Abstract
Cerebral cavernous malformations (CCMs) are relatively common vascular malformations, characterized by increased Rho kinase (ROCK) activity, vascular hyper-permeability and the presence of blood degradation products including non-heme iron. Previous studies revealed robust inflammatory cell infiltration, selective synthesis of IgG, in situ antigen driven B-cell clonal expansion, and deposition of immune complexes and complement proteins within CCM lesions. We aimed to evaluate the impact of suppressing the immune response on the formation and maturation of CCM lesions, as well as lesional iron deposition and ROCK activity. Two murine models of heterozygous Ccm3 (Pdcd10), which spontaneously develop CCM lesions with severe and milder phenotypes, were either untreated or received anti-mouse BR3 to deplete B cells. Brains from anti-mouse BR3-treated mice exhibited significantly fewer mature CCM lesions and smaller lesions compared to untreated mice. B cell depletion halted the progression of lesions into mature stage 2 lesions but did not prevent their genesis. Non-heme iron deposition and ROCK activity was decreased in lesions of B cell depleted mice. This represents the first report of the therapeutic benefit of B-cell depletion in the development and progression of CCMs, and provides a proof of principle that B cells play a critical role in CCM lesion genesis and maturation. These findings add biologics to the list of potential therapeutic agents for CCM disease. Future studies would characterize the putative antigenic trigger and further define the mechanism of immune response in the lesions.
Keywords: Cerebral cavernous malformation, B cells, B-cell depletion, Inflammation, Immune response, Stroke
Introduction
Cerebral cavernous malformations (CCMs) are low-flow vascular malformations of the brain (Robinson et al. 1993) affecting around 0.5 % of the population (Al-Shahi Salman et al. 2012). They account for about 10 % of all vascular malformation and are only second in frequency to developmental venous anomalies with which they sometimes co-exist (Maraire and Awad 1995; Moriarity et al. 1999). A CCM lesion might be completely asymptomatic and clinically quiescent (Kondziolka et al. 1995; Maraire and Awad 1995) or might undergo growth and hemorrhage manifesting clinically as headaches, seizures or neurological deficits (Zabramski et al. 1994).
Hemorrhage is associated with a 6 % per year rebleed rate, and 42.4 % rate of recurrent strokes and/or persistent neurologic deficits within 5 years (Al-Shahi Salman et al. 2012). Important for clinical trials, the definition of symptomatic hemorrhage in CCM has been carefully adjudicated (Al-Shahi Salman et al. 2008). In the United States alone, more than one and half million patients are likely living with CCM. Hence, more than 200,000 adults in the United States are living with a CCM that has led to at least one stroke, and nearly half of those will suffer further sequelae within 5 years. This does not include CCMs in children and adolescents, who often have an even more aggressive disease course (Al-Holou et al. 2012; Shenkar et al. 2015). Childhood CCM, usually caused by mutations in the CCM3 gene often is diagnosed in the first 5 years of life, and results in frequent disability before adulthood. There is currently no therapy to prevent CCM lesion formation or CCM enlargement. Further, there is no treatment of the symptomatic progression to a first stroke and no treatment to prevent re-hemorrhages.
CCM disease exists in two forms, sporadic (70–80 % of cases) and familial (20–30 %), with the latter manifesting an autosomal dominant Mendelian inheritance with mutations at three known gene loci (KRIT1/CCM1, CCM2 and PDCD10/ CCM 3) (Riant et al. 2010). The three CCM genes encode respective proteins KRIT1, MGC4607 and PDCD10, involved in maintaining endothelial barrier integrity via suppression of RhoA kinase (ROCK) activity (Whitehead et al. 2009; Stockton et al. 2010; Shenkar et al. 2015). The CCM lesions are associated with a defective blood-brain barrier (Wong et al. 2000; Stockton et al. 2010; McDonald et al. 2011; Jakimovski et al. 2014; Mikati et al. 2015) and lesions harbor blood breakdown products including non-heme iron deposition (Rigamonti et al. 1987; Steiger et al. 1987; Tan et al. 2014).
Our group had described a defined immune response in human CCM lesions. Surgically excised specimens from sporadic and familial cases had a robust infiltration of inflammatory cells, with abundance of CD20+ B-cells, CD138+ plasma cells, CD3+ T-cells and CD68+ antigen presenting macrophages (Shi et al. 2009). Immunoglobulins were concentrated in CCM lesions, with oligoclonality as compared to peripheral blood (Shi et al. 2007). The CDR3 regions of the IgG heavy chains analyzed from CCM lesions by spectratyping, cloning, and sequencing also confirmed oligoclonality, distinct from those of peripheral blood (Shi et al. 2009). The same inflammatory cell infiltrates were documented in mature CCM lesions, but not in capillary ectasia (primordial or pre-lesions) in murine models (McDonald et al. 2012). Recently, proinflammatory genotype was associated with more aggressive CCM disease severity (Choquet et al. 2014). We demonstrated in situ antigen driven B-cell clonal expansion, B-T cells clusters and immune complexes containing local antibodies and complement proteins in CCM lesions resected from patients (Shi et al. 2014). While this suggested a pathogenic mechanism as in other autoimmune diseases and vasculitis (Tulamo et al. 2006; Xing et al. 2009), it remained unclear if the immune response plays a causative role in CCM development. In this study, we utilized anti-BR3 antibodies to induce B-cell depletion in mouse models of CCM disease and assess the impact of such intervention as a potential therapeutic target. We utilized murine models of heterozygous CCM3 disease, which spontaneously develop CCM lesions throughout the brain during life, recapitulating the human disease (McDonald et al. 2011; Shenkar et al. 2015). These include heterozygous Pdcd10+/−(Ccm3+/−) transgenic mice known to develop a milder pheno-type, and Pdcd10+/−Trp53−/− mice sensitized to form a heavier lesion burden through the loss of tumor suppressor gene p53 (Shenkar et al. 2015).
