), M (75.7 ± 17.2 SFCs/1 × 105 cells), E (259.5 ± 115.5 SFCs/1 × 105 cells), and NS1 (282.7 ± 146.2 SFCs/1 × 105 cells), compared to irrelevant antigen actin (1.1 ± 0.4 SFCs/1 × 105 cells) (Figure 1B). The frequency of circulating ZIKV-specific T-cells in pre-expansion PBMCs was low which was in sharp contrast to the frequency of ZIKV-specific T-cell populations detected in the ex vivo expanded ZIKV-specific T-cell products (Supplementary Figure S2).
Figure 1. ZIKV-specific T-cells can be expanded from ZIKV-exposed, ZIKV-naïve adult donors and umbilical cord blood.
(A) Fold expansion of ZIKV-specific T-cell products generated from ZIKV-exposed donors (n = 3), ZIKV-naïve adult donors (n = 6), and cord blood (n = 7) based on the fold increase in the total cell numbers (mean ± SEM). (B) Specificity of ZIKV-specific T-cells from ZIKV-exposed donors (n = 3), ZIKV-naïve adult donors (n = 6), and cord blood (n= 7) with response to ZIKV antigens stimulation by IFN-γ ELISpot assay. Stimulation with actin was used as a negative control. Results are presented as SFC/1 × 105 cells. Bars denote the means. The same figure/color plot is from the same individual donor. (C) Specificity of ZIKV-specific T-cells from all cohort donors (n = 16) with response to ZIKV antigens (mean ± SEM; *P = 0.0002, **P < 0.0001; one-way ANOVA with Tukey’s post hoc test).
Next, to evaluate whether ZIKV-specific T-cells can be generated from seronegative donors using the similar methodology as HIV-specific T-cells [33,35], PBMCs from ZIKV-naïve adult donors were stimulated with autologous DCs pulsed with C, M, E, and NS1 overlapping peptides in the presence of IL-7, IL-12, and IL-15. After two more rounds of stimulation using autologous peptide-pulsed PHA-blasts, T-cell products were successfully grown with a mean expansion of 90.8 ± 16.7-fold (range, 40.0–155.9) after 21 days of stimulation (Figure 1A). The resultant T-cell products specifically released IFN-γ against ZIKV antigens C (mean ± SEM, 39.6 ± 20.3 SFCs/1 × 105 cells), M (82.4 ± 48.1 SFCs/1 × 105 cells), E (62.9 ± 39.6 SFCs/1 × 105 cells), and NS1 (139.1 ± 69.8 SFCs/1 × 105 cells), compared to irrelevant antigen actin (8.0 ± 2.7 SFCs/1 × 105 cells) (Figure 1B).
To further evaluate whether ZIKV-specific T-cells can be generated from virus-naïve donors, we generated ZIKV-specific T-cell products from umbilical cord blood. CBMCs were stimulated with autologous peptide-pulsed DCs in the first stimulation and peptide-pulsed LCLs in the second and third stimulation based on the success of generating HIV-specific T-cells [35]. T-cell products showed a mean expansion of 43.9 ± 18.2-fold (range, 5.7–123.5) after 21 days of stimulation (Figure 1A). T-cells specifically released IFN-γ against ZIKV antigens C (mean ± SEM, 160.4 ± 116.6 SFCs/1 × 105 cells), M (83.5 ± 39.5 SFCs/1 × 105 cells), E (136.6 ± 49.1 SFCs/1 × 105 cells), and NS1 (172.6 ± 108.8 SFCs/1 × 105 cells), compared to irrelevant antigen actin (4.9 ± 1.6 SFCs/1 × 105 cells) (Figure 1B). Together, these data indicate that T-cell products targeting multiple ZIKV antigens can be generated from ZIKV-exposed donors, ZIKV-naïve adult donors, and umbilical cord blood to clinically relevant numbers. Furthermore, when the magnitude of the response was compared between donor sources, ZIKV-specific T-cell products from ZIKV-exposed donors showed the highest activity against ZIKV antigens (Supplementary Figure S3).
To identify immunodominant antigens, the IFN-γ ELISpot data from the three cohorts were combined and analyzed. The T-cell products showed significantly higher specific responses to C, M, E, and NS1 antigens compared with actin control (Figure 1C). Among the ZIKV antigens we tested, NS1 generated the highest frequency of specific T-cells (mean ± SEM, 180.8 ± 58.2 SFCs/1 × 105 cells; P < 0.0001) followed by C (142.4 ± 57.7 SFCs/1 × 105 cells; P = 0.0002), E (132.0 ± 35.7 SFCs/1 × 105 cells; P < 0.0001), and M (81.6 ± 23.9 SFCs/1 × 105 cells; P = 0.0002).
