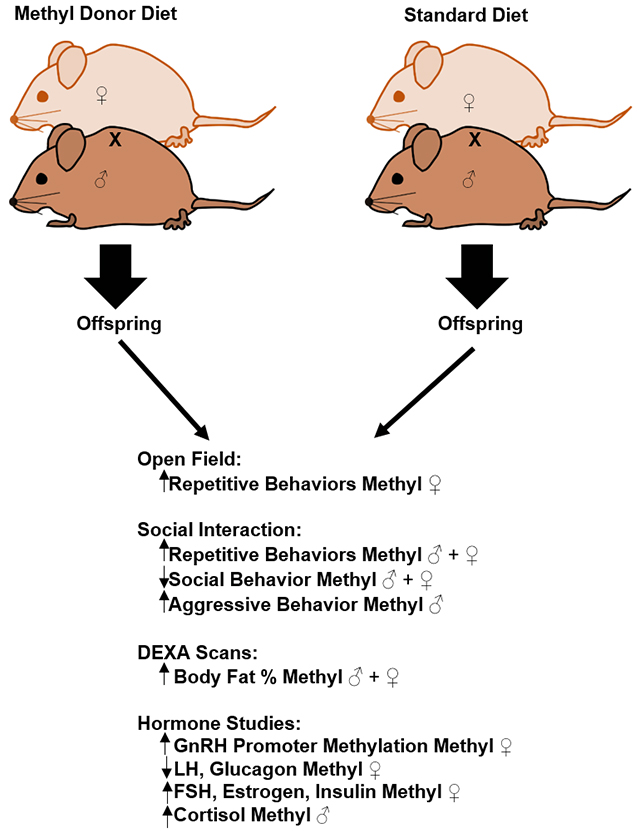
Abstract
Folic acid and other dietary methyl donors are widely supplemented due to their ability to prevent neural tube defects. Dietary methyl donors are also added to other consumables such as energy drinks due to energy-promoting attributes and other perceived benefits. However, there is mounting evidence that indicates developmental exposure to high levels of dietary methyl donors may have deleterious effects. We assessed whether behavior was affected in the social North American rodent species Peromyscus polionotus exposed to a diet enriched with folic acid, Vitamin B12, choline, and betaine/trimethylglycine(TMG). P. polionotus (PO) animals are very social and exhibit little repetitive behavior, particularly compared to their sister species, P. maniculatus. We assayed the effects of dietary methyl-donor supplementation on anxiety-like repetitive and social behaviors by testing young adult animals for novel cage behavior and in social interaction tests. Animals of both sexes exposed to the diet had increased repetitive behaviors and reduced social interactions. Males exposed to the diet became more aggressive compared to their control counterparts. Since methyl-diet animals were larger than control animals, DEXA scans and hormone analyses were performed. Animals exposed to the diet had increased body fat percentages and experienced hormonal changes typically associated with excess fat storage and anxiety-like behavior changes. Therefore, these data suggest the wide use of these dietary supplements makes further investigation imperative.
Keywords: folic acid, methyl-donor pathway, anxiety-like behavior, repetitive behavior, social behavior, Peromyscus
Graphical Abstract
1. Introduction
Folic acid and other B vitamins are widely supplemented during pregnancy due to their ability to prevent offspring neural tube defects [1] and purported ability to increase energy. Consumption of B vitamins has increased since the mid-1990’s due to the consumption of vitamin tablets and capsules, enriched grain products, and other products like fortified energy drinks [2]. B vitamins, particularly folic acid and Vitamin B12, contribute to the 1-carbon metabolic pathway that is necessary for nucleotide production as well as DNA and histone methylation [3]. The latter processes are important in regulating gene expression, and hence organismal physiology.
Well-known studies on the effects of dietary B vitamin supplementation on gene regulation include experiments on the viable yellow allele of the agouti locus (Avy) and on the Axinl locus (Axin1Fu) in Mus musculus [4–7]. Methyl donor pathway components have beneficial effects under certain circumstances, but a growing body of evidence suggests there is cause for concern [8–11]. Animal studies have investigated the effects of methyl donor supplementation on cognitive functions and depression in Mus musculus and Rattus norvegicus domesticus, respectively, but the effects of methyl donor supplementation on social and repetitive behaviors remain relatively under-explored [12–14].
Previously, we investigated the effects of a methyl donor diet using a natural variant of the North American deer mouse that overexpresses agouti (Peromyscus maniculatus- BW.ANb) [15,16]. The BW.ANb variant animals, when exposed to high methyl donors, had increased repetitive behaviors, increased body weight, and other abnormalities (e.g. cataracts) [15]. Since overexpression of agouti influences weight and behavior in Mus musculus and Peromyscus [4,17], we undertook tests in our current study utilizing P. polionotus (PO) animals that perform low levels of repetitive behaviors, high levels of social behaviors, and low levels of aggressive behaviors in social interaction tests [17] so that agouti expression would not be a significant factor in detected behavioral or weight changes. The PO colony at the Peromyscus Genetic Stock Center was derived from wild-caught progenitors and have been maintained as an outbred stock for many generations. As presented in a review by Bedford and Hoekstra, PO animals would still carry those traits that have been selected for under natural environmental conditions [18]. Thus, PO animals can be referred to as a naturalistic model [18].
Further, in this study, we sought to assess whether hormonal changes could be correlated with behavioral and body fat changes. High stress and anxiety are associated with increased repetitive behaviors [19] and decreased sociability [20]. Increased stress and anxiety are based in excess communication between the amygdala and the hypothalamus-pituitary-adrenal (HPA) axis that regulates production of hormones like cortisol. Increased cortisol levels have long been linked with stress and anxiety-related behavior and increases in weight and body fat [21–22]. Increased cortisol also leads to insulin dysregulation, metabolic syndromes, and estrogen dysregulation, which lead to obesity [23]. Estrogen imbalances also can induce insulin resistance and dysregulation of Gonadotropin Releasing Hormone, or GnRH, production in the hypothalamus [24–26]. GnRH stimulates pituitary production of Luteinizing Hormone (LH) and Follicle Stimulating Hormone (FSH) which regulate estrogen and testosterone production in gonads, though actions of insulin are thought to also regulate LH and FSH production [24]. The effects of altered estrogen and altered testosterone on anxiety and depression-related behaviors have been documented in prior experiments [26–27].
Therefore, we examined the effects of the methyl donor diet on behavior and physiology of PO animals using (1) Behaviors scored during adjustment to a novel cage to test for changes to general behaviors, (2) Social Interaction Tests to tests for a change in reactions to a novel animal of the same sex, species, and age, (3) DEXA Scans, and (4) Analysis of hormones associated with behavior and weight changes in animals.
