Abstract
Cisplatin and other widely employed platinum-based anticancer agents produce chemotherapy-induced peripheral neuropathy (CIPN) that often results in pain and hyperalgesia that are difficult to manage. We investigated the efficacy of a novel bivalent ligand, MCC22, for the treatment of pain arising from CIPN. MCC22 consists of mu opioid receptor (MOR) agonist and chemokine receptor 5 (CCR5) antagonist pharmacophores connected through a 22-atom spacer and was designed to target a putative MOR-CCR5 heteromer localized in pain processing areas. Mice received once daily intraperitoneal (i.p.) injections of cisplatin (1 mg/kg) for seven days and behavior testing began 7 days later. Cisplatin produced mechanical hyperalgesia that was decreased dose-dependently by MCC22 given by intrathecal (ED50 = 0.004 pmol) or i.p. (3.07 mg/kg) routes. The decrease in hyperalgesia was associated with decreased inflammatory response by microglia in the spinal cord. Unlike morphine, MCC22 given daily for nine days did not exhibit tolerance to its analgesic effect and its characteristic antihyperalgesic activity was fully retained in morphine-tolerant mice. Furthermore, MCC22 did not alter motor function and did not exhibit rewarding properties. Given the exceptional potency of MCC22 without tolerance or reward, MCC22 has the potential to vastly improve management of chronic pain due to CIPN.
Keywords: Chemotherapy, neuropathy, hyperalgesia, chemokines, heteromer
1. Introduction
Cisplatin and platinum-based chemotherapy are employed to treat a variety of cancers, including breast, ovarian, and prostate (Kelland, 2007). Cisplatin causes peripheral neuropathic pain in ~30–40% of treated patients (Joseph and Levine, 2009; Ta et al., 2009; Windebank and Grisold, 2008). This syndrome can often be severe and persist for months to years after treatment has ended (McWhinney et al., 2009). Analgesics or agents typically used to treat neuropathic pain, including opioids (Cherny et al., 1994), gabapentin (Rao et al., 2007), and amitriptyline (Kautio et al., 2008), appear to be relatively ineffective in alleviating pain from CIPN treatment (Wolf et al., 2008), and exhibit their own dose-limiting adverse side effects such as motor impairment, tolerance, and dependence.
It is well known that pro-inflammatory chemokines that are released by activated microglia contribute to chronic pain and hyperalgesia, including neuropathic pain (Ramesh et al., 2013), by increasing synaptic strength (Clark et al., 2015) that leads to sensitization of nociceptive neurons in the spinal cord dorsal horn (Latremoliere and Woolf, 2009). Through this process, pro-inflammatory cytokines and chemokines counteract the analgesia produced by opioids (Vallejo et al., 2010; Watkins et al., 2005). It is known that functional interactions occur between the MOR and chemokine receptor 5 (CCR5) (Chen et al., 2004; El-Hage et al., 2006; Mahajan et al., 2002; Song et al., 2011), and there is evidence that MOR-CCR5 heteromers exist in vivo. Previous studies have shown both receptors are present in neurons and glia in the dorsal horn, and are co-localized in other areas of pain processing (Happel et al., 2008; Lee et al., 2013).
The bivalent ligand, MCC22, was developed to target the putative MOR-CCR5 heteromer (Akgün et al., 2015). The pharmacophores in MCC22 are derived from the MOR agonist oxymorphone (Weiss, 1955), and the CCR5 antagonist, TAK-220 (Takashima et al., 2005). The pharmacophores are linked by a 22-atom spacer which confers optimal antinociception in mouse models of inflammatory pain (Akgün et al., 2015). In this regard, MCC22 was orders of magnitude more potent than any of its homologs or a mixture of the monovalent mu agonist and CCR5 antagonist ligands. It was over 2,000 times more potent than morphine without exhibiting tolerance. Also, in a model of inflammation, the analgesic effect of MCC22 was orders of magnitude more potent when administered in the spinal cord as compared to the brain (Akgün et al., 2015), suggesting that the spinal cord is the primary site of action for its analgesic effects. It is therefore possible that MOR-CCR5 heteromers exist in vivo and may be located on spinal microglia. In this regard, activated microglia are essential for the extraordinary antihyperalgesic potency of MCC22.
In the present study, we investigated the antinociceptive profile of MCC22 in a murine model of CIPN produced by cisplatin. The data reveal that MCC22 is highly effective in reducing hyperalgesia associated with neuropathic pain without tolerance or producing other adverse effects. The studies further demonstrate that MCC22 reduced the inflammatory activation of microglia in the spinal cord associated with neuropathic pain.
2. Materials and methods
2.1. Subjects
Adult male C3H/HeJ mice (8–17 weeks old, 25–35 g, Charles River Laboratories, Wilmington, MA) were housed in cages of four mice per cage and maintained on a 12-h light-dark cycle. Animals had access to food and water ad libitum. Mice were randomly selected to treatments and conditions. The University of Minnesota Institutional Animal Care and Use Committee approved all protocols and procedures.
2.2. Cisplatin treatment
The platinum chemotherapy agent cis-dichlorodiammineplatinum (II) (LKT Laboratories, St. Paul, MN) was prepared in a 1 mg/mL solution in normal saline and administered at 1 mL/kg intraperitoneal (i.p.). Mice received one injection of cisplatin per day for 7 consecutive days. Behavioral testing began 7 days following the end of cisplatin treatment.
2.3. Drugs and administration
All compounds, including the bivalend ligand, MCC22, were synthesized as described previously (Akgün et al., 2015). The opioid pharmacophore MA19 (oxymorphone; 4, 5α-epoxy-3, 14-dihydroxy-17-methylmorphinan-6-one hydrochloride) with attached spacer, and the CCR5 antagonist, TAK220 (1-acetyl-N-[3-[4-[(4-carbamoylphenyl)methyl]piperidin-1-yl]propyl]-N-(3-chloro-4-methylphenyl)piperidine-4-carboxamide), were dissolved in 10% dimethyl sulfoxide (DMSO) (Fisher Scientific, Hampton, NH) then diluted to less than 1% DMSO in the test solutions. Homologs of MCC22 with spacer lengths of 14- and 24-atoms served as controls. Morphine (Mallinckrodt Inc., Hazelwood, MO) was dissolved in distilled water. Compounds were administered intrathecal (i.t.) in a 5-μL volume in conscious mice (Hylden and Wilcox, 1980) or i.p. to determine peak time and ED50/80. For i.t. injections, compounds were delivered using a 10-μl syringe with a 30 gauge needle. Mice were held firmly by the pelvic girdle and the needle was inserted into one side of the L5 or L6 spinous process so that it was placed into the groove between the spinous and transverse processes. The needle was moved forward to the intervertebral space so that approximately 0.5 cm of the needle was within the vertebral column. The irreversible mu opioid antagonist, beta-funaltrexamine (beta-FNA) was also prepared in distilled water and given i.p. to assess the contribution of MOR to the antonocice[ption produced by MCC22.
