Abstract
Radiotherapy (RT) has become an indispensable part of oncologic treatment protocols for a range of malignancies. However, a serious side effect of RT is radiodermatitis; almost 95% of patients develop moderate to severe skin reactions following radiation treatment. In the acute setting, these can erythema, desquamation, ulceration, and pain. Chronically, soft tissue atrophy, alopecia, and stiffness can be noted. Radiodermatitis can delay oncologic treatment protocols and significantly impair quality of life. There is currently a paucity of effective treatment options and prevention strategies for radiodermatitis. Importantly, recent preclinical and clinical studies have suggested that fat grafting may be of therapeutic benefit, reversing detrimental changes to soft tissue following radiation therapy. This review outlines the damaging effects of RT on the skin and soft tissue as well as discusses currently available treatment options for radiodermatitis. Emerging strategies to mitigate detrimental, chronic radiation-induced changes are also presented.
Introduction
Radiation therapy or radiotherapy (RT) has become an essential part of curative as well as palliative oncologic treatment protocols for a range of malignancies; currently RT is used as an adjunct therapy in over 50% of cancer patients1,2. While delivery methods for RT have been developed to combat cancer more effectively, collateral damage to healthy tissue in the radiation field surrounding the area of malignancy remains a serious adverse outcome. Skin is particularly radiosensitive, and over 95% of patients receiving RT develop moderate to severe skin reactions3,4. In the acute phase following radiation exposure, the skin typically becomes erythematous and may desquamate or ulcerate. On the molecular level, cytokine cascades and fibro-inflammatory pathways are up-regulated due to radiation which can progress for many years leading to substantial fibrosis, the hallmark of chronic RT damage5. Cutaneous fibrosis alters form, function, and aesthetic appearance of the skin, and the consequences can significantly impact quality of life. Although a number of treatment options have been described, none has proven to be effective in preventing or reversing radiation-induced fibrosis (RIF) of the skin. Recent clinical and preclinical studies have demonstrated the benefit of autologous fat grafting (AFG) in the treatment of RIF6,7. First used for reconstructive purposes, fat is increasingly recognized to exert regenerative effects upon the tissue into which it is transplanted8–10. In irradiated skin, fat grafts can attenuate acute inflammation and slow/reverse the progression of chronic RIF6. The mechanisms by which fat regenerates the overlying skin and soft tissue remains to be elucidated but is thought to be driven by the adipose derived stromal cells (ASCs) of the stromal vascular fraction (SVF) of adipose tissue. ASCs have potent paracrine signaling action and are also multipotent and able to differentiate into a number of mesenchymal cell lineages. In this review, we outline the current understanding of RIF, the current treatment options, and the benefit of AFG within this setting. We also delve into alternative emerging strategies to mitigate RIF.
Radiation-induced cell death
Radiation therapy is the process of delivering lethal doses of radiation to areas of malignancy to kill cancer cells. Radiation therapy has evolved to allow for more specific targeting of cancer cells and reduction of the “bystander response” in neighboring healthy tissue11. There are three main ways to deliver RT: 1) External beam radiation therapy directs radiation beams from outside of a patient’s body in the direction of the tumor; 2) Brachytherapy delivers radiation internally with the insertion of radioactive materials inside the body; and 3) Radioisotope therapy systemically circulates radiation throughout the bloodstream via injection of a targeted radioisotope12–14 RT can utilized alone or can be combined with other treatment modalities–such as chemotherapy or surgery–to treat primary malignancies as well as metastatic disease15.
Radiation therapy is based on the concept that malignant cells are more sensitive to radiation and cannot repair damage as efficiently as healthy cells. The molecular mechanisms of radiation-induced cell death are not completely understood16, and several mechanisms may be at play. Within hours of radiation, a number of cytokine signaling and inflammatory cascades are initiated. Radiation therapy forms ions that pass through tissues which can directly induce double-stranded breaks in genetic material17. Cell death ensues via apoptosis, mitotic cell death, necrosis, and/or senescence12 including the release of damage-associated molecule pattern (DAMP) molecules18,19. Release of DAMPs activates the innate and adaptive immune systems that allows for additional antitumor responses20,21. Energy from ionizing radiation also acts on other molecules within cells, such as water, to generate reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, and hydroxyl radical, which indirectly cause further damage of the DNA and other cellular components (e.g. proteins, lipids)22,23. Generation of ROS are thought to account for more than 60% of the total radiation induced damage24,25.
To improve targeting of malignant cells with radiation therapy, Begg and colleagues have described several approaches to modulate cellular response to radiation. These include inhibiting additional DNA repair mechanisms, cell cycle checkpoints, and signal transduction pathways26. For example, breast cancer cells with BRCA1 or BRCA2 mutations already have an impaired ability to repair double-stranded breaks in DNA via homologous recombination and rely on other mechanisms of DNA repair, such as base excision repair and single-strand break repair, to survive26. Farmer et al. found that exposure of BRCA1- or BRCA2-deficient embryonic stem cells to an inhibitor of poly(ADP-ribose) polymerase–an enzyme involved in base excision repair–resulted in cell cycle arrest and apoptosis27. Inhibition of alternative survival and signaling pathways would thus render cancer cells more vulnerable to radiation-induced DNA damage while sparing normal cells that retain other mechanisms of repair.
