Abstract
Objective
To validate the Genot-PA score, a clinical-genetic logistic regression score that stratifies the thrombolytic therapy safety, in a new cohort of patients with stroke.
Methods
We enrolled 1,482 recombinant tissue plasminogen activator (rtPA)-treated patients with stroke in Spain and Finland from 2003 to 2016. Cohorts were analyzed on the basis of ethnicity and therapy: Spanish patients treated with IV rtPA within 4.5 hours of onset (cohort A and B) or rtPA in combination with mechanical thrombectomy within 6 hours of onset (cohort C) and Finnish participants treated with IV rtPA within 4.5 hours of onset (cohort D). The Genot-PA score was calculated, and hemorrhagic transformation (HT) and parenchymal hematoma (PH) risks were determined for each score stratum.
Results
Genot-PA score was tested in 1,324 (cohort A, n = 726; B, n = 334; C, n = 54; and D, n = 210) patients who had enough information to complete the score. Of these, 213 (16.1%) participants developed HT and 85 (6.4%) developed PH. In cohorts A, B, and D, HT occurrence was predicted by the score (p = 2.02 × 10−6, p = 0.023, p = 0.033); PH prediction was associated in cohorts A through C (p = 0.012, p = 0.034, p = 5.32 × 10−4). Increased frequency of PH events from the lowest to the highest risk group was found (cohort A 4%–15.7%, cohort B 1.5%–18.2%, cohort C 0%–100%). The best odds ratio for PH prediction in the highest-risk group was obtained in cohort A (odds ratio 5.16, 95% confidence interval 1.46–18.08, p = 0.009).
Conclusion
The Genot-PA score predicts HT in patients with stroke treated with IV rtPA. Moreover, in an exploratory study, the score was associated with PH risk in mechanical thrombectomy-treated patients.
IV recombinant tissue plasminogen activator (rtPA) is the first-line treatment for acute ischemic stroke (AIS). Despite its benefits, only 15.6% of patients are treated with rtPA1 because of the narrow therapeutic time window2 and side effects such as hemorrhagic transformation (HT)3–5 that influence patient outcomes.6 Mechanical thrombectomy has demonstrated its efficacy in patients with large vessel occlusion; however, according to current guidelines, patients eligible for IV rtPA should receive this drug even if other treatments are considered.7
Individualizing thrombolytic therapy is a challenge. Clinical predictors,8 plasma biomarkers,9–11 imaging parameters,12 and risk models13,14 of postthrombolytic HT have been described. Two single nucleotide polymorphisms (SNPs), rs669 in α-2-macroglobulin (A2M) and rs1801020 in coagulation factor XII (F12), were associated with rtPA safety.15 In addition, a logistic regression-based score (Genot-PA score) generated with medical history of atrial fibrillation (AF), admission diastolic blood pressure (DBP), baseline NIH Stroke Score (NIHSS) score,16 onset-to-treatment (OTT) time, and the presence of rs669 and rs1801020 risk alleles predicted HT in the Spanish population.15 The score stratified the HT risk into 4 groups (G0–G3); G0 represented the lowest-risk and G3 the highest-risk group.15 The Genot-PA score was the first study that combined genetics and clinical variables to detect patients at high risk of a hemorrhagic event.
Our aim was to validate the Genot-PA score in a new cohort of rtPA-treated patients with AIS to determine its predictive capability. Furthermore, as an exploratory aim, we tested the predictive value of the score in a cohort of patients who received thrombolysis and mechanical thrombectomy.
Methods
Study population
Our target group was white patients with an AIS admitted to an emergency room treated with IV rtPA alone or in combination with mechanical thrombectomy. IV rtPA was administered in a standard dose of 0.9-mg/kg dose (10% bolus, 90% continuous 1-hour infusion), initiated within 4.5 hours after symptom onset. Endovascular treatment decision was considered according to the Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours (REVASCAT) trial eligibility criteria.17
Patients with AIS treated with rtPA were collected. We excluded patients with remote parenchymal hematoma (PH) and without HT occurrence data. Cohorts A (n = 726) and B (n = 334) consisted of patients with ischemic stroke treated with IV thrombolysis in Spanish hospitals (table 1) between 2003 and 2016; overlapping with previous participants, for whom the score was tested previously, was avoided. OTT time was not available in cohort B, so onset-to-door (OTD) time for calculating the score was used instead. Cohort C (n = 36) was formed by patients with AIS with eligibility for endovascular therapy admitted to Vall d’Hebron University Hospital from 2012 to 2015. Cohort D (n = 210) was part of the Helsinki 2000 Ischemic Stroke Genetics Study. All ischemic stroke cases with positive neuroimaging for the presence of a new infarction relevant to the symptoms were recruited at the Helsinki University Central Hospital, which is the only neurologic emergency unit for a population of 1.5 million inhabitants.18 Patients treated exclusively with IV rtPA were selected in this cohort.
