Abstract
The international, randomized phase 3 HD15 trial established 6xeBEACOPP as standard therapy for patients with newly diagnosed advanced-stage Hodgkin lymphoma (HL) within the German Hodgkin Study Group (GHSG). We performed a follow-up analysis to assess long-term efficacy and safety of this approach. Between 2003 and 2008, 2182 patients aged 18 to 60 years were recruited and randomized in a 1:1:1 ratio between 8 or 6 cycles of eBEACOPP or 8 cycles of the dose-dense BEACOPP-14 regimen, each followed by 30 Gy radiotherapy in case of positron emission tomography (PET)-positive residual lesions ≥2.5 cm. The study aimed at demonstrating non-inferiority regarding efficacy of the 2 experimental arms on a significance level of 2.5% each. The intention-to-treat analysis comprised 2126 patients with a median follow-up of 102 months. Ten-year progression-free survival was 81% (97.5% CI 77–85) with 8xeBEACOPP, 84% (80–87) with 6xeBEACOPP, and 84% (80–87) with 8xBEACOPP-14; the non-inferiority margin of 1.51 for the hazard ratio (HR) could be excluded for both comparisons (6xeBEACOPP, HR = 0.7, 97.5% CI 0.5–1.0; 8xBEACOPP-14, HR = 0.9, 97.5% CI 0.7–1.2). Overall survival at 10 years was 88% (85–91), 90% (88–93), and 92% (89–94), respectively. A total of 142 second malignancies corresponding to 10-year cumulative incidences of 10%, 7%, and 7% and standardized incidence ratios of 4.3, 2.5, and 2.8 were reported for 8xeBEACOPP, 6xeBEACOPP, and 8xBEACOPP-14, respectively. This updated analysis of the HD15 trial thus confirms the efficacy and reports on the long-term safety of a shortened first-line chemotherapy consisting of 6xeBEACOPP followed by PET-guided radiotherapy in advanced-stage HL.
Keywords: Advanced stages, Hodgkin lymphoma, long-term outcome
Introduction
The prognosis of patients with Hodgkin lymphoma (HL) has improved with the introduction of multiagent chemotherapy such as MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) or ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), resulting in a 5-year tumor control of about 65% and an overall survival of 75% at 5 years.1,2 In an attempt to further improve the prognosis of this patient cohort, the German Hodgkin Study Group (GHSG) developed a more intensive protocol termed escalated BEACOPP (eBEACOPP; bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated dose). In the randomized HD9 trial, this regimen was compared with the GHSG standard at that time, 8xCOPP/ABVD (cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine, dacarbazine), and a lower intensity BEACOPP variant termed baseline BEACOPP (bBEACOPP). Freedom from treatment failure (FFTF) at 5 years was 69% for COPP/ABVD, 76% for bBEACOPP, and 87% for eBEACOPP, respectively.3 Consequently, 8 cycles of eBEACOPP were established as standard of care for advanced-stage HL in the following GHSG trial HD12.4 In this trial, a dose reduction to 4 cycles of eBEACOPP followed by 4 cycles of bBEACOPP did not result in a meaningful reduction of treatment-associated toxicity. The subsequent randomized phase 3 HD15 trial showed the noninferiority of 6 cycles of eBEACOPP in terms of tumor control as compared to 8 cycles of eBEACOPP, with 5-year FFTF of 89% and 84%, respectively.5 As a consequence of HD15, the GHSG standard of care for advanced-stage HL changed to 6 cycles of eBEACOPP followed by positron emission tomography (PET)-guided radiotherapy. The original report of the HD15 trial had a median follow-up of 48 months with 2126 patients included. To better understand the long-term outcomes and late side effects of treatment, we performed an update analysis on HD15 with substantially longer follow-up of 102 months.
Material and methods
Study design and patients
This open-label, multicenter, international, randomized phase 3 non-inferiority trial was conducted in more than 400 hospitals and private practices in Germany, the Czech Republic, Switzerland, the Netherlands, and Austria. We recruited patients aged 18 to 60 years with newly diagnosed, histology-proven, classical, or nodular lymphocyte-predominant HL in advanced stages (ie, clinical stage III-IV or stage II with B symptoms and 1 or both risk factors of large mediastinal mass [≥1/3 of the maximal thoracic diameter] or extranodal lesions). All patients provided written informed consent before study entry according to the Good Clinical Practice guidelines of the International Conference on Harmonisation and national regulations. The trial was designed by the GHSG steering committee, approved by the ethics committees of all participating countries and centers, and registered with Current Controlled Trials (IRCTN32443041).
