Abstract
Accelerating innovation translation is a priority for improving healthcare and health. Although dissemination and implementation (D&I) research has made significant advances over the past decade, it has attended primarily to the implementation of long-standing, well-established practices and policies. We present a conceptual architecture for speeding translation of promising innovations as candidates for iterative testing in practice. Our framework to Design for Accelerated Translation (DART) aims to clarify whether, when, and how to act on evolving evidence to improve healthcare. We view translation of evidence to practice as a dynamic process and argue that much evidence can be acted upon even when uncertainty is moderately high, recognizing that this evidence is evolving and subject to frequent reevaluation. The DART framework proposes that additional factors – demand, risk, and cost, in addition to the evolving evidence base – should influence the pace of translation over time. Attention to these underemphasized factors may lead to more dynamic decision-making about whether or not to adopt an emerging innovation or de-implement a suboptimal intervention. Finally, the DART framework outlines key actions that will speed movement from evidence to practice, including forming meaningful stakeholder partnerships, designing innovations for D&I, and engaging in a learning health system.
Key words: Designing for dissemination and implementation, stakeholder partnerships, learning health system, implementation science, precision medicine, speed of translation
The Current and Evolving Role of Translational Research (“Where We Are”)
The Current Landscape
Translational research is challenged to speed the advancement of innovations likely to improve clinical and population health. Within this enterprise, dissemination and implementation (D&I) research is well positioned to optimize the adoption and implementation of emerging evidence within practice settings. However, D&I research has often attended only to the implementation of long-standing, well-established practices and programs. This has situated D&I research as the final step within a linear sequence of phased trials (i.e., discovery, efficacy, effectiveness, and implementation) and translational science paradigm (i.e., T0→T4 research) – a constrained role creating unnecessary lags in translation.
To advance a more robust and expediting role for D&I research, we acknowledge two well-documented foundations (premises) in the translation of evidence to practice followed by a select set of more provocative viewpoints (hot-takes; See Table 1).
Table 1.
Premises and hot-takes in translating evidence to practice
| Key Premise #1 | Translation of evidence to practice is unnecessarily slow. |
|---|---|
| Hot-Take #1 | Dissemination and implementation research should not be viewed merely as a final step in the translational process. |
| Hot-Take #2 | Without radically different approaches to accelerating translation, implementation of evidence to practice will remain slow. |
| Key Premise #2 | Translation of evidence to practice is a dynamic process. |
| Hot-Take #3 | Researchers are responsible for considering implementation needs “early and often” for accelerated and dynamic translation. |
| Hot-Take #4 | All health research should aim to address an actual problem or need, with an expectation of ongoing iterative improvements. |
| Hot-Take #5 | Much evidence can be acted upon even when uncertainty is moderately high, recognizing that this evidence is evolving and subject to frequent reevaluation. |
An established goal of D&I research is to ensure more timely translation of evidence to practice, largely in response to the documented 17-year translational gap [1]. Despite working toward this end for more than a decade, evidence of clear progress is scant. The gap continues to widen when implementation is merely “left as a posthoc procedure” [2]. The translational science community must also reconcile the need for scientific rigor with the recognition that evidence is always evolving and imperfect. Via paradigms such as the “lean startup” concept [3], entrepreneurship may provide guidance on balancing this tension between rigor and a learning mindset.
Furthermore, D&I research is generally reactive, with an eye toward identifying barriers to then address. However, reactivity creates an inherent lag. More efficient approaches are anticipatory, designing strategies to fit the context in which the innovation will be implemented [4]. Making these anticipatory strategies more adaptive, robust, and sustainable in complex healthcare systems can maximize the clinical and population health benefit of promising innovations.
Designing for Accelerated Translation (DART) of Emerging Innovations (“Where We Want to Be”)
Demand, Risk, Cost, and the Pace of Translation
A perennial question among the translational science community is “When can we begin acting on the evidence we have?” For D&I research, this is often interpreted as “What is the minimum level of evidence needed for implementation?” This question reflects an assumption that a minimum threshold of evidence is the primary impetus of translation. We assert that additional factors – demand, risk, and cost, in addition to the evolving evidence base – should influence the pace of translation over time. Attention to these underemphasized factors leads to more dynamic decision-making about whether or not to adopt an emerging innovation or de-implement a suboptimal intervention.
