Abstract
Substance use disorders (SUD) are serious public health problems worldwide. Although significant progress has been made in understanding the neurobiology of drug reward and the transition to addiction, effective pharmacotherapies for SUD remain limited and a majority of drug users relapse even after a period of treatment. The United States Food and Drug Administration (FDA) has approved several medications for opioid, nicotine, and alcohol use disorders, whereas none are approved for the treatment of cocaine or other psychostimulant use disorders. The medications approved by the FDA for the treatment of SUD can be divided into two major classes – agonist replacement therapies, such as methadone and buprenorphine for opioid use disorders (OUD), nicotine replacement therapy (NRT) and varenicline for nicotine use disorders (NUD), and antagonist therapies, such as naloxone for opioid overdose and naltrexone for promoting abstinence. In the present review, we primarily focus on the pharmacological rationale of agonist replacement strategies in treatment of opioid dependence, and the potential translation of this rationale to new therapies for cocaine use disorders. We begin by describing the neural mechanisms underlying opioid reward, followed by preclinical and clinical findings supporting the utility of agonist therapies in the treatment of OUD. We then discuss recent progress of agonist therapies for cocaine use disorders based on lessons learned from methadone and buprenorphine. We contend that future studies should identify agonist pharmacotherapies that can facilitate abstinence in patients who are motivated to quit their illicit drug use. Focusing on those that are able to achieve abstinence from cocaine will provide a platform to broaden the effectiveness of medication and psychosocial treatment strategies for this underserved population.
Keywords: Substance Use Disorders, Addiction, Agonist replacement therapy, Opioids, Methadone, Cocaine, Dopamine transporter, Atypical dopamine uptake inhibitor
1. Introduction
Substance use disorder (SUD) is a serious public health problem that affects millions of people worldwide. Substances having the highest addiction liability include opioids (most recently prescription and other new synthetic opioids, such as fentanyl), psychostimulants (such as cocaine and methamphetamine), and nicotine. Nearly 13.5 million people use opioids worldwide (WHO, 2018). In the United States, over two million people are diagnosed with opioid use disorder (OUD), resulting in economic costs that exceed 500 billion dollars each year (HHS, 2018). Notably, synthetic opioids have been driving the recent increase in cocaine overdose-induced deaths (Khatri et al., 2018). Cocaine-related overdose deaths increased nearly 60% from 2010 to 2015 (1.35 to 2.13 per 100,000 individuals; McCall Jones et al., 2017). Today, cocaine remains the leading cause of overdose deaths among African Americans (CDC, 2018). Although many patients with SUD manage to remit without pharmacotherapy, many still require or benefit from medication assistance. Despite extensive research, pharmacotherapies for SUD have advanced slowly and only a handful of drugs have been approved by the U. S. Food and Drug Administration (FDA) for the treatment of opioid, nicotine, and alcohol use disorders (FDA, 2018). Currently, there are no approved medications for the treatment of cannabis, cocaine or other psychostimulant use disorders.
Medications currently approved by the U.S. FDA for SUD can be classified into two major categories – agonist replacement therapies (such as methadone and buprenorphine for OUD, and nicotine replacement therapy [NRT] and varenicline for smoking cessation) and antagonist therapies (such as naloxone and naltrexone; FDA, 2018). Of these, methadone is a mu opioid receptor full agonist, while buprenorphine and varenicline are partial agonists that bind at mu opioid and α4β2 nicotinic acetylcholine receptors, respectively. Full agonist therapies for tobacco use disorder, in the form of nicotine replacement (e.g., nasal sprays, patches, chewing gum, and inhalers that deliver nicotine) have also shown efficacy in promoting smoking cessation (Farsalinos and Niaura, 2019; Hajek et al., 2019; Stead et al., 2012). Although agonists themselves may have inherent abuse potential, they are highly effective in the prevention of withdrawal and the reduction of drug craving and relapse. Abuse potential can be further minimized by extended release and depot medication formulations (Blanco-Gandia and Rodriguez-Arias, 2018). As such, methadone and buprenorphine have become first-line pharmacological maintenance treatments for OUD (Stotts et al., 2009).
In contrast to agonist therapies, the opioid receptor antagonists naloxone and naltrexone lack abuse potential. Naloxone in particular is a first-line treatment for opioid overdose due to its efficacy in reversing opioid-induced respiratory depression. However, naloxone and naltrexone precipitate acute opioid withdrawal (Buajordet et al., 2004; Kim and Nelson, 2015) and thereby increase the risk of relapse. Clinical observations indicate low clinical success rates and poor compliance to naltrexone as a treatment for opioid abuse, as well as mecamylamine and DHβE (dihydro-β-erythroidine hydrobromide) nicotinic receptor antagonist treatments for nicotine use disorder (Blanco-Gandia and Rodriguez-Arias, 2018; Jordan and Xi, 2018).
In this review, we focus primarily on agonist therapeutic strategies in the treatment of OUD with the assertion that this strategy may be particularly cogent for developing medications to treat cocaine use disorders. We first describe the neural mechanisms underlying opioid reward and addiction and the rationale of agonist therapy in treatment of OUD, particularly agonist therapies that produce slow-onset, long-lasting effects that achieve functional outcomes such as limiting the rewarding efficacy and withdrawal effects of these drugs of abuse while producing minimally reinforcing effects by themselves. We then describe the preclinical and clinical evidence supporting the utility of agonist-based medications for OUD, and, briefly, the successful use of agonist replacement therapy with varenicline in the treatment of nicotine use disorder. Finally, we discuss recent progress in medication development of agonist-like therapies for cocaine use disorders based on lessons learned from methadone and buprenorphine. We contend that the successful agonist approach to treatments of OUD can be applied to the development of new treatment strategies for cocaine use disorders, for which no approved pharmacotherapeutics currently exist.
2. Neural Substrates of Opioid Reward
2.1. Opioid receptor mechanisms
The ideal strategy for treatment discovery in SUD is to understand the neural mechanisms underlying drug reward and addiction and subsequently develop mechanism-based pharmacotherapies that target those same neural substrates. Opioids act by binding to opioid receptors located in the peripheral and central nervous systems. There are four primary types of opioid receptors: mu (μ), kappa (κ), delta (δ), and opioid-receptor like-1 (ORL1) or the nociception (NOP) receptor. Each receptor has seven transmembrane domains and is coupled to inhibitory G-proteins (Gαi) which, when activated, inhibit neuronal activity (Al-Hasani and Bruchas, 2011). Recent insights into biased agonism or functional selectivity indicate that some opioid receptor ligands preferentially recruit intracellular G-protein vs. β-arrestin signaling pathways. With respect to the mu opioid receptor, the β-arrestin pathway is associated with adverse effects of exogenous opioids, including tolerance, reduced analgesia, enhanced respiratory suppression, and constipation (Bohn et al., 2000; Bohn et al., 1999; Madariaga-Mazon et al., 2017; Raehal et al., 2005). The delta and kappa opioid receptors also involve β-arrestin signaling that promotes tolerance and receptor internalization (Ho et al., 2018; Vicente-Sanchez et al., 2018). All three opioid receptors, as well as opioid receptor-like 1 (ORL1) may play a role in the induction and development of addiction by differentially mediating the rewarding and euphoric vs. aversive and withdrawal effects of opioids that drive the stages of compulsive drug use and relapse. For the purposes of this review we focus primarily on the mu opioid receptor, but refer readers to additional reviews of kappa and delta opioid receptors, as well as ORL1 involvement, in addiction elsewhere (Castro and Berridge, 2014; Helal et al., 2017; Karkhanis et al., 2017; Margolis et al., 2017; Poznanski et al., 2017; Schank et al., 2012).
2.2. Neural circuits underlying opioid reward
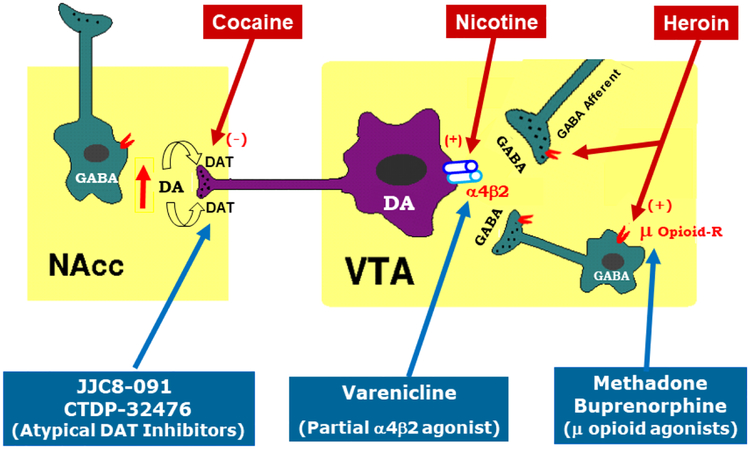
Mu opioid receptors are the primary mediators of the euphoric effects of opioids and are also involved in the rewarding efficacy of other drugs of abuse, including cannabinoids, alcohol and nicotine (Madariaga-Mazon et al., 2017). Mu opioid receptors are highly expressed on GABAergic neurons in regions of the brain implicated in reward and addiction, including the thalamus, amygdala, anterior cingulate cortex, striatum (including the nucleus accumbens or NAc) and midbrain (Jaferi et al., 2011; Svingos et al., 1997; Xi and Stein, 2000). In the ventral tegmental area (VTA) of the midbrain, GABAergic interneurons and afferents tonically modulate dopamine (DA) neuron firing (Figure 1; Li, 2016). By binding to mu opioid receptors expressed on GABAergic interneurons or afferents within the VTA, opioids are assumed to disinhibit VTA DA neurons and increase DA release to the NAc, an effect that underlies the subjective experience of reward and euphoria induced by all drugs of abuse (Chartoff and Connery, 2014; Fields and Margolis, 2015; Kosten and George, 2002; Wise, 2008). In addition, activation of mu opioid receptors on GABAergic medium spiny neurons in the NAc, which share reciprocal projections with the VTA, may further release GABAergic inhibition of VTA DA neurons and augment DA release (Li, 2016; Matsui et al., 2014; Ross and Peselow, 2009; Xi and Stein, 2000). Opioids also act on other areas of the brain involved in reward-related learning and memory, drug craving, tolerance and relapse that contribute to the cycle of compulsive drug abuse (Kosten and George, 2002).
Figure 1:
A summary diagram illustrating the mesolimbic DA reward system, and targets of heroin, nicotine, cocaine, and compounds used as agonist therapies for the treatment of substance use disorder.
Evidence for mu opioid receptor involvement in addiction is derived from both in vitro and in vivo studies. In vivo, opioid conditioned place preferences (CPP) and opioid self-administration are blocked by mu opioid receptor antagonists, administered either systemically or directly within the VTA (Britt and Wise, 1983; Olmstead and Franklin, 1997). Accordingly, selective genetic deletion of mu opioid receptors in the VTA abolishes opioid CPP (Zhang et al., 2009). Further, mu opioid receptor agonists sustain CPP and are voluntarily self-administered into the VTA, NAc shell, hypothalamus, amygdala, and periaqueductal grey, but not the NAc core or dorsal striatum (Bals-Kubik et al., 1993; Bozarth and Wise, 1981; David and Cazala, 1994; Olmstead and Franklin, 1997; Phillips and LePiane, 1980; Steidl et al., 2015; Zangen et al., 2002), suggesting mu expression in regions beyond the VTA also participate in opioid reward. In sum, these results strongly implicate mu opioid receptors as attractive targets for the treatment of OUD.
