Abstract
Objective:
To describe the incidence of and risk factors for overweight and obesity following antiretroviral therapy (ART) initiation.
Methods:
We used Cox proportional hazards models to investigate risk factors for incident overweight and obesity in 79 074 individuals aged 15 years or older who initiated ART in Dar es Salaam, Tanzania.
Results:
Twenty-five percent of the patients became overweight and 10% became obese. The incidence rate of obesity was 3.2 per 100 person-years (95% confidence interval [CI]: 3.1-3.3) in patients who were of normal weight before starting ART and 22.6 per 100 person-years (95% CI: 21.9-23.3) in those who were overweight. Lower CD4 count was associated with a higher risk of overweight and obesity (P value for trend < .0001).
Conclusion:
There is a high burden of overweight and obesity after starting ART, leading to proportions of these 2 conditions that are similar to those in the general population.
Keywords: antiretroviral therapy, overweight, obesity, incidence, risk factors
Introduction
The scale-up of HIV treatment and care services in sub-Saharan Africa (SSA) has resulted in a large increase in the number of patients on antiretroviral therapy (ART).1 In Tanzania, over 470 000 (31%) of the 1.5 million HIV-positive adults had been started on ART by 2014.2 Wider access to ART in similar settings has led to fewer deaths due to HIV/AIDS and has also been shown to result in increased life expectancy at birth.3 Additionally, many patients now start ART before progressing to advanced stages of HIV due to changes in treatment guidelines.4
Increased survival predisposes HIV-positive individuals to conditions associated with aging. Several studies have described increasing proportions of overweight and obesity in people living with HIV/AIDS in SSA.5-7 In a large cross-sectional study in Dar es Salaam, Tanzania, we found that 18% of HIV-positive individuals were overweight (25 ≤ body mass index [BMI] < 30) and 7% were obese (BMI ≥ 30).5 Population-based studies comparing overweight and obesity prevalence in HIV-positive individuals to HIV-negative individuals are limited in SSA. Demographic surveillance data from rural South Africa showed a similar prevalence of overweight in HIV-positive and HIV-negative individuals.7 With more HIV-positive patients becoming overweight and obese, there is a need for improvements in monitoring and managing excessive weight gain in patients on ART. This requires a better understanding of risk factors for overweight and obesity in this population. There is also limited data on progression to overweight and obesity in patients started on ART in SSA. More studies have instead evaluated short-term trends in weight changes, with a specific focus on monitoring clinical outcomes of ART.7-9 A few studies have evaluated long-term trends, but none specifically looked at predictors of incident overweight and obesity.8-11 Data outside SSA are also limited. Tate et al followed up ART-naive patients receiving care in the United States and found that 20% became overweight or obese within 2 years of being on ART.12 Another study in a Swiss HIV cohort found that weight gain after ART initiation was biphasic, with the first and more rapid phase occurring within a year of being on treatment.13 This is followed by a slower phase of weight gain over the next 3 years. Although these studies explain some trends in weight gain, none explicitly describes the incidence of obesity and overweight and their risk factors. The observed weight trends in these studies may also not be generalizable to SSA because of environmental and socioeconomic differences that influence weight gain.14 The goal of this study was to describe the incidence of overweight and obesity after initiation of ART in an urban setting in SSA and to explore risk factors for the 2 conditions.
Methods
Study Population
We used data that are prospectively collected at HIV Care and Treatment Clinics in Dar es Salaam, Tanzania. These clinics are funded by the US President’s Emergency Plan for AIDS Relief through one of its recipient agencies, Management and Development for Health (MDH). Treatment protocols follow national guidelines as set by the Tanzanian National AIDS Control Program.15 All patients aged 15 years or older (N = 113 468) who had been started on ART between November 2004 and September 2014 were considered for the analysis. This number includes patients who had started ART at other clinics and later transferred to MDH-supported clinics. We excluded the following categories of patients: pregnant at baseline (n = 13 579), missing baseline BMI (n = 16 170), and those who were obese at baseline (n = 4645). The final analysis sample included 79 074 patients.
Data Collection
Patients were routinely followed up at the clinics after enrollment into care and after being started on ART. Height and weight were measured by a trained nurse at the time of enrollment according to standard techniques.16 Weight was then updated at subsequent clinic visits. Where necessary, demographic, clinical, and laboratory data were updated on case report forms. Laboratory tests including patients’ CD4 counts and hemoglobin levels were done at the time of ART initiation and updated every 6 months. All data were entered into a secure computerized database that underwent regular data quality checks.
The outcomes of interest were incident overweight and obesity after ART initiation. Body mass index was calculated as the patient’s weight in kilograms divided by the square of their height in meters. We categorized BMIs <18.5 kg/m2 as underweight, 18.5 ≤ BMI < 25.0 kg/m2 as normal weight, 25 ≤ BMI < 30 kg/m2 as overweight, and BMI ≥30 kg/m2 as obese. For the analysis on incident overweight, we included patients who were either underweight or normal weight at ART initiation. All patients with a BMI <30 kg/m2 were assessed for incident obesity. Patients were censored at the time of event, death, and pregnancy and at the end of follow-up. Women who became pregnant were not reentered into the analysis after the end of their pregnancies.
We assessed several demographic and clinical indicators as potential risk factors for overweight and obesity. These included sex, marital status, parity, year of ART initiation, and age. Clinical data included the patient’s baseline BMI category, time-varying CD4 count, hemoglobin level, and previous opportunistic infections. We also controlled for previous ART exposure, whether a patient was on a first- or second-line regimen of ART, and their adherence to ART. We defined nonadherence as ≥5% noncompliance with scheduled ART pickup visits, a measure that has been shown to be predictive of virologic failure.17 Additionally, we included the type of nucleoside reverse transcriptase inhibitor (NRTI) in the ART regimen and the type of non-nucleoside reverse transcriptase inhibitor.
