Abstract
Global climatic fluctuations and the increasing population have been responsible for the decline in the crop productivity. The chemical fertilizers, pesticides, and suitable genetic resources are commonly used for improving the crop yield. Magnetic field (MF) therapy for plants and animals has been found to be an effective and emerging tool to control diseases and increase tolerance against the adverse environment. Very limited studies have been attempted to determine the role of MF on plant tolerance against various stress conditions. This review aims to highlight the mitigating effect of MF on plants against abiotic and biotic stresses. MF interacts with seeds and plants and accelerates metabolism, which leads to an improved germination. The primary and secondary metabolites, enzyme activities, uptake of nutrient and water are reprogrammed to stimulate the plant growth and yield under favorable conditions. During adverse conditions of abiotic stress such as drought, salt, heavy metal contamination in soil, MF mitigates the stress effects by increasing antioxidants and reducing oxidative stress in plants. The stunted plant growth under different light and temperature conditions can be overcome by the exposure to MF. An MF treatment lowers the disease index of plants due to the modulation of calcium signaling, and proline and polyamines pathways. This review explores the basic and recent information about the impact of MF on plant survival against the adverse environment and emphasizes that thorough research is required to elucidate the mechanism of its interaction to protect the plants from biotic and abiotic stresses.
Keywords: Diseases, Drought, Heavy metals, Magnetic field, Pant growth, Salt
Introduction
The earth is a giant magnet and its geomagnetic field (GMF) has a huge impact on the productivity of crops. Specifically, the electromagnetic radiations coming from the sun stimulate the growth and development of plants through the process of photosynthesis. The other possibility to increase plant growth could be a alteration in electrostatic balance of the plant system at the cell membrane level, as it is the primary of plant growth. The GMF can influence basic biological functions such as rhythmicity (Wever 1968), orientation (Brown 1962), and development (Asashima et al. 1991). The effects of the magnetic field (MF) on plants, fungi, and microbes can be elucidated by ion-cyclotron-resonance (ICR) and the radical-pair model. These two mechanisms also play an essential role in the magneto-reception of organisms.
Many scientists have proposed various theories about the biological effects of MF, these are as follows: a moving electric charge generates an MF around it (like an electron, ion or polarized particle). The organic material, constituting living organisms, has a polar structure due to various polarized chemical bonds, which may be linked to water molecules and dissociated mineral salts conferring magnetic properties (Chepts et al. 1985). The hypothetical interaction of weak magnetic field (WMF) and the living organism has been proposed by Binhi (2001). The experimental analysis of electromagnetic fields (EMF) applied to biological systems has gained a rapid interest in the past few years. The applications of MF are being explored in several areas, particularly in the agricultural science. The effects of MF on seed germination, biochemical, hormonal changes, plant growth, and yield have been subject to several investigations. The enhancement of growth in crops under precise magnetic conditions has been confirmed but a systematic and extensive study is still necessary to delineate the mechanisms of magnetic action in cells and tissues. Although attempts have been made to understand the mechanisms of action of extremely low-frequency EMFs in biological systems, still more detailed studies need to be undertaken (Belyavskaya 2004).
An application of 20–30 mT of MF on crop plants revealed an enhancement in their growth. The plant cells contain ferritin and each cell has about 4500 iron atoms involved in growth and metabolism. The magnetic rotator moment of ultimate iron atoms creates an external MF, and collectively generates oscillations, which generate energy and finally re-position the atoms in the direction of MF. This increases the temperature in plants, which depends upon the duration and the frequency of MF treatment (Vaezzadeh et al. 2006). WMF modulates cryptochrome and phytochrome mediated plant responses in plants (Dhiman and Galland 2018). Very limited information is available on the molecular basis and the function of the putative WMF receptors and their activation by physiological signals, therefore their involvement in directing the overall response in different plant organs is yet to be determined.
Savostin (1930) first reported a two-fold increase in wheat seedling elongation under MF. Murphy (1942) observed the positive effects of MF on seed germination. Audus (1960) and Pittman (1965) also studied a strong magnetotropic effect on root development. MF influences the normal tendency of Fe and Co atoms and utilizes their energies to continue the translocation of microelements in root meristems, which leads to an increased plant growth (Mericle et al. 1964). The different dosage of MF alters the root biomass, stems girth, and leaf size. Further, the root growth is more sensitive than shoots to MF (Kato 1988; Kato et al. 1989; Smith et al. 1993). The pretreatment of seeds by MF resulted in seedling growth, seed vigor, and increased crop yield (Pieturszewski 1993). MF accelerates growth by triggering the protein synthesis and activates the root tropism by altering the intracellular movement of amyloplasts in the statocyst of root cap cells (Kuznetsov et al. 1999; Pieturszewski 1999). A positive effect on seed germination, uptake of nutrients, flowering, and crop yield can be achieved by applying MF (Duarte-Diaz et al. 1997; Samy 1998; Souza-Torres et al. 1999). MF treatments also affect the plant metabolisms that involve free radicals and stimulate the activity of proteins and enzymes to enhance seed vigor (Morar et al. 1993).
The effects of continuous as well as pulsed MF on plant growth and development have been investigated in a large number of plant species (Yano et al. 2001). Aladjadjiyan (2002) revealed that the exposure of MF (150 mT) stimulated shoot development which led to an increase in the germination, fresh weight, and shoot length in maize. The mechanism of action of MF on plant growth promotion is still not very clearly understood, therefore an optimal external EMF may accelerate the plant growth, especially seed germination (Esitken and Turan 2004). Yinan et al. (2005) observed a positive effect of MF pretreatment on cucumber seedlings by stimulating seedling growth and development. The promotion of seed germination and the growth of plants depend on the magnetic flux densities, frequencies, and pretreatment of the plant material (Davies 1996).
Several harsh environmental conditions such as drought, salinity, low or high temperatures, flood, pollution, radiation, and diseases are the important stress factors that adversely affect the growth, metabolism, and the yield of plants and thereby limit the productivity of crops (Lawlor 2002).The productivity of plants can be increased by the application of plant growth promoting substances, microbial inoculation to the soil, organic and inorganic manure and several other non-conventional approaches such as plant breeding and genetic engineering (Radhakrishnan and Lee 2013; Radhakrishnan et al. 2014; Radhakrishnan et al. 2015). The application of MF is a novel approach to improve plant growth and the overall productivity (Radhakrishnan and Ranjitha-Kumari 2012; Maffei 2014).
Plant growth promoting effect of MF on plant physiology under favorable condition: seed germination
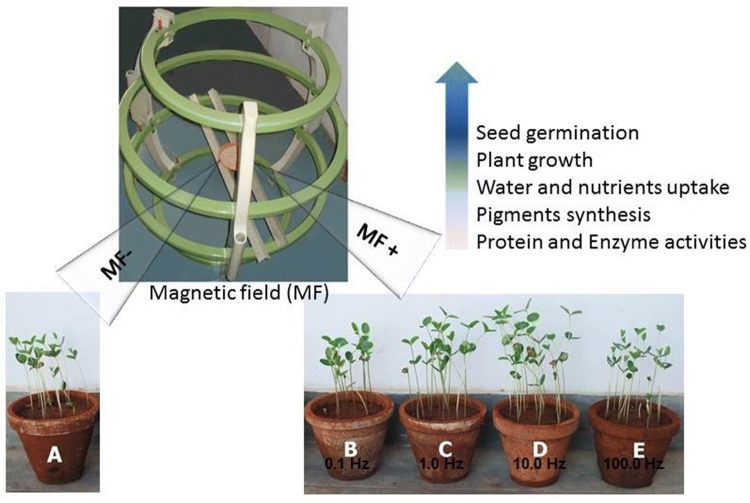
Many researchers reported an increase in seed germination under MF exposure. MF stimulates the initial growth stages and early sprouting of seeds (Carbonell et al. 2000). Recently, Radhakrishnan and Ranjitha-Kumari (2012) observed an increased rate of seed germination in soybean under pulsed MF. Morar et al. (1993) also reported that MF influences the free radical formation and stimulates the activity of proteins and enzymes to enhance the seed vigor. The paramagnetic properties of plastid may be responsible for the enhanced seed vigor. MF increases the energy in plants and disperses this energy to biomolecules, which in turn stimulates the metabolism to enhance the seed germination. A metabolically active plant cell contains free radicals that play a vital role in electron transfer and the kinetics of biochemical reactions. These free radicals possess non-paired electrons with magnetic activities that can be oriented under an external MF. The microwave energy is absorbed as a result of the interaction between the external MF and the magnetic action of unpaired electrons. Finally, this energy is converted into a chemical form and accelerates the fundamental processes in seeds (Commoner et al. 1954). The overall effects of MF on crop plants are summarized in Fig. 1 and Table 1.
