Abstract
BACKGROUND:
Plant-based diets have been associated with lower risk of type 2 diabetes and cardiovascular disease (CVD) and are recommended for both health and environmental benefits. However, the association between changes in plant-based diet quality and mortality remains unclear.
METHODS:
We investigated the associations between 12-year changes (from 1986 to 1998) in plant-based diet quality assessed by three plant-based diet indices (score range: 18 to 90)—an overall plant-based diet index (PDI), a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI)—and subsequent total and cause-specific mortality (from 1998 to 2014). Participants were 49,407 women in the Nurses’ Health Study (NHS) and 25,907 men in the Health Professionals Follow-Up Study (HPFS) who were free from CVD and cancer at 1998. Multivariable-adjusted Cox proportional-hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
RESULTS:
We documented 10,686 deaths including 2,046 CVD deaths and 3,091 cancer deaths in the NHS over 725,316 person-years of follow-up, and 6,490 deaths including 1,872 CVD deaths and 1,772 cancer deaths in the HPFS over 371,322 person-years of follow-up. Compared with participants whose indices remained stable, among those with the greatest increases in diet scores (highest quintile), the pooled multivariable-adjusted HRs for total mortality were 0.95 (95% CI, 0.90-1.00) for PDI, 0.90 (95% CI, 0.85-0.95) for hPDI, and 1.12 (95% CI, 1.07-1.18) for uPDI. Among participants with the greatest decrease (lowest quintile), the multivariable-adjusted HRs were 1.09 (95% CI, 1.04-1.15) for PDI, 1.10 (95% CI, 1.05-1.15) for hPDI, and 0.93 (95% CI, 0.88-0.98) for uPDI. For CVD mortality, the risk associated with a 10-point increase in each plant-based diet index was 7% lower (95% CI, 1-12%) for PDI, 9% lower (95% CI, 4-14%) for hPDI, and 8% higher (95% CI, 2-14%) for uPDI. There were no consistent associations between changes in plant-based diet indices and cancer mortality.
CONCLUSIONS:
Improving plant-based diet quality over a 12-year period was associated with a lower risk of total and CVD mortality, whereas increased consumption of an unhealthful plant-based diet was associated with a higher risk of total and CVD mortality.
Keywords: Plant-based diet, diet quality, epidemiology, all-cause death, cardiovascular death
INTRODUCTION
Plant-based diets have been associated with lower risk of type 2 diabetes, cardiovascular disease (CVD), and other cardiometabolic risk factors.1-4 The 2015-2020 US Dietary Guidelines recommends several dietary patterns for chronic disease prevention including a healthy vegetarian pattern, healthy Mediterranean-style pattern, and healthy US-style pattern. At the same time, they raised the alarm on the current excessive intake of refined grains and added sugars.5 In addition, the EAT-Lancet report recommends a global shift towards plant-based diets not only for their health benefits but also for environmental sustainability.6
Previous studies on plant-based diets are somewhat limited because most defined a plant-based diet dichotomously as either vegetarian or non-vegetarian, excluding some or all animal foods, and importantly, without differentiation for the quality of plant foods. While a higher intake of healthy plant foods, such as whole grains, vegetables, fruits, and nuts has been associated with lower all-cause and CVD mortality, a higher intake of less healthy plant foods, such as potatoes and added sugars has been associated with a higher cardiometabolic disease risk.7-11 Therefore, we previously developed three plant-based diet indices, an overall plant-based diet index (PDI), a healthful PDI (hPDI), and an unhealthful PDI (uPDI).12, 13 We found that higher scores on the hPDI were associated with a lower risk of coronary heart disease, type 2 diabetes, and favorable adiposity-associated biomarker profiles while higher scores on the uPDI were associated with a higher risk of these outcomes.12-14 In the Third National Health and Nutrition Examination Survey (NHANES III), a higher hPDI was found to be associated with lower risk of all-cause mortality.15 Yet, it remains unknown whether changes in plant-based diet quality are associated with subsequent risk of death. From a public health standpoint, evaluating the effects of dietary change is meaningful as it reflects the risk associated when individuals make changes to their diet in the real world. We previously reported that changes in several dietary patterns including the Alternate Healthy Eating Index-2010, the Alternate Mediterranean Diet, and the Dietary Approaches to Stop Hypertension were associated with risk of mortality.16 While a healthy plant-based diet closely aligns with the principles of these dietary patterns, our plant-based diet indices are different from these other diet indices in several aspects. First, plant-based diet indices focus solely on the quality of plant foods included in a person’s diet. Second, the plant-based diet indices score all animal foods negatively, including animal foods known to be associated with better health outcomes such as fatty fish and poultry. Therefore, examining how changes in plant-based diet quality is associated with mortality risk is essential especially given the global concerns for environmental sustainability.
In the present study, we evaluated associations between 12-year changes in plant-based diets and subsequent total and cause-specific mortality among participants in the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS).
METHODS
The data, analytical methods, and study materials will be available to other researchers from the corresponding author on reasonable request for purposes of reproducing the results or replicating the procedure.
Study Population and Design
The NHS was established in 1976 among 121,700 US female registered nurses aged 30 to 55 years. The HPFS was established in 1986 among 51,529 US male health professionals aged 40 to 75 years. Baseline and follow-up questionnaires were sent to participants every two years to update lifestyle factors and medical history over the follow-up period.17, 18 In both cohorts, follow-up rates exceeded 90%.
For both the NHS and HPFS, the initial cycle was set at 1986 to have comparable follow-up in both cohorts, the baseline was set at 1998 and the end of follow-up was 2014. We excluded participants who either died before baseline, had a history of CVD or cancer at or before baseline, had missing dietary information, or had implausible energy intakes (<800 kcal or >4200 kcal per day in men and <500 kcal or >3500 kcal per day in women).
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. The completion of self-administered questionnaires was considered to imply informed consent.
Exposure Assessment
From 1986, dietary data were collected every four years using a semi-quantitative food frequency questionnaire (FFQ). The reliability and validity of the FFQ have been described elsewhere.19, 20 Participants reported how often, on average, they had consumed each food of a standard portion size in the past year. The development of three plant-based diet indices have been described previously.12, 13 We first created 18 food groups based on nutrients and culinary similarities. We further classified these 18 food groups into three larger categories of healthy plant foods (n=7; whole grains, fruits, vegetables, nuts, legumes, vegetable oils, tea/coffee), less healthy plant foods (n=5; fruit juices, refined grains, potatoes, sugar-sweetened beverages, sweets/desserts), and animal foods (n=6; animal fat including butter or lard, dairy, eggs, fish/seafood, meat, miscellaneous animal-based foods). The categorization of healthy and less healthy plant foods was based on the most recent empirical evidence.5, 7-11, 21, 22 All healthy and less healthy plant foods were given equal weight regardless of the strength of the evidence or the association of the individual foods with chronic disease risk. Foods constituting the 18 food groups along with their corresponding serving sizes are shown in Supplemental Table 1 in the online-only Data Supplement. Intake of 18 food groups (servings/day) was categorized into quintiles (Q) and each quintile was assigned a score between 1 and 5. For creating PDI, healthy and less healthy plant food groups were given positive scores (ex: Q1=1, Q2=2, Q3=3, Q4=4, Q5=5) while animal food groups were given reverse scores (ex: Q5=1, Q4=2, Q3=3, Q2=4, Q1=5). For hPDI, we assigned positive scores to healthy plant food groups and reverse scores to less healthy plant and animal food groups. For uPDI, we gave positive scores to less healthy plant food groups and reverse scores to healthy plant and animal food groups (Supplemental Table 1 in the online-only Data Supplement). Because alcohol has different associations with various health outcomes, we did not include this as a food group but rather adjusted for it in the analyses. Likewise, because the fatty acid composition of margarine has changed over time from high-trans to high-unsaturated fats, we included this variable as a covariate in the analyses. While dark chocolate might be neutral or beneficial for health outcomes, we included chocolate in less healthy plant food because our data did not distinguish chocolate type.23 Mixed dishes that have substantial amounts of animal foods (such as cheese or heavy cream) or where meat was the main component were classified as animal foods. We summed scores of 18 food groups to derive PDI, hPDI, and uPDI, ranging from 18 to 90. We assessed changes in plant-based diet scores from 1986 to 1998 as the main exposures to evaluate their associations with subsequent risk of death.
