Abstract
Introduction
Cancer care has expanded from a disease-focused, survival-oriented model to an approach that now considers how survivors can live well in the aftermath of intensive therapy, where they may deal with significant changes to their bodies, mental health or emotional well-being. Research evidence supports the benefit of exercise during and following cancer treatments for cancer-related symptoms, physical functioning and fitness, and health-related quality of life. To move this efficacy evidence into practice, we designed and launched a 5-year study to evaluate the relative benefit from implementing a clinic-to-community-based cancer and exercise model of care.
Methods and analysis
A hybrid effectiveness and implementation trial design is being used to evaluate the effectiveness of delivery of community-based exercise and to collect data on implementation of the programme. The study opened in January 2017, with estimated completion by January 2022. The programme will be delivered in seven cities across the province of Alberta, Canada, with sites including three academic institutions, six YMCA locations, Wellspring Edmonton and Calgary, and six municipal fitness centres. Participants are adult cancer survivors (n=2500) from all tumour groups and stages and at any time point along their cancer treatment trajectory, up to 3 years post treatment completion. Survivors take part in a minimum of 60 min of mild-to-moderate intensity full body exercise twice weekly for a 12-week period. The primary effectiveness outcome is the proportion of participants meeting or exceeding 150 min of moderate intensity exercise per week at 1-year follow-up. The Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework will be utilised to capture individual-level and organizational-level impact of the exercise programme at 12 and 24 weeks and 1-year follow-up. The cohort of survivors participating in the study will allow for long-term (>5-year) evaluation of rates of cancer recurrence and secondary cancers beyond the funding period.
Ethics and dissemination
The study was approved by the Health Research Ethics Board of Alberta. The study is funded by Alberta Innovates and the Alberta Cancer Foundation. The study will help to answer critical questions on the effectiveness of cancer-specific community-based exercise programming in both the short-term and the long-term. Collectively, the findings will help to inform the acceptability, adoption, feasibility, reach and sustainability of community-based exercise.
Trial registration number
NCT02984163; Pre-results.
Keywords: cancer survivorship, exercise, physical activity, quality of life, supportive care, implementation, knowledge translation
Strengths and limitations of this study.
The study involves patients and other stakeholders in the design and ongoing delivery of exercise programming.
External validity of the program is supported by the community-based implementation focus, with novel aspects of supervision by cancer-trained exercise specialists and support provided by study personnel.
We will determine both short-term and long-term effectiveness of community-based exercise and identify important intervention-implementation interactions.
The main limitation of the Alberta Cancer Exercise hybrid effectiveness-implementation study is related to the single-group design that does not allow for comparison of findings to usual care.
Introduction
In 2019, there will be an estimated 20 473 new cancer cases diagnosed in Alberta, Canada. By 2030, this number is expected to exceed 27 000.1 The growing population of individuals living with or beyond a diagnosis of cancer highlights the long-term impact of cancer and its therapies on the body, the mind and overall health of survivors. This necessitates an expansion of focus from merely survival to how to live in the aftermath of intensive therapy with an altered body and attendant psychological changes. There is an immediate and emergent need to disseminate strategies that can improve the health of cancer survivors.
Exercise is a low-cost and safe intervention for cancer survivors with beneficial effects on physical functioning and all aspects of health-related fitness, including aerobic and muscular fitness, and body composition.2–4 Exercise reduces the severity of treatment-related side effects such as pain, fatigue and lymphoedema5–8 and also benefits psychosocial well-being, including mental and emotional health, and overall quality of life (QoL).4 Evidence from randomised controlled trials has shown that supervised exercise results in better chemotherapy completion rates, thus potentially optimising treatment outcomes.5 6 Importantly, for three of the four most common cancers, representing 50% of all cancer survivors, exercise may prove valuable for secondary cancer prevention.7–11 Despite the known benefits of exercise, including the prevention of secondary cancers, less than one third of cancer survivors self-report that they are meeting the public health guideline recommendations for physical activity.3 This proportion is lower than the self-reported estimates of the general population (52%) in Canada.12
In recent years, strong evidence supporting the efficacy of exercise for cancer survivors has resulted in the development of cancer-specific exercise guidelines.3 13 14 As a result, implementation of programming in the community-based setting and preliminary data evaluating effectiveness of programming have begun to emerge.4 15–20 While positive results have been seen with laboratory-based studies,4 these results may not translate into the same benefits when implemented in a community-based setting.21 To date, published cancer-specific exercise implementation studies report significant short-term benefit from exercise for physical activity,22 6 min walk test distance,17 22 fatigue,23 QoL22 23 and medical costs.23 However, high programme attrition19 24–26 suggests the need for further exploration on the extent and nature (random or non-random) of programme dropouts and withdrawals. Moreover, the overall uptake of community-based exercise by cancer survivors relative to the larger population of survivors appears low. Finally, there is a lack of data from implementation studies supporting the long-term effectiveness of programming for physical fitness and QoL outcomes, overall health including healthcare utilisation and long-term survivorship, including survival rates.27
In order to move the efficacy evidence into practice, we designed and launched a 5-year hybrid effectiveness and implementation study to evaluate the relative benefit from an Alberta-wide clinic-to-community-based cancer and exercise model of care—the Alberta Cancer Exercise (ACE) programme and to evaluate the implementation of such an initiative. The overarching goal of the ACE programme is to provide and support high-quality, timely and personalised exercise for the survivor after a cancer diagnosis. In addition to implementing exercise programming, our hybrid effectiveness-implementation study was designed to better evaluate exercise effectiveness on overall health, considering both physical and psychosocial outcomes. At a pragmatic and policy level, we will aim to capture the costs, and potential for cost savings, of such a programme.28 To achieve widespread adoption, we acknowledge that our programme must benefit participants and must be cost-effective and reduce healthcare utilisation. At present, there are limited data on these key aspects of community-based exercise programming.