2. Methods & Materials
2.1. Ethics Statement
All procedures were conducted in accordance with the NIH guidelines using protocols approved by the University of South Carolina Institutional Animal Care and Use Committee prior to conducting any experiments (IACUC; protocol #1809-100340-061011).
2.2. Animal Husbandry & Mating Schemes
Animals used in these experiments were obtained from longstanding stocks maintained at the Peromyscus Genetic Stock Center (http://stkctr.biol.sc.edu/). Breeding pairs and their offspring were maintained on a 16:8 hour light-dark cycle and food and water was provided ad libitum. PO breeding pairs were established and maintained on either the methyl-supplemented diet (Table 1) or normal rodent chow (i.e. controls). Offspring were weaned at approximately 25 days of age and maintained on the same diets as their parents (either methyl donor diet or normal rodent chow) until they were four months of age (young adult; note that these animals live >4 years). Animals were maintained on ALPHA-dri +PLUS bedding (http://www.ssponline.com/alpha_dri_PLUS.htm) with additional nest building material added (http://www.andersonslabbedding.com/irradiated/bed-rnest/).
Table 1.
Comparison of differing components in Harlan-Teklad (http://www.harlan.com/) Standard rodent (8604) vs. Methyl-Donor (7517) diet with fold change of each supplement.
| Standard (8604) | Methyl Donor (7517) | Fold Change | |
|---|---|---|---|
| TMG (g/kg chow) | 0 | 5 | N/A |
| Choline (g/kg chow) | 2.53 | 7.97 | 3.15x |
| Folic Acid (mg/kg chow) | 0.0027 | 0.0043 | 1.6x |
| Vitamin B12 (mg/kg chow) | 0.051 | 0.53 | 10x |
2.3. Animals Tested
Behavioral testing was done on 3-6 month old animals in the following order: 1. Novel cage adjustment, and 2. Social Interaction (SI) Test (immediately after the novel cage adjustment period). Experiments were consistently performed at the same time of day with the same personnel present. The number of animals in the different groups were used the tests were: controls (n=26; 14 females, 12 males) and methyl-diet (n=69, 37 females, 32 males).
2.4. Novel Cage Behaviors/Social Interaction Testing
Offspring (methyl and control groups) were evaluated during a novel cage adjustment period and during Social Interaction (SI) tests at 4-6 months of age, as described previously [15,17]. These two tests were always conducted during mid-light cycle. All behaviors were recorded with a Sony handycam HDR-CX160 digital camcorder. The Noldus Observer XT software (Noldus, Leesburg, VA) was used to score behaviors from the archived video data.
Briefly, a single animal was placed into a cage with a floor space of 1,250cm2 with aspen shavings and ventilated transparent cover. Peromyscus home cages have a floor space of 610cm2 with aspen shavings and a ventilated lid, and the home cage houses up to 6 animals, making the novel cage much larger than a home cage. The incidence of the following behaviors were scored for the novel cage adjustment period: burrowing, freezing, jumping, back-flipping, running in circles, and grooming; see [17] for examples. Collectively, straight vertical jumping, back-flipping, and circle running are considered repetitive behaviors in Mus musculus [27,28] and Peromyscus [29] and were therefore grouped together during the analyses. After five minutes of observation in the novel cage, a novel animal of the same sex and species was introduced for five minutes.
The subsequent five-minute period constituted the social interaction test. The novel animal’s tail was marked with a non-toxic marker to distinguish it from the animal being tested. The cage was cleaned and disinfected with 70% ethanol between each animal tested and new bedding was placed in the social interaction cage between trials. For the social interaction test videos, the same behaviors were scored as in the novel cage adjustment period with the addition of social and aggressive behaviors. Note that we assessed behaviors in the SI test only for the original animal (i.e. rather than the introduced animal). General social behaviors included sniffing, following, and allogrooming. Aggressive behaviors included biting, chasing, boxing, and mounting (see [17] for video examples of each behavioral type).
All behaviors were scored by incidence; behavior type was assessed at five second intervals throughout the video. Three people scored each video; overall inter-rater reliability was at least 80 percent. At least two scorers were blind to the diet of the animals being scored. In the rare cases when specific behavioral assessments disagreed, we alternated accepting the assessment of the three scorers. We utilized Minitab and SPSS software packages to perform statistics. Note that Kruskal-Wallis one-way analysis of variance was used for statistical analysis in cases of non-normal distributions; otherwise, ANOVA was used. Behaviors are reported as percentage of incidence of behavior.
2.5. Dual Energy X-Ray Absorptiometry (DEXA) Scans and Tissue Collection
DEXA scans were performed on a random cohort of the mice that participated in behavior testing in order to determine body fat composition and bone mineral density. At least two weeks after behavioral testing was complete, 9 male controls, 14 methyl-diet males, 8 female controls, and 18 methyl-diet females were scanned for body fat percentage and bone mineral density using a DEXA scanner at the University of South Carolina. DEXA scans were completed by first anesthetizing the mice for about 2 minutes prior to the scan and for about 5 minutes during the scan by exposure to 2-3% isoflurane-oxygen gas via a nose cone. Each animal was placed on the scanner bed in the prone position with their limbs and tail stretched away from the body. The head was excluded from calculation using a manual region of interest (ROI). Two tailed t-tests were used to determine significance.
Blood samples were taken from animals by retro-orbital bleed approximately two weeks after DEXA Scans took place. Immediately following retro-orbital bleed, animals were sacrificed by decapitation. A small portion of blood was tested by glucometer to measure glucose levels while the large portion of blood was centrifuged in serum separator tubes to obtain serum. The brain was extracted from each animal and was kept on the lid of a glass petri dish that was filled with ice (−20°C water) to keep the glass cold (around 2°C) during dissection. The glass was sterilized with 70% ethanol between brain samples. The hypothalamus was extracted from each brain and was placed into a cryogenic vial which was then flash frozen in liquid nitrogen. Other brain regions (e.g. hippocampus) and other tissues (e.g. liver) were collected and were flash-frozen in liquid nitrogen but were not utilized in this study. Each brain dissection took no more than 90 seconds.