Ascertainment of Deaths
Deaths were identified from state vital statistics records and the National Death Index, or were reported by the participants’ families and the U.S. postal system.24 Using these methods, 98% of the deaths in each cohort were ascertained.24 We attempted to obtain the death certificate of each participant who had died, and when appropriate, requested permission from the participant’s next of kin to review medical records. The underlying cause of death was assigned by physicians after they had reviewed death certificates and medical records. Cardiovascular deaths were determined by ICD-8 codes 390-458, and cancer deaths were defined by ICD-8 codes 140-207.
Ascertainment of Covariates
Every two years, participants returned a mailed validated questionnaire that obtained updated information on their lifestyle and other CVD risk factors including age, body weight, smoking status, physical activity, aspirin use, multivitamin use, menopausal status and postmenopausal hormone use in women, and physician diagnosis of chronic diseases. Baseline history of hypertension, hypercholesterolemia, and type 2 diabetes were determined through self-report. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Detailed descriptions of the validity and reproducibility for body weight, physical activity, and alcohol consumption have been published previously.25-27
Statistical Analysis
We categorized changes in PDI, hPDI, and uPDI into quintiles from the largest decrease (quintile 1) to the largest increase (quintile 5). We evaluated the distribution of initial (1986) and changes in characteristics of participants by applying direct standardization to the age distribution of the cohort. Person-years were calculated from the date of return of the baseline questionnaire to the date of death or the end of follow-up (2014), whichever occurred first. We used cause-specific Cox proportional-hazards models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) associated with changes in PDI, hPDI, uPDI, and total and cause-specific mortality. In the basic model, we used calendar year as the underlying time scale and adjusted for age and the initial (1986) plant-based diet index score (in quintiles). In multivariable-adjusted model 1, we further adjusted for race, family history of myocardial infarction, diabetes, or cancer, baseline (1998) aspirin use and multivitamin use, initial BMI categories (<23.0, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≤35 kg/m2), menopausal status and postmenopausal hormone use in women (premenopausal, postmenopausal without hormone use, postmenopausal with current hormone use, postmenopausal with past hormone use), smoking status (never-never, current-past, past-current, never-current, past-past, current-current), and initial and changes in each of smoking pack-years among ever smokers (continuous variables), alcohol consumption (in quintiles), margarine intake (in quintiles), physical activity (in quintiles), and total energy intake (in quintiles). Multivariable-adjusted model 2 was further adjusted for weight change (in quintiles), history of hypertension, hypercholesterolemia, or type 2 diabetes, antihypertensive medication use, and cholesterol-lowering medication use. Tests for trend were computed by assigning the median value to each quintile. We estimated the risk of death per 10-point increase in PDI, hPDI, and uPDI by treating the scores as continuous variables. We used restricted-cubic-spline regression analyses with 4 knots to flexibly model the associations of PDI, hPDI, and uPDI with risk of mortality, excluding participants in the top and bottom 0.5% of the corresponding index as outliers. To test for potential non-linear associations between plant-based diet indices (continuous) and mortality risk, we compared the model with the linear term to the model with both the linear and the cubic spline terms and performed a likelihood ratio test. A P value <0.05 denoted significant non-linearity. In secondary analyses, we examined associations of 8-year (from 1986 to 1994; baseline=1994) and 16-year (from 1986 to 2002; baseline = 2002) changes in PDI, hPDI, and uPDI with subsequent total and cause-specific mortality. We examined the pooled least squares mean (95% CIs) initial (1986) and changes (1986 to 1998) in individual 18 food group intakes according to the quintiles of 12-year changes in PDI, hPDI, and uPDI from general linear model adjusted for age, sex, initial and changes in total energy intake, and initial intakes of the corresponding food.
We conducted several sensitivity analyses to test the robustness of our findings. First, we adjusted for mammographic screening (women only) and physical checkups as early detection and treatment of disease could confound results.28 Second, we conducted 4-year lag analyses to minimize reverse causation due to existing diseases. Third, we conducted subgroup analyses and assessed statistical interactions according to several potential risk factors such as initial (1986) plant-based diet index scores, sex, baseline (1998) age, baseline BMI, baseline physical activity, baseline smoking status, weight change, and smoking status change. Fourth, in order to assess whether any observed associations were driven by changes in a single food group, we created modified plant-based diet indices by individually excluding each of the 18 food groups from the indices and assessed associations of changes in each of the modified indices and risk of death with further adjustment for the initial and changes in the excluded food intakes. Fifth, based on previously reported neutral or beneficial effects of certain animal foods on health outcomes, we created additional healthy plant-based diet indices by assigning positive scores individually to fish/seafood, poultry, fermented dairy, low-fat dairy, high-fat dairy, egg, and simultaneous positive coding for fish/seafood, poultry, fermented dairy, low-fat dairy, and egg. 29-31All additionally created index scores were scaled to be between 0-90 to allow for comparisons with the original PDI, hPDI, and uPDI.
Analyses were performed separately for each cohort and pooled with the use of an inverse, variance-weighted meta-analysis with a fixed effects model. However, we pooled the data for the restricted cubic-spline analyses, the analyses examining pooled initial and changes in food groups according to the changes in plant-based diet indices, and the subgroup analysis for sex. All analyses were executed using SAS for UNIX version 9.4 (Cary, NC). Statistical tests were two-sided with P values <0.05 indicating statistical significance.
RESULTS
Participant Characteristics
Initial and 12-year changes in characteristics of participants according to quintiles of PDI change are shown in Table 1. In both cohorts, participants with the greatest increase in PDI had a lower initial PDI score, a greater increase in physical activity, a lower initial energy intake, and a lower baseline prevalence of diabetes than participants whose PDI scores were relatively stable (Table 1). Participants with the greatest hPDI increase were younger, had a lower initial hPDI score, a lower weight gain, a greater increase in physical activity, a higher initial energy intake, and a higher baseline prevalence of diabetes. Participants with the greatest uPDI increase were older, had a lower initial uPDI score, a smaller increase in physical activity, a higher initial energy intake, and a lower baseline prevalence of diabetes than those whose scores were relatively stable (Supplemental Table 2 in the online-only Data Supplement).
Table 1.