2.4. Mechanical hyperalgesia
Mechanical hyperalgesia was assessed by determining the frequency of paw withdrawal evoked by a calibrated von Frey monofilament (North Coast Medical Inc., Gilroy, CA) with a bending force 3.9 mN applied to the plantar surface of each hind paw for 1–2 seconds. Mice were placed beneath individual glass containers on a raised wire mesh surface and allowed to habituate for 30 min. The monofilament was applied to each hind paw 10 times at intervals of at least 3 seconds, and the total number of withdrawal responses was recorded for each paw and then averaged. The experimenter was blinded to the treatment condition.
In some experiments, we determined paw withdrawal threshold. Mice were placed on a wire mesh grid under a glass enclosure and allowed to acclimate for 30 minutes before testing. Paw withdrawal threshold was determined using an electronic Von Frey device (Life Sciences, IITC). The tip of the stimulator was applied to the plantar surface of both hind paws with enough force to cause paw withdrawal. The amount of force (g) required to evoked a withdrawal response response was averaged for both paws.
2.5. Rotarod test for motor coordination
The retention time on an accelerating rotarod (Ugo Basile, Monvalle VA, Italy) was used to determine whether MCC22 caused motor impairment and/or sedation. Animals were trained until retention times exceeded 240 seconds with a maximum cutoff of 5 minutes. Once trained, animals were tested before and at 30 min after administration of vehicle (1% DMSO, i.p.), MCC22 (10 mg/kg, i.p.), or morphine (40 mg/kg, i.p.).
2.6. Conditioned place preference (CPP)
An operant behavioral paradigm designed to test the rewarding effects of drugs was used as a screening tool for abuse potential. CPP was conducted in three phases: preconditioning, conditioning, and test day. During the pre-conditioning phase, mice were introduced to the activity boxes for 30 minutes and allowed access to both chambers through an opening in a partition separating the two. The left and right sides of the activity box were lined with horizontal or vertical black and white striped patterns respectively, and the time spent in each chamber recorded by computer. Mice showing a baseline preference for one chamber over another (spending >18 minutes on one side) were not used. During conditioning (3 days), mice were administered vehicle in the morning and placed in one side of the activity box with a closed partition for 30 minutes (vehicle paired chamber). In the afternoon, mice were administered drug (MCC22 or morphine at 10mg/kg, i.p.) and placed in the opposite chamber for an additional 30 minutes (drug paired chamber). On test day, mice were placed in the activity boxes with access to both chambers in the absence of any drug administration.
2.7. Immunohistochemistry
Mice were deeply anesthetized with 4% isoflurane and underwent cardiac perfusion with 4% paraformaldehyde. The lumbar spinal cord was removed and frozen in OCT before sectioning into 10 micron sections on a cryostat. The sections were blocked with 1% bovine serum albumin in PBST before incubating with antibody for MOR (1:1000 Ray Biotec), CCR5 (1:100 Abcam), and Iba1 (1:1000 Wako). The sections were washed before incubating with secondary antibodies Cy3 anti-guinea pig (1:400 Jackson Immunoresearch Labs), Cy5 anti-rabbit (1:400 Jackson Immunoresearch Labs), and Alexa488 anti-rat (1:1000 Abcam). The sections were mounted with Vectashield (Vector Laboratories) before imaging with an Olympus BX51 confocal microscope.
2.8. RNA isolation and real time PCR
Mice were deeply anesthetized with 4% isoflurane and underwent cardiac perfusion with phosphate buffered saline (PBS). The spinal cord was removed and RNA was isolated (Trizol). The RNA was DNAse treated (Invitrogen) and converted to cDNA with oligo(dT)12–18 primers with the Advantage RT for PCR Kit (Takara). Real time PCR reactions were conducted with Rotor-Gene SYBR Green PCR kit (Qiagen). Briefly, 0.5 μM primers, 1X SYBR Green Master Mix, and 2 μL diluted cDNA were combined, and reactions were conducted in triplicates. The primers sequences have been previously published (Bowen and Olson, 2009; Olson and Miller, 2004). Real time PCR was conducted on a Qiagen Q real time PCR machine using a hot start with cycle conditions, 40 cycles; 95°C 15 seconds, 60°C 10 seconds, and 72°C 15 seconds; followed by a melt from 75°C to 95°C. Quantitation of the mRNA was based on standard curves derived from cDNA standards for each primer set from 1fg/μ of RNA to 100ng/μg of RNA. Positive and negative cDNA controls were used for each primer set derived from known cell sources for each cytokine. Samples were normalized to the expression of β-actin.
2.9. Experimental design
Anti-hyperalgesia.
Withdrawal response frequencies were determined for two consecutive days before cisplatin treatment, at post-injection day (PID) 3, 5, and 7, as well as at one week after treatment. In separate groups of mice, ED50/80 doses were determined for both i.t and i.p. administration. For each injection, withdrawal frequencies were determined at various times after injection to determine the duration of anti-hyperalgesia produced by each dose. In some experiments, MCC22 was given weekly for up to 16 weeks to determine if the potency of MCC22 changed over time.
Tolerance.
Cisplatin-treated mice received two injections daily (morning and afternoon separated by 6 hours) of MCC22 (10 mg/kg, i.p.) or morphine (40 mg/kg, i.p.) for 9 consecutive days and withdrawal response frequencies were determined before injection, and on treatment days 1, 2, 3 and 9 at 30 min after the second daily injection, as described previously (Cataldo et al., 2018; Dutta et al., 2018). This time course for drug administration was chosen based on the time it takes for mice to become tolerant to the analgesic effect of morphine. Mice given morphine for 9 days were also administered (MCC22 10 mg/kg, i.p.) after the behavioral testing following the final injection of morphine on treatment day 9. Withdrawal response frequencies were determined before and at 30 min after MCC22.
Immunohistochemistry.
Cisplatin-treated mice received no treatment or a daily injection of MCC22 (10mg/kg, i.p.) for 7 consecutive days. The spinal cords were removed from cisplatin-treated and control (naïve) mice (n=3–4 per group), sectioned, and immunostained to determine expression of MOR and CCR5 on microglia. Five sections were analyzed for each mouse on a confocal microscope by a blinded reviewer.
mRNA expression.
Cisplatin-treated mice received an injection daily of MCC22 (10mg/kg, i.p.) or control treated for 7 consecutive days. The spinal cords were removed from cisplatin-treated and control treated mice. The RNA was isolated and used in real time PCR to determine the expression of cytokines IL-1β, IL-6, IL-10, TNFα, chemokine CCL3, and effector molecule Inducible nitric oxide synthase (iNOS).
2.10. Data analyses
Mean (±SEM) values are shown for all data unless otherwise stated. Mean withdrawal response frequencies, mean amount of time (seconds) spent on the treadmill and the mean time spent on preferred side in CPP paradigm were compared between groups using a one- or two-way ANOVA with Bonferroni t-tests for post-hoc comparisons between groups. For immunohistochemistry, a minimum of five spinal cord sections were analyzed from each mouse. A representative image was chosen for each experimental group. One-way ANOVAs and Bonferroni t-tests were used to determine differences between groups in the expression of cytokines, chemokines, and effector molecules. For all statistical analyses, a probability value of <0.05 was considered significant.
3. Results
3.1. MCC22 produced potent dose-dependent anti-hyperalgesia in a model of CIPN
Intrathecal administration.