Table 1.
Spanish university hospitals that participated in the study

Clinical protocol
A detailed history of vascular risk factors and current medication was obtained from each patient. On admission, clinical examination and stroke severity were assessed with the NIHSS.16 Participants who underwent endovascular therapy were registered. Presence of HT was excluded by CT scan before rtPA administration. HT was evaluated in a follow-up CT scan performed 24 hours after symptom onset or whenever a neurologic worsening was detected, defined as an increase of ≥4 points on NIHSS score. Neuroradiologists or neurologists evaluated the CT scans at each center.
Endovascular treatment decision was considered in selected patients with AIS with persistent arterial occlusion determined by CT angiography or MR angiography after 30 minutes of rtPA therapy. Eligible patients for mechanical thrombectomy consisted of patients with AIS with carotid artery occlusion at T or M1 vessels, an Alberta Stroke Program Early Computed Tomography Score >6 on CT scan, prestroke functional disability assessed with the modified Rankin Scale score of ≤1, and baseline NIHSS score of at least 6 points.
HT was radiologically classified according to the European Cooperative Acute Stroke Study (ECASS) criteria.19 Hemorrhagic infarct (HI) was defined as small or confluent petechiae along the periphery of the infarct without space-occupying effect (HI1 and HI2). PH was established as a bleeding in <30% of the infarcted area with mild space-occupying effect (PH1) or hematoma in >30% of this area and a significant mass effect (PH2). Symptomatic intracerebral hemorrhage (sICH) was defined according to the ECASS-II criteria20 as an increase of ≥4 points in the NIHSS score due to hemorrhage on CT brain scan within the first 24 to 36 hours after thrombolytic therapy. The radiologic, clinical, and genetic evaluations were blinded to each other.
Standard protocol approvals, registrations, and patient consents
The study was reviewed and approved by the appropriate Ethics Committee of each center (Hospital Vall d'Hebron Clinical Research Ethics Committee reference IFC-ALT-2011-01), and all patients or relatives gave informed written consent.
Genetic analysis
We collected 1,482 patients with stroke. Genotyping of 1,210 participants was conducted with commercial panels of SNPs: the Human Core Exome, Human Omni Quad 5 M from Illumina (San Diego, CA, SA) and Axiom Biobank from Affymetrix (Santa Clara, CA) platforms. In addition, direct genotyping for A2M rs669 and F12 rs1801020 SNPs was performed in 272 strokes from cohort A with Sequenom iPLEX technology at the Spanish National Genotyping Centre (Santiago de Compostela, Spain).
Before the association analysis, all the samples (cohorts A–D) genotyped with commercial panels underwent a rigorous quality control with PLINK version 1.7. Sample cleaning process included sex mismatch, duplicate or kinship among the participants (Pihat > 0.18), missing filter (95%), and batch effects. Samples were projected onto the HapMap3 data with principal component analysis to confirm European ancestry.21 High-quality genotype call rates (>95%), minor allele frequency (MAF 0.01), duplicate errors, and Hardy-Weinberg equilibrium (p = 1 × 10−4) were used. As a result of a call rate <95% of F12 rs1801020 in the cohort genotyped in the Axiom Biobank array, imputation was performed in 110 samples. After estimation of the phased haplotypes, imputation was performed with Impute2 version 2.3.022 and 1000 Genomes Project data (March 2012 release). Postimputation quality controls included information >0.5 and MAF >0.01.23 In addition, 99.99% concordance was found between a random selection of 58 paired samples sequenced by genome-wide platforms and PCR-Sanger technique.
We assigned the 272 sequenom approach samples from cohort A in 96 well plates. HapMap samples (NA10830, NA10831, and NA12147) were included as a quality control for validating genotype accuracy; Hardy Weinberg equilibrium (p = 0.001) and a mean call rate of 95% were used. MAFs were compared with the reference population groups from the 1000 Genomes Project: Iberian in Spain and Finnish in Finland.