Fig. 1.
Effect of magnetic field on functional changes in plants for their growth improvement. Magnetic field (MF+) treatment triggers seed germination, plant growth, water and nutrient uptake, pigments synthesis, protein and enzyme activities as compared to the control (MF−)
Table 1.
MF induced biochemical and physiological changes improve crop production at different environmental conditions
| MF treatments | Crops | Plant functions | References |
|---|---|---|---|
| Plant growth promoting activity | |||
| 150 mT- 250 mT of SMF | Oryza sativa L. | Improve seed germination | Carbonell et al. (2000) |
| 1500 nTat 10.0 Hz of PMF | Glycine max L. |
Increase plant height, biomass, number of leaves, pods, seeds, weight of seeds, proteins, β-amylase, acid phosphatase, polyphenol oxidase, catalase, Fe, Cu, Mn, Zn, Mg, K and Na contents Decrease α-amylase, alkaline phosphatase, protease and nitrate reductase activities and Ca content |
Radhakrishnan and Ranjitha-Kumari (2012) |
| 500 gauss -1550 gauss MF | Lycopersicum esculentum L. | Increase branches of shoots | Dayal and Singh (1986) |
| 125 mT-250 mT SMF | Zea mays L. | Faster seed germination, increase length and biomass of plants | Florez et al. (2007) |
| 7 T EMF | Zea mays L | Accelerate meristem activity and cell division in roots | Bitonti et al. (2006) |
| 20 µT at 16 2/3 Hz SSMF | Helianthus annuusL. and Triticum aestivumL. | Increase germination rate and growth of plants | Fischer et al. (2004) |
| 100 mT -170 mT SSMF | Lycopersicum esculentum L. | Enhance plant growth, pigments synthesis and fruit yield | Souza-Torres et al. (1999, 2006) |
| 1500 nT at 100 Hz SSMF | Gossypium species | Increase germination percentage, growth, pigments synthesis and yield | Leelapriya et al. (2003) |
| 0.096 T-0.384 T AMF | Fragaria × ananassa cv. camarosa |
Increase fruit yield, N, K, Ca, Mg, Cu, Fe, Mn, Na and Zn in plants Reduce P and S content |
Esitken and Turan (2004) |
| 403 A/m WMF | Allium cepa L. | Increase chlorophylls, proteins and enzyme activities in plants | Novitsky et al. (2001) |
| Drought tolerance | |||
| Magnetic funnel | Lycopersicum esculentum L. | Stimulate plant growth, cambium differentiation activity, thickness of mesophyll tissue, water uptake, proline concentration and photosynthetic pigments | Selim and El-Nady (2011) |
| 100 mT-150 mT EMF | Zea mays L. | Improve plant growth, chlorophyll, photosynthesis rate, transpiration rate, stomatal conductance, substomatal CO2 concentration, photochemical quenching and nonphotochemical quenching reactions | Javed et al. (2011) |
| 100 mT-200 mT SMF | Zea mays L. |
Increase plant growth, leaf water potential, turgor potential, water content, photosynthesis and stomatal conductance Decrease H2O2, POX,CAT and SOD activities |
Anand et al. (2012) |
| 2.9 mT-4.7 mT SMF | Triticum aestivum L. |
Increase chlorophyll and carotenoids Decrease SOD, POX, APX and CAT activities |
Sen and Alikamanoglu (2014) |
| Salinity tolerance | |||
| 4 mT-7mT SMF | Triticum aestivum L. and Phaseolus vulgaris L. | Increase seed germination, biomass and growth of plants | Cakmak et al. (2010) |
| 1500 nT at 0.1,1.0,10.0 and 100.0 Hz PMF | Glycine max L. | Enhance the frequency of shoot and root regeneration, length and number of roots | Radhakrishnan and Ranjitha-Kumari (2013) |
| 200 mT SMF | Glycine max L. and Zea mays L. | Increase seed germination, seedling growth, α-amylase, protease and free-radicals | Kataria et al. (2017) |
| 200 mT SMF | Glycine max L. | Enhance root nodules, biomass, yield, pigments synthesis, photosynthetic rate, stomatal conductance, transpiration, internal CO2 concentration, carbon metabolism, nitrogen metabolism, leghemoglobin and hemechrome content in root nodules | Baghel et al. (2016) |
| 1500 nT at 1.0 Hz PMF | Glycine max L. |
Increase callus biomass, sugars, proteins, phenols, flavonoids, flavonoles, alkaloids and saponins Decrease lipid peroxidation and CAT activity |
Radhakrishnan et al. (2012) |
| Heavy metal tolerance | |||
| 600 mT MF | Vigna radiata L. |
Increase plant growth, photosynthesis, nitric oxide synthase and nitric oxide Decrease lipid peroxidation, H2O2, O2−and electrolyte leakage |
Chen et al. (2011) |
| Temperature and light stress tolerance | |||
| 150 mT MF | Zea maysL. |
Increase chilling tolerance, plant growth, chlorophyll, total phenolics, gaseous exchange, seed protein, and oil Reduce membrane permeability |
Afzal et al. (2015) |
| 400 A/m WMF | Raphanus sativus L. | Increase polar lipids at light and chilling stresses | Novitskaya et al. (2010) |
| Biotic stress resistance | |||
| 10 kHz WMF | Citrus aurantifoliaL. |
Increase biomass of leaves, MDA, proline and protein content Decrease H2O2 and carbohydrates |
Abdollahi et al. (2012) |
| -17 to 13 µT (SMF) + 10 Hz at 25.6 to 28.9 µT (SSMT) | Nicotiana tabacumL. |
Decrease number and area of lesions Increase ODC and PAL activities |
Trebbi et al. (2007) |
AMF alternative magnetic field, EMF electro magnetic field, MF magnetic field, PMF pulsed magnetic field, SMF static magnetic field, SSMF sinusoidal magnetic field, T tesla
Vegetative growth phase
MF positively influences the growth of plants by increasing shoot and root length (Dayal and Singh 1986; Florez et al. 2007). Root growth depends upon the cell division in the root meristems and subsequent differentiation and elongation of the descendant cells (Beemster and Baskin 1998). The root cap cells were notably larger and the metaxylem cells became significantly longer starting from the quiescent center to periphery in MF treated plants. The induction of metaxylem cells by EMF is an important component of the increase in the rate of root elongation (Bitonti et al. 2006). MF exposure to seeds accelerates their growth, activates protein formation and the root growth (Pieturszewski 1999). In an experiment, sunflower seedlings exposed to MF showed a substantial increase in the shoot and root fresh weight (Fischer et al. 2004). MF treated plants also showed, at the vegetative stage, a significantly larger leaf area and higher leaf dry weight than the controls. This effect may be attributed to the increased photosynthetic rates due to the better perception of light and nutrients available for vegetative growth (Souza-Torres et al. 1999, 2006).
Reproductive growth phase
Very limited studies have documented the effect of MF on reproductive development in crops. Matsuda et al. (1993) reported that MF enhanced the yield in strawberry. Similar effects were also witnessed for flax, buckwheat, pea, wheat, tomato, pepper, soybean and cotton by Gubbels (1982), Grabrielian (1996), Phirke and Umbarkar (1998), Pieturszewski (1993), Ogolnej et al. (2002), Vasilevski (2003), Leelapriya et al. (2003) and Esitken and Turan (2004), respectively, and it was suggested that the enhancement in growth and yield of the tomato plants may be attributed to an MF-induced energetic excitement of cellular proteins and carbohydrates and/or water inside the dry seeds.
Endogenous bio-molecular changes
The plant growth is regulated by various biochemical processes. MF may cause changes in one or more parameters that affect the enzymatic activity, the transportation of metabolites, growth regulators, ions, and water, thereby regulating the overall plant growth (Leelapriya et al. 2003). The transport of carbohydrate and plant growth hormones from the site of synthesis to the distant growth zones (fruits) could be stimulated at lower MF intensity (Esitken and Turan 2004). Hirano et al. (1998) also observed that the increase in MF intensity from 0.0005 to 0.1 T showed a positive effect on the growth and photosynthesis in Spirulina platensis. MF showed an increase in the chlorophyll content in onion (Novitsky et al. 2001), cotton (Leelapriya et al. 2003), potato and wild Solanum species (Tican et al. 2005).