Patients were randomly assigned to receive either 8 cycles of eBEACOPP, 6 cycles of eBEACOPP, or 8 cycles of the more dose-dense BEACOPP-14 in a 1:1:1 ratio. In all 3 groups, radiotherapy was recommended in case of lesions of at least 2.5 cm in the largest diameter with residual FDG uptake after chemotherapy. Follow-up examinations were performed and documented at increasing intervals after therapy completion, with computed tomography only performed at the investigators’ discretion. Relapse of HL or occurrence of a second malignancy was confirmed by biopsy locally, and reports were verified by GHSG trial physicians before database entry. Details of inclusion criteria, procedures and statistics as well as final results of the trial have been published.5
Statistical analysis
In order to assess long-term treatment outcome, we used progression-free survival (PFS), defined as the time from completion of staging until progressive disease, relapse, or death from any cause. If none of these events had occurred, PFS was censored at the date of last information on the disease status. Overall survival was defined as time from completion of staging until death from any cause, or censored at the date of last information on the patient being alive. In cases of an information lag of more than 1 year, information on survival status was obtained from residents’ registration offices wherever possible. To assess the long-term outcome of patients with progression or relapse, within this subgroup we report second PFS, defined as time from first progression or relapse to further relapse or death from any cause, or censored at the date of last information on the disease status. Survival outcomes were analyzed according to Kaplan-Meier and compared between treatment groups using hazard ratios (HR) with CIs obtained from Cox regression models, whereby models for PFS and overall survival were adjusted for the stratification factors age (<50 years vs ≥50 years), sex, Ann Arbor stage (IIB or IIIA vs IIIB or IV), international prognostic score (IPS, <3 vs ≥3) and hemoglobin level (>12 vs ≤12 g/dL). Cumulative incidences of second malignancies were estimated according to Kaplan-Meier accounting for death as a competing risk, and compared between treatment groups using subdistribution Hazard Ratios (sHR) and CIs obtained from Cox regression models. We estimated standardized incidence ratios (SIR) for second malignancies by dividing observed by expected event numbers with respect to age- and sex-specific reference values for the German population,6 taking into account the observed person years in each sex/age group throughout the total study and follow-up period. Standardized mortality ratios (SMR) were estimated accordingly.7 In addition, we restricted mortality analyses to the long-term follow-up period starting 5 years after first diagnosis (SMRlong-term), including only patients having survived and being observed for at least 5 years. Patient characteristics were analyzed descriptively. All analyses were performed according to the intention-to-treat (ITT) principle and on a per-protocol analysis set, which was based on data from the final analysis. The significance level was set to 5% for single comparisons and adjusted to 2.5% in case of 2 parallel tests (8xeBEACOPP vs 6xeBEACOPP and 8xeBEACOPP vs 8xBEACOPP-14). We used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) for all analyses.
Results
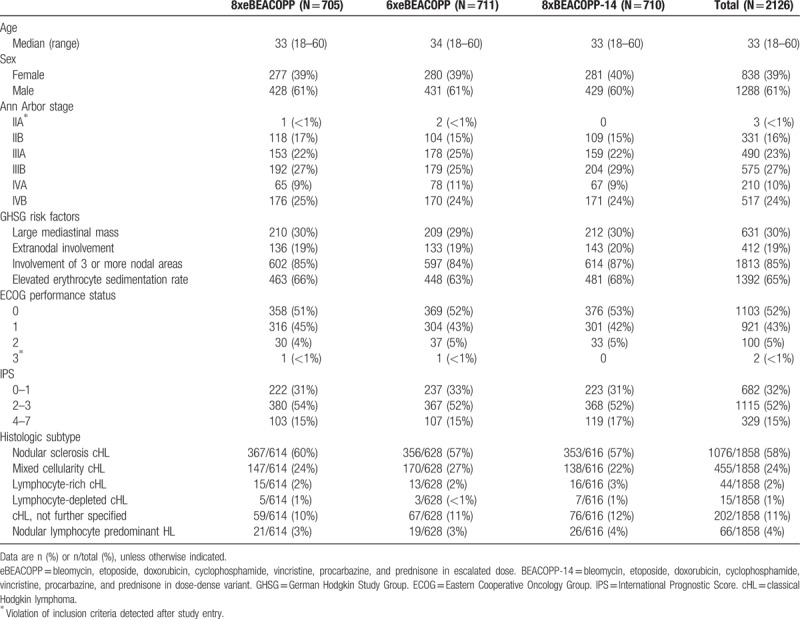
Out of 2182 patients enrolled between January 28, 2003 and April 18, 2008, 56 were excluded from the ITT analysis due to disconfirmation of their HL diagnosis (n = 44), withdrawal of consent (n = 3) or revision of staging and participation in another study before start of treatment (n = 9), resulting in 2126 patients finally analyzed (Fig. 1). Baseline characteristics are presented in Table 1 and were similar between randomized treatment groups. The median age was 33 years (range 18–60), 61% were male, and 84% had stage III or IV disease.
Figure 1.