Given complexities in decision-making based on the risk–benefit ratio for promising innovations, it is useful to generate testable hypotheses about circumstances that drive use of evidence to inform next steps prior to large-scale implementation. We suggest that the pace of translation (P) is a function of the strength of evidence supporting the effectiveness (E) of the innovation, multiplied by the sum of stakeholder estimates of the demand (D, factoring in urgency, existing alternatives, and stakeholder pull), divided by the sum of risks (R, viewed here as a ratio of risk of potential clinical harms of the innovation (R Innovation) balanced by risks associated with not acting on the available evidence or with maintaining the status quo (R Status quo); for instance, R = RInnovation/R Status quo), and costs (C, factoring in financial expense, resource intensiveness, and disruptive effects) of the innovation:
The equation may serve as a heuristic, indicating that if either the evidence of effectiveness is very weak or if demand for the innovation is very low, then movement toward implementation should come to a halt. The influence of these factors is moderated by a denominator that includes risk and cost, such that the pace of translation is relatively unaffected when the risks and costs of implementation are low yet the pace of translation is substantially reduced when risks and costs are high. One might hypothesize that an innovation with limited existing evidence of effectiveness yet high demand and low risk and cost may be a better candidate for accelerated translation than one with strong evidence yet low demand and high risk and cost. We encourage investigators to test this type of hypothesis through observation of naturally occurring implementation efforts involving innovations with different profiles of effectiveness evidence, demand, risk, and cost. These factors could also be varied prospectively to test the effects on the pace of implementation. Finally, we expect that other factors could and should be added to the proposed equation as hypothesized drivers of implementation.
A Paradigm Shift
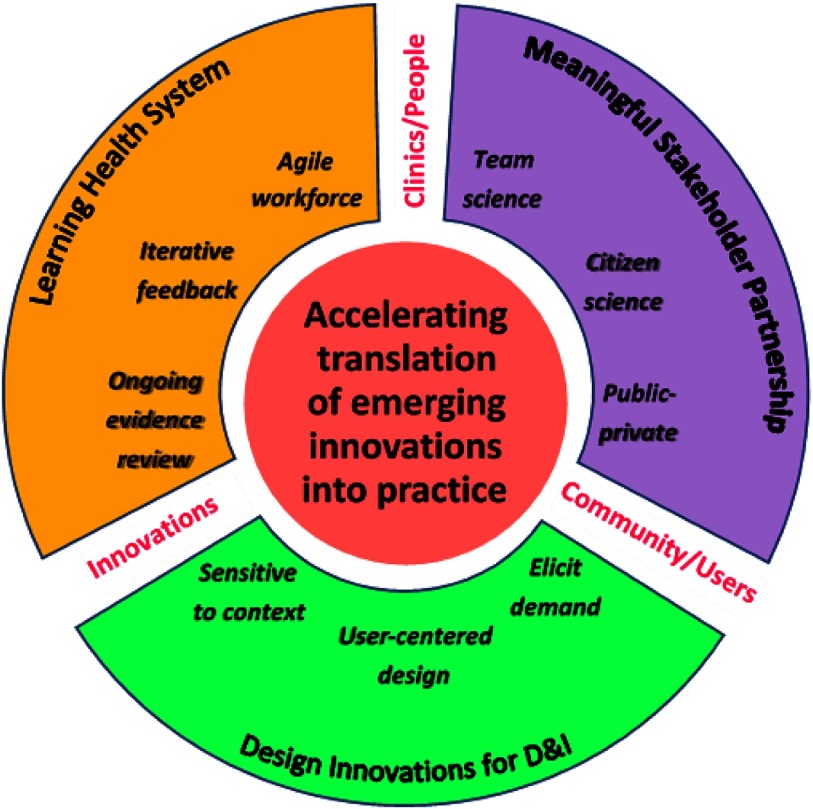
Rapid advancements in technology and precision medicine (e.g., genomics) indicate the need for a faster pace of translational research [5–7]. Still, few heuristics exist to guide scientific approaches to accelerating research to practice. We present a conceptual architecture for speeding the translation potential for emerging innovations that demonstrate promising utility. Our framework to Design for Accelerated Translation (DART) is the product of synthesizing related yet inadequately linked areas of science into a cohesive three-pronged agenda focused on meaningful stakeholder partnership, designing innovations for D&I, and a learning health system (see Fig. 1 and Table 2):
Forming meaningful stakeholder partnerships, including participation in team science, citizen science, and public–private partnerships.