3. Agonist Therapies for Opioid Use Disorder
3.1. Rationale of agonist therapy
Pharmacological manipulations of mu opioid receptors can be accomplished using full agonists, partial agonists, or antagonists. While antagonists can block the effects of opioids and are effective overdose reversal agents, they also precipitate withdrawal symptoms and are used less frequently for long term abstinence, due to lack of adherence (Ndegwa et al., 2016). Nonetheless, there remain strong advocates for the use of extended-release naltrexone (XR-NTX) for the treatment of OUD, a formulation which has improved treatment retention (Sullivan et al., 2019). In contrast, the use of agonists and partial agonists (known as maintenance or substitution therapy) for OUD is based on the rationale that replacement of abused opioids with pharmacotherapeutics that exert a similar mechanism of action in the brain (Figure 1) will facilitate abstinence and relapse prevention efforts by mitigating withdrawal symptoms and craving. Notably, pharmacotherapeutics that are mildly-to-moderately reinforcing mediate increased attendance/adherence with treatment. Because full agonists effectively mimic the effects of abused opioids, they may carry significant abuse liability. Partial agonists bind to their receptor targets, but do not elicit the maximum receptor response of a full agonist. Therefore, in the presence of a full agonist, such as heroin, a partial mu agonist will behave as an antagonist and attenuate heroin’s effects. However, in the absence of other opioids, a partial agonist will function as an agonist and mitigate withdrawal symptoms. In addition, cross-tolerance may represent an additional mechanism by which agonist therapies reduce illicit opioid abuse. For example, full mu opioid receptor agonists such as methadone tend to induce rightward shifts in heroin dose-response curves, suggesting cross-tolerance (Volavka et al., 1978; Zaks et al., 1971), whereas partial mu opioid receptor agonists, such as buprenorphine, can produce both rightward and downward shifts in heroin dose-response functions, suggesting antagonist effects (Greenwald et al., 2003). Partial agonists can therefore block opioid reward, minimize craving and reduce withdrawal, thereby promoting abstinence and relapse prevention for OUD.
Converging preclinical and clinical evidence point to the utility of three opioids as efficacious medication-assisted therapies for OUD: 1) methadone, a full opioid agonist, 2) buprenorphine, a partial opioid agonist, and 3) levo-alpha-acetylmethadol (LAAM). LAAM is also a mu opioid receptor full agonist approved by the U.S. FDA in 1993 for the treatment of OUD. However, LAAM’s indication has since been revised due to hERG (human Ether-à-go-go-Related Gene) potassium channel activity and adverse side effects, such as prolonged QTc-intervals and potentially fatal cardiac arrhythmia (Clark et al., 2002; Kang et al., 2003; Wieneke et al., 2009). As such, marketing in the U.S. ceased in 2003 and LAAM is currently not available in Canada or the European Union. Therefore, in the present review we focus on evidence from animal and human studies supporting the utility of methadone and buprenorphine medications in the treatment of OUD.
3.2. Methadone for OUDs
3.2.1. Historical discovery of methadone agonist therapy
For centuries, OUD was considered a disease of the mind, due to criminal or deviant behavior and a weak personality. As a result, a major approach to managing OUD was incarceration and punishment. The 1960’s heralded a revolution in addiction management as a “metabolic disease” of the brain with resultant behaviors of “drug hunger” and drug self-administration, which required pharmacological intervention rather than punishment (Dole and Nyswander, 1966a; Dole and Nyswander, 1966b). Beginning in 1959, the Canadian Department of Health approved a series of experiments by Vancouver specialist Dr. Robert Halliday, to utilize methadone, an orally effective synthetic opioid, in the management of acute opioid withdrawal (Fischer, 2000). This short-term experiment quickly transitioned to a “prolonged withdrawal” program, which emphasized psychosocial support alongside methadone treatment with the goal of harm reduction rather than abstinence. Halliday likened his opioid maintenance program to insulin treatment for diabetes (Fischer, 2000). With a resurgence of heroin abuse in New York in the 1960s, Dole and Nyswander soon afterwards began prescribing methadone, which was initially assumed to be a short-acting opioid, for opioid detoxification by administering multiple doses per day, followed by rapid tapering without further treatment. Due to nursing limitations, a handful of patients were given only one or two doses of methadone in a day. Surprisingly, these patients showed significant reductions in opioid withdrawal severity, suggesting that methadone might have a longer-acting profile than previously assumed (Kreek, 2000; Kreek et al., 2004). Nonetheless, these anecdotal findings inspired researchers to study whether methadone would be effective in the treatment of heroin abuse.
3.2.2. Pharmacology and preclinical studies with methadone
Methadone is a mu opioid receptor full agonist:
In vitro receptor binding and functional assays indicate that methadone itself is a mu opioid receptor full agonist with Ki values of 1.7, 435, and 405 nM for mu, delta, and kappa opioid receptors, respectively, similar to morphine (Ki values of 1.4, 145, 23.4 nM for mu, delta, and kappa opioid receptors, respectively (Codd et al., 1995). Of note, methadone also acts as a glutamate NMDA receptor antagonist (Ebert et al., 1998; Oxenham and Farrer, 1998), although it is unclear whether or not this off target action is relevant to its therapeutic utility for treating OUD. Heroin itself exhibits relatively low affinity for the mu opioid receptor (Ki = 483 nM) (Inturrisi et al., 1983), but when administered systemically heroin works as a prodrug, rapidly entering the brain and metabolizing from 6-acetyl-morphine to morphine itself, thereby producing euphoric, analgesic, and anxiolytic effects (Sawynok, 1986). In vivo, both morphine and methadone increase expression of c-Fos, an immediate early gene indicating neuronal activation, in the somatosensory and insular cortices (Taracha et al., 2008). Systemic administration of heroin or methadone produces similar increases in NAc DA and locomotor hyperactivity in a dose-dependent manner (Fig. 2 A–D). Chronic administration of methadone also produces physical dependence and withdrawal symptoms that mirror those associated with morphine, including wet dog shakes, weight loss, diarrhea, ptosis and teeth chattering (Ling et al., 1984). However, methadone withdrawal has more profound effects than morphine on gene expression in the pineal gland, melatonin synthesis, and regulation of circadian rhythms (Pacesova et al., 2016). Methadone overdose also causes respiratory depression similar to morphine and heroin (Lewanowitsch et al., 2006). Together, these findings support comparable mu opioid receptor agonist profiles of heroin, morphine and methadone, both in vitro and in vivo.
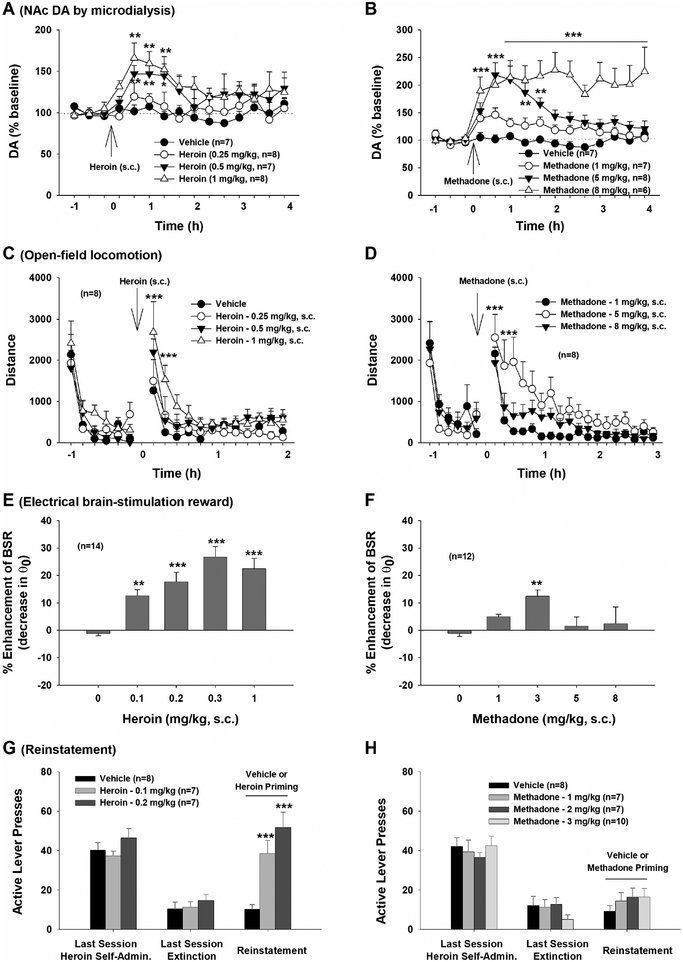
Figure 2.
Characterization of the neurochemical and behavioral effects of heroin and methadone in vivo in rats. A, B: Systemic administration of heroin or methadone produced significant and dose-dependent increases in extracellular NAc DA, with methadone displaying a longer-duration of action than heroin. C, D: Systemic administration of heroin or methadone dose-dependently increased open-field locomotor activity. Again, methadone displays a long-acting profile. E, F: Systemic administration of heroin produced a dose-dependent increase in intracranial brain-stimulation reward (BSR) maintained by electrical stimulation of the medial forebrain bundle of the hypothalamus, while methadone produced a modest increase in BSR only at 3 mg/kg. G, H: Heroin priming induced robust reinstatement of heroin-seeking behavior in rats extinguished from previous heroin self-administration, while methadone did not induce reinstatement at either dose tested. *p<0.05, **p<0.01, ***p<0.001, compared to baseline before heroin or methadone injection or compared to vehicle control group. (Some data are replotted from Peng et al., 2010).
Methadone displays less rewarding and addictive potential than heroin:
Preclinical studies in experimental animals indicate that methadone is less rewarding and has lower addictive potential than heroin. As shown in Figure 2 (A, B), systemic administration of heroin produces a rapid increase in extracellular NAc DA, while methadone produces a slow-onset, long-lasting increase in extracellular NAc DA (Peng et al., 2010; Preshaw et al., 1982). Behaviorally, methadone induces dose-dependent increases in open-field locomotor activity with a longer duration of action than heroin (Fig. 2 C, D), possibly due to methadone’s relatively long half-life (24–36 h), slow metabolism and high fat solubility (Eap et al., 2002).
In intracranial self-stimulation (ICSS) maintained by electrical stimulation of the medial forebrain bundle of the hypothalamus, heroin produces a robust, dose-dependent increase in brain-stimulation reward, while methadone does not, except at a moderate 3 mg/kg dose (Fig. 2 E, F). Following extinction from heroin self-administration, methadone priming fails to reinstate drug-seeking behavior in rats, in contrast to morphine, heroin and oxycodone (Fig. 2 G, H; Leri et al., 2004; Stewart et al., 1996; Werner et al., 1976; You, 2018; You et al., 2017). During substitution testing following heroin self-administration, methadone sustains a higher rate of self-administration but elicits progressive decreases in drug intake over time, suggesting that methadone may have lower reinforcing value than heroin and that the higher rate of methadone self-administration could be a compensatory response to reduced reward (Peng et al., 2010). Methadone also appears to have lower transgenerational abuse liability than morphine. Offspring of dams exposed to chronic morphine voluntarily drink more morphine solution than controls and show greater reinstatement to morphine consumption after an abstinence period. In contrast, offspring of methadone-exposed dams show no differences in methadone consumption (Hovious and Peters, 1985). Together, these findings suggest that methadone not only prevents opioid withdrawal but is less rewarding and addictive than other opioids such as heroin (Peng et al., 2010), supporting its utility in the treatment of OUD.