Objectives
The specific objectives of this study are to:
Determine the utility of facilitated referral of survivors, where participants are screened for inclusion in exercise programming within their respective communities, as a strategy for increasing adoption of exercise, with the primary aim to increase physical activity levels of participating cancer survivors.
Determine the immediate and long-term effectiveness of community-based programming on the survivors’ health-related QoL, physical fitness, patient-reported symptoms including fatigue and distress, as well as healthcare utilisation.
Identify strategic opportunities for enhancing implementation of the ACE clinic-to-community strategy by formalising screening methods, referral processes and incorporating clinical evaluation of physical function.
Methods and analysis
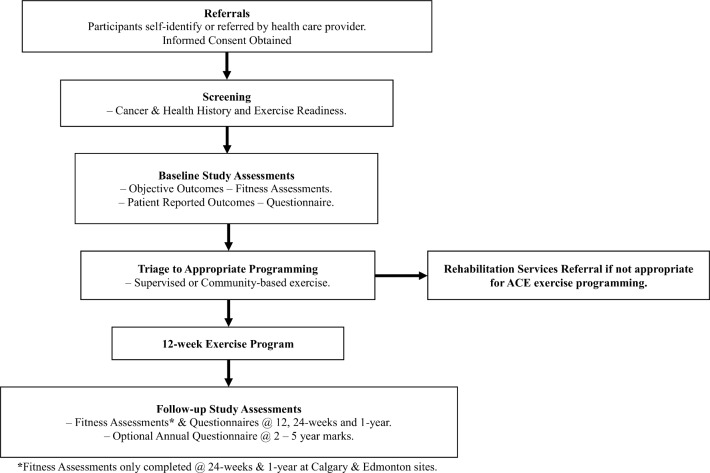
A hybrid effectiveness and implementation trial design is being used to evaluate the effectiveness of delivery of community-based exercise and to collect data on implementation of the programme.29 The study opened in January 2017 and will run for a 5-year period to January 2022. We chose this trial design because: (1) there is strong evidence from efficacy trials supporting the benefit of exercise for survivors both during and following cancer treatment, (2) there is a limited body of evidence supporting implementation of programming in the community and evidence supporting objective outcomes and long-term adoption is currently lacking and (3) with appropriate pre-exercise evaluation and screening, there is minimal risk in implementing a community-based exercise intervention. The hybrid design provides important data on the effectiveness of community exercise programming while fast-tracking translation of research findings into clinical practice and survivorship care pathways (figure 1: study schema).
Figure 1.
Study schema. ACE, Alberta Cancer Exercise.
Participants
Participants are adult cancer survivors from all tumour groups and stages and at any time point along their cancer treatment trajectory into the survivorship post-cancer treatment period, up to 3 years post treatment completion. Participants can self-refer to the programme or be referred by their healthcare professional (HCP). This inclusionary focus will allow us to build a clinic-to-community model that is sustainable and meets the needs of most cancer survivors.
We will aim to recruit a minimum of 60% of survivors from the three target cancer types with evidence supporting secondary prevention: breast, prostate and colorectal. These samples will allow for subgroup analyses across sites and cancer groups. This cohort of survivors participating in the study will allow for long-term evaluation of rates of cancer recurrence, secondary cancers and other chronic diseases (eg, cardiovascular diseases, diabetes) beyond the funding period.
Setting
The exercise programming intervention takes place at six YMCAs and six municipal fitness centres, three Wellspring locations (a non-profit cancer support organisation) in Calgary (two sites) and Edmonton (one site), as well as three academic fitness facilities (two of which are cancer-specific facilities). See figure 2: ACE programming sites map.
Figure 2.
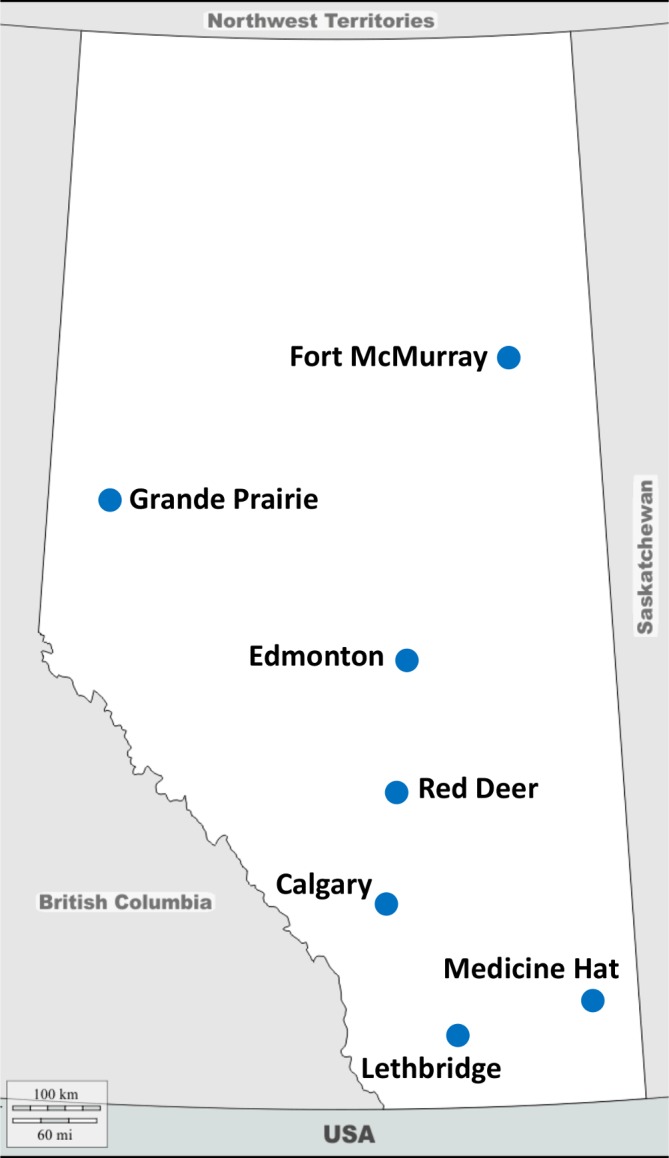
Alberta Cancer Exercise (ACE) programming sites.