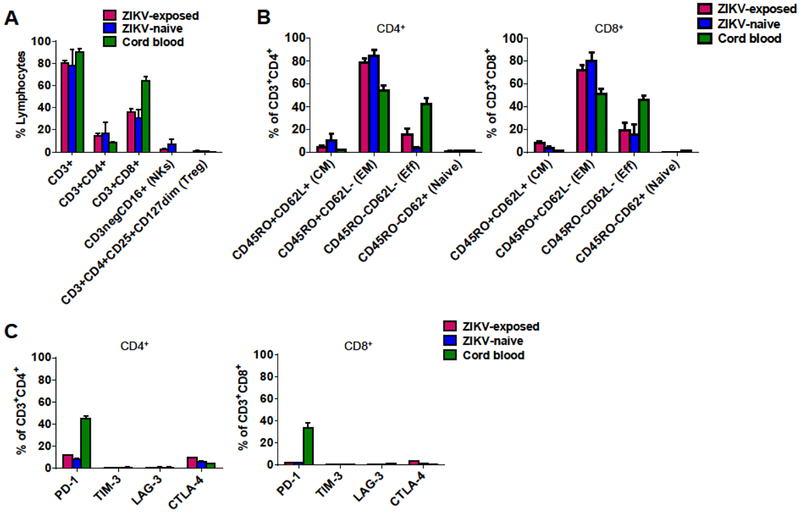
ZIKV-Specific T-Cell Products Comprise Both CD4+ and CD8+ and Are Predominately Effector Memory Phenotype
Phenotyping analysis revealed that the ZIKV-specific T-cell products comprised CD3+ T-cells (ZIKV-exposed, mean ± SEM, 80.2 ± 1.2%; ZIKV-naïve, 77.8 ± 6.5%; cord blood, 90.5 ± 1.2%) with a mixture of CD3+CD4+ T-cells (ZIKV-exposed, 14.9 ± 1.0%; ZIKV-naïve, 16.9 ± 4.5%; cord blood, 8.7 ± 0.2%) and CD3+CD8+ T-cells (ZIKV-exposed, 35.9 ± 1.8%; ZIKV-naïve, 30.3 ± 3.4%; cord blood, 64.3 ± 1.6%) (Figure 2A). There was minimal outgrowth of NK cells (CD3−CD16+) and no evidence of regulatory T-cell (CD3+CD4+CD25+CD127dim) outgrowth (Figure 2A). Both CD4+ and CD8+ T-cell subsets consisted predominantly of effector memory T-cells (CD45RO+CD62L−: ZIKV-exposed, 73.7 ± 4.5%; ZIKV-naïve, 82.2 ± 6.1%; cord blood, 51.6 ± 4.1%) with a small population of central memory T-cells (CD45RO+CD62L+: ZIKV-exposed, 7.3 ± 1.3%; ZIKV-naïve, 7.6 ± 3.1%; cord blood, 1.4 ± 0.1%) with no difference between CD4+ and CD8+ populations (Figure 2B). Gating strategies for T-cell differentiation and example flow plots are shown in Supplementary Figure 4. We measured co-inhibitory receptors including PD-1, TIM-3, LAG-3, and CTLA-4 on CD4+ and CD8+ T-cell products. Despite multiple stimulations, flow analysis revealed low expression levels of inhibitory molecules in all three cohorts with exception of PD-1 on cord blood-derived products (CD3+PD-1+: ZIKV-exposed, 4.9 ± 0.1%; ZIKV-naïve, 3.6 ± 0.2%; cord blood, 34.9 ± 4.3%) (Figure 2C).
Figure 2. Phenotyping of ZIKV-specific T-cell products.
(A) Surface marker phenotype of ZIKV-specific T-cell products from ZIKV-exposed donors (n = 3), ZIKV-naïve adult donors (n = 5), and cord blood (n = 5) (mean ± SEM). NKs = natural killer cells; Treg = regulatory T-cells. (B) T-cell subsets of ZIKV-specific T-cell products from ZIKV-exposed donors (n = 3), ZIKV-naïve adult donors (n = 5), and cord blood (n = 5) by CD4+ and CD8+ T-cells (mean ± SEM). CM = central memory T-cell; EM = effector memory T-cell; Eff = effector T-cell; Naïve = naïve T-cell. (C) Expression of inhibitory markers of ZIKV-specific T-cell products from ZIKV-exposed donors (n = 3), ZIKV-naïve adult donors (n = 5), and cord blood (n = 5) by CD4+ and CD8+ T-cells (mean ± SEM).
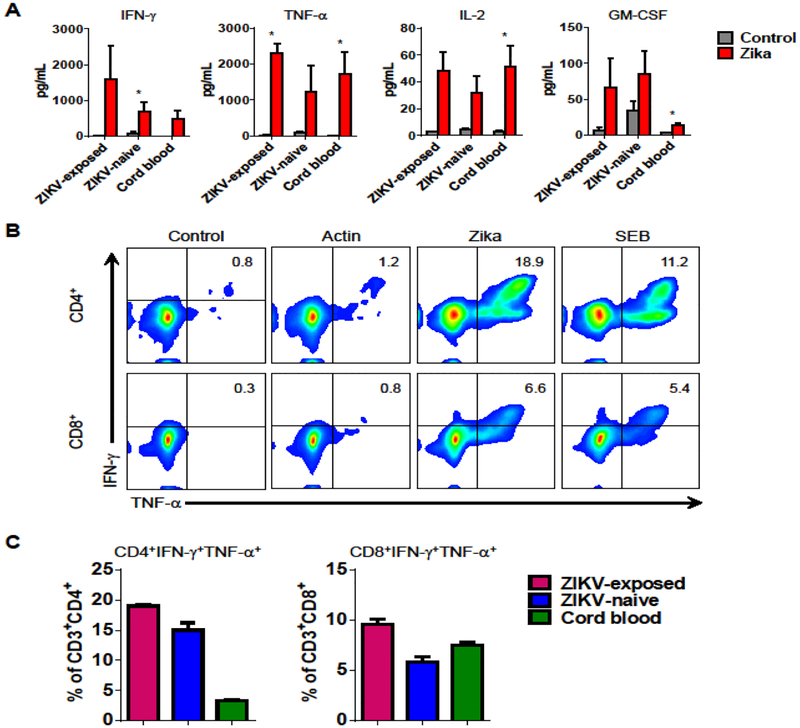
ZIKV-Specific T-Cell Products Are Polyfunctional
Recent studies suggest that polyfunctional antigen-specific T-cells have superior in vivo activity. To determine the polyfunctionality of ZIKV-specific T-cell products, we evaluated the production of multiple pro-inflammatory cytokines by Luminex assay. T-cell products from all three cohorts released IFN-γ, TNF-α, IL-2, and GM-CSF in response to ZIKV antigens stimulation compared to actin control (Figure 3A). Overall, the products produced more Th1 cytokines (IFN-γ, TNF-α, and IL-2; P < 0.01, P < 0.01, and P < 0.001, respectively) than Th2 cytokines (IL-6 and IL-10; P = n.s. [not significant]), suggesting that the T cell products were Th1-polarized (Supplementary Figure S5).