Statistical analysis
Statistical analyses were performed with the SPSS statistical package, version 17.0 (IBM, Chicago, IL). Univariate analysis for cases-controls was evaluated by χ2 for categorical variables. The t test or Mann-Whitney U test was used for continuous variables. The Genot-PA score was created from logistic regression β coefficients15 and tested with the previously described formula15: score = 0.053 × baseline NIHSS + 0.534 × AF + 0.039 × OTT (10 minutes) + 0.0149 × DBP (10 mm Hg) + 0.711 × rs18010120 F12 (C allele) + 0.784 × rs669 A2M (A allele). Risk categories from the lowest-risk group G0 to the highest-group G3 (G0, <3.95 points; G1, 3.95–5.10 points; G2, 5.10–6.10 points; G3, >6.10 points) were stablished by the mathematic algorithm Chi-Square Automatic Interaction Detector algorithm as detailed previously.15 We can better explain how to calculate the score with a clinical case. For example, a patient evaluated in the emergency room registered the following: baseline NIHSS score 13, presence of AF, OTT 170 minutes, DBP 85 mm Hg, and the absence of any of the risk alleles for rs1801020 and rs669. To estimate the HT risk, the Genot-PA score is calculated from the following: 0.053 × 13 (NIHSS) + 0.534 × 1 (AF) + 0.039 × 17 (OTT) + 0.0149 × 8.5 (DBP) + 0.711 × 0 (F12 C allele) + 0.784 × 0 (A2M A allele) = 3.15, identifying this patient as being in the lowest-risk group G0. However, under the same clinical presentation but with the presence of 2 risk alleles in both genotyped genes, the score generated is 6.14, leading to the highest-risk group G3.
The area under the receiver operating characteristic curve (AUC-ROC) was assessed. Sensitivity and specificity were calculated with MedCalc software (medcalc.org/calc/diagnostic_test.php).
Data availability
Anonymized data will be shared by request from any qualified investigator for purposes of replicating procedures and results only.
Results
Among 1,482 rtPA-treated strokes, 8 AIS without HT reported and 22 with remote PHs were excluded. Sixty-eight participants did not pass the quality controls previously described. Moreover, F12 and A2M variants were not successfully genotyped or imputed in 9 participants; 51 participants did not fulfill all the clinical predictors needed for the score. After clean-up, 1,324 participants were included in the study.
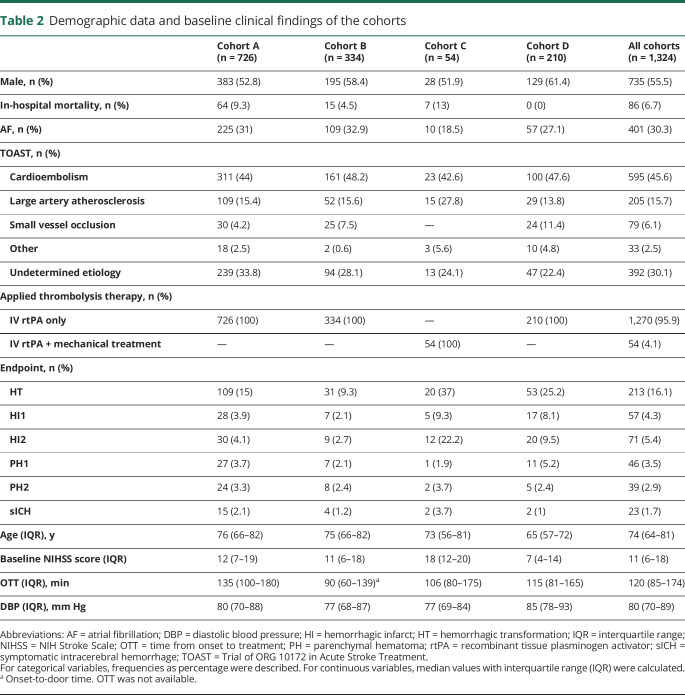
Briefly, in the whole study 55.5% (735) participants were men with a median age of 74 years. The prevalence of all HT was 16.1% (213): HI1, 4.3% (57); HI2, 5.4% (71); PH1, 3.5% (46); and PH2, 2.9% (39). Meanwhile, the prevalence of sICH was 1.7% (23) (table 2). MAFs did not differ significantly from their respective reference population (1000 Genomes Project).
Table 2.
Demographic data and baseline clinical findings of the cohorts
Cohort A (n = 726) showed a prevalence of global HT of 15% (109) with an occurrence of PH of 7.1% (51) and moderate to severe strokes (median baseline NIHSS score 12). Cohort B (n = 334) presented a global HT and PH prevalence of 9.3% (31) and 4.5% (15), respectively, with moderate to severe AIS (median baseline NIHSS score 11); an OTD median time of 90 minutes was used instead of OTT for calculating the score. Cohort C (n = 54), which included patients who received rtPA in combination with mechanical thrombectomy, reported 37% (20) hemorrhagic events, 4.5% (3) PH, and severe strokes (median baseline NIHSS score 18). Meanwhile, cohort D (n = 210) showed 25.2% (53) HT, 7.6% (16) PH, and mild strokes (baseline NIHSS score 7). (table 2).