Statistical Analysis
We used the Andersen-Gill formulation of Cox proportional hazards models18 to examine associations between time-varying risk factors with incident overweight and obesity. We used restricted cubic spline models to assess the possibility of nonlinear relationships between the outcomes of interest with continuous covariates. Missing indicators were created for missing values of potential risk factors. All covariates with P values ≤0.20 in univariate analyses were included in the multivariate model. All analyses were performed using SAS software version 9.3 (SAS Institute, Inc).
Ethical Consideration
The study was approved by the institutional review boards of the Tanzanian National Institute of Medical Research, at Muhimbili University of Health and Allied Sciences, and of the Harvard T.H. Chan School of Public Health. No patient consent was required for this study because data that were used are routinely collected during health care delivery. Anonymized data were used in all analyses.
Results
Baseline data of 79 074 patients who were included in this analysis are shown in Table 1. The median age was 37 years (interquartile range [IQR], 31-44 years); 65% were female and 43% were married. At the time of ART initiation, 22 203 (28%) were underweight, 45 231 (57%) normal weight, and 11 640 (15%) overweight. The median CD4 count at the time of ART initiation was 149 cells/μL (IQR: 64-256 cells/mm3) and 81% of the patients were ART naive. Twenty-three percent had a history of tuberculosis (TB) infection, and 47% were started on zidovudine (ZDV)-containing regimen and 61% on efavirenz (EFV)-containing regimen.
Table 1.
Patient Characteristics at Initiation of Antiretroviral Therapy in Dar es Salaam, Tanzania.a
| Variable | Underweight (n = 22 203) | Normal Weight (n = 45 231) | Overweight (n = 11 640) | Total Sample (N = 79 074)b |
|---|---|---|---|---|
| Age, median (IQR), years | 36.4 (30.4-43.1) | 36.9 (31.2-43.8) | 38.0 (32.4-44.9) | 36.9 (31.1-43.8) |
| Age category, years | ||||
| 15 to <30 | 5065 (22) | 8948 (20) | 1852 (16) | 15 865 (20) |
| 30 to <40 | 9206 (42) | 19 278 (42) | 4891 (42) | 33 375 (42) |
| 40 to <50 | 5416 (24) | 11 687 (26) | 3357 (29) | 20 460 (26) |
| 50+ | 2493 (11) | 5271 (12) | 1525 (13) | 9289 (12) |
| Sex | ||||
| Male | 8662 (39) | 16 409 (36) | 2620 (22) | 27 691 (35) |
| Female | 13 541 (61) | 28 822 (64) | 9020 (78) | 51 383 (65) |
| Married | ||||
| No | 13 410 (60) | 25 442 (56) | 6336 (54) | 45 188 (57) |
| Yes | 8793 (40) | 19 789 (44) | 5304 (46) | 33 886 (43) |
| District | ||||
| Ilala | 8429 (38) | 17 252 (38) | 4987 (42) | 30 668 (39) |
| Kinondoni | 6759 (31) | 15 058 (33) | 3893 (34) | 25 710 (33) |
| Temeke | 6990 (32) | 12 854 (29) | 2742 (24) | 22 586 (29) |
| Facility level | ||||
| Hospital | 15 853 (73) | 31 444 (72) | 8071 (74) | 55 368 (73) |
| Health center | 2434 (11) | 4279 (10) | 973 (8) | 7686 (10) |
| Dispensary | 3417 (16) | 7706 (18) | 1823 (17) | 12 946 (17) |
| Year started on ART | ||||
| 2004-2005 | 543 (3) | 1148 (3) | 351 (3) | 2042 (3) |
| 2006 | 1567 (7) | 3382 (8) | 893 (8) | 5842 (7) |
| 2007 | 2576 (12) | 4369 (10) | 943 (8) | 7888 (10) |
| 2008 | 3103 (13) | 5376 (11) | 1152 (10) | 9631 (12) |
| 2009 | 2936 (13) | 5378 (12) | 1150 (10) | 9464 (12) |
| 2010 | 2735 (12) | 5344 (12) | 1483 (13) | 9562 (12) |
| 2011 | 2240 (10) | 4353 (10) | 1175 (10) | 7768 (10) |
| 2012 | 2573 (11) | 6097 (14) | 1650 (14) | 10 320 (13) |
| 2013 | 2229 (10) | 5404 (12) | 1484 (13) | 9117 (12) |
| 2014 | 1701 (8) | 4380 (10) | 1359 (12) | 7440 (9) |
| BMI, median (IQR), kg/m2 | 16.9 (15.7-17.7) | 21.1 (19.8-22.7) | 26.8 (25.8-28.1) | 20.5 (18.2-23.3) |
| Hemoglobin category, g/dL | ||||
| <8.5 | 3570 (30) | 3567 (16) | 462 (10) | 7599 (20) |
| 8.5-10.9 | 5230 (43) | 9090 (42) | 1719 (36) | 16 039 (42) |
| ≥11 | 3277 (27) | 9055 (41) | 2602 (54) | 14 934 (38) |
| CD4 count, median (IQR), cells/mm3 | 110 (40-216) | 158 (73-261) | 186 (104-297) | 149 (64-256) |
| CD4 count category, cells/mm3 | ||||
| <50 | 5266 (29) | 6435 (18) | 1112 (12) | 12 807 (20) |
| 50-99 | 3221 (18) | 5341 (14) | 1015 (11) | 9577 (15) |
| 100-199 | 4533 (25) | 10 902 (30) | 2795 (31) | 18 230 (29) |
| ≥200 | 5086 (28) | 1 3363 (37) | 4026 (44) | 22 475 (36) |
| History of tuberculosis | ||||
| No | 8754 (71) | 19 067 (78) | 5061 (85) | 32 882 (77) |
| Yes | 3648 (29) | 5395 (22) | 877 (15) | 9920 (23) |
| Previous ART use | ||||
| No | 19 159 (86) | 36 277 (80) | 9002 (77) | 64 438 (81) |
| For HAART | 3024 (13) | 8873 (20) | 2599 (22) | 14 496 (18) |
| For PMTCT | 20 (0) | 81 (0) | 39 (0) | 140 (1) |
| ARV regimen | ||||
| First line | 22 106 (99) | 45 086 (99) | 11 597 (99) | 78 789 (99) |
| Second line | 97 (1) | 145 (1) | 43 (1) | 285 (1) |
| NRTI | ||||
| Zidovudine | 9958 (45) | 21 505 (48) | 5557 (48) | 37 020 (47) |
| Stavudine | 7715 (35) | 13 855 (31) | 3262 (28) | 24 832 (31) |
| Tenofovir | 4426 (20) | 9697 (22) | 2778 (24) | 16 901 (22) |
| NNRTI | ||||
| Nevirapine | 8040 (36) | 17 655 (39) | 4894 (42) | 30 589 (39) |
| Efavirenz | 14 091 (66) | 27 442 (61) | 6700 (58) | 48 233 (61) |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; BMI, body mass index; HAART, highly active antiretroviral therapy; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PMTCT, prevention of mother-to-child transmission of HIV.