Figure 3. ZIKV-specific T-cell products are polyfunctional.
(A) ZIKV-specific T-cell products from ZIKV-exposed donors (n = 2), ZIKV-naïve adult donors (n = 6), and cord blood (n = 5) were tested for secretion of pro-inflammatory cytokines in response to stimulation with ZIKV antigens (mean ± SEM; *P < 0.05; t-test). Cytokines were measured in cell supernatant after 24-hour stimulation by Luminex assay. Stimulation with actin was used as a negative control. (B) Polycytokine (IFN-γ and TNF-α) production in response to ZIKV antigen stimulation as evaluated by intracellular cytokine staining from CD4+ and CD8+ T-cells in one representative donor (gated on CD3+). No stimulation (control) and stimulation with actin was used as negative control. Stimulation with SEB was used as a positive control. (C) Summary results of double cytokine (IFN-γ and TNF-α) production cells from ZIKV-exposed donors (n = 3), ZIKV-naïve adult donors (n = 4), and cord blood (n = 6) in CD4+ and CD8+ T-cell populations (mean ± SEM).
To further evaluate whether the T-cell products produce more than one cytokine, we performed intracellular IFN-γ and TNF-α staining, gating on CD4+ and CD8+ T-cells (Figure 3B–C). After stimulation with ZIKV antigens, 18.9% of CD4+ and 6.6% of CD8+ cells in the T-cell product were IFN-γ+TNF-α+, demonstrating that the majority of IFN-γ-producing cells also produced TNF-α. Summary data for by donor source demonstrated a large proportion of bifunctional CD4+ T-cells in adult donors compared with cord blood (CD4+IFN-γ+TNF-α+, mean ± SEM, ZIKV-exposed: 18.9 ± 0.2%; ZIKV-naïve: 15.0 ± 1.2%; cord blood: 3.3 ± 0.1%), and similar levels of bifunctional CD8+ T-cells (CD8+IFN-γ+TNF-α+: ZIKV-exposed: 9.6 ± 0.4%; ZIKV-naïve: 5.8 ± 0.5%; cord blood: 7.5 ± 0.2%). Together, these data indicate that the ex vivo expanded ZIKV-specific T-cell products are polyfunctional.
ZIKV-Specific T-Cell Products Kill Virus-Infected Targets
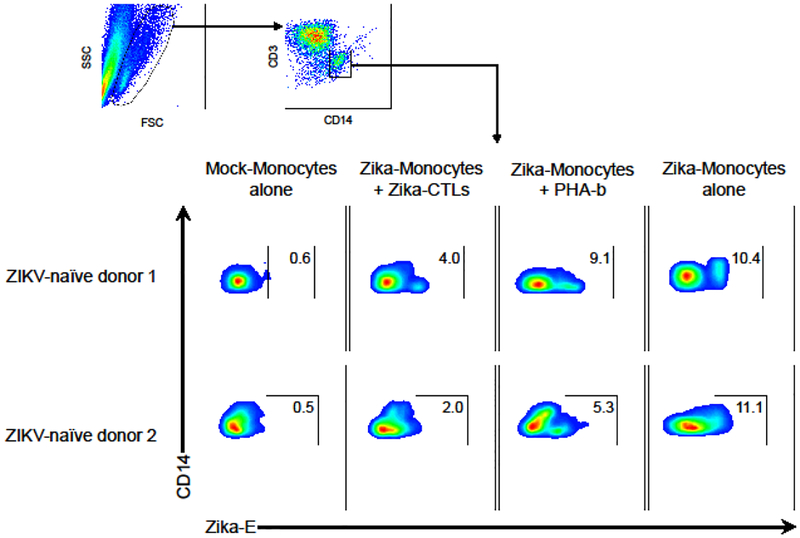
Given that monocytes are known to be the primary cellular target of ZIKV infection in human blood [40,41], ZIKV-infected monocytes were used as target cells to evaluate the cytolytic activity of the ex vivo expanded ZIKV T-cell products. Monocytes (CD14+) infected with ZIKV strain PRVABC59 were cocultured with autologous ZIKV-specific T-cells versus non-specific activated autologous PHA-blasts (CD3+). Intracellular staining for monoclonal antibody 4G2, that recognizes ZIKV E, was used to assess the presence of residual ZIKV-infected target cells. A 24-hour coculture experiment with ZIKV-specific T-cells derived from two ZIKV-naïve donors showed 4.0% and 2.0% residual virus-infected monocytes compared to 9.1% and 5.3% with control PHA-blasts, respectively (Figure 4). Additionally, we evaluated the cytolytic activity using 51Chromium-labeled, peptide-pulsed autologous cells as targets. ZIKV peptide-loaded PHA-blasts were specifically lysed by ZIKV-specific T-cell products from cord blood (Supplementary Figure S6). These results confirmed that ZIKV-specific T-cell products kill not only targets pulsed with ZIKA peptides but also ZIKV-infected targets in vitro.
Figure 4. ZIKV-specific T-cell products kill ZIKV-infected targets.
CD14-selected monocytes were infected with ZIKV (strain PRVABC59) and used as target cells in the coculture cytotoxicity assay. ZIKV-specific CTLs (CD3+) were cocultured with autologous ZIKV-infected CD14+ monocytes at an effector/target ratio of 2:1 for 24 hours. Autologous PHA-blasts were used as a negative control. CD3− and CD14+ fraction was gated and the presence of ZIKV-infected targets (Zika-E+) were assessed by flow cytometry. Uninfected monocytes (mock-monocytes alone) were used as negative control of accessing infected targets and infected monocytes (Zika-monocytes alone) were used as positive control.