HT study
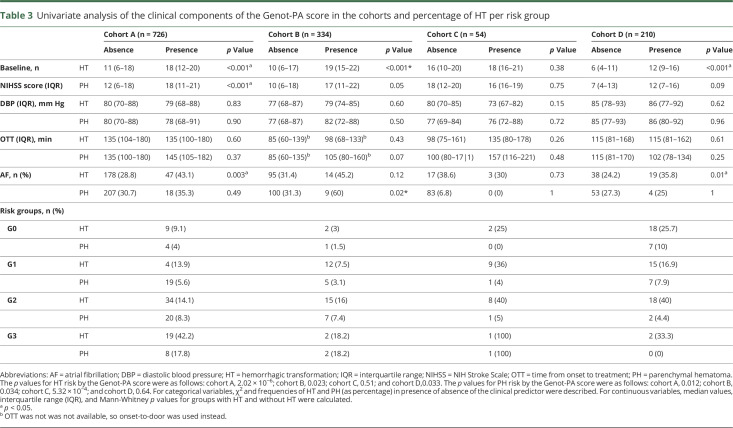
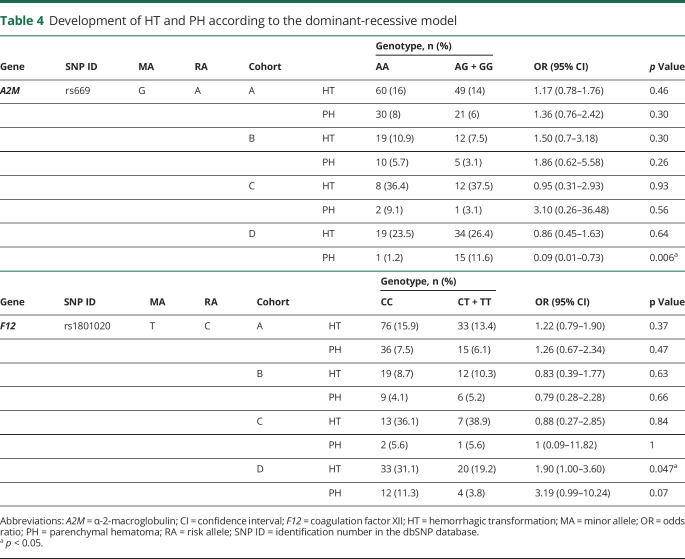
Analysis of the Genot-PA score components showed high baseline NIHSS score to be associated with the occurrence of HT in 3 cohorts (cohorts A, B, and D). AF was statistically significant in cohorts A and D (p < 0.05). Other clinical predictors of the score such as DBP, OTT, or OTD did not show significant association in our study (table 3). The variant rs669 was not significantly associated with HT occurrence in the dominant-recessive or the allelic model in any cohorts, whereas rs1801020 was significantly associated only in cohort D in the dominant-recessive model with HT (CC 31.1%, CT + TT = 19.2%, p = 0.047, odds ratio [OR] 1.90, 95% confidence interval [CI] 1.00–3.60) (tables 4 and 5).
Table 3.
Univariate analysis of the clinical components of the Genot-PA score in the cohorts and percentage of HT per risk group
Table 4.
Development of HT and PH according to the dominant-recessive model
Table 5.
Development of HT and PH according to the allele model
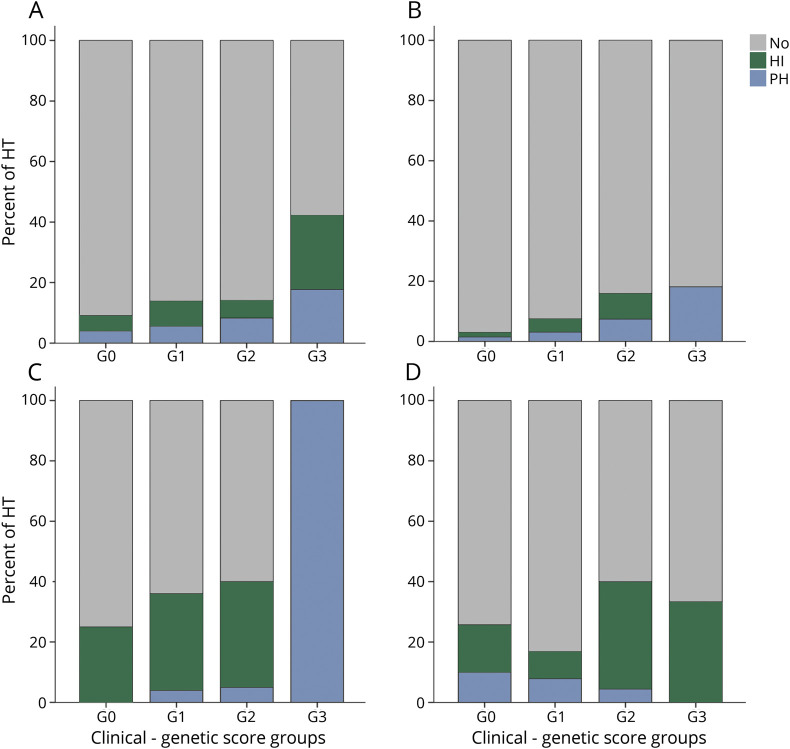
Increasing rates of HT in the 4 risk groups of each cohort were found. Global HT risk was predicted by Genot-PA score in the cohorts treated with IV rtPA exclusively (cohorts A, B, and D) (cohort A 9.1% [G0]–42.2% [G3], p = 2.02 × 10−6; cohort B 3% [G0]–18.2% [G3], p = 0.023; cohort C: 25% [G0]–100% [G3], p = 0.51; cohort D 25.7% [G0]–33.3% [G3], p = 0.033). However, hemorrhagic events in the Finnish cohort (cohort D) were more prevalent in the lowest-risk groups (G0 and G1) compared to the other cohorts (figure and table 3). Analysis of sICH was not performed due to the small number of cases in each cohort.