aExcept where indicated, figures are for n (%) of patients.
bTotal numbers might not add up to 79 074 due to missing data.
Incidence of Obesity and Overweight
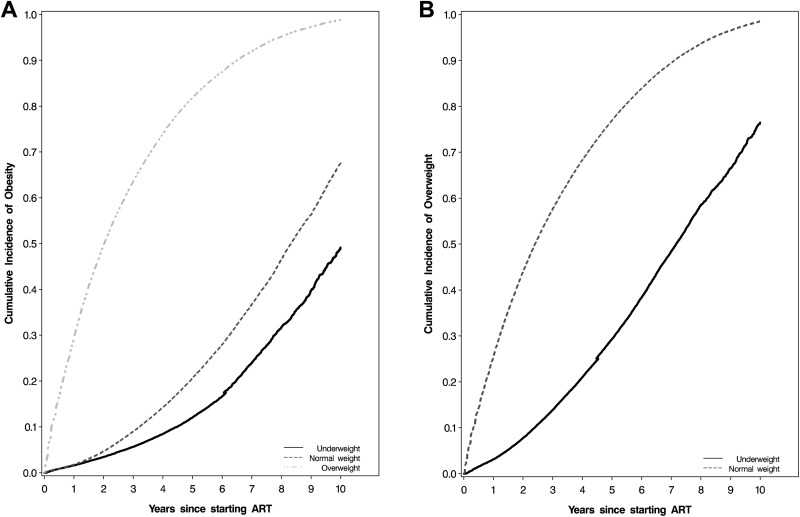
During 160 951 person-years of follow-up, 7848 (10%) patients became obese with incidence rate (IR) of 4.9 per 100 person-years (95% confidence interval [CI]: 4.8-5.0). The median follow-up time of patients assessed for incident obesity was 1.3 years (IQR: 0.3-3.3 years). Covariate-adjusted Kaplan-Meier plots for incident obesity by baseline BMI category are presented in Figure 1, panel A. Overweight patients had a higher incidence of obesity (IR: 22.6 per 100 person-years, 95% CI: 21.9-23.3) than those who were normal weight at baseline (IR: 3.2 per 100 person-years, 95% CI: 3.1-3.3) and those who were underweight (IR: 1.5 per 100 person-years, 95% CI: 1.4-1.7).
Figure 1.
Cumulative incidence of overweight (A) and obesity (B) after ART initiation. ART indicates antiretroviral therapy.
During 107 858 person-years of follow-up, 16 503 (25%) patients became overweight with an IR of 14.9 per 100 person-years (95% CI: 14.7-15.2). The median follow-up time for patients assessed for incident overweight was 0.8 years (IQR: 0.2-2.3 years). Normal weight patients had a higher incidence of overweight (IR: 20.9 per 100 person-years, 95% CI: 20.6-21.2) than those who were underweight at baseline (IR: 5.1 per 100 person-years, 95% CI: 4.8-5.3; Figure 1, panel B). Sixteen percent (n = 2612) of patients who became overweight after starting ART progressed to obese states at later date. The median follow-up time to obesity in this subgroup was 2.7 years (IQR: 1.3-5.0 years) and a majority (92%) had been normal weight at the time of ART initiation.
Risk Factors for Overweight and Obesity
Univariate and multivariate analyses of risk factors for obesity are presented in Table 2. After adjusting for potential confounders, women had double the risk of obesity than men (Relative Risk [RR]: 2.16, 95% CI: 2.04-2.30). Being married was not associated with an increased risk of obesity (RR: 0.97, 95% CI :0.92-1.01) and neither was age (P value for trend = .8). Patients from the most affluent district, Kinondoni, had a higher risk of obesity than those from Ilala, a less affluent area (RR: 1.05, 95% CI: 1.00-1.11). Those from Temeke, the least affluent district, however did not have a lower risk of obesity than patients from Ilala (RR: 0.98, 95% CI: 0.93-1.04).
Table 2.