Eligibility: inclusion criteria
Participants are screened for eligibility over the phone by the respective site coordinator (Alberta north or Alberta south) and must: (1) have a diagnosis of cancer of any type; (2) be over the age of 18 years; (3) be able to participate in mild levels of activity at minimum; (4) be pretreatment or receiving active cancer treatment (eg, surgery, systemic therapy and/or radiation therapy) or have received cancer treatment within the past 3 years or have existing long-term or late presenting effects of their cancer treatment (eg, radiation fibrosis syndrome, lymphoedema, communication deficits related to cancer treatment or incontinence) and (5) be able to provide informed written consent in English.
Screening
Two Certified Exercise Physiologists (CEPs), with graduate level training or certification in exercise physiology,30 and >5 years of experience in the cancer field, perform the screening for exercise safety (one CEP north, one CEP south). The CEPs report to the respective study principal investigators at the tertiary centres in the north and south of Alberta. For screening purposes, consenting participants complete a cancer-specific intake form and Physical Activity Readiness Questionnaires (PAR-Q+) online to determine appropriateness for community-based exercise programming. If any clarification on responses or status is needed, the CEP contacts the participant via telephone or meets with them in-person. Data are collected on exercise preferences as well as the participant’s Physical Activity Stages of Change to inform the participant’s status in terms of preferences, attitudes and behaviours towards increasing physical activity. The CEP oversees baseline objective assessments and evaluates testing results. The CEP then triages the participant to local programming based on his/her current health, findings of baseline objective assessment, cancer-related symptoms and exercise and location preferences. If safety issues emerge during screening (eg, uncontrolled seizures, history of falls, presence of metastatic disease, recent surgery or hospitalisation), the CEP consults with the participant’s oncologist or family physician on the need for further evaluation and/or referral to rehabilitation services or medically supervised exercise programming.
Implementation components and framework
Cancer-specific education and support for community-based exercise specialists
All community-based exercise programming is administered by exercise specialists (ie, certified personal trainer, kinesiologist or group exercise instructor) who have undergone the ACE Cancer and Exercise: Training for Fitness Professionals online course offered through the University of Calgary. The training involves 16 hours of cancer-specific content related to cancer biology, cancer incidence, treatment and treatment-related effects, exercise evidence and prescription for cancer survivors and health behaviour change. The ACE CEP provides additional in-person training to ensure community-based exercise professionals have the skills and knowledge required to work with the cancer population, as well as ongoing support to ensure success of the programme implementation. This training aids in the dissemination of the ACE programme’s critical knowledge to key community fitness partners.
Screening, referral and support for community-based exercise programming
The ACE programme bridges the gap between HCPs and community exercise programming by facilitating the referral of survivors to appropriate cancer-specific exercise programming. The CEPs provide education and onsite support to HCPs within the tertiary centres (Calgary and Edmonton) and via online and telephone-based support to HCPs working with survivors in smaller communities.
Patient and public involvement
Our ACE clinic-to-community-based exercise programme works with survivors and families, community exercise specialists, HCPs and end-users to improve the survivor exercise experience. All stakeholders, including cancer survivors, contributed to the design and delivery of ACE from inception, including providing input towards the funding application and during pilot testing. Survivors informed the format of the study (eg, no control group, implementation focus), recruitment (eg, self-referral option), eligibility (eg, including all cancer types and stage of disease) and intervention design in terms of preferences for exercise location (eg, community locations, ease of parking), format (eg, supervised programme, group class, mild-to-moderate intensity exercise, instructors with knowledge in cancer), days per week (ie, two) and time commitment (ie, 60–90 min per session). A series of future focus groups and semistructured interviews are planned to elicit feedback from participants, HCPs and exercise specialists over the course of ACE implementation.
Exercise intervention
Intervention options are geared to the various settings where ACE is being implemented. Participants take part in a combination of aerobic, resistance, balance and flexibility exercises delivered in a standardised circuit-type class setting or group personal training format, twice weekly for a minimum of 60 min per session (approximately 3–4 metabolic equivalent (MET) units per session) for a 12-week period. The exercise sessions are conducted in small groups of 8–15 participants under the direct supervision of the community-based ACE-trained exercise specialist. Two options for community-based exercise programming exist: group fitness classes or supervised fitness centre access. The programme includes options for low-to-moderate intensity exercise set at 3–4 MET units per session (360–480 MET-minutes per week) and is progressed in intensity to 4–5 METs over the 12-week programme duration (480–600 MET-minutes per week) as a means to progress participants towards recommended physical activity levels (500–1000 MET-minutes per week).31 In terms of intensity, this would be similar to prescribing walking at a comfortable pace (4 km per hour) initially and then slowly progressing to a brisk walking pace (6 km per hour) over a 12-week period. Participating community sites offer one or more of these options depending on available resources and demand. Attendance at the exercise sessions is tracked as a marker of acceptability. Reasons for missed sessions are recorded. Exercise adherence includes attendance at supervised exercise sessions and average exercise minutes per week over the study period. Intensity is monitored using the 10-point Borg Rating of Perceived Exertion scale.32 33 Active support and ongoing mentoring by the CEP are provided to community-based exercise specialists in the participating community programme for the duration of ACE programming. Fidelity checks are performed by the respective CEP at scheduled times during the 12-week exercise session. Participants record exercise sessions in minutes and intensity in their training log and other physical activity in their exercise diary. To encourage longer-term exercise adherence, participants are offered a second 12-week optional maintenance programme, where possible, at low to no cost to survivors.
Participants assessed as having high needs (eg, mobility issues, high risk of falling, risk of bone fracture, cognitive issues) due to active cancer, metastatic disease or with severe symptoms (where their disease or symptoms pose a risk in terms of safety of community-based exercise participation) are referred to ACE medically supervised programming or local cancer rehabilitation services.
