Abstract
Ketamine shows promise as a rapidly-acting treatment for depression and suicidal ideation, but side effects and abuse potential limit its use. Understanding its mechanism of action could help develop analogous but safer drugs. This post hoc study explored relationships of ketamine and metabolites, including hydroxynorketamine enantiomers, (2S,6S)- and (2R,6R)-HNK, to clinical response in a subgroup from a published trial in suicidal depression. Depressed adults with clinically significant suicidal ideation were randomized to double-blind infusion of sub-anesthetic ketamine or midazolam. Ketamine and metabolites were measured after infusion (N=53). Plasma (2R,6R)-HNK was associated with change (higher levels correlated with less clinical improvement) from baseline to 24h post-infusion of depression (HDRS-24: Spearman r=0.37, p=0.009) and suicidal thoughts (SSI: Spearman r=0.29, p=0.041). There were similar correlations with weekly follow-up clinical rating scores for both HNK enantiomers and dehydronorketamine (DHNK). Ketamine and norketamine were not associated with change in depression or suicidal ideation (unadjusted p>0.28).
Keywords: ketamine, metabolite, major depressive disorder, suicidal ideation, clinical trial
Introduction
Despite modest advantages over placebo, slow onset of action, and troubling side effects like sexual dysfunction, antidepressants are the third most-prescribed drug class in U.S. medical office visits (Cipriani et al., 2018; Mann et al., 2005; Pratt et al.). This likely relates to the fact that depression is among the most disabling of human maladies (WHO, 2017). It remits with antidepressant treatment in a third or fewer patients and a minority experience even 50% improvement with a first-line drug (Trivedi et al., 2006). An estimated 30–50% of patients don’t respond sufficiently to current therapies – termed treatment resistant depression (TRD) (Akil et al., 2018; Fava and Davidson, 1996). Because depression is the major contributor to suicidal states, shortcomings of treatment may factor in the more than 30% increase from 1999 – 2017 in US suicide rates (Hedegaard, 2018; Mann et al., 2005). The level of interest in ketamine treatment for depression is further evidence of the need for antidepressants that work more quickly, effectively, with fewer side effects, and for more patients.
Clinical trials (Grunebaum et al., 2017; Grunebaum et al., 2018; Milak et al., 2016; Murrough et al., 2013) show relief of depressive symptoms including suicidal thoughts within hours of treatment with sub-anesthetic ketamine, a non-competitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist approved in 1970 as an anesthetic (reviewed in (Newport et al., 2015)). We found that ketamine reduced suicidal thoughts within hours in suicidal depressed patients (N=80) and the improvement appeared to persist for up to six weeks with optimized, standard pharmacotherapy (Grunebaum et al., 2018). Only about one-third of ketamine’s effect on suicidal ideation was explained by its overall antidepressant effect (Grunebaum et al., 2018). There is no current approved treatment for rapid relief of suicidal thoughts in depressed patients.
Despite recent FDA approval of intra-nasal (S)-ketamine for treatment resistant depression, its dissociative effects, addictive potential and lack of long-term safety data on potential toxicity such as cystitis (Jhang et al., 2015) and white matter damage (Edward Roberts et al., 2014), limit its use (Caddy et al., 2015; Newport et al., 2015; Sanacora et al., 2017). It targets multiple receptors (NMDA, nicotinic, dopaminergic, opioid), and neurotransmitter systems (glutamate, GABA, serotonin) all of which could contribute to its clinical effects (Gupta et al., 2011; Morgan and Curran, 2006; Williams et al., 2018). Elucidating ketamine’s antidepressant and anti-suicidal ideation mechanisms of action could aid development of safer alternatives. Pre-clinical research suggests that ketamine’s antidepressant mechanism of action may involve blockade of ionotropic NMDA receptors, enhanced transmission via α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) (Machado-Vieira et al., 2009; Maeng et al., 2008; Trullas and Skolnick, 1990), and trophic effects via mechanistic target of rapamycin (mTOR) and brain derived neurotrophic factor (BDNF) stimulating or maintaining dendritic spinogenesis (Ardalan et al., 2017; Autry et al., 2011; Duman and Duman, 2015; Li et al., 2010; Moda-Sava et al., 2019).