Designing innovations for D&I, including eliciting demand, incorporating user-centered design, and prioritizing adoption potential.
Engaging in a learning health system, including fostering an agile workforce, monitoring the effect of a new innovation and providing iterative feedback, and performing ongoing evidence review.
Fig. 1.
Design for Accelerated Translation (DART) framework. Forming meaningful stakeholder partnerships, designing innovations for dissemination and implementation (D&I), and engaging in a learning health system as a three-pronged approach to accelerate translation of emerging innovations in practice.
Table 2.
Design for Accelerated Translation (DART) strategies to optimize the implementation of emerging health innovations
| Current State: “Where We Are” |
Optimal State: “Where We Want to Be” |
Improvement Strategies: “What It Will Take” |
|
|---|---|---|---|
| Meaningful Stakeholder Partnership | Disconnected from customers; Lab silos | Utilizing team science | Develop partnerships between investigators across the translational spectrum early in design/development |
| Small/restrictive samples | Leveraging citizen science | Harness power of public for scientific activities | |
| Disconnected from industry | Partnering with private industry | Partner with those primed to bring innovations to market | |
| Design Innovations for D&I | Pushing out innovations | Eliciting demand and performance needs from users | Understand user motives and context; demonstrate value added and simplicity |
| Researcher-driven development | Engaging in human/user-centered design | Involve diverse group of end-users as partners throughout design/development | |
| Efficacy over effectiveness | Implementing robust, context-sensitive innovations | Better packaging of research evidence for translation to practice and policy; focus on pragmatic and adaptive trials to optimize adoption potential | |
| Learning Health System | Rigid/narrow use of evidence | Ongoing and efficient review of evidence | Use existing data to add to evidence on intervention impact; conduct rapid reviews; use create–trial–sustain approaches to guide ongoing adaptation |
| Static delivery systems | Supporting the use of iterative feedback | Give real-time feedback on key outcomes to providers | |
| Resistant to change | Promoting an agile workforce with change-oriented mindset | Train workforce in core concepts that apply across technologies |
D&I, Dissemination and Implementation.
The DART framework aims to clarify whether, when, and how to act on evolving evidence to address identified needs in healthcare. We challenge the field to embrace a new way of approaching D&I research that prioritizes acceleration for innovations that can provide immediate clinical benefit and be improved over time. To achieve the goals envisioned by the DART framework, the D&I and translational science communities will need to engage in a set of improvement strategies to move from a system characterized as siloed, slow, and static toward an optimized system featuring powerful stakeholder engagement, innovations designed for D&I, and learning health system principles.
Strategies to Achieve DART (“What It Will Take”)
Meaningful Stakeholder Partnership
The scientific enterprise is criticized for being overly homogenous in collaboration, disconnected from consumers (e.g., patients, caregivers, providers, manufacturers), and slow to translate evidence to practice. To dismantle these perceptions, team science should be stretched to the fullest extent, fostering teamwork between D&I scientists and discovery scientists engaged in research pertinent to elucidation of disease processes which might lead to novel approaches to prevention, diagnosis, or treatment of disease. Bench scientists might consider the direction and translation potential of their discoveries, in partnership with both translational researchers and customers. Intervention developers might prioritize identifying an important problem to address with an appropriate solution rather than pushing their developed tool toward the next possible target. Effectiveness researchers might address D&I research questions (e.g., what disruption is caused). Finally, D&I scientists might feed insights back to bench scientists and community partners to inform future directions of early-stage translational science. At the point of bringing discovery science into clinical investigation, a team approach spanning the translational science spectrum could accelerate overall success.
