Abstract
Chronic myelomonocytic leukemia (CMML) is a disease of the elderly, and by far the most frequent overlap myelodysplastic/myeloproliferative neoplasm in adults. Aside from the chronic monocytosis that remains the cornerstone of its diagnosis, the clinical presentation of CMML includes dysplastic features, cytopenias, excess of blasts, or myeloproliferative features including high white blood cell count or splenomegaly. Prognosis is variable, with several prognostic scoring systems reported in recent years, and treatment is poorly defined, with options ranging from watchful waiting to allogeneic stem cell transplantation, which remains the only curative therapy for CMML. Here, we present on behalf of the European Hematology Association and the European LeukemiaNet, evidence- and consensus-based guidelines, established by an international group of experts, from Europe and the United States, for standardized diagnostic and prognostic procedures and for an appropriate choice of therapeutic interventions in adult patients with CMML.
Introduction
Chronic myelomonocytic leukemia (CMML) is, by far, the most frequent of myelodysplastic/myeloproliferative entities recognized by World Health Organization (WHO) classifications1 with an incidence of about 1/100,000 per year. It is a very heterogeneous disease, with hematological characteristics ranging from those of a myelodysplastic syndrome (MDS) with peripheral monocytosis, to very proliferative forms, characterized by high white blood cell (WBC) counts, splenomegaly, and/or other forms of extramedullary disease. Its diagnosis remains largely based on morphology, though recent advances in flow cytometry of blood monocytes may contribute in difficult cases. Somatic mutations in a small subset of recurrently mutated genes can be detected in almost all patients, some carrying a poor prognostic value. Treatment choices remain poorly supported, since, until recently, CMML patients were included in MDS series, whereas only 1 CMML-specific randomized clinical trial (RCT) has ever been published to date.2
The European Hematology Association and the European LeukemiaNet have convened an international program, involving experts from Europe and the United States, aimed at developing evidence- and consensus-based guidelines that provide clinical practice recommendations for1 standardized diagnostic and prognostic procedures and for2 an appropriate choice of therapeutic interventions in adult patients with CMML. Herein, we present our results.
Design and methods
Systematic review of the literature and synthesis of evidence
English-language original and review articles published between 1985 and 2017 were systematically extracted from PubMed and reviewed in working groups. The level of evidence was rated according to the Revised Grading System for Recommendations in Evidence-Based Guidelines of the Scottish Intercollegiate Guidelines network Grading Review Group. Briefly, meta-analyses and systematic reviews of RCTs, or RCT were graded 1, systematic reviews of case-control or cohort studies, case-control or cohort studies were graded 2, nonanalytic studies (eg, case reports, case series) were graded 3, and expert opinion was graded 4.
Consensus phase
Opinions between experts from Europe and the United States were exchanged including via face-to-face meeting held twice a year for 2 years from 2016 to 2018, and conflicts in recommendations were resolved by case scenario studies followed by anonymous electronic votes.
Recommendations were formulated and ranked according to the supporting level of evidence. The level of recommendation was graded according to the criteria of the Scottish Intercollegiate Guidelines Network Grading Review Group. A recommendation was rated as: A, when based on at least 1 meta-analysis, systematic review, or RCT and directly applicable to the target population and demonstrating overall consistency of results; B, when based on a body of evidence including systematic reviews of case-control or cohort studies, case-control or cohort studies directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from meta-analysis, systematic review or RCT; C, when based on extrapolated evidence from studies rated as systematic reviews of case-control or cohort studies, case-control or cohort studies; and D, when based on evidence level 3 or 4. Recommendations for diagnostic work-up were based on expert consensus (recommendation level D) and graded as mandatory, recommended or suggested.
Diagnostic procedures
The differential diagnosis of CMML includes all conditions that cause a sustained monocytosis of >1 × 109/L in the peripheral blood. These include reactive causes such as a number of chronic infections3 and autoimmune disorders.4–6 Multiparameter flow cytometry analysis of peripheral blood monocytes can help distinguish reactive monocytosis from CMML. The presence of an autoimmune condition should, however, not exclude the diagnosis of CMML, as these entities can present concomitantly.7,8
Morphology
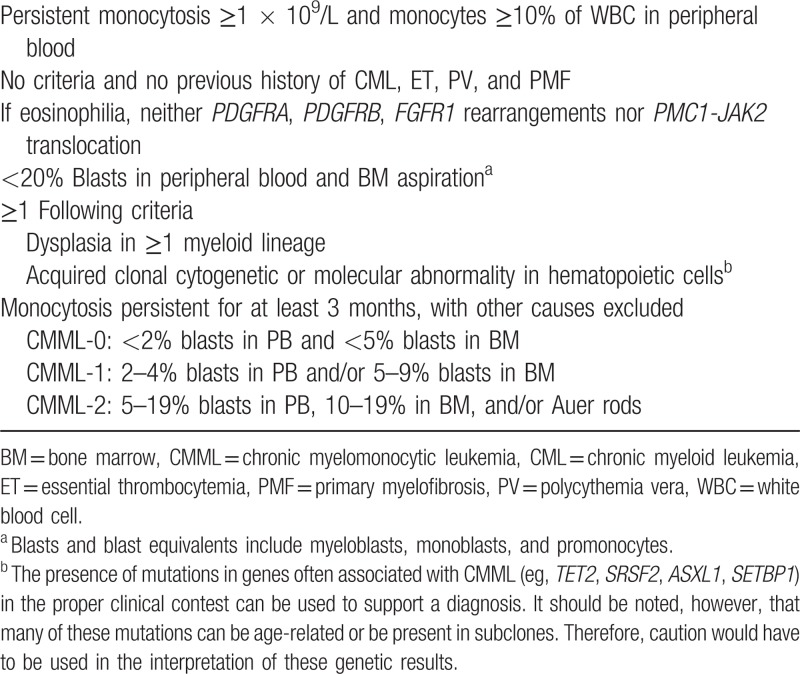
A precise diagnostic workup of patients with suspected CMML starts with blood counts, a manual differential count with the assessment of percentage and absolute number of monocytes and blasts (including promonocytes) and immature myeloid cells (IMC)) (metamyelocytes, myelocytes, and promyelocytes). An absolute monocyte count of >1 × 109/L, accounting for more than 10% of leukocytes, is a prerequisite for the diagnosis of any type of CMML.9,10 The latter is important to differentiate CMML from atypical chronic myeloid leukemia (CML). If available, antecedent blood counts should be collected to document that monocytosis has been sustained for more than 3 months.
Bone marrow (BM) cytology using May-Grünwald or Wright-Giemsa staining of marrow aspirates, accompanied by iron staining, assessment of dysplasia in all lineages, calculation of percentage of monocytes and blasts (including promonocytes)11,12 are mandatory. Peroxidase and esterase can also be useful. The percentage of marrow and peripheral blasts allows classification of CMML according to WHO 2016 criteria as CMML 0 or 1 or 2 (Table 1), and exclusion of acute myeloid leukemia (AML), especially M4 AML.13 Marrow monocytosis per se is not sufficient for diagnosis but should be assessed, as there is generally a correlation between blood and marrow monocytosis. Distinguishing monocytes, promonocytes, and monoblasts only on morphological grounds can sometimes be extremely difficult, but it is formally necessary, and efforts should be made to distinguish them as much as possible.12 In some cases, the degree of dysplasia of blood and marrow can help distinguish between CMML and AML, as in CMML dysplastic features are more pronounced in the megakaryocytic and granulocytic lineages. In addition, degranulated myelocytes sometimes cannot be easily distinguished from monocytes, potentially leading to confusion. In these cases, peroxidase and esterase staining can be helpful.
Table 1.
Diagnostic criteria for CMML according to World Health Organization 20161
Although cytology of marrow aspirates can often better assess signs of single cell dysplasia, marrow biopsy is useful in the diagnosis of CMML.14–16 It allows the assessment of cellularity, the description of stromal changes, of fibrosis,17,18 and a marrow description in cases of dry tap. Finally, it may allow detection of infiltration with mast cells, in patients with concomitant systemic mastocytosis and CMML.
PANEL RECOMMENDATION. Complete blood count (CBC), marrow aspirate with routine staining using May-Grünwald or Wright-Giemsa staining, and iron staining should be mandatorily performed, while BM biopsy with Hematoxylin-Eosin and/or Gomori's Silver staining, is strongly recommended. Immunohistochemistry can be added including CD34 and monocytic markers like CD68, CD163, CD14, and CD16. A final BM report should be made including both cytology and histology (recommendation level D).
Flow cytometry immunophenotyping
Flow cytometry analysis of BM cells may contribute to CMML diagnosis, and potentially to prognosis, and treatment monitoring by detecting subtle changes in antigen expression at the surface of myelomonocytic cells and in the erythroid lineage.19–23 BM cell immunophenotyping can similarly detect aberrations in CMML BM.24 Recent data suggest that flow analysis of peripheral blood cells can greatly contribute to the diagnosis and follow-up of CMML.25–28
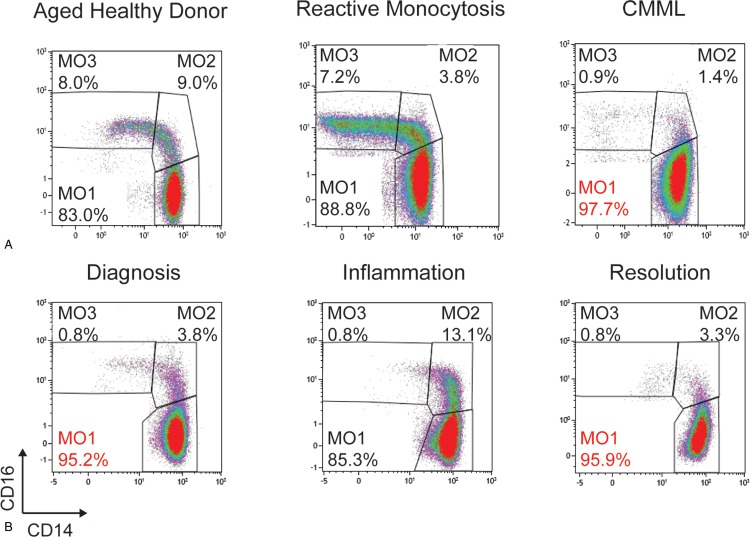
In patients with peripheral blood monocytosis ≥1 × 109/L, flow cytometry analysis of monocyte subset distribution readily distinguishes CMML from benign reactive monocytosis. The fraction of classical monocytes (CD14+/CD16−) referred to MO1 can be distinguished from intermediate (CD14+/CD16+, MO2) and nonclassical (CD14low/CD16+, MO3) monocytes according to the current nomenclature of normal human monocyte subsets.29,30 The proportion of MO1 is increased in CMML patients, while being decreased in those with a reactive condition.25,26 A proportion of MO1 ≥ 94% provides a specificity and sensitivity both higher than 90% to distinguish CMML for reactive monocytosis. This 94% cutoff has been validated by independent groups25–28 and this assay has been approved as a Clinical Laboratory Improvement Amendments-certified clinical test in the United States.26 Similar standardization in European countries should be promoted.
