Ureaplasma spp. are a genus of bacteria for which two human-associated species exist: Ureaplasma urealyticum and Ureaplasma parvum. Their definition as a pathogen in the context of nongonococcal urethritis (NGU) and infertility among males remains highly controversial, largely due to historically high rates of isolation of these bacteria from the urethra of seemingly healthy men.
KEYWORDS: Ureaplasma parvum, Ureaplasma urealyticum, infertility, nongonococcal urethritis
SUMMARY
Ureaplasma spp. are a genus of bacteria for which two human-associated species exist: Ureaplasma urealyticum and Ureaplasma parvum. Their definition as a pathogen in the context of nongonococcal urethritis (NGU) and infertility among males remains highly controversial, largely due to historically high rates of isolation of these bacteria from the urethra of seemingly healthy men. This review summarizes the emerging evidence suggesting a true pathogenic role of these bacteria under specific conditions, which we term risk factors. We examine the historical, clinical, and experimental studies which support a causal role for Ureaplasma spp. in the development of NGU as well as some of the proposed mechanisms behind the association of Ureaplasma spp. and the development of infertility. Finally, we discuss the potential for developing a case-by-case risk-based approach toward the management of men who present with seemingly idiopathic NGU but who are positive for Ureaplasma spp.
INTRODUCTION
Nongonococcal urethritis (NGU) is a leading sexually acquired condition among men. It is defined by inflammation of the urethra in the absence of Neisseria gonorrhoeae and includes signs and symptoms such as penile discharge, dysuria, as well as irritation inside and around the urethra. Chlamydia trachomatis has long been regarded as the predominant infectious agent among patients suffering from NGU, with 20 to 50% of individuals being positive for the pathogen, whereas more recently, Mycoplasma genitalium has achieved recognition as a pathogen and may be isolated from 10 to 30% of NGU patients (1). Although C. trachomatis and M. genitalium account for many cases of NGU, of concern is the high prevalence of up to 45% of idiopathic NGU cases in which classic pathogens are not identified (2).
Bacteria from the genus Ureaplasma are leading candidates to fill the void presented by this idiopathic condition among NGU patients. The first documented isolation of these bacteria was from male patients experiencing NGU (3). Many reports have followed up this observation with a view to gather evidence to support the idea of Ureaplasma spp. being an etiological agent of NGU, but the combination of inconsistencies in reporting and study design and the high prevalence of between 5 and 15% among healthy males aged 16 to 44 years has prevented the acknowledgment of these organisms as true pathogens in the context of genitourinary medicine (GUM) (4). For these reasons, the idea of Ureaplasma spp. as GUM pathogens remains controversial among GUM practitioners. Additionally, the potential role of Ureaplasma spp. as agents with a causal role in male infertility has been debated. Many of the recognized GUM pathogens, such as N. gonorrhoeae and C. trachomatis, have been implicated in complications, such as male infertility, but more work is required to gain a clear understanding of the implications associated with a failure to clear Ureaplasma spp. from the urethra (5).
In this review, we present an update from the current literature to discuss the potential of Ureaplasma spp. to be a risk factor for male genital tract infections, with specific reference to NGU and infertility. In the context of NGU, we present the arguments for and against a role for these bacteria in disease development with a focus on some of the unique risk factors which have been overlooked historically. Increasing interest has focused on Ureaplasma spp. and their potential role in the development of male infertility. We discuss the clinical evidence as well as the proposed mechanisms which have been neglected when taking into account markers for infertility. Finally, the potential therapeutic considerations are evaluated, and we discuss the potential for risk-based screening approaches as an effective means to manage patients with seemingly idiopathic NGU in the face of growing concerns over antimicrobial resistance among GUM pathogens.
Background Biology of Ureaplasma spp.
Ureaplasma spp. are recognized as some of the smallest self-replicating, free-living microorganisms. They are a unique genus of bacteria due to their essential requirement for urea in the synthesis of ATP, with further defining characteristics being shared with the closely related mycoplasmas, including a low G+C genomic content, the lack of a peptidoglycan-containing cell wall, and a requirement of cholesterol for growth (6, 7).
Ureaplasmas were first isolated from male NGU patients in 1954, and due to the tiny colony size upon agar plates, these bacteria were originally referred to as “T-strain” or “tiny” mycoplasmas (3). Following the establishment of the essential requirement for urea, the genus name Ureaplasma was adopted (8). A single species of human-associated Ureaplasma, Ureaplasma urealyticum, was initially recognized, and was further subdivided into two biovars. The nomenclature of U. urealyticum for describing all human-associated isolates was embedded until the work by Robertson et al. in 2002, which made a substantial contribution to redefining these bacteria into two antigenically distinct human-associated species (9). These were defined as U. urealyticum and Ureaplasma parvum. The two species are divided into 14 serovars, with serovars 1, 3, 6 and 14 being assigned to U. parvum and the remaining serovars (serovars 2, 4, 5, and 7 to 13) being defined as U. urealyticum.
Numerous clinical manifestations have been associated with Ureaplasma spp. Among the most notable is the role of Ureaplasma spp. in adverse pregnancy outcomes, such as chorioamnionitis and preterm premature rupture of membranes leading to preterm birth (10, 11). The subsequent colonization of Ureaplasma spp. within the lungs of premature neonates has been associated with a 7.9-fold increased risk of bronchopulmonary dysplasia, a 3.3-fold increased risk of intraventricular hemorrhage, and a 2.5-fold increased risk of necrotizing enterocolitis (12). In adults, attention has been drawn to the development of an atypical hyperammonemia, in which lung transplant patients and, potentially, kidney transplant patients have increased serum ammonia levels as a result of systemic Ureaplasma species infection (13–15). If left untreated with antibiotics, such increased serum ammonia levels can lead to delirium, cerebral edema, and, eventually, death.
Historically, the link between Ureaplasma spp. and the development of NGU, as well as infertility, prostatitis, and epididymitis, among men has been inconsistent. The reason for this, however, is certainly not from a lack of studies examining potential associations between Ureaplasma spp. and NGU (2, 16–21). Rather, the lack of conclusive evidence may reflect the complex interaction between host and pathogen, as discussed later, combined with the high prevalence of Ureaplasma spp. among control groups, which suggests that they are innocent bystanders present at the time of screening. Although Ureaplasma spp. were isolated approximately 30 years prior to the isolation of Mycoplasma genitalium, the latter has risen to prominent pathogen status more rapidly, and new guidelines for its management are now in place in the United Kingdom (3, 22, 23).
THE PROINFLAMMATORY POTENTIAL OF UREAPLASMA SPP.
Human Volunteer Experiments with Ureaplasma Species Infection of the Urethra
To demonstrate the pathogenicity of Ureaplasma spp., several investigators have undertaken human participant experiments (24, 25). The first such experiment, by Jänsch in 1972, identified a polymorphonuclear leukocyte (PMN) response following inoculation with an unknown and poorly defined Ureaplasma sp. (24). Although the experiment was poorly designed and controlled for, this gave an initial insight into the inflammatory nature of a human infection with a Ureaplasma sp. A second, more defined experiment was conducted with two human participants. The first participant received an intraurethral inoculation of a clinically relevant titer of a low-passage clinical isolate of U. urealyticum serovar 5 isolated from a patient experiencing NGU in which no other organisms were present (25). The participant subsequently developed symptoms of dysuria and signs of urethritis in the form of a PMN response. The serum recovered from the volunteer demonstrated seroconversion with high titers of specific antibodies. Upon administration of tetracycline, both signs and symptoms resolved. The second participant received an alternative isolate of U. urealyticum serovar 5 isolated from a second patient presenting with NGU. Again, signs and symptoms ensued, but upon administration of tetracycline, signs, such as urinary threads, persisted in the absence of viable cultures. Seminal samples collected after antibiotic treatment indicated that the U. urealyticum isolate had disseminated, suggesting the potential involvement of the prostate and highlighting the potential adverse sequelae associated with exposure to Ureaplasma spp. Although such experiments are ethically questionable by today’s standards, these studies provided initial evidence that exposure of the male urethra to clinically relevant titers of U. urealyticum has the capacity to elicit a PMN immune response in the presence of symptoms which reflect those seen among NGU patients. It should be noted, however, that what a clinically relevant titer of U. urealyticum is was not defined in this study and is a notable limitation for interpretation of these data.