Materials and Methods
Pdcd10 Heterozygous Murine Model
Animal procedures were approved by the Duke University Institutional Animal Care and Use Committee (IACUC). Pdcd10+/− (Ccm3+/−) mice were obtained through a material transfer agreement with Yale University (Louvi et al. 2011). Animals bred in a sensitized background (with loss of Trp53) designed to promote second-hit somatic mutations and a heavier lesion burden were examined, as were nonsensitized heterozygotes (Shenkar et al. 2015). The B6.129S2-Trp53tm1Tyi mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The Pdcd10+/−Trp53−/− model was developed by a similar strategy as was done for the Krit1+/−Trp53−/− model as previously reported (Plummer et al. 2004). Twenty seven Pdcd10+/−Trp53−/− mice (16 male, 11 female) and 19 non-sensitized Pdcd10+/− mice (7 male, 12 female) were included in the experiments. Of these, 21 Pdcd10+/− Trp53−/− mice (14 male, 7 female) and 9 Pdcd10+/−mice (2 male, 7 female) were untreated controls, while 5 Pdcd10+/−Trp53−/− mice (2 male, 3 female) and 10 Pdcd10+/−mice (5 male, 5 female) received treatment to deplete B cells.
B-Cell Depletion Treatment
Mice to be depleted of B cells received intraperitoneal injections once a week commencing at 3–4 weeks of age for a total of 12–15 weeks with anti-BR3 antibody (5 mg/kg/week), by a procedure previously described that depleted B cells in mice (Lin et al. 2007). The anti-BR3 antibody was a gift from Flavius Martin (Genentech Corporation).
The first feasibility experiment with the severe phenotype sensitized Pdcd10+/−Trp53−/− mice was an open label study without randomization or blinded assessment of lesion burden (Landis et al. 2012). Hence we further tested whether therapeutic benefit would remain in non-sensitized heterozygous Pdcd10+/− mice (with milder phenotype in absence of Trp53 loss), and with more rigorous prospective randomization and blinded assessment of lesion burden.
Organ Harvesting
The animals were euthanized at 4 months of age unless the overall health status of the animals was poor, requiring earlier euthanasia. The brains were removed, immersed in 10 % formalin and sent to the University of Chicago for lesion burden assessment and immunohistochemistry. Peripheral blood was collected from the mice using a preservative that stabilizes leukocytes (TransFix®, Invitrogen) according to the manufacturer’s protocol, and sent to the University of Chicago overnight on ice for analysis by flow cytometry.
Spleens were removed from the animals and cut into halves. One half was placed into 10 % formalin and sent to the University of Chicago. The other half was cut into several pieces and placed into RPMI-5 medium in a petri dish. After crushing with a syringe plunger, the clumps were dispersed by suspending several times through a syringe with a 19G needle. After filtering and washing with RPMI-5 medium with 5 % fetal calf serum through a 200 μm mesh nylon screen, the suspension is centrifuged twice for 10 min at 200×g with serum containing RPMI. After the supernatant is discarded, red blood cells are lysed by resuspension of the pellet with 5 ml of 0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2–7.4 and incubation for 5 min at room temperature with occasional shaking. Leukocytes from spleens were stabilized and sent to the University of Chicago as described above for peripheral blood.
Quantification of B and T Cells in CCM Murine Models
B cells and T cells from splenic and peripheral blood leukocytes were quantified by flow cytometry by the University of Chicago Flow Cytometry Facility after leukocytes were fluorescently labeled with anti-CD45R/B220, anti-CD19 and anti-CD22 antibodies by the University of Chicago Fitch Monoclonal Antibody Facility.
CCM Lesion Analysis
At approximately 4 months of age, both Pdcd10+/−Trp53−/−and Pdcd10+/− murine models were assessed for lesion burden and the lesional area on serial histologic sections by a procedure previously described (McDonald et al. 2011; McDonald et al. 2012). Briefly, formalin fixed brains were cut into 1-mm thick coronal slices. After embedding the slices in paraffin, the slices were cut into 5-μm thick sections with a microtome. After staining with hematoxylin and eosin, the sections were assessed for number of stage 1 pre-lesions (single ballooned capillaries >100 μm in diameter) and stage 2 lesions (mature, multicavernous), and the lesional area was determined by using the polygon area function of a microscope digital camera DP21 (Olympus).
Immunohistochemistry
Blank sections from spleens were stained for CD45R/B220 positive B lymphocytes and CD3 positive T lymphocytes, and from brains with CCM stage 2 lesions for non-heme iron deposition by Perl Prussian blue, ROCK activity by intensity of phosphorylated myosin light chain (pMLC) and also for B cells through methods previously described (McDonald et al. 2011; McDonald et al. 2012). Sections with tumors were stained for CD31 positive endothelium (angiosarcoma) and CD3 positive T cells (lymphoma), and identified by University of Chicago neuropathologist Peter Pytel.