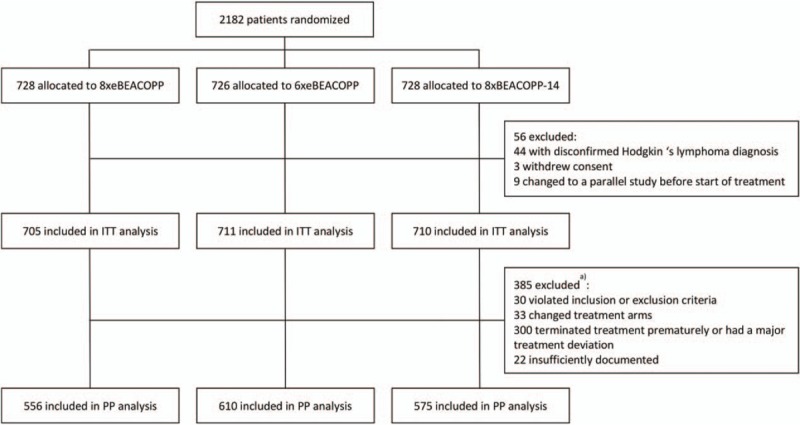
Trial profile. ITT is defined as the set of all patients except for those with disconfirmed diagnosis of Hodgkin lymphoma, withdrawal of consent or revision of staging and participation in another study before start of treatment. PP contains all ITT patients without severe protocol deviation, having complete therapy documentation or progressive disease or death during therapy. BEACOPP-14 = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in dose-dense variant, eBEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated dose, ITT = intention-to-treat, PP = per-protocol. (a) Analysis set definition based on data from final analysis.
Table 1.
Baseline Characteristics of the Intention-to-Treat Set
Among 1987 patients who were alive at the time of the last analysis published in 2012, updated follow-up information was available for 89%. The last information was obtained from residents’ registries in 17% of patients. Median follow-up was 112 months for survival and 102 months for disease status, respectively, and did not differ between trial arms.
With 10-year PFS estimates of 81% (97.5% CI 77%–85%), 84% (80%–87%), and 84% (80%–87%) for 8xeBEACOPP, 6xeBEACOPP, and 8xBEACOPP-14, respectively, noninferiority of both experimental treatment regimens could be confirmed with longer follow-up (Fig. 2A): the HR and respective 97.5% CI were 0.7 (0.5–1.01) for 6xeBEACOPP and 0.9 (0.7–1.2) for 8xBEACOPP-14, respectively, versus 8xeBEACOPP, and both excluded the noninferiority margin of 1.51. In the per-protocol analysis comprising 1741 patients (Fig. 3A), 6xeBEACOPP proved to be significantly superior to 8xeBEACOPP, with a HR of 0.7 (97.5% CI 0.5–0.9). PFS according to response to chemotherapy is displayed in Figure 4; 10-year estimates were 85% for those with complete remission, 88% for those with partial remission including residues of at least 2.5 cm in the largest diameter and a negative PET, and 85% for those receiving consolidating radiotherapy due to partial remission including residues of at least 2.5 cm in the largest diameter and a positive PET.
Figure 2.
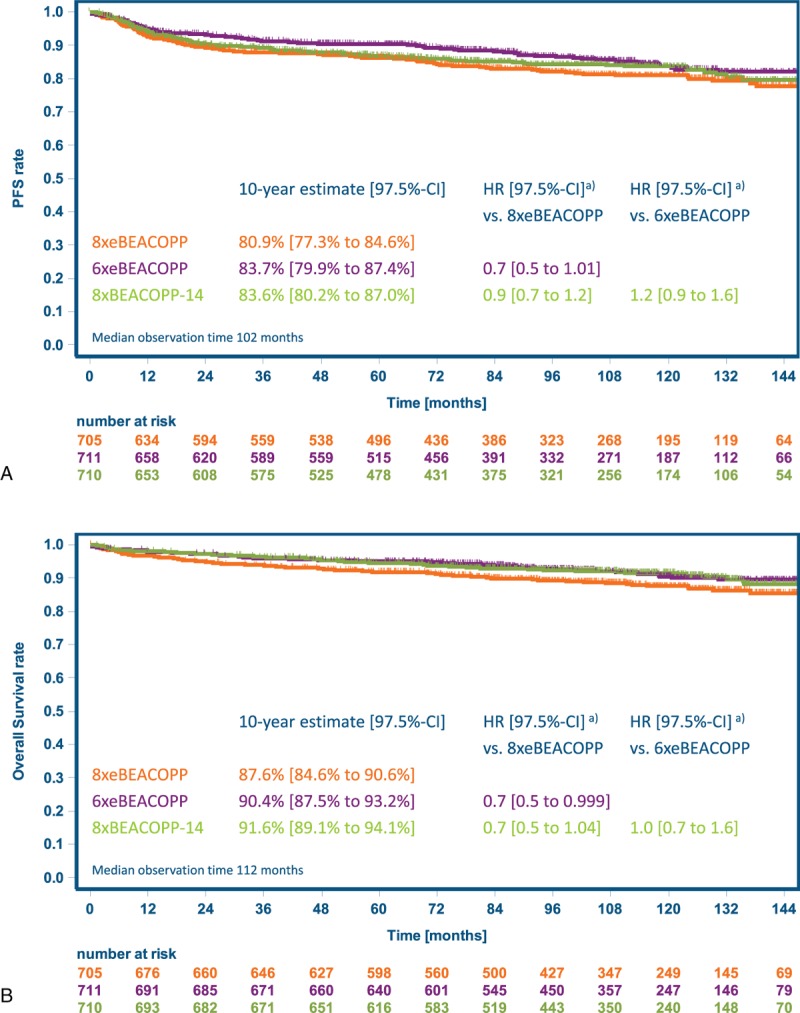
Kaplan-Meier estimates for the intention-to-treat set. (A) Progression-free survival. (B) Overall survival. BEACOPP-14 = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in dose-dense variant, eBEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated dose, HR = hazard ratio, PFS = progression-free survival. (a) Hazard ratios adjusted for stratification factors age, sex, Ann Arbor stage, international prognostic score and hemoglobin level.