Animal Models of Ureaplasma Species-Induced Urethritis
Due to the substantial ethical implications of human volunteer studies, investigators turned to model urethral infection caused by Ureaplasma spp. utilizing animal models. Like Neisseria gonorrhoeae, Ureaplasma spp. are host specific, resulting in an early reliance upon chimpanzee models due to the close ancestry with humans. Initial experiments with intraurethral inoculation saw the rapid multiplication of the bacteria within the urethra, but in the absence of a PMN response (26). A possible explanation for this lack of an immune response was suggested to be a loss of virulence from in vitro passage. To examine this hypothesis, a second study was conducted with a larger number of chimpanzees (27). The inoculum for this study consisted of Ureaplasma spp. from men with NGU resuspended in a transport medium which was directly inoculated into the chimpanzees via intraurethral inoculation. Unlike the first study, a substantial PMN response was noted in conjunction with an increase in the Ureaplasma species titer. For reasons which are unknown, the species of Ureaplasma which was inoculated during this study was not determined.
Due to the lack of availability of chimpanzee models, investigators have moved to murine models to investigate colonization of the genital tract (28–30). Although many of these models have relied upon female mice and vaginal colonization, due to the physiology of the male mouse urethra, an inflammatory response characterized by increased tumor necrosis factor alpha (TNF-α) and PMN recruitment has been described. A key confounding variable has been the essential requirement to pretreat the mice with estradiol to allow colonization to establish. This requirement for estradiol is likely due to suppression of the innate immune system, but the presence of estradiol binding proteins, as seen in other pathogens, is yet to be ruled out (31, 32).
In Vitro Cell Line Models of Ureaplasma Species-Induced Inflammation
The difficulty in assessing Ureaplasma species infection of the urethra has resulted in a reductionist approach utilizing specific cell lines in isolation in vitro to look at cytokine responses. Some studies have focused on immune cells, such as THP-1 monocytes, phorbol myristate acetate (PMA)-differentiated macrophages, and primary human macrophages derived from lung fluid, which were then stimulated with U. urealyticum serovar 8 (33). In all cell types examined, stimulation with U. urealyticum resulted in a dose-dependent increase in the levels of interleukin-6 (IL-6) and TNF-α at both the mRNA and the protein levels. However, it should be noted that studies examining cytokine expression in relation to stimulation by Ureaplasma spp. tend to examine a single bacterial isolate and therefore do not give a true representation of the diversity of stimulating properties of Ureaplasma spp. It has been suggested that the predominant antigen found on the surface of Ureaplasma spp., known as the multiple-banded antigen (MBA), may account for differences in the inflammatory response (34). Sweeney et al. noted that the size and number of MBA repeats had an effect upon the levels of IL-8, which is a primary chemoattractant of PMNs, such as neutrophils (34). Although many of these studies were generalized for the immunogenic properties of Ureaplasma spp., they provide evidence for the inflammatory potential for these bacteria. An obvious limitation of these studies is the lack of consideration of the complexities of a full biological system, such as an adaptive immune response, the presence of other microorganisms which may permit infection, as well as the response to chronic exposure over time.
RISK FACTORS LINKED WITH DEVELOPMENT OF UREAPLASMA SPECIES-ASSOCIATED NGU
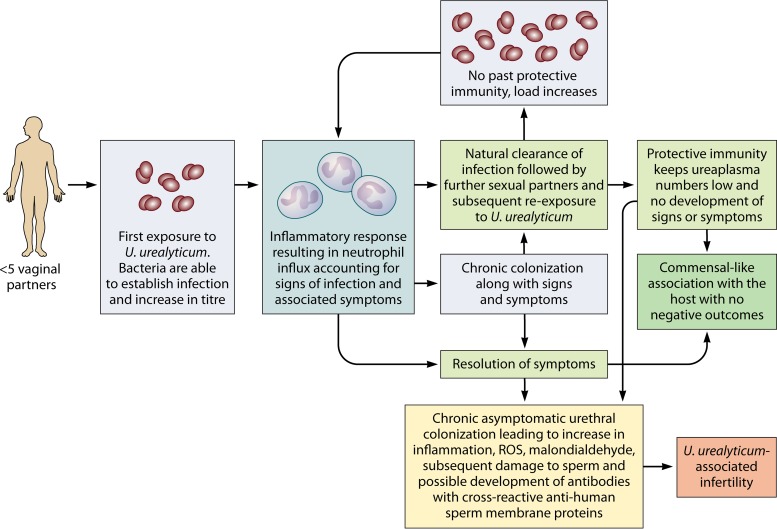
Ureaplasma spp. can be detected in genital samples from men with NGU as well as healthy controls, which has fueled much of the controversy surrounding the role of Ureaplasma spp. in NGU (Table 1). Much of this historic evidence may now be questioned due to developments in the reclassification of Ureaplasma spp., better techniques for species differentiation, fully quantitative reporting of sample titer, as well as a better understanding of patient sexual histories. A proposed overview of the natural history of Ureaplasma spp. taking into account these risk factors is presented in Fig. 1.
TABLE 1.
Published studies examining the relationship between Ureaplasma spp. and NGUa
| Authors (yr) | Reference | Country of study | Patient group | Sample type | No. of participants | Method of identification | Differentiation of Ureaplasma spp. | Key findings relating to Ureaplasma spp. |
|---|---|---|---|---|---|---|---|---|
| Frolund et al. (2016) | 45 | Sweden | Male patients attending STD clinic | First-void urine | 187 men with acute NGU, 24 men with chronic NGU, and 73 men without NGU | Species-specific qPCR | Yes | The number of lifetime sexual partners was negatively associated with the U. urealyticum load. |
| Urine containing U. urealyticum with >1.3 × 103 genome equivalents/ml was associated with NGU. | ||||||||
| Cox et al. (2016) | 41 | UK | Male patients attending a GUM clinic | Urine | 75 men with NCNGU and 90 men without NCNGU | Species-specific real-time PCR | Yes | The prevalence of U. parvum was significantly higher in the NCNGU group. |
| There was no association between U. urealyticum and NCNGU. | ||||||||
| Khatib et al. (2015) | 19 | UK | Males attending an urban sexual health clinic | Urine | 83 men with urethritis | Multiplex PCR | Yes | Only four patients were positive for U. urealyticum. |
| Deguchi et al. (2015) | 48 | Japan | Retrospective study of men attending a urology clinic | First-void urine | 15 symptomatic men and 38 asymptomatic men | qPCR | Yes | A U. parvum load of >5 × 103 cells/ml was significantly associated with >12.5 leucocytes/μl of urine. |
| 83% of subjects had <5 × 103 cells/ml, suggesting a low bacterial load and a lack of signs of inflammation. | ||||||||
| Zhang et al. (2014) | 40 | Multiple countries | Meta-analysis | NA | 1,507 men with NGU and 1,223 men without NGU | NA | Yes | No significant difference in the undifferentiated Ureaplasma species-positive rate was found between the NGU and control groups. |
| When the species was differentiated, U. urealyticum was significantly associated with the NGU group, whereas U. parvum was significantly associated with the control group. | ||||||||
| Shimada et al. (2014) | 47 | Japan | Retrospective study of men attending a urology clinic | First-void urine | 25 symptomatic men and 26 asymptomatic men | Species-specific qPCR | Yes | The bacterial load of U. urealyticum was positively correlated with NGU and the number of leukocytes in urine. |
| Wetmore et al. (2011) | 17 | USA | Men attending an STD clinic | Urine | 329 men with NGU, control group 1 consisting of 191 males without NGU attending an STD clinic, and control group 2 consisting of 193 males without NGU attending an emergency room | Culture | Yes | U. urealyticum was associated with NGU. |
| The association was significantly stronger when analyzing men with <10 vaginal partners. | ||||||||
| The association was further strengthened when analyzing men with <5 vaginal partners. | ||||||||
| U. parvum was not associated with NGU. | ||||||||
| Couldwell et al. (2010) | 38 | Australia | Men attending a sexual health clinic | First-void urine | 237 men with NGU and 268 controls | PCR | Yes | U. urealyticum was significantly associated with NGU in the absence of another urethral pathogen. |
| Ondondo et al. (2010) | 21 | USA | Archived samples from heterosexual males attending an STD clinic | Urine | 119 men with NGU and 117 controls | PCR | Yes | U. urealyticum was strongly associated with NGU. |
| This association was the strongest in men <28 yr of age. | ||||||||
| U. parvum was not associated with NGU. | ||||||||
| Yu et al. (2008) | 18 | Hong Kong | Males attending a government STD clinic | Urine | 98 men with NGU and 235 controls | Real-time PCR targeting the urease gene | No | Neither Ureaplasma nor M. genitalium was associated with symptomatic NGU. |
| Bradshaw et al. (2006) | 39 | Australia | Men attending a sexual health clinic | First-stream urine | 329 men with NGU and 307 controls | PCR | Yes | Neither U. urealyticum nor U. parvum was associated with NGU. |
| Povlsen et al. (2002) | 36 | Sweden | Men attending a sexual health clinic | Urethral swab | 125 men with NGU and 205 without NGU | PCR | Yes | No difference was found between the NGU and non-NGU group if ureaplasmas were not differentiated to the species level. |
| When differentiated, significantly more U. urealyticum bacteria were associated with males with NGU than those without. | ||||||||
| Horner et al. (2001) | 16 | UK | Heterosexual men with NGU and a control group | First-pass urine | 114 men with NGU and 64 without NGU | Culture | No | Ureaplasmas were not associated with acute NGU. |
| Ureaplasmas were associated with NGU during follow-up. | ||||||||
| Ureaplasmas were associated with chronic NGU. |
qPCR, quantitative PCR; NCNGU, nonchlamydial nongonococcal urethritis; NA, not applicable.