Figure. Occurrence of HT after recombinant tissue plasminogen activator treatment per increasing group of Genot-PA score.
(A) Cohort A, (B) cohort B, (C) cohort C, and (D) cohort D. Score groups: G0 ≤3.95 points; G1, 3.95 to 5.10 points; G2, 5.10 to 6.10 points; and G3 ≥6.10 points. HI = hemorrhagic infarct; HT = hemorrhagic transformation; PH = parenchymal hematoma.
Furthermore, the mean value of the score in the hemorrhagic event group was higher compared with the group without HT in all the cohorts (cohort A: 5.14 ± 0.92 HT vs 4.80 ± 0.80 non-HT, p = 0.001; cohort B: 5.09 ± 0.69 HT vs 4.61 ± 0.85 non-HT, p = 0.002; cohort C: 1.40 ± 0.75 HT vs 1.17 ± 0.72 non-HT, p = 0.28; cohort D: 4.67 ± 0.89 HT vs 4.28 ± 0.88 non-HT, p = 0.006).
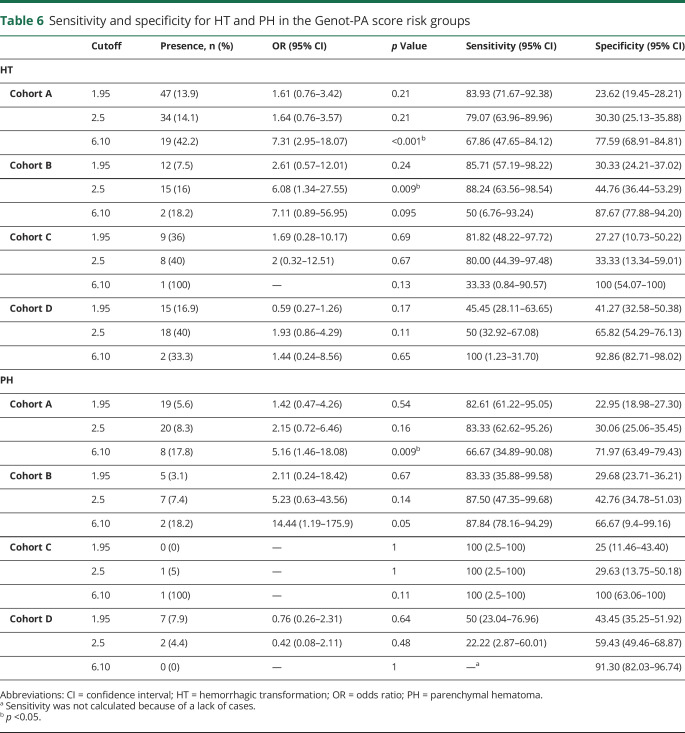
Overall, the performance of the score over the HT risk in all cohorts was comparable to the previously reported AUC-ROC of 0.72 with a 95% CI of 0.66 to 0.77 (cohort A: AUC-ROC 0.68, 95% CI 0.62–0.74; cohort B: AUC-ROC 0.74, 95% CI 0.65–0.83,; cohort C: AUC-ROC 0.66, 95% CI 0.46–0.82; cohort D: AUC-ROC 0.74, 95% CI 0.67–0.82). Risk group G3 (cutoff 6.10) showed the highest risk of hemorrhagic events compared to the G0 group, with cohort A having the best OR (OR 7.31, 95% CI 2.95–18.07, p = 3.22 × 10−6) with HT prediction specificity of 77.59% (95% CI 68.91–84.81), whereas the sensitivity was 67.86% (95% CI 47.65–84.12) (table 6).
Table 6.
Sensitivity and specificity for HT and PH in the Genot-PA score risk groups
PH study
PH phenotype was associated with stroke severity (baseline NIHSS score) in cohort A and AF in cohort B (p < 0.05). Other predictors of the score were not significantly associated (table 3).