Initial (1986) and 12-Year Changes* in Characteristics of Participants according to Quintiles of Changes in Overall Plant-Based Diet Index (PDI)
| Nurses’ Health Study | Health Professionals Follow-Up Study | |||||
|---|---|---|---|---|---|---|
| Quintile 1 (No.=10,861) |
Quintile 3 (No.=11,735) |
Quintile 5 (No.=9,550) |
Quintile 1 (No.=5,517) |
Quintile 3 (No.=4,664) |
Quintile 5 (No.=5,128) |
|
| PDI score | ||||||
| Initial | 59.0 (55.0, 63.0) | 55.0 (51.0, 58.0) | 50.0 (46.0, 54.0) | 59.0 (55.0, 63.0) | 55.0 (51.0, 58.0) | 50.0 (46.0, 54.0) |
| Change | −9.0 (−11.0, −7.0) | 0.0 (−1.0, 1.0) | 8.0 (7.0, 11.0) | −9.0 (−11.0, −7.0) | 0.0 (−1.0, 1.0) | 9.0 (7.0, 11.0) |
| Age at 1998 (yr) | 64 (58, 71) | 63 (58, 70) | 63 (58, 69) | 62 (56, 71) | 63 (56, 70) | 63 (56, 70) |
| Initial body-mass index† | 24.2 (22.0, 27.5) | 24.1 (21.9, 27.4) | 24.0 (21.8, 27.2) | 25.1 (23.4, 27.0) | 25.0 (23.2, 26.6) | 25.0 (23.2, 26.7) |
| Weight change (kg) | 3.6 (0.0, 7.7) | 3.6 (0.0, 7.7) | 3.2 (0.0, 7.3) | 2.3 (−0.5, 5.9) | 2.3 (−0.9, 5.4) | 1.8 (−0.9, 5.0) |
| Physical activity (metabolic equivalents/wk) | ||||||
| Initial | 7.4 (2.0, 18.2) | 7.5 (2.0, 17.7) | 7.5 (2.0, 18.8) | 13.4 (4.6, 30.8) | 13.1 (4.4, 29.7) | 12.5 (4.2, 29.1) |
| Change | 1.4 (−4.8, 10.5) | 2.0 (−3.8, 11.2) | 3.3 (−3.2, 14.4) | 0.3 (−8.4, 13.0) | 1.3 (−7.1, 14.1) | 1.8 (−6.7, 15.4) |
| Alcohol intake (g/day) | ||||||
| Initial | 1.8 (0.0, 7.4) | 1.8 (0.0, 7.6) | 1.8 (0.0, 8.4) | 6.0 (1.0, 14.8) | 6.4 (1.0, 15.2) | 6.4 (1.0, 15.8) |
| Change | 0.0 (−1.9, 0.1) | 0.0 (−1.8, 0.6) | 0.0 (−1.9, 0.9) | 0.0 (−3.0, 2.8) | 0.0 (−2.6, 3.2) | 0.0 (−2.9, 3.6) |
| Margarine Intake (servings/day) | ||||||
| Initial | 0.8 (0.1, 1.0) | 0.8 (0.1, 1.0) | 0.4 (0.1, 1.0) | 0.4 (0.1, 1.0) | 0.4 (0.0, 0.8) | 0.4 (0.0, 0.8) |
| Change | −0.2 (−0.9, 0.0) | −0.1 (−0.8, 0.0) | 0.0 (−0.6, 0.0) | 0.0 (−0.4, 0.0) | 0.0 (−0.4, 0.0) | 0.0 (−0.4, 0.1) |
| Initial Energy Intake (kcal/day) | 1830 (1493, 2200) | 1722 (1399, 2095) | 1611 (1313, 1963) | 2050 (1680, 2488) | 1947 (1559, 2394) | 1816 (1464, 2218) |
| White race (%)‡ | 98 | 98 | 98 | 96 | 96 | 97 |
| Smoking status (%) | ||||||
| Remained a never smoker | 45 | 46 | 45 | 46 | 49 | 47 |
| Change from current to former or remained former smoker | 44 | 43 | 46 | 44 | 42 | 44 |
| Change from never or past to current, or remained current smoker | 10 | 10 | 9 | 5 | 5 | 4 |
| Smoking history among ever smokers (no. of pack-yr)§ | ||||||
| Initial | 18.0 (6.0, 32.0) | 16.0 (6.0, 31.0) | 16.0 (6.0, 32.0) | 18.0 (10.0, 30.0) | 20.0 (10.0, 30.0) | 19.0 (10.0, 30.0) |
| Change | 0.0 (0.0, 4.0) | 0.0 (0.0, 4.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Past diagnoses (%) | ||||||
| Hypertension | 44 | 42 | 43 | 37 | 34 | 33 |
| High cholesterol | 56 | 56 | 59 | 46 | 45 | 47 |
| Diabetes | 9 | 6 | 5 | 8 | 5 | 4 |
| Current use of medication or supplements (%) | ||||||
| Aspirin | 53 | 53 | 54 | 64 | 65 | 65 |
| Multivitamin | 62 | 61 | 63 | 61 | 59 | 60 |
| Antihypertensive medication | 37 | 35 | 35 | 28 | 27 | 25 |
| Cholesterol-lowering medication | 14 | 15 | 15 | 15 | 13 | 13 |
| Family history (%) | ||||||
| Diabetes | 30 | 30 | 30 | 23 | 22 | 22 |
| Myocardial infarction | 25 | 25 | 25 | 16 | 16 | 15 |
| Cancer | 58 | 59 | 59 | 37 | 38 | 39 |
The initial of the study was setup at 1986, and the baseline was setup at 1998; the change means 12-year difference between 1986 and 1998; values are medians (25th and 75th percentiles) for continuous variables and percentages for categorical variables. All variables except age are standardized to the age distribution of the study population.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Race was reported by the participants.
A pack-year is defined as the equivalent of smoking one pack of cigarettes (20 cigarettes) per day for one year. PDI indicates overall plant-based diet index.
In the NHS, over 725,316 person-years of follow-up, we documented 10,686 deaths including 2,046 deaths from CVD and 3,091 deaths from cancer. In the HPFS, during 371,322 person-years of follow-up, we documented 6,490 deaths including 1,872 deaths from CVD and 1,772 deaths from cancer.
Changes in Plant-Based Diet Quality and Risk of Total and Cause-Specific Mortality
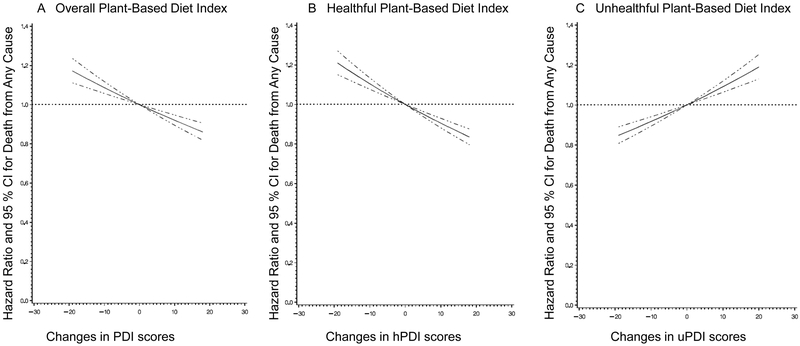
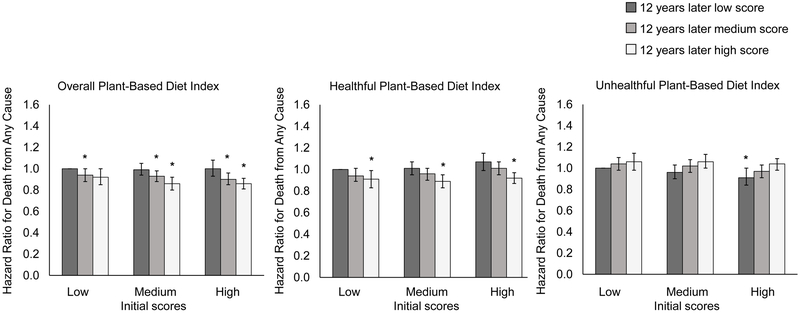
In pooled multivariable analyses, compared with participants whose PDI scores were relatively stable, those with the greatest increase (quintile 5) had a marginally lower risk of all-cause mortality (5% [95% CI, 0% to 10%]) (Table 2). Participants with the greatest increase in hPDI had a 10% lower all-cause mortality risk (95% CI, 5% to 15%), whereas those with the greatest increases in uPDI had a 12% higher risk of death (95% CI, 7% to 18%). In contrast, compared with participants whose diet scores were relatively stable, participants with the greatest decrease (quintile 1) in the corresponding diet scores had a 9% (95% CI, 4% to 15%) higher risk of death for PDI, a 10% (95% CI, 5% to 15%) higher risk of death for hPDI, and a 7% (95% CI, 2% to 12%) lower risk of death for uPDI. Restricted-cubic-spline analyses showed no evidence of nonlinearity for the associations between plant-based diet indices and total mortality (P >0.05 for test of curvature and P <0.001 for linearity in all plant-based diet indices, Figure 1). When we examined the joint association of scores at initial and 12 years later, compared with participants who had consistently low hPDI scores over time, participants with the largest increase in hPDI (low to high) had a 9% (95 % CI, 1% to 17%) lower risk of total mortality while those with consistently high hPDI scores over time had an 8% (95% CI, 3% to 13%) lower risk of total mortality (Figure 2). Compared with participants who had consistently low uPDI scores over time, the HR for those with consistently high uPDI was 1.04 (95% CI, 0.98 to 1.09), while the HR for those with the greatest decrease in uPDI (high to low) was 0.91 (95% CI, 0.84 to 1.00) (Figure 2).