2.6. Gonadotropin Releasing Hormone (GnRH) RNA and DNA Methylation Studies
RNA and DNA were extracted from hypothalamus samples from methyl-diet and control animals of both sexes using a DNA and RNA extraction kit from Qiagen. Samples were chosen based on measured body fat percent. Hypothalamus samples from high body fat % methyl-diet animals and from low body fat % control animals were used as representative populations from the two groups since body fat % was much higher in methyl donor animals. DNA was subjected to bisulfite treatment followed by methylation-specific qPCR using a Bisulfite Conversion Kit from Epigentek. Methylation analysis was completed by using methylation specific priming (MSP) during qPCR. Primers specific to methylated Peromyscus GnRH and unmethylated Peromyscus GnRH were designed using MethPrimer (http://www.urogene.org/cgibin/methprimer/methprimer.cgi). The primer sequences were as follows: Methylated GnRH F: AGTTAGTATTGGTTTTATGGATTGC; Methylated GnRH R: AAATTCTACCAACTAATCCACGTC; and Unmethylated GnRH F: TTAGTATTGGTTTTATGGATTGTGT; Unmethylated GnRH R: TAAAATTCTACCAACTAATCCACATC. The amplified region covers five CpGs. Two sets of qPCR reactions were run for each bisulfite-converted DNA sample: each DNA sample was input at 20ng DNA per reaction and was incubated with SYBR green qPCR mix (Qiagen) for 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of [95°C for 15 seconds followed by 60°C for 1 minute]. All DNA samples were amplified in two separate reactions: one using the unmethylated GnRH promoter primer set and the other using the methylated GnRH promoter primer set. Each individual reaction was performed in triplicate and threshold levels for each sample were calculated by averaging the triplicate threshold cycle (Ct) readings. The ΔΔCt method was used to obtain relative abundance of methylated or unmethylated GnRH. Two tailed t-tests were utilized to determine significance of MSP qPCR data.
RNA was converted to cDNA using a High Capacity cDNA Synthesis Kit from ThermoFisher during RT-PCR. Thermal cycling for RT-PCR was performed according to kit instructions. CDNA was then used in qPCR by inputting 20ng/reaction with SYBR green mix (Qiagen). Primers designed to amplify one exon of Peromyscus GnRH were also utilized in the gene expression qPCR reaction. Primers were designed using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/). Primer sequences were as follows: GnRH F: 5’ GCTGTTCTTCTGCTGAGTCTAT 3’ and GnRH R: 5’ TTCTGCCTGCTTTCCTCTTC 3’. The qPCR primer set for gene expression was ordered from Integrative DNA Technologies (IDT) as PrimeTime qPCR primers. Each cDNA sample was tested in triplicate. QPCR reactions were incubated for 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of [95°C for 15 seconds followed by 60°C for 1 minute]. The ΔΔCt method was used to determine relative abundance of GnRH in each sample. Two tailed t-tests were used to determine significance.
2.7. ELISA-Based Hormone Assays
The following ELISA-based assays were purchased: Estradiol (VWRCat. No. 102966-728), Free Testosterone (VWR Cat. No. 75871-402), Total Testosterone (VWR Cat. No. 75871-120) Insulin (EMD Millipore Cat. No. EZRMI-13K), Glucagon (FisherScientific Cat. No. 50-104-3269), Cortisol (FisherScientific Cat. No. EIAHCOR), LH (FisherScientific Cat. No. EHLH), and FSH (FisherScientific Cat. No. 50-148-7181). Serum samples randomly chosen from 6 male controls, 11 methyl-diet males, 7 female controls, and 17 methyl-diet females were tested in each ELISA assay. Serum samples were loaded into 96 well plates containing antibodies specific to the hormone of interest. Wells were washed following incubation, and a secondary antibody with a conjugated reporter was added. Wells were washed again after incubation with the secondary antibody, and a substrate solution was added for 10 minutes. The reactions were stopped and the assays were analyzed by a spectrophotometric plate reader at 450nm. Two tailed t-tests were used to determine if there were significant changes in hormones between male controls and methyl-diet males as well as between control females and methyl-diet females.
3. Results
3.1. Novel Cage Behaviors & Social Interaction (SI) Tests
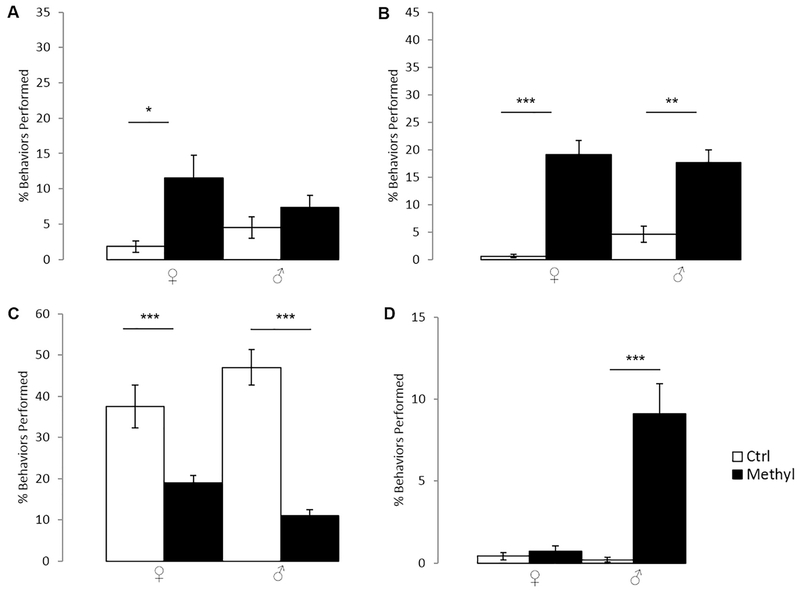
During novel cage adjustment periods, repetitive behaviors (jumping, backflips, and circle running) were significantly increased by about four-fold in methyl-diet females compared to control PO females (Fig. 1A; p=0.043; Kruskal-Wallis). No other behavioral differences were observed in PO methyl-diet animals relative to controls during the novel cage adjustment period. The SI tests revealed a dramatic increase in repetitive behaviors in the methyl-diet PO animals in both sexes when compared to their control diet counterparts (Fig. 1B; p=0.000030 for females; p=0.002 for males; Kruskal-Wallis), including an approximately ten-fold increase in females and four-fold increase in males. In contrast, non-aggressive social interaction behaviors were dramatically decreased in the methyl-diet animals in both sexes when compared to their control counterparts (Fig. 1C; p=0.00035 for females; p=0.000000027 for males; Kruskal-Wallis). Methyl-diet males also exhibited a nearly ten-fold increase in aggressive behavior compared to control males (Fig. 1D; p=0.59 for females; p=0.0000040 for males; Kruskal-Wallis).
Figure 1.