Citizen science, or collaboration between members of the public and professional scientists to collect and process large quantities of data, offers distinct opportunities to harness the power, bandwidth, and unique insights of the public for scientific activities [8]. Wearable technologies (e.g., activity trackers) and direct-to-consumer genetic testing (e.g., 23andMe) are now ubiquitous and poised to integrate with routine clinical care. In addition, families are leveraging social media to study rare diseases [9]. Community-driven research approaches prioritize focus in areas of true need and in partnership with stakeholders well positioned to apply context-appropriate solutions. Finally, private industry is primed to bring innovations to market, and entrepreneurs and technology development firms can help researchers rapidly move tested products into the hands of consumers.
Designing Innovations for D&I
Knowing the problem and context for implementation, including stakeholder needs, motives, and preferences, is a critical precondition to effectively designing solutions primed for D&I [10]. Potential users will only “pull” for emerging innovations demonstrated to be more effective or easier to use than alternatives. Innovators must also address financial issues of cost for consumers and reimbursement options for providers (e.g., pay for performance, prospective payment systems). Innovation design should prioritize meeting identified user needs, using iterative feedback to optimize design [11]. However, diverse groups of potential end-users and ancillary stakeholders should be engaged throughout the process, not simply in refinement or optimization stages. In general, innovation translation is enhanced by executing user-driven priorities, not researcher-driven assumptions. Researchers should be trained to package evidence in ways that meet practitioner and policymaker needs. Concerted efforts to improve packaging of evidence for translation will make this information more actionable to those driving real-world change [12–14].
Learning Health System
Shaping a dynamic, agile, and efficient healthcare system requires researchers, providers, patients, and administrators to think differently about how they plan, collect, and respond to data. This transformation encourages replacing lengthy and tightly controlled trials with opportunities for timely learning, and a willingness to act on equivocal evidence recognizing that evidence best evolves through testing in externally valid contexts. With continuous learning as a priority, ongoing review of an ever-evolving evidence base is essential. When higher-burden systematic reviews are considered infeasible, organizational leaders may opt for rapid reviews to inform decision-making, focusing on real-world data from highly pragmatic comparative effectiveness research. Providers should also have access to personalized, near real-time performance data relative to benchmarks to inform, reinforce, and motivate optimal service delivery [15]. Create–trial–sustain approaches prioritize first solving a tangible problem with an unrefined innovation in a limited context [16]; this ensures feasibility and potential for scale-up and sustainability prior to investing resources in further development, testing, and optimization. Lastly, healthcare systems that expect support and reward responsiveness to new evidence help to foster a more agile, change-oriented workforce adaptable to frequent shifts in technology. An agile healthcare workforce can accelerate the impact of emerging innovations by anticipating and planning for the future, rather than current, healthcare system.
Contextual Domains of Activity
To effectively execute the above three-pronged approach, each DART component – meaningful stakeholder partnership, innovations designed for D&I, and a learning health system – is greatly influenced by the context for implementation, or the constellation of interacting factors and circumstances that surround a particular implementation effort [17]. Frameworks to understand implementation context abound and most include the organizational setting (e.g., clinic, community), individuals involved (e.g., healthcare providers, end-users), and characteristics of the innovation itself (e.g., design quality and packaging, adaptability) among the important contextual domains [17–19]. As illustrated in Fig. 1, one or more of these contextual domains reside at the nexus between each DART component. For instance, efforts to conduct meaningful stakeholder partnership and/or design innovations for D&I rely on a robust understanding of both the potential end-users of the innovation and the broader communities involved in the implementation effort. Designing innovations for D&I and/or employing a learning health system requires extensive focus on the characteristics of the innovation and necessary adaptations to enhance fit with the local context. Finally, engaging in successful learning health system and meaningful stakeholder partnership activities depends upon being sensitive and responsive to the needs of the clinical or other organizational setting and the teams of individuals (e.g., decision-makers, care teams, patients) actively involved in the implementation process. Active consideration of each of these contextual domains is likely to help synergize the joint efforts of each DART component. As such, context can be viewed as the “glue” that binds the other elements of the DART framework.
Potential Approaches to DART
How can researchers and consumers accelerate the evidence translation? We offer three potential approaches: anticipatory, simultaneous, and hybrid.
Anticipatory approaches focus on taking steps to prevent implementation barriers before they arise. Key stakeholders, champions, and change agents are engaged early and throughout the process. Intervention designers learn the local practice context while end-users inform design and testing; together they align the intervention with local and national priorities, including those pertaining to policy and payment. Stakeholders may also leverage networks and social media to build urgency and demand.