Quantification of the MO1 fraction as CD16-negative monocytes by means of the HematoFlow technology (Beckman-Coulter, Brea, CA) could potentially be used in routine practice.31 In patients with a suspected CMML but with a normal monocyte subset distribution (and fewer than 94% MO1s), molecular analyses and follow-up are required to confirm CMML diagnosis according to WHO 2016 criteria. Alternatively, a decreased MO3 subset combined with an increased MO2 fraction suggests the combination of CMML with an inflammatory condition.27,32 In the latter situation,8 correction of the inflammatory manifestation (eg, with steroids) will reveal a typical increase in MO1 fraction32 (Fig. 1).
Figure 1.
Flow cytometry panel of CD14 and CD16 expression on gated monocytes in (A) an aged healthy donor, a patient with reactive monocytosis, and a CMML case, showing in the latter a proportion of CD14+/CD16− monocytes >94% (reproduced with permission from Selimoglu-Buet et al25) and (B) in a CMML patient before, during, and after occurrence of a chondritis inflammatory episode (reproduced with permission from Selimoglu-Buet et al32). CMML = chronic myelomonocytic leukemia.
Flow cytometry can also help distinguish CMML from myeloproliferative neoplasms (MPNs) with monocytosis, especially polycythemia vera33 and primary myelofibrosis.34 Such a distinction may have therapeutic implications.27 The distinction between CMML and MDS can be more complex and semantic, as MDS with marrow monocytosis, peripheral blood monocyte count neighboring the threshold of monocytosis, and MO1 accumulation frequently evolve to genuine CMML.32 The combined analysis by flow cytometry focusing on granulocytic and erythroid dysplasia by an integrated analysis might discriminate these MDS subgroups from CMML.22 Ongoing prospective investigation may clarify the role and interest of monocyte subset distribution analysis in MDS.
Finally, flow analysis of monocyte subset distribution in the peripheral blood can possibly be used as a biomarker to monitor CMML response to standard and novel therapeutic regimen, for example, patients who respond to hypomethylating agents (HMAs) have normalization of the MO1 fraction,25 although those results need confirmation.
PANEL RECOMMENDATION. Analysis of peripheral blood monocyte subset distribution by a multiparameter flow cytometry assay to distinguish CMML from reactive monocytosis is recommended. Its diagnostic robustness in the context of CMML and concomitant inflammatory manifestations remains to be validated (recommendation level D).
Cytogenetics
Chromosomal abnormalities in CMML have been reported in 10% to 40% of patients in published reports, the variability been largely due to small numbers, inclusion criteria, and referral patterns. Cytogenetic aberrations are not specific of CMML. The most frequent are trisomy 8, and monosomy 7, while complex karyotypes are infrequent.35–37 Presence of chromosomal abnormalities is more common in patients with CMML-2.37
There is a strong association between specific cytogenetic abnormalities and the risk of AML evolution and overall survival (OS).38–40 The Spanish cytogenetic risk stratification divided karyotypic abnormalities into 3 risk groups according to OS: patients with trisomy 8, chromosome 7 abnormalities or complex karyotype with a very poor outcome (poor-risk category); patients with normal karyotype or isolated loss of Y chromosome with better OS (good-risk category); the remaining specific chromosomal abnormalities being merged into an intermediate-risk category.39 The prognostic significance of isolated trisomy 8 is, however, controversial. Investigators at MD Anderson Cancer Center have proposed to reassign trisomy 8 to the intermediate-risk category based on the better OS of these patients, but around 50% of them evolved to AML.39,40 A Mayo Clinic-French Consortium reassigned cases with monosomal karyotype as high-risk (85% of cases with complex karyotype also have a monosomal karyotype) and isolated der(3) as low-risk abnormalities.41
PANEL RECOMMENDATION. Cytogenetic analysis is mandatory in the diagnostic work-up of CMML, with analysis of at least 20 mitoses. If an insufficient number of mitoses is obtained, or if only 1 or 2 metaphases with +8 or −7 are seen, fluorescence in situ hybridization (FISH) analysis with centromeric probes for chromosomes 7 and 8 is recommended (recommendation level D).
Molecular genetics
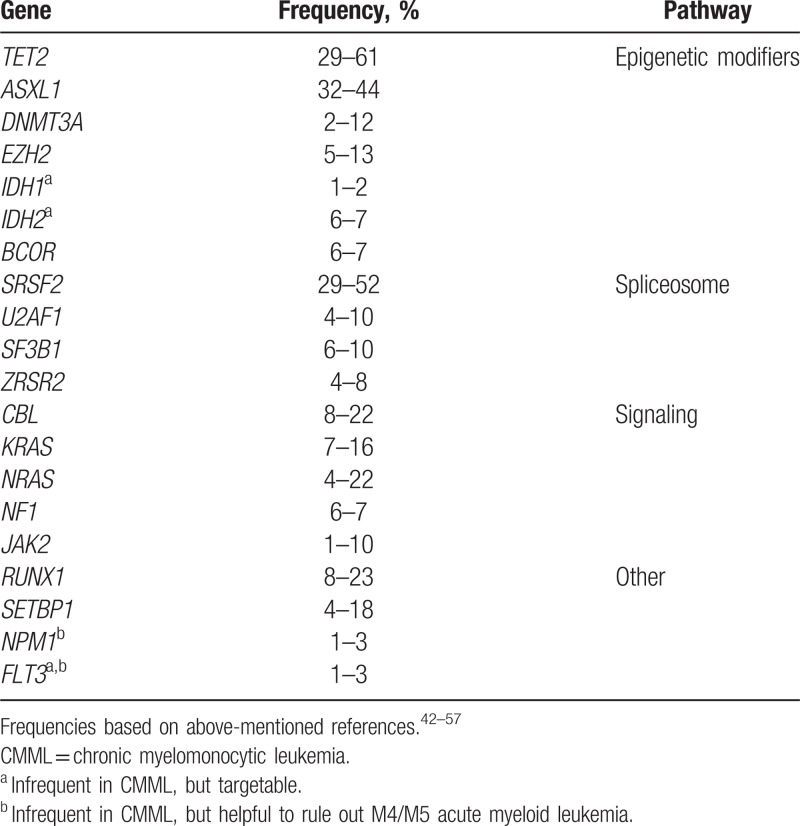
An average of 10 to 15 somatic mutations can be found in the coding regions of the genome in CMML patients.42 Compared to MDS and AML,43 the mutational spectrum of CMML is more homogeneous and sequencing of 20 genes can detect a clonal abnormality in > 90% of cases44,45 (Table 2). The prototypical molecular fingerprint combines a mutation in a gene encoding an epigenetic regulator (mainly TET2 and ASXL1) with a mutation affecting the spliceosome machinery (SRSF2, less often SF3B1, ZRSR2) with or without a mutation in the RAS/MAPK signaling pathway (NRAS, KRAS, CBL, JAK2). In particular, the combination of TET2 and SRSF2 mutations is very frequently observed in CMML and was shown to be highly specific for myeloid neoplasm with monocytosis.46 Signaling mutations are more commonly seen in association with a proliferative phenotype.44,45,47–65 Although prototypical patterns of co-occurrence of mutations have been reported,44,45,48–50 the diagnostic46 or prognostic51 roles of mutation combinations in CMML have yet to be validated.
Table 2.
Recommended minimal Next Generation Sequencing panel in CMML
According to the WHO 2016 criteria, molecular genetics is required to exclude other myeloid neoplasms including CML, the very rare myeloid/lymphoid neoplasms with eosinophilia (MLN-eo), and “classical” MPNs. 1 Of note, JAK2 mutations can be found in ∼5% CMML cases,52 with a phenotypic continuum between JAK2-mutated CMML and classical MPN.34,53,54 A similar genotypic continuum exists between CMML and atypical CML with respect to SETBP1 mutations.55 Although not AML-defining stricto sensu, NPM1 mutations in CMML tend to be associated with rapid progression to AML,45,56,57 suggesting that NPM1-mutated CMML is in fact very close to AML with monocytic differentiation (M4/5 AML). Conversely, the presence of FLT3 ITD/TKD, present in <5% CMML, does not necessarily herald transformation to AML, but may be important to know for therapeutic purposes with the recent advent of FLT3 inhibitors.58
Molecular genetics can also be used to document the clonal origin of monocytosis and thus contribute to the definitive diagnosis of CMML in patients with uninformative cytogenetics. Identification of somatic mutations may support the diagnosis of CMML if 2 or more somatic mutations are present, or at least one of them has high variant allele frequency (VAF), thereby reducing the possibility of “clonal hematopoiesis of indeterminate potential” (CHIP)59 occurring in the context of reactive monocytosis. Because dysplasia is often subtle or absent in CMML, it is important to emphasize the negative predictive value of normal findings by both cytogenetic and molecular analyses with a large-enough gene panel (Table 2) to help exclude a diagnosis of CMML.
Mutational analysis of the 20 genes listed in Table 2 is best achieved by targeted Next Generation Sequencing (NGS) panels. Studies carried out in MDS, which are likely relevant in CMML, indicate that peripheral blood is as reliable as BM for mutational analysis.60 To date, there is no consensus on the requirement or type of germline controls, analysis pipeline and VAF cutoffs for the consideration of a pathogenic somatic variant by NGS in myeloid neoplasms. The NGS report should exclude polymorphisms or platform-specific sequencing artifacts. Of note, some platforms report low-level false positive ASXL1 c.1934dupG (p.Gly646TrpfsX12) while others fail to detect it, but this frequent lesion (up to 20% of patients) represents a bona fide genetic lesion in CMML.45 A recent study has suggested that this variant is a real mutation when present at a ≥VAF 15%.61 More extensive gene panels may find additional mutations, but their significance is uncertain and thus these panels should still be considered mainly for research purposes.
The NGS report should mention the VAF, nucleic acid and amino acid changes according to HGVS nomenclature62 of all likely pathogenic variants. The nature of the somatic variant can influence its prognostic relevance. In particular, missense mutations in ASXL1 do not seem to carry the same prognostic relevance as nonsense and frameshift mutations.45,63 Attention should be paid to variants with allele frequencies compatible with a germline origin when they involve genes predisposing to myeloid neoplasms (eg, RUNX1), especially in patients diagnosed with CMML at an early age (younger than 50) and/or in those with a family history of myeloid neoplasm. In addition, the possibility of CHIP should be considered for cases with single gene mutations and low VAF, particularly when involving DNMT3A, TET2, or ASXL1.