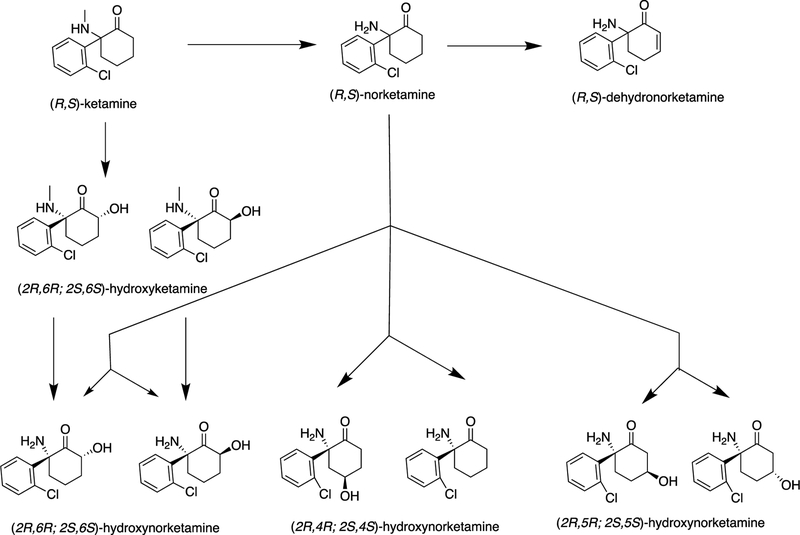
There is debate in pre-clinical literature on whether specific (R)- or (S)-enantiomers of ketamine or its metabolites - especially (2R,6R)-hydroxynorketamine (HNK) - may have antidepressant effects but with less potential toxicity and side effects. Ketamine is rapidly metabolized by liver enzymes to norketamine and hydroxyketamines, and then to the secondary metabolites dehydronorketamine and HNKs (Figure 1) (Zanos et al., 2018; Zarate et al., 2012). The (2S,6S)- and (2R,6R)-HNK metabolites of, respectively, (S)-ketamine and (R)-ketamine, do not inhibit NMDARs at antidepressant-relevant concentrations, but demonstrate antidepressant-like effects in pre-clinical studies (Zanos et al., 2018). One group found (2R,6R)-HNK had antidepressant-related effects in mice associated with NMDA-independent AMPAR activation and without side effects seen in mice given racemic ketamine at pharmacologically relevant doses (Zanos et al., 2016). Another group using a learned helplessness model in rats found an antidepressant effect of (R)-ketamine but not (2R,6R)-HNK (Shirayama and Hashimoto, 2018). Adding complexity, (2S,6S)-HNK upregulates mTOR in rats (Li et al., 2010; Paul et al., 2014).
Figure 1.
Major metabolic pathways of ketamine
Refs: Zarate J et al. Biol. Psychiatry, 2012, 72(4), 331; Zanos et al. Pharmacological reviews, 2018, 70, 621–660
There are limited human data to add perspective. A study of ketamine metabolites after a 40-minute infusion in 45 patients with a unipolar major depressive episode and 22 with bipolar depression did not report results for individual HNK enantiomers (Zarate et al., 2012). To our knowledge there is no published data on each enantiomer’s antidepressant and anti-suicidal ideation effects in humans. We conducted a post hoc pilot study of ketamine, and its metabolites norketamine (NK), dehydronorketamine (DHNK), (2S,6S)-HNK, and (2R,6R)-HNK in a subgroup (N=53) of a published clinical trial in suicidal depression (Grunebaum et al., 2018). We explored relationships with clinical response, and hypothesized that plasma (2R,6R)-HNK would correlate with improvement in depression and suicidal ideation.
METHOD
Participants
Detailed trial methods, CONSORT material, and clinical results are published (Grunebaum et al., 2018). Adults (N=80) with current major depressive disorder (MDD) and a score ≥4 on the Beck Scale for Suicidal Ideation (SSI), of whom 54% (N=43) were taking antidepressant medication, were randomly assigned to double-blind ketamine (0.5 mg/kg) or midazolam (0.02 mg/kg) in 100 mL normal saline infused intravenously over 40 minutes. The primary outcome was the SSI score at 24h post-infusion. Non-responders were un-blinded and if they had received midazolam were offered a second infusion the following day with open label ketamine. Other outcomes included global depressive symptoms and adverse effects. The main results at 24h were greater reduction with ketamine compared to midazolam in SSI (95% CI=2.33, 7.59; Cohen’s d=0.75), and Profile of Mood States (POMS) depression score (95% CI=1.36, 13.94) (Grunebaum et al., 2018). The protocol was approved by the NYSPI IRB, and written informed consent was obtained from all participants.
