Supplemental Digital Content is available in the text
Abstract
Current treatment of patient with mantle cell lymphoma (MCL) is insufficient and does not result in cure. To assess the efficacy and safety of maintenance therapy for patients with MCL, we performed a systematic review and meta-analysis of randomized controlled trials. Six trials randomizing 858 patients were included in the meta-analysis. In 5 trials, maintenance therapy consisted of rituximab. The pooled hazard ratio (HR) of death with rituximab maintenance compared to observation was 0.79, 95% CI 0.58 to 1.06 (4 trials, 737 patients). Progression free survival was longer with rituximab maintenance in each of the trials and in the pooled analysis (HR 0.58, 95% CI 0.45–0.73). The risk of neutropenia was higher with maintenance compared to observation risk ratio (RR) 1.31, 95% CI 1.03 to 1.66. None of the trials reported on quality of life outcomes. The grade 3 to 4 infection rate was 7% in each of the treatment groups. The risk of grade 3 to 4 infection was not affected by allocation to maintenance. Rituximab maintenance is recommended after R-CHOP or R-cytarabine-containing induction in the frontline setting for transplant eligible and ineligible patients, and after R-CHOP in the relapse setting. It is unclear if maintenance is of benefit after different induction chemotherapy such as bendamustine or fludarabine. It is too early to conclude on other type of maintenance for MCL patients.
Introduction
The standard therapy for patients with mantle cell lymphoma (MCL) is chemotherapy and rituximab.1 The type of chemotherapy depends primarily upon patient eligibility to transplantation. Younger and fit patients are usually treated with rituximab and cytarabine-containing induction followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT), and older patients are treated with rituximab and a less intensive chemotherapy as cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP), or bendamustine. After responding to initial therapy patients with MCL inevitably relapse, and their overall survival curve shows no plateau. Median overall survival with intensive therapy is about 8 years, but with less intensive regimens survival is <5 years.2,3 Maintenance therapy has the potential to improve disease control, duration of response, and survival. The introduction of nonchemotherapy drugs for MCL as monoclonal antibodies (ie, rituximab), proteasome inhibitors (ie, bortezomib), Bruton tyrosine kinase inhibitors, and immunomodulatory drugs as lenalidomide and interferon-alfa enabled their incorporation as maintenance therapy. To assess the efficacy and safety of any maintenance treatment for patients with mantle cell lymphoma, we conducted a systematic review and meta-analysis of randomized controlled trials.
Methods
Study selection
We included randomized controlled trials comparing any maintenance treatment to no maintenance (observation or placebo) or to another type of maintenance treatment in patients with mantle cell lymphoma, after induction treatment. Maintenance was defined as any treatment given repeatedly after end of induction therapy. We included trials regardless of publication status, date of publication, and language of the manuscript.
The primary outcome was overall survival (OS). Secondary outcomes were progression free survival (PFS), quality of life, response rates, and toxicity.
Data sources
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 4, 2017), PubMed (1966 to December 2017), and conference proceedings of the American Society of Clinical Oncology Annual Meeting (1995–2017), the American Society of Hematology (1995–2017), and the European Hematology Association (2002–2017). We searched databases of ongoing trials: http://www.controlled-trials.com/, http://www.clinicaltrials.gov/ct. We cross-searched “mantle cell lymphoma” with “maintenance.” We combined the search terms with the highly sensitive search strategy for identifying reports of randomized controlled trials in the MEDLINE search and checked the citations of included trials to capture all studies.
Data extraction and quality assessment
Two reviewers (LV and RG) independently extracted data regarding case definitions, characteristics of patients and outcomes. Risk of bias was evaluated to 5 domains and assessed allocation concealment, generation of the allocation sequence, blinding, selective outcome reporting and incomplete outcome reporting according to the criteria specified in the Cochrane Handbook version 5.1.0.24 In the event of disagreement between the 2 reviewers in any of the above, a third reviewer (AG) also extracted the data. We contacted the first or corresponding author of each included trial in order to obtain additional information on the trials.