We used our previously-developed model of CIPN (Khasabova et al., 2012) in which mice receive daily injections of the chemotherapeutic agent cisplatin to determine whether the bivalent ligand MCC22 reduces neuropathic pain produced by chemotherapy. Cisplatin produced a robust mechanical hyperalgesia, defined as an increase in the frequency of withdrawal to a 3.9 mN force applied to the hind paw that peaked within 5 days of treatment. In pilot studies, it was found that the peak antihyperalgesic effect of MCC22 occurred 20 min after injection. Figure 1 shows that i.t. administration of MCC22 decreased cisplatin-mediated hyperalgesia in a dose- dependent manner (Two-way ANOVA, p<0.0001). The lowest effective dose of MCC22 was 0.0001 pmol, which decreased cisplatin-mediated hyperalgesia 19%. The highest dose, 10 pmol, reduced the frequency of withdrawal to baseline (pre-cisplatin) levels (3.75 ± 3.75).
Figure 1. Intrathecal MCC22 reduced cisplatin-evoked mechanical hyperalgesia dose-dependently.
Cisplatin treatment produced robust mechanical hyperalgesia, which was reduced by MCC22. Data show mean paw withdrawal frequencies before cisplatin treatment, after cisplatin treatment, and at 20 minutes following various intrathecal doses of MCC22; the time of its peak effect. # indicates a difference in paw withdrawal frequency as compared to baseline; * indicates a difference in paw withdrawal frequency as compared to values following cisplatin. Data are presented as means (±SEM); n = 8 mice per group; (**p<0.01, ***p<0.001, ###p<0.001).
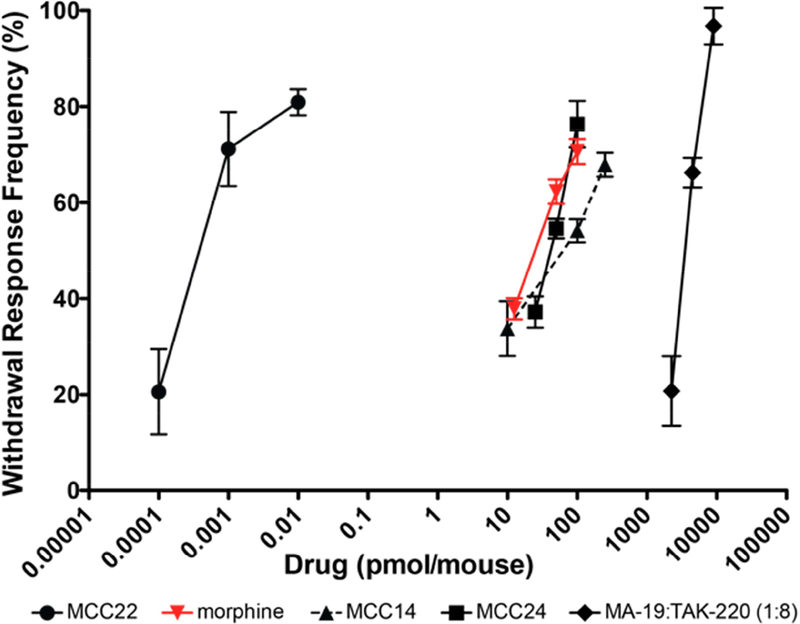
To determine the potency of MCC22 following i.t. administration, we determined its ED50 for decreasing the frequency of withdrawal after cisplatin. We also obtained the ED50 for morphine to compare the potency of MCC22 with that of morphine. To investigate the importance of the pharmacophore spacer length on the potency of MCC22, the ED50 for MCC14 (short spacer) and for MCC24 (long spacer) were also determined. MCC22 was orders of magnitude (>5000-fold) more potent than morphine as well as the other MCC homologs with shorter spacer length (Figure 2). The ED50 (with 95% confidence interval) of MCC22, morphine, MCC14, and MCC24 were: MCC22: 0.00022 (7.8e−5-6.02e−4) pmol; morphine: 25.44 (20.65–31.35) pmol; MCC14: 53.05 (32.01–87.83) pmol; MCC24: 40.19 (34.28–47.1).
Figure 2. MCC22 is more potent than morphine, spacer length homologs MCC14 and MCC24, and its monovalents: MA19 (mu agonist) + TAK220 (CCR5 antagonist).
ED50 values (pmol) and confidence intervals are: MCC22: 0.00022 (7.8e−5-6.02e−4) pmol; morphine: 25.44 (20.65–31.35) pmol; MCC14: 53.05 (32.01–87.83) pmol; MCC24: 40.19 (34.28–47.1). Data are presented as maximum possible effect at multiple doses from which ED50 values with 95% confidence intervals are computed by using nonlinear regression. A minimum of three groups employing n = 8–10 mice for each dose.
Figure 2 also shows no synergy when MA19 (the MOR agonist) was administered with TAK220 (the CCR5 antagonist). MA19 and TAK220 were administered at a ratio of 1:8 based on their individual ED50 values. This further suggests that MCC22 is acting via association of each of its pharmacophores with the protomers of the MOR-CCR5 heteromer rather than a mixture of receptor homomers.
In earlier studies (Smeester et al., 2014), a different bivalent compound, MMG22, was shown to become more potent over time in reducing hyperalgesia in a model of bone cancer pain. We therefore determined whether the efficacy of MCC22 increased over time in the cisplatin model. A sub-therapeutic i.t. dose of MCC22, 0.1 fmol, was administered chronically once per week for 16 weeks after cisplatin treatment in one group of animals whereas in another group this same dose was administered acutely only once a week 16. Withdrawal responses were determined before and at 20 min after injection. As shown in Figure 3, 0.1 fmol MCC22 does not alter mean paw withdrawal frequency in the chronic group during the first 5 weeks. However, paw withdrawal frequencies were found to decrease at week 9. Both groups had significantly lower paw withdrawal frequencies at week 16. This suggests that the increased potency of MMC22 over time was not due to repeated, or cumulative administration of MCC22 (i.e. no hysteresis). Rather, this appears to be due to other factors that may occur over time following administration of cisplatin.
Figure 3. The efficacy of MCC22 increased over time.
A sub-therapeutic dose of MCC22 (0.1 fmol) given intrathecally was administered once per week for 16 weeks after cisplatin treatment. Paw withdrawal frequencies were determined before (baseline) and at 20 min after MCC22 injection at weeks 2, 5, 9, and 16 after cisplatin treatment; *p<0.001. A single 0.1 fmol dose of MCC22 in week 16 (day 112) to MCC22 naïve mice shows no hysteresis of action. Data are presented as means (±SEM); n = 8–20 mice. # indicates a difference in paw withdrawal frequency in response to cisplatin as compared to baseline; * indicates a difference in paw withdrawal frequency when treated with MCC22 as compared to values following cisplatin; (**p<0.01, ***p<0.001, ###p<0.001).
Intraperitoneal administration.