Identification of NS1 T-Cell Epitopes
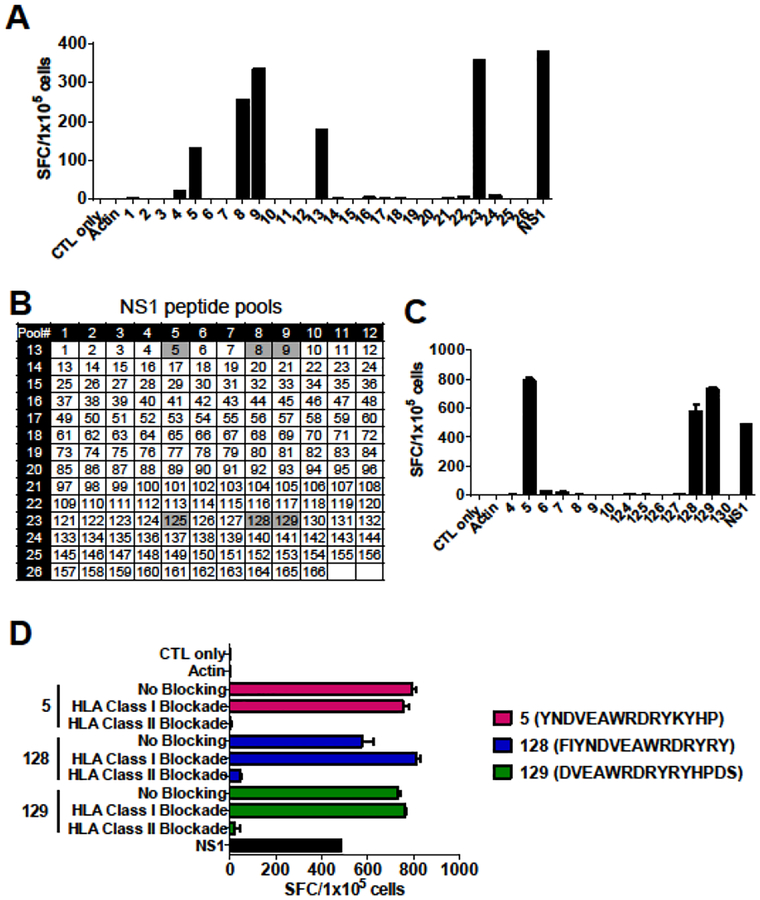
In our all donor cohorts, the strongest T-cell response observed was against NS1 protein, which has not previously been characterized, and is known to be essential for viral replication and immune evasion for ZIKV (Figure 1C). Therefore, we selected NS1 for further epitope mapping. An example of mapping a ZIKV-specific T-cell product is shown in Figure 5. In this approach, the NS1 protein was dissected into 15mer peptides overlapping by 11 amino acids, which were then divided into 26 mini-pools such that each peptide is uniquely present in two pools (Supplementary Figure S1). Using this method, the T-cell product recognized pools 5, 8, 9, 13 and 23 (Figure 5A), which suggests that potential epitopes laid within individual 15mer peptides 5, 8, 9, 125, 128, and 129 (Figure 5B). Testing of these single peptides by IFN-γ ELISpot revealed recognition of the single peptides 5, 128, and 129 (Figure 5C). HLA-restriction of these peptides were evaluated with HLA class I and II blocking antibodies. The responses to peptides 5, 128, and 129 decreased in the presence of HLA class II blockade, indicating that these peptides were HLA class II restricted epitopes (Figure 5D). The complete NS1 epitope mapping data is summarized in Table 1. As shown, we identified two novel HLA class I restricted epitopes and nine HLA class II restricted epitopes including variant sequences from multiple ZIKV subtypes within NS1 antigen. Analysis of restricted HLA alleles using predictive algorithms (IEDB MHC Predictor [www.iedb.org], NetMHC [www.cbs.dtu.dk/services/NetMHCpan/]) was performed (Table 1). Finally, analysis of conservation of identified NS1 epitopes among the Flaviviridae family including DENV, JEV, WNV, and YFV demonstrated limited similarity, suggesting that T-cell recognition of these epitopes represent ZIKV-specific immune responses (Supplementary Table S2). These data suggest that ZIKV-specific T-cell products are polyclonal containing both HLA class I and class II restricted T-cells recognizing multiple epitopes within NS1.
Figure 5. Identification of novel HLA class II-restricted NS1 epitopes.
Epitope mapping for NS1 protein was performed in five donors and results from one representative donor is shown. (A) A ZIKV-specific T-cell product from a ZIKV-exposed donor were stimulated with an NS1 peptide library pooled into 26 pools and the T-cell responses were measured by IFN-γ ELISpot. (B) Peptide matrix for NS1. NS1 peptides were divided into 26 mini-pools such that each peptide is present in two pools. Responses to pools 5 and 13, 5 and 23, 8 and 13, 8 and 23, 9 and 13, and 9 and 23 may be induced by individual peptides 5, 8, 9, 125, 128, and 129, respectively. (C) Testing of these single peptides by IFN-γ ELISpot revealed recognition of the single peptides of 5, 128, and 129 (mean ± SEM). (D) Blocking experiments with HLA class I and II blocking antibodies by IFN-γ ELISpot (mean ± SEM).
Table 1.