Table 2.
Hazard Ratios (95% CIs) for All-Cause Mortality according to 12-Year Changes in Quintiles of Plant-Based Diet Indices*
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
P Value for Trend |
|
|---|---|---|---|---|---|---|
| Overall Plant-Based Diet Index (PDI) | ||||||
| Nurses’ Health study | ||||||
| Median (range) | −9 (−19, −6) | −4 (−5, −3) | 0 (−2, 1) | 3 (2, 5) | 8 (6, 18) | |
| Cases/person-years | 2,632/157,294 | 1,669/110,383 | 2,514/172,758 | 1,971/143,651 | 1,900/141,232 | |
| Basic model | 1.18 (1.12, 1.25) | 1.05 (0.98, 1.11) | 1.00 | 0.91 (0.86, 0.97) | 0.85 (0.80, 0.91) | <0.001 |
| Multivariable-adjusted model 1 | 1.10 (1.04, 1.17) | 1.03 (0.97, 1.09) | 1.00 | 0.95 (0.90, 1.01) | 0.93 (0.87, 0.99) | <0.001 |
| Multivariable-adjusted model 2 | 1.07 (1.01, 1.14) | 1.02 (0.96, 1.09) | 1.00 | 0.96 (0.91, 1.02) | 0.95 (0.89, 1.01) | <0.001 |
| Health Professionals Follow-Up Study | ||||||
| Median (range) | −9 (−20, −6) | −3 (−5, −2) | 0 (−1, 1) | 3 (2, 5) | 9 (6, 18) | |
| Cases/person-years | 1,486/78,147 | 1,315/76,070 | 1,155/66,918 | 1,238/76,576 | 1,296/73,611 | |
| Basic model | 1.22 (1.13, 1.33) | 1.05 (0.97, 1.14) | 1.00 | 0.92 (0.85, 1.00) | 0.93 (0.85, 1.01) | <0.001 |
| Multivariable-adjusted model 1 | 1.15 (1.06, 1.25) | 1.04 (0.96, 1.13) | 1.00 | 0.92 (0.85, 1.00) | 0.95 (0.87, 1.03) | <0.001 |
| Multivariable-adjusted model 2 | 1.13 (1.04, 1.23) | 1.03 (0.95, 1.12) | 1.00 | 0.93 (0.85, 1.01) | 0.96 (0.88, 1.04) | <0.001 |
| Pooled Results | ||||||
| Basic model | 1.20 (1.14, 1.25) | 1.05 (1.00, 1.10) | 1.00 | 0.91 (0.87, 0.96) | 0.88 (0.84, 0.92) | <0.001 |
| Multivariable-adjusted model 1 | 1.12 (1.06, 1.17) | 1.03 (0.98, 1.08) | 1.00 | 0.94 (0.90, 0.99) | 0.93 (0.89, 0.98) | <0.001 |
| Multivariable-adjusted model 2 | 1.09 (1.04, 1.15) | 1.03 (0.98, 1.08) | 1.00 | 0.95 (0.91, 1.00) | 0.95 (0.90, 1.00) | <0.001 |
| Healthful Plant-Based Diet Index (hPDI) | ||||||
| Nurses’ Health study | ||||||
| Median | −10 (−20, −7) | −4 (−6, −3) | 0 (−2, 1) | 3 (2, 5) | 9 (6, 18) | |
| Cases/person-years | 2,606/145,377 | 2,140/139,319 | 2,354/164,265 | 1,880/136,793 | 1,706/139,562 | |
| Basic model | 1.13 (1.06, 1.19) | 1.02 (0.96, 1.08) | 1.00 | 0.98 (0.92, 1.04) | 0.92 (0.86, 0.98) | <0.001 |
| Multivariable-adjusted model 1 | 1.08 (1.02, 1.14) | 1.00 (0.94, 1.06) | 1.00 | 0.98 (0.92, 1.04) | 0.94 (0.88, 1.00) | <0.001 |
| Multivariable-adjusted model 2 | 1.09 (1.03, 1.16) | 1.00 (0.95, 1.07) | 1.00 | 0.97 (0.91, 1.03) | 0.90 (0.85, 0.96) | <0.001 |
| Health Professionals Follow-Up Study | ||||||
| Median | −9 (−19, −6) | −3 (−5, −2) | 0 (−1, 2) | 4 (3, 6) | 10 (7, 19) | |
| Cases/person-years | 1,564/72,596 | 1,343/72,573 | 1,478/84,315 | 1,116/73,239 | 989/68,599 | |
| Basic model | 1.09 (1.01, 1.18) | 1.02 (0.94, 1.10) | 1.00 | 0.90 (0.83, 0.97) | 0.92 (0.84, 1.00) | <0.001 |
| Multivariable-adjusted model 1 | 1.07 (0.99, 1.16) | 1.02 (0.94, 1.10) | 1.00 | 0.93 (0.86, 1.00) | 0.92 (0.84, 1.00) | <0.001 |
| Multivariable-adjusted model 2 | 1.10 (1.02, 1.19) | 1.04 (0.96, 1.12) | 1.00 | 0.92 (0.85, 1.00) | 0.90 (0.82, 0.98) | <0.001 |
| Pooled Results | ||||||
| Basic model | 1.12 (1.06, 1.17) | 1.02 (0.97, 1.06) | 1.00 | 0.95 (0.90, 0.99) | 0.92 (0.87, 0.97) | <0.001 |
| Multivariable-adjusted model 1 | 1.08 (1.03, 1.13) | 1.01 (0.96, 1.06) | 1.00 | 0.96 (0.92, 1.01) | 0.93 (0.89, 0.98) | <0.001 |
| Multivariable-adjusted model 2 | 1.10 (1.05, 1.15) | 1.02 (0.97, 1.07) | 1.00 | 0.95 (0.90, 1.00) | 0.90 (0.85, 0.95) | <0.001 |
| Unhealthful Plant-Based Diet Index (uPDI) | ||||||
| Nurses’ Health study | ||||||
| Median | −10 (−20, −7) | −4 (−6, −2) | 0 (−1, 2) | 4 (3, 6) | 10 (7, 21) | |
| Cases/person-years | 1,439/141,700 | 1,998/165,308 | 2,091/150,238 | 2,111/122,957 | 3,047/145,114 | |
| Basic model | 0.96 (0.89, 1.03) | 0.99 (0.93, 1.05) | 1.00 | 1.10 (1.03, 1.16) | 1.18 (1.11, 1.24) | <0.001 |
| Multivariable-adjusted model 1 | 0.97 (0.90, 1.04) | 0.99 (0.93, 1.06) | 1.00 | 1.07 (1.01, 1.14) | 1.10 (1.05, 1.18) | <0.001 |
| Multivariable-adjusted model 2 | 0.91 (0.85, 0.98) | 0.97 (0.91, 1.03) | 1.00 | 1.07 (1.01, 1.14) | 1.14 (1.08, 1.21) | <0.001 |
| Health Professionals Follow-Up Study | ||||||
| Median | −10 (−19, −7) | −4 (−6, −3) | −1 (−2, 1) | 3 (2, 5) | 9 (6, 19) | |
| Cases/person-years | 1,078/68,229 | 1,226/72,360 | 1,459/85,004 | 1,250/72,673 | 1,477/73,056 | |
| Basic model | 1.01 (0.93, 1.09) | 1.06 (0.98, 1.14) | 1.00 | 1.02 (0.94, 1.10) | 1.10 (1.02, 1.18) | 0.13 |
| Multivariable-adjusted model 1 | 1.01 (0.93, 1.10) | 1.07 (0.99, 1.16) | 1.00 | 1.03 (0.95, 1.11) | 1.06 (0.98, 1.15) | 0.47 |
| Multivariable-adjusted model 2 | 0.96 (0.88, 1.05) | 1.05 (0.97, 1.13) | 1.00 | 1.03 (0.95, 1.12) | 1.09 (1.00, 1.17) | 0.03 |
| Pooled Results | ||||||
| Basic model | 0.98 (0.93, 1.03) | 1.01 (0.96, 1.06) | 1.00 | 1.06 (1.01, 1.12) | 1.15 (1.09, 1.20) | <0.001 |
| Multivariable-adjusted model 1 | 0.98 (0.93, 1.04) | 1.02 (0.97, 1.07) | 1.00 | 1.05 (1.00, 1.10) | 1.09 (1.04, 1.15) | <0.001 |
| Multivariable-adjusted model 2 | 0.93 (0.88, 0.98) | 1.00 (0.95, 1.05) | 1.00 | 1.06 (1.01, 1.11) | 1.12 (1.07, 1.18) | <0.001 |
The Basic model was adjusted for age and initial plant-based diet index score. The multivariable-adjusted model 1 was adjusted for age, initial plant-based diet index score, race, family history of myocardial infarction, diabetes, or cancer, aspirin use, multivitamins use, initial body mass index, menopausal status and hormone use in women, smoking status, initial and changes in each of smoking pack-years, physical activity, total energy intake, alcohol consumption, and margarine intake. The multivariable-adjusted model 2 had the same adjustments as multivariable-adjusted model 1 plus weight change, history of hypertension, hypercholesterolemia, or type 2 diabetes, antihypertensive medication use, and cholesterol-lowering medication use. Results from the Nurses’ Health Study and the Health Professionals Follow-Up Study were combined with the use of the fixed effects model. CI indicates confidence interval; PDI, overall plant-based diet index; hPDI, healthful plant-based diet index; and uPDI, unhealthful plant-based diet index.