Risk Factors for Obesity (BMI ≥30) after ART Initiation.a
| Variable | Univariate | Multivariate | Multivariate Excluding Underweight at Baselineb | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P Valuec | RR (95% CI) | P Valuec | RR (95% CI) | P Valuec | |
| Sex | <.0001 | <.0001 | <.0001 | |||
| Male | Reference | Reference | Reference | |||
| Female | 2.77 (2.62-2.93) | 2.16 (2.04-2.30) | 2.20 (2.07-2.35) | |||
| Age category | .8 | .8 | .8 | |||
| 15-30 | Reference | Reference | Reference | |||
| 30 to <40 | 1.28 (0.84-1.95) | 1.29 (0.86-1.96) | 1.31 (0.85-2.04) | |||
| 40 to <50 | 1.13 (0.66-1.93) | 1.06 (0.62-1.79) | 1.10 (0.62-1.93) | |||
| 50+ | 1.07 (0.49-2.32) | 1.09 (0.51-2.35) | 1.09 (0.49-2.40) | |||
| Married | .001 | .2 | .3 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.93 (0.89-0.97) | 0.97 (0.92-1.01) | 0.98 (0.93-1.03) | |||
| Year started ART | <.0001 | <.0001 | <.0001 | |||
| 2004-2005 | Reference | Reference | Reference | |||
| 2006 | 1.17 (1.03-1.32) | 1.10 (0.97-1.24) | 1.11 (0.98-1.26) | |||
| 2007 | 1.60 (1.42-1.80) | 1.45 (1.29-1.63) | 1.45 (1.28-1.64) | |||
| 2008 | 1.74 (1.55-1.96) | 1.36 (1.21-1.54) | 1.38 (1.22-1.57) | |||
| 2009 | 2.24 (1.99-2.53) | 1.46 (1.28-1.65) | 1.45 (1.27-1.65) | |||
| 2010 | 2.65 (2.35-3.00) | 1.26 (1.10-1.43) | 1.25 (1.09-1.43) | |||
| 2011 | 3.55 (4.60-5.94) | 1.40 (1.22-1.60) | 1.43 (1.24-1.65) | |||
| 2012 | 5.23 (4.60-5.94) | 1.67 (1.46-1.91) | 1.72 (1.50-1.98) | |||
| 2013 | 11.72 (10.23-13.41) | 2.36 (2.04-2.74) | 2.53 (2.17-2.94) | |||
| 2014 | 30.42 (25.24-36.66) | 2.91 (2.38-3.56) | 3.05 (2.48-3.75) | |||
| District | <.0001 | .0007 | .04 | |||
| Ilala | Reference | Reference | Reference | |||
| Kinondoni | 1.08 (1.02-1.13) | 1.05 (1.00-1.11) | 1.03 (0.97-1.09) | |||
| Temeke | 0.89 (0.84-0.94) | 0.98 (0.93-1.04) | 0.97 (0.91-1.03) | |||
| Facility level | <.0001 | <.0001 | <.0001 | |||
| Hospital | Reference | Reference | Reference | |||
| Health center | 0.78 (0.71-0.85) | 0.83 (0.75-0.91) | 0.86 (0.78-0.94) | |||
| Dispensary | 0.73 (0.68-0.77) | 0.81 (0.76-0.87) | 0.83 (0.77-0.89) | |||
| Baseline BMI (kg/m2) | <.0001 | <.0001 | <.0001 | |||
| <18.5 | Reference | Reference | Excluded | |||
| 18.5-24.9 | 2.15 (1.98-2.34) | 2.17 (1.99-2.36) | Reference | |||
| 25.0-29.9 | 17.80 (16.41-19.32) | 14.72 (13.54-16.00) | 6.75 (6.44-7.08) | |||
| CD4 count, cells/mm3 | <.0001 | <.0001 | <.0001 | |||
| <50 | 1.42 (1.30-1.55) | 1.53 (1.40-1.68) | 1.56 (1.41-1.71) | |||
| 50-99 | 1.43 (1.31-1.57) | 1.47 (1.34-1.61) | 1.51 (1.37-1.66) | |||
| 100-199 | 1.31 (1.24-1.39) | 1.25 (1.18-1.33) | 1.28 (1.21-1.36) | |||
| ≥200 | Reference | Reference | Reference | |||
| Previous ART use | .4 | <.0001 | .0003 | |||
| No | Reference | Reference | Reference | |||
| For HAART | 0.84 (0.80-0.88) | 0.92 (0.87-0.97) | 0.94 (0.88-0.99) | |||
| For PMTCT | 0.66 (0.31-1.38) | 0.36 (0.17-0.75) | 0.32 (0.14-0.72) | |||
| Adherence | .5 | .03 | <.0001 | |||
| High | Reference | Reference | Reference | |||
| Low | 1.01 (0.97-1.06) | 0.95 (0.91-1.00) | 0.94 (0.90-0.99) | |||
| History of tuberculosis | <.0001 | 0.4 | 0.8 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.68 (0.62-0.74) | 0.96 (0.87-1.05) | 0.99 (0.89-1.09) | |||
| NRTI | <.0001 | <.0001 | <.0001 | |||
| Zidovudine | Reference | Reference | Reference | |||
| Stavudine | 0.86 (0.81-0.91) | 1.26 (1.18-1.34) | 1.27 (1.19-1.37) | |||
| Tenofovir | 0.70 (0.59-0.83) | 0.80 (0.74-0.86) | 0.80 (0.74-0.87) | |||
| NNRTI | .4 | <.0001 | <.0001 | |||
| Nevirapine | Reference | Reference | Reference | |||
| Efavirenz | 1.02 (0.96-1.09) | 1.16 (1.01-1.24) | 1.17 (1.10-1.25) | |||
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HAART, highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PMTCT, prevention of mother-to-child transmission of HIV; RR, relative risk.
aN = 79 074.
bn = 56 871.
cWald tests for trend for median scores of categorized continuous variables; Wald test for categorical and binary variables.
Compared to patients who were underweight at baseline, normal weight patients had double the risk of becoming obese (RR: 2.17, 95% CI: 1.99-2.36), while those who were overweight had a 14.72-fold increase in risk (95% CI: 13.54-16.00). More recent year of ART initiation was associated with a higher risk of obesity (P < .0001). Patients started on ART in 2014 had almost 3 times the risk of becoming obese as compared to those who started ART between 2004 and 2005 (RR: 2.91, 95% CI: 2.38-3.56). Those with previous exposure to ART for highly active antiretroviral therapy (HAART) had an 8% lower risk of becoming obese than those who were ART naive (RR: 0.92, 95% CI: 0.87-0.97). Using the same reference category, those who had used ART for prevention of mother-to-child transmission of HIV (PMTCT) had a less than half the risk for obesity (RR: 0.36, 95% CI: 0.17-0.75).