MCC22 given i.p., reduced cisplatin-evoked hyperalgesia dose-dependently. The ED50 for MCC22 was 3.07 mg/kg (2.45 – 3.84), which was significantly lower than that of morphine (14.28; 12.70 – 16.07) (Figure 4A). The reduction in paw withdrawal frequency produced by 10 mg/kg, i.p. of MCC22 (ED80 dose), occurred 10 min after injection and peaked at 20 min. MCC22 produced a maximal reduction in paw withdrawal frequency of more than 89%, and persisted for at least 8 h after injection (Figure 4B) at which time there was still approximately 42% inhibition of hyperalgesia. Paw withdrawal frequency returned to pre-injection values at 24 h. In contrast, anti-hyperalgesia following the ED80 dose for morphine (40 mg/kg, i.p.) peaked at 30 min and produced a maximal reduction in paw withdrawal frequency of approximately 74%. Following morphine, hyperalgesia recovered more quickly as compared to MCC22, and 45% inhibition of hyperalgesia occurred 1 h after injection, as compared to 42% at 8 h after MCC22. Since hyperalgesia recovered by 75% at 120 min after injection of morphine, additional time points between this and 24 h were not tested.
Figure 4. Comparisons of potency and time course for MCC22 and morphine.
A) Potency: Groups of cisplatin-treated mice received various doses of MCC22 or morphine and withdrawal response frequencies were determined before and at 30 min after injection; ED50 values were determined. ED50 values and confidence intervals were 3.07 mg/kg (2.45–3.84) for MCC22 and 14.28 mg/kg (12.70–16.07) for morphine. Data are presented as maximum possible effect at multiple doses from which ED50 values with 95% confidence intervals are computed by using nonlinear regression. A minimum of three groups n = 8–10 mice for each dose. B) Time-course: Groups of cisplatin-treated mice developed hyperalgesia and were given the ED80 dose of MCC22 (10 mg/kg) or morphine (40 mg/kg). # indicates a significant difference from pre-cisplatin baseline; * indicates a significant difference from paw withdrawal frequency post-cisplatin treatment and before injection. Data are presented as means (±SEM); n = 8 mice per group; (Bonferroni t-tests; *p<0.01, ***p<0.001, ###p<0.001).
3.2. The anti-hyperalgesia produced by MCC22 was not due to loss of motor function or sedation
Three groups of naïve mice were treated with i.p. injection of vehicle, 10 mg/kg MCC22 or 40 mg/kg of morphine and placed on a rotarod treadmill 30 min after injection. There was no significant difference between groups in the time (mean ±SEM) spent on the rotarod before any injection and this was not significantly altered after vehicle (before = 281 ± 6.8 sec; after = 270 ± 13.3 sec), morphine (before = 279 ± 9.2 sec; after = 266 ± 10.2 sec.), or MCC22 (before = 273 ± 8.6 sec; after = 259 ± 6.5 sec) injection. These results indicate that the potent anti-hyperalgesia produced by MCC22, did not produce motor deficit.
3.3. MCC22 did not produce tolerance to its anti-hyperalgesic effect
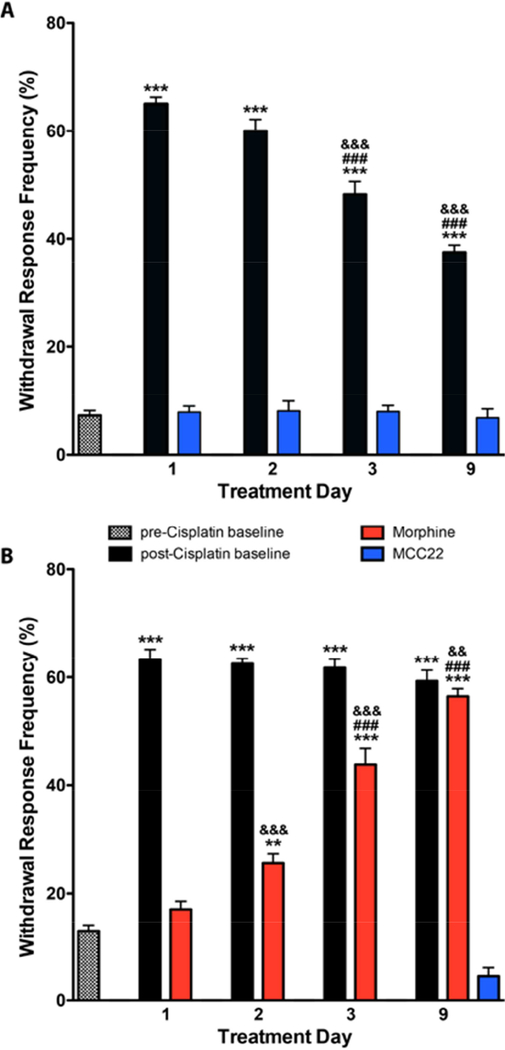
It is well known that opioids produce tolerance with repeated administration, resulting in the need for higher doses that limit their use. Therefore, we examined the extent to which tolerance occurred following repeated administration of MCC22. We compared the anti-hyperalgesia produced by repeated administration of the ED80 dose for MCC22 (10 mg/kg, i.p.) to that produced by repeated administration of morphine (40 mg/kg, i.p.) in separate groups of mice. Mice were given twice-daily injections of either MCC22 or morphine for 9 consecutive days beginning one week after cisplatin treatment. Withdrawal response frequencies were determined before and at 30 min after injection of MCC22 (Figure 5A) or morphine (Figure 5B) on day 1, 2, 3, and 9 of treatment. Tolerance was not observed following MCC22, which consistently produced maximal anti-hyperalgesia over the 9-day time-course. Unexpectedly, baseline withdrawal response frequencies gradually decreased during this period. In contrast, morphine produced tolerance as early as day 3 and did not produce significant anti-hyperalgesia by day 9 of treatment. This group of mice was then given an injection of MCC22 (10 mg/kg, i.p.) to determine whether mice made completely tolerant to the antihyperalgesic effects of morphine would be responsive to MCC22. As shown in Figure 5B, MCC22 produced its characteristic anti-hyperalgesia and withdrawal response frequencies after morphine that were not different from those obtained before cisplatin treatment. These results show that unlike morphine, MCC22 does not produce tolerance to its antihyperalgesic properties and is effective in morphine-tolerant mice.
Figure 5. Comparisons of the development of tolerance following MCC22 (10 mg/kg, i.p.) and morphine (40 mg/kg, i.p.) in cisplatin-treated mice.
Separate groups of mice received twice daily injections of either MCC22 or morphine for 9 consecutive days and withdrawal frequencies were determined before and at 30 min after injection of MCC22 (n=8–20) or morphine (n=8–17) on days 1, 2, 3, and 9 of treatment. A) Tolerance was not observed following MCC22. B) Morphine exhibited tolerance as early as day 3 and did not produce any anti-hyperalgesia by day 9 of treatment. These same mice were then given an injection of MCC22. * indicates a significant difference from pre-injection baseline; # indicates a significant difference from withdrawal response frequencies as compared to day 1; & indicates a significant decrease from baseline withdrawal response frequencies obtained on the previous testing day. Data are presented as mean (± SEM); (Bonferroni t-tests ***p<0.01; ###p<0.001; &&&p<0.001).