Radiodermatitis
While it is the aim of RT to deliver sufficient levels of radiation to induce death of cancer cells, damaging effects on surrounding healthy cells should be minimized. Substantial progress has been made towards this goal, but damage to healthy soft tissue within the radiation field remains a significant problem. The proliferative nature, high oxygen requirement, and superficial nature of the skin make it the most frequently damaged tissue following RT28,29. Collectively, damage to the skin following RT is known as radiodermatitis and is typically categorized into acute and chronic stages. In the early phase following radiation exposure, the skin appears discolored, erythematous, and inflamed. Severely damaged skin may desquamate, atrophy and/or ulcerate30–32. The chronic phase of radiation damage is marked by radiation-induced fibrosis–the final common pathology across multiple tissue types. Skin RIF involves substantial dermal and epidermal induration, scarring and retraction, with histological evidence of extensive hyalinization of reticular collagen. There may be associated permanent scarring alopecia or loss of hair pigmentation33–38, suggesting an irreversible loss of hair follicle stem cells and melanocyte stem cells. The epidermis may be hyperplastic or atrophic and develop chronic ulcers and/or skin tumors.39,40 Chronic radiation-induced fibrosis typically develops within 4 to 12 months after therapy but may continue for many years in a progressive fashion40.
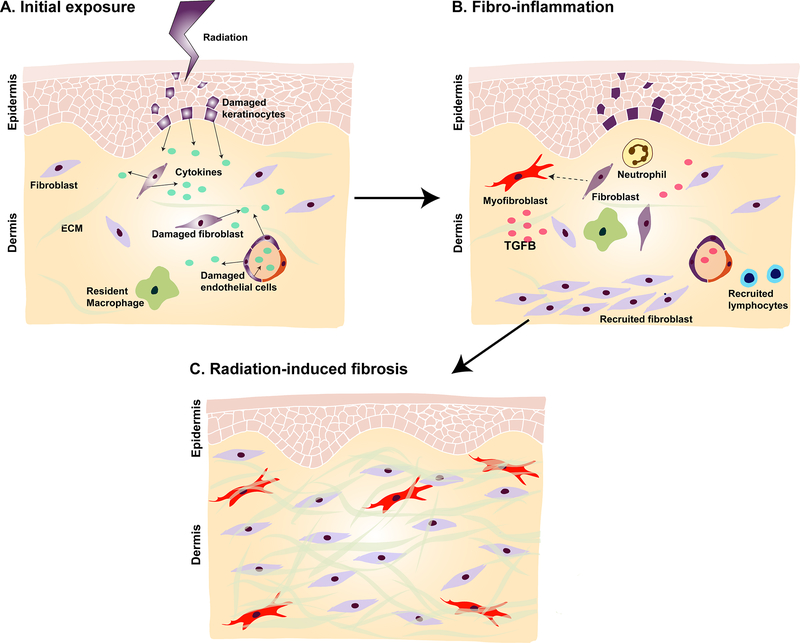
The same mechanisms at play in killing cancer cells are also responsible for causing radiodermatitis (Fig. 1). Immediately following exposure there is an inflammatory response, and neutrophils are attracted to the site of irradiation by cytokines that are released by damaged skin and endothelial cells. Upon entry to the irradiated area, neutrophils are stimulated further and release pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6, which perpetuate inflammation and increase formation of ROS. Monocytes and lymphocytes subsequently migrate to irradiated skin. Upon entry into irradiated tissue, monocytes differentiate into macrophages, and release platelet-derived growth factor (PDGF) which stimulates angiogenesis and the migration of fibroblasts41. Finally, macrophages, along with the native endothelial cells, fibroblasts, and epidermal cells, secrete transforming growth factor-beta (TGF-β)5,42, a potent pro-fibrotic factor which is elevated in the early phases of radiation damage5 and heavily implicated in the pathogenesis of RIF. TGF-β binds the TGβRI receptor and thus induces phosphorylation, and activation of the intracellular receptor-associated Smads (R-Smads). Activated R-Smads form heteromeric complexes with a co-Smad (Smad4), translocate to the nucleus, and induce pro-fibrotic gene transcription, either by directly binding DNA or by associating with other transcription factors43–45.
Fig. 1. The proposed mechanism underlying radiation-induced fibrosis (RIF).
A) RT delivers ions that directly induces DNA damage and generates reactive oxygen species (ROS). Damaged cells in the skin and endothelium (colored purple) release cytokines, leading to activation of the innate and adaptive immune systems and recruitment of inflammatory cells. B) Once recruited to the irradiated area, neutrophils release additional inflammatory mediators to sustain inflammation. Lymphocytes and monocytes also migrate to the location of injury, the latter of which differentiate into macrophages. Macrophages, fibroblasts, native endothelial cells, and epidermal cells release transforming growth factor-beta (TGF-β) which stimulates fibroblasts to differentiate into myofibroblasts. C) Myofibroblasts secrete excess amounts of extracellular matrix proteins, leading to increased tissue stiffness and thickness. Over time, RIF ensues and may persist even decades after radiation therapy.