Outcomes to support effectiveness of programming
The CEPs perform the objective assessments at the university sites or at the respective fitness facilities offering the programming both before (baseline) and after the exercise programme (at week 12), with further follow-up objective testing at 24 weeks and 1 year at the tertiary sites. The respective CEPs travel to the smaller cities in the north and south to conduct the baseline and 12-week assessments.
Objective and subjective physical outcome measures with demonstrated validity and reliability include:
Physical activity level: Godin Leisure-Time Physical Activity Questionnaire.34–36
Height, weight (calculation of body mass index).
Waist and hip circumference.37
Six-minute walk test.38
Other objective measures: grip strength,39–41 timed sit-to-stand,42 shoulder flexion43 (flexibility) and one-legged stance (balance).44
Cancer-related symptoms: Edmonton Symptom Assessment Scale and Screening for Distress.45
Health-related QoL is assessed using the Functional Assessment of Cancer Therapy-General46 and Fatigue scales,47 RAND Short Form Instrument (SF-36)48 and EQ5D-5L49 at baseline, 12 weeks, 24 weeks and 1 year for all participants. Participants will have the option for further follow-up yearly for the duration of the study. The study database was created in the REDCap system provided by the Women and Children’s Health Research Institute (WCHRI) and hosted in the University of Alberta’s Faculty of Medicine and Dentistry’s data centre. Data collection and storage will comply with the measures outlined in WCHRI’s REDCap privacy document.
Additional tests performed where equipment, time and resources are available: (1) one or eight repetition maximum bench press and one or eight repetition maximum leg press to determine muscular strength; (2) sit-and-reach test to assess flexibility; (3) plank muscular endurance test; (4) push-up test. A priori targets for objective outcomes, symptoms and QoL outcomes will be used to inform effectiveness and safety of the intervention (table 1).
Table 1.
Effectiveness outcomes
| Outcome measure/measurement | Minimal clinically important difference*/established cut-point | Study target for improvement in outcome score |
| Godin Leisure-Time Questionnaire | 10% change in physical activity behaviour at 1 year | +10% or more of survivors are engaging in >150 min of moderate intensity physical activity at 1 year |
| Waist circumference | Cut-points for health64: Men: 102 cm Women: 88 cm |
+10% survivors with reduction to below disease risk cut-point based on age and gender |
| 6 min walk test distance | 24 to 30.5 m65 | +30 m |
| Hand-grip dynamometry | 6.5 kg66 67 | +10% meeting or exceeding age-specific average score |
| 30 s sit-to-stand | Not established in cancer | +10% in the number of participants meeting age-specific functional level |
| Shoulder Flexion Range Goniometry |
>10 degrees68 | +10% meeting or exceeding age-specific average score |
| Sit and reach test | Population values67 69
Men 0 to +5 cm Women 0 to +10 cm |
+10% meeting or exceeding age-specific average score |
| Single leg balance: | 24 s70 | +10% meeting 45 s maximum time |
| One repetition maximum test | MCID: 1%–3% | +10% increase |
| Functional Assessment of Cancer Therapy (FACT)—General Scale | Population value46: score 88 MCID: 3 points |
+3 points |
| FACT-Fatigue subscale | Population value47: score of 40 MCID: 3–6 points |
+6 points |
| RAND Short Form-36 | Population value71: 67–87/100 across domains; MCID 6–7 points |
12% change from baseline |
| EQ5D-5 L | EQ5D index: 0.0649 72 73 | +0.06 from baseline |
| Attendance at sessions | Population values in older adults: 58% to 77%74 |
>70% attendance at exercise sessions |
*The minimum clinically important difference (MCID) is the minimum difference that the patient is able to recognise and appreciate.75
Outcomes to support implementation
The Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework will be utilised to evaluate and enhance the external validity of the ACE programme and presents a means to evaluate the impact of a community-based intervention as a function of these five factors. This framework has been used to evaluate health and lifestyle behaviours to determine the public health impact of the intervention.27 50 Embedded within RE-AIM is a cost description analysis pertaining to the survivor (individual costs) and the institutions (community fitness facilities, universities, and CancerControl Alberta). A proposed RE-AIM evaluation plan to assess the impact of the ACE programme has been developed based on existing research (table 2).27
Table 2.
RE-AIM framework
| Components/categories | Reporting outcomes |
| Reach (Individual Level) |
|
| Effectiveness (Individual and Institutional Level) |
|
| Adoption (Institutional Level) |
|
| Implementation (Community) |
|
| Maintenance (Individual, Institutional and Community) |
|
HCP, healthcare professional; RE-AIM, Reach, Effectiveness, Adoption, Implementation and Maintenance.
Healthcare utilisation evaluation
The proposed methods for healthcare utilisation include evaluation of usage among participants compared with that of matched controls, before and after the exercise programme. Using personal health numbers (PHN) for consenting participants who provide permission, the PHN will be linked to the Cancer Registry to obtain: tumour type, sex, year of diagnosis, age and stage at diagnosis and provincial zone of residence. These six variables will be used to match each participant (1:1, as closely as possible) to a control identified from the Cancer Registry. For each matched pair, ‘time 0’ will be the date the participant joined the exercise programme. Relative to this date, the Cancer Registry records will be linked to administrative data sources to capture all physician visits, emergency room visits and hospitalisations, 1 year prior to and 1 year following ‘time 0’. For physician visits, we will link to Alberta Health physician claims data. The following variables will be collected: date of visit, health service type code and category, primary/secondary/tertiary diagnoses and health service(s) performed. Healthcare utilisation will be examined overall (costs summed for each service component and each database) and by subgroups of interest (eg, diagnostic groupings, services provided, resource intensity weights), before and after ‘time 0’, separately for cases and controls. Differences in healthcare utilisation across the two time periods will be described for both groups. The analysis will be performed for all participants and stratified by tumour type.