3.4. MCC22 did not exhibit rewarding properties
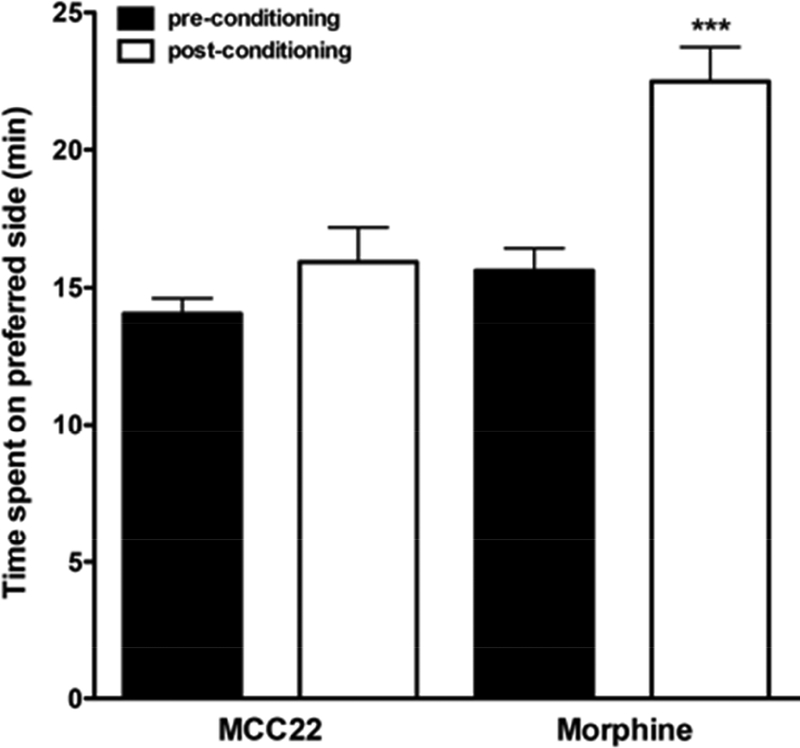
Morphine and other opioids are highly abused and addictive. Given the current opioid epidemic, we compared the rewarding properties of morphine and MCC22 using a CPP paradigm. In pre-conditioning, separate groups of mice were first placed in the center of a dual sided activity chamber for 30 minutes. These animals initially showed no preference and spent a similar amount of time (mean 15 minutes ± 1) on either side. CPP was then performed over 3 days where one group was conditioned with morphine (10.0 mg/kg, i.p.) and another with MCC22 (10.0 mg/kg, i.p.). Post-conditioning, it was found that morphine mice spent significantly more time (mean 21.9 minutes ± 1.4, p<0.001) on the side paired with drug (preferred side) while mice conditioned with MCC22 did not differ from pre-conditioning (mean 15.6 minutes ± 1.2) (Figure 6). Thus, mice conditioned with morphine displayed place preference as expected, whereas mice conditioned with MCC22 did not.
Figure 6. MCC22 did not produce conditioned place preference.
Time spent on the preferred side (minutes) pre- and post-conditioning are shown for MCC22 and Morphine. Data are presented as mean (± SEM); n = 6 mice per group; (*** indicates a significant difference from pre-conditioning, p<0.001, Bonferroni t-test).
3.5. MCC22 targets microglia in the spinal cord
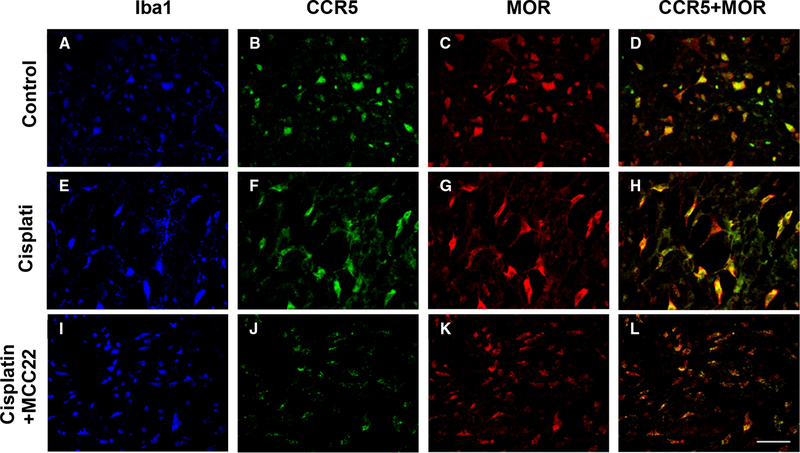
Since MCC22 contains a CCR5 antagonist and MOR agonist pharmacophores, we wanted to determine which cells in the spinal cord express these receptors. The expression of CCR5 and MOR were examined in naive mice as well as cisplatin-treated mice with and without MCC22 (Figure 7). CCR5 and MOR are both expressed on microglia (Iba1 positive cells) in the spinal cord of naïve mice as well as in cisplatin-treated mice. MCC22 altered the expression pattern of CCR5 and MOR on microglia in the cisplatin-treated mice into small clumps suggesting the formation of heteromers. These results show that CCR5 and MOR co-localize on microglia in the spinal cord, and MCC22 can promote heteromer formation of CCR5 and MOR on microglia.
Figure 7. CCR5 and MOR are expressed on microglia.
Mice were treated with cisplatin for 7 days. Cisplatin-treated mice received no treatment (E-H) or were administered MCC22 (10mg/kg) (I-L) for 7 days. Naïve mice served as control (A-D). The spinal cords were removed from the mice (3–4 mice per group), sectioned, and stained with antibodies for Iba1 (blue), CCR5 (green), and MOR (red). Sections were imaged with a confocal microscope and representatives from each group are shown. Scale bar = 40 microns. Panels D, H, L show the merged florescence for CCR5 (green) and MOR (red).
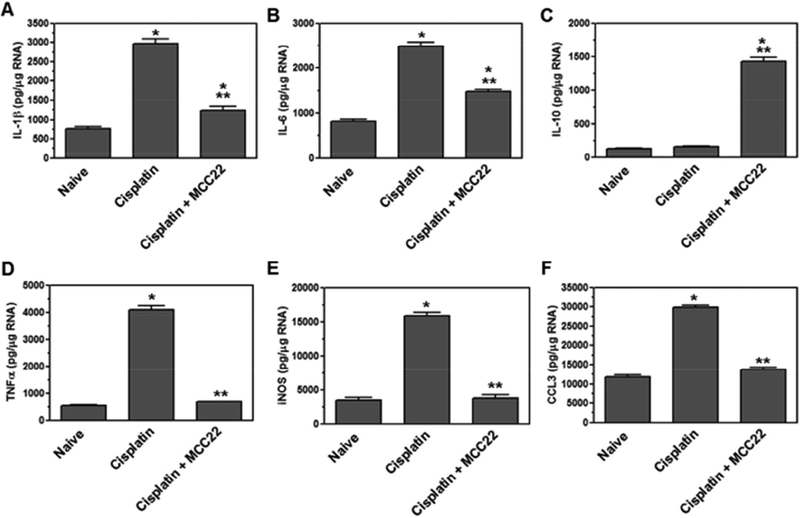
Microglia become activated in the spinal cord after cisplatin treatment induces the expression of pro-inflammatory cytokines IL-1β, IL-6, and TNFα, as well as the chemokine, CCL3, and effector molecule, iNOS. The expression of pro-inflammatory cytokines, chemokines, and iNOS in the spinal cord has been associated with inflammation and pain. Therefore, we wanted to determine whether MCC22 could alter the activation of microglia following cisplatin treatment. Naïve, cisplatin- treated, and cisplatin-treated mice given MCC22 mice were analyzed for the expression of IL-1β; F(2–6) = 409.3, p<0.0001; IL-6; F(2–6) = 526.3, p<0.0001; IL-10; F(2–6) = 1012, p<0.0001; TNFα; F(2–6) =1471, p<0.0001; iNOS; F(2–6) = 921.3, p<0.0001; and CCL3; F(2–6) = 903.9, P<0.0001. Cisplatin-treated mice expressed increased levels of pro-inflammatory cytokines, chemokines, and iNOS, which were decreased when the mice were administered MCC22 (Figure 8). Significantly, MCC22 treatment increased the expression of anti-inflammatory cytokine IL-10 in the spinal cord. The combined reduced levels of pro-inflammatory mediators and enhanced production of IL-10 provide compelling evidence that MCC22 inhibits activated microglia in hyperalgesic cisplatin-treated mice.
