The skin is an organ harboring several types of immune cells that participate in innate and adaptive immune responses. The immune system of the skin comprises both skin cells and professional immune cells that together constitute what is designated skin-associated lymphoid tissue (SALT). In this review, I extensively discuss the organization of SALT and the mechanisms involved in its responses to infectious diseases of the skin and mucosa.
KEYWORDS: skin, cytokines, immune response, infections, innate immunity, keratinocytes, lymphocytes
SUMMARY
The skin is an organ harboring several types of immune cells that participate in innate and adaptive immune responses. The immune system of the skin comprises both skin cells and professional immune cells that together constitute what is designated skin-associated lymphoid tissue (SALT). In this review, I extensively discuss the organization of SALT and the mechanisms involved in its responses to infectious diseases of the skin and mucosa. The nature of these SALT responses, and the cellular mediators involved, often determines the clinical course of such infections. I list and describe the components of innate immunity, such as the roles of the keratinocyte barrier and of inflammatory and natural killer cells. I also examine the mechanisms involved in adaptive immune responses, with emphasis on new cytokine profiles, and the role of cell death phenomena in host-pathogen interactions and control of the immune responses to infectious agents. Finally, I highlight the importance of studying SALT in order to better understand host-pathogen relationships involving the skin and detail future directions in the immunological investigation of this organ, especially in light of recent findings regarding the skin immune system.
INTRODUCTION
The concept of organ-specific immunity and its implications on the evolution of diseases, especially infectious diseases, were proposed by Engwerda and Kaye in 2000 (1). In this context, it is possible to divide the pattern of tissue immune responses into three major groups: barrier epithelium immune responses, complex organ immune responses, and immunologically privileged organ immune responses (2). The skin is an archetype of organs involved in mechanical and immunological barriers to pathogens and in body homeostasis (3–7). Tissues that form host–environment interfaces, including mucosal surfaces, represent the primary stage of defense against pathogenic microorganisms (8–11). The skin has a similar organization over most of the body, but certain regions, such as the feet, scalp, and genitalia, have specific and highly specialized adaptations. The skin comprises the epidermis, dermis, cutaneous appendages, and subcutaneous tissue (12). It has a complex immune system that is histologically represented by skin-associated lymphoid tissue (SALT), which includes dendritic cells (DCs), mast cells, B and T lymphocytes, and keratinocytes (13). In certain situations, such cells may modulate the cascade of the local immune responses (14, 15). SALT acts by protecting the body against foreign microorganisms and plays a role in the pathophysiology of inflammatory diseases such as autoimmune and hypersensitivity disorders (8–13). Such diseases have pathogenetic mechanisms that are generally mediated by T lymphocytes and respond to immunosuppressive or anti-inflammatory therapy (7).
The epidermis is constituted by three major cell populations: keratinocytes, melanocytes, and Langerhans cells (LCs). It has four distinct layers: the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum (12, 13). The stratum basale (or basal stratum) is composed of a layer of undifferentiated cells in constant mitotic activity (16). From the basal stratum, cells differentiate to form the stratum spinosum (or spinous stratum), which produces keratin. The stratum granulosum is composed of keratinocytes that have blackened granules in their cytoplasm and produce keratin-forming proteins, in addition to lipids (17, 18). Finally, the stratum corneum contains keratinocytes in later stages of maturity. It eliminates most agents that are potentially toxic to the organism and helps maintain hydroelectrolytic homeostasis (18). In the basal stratum, melanocytes help protect DNA from UV radiation by producing melanin and by promoting expression of major histocompatibility complex class II (MHC-II) proteins (19–24). Merkel cells in the epidermis are responsible for skin somesthesia (sensibility), along with LCs, whose functions are discussed below. In addition to these cells, in the basal and corneum layers are T lymphocytes, most of which are CD8+ lymphocytes (4, 7, 9, 14, 17, 18).
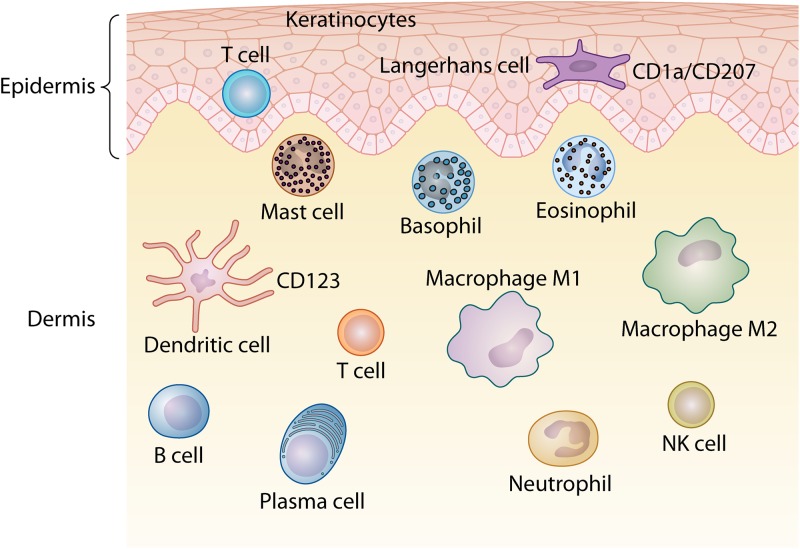
The dermis is characterized by a complex organization and a diverse immune cell population. This region contains specialized immune cells such as DCs, CD4+ T lymphocytes, γδ T lymphocytes, natural killer (NK) cells, mast cells, macrophages, neurons, lymphatic vessels, and blood vessels, which are responsible for the efflux of cells from the blood into the skin (Fig. 1) (7, 9, 13, 15, 16).
FIG 1.
Schematic representation of the distribution of immune cells that form the skin-associated lymphoid tissue (SALT) in the epidermis and dermis of the skin.
IMMUNE SYSTEM AND THE SKIN
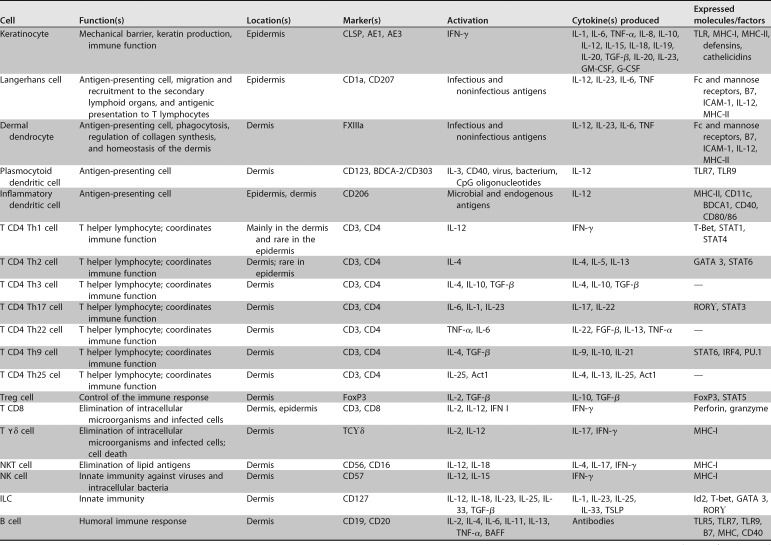
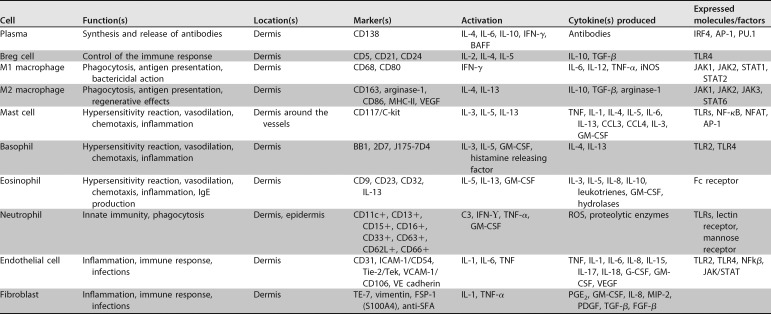
The skin has a complex network of cells that constitute SALT. Table 1 presents the various cellular components that make up the skin immune system (13). In addition to the immune cells themselves, certain skin cells, such as keratinocytes and melanocytes, may also affect local immune responses by releasing cytokines, which can modulate inflammatory responses (13). Therefore, the skin is an immunologically complex organ that has the ability to respond to infectious and noninfectious aggressive agents through innate and adaptive immunity mechanisms (9, 14, 15).
TABLE 1.
Cells that make up the immune system of the skin, its specific markers, activation factors and factors released in the cascade of immune responsea
G-CSF, granulocyte colony-stimulating factor; VEGF, vascular endothelial growth factor; PGE2, prostaglandin E2; PDGF, platelet-derived growth factor; FSP-1, fibroblast-specific protein 1.
Keratinocytes
Keratinocytes are epithelial cells that are interconnected by intercellular bridges (tight junctions). They play important roles in maintaining the mechanical and barrier functions of the epidermis, and they contribute to the pathophysiology of infectious and inflammatory processes by producing cytokines and by expressing MHC class I and II glycoproteins (13, 15, 25, 26). Similar to the epithelial cells of the gastrointestinal tract, keratinocytes can distinguish between the microbiota and potentially pathogenic agents, and they can recognize highly conserved structures known as pathogen-associated molecular patterns (PAMPs) (13, 27–29). PAMPs are often important for the survival, colonization, or invasion of microorganisms and are generally composed of lipopolysaccharides (LPSs), flagellins, nucleic acids, unmethylated CpG DNA sequences, teichoic acids, mannose-rich oligosaccharides, and other compounds (29, 30). The recognition of PAMP ensures that there is no activation of mechanisms to escape the host immune response, as is observed in the pathophysiology of some infections (31, 32). Molecules that bind to PAMPs are known as pattern recognition receptors (PPRs) and include the Toll-like receptors (TLRs) (29). The activation of TLR pathways occurs upon binding of PAMPs and induces a cascade of innate and adaptive immunity. Several TLRs are expressed in epidermal keratinocytes and are located on the cell surface (TLR1, TLR2, TLR4, TLR5, and TLR6) as well as in endosomes (TLR3 and TLR9). Moreover, TLR7 expression may be induced by the activation of TLR3 by double-stranded RNA molecules involved in immune responses to viruses (29). These factors indicate that the activation of TLRs by keratinocytes is crucial for triggering dermal immune responses, leading to a predominantly T helper cell type 1 (Th1) response accompanied by the activation of type I interferons (IFNs), including interferon alpha (IFN-α) and beta (IFN-β) (29, 30).
Similar to TLRs, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) can recognize PAMPs and endogenous PAMP analogs, danger-associated molecular patterns (DAMPs) (29, 33). They play important roles in the recognition of substances such as irritants and toxins. The induction of NLRs results in the activation of proinflammatory signaling pathways through inflammasomes, which are protein complexes composed of an NLR, an adapter protein termed ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain [CARD]), and pro-caspase-1 (29, 33). Activation of caspase-1 through inflammation leads to the cleavage of pro-interleukin-1β (pro-IL-1β) and pro-IL-18, which, in turn, leads to the expression of proinflammatory cytokines that activate immune cells and induce an elaborate immune response to harmful agents (34–36).
Keratinocytes produce antimicrobial peptides (AMPs) called defensins and cathelicidins, which are part of a highly conserved eukaryotic cell defense mechanism. AMPs are expressed on the injured epithelium to counteract the invasion of microorganisms by killing pathogens, activating immune cells, or modulating cytokine profiles (37). Keratinocytes produce cytokines such as IL-1, IL-6, IL-10, IL-17, IL-18, IL-22, and tumor necrosis factor alpha (TNF-α). In the presence of an infection, IL-17 and IL-22 can activate the production of AMPs by keratinocytes, constituting a form of innate immune response (38–44). Particular importance has been attached to the pleotropic cytokine IL-1, which is capable of inducing a broad spectrum of biological effects, including the activation of helper T cells and DCs and the maturation and clonal expansion of B cells (43). Under physiologic conditions, keratinocytes secrete both IL-1α and IL-1β in biologically inactive forms. IL-1β is released following the activation of inflammasomes in response to UV radiation. IL-1α appears to be involved in the induction of inflammatory lesions in psoriasis, with neutrophil infiltration into the epidermis, by increasing the expression of chemokine (C-C motif) ligand 20 (CCL20) by keratinocytes. Studies have demonstrated that a reduction in the level of caspase-8 leads to active IL-1α secretion from the pro-IL-1α pool in mouse keratinocytes (43).
The activation of chemokine C-X-C motif ligand 9 (CXCL9), CXCL10, CXCL11, and CCL20 by keratinocytes can attract effector T cells to the skin in diseases characterized by T cell infiltration, such as psoriasis (44). The expression of CXCL1 and CXCL8 by keratinocytes may attract neutrophils to the epidermis in inflammatory diseases such as psoriasis, and furthermore, CCL20 may influence the trafficking to the epithelium of LC precursors (45, 46). Keratinocytes can express MHC-II in various skin diseases characterized by leukocyte infiltration, which implies that they function as nonspecific antigen-presenting cells (APCs). Keratinocytes can also induce CD4+ and CD8+ T lymphocyte responses by expressing Th1 or Th2 cytokines (46, 47). The activation of keratinocytes is important in inflammation and precedes the influx of lymphocytes into the epidermis following skin reactions to inflammatory mediators. The participation of keratinocytes in various skin immune response mechanisms implies their significant roles in the induction of inflammatory processes and in the control of skin conditions, such as cutaneous leishmaniasis, human papillomavirus (HPV), and staphylococcus infections (48–52).