The GMF may affect a variety of enzymes in many living organisms. The activity of Ca2+/calmodulin dependent cyclic nucleotide phosphodiesterase at 20 μT (Liboff et al. 2003) and cytochrome C oxidase at 50 Hz (Nossol et al. 1993) were altered by MF. It is well known that MF can influence biological processes involving photochemical reactions (Boxer et al. 1982), the biological effects of MF are still debatable (Azanza and Del-Moral 1994; Grissom 1995). However, the mechanisms of some of the alterations in enzyme activity during MF exposure have been identified (Grissom 1995). MF effects are exerted by the inter-conversion of singlet and triplet rotatory states of the radical pair of bio-molecules (Salikhov et al. 1984). Some enzyme reactions are sensitive and their kinetics are affected by MF.
MF treatments are expected to enhance seed vigor by influencing activity of proteins and enzymes and the biochemical processes that involve free radicals (Jia-Ming 1988; Kurinobu and Okazaki 1995; Morar et al. 1993), auxin content (Mitrov et al. 1988), nutrient (Duarte-Diaz et al. 1997), and water uptake (Reina et al. 2001). Auxin is a signaling molecule, present in root apices, which manages the activities of adjacent cells via electrochemical signaling. The transport of auxin in plants is associated with environmental factors such as gravity, MF, and light (Baluska et al. 2005). MF increases the auxin content as well as enzymes activities that regulate the elongation of the plant cell wall (Mitrov et al. 1988). The studies on the influence of MF on the modifications in protein profile and enzyme activity are scarce and no information is available on its chemical constituents so far (Novitsky et al. 2001). The total protein contents of onion leaves were increased in MF treated plants. MF at different levels altered distribution of polypeptide in eukaryotic and bacterial cells (Blank et al. 1994; Goodman et al. 1994; Radhakrishnan and Ranjitha-Kumari 2012).
Xiao-ju and Guo (1999) found an increase in the activity of catalase and peroxidase enzymes in tomato seeds pretreated with MF. The amplitude, gradient and high frequency of the non-uniform MF together cause a combined effect on dry seeds and induced the changes in living matter and was called as “ponderomotive effects”. This effect reprograms the enzymatic activity, transport of the metabolites including growth regulators, and also the transport of charged solutes possibly through “Hall” effect for plant growth improvement (Balcavage et al. 1996; Souza-Torres et al. 2006). The stationary MF (150 and 200 mT) stimulates reactive oxygen species in germinating seeds to enhance plant growth (Shine et al. 2012). The changes in amylase and nitrate reductase activities were detected in germinating seeds treated with different levels of EMF (Levedev et al. 1975; Bathnagar and Deb 1978) and many authors have reported the effect of static MF on the metabolism and growth of different plants (Kato 1988; Kato et al. 1989; Peteiro-Cartelle and Cabezas-Cerato 1989). An extremely low MF (0.2–0.3μT) stimulates the activity of Na and K-ATPases (Blank and Soo 1996), whereas a weak and moderate MF influences the redox activity of cytochrome C oxidase (Nossol et al. 1993). A treatment of 30 mT increased the esterase activity in Triticum aestivum (Aksenov et al. 2000) and 1 mT influenced the activity of horseradish peroxidase (Portaccio et al. 2005). Strong MF (6 T) reduced L-glutamate dehydrogenase and catalase activity (Haberditzl 1967), but 2 T substantially enhanced the activity of carboxydismutase in Spinacia oleracea (Akoyunoglou 1964). The strong MF also enhanced the activity of trypsin (Cook and Smith 1964) and ornithine decarboxylase (Mullins et al. 1999); so that the changes in the enzyme activity may depend on strength, the frequency of the MF and the plant species.
A study on tomato plants showed that the irrigation water exposed to MF increases the nutrient uptake in plants (Duarte-Diaz et al. 1997). Radhakrishnan and Ranjitha-Kumari (2012) reported that the MF increases the Fe, Cu, Mn, Zn, Mg, K, and Na content and decreases the Ca content in soybean seedlings. Another study showed that the levels of N, K, Ca, Mg, Fe, Mn, and Zn significantly increased but Cu and Na remained unchanged in the leaves of MF treated strawberry plants (Esitken and Turan 2004). MF affects the membranes and Ca2+ signaling in plant cells, and many magnetic effects in living organisms are probably due to the alterations in membrane-associated Ca2+ flux (Galland and Pazur 2005). Na-channels are less affected than Ca2+ channels (Rosen 2003) and due to the changes of Ca2+ channels, the Ca content might be reduced in MF treated plants. However, MF treatment in seeds induces the changes in protein and lipid profile in harvested seeds (Radhakrishnan 2018).
Mitigation effect of MF on crops against unfavorable environments
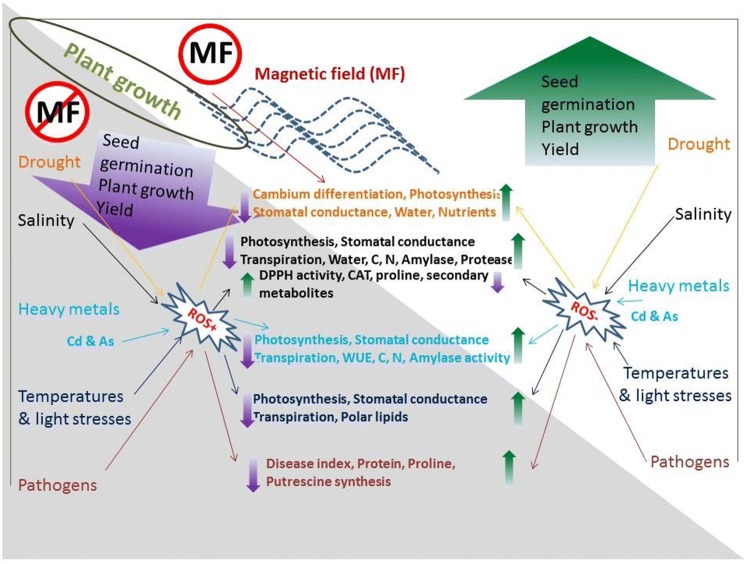
The adverse environmental conditions including drought, salinity and heavy metal accumulation in soil, and light, temperature, insects, and pathogens affect the growth and yield of agricultural crops. MF induced changes in the metabolism of plants during those unfavorable environments are given in Fig. 2 and Table 1.
Fig. 2.
Magnetic field induced metabolic alteration and tolerance of plants against adverse environmental conditions. Adverse stress conditions including drought, salinity, heavy metals, high/low temperatures, high/low light and pathogens infections reduce seed germination, plant growth and yield due to the molecular and physiological changes in plants. MF treatment reduces the ROS production in plants at stress conditions, and enhances cambium differentiation, photosynthesis, stomatal conductance, water and nutrient uptake in drought affected plants. MF induced salinity tolerance is achieved by an increase of photosynthesis, stomatal conductance, transpiration, water uptake, carbon, nitrogen, amylase and protease activities and while reducing the DPPH activity, CAT, proline and some secondary metabolites. Cadmium (Cd) and Arsenic (As) affected plants are possibly recovered by promoting photosynthesis, stomatal conductance, transpiration, water use efficiency (WUE), carbon, nitrogen, amylase activities due to the treatment of MF. Whereas plants that suffered at temperature and light stresses are mitigated by MF treatment, which accelerate photosynthesis, stomatal conductance, transpiration and polar lipids. In addition, MF recovers the pathogen infected plants by increasing protein, proline, putrescine synthesis and disease index
Drought
Drought is a very serious problem in agriculture. Few researchers have studied the application of MF to overcome the detrimental effect of drought stress (Vashisth and Nagarajan 2010; Javed et al. 2011; Selim and El-Nady 2011; Anand et al. 2012; Karimi et al. 2012; Sen and Alikamanoglu 2014). The drought-induced reduction in plant growth can be mitigated by the application of MF as it stimulates cambium differentiation activity to form more xylem and phloem tissues for improving the absorption and transport of water and nutrients to enhance plant growth under drought condition (Selim and El-Nady 2011). The plant cell membrane permeability and free water flow are increased in MF pretreated seeds (Bondarenko et al. 1996). Owing to the variability in ionic flow through the cell membrane, the osmotic potential is changed under drought condition. In MF treated plants, Ca2+ enhancement is found to play a significant role in plant drought tolerance, it prevents the impairment of plasma membrane and photosynthetic apparatus and regulates the hormonal metabolism in drought affected plants (Blum 1993; Song et al. 2008, Selim and El-Nady 2011). MF enhanced the chlorophyll and carotenoid synthesis in leaves, which might be due to the increase in proline and GA3, which trigger the accumulation of Mg2+ for chlorophyll synthesis (Shaddad 1990) and K+ to increase the number of chloroplasts (Garcia-Reina and Arza-Pascual 2001). This might eventually lead to increase in the thickness of mesophyll tissue (Selim and El-Nady 2011). In addition, it also increases stomatal conductance, sub-stomatal CO2 concentration, and photochemical and non-photochemical reducing reactions to moderate the effect of drought in plants (Javed et al. 2011). MF prevents oxidative stress damage in drought affected plants by reducing H2O2, SOD, POD and CAT activities and, the metabolic energy used for scavenging the free radicals and ultimately improves the plant growth (Anand et al. 2012; Sen and Alikamanoglu 2014).