Data synthesis and statistical analysis
Hazard ratios (HRs) and variances for time-to-event outcomes were estimated and pooled according to the inverse of variance method4,5 (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). A HR <1.0 was regarded as being in favor of maintenance. Relative risks (RRs) and 95% confidence intervals (CIs) for dichotomous data were estimated using the Mantel–Haenszel method.6 We used a fixed-effect model to pool the outcomes. We assessed heterogeneity of trial results by the chi-squared test of heterogeneity and the I2 statistic of inconsistency.7 Statistically significant heterogeneity was defined as P value <0.1 or an I2 statistic >50%. We explored potential sources of heterogeneity through stratifying the patient subgroups given below, allocation concealment, blinding, and size of studies. We used random-effect model instead of fixed-effect model in a sensitivity analysis.
We planned to perform subgroup analysis by treatment line (first line; ≥second line); induction regimen; and transplant eligibility (high dose chemotherapy and ASCT before maintenance). Differences between subgroups were assessed using the chi-squared test for difference between subgroups. All statistical tests were 2-sided. We examined the funnel plot for OS to estimate the effect of small study size (ie, publication bias).7 We used the GRADE system for assessment of quality of the evidence.8
Results
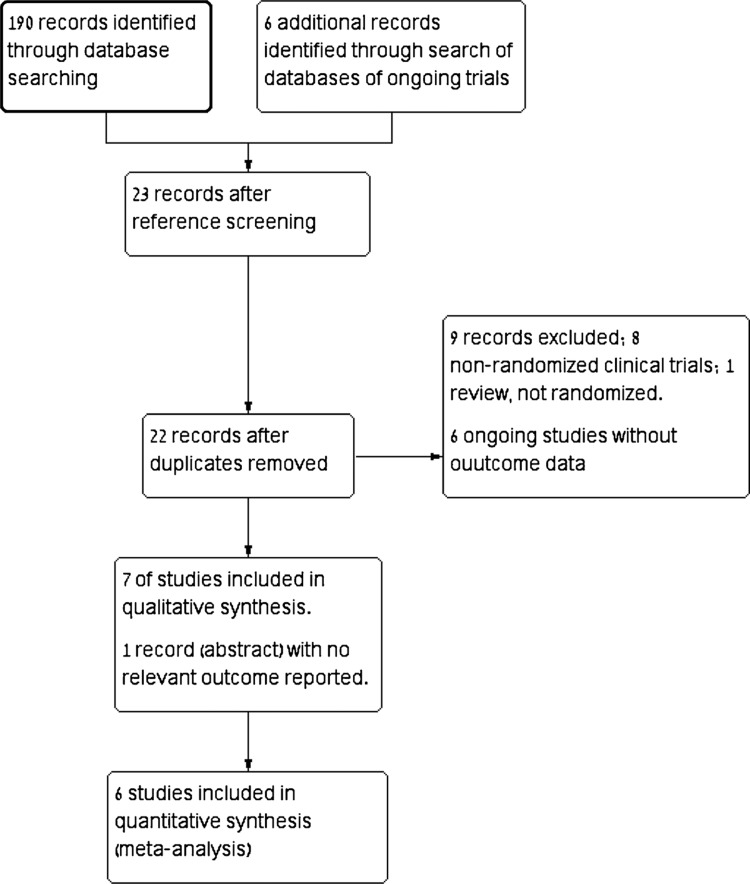
Of 190 titles and abstracts screened, 16 were relevant and retrieved for full details, of them 9 publications were excluded (Supplemental Digital Content). Additional 6 ongoing trials were identified but none had outcome data.9–13 Seven trials fulfilled inclusion criteria and were included in the systematic review,14–21 one of which did not report relevant clinical outcomes (Fig. 1).22 Thus 6 trials were included in the meta-analysis.
Figure 1.
Flow diagram of included trials.