Sample size
The overall sample size goal is to accrue up to 2500 survivors via the ACE 5-year roll out across the Province of Alberta (seven cities: 18 sites) to inform implementation. The primary objective outcome to assess study effectiveness is the number of participants meeting public health guidelines for physical activity at 1-year follow-up. The current estimate for the number of cancer survivors meeting public health guidelines of 150 min or more of moderate intensity exercise per week is 25.8%.51 According to the Conference Board of Canada, by simply getting 10% of Canadians with suboptimal levels of physical activity to exercise more would reduce incidence rates for major chronic conditions including cancer and result in significant savings in healthcare costs.52 Thus, assuming a 10% increase in the proportion of participants meeting the guidelines for physical activity (minimally important difference of 10%) at 1 year (p<0.01; 90% power), a sample size of approximately 305 survivors would be required. As the aim of the study is to evaluate both effectiveness and implementation, evaluating site-specific effects and implementation issues is of utmost importance, and thus our sample will allow adequate power for subgroup analyses given the number of sites and outcomes, and the anticipated variability among participants, cancer types and disease stages.
Statistical analysis plan
Descriptive analyses will be performed to evaluate participant demographic, medical and exercise-related variables, as well as RE-AIM components including an economic evaluation of the programme. We will perform checks of data integrity including evaluating statistical power, test assumptions and missing data. A single proportion inference test and CI will be performed to determine the proportion of eligible survivors who provide informed consent and complete the programme, as well as adherence rates to the programme. Generalised linear mixed models will be utilised to examine the changes over time in the programme participants on the patient-reported outcomes, including objective outcomes, activity levels and indices of QoL (ie, baseline, 12 weeks, 24 weeks and 1 year). The cohort of survivors participating in the study will allow for long-term evaluation of rates of cancer recurrence and secondary cancers beyond the study period.
Safety
Safety is monitored during exercise testing and training by the CEP and the ACE-trained exercise specialists in community locations. Participants are asked to report any issues, injuries or falls, related and unrelated to exercise participation to the ACE exercise specialist at the respective site. Where necessary, the medical advisor and rehabilitation team at the cancer centre are consulted. The CEPs and ACE exercise specialist record rates of adverse events (minor to serious adverse events including cardiovascular events, falls or musculoskeletal injuries) on the REDCap database with serious adverse events also reported to the Research Ethics Board.
Dissemination
We propose that our hybrid effectiveness-implementation study will help to answer critical questions on the value of cancer-specific community-based exercise programming. The ACE study will allow us to determine both the short-term and long-term effectiveness of exercise and enhance our ability to identify important intervention-implementation interactions. Collectively, the findings will help to inform the acceptability, adoption, feasibility, reach and sustainability of community-based exercise and simultaneously evaluate integration of exercise into clinical care.53
The end-of-grant knowledge translation (KT) will focus on dissemination of the long-term effectiveness of programming on outcomes of survivors, including markers supporting secondary cancer prevention and healthcare utilisation. Initial KT efforts will utilise academic peer-reviewed publications and conference presentations to disseminate new knowledge to academic audiences working in the field of exercise and cancer survivorship. Further dissemination and utilisation of our research findings will involve partnering with cancer groups such as Canadian Cancer Survivorship Network, Prostate Cancer Canada, the Canadian Cancer Society, the Canadian Partnership Against Cancer, the Canadian Physiotherapy Association Oncology Division and the Psychosocial and Palliative Oncology Network. Collaboration with these agencies will ensure that information from the study will be widely disseminated to local as well as the broader cancer survivor community across Canada.
Discussion
In recent years, the focus of research in the oncology exercise field has expanded from determining efficacy through randomised controlled trial designs to include ‘real world’ effectiveness studies focusing on implementation of exercise into cancer care.17 19 20 23 A wide variety of approaches to promote exercise among cancer survivors are available, including programmes that are medically supervised, community-based or self-directed/home-based.21 Advantages of community-based programmes include high accessibility, safety and supervision of exercise and social interaction.18 Importantly, systematic review evidence supports greater and more consistent benefits when exercise is delivered in a group or supervised setting when compared with a home-based or unsupervised setting.54 Moreover, surveys of cancer survivors show a high interest in exercise, with reported preference for exercise programmes that are offered in a supportive environment where treating and managing cancer are understood and at a location that focuses on health promotion rather than illness.55–58 Community-based studies performed to date, while demonstrating short-term effectiveness, are lacking data supporting long-term effectiveness. Moreover, studies commonly report low adherence and high dropout rates.21 27 Given the infancy of implementation efforts with regard to community-based programming, further research with greater attention to implementation science aspects appears warranted.
Our ACE-integrated KT strategy involves stakeholders in the design and ongoing delivery of ACE (ie, survivors, end-users, administrators and policy-makers) and aims to address HCP barriers and facilitators to exercise counselling and referral within the local cancer clinical setting. To address issues seen with less than optimal adherence and completion rates in previous implementation studies, key strategies built into ACE include monitoring of exercise adherence and behaviour change support for exercise.17 The primary behavioural supports within the ACE programme are the supervised and supportive aspects of the programming, along with exercise behaviour change education, goal setting and self-monitoring of activities. An ACE-trained exercise specialist at the community site leads exercise classes and sessions. An ACE CEP and physical therapist are available to provide additional support to the survivor to address issues related to cancer treatment effects. This supportive format allows for modification and tailoring of the exercise, as needed, to the survivor’s cancer type, capabilities and preferences.59 In theory, if the programme meets the needs of survivors, then adherence and completion rates should be high, reflecting programme acceptability.
Consistent with the design of an effectiveness study, the ACE programme is a cancer-specific exercise intervention with broad eligibility criteria that reflect ‘real-world’ conditions. As many survivors report feeling neither physically nor psychologically prepared to engage in community-based exercise programmes designed for the general public,58 a feature of ACE is the built-in flexibility of the exercise prescription such that participants self-select the exercise intensity based on presenting symptoms, ‘down days’ or personal preference. While participants are expected to meet a minimal goal of 2 hours per week of at least light intensity exercise, the participant is encouraged to exceed this goal if able and desired.