Clinical Measures
Raters were PhD or masters level psychologists. Axis I and II diagnoses were made using the Structured Clinical Interview for DSM-IV,(First et al., 1994; First et al., 1996) in a weekly consensus conference of psychologists and psychiatrists. Raters participated in weekly reliability monitoring. Instruments included the clinician-rated SSI(Beck et al., 1979) to assess suicidal ideation with 19 items scaled 0 (least severe) to 2 (most severe) and total from 0 to 38 (Beck et al., 1979). It was assessed at screening, baseline (day before infusion), 230 minutes post-infusion, 24h post-infusion (day1), and weeks 1–6 of follow-up. Response was defined as at least 50% reduction in SSI from baseline to day1 and day1 score <4 (i.e. the subject would no longer be study-eligible). Depressive symptoms were assessed with the 24-item Hamilton Depression Rating Scale (HDRS-24) (Hamilton, 1960), Profile of Mood States (POMS)(McNair et al., 1981), and anxiety was measured with a 5-level Likert scale asking patients to self-rate from 0 (not at all) to 4 (extremely anxious). Medication side effects were measured with the Clinician-Administered Dissociative States Scale (CADSS)(Bremner et al., 1998), and positive symptom subscale of the Brief Psychiatric Rating Scale (BPRS)(Overall and Gorham, 1962).
Ketamine and metabolite levels
The pilot study of plasma ketamine (KET) and its metabolites norketamine (NK), dehydronorketamine (DHNK), and (2S,6S)- and (2R,6R)-hydroxynorketamine (HNK), immediately post-infusion, was initiated at approximately the midpoint of the parent trial (N=80) and included all subsequent participants (N=53). Plasma KET, NK, and DHNK were quantified using liquid/liquid extraction followed by validated HPLC separation and UV detection at 210nm. HNK enantiomers (2S,6S)-HNK and (2R,6R)-HNK were quantified using a validated modified LC-MS method (Hasan et al., 2017).
Statistical Analysis
SAS version 9.4 (SAS Institute, Cary, N.C.) and SPSS 25 (IBM, Armonk, NY) were used for analyses. Histograms and pairwise scatterplots of biomarkers and clinical outcomes were inspected for outliers and inconsistent values. The distribution of several biomarkers was skewed, and/or had outliers, which is reflected in the choice of analytic method. Imputation of square root(2) was used for the 15 (28%) non-zero (2R,6R)-HNK enantiomer levels that were below the detection threshold. Wilcoxon signed rank test (paired) was used to compare plasma levels of the (2S,6S)- versus (2R,6R)-HNK enantiomers. Spearman correlation coefficients were used to test for associations of plasma KET, NK, DHNK, (2S,6S)-HNK and (2R,6R)-HNK with change from pre- to 24h post-infusion and weeks 1–6 of follow-up clinical treatment, in SSI, HDRS-24 and POMS. Pre-clinical data suggests a greater antidepressant-like effect of ketamine in female compared to male mice in association with higher brain levels of (2S,6S;2R,6R)-HNK but not KET or NK (Zanos et al., 2016), so we explored this by testing models of change in SSI or HDRS-24 score from baseline to 24h post-infusion with (2R,6R)-HNK level, sex, and the interaction of these as predictor variables. Since this was an exploratory pilot study, we did not correct for multiple comparisons.
Results
Associations with 24h Post-Infusion Ratings
(2R,6R)-HNK levels were missing for 5 (9%) subjects and (2S,6S)-HNK levels were missing for 8 (15%). The distribution of (2R,6R)-HNK levels was not associated with infusion type (randomized vs. open) (Wilcoxon W=604.0, p=0.737), and the same was found for (2S,6S)-HNK (Wilcoxon W=507.0, p=0.617). Thus randomized and open ketamine infusion data were pooled. The interaction of sex with (2R,6R)-HNK was not associated with change from baseline to 24h post-infusion in SSI (p=0.931) or HDRS-24 (p=0.413).