Attrition
Seven Pdcd10+/−Trp53−/− mice (all untreated controls) and no Pdcd10+/− mice were excluded from analysis because of attrition. Two Pdcd10+/−Trp53−/− mice died before euthanasia from unknown causes. Nine Pdcd10+/−Trp53−/− mice (6 untreated, 3 treated with anti-BR3 antibody) were euthanized before the end of the study, to prevent suffering as determined by the IACUC, including five mice harboring tumors (2 untreated, 3 treated with anti-BR3 antibody), one mouse exhibiting lethargy and weight loss from unknown causes and one mouse with an injury. Two untreated Pdcd10+/−Trp53−/− mice that also had tumors and euthanized at ≥112 days of age were included in the analysis.
Statistical Analysis
The F test was used to evaluate the variances between two unpaired groups. The differences between these two groups were compared using Student’s t-test with equal variances and Welch’s t test with unequal variances. The lesion area was normalized using log-transformation.
The lesions per animal between B-cell depleted and untreated groups were compared for stage 1, stage 2 and all lesions using Negative Binomial Regression if the outcome is over-dispersed and Poisson regression analysis if the mean and variance are equal.
The Mann-Whitney test was used to compare integrated density of iron per lesion and integrated density of iron per lesional area between B-cell depleted and untreated groups.
Chi-square test was conducted to compare the prevalence of stage 2 lesions and endothelial cell ROCK activity between B-cell depleted and untreated groups.
Statistical analyses were performed using SAS9.4 (SAS Institute Inc., Cary, NC) and GraphPad Prism 4.0 (Graphpad Software Inc., La Jolla, CA). All P values were considered to be statistically significant at P < 0.05.
Results
Confirmation of B-Cell Depletion in a CCM Murine Model
Flow cytometry indicated over an 80 % reduction in B lymphocytes in both peripheral blood and spleens in Pdcd10+/−Trp53−/− mice treated with anti-BR3 antibody compared to the same mice not treated with antibody (Supplementary Fig. 1a). Most of B cells were CD45R, CD19, and CD22 triple positive cells. In contrast, treatment with anti-BR3 antibody did not reduce T lymphocytes in this model. Depletion of B cells, but not T cells, in spleens by the anti-BR3 antibody in the same murine models was confirmed by immunohistochemistry (Supplementary Fig. 1b).
CCM Lesion Burden and Lesional Area after B-Cell Depletion in Pdcd10+/−Trp53−/− (Sensitized) Mice
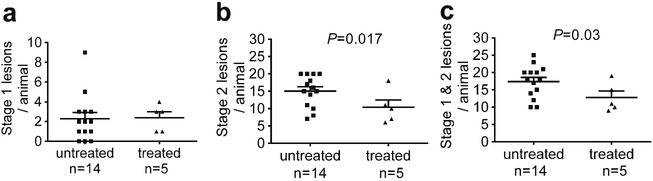
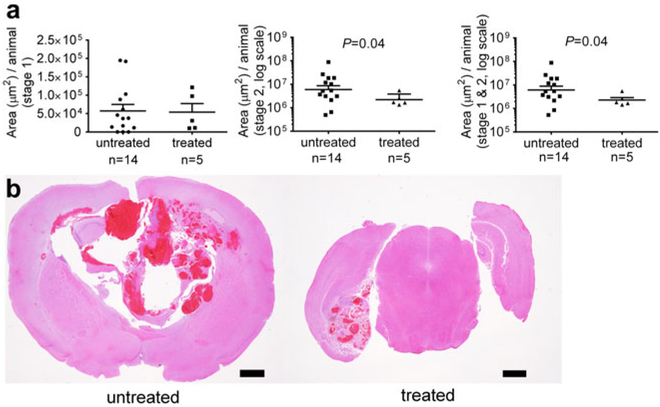
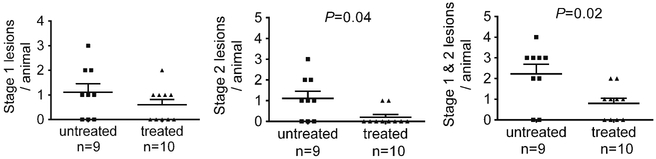
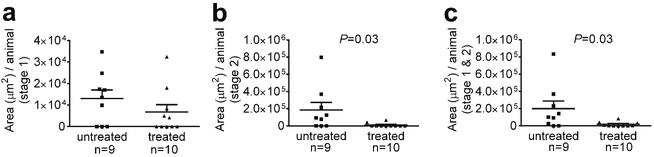
Anti-BR3 antibody treatment of Pdcd10+/−Trp53−/− mice reduced the number of mature stage 2 lesions per animal, by about a third as compared to the same untreated mice (Fig. 1). In contrast, the amount of stage 1 pre-lesions were unaffected by B cell depletion. Similarly, B cell therapy halved the stage 2 lesional area in the same murine model, but did not affect the size of the stage 1 pre-lesions (Fig. 2).
Fig. 1.
Lesion burden in a sensitized CCM murine model after B cell depletion. a) Treatment of Pdcd10+/−Trp53−/− mice with anti-BR3 antibody did not affect the number of stage 1 pre-lesions. B-cell depletion significantly decrease b) the number of mature stage 2 lesions and c) the total number of lesions in these animals. Horizontal lines indicate the mean (longer lines) and standard error of the mean (shorter lines)
Fig. 2.