Behavior during novel cage adjustment and Social Interaction Tests of PO animals of both sexes on either control- or methyl-diets. Open bars are control animals while filled-in bars are methyl-diet animals. Female is indicated by “♀,” and male is indicated by “♂.” A) Percent of total behavior that is repetitive during novel cage adjustment (p=0.043 for females; p=0.18 for males; Kruskal-Wallis). B) Percent of total behaviors that are repetitive behaviors in the Social Interaction Test (p=0.000030 for females; p=0.002 for males; Kruskal-Wallis). C) Percent of total behaviors that are social behaviors in the Social Interaction Test (p=0.00035 for females; p=0.000000027 for males; Kruskal-Wallis). D) Percent of total behaviors that are aggressive behaviors in the Social Interaction Test (p=0.59 for females; p=0.0000040 for males; Kruskal-Wallis). Error bars indicate +/−1 standard error. (*p<0.05; **<p.0.01; ***p<0.001, Kruskal-Wallis; open columns are control-diet animals; filled-in columns are methyl-diet animals). Methyl-diet females had a significant increase in repetitive behaviors in novel cage adjustment periods whereas males remained relatively unchanged. A significant increase in repetitive behaviors was indicated in both methyl-diet males and females while a significant decrease in social behaviors was seen in methyl-diet males and females in Social Interaction Tests. A significant increase in aggressive behaviors was seen in methyl-diet males in Social Interaction Tests.
3.2. DEXA Scans
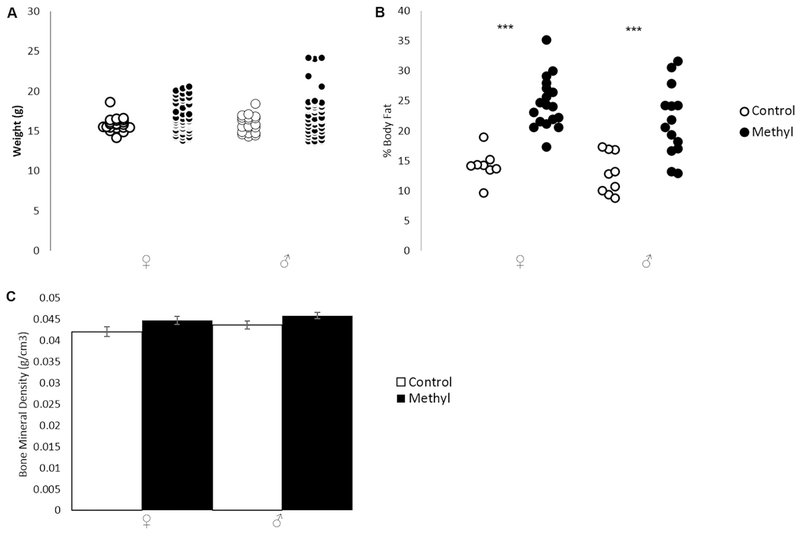
We noted that methyl-diet animals were larger in size than control diet animals. However, there were no measurable differences in amounts of food consumed between control and methyl-diet cages. We weighed each animal after behavioral testing, but noted there was no overall significant change in weight though the methyl-diet range extended higher than that of the controls (Fig. 2A; p=0.072 for females; p=0.060 for males; two-tailed t-test). DEXA scans, however, revealed methyl-diet animals had significantly higher body fat percentages than control animals (Fig. 2B; p=0.0000013 for females; p=0.00061 for males; two-tailed t-test). Bone density (as measured by DEXA scan) was unchanged between methyl-diet and control diet animals (Fig. 2C; p=0.25 for females, p=0.07 for males; two-tailed t-test).
Figure 2.
Weight and DEXA Scan data for PO animals on control- or methyl-diets. Open shapes are control animals while filled-in shapes are methyl-diet animals. Female is indicated by “♀,” and male is indicated by “♂.” A) Distribution of weights in grams (p=0.072 for females; p=0.060 for males; two-tailed t-test). B) Distribution of percent body fat (p=0.0000013 for females; p=0.00061 for males; two-tailed t-test. C) Average bone mineral density in g/cm3for each experimental group (p=0.25 for females; p=0.07 for males; two-tailed t-test). Error bars indicate +/−1 standard error (*p<0.05; **p<0.01; ***p<0.001; two-tailed t-test). Weight was not significantly changed in either sex. Percent body fat was significantly higher in methyl-diet females compared to control females and in methyl-diet males compared to control males. Bone mineral density was unchanged in either sex.
3.3. Hormone Studies
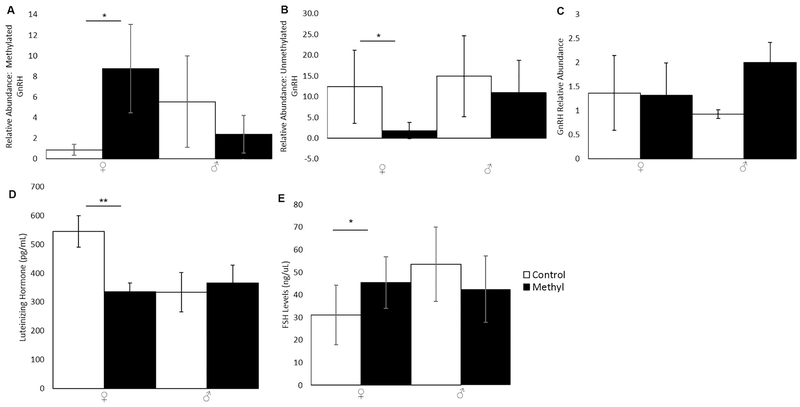
DNA methylation at the GnRH promoter increased significantly in methyl-diet females compared to control females (Fig. 3A; p=0.048; two-tailed t-test) but was unchanged in males (p=0.27; two-tailed t-test). Consequently, unmethylated GnRH promoter decreased significantly in methyl-diet females compared to control females (Fig. 3B; p=0.043; two-tailed t-test) and was unchanged in males (p=0.22; two-tailed t-test). Due to increased DNA methylation at the GnRH promoter in methyl-diet females, we expected that the GnRH mRNA level would be lower in methyl-diet females compared to control-females since an increase in DNA methylation is typically associated with inhibition of gene expression. The quantitative PCR (qPCR) to examine mRNA levels of GnRH revealed no significant change in GnRH expression at the mRNA level (Fig. 3C; p=0.85 for females; p=0.16 for males; two-tailed t-test).
Figure 3.