TGF-β activates a number of pro-fibrotic pathways that drive the pathogenesis of radiodermatitis. Following radiation, TGF-β stimulates the differentiation of fibroblasts into myofibroblasts46, which in turn secrete excessive amounts of ECM proteins including collagen, fibronectin, and proteoglycans47. Increased ECM production is further compounded by impaired matrix degradation. TGF-β decreases the activity of matrix metalloproteinase (MMP) activity, specifically MMP-2, MMP-9, and MMP-1, within fibroblasts30,31,48. Consequently, there is net gain of ECM that amounts to increased tissue stiffness and thickness, characteristic of chronic RIF. TGF-β in radiation also activates the process of epithelial to mesenchymal transition (EMT) and the interferon (IFN)-γ response49 which can also contribute to soft tissue fibrosis. Chronic activation of many of these fibrotic pathways is thought to persist for years after initial exposure. Indeed, elevated levels of collagen type I, collagen type III, and TGFβ1 are detectable in breast biopsies even 20 years post-radiotherapy5.
The fibrotic changes in skin are also accompanied by damage to the vasculature. Histologically there is evidence of decreased microvascular network density and alterations to the morphology of blood vessels50. Acutely following radiation exposure, the vessels of mice become plugged with fibrin and leukocytes, with evidence of endothelial swelling and hyperplasia and perivascular fibrosis.46,51 These changes decrease blood supply to the tissue and lower oxygen tension, which further stimulates fibrosis by increasing expression of collagen type 1 alpha 1 (COL1A1)52.
The consequences of RIF are profound, and up to 30% of patients that receive RT to the breast or chest wall experience severe RIF53–58. RIF reduces tissue perfusion and further worsens the quality and function of the irradiated skin50. Tissue fibrosis can disrupt lymphatic and vascular drainage, which produces hypoxia and predisposes to ulceration and impaired wound healing59,60. This often results in severe soft tissue defects that may require coverage with vascularized tissue. Furthermore, implant-based breast reconstructions in the irradiated setting show significantly higher complication rates such as capsular contracture or infection necessitating implant removal or replacement61. As increasing numbers of individuals are surviving cancer, more patients are living with the long-term effects of radiation treatment62. Radiation-induced fibrosis is therefore especially undesirable for patients with malignancies where treatment can be curative63.
Current treatment options
Although some therapies have been shown to delay the onset or reduce the risk of developing RIF, a key step of prevention is to minimize radiation doses to areas of exposed healthy skin. Current treatment options, while limited, range from physical therapy to oral and topical medications. Recent advances in surgical treatment options with autologous fat grafting have also been reported, with some success noted in reversing detrimental changes seen in chronically damaged skin and soft tissue64.
Physical therapy with massage is a non-medical option that, in two studies, has been shown to have some promising results. In one study, twenty patients who had been treated for breast cancer with surgery and radiotherapy were enrolled in a randomized control trial that assessed mechanical massage compared with medical observation alone. Mechanical massage was found to be superior at reducing erythema, pain, pruritus, skin induration, and skin softening65. In a second study, deep friction massage was found to reduce RT-associated muscle spasms, though it did not have any effect on skin appearance66. Massage may have the potential to break down fibrotic tissues, particularly in the thoracic region following breast cancer radiation therapy, interrupting the progressive nature of radiation-induced fibrosis. However, larger, more rigorous studies have yet to be performed to confirm these findings.
Antioxidants have also been studied for their preventative and therapeutic effects in protecting healthy cells from radiation-induced DNA damage67. Silymarin, an extract from milk thistle with antioxidant and anti-inflammatory effects, was noted to delay the onset of radiodermatitis in a randomized trial of forty breast cancer patients when applied topically as a gel for five weeks at the onset of RT68. In a prospective, nonrandomized study of 112 patients post-mastectomy, daily subcutaneous administration of the antioxidant amifostine throughout radiation treatment was associated with reduced erythema, edema, and moist skin desquamation compared with patients who did not receive antioxidant treatment68. Finally, the hemorrheologic agent pentoxifylline has also been shown to have antioxidant effects. Along with improving blood flow, this medication may also inhibit fibroblast proliferation and has been shown to both prevent and treat RIF69. Randomized control trials have shown that the combination of oral pentoxifylline with alpha-tocopherol (vitamin E) improves tissue compliance in breast cancer patients when taken daily for six months post-RT and reduces the RIF surface area even when administered years after RT for breast or head and neck cancer70,71. However, recent studies have found compliance with pentoxifylline and vitamin E therapy to be poor in almost 40% of patients, with nausea the most frequently reported indication for treatment dose reduction or discontinuation of therapy72.