Melanocytes
Melanocytes produce melanin, a pigment that is responsible for skin color and protection of keratinocytes against the harmful action of UV radiation, which can induce DNA damage (19–21). These cells derive from the neural crest and can control innate and adaptive local immune responses, promoting pathogen phagocytosis and the production of cytokines such as IL-1β, IL-6, TNF-α, and chemokines. In the pathogenesis of autoimmune diseases such as vitiligo, melanocytes express MHC-II, and possibly also molecules recognized by cytotoxic T lymphocytes (19–22). A number of studies have shown that skin melanocytes can be compromised by infectious viral agents such as alphavirus, varicella-zoster virus, and parvovirus and by bacteria such as Mycobacterium leprae and Leptospira, among others (3, 23). This dual role of melanocytes, functioning both as targets and as a source of immune factors, along with their involvement in the pathophysiology of important infectious diseases, such as HIV and hepatitis C virus (HCV), makes the alteration of skin pigmentation an important symptom observed in the clinical course of these infections (24, 53). Upon contact with infectious agents of diverse nature, melanocytes can also express a variety of TLRs, including TLR1, -2, -3, -4, -6, -7, and -9 (22), with release of IL-6 and IL-8, and show increased chemokine mRNA expression involved in the recruitment of leukocytes, such as CCL2, CCL3, and CCL5 (3, 20, 22). Finally, similar to keratinocytes and other cells with immune functions in skin, melanocytes may express IFN type I and constitute the first line of defense against viruses in the skin (54, 55).
APCs and Skin Immunology
In general, skin APCs are localized in the epidermis and dermis (Table 1). Skin APCs have been classified according to their embryogenic origin. In this sense, we can classify subpopulations of APCs as classic APCs or classical DCs, which are represented by a cell population with stellate morphology, high expression of MHC-II, and the ability to migrate to the lymph nodes, activating naive T lymphocytes (56–60). A second population of APCs of the skin is represented by tissue-resident macrophages, monocytes, and other monocyte-derived cells (58, 59).
More recent ontogeny studies have shown that LCs are resident macrophages of the epidermis. However, despite this finding, LCs have functional characteristics similar to those of classic DCs, especially the ability to migrate to the lymph nodes and stimulate T lymphocytes responses (57). Studies of ontogeny, coupled with the functional characterization of LCs, have led to a great controversy in recent years concerning the identity of LCs, which were reinforced by transcriptomic analysis (56, 57). These analyses indicated that LCs are a distinct population of both DCs and macrophages, suggesting that the epidermal tissue microenvironment acts in the early stages of development of LCs, modulating their biological characteristics, despite their ontogeny (57). In this way, we can functionally consider LCs a subtype of DCs for the purpose of our discussion (59, 60).
LCs are the major DCs dispersed in the epidermis, whereas dermal DCs are located in the skin, immediately below the dermal-epidermal junction (13, 61–65).
DCs consist of several subpopulations of APCs that play important roles in the activation of immune responses (Table 1). They are produced continuously from hematopoietic precursors in the bone marrow and are distributed as immature cells in both lymphoid and nonlymphoid tissues (65, 66). The term dendritic cells was first used in 1973 by Steinman and Cohn based on their studies of the morphology of cells found in the lymph nodes of mice (66).
Four distinct human DC subtypes have been delineated based on biological studies of these cells in human skin: LCs, dermal dendrocytes (DDs), plasmocytoid dendritic cells (pDCs), and inflammatory dendritic cells (iDCs) (Table 1). LCs and DDs express myeloid markers, including CD13 and CD33 (13, 61–72). However, LCs exclusively express CD1a, Birbeck granules, langerin, and E-cadherin adhesion molecule (73). In contrast, DDs exclusively express the blood coagulation factor XIIIa. LCs and DDs may activate naive CD4+ and CD8+ T cells, with subsequent secretion of IL-12 (74–76). However, DDs can induce the differentiation of naive B cells into immunoglobulin-secreting plasma cells, whereas LCs cannot (Table 1) (77, 78).
pDCs resemble immunoglobulin-secreting plasma cells when visualized by ultrastructural microscopy. They are found in the thymus, blood tissues, and the lymph node T cell zone. pDCs secrete large quantities of interferon alpha following viral stimulation (13, 71, 79). In this context, precursor pDCs in blood correspond to natural IFN-α-producing cells, suggesting that they play an important role during viral infections. Unlike LCs and DDs, IL-3 induces pDC differentiation and promotes the expression of the myeloid markers CD14, CD13, and CD33. pDCs are devoid of mannose receptors and express high levels of CD123. However, like LCs and DDs, pDCs also activate CD4+ and CD8+ antigen-specific T lymphocytes and secrete IL-12 during viral infections (Table 1) (13, 71, 79, 80).
iDCs are characterized by the expression of CD206 (macrophage mannose receptor) and are observed in inflamed epidermal tissues of patients with various inflammatory skin diseases. These cells express an Fc-like receptor that has a high affinity for immunoglobulin E (IgE) and is involved in allergen-specific inflammatory processes. Although these cellular activities have been described to occur in the epidermis, they are involved in inflammatory processes in both the epidermis and dermis (Table 1) (67, 80).
Three main characteristics are attributed to DCs: capture, processing, and antigen presentation to T cells (67).
Endocytic activity is high in immature DCs, whereas this characteristic is less apparent in mature cells. DCs degrade antigens within the MHC class II endosomal compartment and subsequently display them on their surface bound to MHC-II (81, 82). Such antigens are captured by phagocytosis involving membrane receptors, and DCs can capture whole cells, including those undergoing necrosis and apoptosis (67). In contact with foreign particles of microbial or endogenous origin, the process of maturation of DCs begins with the expression of MHC class II glycoproteins, with increased ability of DCs to capture antigens. Following antigen incorporation, molecules that facilitate migration to the lymph nodes adhere to DCs (68–72). The DCs then interact with lymphocytes, and there is an increase in the expression of cytokines and costimulatory molecules. The activation of costimulatory molecules in DCs induces signals that cooperate with T cell receptors (TCRs) to promote T cell activation. Among these costimulatory molecules, B7-1 (CD80) and B7-2 (CD86) molecules are expressed by DCs, macrophages, and lymphocytes. B7 stimulates molecules present on T lymphocytes, known as CD28, which are responsible for the secondary signs of T lymphocyte activation (83). Programmed cell death-1 (PD-1), encoded by the PDCD1 gene, is a 288-amino-acid receptor that belongs to the B7/CD28 superfamily and is involved in the regulation of T cell activity. It is expressed on the surface of T, B, and NK cells and some subclasses of DCs and monocytes, as well as in the cytoplasm of T CD4+ and Treg lymphocytes (84–87). There is also an increase in the level of MHC-II expression in DCs. DC maturation is complete when they receive a stimulus to migrate to lymphatic tissues. DCs provide three signals that allow antigen-specific CD4+ T cells to initiate proliferation and differentiation (68–72). They capture antigens and readily degrade them to produce antigenic linear peptides of at least 13 amino acids in length that are capable of binding to MHC class II molecules and are trimmed by peptidases to no more than 17 amino acids in length (88). Subsequently, DCs express on their surface a high density of MHC class II/peptide complexes for TCR recognition expressed by T CD4+ cells (signal 1) and costimulation receptors CD40 and B7 to induce the proliferation of CD4+ T lymphocytes (signal 2) (89). IL-12 secretion by DCs (signal 3) induces CD4+ lymphocyte maturation in cell types 1 and 2. Dendritic cells are quite heterogeneous in the skin, but individual subsets can be identified by the careful use of markers. However, a successful functional analysis of these subsets of dendritic cells requires systems that allow the analysis of individual subsets, with the use of techniques involving separation of cell populations, culture in vitro, and antigenic challenge of these cells with specific antigens (Table 1) (57, 90).
Inflammasomes and Infectious Skin Diseases
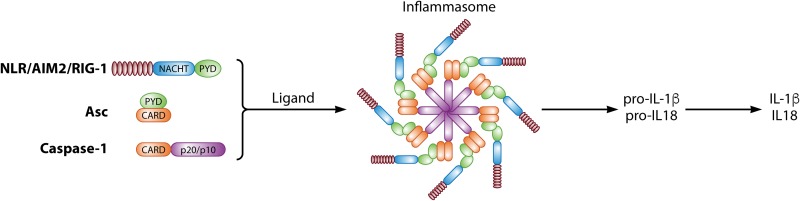
The inflammasomes are constituted by a group of complex proteins of innate immunity (Fig. 2). They act as mediators of inflammatory responses to PAMPs and DAMPs. Inflammasomes induce the activation of caspase-1, the secretion of IL-1β and IL-18, and pyroptotic cell death (91, 92). Depending on the type of caspase involved, they can be classified into one of two types of signaling pathways: classical/canonical, which requires the activation of caspase-1, and noncanonical, which uses other caspases to cause inflammation (91–93).
FIG 2.
Schematic representation of the components of the inflammasome and its role in the production of proinflammatory cytokines. NLRP inflammasome activation can be induced by cytosolic PAMPs and DAMPs, leading to IL-1β and IL-18 activation. Activation of caspase-1 by the inflammasome may also induce pyropitosis.
The PRRs involved in the formation of inflammasomes include the NLRs. Their basic structure consists of a variable N-terminal effector domain, a central NACHT (central nucleotide binding domain/NOD) domain, and a leucine-rich repeat (LRR) C-terminal region (92). There are 22 types and four subfamilies of NLRs in humans, classified according to their N-terminal acid activation domain (NLRA), BIR (baculovirus-inhibitor-repeat)-type domain (NLRB), activation and recruitment of caspase domain (NLRC), and pyrin domain (NLRP) (91). The accessory molecule ASC is an adapter protein common to several inflammasomes. Two protein domains constitute this molecule: an N-terminal PYD (pyrin domain) and a C-terminal CARD. These molecules actively participate during the development of the inflammasome, establishing the connection between the NLR and pro-caspase (Fig. 2). Twelve caspases are known in humans and are classified into inflammatory caspases (caspase-1, -4, and -5), and apoptotic caspases (which are further divided into initiator caspase-2, -8, -9, and -10 and executioner caspase-3, -6, and -7). Caspase-1 is the key inflammatory caspase that activates IL-1β and IL-18 cytokines and the pyroptotic mediator gasdermin D (91, 93). Other caspases may also be involved in the release of cytokines and pyroptosis. Caspase-4 and -5 are activated by Gram-negative bacteria, which may cleave gasdermin D, contributing to pyroptosis. Caspase-8 can induce inflammatory responses and apoptotic cell death; it forms the inflammasome and leads to the activation of IL-1β, IL-18, and gasdermin D. Caspase-8 participates in the induction of a noncanonical pathway that activates IL-1β independently of caspase-1, through the dectin-1 receptor (also known as a lectin C receptor family member) (91, 93).
The inflammasome canonical pathway activation begins when NLRs recognize an intracellular PAMP or DAMP, which leads to the recruitment of the adapter ASC in a PYD-PYD binding step. Subsequently, via a CARD-CARD linkage, pro-caspase-1 binds to the ASC (Fig. 2). This complex leads to the canonical activation of the inflammasome, resulting in the production of IL-1β and IL-18 and induction of pyroptosis (93).
The inflammasome noncanonical pathway can follow two routes. In the first, caspases-4 and -5 are activated by LPS from Gram-negative bacteria, activating gasdermin D, causing pyroptosis, which triggers the activation of the NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome. This attracts caspase-1, resulting in production of IL-1β. The second noncanonical pathway involves the activation of caspase-8 following the recognition of PAMPs by C-type lectin receptors, which leads to the production of IL-1β independently of caspase-1 (Fig. 2) (91, 93).
Inflammasomes have an important role in the host immune response to certain skin infectious diseases, such as cutaneous leishmaniasis and leprosy (93–95). In cutaneous leishmaniasis, the response of macrophages begins when the parasite invades and multiplies within the macrophage, which can kill the parasite through the production of free radicals (94). Macrophages may also act as APCs for T lymphocytes. The role of the inflammasome in macrophages infected with species of the genus Leishmania was evaluated by Lima-Junior and colleagues (95). These authors demonstrated that, in macrophages infected by Leishmania amazonensis, caspase-1 and IL-1β are activated (95). IL-1β production is caspase-1 dependent, because casp1−/− animals do not produce caspase-1 or IL-1β upon infection with L. amazonensis. IL-1β expression and caspase-1 activation were lower in cells treated with L. amazonensis than in cells treated with LPS and nigericin (NLRP3 activators), indicating that the parasite inhibits inflammasome activation in some cases (95, 96). The functions of NLRP3 in parasite elimination were demonstrated in an assay in which macrophages of Nos2−/−, Asc−/−, casp1−/−, or NLRP3−/− mice, when infected with Leishmania, had a higher number of infected cells and more amastigotes (the tissue stage of the parasite) than macrophages from normal animals. Furthermore, potassium, cathepsins, and K+ channels are required for L. amazonensis to activate NLRP3 (97, 98). Inflammasome-deficient cells did not show increased leishmanicidal activity or nitric oxide (NO) production when treated with exogenous IL-1β. That is, inflammasomes seem to be involved in the process of macrophage resistance (99–101). L. amazonensis-infected macrophages show decreased expression of nitric oxide synthase 2 (NOS2) and IFN-γ, but not of TNF-α, indicating that inflammasomes may contribute to the efficient production of IFN-γ and IL-1β. NO production by inflammasomes may also restrict the multiplication of the parasite via a mechanism involving IL-1β and IFN-γ (96–98).