Genotype/phenotype correlations of recurrently mutated genes have been reported45,64,65 and the prognostic role of gene mutations interacts with that of clinical features.45,66 Nonsense and frameshift mutations of ASXL1 have been invariably associated with a poor prognosis.45,50,63,67–69 Other genes reported to be associated with an adverse prognosis are TET2,51,70,71SETBP1,50,72,73SRSF2,44,45RUNX1,44,45,50NRAS,45,47,50CBL,45,69 and EZH2.45,71,74 However, these published series must be interpreted with caution, because of the limited power and heterogeneous therapeutic interventions in these cohorts. In addition to genes frequently encountered in CMML, analysis of IDH2 and IDH1 (further to that of FLT3) may be useful for the rare CMML-2 cases harboring these mutations because of emerging inhibitors (eg, enasidenib and ivosidenib, respectively) for these abnormalities. The presence of an FLT3-ITD or an NPM1 mutation should in fact lead to reconsider the diagnosis of CMML, as M4/M5 AML can masquerade initially as CMML, and intensive chemotherapy could be considered. Despite emerging data in the context of allogeneic stem cell transplantation (SCT)75 or HMAs,76 molecular genetics cannot be currently used as a biomarker for available therapies in CMML.
PANEL RECOMMENDATION. Analysis of 4 genes is mandatory for risk assessment according to accepted risk scoring systems (ASXL1, NRAS, RUNX1, and SETBP1; Table 3) in patients eligible for transplant. Analysis of a minimum of 20 genes (Table 2) is recommended for patients being considered for active treatments including transplant. It is suggested in patients only eligible for hydroxyurea (HY) and supportive care to inform prognosis and/or reveal actionable targets. The list includes IDH1, IDH2, NPM1, and FLT3 that are mutated in <5% CMML but have practical therapeutic implications (recommendation level D).
Table 3.
Recommended prognostic models
Differential diagnosis and borderline diseases
As mentioned above, WHO 2016 criteria include checking for the absence of BCR-ABL1 rearrangement (including atypical breakpoints) to rapidly exclude CML, although CML very rarely presents with >10% monocytes in the WBC differential count, and generally has a higher percentage of circulating IMC than CMML. Classical MPN can also include monocytosis, which typically does not show the abnormal distribution of monocyte subsets observed in CMML.27
In the very rare cases with eosinophilia, FIP1L1-PDGFRA should be excluded by polymerase chain reaction or FISH.77 Other tyrosine kinase (TK) fusion genes that predict sensitivity to imatinib78–80 or other inhibitors81 are almost always associated with detectable cytogenetic rearrangements, whose finding direct further molecular investigations.77 The very rare patients positive for TK fusions should be reclassified as “Myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB, or FGFR1, or with PCM1-JAK2” even if they otherwise fulfill the diagnostic criteria for CMML.1 Testing for KIT D816V mutation (and serum tryptase) helps to identify cases with systemic mastocytosis associated with CMML if morphological analysis suggests this association.82 Differentiating CMML from M4 AML can be challenging and requires expert pathologic and/or consensus review to accurately enumerate the promonocytes, which should be added to the total blast percentage.12 Presence of Auer rods or of an NPM1 mutation would favor diagnosis of M4 AML and suggest treatment with classical intensive chemotherapy.
Lastly, care must be taken to ensure that the monocyte count constitutes >10% of the total peripheral differential as proliferative cases of MDS/MPN-unclassifiable, chronic neutrophilic leukemia, and atypical CML (sometimes also typical CML) can present with clonal, sustained monocytosis of >1 × 109/L.
PANEL RECOMMENDATION. BCR-ABL1 rearrangement should be excluded in all cases. The very rare cases of monocytosis with eosinophilia should be tested for FIP1L1-PDGFRA rearrangement and other very rare TKs fusion genes should be investigated if cytogenetic analysis indicates a relevant rearrangement. M4 AML is also important to rule out when CMML2 is suspected. Borderline cases must be analyzed with NGS (including NPM1 gene status, which must be urgently assessed when M4 AML is suspected) and flow cytometry (recommendation level D).
Diagnostic classification
Clinical examination, morphology, cytogenetics, and, whenever possible, flow cytometry and molecular biology should be integrated to classify patients according to WHO 2016 categories. WHO classification (Arber et al Blood 20161,9 includes CMML-0 for cases with <2% blasts in PB and <5% blasts in BM; CMML-1 for cases with 2% to 4% blasts in PB and/or 5% to 9% blasts in BM; and CMML-2 for cases with 5% to 19% blasts in PB, 10% to 19% in BM, and/or when Auer rods are present (Table 1). This stratification holds prognostic significance.13
The distinction between “dysplastic” CMML (MD-CMML) and “proliferative” CMML (MP-CMML) initially proposed by the French-American-British (FAB) classification83 based on a WBC cutoff of 13 × 109/L) remains useful, as their clinical features differ (cytopenias vs organomegaly, high WBC, and constitutional symptoms), and consequently, their clinical management.84–86 MD-CMML, however, often progresses to MP-CMML, and this progression is frequently concomitant with occurrence of signal transduction mutations such as in the RAS and CBL genes (Table 2).47 Organomegaly should be assessed at least clinically, and if splenomegaly is suspected, measured, for example, by ultrasound, to monitor spleen size and volume. Organomegaly is infrequent in patients with MD-CMML. A systematic search for the optimal definition of MP-CMML remains to be undertaken but reports from prognostication studies indicate that the 13 × 109/L remains relevant.45,87 Extramedullary leukemia, apart from splenomegaly and hepatomegaly, mainly includes specific serous effusions (pleural and less often pericardial or peritoneal) and specific cutaneous infiltration, all associated to worse prognosis.88
PANEL RECOMMENDATION. CMML should be classified according to the WHO criteria, as revised in 2016, including MD/MP-CMML categorization (recommendation level D).
Risk assessment
Disease-related factors
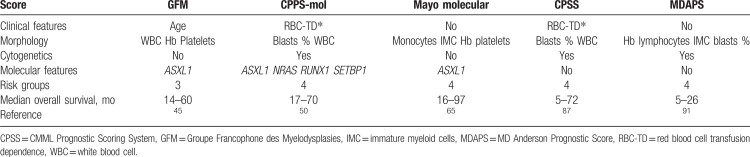
Risk assessment is a critical aspect of CMML management because the disease is very heterogeneous, and median OS of patients with CMML may range from over 50 months to <1 year.63,65 However, the prognostication of CMML patients is challenging due to the large number of prognostic tools available in the literature, including 9 models with external validation,35,50,63,65,87–91 and the wide prediction range when applying several of these scores at the patient level. Most of these scores combine “MDS-type” factors (including cytopenias, marrow blast percentage, and karyotype), “MPN-type” factors (including splenomegaly and other extramedullary disease, WBC count, and presence of circulating immature cells), and, more recently, somatic mutations. Although not extensively validated, some risk stratification can be achieved by determining the WHO subtype (CMML-0, CMML-1, and CMML-2).1,13 The FAB classification can also be applied, as MD-CMML generally have a better survival compared with MP-CMML patients.92 Although the 9 validated models all have slightly different variables and cut-points incorporated, they generally include cytopenias (anemia, thrombocytopenia), leukocytosis (monocytosis), and circulating and/or marrow blasts. Complex karyotype and aberrations of chromosome 7, and, more controversially, trisomy 8, are associated with an adverse outcome.38,41 Regarding somatic mutations, ASXL1 mutations have consistently and independently been associated with shorter OS.45,50,65,69ASXL1 status is thus part of the Groupe Francophone des Myelodysplasies (GFM) prognostic model.45 The number of mutations also worsens prognosis.45 Recently RUNX1, NRAS, and SETBP1 mutations were found to be independent adverse prognostic factors of unfavorable survival and incorporated into a molecular CMML Prognostic Scoring System (CPSS-mol).50 Of note, prognostic scores in CMML have been established in patients with a median age of at least 70 years, whose life expectancy is influenced not only by the hematological disease but also other causes. They should therefore be interpreted with caution in younger patients, who have a longer life expectancy, but in whom on the other hand CMML may induce a greater loss of survival years, potentially prompting more intensive treatment.93 Additional work is required to propose a consensus prognostic model for CMML and to overcome the limited predictive power of current models.89
PANEL RECOMMENDATION. All patients should have a detailed risk stratification assessment with any of the following CMML-specific models incorporating mutational analysis: (a) the GFM CMML model, (b) the CPSS-mol, or (c) the Mayo Molecular Model (Table 3). If mutational profiling is not available, we recommend any of the clinical CMML-specific scores including (1) CPSS or (2) MD Anderson Prognostic Score (recommendation level D).
Patient-related factors
Besides clinically relevant parameters, different factors related both to individual general health status and to individual expectations may affect clinical outcome and should be considered for risk assessment and treatment allocation in patients with CMML. These include age, functional ability (performance status), comorbidities, physical reserves (frailty), nutritional status and cognition, and quality of life.
Older age is an independent adverse prognostic factor in CMML,45,69,91,94 and has been associated with adverse outcome after treatment, including HMAs and allogeneic hematopoietic stem cell transplantation (HSCT). Because CMML is a malignancy typically occurring in elderly people (median age around 77 years)95 favorable influence of younger age for longer survival, together with good performance status and absence of major comorbidities, currently appears to be mainly related to the patient feasibility for an HSCT, which still represents the only potentially curative treatment option.93 However, chronological age may be distinct from biological or functional age, and additional factors should be considered when evaluating the eligibility of patients to disease-modifying treatments. Geriatric assessment tools should be evaluated in CMML patients.96
Measurement of individual performance status has been applied to patients with hematologic malignancy, including CMML, and used as a selection criterion to undergo intensive treatments or to enter clinical trials.97 A high prevalence of comorbidities has been reported in patients with MDS or MDS/MPN. Sorror et al developed the Hematopoietic Cell Transplantation Comorbidity Index as an instrument to assess pretransplantation comorbidities.98 This scoring system was validated in independent cohorts of patients with CMML and can be used in predicting post-transplantation outcomes and stratifying CMML patients.99–101 Several comorbidity scores have been tested in the general MDS patient population. These include general measures, such as the Charlson comorbidity index or the Adult Comorbidity Evaluation-27, and disease-specific measures, such as the MDS-Specific Comorbidity Index. Although none of these indices was validated in independent cohorts of CMML patients, general criteria issued for MDS may be adopted as translated evidence.102,103
PANEL RECOMMENDATION. Based on the available evidence, the Expert Panel agreed that the prognostic relevance of factors related to individual general health status may have important implications in the management of patients with CMML, and accounting for both disease- and patient-related factors improves risk stratification and clinical decision making (recommendation level D).