Soil salinity
About one-third of the agricultural land is affected by salinity (Flowers and Yeo 1995) due to in appropriate irrigation practices and natural factors (Chinnusamy and Zhu 2003). A high salt content in soil disrupts the homeostasis in water potential and ion distribution in plant cells (Zhu 2001; Munns et al. 2002). An excessive accumulation of Na+ and Cl–ions changes the protein structures, which lead to the loss of turgidity of the cell (Chinnusamy and Zhu 2003). MF pretreatment enhances the water absorption in seeds and promotes the seed germination and growth of plants in saline or non-saline soil conditions (Cakmak et al. 2010; Radhakrishnan and Ranjitha-Kumari 2013; Karimi et al. 2017). In addition, α-amylase and protease activities are also increased in MF treated seeds due to the faster utilization of reserve materials required for a higher rate of germination (Kataria et al. 2017). MF treated seeds absorb water faster due to the electrophysiological changes in cells (Reina et al. 2001) and may help to alleviate the salt stress. Nevertheless, the photosynthetic rate, stomatal conductance, transpiration, and internal CO2 concentrations were enhanced in salt affected plants pretreated with MF (Baghel et al. 2016; Rathod and Anand 2016). To achieve salt tolerance, plant cells have evolved several biochemical and physiological pathways, which include the exclusion of Na+ and their trans-localization into vacuoles, and also the accumulation of compatible solutes such as proline, glycine, betaine, and polyols (Kameli and Losel 1996; Hasegawa et al. 2000; Chinnusamy and Zhu 2003; Parida and Das 2005). However, the precise mechanism underlying these effects has not yet been fully understood because salinity tolerance is a multigenic trait (Parida and Das 2005). MF exposure increases the sugar and protein content in salt affected calli to overcome the stress effects (Radhakrishnan et al. 2012). These compounds accumulate in high volumes in the cytoplasm of stressed cells without interfering with other macromolecules and act as osmoprotectants (Yancey 1994). It has been shown that proline plays a key role in stabilizing the cellular membrane and proteins (Rudolph et al. 1986; Yancey 1994). The higher proline accumulation in roots may be due to the increased rate of inhibition of proline dehydrogenase and proline oxidase (Veeranjaneyullu and Ranjitha-Kumari 1989). The production, along with accumulation, of proline in plant tissue during salt stress is an adaptive response and it has been proposed as a stress-related metabolic marker (Burton 1991). The osmotic potential in the cytoplasm is adjusted by proline which acts as a compatible solute (Bartels and Sunkar 2006). It signals protein synthesis immediately after the salt stress that protects the plasma membrane (Santoro et al. 1992). Ahmad and Wyn Jones (1979) reported that during recovery period, tissue rehydration is associated with decline. Hence, subsequent to relief of stress, it acts as reserve of organic nitrogen for maintaining amino acid and protein synthesis (Trotel et al. 1996; Sairam and Tygai 2004). MF ameliorates the salt effects by reducing the overproduction of proline (Radhakrishnan et al. 2012).
According to Mittler (2002) high level of H2O2 accelerates the Haber–Weiss reaction and results in OH· formation and consequently lipid peroxidation. Several studies showed that the lipid peroxidation activity is enhanced during high salinity (Hernandez et al. 2000; Davenport et al. 2003). MF also increased lipid peroxidation in tobacco cell suspension cultures (Sahebjamei et al. 2007). On the contrary, during a salt stress condition, MF pretreatment resulted in the decline of lipid peroxidation in soybean callus culture (Radhakrishnan et al. 2012). Salt stress increases catalase (CAT) in plants (Manchandia et al. 1999), but MF pretreated seeds showed resistance toward the salinity and decreased the CAT and DPPH scavenging activity due to the reduction of oxidative stress (Radhakrishnan et al. 2012; Roshandel and Azimian 2015).
Alkaloids, saponins, flavonoids, flavones, and flavonols are generally increased in salt affected cells, while MF exposure reduced the accumulation of these secondary metabolites and alleviated the salt stress (Radhakrishnan et al. 2012). Saponins are glycosides occurring commonly in plants, which are derived from tri-terpenoids and exhibit a wide range of biological functions (Osborn 2003). The decrease in saponins at high Cu concentration provides an intrinsic defense to resist Cu-induced oxidative damage in Panax ginseng (Ali et al. 2006). Russo et al. (2002) reported that flavones and flavonols have antioxidant property, and isoflavones also possess antioxidant and antifungal activities that protect the plant against insect attack (Burden and Norris 1992). The salt stress induces the accumulation of isoflavones such as genistein and daidzein, while MF pretreatment resulted in the lowering of their levels. The high amount of total polyphenols increases the antioxidant potential in plants and MF results in the enhancement of total polyphenol in callus tissue grown under saline condition (Radhakrishnan et al. 2012).
Heavy metals
The heavy metals from the industry, fertilizers, and pesticides enter the water bodies and soil and subsequently reach to humans through the food chain (Wagner 1993; Pinto et al. 2014). The excessive deposition of heavy metals in soil limits the plant productivity. Recently, Chen et al. (2011) and Flores-Tavizon et al. (2012) reported that the toxic effects of cadmium (Cd) and arsenic (As) in plants were mitigated by an MF exposure. Due to the heavy metal toxicity, plants produce reactive oxygen species (ROS), which damage the cellular membranes and inhibit the photosynthesis and other metabolic processes (Prasad 1995). MF triggers nitric oxide (NO) signaling, which activates cell division, photosynthesis, and growth of Cd affected plants. The mung-bean seedlings treated with MF (600 mT) showed a lower level of ROS such as H2O2, O2−, and malondialdehyde (MDA) but a higher level of total chlorophyll, photosynthetic rate, stomatal conductance, transpiration rate, intercellular CO2 concentration, and water use efficiency in Cd stress conditions. In addition, MF increased the C and N concentrations in Cd-stressed plants (Chen et al. 2011). Another toxic metal, As is a non-essential metal for plant growth and inhibits enzyme activities in plants (Liu et al. 2005). MF pretreatment increased resistance towards As toxicity in the plants by the regulation of ionic flow in plant cell membranes (Galland and Pazur 2005). The seed germination, growth, amylolytic activity, and As uptake was increased in As stressed plants treated with MF (Flores-Tavizon et al. 2012).
Temperature and light stresses
Crop productivity is affected by a wide range of temperature and light regimes. MF exposure alleviates the inhibitory effect of heat shock by eliciting heat shock proteins under thermal stress (Goodman and Blank 1998; Ruzic and Jerman 2002). Low temperature (cold) stress limits the yield and geographical distribution of several crops (Gai et al. 2008). Afzal et al. (2015) proved that chilling stress reduces the seed germination in maize, but MF treatment stabilizes the membrane permeability and regulates ion transport in stressed seeds to alleviate the chilling stress. In addition, MF accelerates the primary metabolic process such as photosynthesis, transpiration, and stomatal conductance during chilling injury in maize plants. The increased synthesis of chlorophylls and phenolics due to the effect of MF in stressed plants could be the reason for averting the ROS production. Similarly, the harvest index, weight, yield, and protein content in grains were significantly higher but the oil contents were lower in MF treated plants than untreated plants.
The role of MF against thermal stress varies under light or dark conditions. At low temperature, cell membranes change the lipid composition by promoting the conversion of unsaturated fatty acids to saturated ones (Kreps 1981). The fatty acids, especially erucic acid, are enhanced by 25% in light and dark grown plants pretreated with MF at 20 °C and declined at 10 °C in the light (Novitskaya et al. 2010). At 20 °C, MF decreased the synthesis of polar lipids (18%) in radish seedlings grown under thelight but it was about 80% higher than non-treated seedlings. MF exposure increased the polar lipid content during chilling (10 °C) temperature and light but had no effects in the plants grown in dark plants. The breakdown process of lipids in germinating seeds is a critical element that provides energy for growing cells (Bewley and Black 1994). The MF treatment can modulate the lipid metabolism and synthesis in plants at the exposure of light and temperature (Novitskaya et al. 2010). The strong light enhances the singlet oxygen production in chloroplast by photosystem II (Telfer 2014) but disrupts the cellular activities and is harmful to plant growth. MF inhibits the formation of singlet oxygen, which reduces the metabolic energy available to the chloroplast (Hakala-Yatkin et al. 2011). The light wavelengths significantly influence the growth and flowering in plants, MF suppresses the flowering in white and blue light but did not affect the flowering in the red light (Xu et al. 2015).