In leprosy infections, dectin-1 acts as a C-type lectin PRR. It is present primarily in DCs and is targeted with the induction of the Th1 and Th17 protective response. According to a study by Gringhuis and coworkers (102), dectin-1 acts as an extracellular sensor for mycobacterial PAMPs and directly activates caspase-8 for pro-IL-1β processing. IL-1β expression in response to the stimulation of DCs with Mycobacterium leprae is induced primarily via dectin-1 and is completely dependent on caspase-8. Thus, recognition of M. leprae by dectin-1 results in the noncanonical activation of caspase-8-dependent inflammasomes and pro-IL-1β processing (103, 104). The pathogen–dectin-1 bond acts as a trigger for the formation and activation of a noncanonical inflammasome complex—consisting of CARD9, Bcl-10, MALT1, caspase-8, and ASC—which converts pro-IL-1β to its biologically active form. While PRRs such as NLRs recognize pathogens intracellularly, dectin-1 detects M. leprae PAMPs extracellularly, enabling a rapid response to the pathogen without the need for internalization. The existence of a caspase-8-dependent noncanonical inflammasome emphasizes the diversity and versatility of immune responses to infection (103–106).
Neutrophils and Macrophages
Blood neutrophils are recruited to the sites of tissue infection or injury by multiple-step processes that are dependent on selectins, integrins, and chemokines (107, 108). When these cells are recruited to a site of infection they express receptors that recognize and bind to microorganisms, ingesting and destroying them by phagocytosis and microbicidal molecules present in phagolysosomes (109, 110). These microbicidal molecules can be grouped into three main classes: reactive oxygen species (ROS), NO, and proteolytic enzymes (111).
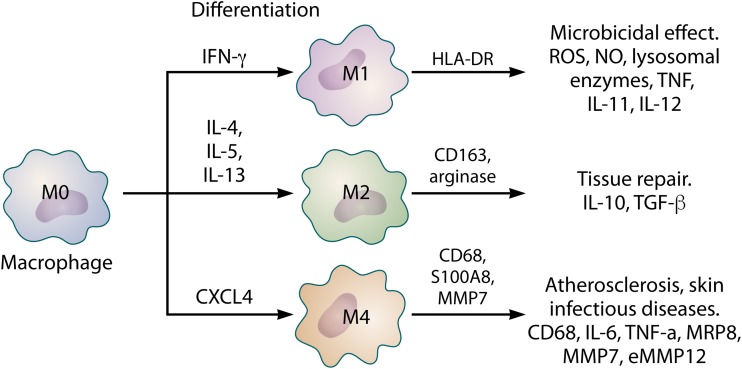
The mononuclear phagocytic system includes monocytes (circulating in blood) and macrophages (resident in tissues) (112, 113). Macrophages play important roles in innate and adaptive immunity and are located in organs and connective tissue. Skin macrophages can migrate to the lymph nodes under immunologic conditions (112, 113). Macrophages are part of a group of cells that undergo phenotypic modification and express receptors, costimulatory molecules such as CD163, CD68, CD80, CD86, and CD206, and cytokines that induce the development of suppressive or proinflammatory responses (Fig. 3) (112–114).
FIG 3.
Phenotypic subpopulations of macrophages and their differentiation based on the specific profile of cytokines and chemokines. Each subpopulation is involved in specific physiological and pathological processes and the expression of a particular profile of cytokines, enzymes, and metalloproteinases, which may contribute to microbicidal, regenerative, and atherosclerotic plaques.
More recent studies have shown that like for DCs, different phenotypes of macrophages can be identified by the expression of HLA-DR, CD163, arginase, and MRP8 (115–118). These studies identified three different macrophage populations: (i) M1 macrophages, which characteristically express HLA-DR, (ii) M2 macrophages, identified by the presence of CD163 and arginase, and (iii) M4 macrophages, characterized by the expression of MRP8 (Fig. 3) (115–118). Macrophages ingest microorganisms by phagocytosis, which is the energy-dependent envelopment of large particles. Microbial destruction in macrophages occurs in phagolysosomes, vesicles formed from the fusion of phagosomes with lysosomes during phagocytosis, necessarily involving enzymes and ROS (112, 119–123). Macrophages have high-affinity receptors that bind to antibody molecules, complement proteins, and lectin, which are essential receptors for the phagocytosis of many different microbes. Increasing evidence suggests a new subpopulation of macrophages known as M4. M0 macrophage differentiation gives rise to M4 macrophages in the presence of CXCL4; M4 macrophages produce IL-6, TNF-α, MRP8, MMP7, MMP12, and CD68 (118, 124, 125). The first reports of M4 macrophages showed that these cells are abundant in atherosclerotic lesions, where the expression of low-density lipoprotein receptor (LDL-R) by macrophages is increased. This provokes the accumulation of fat in phagocytes, causing the development of atheroma plaques and consequent oxidative lesions. However, recent data have pointed to the expression of LDL-R in infectious skin lesions such as leishmaniasis and leprosy, which demonstrates a possible association and role of M4 macrophages in immune responses to these diseases in the skin (Fig. 3) (118, 124–130).
M17 and Mreg (regulatory) macrophages have also been proposed, in addition to the subpopulations described above. However, their characterization and involvement in the physiological and pathological processes of the skin and other organs require further investigation (115, 117).
Finally, it is important to emphasize that both macrophages and DCs of the skin are characterized by a marked phenotypic heterogeneity that reflects their diversity and involvement in the various types and stages of cutaneous immune responses.
Basophils and Mast Cells
The morphology and physiology of basophils and mast cells are similar; however, basophils are found predominantly in blood, while mast cells are mainly found in tissue (131, 132). According to their tissue distribution and enzymatic composition of their granules, it is possible to distinguish at least two subpopulations of mast cells: mucosal mast cells, which contain trypsin, and connective tissue mast cells, which contain chymotrypsin. A high-affinity receptor for IgE is expressed by basophils and mast cells, which is strongly bound to their surfaces, leading to degranulation and the release of various substances, including serotonin, histamine, platelet-activating factor, and leukotrienes (Table 1) (133, 134).
Mast cells constitute one of the most abundant cell populations of the skin and express complement C5a (CD88) receptors (132). Their location in the dermis, their ability to release vasoactive and proinflammatory substances, and the expression of IgE receptors on their surfaces imply that they have important roles in the immunity of the skin to infectious or harmful agents (132–134). Mast cells are probably involved in the mechanisms of fibroblast proliferation, blood flow control, and angiogenesis (135–139). These functions are implicated in extracellular matrix remodeli and in the cicatricial (scarring) and fibrotic processes associated with skin lesions (Table 1) (139).
Eosinophils
Eosinophils are abundant cells in the inflammatory infiltrates of late-phase reactions. These cells are granulocytes derived from bone marrow and are involved in the pathogenesis of allergic diseases and infections (140, 141). Th2 profile cytokines promote eosinophil activation and recruitment at sites of inflammation, stimulating the release of granular proteins that are toxic to parasitic helminths, particularly nematodes, and actively participate in the inflammatory cascade underlying allergic processes (141–146). The presence of such parasites is linked to the production of IgEs, which bind to parasites and trigger the release of various eosinophil-derived microbicidal substances, such as cationic proteins, peroxidase, and neurotoxins (147, 148). Eosinophils can also release prostaglandins that may induce vasodilation and chemotaxis by neutrophils, leukotrienes involved in allergic skin diseases, and cytokines (Table 1) (148).
NK and Natural Killer T (NKT) Cells
NK cells are lymphocytes that do not express T or B cell receptors (149). They can participate in both innate immunity and adaptive immunity via antibody-dependent cytotoxicity, the downregulation of MHC class I expression, or IL-12 stimulation with IFN-γ release (Table 1) (150–152). They can also produce granzyme, perforin, and granulysin and thereby participate in the direct destruction of microorganisms and neoplastic cells. Furthermore, they play key roles in the pathogenesis of inflammatory and infectious skin diseases (153). They are detected primarily by the expression of CD16 (phagocytosis and antibody-dependent cellular cytotoxicity), CD56 (an NCAM-neural cell adhesion molecule), and CD57 (HNK-1 sulfated carbohydrate chain). NK cells also release TNF-α, GM-CSF (a growth-stimulating factor for granulocyte and monocyte colonies), and IL-3. These cells also promote macrophage activation, leading to phagocytosis and the destruction of microorganisms (150, 151).
Another type of NK cell is the NKT cell. NKT cells have both NK and T cell characteristics and are also capable of releasing cytokines such as IFN-γ, IL-22, and IL-17; these cytokines are involved in the activation of macrophages and in the maintenance of epithelial barrier integrity, which stimulates the generation of antimicrobial peptides and production of chemokines in the skin (Table 1) (154). Moreover, they can express chemokine receptors such as CXCR3, CCR5, and CCR6, which facilitate the recruitment of lymphocytes. Despite their role in inducing skin inflammation in experimental mouse models of psoriasis, further studies are needed to elucidate the role of NKT cells in this process (155–157).
ILCs
During hematopoiesis, lymphoid precursors give rise to T and B cells, which have specific antigen receptors that are characteristic of each of these subpopulations (158). Until recently, it was thought that there were only two subpopulations that had no characteristic antigen receptors. The first subpopulation has already been described as NK cells. The second subtype comprises lymphoid tissue-inducing (LTI) cells, which are important for lymphoid tissue development, including Peyer’s patches, lymph nodes, and other lymphoid cells (159). However, more recently, cells resembling LTI cells have been identified and termed NK22 cells. These cells concomitantly present markers of NK cells and express the cytokine IL-22 (160, 161). Both LTI and NK22 cells are now considered a subtype of innate lymphoid cells (ILCs), referred to as ILC3 cells (Table 1).
Studies have indicated the importance of ILCs in responses to infections, especially with helminths. These cells are observed in tissues such as the gut and appear to play key roles in the immunopathogenesis of psoriasis and skin infections. Recently, they were named innate lymphoid cells (108) and were divided into three categories according to the adaptive response phenotype mediated by T CD4+ helper lymphocytes—namely, ILC1 (Th1), ILC3 (Th17), and ILC2 (Th2)—which are, respectively, directed against intracellular agents, extracellular microbes, and large parasites. Furthermore, they appear to play a central role in the control of adaptive immune responses (108, 161).
B Lymphocytes
B lymphocytes typically play a central role in the humoral immune response because they synthesize antibodies (162). In addition to their central role in antibody production, B lymphocytes can function as APCs and produce cytokines that affect tissue and systemic immunity (163) (Table 1). Moreover, recent evidence suggests a role for B lymphocytes in both homeostasis and pathological processes involving the skin associated with local immune responses in neoplastic and infectious processes. B lymphocytes are rarely observed in normal skin, and although several techniques have been used to characterize this population, the specific roles of B cells in dermal homeostasis and immunological surveillance have not been fully clarified (164).
Nevertheless, B lymphocytes are known to migrate to the skin through the endothelium and interact with receptors of selectins, chemokines, and integrins (165). B lymphocytes migrate toward the antigenic focus, where APCs activate naive lymphocytes present in the tissue microenvironment (166).
In the pathophysiology of infectious and inflammatory skin processes, B cells can exhibit both proinflammatory and suppressive effects. Infiltration by B lymphocytes has been observed in cutaneous lesions in leishmaniasis, as well as in inflammatory diseases such as atopic dermatitis (166–168). The mechanisms by which B cells contribute to inflammatory skin pathogenesis occur through their interaction with components of innate immunity and with T lymphocytes (166). In Staphylococcus aureus skin infections, B lymphocytes play an important role in the synthesis of specific antibodies, as well as in bacterial opsonization and in the induction of phagocytosis by macrophages and neutrophils (169, 170). More recently, regulatory B lymphocytes (Breg) have been identified and appear to play an important role in attenuating inflammation and inducing the maintenance of immune tolerance mechanisms through the release of IL-10 (171, 172) (Table 1).