FIG 1.
Proposed natural history of U. urealyticum urethral infection in men following initial exposure. A hypothetical scenario in an immunologically naive male when exposed to U. urealyticum for the first time is described. The lack of prior exposure to U. urealyticum results in an increased bacterial titer and subsequent polymorphonuclear neutrophil influx (signs of infection) with accompanying symptoms. Depending on the adaptive immunological response to U. urealyticum, the infection may clear without intervention or result in persistent urethral colonization. With an increase in the number of sexual contacts, the presence of an adaptive immune response is able to keep the titer of any newly acquired U. urealyticum bacteria below the threshold which results in inflammation. In the absence of an adaptive immune response, signs and symptoms may be present again. Persistent urethral colonization may result in a commensal-like association with the host, accounting for the high prevalence among healthy individuals, or, alternatively, may result in the factors which are associated with the development of infertility.
Risk Factor 1: the Species of Ureaplasma Present within the Urethra
Until 2002, human ureaplasmas were recognized as a single species subdivided into two biovars. The result of phenotypic and genotypic analysis later saw the official recognition of two independent species, U. parvum and U. urealyticum (9). This absence of species differentiation meant that studies prior to 2002 solely reported results as U. urealyticum and therefore may have overrepresented this species among clinical samples from both cases and control groups. The legacy of the original nomenclature is still evident today, with publications still referring to U. urealyticum or just Ureaplasma spp., and may be partially attributed to the use of culture-based rapid diagnostic kits which are commercially available (35). Over time, studies have begun to differentiate the Ureaplasma spp. found into the respective species, with some studies identifying the presence of U. urealyticum more often among NGU patients than among controls, whereas the inverse of this was found for U. parvum (17, 21, 36–39). In many of these studies, patient numbers in both NGU and control groups were seen to be low and therefore lacked the power to confidently associate U. urealyticum with NGU. For this reason, Zhang et al. performed a meta-analysis which included seven eligible case-control studies encompassing 1,507 NGU patients and 1,223 controls from four separate continents (40). The findings identified that U. urealyticum was more prevalent among NGU patients than among controls and that U. parvum was significantly more associated with the control group than with those with NGU. This analysis gave significant weighting toward the idea that U. urealyticum is the most commonly associated species of Ureaplasma among NGU patients and presents as a substantial risk factor for the development of disease, although some studies have found the inverse result (41).
Due to the link between species and disease, it is essential that future studies differentiate Ureaplasma spp. from clinical samples to the species level using molecular methods to aid in epidemiological studies, which will either support or refute the role of these bacteria in the development of NGU. One of the limiting factors inhibiting this is the use of culture-based rapid diagnostic tests available commercially, which are able to yield semiquantitative data with regard to the titers within a sample, as well as an indication of antibiotic susceptibility, but which are of limited diagnostic use in the instance of NGU due to the failure to differentiate between species (35). If reference or research facilities are accessible, molecular-based techniques are available which can differentiate the two species based on the size of the amplicons generated following a PCR that targets the 5′ region of the mba gene or that uses real time-based molecular probes (42–44). Additionally, multiplex molecular assays are also commercially available and may play an important role in the species-level identification of Ureaplasma spp., alongside more traditional pathogens responsible for sexually transmitted infections (STI). As discussed below, the presence of Ureaplasma spp. alone may not be sufficient to warrant clinical intervention and the results of such tests being reported back to the clinician may result in inappropriate use of antibiotics, and therefore caution should be taken in reporting and/or interpretation of these results.
Risk Factor 2: the Sexual History of the Patient
It would be very simplistic to assume that the species of Ureaplasma was the sole differential which accounts for NGU among men and the inconsistency in reporting in previous cases. A fascinating insight most likely relating to the immune response of the host and the transition from pathogen to commensal has instead been indicated. For many STIs, the risk of symptomatic disease is proportional to the number of sexual partners (36). In contrast to this, the relationship between Ureaplasma spp. and the number of sexual partners is inversely correlated (17, 45). Some of the pioneering work in this area was identified by Wetmore et al., who examined 329 patients with NGU, defined as ≥5 PMNs per high-powered field and/or a visible discharge (17). In this study, two control groups, consisting of 191 attendees to a sexually transmitted disease (STD) clinic and 193 patients who were attending the emergency department who did not have NGU, were also assessed. Upon initial analysis, U. urealyticum was only marginally associated with NGU compared with the association found for the STD control group (adjusted odds ratio, 1.6) or the emergency department group (adjusted odds ratio, 1.7), but when the analysis considered the number of sexual partners, the adjusted odds ratio rose to 2.9 for the STD group and 3.2 for the emergency department group when focusing on less than 10 vaginal partners. This association was even greater when the number of vaginal partners was restricted to less than five, with the adjusted odds ratios increasing to 6.2 and 5.2, respectively. When the same analysis was performed on patients positive for U. parvum, there was no association in any group, adding further weighting to the argument to differentiate the species of Ureaplasma. A similar finding was noted by Frolund et al., who examined a Danish cohort of 211 NGU patients and 73 asymptomatic controls (45). Again, a similar finding was observed, with the increase in the number of sexual partners being associated with a reduced likelihood of disease.
These studies suggest that Ureaplasma species infections resulting in NGU are associated with patients with fewer sexual contacts. At first, this may seem counterintuitive, considering that other sexually transmitted infections are positively associated with the number of sexual contacts, which therefore represents a significant risk factor. The scenario with Ureaplasma spp. suggests a significant role for the adaptive immune system in the presentation of disease. Early work by Brown et al. examined the serological response to Ureaplasma spp. among NGU patients in acute- and convalescent-phase serum (46). They noted that a change in antibody titer was identified in 68% of patients, and greater than 80% of these patients saw a change in IgM titer, suggesting an active infection. When examining the titers of IgG and IgA, the immunoglobulins responsible for protective immunity, only 10% of patients had an increase in titer. When the data were stratified by prior NGU or not, there was no significant difference in IgG levels in either acute- or convalescent-phase serum, whereas prior NGU accounted for a greater IgA response. These data suggest that some patients gained protective immunity following a previous NGU, whereas others did not. This ability to develop protective immunity may account for why some individuals do not develop NGU on future reexposure, whereas others may.