DNA methylation of the GnRH promoter, GnRH expression, and LH and FSH levels in PO animals on control- or methyl-diets. Open bars are control animals while filled-in bars are methyl-diet animals. Female is indicated by “♀,” and male is indicated by “♂.” A) Relative abundance of methylated GnRH promoter (p=0.048 for females; p=0.27 for males; two-tailed t-test). B) Relative abundance of unmethylated GnRH promoter (p=0.043 for females; p=0.22 for males; two-tailed t-test). C) Relative abundance of GnRH mRNA (p=0.85 for females; p=0.16 for males; two-tailed t-test). D) Levels of LH in pg/mL (p=0.0010 for females; p=0.74 for males; two-tailed t-test). E) FSH levels in ng/uL (p=0.040 for females; p=0.30 for males; two-tailed t-test). Error bars indicate +/−1 standard error (*p<0.05; **p<0.01; ***p<0.001; two-tailed t-test). Methylation of the GnRH promoter was significantly higher in methyl-diet females compared to control females. Unmethylated GnRH promoter was significantly reduced in methyl-diet females compared to control females. mRNA expression of GnRH was unchanged. LH was significantly lower in methyl-diet females compared to control females. FSH was significantly higher in methyl-diet females compared to control females.
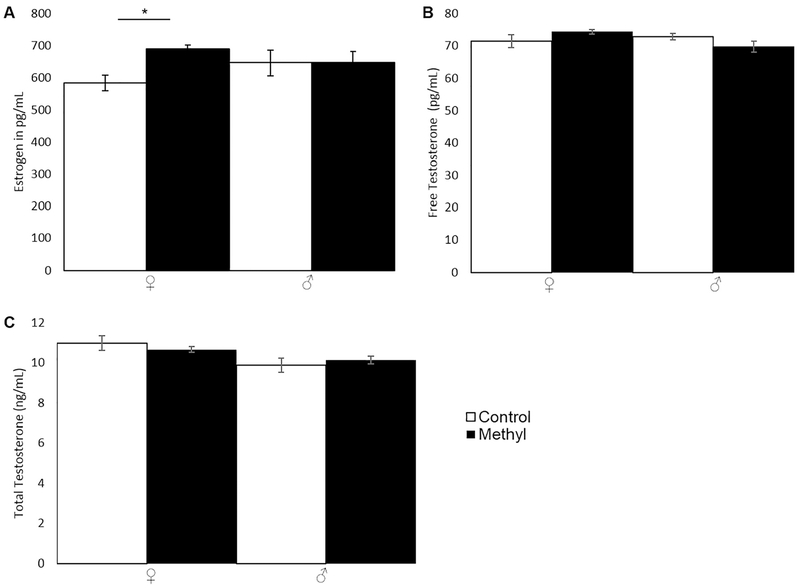
LH was significantly decreased in methyl-diet females compared to control females (Fig. 3D; p=0.0010; two-tailed t-test) as was FSH (Fig. 3E; p=0.040; two-tailed t-test). LH and FSH were unchanged in males (Figs. 3D and 3E, respectively; p=0.74 for LH; p=0.30 for FSH; two-tailed t-test). Since LH and FSH affect estrogen and testosterone production, estrogen, free testosterone, and total testosterone were assayed. Estrogen was significantly increased in methyl-diet females compared to control females (Fig. 4A; p=0.019; two-tailed t-test) but was unchanged in males (Fig. 4A; p=0.98; two-tailed t-test). Free testosterone was unchanged in males and females (Fig. 4B; p= 0.20 for females; p= 0.89 for males; two-tailed t-test). Total testosterone was also unchanged in males and females (Fig. 4C; p= 0.32 for females; p= 0.47 for males; two-tailed t-test).
Figure 4.
Estrogen, Free Testosterone, and Total Testosterone in PO animals on control- or methyl-diets. Open bars are control animals while filled-in bars are methyl-diet animals. Female is indicated by “♀,” and male is indicated by “♂.” A) Estrogen levels in pg/mL (p=0.019 for females; p=0.98 for males; two-tailed t-test). B) Free Testosterone in pg/mL (p=0.20 for females; p=0.89 for males; two-tailed t-test). C) Total Testosterone in ng/mL (p= 0.32 for females; p= 0.47 for males; two-tailed t-test). Error bars indicate +/− 1 standard error (*p<0.05; **p<0.01; ***p<0.001; two-tailed t-test). Estrogen (estradiol) was significantly increased in methyl-diet females compared to control females. Free and total testosterone were unchanged.
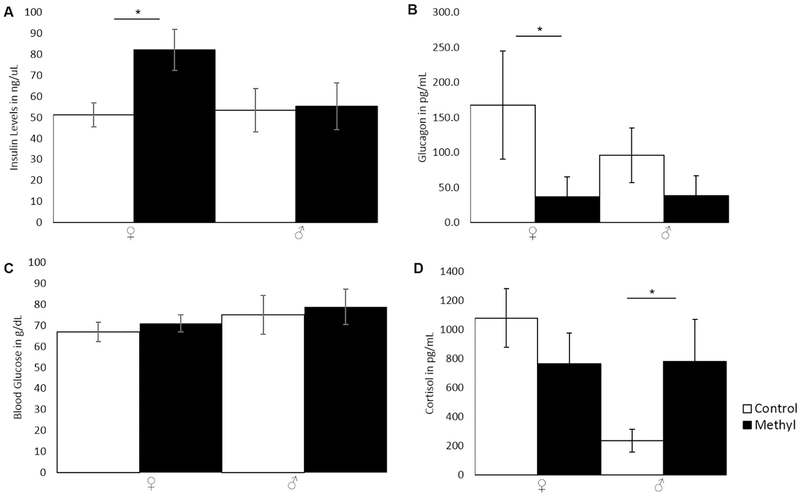
Estrogen is known to affect insulin levels [19,20], and insulin was significantly increased in methyl-diet females (Fig. 5A; p=0.048; two-tailed t-test) compared to control-females. Insulin was unchanged in males (Fig. 5A; p=0.89; two-tailed t-test). Insulin’s physiological function opposes that of glucagon’s in glucose regulation, and glucagon was significantly decreased in methyl-diet females compared to control females (Fig. 5B; p=0.024; two-tailed t-test). Glucagon was unchanged in the males (Fig. 5B; p=0.14; two-tailed t-test). Blood glucose levels (non-fasting) did not differ between methyl-diet animals and controls (Fig. 5C; p=0.41 for females; p=0.70 for males; two-tailed t-test). Cortisol levels were significantly higher in methyl-diet males compared to control males (Fig. 5D; p=0.047; two-tailed t-test) and were unchanged in females (Fig. 5D; p=0.21; two-tailed t-test).
Figure 5.