Figure 3.
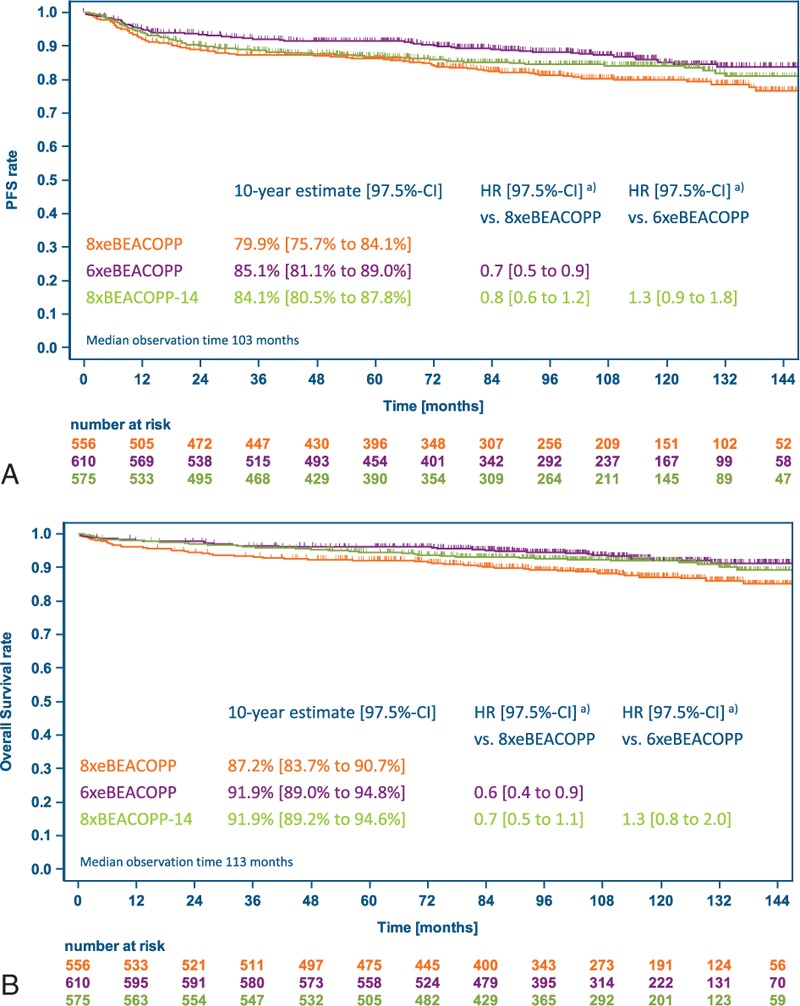
Kaplan-Meier estimates for the per-protocol set. (A) Progression-free survival. (B) Overall survival. BEACOPP-14 = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in dose-dense variant, eBEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated dose, HR = hazard ratio, PFS = progression-free survival. (a) Hazard ratios adjusted for stratification factors age, sex, Ann Arbor stage, international prognostic score and hemoglobin level.
Figure 4.