Risk Factor 3: the Bacterial Load of Ureaplasma spp. within the Male Urethra
The third significant risk factor linking U. urealyticum and, in some cases, U. parvum to the development of NGU is the bacterial load within the urethra. As mentioned previously, in vitro stimulation of monocytic cell lines with U. urealyticum resulted in a dose-dependent response between the U. urealyticum titer added and mRNA and cytokine production for the proinflammatory cytokines IL-6 and TNF-α (33). This in vitro evidence is supported by clinical findings from a number of studies (45, 47, 48). Frolund et al. reported that in the presence of U. urealyticum at concentrations of ≥1.3 × 103 genome equivalents/ml of urine, corresponding to approximately 1 × 103 bacteria/ml, there was a significant association with the development of NGU (45). This figure was similar to that in earlier papers which looked at cutoff points for the bacterial load for both U. urealyticum and U. parvum (47, 48). It is important to note that the study by Deguchi et al. reported that 83% of subjects which were positive for U. parvum had less than 5 × 103 bacteria/ml urine, and 80% of these subjects had less than 12.5 leukocytes/ml (48). This gives further weighting to the idea that U. parvum is less proinflammatory, but in situations in which high titers of U. parvum are present, it has the capacity to generate an inflammatory response. An observation by Frolund et al. ties in risk factor 2 (sexual history of the patient) with risk factor 3 (bacterial load) (45). Analysis of their cohort identified that as the number of vaginal sexual partners increased, the load of Ureaplasma isolated decreased, with a predicted drop by 2.2% with each additional sexual partner. It is conceivable that the host immune response which develops due to multiple exposures to Ureaplasma spp. may have a direct impact on keeping the titer of Ureaplasma spp. low and therefore under the threshold to mount a significant proinflammatory response like that which would result in a PMN response, but these findings needs to be expanded by future studies.
The data from the studies presented here suggest that a simple qualitative result would not be enough to predict a causal relationship between the presence of Ureaplasma spp. and NGU, a factor which has been overlooked by previous studies. From these data it may be possible to develop an objectively determined titer of Ureaplasma spp. which clinical laboratories could use to differentiate between causality and association.
IMPACT OF UREAPLASMA SPP. ON MALE FERTILITY
The link between sexually transmitted pathogens and a negative impact on fertility in females is well established. In males, however, such a link is less well defined, yet it has been controversially suggested by some that Ureaplasma spp. may be associated with male infertility (Table 2). The chronic and often asymptomatic carriage of Ureaplasma spp. may therefore have important implications on the development and progression of infertility among men. In this section, we discuss the studies which have contributed to this argument and examine the clinical and mechanistic studies which have contributed to the argument for a causal role of Ureaplasma spp. in impaired male fertility.
TABLE 2.
Published studies examining the relationship between Ureaplasma spp. and male infertility
| Authors (yr) | Reference | Country of study | Patient group | Sample type | No. of participants | Method of identification | Species differentiated | Key findings relating to Ureaplasma spp. |
|---|---|---|---|---|---|---|---|---|
| Huang et al. (2016) | 57 | China | Men attending a reproductive center | Semen | 19,098 infertile men and 3,368 fertile men | Culture | No | Ureaplasma spp. were significantly associated with infertility. |
| Ureaplasma spp. were significantly associated with reduced motility and normal forms in infertile men compared with fertile controls. | ||||||||
| Huang et al. (2015) | 56 | Multiple countries | Meta-analysis | NAa | 611 infertile men and 506 fertile men | NA | Yes | U. urealyticum was significantly associated with infertility. |
| U. parvum was not associated with infertility. | ||||||||
| Zhang et al. (2014) | 54 | China | Men attending an infertility clinic | Semen | 223 infertile men and 146 fertile men | Culture | Yes | U. urealyticum was significantly associated with infertility compared with U. parvum. |
| Semen positive for U. urealyticum showed a decreased concentration of spermatozoa and decreased motility. | ||||||||
| Abusarah et al. (2013) | 55 | Jordan | Men attending a urology clinic | Semen and first-void urine | 93 infertile men and 70 fertile men | PCR | Yes | Ureaplasmas were found more frequently among samples from infertile men (10.8%) than among those from fertile men (5.7%). |
| Zeighami et al. (2009) | 53 | Iran | Men attending an infertility center | Semen | 100 infertile men and 100 fertile controls | PCR | Yes | Ureaplasmas were detected significantly more often in semen from infertile men than in semen from controls. |
| U. urealyticum was detected in 9% of infertile men vs 1% of control men. | ||||||||
| U. parvum was detected in 3% of infertile men vs 2% of control men. |
NA, not applicable.
Clinical Studies Associating Ureaplasma spp. with Infertility
As discussed above, Ureaplasma spp. can be found in the urethra of seemingly healthy males, but they have also been isolated from expressed prostatic secretions, urine following prostatic massage, and prostate tissue (49–53). This colonization therefore permits a source to contaminate semen during ejaculation and serves as a means to impact male fertility.
Many studies have examined the clinical association between the presence of Ureaplasma spp. in men who are infertile and that in a control group of men without signs of infertility (53–57). In one relatively small study of 100 infertile and 100 control individuals, the authors identified 12% of infertile men but only 3% of fertile men to be Ureaplasma species positive by PCR (53). The individuals which were Ureaplasma species positive and infertile had significantly lower volumes of seminal fluid, lower concentrations of sperm cells, and higher levels of sperm cells with an abnormal morphology than the Ureaplasma species-negative infertile patients. Of significance was the finding that the frequency of U. urealyticum in the semen of infertile men was higher (9%) than that in the healthy controls (1%), whereas the frequency of the presence of U. parvum was 3% in the infertile group and 2% in healthy men, suggesting that U. parvum may not have a causal role in infertility in this patient group. In a fate similar to that of previous NGU studies, many studies have neglected to differentiate between species (and in one instance, in excess of 19,000 samples were examined), and although a significant negative impact of Ureaplasma spp. on semen quality was identified, further power may have been afforded if species-level discrimination had been conducted (57, 58). To further investigate the species-specific association with infertility, a meta-analysis which examined 14 studies comprising 611 cases and 506 controls was conducted (56). These studies suggested an association between U. urealyticum and a negative impact on fertility, whereas there was little evidence for a role of U. parvum, which draws a parallel to the findings for NGU patients discussed above.
Proposed Mechanisms of Ureaplasma Species-Associated Infertility
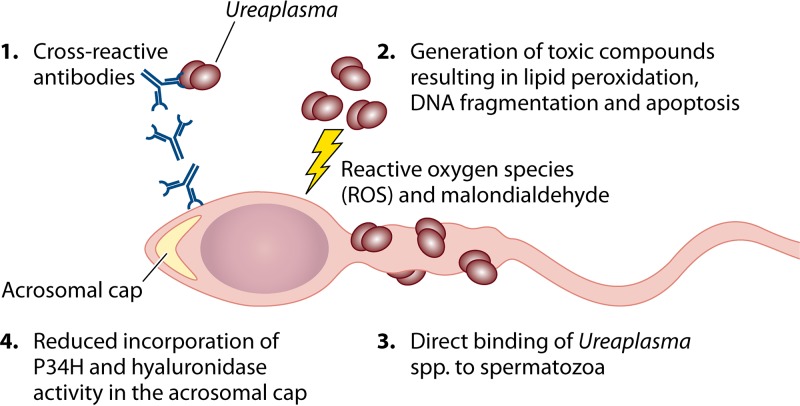
It is important to discuss the proposed mechanisms behind these observations that Ureaplasma spp. contribute to infertility among men (Fig. 2). Sexually transmitted pathogens are known to affect sperm quality by reducing motility, negatively affecting sperm morphology, and inducing apoptosis (5). Several mechanisms have been proposed to account for infertility in men because of Ureaplasma species colonization. These include the direct binding of Ureaplasma spp. to spermatozoa, which may impede swimming motility (59–62); the production of toxic metabolites, which can damage spermatozoon membranes and result in DNA fragmentation (54, 63); as well as the host generation of cross-reactive antibodies between Ureaplasma spp. and sperm surface proteins (64–66).