Insulin, Glucagon, blood glucose, and Cortisol levels in PO animals on control- or methyl-diets. Open bars are control animals while filled-in bars are methyl-diet animals. Female is indicated by “♀,” and male is indicated by “♂.” A) Insulin levels in ng/uL (p=0.048 for females; p=0.89 for males; two-tailed t-test). B) Glucagon levels in pg/mL (p=0.024 for females; p=0.14 for males; two-tailed t-test). C) Average blood glucose in mg/dL in each group (p=0.41 for females; p=0.70 for males; two-tailed t-test). D) Cortisol levels in animals on control or methyl diets (p=0.21 for females; p=0.047 for males; two-tailed t-test). Error bars indicate +/− 1 standard error (*p<0.05; **p<0.01; ***p<0.001; two-tailed t-test). Insulin was significantly higher in methyl-diet females compared to control females. Glucagon was significantly lower in methyl-diet females compared to control females. Blood glucose was not significantly changed. Cortisol was significantly higher in methyl-diet males compared to control males.
4. Discussion
This study had two major goals: the first goal was to assess the effects of the methyl donor diet on social behavior, repetitive behaviors, and anxiety-like behaviors in PO animals, and the second was to determine if the observed body size changes and/or behavioral changes in methyl-diet PO animals could be attributed to hormonal changes. It is evident the methyl donor diet influenced behavior, body fat percentage, and hormone levels in methyl-diet exposed PO animals.
First, the methyl-diet affected PO animals during novel cage adjustment periods: PO females exhibited a 5x increase in repetitive behaviors. The methyl-diet also affected methyl-diet animals in SI tests as well. The SI testing revealed a dramatic increase in repetitive behavior and a decrease in non-aggressive social behavior in methyl-diet exposed animals of both sexes. Additionally, methyl-diet males exhibited an increase in aggressive social behaviors in comparison to their control counterparts.
We noted that behavior was not the only phenotype apparently affected by the methyl donor diet. Methyl-diet animals, despite remaining the same overall average weight as their controls, had significantly higher body fat percentages as indicated by the DEXA scans. Higher estrogen levels in methyl-diet females may have contributed to the increased insulin, decreased glucagon, and altered LH and FSH production in methyl-diet females [25]. Methyl-diet females also had higher DNA methylation levels at the GnRH promoter, which should be associated with lower GnRH mRNA levels. While we did not observe changes in GnRH mRNA levels, we speculated that its temporal or spatial expression pattern may be subtly altered. LH, FSH, estrogen, insulin, and free and total testosterone were not changed in methyl-diet males. The increase in estrogen in methyl-diet females may have led to increased repetitive and social behaviors in methyl-diet females through affecting estrogen receptors [27]. Further investigation is warranted to determine the etiology of these diet-induced phenotypes findings.
Methyl-diet males had higher cortisol production, which might be linked to the increase in anxiety-like behaviors such as decreased social interaction and increased repetitive behaviors [31,32]. Additionally, higher cortisol levels are known to affect body fat levels in humans. It seems through methyl donor supplementation, higher cortisol in methyl-diet males and higher estrogen and insulin in methyl-diet females could have led to the increases in repetitive and social behaviors as well as increases in body fat percentages. The effects of the methyl diet on behavior and body fat percent may not be limited to the scope of this research and its findings. For example, gut microbiomes have a profound effect on metabolism and weight regulation; perhaps the microbiomewas affected in methyl-diet animals as was shown after methionine supplementation in Mus musculus [33]. Alternatively, we do not know the extent of epigenome alterations in the methyl-diet animals. Genome wide assessment of DNA methylation and histone methylation modifications might reveal epigenetic alterations at sites that are particularly sensitive to dietary supplementation.
Though some research has indicated a protective effect of methyl donor supplementation in high-fat-fed mice [34–35], other research has indicated high folate intake leads to obesity in male mice offspring [36]. We did not detect differences in food intake among methyl-diet PO animals in comparison to control PO animals, increased methyl donor content, particularly folic acid, has been shown to alter hypothalamic regulation of food intake in rats [37]. This indicates hypothalamic dysregulation may occur due to higher methyl donor intake, and hypothalamic dysregulation could lead to hormonal changes—rather than food consumption level changes—in the methyl-diet PO animals.
Due to the differences in findings between our previous study using BW.ANb animals [15] and the current findings using PO animals, the Peromyscus model could provide a rare opportunity to identify genetic variants that respond differently to methyl donor diet compounds. Possible variants include genes that encode enzymes involved in the one-carbon/methyl donor pathway as well as epigenetic regulators. However, variation at target loci may also play a role by being more susceptible to epigenetic modifications. For example, these nutrients have been shown to affect DNA methylation at specific sites in both humans and other mammals [5,7,38,39].
In our study, the observed increase in anxiety-like behaviors, altered hormone levels, and increases in body fat percentages seem to be a consequence of increased methyl donor supplementation in P. polionotus. As the methyl donor diet includes multiple components of the methyl donor pathway, it is unclear whether behavioral, hormonal, or body fat percentage changes are a combined effect of the compounds or if observed changes are attributable to a single methyl donor compound.
Methyl donors have been the focus of many studies given the recommendations to pregnant women and mandatory grain fortification in the US and other countries [2]. Methyl donor compounds clearly have major beneficial health effects in humans. Primary among these positive effects is the reduction in neural tube defects. However, it seems unlikely that epigenetic (or other) changes induced by these compounds are universally beneficial. Recent studies indicate high maternal levels of folic acid and vitamin B12 consumption may be linked to autism spectrum disorders (ASD) [40] though there are conflicting studies, likely due to differences in genetics of the examined populations or differences in how the studies were conducted [41].
We note several changes we observed in PO animals (reduced social behavior, increased repetitive behavior) mimic those of ASD behavioral diagnostic criteria [42]. Additionally, changes we observed in body fat percent and hormonal levels in PO females mimic changes seen in metabolic syndrome, insulin resistance, and type 2 diabetes. Hence, the effects of these common food additives on brain development, behavior, and physiology require further study. We suggest individuals with certain genotypes might be more susceptible to deleterious epigenetic changes facilitated by overexposure to methyl donor molecules.
Highlights.
The methyl donor diet in Peromyscus polionotus induces an increase in repetitive behavior and a decrease in social behavior in both males and females.
The methyl donor diet in P. polionotus males induces an increase in aggressive behaviors as well as increased cortisol levels.
Methyl donor diet exposed P. polionotus males and females have higher body fat percentages.
Methyl donor diet exposed P. polionotus females had increased estrogen, insulin, GnRH promoter methylation, and FSH as well as decreased LH and glucagon.