Lower CD4 count was associated with a higher risk of obesity (P value for trend <.0001). Patients with CD4 counts of less than 50 cells/mm3 had a 1.53-fold increased risk of becoming obese as compared to those with counts of 200 cells/mm3 or higher (95% CI: 1.40-1.68). Using the same reference category, those with a CD4 count between 50 and <100 cells/mm3 had a 1.47-fold increased risk (95% CI: 1.34-1.61) and those with a CD4 count between 100 and <200 cells/mm3 had a 1.25-fold increased risk (95% CI: 1.18-1.33). We found a similar trend after excluding patients who were underweight at baseline from the analysis.
Previous opportunistic infections were not associated with lower risk of obesity. In the univariate analysis, previous TB infection was associated with a lower risk of obesity (RR: 0.68, 95% CI: 0.62-0.74), but this association was not statistically significant after controlling for other factors (RR: 0.96, 95% CI: 0.87-1.05). Patients who were nonadherent to ART had a 5% lower risk of becoming obese (RR: 0.95, 95% CI: 0.91-1.00). Being on a tenofovir (TDF)-based regimen was associated with a 20% lower risk of becoming obese as compared to being on ZDV-based regimen (RR: 0.80, 95% CI: 0.74-0.86). Additionally, being on an EFV-based ART regimen was associated with a 1.16-fold increased risk of obesity as compared to being on a nevirapine (NVP)-based regimen (95% CI: 1.01-1.24).
Risk factors for becoming overweight are shown in Table 3. Normal weight patients had a 3.96-fold higher risk of becoming overweight when compared to those who were underweight at baseline (95% CI: 3.78-4.15). Risk factors for overweight were mostly similar to those of obesity, but with the exception of age and history of infection with TB. Contrary to the observed nonsignificant association between age and risk of obesity, older patients were more likely to become overweight (P value for trend <.0001). Similarly, patients with previous TB infection had a 6% lower risk of becoming overweight (RR: 0.94, 95% CI: 0.89-1.00), despite a null association for risk of obesity.
Table 3.
Risk Factors for Overweight (25 ≤ BMI < 30) after ART Initiation.a
| Variable | Univariate | Multivariateb | Multivariate Excluding Underweight at Baselineb | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P Valuec | RR (95% CI) | P Valuec | RR (95% CI) | P Valuec | |
| Sex | <.0001 | <.0001 | <.0001 | |||
| Male | Reference | Reference | Reference | |||
| Female | 2.00 (1.93-2.07) | 1.91 (1.84-1.98) | 1.91 (1.84-1.98) | |||
| Age category, years | <.0001 | <.0001 | <.0001 | |||
| 15-30 | Reference | Reference | Reference | |||
| 30 to <40 | 1.18 (0.91-1.52) | 1.20 (0.93-1.55) | 1.16 (0.88-1.53) | |||
| 40 to <50 | 1.91 (1.32-2.77) | 2.03 (1.40-2.93) | 2.22 (1.48-3.35) | |||
| 50+ | 3.31 (1.85-5.93) | 3.76 (2.11-6.71) | 4.27 (2.30-7.95) | |||
| Married | .1 | .7 | .4 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.97 (0.94-1.00) | 1.01 (0.97-1.04) | 1.01 (0.98-1.05) | |||
| Year started ART | <.0001 | <.0001 | <.0001 | |||
| 2004-2005 | Reference | Reference | Reference | |||
| 2006 | 1.16 (1.06-1.27) | 1.01 (0.92-1.10) | 1.03 (0.94-1.14) | |||
| 2007 | 1.57 (1.44-1.72) | 1.31 (1.20-1.43) | 1.31 (1.19-1.44) | |||
| 2008 | 1.79 (1.65-1.95) | 1.27 (1.17-1.39) | 1.29 (1.17-1.41) | |||
| 2009 | 2.06 (1.89-2.25) | 1.39 (1.27-1.53) | 1.41 (1.27-1.55) | |||
| 2010 | 2.27 (2.08-2.48) | 1.31 (1.20-1.44) | 1.32 (1.19-1.46) | |||
| 2011 | 2.77 (2.52-3.04) | 1.44 (1.31-1.59) | 1.48 (1.34-1.65) | |||
| 2012 | 3.93 (3.59-4.30) | 1.86 (1.69-2.05) | 1.95 (1.75-2.16) | |||
| 2013 | 7.99 (7.26-8.78) | 2.70 (2.43-3.00) | 2.88 (2.58-3.23) | |||
| 2014 | 19.77 (17.47-22.38) | 3.71 (3.23-4.26) | 4.12 (3.56-4.76) | |||
| District | <.0001 | <.0001 | .0003 | |||
| Ilala | Reference | Reference | Reference | |||
| Kinondoni | 1.11 (1.07-1.15) | 1.03 (0.99-1.07) | 1.02 (0.98-1.06) | |||
| Temeke | 0.98 (0.95-1.02) | 0.94 (0.91-0.98) | 0.92 (0.88-0.96) | |||
| Facility level | <.0001 | <.0001 | <.0001 | |||
| Hospital | Reference | Reference | Reference | |||
| Health center | 0.82 (0.77-0.87) | 0.82 (0.77-0.87) | 0.84 (0.79-0.90) | |||
| Dispensary | 0.67 (0.64-0.70) | 0.71 (0.68-0.74) | 0.72 (0.69-0.76) | |||
| Baseline BMI (kg/m2) | <.0001 | <.0001 | – | |||
| <18.5 | Reference | Reference | Excluded | |||
| 18.5-24.9 | 4.54 (4.34-4.75) | 3.96 (3.78-4.15) | ||||
| CD4 count (cells/mm3) | <.0001 | <.0001 | <.0001 | |||
| <50 | 1.67 (1.58-1.76) | 1.65 (1.56-1.74) | 1.81 (1.70-1.92) | |||
| 50-99 | 1.60 (1.51-1.69) | 1.49 (1.40-1.57) | 1.60 (1.51-1.70) | |||
| 100-199 | 1.48 (1.42-1.53) | 1.34 (1.29-1.40) | 1.42 (1.36-1.48) | |||
| ≥200 | Reference | Reference | Reference | |||
| Previous ART use | <.0001 | .001 | .003 | |||
| No | Reference | Reference | Reference | |||
| For HAART | 0.84 (0.81-0.87) | 0.96 (0.93-1.00) | 0.96 (0.93-1.00) | |||
| For PMTCT | 0.66 (0.41-1.08) | 0.49 (0.30-0.80) | 0.50 (0.30-0.83) | |||
| Adherence | .1 | <.0001 | <.0001 | |||
| High | Reference | Reference | Reference | |||
| Low | 0.98 (0.95-1.01) | 0.91 (0.88-0.94) | 0.91 (0.88-0.94) | |||
| History of tuberculosis | <.0001 | .04 | <.0001 | |||