Simultaneous approaches use rapid-cycle and iterative design to accelerate and optimize an innovation. Intervention researchers can employ user/human-centered design principles to shape minimally viable products, continuously refined through stakeholder feedback. Simultaneous work on design and translation may function best in learning health systems marked by frequent efforts to align innovations and settings.
Finally, hybrid approaches combine anticipatory and simultaneous principles, often involving early-stage trialing at local or system levels. These approaches afford intervention developers the opportunity to fail “safely,” identify contextual fault lines, and continually refine processes for larger-scale implementation.
Areas Primed for DART Approaches
DART approaches are widely applicable to the majority of research domains. While it is “never too soon” to think about accelerating translation, it is also “never too late” to make translation more dynamic through ongoing evaluation and improvements for a broad range of innovations. We highlight certain areas – including genomic medicine and mHealth – that are particularly ripe for utilizing DART approaches. Such examples are both emergent and evolving in nature, as vast advancements have recently been made and are likely to continue fluctuating.
Emergent Considerations
Genomic medicine and mHealth tools afford unique opportunities to design for D&I at the outset, drive learning health system efforts with new windfalls of data, and engage transdisciplinary groups of stakeholders in novel ways. Rapid-cycle and iterative design with innovation developers and users is most naturally conducted with newer innovations that have not yet established stable patterns of use. Even when the rate of implementation must be moderated by forthcoming effectiveness data, innovation teams can maintain the pace of translation by utilizing anticipatory DART approaches.
For instance, many promising genomic innovations have demonstrated solid analytic and clinical validity with uncertain clinical utility. Researchers can accelerate translation here through anticipatory approaches, collecting pre-implementation data on local context and end-users alongside clinical effectiveness and utility data. Relatedly, although evidence clearly supports genetic testing for high risk of colon cancer, this innovation cannot have any major public health impact in the absence of a system that delivers colon cancer screening and subsequent therapy in a reliable and efficient manner, ideally also addressing equity. Therefore, joint consideration of implementation and health system approaches while developing the test would accelerate adoption. Thinking ahead about the full continuum of care involved with genomic innovations may also speed translation. Instead of focusing narrowly on genetic testing, researchers can impact the broader cascade of care, including interpretation, follow-up, preventative care, and treatment informed by genomic information. Ultimately, a comprehensive understanding of this full continuum is needed to enable implementation, and holistic approaches will likely advance translation more quickly than piecemeal ones.
Evolutionary Considerations
As genomics and mHealth innovations are in flux, we must manage the uncertainty of forthcoming evidence and ongoing iterative refinement. In tandem with the emphasis on rapid acceleration, there is an important opportunity to focus on evolution in these areas over time, rather than the traditional view of an intervention that moves unidirectionally from bench to bedside.
Technology can be a key component of treatment support for many people. However, consumer interaction with mHealth interventions varies and may wane quickly upon experiencing diminished returns. However, given vast learning capacities of GPS functionality, smartphone engagement patterns, ecological momentary assessment and intervention, mHealth tools can respond to evolving user needs and preferences and guide ongoing treatment adaptation to provide the right type of support at the right time and place.
Conclusion
The DART framework offers a contrast to the traditional linear view of the translational pathway, recognizing that in the dynamic, rapidly evolving healthcare ecosystem, we can no longer wait for the slow, steady progress from intervention development, through testing, and ultimately into real-world implementation. Instead, we must look across the translational spectrum for ways to refine and redefine each step along the path.
We propose DART as a framework that applies across multiple domains of healthcare and health (clinical, community/public health), with specific use for areas of biomedical research in which rapid progress is expected (e.g., genomic medicine, mHealth). It has, at its core, four key objectives that reshape our view of the translational pathway:
Better linkages between research teams (team science) and between researchers and those who can drive, steer, and land one’s innovation (industry, community, citizen science).
Improved design of innovations for optimal use/actionability (design for D&I).
Simultaneous learning about adapting interventions in response to evolving evidence (learning health system).
Consideration of demand, risk (including risk of not implementing), and cost of the innovation to optimize the rate of implementation.