Monitoring patients and criteria for response to treatment
Most clinical studies performed in CMML patients have evaluated response according to the MDS International Working Group (IWG) 2000 and 2006 criteria.104,105 These criteria can accurately capture correction of BM blast excess and cytopenias, but not changes in myeloproliferative features such as correction of hyperleukocytosis and monocytosis, reduction in spleen size, and/or regression of extramedullary disease. An international panel has proposed MDS/MPN response criteria that take myeloproliferative features in consideration.106 They also take into account myelofibrosis,14 and disease-related symptoms via the MPN-symptom assessment form (SAF) scoring system.107 Overall, the MDS/MPN-specific criteria have more stringent definitions of complete remission and progressive disease than the MDS IWG criteria. Though these criteria represent an improvement for CMML and have been validated in a small retrospective cohort,108 future prospective validation is warranted. The relevance of improvements in hyperleukocytosis and monocytosis on long-term outcome and/or quality of life, and the adequacy of the MPN-SAF scale for general symptoms assessment in CMML require detailed investigation. Further investigation of symptom assessment and quality of life tools are warranted in CMML. In clinical practice, we recommend monitoring patients with a CBC and differential, assessment of splenomegaly and extramedullary disease (serous effusions, skin lesions, etc.), and evaluation of general symptoms with a standardized questionnaire such as the MPN-SAF. If splenomegaly is present, repeated measures of spleen volume with the same morphological test are preferable by ultrasound, magnetic resonance imaging, or computed tomography scan (considering the risk of radiation exposure). Finally, a BM examination with cytogenetic profiling should be performed if a change in the above evaluation is identified.39 There is currently no formal data to recommend a repeat mutational panel during follow-up.75 With respect to pivotal phase 3 clinical trials, we recommend as primary endpoints robust criteria such as OS, progression-free survival, or event-free survival and incorporation of the MDS/MPN criteria as secondary endpoints. Phase 2 clinical trials utilizing the MDS/MPN IWG criteria as the primary endpoint should be analyzed with caution, particularly when interpreting the proportion of patients whose response is improvement of myeloproliferative symptoms.
PANEL RECOMMENDATION. While response to treatment can be evaluated by IWG 2006 criteria in MD-CMML, recently proposed ad hoc MDS/MPN criteria should be preferably adopted (recommendation level D). With respect to pivotal phase 3 clinical trials, we recommend robust primary endpoints such as OS, progression-free survival, or event-free survival, and incorporation of the MDS/MPN criteria as secondary endpoints (recommendation level D).
Treatment
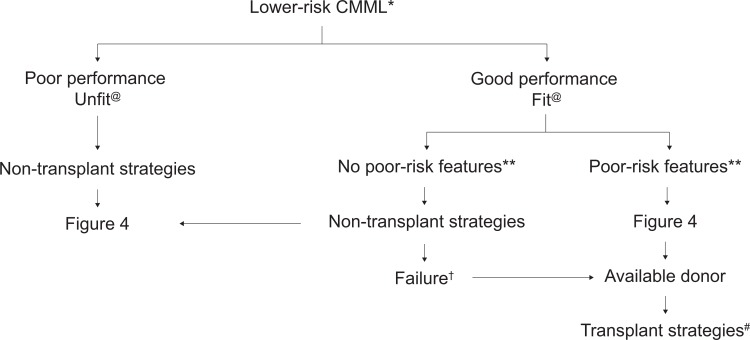
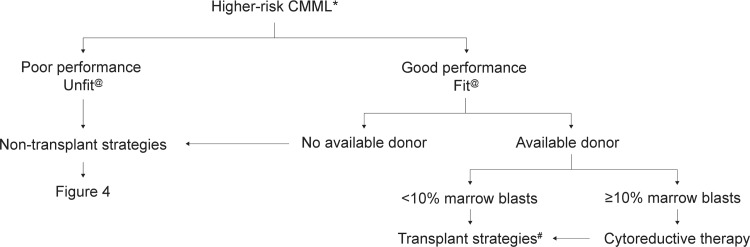
After risk assessment, the patient's eligibility for allogeneic SCT should first be assessed (Figs. 2 and 3). Nontransplant treatment strategies discussed below are summarized in Figure 4.
Figure 2.
Therapeutic algorithm for lower-risk CMML patients. ∗According to proposed CMML prognostic scores.45,50,65,87,91@ Indicates nonfit (patients with multiple comorbidities and/or poor performance) or fit (patients with no comorbidities and good performance status). † Indicates failure of nontransplant strategies. Nontransplant interventions may include >1 line of nontransplant intervention. ∗∗ Indicates poor-risk features (defined as poor-risk cytogenetics, persistent blast increase [>50% or with >15% BM blasts], life-threatening cytopenias [neutrophil counts, <0.3 × 109/L; platelet counts, <30 × 109/L], high red blood cell transfusion intensity ≥2 units per months for 6 months; poor-risk molecular features). # Indicates transplant strategies (all forms of hematopoietic stem cell transplantation, see text). BM = bone marrow, CMML = chronic myelomonocytic leukemia.
Figure 3.
Therapeutic algorithm for higher-risk CMML patients. ∗According to proposed CMML prognostic scores. 45,50,65,87,91@ Indicates nonfit (patients with multiple comorbidities and/or poor performance) or fit (patients with no comorbidities and good performance status). † Indicates failure of nontransplant strategies. Nontransplant interventions may include >1 line of nontransplant intervention. ∗∗ Indicates poor-risk features (defined as poor-risk cytogenetics, persistent blast increase [>50% or with >15% BM blasts], life-threatening cytopenias [neutrophil counts, <0.3 × 109/L; platelet counts, <30 × 109/L], high red blood cell transfusion intensity ≥2 units per months for 6 months; poor-risk molecular features). # Indicates transplant strategies (all forms of hematopoietic stem cell transplantation, see text). BM = bone marrow, CMML = chronic myelomonocytic leukemia.
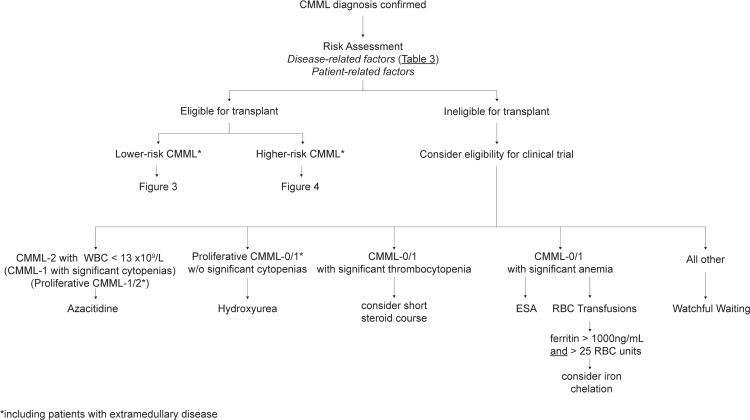
Figure 4.
Treatment strategies in patients not candidates for transplant.
Watchful-waiting strategy
Many CMML patients without (or with only mild asymptomatic) cytopenias or major signs of myeloproliferation may, like MDS, be observed without treatment. There are no demonstrated thresholds to start treatment for cytopenias. For anemia, as for MDS, Hb levels <10 g/dL are generally poorly tolerated by elderly patients and tend to trigger treatment onset. For thrombocytopenia, treatment is generally triggered when the platelet count falls below 30 × 109/L or in case of bleeding symptoms. There is no demonstrated WBC threshold to start treatment in case of myeloproliferation. Most physicians start therapy in case of major, symptomatic splenomegaly, or in the presence of other extramedullary disease, typically cutaneous involvement109 or serous effusions. Finally, constitutional symptoms should be investigated in MP-CMML, and could also trigger therapeutic interventions.
PANEL RECOMMENDATION. CMML patients without excess of marrow blasts and without (or with only mild asymptomatic) cytopenias or major signs of myeloproliferation may observed without treatment (recommendation level D).
Allogeneic stem cell transplantation
Currently available therapeutic agents can lead to survival prolongation but no cure of CMML. Therefore, allogeneic HSCT is increasingly used as a curative treatment option.110 Moreover, nonrelapse mortality after HSCT has decreased significantly in more recently performed HSCT, including patients up to the age of 70 years.111 The main questions are which patients with CMML might benefit from HSCT and when should transplant be recommended. Patient-related and disease-related factors should be considered. We refer to the recently published review on HSCT in MDS and CMML for the general patient-related factors, including age, performance status (functional ability), frailty (reduced physical fitness or physical reserve), and comorbidities.112,113 Prognostic tools, including performance status (eg, Karnofsky score), and HSCT-specific comorbidity index should be considered as well.112,114 We agreed to use the CMML-specific scoring system (CPSS)87 for the recommendation of HSCT for all CMML patients, but IPSS-R may also be used for patients with MD-CMML. The relatively poor survival after HSCT in CMML (compared with MDS) suggests that new transplantation strategies must be developed for these patients, including post-transplant strategies to prevent relapse.
Remission induction therapy before allogeneic hematopoietic SCT
Lower tumor burden prior to HSCT minimizes the risk of post-HSCT relapse and improves disease-free survival. Large retrospective analyses have demonstrated improved outcomes for patients transplanted in complete remission compared to those with active disease at the time of HSCT,111 although these analyses are hampered by a certain selection bias for patients with chemo-sensitive disease and do not take into consideration patients who did not undergo HSCT because of therapy-related toxicity. Therefore, the value of prior induction chemotherapy (IC) is still not clear, considering the absence of randomized prospective trials. Two recent retrospective studies have demonstrated that pre-HSCT therapy with azacitidine (AZA) in MDS patients, including CMML, may allow for similar outcomes after HSCT compared to pretreatment with IC.115,116 Nevertheless, as the rate of complete remissions is generally higher with IC compared to HMA,111 IC might be the best option in selected, medically fit patients, with a high disease burden. Treatment with HMA before HSCT might be considered mainly for unfit and “comorbid” patients and as a “bridging strategy” to HSCT in those where no donor has been identified yet.117 HMA may also be considered as a preferable option in patients with mutated TET2 and wild type ASXL1 who appear to have a higher response rate to HMAs, including in CMML.76,118
Source of hematopoietic stem cells
G-CSF stimulated blood stem cells (PBSC) are preferred and predominantly used in current practice.110 PBSC are associated with a faster engraftment and a lower relapse rate through graft-versus-leukemia effect, caused by the higher number of T cells in the apheresis products. However, PBSCs are associated with higher incidence of chronic graft versus host disease (GvHD) compared with BM. According to a recent randomized study in patients with hematological malignancies (including MDS) undergoing BM transplantation with unrelated donors, the rate of severe chronic GvHD was significantly reduced compared with PBSCs.119 This approach might still be an option, especially in patients who are expected to be sensitive to GVHD-related morbidity. In the absence of a matched related or unrelated donor, alternative donors may be considered in the context of a clinical trial, including haploidentical donors that have emerged as an interesting option.120
Preparative regimen for allogeneic SCT
Myeloablative (MAC), for example, busulfan/cyclophosphamide containing or total body irradiation-based, regimens are considered standard of care mainly in fit CMML patients.112 Addition of ATG is recommended in case of matched unrelated donor and MAC according to a randomized study.121 The introduction of reduced intensity conditioning (RIC) has broadened the use of allogeneic SCT for CMML patients with advanced age and comorbidities through reduced tissue damage, toxicity, and the risk of acute GvHD. However, RIC is associated with less effective reduction of “CMML burden” resulting in an increased rate of relapse.122,123 A randomized study comparing RIC versus MAC in MDS and CMML showed similar outcome after both conditioning regimens.124,125
PANEL RECOMMENDATION. HSCT is recommended for patients below the age 70 with a donor and no major contraindication to transplant who have higher-risk CMML according to CMML-specific prognostic scoring systems described above (Figs. 2 and 3). Transplant may be considered in selected lower-risk patients with poor prognostic factors that include severe cytopenias, several poor-risk somatic mutations (especially ASXL1, RUNX1, SETBP1, NRAS) (recommendation level B). Cytoreductive treatment before HSCT is generally advocated in CMML-2 (level of evidence 2, recommendation level D).