Topical treatments including steroids, gels, and creams have also been studied extensively in randomized trials for treatment of radiation-induced fibrosis. Use of a topical corticosteroid (0.1% methylprednisolone) in concert with 0.5% dexpanthenol, a derivative of pantothenic acid which is an essential component of normally functioning epithelium, delayed the emergence of clinical and functional symptoms of radiation dermatitis by one week in 15 breast cancer patients compared with untreated controls.73 Similarly, a randomized trial of 51 breast cancer patients revealed that topical 0.1% betamethasone delayed, but did not prevent, the occurrence of radiation dermatitis74. Daily use of topical 0.1% mometasone furoate reduced symptoms of radiation-induced skin toxicity, which included assessments of pruritis, irritation, and burning, compared with placebo cream in 176 patients treated with breast or chest wall RT75. Furthermore, creams containing hyaluronic acid or urea may delay or reduce the severity of RT-induced skin effects. In a prospective observational study, 98 breast cancer patients received treatment with lotion containing 3% urea, polidocanol, and hyaluronic acid two to three weeks prior to starting RT. Fewer developed radiodermatitis and of those that did, skin toxicity was reduced compared with controls who did not receive the lotion76. Finally, in a randomized, double-blind, placebo-controlled study, 152 patients with head and neck, breast, or pelvic cancer who received treatment with hyaluronic acid cream for six weeks had delayed and reduced skin reactions to the radiation therapy77.
Fat grafting
The challenges presented by the treatment of RIF have popularized the view that RIF is irreversible. Recently, this concept has been questioned with increasing attention turned to autologous fat grafting and its ability to improve post-irradiated, fibrotic skin7,78. Originally used for volume restoration in reconstructive surgery, fat has become increasingly recognized for its ability to regenerate damaged tissue7,50,79,80. Autologous fat grafting has also been reported to potentially antagonize the effects of aging8–10. In 2007, Rigotti and colleagues7 first demonstrated that fat grafting resulted in visible and symptomatic improvements in 20 patients with RIF following previous radiation treatment for breast cancer. One year after grafting, tissue biopsies showed well vascularized tissue and evidence of fibrosis regression. Since this landmark finding, AFG has been used by more surgeons to reconstruct previously irradiated tissue8,50,81–83. Salgarello et al. reported that fat grafting reduced the radiation-related complications in two patients undergoing breast reconstruction79, and subsequently in a retrospective review of 16 patients84. In a large prospective clinical study, 65 previously irradiated post-mastectomy patients received tissue expanders and AFG as part of their breast reconstruction. With this approach, patients were found to have improved skin quality of the reconstructed breast with reduced capsular contracture (Baker grade ≤1) at 6 months, and this was accompanied by high patient and surgeon satisfaction85. Similarly, Phulpin et al. reported functional and aesthetic benefits in 10 out of 11 patients in whom AFG was used for head and neck reconstruction after RT. Specifically, patients had improved phonation, swallowing, and breathing, with histologic evidence of increased vascularization and normal tissue composition without areas of necrosis50. These reports have been supported by multiple preclinical studies, showing that fat grafting in the irradiated mouse decreases disordered collagen content and thickening of the overlying dermis6, and can increase skin perfusion, as measured by Laser Doppler and immunofluorescence staining51.
While the mechanisms driving these beneficial clinical findings with AFG remain to be elucidated, it is believed that cells within the SVF of adipose tissue, in particular the ASCs, are largely responsible. Adipose tissue is rich in ASCs, which possess multi-lineage potential and the ability to release potent proangiogenic and anti-apoptotic growth factors8,86,87. ASCs may promote angiogenesis by releasing pro-angiogenic growth factors in the recipient site. Consistent with this hypothesis, grafted fat in the irradiated skin of mice was found to increase expression of pro-angiogenic growth factors, such as vascular endothelial growth factor and stromal cell-derived factor 1, and decrease expression of COL1A1 and TGFβ51. Alternatively, ASCs may directly differentiate along various mesenchymal lineages forming endothelial cells which can integrate themselves into newly formed vessels88. Interestingly, a recent study performed whole genome expression analyses on human adipose tissue biopsies harvested from the irradiated and non-irradiated breasts of 10 patients before and 1-year after AFG49. The results indicated that RT causes dysregulated expression of fibrosis-related pathways in human adipose tissue including two canonical pathways: interferon-γ response and hypoxia response. Macrophages were also recruited to the irradiated tissue. Importantly, the dysregulated genes returned to nearly normal expression levels following AFG, supporting the use of AFG in the use of RIF.
Overcoming the challenges of fat-grafting in irradiated tissue
Though fat grafting has shown incredible promise with treatment of radiation-induced soft tissue injury, there remains a number of challenges to address, particularly with grafting into hostile irradiated tissue. Fat retention is already variable even at non-irradiated recipient sites, and resorption rates may range between 40 and 60%89,90. Delivery of small aliquots of adipose tissue into well-vascularized sites can increase survival91–93, but irradiated tissue is hypovascular, inflamed, and fibrotic51,78. This can lead to fat necrosis and stimulate an inflammatory reaction resulting in fibrosis, cyst formation, calcification, or local infection94–97. When used for breast reconstruction, AFG has been reported to show increased rates of fat necrosis and infection in the irradiated compared to non-irradiated breast79,98,99. However, several strategies have been recently developed to help overcome some of the limitations of fat-grafting into irradiated tissue as related to the processing of harvested fat and preconditioning of the recipient site.