Biotic stresses
The application of MF can reduce the detrimental effect of pathogenic microbes and increase the growth and yield of plants (Galland and Pazur 2005). For example, citrus plants intermittently exposed to 10 Hz MF showed a substantial enhancement in fresh and dry leaf weight in healthy as well as Phytoplasma aurantifolia infected plants (Abdollahi et al. 2012). It proved that MF could also increase the resistance against pathogens. Biochemical analysis revealed that the accumulation of proteins was higher but carbohydrates were lesser in infected plants treated with MF. The synthesis of proline (a protective osmolyte) is notably activated by MF thus supporting cellular structures (Resenburg et al. 1993). The biotic stress alleviation mechanism of MF was determined by reduced H2O2 production in infected plants exposed to MF. On the other hand, scavenging enzymes control the free radicals, which alter membrane integrity and increase the resistance in plants against pathogen infection. However, Trebbi et al. (2007) studied the hypersensitive response (HR) in tobacco mosaic virus infected tobacco plants during the MF exposure and proved that MF treatment decreases the number and area of lesions in the diseased plants and it also regulates the calcium (Ca2+) signaling pathway in the cell. During the HR, the Ca2+ influx into the cytosol is stimulated that increases the resistance (Baureus-Koch et al. 2003). Similarly, MF influences the polyamine pathway enzymes such as ornithine decarboxylase (ODC) and phenylalanine ammonia lyase (PAL). The ODC and PAL activities enhanced by an MF exposure during infection suggest that putrescine synthesis helps the plant withstand the biotic stress (Trebbi et al. 2007).
Conclusion
Magnetic field (MF) therapy has been claimed to be effective for human ailments. Very few studies have been undertaken to understand the positive effect of MF on crop plants during environmental stress conditions. This review explores the current knowledge and future prospects of MF-induced physiological changes in plants toward enhancing the growth and yield under favorable and adverse conditions. The application of MF accelerates the seed germination, vegetative as well as reproductive growth in plants due to an increase in energy and its distribution to biomolecules in the cell. The enhancement of water and nutrient uptake, photosynthesis, carbohydrates, protein and enzyme metabolisms would impact the promotion of plant growth and yield. Unfavorable environments such as drought, salinity, heavy metal contamination in soil, cold and/or hot conditions drastically decrease the crop productivity. MF exposed plants tolerate these adverse environments by reducing oxidative stresses. MF treatment can enhance plants drought tolerance by stimulating water and Ca2+ uptake, cell membrane permeability, cambial differentiation, pigment synthesis, stomatal conductance. Similarly, MF protects the plants against salinity by increasing water uptake, stomatal conductance, sugar, and protein synthesis, and also by regulating the antioxidants and defense metabolites. Heavy metals in soil suppress the plant growth but MF treatment alleviates these metal stresses through the increased water flow, nitrogen, carbon, endogenous NO accumulation, photosynthesis, stomatal conductance, transpiration, and cell division. In addition, the production of heat shock proteins in MF exposed plants confers protection against the hyperthermic stresses. During low temperature, MF triggers ion transport, membrane permeability, photosynthesis, stomatal conductance, and transpiration, and regulates the polar lipids and erucic acids, irrespective of the presence or absence of light conditions to enhance the plant tolerance against temperature stresses. However, a reduced area of infection in leaves showed the control of plant diseases by MF exposure and this resistance may be due to the accumulation of Ca2+, proteins, and proline in plants.
Future prospectus
The MF-induced changes in the fundamental physiological process of crop plants against adverse environmental conditions have been investigated by only few researchers. A comprehensive bio-stimulatory activity of MF in several cellular metabolisms and their subsequent effects on tissue proliferation and organization need to be elucidated to decipher the mitigation mechanism of MF and plant interaction under stress environments. The future studies are required to confirm the positive effects of MF on crop yield by answering the following: (1) Whether MF treatment influences the next generation of crop growth and yield? (2) Is there any toxicity due to the consumption of MF treated foods? (3) Does it affect the micro and macro flora of soil during plant growth? In addition, the comprehensive genomic and proteomic analyses in MF treated plants would also bridge the space between current understanding and future perspective of biological effects of the magnetic field in plants.
Acknowledgements
I thank Karpagam Academy of Higher Education, Coimbatore, India for providing financial support through Seed money project.
Compliance with ethical standards
Conflict of interest
The author has no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdollahi F, Niknam V, Ghanati F, Masroor F, Noorbakhsh SN. Biological effects ofweak electromagnetic field on healthy and infected lime (Citrus aurantifolia) trees with phytoplasma. Sci World J. 2012;2012:1–6. doi: 10.1100/2012/716929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal I, Noor MA, Bakhtavar MA, Ahmad A, Haq Z. Improvement of spring maize (Zea mays) performance through physical and physiological seed enhancements. Seed Sci Technol. 2015;43:1–12. [Google Scholar]
- Ahmad N, Wyn Jones RG. Glycinebetaine, proline and inorganic ion levels in barley seedlings following transient stress. Plant Sci Lett. 1979;15:231–237. [Google Scholar]
- Akoyunoglou G. Effect of a magnetic field on carboxydismutase. Nature. 1964;202:452–454. doi: 10.1038/202452a0. [DOI] [PubMed] [Google Scholar]
- Aksenov SI, Bulychev AATI, Turovetskii VB. Effect of a low-frequency magnetic field on esterase activity and change in pH in wheat germ during swelling of wheat seeds. Biofizika. 2000;45:737–745. [PubMed] [Google Scholar]
- Aladjadjiyan A. Study of the influence of magnetic field on some biological characteristics of Zea mays. J Cent Eur Agric. 2002;3:89–94. [Google Scholar]
- Ali MB, Hahn EJ, Paek KY. Copper-induced changes in the growth, oxidative metabolism and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep. 2006;25:1122–1132. doi: 10.1007/s00299-006-0174-x. [DOI] [PubMed] [Google Scholar]
- Anand A, Nagarajan S, Verma AP, Joshi DK, Pathak PC, Bhardwaj J. Pre-treatment of seeds with static magnetic field ameliorates soil water stress in seedlings of maize (Zea mays L.) Indian J Biochem Biophys. 2012;49(1):63–70. [PubMed] [Google Scholar]
- Asashima M, Shimada K, Pfeiffer CJ. Magnetic shielding induces early developmental abnormalities in the newt, Cynopspyrrhogaster. Bioelectromagnetics. 1991;12:215–224. doi: 10.1002/bem.2250120403. [DOI] [PubMed] [Google Scholar]
- Audus LJ. Magnetotropism: a new plant growth response. Nature. 1960;185:132–134. [Google Scholar]
- Azanza MJ, Del-Moral A. A cell membrane biochemistry and neurobiological approach to biomagnetism. Prog Neurobiol. 1994;44:517–601. doi: 10.1016/0301-0082(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Baghel L, Kataria S, Guruprasad KN. Static magnetic field treatment of seeds improves carbon and nitrogen metabolism under salinity stress in soybean. Bioelectromagnetics. 2016;37:455–470. doi: 10.1002/bem.21988. [DOI] [PubMed] [Google Scholar]
- Balcavage WX, Alvager T, Swez J, Goff CW, Fox MT, Abdullyava S, King MW. A mechanism for action of extremely low frequency electromagnetic fields on biological systems. Biochem Biophys Res Commun. 1996;222:374–378. doi: 10.1006/bbrc.1996.0751. [DOI] [PubMed] [Google Scholar]
- Baluska F, Barlow PW, Baskin TI, Chen R, Feldman L, Forde BG, Geisler M, Jernstedt J, Menzel D, Muday GK. What is apical and what is basal in plant root development? Trends Plant Sci. 2005;10:409–411. doi: 10.1016/j.tplants.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2006;24:23–28. [Google Scholar]