Figure 1. Restricted cubic spline curves for associations between 12-year changes in plant-based diet indices and total mortality.
A. Overall plant-based diet index (PDI). B. Healthful plant-based diet index (hPDI). C. Unhealthful plant-based diet index (uPDI). Multivariable-adjusted hazard ratios are calculated by restricted cubic spline regression adjusted for age, sex, race, initial plant-based diet index score, family history of myocardial infarction, diabetes, or cancer, aspirin use, multivitamin use, initial body mass index, weight change, menopausal status and hormone use in women, smoking status, history of hypertension, hypercholesterolemia, or type 2 diabetes, antihypertensive medication use, cholesterol-lowering medication use, and initial and changes in each of smoking pack-years, physical activity, total energy intake, alcohol consumption, and margarine intake. Solid curves represent point estimates and dashed curves represent 95% confidence intervals (CIs).
Figure 2. Risk of death from any cause according to initial (1986) and 12 years later plant-based diet index scores.
Initial plant-based diet index scores are shown as low, mediun, and high. At 12 years later, participants may have had a consistenty low plant-based diet index scores over time, a change from a low score to a medium or high score, a consistently medium score over time, a change from a medium score to a low or high score, a consistetly high score over time, or a change from a high score to a low or medium score. The multivariable-adjusted hazard ratios were calculated with adjustments for age, race, family history of myocardial infarction, diabetes, or cancer, aspirin use, multivitamin use, initial body mass index, weight change, menopausal status and hormone use in women, smoking status, history of hypertension, hypercholesterolemia, or type 2 diabetes, antihypertensive medication use, cholesterol-lowering medication use, and initial and changes in each of smoking pack-years, physical activity, total energy intake, alcohol consumption, and margarine intake. Results from the Nurses’ Health Study and the Health Professionals Follow-Up Study were combined with the use of the fixed effects model. Error bars represent 95% confidence intervals; asterisks represent P <0.05 for the associations of hazard ratios compared with participants who had consistently low plant-based diet index scores over time.
For each 10-point increase in 12-year changes in PDI and hPDI, CVD mortality risk was lower by 7% (95% CI, 1% to 12%) and 9% (95% CI, 4% to 14%), respectively. By contrast, for each 10-point increase in uPDI, risk of CVD mortality was higher by 8% (95% CI, 2% to 14%) (Table 3). A 10-point PDI increase was associated with a 7% lower risk of cancer mortality (95% CI, 2% to 11%). There were no significant associations between changes in hPDI or uPDI and cancer-related deaths. When examining associations of changes in PDI, hPDI, and uPDI with subtypes of CVD and cancer mortality, we found a significant inverse association between a 10-point increase in hPDI and coronary heart disease mortality but not stroke mortality (Supplemental Table 3). For cancer subtype mortality, given the small number of cases, we did not document consistent associations.
Table 3.
Pooled Hazard Ratios (95% CIs) for the Associations between 12-Year Changes in Plant-Based Diet Indices and Risk of Death from Any Cause, Cardiovascular Disease, and Cancer per 10-Point Increase in Plant-Based Diet Indices*
| Plant-Based Diet Indices | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Overall plant-based diet index (PDI) | Death from Any Cause | Death from Cardiovascular Disease | Death from Cancer |
| Basic model | 0.84 (0.82, 0.86) | 0.83 (0.78, 0.87) | 0.86 (0.82, 0.91) |
| Multivariable-adjusted model 1 | 0.90 (0.88, 0.93) | 0.90 (0.85, 0.95) | 0.92 (0.88, 0.97) |
| Multivariable-adjusted model 2 | 0.92 (0.89, 0.95) | 0.93 (0.88, 0.99) | 0.93 (0.89, 0.98) |
| Healthful plant-based diet index (hPDI) | |||
| Basic model | 0.90 (0.88, 0.93) | 0.91 (0.87, 0.96) | 0.95 (0.91, 1.00) |
| Multivariable-adjusted model 1 | 0.93 (0.91, 0.95) | 0.94 (0.89, 0.99) | 0.98 (0.93, 1.02) |
| Multivariable-adjusted model 2 | 0.90 (0.88, 0.93) | 0.91 (0.86, 0.96) | 0.98 (0.93, 1.02) |
| Unhealthful plant-based diet index (uPDI) | |||
| Basic model | 1.08 (1.05, 1.11) | 1.07 (1.02, 1.12) | 1.00 (0.96, 1.05) |
| Multivariable-adjusted model 1 | 1.05 (1.02, 1.07) | 1.02 (0.96, 1.07) | 0.97 (0.93, 1.02) |
| Multivariable-adjusted model 2 | 1.09 (1.06, 1.12) | 1.08 (1.02, 1.14) | 0.98 (0.93, 1.03) |
The Basic model was adjusted for age and initial plant-based diet index score. The multivariable-adjusted model 1 was adjusted for age, initial plant-based diet index score, race, family history of myocardial infarction, diabetes, or cancer, aspirin use, multivitamins use, initial body mass index, menopausal status and hormone use in women, smoking status, initial and changes in each of smoking pack-years, physical activity, total energy intake, alcohol consumption, and margarine intake. The multivariable-adjusted model 2 had the same adjustments as multivariable-adjusted model 1 plus weight change, history of hypertension, hypercholesterolemia, or type 2 diabetes, antihypertensive medication use, and cholesterol-lowering medication use. Results from the Nurses’ Health Study and the Health Professionals Follow-Up Study were combined with the use of the fixed effects model. CI indicates confidence interval; PDI, overall plant-based diet index; hPDI, healthful plant-based diet index; and uPDI, unhealthful plant-based diet index.