ZIKV-specific T-cell products epitope mapping for NS1
| Donor Source | HLA Class I | HLA Class II | NS1 Epitope | HLA Restriction |
IEDB prediction (predicted minimal epitope)a |
NetMHC prediction (predicted minimal epitope)a |
|---|---|---|---|---|---|---|
| ZIKV-exposed 1 | A 03:01, 33:01 | DRB1 01:02, 13:01; DRB3 01:01 | YNDVEAWRDRYKYHP | Class II | DQA1 *01:01/DQB1 *05:01 | DRB1*13:01 |
| B 14:02, 18:01 | DQA1 01:01, 01:03; DQB1 05:01, 06:03 | FIYNDVEAWRDRYRY | Class II | DRB3*01:01 | DRB3*01:01 | |
| C 02:02, 08:02 | DPA1 01:03, 01:03; DPB1 06:01, 104:01 | DVEAWRDRYRYHPDS | Class II | DRB1*13:01 | DRB1*13:01 | |
| ZIKV-exposed 2 | A 03:01, 33:01 | DRB1 01:02, 13:01; DRB3 01:01 | WCCRECTMPPL SFRA | Class II | DRB3*01:01 | DQA1*01:03/DQB1*05:01 |
| B 14:02, 18:01 | DQA1 01:01, 01:03; DQB1 05:01, 06:03 | |||||
| C 02:02, 08:02 | DPA1 01:03, 01:03; DPB1 06:01, 104:01 | |||||
| ZIKV-naïve 1 | A 02:01, 02:01 | DRB1 13:02, 15:01; DRB3 03:01 | LPHGWKAWGKSYFVR | Class II | DRB1*15:01 | DRB1*15:01 |
| B 07:02, 40:02 | DQA1 01:02, 01:02; DQB1 06:02, 06:09 | PHGWKAWGKSYFVRA | Class II | DRB1*15:01 | DRB1*15:01 | |
| C 02:02, 07:02 | DPA1 01:03, 01:03; DPB1 03:01, 04:01 | WKAWGKSYFVRAAKT | Class II | DPA1 *01:03/DPB 1*04:01 | DRB1*15:01 | |
| GFGVFHTSVWLKVRE | Class II | DPA1 *01:03/DPB 1*04:01 | DPA1 *01:03/DPB1 *04:01 | |||
| GFGIFHTSVWLKVRE | Class II | DPA1 *01:03/DPB 1*04:01 | DPA1 *01:03/DPB1 *04:01 | |||
| HGWKAWGKSYFVRAA | Class II | DPA1 *01:03/DPB 1*04:01 | DRB1*15:01 | |||
| ZIKV-naïve 2 | A 24:02, 29:02 | DRB1 13:03, 07:01; DRB3 01:01 | ISSVSRMENIMWKSV | Class I | B*41:02 (MENIMWKSV) | B*41:02 (MENIMWKSV) |
| B 41:02, 44:03 | DQA1 02:01, 05:05; DQB1 02:02, 03:01 | |||||
| C 16:01, 17:03 | DPA1 01:03 | |||||
| Cord blood 1 | A 33:03, 36:01 | DRB1 11:01, 15:03; DRB3 02:02 | FVYNDVDAWRDRYKY | (−)b | A*33:03 (FVYNDVDAWR) | B*58:01 (FVYNDVDAW) |
| B 53:01, 58:01 | DQA1 01:02, 01:02; DQB1 06:02, 06:02 | IS SVSRMENIMWRS V | Class I | B*58:01 (ISSVSRMENIMW) | B*58:01 (VSRMENIMW) | |
| C 03:02, 04:01 | DPA1 01:03, 03:01; DPB1 04:01, 105:01 | SRMENIMWRSVEGEL | Class I | C*04:01 (MWRSVEGEL) | A*33:03 (SRMENIMWR) |
HLA with the highest affinity score (lowest percentile rank) and predicted minimal epitopes by in silico prediction algorithms are shown.
HLA-restriction could not be determined by HLA blocking experiment.
DISCUSSION
The recent ZIKV outbreak has created an urgent need for the development of novel therapeutic as well as preventive approaches against ZIKV infection in pregnant women as well as healthy adults. In the present study, we show that it is possible to generate ZIKV-specific T-cell products ex vivo that comprised CD4+ and CD8+ T-cells with predominance of effector memory phenotype from ZIKV-exposed donors, naïve T-cells derived from adult donors and umbilical cord blood using GMP-applicable reproducible platform. These T-cell products showed reactivity to multiple ZIKV antigens with polyfunctional cytokine profile and demonstrated killing activity against ZIKV-infected monocytes. Finally, we were able to successfully map HLA class I and II restricted novel epitopes within NS1 antigen, which quantitatively induced the highest frequency of specific cells in our donors tested. This is the first report describing the manufacturing of ZIKV-specific T-cell products as a proof of principle for the development of an “off the shelf” T-cell immunotherapeutic targeting ZIKV.
Identification of immunogenic viral antigens is essential for developing immunotherapies, including vaccines and T-cell based therapies. To date, there is limited information about the specificity of the T-cell immune response against ZIKV antigens and comprehensive epitope mapping data are lacking in human ZIKV infection although there is some evidence to suggest the importance of cell mediated immunity [42–46]. Recently, some ZIKV T-cell epitopes have been mapped within structural and nonstructural proteins such as C, E, NS3, and NS5 in exposed and unimmunized donors [45,47]. In the current study, we intentionally focused on preparing T-cells specific for ZIKV antigens C, M, E, and NS1 because all four of these antigens have been correlated with viral pathogenicity and are primary targets for ZIKV vaccines [48–50]. The C protein plays a role in virus budding by binding to the cell membrane and gathering the viral RNA into a nucleocapsid that forms the core of the mature virus particle [51]. The E protein, which forms a heterodimer with M protein, binds to host cell surface receptor and mediates fusion between viral and cellular membranes [52]. The NS1 protein plays an essential role in viral RNA replication and immune evasion [53]. Among the four antigens, the NS1 induced the highest magnitude of the response. Epitope mapping within NS1 revealed that not only HLA class I restricted epitopes but also class II binding epitopes were recognized by T-cell products. Moreover, T-cell products composed of a predominance of CD8+ and a minor CD4+ T-cell population and both CD4+ and CD8+ ZIKV-specific T-cells were polyfunctional as demonstrated by producing IFN-γ and TNF-α simultaneously. CD4+ helper T-cells are known to be essential in the formation of protective memory CD8+ T-cells, which in turn show long term persistence and effector function in vivo [54]. Thus, the T-cell products should favor the subsequent improved cytolytic activity and clinical efficacy for ZIKV infection. Although we selected only four of ten ZIKV antigens for the generation of ZIKV-specific T-cell products, our data demonstrated the immunogenicity of both structural and nonstructural ZIKV proteins.