T Lymphocytes
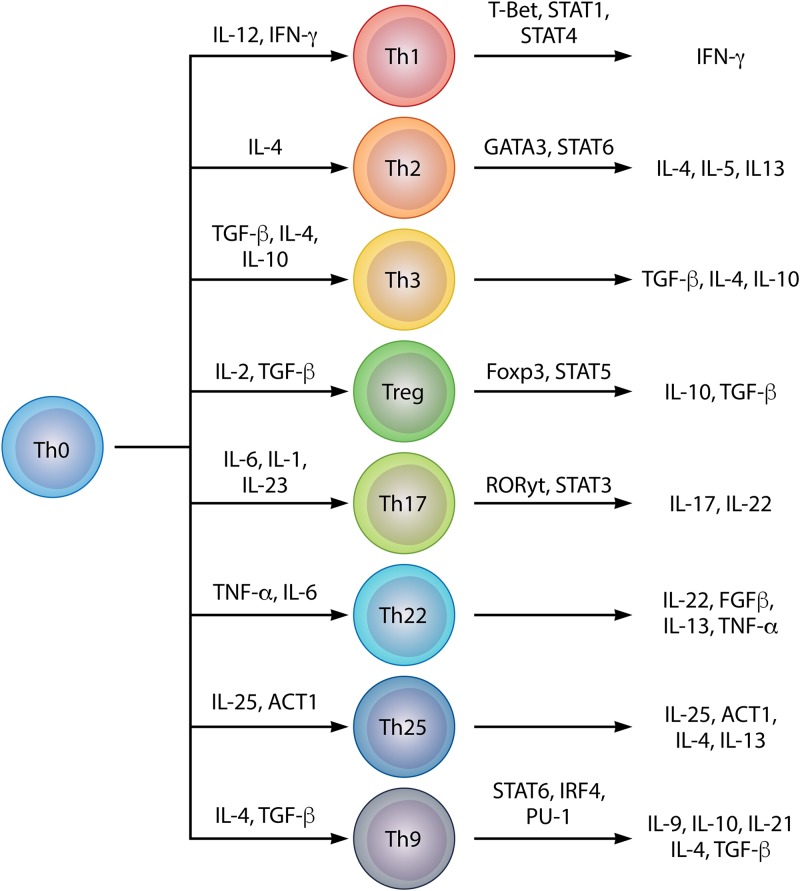
There are approximately twice as many local lymphocytes in normal skin as there are in the blood. Although the presence of T cells in the skin was first described some 70 years ago, this population of lymphocytes has attracted scientific attention only within the last 25 years (173, 174). The skin has a phenotypically heterogeneous population of lymphocytes, mainly comprised of memory T cells (Fig. 4 and Table 1) (175, 176). In the epidermis, T cells are located in the suprabasal and basal strata, close to LCs (Fig. 1) (176). In the dermis, T cells are distributed in clusters around the capillaries; they are also frequently found at the dermo-epidermal junction and around cutaneous appendages. CD4+ and CD8+ T lymphocytes are present in the skin in similar proportions, and most of these are memory T cells (177). The skin has a heterogeneous population of T lymphocytes that participate in classical immune responses, including Th1, Th2, Th3, Th17, and regulatory T (Treg) cells, which have been found in the skin in various infectious and inflammatory diseases (13). More recently, differentiated subpopulations involved in specific physiological and pathological processes have been identified, including Th9, Th22, and Th25 lymphocytes (Fig. 4 and Table 1) (178–180). In the case of infections by intracellular microorganisms, Th1 cells predominantly release IFN-γ and lymphotoxins, leading to the activation of macrophages with microbicidal activity (181). Classically, Th1 lymphocytes in the skin are associated with the resolution of infections and the pathogenesis of autoimmune or immunomediated diseases such as psoriasis (181, 182). In immunocompromised patients, Th2 cells participate in the inflammatory response to infectious agents and are referred to in the context of allergic diseases such as atopic dermatitis and psoriasis (181, 183). Th3 lymphocytes were primarily identified in oral tolerance models (184–186). They are characterized predominantly by the production of transforming growth factor β (TGF-β) and secondarily by the production of IL-4 and IL-10, which are involved in the class switching of B cells to produce IgA, with inhibitory effects on Th1 and Th2 lymphocytes (185–195). In view of the ability of Th3 profile lymphocytes to stimulate the production of antigen-specific lymphocytes, and to inhibit the production of non-antigen-specific lymphocytes, these cells can be considered a subtype of Treg lymphocytes (Fig. 4 and Table 1) (181–195).
FIG 4.
Diversity in helper T lymphocyte profiles, their differentiation factors, and characteristic cytokine profiles.
The Treg cell population consists of Th3 T CD4+ cells and regulatory T cells (Tr1). Th3 CD4+ cells are characterized by their ability to express primarily TGF-β, whereas Tr1 lymphocytes are characterized by high expression of IL-10 (188–193).
Tr1 lymphocytes are a subset of CD4+ T lymphocytes, whose function is to suppress immune responses and maintain self-tolerance (7, 189). Although other types of T cells with suppressor activities have been described, Tr1 lymphocytes are characterized by CD4+, FoxP3+, and CD25+ markers (189). These cells are produced primarily through the recognition of autoantigens in the thymus or of external antigens in peripheral lymphoid organs, in which these cells do not express FoxP3 constitutively but acquire the function of Tr1 cells by increasing FoxP3 expression in response to the presence of IL-2 and TGF-β, or stimulated by the presence of small molecules such as retinoic acid (188, 189). The production of some Tr1 cells requires the cytokine TGF-β, and the maintenance and effectiveness of this profile is dependent on IL-2 (190). Tr1 cells suppress immune responses at multiple stages of their life cycle, and appear to be capable of inhibiting B cell activation, as well as differentiation and proliferation of NK cells (191). Several mechanisms of action have been proposed for Tr1 cells, including the production of immunosuppressive cytokines such as TGF-β and IL-10 (191). Another mechanism involves the inhibition of T cell stimulation by APCs via the binding of CTLA-4 (CD152)—a Tr1 cell surface molecule—to B7 (CD80/CD86), which is expressed on the surface of APCs. This ultimately increases IL-2 cytokine consumption through an increase in IL-2 receptor expression on Tr1 cells (7).
In normal, noninflamed skin, Treg lymphocytes are preferentially distributed close to and in close association with hair follicles, with fewer cells located in the dermis between these structures (196, 197). In general, because of the role of Treg cells in the control of skin immune responses, the biological and functional characterization of these cells in the cutaneous tissue environment is of vital importance for the understanding of inflammatory diseases of the skin (198–200). Because of their close relationship with hair follicles, these structures play a key role in the control of skin homeostasis and in the activation of Treg cells (197, 201–203). Dysregulation of follicle stem cell function has been shown to be linked to Treg cell function, and studies of polymorphisms have linked this to high-affinity IL-2α receptor genes (IL-2RA and CD25), FOXP3, and cytotoxic-T-lymphocyte–associated antigen (CTLA), among others (197, 202, 203). These aspects are confirmed by the observation of the presence of Treg lymphocytes in lesions of alopecia areata undergoing regeneration after institution of specific therapeutic treatments. In addition to the cellular components of the hair follicles, other cell types, including fibroblasts, DCs and LCs, also play important roles in controlling the Treg lymphocyte population of the skin (196, 197).
Th17 cells are implicated in the pathogenesis of both psoriasis and atopic dermatitis and are a key cell type in the body’s first line of defense against infection by certain fungi and bacteria (40, 183, 204, 205). Studies have pointed to important roles of IL-17 and IL-22, including increasing the expression of keratinocyte antimicrobial peptides (38). Therefore, Th17 lymphocytes may form a bridge between immune cells and epithelial cells, thus improving the efficiency of the skin’s immune responses to pathogens (Fig. 4 and Table 1) (199, 200).
Th22 cells constitute another recently identified subgroup of T cells that express IL-22 but not IL-17 or IFN-γ (180). pDCs are able to induce the Th22 profile by releasing TNF-α and IL-6. These cells were identified in cultures from patients with atopic dermatitis, and the authors of several other studies have investigated the role of this profile in the skin under normal and pathological conditions, including psoriasis, lupus erythematosus, lymphoma, allergic and infectious diseases, epidermal skin immunity, and tissue remodeling (Fig. 4 and Table 1) (180, 206–212).
Th9 and Th25 lymphocytes have also been described recently. Th9 lymphocytes are distinguished by the expression of IL-4, IL-9, IL-10, IL-21, and TGF-β and differentiate from Th0 lymphocytes in the presence of IL-4 and TGF-β (213–215). In the skin, Th9 cells appear to be involved in autocrine and paracrine control of local inflammatory responses and in Candida albicans infections. In addition to stimulating IL-9 expression, these cells also induce the release of TNF-α and granzyme B (216, 217). Th25 lymphocytes are characterized by the expression of Act1, IL-4, IL-13, and IL-25 (IL-17E) and are involved in IgE release and mast cell degranulation. Recent data have implicated Th25 in the immunopathogenesis of skin diseases, namely, coccidioidomycosis, chronic mucocutaneous candidiasis, and lepromatous leprosy (Fig. 4 and Table 1) (178, 218–222).
γδ T lymphocytes are so-called nonconventional immune cells (223, 224). These cells have receptors composed of γ and δ heterodimers that are homologous to the α and β chains of TCRs found in CD4+ and CD8+ T lymphocytes, and they are identified through the CD3 and TCγδ markers (Table 1). γδ T lymphocytes do not recognize peptide antigens associated with MHC-II and are not restricted to the MHC. γδ T lymphocytes recognize alkyl amines, small phosphorylated molecules, lipids not commonly found in mycobacteria and other microorganisms (presented by nonclassical molecules such as MHC class I glycoproteins), and protein and nonprotein antigens that do not require any kind of processing by APCs (223–227). In human skin, γδ T lymphocytes constitute a small percentage of T cells in the dermis and epidermis (2 to 9% and 1 to 10%, respectively) (170, 171, 228). Little is known about their roles in human skin, but an increase in the population of γδ T lymphocytes has been observed in patients with melanoma, LC histiocytosis, leishmaniasis, and leprosy, among other disorders, suggesting their involvement in the pathogenesis of these skin diseases (Table 1) (229–234).
Endothelial Cells
Endothelial cells play key roles in controlling the flow of immune cells from blood to tissue through the expression of various adhesion molecules, cytokines, and chemokines, which serve as chemoattractants for leukocyte migration (Table 1) (235, 236). The endothelium is one of the first lines of defense against harmful and infectious agents in blood, and it directly affects innate and adaptive immune responses (236). During the initial phase of inflammation, when the innate response is more apparent, endothelial cells activate TLR proteins (mainly TLR2 and TLR4), which mediate immune responses (236). TLR2 is a crucial agent in the inflammatory response against bacteria and other pathogens, whereas TLR4 is a strong regulator of the innate and adaptive immune responses (Table 1) (236–239). However, these molecules are present at low levels before the activation of endothelial cells, and activation of the endothelial system leads to elevated expression of TLRs. In addition to providing standard receptors for TLR2 and TLR4, the endothelium is responsible for interacting with mediators from the immunoglobulin family such as ICAM-1 and VCAM-1 (240–242). Other adhesins, such as E- and P-selectins, are also present in greater quantities during the inflammatory response and are mainly mediated by cytokine stimuli (243). Such adhesin molecules are constitutively expressed in the membranes of endothelial cells, but when the levels of proinflammatory agents increase, they induce elevated expression of adhesins (240–242). This leads to greater interaction between blood leukocytes and their endothelial ligands, which involves these cells in the cascade that leads to rolling, firm adhesion, and diapedesis (Table 1) (236, 243).
In inflammation, endothelial cells are directly influenced by several factors that facilitate the formation of inflammatory infiltrate (243, 244). The mechanisms responsible for this alteration include oxidative stress, which results from immune responses and increases the expression of adhesion molecules such as VCAM-1, ICAM-2, and selectins CD62E and CD62P. However, the cytokines of the immune system act as direct agents in mediating oxidative stress and influence leukocyte adhesion (243).
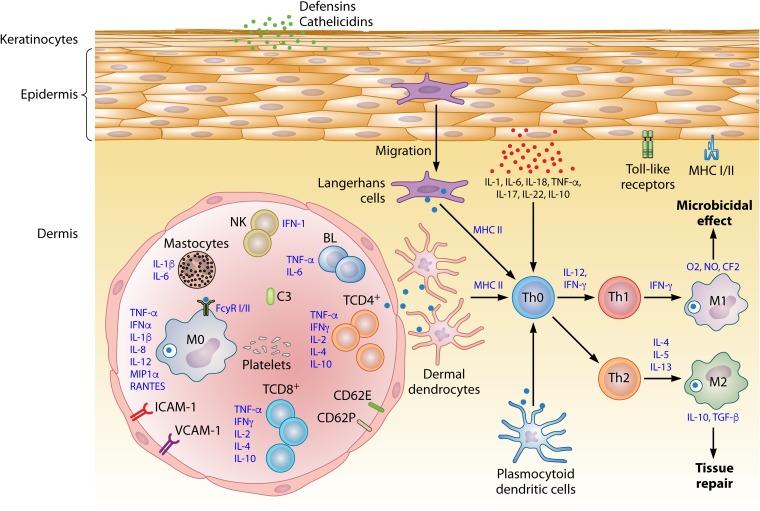
The endothelial immune response is associated with increased expression of cytokines such as IFN-γ, TNF-α, IL-1α, and IL-1β, which increase the expression of adhesion molecules (243, 244). IFN-γ appears to be one of the most important factors inducing MHC class II expression and is an important agent in the formation of costimulatory molecules, including adhesion molecules such as ICAM-1 and E-selectin (245). TNF-α leads to the increased expression of molecules such as VCAM-1 and ICAM-1. Depending on the phenotype and the intensity of the inflammatory stimuli, it is possible to detect various adhesion molecules in the membranes of endothelial cells (Table 1) (246). Such molecules modify the processes of cell rolling and tissue transmigration in leukocytes. Cell transmigration through the endothelium is possible only following rupture of the endothelial wall, which is mainly mediated by cell changes induced by proinflammatory cytokines (246–248). A cascade of intracellular events leads to changes in the cytoskeleton which allow the extravasation of fluid, chemical mediators, and cells toward the cytokine source. This process results in the formation of perivascular edema and directly combats microbial agents at the site of infection (Fig. 5) (215).
FIG 5.