FIG 2.
Mechanisms associated with Ureaplasma species-induced infertility in men. A number of mechanisms have been proposed to account for the clinical observational studies showing decreased fertility among men who experience urethral colonization with Ureaplasma spp. These include (i) cross-reactivity of host-generated antibodies against the UreG protein of Ureaplasma spp. to the autoantigenic sperm protein; (ii) the generation of toxic compounds, such as reactive oxygen species (ROS), which contributes to lipid peroxidation, DNA fragmentation, and subsequent apoptosis; (iii) direct binding to spermatozoa, which may result in reduced motility; and (iv) reduced incorporation of P34H and hyaluronidase activity in the acrosomal cap, which may reduce the capacity of spermatozoa to penetrate the egg.
Work by Potts et al. identified 17 out of 50 chronic prostatitis patients to be positive for Ureaplasma spp., and the levels of reactive oxygen species (ROS) were significantly higher among the Ureaplasma-positive infertile patients than among the Ureaplasma-negative infertile patients and the control group (63). ROS have the potential to induce lipid peroxidation, therefore compromising the integrity of sperm membranes and leading to impaired fertilization capabilities. Of interest was the finding that only 1 out of 17 of the positive samples in which ROS levels were elevated showed signs of leukocytospermia, suggesting that in some cases traditional signs of prostatitis, such as leukocytospermia, may not be indicative of Ureaplasma species infection. The potential for Ureaplasma spp. to contribute to lipid peroxidation through the generation of ROS, as well as malondialdehyde formation, by either U. urealyticum or U. parvum was further developed and stratified (54). The levels of ROS, malondialdehyde formation, and DNA fragmentation were all significantly higher in U. urealyticum-positive samples than in U. parvum-positive samples. The high levels of ROS could therefore result in DNA fragmentation and subsequent apoptosis (67).
Some studies have suggested that the presence of Ureaplasma spp. in seminal fluid has no real impact on semen quality (68, 69). One possibility is that the mechanism associated with infertility is one which cannot be identified by classic markers of infertility but is one which may impact the interaction between the sperm and the egg. P34H is a key membrane-bound protein which is essential for sperm-zona pellucida interactions. P34H is incorporated into membranes covering the acrosomal cap as it transits across the epididymis and therefore can serve as a marker for epididymal function (67). The levels of P34H were significantly lower in the Ureaplasma species-positive group than in the control groups, as determined by Western blotting and immunofluorescence imaging, which identified that 38% of the sperm in the Ureaplasma species-positive group and 73% of the sperm in the control group had P34H. These data suggest a potential impact of chronic asymptomatic infection of the epididymis. The acrosomal cap also contains the enzyme hyaluronidase (HYD), which is essential for the sperm to penetrate the egg. In the Ureaplasma species-positive group, the levels of HYD activity were significantly different from those of the infertile Ureaplasma species-negative group and the fertile controls (67). By reducing the activity of HYD, the likelihood of successful sperm penetration into the egg is therefore reduced.
An alternative mechanism to Ureaplasma-related infertility is the development of cross-reactive antibodies to human sperm membrane proteins following exposure to Ureaplasma spp. (64–66). Shi et al. demonstrated that antibodies raised against the UreG protein of Ureaplasma spp. were able to cross-react with human nuclear autoantigenic sperm protein (NASP) (66). A higher titer of anti-UreG antibody was found in the serum of the infertile men than in that of the fertile controls. In an in vitro fertility assay, sperm which had been pretreated with anti-UreG antibodies had significantly lower binding and fusion to eggs than nontreated control sperm.
The evidence presented here suggests that the impact of Ureaplasma spp. on male infertility is similar to that described for NGU, although unlike in the context of NGU, the effect of the bacterial titer has yet to be investigated. The lack of species differentiation has hindered studies, but association as well as mechanistic studies are pointing toward a potential for U. urealyticum to be the primary Ureaplasma spp. associated with infertility in men. Furthermore, some of the traditional markers for infertility may not indicate a causal role for Ureaplasma spp. in male infertility.
TREATMENT OF GENITAL TRACT INFECTIONS IN MEN CAUSED BY UREAPLASMA SPP.
A position statement from the European STI Guidelines Editorial Board states that routine testing of asymptomatic or symptomatic men for the presence of Ureaplasma spp. is not recommended; however, one of the key messages that the authors made states that Ureaplasma urealyticum at high bacterial loads can cause a small proportion of cases of male NGU (3 to 11% of NGU cases) (4). The authors also noted that NGU caused by U. urealyticum was more likely to develop in younger men and men with fewer lifetime sexual partners. They highlight that there is a paucity of well-designed large controlled studies which investigate the role of Ureaplasma spp. in STI syndromes and NGU.
In the light of mounting evidence of a causal role for Ureaplasma spp. in the development of NGU and male infertility, the question remains whether we should treat individuals who are Ureaplasma spp. positive with symptoms. Currently, a position statement from the European STI Guidelines Editorial Board does not recommend routine testing or treatment of either asymptomatic or symptomatic men for any Ureaplasma spp.; however, this position statement also suggests that U. urealyticum is causal in up to 11% of NGU cases, which contradicts this recommendation (4). The evidence presented in this review suggests that in a subset of men with symptoms of NGU and the absence of other etiological factors, a risk-based approach could be used to guide the treatment of these patients. For example, symptomatic NGU patients with the absence of other sexually transmitted infections, younger age, a low number of partners, and high titers of U. urealyticum may benefit from treatment. Currently, it is clinically difficult to implement a risk-based approach in countries such as the United Kingdom, as sexual health care settings do not widely test for Ureaplasma spp., and when testing is done, it almost never involves the differentiation of U. urealyticum and U. parvum or determination of the bacterial load.
Currently, guidelines set out by the British Association for Sexual Health and HIV (BASHH) suggest treatment of a first episode of NGU with a 7-day course of doxycycline at 100 mg twice daily or, if contraindicated, azithromycin at 1 g stat., followed by 500 mg once daily for 2 days, or ofloxacin at 200 mg twice daily or 400 mg once daily for 7 days (70). With recurrent episodes of NGU where the possibility of reinfection has been excluded, the recommended first-line regimen is azithromycin at 1 g stat. and then at 500 mg once daily for the next 2 days plus metronidazole at 400 mg twice daily for 5 days. If symptoms still persist, treatment with moxifloxacin at 400 mg once daily for 10 to 14 days plus metronidazole at 400 mg twice daily for 5 days is the recommended regimen. It is considered reasonable to provide epidemiological treatment to the partners of men with NGU using the same antimicrobial regimen that resulted in cure in the index case. In practice, if Ureaplasma spp. were present and responsible for the symptoms of NGU, the first-line treatment recommended (a short course of doxycycline at 100 mg twice a day for 7 days) would be adequate to treat it in the United Kingdom at the moment, in light of the low levels of tet(M)-mediated doxycycline resistance among these organisms (71). Any decision to treat would need to be carefully weighed with the risk of inappropriate prescribing. Antibiotic resistance, in particular, azithromycin resistance, among recognized GUM pathogens, such as M. genitalium and N. gonorrhoeae, is of growing concern (72, 73).