The strategy we used to generate ZIKV-specific T-cell products was based on the previous success of generating CMV and HIV-specific T-cells [17,33,35]. We used different expansion methods for the manufacture of ZIKV-specific T-cell products derived from the three donor groups. Of particular importance was the requirement to use autologous DCs as APCs and the requirement for three stimulations when expanding ZIKV-specific T-cells from ZIKV-naïve donors including cord blood. As a result, priming and expanding ZIKV-specific T-cells from virus naïve donors takes a longer period of time compared to the 10-day rapid manufacture protocol used for the expansion of ZIKV-specific T-cells from ZIKV-exposed donors. Hence, we posit that a higher fold expansion in the ZIKV-naïve healthy donors and umbilical cord blood would be attributed to the different methods of expansion.
The GMP-compliant overlapping peptide based method has several advantages over approaches for the direct selection of donor T-cells and genetic modification of T-cell receptors [55,56]. First, this method does not need live virus and viral vectors as the source of viral antigens, and is safe and cost effective for clinical application. Second, the use of overlapping peptide libraries does not require knowledge of immunogenic epitopes of target viral antigens and their HLA-binding characteristics. Third, since multi pathogen or antigen-specific T-cell products can be generated, this can prevent and minimize the likelihood of viral immune escape and may have superior clinical efficacy than targeting a single antigen. It has been suggested in viral infection, similar to the cancer setting, that targeting multiple antigens may improve clinical efficacy and reduce the risk of antigenic escape [57,58]. Finally, we were able to produce ZIKV-specific T-cell products from not only ZIKV seropositive donors but also from naïve donors including seronegative healthy donors and umbilical cord blood after multiple stimulations with peptide-pulsed APCs. This is important for third party derived “off the shelf” adoptive immunotherapy because the seroprevalence of ZIKV is limited especially in non-endemic countries [31]. Although T-cell exhaustion is a concern with the use of prolonged ex vivo culture and expansion, our products showed minimal expression levels of inhibitory molecules including PD-1, TIM-3, LAG-3, and CTLA-4 even after third stimulation, which is consistent with our previous report using similar expansion methods [35].
Recently, blood monocytes have been identified as the primary cellular target of ZIKV infection within circulating human blood [40,41]. ZIKV-infected monocytes function as a Trojan horse and disseminate ZIKV from the initial infection site into immune privileged organs, such as placenta, testes and brain, suggesting that infected monocytes could serve as reservoir of ZIKV [59]. Additionally, ZIKV also infects and replicates in primary human placental macrophages in a murine model in vivo and humans in vitro, suggesting a route for ZIKV to cross the placental barrier [60,61]. Therefore, it is critical for the T-cell products to target ZIKV-infected monocytes and tissue resident macrophages to prevent and reduce the virus dissemination to the fetal brain. In the current study, our T-cell products were successfully able to kill ZIKV-infected monocytes, indicating a potential future clinical utility.
We envision several platforms for applying the use of these novel ZIKV-specific T-cell products to the clinical setting. Although immune responses during pregnancy are complex and remain poorly understood, a recent murine study reported that activation and proliferation of CD4+ and CD8+ T-cells in infected pregnant dams were reduced compared with those in nonpregnant controls [62]. In addition, another murine study showed the contribution of CD8+ T-cells induced via peptide immunization or adoptive transfer to protect against ZIKV infection during pregnancy [63]. Thus, pregnant women with early diagnosis of ZIKV infection could receive ZIKV-specific T-cell products from partially HLA-matched third party donors for therapeutic use to reduce viral loads and potentially prevent neurological birth defects. In male mice, ZIKV have been shown to damage testicular cells and lead to male infertility [13,14]. Low sperm counts have been observed in ZIKV infected men, indicating that infection in the testis may affect sperm production [15]. Therefore, another therapeutic application could be men with ZIKV infection in whom ZIKV RNA can be detected for longer periods of time in semen to prevent male infertility. Since cryopreserved banks of “off the shelf” third party T-cell products can be available for immediate use, this approach would be feasible and applicable to these urgent settings. One possible concern with an adoptive T-cell therapy approach for ZIKV infection might be potential pathogenicity stemming from the CD8+ T-cell response. A recent study in mice suggests a potential involvement of CD8+ T-cells in the process of ZIKV-induced neurological complications induced by apoptosis of infected neurons [64]. However, the clinical relevance of pathogenic role of CD8+ T-cells in human ZIKV infection remains to be determined. Further work will therefore be necessary to better understand the timing and role of T-cell immunity in disease manifestations versus clearance of ZIKV, which may help predict whether T-cell immunotherapy targeting ZIKV infection will be successful, as well as the ideal setting and timing to apply such an approach for the treatment of at-risk and/or infected individuals.
In summary, we show that functionally active ZIKV-specific T-cells can be generated from ZIKV-exposed, ZIKV-naïve healthy adult donors, and umbilical cord blood. These products have wide ZIKV antigen recognition, show polyfunctionality, and kill ZIKV-infected targets. This study provides a rationale for the creation of third party banks of ZIKV-specific T-cell products derived from ZIKV-naïve donors and manufactured using GMP-compliant technologies. Such products could be ultimately used as an “off the shelf” therapeutic to treat patients either at risk of or with ZIKV infection. Therefore, we envision that this strategy could translate to future clinical studies to evaluate the safety and efficacy of adoptively transferred third party ZIKV-specific T-cell products.
Supplementary Material
Acknowledgments
This work was supported by grants from Be The Match and the American Society for Blood and Marrow Transplantation (Amy Strelzer Manasevit Award to P.J.H.), the National Institutes of Health (K23-HL136783–01 to M.D.K.), the Jeffrey Modell Foundation, and the Board of Visitors of the Children’s National Health System.