Recently published guidelines from Australia endorse the integration of exercise into cancer care as a means to lessen some of the negative effects of cancer and its treatment.13 Importantly, the guidelines identify the need for cancer HCPs to discuss the role of exercise in cancer recovery and recommend referral of survivors to a CEP and/or physical therapist with experience in cancer care.13 Implementation studies, to date, have largely focused on the delivery of an exercise intervention rather than studying the processes and outcomes associated with implementation within the healthcare system. Despite guidelines supporting exercise,3 14 60 challenges exist with implementing exercise counselling and referral into practice due to the existing complexity and competing priorities in the cancer clinical setting.61 Embedding CEP positions within our interprofessional supportive care team has the potential to address these challenges and is seen as a sustainable care model that will add measurable value to our efforts to integrate exercise into clinical care.62 63
Limitations
There are important limitations to note in the design of the ACE hybrid effectiveness-implementation study related to the single-group design that does not allow for comparison of findings to usual care. As such, threats to internal validity exist including maturation, history, testing and regression to the mean. To address these concerns, specific objective outcome targets were determined, a priori, based on previous randomised controlled trial findings. Moreover, to reduce bias associated with testing, ACE assessors, who are specially trained and blinded to previous results, conduct the evaluations and the participants complete the patient-reported outcomes electronically at home. External validity of the programme is supported by the community-based implementation focus, with novel aspects of supervision by cancer-trained exercise specialists and support provided by ACE CEPs and physical therapists. Importantly, evaluation of the programme is guided by the RE-AIM framework and includes a robust suite of endpoints.
Through this research, we will better understand the effectiveness of the programme at the level of the individual and institution and evaluate processes to support future implementation and sustainability. Supporting improved rates of exercise adoption and sustained adherence to an active lifestyle among survivors of cancer will improve physical fitness and QoL and may lower rates of cancer recurrence, secondary cancers and other chronic diseases for cancer survivors in Alberta.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contribution of all those involved with implementing and evaluating the Alberta Cancer Exercise Program, including staff and graduate students in the Cancer Rehabilitation Clinic at the University of Alberta and the Thrive Centre at the University of Calgary. We specifically recognise the contributions of our patient advisors on the study protocol: Kevin Power, Diane Cook and Ken Roth.
Footnotes
Contributors: MM, CS, TW, MS-B, AAJ, HYL, JCE, ADM, JV, KC, JRM, MP and NC-R developed the study concept and protocol. MM, NC-R, CS and TW assisted in further development of the exercise and implementation protocol. All authors will oversee the implementation of the protocol and contribute to the acquisition, analysis and interpretation of data. MM and NC-R drafted the manuscript; MM, CS and NC-R contributed to revisions and MM, CS, TW, MS-B, AAJ, HYL, JCE, ADM, JV, KC, JRM, MP and NC-R approved the final manuscript.
Funding: We acknowledge the support and funding received from the Alberta Innovates Cancer Prevention Research Opportunity and the Alberta Cancer Foundation.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was received from the Health Research Ethics Board of Alberta: Cancer Committee and all participants are required to provide written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Surveillance and Reporting The 2019 report on cancer statistics in Alberta. Edmonton: Cancer Control Alberta, 2019. [Google Scholar]
- 2. Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 2010;4:87–100. 10.1007/s11764-009-0110-5 [DOI] [PubMed] [Google Scholar]
- 3. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:242–74. 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 4. Cormie P, Zopf EM, Zhang X, et al. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev 2017;39:71–92. 10.1093/epirev/mxx007 [DOI] [PubMed] [Google Scholar]
- 5. van Waart H, Stuiver MM, van Harten WH, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. JCO 2015;33:1918–27. 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 6. Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–404. 10.1200/JCO.2006.08.2024 [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 2011;28:753–65. 10.1007/s12032-010-9536-x [DOI] [PubMed] [Google Scholar]
- 8. Kenfield SA, Stampfer MJ, Giovannucci E, et al. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol 2011;29:726–32. 10.1200/JCO.2010.31.5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:815–40. 10.1093/jnci/djs207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 2006;24:3527–34. 10.1200/JCO.2006.06.0855 [DOI] [PubMed] [Google Scholar]
- 11. Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006;24:3535–41. 10.1200/JCO.2006.06.0863 [DOI] [PubMed] [Google Scholar]
- 12. Colley RC, Garriguet D, Janssen I, et al. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian health measures survey. Health Rep 2011;22:7–14. [PubMed] [Google Scholar]
- 13. Cormie P, Atkinson M, Bucci L, et al. Clinical oncology Society of Australia position statement on exercise in cancer care. Med J Aust 2018;209:184–7. 10.5694/mja18.00199 [DOI] [PubMed] [Google Scholar]
- 14. Schmitz KH, Courneya KS, Matthews C, et al. American College of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409–26. 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- 15. Leach HJ, Danyluk JM, Culos-Reed SN. Design and implementation of a community-based exercise program for breast cancer patients. Curr Oncol 2014;21:267–71. 10.3747/co.21.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leach HJ, Danyluk JM, Nishimura KC, et al. Evaluation of a community-based exercise program for breast cancer patients undergoing treatment. Cancer Nurs 2015;38:417–25. 10.1097/NCC.0000000000000217 [DOI] [PubMed] [Google Scholar]