Acknowledgements
This study was supported by NIH P400D010961 to MRF. Partial support was also provided by NSF 1736150 to Hippokratis Kiaris at the University of South Carolina College Of Pharmacy, a University of South Carolina Upstate Scholarly Startup Grant award to KRS, and by a University of South Carolina VP for Research “Support to Promote Advancement of Research and Creativity” (SPARC) award to KRS. We thank Dr. Jeannie Chapman and Dr. Benjamin Montgomery for research support as Division of Natural Sciences and Engineering chairs at the University of South Carolina Upstate. We thank Sandra Kelly and Brian Trainor for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jägerstad M, Folic acid fortification prevents neural tube defects and may also reduce cancer risks, Acta Paediatr. Int. J. Paediatr. 101 (2012) 1007–1012. 10.1111/j.1651-2227.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- [2].Greenberg JA, Bell SJ, Guan Y, Yu YH, Folic acid supplementation and pregnancy: more than just neural tube defect prevention, Rev in Obstet. Gynecol. 4 (2011) 52–59. 10.3909/riog0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shorter KR, Felder MR, Vrana PB, Consequences of dietary methyl donor supplements: Is more always better?, Prog. Biophys. Mol. Biol. 118 (2015). 10.1016/j.pbiomolbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- [4].Wolff CA, Kodell GL, Moore RL, Cooney SR, Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice, FASEB J. 12 (1998) 949–957. 10.1096/fasebj.12.11.949. [DOI] [PubMed] [Google Scholar]
- [5].Cooney CA, Dave AA, Wolff GL, Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring, J. Nutr. 132 (2002) 2393S–2400S. 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- [6].Waterland RA, Jirtle RL, Transposable elements: Targets for early nutritional effects on epigenetic gene regulation, Mol. Cell. Biol. 23 (2003) 5293–5300. 10.1128/mcb.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Waterland KG, Dolinoy RA, Lin DC, Smith JR, Shi CA, Tahiliani X, Maternal methyl supplements increase offspring DNA methylation at Axin Fused, Genesis. 44 (2006) 401–406. 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- [8].Kelly KB, Kennelly JP, Ordonez M, Nelson R, Leonard K, Stabler S, Gomez-Muñoz A, Field CJ, Jacobs RL, Excess folic acid increases lipid storage, weight gain, and adipose tissue inflammation in high fat diet-fed rats, Nutrients. 8 (2016). 10.3390/nu8100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pastinen T, Bourque G, MacFarlane AJ, Behan NA, San Gabriel MC, Zini A, Chan D, Trasler J, Aarabi M, Caron M, High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism, Hum. Mol. Genet. 24(2015) 6301–6313. 10.1093/hmg/ddv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang L, Yang L, Wang J, Yao Y, He C, Wang Y, Wang Z, Bi M, Jia X, High dose of maternal folic acid supplementation is associated to infant asthma, Food Chem. Toxicol. 75 (2014) 88–93. 10.1016/j.fct.2014.11.006. [DOI] [PubMed] [Google Scholar]
- [11].Sawaengsri H, Wang J, Reginaldo C, Steluti J, Wu D, Meydani SN, Selhub J, Paul L, High folic acid intake reduces natural killer cell cytotoxicity in aged mice, J. Nutr. Biochem. 30 (2016) 102–107. 10.1016/j.jnutbio.2015.12.006. [DOI] [PubMed] [Google Scholar]
- [12].Sahara Y, Matsuzawa D, Ishii D, Fuchida T, Goto T, Sutoh C, Shimizu E, Paternal methyl donor deficient diets during development affect male offspring behavior and memory-related gene expression in mice, Dev. Psychobiol. 61 (2019) 17–28. 10.1002/dev.21801. [DOI] [PubMed] [Google Scholar]
- [13].McKee SE, Grissom NM, Herdt CT, Reyes TM, Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet-fed dams, FASEB J. 31 (2017) 2352–2363. 10.1096/fj.201601172R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paternain L, Martisova E, Campion J, Martinez JA, Ramirez MJ, Milagro FI, Methyl donor supplementation in rats reverses the deleterious effects of maternal separation on depression-like behavior, Behav. Brain Res. 299 (2016) 51–58. 10.1016/j.bbr.2015.11.031. [DOI] [PubMed] [Google Scholar]
- [15].Shorter KR, Anderson V, Cakora P, Owen A, Lo K, Crossland J, South ACH, Felder MR, Vrana PB, Pleiotropic effects of a methyl donor diet in a novel animal model, PLoS One. 9 (2014). 10.1371/journal.pone.0104942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kenney-Hunt J, Lewandowski A, Glenn TC, Glenn JL, Tsyusko OV, O’Neill RJ, Brown J, Ramsdell CM, Nguyen Q, Phan T, Shorter KR, Dewey MJ, Szalai G, Vrana PB, Felder MR, A genetic map of Peromyscus with chromosomal assignment of linkage groups (a Peromyscus genetic map), Mamm. Genome. 25 (2014). 10.1007/s00335-014-9500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shorter KR, Owen A, Anderson V, Hall-South AC, Hayford S, Cakora P, Crossland JP, Georgi VRM, Perkins A, Kelly SJ, Felder MR, Vrana PB, Natural genetic variation underlying differences in Peromyscus repetitive and social/aggressive behaviors, Behav. Genet. 44 (2014). 10.1007/s10519-013-9640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bedford NL, Hoekstra HE, Peromyscus mice as a model for studying natural variation, Elife 4(2015) e06813 10.7554/eLife.06813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Joyce C, Honey E, Leekam SR, Barrett SL, Rodgers J, Anxiety, intolerance of uncertainty and restricted and repetitive behaviour: Insights directly from young people with ASD, J. Autism Dev. Disord. 47 (2017) 3789–3802. 10.1007/s10803-017-3027-2 [DOI] [PubMed] [Google Scholar]
- [20].Kabir ZD, Che A, Fischer DK, Rice RC, Rizzo BK, Byrne M, Glass MJ, De Marco Garcia NV, Rajadhyaksha AM, Rescue of impaired sociability and anxiety-like behavior in adult cacna1c-deficient mice by pharmacologically targeting eIF2a, Mol. Psychiatry. 22 (2017) 1096–1109. 10.1038/mp.2017.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Redfern JC, Cooke SJ, Lennox RJ, Nannini MA, Wahl DH, Gilmour KM, Effects of maternal cortisol treatment on offspring size, response to stress, and anxiety-related behavior in wild largemouth bass (Micropterus salmoides), Physiol. Behav. 180 (2017) 15–24. 10.1016/j.physbeh.2017.08.001. [DOI] [PubMed] [Google Scholar]