- Bathnagar D, Deb AR. Some aspects of pregermination exposure of wheat seeds to magnetic field II. Effect on some physiological processes. Seed Res. 1978;6:14–22. [Google Scholar]
- Baureus-Koch CLM, Sommarin M, Persson BRR, Salford LG, Eberhardt JL. Interaction between low frequencymagnetic fields and cell membranes. Bioelectromagnetics. 2003;24:395–402. doi: 10.1002/bem.10136. [DOI] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 1998;116:515–526. doi: 10.1104/pp.116.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyavskaya NA. Biological effects due to weak magnetic field on plants. Adv Space Res. 2004;34:1566–1574. doi: 10.1016/j.asr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M. Cellular events during germination and seedling growth. In: Bewley JD, Black M, editors. Seeds physiology of development andgermination. 2. New York: Plenum press; 1994. pp. 147–191. [Google Scholar]
- Binhi VN. Theoretical concepts in magnetobiology. Electromagn Biol Med. 2001;20:43–58. [Google Scholar]
- Bitonti MB, Mazzuca S, Ting T, Innocenti AM. Magnetic field affects meristem activity and cell differentiation in Zea mays roots. Plant Biosyst. 2006;140:87–93. [Google Scholar]
- Blank M, Soo L. The threshold for Na, K-ATPase stimulation by electromagnetic fields. Bioelectrochem Bioenerg. 1996;40:63–65. [Google Scholar]
- Blank M, Khorkova O, Goodman R. Changes in polypeptide distribution stimulated by different levels of electromagnetic and thermal stress. Bioelectrochem Bioenerg. 1994;33:109–114. [Google Scholar]
- Blum A. Selection for sustained production in water-deficit environment. Int Crop Sci. 1993;1:343–347. [Google Scholar]
- Bondarenko NF, Rokhinson EE, GakEZ Klygina LF. Magnetic equipment in agriculture. Russ Agric Sci. 1996;2:30–34. [Google Scholar]
- Boxer SG, Chidsey CED, Roelofs MG. Magnetic field effects on reaction yields in the solid state an example from photysynthetic reaction centers. J Am Chem Soc. 1982;104:1452–1454. [Google Scholar]
- Brown FA. Responses of the Planarium, Dugesia, and the protozoan, Paramecium, to very weak horizontal magnetic fields. Biol Bull. 1962;123:264–281. [Google Scholar]
- Burden BJ, Norris DM. Role of isoflavonoidcoumestrol in the constitutive antixenosic properties of soybean against an oligophagous insect, the Mexican Bean Beetle. J Chem Ecol. 1992;18:1069–1081. doi: 10.1007/BF00980063. [DOI] [PubMed] [Google Scholar]
- Burton RS. Regulation of proline synthesis during osmotic stress in the copepod Tigriopuscalifornicus. J Exp Zool. 1991;259:166–173. [Google Scholar]
- Cakmak T, Dumlupinar R, Erdal S. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics. 2010;31:120–129. doi: 10.1002/bem.20537. [DOI] [PubMed] [Google Scholar]
- Carbonell MV, Martynez E, Amaya JM. Stimulation of germination in rice (Oryza sativa L.) by a static magnetic field. Electro Magnetobiol. 2000;19(1):121–128. [Google Scholar]
- Chen YP, Li R, He JM. Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicol. 2011;20:760–769. doi: 10.1007/s10646-011-0620-6. [DOI] [PubMed] [Google Scholar]
- Chepts AD, Morozowa ZN, Tkacheva NA (1985) Wheat and sorgum yield and grain quality as affected by magnetic field, Monograph: methods for improved yields of winter wheat and spring barley, USSR
- Chinnusamy V, Zhu JK. Plant salt tolerance. Top Curr Gen. 2003;4:241–270. [Google Scholar]
- Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174(4432):689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- Cook ES, Smith MJ. Increase of trypsin activity. In: Barnothy MF, editor. Biological effects of magnetic fields. New York: Plenum Press; 1964. pp. 246–254. [Google Scholar]
- Davenport SB, Gallego SM, Benavides MP, Tomaro ML. Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annuusL. cells. Plant Growth Regul. 2003;40:81–88. [Google Scholar]
- Davies MS. Effect of 60 Hz electromagnetic fields on early growth in three plant species a replication of previous results. Bioelectromagnetics. 1996;17:154–161. doi: 10.1002/(SICI)1521-186X(1996)17:2<154::AID-BEM10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Dayal S, Singh RP. Effect of seed exposure to magnetic field on the height of tomato plants. Indian J Agric Sci. 1986;56:483–486. [Google Scholar]
- Dhiman SH, Galland P. Effects of weak static magnetic fields on the gene expression of seedlings of Arabidopsis thaliana. J Plant Physiol. 2018;231:9–18. doi: 10.1016/j.jplph.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Duarte-Diaz CE, Riquenes JA, Sotolongo B, Portuondo MA, Quintana EO, Perez R. Effects of magnetic treatment of irrigation water on the tomato crop. Hortic Abstr. 1997;69:494. [Google Scholar]
- Esitken A, Turan M. Alternating magnetic field effects on yield and plant nutrient element composition of strawberry (Fragaria × ananassa cv. Camarosa) Acta Agric Scand Sect B Soil Plant Sci. 2004;54:135–139. [Google Scholar]
- Fischer G, Tausz M, Kock M, Grill D. Effects of weak 16 2/3 Hz magnetic fields on growth parameters of young sunflower and wheat seedlings. Bioelectromagnetics. 2004;25(8):638–641. doi: 10.1002/bem.20058. [DOI] [PubMed] [Google Scholar]
- Flores-Tavizon E, Mokgalaka-Matlala NS, Galindo JTE, Castillo-Michelle H, Peralta-Videa JR, Gardea-Torresdey JL. Magnetic field effect on growth, arsenic uptake, and total amylolytic activityon mesquite (Prosopisjuliflora × P. velutina) seeds. J Appl Phys. 2012;111:07B321. [Google Scholar]
- Florez M, Carbonell MV, Martinez E. Exposure of maize seeds to stationary magnetic fields: effects on germination and early growth. Environ Exp Bot. 2007;59:68–75. [Google Scholar]
- Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants: where next? Aust J Plant Physiol. 1995;22:875–884. [Google Scholar]
- Gai YP, Li XZ, Ji XL, Wu CA, Yang GD, Zheng CC. Chilling stress acceleratesdegradation of seed storage protein and photosynthetic protein during cotton seed germination. J Agron Crop Sci. 2008;194:278–288. [Google Scholar]
- Galland P, Pazur A. Magnetoreception in plants. J Plant Res. 2005;118:371–389. doi: 10.1007/s10265-005-0246-y. [DOI] [PubMed] [Google Scholar]
- Garcia-Reina F, Arza-Pascual L. Influence of a stationary magnetic field on water relations in lettuce seeds. Part I: theoretical considerations. Bioelectromagnetics. 2001;22:589–595. doi: 10.1002/bem.88. [DOI] [PubMed] [Google Scholar]
- Goodman R, Blank M. Magnetic field stress induces expression of hsp70. Cell Stress Chaperones. 1998;3(2):79–88. doi: 10.1379/1466-1268(1998)003<0079:mfsieo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman EM, Greenebaum B, Marron MT. Magnetic fields alter translation in Escherichia coli. Bioelectromagnetics. 1994;15:77–83. doi: 10.1002/bem.2250150111. [DOI] [PubMed] [Google Scholar]
- Grabrielian S (1996) The sowing qualities of seeds and productivity of agricultural plants at action by magnetic fields. Ph.D thesis, Thestavropol agriculture academy, Stavropol
- Grissom CB. Magnetic field effects in biology—a survey of possible mechanisms with emphasis on radical pair recombination. Chem Rev. 1995;95:3–24. [Google Scholar]
- Gubbels GH. Seedling growth and yield response of flax, buckwheat, sunflower and field pea after preseedling magnetic treatment. Can J Plant Sci. 1982;62:61–64. [Google Scholar]
- Haberditzl W. Enzyme activity in high magnetic fields. Nature. 1967;213:72–73. [Google Scholar]
- Hakala-Yatkin M, Sarvikas P, Paturi P, Mantysaari M, Mattila H, Tyystjarvi T, Nedbal L, Tyystjarvi E. Magnetic field protects plants against high light by slowingdown production of singlet oxygen. Physiol Plant. 2011;142:26–34. doi: 10.1111/j.1399-3054.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa P, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Plant Physiol Rev Plant Mol Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Hernandez JA, Jimenez A, Mullineaux PM, Sevilla F. Tolerance of pea (Pisumsativum L.) to long term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ. 2000;23:853–862. [Google Scholar]