Lesional area in a sensitized CCM murine model after B cell depletion. a Anti-BR3 antibody treatment of Pdcd10+/−Trp53−/− mice significantly reduced the area of mature stage 2 lesions, but did not affect the area of stage 1 pre-lesions. Horizontal lines indicate the mean (longer lines) and standard error of the mean (shorter lines). b Representative largest lesions are shown from treated and untreated mice at the same magnification (these do not represent the same brain regions). Scale bars are 1 mm
Lesional B Cells after Anti-BR3 Antibody Therapy
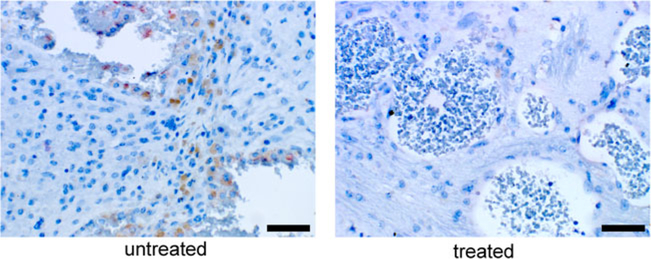
No B cells were detected in the lesions of Pdcd10+/−Trp53−/−mice treated with anti-BR3 antibody in contrast to B cell infiltration observed in stage 2 lesions of untreated mice (Fig. 3).
Fig. 3.
Absence of B lymphocytes in CCM lesions in a sensitized murine model after B cell depletion. While CCM lesions harbored numerous brown stained CD45R/B220 positive B cells in untreated Pdcd10+/−Trp53−/− mice (left panel), lesions from the same murine model treated with anti-BR3 antibody contained no B cells (right panel). Scale bars are 50 μm
Non-Heme Iron Deposition in CCM Lesions after Anti-BR3 Antibody Therapy in Sensitized Mice
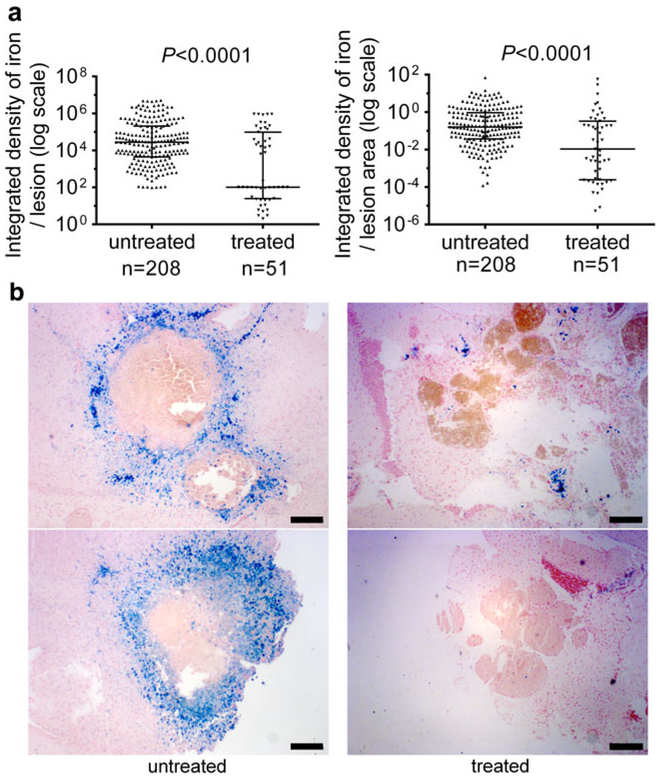
Anti-BR3 antibody treatment of Pdcd10+/−Trp53−/− mice reduced the amount non-heme iron deposition per lesion and the amount non-heme iron deposition per lesional area in mature stage 2 lesions by about two thirds as compared to that found in the same untreated model (Fig. 4).
Fig. 4.
Non-heme iron deposition in mature CCM lesions in a sensitized murine model after B cell depletion. a Anti BR3 antibody treatment of Pdcd10+/−Trp53−/− mice reduced the integrated density of nonheme iron deposition per lesion (left) and the integrated density of non-heme iron deposition per lesional area in stage 2 lesions (right) as compared to the same untreated mice. Horizontal lines indicate the median (longer lines) with interquartile range (shorter lines). b Representative nonheme iron deposition (Perl blue stain) in mature multicavernous lesions is shown for 2 untreated mice (left panels) and 2 mice treated with anti-BR3 antibody (right panels). Scale bars are 200 μm
Tumors in B-Cell Depleted Sensitized Mice
Seven Pdcd10+/−Trp53−/− mice harbored tumors (4 untreated, 3 treated with anti-BR3 antibody). From the three animals with tumors (heart, facial and shoulder) that were treated with anti-BR3 antibody, the tumors were either underdetermined or identified as CD31 positive angiosarcoma or CD3 positive lymphoma (Supplementary Fig. 2).
CCM Lesion Burden and Lesional Area after B-Cell Depletion in Pdcd10+/− (Non-Sensitized) Mice
Contemporaneously raised mice, randomly assigned to treatment or control, had their brains examined blindly for lesion burden at the Chicago site. Anti-BR3 antibody treatment of Pdcd10+/− mice reduced the amount of mature stage 2 lesions by about 80 % as compared to untreated Pdcd10+/− mice (Fig. 5). In contrast, the prevalence of stage 1 pre-lesions were unaffected by B cell depletion. Similarly, B cell therapy reduced the stage 2 lesional area by over 90 % in the same murine model, but did not affect the size of the stage 1 prelesions (Fig. 6).