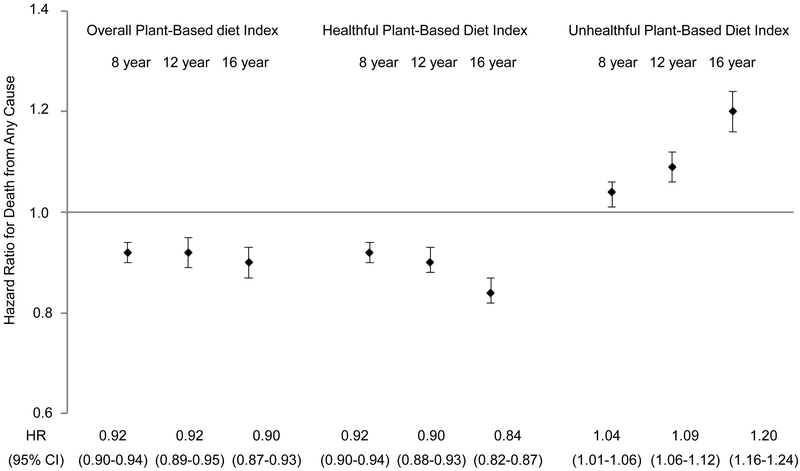
When we additionally examined 8-year and 16-year changes in plant-based diet indices, a 10-point hPDI increase over a 16-year period was associated with a 16% lower risk of total mortality (95% CI, 13% to 18%), whereas a 10-point uPDI increase over a 16-year period was associated with 20% higher risk of total mortality (95% CI, 16% to 24%) (Figure 3). Similar associations were observed for CVD mortality (Supplemental Figure 1A in the online-only Data Supplement). There were no consistent associations between shorter or longer improvement in plant-based diet quality and cancer mortality (Supplemental Figure 1B in the online-only Data Supplement).
Figure 3. Risk of death from any cause per 10-point increase in plant-based diet indices for preceding 8-, 12-, 16-year changes.
The multivariable-adjusted hazard ratios of death from any cause per 10-point increase in three plant-based diet indices for preceding 8-, 12-, and 16-year are shown. Error bars represent 95% confidence intervals (CIs).
Food Intake and Mortality
The initial and 12-year mean changes in each food group intake according to the quintiles of changes in PDI, hPDI, and uPDI are shown in Supplemental Table 4 in the online-only Data Supplement. After accounting for differences in initial food intake levels, changes in hPDI were primarily driven by increased consumption of whole grains, fruits, vegetables, and tea and coffee. On the other hand, changes in uPDI were mainly driven by decreased consumption of fruits, vegetables, and tea and coffee, and increased consumption of refined grains and sweets and desserts (Supplemental Table 4 in the online-only Data Supplement).
When changes in intakes of servings of foods (healthy plant foods, less healthy plant foods, and animal foods) were included simultaneously in the model in place of the plant-based diet indices, compared to participants whose healthy plant foods intake remained relatively stable, those with the greatest increase in intake of healthy plant foods (median change=4.5 servings/day for women and 5.6 servings/day for men) had a 7% lower risk (95% CI, 2% to 12%) while those with the greatest decrease (median change=5.1 servings/day for women and 3.9 servings/day for men) had a 16% higher risk (95% CI, 10% to 22%) of total mortality (Supplemental Figure 2 in the online-only Data Supplement). Participants with the greatest increase in animal foods (median change=2.0 servings/day for women and 2.1 servings/day for men) had a 7% higher risk (95% CI, 2% to 13%) of total mortality (Supplemental Figure 2 in the online-only Data Supplement). We found no associations between changes in intake of less healthy plant foods intake and total mortality.
Sensitivity Analyses
The significant associations between changes in plant-based diet indices and mortality persisted when we further adjusted for mammographic screening (for women) and physical checkups (Supplemental Table 5 in the online-only Data Supplement), when we excluded deaths in the first 4-years of follow-up (Supplemental Table 6 in the online-only Data Supplement), and when we stratified by major confounding factors (Supplemental Figure 3 in the online-only Data Supplement). Changes in hPDI were more strongly associated with total mortality among participants with smaller weight increase than those with larger weight increase (P for interaction <0.001, Supplemental Figure 3 in the online-only Data Supplement). Further, when we excluded the 18 food groups one at a time from PDI, hPDI, and uPDI and adjusted for the initial and changes in the excluded food group intake, the HRs for total mortality per 10-point increases in index scores were not materially altered (Supplemental Table 7 in the online-only Data Supplement). In addition, when we modified hPDI to score certain animal foods positively (fish, poultry, fermented diary, low-fat dairy, high-fat dairy, and eggs individually or simultaneous positive coding for fish, poultry, fermented dairy, low-fat dairy, and eggs), the HRs remained similar (Supplemental Table 8 in the online-only Data Supplement).
DISCUSSION
In the current analyses, we found that improving plant-based diet quality over a 12-year period was associated with a subsequent lower risk of death. A 10-point increase in hPDI over a 12-year period was associated with a 10% lower risk of total mortality and a 9% lower risk of CVD mortality, whereas a 10-point increase in uPDI was associated with an 9% higher risk of total mortality and an 8% higher risk of CVD mortality. There was no consistent evidence for an association between changes in plant-based diet quality and cancer mortality.
Previous studies that examined associations between plant-based dietary patterns and mortality have been somewhat inconclusive and the discrepancies in these findings can be attributed to differences in the quality of plant foods.32-34 In two cohorts of Seventh-day Adventists, vegetarian diets were associated with a 12%-20% lower risk of total mortality.32, 33 In contrast, findings from the European Prospective Investigation into Cancer and Nutrition study found no associations between vegetarian diets and total and CVD mortality.34 Consistent with our finding of a lower mortality risk with a greater hPDI increase, using NHANES III data, Kim et al found that among participants with hPDI scores above the median, each 10-point higher hPDI was associated with a 5% lower total mortality risk.15 However, they found no evidence for associations between PDI, uPDI, and risk of total and CVD mortality. These inconsistencies may be due to the smaller NHANES sample size and our strategy to assess dietary changes. To our knowledge, this is the first study to investigate the associations between changes in plant-based diet quality and subsequent risk of death.
The results on hPDI change in the current study are in line with previous studies that examined changes in other healthy dietary patterns such as the Healthy Eating Index, the Alternate Healthy Eating Index-2010, the Alternate Mediterranean Diet, and the Dietary Approaches to Stop Hypertension diet score and mortality risk.16, 35 While these dietary patterns focus on the overall diet quality, plant-based diet indices focus on the quality of plant foods and score all animal foods negatively. A significant finding from our study is that decreases in consumption of healthy plant foods were associated with a higher risk of death. This has important public health implications as future nutrition policies and public health efforts to lower chronic disease risk should account for the quality of plant foods. Further, when we individually excluded the 18 food groups from the diet scores, results remained unchanged signifying the importance of assessing the overall plant-based diet quality rather than a specific food. Our findings that HRs for the association between a 10-point increase in hPDI and mortality risk remained unchanged after positive coding for fish, poultry, fermented dairy, low-fat dairy, and eggs suggest that the inverse association was not driven by changes in these animal foods but rather by increases in healthy plant foods, decreases in less healthy plant foods and/or other animal foods, or a combination of the above. Because the focus of this study is on examining changes in plant-based diet quality, we are unable to draw conclusions about specific plant-for-animal substitutions that may lead to lower mortality. However, our recent analysis suggested that replacing red and processed meats with healthy plant foods such as whole grains, nuts, legumes, and vegetables was associated with lower risk of mortality31.
A central issue in examining changes in diet quality and subsequent risk of mortality is to adequately control for concomitant changes in other behaviors (such as changes in physical activity and smoking), initial diet quality, and time-varying measures of chronic diseases. For example, in our study, participants with the greatest increase in hPDI had lower initial hPDI scores, had lower weight gain, greater increases in physical activity, and were more likely to be diagnosed with high blood cholesterol or diabetes. Therefore, we adjusted not only for initial and changes in covariates but also for initial diet scores as participants with lower initial scores tended to increase their diet scores. Our findings show that after adjusting for initial diet scores, those with the greatest increases in hPDI had a lower risk of death, suggesting that even improving one’s diet later in life can have health benefits. While we only documented marginally significant associations with decreases in less healthy plant foods and mortality, the existing evidence supports the replacement of less healthy plant foods with healthy plant foods for chronic disease risk reduction. For example, strong evidence from intervention trials of substituting whole grains for refined grains indicates favorable effects on cardiovascular risk factors, the gut microbiome, and immune and inflammatory responses.36, 37 Likewise, epidemiological data consistently show that regular consumption of coffee or tea instead of sugar-sweetened beverages is associated with lower risk of chronic disease and premature death.22, 38
A diet pattern consistent with a high hPDI score has the added advantage of being more environmentally sustainable. Most recently, the EAT-Lancet commission on Food, Planet, and Health recommended the universal healthy reference diet which largely consists of whole grains, vegetables, fruits, legumes, nuts, and unsaturated oils, includes a low to moderate amount of seafood and poultry, and includes no or a low quantity of red meat, processed meat, added sugar, refined grains, and starchy vegetables.6 To assess the environmental benefits of a healthy plant-based diet, Springmann et al. used an integrated health and environmental modeling framework for more than 150 countries and concluded that adopting a public health strategy that is focused on improving energy-balance and dietary changes towards low-meat and predominantly plant-based diets could not only reduce premature mortality but also markedly reduce environmental impacts globally.39 Future work should consider the role of healthy plant-based diet and a planetary health diet in altering the gut microbiome and other biological systems that may influence disease risk.