Remission induction chemotherapy
There is a limited published dataset for intensive chemotherapy (IC) in patients with CMML. The main goal of IC is to reduce BM blasts and aim for complete remission.105 CMML is a fundamentally chemoresistant disease which does not appear to be curable with IC alone. Single center series describe poor long-term outcomes irrespective of the IC regimen, despite achieving up to 40% complete response rate. Remission duration is short, and relapse appears inevitable, even when regimens are intensified.126
On the other hand, as mentioned above, IC is generally recommended before HSCT for “short-term control” in patients with an excess of marrow blasts (especially CMML-2), despite the absence of prospective trials clearly demonstrated the role of IC in this context. When considering intensive chemotherapy as a “bridge to transplant,” however, the benefits should be weighed against the risks of complications (eg, infections) or organ damage that may delay or definitively impair HSCT. IC may also be considered in CMML-2 with severe cytopenias, rapidly evolving disease, especially when the differential diagnosis between CMML-2 and M4 AML appears difficult, such as the presence of Auer rods and/or an NPM1 mutation.
PANEL RECOMMENDATION. Intensive IC is not recommended in patients with CMML, except in CMML-2 patients as a bridge to HSCT, or when the disease appears to be very close to M4 AML (presence of Auer rods, NPM1 mutation; evidence level 3, recommendation level D).
Hypomethylating agents
The HMAs, AZA, and decitabine (DAC) have been approved in CMML in the United States based on pivotal MDS phase 3 trials including <20 CMML patients each.127–129 Most patients had WBC lower than 13 × 109/L. In Europe, only AZA is licensed in CMML and its labeling restricted to CMML-2 patients with WBC < 13 × 109/L on the basis of a phase 3 MDS trial where only 11 CMML patients were randomized.130
Following these licenses, a number of retrospective series of 10 to 150 patients have been reported with AZA and DAC.131–137 Phase 2 trials have also explored prospectively the activity of HMAs in CMML in cohorts of 10 to 40 patients.127,138–144 Overall, these series have reported a weighted mean of ∼50% overall and ∼25% complete response rates by MDS IWG criteria, and a median OS of ∼20 months. The proportion of MP-CMML in these trials was highly variable and no meta-analysis has been performed. Several studies indicate that MP-CMML still has shorter survival than MD-CMML when treated with HMAs,132,133 but there is no obvious trend correlating response to HMAs in CMML with the extent of myeloproliferation.134,137 Indeed, retrospective and prospective data indicate that both AZA and DAC can reduce myeloproliferative features, including normalization of WBC, improvement of splenomegaly, and extramedullary skin lesions.108,131,138,145 Retrospective comparisons of patients treated with AZA versus DAC do not provide data to support the choice of one HMA over the other.76,134
Grades 3 to 4 hematologic toxicity has been reported in 15% to 55% of the patients, including up to 15% infections.137,139 These figures compare favorably with those seen in MDS.130 This could be due to lower toxicity notably the less frequent occurrence of neutropenia in patients with MP-CMML, although there is no data correlating myeloproliferative features and toxicity to HMAs in CMML.
In patients eligible for HSCT, as mentioned above, the role of HMA prior to transplant is disputed, especially as CMML were often analyzed together with MDS.
In MP-CMML patients ineligible for HSCT, a retrospective comparison suggests a survival benefit of HMA over HY, and previous HY exposure seems to reduce the response rate to HMAs137 but this requires prospective confirmation, especially with the ongoing DACOTA trial (NCT02214407, EudraCT: 2014-000200-10), a European phase III trial randomizing frontline DAC versus HY in MP-CMML with adverse features (significant cytopenias, high neutrophil count, blast excess or splenomegaly, defined according to a previous randomized trial of HY in MP-CMML2).
Biomarkers of HMA activity are scarce and few studies have specifically explored CMML cases. The impact of genomic, epigenomic, or transcriptomic features have yet to be validated and cannot yet guide routine practice.138,146–148 In a retrospective series of 174 patients, patients with TET2mut/ASXL1wt genotypes had the highest rates of complete and overall response, but with limited survival benefit. Conversely, patients with RUNX1 or CBL mutations had shortened survival compared with other genotypes.76 The experience with novel HMAs such as oral AZA149 and guadecitabine150 is too limited in CMML to recommend their use outside of clinical trials. Half of patients with primary or secondary HMA failure transform to AML and overall, the prognosis of CMML after HMA failure is very poor, with a median survival of around 7 months. Data on a very small cohort of CMML patients suggest that there is no benefit in switching from AZA to DAC after AZA failure.151
In patients ineligible for transplant, AZA should be used according to its label in MD-CMML-2. Off-label use of AZA in patients with CMML-1 according to WHO 2016 with significant cytopenias, notably thrombocytopenia, should be considered. In MP-CMML, HMAs should be envisaged in patients with a blast excess (CMML-1/2) and cytopenias or significant myeloproliferation, including extramedullary disease, until results of the ongoing DACOTA trial are available. In patients eligible for transplant, it is unclear when HMA should be used before transplant, especially compared with IC.
PANEL RECOMMENDATION. In patients ineligible for transplant, AZA should be considered according to its label in MD-CMML with more than 10% of blasts (evidence level 2, recommendation level B). Use of AZA in patients with a blast excess >5% and significant cytopenias or myeloproliferation should be considered, preferably within clinical trials (evidence level 3, recommendation level D). In patients eligible for transplant, it is unclear when HMA should be used before transplant, especially compared with IC.
Low-dose chemotherapy
Most MP-CMML patients with significant leukocytosis or organomegaly are still treated with low-dose cytoreductive therapy, mainly HY. Despite its very limited disease-modifying activity, HY remains the reference of cytoreductive therapy used in this setting, notably following a randomized study that showed its superiority over oral etoposide in elderly patients with MP-CMML and high-risk features. In that trial, low-dose HY (1 g/d) gave higher response rates and better survival than etoposide (150 mg/wk), with median OS of 20 and 9 months, respectively.2
The decision to introduce HY therapy requires an assessment of the potential benefit of controlling the white cell count (and perhaps the catabolic symptoms associated with this high count), versus the potential worsening of cytopenias, especially in patients with elevated but stable WBC. Development and validation of dedicated symptom scores would be instrumental in that aspect. HY should be tapered or withheld in case of onset or aggravation of transfusion dependency.
Although it has no obvious impact on the abnormal clone(s) size,53 HY is still widely employed in proliferative CMML, especially before the use of HMA. As mentioned above, an ongoing European randomized study compares HY with DAC in MP-CMML with high-risk features (DACOTA trial, NCT02214407, EudraCT: 2014-000200-10), and its results are not yet available. Finally, therapy with HY does not seem to affect the outcome of patients who later undergo HSCT.
PANEL RECOMMENDATION. HY is recommended in proliferative CMML, in the absence of major cytopenias or excess of marrow blasts (evidence level 2, recommendation level A). No single level of WBC count or spleen size can be recommended as being the optimal level to introduce treatment. The decision should be based on the patient's symptoms and comorbidity.
Other treatments including hematopoietic growth factors
While erythropoietic stimulating factors (ESA) are generally the first-line treatment of lower-risk MDS, with known prognostic factors (low endogenous serum erythropoietin [EPO] level and low red blood cell [RBC] transfusion requirements), the only published series on the use of ESAs specifically in CMML is that of the Spanish and German MDS groups including 94 CMML patients.152 Erythroid response (ER) was observed in 64% of patients and RBC transfusion independence in 31%, in keeping with the results of ESAs in lower-risk MDS.153,154 The median duration of ER was 7 months, which seems shorter than responses obtained in MDS.153,154 CPSS and EPO levels were significantly associated with ER in multivariate analysis. Considering only patients with CPSS low- or intermediate-1-risk group, the absence of RBC transfusion dependence (RBC-TD) and EPO level predicted ER. Achievement of ER correlated with a better survival.152 Challenging neutropenia is extremely infrequent in untreated CMML. Severe neutropenia in patients treated with cytoreductive drugs should be addressed by tapering and/or interrupting the drug. There is no data to support the safe use of G-CSF in CMML patients with HMA-induced neutropenia, although its administration could be considered in febrile patients not responding to antibiotics.
PANEL RECOMMENDATION. Specific CMML scoring systems, RBC transfusion requirement, and serum EPO levels are adequate tools to select CMML patients with symptomatic anemia who may benefit from treatment with ESA. A significant ER to ESA is expected in anemic patients with lower risk per CMML scoring systems and a low endogenous serum EPO level (evidence level 2, recommendation level B).
Red cell transfusion and iron chelation therapy
Red cell transfusion
Registry data show that 35% CMML patients at diagnosis have a hemoglobin concentration <10 g/dL, with 20% meeting the “MDS” definition of RBC-TD.87 Surveillance, Epidemiology and End Results (SEER) data demonstrate that 60% patients with CMML receive red cell transfusions.155 Red cell transfusion is indicated for the management of symptomatic anemia in CMML, either in the absence of suitable disease-modifying therapy or in conjunction with active therapeutic intervention. In general, guidelines for red cell transfusion in CMML reflect the recommendations for MDS,154 particularly for MD-CMML which often has a natural history that mirrors lower-risk subtypes of MDS. However, for MP-CMML, some specific features may influence red cell transfusion strategy including:
-
1.
The catabolic symptoms associated with proliferative CMML are analogous to those of myeloproliferative disease. As such fatigue and weight loss may contribute to general malaise independently of the symptoms of anemia.
-
2.
Proliferative CMML may be associated with splenomegaly. Splenic pooling may necessitate larger volume red cell transfusion to achieve symptomatic benefit at any given hemoglobin concentration.
-
3.
Cytoreductive therapy aimed at controlling myeloproliferation may worsen anemia thereby necessitating supportive red cell transfusion independent of the natural history of the disease-associated anemia.
All 3 of these features may render red cell transfusion less effective for symptomatic relief of anemia in the proliferative CMML subtype, but definitive data are lacking.
PANEL RECOMMENDATION. Best practice currently individualizes hemoglobin thresholds based on a combination of patient comorbidities (cardiac, respiratory), symptoms at a given Hb concentration, observed symptomatic benefit from a (series of) transfusion episodes and patient preference. (i) Hemoglobin <80 g/L is typically symptomatic and should be considered to trigger RBC transfusion, but no consistent single hemoglobin threshold can be recommended. (ii) No consistent single hemoglobin target value can be recommended. (iii) Transfusion frequency should reflect the duration of symptomatic benefit between transfusion episodes. (iv) If transfusion frequency and number of units per transfusion episode is steadily increasing, consideration should be given to other causes of anemia (eg, hypersplenism, bleeding, hemolysis) or to disease progression (recommendation level D).
Iron chelation therapy
There are no studies specifically assessing the role of iron chelation therapy (ICT). As such it seems reasonable to recommend the same options as for MDS, adapted for CMML.