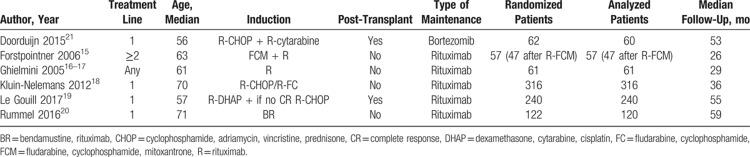
Eight hundred fifty-eight patients were randomized in 6 trials published between 2004 and 2017. Median follow up ranged from 26 to 59 months. Median age of patients ranged between 56 and 70 years. Patients receiving maintenance after first-line induction therapy were included in 4 trials,14,18–21 patients receiving maintenance after at least second-line induction (ie, with relapse/refractory disease) were included in 1 trial,15 and 1 trial included patients with any line of induction therapy.17 Two trials included only transplant ineligible patients,18,20,23 and 2 trials included patients after ASCT.19,21
Induction chemotherapy consisted of bendamustine,20 CHOP or fludarabine/cyclophosphamide (FC),18 FCM,15 and cytarabine in 2 trials.19,21 Rituximab was added to chemotherapy in 4 trials,14,18–21,23 patients were randomized to rituximab or no rituximab in addition to chemotherapy in 1 trial,15 and induction consisted only of rituximab in 1 trial (Table 1).17
Table 1.
Characteristics of Included Trials
In all the trials, patients who responded (complete or partial response) to induction therapy were randomized after the end of induction. Maintenance therapy included rituximab in 5 trials and bortezomib in 1 trial.21 The schedule of rituximab was 1 dose of 375 mg/m2 every 2 months for 8 months (4 doses),17 for 2 years (12 doses),20 for 3 years (18 doses),19 until progression,18 and 2 courses of 4 weekly doses 6 months apart.15 Bortezomib 1.3 mg/m2 was given every 2 weeks for 2 years. Maintenance was compared to observation (4 trials)15,17,19–21,23 or to interferon-alfa (1 trial).14,18
Risk of bias in included studies
Risk of bias of included trials by domains of the Cochrane's tool is presented in supplement material (Supplemental Digital Content) and detailed below.
Three trials were judged at low risk of selection bias,15,17,18 in the 3 remaining trials methods of allocation concealment and generation were not reported. Two of them were reported as abstracts.20,21,23 Blinding of patients and personnel was not done in all the trials.
Five trials were judged at low risk of attrition bias as they reported adequately on drop outs after randomization (number in each group and reasons for drop out). All trials were judged at low risk of reporting bias as clinically important outcomes including overall survival or death were addressed.
Overall survival
Rituximab maintenance vs observation or interferon-alfa
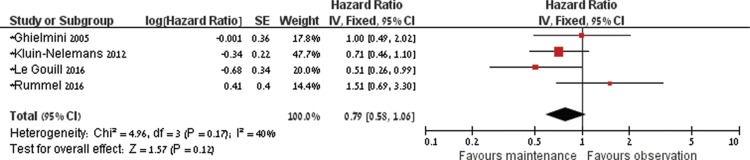
Four of the 5 trials that evaluated rituximab maintenance (737 patients) reported enough data for estimation of death as survival analysis. One trial in which MCL patients represent a subgroup did not report enough data to be included in the analysis. HR of death with rituximab maintenance versus observation or interferon-alfa was 0.79, 95% CI 0.58 to 1.06, I2 of heterogeneity = 40%, P = 0.17 (Fig. 2).15–17,19,20 In 5 of the trials14–19 the point estimate of overall survival was in favor of rituximab maintenance, and in 1 trial it was against it.20
Figure 2.
Forest plot of overall survival with maintenance treatment compared to no maintenance.
In sensitivity analysis of death as dichotomous data that allowed pooled analysis of all 6 trials, the RR of death was 0.71, 95% CI 0.57 to 0.88, I2 of heterogeneity = 55%, P = 0.06.
The quality of overall survival result was graded as moderate (downgraded due to the heterogeneity in some patient characteristics and induction regimens). Visual inspection of the funnel plot of the primary outcome did not support publication bias.
Bortezomib maintenance vs observation
One trial evaluated bortezomib maintenance for patients with MCL. Two of 30 patients died in the maintenance group, and 3 out of 30 patients died in the observation group (estimated HR 0.67, 95% CI 0.12–3.71).