| No | Reference | Reference | Reference | |||
| Yes | 0.78 (0.74-0.83) | 0.94 (0.89-1.00) | 0.92 (0.87-0.98) | |||
| NRTI | <.0001 | <.0001 | <.0001 | |||
| Zidovudine | Reference | Reference | Reference | |||
| Stavudine | 1.23 (1.19-1.27) | 1.39 (1.33-1.46) | 1.36 (1.30-1.44) | |||
| Tenofovir | 1.01 (0.97-1.06) | 0.83 (0.79-0.87) | 0.82 (0.78-0.87) | |||
| NNRTI | <.0001 | <.0001 | <.0001 | |||
| Nevirapine | Reference | Reference | Reference | |||
| Efavirenz | 0.93 (0.90-0.96) | 1.10 (1.06-1.15) | 1.09 (1.05-1.14) | |||
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HAART, highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PMTCT, prevention of mother-to-child transmission of HIV; RR, relative risk.
aN = 67 434.
bn = 45 231.
cWald tests for trend for median scores of categorized continuous variables; Wald test for categorical and binary variables.
Discussion
This study found that a large proportion of HIV-positive individuals progressed to overweight and obese states after being started on ART (25% and 10%, respectively). To our knowledge, this is the first study to prospectively assess these 2 outcomes in this population. Taking into account that 14% of patients were overweight and 6% obese at ART initiation, the observed proportions of overweight and obese patients during follow-up are similar to those that have been reported in Tanzania’s general population.19
The proportion of patients that progress to overweight and obesity in this urban setting is likely to be higher than that of rural areas that have been shown to have lower a prevalence of the 2 conditions.20,21 Our findings also show that the risk of overweight and obesity in this population is increasing with each subsequent year, an association that may be explained by secular trends of the 2 conditions in the general population.19 This trend is more likely to be a result of return to the baseline risk of overweight and obesity in the general population, as opposed to higher risk of these conditions in patients on ART when compared to HIV-negative individuals.
The increasing rates of nonideal BMI are more concerning in HIV-positive individuals because of the challenge of managing HIV and comorbidities associated with high BMI.22 Additionally, HIV-positive individuals are at higher risk than the general population for conditions linked to high BMI, such as cardiovascular diseases and chronic kidney disease.23,24 Despite the increased risk, there are opportunities of being diagnosed and receiving care for these conditions during patients’ HIV clinic visits. Models for delivery of this care have been described in theory but are yet to be demonstrated in most low-income settings.25
Several risk factors for overweight and obesity in the general population were also associated with excessive weight gain in HIV-positive individuals who are started on ART. There are several plausible explanations for the higher risk in women, an association that has been reported in several studies.26,27 Women in this setting seek HIV care earlier than men and are started on ART before progressing to advanced stages of HIV.28 However, given that we control for baseline BMI and time-varying CD4 counts, which are indicators of immune status, the higher risk is likely be due to nonclinical factors. Larger female body sizes are socially more ideal in some African settings and this preference has been associated with overnutrition.26,29 It is also possible that some of these women intentionally gain more weight as has been shown in studies that found significantly higher BMI in HIV-positive women on ART than in matched HIV-negative controls.30,31 Hurley et al also found larger increases in body weight in HIV-positive women who wished to gain weight than in those who did not wish to.10 More research is needed on social influences on weight among HIV-positive individuals.
Several studies have reported higher weight gain in patients with low immunity at baseline, but reasons for this association have not been discussed.10,11,13,30 One study found that this trend is sustained throughout the 2 phases of weight recovery after ART initiation.13 One study described larger increases in lean body mass (LBM) in patients with low immunity at baseline.33 Recovery of lost LBM could in part explain the higher risk of overweight and obesity in patients with lower immunity. A large proportion of patients in our study are likely to have lost a lot of LBM including those who were normal weight and overweight at baseline. However, those with lower CD4 counts are likely to have lost more LBM leading to larger increases in weight after starting ART.
Previous studies have found conflicting associations between age and weight gain in patients on ART with some finding no association,11-13 and others reporting statistically significant higher weight gain in older patients.30,32 This difference in findings is possibly be due to heterogeneity in the association between age and weight gain.13 We found that older patients were more likely to become overweight than younger ones but did not find age association for obesity.