Change in SSI score from pre- to 24h post-infusion was not correlated with post-infusion plasma KET (Spearman r=0.12, p=0.382, N=53), NK (Spearman r=0.06, p=0.672, N=53), or DHNK (Spearman r=0.05, p=0.739, N=53) (ng/ml). Change in HDRS-24 from pre- to 24h post-infusion also did not correlate with post-infusion plasma KET (Spearman r=−0.07, p=0.636, N=53), NK (Spearman r=−0.09, p=0.501, p=53), or DHNK (Spearman r=0.06, p=0.645, N=53).
Plasma levels (ng/ml) of (2S,6S)-HNK (N=45; Mean(SD)=20.8(9.9); Median=20.0, Range 6.0–52.0) were higher compared with (2R,6R)-HNK (N=48; Mean(SD)=4.0(2.9); Median=3.0, Range 1.4–11.0; Wilcoxon signed rank p<0.001), although levels of the two enantiomers were moderately correlated (N=45, Spearman r=0.52, p<0.001). Baseline psychotropic medication status (taking vs. not) was not associated with (2R,6R)-HNK level (Mann-Whitney 11=240.0, p=0.362), but (2S,6S)-HNK levels were higher in subjects taking psychiatric medications (Mean=23.1 ng/ml; SD=10.1) compared to those off meds (Mean=13.9 ng/ml; SD=5.4) (Mann-Whitney U=293.0, p=0.004).
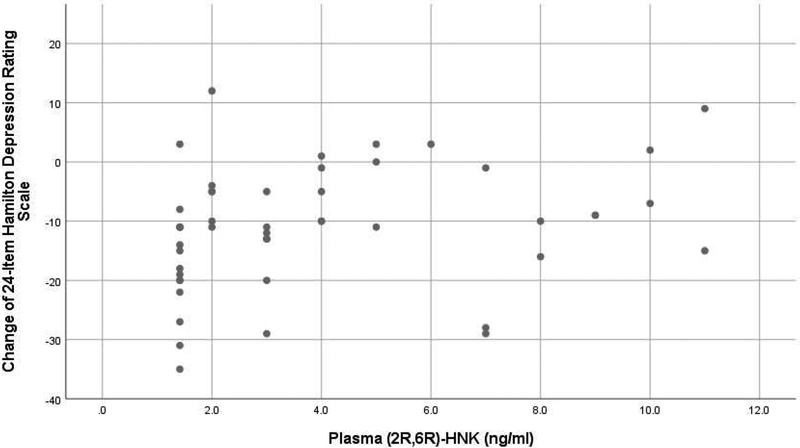
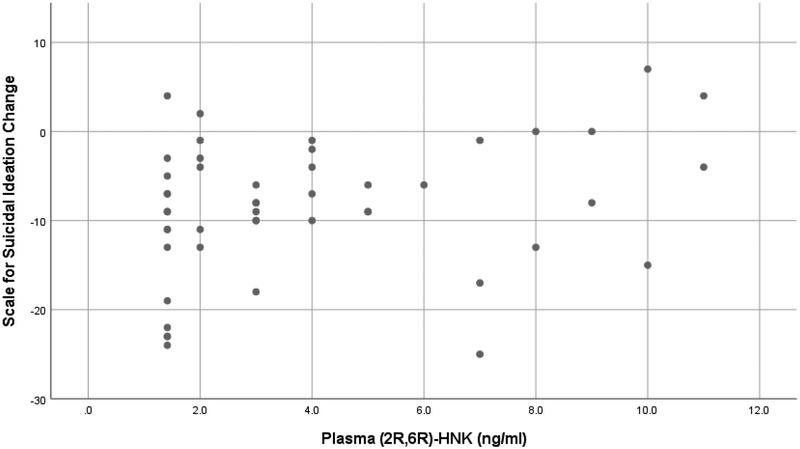
Higher post-infusion plasma level of the (2R,6R)-HNK enantiomer correlated inversely with improvement in depressive symptoms from pre- to 24h post-infusion using the HDRS-24 (Spearman r=0.37, p=0.009) (Figure 2). Similarly, post-infusion (2R,6R)-HNK plasma level correlated inversely with improvement in suicidal ideation (SSI score) from pre- to 24h post-infusion (Spearman r=0.29, p=0.041)(Figure 3).
Figure 2.
Scatterplot of change in 24-item Hamilton Depression Rating Scale (24h post-infusion score minus pre-infusion score) vs. plasma (2R,6R)-HNK (ng/ml)*
*Higher post-infusion plasma (2R,6R)-HNK correlated inversely with improvement in depressive symptoms from pre- to 24h post-infusion (Spearman rank correlation r=0.37, p=0.009).