The mechanisms underlying the differential addictive liability of heroin and morphine vs. methadone are unclear. One reason may be that chronic morphine induces minimal, while methadone induces robust mu opioid receptor internalization in a dose-dependent manner (Liao et al., 2007). A second reason may be related to the dynamic changes in extracellular DA after heroin versus methadone administration, since a drug’s rewarding efficacy is positively correlated with the dynamic change induced by the drug in extracellular DA. The faster the rise and subsequent fall in extracellular DA, the higher the presumed drug-induced reward and locomotor activation (Busto and Sellers, 1986; Kimmel et al., 2008; Kimmel et al., 2007; Volkow et al., 1995; but see Li et al., 2011; Peng et al., 2010). As shown in Figure 2, systemic administration of methadone leads to a slow-onset, long-lasting increase in extracellular NAc DA compare to heroin (Peng et al., 2010), which may be related to its unique pharmacokinetic profiles such as high lipophilicity with rapid GI absorption, large initial volume of distribution and slow tissue release, and long half-life (Ayonrinde et al., 2000; Eap et al., 2002). The unique pharmacokinetic profile of methadone may not only explain in part why oral administration of methadone has reduced addictive liability in humans compared to heroin, but also why systemic (i.p.) administration of methadone is less rewarding than heroin in rats.
Methadone treatment attenuates illicit opioid action:
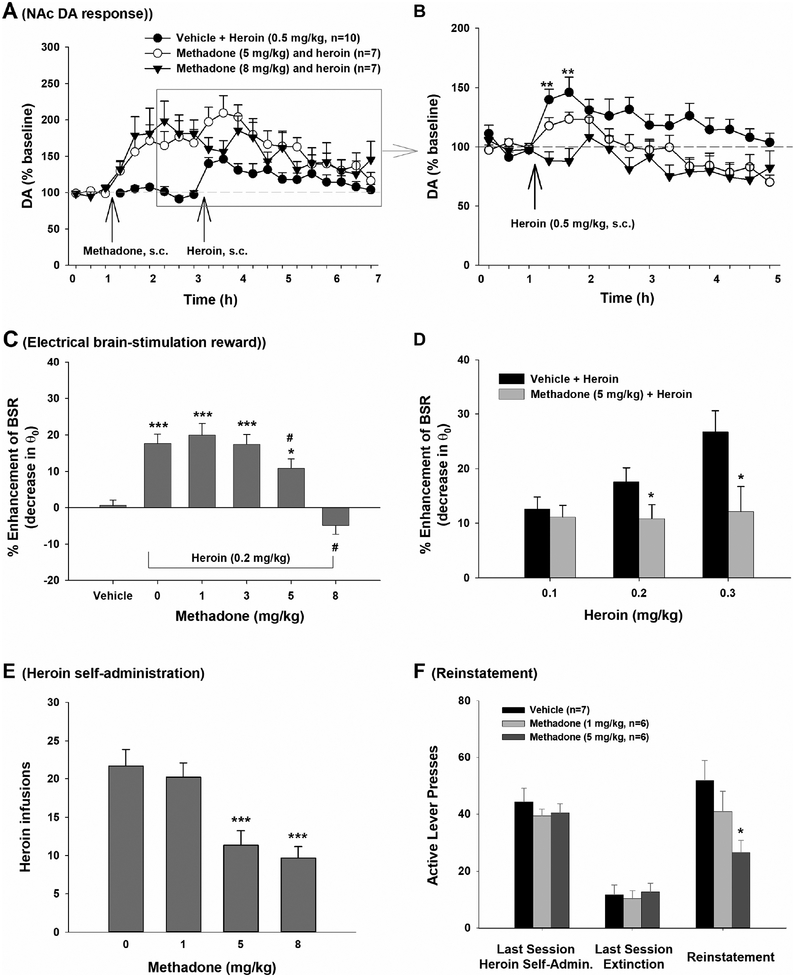
In vitro and in vivo evidence demonstrates that co-administration or pretreatment with methadone attenuates the pharmacological and behavioral effects of illicit opioids such as morphine and heroin. At the cellular level, co-administration of methadone blocks morphine-induced inhibition of adenyl cyclase, desensitizes the mu opioid receptor response to morphine, and inhibits morphine-enhanced cAMP formation and accumulation caused by forskolin (Blake et al., 1997). In vivo, pretreatment with methadone dose-dependently blocks heroin-enhanced extracellular DA in the NAc (Fig. 3 A, B) and heroin-enhanced electrical brain-stimulation reward in rats (Fig. 3 C, D) (Peng et al., 2010; Preshaw et al., 1982). Methadone also reduces intravenous heroin self-administration under both fixed-ratio (FR1; Fig. 3 E) and progressive-ratio reinforcement schedules (Peng et al., 2010), and blocks heroin-induced reinstatement of drug-seeking behavior in rats (Fig. 3 F; Leri et al., 2004). In rhesus monkeys, methadone blocks the shift to heroin choice over food during heroin withdrawal, while other medications, including dopamine agonists and corticotrophin releasing factor (CRF) or kappa receptor antagonists, fail to reliably reduce heroin choice (Negus and Banks, 2018). Such attenuation produced by methadone may be partially due to cross-tolerance to illicit opioids, competitive receptor binding and the relatively long half-life of methadone (Eap et al., 2002).
Figure 3:
Methadone pretreatment attenuates heroin action in animal models of addiction. A: Effects of methadone pretreatment on heroin-induced increase extracellular NAc DA; B: Heroin-induced increases in extracellular DA are blocked by methadone pretreatment (data highlighted in gray were normalized over the baseline before heroin injection); C: Methadone dose-dependently attenuated heroin-enhanced BSR; D: Methadone, at 5 mg/kg, attenuated heroin-enhanced BSR produced by multiple heroin doses; E: Methadone inhibited intravenous heroin self-administration in rats in a dose-dependent manner; F: Methadone, administered 30 min prior to heroin, dose-dependently attenuated 0.25 mg/kg heroin-induced reinstatement of drug-seeking behavior. *p<0.05, ***p<0.001, compared to vehicle control group. (Some data are replotted from Peng et al., 2010).
3.2.3. Clinical studies with methadone
The systematic study of methadone treatment for OUD began in the 1960’s. Early reports from Canada and the U.S. identified significant reductions in opioid craving and withdrawal symptoms in subjects given only one to two doses (5–10 mg) of methadone (Ferguson et al., 1965; Kreek, 2000; Kreek et al., 2004), providing a foundation for the first large-scale studies on methadone for the treatment of heroin abuse. This seminal 1963 study, involving 214 heroin-addicted patients, found that methadone achieved three key hallmarks: 1) prevention of opioid withdrawal, 2) reduction of opioid craving, and 3) normalization of physiological functions (e.g. gastrointestinal) that were perturbed by chronic opioid abuse. These observations led to a decade of clinical studies demonstrating the safety and efficacy of methadone in reducing opioid use and relapse (for comprehensive reviews, see Kreek et al., 2000, 2004). Around the same time, the Ontario Addiction Research Foundation began the first methadone treatment program (Ferguson et al., 1965; Fischer, 2000). By the late 1960’s, methadone was a widely accepted treatment for OUD in Canada (Fischer, 2000). Methadone was approved later by the U.S. FDA as an agonist therapy for OUD in 1972 (Blanco-Gandia and Rodriguez-Arias, 2018; Joseph and Woods, 2018; Kreek, 2000; Kreek et al., 2004; Kreek and Vocci, 2002). Since then, a great number of studies have further confirmed the safety and effectiveness of methadone pharmacotherapyfor OUD (Ali et al., 2017; van Dorp et al., 2007).
Today, tapered methadone treatment (the process of slowly decreasing a replacement opioid agonist) is used for opioid detoxification to mitigate withdrawal and craving during the onset of long-term abstinence and treatment programs (Li, 2016). Typically, methadone is administered in progressively decreasing doses over a long period of time. The detoxification and/or tapering process is associated with fewer withdrawal effects, reduced heroin use, and improved treatment retention compared to non-pharmacological detoxification programs (Amato et al., 2013; Mattick et al., 2009), although psychosocial support is almost always necessary to sustain long-term abstinence (Lobmaier et al., 2010; Mattick et al., 2009; Veilleux et al., 2010).
In addition to detoxification, methadone maintenance therapy is frequently used as a first-line treatment for heroin abuse, and substantially reduces healthcare costs compared to non-pharmacologic therapies (Blanco-Gandia and Rodriguez-Arias, 2018). However, methadone maintenance programs are associated with high degrees of social stigma (Woods and Joseph, 2018), variable attrition rates that are contingent upon dosage (Maxwell and Shinderman, 2002), and withdrawal symptoms upon cessation of use. Although beyond the scope of the current review, behavioral and psychosocial support is a key aspect in maintaining remission during maintenance programs (Dugosh et al., 2016). In addition, as in experimental animals, methadone alone produces euphoric effects in human subjects and is susceptible to abuse and overdose (Jasinski and Preston, 1986; Li, 2016). Thus, although methadone may be less rewarding compared to heroin or morphine, it nonetheless remains a Schedule II drug with relatively high abuse liability. For these reasons, partial agonist therapy, such as that provided by buprenorphine, may be superior in long-term treatment plans.
3.3. Buprenorphine for OUDs
Buprenorphine was discovered in 1966 by chemists at Reckitt & Colman located in Hull, England, and was introduced in phase 1 clinical safety trials in the late 1960’s (Campbell and Lovell, 2012). Reckitt subsequently supplied buprenorphine to the U.S. Addiction Research Center located in Lexington, Kentucky for efficacy testing in opioid-dependent humans, leading to the first publication in 1978 on buprenorphine’s improved safety and reduced addiction liability compared to methadone (Campbell and Lovell, 2012; Jasinski et al., 1978). Thereafter, buprenorphine slowly attained approval in other countries for pain and later the treatment of OUD (see Campbell and Lovell, 2012 for detailed history; Kumar et al., 2009; Lintzeris et al., 2004). The U.S. FDA approved buprenorphine in 2002 as an office-based treatment for OUD (Campbell and Lovell, 2012; FDA, 2002).