The null association between previous TB infection with risk of obesity is indicative of absence of long-term effects of HIV-related immunosuppression on weight gain. Our findings also suggest that adequate viral load suppression is required for weight recovery. This is supported by the lower risk of overweight and obesity in patients with poor adherence, those with previous exposure to ART for PMTCT, and those who were initiated on HAART at other sites.
Earlier recommendations for PMTCT have been linked to virologic failure resulting from use of regimen with less than 3 antiretroviral drugs, as well as from withdrawing ART at the end of the pregnancy.34-36 The lower risk of overweight and obesity in patients initiated on HAART at other treatment sites can be explained by drug resistance that develops when patients are off ART before enrolling at other HIV treatment sites.35 Alternatively, some of these patients have been on ART for a while and regained some weight before transferring to MDH-supported clinics.
The higher risk of overweight and obesity in patients on EFV has previously been described.32,37 One of these studies also showed that this trend is reversed in the second year, with patients on NVP gaining more weight than those on EFV.32 We found no studies that compared body fat changes for these 2 drugs which could explain these differences. Our findings on associations between several NRTIs with overweight and obesity are not consistent with those from previous studies. Patients on TDF and other contemporary drugs have been shown to gain more weight than those on thymidine analogues like stavudine (d4T) or ZDV.13,38,39 The lower risk of overweight and obesity in patients on TDF in this analysis could have resulted from the shorter follow-up duration of patients who were started on TDF as compared to those on ZDV or d4T. Most of patients on TDF (88%) have been on ART for less than 2 years because the other 2 NRTIs were the available first-line choice of drugs in this setting before 2012. Also, the higher risk of overweight and obesity in patients on d4T in our study is suggestive of worse lipodystrophy in patients on d4T, an association that has been found in other studies.40 We however did not have data on waist–hip ratios to ascertain worse central obesity in patients on d4T. A more conclusive comparison of the effects of NRTIs in this population would require a longer follow-up of patients on TDF and other anthropometric measures besides weight gain.
Our analysis has several strengths. First, we prospectively followed up a large cohort of HIV-positive patients for 2 outcomes that have not been studied in this or similar settings. Second, we controlled for several baseline and time-varying covariates in a setting with limited prospective data on HIV-positive patients and for a duration that covers most of the period that ART has been available in SSA. Third, our analysis included patients who were underweight at baseline, a subset of HIV-positive patients that constitutes a large proportion of patients started on ART in SSA (28% in this population). We also show that our findings are unchanged when we exclude patients who were underweight when initiating ART.
One limitation of this study was the lack of time-varying data on plasma HIV viral loads, a known predictor of weight gain in patients on ART.27,31,33 We however controlled for other predictors of treatment failure including adherence ART and time-varying CD4 count. Another limitation was the short follow-up duration of patients who were started on TDF-containing regimen and the lack of data on other anthropometric measures that limited our ability to conclusively examine the effects of NRTIs. We were also unable to include lamivudine (3TC) in the NRTI comparisons because almost all patients in the study were taking it or emtricitabine, a drug with similar properties as 3TC.
Last, we had no data on socioeconomic factors that are known to influence weight gain such as household income and diet. We however control for district of residence, which in this setting is correlated with affluence and find that patients from the wealthiest district were more likely to become overweight and obese.
In conclusion, HIV-positive patients are increasingly progressing to overweight and obesity after starting ART. This trend will become of more concern in part due to the secular increases of overweight and obesity in low-income setting and also as a result of earlier initiation of ART as per World Health Organization recommendations. Routine HIV care should include screening and interventions for overweight and obesity in patients with identified risk factors. The high proportion of HIV-positive patients with overweight and obesity also presents a challenge to offering health care in settings that have not yet fully met treatment needs for HIV and other infectious diseases. More research is needed on long-term trends and clinical effects of overweight and obesity in HIV-positive patients.
Acknowledgments
The authors are grateful to all the patients and MDH-supported staff who have contributed to these findings. The authors also thank Management and Development for Health, Muhimbili University of Health and Allied Sciences, the Dar es Salaam City Council, and Tanzania’s Ministry of Health and Social Welfare for the support offered in implementing HIV treatment and care services in Dar es Salaam.
Authors’ Note: W.F. jointly conceived the study with A.K. A.K analyzed the data and wrote the manuscript with supervision from W.F., T.B., and D.S. All authors discussed results and contributed to the manuscript at different stages.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This analysis was supported by Grant Number 5U2GPS001966-05, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
References
- 1. Joint United Nations Programme on HIV/AIDS. Global HIV Statistics. 2014. http://www.unaids.org/en/resources/fact-sheet. Accessed February 9, 2018.