Consuming a plant-based diet without complete exclusion of animal foods is desirable as such an approach is flexible and allows individuals to make gradual changes to their diets. In addition, excluding all animal foods may not be appropriate for all demographics as moderate intakes of animal foods such as fish, poultry, and dairy have been shown to confer some health benefits.29-31 On the other hand, diets based entirely on plant foods may lead to deficiencies in nutrients such as vitamin B12 in some individuals.40 From this perspective, the hPDI supports gradual changes to healthy plant-based diet without necessarily excluding an entire food group (e.g. certain animal foods). For example, a 10-point increase in hPDI could be achieved by increasing healthy plant foods (such as fruits, vegetables and whole grains) by about 3 servings/day and decreasing less healthy plant foods (such as refined grains and added sugars) and some animal foods (such as processed meats) by about 2 servings/day.
Based on evidence from previous studies, several mechanisms can be speculated through which a healthy plant-based diet is associated with lower mortality. In the current study, the largest contributors to positive changes in the hPDI were whole grains, fruits, vegetables, tea, and coffee. Whole grains, fruits, and vegetables consumption have been associated with lower total and CVD mortality.8, 9, 41 These associations may be partially explained through anti-inflammatory and antioxidant effect of dietary fibers and polyphenols.42-44 Tea and coffee consumption also have been associated with lower total and CVD mortality, explained by polyphenols such as catechins and theaflavins in tea and chlorogenic acid in coffee with antioxidant effects.38, 45-47 Although improvements in the hPDI were previously shown to be favorably associated with adiposity-associated biomarker profiles and less weight gain,14, 48 the statistical interaction between hPDI and weight change and mortality risk suggests that greater amounts of weight gain may attenuate the health benefits of increasing hPDI on mortality risk.
The strengths of this study include the prospective design, the large sample size, the high rate of follow-up, repeated assessments of diet and lifestyle, and the evaluation of changes in plant-based diet quality. However, several limitations should be noted. First, generalizability may be limited because participants in our study were all health professionals and were predominantly white. However, the high educational status of our participants should be considered as an advantage because it allowed us to collect detailed and accurate information on diet, lifestyle, and other health variables and minimize confounding by socioeconomic status. It is also unlikely that the benefits of a healthy plant-based diet would differ by race and ethnicity. Second, although we controlled for several lifestyle factors and excluded participants with CVD, cancer, or implausible energy intakes, the possibility of residual confounding cannot be excluded due to the observational nature of the study. Third, as the plant-based diet indices were established to distinguish the quality of plant foods, all animal foods were scored negatively while certain animal foods have been shown to have either beneficial or null effects to health outcomes such as fish/seafood, poultry, and dairy. However, sensitivity analyses by scoring some of these foods such as fish/seafood, poultry, and fermented and low-fat dairy positively did not materially alter the findings. Further, certain mixed dishes (such as pizza and creamy soups) were classified as animal foods because they contain a substantial amount of animal foods. Such a broad classification may not fully capture certain healthful or unhealthful plant-based components of these mixed dishes. Fourth, our dietary assessment was based on self-reported questionnaires, which may produce measurement errors. However, the FFQs used in the current study were extensively validated against diet records and biomarkers.19, 20 In addition, the consistency of our results with other previous studies on healthy dietary patterns, and the effects of controlled feeding studies on cardiovascular risk factors, suggests that observed associations were unlikely to be explained by residual confounding and measurement errors.16, 49, 50
In conclusion, we found that improving plant-based diet quality over a 12-year period was associated with a lower risk of total and CVD mortality, whereas increased consumption of an unhealthful plant-based diet was associated with a higher risk of total and CVD mortality. Our results support shifts toward diets that emphasize healthy plant foods for improved health outcomes.
Supplementary Material
Clinical Perspective.
1). What Is New?
Whether improving plant-based diet quality is associated with a lower risk of mortality remains unclear.
In two large US cohorts, we investigated the associations between 12-year changes in quantity and quality of plant foods and subsequent risk of total and cause-specific mortality.
Improving plant-based diet quality was associated with lower risk of total and cardiovascular disease mortality, whereas increased consumption of an unhealthful plant-based diet was associated with a higher risk of total and cardiovascular disease mortality.
There were no consistent associations between changes in plant-based diet quality and cancer mortality.
2). What Are the Clinical Implications?
Our results support current recommendations to shift towards a diet that emphasizes healthy plant foods for improved health outcomes.
Increasing intakes of healthy plant foods and decreasing intakes of less healthy and/or animal foods can lower the future risk of mortality.
Given the health and environmental benefits associated with healthy plant-based diets, nutrition policies and public health efforts should consider the quality of plant foods to promote reductions in chronic disease risk.
Acknowledgements
We would like to thank the participants, the Channing Division of Network Medicine, and staff of the NHS and the HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
These results were presented at the American Heart Association’s Epidemiology and Prevention, Lifestyle and Cardiometabolic Health Scientific Sessions 2019 on March 6, 2019.
Sources of Funding
This work was supported by grants UM1 CA186107, UM1 CA167552, P01 CA87969, R01 HL034594, HL088521, HL35464, HL60712, and P30 DK46200 from the National Institutes of Health. Dr. Baden was supported by a fellowship from the Manpei Suzuki Diabetes Foundation. Dr. Satija was supported by American Heart Association Grant #16POST29660000. Dr. Bhupathiraju was supported by a Career Development Grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107804).
Non-standard Abbreviations and Acronyms
- CVD
cardiovascular disease
- PDI
overall plant-based diet index
- hPDI
healthful plant-based diet index
- uPDI
unhealthful plant-based diet index
- NHS
Nurses’ Health Study
- HPFS
Health Professionals Follow-Up Study
- HR
hazard ratio
- CI
confidence interval
- NHANES
National Health and Nutrition Examination Survey
- FFQ
food frequency questionnaire
- Q
quintile
- BMI
body mass index
Footnotes
Disclosures
Dr. Li and Dr. Hu reported receiving research support from California Walnut Commission. Dr. Satija is currently employed at Analysis Group, Inc. All other authors have no conflict of interest to disclose.