3.3.1. Pharmacology and preclinical studies with buprenorphine
Buprenorphine is a long-acting partial agonist at mu opioid receptors due to a unique slow receptor association/dissociation profile, and is therefore anticipated to have lower abuse liability. As a partial agonist, buprenorphine functions as an antagonist in the presence of other opioids by competitively binding to the mu opioid receptor. However, in the absence of opioids, buprenorphine elicits partial activation of the mu opioid receptor and therefore mitigates withdrawal symptoms in chronic users. Notably, buprenorphine also acts as an antagonist at kappa opioid receptors, which may contribute to its antidepressant effects (Falcon et al., 2016). Like methadone, in HEK cells expressing cloned mouse mu opioid receptors, buprenorphine blocks morphine inhibition of adenyl cyclase, desensitizes the mu opioid receptor, and blocks forskolin-induced cAMP increases by morphine, providing a cellular basis by which buprenorphine may help to treat OUDs (Blake et al., 1997). Buprenorphine alone activates VTA DA neurons in a manner similar to morphine and enhances basal extracellular DA release in the NAc, but attenuates NAc DA responses to heroin, consistent with a partial agonist profile (Grant and Sonti, 1994; Sorge et al., 2005; Sorge and Stewart, 2006). Buprenorphine, like methadone, also reverses altered brain glucose metabolism during morphine withdrawal in the thalamus, insular cortex and periaqueductal gray, albeit in a sex-dependent manner (Santoro et al., 2017).
In behaving animals, buprenorphine induces locomotor excitation similar to morphine, and morphine-treated rats show cross-tolerance and cross-sensitization to buprenorphine (Bartoletti et al., 1999; Bartoletti et al., 1993; Galici et al., 2005). At low doses buprenorphine produces modest conditioned place preference, but at high doses buprenorphine produces modest conditioned place aversion, suggesting some biphasic effects (Stinus et al., 2005). Like methadone and other commonly abused opioids, buprenorphine increases sensitivity to electrical ICSS, particularly in opioid-dependent subjects (Bruijnzeel et al., 2007; Hubner and Kornetsky, 1988). These findings suggest buprenorphine has minimal abuse liability but may also alleviate dysphoria associated with opioid withdrawal. Accordingly, buprenorphine prevents spontaneous withdrawal from fentanyl and reduces naloxone-precipitated physical withdrawal symptoms in fentanyl-dependent rats (Bruijnzeel et al., 2007). In morphine-dependent adults as well as rat pups, buprenorphine blocks withdrawal syndrome and withdrawal-induced conditioned place aversion (Stinus et al., 2005; Stoller and Smith, 2004). Chronic buprenorphine delivered via osmotic minipump (1.5 or 3.0 mg/kg/day) is ineffective in reducing ongoing heroin self-administration under fixed-ratio or progressive-ratio schedules over the course of daily, 3-hour tests, but buprenorphine does increase the latency to respond to heroin-associated cues at the onset of self-administration sessions (Sorge and Stewart, 2006) and reduces heroin seeking during extinction and heroin-primed reinstatement (Sorge et al., 2005). In contrast, buprenorphine dose-dependently reduces heroin intake in rats with unlimited heroin self-administration access (Chen et al., 2006), indicating that the animals’ self-administration history impacts the efficacy of buprenorphine in reducing heroin intake. Notably, the effective dose of buprenorphine capable of reducing opioid withdrawal in rats (80 μg/kg) may be different than that required to suppress opioid self-administration (40 μg/kg) (Chen et al., 2006; Sorge and Stewart, 2006), although comparisons across these preclinical studies may be limited due to methodological differences in dosing regimens, timing, rat handling, etc.
Rhesus monkeys voluntarily self-administer buprenorphine over saline, but do not show withdrawal symptoms or physical dependence upon cessation of buprenorphine use (Mello et al., 1981; Mello and Mendelson, 1985; Yanagita et al., 1982). Buprenorphine also blocks monkeys’ shift to heroin choice over food during withdrawal, although methadone is more effective than buprenorphine in blocking heroin choice (Negus, 2006). In macaques, buprenorphine produces dose-dependent reductions in intravenous heroin self-administration, whereas methadone is ineffective in suppressing heroin intake in 4 out of 5 subjects. Buprenorphine also has fewer toxic side effects (e.g., seizures, respiratory suppression, sedation) than methadone (Mello et al., 1981). While other studies in macaques have reported that buprenorphine is a more potent reinforcer than methadone at low doses under a progressive-ratio schedule of reinforcement, both medications are less efficacious reinforcers than heroin (Mello et al., 1984; Mello et al., 1988).
3.3.2. Clinical studies with buprenorphine
In human subjects, buprenorphine produces mild analgesia and subjectively reinforcing effects. However, unlike methadone, heroin, and other opioids, buprenorphine produces fewer physical dependence or withdrawal symptoms upon cessation of use (Comer et al., 2002; Houde, 1979; Jasinski et al., 1978; Mello et al., 1984; Mello et al., 1982), although some studies have reported difficulties with buprenorphine tapering (Fiellin et al., 2014), and in practice adjunctive medications (such as clonidine or sleep-promoting agents) are often co-prescribed to attenuate opioid withdrawal during buprenorphine dose tapering.
In early studies involving human heroin users, buprenorphine was efficacious as a maintenance therapy for OUDs, reducing heroin intake by 69–98% compared to controls (Mello and Mendelson, 1980; Mello et al., 1982), and these findings have been replicated since (Burchenal, 1977; DiPaula et al., 2002; Kakko et al., 2003; Sung and Conry, 2006). Moreover, unlike methadone, prolonged tapering of buprenorphine treatment (7 days vs. 28 days) does not convey additional benefits in promoting abstinence, potentially reducing the need for long-term maintenance programs (Ling et al., 2009). In contrast, a later review of 28 studies suggested that buprenorphine taper duration (ranging from 0 to 365 days) is positively associated with varying degrees of abstinence (Dunn et al., 2011). However, when medium to high doses are used, buprenorphine and methadone appear to be equally effective in treatment retention and reducing illicit opioid use (Mattick et al., 2014). Buprenorphine is less efficacious than methadone in treatment retention when flexible or low fixed doses are used, and in subjects with high opioid tolerance (Mattick et al., 2014).
Although buprenorphine has modest abuse potential in recently-detoxified opioid users and in non-opioid-dependent subjects (Comer et al., 2002; Comer et al., 2005), both animal and human studies indicate reduced abuse liability of buprenorphine compared to other opioids. Combination therapies of buprenorphine and naloxone have emerged to reduce illicit abuse and also have significant efficacy in reducing opioid withdrawal symptoms, craving and relapse (Wang et al., 2018), although combination therapy appears to have similar abuse liability as buprenorphine alone in recently detoxified heroin users (Comer and Collins, 2002). Alternative formulations of buprenorphine, including depot injections, sustained-release subdermal implants and sublingual tablets, are now promoting the convenience, utility and availability of buprenorphine in the treatment of OUD (Haight et al., 2019; Harricharan and Farah, 2017; Saxon et al., 2013; Strain et al., 2011; Walsh et al., 2017).
Co-administration of buprenorphine in emergency room visits for opioid overdose may also improve long-term treatment outcomes (Johns et al., 2018). Systematic comparison of buprenorphine to methadone and other pharmacotherapies illustrates the superiority of buprenorphine in detoxification, treatment retention, reduction of illicit drug use and drug cravings, and minimizing adverse reactions and side effects (Ling et al., 2005; Ling and Wesson, 2003). In addition, total healthcare costs of patients maintained on buprenorphine are up to 49% lower than those of patients maintained on methadone (Baser et al., 2011). Therefore, individual differences and needs must be considered when choosing the best pharmacotherapeutic strategy for OUD.
4. Agonist Therapies for Other Substance Use Disorders
4.1. Varenicline for tobacco use disorder
Like buprenorphine treatment for OUD, partial agonist therapies have strong potential for the treatment of other drug use disorders, including tobacco use (Jordan and Xi, 2018). The euphoric and addictive properties of tobacco are attributed to nicotine’s effects in the mesolimbic reward system, where nicotine is thought to increase VTA DA neuron activity by binding to and activating α4β2 nicotinic acetylcholine receptors (nAChRs). In addition to various formulations of nicotine itself (e.g. NRT; nicotine patch, gum), Varenicline (Chantix) was developed as a smoking cessation agent that selectively targets α4β2 nAChRs. As a potent partial agonist, varenicline attenuates nicotine-induced DA release in the NAc and thereby reduces the reinforcing value of nicotine. Because varenicline elicits partial activation of the α4β2 nAchR in the absence of nicotine, it also mitigates withdrawal symptoms during abstinence. Varenicline therefore conveys significant advantages over full agonists such as nicotine replacement therapies, which do not eliminate nicotine use disorder, and antagonists, which precipitate withdrawal symptoms in the presence of nicotine. Both preclinical and clinical evidence support the efficacy of varenicline in reducing tobacco use and promoting smoking cessation (for review see Jordan and Xi, 2018). It is worth noting that bupropion, a medication that is not a nAChR agonist or antagonist, is also prescribed for tobacco use disorder, although it is typically less efficacious than agonist therapies such as varenicline (Jordan and Xi, 2018).
4.2. Agonist therapy for cocaine use disorder
4.2.1. Classical DAT inhibitors
Cocaine is the third most commonly abused illicit drug (behind opioids and cannabinoids), and currently, there are no FDA-approved medications available for the treatment of cocaine or other psychostimulant use disorders. Cocaine’s abuse potential derives from blockade of monoamine transporters, most notably dopamine (DA) transporters (DAT), which leads to rapid and dramatic increases in extracellular NAc DA. Based on the rationale that methadone and buprenorphine are long-acting mu opioid receptor agonists successful in the treatment of opioid dependence, low dose, slow-release monoamine transporter or DAT inhibitors have been proposed as agonist therapies for cocaine use disorder (Fig. 1). To this end, oral cocaine has shown efficacy in reducing the subjective and physiological effects of low doses of intravenously administered cocaine (Walsh et al., 2000). Accordingly, several classical stimulants that have similar DAT binding profiles as cocaine have since been tested in humans and experimental animals.
d-Amphetamine:
Dextroamphetamine (d-Amphetamine) is the active enantiomer of amphetamine, a potent psychostimulant that is prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. Pharmacologically, d-Amphetamine is a full agonist at the trace amine-associated receptor 1 (TAAR1) and a vesicular monoamine transporter 2 (VMAT2) inhibitor that causes release of DA, serotonin, and norepinephrine (Sitte and Freissmuth, 2015). In early clinical studies, sustained-release d-Amphetamine reduced illicit cocaine use and improved treatment retention (Grabowski et al., 2004). However, in a randomized, placebo-controlled, double-blind clinical trial in treatment-seeking individuals with methamphetamine-use disorder, oral d-Amphetamine reduced withdrawal symptoms and craving but failed to reduce methamphetamine use (Galloway et al., 2011). In turn, sustained-release methamphetamine itself was found to reduce cocaine use and craving (Mooney et al., 2009). Given that both d-Amphetamine and methamphetamine are highly potent psychostimulants that carry significant abuse liability, their viability and potential for attaining FDA approval for the treatment of cocaine use disorder is limited.
Methylphenidate:
Methylphenidate (Ritalin) is an FDA-approved psychostimulant used for treatment of ADHD and narcolepsy. Pharmacologically, methylphenidate acts as a DA-norepinephrine reuptake inhibitor (Childress and Sallee, 2013). Early studies suggested that methylphenidate was not effective in reducing cocaine use (Grabowski et al., 1997), and more recent studies have confirmed the inability of methylphenidate to reduce psychostimulant abuse or improve addiction treatment retention (Miles et al., 2013).