- 2. The United Republic of Tanzania. Global AIDS Response Country Progress Report. Dar es Salaam, Tanzania: UNAIDS; 2014. [Google Scholar]
- 3. Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–965. doi:10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO. Antiretroviral therapy for HIV infection in adults and adolescents. WHO; http://www.who.int/hiv/pub/arv/adult2010/en/. Accessed February 10, 2018. [Google Scholar]
- 5. Semu H, Zack RM, Liu E, et al. sPrevalence and risk factors for overweight and obesity among HIV-infected adults in Dar es Salaam, Tanzania. J Int Assoc Provid AIDS Care. 2016;15(6):512–521. doi:10.1177/2325957414542574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duncan PR, Howe LD, Manukusa Z, Purdy S. Determinants of obesity and perception of weight in hypertensive patients in rural South Africa. South Afr J Clin Nutr. 2014;27(2):56–62. doi:003-8-2469. [Google Scholar]
- 7. Malaza A, Mossong J, Barnighausen T, Newell ML. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One. 2012;7(10):e47761 doi:10.1371/journal.pone.0047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hosseinipour MC, Kanyama C, Nkhalamba T, et al. Safety and efficacy of D4T/3TC/NVP among HIV-positive adults in Lilongwe, Malawi. Poster Exhibition: The XV International AIDS Conference Abstract no. TuPeB4522 Bangkok, Thailand; 2004. [Google Scholar]
- 9. Huis in ‘t Veld D, Balestre E, Menten J. Body weight evolution in HIV-positive patients over two years of antiretroviral treatment: a multiregional analysis of the IeDEA collaboration. In: 19th International AIDS Conference: Abstract no. MOPE104 Washington DC, USA; 2012. [Google Scholar]
- 10. Hurley E, Coutsoudis A, Giddy J, Knight SE, Loots E, Esterhuizen TM. Weight evolution and perceptions of adults living with HIV following initiation of antiretroviral therapy in a South African urban setting. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2011;101(9):645–650. [PubMed] [Google Scholar]
- 11. Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 2013;29(3):435–440. doi:10.1089/AID.2012.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tate T, Willig AL, Willig JH, et al. HIV infection and obesity: where did all the wasting go? Antivir Ther. 2012;17(7):1281–1289. doi:10.3851/IMP2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasse B, Iff M, Ledergerber B, et al. Obesity trends and body mass index changes after starting antiretroviral treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis. 2014;1(2):ofu040 doi:10.1093/ofid/ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi:mxm007 [pii]. [DOI] [PubMed] [Google Scholar]
- 15. National AIDS Control Program. National Guidelines for the Management of HIV and AIDS. Dar es Salaam, Tanzania: Ministry of Health; 2012. [Google Scholar]
- 16. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 17. El-Khatib Z, Katzenstein D, Marrone G, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS One. 2011;6(3):e17518 doi:10.1371/journal.pone.0017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10(4):1100–1120. doi:10.1214/aos/1176345976. [Google Scholar]
- 19. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi:10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shayo GA, Mugusi FM. Prevalence of obesity and associated risk factors among adults in Kinondoni municipal district, Dar es Salaam Tanzania. BMC Public Health. 2011;11:365 doi:10.1186/1471-2458-11-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aspray T. Rural and urban differences in diabetes prevalence in Tanzania: the role of obesity, physical inactivity and urban living. Trans R Soc Trop Med Hyg. 2000;94(6):637–644. doi:10.1016/S0035-9203(00)90216-5. [DOI] [PubMed] [Google Scholar]
- 22. Lu Y, Hajifathalian K, Rimm EB, Ezzati M, Danaei G. Mediators of the effect of body mass index on coronary heart disease: decomposing direct and indirect effects. Epidemiol Camb Mass. 2015;26(2):153–162. doi:10.1097/EDE.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 23. Phillips AN, Carr A, Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13(2):177–187. [DOI] [PubMed] [Google Scholar]
- 24. Wyatt CM. Kidney disease and HIV infection. Top Antivir Med. 2017;25(1):13–16. [PMC free article] [PubMed] [Google Scholar]
- 25. UNAIDS. Chronic care of HIV and noncommunicable diseases - How to leverage the HIV experience. http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110526_JC2145_Chronic_care_of_HIV.pdf. Accessed February 10, 2018.
- 26. Crum-Cianflone N, Roediger MP, Eberly L, et al. ; Infectious Disease Clinical Research Program HIV Working Group. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106 doi:10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma A, Bynum SA, Schneider MF, et al. Changes in body mass index following HAART initiation among HIV-infected women in the women’s interagency HIV study. J AIDS Clin Res. 2014;5:1000323 doi:1000323 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muula AS, Ngulube TJ, Siziya S, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63 doi:10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor BS, Liang Y, Garduno LS, et al. High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr. 2014;65(2):e33–e40. doi:10.1097/QAI.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flys T, Nissley DV, Claasen CW, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192(1):24–29. doi:JID34102 [pii]. [DOI] [PubMed] [Google Scholar]
- 31. Koethe JR, Jenkins CA, Lau B, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32(1):50–58. doi:10.1089/aid.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huisin’t Veld D, Balestre E, Buyze J, et al. ; International Epidemiologic Databases to Evaluate AIDS (IeDEA). Determinants of weight evolution among HIV-positive patients initiating antiretroviral treatment in low-resource settings. J Acquir Immune Defic Syndr. 2015;70(2):146–154. doi:10.1097/QAI.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shikuma CM, Zackin R, Sattler F, et al. ; AIDS Clinical Trial Group 892 Team. Changes in weight and lean body mass during highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;39(8):1223–1230. doi:10.1086/424665. [DOI] [PubMed] [Google Scholar]
- 34. van Griensven J, Zachariah R, Mugabo J, Reid T. Weight loss after the first year of stavudine-containing antiretroviral therapy and its association with lipoatrophy, virological failure, adherence and CD4 counts at primary health care level in Kigali, Rwanda. Trans R Soc Trop Med Hyg. 2010;104(12):751–757. doi:10.1016/j.trstmh.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 35. Boulle A, Orrel C, Kaplan R, et al. ; International Epidemiological Databases to Evaluate Aids in Southern Africa Collaboration Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12(5):753–760. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen A, Calmy A, Schiffer V, et al. ; Swiss HIV Cohort Study. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000-2006. HIV Med. 2008;9(3):142–150. doi:10.1111/j.1468-1293.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 37. Adverse Effects of ARV Limitations to Treatment Safety and Efficacy Adult and Adolescent ARV. AIDSinfo https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/31/adverse-effects-of-arv. Accessed February 10, 2018.
- 38. Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes—a 96-week analysis. J Acquir Immune Defic Syndr 1999. 2006;43(5):535–540. doi:10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 39. McComsey GA. Visceral Adiposity in the Modern HIV Treatment Era. Conference on Retrovirals and Opportunistic Infections In: Pathogenesis and Consequences of Metabolic Complications. Abstract no. 123 Boston, MA USA; 2016. [Google Scholar]
- 40. Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS Lond Engl. 2000;14(10):1309–1316. [DOI] [PubMed] [Google Scholar]