- Hirano M, Ohta A, Abe K. Magnetic field effects on photosynthesis and growth of the cyanobacterium Spirulina platensis. J Ferment Bioeng. 1998;86:313–316. [Google Scholar]
- Javed N, Ashraf M, Akram NA, Al-Qurainy F. Alleviation of adverse effects of drought stress on growth and somepotential physiological attributes in maize (Zea mays L.) by seedelectromagnetic treatment. Photochem Photobiol. 2011;87:1354–1362. doi: 10.1111/j.1751-1097.2011.00990.x. [DOI] [PubMed] [Google Scholar]
- Jia-Ming Y (1988) Effects of high-voltage electrostatic field on growth in plants. In: Proceedings of international conference on modern electrostatics, Beijing, China, pp 140–143
- Kameli A, Losel DM. Growth and sugar accumulation in Durum wheat plants under water stress. New Phytol. 1996;132:57–62. doi: 10.1111/j.1469-8137.1996.tb04508.x. [DOI] [PubMed] [Google Scholar]
- Karimi S, Hojati S, Eshghi S, Moghaddam RN, Jandoust S. Magnetic exposure improves tolerance of fig ‘Sabz’ explants to drought stressinduced in vitro. Sci Hortic. 2012;137:95–99. [Google Scholar]
- Karimi S, Eshghi S, Karimi S, Hasan-Nezhadian S. Inducing salt tolerance in sweet corn by magnetic priming. Acta Agric Slov. 2017;109:89. [Google Scholar]
- Kataria S, Baghel L, Guruprasad KN. Pre-treatment of seeds with static magnetic field improves germination and early growth characteristics under salt stress in maize and soybean. Biocatal Agric Biotechnol. 2017;10:83–90. [Google Scholar]
- Kato R. Effects of magnetic fields on the growth of primary roots of Zea mays. Plant Cell Physiol. 1988;29:1215–1219. [Google Scholar]
- Kato R, Kamada H, Asashma M. Effects of high and very low magnetic fields on the growth of hairy roots of Daucuscarottaand Atropa belladonna. Plant Cell Physiol. 1989;30:605–608. [Google Scholar]
- Kreps EM. Lipidykletochnykhmembran (the lipidsof the cell membranes) Leningrad: Nauka; 1981. [Google Scholar]
- Kurinobu S, Okazaki Y (1995) Dielectric constant and conductivity of one seed in the germination process. In: Annual conference record of IEEE/IAS, pp 1329–1334
- Kuznetsov OA, Schwuchow J, Sack FD, Hasenstein KI. Curvature induced by amyloplastmagnetophoresis in protonemata of the moss Ceratodonpurpureus. Plant Physiol. 1999;19:645–650. doi: 10.1104/pp.119.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW. Limitation to photosynthesis in water stressed leaves: stomata vs. metabolism and the role of ATP. Ann Bot. 2002;89:1–15. doi: 10.1093/aob/mcf110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelapriya T, Dilip KS, Sanker-Narayan PV. Effect of weak sinusoidal magnetic field on germination and yield of cotton (Gossypiumsp.) Electromagn Biol Med. 2003;22:117–125. [Google Scholar]
- Levedev SI, Baranskil PI, Limitrenko LG, Shiyan LT. Physiobiochemical characteristics of plants after presowing treatment with a permanent magnetic field. Sov Plant Physiol. 1975;22:84–90. [Google Scholar]
- Liboff AR, Cherng S, Jenrow KA, Bull A. Calmodulin dependent cyclic nucleotide phosphodiesterase activity is altered by 20 μT magnetostatic fields. Bioelectromagnetics. 2003;24:2–38. doi: 10.1002/bem.10063. [DOI] [PubMed] [Google Scholar]
- Liu X, ZangS Shan X, Zhu Y. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere. 2005;61:293. doi: 10.1016/j.chemosphere.2005.01.088. [DOI] [PubMed] [Google Scholar]
- Maffei ME. Magnetic field effects on plant growth, development, and evolution. Front Plant Sci. 2014;5:445. doi: 10.3389/fpls.2014.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchandia AM, Banks SW, Gossett DR, Bellaire BA, Lucas MC, Millhollon EP. The influence of α-amanitin on the NaCl induced up-regulation of antioxidant enzyme activity in cotton callus tissue. Free Radic Res. 1999;30:429–438. doi: 10.1080/10715769900300471. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Asou H, Kobayashi M, Yonekura M. Influences of magnetic fields on growth and fruit production of strawberry. Acta Hortic. 1993;348:378–380. [Google Scholar]
- Mericle RP, Mericle LW, Smith AC, Campbell WF, Montgomery DJ. Plant growth responses. In: Barnothy MF, editor. Biological effects of magnetic fields. New York: Plenum Press; 1964. pp. 183–195. [Google Scholar]
- Mitrov PP, Kroumova Z, Baidanova VD. Auxin content of corn and tomato plants following magnetic field treatments. Fiziol No Rastenyata. 1988;14:18–23. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Morar R, Iluga A, Dascalescu L, Munteanu I (1993) Electric field influence on the biological processes of seeds. In: Proceedings of international symposium on high-voltage engineering, Yokohama, p 286
- Mullins JM, Penafiel LM, Juutilainen J, Litovitz TA. Dose-response of electromagnetic field-enhanced ornithine decarboxylase activity. Bioelectrochem Bioenerg. 1999;48:193–199. doi: 10.1016/s0302-4598(98)00229-3. [DOI] [PubMed] [Google Scholar]
- Munns R, Husain S, Rivelli AR, Richard A, James RA, Condon AGT, Lindsay MP, Lagudah ES, Daniel P, Schachtman DP, Hare RA. Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant Soil. 2002;247:93–105. [Google Scholar]
- Murphy JD. The influence of magnetic fields on seed germination. Am J Bot. 1942;29:155. [Google Scholar]
- Nossol B, Buse G, Silny J. Influence of weak static and 50 Hz magnetic fields on the redox activity of cytochrome-C oxidase. Bioelectromagnetics. 1993;14:361–372. doi: 10.1002/bem.2250140408. [DOI] [PubMed] [Google Scholar]
- Novitskaya GV, MolokanovDR Kocheshkova TK, Novitskii YI. Effect of weak constant magnetic field on the composition and content of lipids in radish seedlings at various temperatures. Russ J Plant Physiol. 2010;57(1):52–61. [Google Scholar]
- Novitsky YI, Novitskaya GV, Kocheshkoiva TK, Nechiporenko GA, Dobrovolskii MV. Growth of green onions in a weak permanent magnetic field. Russ J Plant Physiol. 2001;48:709–715. [Google Scholar]
- Ogolnej K, Uprawy R, Rolnieza A. The effect of magneticalbiostimulation of sowing material, spring wheat on its development and crops. Folia Univ Agric Stetin Agric. 2002;226:77–82. [Google Scholar]
- Osborn AE. Molecule of interest, saponins in cereals. Phytochemistry. 2003;62:1–4. doi: 10.1016/s0031-9422(02)00393-x. [DOI] [PubMed] [Google Scholar]
- Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotox Environ Saf. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Peteiro-Cartelle FJ, Cabezas-Cerato J. Influence of static magnetic field on mitosis in meristematic cells of Allium cepa. J Bioelectr. 1989;8:167–178. [Google Scholar]
- Phirke PS, Umbarkar SP. Influence of magnetic treatment of oil seed on yield and dry matter. PKV Res J. 1998;22:130–132. [Google Scholar]
- Pieturszewski S. Effect of magnetic biostimulation on wheat. Seeds Sci Technol. 1993;21:621–626. [Google Scholar]
- Pieturszewski S. Effect of alternating magnetic field on germination, growth and yield of plant seeds. Inzynieriarolnicza. 1999;5:209–215. [Google Scholar]
- Pinto E, Ana ARMA, Isabel MPLVOF. Influence of soil chemistry and plant physiology inthe phytoremediation of Cu, Mn, and zinc. Crit Rev Plant Sci. 2014;33:351–373. [Google Scholar]
- Pittman UJ. Magnetism and plant growth. II. Effect on germination and early growth of corn and beans. Can J Plant Sci. 1965;45:549–555. [Google Scholar]
- Portaccio M, De-Luca P, Durante D, Grano V, Rossi S, Bencivenga U, Lepore M, Mita DG. Modulation of the catalytic activity of free and immobilized peroxidase by extremely low frequency electromagnetic fields: dependence on frequency. Bioelectromagnetics. 2005;26:145–152. doi: 10.1002/bem.20059. [DOI] [PubMed] [Google Scholar]
- Prasad MNV. Cadmium toxicity and tolerance in vascular plants. Environ Exp Bot. 1995;35:525–545. [Google Scholar]
- Radhakrishnan R. See pretreatment with magnetic field alters the storage proteins and lipid profiles in harvested soybean seeds. Physiol Mol Biol Plant. 2018;24(2):343–347. doi: 10.1007/s12298-018-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Lee IJ. Regulation of salicylic acid, jasmonic acid and fatty acids in cucumber (Cucumissativus L.) by spermidine promotes plant growth against salt stress. Acta Physiol Plant. 2013;35:3315–3322. [Google Scholar]