References
- 1.Huang T, Yang B, Zheng J, Li G, Wahlqvist ML and Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab. 2012;60:233–240. doi: 10.1159/000337301 [DOI] [PubMed] [Google Scholar]
- 2.Kwok CS, Umar S, Myint PK, Mamas MA and Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;176:680–686. doi: 10.1016/j.ijcard.2014.07.080 [DOI] [PubMed] [Google Scholar]
- 3.Tonstad S, Stewart K, Oda K, Batech M, Herring RP and Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23:292–299. doi: 10.1016/j.numecd.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, Okamura T and Miyamoto Y. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174:577–587. doi: 10.1001/jamainternmed.2013.14547 [DOI] [PubMed] [Google Scholar]
- 5.Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML, Perez-Escamilla R, Siega-Riz AM, Story M and Lichtenstein AH. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv Nutr. 2016;7:438–444. doi: 10.3945/an.116.012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willett W, Rockstrom J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood A, Jonell M, Clark M, Gordon LJ, Fanzo J, Hawkes C, Zurayk R, Rivera JA, De Vries W, Majele Sibanda L, Afshin A, Chaudhary A, Herrero M, Agustina R, Branca F, Lartey A, Fan S, Crona B, Fox E, Bignet V, Troell M, Lindahl T, Singh S, Cornell SE, Srinath Reddy K, Narain S, Nishtar S and Murray CJL. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–492. doi: 10.1016/s0140-6736(18)31788-4 [DOI] [PubMed] [Google Scholar]
- 7.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC and Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–2011. doi: 10.1056/NEJMoa1307352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E and Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W and Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muraki I, Rimm EB, Willett WC, Manson JE, Hu FB and Sun Q. Potato Consumption and Risk of Type 2 Diabetes: Results From Three Prospective Cohort Studies. Diabetes Care. 2016;39:376–384. doi: 10.2337/dc15-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R and Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174:516–524. doi: 10.1001/jamainternmed.2013.13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q and Hu FB. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016;13:e1002039. doi: 10.1371/journal.pmed.1002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB and Hu FB. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol. 2017;70:411–422. doi: 10.1016/j.jacc.2017.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baden MY, Satija A, Hu FB and Huang T. Change in Plant-Based Diet Quality Is Associated with Changes in Plasma Adiposity-Associated Biomarker Concentrations in Women. J Nutr. 2019;149:676–686. doi: 10.1093/jn/nxy301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Caulfield LE and Rebholz CM. Healthy Plant-Based Diets Are Associated with Lower Risk of All-Cause Mortality in US Adults. J Nutr. 2018;148:624–631. doi: 10.1093/jn/nxy019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB and Hu FB. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. N Engl J Med. 2017;377:143–153. doi: 10.1056/NEJMoa1613502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colditz GA, Manson JE and Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B and Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH and Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 20.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Rood JC, Harnack LJ, Sampson LK and Willett WC. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol. 2018;187:1051–1063. doi: 10.1093/aje/kwx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L and Whelton PK. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med. 2001;161:2573–2578. [DOI] [PubMed] [Google Scholar]
- 22.de Koning L, Malik VS, Rimm EB, Willett WC and Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321–1327. doi: 10.3945/ajcn.110.007922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerimi A and Williamson G. The cardiovascular benefits of dark chocolate. Vascul Pharmacol. 2015;71:11–15. doi: 10.1016/j.vph.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 24.Rich-Edwards JW, Corsano KA and Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB and Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L and Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. [DOI] [PubMed] [Google Scholar]
- 27.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A and Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. [DOI] [PubMed] [Google Scholar]
- 28.Zahl PH, Jorgensen KJ, Maehlen J and Gotzsche PC. Biases in estimates of overdetection due to mammography screening. Lancet Oncol. 2008;9:199–201; author reply 201-192. doi: 10.1016/s1470-2045(08)70049-4 [DOI] [PubMed] [Google Scholar]
- 29.Abete I, Romaguera D, Vieira AR, Lopez de Munain A and Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr. 2014;112:762–775. doi: 10.1017/s000711451400124x [DOI] [PubMed] [Google Scholar]
- 30.Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Bechthold A, Schlesinger S and Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105:1462–1473. doi: 10.3945/ajcn.117.153148 [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Li Y, Satija A, Pan A, Sotos-Prieto M, Rimm E, Willett WC and Hu FB. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019;365:l2110. doi: 10.1136/bmj.l2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Key TJ, Fraser GE, Thorogood M, Appleby PN, Beral V, Reeves G, Burr ML, Chang-Claude J, Frentzel-Beyme R, Kuzma JW, Mann J and McPherson K. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999;70:516s–524s. doi: 10.1093/ajcn/70.3.516s [DOI] [PubMed] [Google Scholar]
- 33.Orlich MJ, Singh PN, Sabate J, Jaceldo-Siegl K, Fan J, Knutsen S, Beeson WL and Fraser GE. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173:1230–1238. doi: 10.1001/jamainternmed.2013.6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinu M, Abbate R, Gensini GF, Casini A and Sofi F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57:3640–3649. doi: 10.1080/10408398.2016.1138447 [DOI] [PubMed] [Google Scholar]
- 35.Schwingshackl L, Bogensberger B and Hoffmann G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J Acad Nutr Diet. 2018;118:74–100.e111. doi: 10.1016/j.jand.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 36.Vanegas SM, Meydani M, Barnett JB, Goldin B, Kane A, Rasmussen H, Brown C, Vangay P, Knights D, Jonnalagadda S, Koecher K, Karl JP, Thomas M, Dolnikowski G, Li L, Saltzman E, Wu D and Meydani SN. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr. 2017;105:635–650. doi: 10.3945/ajcn.116.146928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds A, Mann J, Cummings J, Winter N, Mete E and Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/s0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 38.Ding M, Satija A, Bhupathiraju SN, Hu Y, Sun Q, Han J, Lopez-Garcia E, Willett W, van Dam RM and Hu FB. Association of Coffee Consumption With Total and Cause-Specific Mortality in 3 Large Prospective Cohorts. Circulation. 2015;132:2305–2315. doi: 10.1161/circulationaha.115.017341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Springmann M, Wiebe K, Mason-D'Croz D, Sulser TB, Rayner M and Scarborough P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: a global modelling analysis with country-level detail. Lancet Planet Health. 2018;2:e451–e461. doi: 10.1016/s2542-5196(18)30206-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzo G, Lagana AS, Rapisarda AM, La Ferrera GM, Buscema M, Rossetti P, Nigro A, Muscia V, Valenti G, Sapia F, Sarpietro G, Zigarelli M and Vitale SG. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients. 2016;8:767. doi: 10.3390/nu8120767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zong G, Gao A, Hu FB and Sun Q. Whole Grain Intake and Mortality From All Causes, Cardiovascular Disease, and Cancer: A Meta-Analysis of Prospective Cohort Studies. Circulation. 2016;133:2370–2380. doi: 10.1161/circulationaha.115.021101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Hebert JR, Li W, Bertone-Johnson ER, Olendzki B, Pagoto SL, Tinker L, Rosal MC, Ockene IS, Ockene JK, Griffith JA and Liu S. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition. 2008;24:941–949. doi: 10.1016/j.nut.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo H, Xia M, Zou T, Ling W, Zhong R and Zhang W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J Nutr Biochem. 2012;23:349–360. doi: 10.1016/j.jnutbio.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 44.Cassidy A, Rogers G, Peterson JJ, Dwyer JT, Lin H and Jacques PF. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am J Clin Nutr. 2015;102:172–181. doi: 10.3945/ajcn.115.108555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishikawa T, Suzukawa M, Ito T, Yoshida H, Ayaori M, Nishiwaki M, Yonemura A, Hara Y and Nakamura H. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am J Clin Nutr. 1997;66:261–266. doi: 10.1093/ajcn/66.2.261 [DOI] [PubMed] [Google Scholar]
- 46.van Dam RM and Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. Jama. 2005;294:97–104. doi: 10.1001/jama.294.1.97 [DOI] [PubMed] [Google Scholar]
- 47.Gardener H, Rundek T, Wright CB, Elkind MS and Sacco RL. Coffee and tea consumption are inversely associated with mortality in a multiethnic urban population. J Nutr. 2013;143:1299–1308. doi: 10.3945/jn.112.173807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satija A, Malik V, Rimm EB, Sacks F, Willett W and Hu FB. Changes in intake of plant-based diets and weight change: results from 3 prospective cohort studies. Am J Clin Nutr. 2019. doi: 10.1093/ajcn/nqz049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ha V, Sievenpiper JL, de Souza RJ, Jayalath VH, Mirrahimi A, Agarwal A, Chiavaroli L, Mejia SB, Sacks FM, Di Buono M, Bernstein AM, Leiter LA, Kris-Etherton PM, Vuksan V, Bazinet RP, Josse RG, Beyene J, Kendall CW and Jenkins DJ. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta-analysis of randomized controlled trials. Cmaj. 2014;186:E252–262. doi: 10.1503/cmaj.131727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guasch-Ferre M, Li J, Hu FB, Salas-Salvado J and Tobias DK. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: an updated meta-analysis and systematic review of controlled trials. Am J Clin Nutr. 2018;108:174–187. doi: 10.1093/ajcn/nqy091 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.