Genetic variant studies revealed that rs669 was significantly associated with PH occurrence in the dominant-recessive and allelic model only in cohort D. Nevertheless, the SNP exhibited a protection effect (AA = 1.2%, AG + GG = 11.6% p = 0.006, OR 0.09, 95% CI 0.01–0.73) (A = 5.2%; G = 11.2%, p = 0.02, OR 0.43, 95% CI 0.21–0.90). Other associations for rs669 and rs1801020 were not found (tables 4 and 5).
The PH occurrence was predicted by the score only in the Spanish cohorts (cohorts A–C) in which increasing numbers of events for each stratum were found. On the other hand, the Finnish cohort (cohort D) exhibited an opposite pattern of PH identification (cohort A 4% [G0]–17.8% [G3], p = 0.012; cohort B 1.5% [G0]–18.2% [G3], p = 0.034; cohort C 0% [G0]–100% [G3], p = 5.32 × 10−4; cohort D [G0] 10%–0% [G3], p = 0.64) (figure and table 3).
The mean value of the score in the case group was significantly higher compared with group without PH in 2 cohorts (cohorts A and B). Moreover, nominal significance association was reported in cohort C (cohort A: 5.22 ± 0.91 patients with PH vs 4.83 ± 0.81 patients without PH, p = 0.001; cohort B: 5.21 ± 0.81 patients with PH vs 4.63 ± 0.84 patients without PH, p = 0.010; cohort C: 2 ± 1 patients with PH vs 1.2 ± 0.70 patients without PH, p = 0.07; cohort D: 4.29 ± 0.66 patients with PH vs 4.39 ± 0.92 patients without PH, p = 0.67).
The performance of the score over the PH risk was as follows: cohort A: AUC-ROC = 0.67, 95% CI 0.59–0.75, cohort B: AUC-ROC = 0.72, 95% CI 0.60–0.84, cohort C: AUC-ROC = 0.79, 95% CI 0–1, and cohort D: AUC-ROC = 0.75, 95% CI 0.63–0.83. The risk group G3 (cutoff 6.10) showed the higher risk of PH events compared to the G0 group, except for the Finnish cohort. The best OR was detected in cohort A (OR 5.16 [95% CI 1.46–18.08, p = 0.009] with PH prediction specificity of 71.97% [95% CI 63.49–79.43], whereas the sensitivity was 66.67% [95% CI 34.89–90.08], table 6).
Discussion
In the present study, we were able to validate the Genot-PA score in new cohorts treated with IV rtPA alone although the use of OTD as one of the components of the risk scale. Moreover, the PH occurrence was predicted by the score in cohorts receiving IV thrombolysis use alone or in addition to mechanical thrombectomy by an exploratory study.
Spanish cohorts treated exclusively with IV thrombolysis (cohorts A and B) exhibited a performance of the score over global HT risk similar to that previously reported.15 Even though cohort B exhibited lower rates of HT than cohort A, the highest-risk group still had the biggest percentage of hemorrhagic events. Most important, PH occurrence in the highest-risk group (G3) was similar in cohorts A and B.
Higher rates of hemorrhagic events in the G0 and G1 risk groups compared to the Spanish cohorts who underwent the same thrombolytic therapy were found in cohort D. Furthermore, the Finnish group (cohort D) was formed by younger age and less severe rtPA-treated strokes. A previous study identified different baseline characteristics involved in HT risk in the Finnish population such as blood sugar levels or radiologic parameters14; we hypothesized that these parameters could generate a big influence over the hemorrhagic risk in the Finnish cohort. Surprisingly, the genetic variant of A2M (rs669) acted as a protector for PH in this cohort, contrary to that described in the original score.15 Previous studies reported a bottleneck effect in this population24,25 in which the genetic architecture differs from the that of the rest of Europeans and could modulate the effect of the SNPs studied in the Genot-PA score.
A small cohort of 54 patients treated with IV thrombolysis in combination with thrombectomy (cohort C) was included in this study. The worrisome percentage of global HT in cohort C was explained by the accumulation of HI subtype events. Previous studies linked HI as a marker of successful recanalization26 that is achievable after the use of endovascular devices in selected patients. On the other hand, the prevalence of PH remains stable and comparable to the clinical trial performed in the same population.17 Moreover, the prediction of PH showed a complete separation between cases and controls; however, the score should be analyzed in a larger cohort.