While we recognize the need for more work to operationalize the framework, we expect that the field can already contribute by chronicling specific biomedical innovation examples in which iterative testing and implementation has sped the translational cycle. We look forward to ongoing dialog about how key stakeholders in translational science can better support these principles in the context of their research programs. In collaboration, we can greatly improve the ongoing innovation, analysis, and improvement of our health and healthcare systems.
Acknowledgments
We thank M. McGovern and S. Bartels for contributing to our panel presentation entitled “Designing to Accelerate Translation (DART) of Emerging Health Innovations” at the 11th Annual Conference on the Science of Dissemination and Implementation in Health. We also thank R. Brownson for his review of this paper. This paper was supported by National Institute on Drug Abuse (NIDA) grants K12DA041449 (ATR) and R01DA036583 (LJB) and National Cancer Institute (NCI) grants U19CA203654 (LJB) and P30CA091842 (LJB).
Author ORCIDs
Alex T. Ramsey, https://orcid.org/0000-0002-3471-3725
Disclosures
Dr. Bierut is listed as an inventor on Issued US Patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. The other authors have no competing interests.
References
- 1. Padek M, et al. Training scholars in dissemination and implementation research for cancer prevention and control: a mentored approach. Implementation Science 2018; 13(1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohr DC, Riper H, Schueller SM. A solution-focused research approach to achieve an implementable revolution in digital mental health. JAMA Psychiatry 2018; 75(2): 113–114. [DOI] [PubMed] [Google Scholar]
- 3. Ries E. The Lean Startup: How Today’s Entrepreneurs Use Continuous Innovation to Create Radically Successful Businesses. Crown Business, New York, NY, 2011. 338 p. [Google Scholar]
- 4. Klesges LM, et al. Beginning with the application in mind: designing and planning health behavior change interventions to enhance dissemination. Annals of Behavioral Medicine 2005; 29(2): 66–75. [DOI] [PubMed] [Google Scholar]
- 5. Chambers DA, Feero W, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA 2016; 315(18): 1941–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dzau VJ, Ginsburg GS. Realizing the full potential of precision medicine in health and health care. JAMA 2016; 316(16): 1659–1660. [DOI] [PubMed] [Google Scholar]
- 7. Khoury MJ, et al. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in Medicine 2007; 9(10): 665–674. [DOI] [PubMed] [Google Scholar]
- 8. Bonney R, et al. Citizen science: a developing tool for expanding science knowledge and scientific literacy. BioScience 2009; 59(11): 977–984. [Google Scholar]
- 9. Schumacher KR, et al. Social media methods for studying rare diseases. Pediatrics 2014; 133(5): e1345–e1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lyon AR, Lewis CC. Designing health information technologies for uptake: development and implementation of measurement feedback systems in mental health service delivery. Administration and Policy in Mental Health 2016; 43(3): 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dearing JW, et al. Designing for diffusion of a biomedical intervention. American Journal of Preventive Medicine 2013; 44(1): S70–S76. [DOI] [PubMed] [Google Scholar]
- 12. Brownson RC, Fielding JE, Green LW. Building capacity for evidence-based public health: reconciling the pulls of practice and the push of research. Annual Review of Public Health 2018; 39(1): 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brownson RC, et al. Getting the word out: new approaches for disseminating public health science. Journal of Public Health Management & Practice 2018; 24(2): 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabak RG, et al. What predicts dissemination efforts among public health researchers in the United States? Public Health Reports 2014; 129(4): 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bickman L, Lyon AR, Wolpert M. Achieving precision mental health through effective assessment, monitoring, and feedback processes. Administration and Policy in Mental Health 2016; 43(3): 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohr DC, et al. Accelerating digital mental health research from early design and creation to successful implementation and sustainment. Journal of Medical Internet Research [Internet] 2017; 19(5). Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5443926/ Accessed August 24, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damschroder LJ, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science 2009; 4: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenhalgh T, et al. Diffusion of innovations in service organizations: systematic review and recommendations. The Milbank Quarterly 2004; 82(4): 581–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldstein AC, Glasgow RE. A Practical, Robust Implementation and Sustainability Model (PRISM) for integrating research findings into practice. The Joint Commission Journal on Quality and Patient Safety 2008; 34(4): 228–243. [DOI] [PubMed] [Google Scholar]