CONCLUSIONS
The role that Ureaplasma spp. play in the development of genitourinary medicine-related infections is still a controversial area for many, but there is mounting evidence that these bacteria, especially U. urealyticum, have a causative role in infection under very specific conditions. Ureaplasma spp. are by no means a leading cause of NGU, but this is not to say that they do not contribute to cases which are currently classified as idiopathic; as such, these patients are no less deserving of attention or correct management. Furthermore, the role of Ureaplasma spp. in the development of infertility among men is beginning to be recognized, but further work exploring the mechanism, as well as appropriate criteria for identifying patients with Ureaplasma species-induced infertility, is required.
Ureaplasma spp. have a proven proinflammatory capacity in cell lines and in animal and human models of disease, but the species of Ureaplasma spp., the sexual history of the patient, and the titer of bacteria present all appear to be key risk factors for the development of disease. A large prospective case-controlled study considering the species and load of Ureaplasma, the presence of other microorganisms, the number of PMNs as a marker of inflammation, and the number of sexual partners will be crucial to confirm or refute the role of Ureaplasma spp. in the development of NGU in men. If a clear link is identified, then current qualitative diagnostic methods may not be appropriate for determining a causal role for Ureaplasma spp. in cases of NGU. In the meantime, in light of the evidence presented in this review, we recommend that among cases of symptomatic NGU in which classic etiological agents have been ruled out, a risk-based approach taking into account patients with a younger age, a low number of partners, and high titers of U. urealyticum should be considered for treatment.
ACKNOWLEDGMENT
We acknowledge The Royal Society for supporting this collaborative project through the International Exchange Scheme.
Biographies

Michael L. Beeton is a Lecturer in Medical Microbiology at Cardiff Metropolitan University and has been working on ureaplasmas for over 10 years. He obtained his Ph.D. from the Cardiff University School of Medicine in 2009, where his research focused on the incidence and molecular mechanisms of antibiotic resistance among Ureaplasma spp. isolated from preterm neonates. With an extensive publication history with regards to Ureaplasma and infectious disease, he currently sits on the Executive Committee for the European Society of Clinical Microbiology and the Infectious Diseases Study Group for Mycoplasma and Chlamydia Infections (ESGMAC). Furthermore, with an interest in sexually transmitted infections, he represents the Microbiology Society on the Public Health England External Advisory Group on Sexual Health, Reproductive Health and HIV. His current research interests are focused on developing rapid diagnostic tests for Ureaplasma as well as understanding the immune response to Ureaplasma infections.

Matthew S. Payne received his Ph.D. from the University of Queensland in 2007. Since then he has conducted microbiological research at Kings College London (London, UK) and La Trobe University (Melbourne, Australia) and is currently at the University of Western Australia (Perth, Australia). He is a molecular microbiologist whose research is focused on the microbiology of the perinatal period. He has specific interests in the perinatal microbiome, in particular, the use of microbial biomarkers to predict women at high risk of preterm birth and developing methods to accurately define microbial communities in low-biomass samples. Dr. Payne has specific interests in ureaplasmas and group B streptococcus (GBS) as pathogens, as well as the protective role of vaginal Lactobacillus spp. in pregnancy. He also has a significant interest in the use of bacteriophages as antimicrobial agents, particularly for use in the perinatal period, for removal of GBS as an alternative to intrapartum antibiotic prophylaxis.

Lucy Jones is an Associate Specialist in Sexual Health at the Cwm Taf University Health Board and an Honorary Lecturer at the Cardiff University School of Medicine. She is Chief Investigator on four clinical studies in the field of sexual health and has a specialist interest in nongonococcal urethritis, recurrent vaginitis, and antimicrobial resistance. She is Secretary to the British Association of Sexual Health and HIV, Wales. She completed her medical training and a doctorate in reproductive medicine at Oxford University before returning to live and work in Wales.
REFERENCES
- 1.Moi H, Blee K, Horner PJ. 2015. Management of non-gonococcal urethritis. BMC Infect Dis 15:294. doi: 10.1186/s12879-015-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetmore CM, Manhart LE, Lowens MS, Golden MR, Whittington WL, Xet-Mull AM, Astete SG, McFarland NL, McDougal SJ, Totten PA. 2011. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-comparison study. Sex Transm Dis 38:180–186. doi: 10.1097/OLQ.0b013e3182040de9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepard MC. 1954. The recovery of pleuropneumonia-like organisms from Negro men with and without nongonococcal urethritis. Am J Syph Gonorrhea Vener Dis 38:113–124. [PubMed] [Google Scholar]
- 4.Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, Unemo M. 2018. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?—a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol 32:1845–1851. doi: 10.1111/jdv.15146. [DOI] [PubMed] [Google Scholar]
- 5.Gimenes F, Souza RP, Bento JC, Teixeira JJ, Maria-Engler SS, Bonini MG, Consolaro ME. 2014. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol 11:672–687. doi: 10.1038/nrurol.2014.285. [DOI] [PubMed] [Google Scholar]
- 6.Rottem S, Pfendt EA, Hayflick L. 1971. Sterol requirements of T-strain mycoplasmas. J Bacteriol 105:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass JI, Lefkowitz EJ, Glass JS, Heiner CR, Chen EY, Cassell GH. 2000. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature 407:757–762. doi: 10.1038/35037619. [DOI] [PubMed] [Google Scholar]
- 8.Shepard MC, Lunceford CD, Ford DK, Purcell RH, Taylor-Robinson D, Razin S, Black FT. 1974. Ureaplasma urealyticum gen. nov., sp. nov.: proposed nomenclature for the human T (T-strain) mycoplasmas. Int J Syst Bacteriol 24:160–171. doi: 10.1099/00207713-24-2-160. [DOI] [Google Scholar]
- 9.Robertson JA, Stemke GW, Davis JW Jr, Harasawa R, Thirkell D, Kong F, Shepard MC, Ford DK. 2002. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol 52:587–597. doi: 10.1099/00207713-52-2-587. [DOI] [PubMed] [Google Scholar]
- 10.Capoccia R, Greub G, Baud D. 2013. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis 26:231–240. doi: 10.1097/QCO.0b013e328360db58. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney EL, Dando SJ, Kallapur SG, Knox CL. 2017. The human Ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev 30:349–379. doi: 10.1128/CMR.00091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viscardi RM. 2014. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed 99:F87–F92. doi: 10.1136/archdischild-2012-303351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharat A, Cunningham SA, Budinger GRS, Kreisel D, DeWet CJ, Gelman AE, Waites K, Crabb D, Xiao L, Bhorade S, Ambalavanan N, Dilling DF, Lowery EM, Astor T, Hachem R, Krupnick AS, DeCamp MM, Ison MG, Patel R. 2015. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med 7:284re3. doi: 10.1126/scitranslmed.aaa8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beeton ML. 2016. Possible missed diagnosis of Ureaplasma spp infection in a case of fatal hyperammonemia after repeat renal transplantation. J Clin Anesth 33:504–505. doi: 10.1016/j.jclinane.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Matson KM, Sonetti DA. 2019. Successful treatment of Ureaplasma-induced hyperammonemia syndrome post-lung transplant. Transpl Infect Dis 21:e13022. doi: 10.1111/tid.13022:e13022. [DOI] [PubMed] [Google Scholar]