CTDP-31,345:
The success of methadone in treating OUD suggests that long-acting monoamine transporter or DAT inhibitors may be similarly useful for treating cocaine use disorder. Our laboratory has examined this hypothesis using the slow-onset long-acting monoamine transporter inhibitor, CTDP-31,345 (trans-[4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydronaphthalen-1-yl]dimethylammonium chloride) in a variety of cocaine abuse-related animal models. This indatraline analog was effective in antagonizing cocaine reward and relapse (Peng et al., 2010; Xi and Gardner, 2008). However, by itself this slow-onset long-acting monoamine transporter inhibitor displayed similar abuse liability to cocaine, as evidenced by increased electrical brain-stimulation reward and extracellular NAc DA after systemic administration and substitution for cocaine in intravenous self-administration protocols. CTDP-31,345 also failed to alter cocaine-induced increases in extracellular NAc DA, but dose-dependently inhibited cocaine self-administration (Peng et al., 2010), suggesting CTDP-31,345 is more cocaine-like with limited translational potential, similar to other slow-onset, long-acting monoamine transporter inhibitors (e.g. CTDP 30,640; Gardner et al., 2006). Ideally, agonist therapies for cocaine abuse should functionally antagonize cocaine’s action while having low addictive potential on their own.
4.2.2. Atypical DAT inhibitors
Significant progress in DAT-based medication development indicates that not all monoamine transporter or DAT inhibitors elicit behavioral effects identical to those of cocaine. While substitution or cocaine-like effects may improve adherence to and/or retention in treatment, ideally new therapeutics for substance use disorders should have limited abuse liability alone while retaining efficacy in reducing illicit drug abuse, ameliorating withdrawal, and promoting abstinence. To this end, atypical DAT inhibitors are defined as exhibiting reduced or in some cases a complete lack of cocaine-like rewarding effects (Tanda et al., 2009). Moreover, pretreatment with these compounds can reduce cocaine-elicited behaviors in rodent models (Reith et al., 2015), suggesting translational potential for the treatment of cocaine use disorder.
JHW 007:
JHW 007 (N-Butyl-3α-[bis(4’-fluorophenyl)methoxy]tropane) emerged as a lead compound out of a series of benztropine analogues (Agoston et al., 1997; Desai et al., 2005). JHW 007 is an atypical DAT inhibitor (Ki = 25 nM, compared to 1330 and 1730 nM for NET and SERT, respectively) with a slow-onset, long-acting profile. In vivo, JHW 007 alone has minimal cocaine-like behavioral effects, while pretreatment with JHW 007 inhibits cocaine self-administration, cocaine-induced hyperactivity and cocaine locomotor sensitization in rats (Desai et al., 2014). JHW 007 also attenuates the rewarding and locomotor-stimulating effects of amphetamine and other psychostimulants (Hiranita et al., 2014; Reith et al., 2015; Velazquez-Sanchez et al., 2013). Although JHW 007 may have had drug development potential (Raje et al., 2003), it was never studied in humans and thus will likely remain as a preclinical research tool, currently available commercially.
CTDP-32476:
CTDP-32476 (2-(1-(4-chlorophenyl)-3-methylbutyl)piperidine) is a methylphenidate analog with a slow-onset, long-acting profile. In vitro binding assays indicate that CTDP-32476 is a potent and selective DAT inhibitor (Ki = 12 nM) and competitive with cocaine at DAT (cocaine Ki = 279 nM; Froimowitz et al., 2007; Xi et al., 2017). Systemic administration of CTDP-32476 alone produces a slow-onset, long-lasting (6–12 h) increase in extracellular NAc DA, locomotor activity, and brain-stimulation reward. Drug-naive rats do not self-administer CTDP-32476. In a substitution test, cocaine self-administering rats displayed a progressive reduction (i.e., extinction) in CTDP-32476 self-administration, suggesting significantly lower addictive liability than cocaine. Pretreatment with CTDP-32476 inhibited cocaine-enhanced extracellular NAc DA, cocaine self-administration, and cue-induced relapse to cocaine seeking (Xi et al., 2017). Therefore, CTDP-32476 appears to be a unique DAT inhibitor that not only satisfies drug craving through slow-onset, long-lasting DAT inhibition, but also renders subsequent administration of cocaine ineffective.
RTI-336:
RTI-336 was a lead compound emerging from a class of 3-phenyltropane cocaine analog, developed for the treatment of cocaine use, that acts as a selective dopamine reuptake inhibitor (Carroll et al., 2006b). Compared to other 3-phenyltropane analogs such as RTI-177, RTI-336 exhibited a higher LD50/therapeutic ratio, favorable oral bioavailability, induced low levels of locomotor sensitization compared to cocaine, and reduced cocaine self-administration in both rats and non-human primates (Carroll et al., 2006a; Carroll et al., 2006b). However, PET imaging revealed that high levels of DAT occupancy (>90%) by RTI-336 were required to reduce non-human primate cocaine self-administration (Carroll et al., 2006b). In another non-human primate study, RTI-336 produced fast-onset stimulant effects similar to cocaine, and a trend towards reliable self-administration, albeit at lower levels than cocaine (Czoty et al., 2010; Howell et al., 2007; Kimmel et al., 2007). Still other studies in rats revealed strain-dependent effects: while Lewis rats showed reduced cocaine intake and cocaine-induced locomotor activity following RTI-336 administration, RTI-336 increased cocaine intake in F344 rats and had no effect on cocaine-induced locomotion (Haile et al., 2005). Double-blind, placebo-controlled studies suggest RTI-336 is well-tolerated and safe in humans, potentially warranting further investigation (Carroll et al., 2018).
Modafinil:
Modafinil is a clinically available atypical DAT inhibitor with low potency (IC50 = 2–4 μM in the DA reuptake assay) and downstream activities that may interfere with its efficacy for the treatment of psychostimulant use disorders (Mereu et al., 2013; Sangroula et al., 2017; Zolkowska et al., 2009). In an early human laboratory study, modafinil reduced cocaine euphoria (Dackis et al., 2003). However, subsequent clinical trials in patients with psychostimulant use disorder failed to demonstrate efficacy of modafinil over placebo (Anderson et al., 2012; Dackis et al., 2012; Shearer et al., 2009). Indeed, a recent meta-analysis indicated no evidence supporting the superiority of modafinil in promoting cocaine abstinence and treatment retention (Sangroula et al., 2017). However, post-hoc findings from these studies suggest that modafinil may be more efficacious in less severe cases of addiction. The more active R-modafinil (Armodafinil, Nuvigil) has also been examined preclinically as a potential treatment for cocaine use disorders (Loland et al., 2012), although clinical evaluation of R-modafinil in cocaine abusers has not been investigated, to our knowledge.
JJC8–016:
More recently, our laboratory developed a series of more soluble, selective and potent modafinil analogs, with the rationale that these modifications might improve effectiveness in treating psychostimulant use disorders. JJC8–016 (N-(2-((Bis(4-fluorophenyl)methyl)thio)ethyl)-3-phenylpropan-1-amine) was an early lead compound from this series with moderately high affinity for DAT (Ki=116 nM) (Okunola-Bakare et al., 2014; Zhang et al., 2017). In rats, JJC8–016 alone failed to alter extracellular NAc DA, locomotor activity, electrical brain-stimulation reward and reinstatement of drug-seeking behavior. Moreover, substitution of JJC8–016 for cocaine did not maintain self-administration in rats, and pretreatment with JJC8–016 significantly inhibited cocaine-taking and cocaine-seeking behaviors (Zhang et al., 2017). Together, these observations suggest that JJC8–016 has low abuse liability, but translational utility for the treatment of cocaine use disorder was limited by poor metabolic and pharmacokinetic profiles.
JJC8–091:
JJC8–091 (1-(4-(2-((Bis(4-fluorophenyl)methyl)sulfinyl)ethyl)piperazin-1-yl)-propan-2-ol) is a recently described modafinil analog and atypical DAT inhibitor (Ki=289 nM; Cao et al., 2016; Tunstall et al., 2018). Systemic administration of JJC8–091 produced a mild slow-onset, long-duration increase in extracellular NAc DA (Keighron et al., 2018). Drug-naïve rats do not self-administer JJC8–091, and JJC8–091 fails to substitute for cocaine in intravenous self-administration studies, suggesting extremely low addictive potential (Newman et al., 2019). Strikingly, pretreatment with JJC8–091 attenuates cocaine self-administration under progressive-ratio schedule of reinforcement and blocks cocaine-induced reinstatement to drug-seeking behaviors. In addition, JJC8–091 attenuates compulsive methamphetamine self-administration and decreases escalation of methamphetamine intake in rats (Tunstall et al., 2018).
Alongside its favorable effects on stimulant abuse in preclinical models, JJC8–091 displays a metabolic and pharmacokinetic profile consistent with a viable drug development candidate. The development of the Multiparameter Optimization (MPO) algorithm to predict CNS penetration has improved prioritization of clinical candidates in drug development (Wager et al., 2010a; Wager et al., 2010b; Wager et al., 2016). Applying the CNS MPO tool to six physicochemical properties of JJC8–091: lipophilicity calculated partition coefficient (ClogP), calculated distribution coefficient at pH7.4 (ClogD), molecular weight (MW), topological polar surface area (TPSA), number of hydrogen bond donors (HBD), and pKa of most basic center, this DAT inhibitor is predicted to have optimal parameters for CNS penetration and drug safety (MPO=5.22; Table 1). Collectively, these data suggest that JJC8–091 may represent an innovative new agonist therapy for the treatment of cocaine or other psychostimulant use disorders, a fundamental and yet unmet public heath need.
Table 1.
In silico ADME calculations for JJC8–091a
| Parameter | Value | T0b |
|---|---|---|
| ClogP | 1.49 | 1 |
| ClogD | 2.32 | 0.84 |
| TPSA | 43.78 | 1 |
| MW | 422.53 | 0.55 |
| HBD | 1 | 0.83 |
| PKa | 6.89 | 1 |
CNS MPO = 5.22
ClogP, TPSA, and MW values were calculated using ChemDraw. ClogD and PKa values were calculated using Chemicalize.
T0 was calculated using the method published in (Wager et al., 2010b).
5. Summary
Methadone and buprenorphine are successful examples of agonist replacement therapies for the treatment of OUD. These medications are effective in reducing illicit opioid use, mitigating opioid craving and withdrawal syndromes, and promoting abstinence. The efficacy of methadone can be attributed to three specific characteristics: 1) a long-acting mu opioid receptor agonist, similar to morphine; 2) lower addictive potential than morphine and heroin; and 3) attenuation of the rewarding and withdrawal-related effects of morphine and heroin. Based on findings with methadone, two partial agonist therapies (buprenorphine, varenicline) have been subsequently developed and successfully used for the treatment of opioid and tobacco use disorders, respectively. Partial agonists convey additional benefits over full agonist therapies, acting as antagonists in the presence of abused substances while mitigating withdrawal symptoms during abstinence through partial activation at the target receptor. Nevertheless, partial agonist therapies may fail patients with severe drug use, in whom full agonist treatment may be required. A “stepped-care” approach may also be indicated in severe OUD, in which patients can be transitioned from buprenorphine to methadone if clinical efficacy is insufficient (Kakko et al., 2007). Nonetheless, the rationale of agonist/partial agonist therapies is currently being applied to the development of new atypical DAT inhibitors for the treatment of cocaine and other psychstimulant use disorders. This approach has yielded significant progress, with several new lead compounds showing promising translational potential.