PANEL RECOMMENDATION. ICT may be considered for red cell transfusion-dependent patients with CMML belonging to lower-risk categories of specific CMML scoring systems and a serum ferritin level higher than 1000 ng/mL after approximately 25 units of red cell, in the absence of patient-related (non-MDS) factors anticipated to reduce life expectancy to <3 years (evidence level 3, recommendation level D).
Management of thrombocytopenia
Hypersplenism may contribute to thrombocytopenia in CMML patients with splenomegaly. Thrombocytopenia can also have a peripheral component in CMML, and this may be suspected notably when there is severe thrombocytopenia contrasting with the absence of anemia or excess of marrow blasts. Immune thrombocytopenia-like treatments, may be attempted in those cases, sometimes with success.156 Thus, a short steroid course may be envisaged in those cases. A trial of the thrombopoietin (TPO) agonist eltrombopag is ongoing in thrombocytopenic CMML-0 patients (EudraCT 2013-001779-19, NCT02323178). Use of TPO mimetics outside of clinical trials in CMML is not recommended. Otherwise, the recommendations for platelet transfusion should be per the guidelines for MDS.154
PANEL RECOMMENDATION. A short steroid course may be attempted in CMML when a peripheral immune component is suspected, notably patients with severe thrombocytopenia contrasting with the absence of anemia in the absence of an excess of marrow blasts (level of evidence 3, recommendation level D).
Other and new drugs
Few phase I/II trials are exploring novel agents selectively in CMML (or in MDS/MPN). These include drug classes such as oral TPO agonists (eltrombopag, NCT02323178), JAK2 inhibitors (ruxolitinib, NCT01776723), farnesyltransferase inhibitors (tipifarnib, NCT02807272), histone deacetylase inhibitors (tefinostat, EudraCT 2015-002281-23), or the GM-CSF cytokine (lenzilumab, NCT02546284). However, a broader range of drug classes are being explored in more advanced, registration trials designed for MDS and/or AML, but that can include CMML subsets. This is for instance the case for the second-generation HMA guadecitabine (NCT02907359). Because the molecular spectrum of CMML is closely related to that of other myeloid neoplasms, trials exploring isocitrate dehydrogenase inhibitors or spliceosome inhibitors (H3B-8800, NCT02841540) may be open to CMML.
PANEL RECOMMENDATION. Inclusion of CMML patients in clinical trials is strongly encouraged at all stages of the disease. Academic, international CMML-specific confirmatory trials of activity signals detected in early phase trials having only registered a minority of CMML patients should be encouraged (recommendation level D).
Conclusion
Despite an increasing knowledge on the molecular and cellular features of CMML, the clinical management of these overlap MDS/MPN syndromes remains poorly codified. The present recommendations often rely on expert opinions, and/or extrapolations of MDS or MPN guidelines.
The inclusion of patients in clinical trials is strongly encouraged to obtain the maximal information on safety and efficacy of new treatments. The inclusion of patients in national and international registries is also encouraged to obtain data on the disease and on the implementation of treatment strategies in everyday clinical practice and to establish an optimal frame for biological and translational studies. The recently established international collaborative networks69,106,157 established in CMML will be instrumental in unifying the existing prognostication tools and in conducting CMML-specific clinical trials, to clarify the management strategy of CMML in the coming years.
Acknowledgments
All authors would like to thank Gabriela Rojková and European Hematology Association executive office for administrative support.
Footnotes
Citation: Itzykson R, Fenaux P, Bowen D, Cross NCP, Cortes J, De Witte T, Germing U, Onida F, Padron E, Platzbecker U, Santini V, Sanz GF, Solary E, Van de Loosdrecht A, Malcovati L. Diagnosis and Treatment of Chronic Myelomonocytic Leukemias in Adults: Recommendations from the European Hematology Association and the European LeukemiaNet. HemaSphere, 2018;2:6. http://dx.doi.org/10.1097/HS9.0000000000000150
Funding/support: None.
Disclosure: RI and PF have received research support from Janssen and Novartis. GFS has received consulting fees from Amgen, Boehringer Ingelheim, and Novartis. All other authors have no relevant conflict of interest.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 2.Wattel E, Guerci A, Hecquet B, et al. A randomized trial of hydroxyurea versus VP16 in adult chronic myelomonocytic leukemia. Groupe Francais des Myelodysplasies and European CMML Group. Blood 1996; 88:2480–2487. [PubMed] [Google Scholar]
- 3.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton JA. Rheumatoid arthritis: opposing actions of haemopoietic growth factors and slow-acting anti-rheumatic drugs. Lancet 1993; 342:536–539. [DOI] [PubMed] [Google Scholar]
- 5.Hilliard BA, Zizzo G, Ulas M, et al. Increased expression of Mer tyrosine kinase in circulating dendritic cells and monocytes of lupus patients: correlations with plasma interferon activity and steroid therapy. Arthritis Res Ther 2014; 16:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brkic Z, Maria NI, van Helden-Meeuwsen CG, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren's syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis 2013; 72:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peker D, Padron E, Horna P, et al. A close association of history of autoimmunity with chronic myelomonocytic leukemia (CMML) in contrast to chronic myelogenous leukemia (CML). Blood 2012; 120:1712. [Google Scholar]
- 8.Mekinian A, Grignano E, Braun T, et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology (Oxford) 2016; 55:291–300. [DOI] [PubMed] [Google Scholar]
- 9.Orazi A, Bennett JM, Germing U. Swerdlow SH, Campo E, Harris NL, et al. Chronic myelomonocytic leukemia. IARC Press, WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon:2017. [Google Scholar]
- 10.Xubo G, Xingguo L, Xianguo W, et al. The role of peripheral blood, bone marrow aspirate and especially bone marrow trephine biopsy in distinguishing atypical chronic myeloid leukemia from chronic granulocytic leukemia and chronic myelomonocytic leukemia. Eur J Haematol 2009; 83:292–301. [DOI] [PubMed] [Google Scholar]
- 11.Germing U, Strupp C, Giagounidis A, et al. Evaluation of dysplasia through detailed cytomorphology in 3156 patients from the Dusseldorf Registry on Myelodysplastic Syndromes. Leuk Res 2012; 36:727–734. [DOI] [PubMed] [Google Scholar]
- 12.Goasguen JE, Bennett JM, Bain BJ, et al. Morphological evaluation of monocytes and their precursors. Haematologica 2009; 94:994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuler E, Schroeder M, Neukirchen J, et al. Refined medullary blast and white blood cell count based classification of chronic myelomonocytic leukemias. Leuk Res 2014; 38:1413–1419. [DOI] [PubMed] [Google Scholar]
- 14.Orazi A, Chiu R, O’Malley DP, et al. Chronic myelomonocytic leukemia: the role of bone marrow biopsy immunohistology. Mod Pathol 2006; 19:1536–1545. [DOI] [PubMed] [Google Scholar]
- 15.Ngo N-T, Lampert IA, Naresh KN. Bone marrow trephine morphology and immunohistochemical findings in chronic myelomonocytic leukaemia. Br J Haematol 2008; 141:771–781. [DOI] [PubMed] [Google Scholar]
- 16.Qubaja M, Marmey B, Le Tourneau A, et al. The detection of CD14 and CD16 in paraffin-embedded bone marrow biopsies is useful for the diagnosis of chronic myelomonocytic leukemia. Virchows Archiv 2009; 454:411–419. [DOI] [PubMed] [Google Scholar]
- 17.Machherndl-Spandl S, Sega W, Bösmüller H, et al. Prognostic impact of blast cell counts in dysplastic bone marrow disorders (MDS and CMML I) with concomitant fibrosis. Ann Hematol 2013; 93:57–64. [DOI] [PubMed] [Google Scholar]
- 18.Thiele J, Orazi A, Kvasnicka HM, et al. European Bone Marrow Working Group trial on reproducibility of World Health Organization criteria to discriminate essential thrombocythemia from prefibrotic primary myelofibrosis. Haematologica 2012; 97:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westers TM, Ireland R, Kern W, et al. Standardization of flow cytometry in myelodysplastic syndromes: a report from an international consortium and the European LeukemiaNet Working Group. Leukemia 2012; 26:1730–1741. [DOI] [PubMed] [Google Scholar]
- 20.Porwit A, van de Loosdrecht AA, Bettelheim P, et al. Revisiting guidelines for integration of flow cytometry results in the WHO classification of myelodysplastic syndromes-proposal from the International/European LeukemiaNet Working Group for flow cytometry in MDS. Leukemia 2014; 28:1793–1798. [DOI] [PubMed] [Google Scholar]
- 21.Westers TM, Cremers EM, Oelschlaegel U, et al. Immunophenotypic analysis of erythroid dysplasia in myelodysplastic syndromes. A report from the IMDSFlow Working Group. Haematologica 2017; 102:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremers EM, Westers TM, Alhan C, et al. Implementation of erythroid lineage analysis by flow cytometry in diagnostic models for myelodysplastic syndromes. Haematologica 2017; 102:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alhan C, Westers TM, Cremers EM, et al. The myelodysplastic syndromes flow cytometric score: a three-parameter prognostic flow cytometric scoring system. Leukemia 2016; 30:658–665. [DOI] [PubMed] [Google Scholar]
- 24.Lacronique-Gazaille C, Chaury MP, Le Guyader A, et al. A simple method for detection of major phenotypic abnormalities in myelodysplastic syndromes: expression of CD56 in CMML. Haematologica 2007; 92:859–860. [DOI] [PubMed] [Google Scholar]
- 25.Selimoglu-Buet D, Wagner-Ballon O, Saada V, et al. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood 2015; 125:3618–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talati C, Zhang L, Shaheen G, et al. Monocyte subset analysis accurately distinguishes CMML from MDS and is associated with a favorable MDS prognosis. Blood 2017; 129:1881–1883. [DOI] [PubMed] [Google Scholar]
- 27.Patnaik MM, Timm MM, Vallapureddy R, et al. Flow cytometry based monocyte subset analysis accurately distinguishes chronic myelomonocytic leukemia from myeloproliferative neoplasms with associated monocytosis. Blood Cancer J 2017; 7:e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarfi S, Harrivel V, Dumezy F, et al. Multicentric validation of the “monocyte assay” for chronic myelomonocytic leukemia diagnosis by flow cytometry. Blood 2017; 130 (suppl 1):4273. [Google Scholar]
- 29.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116:e74–e80. [DOI] [PubMed] [Google Scholar]
- 30.Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011; 118:e16–e31. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez R, Roussel M, Badaoui B, et al. High sensitivity of the hematoflow solution for chronic myelomonocytic leukemia screening. Cytometry B Clin Cytom 2018; 94:658–661. [DOI] [PubMed] [Google Scholar]
- 32.Selimoglu-Buet D, Badaoui B, Benayoun E, et al. Accumulation of classical monocytes defines a subgroup of MDS that frequently evolves into CMML. Blood 2017; 130:832–835. [DOI] [PubMed] [Google Scholar]
- 33.Barraco D, Cerquozzi S, Gangat N, et al. Monocytosis in polycythemia vera: clinical and molecular correlates. Am J Hematol 2017; 92:640–645. [DOI] [PubMed] [Google Scholar]