Figure 8. MCC22 reduced inflammation in the spinal cord.
Mice were treated with cisplatin for 7 days or untreated. Cisplatin-treated mice were administered MCC22 (10mg/kg) or control for 7 days. The spinal cords were removed from the mice (4 mice per group), lysed, and RNA was isolated. The RNA was DNAse treated, converted to cDNA, and used in real time PCR with primers for IL-1β (A), IL-6 (B), IL-10 (C), TNFα (D), iNOS (E), and CCL3 (F). The samples were normalized to β-actin expression, and concentration was based on a standard curve for each primer pair. The significant difference in expression from naïve animals (*) from cisplatin-treated animals (**) was determined using Bonferroni t-tests (p<0.5).
3.6. The role of MOR in the antinociception produced by MCC22
We evaluated the contribution of MOR in the actions of MCC22 by administering beta-FNA, which covalently binds to MOR producing an irreversible antagonism. One group of mice was given beta-FNA (1.0 nmol, i.t.) only 1x on day 14 after cisplatin, another was given beta-FNA 5 times, one day before cisplatin treatment and every 3 days thereafter. A separate group was given vehicle on day 14. All groups were administered MCC22 on day 15 post-cisplatin which produced significant reductions in hyperalgesia at 30 minutes (Figure 9). The anti-hyperalgesia produced by MCC22 was significantly attenuated following a single administration of beta-FNA and was further reduced following multiple injections of beta-FNA as compared to vehicle. Similar reductions were also seen at 60, 120, and 240 minutes after injection. Importantly, beta-FNA alone did not alter mechanical hypersensitivity produced by cisplatin. Separate groups of mice received either a single injection of beta-FNA or five injections as described above. For both groups, paw withdrawal thresholds after cisplatin did not differ at any time point, and mean thresholds ranged from 2.1 ± 0.4 g to 2.4 ± 0.4 g. These data are consistent with earlier studies showing that intrathecal administration of beta-FNA did not alter mechanical hypersensitivity (Rosen et al., 2017; Zhang et al., 2018). These data show the importance of MOR activation to the antinociception produced by MCC22.
Figure 9. Anti-hyperalgesia produced by MCC22 was decreased by beta-FNA.
Paw withdrawal frequencies were determined before (BL) and at 30, 60, 120, and 240 min after injection of MCC22 in cisplatin-evoked hyperalgesic mice pretreated with beta- FNA (1.0 nmol, i.t.) or vehicle. * indicates a significant increase from pre-cisplatin; # indicates a significant decrease compared to BL. Data are presented as mean (±SEM); n = 8 mice per group; (Bonferroni t-tests, ***p<0.001; ###p<0.001).
4. Discussion
The detailed mechanism by which MCC22 produces potent anti-hyperalgesia have not been established, but one possibility is that MCC22 induces the formation of a MOR-CCR5 heteromer (Portoghese et al., 2017). It is known that MOR interacts with other G protein-coupled receptors (GPCR) through the formation of heteromers (Gomes et al., 2013). For example, MOR-delta opioid receptor heteromers have been identified (George et al., 2000; Gomes et al., 2000) and a selective ligand (MDAN-21) targeting this heteromer affords potent antinociception (Aceto et al., 2012; Daniels et al., 2005; Yekkirala et al., 2012). MMG22, another bivalent ligand that contains mu opioid agonist and metabotropic glutamate receptor 5 antagonist pharmacophores was developed (Akgün et al., 2013; Smeester et al., 2014) which displayed exceptionally potent antinociception following i.t. administration in an acute inflammatory pain model in mice without the side effects associated with clinically employed opioids.
It is widely accepted that interactions between microglia and neurons in the spinal cord play a prominent role in the development of hyperalgesia associated with neuropathic pain (Abbadie, 2005; Milligan and Watkins, 2009; Tsuda et al., 2005; Watkins and Maier, 2003). Recent studies have shown that the CC chemokine ligand 3 (CCL3) and its receptor CCR5 were upregulated in the spinal cord following nerve injury (Matsushita et al., 2014). Furthermore, i.t. administration of CCL3 produced hyperalgesia and i.t. administration of a CCR5 antagonist (Maraviroc) decreased tactile allodynia following nerve injury (Matsushita et al., 2014). The presence of MOR-CCR5 heteromers on Chinese hamster ovary cells (Chen et al., 2004) and on membranes of monkey and human lymphocytes co-expressing MOR and CCR5 receptors (Suzuki et al., 2002) suggests that such a heteromer may exist in vivo. Interaction between MOR and CCR5 receptors on microglia and/or neurons may contribute to the development of hyperalgesia and chronic pain due to desensitization of MOR upon release of chemokines. Since activation of spinal microglia is a key step leading to hyperalgesia via sensitization of neuronal circuitry, targeting a MOR-CCR5 heteromer with concomitant activation of MOR and blockade of CCR5 was considered as an approach to inhibiting activated microglia and activating neuronal MOR as an approach to developing effective analgesics for chronic pain (Akgün et al., 2015). We have conducted in vitro studies using microglia activated with lipopolysaccharide (LPS) to demonstrate that MCC22 is more effective in reducing the expression of pro- inflammatory cytokines and chemokines than MA19 or Tak220 alone or MA19 and Tak220 combined (Olson et al., manuscript in preparation).
The homologous MCC series of bivalent ligands contain mu opioid agonist and CCR5 antagonist pharmacophores that are linked by homologous spacers of different lengths in an effort to optimize activity (Akgün et al., 2015). The selection of spacer length was based on prior studies that suggested effective bridging of heteromeric GPCR protomers in the range of 18–22 atoms (Sham YY; Zheng Y, 2009). The original study of MCC22 in LPS-inflamed mice revealed that a 22-atom spacer length afforded orders of magnitude greater potency relative to homologs with shorter or longer spacers, and molecular simulation studies of the interaction of MCC22 with MOR-CCR5 heteromer are consistent with bridging of the MOR and CCR5 protomers in a MOR-CCR5 heteromer (Akgün et al., 2015).
Remarkably, the magnitude and duration of anti-hyperalgesia produced by MCC22 given by i.t. or i.p. administration in the present CIPN study was substantially greater than that of morphine. Importantly, this was not attributed to motor impairment since MCC22 did not alter performance on the rotarod test. The three orders of magnitude greater potency between MCC22 and its shorter (MCC14) or longer (MCC24) homologs strongly suggest that the anti-hyperalgesia produced by MCC22 occurs through interactions with protomers of a MOR-CCR5 heteromer.