Subgroup and sensitivity analyses of overall survival of rituximab maintenance vs no maintenance or interferon-alfa
Transplant eligibility. Pooling data of 554 patients (3 trials) not eligible for ASCT, the HR of death with maintenance was 0.88, 95% CI 0.63 to 1.23, I2 of heterogeneity = 30%, P = 0.24. One trial compared rituximab maintenance to no maintenance for MCL patients after ASCT. The HR of death was 0.51, 95% CI 0.26 to 0.99.
Treatment line. Treatment with maintenance for patients with previously untreated disease was assessed in 619 patients (HR 0.75, 95% CI 0.54–1.04, I2 of heterogeneity = 55%, P = 0.11). Maintenance treatment for patients with a relapsed or refractory disease was assessed in 92 patients of 2 trials.15,17 Due to the paucity of data and the high statistical heterogeneity (I2 = 88%, P = 0.004) that may be attributed to different disease characteristics and induction regimens (chemotherapy with or without rituximab in 1 trial, and rituximab alone in another trial) we did not pool the results.
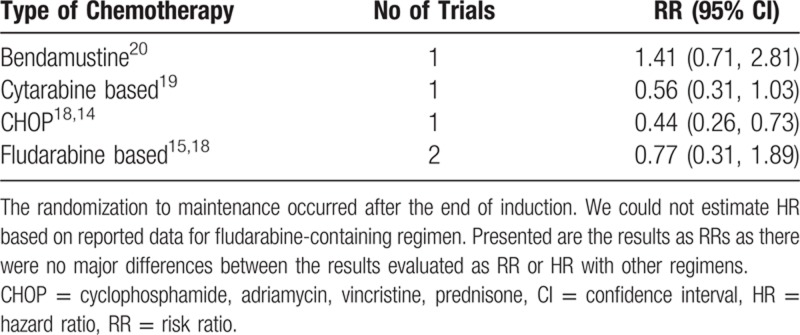
Induction protocol. The effect of maintenance differed with different induction therapy (between subgroups difference chi-squared; P = 0.02). The point estimates of death by the type of chemotherapy are specified in Table 2.
Table 2.
Effect of Maintenance on Death by the Type of Induction Chemotherapy
Control arm. We also performed a post hoc analysis by of trials in which the control group was observed without further treatment the results did not changed significantly.
Methodological quality of trials. We did not perform sensitivity analysis by allocation concealment or sequence generation, since most trials were judged at low risk of bias. Including only trials with at least 100 patients the HR of death was 0.78, 95% CI 0.46 to 1.32.18–20,23
Pooling data using a random-effect model. When overall survival results were pooled using a random-effect model, there was no evidence of effect of maintenance on survival (HR of death 0.81, 95% CI 0.56–1.19).
Secondary outcomes
A beneficial effect of rituximab maintenance on PFS was shown in each of the 5 trials that reported PFS results. In the pooled analysis, PFS was longer with rituximab maintenance compared to observation or interferon-alfa (HR of progression or death 0.58, 95% CI 0.45–0.73). There was no evidence of effect on event-free survival following bortezomib maintenance (HR 0.84, 95% CI 0.32–2.20).21
We graded the quality of PFS estimates as low (downgraded due to clinical heterogeneity and lack of blinding that may affect assessment of subjective outcomes) (detection bias).
The rate of complete response beyond the maintenance phase was higher for patients who received rituximab maintenance compared to those who were observed or received interferon-alfa, with an RR of 2.01, 95% CI 1.52 to 2.64 (3 trials, 622 patients).
Quality of life was not reported in the included trials.
Adverse events
There was no statistically significant difference in the rate of grade 3 to 4 anemia, and thrombocytopenia between maintenance and observation (RR 0.71, 95% CI 0.23–2.22, and RR 1.32, 95% CI 0.67–2.60, respectively, 392 patients)15,17 but the risk of neutropenia was higher with maintenance compared to observation (RR 2.02, 95% CI 1.50–2.73). In 1 trial, the rate of thrombocytopenia and neutropenia were lower among patients who received rituximab maintenance compared to those who received interferon-alfa.18
Of 649, 46 (7%) randomized patients experienced an infection.15,18,19 The risk of grade 3 to 4 infection was not affected by rituximab maintenance (RR 1.30, 95% CI 0.72–2.35).