Integrated view of the association between the host cells and infectious agents in the context of the skin’s in situ immune response. Note that the triggered mechanisms culminate in the activation of professional and nonprofessional immune cells, such as keratinocytes, which can, however, interfere with the immune response cascade against various harmful agents. These cells act as a mechanical barrier and can interfere with the immune response in the skin. Endothelial activation is required for the migration of immune cells from the blood to the tissue.
Other Cellular Components of the Skin
Other cells are also part of this complex organization of the skin, and although they may not act as the first line of host defense, they cooperate with the immune system during immune responses (249–253). The key function of fibroblasts is the production of structural proteins that constitute cutaneous tissue. They also coordinate with immune responses, allowing the migration of blood cells to sites of injury, and interact with other local cells, thereby potentiating the signaling cascade of host responses to infectious agents (254–257). Fibroblasts are relatively abundant cells in skin, and more recent work has shown that these cells release a chemoattractant that is sensed by dermal lymphocytes. In addition, fibroblasts possibly play an important role in attracting and maintaining Treg cells in this environment (258–260). The authors of several studies have discussed the importance of the extracellular matrix of the skin in the pathogenesis of infectious diseases and have characterized the importance of these tissue components in the evolution of infections (261). Moreover, similar to keratinocytes, fibroblasts can produce cytokines such as TGF-β and FGF-β, among others, and thus may influence the local immune response pattern (261–264).
Cutaneous appendages are of fundamental importance in maintaining the physiological homeostasis of the skin. Hairs are keratinized structures formed by invagination of the epidermis in the dermis (265). A terminal dilatation is observed in the process of hair follicle growth—the hair bulb—and, in its central portion, a dermal papilla that induces hair growth (249–253). The cells that cover the papilla and form the root of the hair include keratinocytes and melanocytes. During the differentiation process of keratinocytes, which occurs in the hair root, they undergo keratinization and incorporate melanin. Hair follicles of human skin are specialized structures that may also be involved in the control of local immune responses in the skin. Activation of stem cells from hair follicles may be involved in the hair growth process and in the control of immune responses, through interaction with Treg lymphocytes, as described previously (197, 201–203).
Sebaceous glands, as with hair follicles, originate from the same epithelial sheath and secrete cholesterol and triglycerides (252, 253, 266, 267). These glands can express TLR2 and TLR4 and secrete immune factors such as defensins, cytokines, chemokines, adipokines, serpin E1, leptin, and resistin, which can act as a bridge between the control of the local inflammatory response and lipid metabolism in the skin (266). This ability to control the tissue immune response pattern of sebaceous glands in close association with lipid metabolism makes these glands fundamental to control of the local microbiome. Consequently, the deregulation of their physiology is linked to the pathophysiology of infectious, metabolic, and inflammatory skin diseases, such as acne, psoriasis, seborrheic dermatitis, and rosacea (266–271). The eccrine sweat glands are entangled tubular structures and comprise cells that eliminate the secreted product, without compromising the cells. They produce sweat, which is directly secreted onto the skin surface through an excretory duct and is composed of an aqueous solution of ions (Na2+, K+, and Cl−), urea, ammonia, uric acid, and very small amounts of protein (253). The apocrine sweat glands are found in the axillary, pubic, and perianal regions. Part of the cytoplasm of the cells that constitute these glands is lost during secretion. The apocrine sweat glands produce a viscous secretion that is discharged into the hair follicle canal, rather than directly onto the surface of the skin (252, 253).
Finally, neurons, which primarily act on the somesthetic processes of the skin, interact with lymphocytes and regulate local immune responses (254, 272, 273). Riol-Blanco et al. have shown a close relationship between DDs and skin nociceptors, as these nerve endings probably induce the production of IL-23 and IL-17, which, in turn, function to control immune responses of the skin in experimental models of psoriasiform inflammation (272).
THE ROLE OF CELL DEATH IN HOMEOSTASIS AND THE CONTROL OF THE SKIN’S IMMUNE RESPONSE
The phenomenon of cell death is intrinsically linked to the cellular life cycle and aging, and it occurs as a homeostatic mechanism under both physiological and pathological conditions (274–279). Cells in general may die by two distinct mechanisms, namely, accidental cell death and regulated cell death (RCD) (274). RCD may occur in contexts that include exogenous or endogenous cellular alteration or disturbance, leading to physiological changes that cannot be reverted, and consequently lead to cell death. It may also occur in the absence of exogenous disorders and, as in this specific case, in the context of programmed cell death (PCD) during the process of cell development and renewal (274, 275). Classical studies have divided the mechanism of cell death into three types: type I cell death or apoptosis, type II cell death or autophagy, and type III cell death or necrosis, based primarily on the morphological characteristics of each of these groups (274).
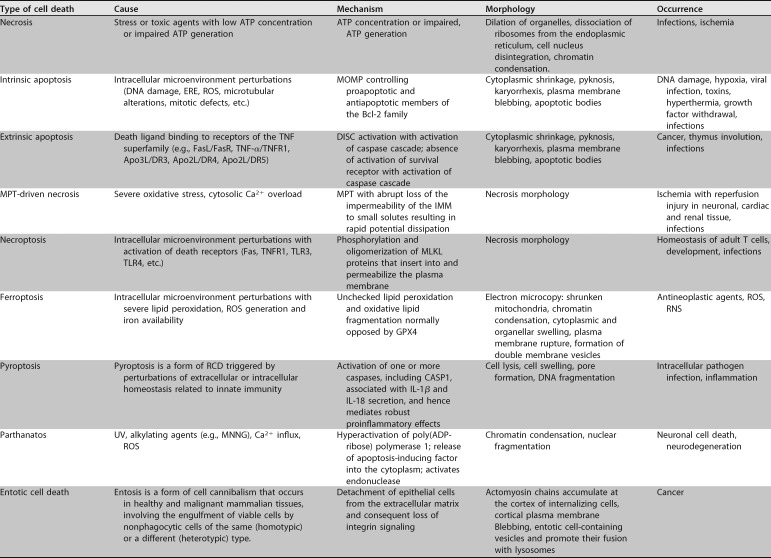
Since 2005, a committee on cell death has formulated the guidelines of a classification system for the different types of cell death, based not only on morphological aspects but also on genetic, biochemical, pharmacological, and functional characteristics. This has resulted in a wider classification for the mechanisms of cell death and has furthered our understanding of the importance of these mechanisms for processes involving homeostasis and disease. This classification scheme proposes the occurrence of at least 12 distinct mechanisms of cell death, which are shown in Table 2 (274, 275).
TABLE 2.
Types of cell death and causes, morphology, mechanisms, or factors that lead to its induction
| Type of skin cell death | Cause |
|---|---|
| Necrosis | Tuberculosis |
| Apoptosis | Leprosy |
| Autophagy | Zika virus infections |
| Pyroptosis | Leishmaniasis |
| Ferroptosis | ROS, RNS, glutathione |
| Necroptosis | Staphylococcus aureus infection |
In the skin, although cell death is related to important physiological and pathological processes, evidence suggests that it also has a prominent role in the control of immunological responses and in the pathophysiology of various skin diseases. Necrosis and apoptosis appear to be at opposite ends of a spectrum, and several mechanisms lead to cell death (257, 262, 280–282). Mechanisms such as necroptosis, pyroptosis, ferroptosis, and autophagy have received increased attention in the study of the pathophysiology of various infectious and noninfectious diseases, including in the cutaneous environment (Table 2) (257, 262, 280–287).
There are many and diverse processes by which infectious agents interact with host cells to induce injury (284). Often, these agents trigger a process that culminates in immunosuppression. Among these mechanisms, apoptosis is commonly used by diverse infectious agents to negatively control immune responses by inducing cell death in inflammatory cells (280–287). The high-affinity recognition of autoantigens by T lymphocytes (when these are constantly stimulated by antigens) can lead to apoptosis (280, 288–297). There are two major apoptotic pathways, and both involve the deletion of peripheral and mature immune cells. Cell death by apoptosis may present mitochondrial changes, which are regulated by the Bcl-2 family of proteins; this family has pro- and antiapoptotic members (284). Changes in the permeability of the outer membranes of mitochondria may be induced by Bcl-2 proteins, or these proteins may interact with a caspase activation inhibitor (280). The extrinsic pathway occurs by activation of death receptors and is the second most important apoptosis activation route in the deletion of immune cells. Death receptors, which are homologous to TNF receptors, may be activated by TNF-like ligands, leading to the oligomerization of an adapter protein (280, 284). This protein activates a caspase cascade or induces the translocation of a proapoptotic member of the Bcl-2 family to the mitochondria, leading to a change in their permeability and to the activation of a caspase (280, 284–287).
Some studies have suggested the occurrence of apoptosis in the control of the immune response in lepromatous leprosy and cutaneous leishmaniasis, as well as in several viral diseases (298–301). Lytic, caseous, or liquefactive necrosis can occur in inflammatory or infectious skin diseases as a consequence of the action of viruses, bacteria, or helminths (302). Moreover, the innate immune response pattern may underlie the occurrence of cell death by pyroptosis, implying that this type of cell death is important in innate immune responses to infectious or noninfectious skin agents (255, 303).
Necroptosis is a type of necrotic cell death that involves fine control of plasma membrane permeability, and it is activated by the formation of RIPK1/RIPK3/MLKL complexes (necrosomes), which activate a pronecroptotic protein termed MLKL (285, 304). After this phase, the propagation of cell death by the release of DAMPs, with activation of cellular immune responses, takes place, leading primarily to the activation of macrophages, DCs, and NK cells (305), inducing the pinocytosis of compromised or infected cells. This leads to the activation of a cascade of cell death where the presence of TNF-α may also contribute to the induction of chronic inflammation and to the presence of more lesions (305, 306). Some infections have been associated with cell death by necroptosis, as reported for infectious processes induced by a suspension of Staphylococcus aureus diluted in phosphate-buffered saline (PBS) and inoculated into mice in experimental models of infections (169, 284, 307, 308).
Cellular autophagy occurs through the accumulation of autophagosomes in the cellular cytoplasm, with the degradation and recycling of cellular components that may have been damaged by aging, chemical agents, infections, or inflammation (257, 262, 309, 310). Experiments on the interaction between Zika virus and skin cells have shown that infected fibroblasts form autophagosomes in the cytoplasm, suggesting that during viral replication, autophagy is stimulated in cutaneous cells (311–313).
Characterized by the occurrence of lipid peroxidation products and ROS release from iron metabolism, ferroptosis is a type of RCD with morphological characteristics such as diminished mitochondria and condensation of mitochondrial membranes that induces reduction in numbers of cristae and the rupture of the outer mitochondrial membrane (314–327). Ferroptosis can be induced in keratinocytes, mainly in processes related to DNA or mitochondrial damage mediated by ROS or nitrogen radicals, as well as by potentially toxic chemical compounds, which can be produced in abundance in the presence of infectious diseases (265, 326, 327). These compounds can cause injury and induce cell death by diverse mechanisms, including apoptosis, necroptosis, and ferroptosis, depending on the activity of xenobiotic biochemical pathways involving glutathione and vitamins C and E (314–327). The types of cell death and their relation to infectious and noninfectious causes, as well as their stimuli, mechanisms, and morphology, are shown in Tables 2 and 3.
TABLE 3.
Mechanisms of cell death in the skin and their relationship with various infectious agents and chemical inducers of irreversible cell damagea
ERE, endoplasmic reticulum stress; MOMP, mitochondrial outer membrane permeabilization; MPT, mitochondrial permeability transition; IMM, inner mitochondrial membrane; DISC, death-inducing signaling complex; MLKL, mixed lineage kinase domain-like; ROS, reactive oxygen species; RNS, reactive nitrogen species; RCD, regulated cell death; DAMP, damage-associated molecular pattern; PRR, pattern recognition receptor.
OVERVIEW OF THE RELATIONSHIP BETWEEN SKIN IMMUNE SYSTEM AND THE EVOLUTION OF INFECTIOUS CUTANEOUS DISEASES
I have already discussed the general organization of the cutaneous immune system, so in the present section I specifically address the mechanisms inherent in compartmentalized responses to infectious agents of diverse nature, with emphasis on local skin immune responses, tissue immune responses, or in situ immune responses.
The in situ pattern of the skin’s response to infections is complex and involves several cellular and humoral factors (Table 1 and Fig. 5). The immune response to various infectious agents in the tissue environment requires the activation of innate and acquired types of immunity, which are closely linked and interregulated (Fig. 5) (328–332). Infectious agents of diverse nature can reach the skin tissue either through direct inoculation at a break in the physical barrier of the epidermis or through blood vessels and activation of the endothelium (5, 333, 334). In the latter context, endothelial cells play an important role in the migration of microorganisms from blood to the cutaneous environment, as well as in the migration of leukocytes to the focus of infection (335). It should be emphasized that endotheliocytes allow the transmigration of cells from the blood into the infected tissue in order to elicit a specific immune response during the course of an infection (Fig. 5) (336–338). It has already been shown that the endothelial phenotype can influence the evolution of chronic and spectral infectious diseases such as leprosy and leishmaniasis, depending on the pattern of cells that migrate to the focus of infection (336–338). Furthermore, the capillaries of the dermis maintain a close anatomical relationship with the DDs and probably participate, together with endotheliocytes, in the first stages of the immune response to infectious agents of the skin (Fig. 5) (339–342). Although LCs are the main type of immune cells in the epidermis, as already mentioned, keratinocytes can participate in the control of the immune response in the tissue microenvironment (343). They do so by releasing antimicrobial peptides, presenting antigens, or participating in innate or adaptive immunity by releasing cytokines that control the cascade of proteins in the immune response to microorganisms (Fig. 5) (343). The first cytokine identified as being released by keratinocytes was IL-1. Subsequently, other cytokines were identified: IL-6, IL-8, IL-10, TNF-α, and TGF-β (38–41, 344). Keratinocytes are also able to express MHC-I and MHC-II and participate in the immunopathogenesis of various infectious viral, bacterial, and protozoal diseases, including leishmaniasis, leprosy, and herpes (Fig. 5) (38–41). In infections with flaviviruses such as Zika virus, West Nile virus (WNV), and dengue virus, keratinocytes possibly play key roles in the early stages of immune responses, which are mediated by IFN-β, IFN-γ, and antimicrobial peptides (345).