Figure 3.
Scatterplot of change in Beck Scale for Suicidal Ideation (24h post-infusion score minus pre-infusion score) vs. plasma (2R,6R)-HNK (ng/ml)*
*Higher post-infusion plasma (2R,6R)-HNK correlated inversely with improvement in suicidal ideation from pre- to 24h post-infusion (Spearman rank correlation r=0.29, p=0.041).
The (2S,6S)-HNK enantiomer was not associated with pre- to 24h post-infusion change in SSI (Spearman r=0.08, p=0.597) or HDRS-24 scores (Spearman r=0.12, p=0.450). In separate models adjusting for baseline medication status, plasma (2S,6S)-HNK was not associated with change in SSI (β=0.19, t=1.21, p=0.235) or HDRS-24 (β=0.26, t=1.59, p=0.119).
POMS and adverse effect rating scales were only administered for the randomized infusion. In this smaller subgroup, plasma KET correlated inversely with improvement from pre- to 24h post-infusion in the POMS Depression subscale (Spearman r=0.41, p=0.025, N=29). Plasma NK correlated positively with dissociative symptoms on the CADSS immediately post-infusion (N=28, Spearman r=0.44, p=0.019) and at day 1 (N=26, Spearman r=0.56, p=0.003), and with anxiety at day 1 at a marginally significant level (N=28, Spearman r=0.37, p=0.050).
Associations with Weeks 1–6 Follow-up Ratings
Higher DHNK correlated with less decrease in SSI score from baseline to weeks 2, 3, 4, and 6 of follow-up clinical treatment (N ranged from 43 – 50, Spearman r ranged from 0.29 – 0.33, p values ranged from 0.026 – 0.044). DHNK was not associated with change in HDRS-24 from baseline to follow-up.
Levels of (2S,6S)-HNK were associated with less decrease in SSI score from baseline to follow-up weeks 3 (N=38, Spearman r=0.36, p=0.027) and 4 (N=37, Spearman r=0.37, p=0.026). Levels of (2S,6S)-HNK correlated with less decrease in HDRS-24 from baseline to weeks 1 (N=41, Spearman r=0.37, p=0.019) and 3 (N=38, Spearman r=0.38, p=0.018).
The (2R,6R)-HNK enantiomer was associated with less decrease in SSI score from baseline to follow-up weeks 1–4 and 6 (N ranged from 39–45, Spearman r ranged from 0.30–0.41, p ranged from 0.008–0.039). Levels of this enantiomer were associated with less decrease from baseline to weeks 1,2,4, and 5 in HDRS-24 (N ranged from 37–45, Spearman r ranged from 0.33–0.39, p ranged from 0.009–0.044). There were no other statistically significant associations of ketamine or metabolites with clinical response.
Discussion
The main finding of this pilot study is the inverse association of post-infusion plasma (2R,6R)-HNK with clinical improvement from pre- to 24h post-infusion in suicidal ideation, and separately, global depressive symptoms. There were similar correlations with change in SSI and HDRS-24 from baseline to follow-up timepoints. Our results contrast with a report that (2R,6R)-HNK had antidepressant-related effects in mice (Zanos et al., 2016), although another study did not find this association in rats (Shirayama and Hashimoto, 2018). This subject remains controversial in the pre-clinical literature. We found correlations of DHNK with less improvement in SSI and of (2S,6S)-HNK with less improvement in SSI and HDRS-24 from baseline to follow-up timepoints. To our knowledge, these are the first clinical trial data published on relationships of the individual (2S,6S)- and (2R,6R)-HNK enantiomers to antidepressant and anti-suicidal ideation response in humans.
Given pre-clinical evidence of antidepressant-like effects of (2R,6R)-HNK (Zanos et al., 2016), the inverse association with clinical improvement that we found in a human trial is counter-intuitive. A plausible explanation is if less ketamine metabolism and higher parent compound levels correlated with clinical improvement, as our group found with NK – precursor to HNK – in a prior study (Milak et al., 2016). However, the current study, with a sample more than fourfold larger, did not find KET or NK levels to be associated with change in SSI or HDRS-24 scores. The lack of these associations is consistent with a study of ketamine and metabolite levels in 45 MDD and 22 BD patients (Zarate et al., 2012). Our results should not be interpreted to mean that (2R,6R)-HNK does not have antidepressant effects as sample timing, assay sensitivity, and dosing may all have contributed. An association of clinical improvement with more rapid absorption of (2R,6R)-HNK into brain, resulting in lower plasma levels, is an alternative explanation requiring further research. The fact that subjects continued current medications at stable doses may have influenced our results, but (2R,6R)-HNK level was not associated with subjects’ baseline psychotropic medication status (taking vs. not), so this seems unlikely.