A variant of fat grafting called cell-assisted lipotransfer (CAL) involves the enrichment of fat graft with cells from the SVF or with culture-expanded ASCs. CAL has been clinically shown to have improved fat retention and cosmetic outcomes100–102. In support of this, recent animal studies have also reported improvement in histologic metrics of fat, as well as decreased dermal thickness, improved structural quality, and greater vascularization with supplemented compared to non-supplemented grafts78. Furthermore, CAL was found to significantly improve stiffness of irradiated mouse skin when compared unenriched fat grafts alone. However, while these results are promising, ASCs are increasingly recognized to be a heterogeneous mix of cells comprised of multiple subpopulations with distinct functions. For example, BMPR-1A+ ASCs have enhanced capacity for adipogenesis103, CD248+ ASCs have augmented angiogenic potential104, and CD105- (endoglin) have enhanced osteogenic capacity105. Future outcomes of fat grafting, especially in the irradiated setting, may thus be potentially enhanced by enrichment for specific subpopulations with increased angiogenic, adipogenic, or antifibrotic qualities.
Fat survival may also be increased by improving the quality of the recipient site prior to transplantation. Deferoxamine (DFO) is an iron chelator that has been FDA-approved for use in iron overload syndrome. This chelating action, however, also stabilizes and thus increases hypoxia-inducible factor-1 alpha which can induce the local expression of angiogenic growth factors106,107. Furthermore, DFO has been shown to possess antioxidant properties, and topical application has been found to reduce reactive oxygen species within the skin106. These properties are thought to mediate the improved vascularization of ischemic wound flaps and enhance wound healing in mice treated with DFO108,109. Deferoxamine may thus also be of benefit in promoting vascularization and reducing ROS-mediated cellular injury following radiation therapy. Indeed, local administration of DFO into irradiated mouse skin has been found to enhance vascularization and subsequent fat graft retention while also reducing dermal thickness110. Furthermore, transdermal delivery of DFO to irradiated rat skin has been shown to reduce collagen fibril disorganization by atomic force microscopy111. These findings highlight an emerging strategy with DFO to mitigate, and possibly prevent, the debilitating soft tissue changes associated with radiation therapy.
Conclusions
Over 1.5 million new cancer cases are diagnosed every year112, and over half of these patients will receive RT. While RT is immensely beneficial, an enhanced understanding of the mechanisms of RIF-induced changes is essential for the development of effective strategies to prevent long-term disability and discomfort following radiation therapy. This has led to emerging strategies including autologous fat grafting and deferoxamine which hold great promise for improving the quality of life in patients suffering from the debilitating sequelae of radiation treatment.
Acknowledgments
Funding: M.R.B. was supported by funding from the Plastic Surgery Research Foundation. A.H.S. was supported by funding from the Sarnoff Cardiovascular Foundation. M.T.L. was supported by NIH grants U24 DE026914, R01 DE026730, R01 DE027323, and R01 GM116892, the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Gunn/Olivier Fund. D.C.W. was supported by NIH grants K08 DE024269 and R01 DE027346 and the Hagey Laboratory for Pediatric Regenerative Medicine.
References
- 1.Mendelsohn FA, Divino CM, Reis ED & Kerstein MD Wound care after radiation therapy. Advances in skin & wound care 15, 216–224 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Delaney G, Jacob S, Featherstone C & Barton M The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence‐based clinical guidelines. Cancer: Interdisciplinary International Journal of the American Cancer Society 104, 1129–1137 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Porock D & Kristjanson L Skin reactions during radiotherapy for breast cancer: the use and impact of topical agents and dressings. European journal of cancer care 8, 143–153 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Porock D, Nikoletti S & Kristjanson L Management of radiation skin reactions: literature review and clinical application. Plastic Surgical Nursing 19, 185 (1999). [PubMed] [Google Scholar]
- 5.Martin M, Lefaix J-L & Delanian S TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? International Journal of Radiation Oncology* Biology* Physics 47, 277–290 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Sultan SM et al. Human fat grafting alleviates radiation skin damage in a murine model. Plastic and reconstructive surgery 128, 363–372 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Rigotti G et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plastic and reconstructive surgery 119, 1409–1422 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Coleman SR Structural fat grafting: more than a permanent filler. Plastic and reconstructive surgery 118, 108S–120S (2006). [DOI] [PubMed] [Google Scholar]
- 9.Illouz Y-G Body contouring by lipolysis: a 5-year experience with over 3000 cases. Plastic and reconstructive surgery 72, 591–597 (1983). [DOI] [PubMed] [Google Scholar]
- 10.Charles-de-Sá L et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plastic and reconstructive surgery 135, 999–1009 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Azzam E, De Toledo S & Little J Stress signaling from irradiated to non-irradiated cells. Current cancer drug targets 4, 53–64 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Baskar R, Lee KA, Yeo R & Yeoh K-W Cancer and radiation therapy: current advances and future directions. International journal of medical sciences 9, 193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffray DA & Gospodarowicz MK Radiation therapy for cancer. Disease Control Priorities, Third Edition, 239 (2015). [Google Scholar]