DCs contribute to the development of an effective and specific adaptive immune response against infectious agents through the processing and presentation of antigens (13, 61–64). Several studies have demonstrated the importance of this group of cells in specific immune responses to bacteria, fungi, protozoa, and viruses in the skin tissue environment (89). These studies have indicated the importance of antigen presentation by DCs in the evolution of infectious diseases such as leprosy, leishmaniasis, paracoccidioidomycosis, lacaziosis, and infections with flaviviruses such as dengue and Zika viruses (89, 313). In several arthropod-borne infections, the interaction of infectious agents with the dermal immune system, in particular with LCs and adjacent keratinocytes, represents the first stage of interaction with the host and one of the determining factors in the clinical evolution of disease, as well as in maintaining transmissibility cycles (345). Several mosquitoes responsible for transmitting infectious agents produce saliva with substances that generally exhibit anticoagulant, angiogenic, and anti-inflammatory properties, inhibiting local T cell proliferation and suppressing the expression of cytokines such as TNF-α, IL-2, and IFN-γ, as well as stimulating the secretion of IL-4 and IL-10, both in the tissue environment and in experimental models (345).
In the process of local immune responses of skin to agents transmitted by arthropods, LCs have the ability to capture the inoculated antigens and migrate to the satellite lymph nodes, where they initiate the process of activation of T cells (345). In dengue virus infection, immunohistochemical and molecular analyses have demonstrated the presence of virus antigens in LCs, whose activation is mainly dependent on TNF-α and to a lesser extent on IL-1-β, contrary to what is observed in WNV, where the activation and migration of LCs are more dependent on IL-1-β than on TNF-α (345–348). In Zika virus diseases, some studies have demonstrated an induction of IFN-β expression by LCs (345). In another study developed by Vielle et al. (349), Zika virus infection was shown to be capable of inducing the expression of type I and II interferons in DC culture, and it was shown that the activation of these cells is mediated by the expression of MHC class II, CD40, CD80/86, or CCR7. Some data have pointed to the importance of DCs in the sexual transmission of infectious agents via mucosal surfaces through the interaction of these agents with the various subpopulations of DCs, including LCs, through the immunomodulation of the activity of these cells, a fact that is well described for the transmission of HIV and is important in the mechanisms of sexual transmission of Zika virus (350, 351). In leishmaniasis and malaria, this ability to modulate the skin can either contribute to the elimination of these microorganisms or create an environment that stimulates the evolution of the disease into milder or more severe clinical forms (344, 352–354). This particular aspect can be observed in the clinical evolution of dengue fever and in its relationship with the phenomenon of antibody-dependent enhancement (ADE) (355). Dengue virus infection can be caused by four serotypes (dengue virus serotypes 1, 2, 3, and 4) and may result in a spectrum of clinical symptoms ranging from mild to hemorrhagic symptoms. Moreover, the pattern of this symptomatology is linked immune responses by the host (355–357). ADE is a process linked to the clinical evolution of severe forms of dengue and occurs in individuals with a history of secondary infection by a virus with a heterologous serotype. ADE triggers the activation of nonneutralizing or subneutralizing antibodies and specific previously sensitized cytotoxic T lymphocytes, which can stimulate a marked cross-immune reaction, contributing to the development of severe forms of infection. Studies have demonstrated the role of DCs in this mechanism (355–357). During ADE, mature DCs require the expression of Fc gamma receptor IIa (Fc RIIa), and the process is inversely related to the high expression levels of DC-SIGN (DC-specific intercellular adhesion molecule-3-grabbing nonintegrin) receptor present in immature DCs (355, 356). DCs stimulate the increased expression of viral proteins as well as the production of cytokines TNF-α and IL-6, which are involved in the pathogenesis of this phenomenon (355).
In cutaneous leishmaniasis and leprosy, populations of dendritic cells in the hyperergic and tuberculoid forms are more frequent than in the anergic and lepromatous forms, indicating a fundamental role for the number of DCs in cutaneous lesions in inducing effective cellular immune responses against intracellular infectious pathogens during the evolution of spectral cutaneous diseases (340, 352). These findings have been further corroborated by results obtained with other cell types such as macrophages, which are important in the presentation of antigens and in microbicidal action against M. leprae and leishmaniasis (358, 359).
The roles of inflammasomes in immunity to infectious agents have been characterized for some skin diseases, such as leprosy, paracoccidioidomycosis, and leishmaniasis (91, 95, 98, 359, 360). The pattern of immune responses is associated with the creation of a marked proinflammatory environment and is also involved in the induction of cell death by pyroptosis, which is related to cell deletion and induction of tissue injury in the inflammatory processes of infectious diseases (255, 303).
Macrophages play important roles in the recognition and elimination of foreign antigens of infectious or noninfectious origin, as well as in cicatrization and in the remodeling of the extracellular matrix (Fig. 3 and 5) (264, 358, 359). In infectious diseases, macrophages are the main cells involved in immunity to infection by M. leprae, Leishmania, Paracoccidioides, and Lacazia loboi and are probably involved in antigen presentation and in the release of ROS and reactive nitrogen species (RNS) with microbicidal effects (361, 362). The microbicidal mechanism employed by macrophages is closely related to gene expression patterns such as natural resistance-associated macrophage proteins (NRAMPs) with regard to the efficiency of the immune response to mycobacteria, such as M. tuberculosis and M. leprae (361).
Mast cells are abundant in the intestinal mucosa and skin. Owing to their preferential location, they play a role as sentinels against microbial agents (bacteria, viruses, helminths, and fungi), triggering local inflammatory responses (132–134). Moreover, along with basophils, mast cells can affect vessels by releasing vasoactive substances that influence the flow of leukocytes between blood and tissue. They may also be associated with the action of IgE during the in situ immune response against infections of the skin (133, 134).
The development of an effective adaptive immune response to infectious skin agents is closely related to the clinical evolution of several diseases, such as leishmaniasis, paracoccidioidomycosis, and leprosy (363–366). The Th1 × Th2 paradigm has been associated with the clinical evolution of several infectious diseases, and studies on leishmaniasis and leprosy have also characterized this process (Fig. 5) (179, 364). In these two diseases, predominance of the expression of Th1 cytokines, such as TNF-α and IFN-γ, is clinically related to more localized forms of lesions, whereas predominance of the expression of Th2 cytokines, such as IL-4 and IL-10, contributes to the clinical evolution of susceptible or anergic forms (Fig. 5) (363–366), with the presence of lesions in greater quantity and more diffusely distributed in the patient’s body. However, the role of recently identified immunological agents in the immune response of the skin has provided a better understanding of the evolution of these and other infectious diseases, as well as of the processes of healing and tissue remodeling (179, 362, 367). Among these new agents and cytokine profiles, Treg cells and Th9, Th17, Th22, and Th25 profile cytokines have provided us with additional data regarding the immunopathogenesis of skin infections and, above all, on complex and spectrally evolving infectious diseases (179, 363–366). For example, cytokines belonging to the Th17 profile, such as IL-6 and IL-17, probably contribute to a response mediated by a more exacerbated and efficient inflammatory profile against intracellular pathogens such as M. leprae and Leishmania (179, 368). In contrast, cytokines such as IL-10 and TGF-β effectively maintain a state of susceptibility to infection. They accomplish this either by inhibiting Th1 profile cytokines, such as IFN-γ and TNF-α, or by contributing to the differentiation of lymphocytes into Treg cells, which also negatively control immune responses (179, 369).
The Th22 profile, characterized by cytokines IL-22, IL-13, and TNF-α, in addition to fibroblast growth factor β (FGF-β), has contributed to the understanding of the complex immunopathogenesis of infections with agents such as M. leprae (180, 336). IL-22 has been implicated as an antimicrobial response cytokine because it interferes with the phagolysosomal maturation process, thereby inhibiting the lytic capacity of macrophages against M. tuberculosis. The increased participation of this cytokine in lepromatous leprosy seems to play a potentiating role in the lytic activity of macrophages, in a possible attempt to revert the susceptibility of this clinical form of the infection (180, 336). Similarly, IL-13 plays a key role in the induction of the humoral response, which is consequently ineffective in the recognition and elimination of intracellular infectious agents (336). Finally, FGF-β is probably involved in tissue regeneration associated with more aggressive infectious diseases and with increased tissue involvement. In addition to participating in the functions of Th22 lymphocytes, FGF-β (along with TGF-β) may help create a regenerative environment in lepromatous leprosy (336). Finally, the importance of Th17 profile cytokines, such as IL-17 and IL-22, should be noted, because they are capable of potentiating the release of AMPs by keratinocytes and mucosal cells. This confers on these cytokines an important role in both innate and adaptive immunity (38–41).
The role of profiles such as Th9 and Th25 in infectious and noninfectious skin inflammatory diseases has not yet been extensively studied and characterized. The few available articles in the literature have indicated the proinflammatory role of Th9 lymphocytes in tuberculoid leprosy. In particular, they have focused on cytokine IL-9, or on anti-inflammatory profiles such as that observed for Th25 lymphocytes, whose cytokine IL-25 (IL-17E) is related to humoral immune responses and is therefore ineffective against intracellular infectious agents. IL-25 belongs to the IL-17 family, and unlike other members of this family such as IL-17A and IL-17F, it inhibits Th1 and Th17 cytokine production (178, 213, 218, 220, 369–372). NF-κB activation is induced by IL-25 and triggers the expression of IL-4, IL-5, IL-9, and immunoglobulin E, as well as the degranulation of mast cells against extracellular pathogens (218).
Several studies have demonstrated the interactions between pathogen and host with regard to various types of infectious agents that have immune escape mechanisms (373, 374). In this scenario, pathogen and host factors may interact to maintain the environment. Studies have shown that factors such as TGF-β and the apoptosis phenomenon may contribute to this process (375, 376). Data collected during the evolution of certain infectious skin diseases suggest that this control is affected by apoptosis. TGF-β, which is a strong inducer of apoptosis, also seems to have a fundamental role in the process. It contributes to the differentiation of the Treg response, inhibits macrophagic responses, and acts as an immunosuppressive cytokine (301, 375–377). Moreover, TGF-β seems to play a major role in the evolution of several infectious diseases of the skin, including leprosy, lacaziosis, cutaneous leishmaniasis, and chromoblastomycosis (96, 359, 375, 376, 378–380). Depending on its relationship with other cytokines and humoral factors of the host’s response, TGF-β has numerous roles in the tissue microenvironment. It has multiple functions and can control the immune response by direct action on the cells of the immune system, inhibiting the effector activity of lymphocytes and macrophages, and by inducing the apoptosis of macrophages (375). Apoptosis has been implicated in the negative control of the immune response because certain infectious pathogens use it as an escape mechanism (301, 375–377, 381–384). In both leprosy and cutaneous leishmaniasis, apoptosis has been identified as the most frequent mechanism of cell death in the lepromatous forms of leprosy and anergic leishmaniasis, which has implicated this phenomenon in a possible mechanism of control of the immune response in these clinical conditions (300, 301, 381–384). In addition, it is worth mentioning that other types of cell death, such as pyroptosis and autophagy, have been implicated in skin tissue responses in viral infections and in the activation of inflammasomes that induce pyroptosis in bacterial infections. This further indicates a possible role of these mechanisms of cell death in the pathogenesis and immunopathogenesis of such infections (Tables 2 and 3) (385–389).
PERSPECTIVES
Finally, it is worth remembering that the skin immune system is complex and acts in very characteristic ways against microbial agents. Moreover, many other factors might be involved in effective immune responses. The identification of new subpopulations of lymphocytes and macrophages and the characterization of other cells that may influence immune responses or act as immunogenic targets are important topics of research. Such research will certainly contribute to the elucidation of the complex mechanisms of skin immune responses and of the evolution of cutaneous diseases. Research concerning the initial stages of systemic infectious diseases with clinical courses that depend on the initial interaction between pathogen and host in the skin, such as in Zika and dengue virus infections, will also be beneficial.
The immune response to various infectious agents in the skin triggers a wide range of defense mechanisms. Nevertheless, this response is sufficiently specific to fulfill its primary role: to act as an interface between host and the environment. In recent years, an increasingly important role has been attributed to the cutaneous immune system because it is in this environment that the mechanisms of immune responses to various infectious agents begin. It is very likely that a series of immune cascades determines the host response pattern according to this first interaction. From this perspective, special emphasis has been placed on DCs, which are components of innate immunity and the first subpopulation to interact with infectious agents that enter the body through epithelia such as skin and mucous membranes. This subpopulation is also the link to the mechanisms of adaptive immunity and determines the pattern of responses to be developed against these specific agents. Recently identified mechanisms and immunological factors involved in the control of immune responses have also attracted attention in recent years, opening up promising research into both cutaneous and general immunity.