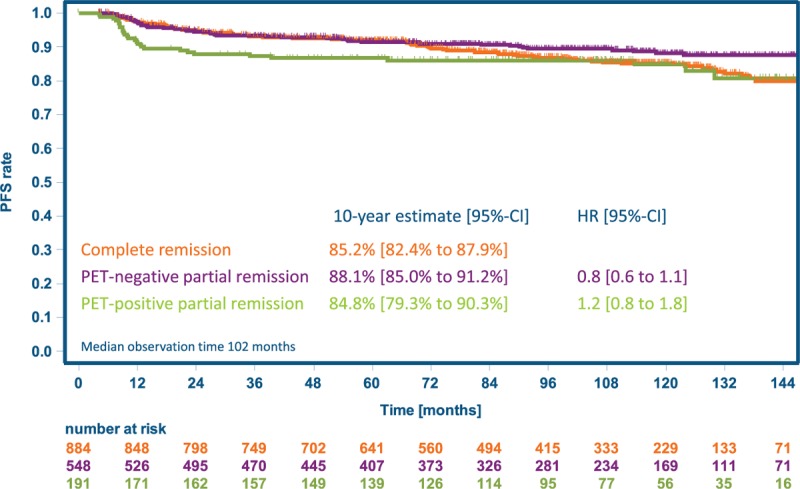
Progression-free survival by outcome after chemotherapy. Analysis includes patients with complete remission after chemotherapy and those with a partial remission, residual lesions of at least 2.5 cm in the largest diameter and centrally reviewed PET available. Radiotherapy was recommended in case of a positive PET scan. HR = hazard ratio, PFS = progression-free survival.
Among 209 patients with progression or relapse (Table 2), second PFS at 5 years was 54% (95% CI 47%–61%, Fig. 5), without differences between treatment groups (data not shown). With a median follow-up of 87 months after first progression or relapse, 84 (40%) of these patients died, including 51 deaths attributed to HL and 9 to toxicity of salvage therapy.
Table 2.
Long-Term Outcomes of the Intention-to-Treat Set
Figure 5.
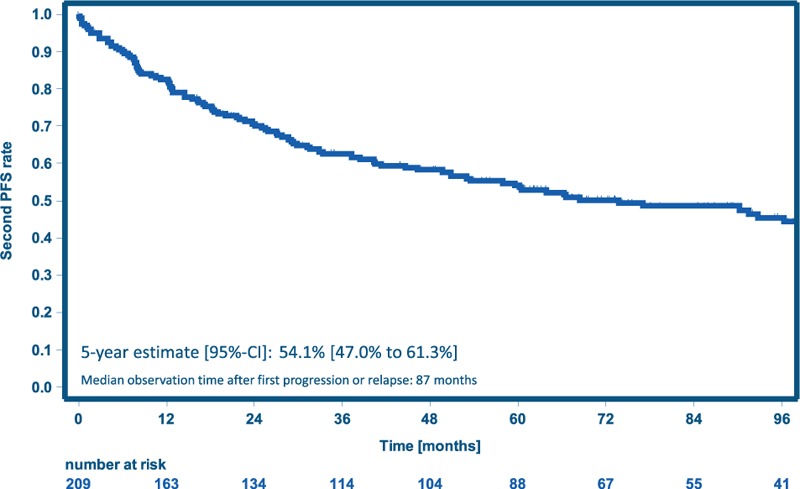
Second PFS for patients with progression or relapse. PFS = progression-free survival.
In total, 202 patients died within the follow-up period (Table 2). The SMR ranged from 4.5 (6xeBEACOPP) to 7.1 (8xeBEACOPP) and thus indicates an increased risk of death with respect to age- and sex-specific German reference data among all groups.7 When restricting the analysis to the long-term follow-up period starting 5 years after initial diagnosis, the most frequent cause of death was second malignancy (34 of 72 events), while at the time of the final analysis, most deaths had been attributed to HL. SMRlong-term, ranging from 3.2 to 4.0 (Table 2), was lower compared with the total study period, but still significantly elevated compared to the reference data.
Ten-year estimates for overall survival were 88% (97.5% CI 85%–91%), 90% (88%–93%), and 92% (89%–94%) with 8xeBEACOPP, 6xeBEACOPP and 8xBEACOPP-14, respectively, with a significant superiority of 6xeBEACOPP over 8xeBEACOPP (HR 0.7, 97.5% CI 0.5–0.999, P = 0.0245) and a trend toward superiority of 8xBEACOPP-14 over 8xeBEACOPP (HR 0.7, 97.5% CI 0.5 to 1.04, P = 0.05, Fig. 2B; per-protocol analysis: Fig. 3B).