6. Future Investigation
Although agonist therapies are effective in the treatment of SUD, a major remaining challenge is the prevention of relapse to drug use after a period of abstinence. Longitudinal studies on heroin users in the Amsterdam Cohort Study have shown that 86% of patients in “low-threshold” harm reduction programs relapse within 5 years of methadone-sustained abstinence (Termorshuizen et al., 2005). A similar relapse rate was reported in tobacco users within a year of combined pharmacological and behavioral treatments (Stead et al., 2012). Alternative medication strategies for future study may involve the combination of multiple pharmacotherapies. For example, dopamine D3 receptor antagonists are highly effective in reducing drug (cocaine or opioid) reward and in preventing relapse to drug-seeking behavior (Heidbreder and Newman, 2010; Sokoloff and Le Foll, 2017; Xi and Gardner, 2007; You et al., 2018; You et al., 2017). Thus, the combination of a full/partial mu opioid receptor agonist with a D3 receptor antagonist may yield promising results in relapse prevention and the promotion of abstinence. In addition to D3 receptor antagonists, other compounds that target brain metabotropic glutamate, GABA, or endocannabinoid systems also show promising results in preventing relapse to drug seeking (Xi and Gardner, 2008). Another emerging strategy for the treatment of OUD is the development of biased mu opioid receptor agonists, which preferentially recruit activation of intracellular G-protein signaling over β-arrestin and therefore convey reduced risk of respiratory suppression and overdose (Bohn et al., 2000; Bohn et al., 1999; Madariaga-Mazon et al., 2017; Raehal et al., 2005; Schmid et al., 2017). However, additional studies are needed to determine whether G-protein signaling preferentially mediates the rewarding or addictive liability of opioids, and whether G-protein-biased agonist therapy is superior to classical (non-biased) agonist therapies for the treatment of OUD.
Human clinical trials will be required to confirm that the “agonist” therapy approach to cocaine use disorders using the atypical DAT inhibitors or other stimulant-like medications can be applied to this patient population. While avoidance of physical withdrawal is the hallmark of opioid relapse, individuals dependent on cocaine do not experience marked physical withdrawal. As a result, it is currently unclear how best to prevent relapse in this population. Nevertheless, identifying new lead molecules that are safe and show promise in animal models is the only way to ultimately determine if this approach is viable. Undoubtedly, there will be no magic bullet for all psychostimulant abusers. Going forward, it is essential to identify pharmacotherapies that can help at least a subpopulation of those patients who are motivated to quit their illicit drug use. We must ultimately broaden the effectiveness of available treatment strategies, especially for those that suffer from cocaine use disorders, for whom there are no therapeutic options.
Highlights.
Agonist replacement therapies have been successfully used for the treatment of opioid and nicotine use disorders, but not yet for addiction to cocaine
Methadone is a long-acting mu opioid receptor full agonist, and buprenorphine is a mu opioid receptor partial agonist
Methadone and buprenorphine have lower addictive liability than morphine or heroin, and pretreatment with either attenuates opioid use
Significant progress has been made in the development of agonist-like atypical DAT inhibitors for the treatment of cocaine use disorder
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (Z1A DA000389), National Institutes of Health, USA. The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, et al. 1997. Novel N-substituted 3 alpha-[bis(4’-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40: 4329–39. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, Bruchas MR. 2011. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115: 1363–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Tahir B, Jabeen S, Malik M. 2017. Methadone Treatment of Opiate Addiction: A Systematic Review of Comparative Studies. Innov Clin Neurosci 14: 8–19. [PMC free article] [PubMed] [Google Scholar]
- Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. 2013. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev: CD003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, et al. 2012. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 120: 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayonrinde OT, Bridge DT. 2000. The rediscovery of methadone for cancer pain management. Med J Austral. 173:536–40. [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. 1993. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–95. [PubMed] [Google Scholar]
- Bartoletti M, Gaiardi M, Gubellini C. 1999. Effects of buprenorphine on motility in morphine post-dependent rats. Pharmacol Res 40: 327–32. [DOI] [PubMed] [Google Scholar]
- Bartoletti M, Gaiardi M, Gubellini C, Bacchi A, Babbini M. 1993. Effects of buprenorphine on motility in chronically morphine treated rats. Neuropharmacology 32: 865–8. [DOI] [PubMed] [Google Scholar]
- Baser O, Chalk M, Fiellin DA, Gastfriend DR. 2011. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care 17 Suppl 8: S235–48. [PubMed] [Google Scholar]
- Blake AD, Bot G, Freeman JC, Reisine T. 1997. Differential opioid agonist regulation of the mouse mu opioid receptor. J Biol Chem 272: 782–90. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandia MC, Rodriguez-Arias M. 2018. Pharmacological treatments for opiate and alcohol addiction: A historical perspective of the last 50 years. Eur J Pharmacol 836: 89–101. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. 2000. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408: 720–3. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. 1999. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495–8. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. 1981. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci 28: 551–5. [DOI] [PubMed] [Google Scholar]
- Britt MD, Wise RA. 1983. Ventral tegmental site of opiate reward: antagonism by a hydrophilic opiate receptor blocker. Brain Res 258: 105–8. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Marcinkiewcz C, Isaac S, Booth MM, Dennis DM, Gold MS. 2007. The effects of buprenorphine on fentanyl withdrawal in rats. Psychopharmacology (Berl) 191: 931–41. [DOI] [PubMed] [Google Scholar]
- Buajordet I, Naess AC, Jacobsen D, Brors O. 2004. Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. Eur J Emerg Med 11: 19–23. [DOI] [PubMed] [Google Scholar]
- Burchenal JH. 1977. The historical development of cancer chemotherapy. Semin Oncol 4: 135–46. [PubMed] [Google Scholar]
- Busto U, Sellers EM. 1986. Pharmacokinetic determinants of drug abuse and dependence. A conceptual perspective. Clin Pharmacokinet 11: 144–53. [DOI] [PubMed] [Google Scholar]
- Campbell ND, Lovell AM. 2012. The history of the development of buprenorphine as an addiction therapeutic. Ann N Y Acad Sci 1248: 124–39. [DOI] [PubMed] [Google Scholar]
- Cao J, Slack RD, Bakare OM, Burzynski C, Rais R, et al. 2016. Novel and High Affinity 2-[(Diphenylmethyl)sulfinyl]acetamide (Modafinil) Analogues as Atypical Dopamine Transporter Inhibitors. J Med Chem 59: 10676–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Fox BS, Kuhar MJ, Howard JL, Pollard GT, Schenk S. 2006a. Effects of dopamine transporter selective 3-phenyltropane analogs on locomotor activity, drug discrimination, and cocaine self-administration after oral administration. Eur J Pharmacol 553: 149–56. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. 2006b. Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse. AAPS J 8: E196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Kosten TR, Buda JJ, Wang L, Walters BB. 2018. A Double-Blind, Placebo-Controlled Trial Demonstrating the Safety, Tolerability, and Pharmacokinetics of Single, Escalating Oral Doses of RTI-336. Front Pharmacol 9: 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Berridge KC. 2014. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci 34: 4239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control (CDC) 2018. CDC WONDER online databases. December 15. 2018. https://wonder.cdc.gov/
- Chartoff EH, Connery HS. 2014. It’s MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol 5: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. 2006. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31: 2692–707. [DOI] [PubMed] [Google Scholar]
- Childress A, Sallee FR. 2013. The use of methylphenidate hydrochloride extended-release oral suspension for the treatment of ADHD. Expert Rev Neurother 13: 979–88. [DOI] [PubMed] [Google Scholar]
- Clark N, Lintzeris N, Gijsbers A, Whelan G, Dunlop A, et al. 2002. LAAM maintenance vs methadone maintenance for heroin dependence. Cochrane Database Syst Rev: CD002210. [DOI] [PubMed] [Google Scholar]
- Codd EE, Shank RP, Schupsky JJ, Raffa RB. 1995. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther 274: 1263–70. [PubMed] [Google Scholar]
- Comer SD, Collins ED. 2002. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther 303: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. 2002. Intravenous buprenorphine self-administration by detoxified heroin abusers. J Pharmacol Exp Ther 301: 266–76. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Walker EA. 2005. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther 315: 1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Carroll FI, Nader MA. 2010. Lower reinforcing strength of the phenyltropane cocaine analogs RTI-336 and RTI-177 compared to cocaine in nonhuman primates. Pharmacol Biochem Behav 96: 274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, et al. 2012. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat 43: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, et al. 2003. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend 70: 29–37. [DOI] [PubMed] [Google Scholar]
- David V, Cazala P. 1994. Differentiation of intracranial morphine self-administration behavior among five brain regions in mice. Pharmacol Biochem Behav 48: 625–33. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services (HHS). 2018. About the U.S. Opioid Epidemic. April 18. 2018. https://www.hhs.gov/opioids/about-the-epidemic/ [Google Scholar]
- Desai RI, Grandy DK, Lupica CR, Katz JL. 2014. Pharmacological characterization of a dopamine transporter ligand that functions as a cocaine antagonist. J Pharmacol Exp Ther 348: 106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. 2005. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25: 1889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaula BA, Schwartz R, Montoya ID, Barrett D, Tang C. 2002. Heroin detoxification with buprenorphine on an inpatient psychiatric unit. J Subst Abuse Treat 23: 163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Nyswander M. 1966a. Study of methadone as an adjunct in rehabilitation of heroin addicts. IMJ Ill Med J 130: 487–9. [PubMed] [Google Scholar]
- Dole VP, Nyswander ME. 1966b. Rehabilitation of heroin addicts after blockade with methadone. N Y State J Med 66: 2011–7. [PubMed] [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. 2016. A Systematic Review on the Use of Psychosocial Interventions in Conjunction With Medications for the Treatment of Opioid Addiction. J Addict Med 10: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. 2011. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend 119: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. 2002. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet 41: 1153–93. [DOI] [PubMed] [Google Scholar]
- Ebert B, Thorkildsen C, Andersen S, Christrup LL, Hjeds H. 1998. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem Pharmacol 56: 553–9. [DOI] [PubMed] [Google Scholar]
- Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, et al. 2016. Antidepressant-like Effects of Buprenorphine are Mediated by Kappa Opioid Receptors. Neuropsychopharmacology 41: 2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Niaura R. 2019. E-cigarettes and smoking cessation in the United States according to frequency of e-cigarette use and quitting duration: analysis of the 2016 and 2017 National Health Interview Surveys. Nicotine Tob Res [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA). Information about Medication-Assisted Treatment (MAT). August 15 2018. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm600092.htm
- Food and Drug Administration (FDA). 2002. Subutex and Suboxone Approval Letter. November 24. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/20732,20733ltr.pdf
- Ferguson JK, Ettinger GH, Joron GE, Lederman JJ, Mackenzie DJ. 1965. Good Medical Practice in the Care of the Narcotic Addict; a Report Prepared by a Special Committee Appointed by the Executive Committee of the Canadian Medical Association. Can Med Assoc J 92: 1040–3. [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Margolis EB. 2015. Understanding opioid reward. Trends Neurosci 38: 217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O’Connor PG. 2014. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med 174: 1947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B 2000. Prescriptions, power and politics: the turbulent history of methadone maintenance in Canada. J Public Health Policy 21: 187–210. [PubMed] [Google Scholar]