- 14.Baskar R, Dai J, Wenlong N, Yeo R & Yeoh K-W Biological response of cancer cells to radiation treatment. Frontiers in molecular biosciences 1, 24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hymes SR, Strom EA & Fife C Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. Journal of the American Academy of Dermatology 54, 28–46 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Rodemann HP & Bamberg M Cellular basis of radiation-induced fibrosis. Radiotherapy and Oncology 35, 83–90 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Jackson SP & Bartek J The DNA-damage response in human biology and disease. Nature 461, 1071 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumniczky K & Sáfrány G The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer letters 356, 114–125 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Brix N, Tiefenthaller A, Anders H, Belka C & Lauber K Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunological reviews 280, 249–279 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Jeong H, Bok S, Hong B-J, Choi H-S & Ahn G Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood research 51, 157–163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Formenti SC & Demaria S Systemic effects of local radiotherapy. The lancet oncology 10, 718–726 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta S, Suhag V, Semwal M & Sharma N Radiotherapy: Basic concepts and recent advances. Medical Journal Armed Forces India 66, 158–162 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura M, Itasaka S, Harada H & Hiraoka M Microenvironment and radiation therapy. BioMed research international 2013. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W & Robbins ME Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Current medicinal chemistry 16, 130–143 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Terasaki Y et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. American Journal of Physiology-Lung Cellular and Molecular Physiology 301, L415–L426 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Begg AC, Stewart FA & Vens C Strategies to improve radiotherapy with targeted drugs. Nature Reviews Cancer 11, 239 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Farmer H et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Hall E & Giaccia A Radiobiology for the Radiologist. 6th Edition, Lippincott Williams and Wilkins, Philadelphia: (2006). [Google Scholar]
- 29.Cox JD & Ang KK Radiation Oncology E-Book: Rationale, Technique, Results. (Elsevier Health Sciences, 2009). [Google Scholar]
- 30.Straub JM et al. Radiation-induced fibrosis: mechanisms and implications for therapy. Journal of cancer research and clinical oncology 141, 1985–1994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Sullivan B & Levin W Late radiation-related fibrosis: pathogenesis, manifestations, and current management In Seminars in radiation oncology. Elsevier,. 274–289 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Delanian S & Lefaix J-L Current management for late normal tissue injury: radiation-induced fibrosis and necrosis In Seminars in radiation oncology. Elsevier; 99–107 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Malkinson FD & Keane JT Radiobiology of the skin: review of some effects on epidermis and hair. Journal of Investigative Dermatology 77, 133–138 (1981). [DOI] [PubMed] [Google Scholar]
- 34.Huang W-Y et al. Mobilizing transit-amplifying cell-derived ectopic progenitors prevents hair loss from chemotherapy or radiation therapy. Cancer research 77, 6083–6096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freites-Martinez A et al. CME Part 1: Hair disorders in cancer patients. Journal of the American Academy of Dermatology (2018). [Google Scholar]
- 36.Freites-Martinez A et al. CME Part 2: Hair disorders in cancer survivors Persistent chemotherapy-induced alopecia, persistent radiotherapy-induced alopecia, and hair growth disorders related to endocrine therapy or cancer surgery. Journal of the American Academy of Dermatology (2018). [Google Scholar]
- 37.Malkinson FD, Griem ML & Morse PH Colchicine synergism of mouse hair root changes produced by x-ray irradiation. Journal of Investigative Dermatology 37, 337–344 (1961). [PubMed] [Google Scholar]
- 38.Chow D, Rothman S, Lorincz A, Malkinson F & Sandburg A Reduction in rate of growth of hair in mice as an indicator of exposure to chronic low dosage ionizing radiation. Nature 203, 847–848 (1964). [DOI] [PubMed] [Google Scholar]
- 39.Archambeau JO, Pezner R & Wasserman T Pathophysiology of irradiated skin and breast. International Journal of Radiation Oncology* Biology* Physics 31, 1171–1185 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Bentzen SM, Thames HD & Overgaard M Latent-time estimation for late cutaneous and subcutaneous radiation reactions in a single-follow-up clinical study. Radiotherapy and Oncology 15, 267–274 (1989). [DOI] [PubMed] [Google Scholar]
- 41.Li M, Jendrossek V & Belka C The role of PDGF in radiation oncology. Radiation Oncology 2, 5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verrecchia F & Mauviel A Transforming growth factor-β and fibrosis. World journal of gastroenterology: WJG 13, 3056 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ten Dijke P & Hill CS New insights into TGF-β–Smad signalling. Trends in biochemical sciences 29, 265–273 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Borrelli MR et al. Fat Chance: The Rejuvenation of Irradiated Skin. Plastic and Reconstructive Surgery–Global Open (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng X-H & Derynck R Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Yarnold J & Brotons M-CV Pathogenetic mechanisms in radiation fibrosis. Radiotherapy and oncology 97, 149–161 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Chithra P, Sajithlal G & Chandrakasan G Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. Journal of ethnopharmacology 59, 179–186 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Yuan W & Varga J Transforming growth factor-β repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. Journal of Biological Chemistry 276, 38502–38510 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Lindegren A et al. Autologous fat transplantation alters gene expression patterns related to inflammation and hypoxia in the irradiated human breast. British Journal of Surgery (2019). [DOI] [PubMed] [Google Scholar]