A total of 142 patients were diagnosed with a second malignancy within the observed follow-up period (Table 2), including 40 patients with therapy-related acute myeloid leukemia or myelodysplastic syndrome (MDS), 3 with acute lymphoblastic leukemia (ALL), 29 with non-Hodgkin lymphoma (NHL) and 71 with solid tumors (1 patient was diagnosed with secondary acute myeloid leukemia and then had a solid tumor). Of the 75 solid tumors diagnosed (4 patients had multiple tumors), most were in the lung (n = 20), digestive organs (n = 17), and skin (n = 14). Patients diagnosed with a second malignancy were significantly older compared with those without a second cancer diagnosis; the median age at study entry was 48 and 32 years, respectively (P < 0.001). The SIR ranged from 2.5 (6xeBEACOPP) to 4.3 (8xeBEACOPP) and was significantly elevated with respect to age- and sex-specific German reference data in all 3 groups (Table 2). In total, 59 of the 142 affected patients (42%) died from their malignancy, with the highest proportion among those diagnosed with secondary leukemia or MDS (26 of 43 patients, 60%). Cumulative incidences of second malignancies after 10 years were 10% (97.5% CI 7%–13%), 7% (4%–9%), and 7% (4%–10%) with 8xeBEACOPP, 6xeBEACOPP and 8xBEACOPP-14, respectively, suggesting a possible trend toward higher incidence in the standard arm (Fig. 6A). Particularly, there were significantly more cases of secondary leukemia or MDS after treatment with 8xeBEACOPP, with 10-year cumulative incidences of 5% (97.5% CI 3%–7%), 1% (0%–1%), and 2% (1%–4%), respectively (Fig. 6B). There were also no differences between treatment arms regarding the occurrence of NHL or solid tumors (Table 2). There were also no differences regarding the occurrence of second malignancies between those 225 patients receiving consolidating radiotherapy and those 1901 patients who did not (Fig. 6C). Of the 3 solid tumors that occurred in patients who had received radiotherapy within our trial, only 1 was located within the radiotherapy field.
Figure 6.
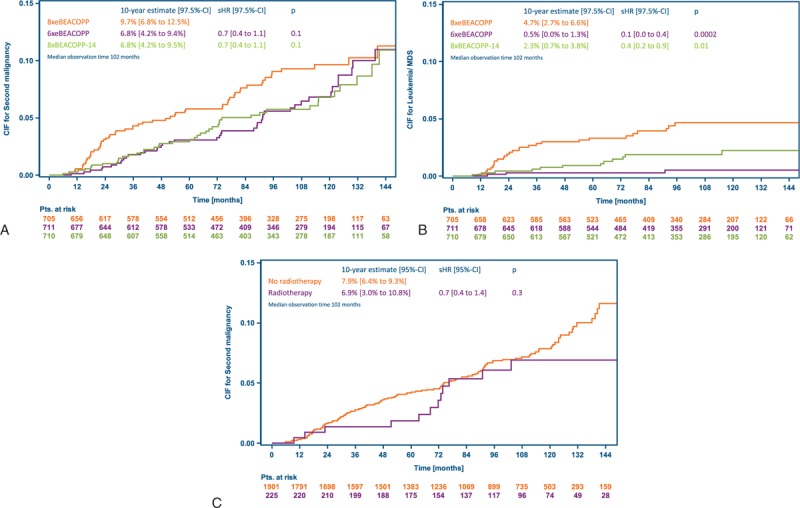
Cumulative incidence of second malignancies accounting for death as a competing risk. (A) Cumulative incidence of any second malignancy by treatment arm. (B) Cumulative incidence of secondary leukemia and myelodysplastic syndrome by treatment arm. (C) Cumulative incidence of any second malignancy by radiotherapy group (radiotherapy was recommended in case of partial remission including residual lesions of at least 2.5 cm in the largest diameter and a positive PET after chemotherapy). BEACOPP-14 = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in dose-dense variant, CIF = cumulative incidence function, eBEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone in escalated dose, MDS = myelodysplastic syndrome, sHR = subdistribution hazard ratio.
Discussion
The prospective randomized HD15 trial enrolled a total of 2182 patients with advanced-stage HL from 5 European countries and is the largest trial performed in this malignancy so far. With 10-year overall survival estimates of 88% and 90%, respectively, a significant benefit of 6xeBEACOPP over 8xeBEACOPP was detected. In addition, there were more cases of secondary leukemia/MDS with 10-year cumulative incidences of 5% with 8xeBEACOPP and 1% with 6xeBEACOPP, respectively; the proportion of patients diagnosed with solid tumors was 3%.
The key objective in the treatment of HL, particularly regarding the first line of therapy, is to find the optimal balance between tumor control and treatment intensity. The HD15 trial can serve as an example, showing that 6 cycles of eBEACOPP are at least equally effective and at the same time less toxic as compared to the prior standard of 8 cycles.5 Following HD15, 6 cycles of eBEACOPP became the new standard in advanced-stage HL. In addition, radiation could be confined to PET-positive residues measuring 2.5 cm or more. The favorable long-term PFS of patients with a PET-negative partial remission after chemotherapy demonstrated in the current update supports this PET-guided radiation strategy.