Pre-clinical evidence shows higher brain levels of KET and NK in female rats (Saland and Kabbaj, 2018) and of (2S,6S;2R,6R)-HNK in female mice, as compared to males (Zanos et al., 2016). A human study also found higher (2S,6S;2R,6R)-HNK and DHNK levels in women compared to men (Zarate et al., 2012). We did not find an interaction of sex with (2R,6R)-HNK in terms of effect on clinical response at 24h post-infusion.
Our finding that (2S,6S)-HNK levels were higher than those of the (2R,6R)-enantiomer, is consistent with pre-clinical data (Moaddel et al., 2015). Unlike the (2R,6R)- enantiomer, the distribution of plasma (2S,6S)-HNK was associated with baseline medication status, but it did not correlate with change in depressive symptoms or suicidal ideation after adjusting for the potential confound.
Our finding that NK levels correlated positively with dissociative side effects is consistent with a prior study that found DHNK correlated inversely with these adverse effects since NK is a precursor to DHNK (Zarate et al., 2012).
The main limitation of this post hoc study is that it was added after the parent trial was underway, and therefore involved a subgroup. However, this seems an unlikely source of bias since the study included all subsequent participants, comprising more than half of the parent trial sample. The substantial number of (2R,6R)-HNK enantiomer levels that were non-zero yet below the detection limit, appeared influential on the result, however non-parametric Spearman rank correlation takes this into account. The latter may partly explain why the 2R,6R enantiomer was more correlated with symptom change compared to the 2S,6S enantiomer. It is possible that any monotonic association here may be restricted to a very small range of the biomarker, after which there is a saturation effect. Of note, (2R,6R)-HNK was associated with antidepressant-like effects in mice at time points after its brain concentration was below detectable levels (Zanos et al., 2016). HDRS-24 and SSI scores before open ketamine infusion were less severe than before the randomized ketamine infusion, but improvement after both infusion types was similar (Grunebaum et al., 2018). The distributions of both HNK enantiomers were not associated with infusion type, suggesting that pooling the plasma ketamine data from randomized and open infusions was reasonable. Measurement of ketamine and metabolites at a single time point immediately post-infusion is a limitation, however, published data show that KET, NK, DHNK, 2S,6S- and 2R,6R-HNK are present at significant levels during the first 230 minutes post-infusion (Zarate et al., 2012).
In contrast to results from a pre-clinical study in mice (Zanos et al., 2016), our findings suggest that higher (2R,6R)-HNK plasma level is associated with less improvement in depression and suicidal thoughts in suicidal depressed persons. These preliminary findings need replication, but at minimum do not suggest a robust benefit of (2R,6R)-HNK for depression or suicidal ideation. They also raise questions about potential differences in response to ketamine and its metabolites in rodent models of depression versus in the human illness.
Funding
This work was supported by the National Institutes of Health [grant number MH-096784]. The funding source had no role in the collection or analysis of the data or preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Drs. Mann and Burke receive royalties for commercial use of the Columbia Suicide Severity Rating Scale which was not used in this work. Dr. Galfalvy’s family owns stock in Illumina, Inc. Dr. Suckow owns stock in Johnson & Johnson. The other authors report no financial relationships with commercial interests.