- 17. Cheifetz O, Park Dorsay J, Hladysh G, et al. CanWell: meeting the psychosocial and exercise needs of cancer survivors by translating evidence into practice. Psychooncology 2014;23:204–15. 10.1002/pon.3389 [DOI] [PubMed] [Google Scholar]
- 18. Santa Mina D, Au D, Brunet J, et al. Effects of the community-based Wellspring cancer exercise program on functional and psychosocial outcomes in cancer survivors. Curr Oncol 2017;24:284–94. 10.3747/co.24.3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas BK, Kimmel G, Hermanns M, et al. Community-Based FitSTEPS for life exercise program for persons with cancer: 5-year evaluation. J Oncol Pract 2012;8:320–4. 10.1200/JOP.2012.000555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heston A-H, Schwartz AL, Justice-Gardiner H, et al. Addressing physical activity needs of survivors by developing a community-based exercise program: LIVESTRONG® at the YMCA. Clin J Oncol Nurs 2015;19:213–7. 10.1188/15.CJON.213-217 [DOI] [PubMed] [Google Scholar]
- 21. Hardcastle SJ, Cohen PA. Effective physical activity promotion to survivors of cancer is likely to be home based and to require oncologist participation. J Clin Oncol 2017;35:3635–7. 10.1200/JCO.2017.74.6032 [DOI] [PubMed] [Google Scholar]
- 22. Irwin ML, Cartmel B, Harrigan M, et al. Effect of the LIVESTRONG at the YMCA exercise program on physical activity, fitness, quality of life, and fatigue in cancer survivors. Cancer 2017;123:1249–58. 10.1002/cncr.30456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Translating exercise oncology research into practice: effectiveness of a community-based exercise program for cancer patients and survivors. MASCC/ISOO annual meeting supportive care in cancer: Copenhagen supportive care in cancer, 2015. [Google Scholar]
- 24. Santa Mina D, Au D, Auger LE, et al. Development, implementation, and effects of a cancer center's exercise‐oncology program. Cancer 2019;3 10.1002/cncr.32297 [DOI] [PubMed] [Google Scholar]
- 25. Cheifetz O, Dorsay JP, MacDermid JC. Exercise facilitators and barriers following participation in a community-based exercise and education program for cancer survivors. J Exerc Rehabil 2015;11:20–9. 10.12965/jer.150183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leach HJ, Danyluk JM, Nishimura KC, et al. Benefits of 24 versus 12 weeks of exercise and wellness programming for women undergoing treatment for breast cancer. Support Care Cancer 2016;24:4597–606. 10.1007/s00520-016-3302-3 [DOI] [PubMed] [Google Scholar]
- 27. White SM, McAuley E, Estabrooks PA, et al. Translating physical activity interventions for breast cancer survivors into practice: an evaluation of randomized controlled trials. Ann Behav Med 2009;37:10–19. 10.1007/s12160-009-9084-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abu-Omar K, Rütten A, Burlacu I, et al. The cost-effectiveness of physical activity interventions: a systematic review of reviews. Prev Med Rep 2017;8:72–8. 10.1016/j.pmedr.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Canadian Society for exercise physiology CSEP certified exercise physiologist Ottawa, Ontario: Canadian Society for exercise physiology, 2019. Available: https://www.csep.ca/view.asp?ccid=534
- 31. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and Met values. Med Sci Sports Exerc 2011;43:1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 32. Marks LE, Borg G, Ljunggren G. Individual differences in perceived exertion assessed by two new methods. Percept Psychophys 1983;34:280–8. 10.3758/BF03202957 [DOI] [PubMed] [Google Scholar]
- 33. Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test*. Int J Sports Med 1982;03:153–8. 10.1055/s-2008-1026080 [DOI] [PubMed] [Google Scholar]
- 34. Amireault S, Godin G. The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills 2015;120:604–22. 10.2466/03.27.PMS.120v19x7 [DOI] [PubMed] [Google Scholar]
- 35. Amireault S, Godin G, Lacombe J, et al. The use of the Godin-Shephard leisure-time physical activity questionnaire in oncology research: a systematic review. BMC Med Res Methodol 2015;15:60 10.1186/s12874-015-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amireault S, Godin G, Lacombe J, et al. Validation of the Godin-Shephard leisure-time physical activity questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J Cancer Surviv 2015;9:532–40. 10.1007/s11764-015-0430-6 [DOI] [PubMed] [Google Scholar]
- 37. Barrios P, Martin-Biggers J, Quick V, et al. Reliability and criterion validity of self-measured waist, hip, and neck circumferences. BMC Med Res Methodol 2016;16:49 10.1186/s12874-016-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med 2013;34:631–6. 10.1055/s-0032-1323746 [DOI] [PubMed] [Google Scholar]
- 39. Bellace JV, Healy D, Besser MP, et al. Validity of the Dexter evaluation system's Jamar dynamometer attachment for assessment of hand grip strength in a normal population. J Hand Ther 2000;13:46–51. 10.1016/S0894-1130(00)80052-6 [DOI] [PubMed] [Google Scholar]
- 40. Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Orthop Sports Phys Ther 1992;16:215–9. 10.2519/jospt.1992.16.5.215 [DOI] [PubMed] [Google Scholar]
- 41. Reuter SE, Massy-Westropp N, Evans AM. Reliability and validity of indices of hand-grip strength and endurance. Aust Occup Ther J 2011;58:82–7. 10.1111/j.1440-1630.2010.00888.x [DOI] [PubMed] [Google Scholar]
- 42. McAllister LS, Palombaro KM. Modified 30-second Sit-to-Stand test: reliability and validity in older adults unable to complete traditional Sit-to-Stand testing. J Geriatr Phys Ther 2019. [DOI] [PubMed] [Google Scholar]
- 43. Kolber MJ, Hanney WJ. The reliability and concurrent validity of shoulder mobility measurements using a digital inclinometer and goniometer: a technical report. Int J Sports Phys Ther 2012;7:306–13. [PMC free article] [PubMed] [Google Scholar]
- 44. Franchignoni F, Tesio L, Martino MT, et al. Reliability of four simple, quantitative tests of balance and mobility in healthy elderly females. Aging Clin Exp Res 1998;10:26–31. 10.1007/BF03339630 [DOI] [PubMed] [Google Scholar]