More recently, PET in advanced-stage HL has been used to discriminate low-risk from high-risk patients.8 The UK RATHL trial showed that after 2 cycles of ABVD, PET-negative HL patients can be safely spared from additional bleomycin.9 The GHSG was also able to further reduce the intensity of treatment in advanced-stage HL: The randomized phase 3 HD18 trial demonstrated that patients with a negative PET after 2 cycles of eBEACOPP need only 2 more cycles, rendering 4 cycles of eBEACOPP the new standard of care in early-responding patients.10 Thus, treatment with 6xeBEACOPP as established in the HD15 trial are still considered standard of care in patients with advanced-stage HL and a positive PET after 2 cycles of eBEACOPP.11
With an intensified front-line treatment particularly in advanced-stage patients, the number of relapses has been substantially reduced over time.4,5,10,11 Long-term follow-up reports in HL are rare but particularly relevant in order to better understand risk factors for late relapses and how to better avoid long-term toxicity in this group of young cancer patients. As far as long-term results are concerned, only smaller case series with rather few patients who relapsed after 5 years in remission have been reported to date.12–14 The GHSG retrospectively analyzed patients included in the HD7 to HD12 trials with a median observation period of 10 years.15,16 Patients who experienced a relapse after more than 5 years in remission were compared with those in continued remission for more than 5 years. From a total of 4953 patients who had achieved CR after first-line treatment and were relapse-free for at least 5 years, 141 patients with late-relapsed HL were identified. Interestingly, the cumulative incidence of late HL relapses rose in a linear fashion with no plateau observed and an estimate of 6.9% late relapses at 20 years after initial diagnosis. More importantly, the risk for developing HL after being free for at least 5 years was about 85-fold increased as compared to an age- and sex-matched general population. On the other hand, more than half of the patients could still be salvaged even if they had received an intensive combination as initial therapy.
Despite the fact that the majority of HL patients achieve lasting complete remission upon first-line treatment, there still is a considerable risk of late events such as second malignancies,17–20 organ damage to lung,21 heart,22 as well as infertility23 and fatigue.24,25 In this update analysis of HD15, there were significantly more cases of secondary leukemia/MDS after treatment with 8xeBEACOPP, with 10-year cumulative incidences of 5% as compared to 1% with 6xeBEACOPP and 2% with 8xBEACOPP-14, respectively. Most secondary leukemia/MDS occurred in the 5-year observation period (n = 29)26 as compared to 14 new cases in years 5 to 10, respectively. In contrast, the number of patients diagnosed with solid tumor rose from a total of 24 (1.2%) at 5 years of follow-up to 71 (3%) at the present 10-year analysis, implying the particular relevance of keeping track of the development of second cancer even beyond this 10-year follow-up report.
As shown in this update of the HD15 trial, HL patients are at increased risk of death compared with an age- and sex-matched general population: the reported SMRs were 7.1 for 8 cycles of eBEACOPP and 4.5 for 6 cycles of eBEACOPP, respectively. With longer follow-up, death from HL has become less common; on the other hand, other causes of death such as second cancers were diagnosed more frequently. Importantly, when the mortality analysis was restricted to the long-term follow-up period, SMRs still implied a significantly elevated risk of death also in patients who survived the first 5 years after their HL diagnosis. Thus, there is clearly a need for more specific new drugs particularly for this young patient population. In addition, the response rates to checkpoint inhibitors such as nivolumab or pembrolizumab in very heavily pretreated HL patients indicate that the Hodgkin and Reed-Sternberg cells are highly susceptible to the blockade of the PD1-PD ligand axis.27,28 These agents might therefore be used to further decrease the intensity of conventional chemo- and or radiotherapy in HL.
The major limitation of the current analysis is the uncertainty related to the number of events to be observed with even longer follow-up. Here, in particular the number of second cancers and their outcomes will be extremely relevant. In addition, we have no comprehensive long-term data on cardiovascular complications such as heart failure. The major strength of this analysis is the large data set and the extended observation period allowing reasonable conclusions. In particular, we gained updated information from nearly 90% of our patients.
The current follow-up analysis of the GHSG HD15 trial clearly underscores the relevance of comprehensive long-term outcome analyses in HL patients and adds relevant data on both, tumor control and safety.
Acknowledgments
We thank participating patients for joining the HD15 study helping to improve the treatment for HL. We also like to thank the families and treating physicians.
We thank all participating GHSG HD15 centers for their continuous support.
Footnotes
Funding/support: The study has been supported by the Deutsche Krebshilfe e.V. (German Cancer Aid) and the Swiss State Secretariat for Education, Research and Innovation (SERI).
Trial registration: The HD15 trial was registered with Current Controlled Trials, number ISRCTN32443041.
Author contributions: AE, JM, TP, JM, JMZ, ZK, DAE, MS and RG directed clinical activities at participating study centers. HS led the reference pathology. HE, CK, MD and MF did the central PET review. HG led the statistical analyses of the data. MF directed activities at the GHSG central office. AE, VD, and PB led the design of the study protocol. AE is the principal investigator of the study. AE and HG wrote the first draft of the report. All authors contributed to data interpretation, reviewed the draft, and approved the final version of this report.