Fig. 5.
CCM lesion burden in a non-sensitized murine model after B cell depletion. Anti-BR3 antibody treatment of Pdcd10+/− mice significantly decreased the amount of mature stage 2 lesions, but did not affect the number of stage 1 pre-lesions, compared to untreated mice run contemporaneously. Horizontal lines indicate the mean (longer lines) and standard error of the mean (shorter lines)
Fig. 6.
Lesional area in a non-sensitized CCM murine model after B cell depletion. Anti-BR3 antibody treatment of Pdcd10+/− mice did not reduce a) the area of stage 1 pre-lesions, but significantly reduced b) the area of mature stage 2 lesions and c) total area of lesions compared to randomly untreated mice raised contemporaneously, and assessed blindly. Horizontal lines indicate the mean (longer lines) and standard error of the mean (shorter lines)
Consistent with their milder phenotype, non-sensitized Pdcd10+/− mice that were either untreated or treated with the anti-BR3 antibody manifested little or no iron deposition.
ROCK Activity in Endothelium of Stage 2 Lesions after B-Cell Depletion in Non-Sensitized Mice
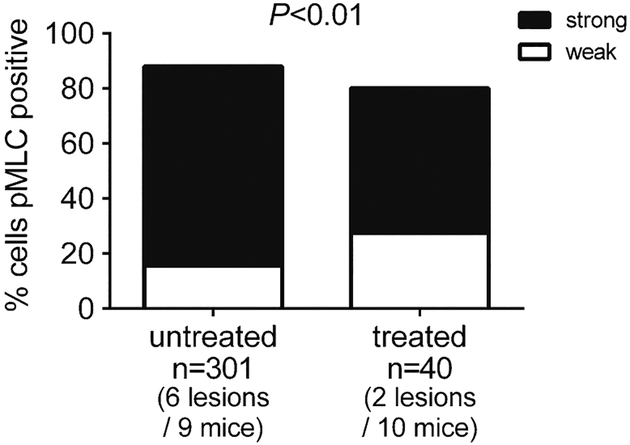
ROCK activity in endothelial cells was assessed by positive brown pMLC immunostaining in two stage 2 lesions in two anti-BR3 antibody treated Pdcd10+/− mice and in six stage 2 lesions in six untreated Pdcd10+/− mice maintained contemporaneously. The ratio of pMLC strong stained to weak/unstained endothelial cells in B cell depleted mice (21 strong stained/19 weak/unstained) was lower (P < 0.01) than in untreated mice (218 strong stained/83 weak/unstained) (Fig. 7).
Fig. 7.
Rho kinase (ROCK) activity in a non-sensitized CCM murine model after B-cell depletion. Strong ROCK activity as measured by phosphorylated myosin light chain staining in the endothelium of mature stage 2 lesions was significantly decreased in B-cell depleted mice compared to untreated mice
Discussion
Our study provides the first definitive proof that B-cell immunomodulation alters the maturation of CCM. Biologics, including B-cell depleting agents, have been used in a wide variety of applications ranging from malignancies such as Non-Hodgkin’s lymphoma (Sehn et al. 2005) to autoimmune conditions including multiple sclerosis, ulcerative colitis, systemic lupus erythematosus and rheumatoid arthritis (Maxwell and Singh 2010; Coles et al. 2012; O’Neill and Scully 2013). B-cell immunotherapy dates back to rituximab’s approval in 1997 and has been growing considerably (Lin et al. 2007). We utilized anti-BR3 antibodies as a method for B cell depletion. BR3 (also known as B-cell activating factor receptor and BLyS receptor) is a receptor within a well-defined and characterized pathway that drives B-cell survival and replication and targeting it has been shown to induce B-cell depletion (Mackay et al. 2003; Vugmeyster et al. 2006).
Anti-BR3 antibodies were chosen as a method of B cell depletion because of their ability to induce B cell depletion more effectively than anti-CD20 or BAFF-dependent survival blockade alone (Lin et al. 2007). Pdcd10+/−Trp53−/−mice with severe phenotype (Shenkar et al. 2015) were used as a proof of concept and as a first feasibility study. We verified the success of B-cell depletion by confirming their relative paucity in the peripheral blood and spleen. As well, CCM lesions in mice treated with anti-BR3 had no B-cells in their lesions. B cell depletion resulted in a significantly lower burden of mature (stage 2), clinically significant CCM lesions, and a dramatic decrease in hemorrhagic iron deposit in the lesions.
However, this first proof of concept presented several confounders. First, the impact of Trp53 loss necessary to induce the severe phenotype may have impacted the effect of immunomodulation. Also, we found extra-cranial tumors in 3 out of 5 B-cell depleted Pdcd10+/−Trp53−/− mice (including 1 angiosarcoma and 1 lymphoma). The presence of such tumors might impact our assessment of lesion burden and response to therapy. As well, the first feasibility experiment was an open label study without randomization or blinded assessment of lesion burden (Landis et al. 2012). Hence we further tested whether therapeutic benefit would remain in non-sensitized heterozygous Pdcd10+/− mice in absence of Trp53 loss, and with more rigorous prospective randomization and blinded assessment of lesion burden. B-cell depletion was shown again to significantly decrease the burden of mature stage 2 lesions in this model with less severe phenotype.