Discussion
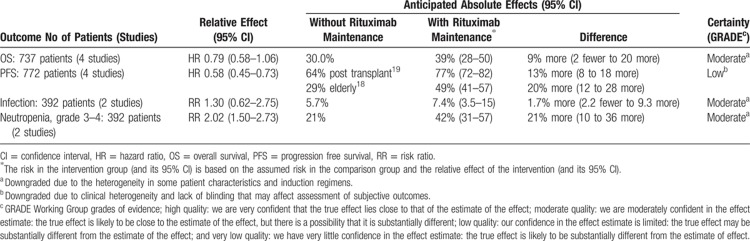
Pooled analysis of 4 trials that assessed rituximab maintenance therapy after a successful induction for patients with MCL demonstrated a statistically significant improved PFS but no clear evidence of effect on overall survival (HR 0.79, 95% CI 0.58–1.06) (Table 3; Summary of Findings). More patients were in complete remission after rituximab maintenance than in the comparator group. The effect of bortezomib maintenance was assessed in 1 trial with a small number of patients with no evidence of effect on PFS and overall survival.
Table 3.
Summary of Findings: Rituximab Maintenance Compared to Observation or Interferon for Patients With Mantle Cell Lymphoma
With rituximab maintenance the risk of grade 3 or higher adverse events was higher compared to observation. The risk of grade 3 to 4 neutropenia was statistically significantly higher with rituximab maintenance compared to observation. However, this did not translate into a higher risk of infection. The overall rate of infection was quite low.
In our search, we used “mantle” crossed with other term to search for trials. This restricted our search to trials that used the REAL classification and later the WHO classification and defined the indication as mantle cell lymphoma. Trials that used other terms (as centrocytic lymphoma) might not been identified through our search.
Despite some variability in patients’ characteristics the effect of rituximab maintenance was a beneficial effect in most trials. Yet, as shown in the trial by Kluin-Nelemans and colleagues,18 the effect of rituximab maintenance may interact with the type of induction. In that trial, rituximab maintenance was of benefit after R-CHOP but not after FCR. In our analysis, there was a differential effect of rituximab maintenance after bendamustine–rituximab and after CHOP or cytarabine-containing induction with no overall survival benefit in the first and a clear benefit in the later settings. A possible explanation for that observation is a different adverse events profile with different induction chemotherapy, as was shown by Marcus et al in the GALLIUM study.23 In that trial, patients treated with bendamustine in combination with rituximab or obinutuzumab had a higher rate of nonrelapse-related fatal adverse events than patients treated with CHOP/CVP in combination with rituximab or obinutuzumab. Likewise, a higher rate of death without progression (vs interferon-alfa), and a higher severe infection rate (vs CHOP) reported by Hoster et al may explain the lack of overall survival benefit with maintenance therapy after fludarabine-based induction.14
There is no doubt that a longer overall survival and quality of life are the most important outcomes. Yet, in the case of MCL, a longer PFS has set some of the treatment standards as ASCT for transplant eligible patients. The combination of a clear longer PFS in each of the trials and in the pooled analysis with a possible trend toward improved overall survival with maintenance in the pooled analysis tips the scales in favor of maintenance treatment for mantle cell lymphoma patients.
To conclude based on our results, rituximab maintenance is recommended after immunochemotherapy with R-CHOP or cytarabine-containing induction in the front-line setting for transplant eligible and ineligible patients, and after R-CHOP in the relapse setting. It is unclear if maintenance is of benefit after different induction chemotherapy such as bendamustine or fludarabine. By contrast, current data does not support improved outcomes with bortezomib maintenance for MCL patients.
Supplementary Material
Footnotes
Citation: Vidal L, Gafter-Gvili A, Dreyling M, Ghielmini M, Witzens-Harig M, Shpilberg O, Unterhalt M, Rummel M, Gurion R. Maintenance Treatment for Patients With Mantle Cell Lymphoma: A Systematic Review and Meta-analysis of Randomized Trials. HemaSphere, 2018;2:4. http://dx.doi.org/10.1097/HS9.0000000000000136
Funding/support: None.
Disclosure: LV is an employee of Syneos Health. The authors have indicated they have no potential conflicts of interest to disclose.