After we reviewed the risk factors for the occurrence of HT after IV thrombolysis, stroke severity measured by the NIHSS appeared to be a strong predictor of hemorrhagic events and its subtype PH, with the greatest risk seen in scores >20.27 The presence of AF in the cohorts who receive IV rtPA alone was higher than the original score group. AF leads to greater hypoperfusion, generating damage to the integrity of blood vessels and increasing the risk of intracerebral bleeding.28 However, we failed to find an association between DBP, OTT time, A2M variant (rs669). and HT. which were predictors in the original score cohort and other previous studies.8 F12 polymorphisms (rs1801020) was associated in the dominant-recessive model with HT occurrence in cohort D. Recently, the contribution of F12 to blood-brain barrier leakage, intracerebral hemorrhage, edema, and infarct volume was confirmed in a murine thrombotic stroke model treated with rtPA.29 In addition, the inhibition of F12 in mice reduced the size of HTs induced by thrombolysis.29
One of the limitations of our study is the small sample size of the combination therapy cohort who underwent mechanical thrombectomy preceded by IV rtPA. However, highly selected patients with strict inclusion criteria were selected, which permitted a more homogeneous sample of patients in whom the therapy outcome is well studied.17 On the other hand, this kind of participants was excluded in previous risk scores.13,14,30,31
Another limitation of the study is the use of OTD time in cohort B. OTD, which was not included in the original score, could introduce heterogeneity and possible reduce points in the score. The Telestroke network on acute stroke care in Catalonia, the same geographic area for most of the participants, reported a mean OTD of 71 minutes and OTT of 120 minutes during AIS treatment.32 A difference of 49 minutes could represent a reduction of 0.191 points in the Genot-PA score (12 − 7.1 × 0.039).
Furthermore, the Genot-PA score is calculated on the basis of the presence of 2 SNPs, so we should consider the genetic variability between populations because polymorphisms could be associated with traits in specific populations.33 The current study includes the participation of only 1 cohort of non-Spanish AIS, which is a limitation, and we cannot fully generalize our findings to groups other than those who participated in the study.
Despite advances in endovascular therapies, the procedure requires highly trained staff, technical equipment, and specialized centers.34 IV rtPA is still the recommended treatment for AIS.7 The implementation of predictive scores may aid in identifying patients with high or low risk of hemorrhagic complications to help physicians in decision-making, avoiding treatment delays, adjusting the rtPA dose,35 initiating mechanical clot removal alone,36 or implementing additional support or therapy.
Considering the interaction of the genetic background and clinical data,37 genetics could improve the effectiveness of the scores to enhance the risk prediction. This could be particularly important in treating patients outside the time window with low HT risk and in the creation of treatments that prevent hemorrhagic events among other circumstances. Nowadays, advances in the molecular diagnosis using point-of-care genotyping devices such as QuantuMDX Q-POC38 would provide a fast, accessible tool with genotyping data in <20 minutes that could be performed in the ambulance before hospital arrival. This short period of time suggests that the use of genetics for the management of therapies during the acute phase of ischemic stroke is possible.
The present study validated the Genot-PA score in new cohorts of patients with stroke treated with IV thrombolysis. Furthermore, in an exploratory study, the score predicted the most severe HT subtype occurrence, PH, in patients with stroke treated with rtPA and mechanical thrombectomy. The score should be analyzed in an extended cohort of endovascularly treated patients selected by the current guidelines from the American Heart Association and American Stroke Association and in other populations.
Glossary
- AF
atrial fibrillation
- AIS
acute ischemic stroke
- A2M
α-2-macroglobulin
- AUC-ROC
area under the receiver operating characteristic curve
- CI
confidence interval
- DBP
diastolic blood pressure
- ECASS
European Cooperative Acute Stroke Study
- F12
coagulation factor XII
- HI
hemorrhagic infarct
- HT
hemorrhagic transformation
- MAF
minor allele frequency
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- OTD
onset-to-door
- OTT
onset-to-treatment
- PH
parenchymal hematoma
- REVASCAT
Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours
- rtPA
recombinant tissue plasminogen activator
- sICH
symptomatic intracerebral hemorrhage
- SNP
single nucleotide polymorphism
Appendix 1. Authors



Appendix 2. Coinvestigators
Study funding
C. Carrera is supported by NIH R01 grant, GENISIS project. C. Cruchaga, L. Heitsch, J.-M. Lee and L. Ibañez are supported by NIH(R01NS085419). The Neurovascular Research Laboratory is supported by the Spanish stroke research network (INVICTUS plus), Pre-Test Stroke Project (PMP15/00022). Stroke Pharmacogenomics and Genetics Laboratory is supported by the Spanish stroke research network (INVICTUS plus), Generation Project (PI15/01978) and Pre-Test Stroke Project (PMP15/00022), Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional (ISCIII-FEDER). Neurovascular Research Group, IMIM (Institut Hospital del Mar d'Investigacions Mèdiques) is supported by INVICTUS plus (RD16/0019/0002). Biomedical Research Institute Hospital de la Santa Creu i Sant Pau, IIB Sant Pau is supported by INVICTUS plus (RD16/0019/0010).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Urra X, Abilleira S, Dorado L, et al. Mechanical thrombectomy in and outside the REVASCAT trial: insights from a concurrent population-based stroke registry. Stroke 2015;46:3437–3442. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–774. [DOI] [PubMed] [Google Scholar]