- 16.Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. 2001. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis 32:995–1003. doi: 10.1086/319594. [DOI] [PubMed] [Google Scholar]
- 17.Wetmore CM, Manhart LE, Lowens MS, Golden MR, Jensen NL, Astete SG, Whittington WL, Totten PA. 2011. Ureaplasma urealyticum is associated with nongonococcal urethritis among men with fewer lifetime sexual partners: a case-control study. J Infect Dis 204:1274–1282. doi: 10.1093/infdis/jir517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu JT, Tang WY, Lau KH, Chong LY, Lo KK, Wong CK, Wong MY. 2008. Role of Mycoplasma genitalium and Ureaplasma urealyticum in non-gonococcal urethritis in Hong Kong. Hong Kong Med J 14:125–129. [PubMed] [Google Scholar]
- 19.Khatib N, Bradbury C, Chalker V, Koh GC, Smit E, Wilson S, Watson J. 2015. Prevalence of Trichomonas vaginalis, Mycoplasma genitalium and Ureaplasma urealyticum in men with urethritis attending an urban sexual health clinic. Int J STD AIDS 26:388–392. doi: 10.1177/0956462414539464. [DOI] [PubMed] [Google Scholar]
- 20.Frolund M, Bjornelius E, Lidbrink P, Ahrens P, Jensen JS. 2014. Comparison between culture and a multiplex quantitative real-time polymerase chain reaction assay detecting Ureaplasma urealyticum and U. parvum. PLoS One 9:e102743. doi: 10.1371/journal.pone.0102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ondondo RO, Whittington WL, Astete SG, Totten PA. 2010. Differential association of Ureaplasma species with non-gonococcal urethritis in heterosexual men. Sex Transm Infect 86:271–275. doi: 10.1136/sti.2009.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tully JG, Taylor-Robinson D, Cole RM, Rose DL. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288–1291. [DOI] [PubMed] [Google Scholar]
- 23.Soni S, Horner P, Rayment M, Pinto-Sander N, Naous N, Parkhouse A, Bancroft D, Patterson C, Fifer H. 2018. 2018 BASHH UK national guideline for the management of infection with Mycoplasma genitalium. British Association for Sexual Health and HIV, Cheshire, United Kingdom. [DOI] [PubMed] [Google Scholar]
- 24.Jänsch HH. 1972. Pathogenicity demonstration for urea splitting Mycoplasma in the human urogenital tract using a self-administered test. Hautarzt 23:558. [PubMed] [Google Scholar]
- 25.Taylor-Robinson D, Csonka GW, Prentice MJ. 1977. Human intra-urethral inoculation of ureplasmas. Q J Med 46:309–326. [PubMed] [Google Scholar]
- 26.Taylor-Robinson D, Purcell RH, London WT, Sly DL. 1978. Urethral infection of chimpanzees by Ureaplasma urealyticum. J Med Microbiol 11:197–201. doi: 10.1099/00222615-11-2-197. [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Robinson D. 2013. Urethral inflammation in male chimpanzees caused by ureaplasmas and Chlamydia trachomatis. J Med Microbiol 62:1609–1613. doi: 10.1099/jmm.0.058446-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhu GX, Lu C, Chen CJ, Feng PY, Ma H, Lu RB, Yuan YL. 2011. Pathogenicity of Ureaplasma urealyticum and Ureaplasma parvum in the lower genital tract of female BALB/c mice. Can J Microbiol 57:987–992. doi: 10.1139/w11-098. [DOI] [PubMed] [Google Scholar]
- 29.Furr PM, Taylor-Robinson D. 1989. The establishment and persistence of Ureaplasma urealyticum in oestradiol-treated female mice. J Med Microbiol 29:111–114. doi: 10.1099/00222615-29-2-111. [DOI] [PubMed] [Google Scholar]
- 30.Furr PM, Taylor-Robinson D. 1993. Factors influencing the ability of different mycoplasmas to colonize the genital tract of hormone-treated female mice. Int J Exp Pathol 74:97–101. [PMC free article] [PubMed] [Google Scholar]
- 31.Rowland SS, Falkler WA Jr, Bashirelahi N. 1992. Identification of an estrogen-binding protein in Pseudomonas aeruginosa. J Steroid Biochem Mol Biol 42:721–727. doi: 10.1016/0960-0760(92)90113-W. [DOI] [PubMed] [Google Scholar]
- 32.Tarry W, Fisher M, Shen S, Mawhinney M. 2005. Candida albicans: the estrogen target for vaginal colonization. J Surg Res 129:278–282. doi: 10.1016/j.jss.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Li YH, Brauner A, Jonsson B, van der Ploeg I, Soder O, Holst M, Jensen JS, Lagercrantz H, Tullus K. 2000. Ureaplasma urealyticum-induced production of proinflammatory cytokines by macrophages. Pediatr Res 48:114–119. doi: 10.1203/00006450-200007000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Sweeney EL, Kallapur SG, Meawad S, Gisslen T, Stephenson SA, Jobe AH, Knox CL. 2017. Ureaplasma species multiple banded antigen (MBA) variation is associated with the severity of inflammation in vivo and in vitro in human placentae. Front Cell Infect Microbiol 7:123. doi: 10.3389/fcimb.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beeton ML, Spiller OB. 2017. Antibiotic resistance among Ureaplasma spp. isolates: cause for concern? J Antimicrob Chemother 72:330–337. doi: 10.1093/jac/dkw425. [DOI] [PubMed] [Google Scholar]
- 36.Povlsen K, Bjornelius E, Lidbrink P, Lind I. 2002. Relationship of Ureaplasma urealyticum biovar 2 to nongonococcal urethritis. Eur J Clin Microbiol Infect Dis 21:97–101. doi: 10.1007/s10096-001-0665-1. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T, Ishiko H, Yasuda M, Takahashi Y, Nomura Y, Kubota Y, Tamaki M, Maeda S, Deguchi T. 2005. Polymerase chain reaction-based subtyping of Ureaplasma parvum and Ureaplasma urealyticum in first-pass urine samples from men with or without urethritis. Sex Transm Dis 32:454–457. doi: 10.1097/01.olq.0000158932.78183.95. [DOI] [PubMed] [Google Scholar]
- 38.Couldwell DL, Gidding HF, Freedman EV, McKechnie ML, Biggs K, Sintchenko V, Gilbert GL. 2010. Ureaplasma urealyticum is significantly associated with non-gonococcal urethritis in heterosexual Sydney men. Int J STD AIDS 21:337–341. doi: 10.1258/ijsa.2009.009499. [DOI] [PubMed] [Google Scholar]
- 39.Bradshaw CS, Tabrizi SN, Read TR, Garland SM, Hopkins CA, Moss LM, Fairley CK. 2006. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis 193:336–345. doi: 10.1086/499434. [DOI] [PubMed] [Google Scholar]
- 40.Zhang N, Wang R, Li X, Liu X, Tang Z, Liu Y. 2014. Are Ureaplasma spp. a cause of nongonococcal urethritis? A systematic review and meta-analysis. PLoS One 9:e113771. doi: 10.1371/journal.pone.0113771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox C, McKenna JP, Watt AP, Coyle PV. 2016. Ureaplasma parvum and Mycoplasma genitalium are found to be significantly associated with microscopy-confirmed urethritis in a routine genitourinary medicine setting. Int J STD AIDS 27:861–867. doi: 10.1177/0956462415597620. [DOI] [PubMed] [Google Scholar]
- 42.Teng LJ, Zheng X, Glass JI, Watson HL, Tsai J, Cassell GH. 1994. Ureaplasma urealyticum biovar specificity and diversity are encoded in multiple-banded antigen gene. J Clin Microbiol 32:1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi J, Yoon BH, Kim EC. 2005. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes 19:255–260. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham SA, Mandrekar JN, Rosenblatt JE, Patel R. 2013. Rapid PCR detection of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum. Int J Bacteriol 2013:168742. doi: 10.1155/2013/168742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frolund M, Lidbrink P, Wikstrom A, Cowan S, Ahrens P, Jensen JS. 2016. Urethritis-associated pathogens in urine from men with non-gonococcal urethritis: a case-control study. Acta Derm Venereol 96:689–694. doi: 10.2340/00015555-2314. [DOI] [PubMed] [Google Scholar]