- 34.Elliott MA, Verstovsek S, Dingli D, et al. Monocytosis is an adverse prognostic factor for survival in younger patients with primary myelofibrosis. Leuk Res 2007; 31:1503–1509. [DOI] [PubMed] [Google Scholar]
- 35.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood 2002; 99:840–849. [DOI] [PubMed] [Google Scholar]
- 36.Solé F, Espinet B, Sanz GF, et al. Incidence, characterization and prognostic significance of chromosomal abnormalities in 640 patients with primary myelodysplastic syndromes. Br J Haematol 2000; 108:346–356. [DOI] [PubMed] [Google Scholar]
- 37.Germing U, Kundgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML). Leuk Lymphoma 2004; 45:1311–1318. [DOI] [PubMed] [Google Scholar]
- 38.Such E, Cervera J, Costa D, et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 2011; 96:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang G, Fu B, Hu S, et al. Prognostic impact of acquisition of cytogenetic abnormalities during the course of chronic myelomonocytic leukemia. Am J Hematol 2015; 90:882–887. [DOI] [PubMed] [Google Scholar]
- 40.Tang G, Zhang L, Fu B, et al. Cytogenetic risk stratification of 417 patients with chronic myelomonocytic leukemia from a single institution. Am J Hematol 2014; 89:813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wassie EA, Itzykson R, Lasho TL, et al. Molecular and prognostic correlates of cytogenetic abnormalities in chronic myelomonocytic leukemia: a Mayo Clinic-French Consortium Study. Am J Hematol 2014; 89:1111–1115. [DOI] [PubMed] [Google Scholar]
- 42.Merlevede J, Droin N, Qin T, et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat Commun 2016; 7:10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball M, List AF, Padron E. When clinical heterogeneity exceeds genetic heterogeneity: thinking outside the genomic box in chronic myelomonocytic leukemia. Blood 2016; 128:2381–2387. [DOI] [PubMed] [Google Scholar]
- 44.Meggendorfer M, Roller A, Haferlach T, et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML). Blood 2012; 120:3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol 2013; 31:2428–2436. [DOI] [PubMed] [Google Scholar]
- 46.Malcovati L, Papaemmanuil E, Ambaglio I, et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood 2014; 124:1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricci C, Fermo E, Corti S, et al. RAS mutations contribute to evolution of chronic myelomonocytic leukemia to the proliferative variant. Clin Cancer Res 2010; 16:2246–2256. [DOI] [PubMed] [Google Scholar]
- 48.Mason CC, Khorashad JS, Tantravahi SK, et al. Age-related mutations and chronic myelomonocytic leukemia. Leukemia 2016; 30:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohlmann A, Grossmann V, Klein HU, et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol 2010; 28:3858–3865. [DOI] [PubMed] [Google Scholar]
- 50.Elena C, Galli A, Such E, et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016; 128:1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patnaik MM, Lasho TL, Vijayvargiya P, et al. Prognostic interaction between ASXL1 and TET2 mutations in chronic myelomonocytic leukemia. Blood Cancer J 2016; 6:e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steensma DP, Dewald GW, Lasho TL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood 2005; 106:1207–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itzykson R, Kosmider O, Renneville A, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood 2013; 121:2186–2198. [DOI] [PubMed] [Google Scholar]
- 54.Beran M, Shen Y, Onida F, et al. Prognostic significance of monocytosis in patients with myeloproliferative disorders. Leuk Lymphoma 2006; 47:417–423. [DOI] [PubMed] [Google Scholar]
- 55.Meggendorfer M, Jeromin S, Haferlach C, et al. The mutational landscape of 18 investigated genes clearly separates four subtypes of myelodysplastic/myeloproliferative neoplasms. Haematologica 2018; 103:e192–e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng J, Zuo Z, Fu B, et al. Chronic myelomonocytic leukemia with nucleophosmin (NPM1) mutation. Eur J Haematol 2016; 96:65–71. [DOI] [PubMed] [Google Scholar]
- 57.Ernst T, Chase A, Zoi K, et al. Transcription factor mutations in myelodysplastic/myeloproliferative neoplasms. Haematologica 2010; 95:1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daver N, Strati P, Jabbour E, et al. FLT3 mutations in myelodysplastic syndrome and chronic myelomonocytic leukemia. Am J Hematol 2013; 88:56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015; 126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohamedali AM, Alkhatabi H, Kulasekararaj A, et al. Utility of peripheral blood for cytogenetic and mutation analysis in myelodysplastic syndrome. Blood 2013; 122:567–570. [DOI] [PubMed] [Google Scholar]
- 61.Thomas M, Sukhai MA, Zhang T, et al. Integration of technical, bioinformatic, and variant assessment approaches in the validation of a targeted Next-Generation Sequencing panel for myeloid malignancies. Arch Pathol Lab Med 2017; 141:759–775. [DOI] [PubMed] [Google Scholar]
- 62.den Dunnen JT, Dalgleish R, Maglott DR, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat 2016; 37:564–569. [DOI] [PubMed] [Google Scholar]
- 63.Patnaik MM, Itzykson R, Lasho TL, et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia 2014; 28:2206–2212. [DOI] [PubMed] [Google Scholar]
- 64.Smith AE, Mohamedali AM, Kulasekararaj A, et al. Next-generation sequencing of the TET2 gene in 355 MDS and CMML patients reveals low-abundance mutant clones with early origins, but indicates no definite prognostic value. Blood 2010; 116:3923–3932. [DOI] [PubMed] [Google Scholar]
- 65.Patnaik MM, Padron E, Laborde RR, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia 2013; 27:1504–1510. [DOI] [PubMed] [Google Scholar]
- 66.Cervera N, Itzykson R, Coppin E, et al. Gene mutations differently impact the prognosis of the myelodysplastic and myeloproliferative classes of chronic myelomonocytic leukemia. Am J Hematol 2014; 89:604–609. [DOI] [PubMed] [Google Scholar]
- 67.Gelsi-Boyer V, Trouplin V, Roquain J, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol 2010; 151:365–375. [DOI] [PubMed] [Google Scholar]
- 68.Lin Y, Zheng Y, Wang ZC, et al. Prognostic significance of ASXL1 mutations in myelodysplastic syndromes and chronic myelomonocytic leukemia: a meta-analysis. Hematology 2016; 21:454–461. [DOI] [PubMed] [Google Scholar]
- 69.Padron E, Garcia-Manero G, Patnaik MM, et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J 2015; 5:e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kosmider O, Gelsi-Boyer V, Ciudad M, et al. TET2 gene mutation is a frequent and adverse event in chronic myelomonocytic leukemia. Haematologica 2009; 94:1676–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grossmann V, Kohlmann A, Eder C, et al. Molecular profiling of chronic myelomonocytic leukemia reveals diverse mutations in >80% of patients with TET2 and EZH2 being of high prognostic relevance. Leukemia 2011; 25:877–879. [DOI] [PubMed] [Google Scholar]
- 72.Laborde RR, Patnaik MM, Lasho TL, et al. SETBP1 mutations in 415 patients with primary myelofibrosis or chronic myelomonocytic leukemia: independent prognostic impact in CMML. Leukemia 2013; 27:2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damm F, Itzykson R, Kosmider O, et al. SETBP1 mutations in 658 patients with myelodysplastic syndromes, chronic myelomonocytic leukemia and secondary acute myeloid leukemias. Leukemia 2013; 27:1401–1403. [DOI] [PubMed] [Google Scholar]
- 74.Ernst T, Chase AJ, Score J, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 2010; 42:722–726. [DOI] [PubMed] [Google Scholar]
- 75.Yoshizato T, Nannya Y, Atsuta Y, et al. Impact of genetic alterations in stem-cell transplantation for myelodysplasia and secondary acute myeloid leukemia. Blood 2017; 129:2347–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duchmann M, Yalniz FF, Sanna A, et al. Prognostic role of gene mutations in chronic myelomonocytic leukemia patients treated with hypomethylating agents. EBioMedicine 2018; 31:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butt NM, Lambert J, Ali S, et al. Guideline for the investigation and management of eosinophilia. Br J Haematol 2017; 176:553–572. [DOI] [PubMed] [Google Scholar]
- 78.Bell GC, Padron E. Detection of a PDGFRB fusion in refractory CMML without eosinophilia: a case for broad spectrum tumor profiling. Leuk Res Rep 2015; 4:70–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheah CY, Burbury K, Apperley JF, et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood 2014; 123:3574–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Apperley JF, Gardembas M, Melo JV, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med 2002; 347:481–487. [DOI] [PubMed] [Google Scholar]
- 81.Schwaab J, Knut M, Haferlach C, et al. Limited duration of complete remission on ruxolitinib in myeloid neoplasms with PCM1-JAK2 and BCR-JAK2 fusion genes. Ann Hematol 2014; 94:233–238. [DOI] [PubMed] [Google Scholar]
- 82.Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia 2015; 30:136–143. [DOI] [PubMed] [Google Scholar]
- 83.Bennett JM, Catovsky D, Daniel MT, et al. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French-American-British Cooperative Leukaemia Group. Br J Haematol 1994; 87:746–754. [DOI] [PubMed] [Google Scholar]
- 84.Michaux JL, Martiat P. Chronic myelomonocytic leukaemia (CMML)—a myelodysplastic or myeloproliferative syndrome? Leuk Lymphoma 1993; 9:35–41. [DOI] [PubMed] [Google Scholar]
- 85.Voglova J, Chrobak L, Neuwirtova R, et al. Myelodysplastic and myeloproliferative type of chronic myelomonocytic leukemia—distinct subgroups or two stages of the same disease? Leuk Res 2001; 25:493–499. [DOI] [PubMed] [Google Scholar]
- 86.Onida F, Iacobelli S, Garcia-Manero G, et al. A new clinically-based subclassification proposal in CMML with significant prognostic implications to overcome the MDS/MPN categorizing dilemma. Blood 2016; 128:4320. [Google Scholar]
- 87.Such E, Germing U, Malcovati L, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood 2013; 121:3005–3015. [DOI] [PubMed] [Google Scholar]
- 88.Worsley A, Oscier DG, Stevens J, et al. Prognostic features of chronic myelomonocytic leukaemia: a modified Bournemouth score gives the best prediction of survival. Br J Haematol 1988; 68:17–21. [DOI] [PubMed] [Google Scholar]
- 89.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120:2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89:2079–2088. [PubMed] [Google Scholar]
- 91.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer 2008; 113:1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol 1976; 33:451–458. [DOI] [PubMed] [Google Scholar]
- 93.Patnaik MM, Wassie EA, Padron E, et al. Chronic myelomonocytic leukemia in younger patients: molecular and cytogenetic predictors of survival and treatment outcome. Blood Cancer J 2015; 5:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanz GF, Sanz MA, Vallespi T, et al. Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: a multivariate analysis of prognostic factors in 370 patients. Blood 1989; 74:395–408. [PubMed] [Google Scholar]
- 95.Roman E, Smith A, Appleton S, et al. Myeloid malignancies in the real-world: occurrence, progression and survival in the UK's population-based Haematological Malignancy Research Network 2004–15. Cancer Epidemiol 2016; 42:186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood 2018; 131:515–524. [DOI] [PubMed] [Google Scholar]