- Froimowitz M, Gu Y, Dakin LA, Nagafuji PM, Kelley CJ, et al. 2007. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem 50: 219–32. [DOI] [PubMed] [Google Scholar]
- Galici R, McMahon LR, France CP. 2005. Cross-tolerance and mu agonist efficacy in pigeons treated with LAAM or buprenorphine. Pharmacol Biochem Behav 81: 626–34. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Buscemi R, Coyle JR, Flower K, Siegrist JD, et al. 2011. A randomized, placebo-controlled trial of sustained-release dextroamphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther 89: 276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Liu X, Paredes W, Giordano A, Spector J, et al. 2006. A slow-onset, long-duration indanamine monoamine reuptake inhibitor as a potential maintenance pharmacotherapy for psychostimulant abuse: effects in laboratory rat models relating to addiction. Neuropharmacology 51: 993–1003. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. 1997. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 17: 485–8. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. 2004. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29: 1439–64. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Sonti G. 1994. Buprenorphine and morphine produce equivalent increases in extracellular single unit activity of dopamine neurons in the ventral tegmental area in vivo. Synapse 16: 181–7. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Moody DE, Woods JH, Kilbourn MR, et al. 2003. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology 28: 2000–9. [DOI] [PubMed] [Google Scholar]
- Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, Greenwald MK, Nadipelli VR, Ling W, Heidbreder C; RB-US-13–0001 Study Investigators. 2019. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 393: 778–790. [DOI] [PubMed] [Google Scholar]
- Haile CN, Zhang XY, Carroll FI, Kosten TA. 2005. Cocaine self-administration and locomotor activity are altered in Lewis and F344 inbred rats by RTI 336, a 3-phenyltropane analog that binds to the dopamine transporter. Brain Res 1055: 186–95. [DOI] [PubMed] [Google Scholar]
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, et al. 2019. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med 380: 629–37. [DOI] [PubMed] [Google Scholar]
- Harricharan S, Farah K. 2017. In Buprenorphine Formulations for the Treatment of Opioid Use Disorders: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness and Guidelines. Ottawa (ON), Canadian Agency for Drugs and Technologies in Health; [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. 2010. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci 1187: 4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helal MA, Habib ES, Chittiboyina AG. 2017. Selective kappa opioid antagonists for treatment of addiction, are we there yet? Eur J Med Chem 141: 632–47. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL. 2014. Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. J Pharmacol Exp Ther 348: 174–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JH, Stahl EL, Schmid CL, Scarry SM, Aube J, Bohn LM. 2018. G protein signaling-biased agonism at the kappa-opioid receptor is maintained in striatal neurons. Sci Signal 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde RW. 1979. Analgesic effectiveness of the narcotic agonist-antagonists. Br J Clin Pharmacol 7 Suppl 3: 297S–308S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovious JR, Peters MA. 1985. Opiate self-administration in adult offspring of methadone-treated female rats. Pharmacol Biochem Behav 22: 949–53. [DOI] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. 2007. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320: 757–65. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Kornetsky C. 1988. The reinforcing properties of the mixed agonist-antagonist buprenorphine as assessed by brain-stimulation reward. Pharmacol Biochem Behav 30: 195–7. [DOI] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. 1983. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci 33 Suppl 1: 773–6. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Zhou P, Pickel VM. 2011. Enhanced dendritic availability of mu-opioid receptors in inhibitory neurons of the extended amygdala in mice deficient in the corticotropin-releasing factor-1 receptor. Synapse 65: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. 1978. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry 35: 501–16. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Preston KL. 1986. Comparison of intravenously administered methadone, morphine and heroin. Drug Alcohol Depend 17: 301–10. [DOI] [PubMed] [Google Scholar]
- Johns SE, Bowman M, Moeller FG. 2018. Utilizing Buprenorphine in the Emergency Department after Overdose. Trends Pharmacol Sci 39: 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Xi ZX. 2018. Discovery and development of varenicline for smoking cessation. Expert Opin Drug Discov 13: 671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph H, Woods JS. 2018. Changing the Treatment Direction for Opiate Addiction: Dr. Dole’s Research. Subst Use Misuse 53: 181–93. [DOI] [PubMed] [Google Scholar]
- Kakko J, Gronbladh L, Svanborg KD, von Wachenfeldt J, Ruck C, et al. 2007. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry 164: 797–803. [DOI] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. 2003. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet 361: 662–8. [DOI] [PubMed] [Google Scholar]
- Kang J, Chen XL, Wang H, Rampe D. 2003. Interactions of the narcotic l-alpha-acetylmethadol with human cardiac K+ channels. Eur J Pharmacol 458: 25–9. [DOI] [PubMed] [Google Scholar]
- Karkhanis A, Holleran KM, Jones SR. 2017. Dynorphin/Kappa Opioid Receptor Signaling in Preclinical Models of Alcohol, Drug, and Food Addiction. Int Rev Neurobiol 136: 53–88. [DOI] [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. 2004. Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309: 650–660. [DOI] [PubMed] [Google Scholar]
- Keighron JDQ JC; Cao J; DeMarco E; Coggiano MA; Gleaves A; Slack RD; Zanettini C; Newman AH; Tanda G 2018. Effects of R-Modafinil and Modafinil Analogs on Dopamine Dynamics Assessed by Voltammetry and Microdialysis in the Mouse Nucleus Accumbens Shell. A.C.S. Chem. Neurosci e-Pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri UG, Viner K, Perrone J. 2018. Lethal Fentanyl and Cocaine Intoxication. N Engl J Med 379: 1782. [DOI] [PubMed] [Google Scholar]
- Kim HK, Nelson LS. 2015. Reducing the harm of opioid overdose with the safe use of naloxone : a pharmacologic review. Expert Opin Drug Saf 14: 1137–46. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, et al. 2008. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav 90: 453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. 2007. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav 86: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, George TP. 2002. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect 1: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ. 2000. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann N Y Acad Sci 909: 186–216. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Bart G, Laforge KS, Butelman ER. 2004. Evolving perspectives on neurobiological research on the addictions: celebration of the 30th anniversary of NIDA. Neuropharmacology 47 Suppl 1: 324–44. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Vocci FJ. 2002. History and current status of opioid maintenance treatments: blending conference session. J Subst Abuse Treat 23: 93–105. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Natale RD, Langkham B, Sharma C, Kabi R, Mortimore G. 2009. Opioid substitution treatment with sublingual buprenorphine in Manipur and Nagaland in Northeast India: what has been established needs to be continued and expanded. Harm Reduct J 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Tremblay A, Sorge RE, Stewart J. 2004. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology 29: 1312–20. [DOI] [PubMed] [Google Scholar]
- Lewanowitsch T, Miller JH, Irvine RJ. 2006. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci 78: 682–8. [DOI] [PubMed] [Google Scholar]
- Li SM, Kopajtic TA, O’Callaghan MJ, Agoston GE, Cao J, et al. 2011. N-substituted benztropine analogs: selective dopamine transporter ligands with a fast onset of action and minimal cocaine-like behavioral effects. J Pharmacol Exp Ther 336: 575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Xi ZX. 2016. Methadone Usage, Misuse, and Addiction Processes: An Overview In Neuropathology of Drug Addiction and Substance Misuse: Elsevier Inc. [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. 2007. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci 35: 456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling GS, Tappe NS, Inturrisi CE. 1984. Methadone induced physical dependence in the rat. Life Sci 34: 683–90. [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, et al. 2005. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction 100: 1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, et al. 2009. Buprenorphine tapering schedule and illicit opioid use. Addiction 104: 256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Wesson DR. 2003. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend 70: S49–57. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Ritter A, Panjari M, Clark N, Kutin J, Bammer G. 2004. Implementing buprenorphine treatment in community settings in Australia: experiences from the Buprenorphine Implementation Trial. Am J Addict 13 Suppl 1: S29–41. [DOI] [PubMed] [Google Scholar]
- Lobmaier P, Gossop M, Waal H, Bramness J. 2010. The pharmacological treatment of opioid addiction--a clinical perspective. Eur J Clin Pharmacol 66: 537–45. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, et al. 2012. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry 72: 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madariaga-Mazon A, Marmolejo-Valencia AF, Li Y, Toll L, Houghten RA, Martinez-Mayorga K. 2017. Mu-Opioid receptor biased ligands: A safer and painless discovery of analgesics? Drug Discov Today 22: 1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fujita W, Devi LA, Fields HL. 2017. Two delta opioid receptor subtypes are functional in single ventral tegmental area neurons, and can interact with the mu opioid receptor. Neuropharmacology 123: 420–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT. 2014. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 82: 1346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. 2009. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev: CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev: CD002207. [DOI] [PubMed] [Google Scholar]
- Maxwell S, Shinderman MS. 2002. Optimizing long-term response to methadone maintenance treatment: a 152-week follow-up using higher-dose methadone. J Addict Dis 21: 1–12. [DOI] [PubMed] [Google Scholar]
- McCall Jones C, Baldwin GT, Compton WM. 2017. Recent Increases in Cocaine-Related Overdose Deaths and the Role of Opioids. Am J Public Health 107: 430–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. 1981. Buprenorphine self-administration by rhesus monkey. Pharmacol Biochem Behav 15: 215–25. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. 1984. Buprenorphine, heroin, and methadone: comparison of relative reinforcing properties. NIDA Res Monogr 49: 172–8. [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. 1988. Progressive ratio performance maintained by buprenorphine, heroin and methadone in Macaque monkeys. Drug Alcohol Depend 21: 81–97. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. 1980. Buprenorphine suppresses heroin use by heroin addicts. Science 207: 657–9. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. 1985. Behavioral pharmacology of buprenorphine. Drug Alcohol Depend 14: 283–303. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC. 1982. Buprenorphine effects on human heroin self-administration: an operant analysis. J Pharmacol Exp Ther 223: 30–9. [PubMed] [Google Scholar]
- Mereu M, Bonci A, Newman AH, Tanda G. 2013. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 229: 415–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles SW, Sheridan J, Russell B, Kydd R, Wheeler A, et al. 2013. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction 108: 1279–86. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. 2009. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 101: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndegwa S, Pant S, Pohar S, Mierzwinski-Urban M. 2016. Injectable Extended-Release Naltrexone to Treat Opioid Use Disorder In CADTH Issues in Emerging Health Technologies. Ottawa (ON) [PubMed] [Google Scholar]