Author contributions: LV, RG, and AG-G designed the research study and performed the research; LV analyzed the data and wrote the first version of the paper; and MD, MG, MW-H, MU, and MR contributed data and participated in study design. All authors reviewed contributed to writing of the manuscript.
References
- 1.Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28:iv62–iv71. [DOI] [PubMed] [Google Scholar]
- 2.Eskelund CW, Kolstad A, Jerkeman M, et al. 15-Year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol 2016; 175:410–418. [DOI] [PubMed] [Google Scholar]
- 3.Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016; 388:565–575. [DOI] [PubMed] [Google Scholar]
- 4.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 6.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748. [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 8.Brozek JL, Akl EA, Compalati E, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy 2011; 66:588–595. [DOI] [PubMed] [Google Scholar]
- 9.Andorsky D, Cataruozolo P, Mouro J, et al. MAGNIFY: a phase 3B, randomized trial of lenalidomide plus rituximab induction and maintenance therapy followed by lenalidomide single-agent versus rituximab maintenance in patients with relapsed/refractory indolent non-Hodgkin lymphoma (NHL). J Clin Oncol 2014; 32: [Google Scholar]
- 10.Cortelazzo S. Study with lenalidomide (revlimid) maintenance vs observation after intensified induction regimen containing rituximab followed by high dose chemotherapy and autologous stem cell transplantation as first line treatment in adult patients with advanced mantle cell lymphoma (MCL-0208). Available at: clinicaltrials.gov; 4 pages. [Google Scholar]
- 11.Dreyling M. R-CHOP + R-HAD vs R-CHOP followed by maintenance lenalidomide + rituximab vs rituximab for older patients with MCL. Available at: clinicaltrials.gov; 4 pages. [Google Scholar]
- 12.Eastern Cooperative Oncology Group. Rituximab, bendamustine hydrochloride, and bortezomib followed by rituximab and lenalidomide in treating older patients with previously untreated mantle cell lymphoma. Available at: clinicaltrials.gov; 4 pages. [Google Scholar]
- 13.Celgene Corporation. A study to evaluate the efficacy of lenalidomide as maintenance therapy after completion of first-line combination chemotherapy in patients with mantle cell lymphoma (MCL) (Renew). Available at: clinicaltrials.gov; 4 pages. [Google Scholar]
- 14.Hoster E, Kluin-Nelemans H, Hermine O, et al. Rituximab maintenance after first-line immunochemotherapy in mantle cell lymphoma: long-term follow-up of the randomized European MCL elderly trial. Blood 2017; 130 (suppl 1):153. [Google Scholar]
- 15.Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006; 108:4003–4008. [DOI] [PubMed] [Google Scholar]
- 16.Ghielmini M, Rufibach K, Salles G, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol 2005; 16:1675–1682. [DOI] [PubMed] [Google Scholar]
- 17.Ghielmini M, Schmitz SF, Cogliatti S, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol 2005; 23:705–711. [DOI] [PubMed] [Google Scholar]
- 18.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012; 367:520–531. [DOI] [PubMed] [Google Scholar]
- 19.Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med 2017; 377:1250–1260. [DOI] [PubMed] [Google Scholar]
- 20.Rummel MJ, Knauf W, Goerner M, et al. Two years rituximab maintenance vs. observation after first-line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: first results of a prospective, randomized, multicenter phase II study (a subgroup study of the StiL NHL7-2008 MAINTAIN trial). J Clin Oncol 2016; 34: [Google Scholar]
- 21.Doorduijn J, Minnema M, Kersten M, et al. Bortezomib maintenance therapy after induction with R-CHOP, ARA-C and autologous stem cell transplantation in newly diagnosed MCL patients, results of a multicenter phase II HOVON study. Blood 2015; 126:339. [Google Scholar]
- 22.Kaplan L, Jung S, Stock W, et al. Bortezomib maintenance (BM) versus consolidation (BC) following aggressive immunochemotherapy and autologous stem cell transplant (ASCT) for untreated mantle cell lymphoma (MCL): CALGB (Alliance) 50403. Blood 2015; 126:337. [Google Scholar]
- 23.Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 2017; 377:1331–1344. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011.]. The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.