No effect was seen on the number or size of isolated capillary ectasia, pre-lesions (stage 1). This indicates that B-cell depletion does not impact the initial lesion genesis, but rather halts the maturation and progression of already formed ectatic blood vessels into multicavernous clinically significant lesions. These results are anticipated given the previous demonstration that stage 1 pre-lesions develop as a result of Knudsonian biallelic somatic mutations in endothelial cells lining the CCM lesions, and these pre-lesions lack hemorrhage and immune cell infiltration (Akers et al. 2009; McDonald et al. 2011). Polymorphism in immune response and inflammatory pathways has also been implicated in disease severity and penetrance (Choquet et al. 2014). The immunomodulation likely comes into play secondary to a leaky blood-brain barrier, sequestration of thrombi at various stages of organizations, and chronic deposition of blood degradation products characterizing the more mature clinically significant stage 2 lesions, creating a niche for a neo-antigenic milieu and immune response activation. This implies that the immune system responds to and exacerbates CCM pathology rather than induce it. Hence, the findings in this study provide proof of principle that B cell mechanism is a critical step in CCM lesion maturation into a clinically relevant phenotype.
We have not detected any tumors in 10 out of 10 B-cell depleted Pdcd10+/− (non sensitized) mice in the absence of Trp53 loss. This indicates that Trp53−/− background induces neoplasia in conjunction with B cell depletion, as previously reported in multiple studies (Kaplan et al. 1998; Smyth et al. 2000; Shankaran et al. 2001; Loser et al. 2005; Swann et al. 2009). B cell depletion alone in conjunction with Pdcd10 heterozygosity did not appear to induce neoplasms in our models.
Our findings here clearly establish CCM as another disorder in a long line of immune-mediated diseases, and suggest potential viability of this line of therapy in humans. ROCK inhibition has already emerged as another promising therapy in CCM models (Borikova et al. 2010; McDonald et al. 2012; Richardson et al. 2013). The specific ROCK inhibitor Fasudil, and the less potent yet more pleiotropic drugs statins have been shown to rescue the CCM phenotype in endothelial cells and in mice (Whitehead et al. 2009; McDonald et al. 2012). In fact, ROCK inhibition and statins have well-recognized anti-inflammatory effects (Jain and Ridker 2005; Mueller et al. 2005), and we have shown decreased ROCK activity in our B-cell depleted lesions. Whether immune-modulation using B-cell depletion is superior to ROCK inhibition therapy should be tested in future studies, and the two treatments might convey an additive or synergistic effect. Investigating biologics with different mechanism of action, such as B-T cell interactions (Ruderman and Pope 2006; Lu et al. 2014) will provide greater mechanistic insight into the immuno-pathogenesis molecular pathways of CCM. Moreover, characterizing the putative antigenic trigger in CCM may provide novel approaches antigen-specific immunotherapy of CCM.
Our study did not examine the impact of B- cell depletion on immunoglobulin or complement deposition in the murine CCM lesions, features previously described in human CCMs (Shi et al. 2014). We did not use an isotype matched control antibody or sham treatment groups in our experimental design. And we did not address the impact of potential T-cell immunomodulation, which could also be effective, given the role of B-T cell interactions in pathogenetic immune response in CCMs (Shi et al. 2014). These confounders will need clarification in future research.
The established experience with a variety of biologics makes it relevant to evaluate further targets of the immune pathway that might be more potent and efficacious, or less risky than B-cell depletion. Several agents with excellent tolerance in human use are already approved for use in a variety of autoimmune diseases, and might be repurposed for CCM therapy. We aim to investigate biologics that have proven efficacy in humans, have an acceptable safety profile and are easy to administer. These characteristics would allow us to translate such drugs into clinical trials and practice in a rapid manner. The treatment appears most likely to stabilize a CCM lesion and prevent its progression through further proliferative growth and hemorrhage, rather initial lesion formation. Repurposing such drugs would hence most likely aim at the treatment of CCMs that had recently bled. Such lesions exert a heavy public health burden, with a high rate of subsequent growth and rebleeding.
Supplementary Material
Acknowledgments
This work was supported by the Scientist Development Grant of the American Heart Association AHA-11DG48900009 (C.S.), a pilot grant from the Angioma Alliance, and the Bill and Judy Davis Fund in Neurovascular Surgery Research at the University of Chicago. We thank Genentech Inc. for providing the anti-BR3 antibody through Material Transfer Agreement to IAA, and Flavius Martin for advice on the dosing regimen.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11481-016-9670-0) contains supplementary material, which is available to authorized users.