- 46.Brown MB, Cassell GH, Taylor-Robinson D, Shepard MC. 1983. Measurement of antibody to Ureaplasma urealyticum by an enzyme-linked immunosorbent assay and detection of antibody responses in patients with nongonococcal urethritis. J Clin Microbiol 17:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimada Y, Ito S, Mizutani K, Sugawara T, Seike K, Tsuchiya T, Yokoi S, Nakano M, Yasuda M, Deguchi T. 2014. Bacterial loads of Ureaplasma urealyticum contribute to development of urethritis in men. Int J STD AIDS 25:294–298. doi: 10.1177/0956462413504556. [DOI] [PubMed] [Google Scholar]
- 48.Deguchi T, Shimada Y, Horie K, Mizutani K, Seike K, Tsuchiya T, Yokoi S, Yasuda M, Ito S. 2015. Bacterial loads of Ureaplasma parvum contribute to the development of inflammatory responses in the male urethra. Int J STD AIDS 26:1035–1039. doi: 10.1177/0956462414565796. [DOI] [PubMed] [Google Scholar]
- 49.Gnarpe H, Friberg J. 1973. T-mycoplasmas as a possible cause for reproductive failure. Nature 242:120–121. doi: 10.1038/242120a0. [DOI] [PubMed] [Google Scholar]
- 50.Irajian G, Sharifi M, Mirkalantari S, Mirnejad R, Jalali Nadoushan MR, Ghorbanpour N. 2016. Molecular detection of Ureaplasma urealyticum from prostate tissues using PCR-RFLP, Tehran, Iran. Iran J Pathol 11:138–143. [PMC free article] [PubMed] [Google Scholar]
- 51.Skerk V, Mareković I, Markovinović L, Begovac J, Skerk V, Barsić N, Majdak-Gluhinić V. 2006. Comparative randomized pilot study of azithromycin and doxycycline efficacy and tolerability in the treatment of prostate infection caused by Ureaplasma urealyticum. Chemotherapy 52:9–11. doi: 10.1159/000090234. [DOI] [PubMed] [Google Scholar]
- 52.Badalyan RR, Fanarjyan SV, Aghajanyan IG. 2003. Chlamydial and ureaplasmal infections in patients with nonbacterial chronic prostatitis. Andrologia 35:263–265. doi: 10.1111/j.1439-0272.2003.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 53.Zeighami H, Peerayeh SN, Yazdi RS, Sorouri R. 2009. Prevalence of Ureaplasma urealyticum and Ureaplasma parvum in semen of infertile and healthy men. Int J STD AIDS 20:387–390. doi: 10.1258/ijsa.2008.008334. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q, Xiao Y, Zhuang W, Cheng B, Zheng L, Cai Y, Zhou H, Wang Q. 2014. Effects of biovar I and biovar II of Ureaplasma urealyticum on sperm parameters, lipid peroxidation, and deoxyribonucleic acid damage in male infertility. Urology 84:87–92. doi: 10.1016/j.urology.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Abusarah EA, Awwad ZM, Charvalos E, Shehabi AA. 2013. Molecular detection of potential sexually transmitted pathogens in semen and urine specimens of infertile and fertile males. Diagn Microbiol Infect Dis 77:283–286. doi: 10.1016/j.diagmicrobio.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, Zhu WB. 2015. Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology 3:809–816. doi: 10.1111/andr.12078. [DOI] [PubMed] [Google Scholar]
- 57.Huang C, Long X, Jing S, Fan L, Xu K, Wang S, Zhu W. 2016. Ureaplasma urealyticum and Mycoplasma hominis infections and semen quality in 19,098 infertile men in China. World J Urol 34:1039–1044. doi: 10.1007/s00345-015-1724-z. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Liang CL, Wu JQ, Xu C, Qin SX, Gao ES. 2006. Do Ureaplasma urealyticum infections in the genital tract affect semen quality? Asian J Androl 8:562–568. doi: 10.1111/j.1745-7262.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 59.Knox CL, Allan JA, Allan JM, Edirisinghe WR, Stenzel D, Lawrence FA, Purdie DM, Timms P. 2003. Ureaplasma parvum and Ureaplasma urealyticum are detected in semen after washing before assisted reproductive technology procedures. Fertil Steril 80:921–929. doi: 10.1016/S0015-0282(03)01125-7. [DOI] [PubMed] [Google Scholar]
- 60.Xu C, Sun GF, Zhu YF, Wang YF. 1997. The correlation of Ureaplasma urealyticum infection with infertility. Andrologia 29:219–226. [DOI] [PubMed] [Google Scholar]
- 61.Núñez-Calonge R, Caballero P, Redondo C, Baquero F, Martínez-Ferrer M, Meseguer MA. 1998. Ureaplasma urealyticum reduces motility and induces membrane alterations in human spermatozoa. Hum Reprod 13:2756–2761. doi: 10.1093/humrep/13.10.2756. [DOI] [PubMed] [Google Scholar]
- 62.Gnarpe H, Friberg J. 1973. T mycoplasmas on spermatozoa and infertility. Nature 245:97–98. doi: 10.1038/245097a0. [DOI] [PubMed] [Google Scholar]
- 63.Potts JM, Sharma R, Pasqualotto F, Nelson D, Hall G, Agarwal A. 2000. Association of Ureaplasma urealyticum with abnormal reactive oxygen species levels and absence of leukocytospermia. J Urol 163:1775–1778. doi: 10.1016/S0022-5347(05)67540-4. [DOI] [PubMed] [Google Scholar]
- 64.Ye YK, Lu DY, Zhu YF, Peng W, Wang YF. 1994. Study on the common antigen between human sperm and Ureaplasma urealyticum. J Androl 8:1–4. [Google Scholar]
- 65.Wu AW, Huang GL, Lin TF, Shen JL. 1996. Analyses of the common antigen between the membrane protein of sperm and Ureaplasma urealyticum. J Androl 10:203–205. [Google Scholar]
- 66.Shi J, Yang Z, Wang M, Cheng G, Li D, Wang Y, Zhou Y, Liu X, Xu C. 2007. Screening of an antigen target for immunocontraceptives from cross-reactive antigens between human sperm and Ureaplasma urealyticum. Infect Immun 75:2004–2011. doi: 10.1128/IAI.01171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma XP, Gao XQ. 2017. The effect of Ureaplasma urealyticum on the level of P34H expression, the activity of hyaluronidase, and DNA fragmentation in human spermatozoa. Am J Reprod Immunol 77:e12600. doi: 10.1111/aji.12600. [DOI] [PubMed] [Google Scholar]
- 68.Andrade-Rocha FT. 2003. Ureaplasma urealyticum and Mycoplasma hominis in men attending for routine semen analysis. Prevalence, incidence by age and clinical settings, influence on sperm characteristics, relationship with the leukocyte count and clinical value. Urol Int 71:377–381. doi: 10.1159/000074089. [DOI] [PubMed] [Google Scholar]
- 69.Soffer Y, Ron-El R, Golan A, Herman A, Caspi E, Samra Z. 1990. Male genital mycoplasmas and Chlamydia trachomatis culture: its relationship with accessory gland function, sperm quality, and autoimmunity. Fertil Steril 53:331–336. doi: 10.1016/S0015-0282(16)53290-7. [DOI] [PubMed] [Google Scholar]
- 70.British Association for Sexual Health and HIV. 2017. Update to the 2015 BASHH UK National Guideline on the management of non-gonococcal urethritis. British Association for Sexual Health and HIV, Cheshire, United Kingdom. [Google Scholar]
- 71.Beeton ML, Chalker VJ, Jones LC, Maxwell NC, Spiller OB. 2016. Antibiotic resistance among clinical Ureaplasma isolates recovered from neonates in England and Wales between 2007 and 2013. Antimicrob Agents Chemother 60:52–56. doi: 10.1128/AAC.00889-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fifer H, Cole M, Hughes G, Padfield S, Smolarchuk C, Woodford N, Wensley A, Mustafa N, Schaefer U, Myers R, Templeton K, Shepherd J, Underwood A. 2018. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: an observational study. Lancet Infect Dis 18:573–581. doi: 10.1016/S1473-3099(18)30122-1. [DOI] [PubMed] [Google Scholar]
- 73.Unemo M, Jensen JS. 2017. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol 14:139–152. doi: 10.1038/nrurol.2016.268. [DOI] [PubMed] [Google Scholar]