- 4.IST-3 Collaborative Group, Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the Third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008;372:1303–1309. [DOI] [PubMed] [Google Scholar]
- 6.Strbian D, Sairanen T, Meretoja A, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology 2011;77:341–348. [DOI] [PubMed] [Google Scholar]
- 7.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 8.Thanvi BR, Treadwell S, Robinson T. Haemorrhagic transformation in acute ischaemic stroke following thrombolysis therapy: classification, pathogenesis and risk factors. Postgrad Med J 2008;84:361–367. [DOI] [PubMed] [Google Scholar]
- 9.Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003;107:598–603. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Guillamon M, Garcia-Bonilla L, Sole M, et al. Plasma VAP-1/SSAO activity predicts intracranial hemorrhages and adverse neurological outcome after tissue plasminogen activator treatment in stroke. Stroke 2010;41:1528–1535. [DOI] [PubMed] [Google Scholar]
- 11.Castellanos M, Leira R, Serena J, et al. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke 2004;35:1671–1676. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Sabin J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol 2013;12:689–705. [DOI] [PubMed] [Google Scholar]
- 13.Lou M, Safdar A, Mehdiratta M, et al. The HAT score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology 2008;71:1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strbian D, Engelter S, Michel P, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol 2012;71:634–641. [DOI] [PubMed] [Google Scholar]
- 15.del Rio-Espinola A, Fernandez-Cadenas I, Giralt D, et al. A predictive clinical-genetic model of tissue plasminogen activator response in acute ischemic stroke. Ann Neurol 2012;72:716–729. [DOI] [PubMed] [Google Scholar]
- 16.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 17.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 18.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke 2009;40:1195–1203. [DOI] [PubMed] [Google Scholar]
- 19.Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke: potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:957–960. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–1251. [DOI] [PubMed] [Google Scholar]
- 21.Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case-control association studies. Nat Protoc 2010;5:1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Leeuwen EM, Kanterakis A, Deelen P, et al. Population-specific genotype imputations using minimac or IMPUTE2. Nat Protoc 2015;10:1285–1296. [DOI] [PubMed] [Google Scholar]
- 23.Verma SS, de Andrade M, Tromp G, et al. Imputation and quality control steps for combining multiple genome-wide datasets. Front Genet 2014;5:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim ET, Wurtz P, Havulinna AS, et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet 2014;10:e1004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina CA, Alvarez-Sabin J, Montaner J, et al. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke 2002;33:1551–1556. [DOI] [PubMed] [Google Scholar]
- 27.Campbell BC, Costello C, Christensen S, et al. Fluid-attenuated inversion recovery hyperintensity in acute ischemic stroke may not predict hemorrhagic transformation. Cerebrovasc Dis 2011;32:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu HT, Campbell BC, Christensen S, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke 2015;10:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, et al. Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism. Blood 2016;128:2423–2434. [DOI] [PubMed] [Google Scholar]
- 30.Cucchiara B, Tanne D, Levine SR, Demchuk AM, Kasner S. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis 2008;17:331–333. [DOI] [PubMed] [Google Scholar]
- 31.Menon BK, Saver JL, Prabhakaran S, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke 2012;43:2293–2299. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Cancio E, Ribo M, Cardona P, et al. Telestroke in Catalonia: increasing thrombolysis rate and avoiding interhospital transfers. Cerebrovasc Dis 2018;46:66–71. [DOI] [PubMed] [Google Scholar]
- 33.Lu X, Peloso GM, Liu DJ, et al. Exome chip meta-analysis identifies novel loci and East Asian-specific coding variants that contribute to lipid levels and coronary artery disease. Nat Genet 2017;49:1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokin M, Snyder KV, Siddiqui AH, Levy EI, Hopkins LN. Recent endovascular stroke trials and their impact on stroke systems of care. J Am Coll Cardiol 2016;67:2645–2655. [DOI] [PubMed] [Google Scholar]
- 35.Anderson CS, Robinson T, Lindley RI, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med 2016;374:2313–2323. [DOI] [PubMed] [Google Scholar]
- 36.Coutinho JM, Liebeskind DS, Slater LA, et al. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: a pooled analysis of the SWIFT and STAR studies. JAMA Neurol 2017;74:268–274. [DOI] [PubMed] [Google Scholar]
- 37.Chuang LC, Kuo PH. Building a genetic risk model for bipolar disorder from genome-wide association data with random forest algorithm. Sci Rep 2017;7:39943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.QuantuMDx: molecular diagnostic in minutes. Available at: quantumdx.com/. Accessed February 20, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator for purposes of replicating procedures and results only.