Abbreviations:
- ZIKV
Zika virus
- DENV
dengue virus
- WNV
West Nile virus
- YFV
yellow fever virus
- JEV
Japanese encephalitis virus
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- GMP
good manufacturing practice
- PBMCs
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- ELISpot
enzyme linked immunospot
- SFCs
spot-forming cells
- DCs
dendritic cells
- CBMCs
cord blood mononuclear cells
- LCLs
lymphoblastoid cell lines
- APCs
antigen presenting cells
- SEB
staphylococcus enterotoxin B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures of Interest
P.J.H. is a founder and director of Mana Therapeutics. C.M.B. is on the scientific advisory boards for Cellectis and has stock options in Neximmune and Torque Therapeutics and has stock or ownership in Mana Therapeutics. The other authors declare that there are no conflicts of interests.
REFERENCES
- [1].Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med 2016;374:1552–63. doi: 10.1128/CMR.00072-15. [DOI] [PubMed] [Google Scholar]
- [3].Pierson TC, Diamond MS. The emergence of Zika virus and its new clinical syndromes. Nature 2018;560:573–81. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- [4].Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, da Rosa AT, Haddow AD, et al. Probable Non-Vector-borne Transmission of Zika Virus, Colorado, USA. Emerg Infect Dis 2011;17:880–2. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, Rocco IM, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 2016;56:1684–8. doi: 10.1111/trf.13681. [DOI] [PubMed] [Google Scholar]
- [6].Shuaib W, Stanazai H, Abazid AG, Mattar AA. Re-Emergence of Zika Virus: A Review on Pathogenesis, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Am J Med 2016;129:879.e7–879.e12. doi: 10.1016/j.amjmed.2016.02.027. [DOI] [PubMed] [Google Scholar]
- [7].dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, et al. Zika Virus and the Guillain–Barré Syndrome — Case Series from Seven Countries. N Engl J Med 2016;375:1598–601. doi: 10.1056/NEJMc1609015. [DOI] [PubMed] [Google Scholar]
- [8].Brasil P, Sequeira PC, Freitas ADA, Zogbi HE, Calvet GA, De Souza RV, et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet 2016;387:1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- [9].de Oliveira WK, de França GVA, Carmo EH, Duncan BB, de Souza Kuchenbecker R, Schmidt MI. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 2017;390:861–70. doi: 10.1016/S0140-6736(17)31368-5. [DOI] [PubMed] [Google Scholar]
- [10].Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 2016;18:587–90. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabié A, et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N Engl J Med 2018;378:985–94. doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- [12].de Araújo TVB, de Ximenes RAA, de Miranda-Filho DB, Souza WV, Montarroyos UR, de Melo APL, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis 2018;18:328–36. doi: 10.1016/S1473-3099(17)30727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, et al. Zika virus infection damages the testes in mice. Nature 2016;540:438–42. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 2016;167:1511–1524.e10. doi: 10.1016/j.cell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- [15].Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 2017;17:1200–8. doi: 10.1016/S1473-3099(17)30444-9. [DOI] [PubMed] [Google Scholar]
- [16].Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 2006;12:1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- [17].Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther 2013;21:2113–21. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014;6. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hanley PJ, Melenhorst JJ, Nikiforow S, Scheinberg P, Blaney JW, Demmler-Harrison G, et al. CMV-specific T cells generated from naïve T cells recognize atypical epitopes and may be protective in vivo. Sci Transl Med 2015;7. doi: 10.1126/scitranslmed.aaa2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013;121:5113–23. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol 2017;35:3547–57. doi: 10.1200/JCO.2017.73.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neuenhahn M, Albrecht J, Odendahl M, Schlott F, Dössinger G, Schiemann M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 2017;31:2161–71. doi: 10.1038/leu.2017.16. [DOI] [PubMed] [Google Scholar]
- [23].Muftuoglu M, Olson A, Marin D, Ahmed S, Mulanovich V, Tummala S, et al. Allogeneic BK Virus–Specific T Cells for Progressive Multifocal Leukoencephalopathy. N Engl J Med 2018;379:1443–51. doi: 10.1056/NEJMoa1801540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lai L, Rouphael N, Xu Y, Natrajan MS, Beck A, Hart M, et al. Innate, T-, and B-Cell Responses in Acute Human Zika Patients. Clin Infect Dis 2018;66:1–10. doi: 10.1093/cid/cix732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hassert M, Wolf KJ, Schwetye KE, DiPaolo RJ, Brien JD, Pinto AK. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLOS Pathog 2018;14:e1007237. doi: 10.1371/journal.ppat.1007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016;534:267–71. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lucas CGO, Kitoko JZ, Ferreira FM, Suzart VG, Papa MP, Coelho SVA, et al. Critical role of CD4+T cells and IFNγ signaling in antibody-mediated resistance to Zika virus infection. Nat Commun 2018;9. doi: 10.1038/s41467-018-05519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science (80- ) 2016;353:823–6. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- [29].Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, et al. Mapping and Role of the CD8+T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017;21:35–46. doi: 10.1016/j.chom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huang H, Li S, Zhang Y, Han X, Jia B, Liu H, et al. CD8 + T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J Virol 2017:JVI.00900–17. doi: 10.1128/JVI.00900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saá P, Proctor M, Foster G, Krysztof D, Winton C, Linnen JM, et al. Investigational Testing for Zika Virus among U.S. Blood Donors. N Engl J Med 2018;378:1778–88. doi: 10.1056/NEJMoa1714977. [DOI] [PubMed] [Google Scholar]