ClinicalTrials.gov identifier:
References
- Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, Meaney MJ, Nestler EJ, 2018. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci Biobehav Rev 84, 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardalan M, Rafati AH, Nyengaard JR, Wegener G, 2017. Rapid antidepressant effect of ketamine correlates with astroglial plasticity in the hippocampus. Br J Pharmacol 174(6), 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P. f., Kavalali ET, Monteggia LM, 2011. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT., Kovacs M, Weissman A, 1979. Assessment of suicidal intention: The scale for suicide ideation:. J.Consult.Clin.Psychol 47(2), 343–352. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM, 1998. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). Journal of Traumatic Stress 11, 125–136. [DOI] [PubMed] [Google Scholar]
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, Hawton K, Cipriani A, 2015. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev(9), CD011612. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR, 2018. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Duman RS, 2015. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett 601, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward Roberts R, Curran HV, Friston KJ, Morgan CJ, 2014. Abnormalities in white matter microstructure associated with chronic ketamine use. Neuropsychopharmacology 39(2), 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Davidson KG, 1996. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 19(2), 179–200. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1994. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York State Psychiatric Institute, Biometrics Research, New York, NY. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JMG, Benjamin L, 1996. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), (Version 2.0) Biometrics Research Department, New York State Psychiatric Institute, New York. [Google Scholar]
- Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ, 2017. Ketamine versus midazolam in bipolar depression with suicidal thoughts: A pilot midazolam-controlled randomized clinical trial. Bipolar Disord 19(3), 176–183. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ, 2018. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am J Psychiatry 175(4), 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Devi LA, Gomes I, 2011. Potentiation of mu-opioid receptor-mediated signaling by ketamine. J Neurochem 119(2), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J.Neurol.Neurosurg.Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Hofstetter R, Fassauer GM, Link A, Siegmund W, Oswald S, 2017. Quantitative chiral and achiral determination of ketamine and its metabolites by LC-MS/MS in human serum, urine and fecal samples. J Pharm Biomed Anal 139, 87–97. [DOI] [PubMed] [Google Scholar]
- Hedegaard HC, C. S; Warner M, 2018. Suicide mortality in the United States, 1999–2017, in: Statistics, N.C.f.H (Ed.). Hyattsville, MD. [Google Scholar]
- Jhang JF, Hsu YH, Kuo HC, 2015. Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. Int J Urol 22(9), 816–825. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, DiazGranados N, Zarate CA, 2009. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol.Ther. 123, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK, 2008. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of +¦− Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol.Psychiatry 63(4), 349–352. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, Hegerl U, Lonnqvist J, Malone KM, Marusic A, Mehlum L, Patton G, Phillips M, Rutz W, Rihmer Z, Schmidtke A, Shaffer D, Silverman M, Takahashi Y, Varnik A, Wasserman D, Yip P, Hendin H, 2005. Suicide prevention strategies: a systematic review. JAMA 294(16), 2064–2074. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, 1981. Manual for the Profile of Mood States Educational and Industrial Testing Service, San Diego. [Google Scholar]
- Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, Mao X, Rodriguez CI, Oquendo MA, Suckow RF, Cooper TB, Keilp JG, Shungu DC, Mann JJ, 2016. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry 21(3), 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Sanghvi M, Dossou KS, Ramamoorthy A, Green C, Bupp J, Swezey R, O’Loughlin K, Wainer IW, 2015. The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol Res Perspect 3(4), e00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C, 2019. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364(6436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV, 2006. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl) 188(4), 408–424. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, 2013. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170(10), 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, 2015. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry 172(10), 950–966. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The brief psychiatric rating scale. Psychological Reports 10, 799–812. [Google Scholar]
- Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O’Loughlin K, Torjman MC, Bernier M, Wainer IW, 2014. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology 121(1), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Gu Q, 2017. Antidepressant Use Among Persons Aged 12 and Over: United States, 2011–2014. https://www.cdc.gov/nchs/products/databriefs/db283.htm. (Accessed January 3 2018). [PubMed]
- Saland SK, Kabbaj M, 2018. Sex Differences in the Pharmacokinetics of Low-dose Ketamine in Plasma and Brain of Male and Female Rats. J Pharmacol Exp Ther 367(3), 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, American Psychiatric Association Council of Research Task Force on Novel, B., Treatments, 2017. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, 2018. Lack of Antidepressant Effects of (2R,6R)-Hydroxynorketamine in a Rat Learned Helplessness Model: Comparison with (R)-Ketamine. Int J Neuropsychopharmacol 21(1), 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland R, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs JT, Balsubramani GK, Fava M, Team SDS, 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163(1), 28–40. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P, 1990. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol 185(1), 1–10. [DOI] [PubMed] [Google Scholar]
- WHO, 2017. Depression and other common mental disorders: Global Health Estimates. World Health Organization, Geneva. [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF, 2018. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am J Psychiatry, appiajp201818020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604), 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., Gould TD, 2018. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev 70(3), 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche NE, Laje G, Luckenbaugh DA, Venkata SLV, Ramamoorthy A, Moaddel R, Wainer IW, 2012. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol.Psychiatry 72, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]