As suggested from earlier studies using LPS-inflamed mice, the primary site of action in the central nervous system for MCC22 appears to be the spinal cord given the ~7500-fold greater potency by the i.t. versus intracerebroventricular (i.c.v.) route of administration (Akgün et al., 2015). This is consistent with the targeting of activated microglia in the dorsal horn that leads to central sensitization and persistent pain (Watkins et al., 2005). Our studies show that CCR5 and MOR co-localize on microglia in the spinal cord. Most interestingly, after MCC22 administration, there were more intense areas of staining for MOR and CCR5, suggesting formation of heteromers on the surface of microglia.
Our results further show that microglia produce pro-inflammatory cytokines, chemokines, and effector molecules after cisplatin treatment that were markedly reduced by MCC22 treatment. The concomitant elevation of anti-inflammatory and CIPN resolving (Krukowski et al., 2016) cytokine, IL-10, would be expected to also contribute to the efficacy of MCC22. Further, our minocycline findings in the cisplatin treated mice show that anti-hyperalgesia produced by MCC22 was prevented by blocking activation of microglia with minocycline, suggesting that MOR-CCR5 heteromers are located on microglia. Thus, the efficacy and duration of anti-hyperalgesia produced by MCC22 are likely due to targeting of the MOR-CCR5 heteromer on activated microglia following injury (Akgün et al., 2018). The increased expression of CCR5 (Gamo et al., 2008) and MOR on activated microglia provide the opportunity to induce formation of MCC22-bound heteromer that leads to reduced microglia activation.
A common side effect associated with clinically employed opioids is tolerance that leads to elevated opioid dosing to achieve the desired effect (Dumas and Pollack, 2008). Significantly, tolerance was not observed following repeated daily administration of MCC22, and baseline paw withdrawal frequency unexpectedly decreased over time. It is unclear why the baseline withdrawal responses decreased over time following repeated administration of MCC22. Importantly, the antihyperalgesic effect of MCC22 was not reduced in mice that were tolerant to morphine, indicating lack of cross- tolerance. This could be related to the presence of different pools of MOR: 1) those that are activated by morphine and 2) a pool of MOR-CCR5 heteromers that interact selectively with MCC22. Also, Intrinsic receptor mechanisms such as receptor internalization and desensitization contribute to opioid tolerance (Williams et al., 2013) and it is unknown whether MCC22 alters these kinetics leading to down-regulation or desensitization of the MOR. As it has been reported that inhibition of microglia leads to reduced opioid tolerance and increased analgesic efficacy (Watkins et al., 2009), the observed lack of cross-tolerance may also be due to the lower effective dose of opioid that is required for analgesia due to blockage of neuronal sensitization.
Another serious side effect of opioids is abuse and addiction. Remarkably, results from our studies using CPP suggest that unlike morphine, MCC22 did not display properties of reward. It has previously been reported that i.c.v. administration of MCC22 does not produce anti-hyperalgesia, suggesting that a supraspinal MOR-CCR5 heteromer may not be involved due to the presence of different microglia subtypes (Akgün et al., 2015).
In conclusion, while morphine and related opioids continue to be the mainstay for treating many chronic pain conditions, side effects such as tolerance and dependence limit their use. Novel approaches to treat chronic pain are needed, and MCC22 exemplifies such an advance, in that its efficacy is related to the inhibition of microglia with concomitant activation of neuronal MOR. Our studies in mice indicate that these compounds produce potent antinociception and are devoid of tolerance and reward. Although preliminary studies suggest that MCC22 inhibits activity of nociceptive dorsal horn neurons (unpublished observations), much more research is needed to further determine mechanism and sites of action. The finding that MCC22 is highly effective in inhibiting hyperalgesia without adverse effects in mice with cisplatin-evoked neuropathy, suggests potential applications for treatment of neuropathic pain arising from a variety of chemotherapeutic agents as well as other types of neuropathies.
Highlights.
Chemotherapy often causes peripheral neuropathy that can be chronic and painful.
The bivalent ligand, MCC22, potently reduced chemotherapy-induced neuropathic pain produced by cisplatin without tolerance or reward.
Acknowledgements
We thank Dr. Glenn Giesler for reading an earlier version of the manuscript and Dr. Richard Bodnar for discussions related to experimental designs for the tolerance studies.
Grant Support
This work was supported by NIH grants HL135895 (DAS) and DA030316 (PSP). Dr. G. Cataldo received support from the National Institute of Dental and Craniofacial Research Grant [T90 DE0227232]. Dr. S. Erb received support from the National Institute of Drug Abuse Grant [2T32 DA007234–31]. N. Luong received support from the National Institute of Drug Abuse Grant [T32 DA007097].
Financial support
Supported by NIH grants HL135895 (DAS) and DA030316 (PSP). Dr. G. Cataldo was supported by the National Institute of Dental and Craniofacial Research (Grant T90 DE0227232). N. Luong received support from the National Institute of Drug Abuse Grant T32 DA007097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest.
References
- Abbadie C, 2005. Chemokines, chemokine receptors and pain. Trends Immunol 26, 529–534. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Harris LS, Negus SS, Banks ML, Hughes LD, Akgün E, Portoghese PS, 2012. MDAN-21: A bivalent opioid ligand containing mu-agonist and delta-antagonist pharmacophores and its effects in Rhesus Monkeys. Int J Med Chem 2012, 327257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgün E, Javed MI, Lunzer MM, Powers MD, Sham YY, Watanabe Y, Portoghese PS, 2015. Inhibition of Inflammatory and Neuropathic Pain by Targeting a Mu Opioid Receptor/Chemokine Receptor5 Heteromer (MOR-CCR5). J Med Chem 58, 8647–8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgün E, Javed MI, Lunzer MM, Smeester BA, Beitz AJ, Portoghese PS, 2013. Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. PNAS 110, 11595–11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgün E, Lunzer MM, Portoghese PS, 2018. Combined glia inhibition and opioid receptor agonism afford highly potent analgesics without tolerance. ACS Chem Neurosci. Aug 24. doi: 10.1021/acschemneuro.8b00323. [DOI] [PubMed] [Google Scholar]
- Bowen JL, Olson JK, 2009. Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler’s virus-induced demyelinating disease. J Immunol 183, 6971–6980. [DOI] [PubMed] [Google Scholar]
- Cataldo G, Lunzer MM, Olson JK, Akgün E, Belcher JD, Vercellotti GM, Portoghese PS and Simone DA, 2018. The bivalent ligand MCC22 potently attenuates nociception in a murine model of sickle cell disease. Pain, 159,1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen L-Y, 2004. Heterodimerization and cross-desensitization between the μ-opioid receptor and the chemokine CCR5 receptor. E J Pharm 483, 175–186. [DOI] [PubMed] [Google Scholar]
- Cherny NI, Thaler HT, Friedlander-Klar H, Lapin J, Foley KM, Houde R, Portenoy RK, 1994. Opioid responsiveness of cancer pain syndromes caused by neuropathic or nociceptive mechanisms: a combined analysis of controlled, single-dose studies. Neurology 44, 857–861. [DOI] [PubMed] [Google Scholar]