- 97.Liu HD, Ahn KW, Hu ZH, et al. Allogeneic hematopoietic cell transplantation for adult chronic myelomonocytic leukemia. Biol Blood Marrow Transplant 2017; 23:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 2015; 21:1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma P, Shinde SS, Damlaj M, et al. Allogeneic hematopoietic stem cell transplant in adult patients with myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) overlap syndromes. Leuk Lymphoma 2016; 58:872–881. [DOI] [PubMed] [Google Scholar]
- 100.Eissa H, Gooley TA, Sorror ML, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia: relapse-free survival is determined by karyotype and comorbidities. Biol Blood Marrow Transplant 2011; 17:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kerbauy DMB, Chyou F, Gooley T, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol Blood Marrow Transplant 2005; 11:713–720. [DOI] [PubMed] [Google Scholar]
- 102.Yucel OK, Saliba RM, Rondon G, et al. Cytogenetics and comorbidity predict outcomes in older myelodysplastic syndrome patients after allogeneic stem cell transplantation using reduced intensity conditioning. Cancer 2017; 123:2661–2670. [DOI] [PubMed] [Google Scholar]
- 103.Buckstein R, Wells RA, Zhu N, et al. Patient-related factors independently impact overall survival in patients with myelodysplastic syndromes: an MDS-CAN prospective study. Br J Haematol 2016; 174:88–101. [DOI] [PubMed] [Google Scholar]
- 104.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000; 96:3671–3674. [PubMed] [Google Scholar]
- 105.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108:419–425. [DOI] [PubMed] [Google Scholar]
- 106.Savona MR, Malcovati L, Komrokji R, et al. An international consortium proposal of uniform response criteria for myelodysplastic/myeloproliferative neoplasms (MDS/MPN) in adults. Blood 2015; 125:1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012; 30:4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duchmann M, Braun T, Micol JB, et al. Validation of response assessment according to international consortium for MDS/MPN criteria in chronic myelomonocytic leukemia treated with hypomethylating agents. Blood Cancer J 2017; 7:e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vitte F, Fabiani B, Benet C, et al. Specific skin lesions in chronic myelomonocytic leukemia: a spectrum of myelomonocytic and dendritic cell proliferations: a study of 42 cases. Am J Surg Pathol 2012; 36:1302–1316. [DOI] [PubMed] [Google Scholar]
- 110.Passweg JR, Baldomero H, Bregni M, et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant 2013; 48:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Symeonidis A, van Biezen A, de Wreede L, et al. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Trans. Br J Haematol 2015; 171:239–246. [DOI] [PubMed] [Google Scholar]
- 112.de Witte T, Bowen D, Robin M, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood 2017; 129:1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kerbauy DM, Lesnikov V, Abbasi N, et al. NF-kappaB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs). Blood 2005; 106:3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol 2014; 32:3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Damaj G, Duhamel A, Robin M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. J Clin Oncol 2012; 30:4533–4540. [DOI] [PubMed] [Google Scholar]
- 116.Potter VT, Iacobelli S, van Biezen A, et al. Comparison of intensive chemotherapy and hypomethylating agents before allogeneic stem cell transplantation for advanced myelodysplastic syndromes: a study of the myelodysplastic syndrome subcommittee of the chronic malignancies working party of the European Society for Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 2016; 22:1615–1620. [DOI] [PubMed] [Google Scholar]
- 117.Robin M, Fenaux P. Hypomethylating agents as bridging therapy before allogeneic hematopoietic stem cell transplantation in patients with chronic myelomonocytic leukemia? Biol Blood Marrow Transplant 2016; 22:1–2. [DOI] [PubMed] [Google Scholar]
- 118.Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014; 124:2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012; 367:1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant 2017; 52:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kroger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med 2016; 374:43–53. [DOI] [PubMed] [Google Scholar]
- 122.Martino R. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood 2006; 108:836–846. [DOI] [PubMed] [Google Scholar]
- 123.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol 2010; 28:405–411. [DOI] [PubMed] [Google Scholar]
- 124.Scott B, Deeg HJ, Storer B, et al. Targeted busulfan and cyclophosphamide as compared to busulfan and TBI as preparative regimens for transplantation in patients with advanced MDS or transformation to AML. Leuk Lymphoma 2004; 45:2409–2418. [DOI] [PubMed] [Google Scholar]
- 125.Kroger N, Iacobelli S, Franke GN, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC trial). J Clin Oncol 2017; 35:2157–2164. [DOI] [PubMed] [Google Scholar]
- 126.Beran M, Estey E, O’Brien S, et al. Topotecan and cytarabine is an active combination regimen in myelodysplastic syndromes and chronic myelomonocytic leukemia. J Clin Oncol 1999; 17:2819–2830. [DOI] [PubMed] [Google Scholar]
- 127.Wijermans PW, Ruter B, Baer MR, et al. Efficacy of decitabine in the treatment of patients with chronic myelomonocytic leukemia (CMML). Leuk Res 2008; 32:587–591. [DOI] [PubMed] [Google Scholar]
- 128.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 2002; 20:2429–2440. [DOI] [PubMed] [Google Scholar]
- 129.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007; 109:52–57. [DOI] [PubMed] [Google Scholar]
- 130.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aribi A, Borthakur G, Ravandi F, et al. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer 2007; 109:713–717. [DOI] [PubMed] [Google Scholar]
- 132.Costa R, Abdulhaq H, Haq B, et al. Activity of azacitidine in chronic myelomonocytic leukemia. Cancer 2011; 117:2690–2696. [DOI] [PubMed] [Google Scholar]
- 133.Ades L, Sekeres MA, Wolfromm A, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res 2013; 37:609–613. [DOI] [PubMed] [Google Scholar]
- 134.Alfonso A, Montalban-Bravo G, Takahashi K, et al. Natural history of chronic myelomonocytic leukemia treated with hypomethylating agents. Am J Hematol 2017; 92:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tendas A, Cupelli L, Siniscalchi A, et al. Azacitidine in chronic myelomonocytic leukemia: an effective and manageable approach. Mediterr J Hematol Infect Dis 2014; 6:e2014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iastrebner M, Jang JH, Nucifora E, et al. Decitabine in myelodysplastic syndromes and chronic myelomonocytic leukemia: Argentinian/South Korean multi-institutional clinical experience. Leuk Lymphoma 2010; 51:2250–2257. [DOI] [PubMed] [Google Scholar]
- 137.Pleyer L, Germing U, Sperr WR, et al. Azacitidine in CMML: matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk Res 2014; 38:475–483. [DOI] [PubMed] [Google Scholar]
- 138.Braun T, Itzykson R, Renneville A, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase II trial. Blood 2011; 118:3824–3831. [DOI] [PubMed] [Google Scholar]
- 139.Drummond MW, Pocock C, Boissinot M, et al. A multi-centre phase 2 study of azacitidine in chronic myelomonocytic leukaemia. Leukemia 2014; 28:1570–1572. [DOI] [PubMed] [Google Scholar]
- 140.Tantravahi SK, Szankasi P, Khorashad JS, et al. A phase II study of the efficacy, safety, and determinants of response to 5-azacitidine (Vidaza(R)) in patients with chronic myelomonocytic leukemia. Leuk Lymphoma 2016; 57:2441–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fianchi L, Criscuolo M, Breccia M, et al. High rate of remissions in chronic myelomonocytic leukemia treated with 5-azacytidine: results of an Italian retrospective study. Leuk Lymphoma 2013; 54:658–661. [DOI] [PubMed] [Google Scholar]
- 142.Thorpe M, Montalvao A, Pierdomenico F, et al. Treatment of chronic myelomonocytic leukemia with 5-Azacitidine: a case series and literature review. Leuk Res 2012; 36:1071–1073. [DOI] [PubMed] [Google Scholar]
- 143.Wong E, Seymour JF, Kenealy M, et al. Treatment of chronic myelomonocytic leukemia with azacitidine. Leuk Lymphoma 2013; 54:878–880. [DOI] [PubMed] [Google Scholar]
- 144.Santini V, Allione B, Zini G, et al. A phase II, multicentre trial of decitabine in higher-risk chronic myelomonocytic leukemia. Leukemia 2018; 32:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Subari S, Patnaik M, Alfakara D, et al. Hypomethylating agents are effective in shrinking splenomegaly in patients with chronic myelomonocytic leukemia. Leuk Lymphoma 2016; 57:1714–1715. [DOI] [PubMed] [Google Scholar]
- 146.Yi JH, Huh J, Kim HJ, et al. Genome-wide single-nucleotide polymorphism array-based karyotyping in myelodysplastic syndrome and chronic myelomonocytic leukemia and its impact on treatment outcomes following decitabine treatment. Ann Hematol 2013; 92:459–469. [DOI] [PubMed] [Google Scholar]
- 147.Meldi K, Qin T, Buchi F, et al. Specific molecular signatures predict decitabine response in chronic myelomonocytic leukemia. J Clin Invest 2015; 125:1857–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Duchmann M, Braun T, Kosmider O, et al. A two-gene classifier for chronic myelomonocytic leukemia (CMML) patients treated with hypomethylating agents (HMA): a report by the GFM. Blood 2015; 126:2872. [Google Scholar]
- 149.Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol 2011; 29:2521–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Issa JP, Roboz G, Rizzieri D, et al. Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose-escalation phase 1 study. Lancet Oncol 2015; 16:1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harel S, Cherait A, Berthon C, et al. Outcome of patients with high risk myelodysplastic syndrome (MDS) and advanced chronic myelomonocytic leukemia (CMML) treated with decitabine after azacitidine failure. Leuk Res 2015; 39:501–504. [DOI] [PubMed] [Google Scholar]
- 152.Xicoy B, Germing U, Jimenez MJ, et al. Response to erythropoietic-stimulating agents in patients with chronic myelomonocytic leukemia. Eur J Haematol 2016; 97:33–38. [DOI] [PubMed] [Google Scholar]
- 153.Hellstrom-Lindberg E, van de Loosdrecht A. Erythropoiesis stimulating agents and other growth factors in low-risk MDS. Best Pract Res Clin Haematol 2013; 26:401–410. [DOI] [PubMed] [Google Scholar]
- 154.Malcovati L, Hellstrom-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood 2013; 122:2943–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zandberg DP, Huang TY, Ke X, et al. Treatment and outcomes for chronic myelomonocytic leukemia compared to myelodysplastic syndromes in older adults. Haematologica 2013; 98:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hadjadj J, Michel M, Chauveheid MP, et al. Immune thrombocytopenia in chronic myelomonocytic leukemia. Eur J Haematol 2014; 93:521–526. [DOI] [PubMed] [Google Scholar]
- 157.Mughal TI, Cross NC, Padron E, et al. An International MDS/MPN Working Group's perspective and recommendations on molecular pathogenesis, diagnosis and clinical characterization of myelodysplastic/myeloproliferative neoplasms. Haematologica 2015; 100:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]