Ethical Approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Akers AL, Johnson E, Steinberg GK, Zabramski JM, Marchuk DA (2009) Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet 18:919–930. doi: 10.1093/hmg/ddn430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Holou WN, O’Lynnger TM, Pandey AS, Gemmete JJ, Thompson BG, Muraszko KM, Garton HJ, Maher CO (2012) Natural history and imaging prevalence of cavernous malformations in children and young adults. J Neurosurg Pediatr 9:198–205. doi: 10.3171/2011.11.PEDS11390 [DOI] [PubMed] [Google Scholar]
- Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA, Angioma Alliance Scientific Advisory B (2008) Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Sci Advis Board Stroke 39:3222–3230. doi: 10.1161/STROKEAHA.108.515544 [DOI] [PubMed] [Google Scholar]
- Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, Counsell CE, Murray GD, Papanastassiou V, Ritchie V, Roberts RC, Sellar RJ, Warlow CP, Scottish Audit of Intracranial Vascular Malformations c (2012) Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 11:217–224. doi: 10.1016/S1474-4422(12)70004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borikova AL, Dibble CF, Sciaky N, Welch CM, Abell AN, Bencharit S, Johnson GL (2010) Rho kinase inhibition rescues the endothelial cell cerebral cavernous malformation phenotype. J Biol Chem 285: 11760–11764. doi: 10.1074/jbc.C109.097220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet H, Pawlikowska L, Nelson J, McCulloch CE, Akers A, Baca B, Khan Y, Hart B, Morrison L, Kim H, Brain Vascular Malformation Consortium S (2014) Polymorphisms in inflammatory and immune response genes associated with cerebral cavernous malformation type 1 severity. Cerebrovasc Dis 38:433–440. doi: 10.1159/000369200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, Skoromets A, Stolyarov I, Bass A, Sullivan H, Margolin DH, Lake SL, Moran S, Palmer J, Smith MS, Compston DA (2012) Alemtuzumab more effective than interferon beta-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 78:1069–1078. doi: 10.1212/WNL.0b013e31824e8ee7 [DOI] [PubMed] [Google Scholar]
- Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4:977–987. doi: 10.1038/nrd1901 [DOI] [PubMed] [Google Scholar]
- Jakimovski D, Schneider H, Frei K, Kennes LN, Bertalanffy H (2014) Bleeding propensity of cavernous malformations: impact of tight junction alterations on the occurrence of overt hematoma. J Neurosurg 121:613–620. doi: 10.3171/2014.6.JNS132775 [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD (1998) Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 95:7556–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, Lunsford LD, Kestle JR (1995) The natural history of cerebral cavernous malformations. J Neurosurg 83:820–824. doi: 10.3171/jns.1995.83.5.0820 [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD (2012) A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490:187–191. doi: 10.1038/nature11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Gong Q, Seshasayee D, Lin Z, Ou Q, Ye S, Suto E, Shu J, Lee WP, Lee CW, Fuh G, Leabman M, Iyer S, Howell K, Gelzleichter T, Beyer J, Danilenko D, Yeh S, DeForge LE, Ebens A, Thompson JS, Ambrose C, Balazs M, Starovasnik MA, Martin F (2007) Anti-BR3 antibodies: a new class of B-cell immunotherapy combining cellular depletion and survival blockade. Blood 110:3959–3967. doi: 10.1182/blood-2007-04-088088 [DOI] [PubMed] [Google Scholar]
- Loser K, Scherer A, Krummen MB, Varga G, Higuchi T, Schwarz T, Sharpe AH, Grabbe S, Bluestone JA, Beissert S (2005) An important role of CD80/CD86-CTLA-4 signaling during photocarcinogenesis in mice. J Immunol 174:5298–5305 [DOI] [PubMed] [Google Scholar]
- Louvi A, Chen L, Two AM, Zhang H, Min W, Gunel M (2011) Loss of cerebral cavernous malformation 3 (Ccm3) in neuroglia leads to CCM and vascular pathology. Proc Natl Acad Sci USA 108: 3737–3742. doi: 10.1073/pnas.1012617108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Nakagawa R, Lazzaro S, Staudacher P, Abreu-Goodger C, Henley T, Boiani S, Leyland R, Galloway A, Andrews S, Butcher G, Nutt SL, Turner M, Vigorito E (2014) The miR-155-PU.1 axis acts on Pax5 to enable efficient terminal B cell differentiation. J Exp Med 211:2183–2198. doi: 10.1084/jem.20140338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Schneider P, Rennert P, Browning J (2003) BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol 21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152 [DOI] [PubMed] [Google Scholar]
- Maraire JN, Awad IA (1995) Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery 37:591–605 [DOI] [PubMed] [Google Scholar]
- Maxwell LJ, Singh JA (2010) Abatacept for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol 37:234–245. doi: 10.3899/jrheum.091066 [DOI] [PubMed] [Google Scholar]
- McDonald DA, Shenkar R, Shi C, Stockton RA, Akers AL, Kucherlapati MH, Kucherlapati R, Brainer J, Ginsberg MH, Awad IA, Marchuk DA (2011) A novel mouse model of cerebral cavernous malformations based on the two-hit mutation hypothesis recapitulates the human disease. Hum Mol Genet 20:211–222. doi: 10.1093/hmg/ddq433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH, Marchuk DA, Awad IA (2012) Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke 43:571–574. doi: 10.1161/STROKEAHA.111.625467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati AG, Khanna O, Zhang L, Girard R, Shenkar R, Guo X, Shah A,Larsson HB, Tan H, Li L, Wishnoff MS, Shi C, Christoforidis GA, Awad IA (2015) Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab 35:1632–1639. doi: 10.1038/jcbfm.2015.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity JL, Clatterbuck RE, Rigamonti D (1999) The natural history of cavernous malformations. Neurosurg Clin N Am 10:411–417 [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N (2005) Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 4:387–398. doi: 10.1038/nrd1719 [DOI] [PubMed] [Google Scholar]