- 50.Phulpin B et al. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plastic and reconstructive surgery 123, 1187–1197 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Garza RM et al. Studies in Fat Grafting: Part III. Fat grafting irradiated tissue: Improved skin quality and decreased fat graft retention. Plastic and reconstructive surgery 134, 249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falanga V, Zhou L & Yufit T Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF‐β1. Journal of cellular physiology 191, 42–50 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Mukesh MB et al. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5-year results confirm superior overall cosmesis. Journal of Clinical Oncology 31, 4488–4495 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Mukesh MB et al. Normal tissue complication probability (NTCP) parameters for breast fibrosis: pooled results from two randomised trials. Radiotherapy and Oncology 108, 293–298 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Mukesh M, Harris E, Jena R, Evans P & Coles C Relationship between irradiated breast volume and late normal tissue complications: a systematic review. Radiotherapy and Oncology 104, 1–10 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Whelan TJ et al. Long-term results of hypofractionated radiation therapy for breast cancer. New England Journal of Medicine 362, 513–520 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Pignol J-P et al. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiotherapy and oncology 121, 414–419 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Haviland JS et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The lancet oncology 14, 1086–1094 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Koerdt S et al. An expression analysis of markers of radiation-induced skin fibrosis and angiogenesis in wound healing disorders of the head and neck. Radiation Oncology 10, 202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Boerma M, Fu Q & Hauer-Jensen M Radiation responses in skin and connective tissues: effect on wound healing and surgical outcome. Hernia 10, 502–506 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Classen J et al. Fibrotic changes after postmastectomy radiotherapy and reconstructive surgery in breast cancer. A retrospective analysis in 109 patients. Strahlenther Onkol 186, 630–636, doi: 10.1007/s00066-010-2158-6 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Cho H, Mariotto AB, Schwartz LM, Luo J & Woloshin S When do changes in cancer survival mean progress? The insight from population incidence and mortality. Journal of the National Cancer Institute Monographs 2014, 187–197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berkey FJ Managing the adverse effects of radiation therapy. Am Fam Physician 82, 381–388 (2010). [PubMed] [Google Scholar]
- 64.Chan RJ et al. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC cancer 14, 53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bourgeois J, Gourgou S, Kramar A, Lagarde J & Guillot B A randomized, prospective study using the LPG® technique in treating radiation‐induced skin fibrosis: clinical and profilometric analysis. Skin Research and Technology 14, 71–76 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Warpenburg MJ Deep friction massage in treatment of radiation-induced fibrosis: rehabilitative care for breast cancer survivors. Integrative Medicine: A Clinician’s Journal 13, 32 (2014). [PMC free article] [PubMed] [Google Scholar]
- 67.Amber KT, Shiman MI & Badiavas EV The use of antioxidants in radiotherapy-induced skin toxicity. Integrative cancer therapies 13, 38–45 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Karbasforooshan H, Hosseini S, Elyasi S, Fani Pakdel A & Karimi G Topical silymarin administration for prevention of acute radiodermatitis in breast cancer patients: A randomized, double‐blind, placebo‐controlled clinical trial. Phytotherapy Research 33, 379–386 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Berman B & Duncan M Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. British Journal of Dermatology 123, 339–346 (1990). [DOI] [PubMed] [Google Scholar]
- 70.Jacobson G et al. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. International Journal of Radiation Oncology* Biology* Physics 85, 604–608 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Delanian S, Porcher R, Balla-Mekias S & Lefaix J-L Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. Journal of Clinical Oncology 21, 2545–2550 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Famoso JM, Laughlin B, McBride A & Gonzalez VJ Pentoxifylline and vitamin E drug compliance after adjuvant breast radiation therapy. Adv Radiat Oncol 3, 19–24, doi: 10.1016/j.adro.2017.09.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmuth M et al. Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double‐blind study. British Journal of Dermatology 146, 983–991 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Omidvari S et al. Topical betamethasone for prevention of radiation dermatitis. Indian Journal of Dermatology, Venereology, and Leprology 73, 209 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Miller RC et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. International Journal of Radiation Oncology* Biology* Physics 79, 1460–1466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masferrer JP et al. Prophylaxis with a cream containing urea reduces the incidence and severity of radio-induced dermatitis. Clinical and Translational Oncology 12, 43–48 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Liguori V, Guillemin C, Pesce GF, Mirimanoff RO & Bernier J Double-blind, randomized clinical study comparing hyaluronic acid cream to placebo in patients treated with radiotherapy. Radiotherapy and Oncology 42, 155–161 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Luan A et al. Cell‐assisted lipotransfer improves volume retention in irradiated recipient sites and rescues radiation‐induced skin changes. Stem Cells 34, 668–673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salgarello M, Visconti G & Farallo E Autologous fat graft in radiated tissue prior to alloplastic reconstruction of the breast: report of two cases. Aesthetic plastic surgery 34, 5–10 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Cihan YB Autologous grafts in radiotherapy received breast cancer patients. J. Radiol. Oncol 2, 1–2 (2018). [Google Scholar]