- [32].Hanley PJ, Cruz CRY, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood 2009;114:1958–67. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Patel S, Lam S, Cruz CR, Wright K, Cochran C, Ambinder RF, et al. Functionally Active HIV-Specific T Cells that Target Gag and Nef Can Be Expanded from Virus-Naïve Donors and Target a Range of Viral Epitopes: Implications for a Cure Strategy after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2016;22:536–41. doi: 10.1016/j.bbmt.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dave H, Luo M, Blaney JW, Patel S, Barese C, Cruz CR, et al. Toward a Rapid Production of Multivirus-Specific T Cells Targeting BKV, Adenovirus, CMV, and EBV from Umbilical Cord Blood. Mol Ther - Methods Clin Dev 2017;5:13–21. doi: 10.1016/j.omtm.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Patel S, Chorvinsky E, Albihani S, Cruz CR, Jones RB, Shpall EJ, et al. HIV-Specific T Cells Generated from Naive T Cells Suppress HIV In Vitro and Recognize Wide Epitope Breadths. Mol Ther 2018;26:1435–46. doi: 10.1016/j.ymthe.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232–9. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ngo MC, Ando J, Leen AM, Ennamuri S, Lapteva N, Vera JF, et al. Complementation of antigen-presenting cells to generate T lymphocytes with broad target specificity. J Immunother 2014;37:193–203. doi: 10.1097/CJI.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pardy RD, Rajah MM, Condotta SA, Taylor NG, Sagan SM, Richer MJ. Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+T Cell Epitope in Immunocompetent Mice. PLoS Pathog 2017;13. doi: 10.1371/journal.ppat.1006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog 2017;13. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Michlmayr D, Andrade P, Gonzalez K, Balmaseda A, Harris E. CD14+CD16+monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat Microbiol 2017;2:1462–70. doi: 10.1038/s41564-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Foo SS, Chen W, Chan Y, Bowman JW, Chang LC, Choi Y, et al. Asian Zika virus strains target CD14+blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol 2017;2:1558–70. doi: 10.1038/s41564-017-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lima NS, Rolland M, Modjarrad K, Trautmann L. T Cell Immunity and Zika Virus Vaccine Development. Trends Immunol 2017;38:594–605. doi: 10.1016/j.it.2017.05.004. [DOI] [PubMed] [Google Scholar]
- [43].Xu X, Vaughan K, Weiskopf D, Grifoni A, Diamond MS, Sette A, et al. Identifying Candidate Targets of Immune Responses in Zika Virus Based on Homology to Epitopes in Other Flavivirus Species. PLoS Curr 2016. doi: 10.1371/currents.outbreaks.9aa2e1fb61b0f632f58a098773008c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, et al. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+T cells. Nat Microbiol 2017;2. doi: 10.1038/nmicrobiol.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Grifoni A, Pham J, Sidney J, O’Rourke PH, Paul S, Peters B, et al. Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J Virol 2017:JVI.01469–17. doi: 10.1128/JVI.01469-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ricciardi MJ, Magnani DM, Grifoni A, Kwon YC, Gutman MJ, Grubaugh ND, et al. Ontogeny of the B- and T-cell response in a primary Zika virus infection of a dengue-naïve individual during the 2016 outbreak in Miami, FL. PLoS Negl Trop Dis 2017;11. doi: 10.1371/journal.pntd.0006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Paquin-Proulx D, Leal FE, Terrassani Silveira CG, Maestri A, Brockmeyer C, Kitchen SM, et al. T-cell Responses in Individuals Infected with Zika Virus and in Those Vaccinated Against Dengue Virus. Pathog Immun 2017;2:274. doi: 10.20411/pai.v2i2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bailey MJ, Duehr J, Dulin H, Broecker F, Brown JA, Arumemi FO, et al. Human antibodies targeting Zika virus NS1 provide protection against disease in a mouse model. Nat Commun 2018;9:4560. doi: 10.1038/s41467-018-07008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li A, Yu J, Lu M, Ma Y, Attia Z, Shan C, et al. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat Commun 2018;9. doi: 10.1038/s41467-018-05276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Poland GA, Kennedy RB, Ovsyannikova IG, Palacios R, Ho PL, Kalil J. Development of vaccines against Zika virus. Lancet Infect Dis 2018;18:e211–8. doi: 10.1016/S1473-3099(18)30063-X. [DOI] [PubMed] [Google Scholar]
- [51].Rana J, Slon Campos JL, Leccese G, Francolini M, Bestagno M, Poggianella M, et al. Role of Capsid Anchor in the Morphogenesis of Zika Virus. J Virol 2018;92. doi: 10.1128/JVI.01174-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fontes-Garfias CR, Shan C, Luo H, Muruato AE, Medeiros DBA, Mays E, et al. Functional Analysis of Glycosylation of Zika Virus Envelope Protein. Cell Rep 2017;21:1180–90. doi: 10.1016/j.celrep.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hilgenfeld R Zika virus NS1, a pathogenicity factor with many faces. EMBO J 2016;35:2631–3. doi: 10.15252/embj.201695871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Whitmire JK. Induction and function of virus-specific CD4+ T cell responses. Virology 2011;411:216–28. doi: 10.1016/j.virol.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 2016;127:3331–40. doi: 10.1182/blood-2016-01-628982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sutrave G, Blyth E, Gottlieb DJ. Cellular therapy for multiple pathogen infections after hematopoietic stem cell transplant. Cytotherapy 2017;19:1284–301. doi: 10.1016/j.jcyt.2017.07.012. [DOI] [PubMed] [Google Scholar]
- [57].Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: Unexpected commonalities. Cell Host Microbe 2014;15:295–305. doi: 10.1016/j.chom.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weber G, Gerdemann U, Caruana I, Savoldo B, Hensel NF, Rabin KR, et al. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia 2013;27:1538–47. doi: 10.1038/leu.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jurado KA, Iwasaki A. Zika virus targets blood monocytes. Nat Microbiol 2017;2:1460–1. doi: 10.1038/s41564-017-0049-7. [DOI] [PubMed] [Google Scholar]
- [60].Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight 2016;1. doi: 10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Winkler CW, Myers LM, Woods TA, Messer RJ, Carmody AB, McNally KL, et al. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J Immunol 2017;198:3526–35. doi: 10.4049/jimmunol.1601949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Regla-Nava JA, Elong Ngono A, Viramontes KM, Huynh AT, Wang YT, Nguyen AVT, et al. Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat Commun 2018;9. doi: 10.1038/s41467-018-05458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jurado KA, Yockey LJ, Wong PW, Lee S, Huttner AJ, Iwasaki A. Antiviral CD8 T cells induce Zika-virus-associated paralysis in mice. Nat Microbiol 2018;3:141–7. doi: 10.1038/s41564-017-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.