- O’Neill ID, Scully C (2013) Biologics in oral medicine: ulcerative disorders. Oral Dis 19:37–45. doi: 10.1111/j.1601-0825.2012.01931.x [DOI] [PubMed] [Google Scholar]
- Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN,Marchuk DA (2004) Loss of p53 sensitizes mice with a mutation in Ccm1 (KRIT1) to development of cerebral vascular malformations. Am J Pathol 165:1509–1518. doi: 10.1016/S0002-9440(10)63409-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riant F, Bergametti F, Ayrignac X, Boulday G, Tournier-Lasserve E (2010) Recent insights into cerebral cavernous malformations: the molecular genetics of CCM. FEBS J 277:1070–1075. doi: 10.1111/j.1742-4658.2009.07535.x [DOI] [PubMed] [Google Scholar]
- Richardson BT, Dibble CF, Borikova AL, Johnson GL (2013) Cerebral cavernous malformation is a vascular disease associated with activated RhoA signaling. Biol Chem 394:35–42. doi: 10.1515/hsz-2012-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF (1987) The MRI appearance of cavernous malformations (angiomas). J Neurosurg 67:518–524. doi: 10.3171/jns.1987.67.4.0518 [DOI] [PubMed] [Google Scholar]
- Robinson JR Jr, Awad IA, Masaryk TJ, Estes ML (1993) Pathological heterogeneity of angiographically occult vascular malformations of the brain. Neurosurgery 33:547–554, discussion 554–545 [DOI] [PubMed] [Google Scholar]
- Ruderman EM, Pope RM (2006) Drug Insight: abatacept for the treatment of rheumatoid arthritis. Nat Clin Pract Rheumatol 2:654–660. doi: 10.1038/ncprheum0345 [DOI] [PubMed] [Google Scholar]
- Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, MacPherson N, O’Reilly S, Spinelli JJ, Sutherland J, Wilson KS, Gascoyne RD, Connors JM (2005) Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia . J Clin Oncol 23: 5027–5033. doi: 10.1200/JCO.2005.09.137 [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ,Schreiber RD (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410:1107–1111. doi: 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- Shenkar R, Shi C, Rebeiz T, Stockton RA, McDonald DA, Mikati AG, Zhang L, Austin C, Akers AL, Gallione CJ, Rorrer A, Gunel M, Min W, Marcondes de Souza J, Lee C, Marchuk DA, Awad IA (2015) Exceptional aggressiveness of cerebral cavernous malformation disease associated with PDCD10 mutations. Genet Med 17:188–196. doi: 10.1038/gim.2014.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Shenkar R, Batjer HH, Check IJ, Awad IA (2007) Oligoclonal immune response in cerebral cavernous malformations. Laboratory investigation. J Neurosurg 107:1023–1026. doi: 10.3171/JNS-07/11/1023 [DOI] [PubMed] [Google Scholar]
- Shi C, Shenkar R, Du H, Duckworth E, Raja H, Batjer HH, Awad IA (2009) Immune response in human cerebral cavernous malformations. Stroke 40:1659–1665. doi: 10.1161/STROKEAHA.108.538769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Shenkar R, Kinloch A, Henderson SG, Shaaya M, Chong AS, Clark MR, Awad IA (2014) Immune complex formation and in situ B-cell clonal expansion in human cerebral cavernous malformations. J Neuroimmunol 272:67–75. doi: 10.1016/j.jneuroim.2014.04.016 [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA (2000) Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 192:755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger HJ, Markwalder TM, Reulen HJ (1987) Clinicopathological relations of cerebral cavernous angiomas: observations in eleven cases. Neurosurgery 21:879–884 [DOI] [PubMed] [Google Scholar]
- Stockton RA, Shenkar R, Awad IA, Ginsberg MH (2010) Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med 207:881–896. doi: 10.1084/jem.20091258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, Smyth MJ (2009) Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood 113:6382–6385. doi: 10.1182/blood-2009-01-198564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Liu T, Wu Y, Thacker J, Shenkar R, Mikati AG, Shi C, Dykstra C, Wang Y, Prasad PV, Edelman RR, Awad IA (2014) Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Invest Radiol 49:498–504. doi: 10.1097/RLI.0000000000000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulamo R, Frosen J, Junnikkala S, Paetau A, Pitkaniemi J, Kangasniemi M, Niemela M, Jaaskelainen J, Jokitalo E, Karatas A, Hernesniemi J, Meri S (2006) Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery 59:1069–1076. doi: 10.1227/01.NEU.0000245598.84698.26, discussion 1076–1067 [DOI] [PubMed] [Google Scholar]
- Vugmeyster Y, Seshasayee D, Chang W, Storn A, Howell K, Sa S, Nelson T, Martin F, Grewal I, Gilkerson E, Wu B, Thompson J, Ehrenfels BN, Ren S, Song A, Gelzleichter TR, Danilenko DM (2006) A soluble BAFF antagonist, BR3-Fc, decreases peripheral blood B cells and lymphoid tissue marginal zone and follicular B cells in cynomolgus monkeys. Am J Pathol 168:476–489. doi: 10.2353/ajpath.2006.050600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Jones CA, Zhu W, Marchuk DA, Davis GE, Li DY (2009) The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med 15: 177–184. doi: 10.1038/nm.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JH, Awad IA, Kim JH (2000) Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery 46:1454–1459 [DOI] [PubMed] [Google Scholar]
- Xing GQ, Chen M, Liu G, Heeringa P, Zhang JJ, Zheng X, E J (2009) Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol 29:282–291. doi: 10.1007/s10875-008-9268-2 [DOI] [PubMed] [Google Scholar]
- Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, Brown B, Rigamonti D, Brown G (1994) The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 80:422–432. doi: 10.3171/jns.1994.80.3.0422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.