- 81.Jackson IT, Simman R, Tholen R & DiNick VD A successful long-term method of fat grafting: Recontouring of a large subcutaneous postradiation thigh defect with autologous fat transplantation. Aesthetic plastic surgery 25, 165–169 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Mojallal A & Foyatier J The effect of different factors on the survival of transplanted adipocytes. Annales de chirurgie plastique et esthetique. 426–436. [DOI] [PubMed] [Google Scholar]
- 83.Kim SS, Kawamoto HK, Kohan E & Bradley JP Reconstruction of the irradiated orbit with autogenous fat grafting for improved ocular implant. Plastic and reconstructive surgery 126, 213–220 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Salgarello M, Visconti G & Barone-Adesi L Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plastic and reconstructive surgery 129, 317–329 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Serra-Renom JM, Muñoz-Olmo JL & Serra-Mestre JM Fat grafting in postmastectomy breast reconstruction with expanders and prostheses in patients who have received radiotherapy: formation of new subcutaneous tissue. Plastic and reconstructive surgery 125, 12–18 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Rehman J et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Kinnaird T et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109, 1543–1549 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Cao Y et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochemical and biophysical research communications 332, 370–379 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Fournier PF Fat grafting: my technique. Dermatologic surgery 26, 1117–1128 (2000). [PubMed] [Google Scholar]
- 90.Ersek RA, Chang P & Salisbury M Lipo layering of autologous fat: an improved technique with promising results. Plastic and reconstructive surgery 101, 820–826 (1998). [DOI] [PubMed] [Google Scholar]
- 91.Del Vecchio D & Rohrich RJ A classification of clinical fat grafting: different problems, different solutions. Plastic and reconstructive surgery 130, 511–522 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Coleman SR Facial recontouring with lipostructure. Clinics in plastic surgery 24, 347–367 (1997). [PubMed] [Google Scholar]
- 93.Rieck B & Schlaak S Measurement in vivo of the survival rate in autologous adipocyte transplantation. Plastic and reconstructive surgery 111, 2315–2323 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Coleman SR Long-term survival of fat transplants: controlled demonstrations. Aesthetic plastic surgery 19, 421–425 (1995). [DOI] [PubMed] [Google Scholar]
- 95.Coleman S Structural fat grafting. (Quality Medical Pub., 2004). [Google Scholar]
- 96.Maillard GF Liponecrotic cysts after augmentation mammaplasty with fat injections. Aesthetic plastic surgery 18, 405–406 (1994). [DOI] [PubMed] [Google Scholar]
- 97.Gutowski KA & Force AFGT Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plastic and reconstructive surgery 124, 272–280 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Panettiere P, Marchetti L & Accorsi D The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic plastic surgery 33, 695–700 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Fodor J, Gulyas G, Polgár C, Major T & Kasler M Radiotherapy and breast reconstruction: the issue of compatibility. Orvosi hetilap 144, 549–555 (2003). [PubMed] [Google Scholar]
- 100.Matsumoto D et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng 12, 3375–3382, doi: 10.1089/ten.2006.12.3375 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Yoshimura K et al. Cell‐assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose‐derived stem cells. Dermatologic Surgery 34, 1178–1185 (2008). [DOI] [PubMed] [Google Scholar]
- 102.Yoshimura K et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic plastic surgery 32, 48–55 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zielins ER et al. Enrichment of adipose-derived stromal cells for BMPR1A facilitates enhanced adipogenesis. Tissue Engineering Part A 22, 214–221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brett E et al. Isolation of CD248‐expressing stromal vascular fraction for targeted improvement of wound healing. Wound Repair and Regeneration 25, 414–422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levi B et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor β1 (TGF-β1) signaling. Journal of Biological Chemistry 286, 39497–39509 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duscher D et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proceedings of the National Academy of Sciences 112, 94–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thangarajah H et al. HIF-1α dysfunction in diabetes. Cell cycle 9, 75–79 (2010). [DOI] [PubMed] [Google Scholar]
- 108.Thangarajah H et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proceedings of the National Academy of Sciences 106, 13505–13510 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang C et al. Local injection of deferoxamine improves neovascularization in ischemic diabetic random flap by increasing HIF-1α and VEGF expression. PloS one 9, e100818 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flacco J et al. Deferoxamine preconditioning of irradiated tissue improves perfusion and fat graft retention. Plastic and reconstructive surgery 141, 655–665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Snider AE et al. Topical Deferoxamine Alleviates Skin Injury and Normalizes Atomic Force Microscopy Patterns Following Radiation in a Murine Breast Reconstruction Model. Annals of plastic surgery 81, 604–608, doi: 10.1097/SAP.0000000000001592 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Society, A. C. Cancer Facts & Figures, <https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf> (2018).