- Clark AK, Gruber-Schoffnegger D, Drdla-Schutting R, Gerhold KJ, Malcangio M, Sandkuehler J, 2015. Selective activation of microglia facilitates synaptic strength. J Neurosci 35, 4552–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS, 2005. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. PNAS 102, 19208–19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas EO, Pollack GM, 2008. Opioid tolerance development: a pharmacokinetic/pharmacodynamic perspective. AAPS J. 10, 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Lunzer MM, Auger JL, Akgün E, Portoghese PS, Binstadt BA, 2018. A bivalent compound targeting CCR5 and the mu opioid receptor treats inflammatory arthritis pain in mice without inducing pharmacologic tolerance. Arthritis Res Ther 20, 154. doi: 10.1186/s13075-018-1661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF, 2006. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia 53, 132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo K, Kiryu-Seo S, Konishi H, Aoki S, Matsushima K, Wada K, Kiyama H, 2008. G-protein coupled receptor screen reveals a role for chemokine CCR5 in supressing microglial neurotoxicity. J Neurosci 28, 11980–11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O’Dowd BF, 2000. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem 275, 26128–26135. [DOI] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Chandrakala MV, Devi LA, 2013. Disease-specific heteromerization of G-protein-coupled receptors that target drugs of abuse. Prog. Mol. Biol Transl Sci 117, 207–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA, 2000. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci 20, RC110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ, 2008. DAMGO- induced expression of chemokines and chemokine receptors: the role of TGF-β1. J Leukocyte Biol 83, 956–963. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL, 1980. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67, 313–316. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD, 2009. Comparison of Oxaliplatin-and Cisplatin-Induced Painful Peripheral Neuropathy in the Rat. J Pain 10, 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautio A-L, Haanpaa M, Saarto T, Kalso E, 2008. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J. Pain Symptom Management 35, 31–39. [DOI] [PubMed] [Google Scholar]
- Kelland L, 2007. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA, Seybold VS, 2012. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J Neurosci 16, 7091–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A, 2016. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 36, 11074–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ, 2009. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10, 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Choi D-Y, Jung Y-Y, Yun YW, Lee BJ, Han SB, Hong JT, 2013. Decreased pain responses of C-C chemokine receptor 5 knockout mice to chemical or inflammatory stimuli. Neuropharmacology 67, 57–65. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MPN, 2002. Morphine regulates gene expression of α- and β-chemokines and their receptors on astroglial cells via the opioid μ receptor. J. Immunology 169, 3589–3599. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Tozaki-Saitoh H, Kojima C, Masuda T, Tsuda M, Inoue K, Hoka S, 2014. Chemokine (C-C motif) Receptor 5 Is an Important Pathological Regulator in the Development and Maintenance of Neuropathic Pain. Anesthesiology 120, 1491–1503. [DOI] [PubMed] [Google Scholar]
- McWhinney SR, Goldberg RM, McLeod HL, 2009. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 8, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR, 2009. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J, Miller S, 2004. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173, 3916–3924. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Akgun E, Lunzer MM, 2017. Heteromer Induction: An Approach to Unique Pharmacology? ACS Chem Neurosci 8, 426–428. [DOI] [PubMed] [Google Scholar]
- Ramesh G, MacLean AG, Philipp MT, 2013. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013, 480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY, 2007. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110, 2110–2118. [DOI] [PubMed] [Google Scholar]
- Rosen SF, Ham B, Drouin S, Boachie N, Chabot-Dore AJ, Austin JS, Diatchenko L, Mogil JS, 2017. T-Cell Mediation of Pregnancy Analgesia Affecting Chronic Pain in Mice. J Neurosci 37, 9819–9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham YY, L. M, Powers MD, Javed MI, Cataldo G, Simone DA, Akgun E, Portoghese PS., Modeling and simulation of MCC22, a bivalent ligand that potently inhibits inflammatory and neuropathic pain in mice. New Frontiers in Computer-Aided Drug Design, Computer Aided Drug Design, Gorden Research Conference, 2015 July 19–24, Mount Snow, VT. [Google Scholar]
- Smeester BA, Lunzer MM, Akgun E, Beitz AJ, Portoghese PS, 2014. Targeting putative mu opioid/metabotropic glutamate receptor-5 heteromers produces potent antinociception in a chronic murine bone cancer model. Eur J Pharmacol 743, 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Rahim RT, Davey PC, Bednar F, Bardi G, Zhang L, Zhang N, Oppenheim JJ, Rogers TJ, 2011. Protein Kinase Cζ Mediates μ-Opioid Receptor-induced Cross-desensitization of Chemokine Receptor CCR5. J Biol Chem 286, 20354–20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY, 2002. Interactions of opioid and chemokine receptors: Oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res 280, 192–200. [DOI] [PubMed] [Google Scholar]
- Ta LE, Low PA, Windebank AJ, 2009. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima K, Miyake H, Kanzaki N, Tagawa Y, Wang X, Sugihara Y, Iizawa Y, Baba M, 2005. Highly potent inhibition of human immunodeficiency virus type 1 replication by TAK-220, an orally bioavailable small-molecule CCR5 antagonist. Antimicrob Agents and Chemother 49, 3474–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter M, 2005. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 28, 101–107. [DOI] [PubMed] [Google Scholar]
- Vallejo R, Tilley DM, Vogel L, Benyamin R, 2010. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 10, 167–184. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF, 2005. Glia: novel counter-regulators of opioid analgesia. Trends in Neurosci 28, 661–669. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF, 2009. The “Toll” of Opioid- Induced Glial Activation: Improving the Clinical Efficacy of Opioids by Targeting Glia. Trends Pharmacol Sci 30, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, 2003. GLIA: A novel drug discovery target for clinical pain. Nat. Rev. Drug Discovery 2, 973–985. [DOI] [PubMed] [Google Scholar]
- Weiss U, 1955. Derivatives of morphine. I. 14-Hydroxydihydromorphinone. J Am Chem Soc 77, 5891–5892. [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ, 2013. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization and tolerance. Pharmacol Rev 65, 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank AJ, Grisold W, 2008. Chemotherapy-induced neuropathy. J Peripher Nerv Syst 13, 27–46. [DOI] [PubMed] [Google Scholar]
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C, 2008. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer 44, 1507–1515. [DOI] [PubMed] [Google Scholar]
- Yekkirala AS, Banks ML, Lunzer MM, Negus SS, Rice KC, Portoghese PS, 2012. Clinically Employed Opioid Analgesics Produce Antinociception via μ-δ Opioid Receptor Heteromers in Rhesus Monkeys. ACS Chem Neurosci 3, 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang N, Zhang R, Zhao W, Chen Y, Wang Z, Xu B, Zhang M, Shi X, Zhang Q, Guo Y, Xiao J, Chen D, Fang Q, 2018. Preemptive intrathecal administration of endomorphins relieves inflammatory pain in male mice via inhibition of p38 MAPK signaling and regulation of inflammatory cytokines. J Neuroinflammation 15, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, L. E, Akgun E, Harikumar KG, Hopson J, Powers MD, Lunzer MM, Miller LJ, Portoghese PS., 2009. Induced association of μ opioid (MOP) and type 2 cholecystokinin (CCK2) receptors by novel bivalent ligands. J Med Chem 52, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]