- [22].Yener S, Baris M, Peker A, Demir O, Ozgen B, Secil M, Autonomous cortisol secretion in adrenal incidentalomas and increased visceral fat accumulation during follow-up, Clin. Endocrinol. 87 (2017) 425–432. 10.1111/cen.13408. [DOI] [PubMed] [Google Scholar]
- [23].Mino D, Amato D, Cuevas ML, Foneseca ME, Burbano G, Wacher N, Lifehitz A, Relationship of insulin resistance and overweight with cortisol and dehydroepiandrosterone-sulfate levels, Arch. Med. Res. 6 (2002) 524–530. 10.1016/S0188-4409(02)00400-9. [DOI] [PubMed] [Google Scholar]
- [24].Xia YX, Weiss JM, Polack S, Diedrich K, Ortmann O, Interactions of insulin-like growth factor-1, insulin, and estradiol with GnRH-stimulating luteinizing hormone release from female rat gonadotrophs, Eur. J. Endocrinol. 144 (2001) 73–79. [DOI] [PubMed] [Google Scholar]
- [25].Gonzalez C, Diaz F, Alonso A, Neuroprotective effects of estrogens: Cross-talk between estrogen and intracellular insulin signalling, Infect. Disord.-Drug Targets. 8(2012) 65–67. 10.2174/187152608784139659. [DOI] [PubMed] [Google Scholar]
- [26].Aikey JL, Nyby JG, Anmuth DM, James PJ, Testosterone rapidly reduces anxiety in male house mice (Mus musculus), Horm. Behav. 42 (2002) 448–460. 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- [27].Zou Y, Lu Q, Zheng D, Chu Z, Liu Z, Chen H, Ruan Q, Ge X, Zhang Z, Wang X Lou W, Huang Y, Wang Y, Huang X, Liu Z, Xe W, Zhou Y, Yao P, Prenatal levonorgestrel exposure induces autism-like behavior in offspring through ERβ suppression in the amygdala, Mol. Autism. 8 (2017) 1–16. 10.1186/s13229-017-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nonneman RJ, Moy SS, Segall SK, Andrade GM, Crawley JN, Nadler JJ, Magnuson TR, Young NB, Social approach and repetitive behavior in eleven inbred mouse strains, Behav. Brain Res. 191 (2008) 118–129. 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS, Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain, Behav. Brain Res. 208 (2010) 178–188. 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lewis MH, Tanimura Y, Lee LW, Bodfish JW, Animal models of restricted repetitive behavior in autism, Behav. Brain Res. 176 (2007) 66–74. 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van den Bos E, Tops M, Westenberg PM, Social anxiety and the cortisol response to social evaluation in children and adolescents, Psychoneuroendocrinology. 78 (2017) 159–167. 10.1016/j.psyneuen.2017.02.003. [DOI] [PubMed] [Google Scholar]
- [32].Sun MJ, Li H, Gong S, Tan JH, Jiao GZ, Luo MJ, Lin J, Miao YL, Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice, PLoS One. 10 (2015) e0117503 10.1371/journal.pone.0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Miousse I, Pathak IR, Garg R, Skinner S, Melnyk CM, Pavliv S, Hendrickson O, Landes H, Lumen RD, Tackett A, Deutz AJ, Hauer-Jensen NEP, Koturbash M, Short-term dietary methionine supplementation affects one-carbon metabolism and DNA methylation in the mouse gut and leads to altered microbiome profiles, barrier function, gene expression, and histomorphology, Genes Nutr. 12 (2017) 22 10.1186/s12263-017-0576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li B, Lu J, Tsuprykov YP, Hasan O, Reichetzeder AA, Tian C, Zhang M, Zhang XL, Sun Q, Guo GY, Gaballa J, Peng MMS, Lin XN, Hocher G, Folate treatment of pregnant rat dams abolishes metabolic effects in female offspring induced by a paternal pre-conception unhealthy diet, Diabetologia. 61 (2018) 1862–1876. 10.1007/s00125-018-4635-x. [DOI] [PubMed] [Google Scholar]
- [35].Li J, Tang W, Ma R, Ouyang F, Liu S, Wu Z, Folic acid supplementation alters the DNA methylation profile and improves insulin resistance in high-fet-diet-fed mice, J Nutr Biochem. 59 (2018) 76–83. 10.1016/j.jnutbio.2018.05.010. [DOI] [PubMed] [Google Scholar]
- [36].Xie X, Fu K, Li Z, Gu H, Cai X, Xu Z, Cui P, You X, Wang L, Zhu X, Ji L, Guo C, High folate intake contributes to the risk of large for gestational age birth and obesity in male offspring, J. Cell Physiol. 233 (2018) 9383–9389. 10.1002/jcp.26520. [DOI] [PubMed] [Google Scholar]
- [37].Yang GH, Pannia NV, Chatterjee E, Kubant D, Ho R, Hammoud M, Pausova R, Anderson Z, Gestational folic acid content alters the development and function of hypothalamic food intake regulating neurons in Wistar rat offspring post-weaning, Nutr Neurosci. (2018) 1–12. 10.1080/1028415X.2018.1479628. [DOI] [PubMed] [Google Scholar]
- [38].Devlieger R, Freson K, Duca RC, Pauwels S, Ghosh M, Langie SAS, Huybrechts I, Koppen G, Bekaert B, Godderis L, Dietary and supplemental maternal methyl-group donor intake and cord blood DNA methylation, Epigenetics. 12 (2016) 1–10. 10.1080/15592294.2016.1257450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Devlieger R, Freson K, Langie SAS, Duca RC, Pauwels S, Ghosh M, Huybrechts I, Koppen G, Bekaert B, Godderis L, Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants, Clin. Epigenetics. 9 (2017) 1–13. 10.1186/s13148-017-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ji Y, Riley AW, Pearson C, Raghavan R, Sices L, Wang X, Stuart EA, Wahl A, Fallin MD, Zuckerman B, Volk H, Wang G, Landa R, Brucato M, Hong X, Hironaka L, Caruso D, Stivers T, Maternal Multivitamin Intake, plasma folate and Vitamin B 12 Levels and Autism Spectrum Disorder risk in offspring, Paediatr. Perinat. Epidemiol. 32 (2017) 100–111. 10.1111/ppe.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Virk B, Liew J, Olsen Z, Nohr J, Catov EA, Ritz JM, Periconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders, Autism. 20 (2016) 710–718. 10.1177/1362361315604076. [DOI] [PubMed] [Google Scholar]
- [42].Ruzzano HM, Borsboom L, Geurts D, Repetitive behaviors in autism and obsessive compulsive disorder: new perspectives from a network analysis, J. Autism Dev. Disord. 45 (2015) 192–202. 10.1007/s10803-014-2204-9. [DOI] [PubMed] [Google Scholar]