- Radhakrishnan R, Ranjitha-Kumari BD. Pulsed magnetic field: a contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol Biochem. 2012;51:139–144. doi: 10.1016/j.plaphy.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Ranjitha-Kumari BD. Protective role of pulsed magnetic field against salt stress effects in soybean organ culture. Plant Biosyst. 2013;147(1):135–140. [Google Scholar]
- Radhakrishnan R, Leelapriya T, Ranjitha-Kumari BD. Effects of pulsed magnetic field treatment of soybean seeds on calli growth, cell damage, and biochemical changes under salt stress. Bioelectromagnetics. 2012;33:670–681. doi: 10.1002/bem.21735. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Kang SM, Baek IY, Lee IJ. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J Plant Interact. 2014;9:754–762. [Google Scholar]
- Radhakrishnan R, Khan AL, Kang SM, Lee IJ. A comparative study of phosphate solubilization and the hostplant growth promotion ability of FusariumverticillioidesRK01 and Humicola sp. KNU01 under salt stress. Ann Microbiol. 2015;65:585–593. [Google Scholar]
- Rathod GR, Anand A. Effect of seed magneto-priming on growth, yield and Na/K ratio in wheat (Triticum aestivum L.) under salt stress. Indian J Plant Physiol. 2016;21:15–22. [Google Scholar]
- Reina FG, Pascual LA, Fundora IA Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: Experimental results. Bioelectromagnetics. 2001;22:596–602. doi: 10.1002/bem.89. [DOI] [PubMed] [Google Scholar]
- Resenburg LV, Kruger GHJ, Kruger H. Prolineaccumulation as drought tolerance selection criterion: its relationshipto membrane integrity and chloroplast ultrastructurein Nicotianatabacum L. J Plant Physiol. 1993;141(2):188–194. [Google Scholar]
- Rosen AD. Mechanism of action of moderate intensity static magnetic fields on biological systems. Cell Biochem Biophys. 2003;39:163–174. doi: 10.1385/CBB:39:2:163. [DOI] [PubMed] [Google Scholar]
- Roshandel P, Azimian F. Effects of magnetic field on growth and antioxidant capacity of Artemisia aucheri in normal or saline conditions. Biol Forum Int J. 2015;7(2):1095–1103. [Google Scholar]
- Rudolph AS, Crowe JH, Crowe LM. Effects of three stabilizing agents: proline, betaine, and trehalose on membrane phospholipids. Arch Biochem Biophys. 1986;245:134–143. doi: 10.1016/0003-9861(86)90197-9. [DOI] [PubMed] [Google Scholar]
- Russo A, Longo R, Venella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73(1):S21–S29. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- Ruzic R, Jerman I. Weak magnetic field decreases heat stress in cress seedlings. Electromagnetobiology. 2002;21(1):69–80. [Google Scholar]
- Sahebjamei H, Abdolmaleki P, Ghanati F. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics. 2007;28:42–47. doi: 10.1002/bem.20262. [DOI] [PubMed] [Google Scholar]
- Sairam RK, Tygai A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–421. [Google Scholar]
- Salikhov KM, Molin YN, Sagdeev RZ, Buchachenko AL. Spin polarization and magnetic effects in radical reactions. Amsterdam: Elsevier; 1984. [Google Scholar]
- Samy CG. Magnetic seed treatment. I. Influence on flowering, siliquae and seed characteristics of cauliflower. Orissa J Hortic. 1998;26:68–69. [Google Scholar]
- Santoro MM, Lau Y, Khan SMA, Hou L, Bolen DW. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochem. 1992;31:5278–5283. doi: 10.1021/bi00138a006. [DOI] [PubMed] [Google Scholar]
- Savostin PW. Magnetic growth relations in plants. Planta. 1930;12:327. [Google Scholar]
- Selim AFH, El-Nady MF. Physio-anatomical responses of drought stressed tomato plants to magnetic field. Acta Astronaut. 2011;69:387–396. [Google Scholar]
- Sen A, Alikamanoglu S. Effects of static magnetic field pretreatment with and without PEG 6000 or NaCl exposure on wheat biochemical parameters. Russ J Plant Physiol. 2014;61(5):646–655. [Google Scholar]
- Shaddad MA. The effect of proline application on physiologyof Raphanus sativus plants grown under salinity stress. Biol Plant. 1990;32(2):104–112. [Google Scholar]
- Shine MB, Guruprasad K, Anand A. Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics. 2012;33(5):428–437. doi: 10.1002/bem.21702. [DOI] [PubMed] [Google Scholar]
- Smith EJ, Neugebauer M, Balogh A, Bame SJ, Erdös G, Forsyth RJ, Goldstein BE, Phillips JL, Tsurutani BT. Disappearance of the heliospheric sector structure at Ulysses. Geophy Res Lett. 1993;20(21):2327–2330. [Google Scholar]
- Song W, Zhang Z, Shao H, Guo X, Cao H, Zhao H, FuZ HuX. Relationship between calcium decoding elements and plant abiotic-stress resistance. Int J BioSci. 2008;4(2):116–125. doi: 10.7150/ijbs.4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Torres E, Porras-Leon E, Casate-Fernandez R. Effects of magnetic treatment of tomato (Lycopersicon esculentum Mill) seeds on germination and seedling growth. Horic Abstr. 1999;70:6892. [Google Scholar]
- Souza-Torres AD, Garcia D, Sueiro L, Gilart F, Porras E, Licea L. Presowing agnetic treatments of tomato seeds increase the growth and yield of plants. Bioelectromagnetics. 2006;27:247–257. doi: 10.1002/bem.20206. [DOI] [PubMed] [Google Scholar]
- Telfer A. Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of β-carotene. Plant Cell Physiol. 2014;55(7):1216–1223. doi: 10.1093/pcp/pcu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tican LR, Auror CM, Morariu VV. Influence of near null magnetic field on in vitro growth of potato and wild solanum species. Bioelectromagnetics. 2005;26:548–557. doi: 10.1002/bem.20134. [DOI] [PubMed] [Google Scholar]
- Trebbi G, Borghini F, Lazzarato L, Torrigiani P, Calzoni GL, Betti L. Extremely low frequencyweak magneticfields enhance resistance of nn tobaccoplants totobacco mosaicvirus and elicitstress-related biochemical activities. Bioelectromagnetics. 2007;28:214–223. doi: 10.1002/bem.20296. [DOI] [PubMed] [Google Scholar]
- Trotel P, Bouchereau A, Niogret MF, Larher F. The fate of osmoaccumulatedproline in leaf discs of rape (Brassica napusL.) incubated in a medium of low osmolarity. Plant Sci. 1996;118:1–45. [Google Scholar]
- Vaezzadeh M, Noruzifar E, Faezeh G, Salehkotahi M, Mehdian R. Excitation of plant growth in dormant temperature by steady magnetic field. J Magnet Magnet Mater. 2006;302:105–108. [Google Scholar]
- Vashisth A, Nagarajan S. Characterization of waterdistribution and activities of enzymes during germination in magnetically-exposed maize (Zea maysL.) seeds. Indian J Biochem Biophys. 2010;47:311–318. [PubMed] [Google Scholar]
- Vasilevski G. Perspectives of the application of biophysical methods in sustainable agriculture. Bulg J Plant Physiol. 2003;2:179–186. [Google Scholar]
- Veeranjaneyullu K, Ranjitha-Kumari BD. Proline metabolism during water stress in mulberrry. J Exp Bot. 1989;40:581–583. [Google Scholar]
- Wagner GJ. Accumulation of cadmium in crop plants and itsconsequences to human health. Adv Agron. 1993;51:173–212. [Google Scholar]
- Wever R. Einflußschwacherelektro-magnetischerfelder auf die Periodik des Menschen. Naturwissenschaften. 1968;55:29–32. doi: 10.1007/BF00593403. [DOI] [PubMed] [Google Scholar]
- Xiao-ju MM, Guo YG. Study on the effect of tomato seeds physiology and biochemistry with magnetic field treatment. Bull Bot Res. 1999;99:1–8. [Google Scholar]
- Xu C, Li Y, Yu Y, Zhang Y, Wei S. Suppression of Arabidopsis flowering by near-null magnetic field is affected by light. Bioelectromagnetics. 2015;36:476–479. doi: 10.1002/bem.21927. [DOI] [PubMed] [Google Scholar]
- Yancey PH. Compatible and counteracting solutes. In: Strange K, editor. Cellular and molecular physiology of cell volume regulation. Boca Raton: CRC Press; 1994. pp. 81–109. [Google Scholar]
- Yano A, Hidaka E, Fujiwara K, Limoto M. Induction of primary root curvature in radish seedlings in a static magnetic field. Bioelectromagnetics. 2001;22:194–199. doi: 10.1002/bem.38. [DOI] [PubMed] [Google Scholar]
- Yinan L, Yuan L, Yongquing Y, Chunyang L. Effect of seed pretreatment by magnetic field on the sensitivity of cucumber (Cucumissativum) seedling to ultraviolet- B radiation. Environ Exp Bot. 2005;54:286–294. [Google Scholar]
- Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]