Important research topics include the role of inflammasomes in the control of local immune responses, the characterization of endoplasmic reticulum stress mechanisms in the effectiveness of the responses by DCs and macrophages, the emergence of the roles of keratinocytes, fibroblasts, and melanocytes in the control of the immune response, and the role of endotheliocytes in the flux of immune cells into the skin. The characterization of this tissue environment will lead to a better understanding of the relationship between the host and the environment.
ACKNOWLEDGMENTS
Juarez A. S. Quaresma is a Research Productivity Fellow and Senior Postdoctoral Fellow at the Brazilian National Council for Scientific and Technological Development—CNPq/Brazil (grant numbers 302553/2015-0 and 116427/2016-7).
I declare that I have no competing interests.
Biography

Juarez Antonio Simões Quaresma received undergraduate training in medicine at School of Medicine of Federal University of Pará—Brazil and obtained a Ph.D. degree in pathology from the Faculty of Medicine of the University of São Paulo—Brazil. His research interests include immunity and immunopathogenesis of infectious diseases. He has studied the mechanisms of impaired host defense in the skin and liver using human and experimental animal models for the past 20 years. More recently, he has been involved in studies of interaction between skin cells and arboviruses of public health importance such as dengue, yellow fever, chikungunya, and Zika viruses. He is an Associate Professor of Tropical Medicine Center of Federal University of Pará/Brazil, Researcher of the Division of Pathology at the Evandro Chagas Institute/Ministry of Health/Brazil, and Collaborator Researcher of Department of Pathology, School of Medicine of São Paulo University/Brazil.
REFERENCES
- 1.Engwerda CR, Kaye PM. 2000. Organ-specific immune responses associated with infectious disease. Immunol Today 21:73–78. doi: 10.1016/S0167-5699(99)01549-2. [DOI] [PubMed] [Google Scholar]
- 2.Quaresma JAS, Sotto MN, Balato A. 2017. Inflammatory and immune-mediated cutaneous diseases. Mediators Inflamm 2017:6793968. doi: 10.1155/2017/6793968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasque P, Jaffar-Bandjee MC. 2015. The immunology and inflammatory responses of human melanocytes in infectious diseases. J Infect 71:413–421. doi: 10.1016/j.jinf.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Handfield C, Kwock J, MacLeod AS. 2018. Innate antiviral immunity in the skin. Trends Immunol 39:328–340. doi: 10.1016/j.it.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd AL, Belkaid Y, Segre JA. 2018. The human skin microbiome. Nat Rev Microbiol 16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 6.Egawa G, Kabashima K. 2018. Barrier dysfunction in the skin allergy. Allergol Int 67:3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Ali N, Rosenblum MD. 2017. Regulatory T cells in skin. Immunology 152:372–381. doi: 10.1111/imm.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias PM, Wakefield JS. 2014. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol 134:781–791. doi: 10.1016/j.jaci.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt D. 1994. Cutaneous immune system. C R Seances Soc Biol Fil 188:207–221. [PubMed] [Google Scholar]
- 10.Lapins J, Gaines H, Lindbäck S, Lidbrink P, Emtestam L. 1997. Skin and mucosal characteristics of symptomatic primary HIV-1 infection. AIDS Patient Care STDS 11:67–70. doi: 10.1089/apc.1997.11.67. [DOI] [PubMed] [Google Scholar]
- 11.Owen JL, Mohamadzadeh M. 2013. Microbial activation of gut dendritic cells and the control of mucosal immunity. J Interferon Cytokine Res 33:619–631. doi: 10.1089/jir.2013.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debeer S, Le Luduec JB, Kaiserlian D, Laurent P, Nicolas JF, Dubois B, Kanitakis J. 2013. Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol 23:456–466. doi: 10.1684/ejd.2013.2060. [DOI] [PubMed] [Google Scholar]
- 13.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. 2009. Skin immune sentinels in health and disease. Nat Rev Immunol 9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono S, Kabashima K. 2016. The role of dendritic cells and macrophages in the skin immunity. Nihon Rinsho Meneki Gakkai Kaishi 39:448–454. doi: 10.2177/jsci.39.448. [DOI] [PubMed] [Google Scholar]
- 15.Tay SS, Roediger B, Tong PL, Tikoo S, Weninger W. 2014. The skin-resident immune network. Curr Dermatol Rep 3:13–22. doi: 10.1007/s13671-013-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousef H, Badri T. 2018. Histology, skin appendages. StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- 17.Alegría-Landa V, Cerroni L, Kutzner H, Requena L. 2017. Paraprotein deposits in the skin. J Am Acad Dermatol 77:1145–1158. doi: 10.1016/j.jaad.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Smith EH, Chan MP. 2017. Inflammatory dermatopathology for general surgical pathologists. Clin Lab Med 37:673–696. doi: 10.1016/j.cll.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Abdayem R, Haftek M. 2018. The epidermal barrier. Ann Dermatol Venereol 145:293–301. doi: 10.1016/j.annder.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Prinz JC. 2017. Melanocytes: target cells of an HLA-C*06:02-restricted autoimmune response in psoriasis. J Invest Dermatol 137:2053–2058. doi: 10.1016/j.jid.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Boniface K, Taïeb A, Seneschal J. 2016. New insights into immune mechanisms of vitiligo. G Ital Dermatol Venereol 151:44–54. [PubMed] [Google Scholar]
- 22.Tremante E, Ginebri A, Lo Monaco E, Benassi B, Frascione P, Grammatico P, Cappellacci S, Catricalà C, Arcelli D, Natali PG, Di Filippo F, Mottolese M, Visca P, Benevolo M, Giacomini P. 2014. A melanoma immune response signature including human leukocyte antigen-E. Pigment Cell Melanoma Res 27:103–112. doi: 10.1111/pcmr.12164. [DOI] [PubMed] [Google Scholar]
- 23.Tsao H, Fukunaga-Kalabis M, Herlyn M. 2017. Recent advances in melanoma and melanocyte biology. J InvestDermatol 137:557–560. doi: 10.1016/j.jid.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Feller L, Chandran R, Kramer B, Khammissa RA, Altini M, Lemmer J. 2014. Melanocyte biology and function with reference to oral melanin hyperpigmentation in HIV-seropositive subjects. AIDS Res Hum Retroviruses 30:837–843. doi: 10.1089/AID.2014.0062. [DOI] [PubMed] [Google Scholar]
- 25.Burgeson RE, Christiano AM. 1997. The dermal-epidermal junction. Curr Opin Cell Biol 9:651–658. doi: 10.1016/S0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- 26.Jakubowicz K. 1969. Role of keratinocytes in the process of keratinization. Przegl Dermatol 56:795–800. [PubMed] [Google Scholar]
- 27.Elbe A, Stingl G. 1995. Dendritic cells as stimulator cells of MHC class I-restricted immune responses. Adv Exp Med Biol 378:341–345. doi: 10.1007/978-1-4615-1971-3_76. [DOI] [PubMed] [Google Scholar]
- 28.Gieryńska M, Schollenberger A, Cespedes IS, Pawlak E. 2009. Dendritic epidermal T cells: sentinels of the skin. Postepy Hig Med Dosw (Online) 63:114–122. [PubMed] [Google Scholar]
- 29.Lai Y, Gallo RL. 2008. Toll-like receptors in skin infections and inflammatory diseases. Infect Disord Drug Targets 8:144–155. doi: 10.2174/1871526510808030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton K, Dixit VM. 2012. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4:a006049. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shisler JL. 2015. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res 92:201–252. doi: 10.1016/bs.aivir.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Ulland TK, Ferguson PJ, Sutterwala FS. 2015. Evasion of inflammasome activation by microbial pathogens. J Clin Invest 125:469–477. doi: 10.1172/JCI75254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA. 2015. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. 2007. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol 17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe H, Gaide O, Pétrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE. 2007. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 36.Keller M, Ruegg A, Werner S, Beer HD. 2008. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 37.Gilliet M, Lande R. 2008. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr Opin Immunol 20:401–407. doi: 10.1016/j.coi.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harder J, Bartels J, Christophers E, Schröder JM. 1997. A peptide antibiotic from human skin. Nature 387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 40.Weaver CT, Hatton RD, Mangan PR, Harrington LE. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 41.Kolls JK, McCray PB Jr, Chan YR. 2008. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol 8:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schütze S, Machleidt T, Krönke M. 1992. Mechanisms of tumor necrosis factor action. Semin Oncol 19:16–24. [PubMed] [Google Scholar]
- 43.Arend WP, Palmer G, Gabay C. 2008. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 44.Albanesi C, Scarponi C, Giustizieri ML, Girolomoni G. 2005. Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy 4:329–334. doi: 10.2174/1568010054022033. [DOI] [PubMed] [Google Scholar]
- 45.Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, Caux C. 2000. Macrophage inflammatory protein 3 alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med 192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickoloff BJ, Turka LA. 1994. Immunological functions of non-professional antigen-presenting cells: new insights from studies of T-cell interactions with keratinocytes. Immunol Today 15:464–469. doi: 10.1016/0167-5699(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 47.Black AP, Ardern-Jones MR, Kasprowicz V, Bowness P, Jones L, Bailey AS, Ogg GS. 2007. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur J Immunol 37:1485–1493. doi: 10.1002/eji.200636915. [DOI] [PubMed] [Google Scholar]
- 48.Kupper TS. 1990. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol 94:146–150. [DOI] [PubMed] [Google Scholar]
- 49.Luger TA, Schwarz T. 1990. Evidence for an epidermal cytokine network. J Invest Dermatol 95:100–104. [DOI] [PubMed] [Google Scholar]
- 50.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. 1991. Keratinocytes as initiators of inflammation. Lancet 337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 51.Bitschar K, Wolz C, Krismer B, Peschel A, Schittek B. 2017. Keratinocytes as sensors and central players in the immune defense against Staphylococcus aureus in the skin. J Dermatol Sci 87:215–220. doi: 10.1016/j.jdermsci.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Moody C. 2017. Mechanisms by which HPV induces a replication competent environment in differentiating keratinocytes. Viruses 19:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sylvestre DL, Disston AR, Bui DP. 2003. Vogt-Koyanagi-Harada disease associated with interferon alpha-2b/ribavirin combination therapy. J Viral Hepat 10:467–470. doi: 10.1046/j.1365-2893.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 54.Natarajan VT, Ganju P, Singh A, Vijayan V, Kirty K, Yadav S, Puntambekar S, Bajaj S, Dani PP, Kar HK, Gadgil CJ, Natarajan K, Rani R, Gokhale RS. 2014. IFN-γ signaling maintains skin pigmentation homeostasis through regulation of melanosome maturation. Proc Natl Acad Sci U S A 111:2301–2306. doi: 10.1073/pnas.1304988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taieb A, Ezzedine K, Seneschal J. 2014. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res 27:398–407. doi: 10.1111/pcmr.12219. [DOI] [PubMed] [Google Scholar]
- 56.Otsuka M, Egawa G, Kabashima K. 2018. Uncovering the mysteries of Langerhans cells, inflammatory dendritic epidermal cells, and monocyte-derived Langerhans cell-like cells in the epidermis. Front Immunol 9:1768. doi: 10.3389/fimmu.2018.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clayton K, Vallejo AF, Davies J, Sirvent S, Polak ME. 2017. Langerhans cells—programmed by the epidermis. Front Immunol 8:1676. doi: 10.3389/fimmu.2017.01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worbs T, Hammerschmidt SI, Förster R. 2017. Dendritic cell migration in health and disease. Nat Rev Immunol 17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 59.Doebel T, Voisin B, Nagao K. 2017. Langerhans cells—the macrophage in dendritic cell clothing. Trends Immunol 38:817–828. doi: 10.1016/j.it.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Ono S, Kabashima K. 2015. Novel insights into the role of immune cells in skin and inducible skin-associated lymphoid tissue (iSALT). Allergo J Int 24:170–179. doi: 10.1007/s40629-015-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. 2007. Identification of a novel population of Langerin+ dendritic cells. J Exp Med 204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med 204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. 2007. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med 204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, Merad M, Udey MC. 2009. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A 106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collin M, Bigley V. 2016. Monocyte, macrophage, and dendritic cell development: the human perspective. Microbiol Spectr 4:MCHD-0015-2015. doi: 10.1128/microbiolspec.MCHD-0015-2015. [DOI] [PubMed] [Google Scholar]
- 66.Malissen B, Tamoutounour S, Henri S. 2014. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol 14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 67.Klechevsky E. 2015. Functional diversity of human dendritic cells. Adv Exp Med Biol 850:43–54. doi: 10.1007/978-3-319-15774-0_4. [DOI] [PubMed] [Google Scholar]