- 45. Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton symptom assessment system. Curr Oncol 2009;16:55 10.3747/co.v16i1.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570–9. 10.1200/JCO.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 47. Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manage 1997;13:63–74. 10.1016/S0885-3924(96)00274-6 [DOI] [PubMed] [Google Scholar]
- 48. Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res 1994;3:7–12. 10.1007/BF00647843 [DOI] [PubMed] [Google Scholar]
- 49. Pickard AS, Wilke CT, Lin H-W, et al. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics 2007;25:365–84. 10.2165/00019053-200725050-00002 [DOI] [PubMed] [Google Scholar]
- 50. Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health 2007;28:413–33. 10.1146/annurev.publhealth.28.021406.144145 [DOI] [PubMed] [Google Scholar]
- 51. Neil SE, Gotay CC, Campbell KL. Physical activity levels of cancer survivors in Canada: findings from the Canadian community health survey. J Cancer Surviv 2014;8:143–9. 10.1007/s11764-013-0322-6 [DOI] [PubMed] [Google Scholar]
- 52. Bounajm F, Dinh T, Theiault L, et al. The economic impact of reducing physical inactivity and sedentary behaviour. Ottawa: The Conference Board of Canada, 2014: 1–37. [Google Scholar]
- 53. Bernet AC, Willens DE, Bauer MS. Effectiveness-implementation hybrid designs: implications for quality improvement science. Implementation Science 2013;8(Suppl 1):S2–2. 10.1186/1748-5908-8-S1-S2 [DOI] [Google Scholar]
- 54. Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a systematic review. Curr Oncol 2017;24:290–315. 10.3747/co.24.3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rogers LQ, Malone J, Rao K, et al. Exercise preferences among patients with head and neck cancer: prevalence and associations with quality of life, symptom severity, depression, and rural residence. Head Neck 2009;31:994–1005. 10.1002/hed.21053 [DOI] [PubMed] [Google Scholar]
- 56. Rogers LQ, Markwell SJ, Verhulst S, et al. Rural breast cancer survivors: exercise preferences and their determinants. Psychooncology 2009;18:412–21. 10.1002/pon.1497 [DOI] [PubMed] [Google Scholar]
- 57. Rogers LQ, Courneya KS, Verhulst S, et al. Factors associated with exercise counseling and program preferences among breast cancer survivors. J Phys Act Health 2008;5:688–705. 10.1123/jpah.5.5.688 [DOI] [PubMed] [Google Scholar]
- 58. Blaney JM, Lowe-Strong A, Rankin-Watt J, et al. Cancer survivors' exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psychooncology 2013;22:186–94. 10.1002/pon.2072 [DOI] [PubMed] [Google Scholar]
- 59. Buffart LM, Ros WJG, Chinapaw MJM, et al. Mediators of physical exercise for improvement in cancer survivors' quality of life. Psychooncology 2014;23:330–8. 10.1002/pon.3428 [DOI] [PubMed] [Google Scholar]
- 60. Tomasone J, Zwaal C, Kim GM, et al. Moving guidelines into action: a report from cancer care Ontario’s event let’s get moving: exercise and rehabilitation for cancer patients. Curr Oncol 2017;24:65–74. 10.3747/co.24.3422 [DOI] [Google Scholar]
- 61. Smith-Turchyn J, Richardson J, Tozer R, et al. Physical activity and breast cancer: a qualitative study on the barriers to and facilitators of exercise promotion from the perspective of health care professionals. Physiother Can 2016;68:383–90. 10.3138/ptc.2015-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fortier MS, Hogg W, O'Sullivan TL, et al. Impact of integrating a physical activity counsellor into the primary health care team: physical activity and health outcomes of the physical activity counselling randomized controlled trial. Appl Physiol Nutr Metab 2011;36:503–14. 10.1139/h11-040 [DOI] [PubMed] [Google Scholar]
- 63. Mina DS, Sabiston CM, Au D, et al. Connecting people with cancer to physical activity and exercise programs: a pathway to create accessibility and engagement. Curr Oncol 2018;25:149–62. 10.3747/co.25.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Government of Canada Canadian guidelines for body weight classification in adults, 2011. Available: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/healthy-weights/canadian-guidelines-body-weight-classification-adults/questions-answers-public.html#a4
- 65. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 2017;23:377–81. 10.1111/jep.12629 [DOI] [PubMed] [Google Scholar]
- 66. Record Owner NLM What is the minimum clinically important difference in grip strength?. [DOI] [PMC free article] [PubMed]
- 67. Statistics Canada Musculoskeletal fitness in Canada 2007 to 2009: government of Canada, 2015. Available: https://www150.statcan.gc.ca/n1/pub/82-625-x/2010001/article/11089-eng.htm [Accessed 5 Jul 2019].
- 68. Muir SW, Corea CL, Beaupre L. Evaluating change in clinical status: reliability and measures of agreement for the assessment of glenohumeral range of motion. N Am J Sports Phys Ther 2010;5:98–110. [PMC free article] [PubMed] [Google Scholar]
- 69. Lemmink KAPM, Kemper HCG, de Greef MHG, et al. The validity of the sit-and-reach test and the modified sit-and-reach test in middle-aged to older men and women. Res Q Exerc Sport 2003;74:331–6. 10.1080/02701367.2003.10609099 [DOI] [PubMed] [Google Scholar]
- 70. Goldberg A, Casby A, Wasielewski M. Minimum detectable change for single-leg-stance-time in older adults. Gait Posture 2011;33:737–9. 10.1016/j.gaitpost.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 71. Wood-Dauphinee S. The Canadian SF-36 health survey: normative data add to its value. CMAJ 2000;163:283–4. [PMC free article] [PubMed] [Google Scholar]
- 72. Pickard AS, De Leon MC, Kohlmann T, et al. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Med Care 2007;45:259–63. 10.1097/01.mlr.0000254515.63841.81 [DOI] [PubMed] [Google Scholar]
- 73. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rivera-Torres S, Fahey TD, Rivera MA. Adherence to exercise programs in older adults: informative report. Gerontol Geriatr Med 2019;5 10.1177/2333721418823604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res 2014;14:221–33. 10.1586/14737167.2014.894462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.