- Negus SS. 2006. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther 317: 711–23. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. 2018. Modulation of drug choice by extended drug access and withdrawal in rhesus monkeys: Implications for negative reinforcement as a driver of addiction and target for medications development. Pharmacol Biochem Behav 164: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Cao J, Keighron JD, Jordan CJ, Bi G-H, Liang Y, Abramyan AM, Avelar AJ, Tschumi CW, Beckstead MJ, Shi L, Tanda G, Xi Z-X In press. Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunola-Bakare OM, Cao J, Kopajtic T, Katz JL, Loland CJ, et al. 2014. Elucidation of structural elements for selectivity across monoamine transporters: novel 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues. J Med Chem 57: 1000–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. 1997. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci 111: 1324–34. [DOI] [PubMed] [Google Scholar]
- Oxenham D, Farrer K. 1998. Methadone: opioid, N-methyl-D-aspartate antagonist or both? Palliat Med 12: 302. [DOI] [PubMed] [Google Scholar]
- Pacesova D, Novotny J, Bendova Z. 2016. The effect of chronic morphine or methadone exposure and withdrawal on clock gene expression in the rat suprachiasmatic nucleus and AA-NAT activity in the pineal gland. Physiol Res 65: 517–25. [DOI] [PubMed] [Google Scholar]
- Peng XQ, Xi ZX, Li X, Spiller K, Li J, et al. 2010. Is slow-onset long-acting monoamine transport blockade to cocaine as methadone is to heroin? Implication for anti-addiction medications. Neuropsychopharmacology 35: 2564–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, LePiane FG. 1980. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav 12: 965–8. [DOI] [PubMed] [Google Scholar]
- Poznanski P, Lesniak A, Korostynski M, Szklarczyk K, Lazarczyk M, et al. 2017. Delta-opioid receptor antagonism leads to excessive ethanol consumption in mice with enhanced activity of the endogenous opioid system. Neuropharmacology 118: 90–101. [DOI] [PubMed] [Google Scholar]
- Preshaw RL, Zenick H, Stutz RM. 1982. Effects of parenteral morphine and oral methadone on self-stimulation in the rat. Pharmacol Biochem Behav 16: 81–5. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM. 2005. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314: 1195–201. [DOI] [PubMed] [Google Scholar]
- Raje S, Cao J, Newman AH, Gao H, Eddington ND. 2003. Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther 307: 801–808. [DOI] [PubMed] [Google Scholar]
- Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, et al. 2015. Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol Depend 147: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Peselow E. 2009. The neurobiology of addictive disorders. Clin Neuropharmacol 32: 269–76. [DOI] [PubMed] [Google Scholar]
- Sangroula D, Motiwala F, Wagle B, Shah VC, Hagi K, Lippmann S. 2017. Modafinil Treatment of Cocaine Dependence: A Systematic Review and Meta-Analysis. Subst Use Misuse 52: 1292–306. [DOI] [PubMed] [Google Scholar]
- Santoro GC, Carrion J, Patel K, Vilchez C, Veith J, et al. 2017. Sex Differences in Regional Brain Glucose Metabolism Following Opioid Withdrawal and Replacement. Neuropsychopharmacology 42: 1841–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J 1986. The therapeutic use of heroin: a review of the pharmacological literature. Can J Physiol Pharmacol 64: 1–6. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Hser YI, Woody G, Ling W. 2013. Medication-assisted treatment for opioid addiction: methadone and buprenorphine. J Food Drug Anal 21: S69–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. 2012. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron 76: 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM. 2017. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171: 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, van Beek I, et al. 2009. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction 104: 224–33. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. 2015. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci 36: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Le Foll B. 2017. The dopamine D3 receptor, a quarter century later. Eur J Neurosci 45: 2–19. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. 2005. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology 30: 1681–92. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. 2006. The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration. Psychopharmacology (Berl) 188: 28–41. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, et al. 2012. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 11: CD000146. [DOI] [PubMed] [Google Scholar]
- Steidl S, Myal S, Wise RA. 2015. Supplemental morphine infusion into the posterior ventral tegmentum extends the satiating effects of self-administered intravenous heroin. Pharmacol Biochem Behav 134: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Grabowski J, Wang NS, Meisch RA. 1996. Orally delivered methadone as a reinforcer in rhesus monkeys. Psychopharmacology (Berl) 123: 111–8. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. 2005. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology 30: 90–8. [DOI] [PubMed] [Google Scholar]
- Stoller DC, Smith FL. 2004. Buprenorphine blocks withdrawal in morphine-dependent rat pups. Paediatr Anaesth 14: 642–9. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. 2009. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother 10: 1727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Harrison JA, Bigelow GE. 2011. Induction of opioid-dependent individuals onto buprenorphine and buprenorphine/naloxone soluble-films. Clin Pharmacol Ther 89: 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Bisaga A, Pavlicova M, Carpenter KM, Choi CJ, et al. 2019. A Randomized Trial Comparing Extended-Release Injectable Suspension and Oral Naltrexone, Both Combined With Behavioral Therapy, for the Treatment of Opioid Use Disorder. Am J Psychiatry 176: 129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Conry JM. 2006. Role of buprenorphine in the management of heroin addiction. Ann Pharmacother 40: 501–5. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM. 1997. mu-Opioid receptors are localized to extrasynaptic plasma membranes of GABAergic neurons and their targets in the rat nucleus accumbens. J Neurosci 17: 2585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Katz JL. 2009. Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol 57: 253–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taracha E, Chrapusta SJ, Lehner M, Skorzewska A, Maciejak P, et al. 2008. Morphine and methadone pre-exposures differently modify brain regional Fos protein expression and locomotor activity responses to morphine challenge in the rat. Drug Alcohol Depend 97: 21–32. [DOI] [PubMed] [Google Scholar]
- Termorshuizen F, Krol A, Prins M, Geskus R, van den Brink W, van Ameijden EJ. 2005. Prediction of relapse to frequent heroin use and the role of methadone prescription: an analysis of the Amsterdam Cohort Study among drug users. Drug Alcohol Depend 79: 231–40. [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Ho CP, Cao J, Vendruscolo JCM, Schmeichel BE, et al. 2018. Atypical dopamine transporter inhibitors attenuate compulsive-like methamphetamine self-administration in rats. Neuropharmacology 131: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp E, Yassen A, Dahan A. 2007. Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf 6: 125–32. [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. 2010. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev 30: 155–66. [DOI] [PubMed] [Google Scholar]
- Velazquez-Sanchez C, Garcia-Verdugo JM, Murga J, Canales JJ. 2013. The atypical dopamine transport inhibitor, JHW 007, prevents amphetamine-induced sensitization and synaptic reorganization within the nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry 44: 73–80. [DOI] [PubMed] [Google Scholar]
- Vicente-Sanchez A, Dripps IJ, Tipton AF, Akbari H, Akbari A, et al. 2018. Tolerance to high-internalizing delta opioid receptor agonist is critically mediated by arrestin 2. Br J Pharmacol 175: 3050–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volavka J, Verebey K, Resnick R, Mule S. 1978. Methadone dose, plasma level, and cross-tolerance to heroin in man. J Nerv Ment Dis 166: 104–9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, et al. 1995. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 52: 456–63. [DOI] [PubMed] [Google Scholar]
- Wager TT, Chandrasekaran RY, Hou X, Troutman MD, Verhoest PR, et al. 2010a. Defining desirable central nervous system drug space through the alignment of molecular properties, in vitro ADME, and safety attributes. ACS Chem Neurosci 1: 420–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TT, Hou X, Verhoest PR, Villalobos A. 2010b. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem Neurosci 1: 435–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TT, Hou X, Verhoest PR, Villalobos A. 2016. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem Neurosci 7: 767–75. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Comer SD, Lofwall MR, Vince B, Levy-Cooperman N, Kelsh D, Coe MA, Jones JD, Nuzzo PA, Tiberg F, Sheldon B, Kim S. Effect of buprenorphine weekly depot (CAM2038) and hydromorphone blockade in individuals with opioid use disorder: a randomized clinical trial. JAMA Psychiatry 74: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Haberny KA, Bigelow GE. 2000. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology (Berl) 150: 361–73. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang H, Zhao M, Li J, Gray F, et al. 2018. Treatment of opioid dependence with buprenorphine/naloxone sublingual tablets: A phase 3 randomized, double-blind, placebo-controlled trial. Asia Pac Psychiatry: e12344. [DOI] [PubMed] [Google Scholar]
- Werner TE, Smith SG, Davis WM. 1976. A dose-response comparison between methadone and morphine self-administration. Psychopharmacologia 47: 209–11. [DOI] [PubMed] [Google Scholar]
- Wieneke H, Conrads H, Wolstein J, Breuckmann F, Gastpar M, et al. 2009. Levo-alpha-acetylmethadol (LAAM) induced QTc-prolongation - results from a controlled clinical trial. Eur J Med Res 14: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. 2008. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14: 169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Saville K, Hamreus K. 2012. Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility. Ther Clin Risk Manag 8: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Joseph H. 2018. From Narcotic to Normalizer: The Misperception of Methadone Treatment and the Persistence of Prejudice and Bias. Subst Use Misuse 53: 323–29. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). 2018. Management of substance abuse: Opiates. April 18. 2018. http://www.who.int/substance_abuse/facts/opiates/en/
- Xi ZX, Gardner EL. 2007. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev 13: 240–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. 2008. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev 1: 303–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Song R, Li X, Lu GY, Peng XQ, et al. 2017. CTDP-32476: A Promising Agonist Therapy for Treatment of Cocaine Addiction. Neuropsychopharmacology 42: 682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. 2000. Increased mesolimbic GABA concentration blocks heroin self-administration in the rat. J Pharmacol Exp Ther 294: 613–9. [PubMed] [Google Scholar]
- Yanagita T, Katoh S, Wakasa Y, Oinuma N. 1982. Dependence potential of buprenorphine studied in rhesus monkeys. NIDA Res Monogr 41: 208–14. [PubMed] [Google Scholar]
- You ZB, Bi GH, Galaj E, Kumar V, Cao JJ, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, Newman AH 2018. Dopamine D¬3R antagonist VK4–116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Under review [DOI] [PMC free article] [PubMed]
- You ZB, Gao JT, Bi GH, He Y, Boateng C, et al. 2017. The novel dopamine D3 receptor antagonists/partial agonists CAB2–015 and BAK4–54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats. Neuropharmacology 126: 190–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaks A, Fink M, Freedman AM. 1971. Duration of methadone induced cross-tolerance to heroin. Br J Addict Alcohol Other Drugs 66: 205–8. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Scaglione A, Keighron JD, Giancola JB, Lin SC, et al. 2018. Pharmacological classification of centrally acting drugs using EEG in freely moving rats: An old tool to identify new atypical dopamine uptake inhibitors. Neuropharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Ikemoto S, Zadina JE, Wise RA. 2002. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci 22: 7225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Yang HJ, He Y, Xue G, et al. 2017. The Novel Modafinil Analog, JJC8–016, as a Potential Cocaine Abuse Pharmacotherapeutic. Neuropsychopharmacology 42: 1871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Landthaler M, Schlussman SD, Yuferov V, Ho A, et al. 2009. Mu opioid receptor knockdown in the substantia nigra/ventral tegmental area by synthetic small interfering RNA blocks the rewarding and locomotor effects of heroin. Neuroscience 158: 474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, et al. 2009. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther 329: 738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]