Disclosure: The Deutsche Krebshilfe reviewed the trial protocol for adherence to good clinical practice. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data and the corresponding author had final responsibility for the decision to submit for publication. The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med 1992;327:1478–1484. [DOI] [PubMed] [Google Scholar]
- 2.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 2013;31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl V, Franklin J, Pfreundschuh M, et al. German Hodgkin's Lymphoma Study Group. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med 2003;348:2386–2395. [DOI] [PubMed] [Google Scholar]
- 4.Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin's lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol 2011;29:4234–4242. [DOI] [PubMed] [Google Scholar]
- 5.Engert A, Haverkamp H, Kobe C, et al. German Hodgkin Study Group; Swiss Group for Clinical Cancer Research; Arbeitsgemeinschaft Medikamentöse Tumortherapie. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet 2012;379:1791–1799. [DOI] [PubMed] [Google Scholar]
- 6.Age and sex standardized incidence rates 2013, Association of Population-based Cancer Registries in Germany (GEKID), www.gekid.de. [Google Scholar]
- 7.Life tables 2013/2015, Federal Statistical Office (DESTATIS), 2017, www.destatis.de. [Google Scholar]
- 8.Juweid ME, Stroobants S, Hoekstra OS, et al. Imaging Subcommittee of International Harmonization Project in Lymphoma. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571–578. [DOI] [PubMed] [Google Scholar]
- 9.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med 2016;374:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borchmann P, Görgen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin. Lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017. [DOI] [PubMed] [Google Scholar]
- 11.Borchmann P, Haverkamp H, Lohri A, et al. Progression-free survival of early interim PET-positive patients with advanced stage Hodgkin's lymphoma treated with BEACOPP escalated alone or in combination with rituximab (HD18): an open-label, international, randomised phase 3 study by the German Hodgkin Study Group. Lancet Oncol 2017;18:454–463. [DOI] [PubMed] [Google Scholar]
- 12.Provencio M, Salas C, Millán I, et al. Late relapses in Hodgkin lymphoma: a clinical and immunohistochemistry study. Leuk Lymphoma 2010;51:1686–1691. [DOI] [PubMed] [Google Scholar]
- 13.Bodis S, Henry-Amar M, Bosq J, et al. Late relapse in early-stage Hodgkin's disease patients enrolled on European Organization for Research and Treatment of Cancer protocols. J Clin Oncol 1993;11:225–232. [DOI] [PubMed] [Google Scholar]
- 14.Meyer RM, Gospodarowicz MK, Connors JM, et al. NCIC Clinical Trials Group; Eastern Cooperative Oncology Group. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med 2012;366:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasse S, Bröckelmann PJ, Görgen H, et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: updated analyses of the GHSG HD7, HD8, HD10 and HD11 trials. J Clin Oncol 2017;35:1999–2007. [DOI] [PubMed] [Google Scholar]
- 16.Bröckelmann PJ, Görgen H, Kohnhorst C, et al. Late relapse of classical hodgkin lymphoma: an analysis of the German Hodgkin Study Group HD7 to HD12 trials. J Clin Oncol 2017;35:1444–1450. [DOI] [PubMed] [Google Scholar]
- 17.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med 2015;373:2499–2511. [DOI] [PubMed] [Google Scholar]
- 18.Engert A, Younes A (eds). Hodgkin Lymphoma: A Comprehensive Overview. Chapter 24: Hodgson DC, van Leeuwen FE. Second Malignancy Risk After Treatment of Hodgkin Lymphoma. 375–409. Springer International Publishing 2015. [Google Scholar]
- 19.Travis LB, Demark Wahnefried W, Allan JM, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol 2013;10:289–301. [DOI] [PubMed] [Google Scholar]
- 20.van Eggermond AM, Schaapveld M, Lugtenburg PJ, et al. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma. Blood 2014;124:319–327. [DOI] [PubMed] [Google Scholar]
- 21.Martin WG, Ristow KM, Habermann TM, et al. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. J Clin Oncol 2005;23:7614–7620. [DOI] [PubMed] [Google Scholar]
- 22.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 23.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 2007;99:206–214. [DOI] [PubMed] [Google Scholar]
- 24.Kreissl S, Müller H, Görgen H, et al. Cancer-related fatigue in patients with and survivors of Hodgkin's lymphoma: A longitudinal study of the German Hodgkin Study Group. Lancet Oncol 2016;17:1453–1462. [DOI] [PubMed] [Google Scholar]
- 25.Behringer K, Görgen H, Müller H, et al. on behalf of the German Hodgkin Study Group. Cancer-related fatigue in patients with and survivors of Hodgkin Lymphoma: the impact on treatment outcome and social reintegration. J Clin Oncol 2016;34:4329–4337. [DOI] [PubMed] [Google Scholar]
- 26.Eichenauer DA, Thielen I, Haverkamp H, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood 2014;123:1658–1664. [DOI] [PubMed] [Google Scholar]
- 27.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016;17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R, Zinzani PL, Fanale MA, et al. KEYNOTE-087. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017;35:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]