- 68.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59–70. doi: 10.1016/S1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 69.Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, Novitskaya I, Carbonaro H, Cardinale I, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Wittkowski KM, Papp K, Garovoy M, Dummer W, Steinman RM, Krueger JG. 2005. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci U S A 102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bedoui S, Greyer M. 2014. The role of dendritic cells in immunity against primary herpes simplex virus infections. Front Microbiol 5:533. doi: 10.3389/fmicb.2014.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baliwag J, Barnes DH, Johnston A. 2015. Cytokines in psoriasis. Cytokine 73:342–350. doi: 10.1016/j.cyto.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwasaki Y, Fujio K, Okamura T, Yamamoto K. 2015. Interleukin-27 in T cell immunity. Int J Mol Sci 16:2851–2863. doi: 10.3390/ijms16022851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. 2006. Epidermal Langerhans cells—changing views on their function in vivo. Immunol Lett 106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Hoyo E, Kanitakis J, Schmitt D. 1993. The dermal dendrocyte. Pathol Biol (Paris) 41:613–618. [PubMed] [Google Scholar]
- 75.Silverman JS, Tamsen A. 1998. CD34 and factor XIIIa-positive microvascular dendritic cells and the family of fibrohistiocytic mesenchymal tumors. Am J Dermatopathol 20:533–536. doi: 10.1097/00000372-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 76.Quatresooz P, Paquet P, Hermanns-Lê T, Piérard GE. 2008. Molecular mapping of factor XIIIa-enriched dendrocytes in the skin. Int J Mol Med 22:403–409. [PubMed] [Google Scholar]
- 77.Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, Rea TH, Brennan PJ, Belisle JT, Blauvelt A, Porcelli SA, Modlin RL. 2004. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest 113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Igyártó BZ, Kaplan DH. 2013. Antigen presentation by Langerhans cells. Curr Opin Immunol 25:115–119. doi: 10.1016/j.coi.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science 294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 80.Segura E, Amigorena S. 2014. Inflammatory dendritic cells. Med Sci (Paris) 30:64–68. doi: 10.1051/medsci/20143001015. [DOI] [PubMed] [Google Scholar]
- 81.Boyman O, Conrad C, Dudli C, Kielhorn E, Nickoloff BJ, Nestle FO. 2005. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br J Dermatol 152:1211–1218. doi: 10.1111/j.1365-2133.2005.06701.x. [DOI] [PubMed] [Google Scholar]
- 82.Angel CE, Lala A, Chen CJ, Edgar SG, Ostrovsky LL, Dunbar PR. 2007. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int Immunol 19:1271–1279. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- 83.Podojil JR, Miller SD. 2013. Targeting the B7 family of co-stimulatory molecules: successes and challenges. BioDrugs 27:1–13. doi: 10.1007/s40259-012-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 85.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. 2003. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp 252:106–114. [DOI] [PubMed] [Google Scholar]
- 86.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riella LV, Paterson AM, Sharpe AH, Chandraker A. 2012. Role of the PD-1 pathway in the immune response. Am J Transplant 12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy K, Weaver C. 2016. Janeway’s immunobiology, 9th ed, p 161 Garland Science, New York, NY. [Google Scholar]
- 89.Peron G, de Lima Thomaz L, Camargo da Rosa L, Thomé R, Cardoso Verinaud LM. 2018. Modulation of dendritic cell by pathogen antigens: where do we stand? Immunol Lett 196:91–102. doi: 10.1016/j.imlet.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Qian C, Cao X. 2018. Dendritic cells in the regulation of immunity and inflammation. Semin Immunol 35:3–11. doi: 10.1016/j.smim.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Zhong Y, Kinio A, Saleh M. 2013. Functions of NOD-like receptors in human diseases. Front Immunol 4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsuchiya K, Hara H. 2014. The inflammasome and its regulation. Crit Rev Immunol 34:41–80. doi: 10.1615/CritRevImmunol.2013008686. [DOI] [PubMed] [Google Scholar]
- 93.Kim JO, Jo E. 2013. NLRP3 inflammasome and host protection against bacterial infection. J Korean Med Sci 28:1415–1423. doi: 10.3346/jkms.2013.28.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yurdakul P. 2005. Immunopathogenesis of Leishmania infections. Mikrobiyol Bul 39:363–381. [PubMed] [Google Scholar]
- 95.Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva AL, Mineo TW, Gutierrez FR, Bellio M, Bortoluci KR, Flavell RA, Bozza MT, Silva JS, Zamboni DS. 2013. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat Med 19:909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 96.Maspi N, Abdoli A, Ghaffarifar F. 2016. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: a review. Pathog Glob Health 110:247–260. doi: 10.1080/20477724.2016.1232042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gurung P, Kanneganti TD. 2016. Immune responses against protozoan parasites: a focus on the emerging role of Nod-like receptors. Cell Mol Life Sci 73:3035–3051. doi: 10.1007/s00018-016-2212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clay GM, Sutterwala FS, Wilson ME. 2014. NLR proteins and parasitic disease. Immunol Res 59:142–152. doi: 10.1007/s12026-014-8544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lima-Junior DS, Mineo TWP, Calich VLG, Zamboni DS. 2017. Dectin-1 activation during Leishmania amazonensis phagocytosis prompts syk-dependent reactive oxygen species production to trigger inflammasome assembly and restriction of parasite replication. J Immunol 199:2055–2068. doi: 10.4049/jimmunol.1700258. [DOI] [PubMed] [Google Scholar]
- 100.Gupta AK, Ghosh K, Palit S, Barua J, Das PK, Ukil A. 2017. Leishmania donovani inhibits inflammasome-dependent macrophage activation by exploiting the negative regulatory proteins A20 and UCP2. FASEB J 31:5087–5101. doi: 10.1096/fj.201700407R. [DOI] [PubMed] [Google Scholar]
- 101.Zamboni DS, Lima-Junior DS. 2015. Inflammasomes in host response to protozoan parasites. Immunol Rev 265:156–171. doi: 10.1111/imr.12291. [DOI] [PubMed] [Google Scholar]
- 102.Gringhuis SI, Wevers BA, Kaptein TM, van Capel TM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TB. 2011. Selective C-Rel activation via malt1 controls anti-fungal T(H)-17 immunity by dectin-1 and dectin-2. PLoS Pathog 7:e1001259. doi: 10.1371/journal.ppat.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia VE, Uyemura K, Sieling PA, Ochoa MT, Morita CT, Okamura H, Kurimoto M, Rea TH, Modlin RL. 1999. IL-18 promotes type 1 cytokine production from NK cells V and T cells in human intracellular infection. J Immunol 162:6114–6121. [PubMed] [Google Scholar]
- 104.Costa RD, Mendonça VA, Lyon S, Penido RA, Costa A, Costa MD, Nishi MP, Teixeira MM, Teixeira AL, Antunes C. 2008. Avaliação da expressão de interleucina 1 beta (IL-1β) e antagonista do receptor de interleucina1 (IL-1Ra) em pacientes com hanseníase. Rev Soc Bras Med Trop 41:99–103. doi: 10.1590/S0037-86822008000700020. [DOI] [PubMed] [Google Scholar]
- 105.Sinsimer D, Fallows D, Peixoto B, Krahenbuhl J, Kaplan G, Manca C. 2010. Mycobacterium leprae actively modulates the cytokine response in naïve human monocytes. Infect Immun 78:293–300. doi: 10.1128/IAI.00816-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang TJ, Lee GS, Kim SK, Jin SH, Chae GT. 2010. Comparison of two mice strains, A/J and C57BL/6, in caspase-1 activity and IL-1β secretion of macrophage to Mycobacterium leprae infection. Med Inflamm 2010:1. doi: 10.1155/2010/708713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marzano AV, Borghi A, Wallach D, Cugno M. 2018. A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol 54:114–130. doi: 10.1007/s12016-017-8621-8. [DOI] [PubMed] [Google Scholar]
- 108.Chiricozzi A, Romanelli P, Olpe E, Borsellino G, Romanelli M. 2018. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci 19:179. doi: 10.3390/ijms19010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taylor PR, Roy S, Leal SM, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. 2014. Activation of neutrophils by autocrine IL-17-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol 15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tamarozzi F, Wright HL, Thomas HB, Edwards SW, Taylor MJ. 2014. A lack of confirmation with alternative assays questions the validity of IL-17 expression in human neutrophils using immunohistochemistry. Immunol Lett 162:194–198. doi: 10.1016/j.imlet.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 111.Gonçalves-de-Albuquerque SDC, Pessoa-E-Silva R, Trajano-Silva LAM, de Goes TC, de Morais RCS, Oliveira CNDC, de Lorena VMB, de Paiva-Cavalcanti M. 2017. The equivocal role of Th17 cells and neutrophils on immunopathogenesis of leishmaniasis. Front Immunol 8:1437. doi: 10.3389/fimmu.2017.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murray PJ, Wynn TA. 2011. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chow A, Brown BD, Merad M. 2011. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 114.Gordon S. 2003. Alternative activation of macrophages. Nat Rev Immunol 3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 115.Broichhausen C, Riquelme P, Geissler EK, Hutchinson JA. 2012. Regulatory macrophages as therapeutic targets and therapeutic agents in solid organ transplantation. Curr Opin Organ Transplant 17:332–342. doi: 10.1097/MOT.0b013e328355a979. [DOI] [PubMed] [Google Scholar]
- 116.Hristodorov D, Mladenov R, Huhn M, Barth S, Thepen T. 2012. Macrophage-targeted therapy: CD64-based immunotoxins for treatment of chronic inflammatory diseases. Toxins (Basel) 4:676–694. doi: 10.3390/toxins4090676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Konttinen YT, Pajarinen J, Takakubo Y, Gallo J, Nich C, Takagi M, Goodman SB. 2014. Macrophage polarization and activation in response to implant debris: influence by “particle disease” and “ion disease”. J Long Term Eff Med Implants 24:267–281. doi: 10.1615/JLongTermEffMedImplants.2014011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erbel C, Wolf A, Lasitschka F, Linden F, Domschke G, Akhavanpoor M, Doesch AO, Katus HA, Gleissner CA. 2015. Prevalence of M4 macrophages within human coronary atherosclerotic plaques is associated with features of plaque instability. Int J Cardiol 186:219–225. doi: 10.1016/j.ijcard.2015.03.151. [DOI] [PubMed] [Google Scholar]
- 119.Lawrence T, Natoli G. 2011. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 120.Shi C, Pamer EG. 2011. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wynn TA, Chawla A, Pollard JW. 2013. Macrophage biology in development, homeostasis and disease. Nature 496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.London A, Cohen M, Schwartz M. 2013. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front Cell Neurosci 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galain CL, Olleros ML, Vesin D, Garcia I. 2015. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Front Immunol 6:263. doi: 10.3389/fimmu.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paoli F, Staels B, Chinetti-Gbaguidi G. 2014. Macrophage phenotypes and their modulation in atherosclerosis. Circ J 78:1775–1781. doi: 10.1253/circj.CJ-14-0621. [DOI] [PubMed] [Google Scholar]
- 125.Colin S, Chinetti-Gbaguidi G, Staels B. 2014. Macrophage phenotypes in atherosclerosis. Immunol Rev 262:153–166. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- 126.Rabhi S, Rabhi I, Trentin B, Piquemal D, Regnault B, Goyard S, Lang T, Descoteaux A, Enninga J, Guizani-Tabbane L. 2016. Lipid droplet formation, their localization and dynamics during Leishmania major macrophage infection. PLoS One 11:e0148640. doi: 10.1371/journal.pone.0148640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nogueira PM, Assis RR, Torrecilhas AC, Saraiva EM, Pessoa NL, Campos MA, Marialva EF, Ríos-Velasquez CM, Pessoa FA, Secundino NF, Rugani JN, Nieves E, Turco SJ, Melo MN, Soares RP. 2016. Lipophosphoglycans from Leishmania amazonensis strains display immunomodulatory properties via TLR4 and do not affect sand fly infection. PLoS Negl Trop Dis 10:e0004848. doi: 10.1371/journal.pntd.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sousa JR, Lucena Neto FD, Sotto MN, Quaresma J. 2018. Immunohistochemical characterization of the M4 macrophage population in leprosy skin lesions. BMC Infect Dis 18:576. doi: 10.1186/s12879-018-3478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mattos KA, Oliveira VC, Berrêdo-Pinho M, Amaral JJ, Antunes LC, Melo RC, Acosta CC, Moura DF, Olmo R, Han J, Rosa PS, Almeida PE, Finlay BB, Borchers CH, Sarno EN, Bozza PT, Atella GC, Pessolani MC. 2014. Mycobacterium leprae intracellular survival relies on cholesterol accumulation in infected macrophages: a potential target for new drugs for leprosy treatment. Cell Microbiol 16:797–815. doi: 10.1111/cmi.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elamin AA, Stehr M, Singh M. 2012. Lipid droplets and Mycobacterium leprae infection. J Pathog 2012:361374. doi: 10.1155/2012/361374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abraham SN, Arock M. 1998. Mast cells and basophils in innate immunity. Semin Immunol 10:373–381. doi: 10.1006/smim.1998.0140. [DOI] [PubMed] [Google Scholar]
- 132.Kabashima K, Nakashima C, Nonomura Y, Otsuka A, Cardamone C, Parente R, De Feo G, Triggiani M. 2018. Biomarkers for evaluation of mast cell and basophil activation. Immunol Rev 282:114–120. doi: 10.1111/imr.12639. [DOI] [PubMed] [Google Scholar]
- 133.Yu R, Igawa K, Handa Y, Munetsugu T, Satoh T, Yokozeki H. 2017. Basophils and mast cells are crucial for reactions due to epicutaneous sensitization to ovalbumin. Exp Dermatol 26:778–784. doi: 10.1111/exd.13279. [DOI] [PubMed] [Google Scholar]
- 134.Iki M, Tanaka K, Deki H, Fujimaki M, Sato S, Yoshikawa S, Yamanishi Y, Karasuyama H. 2016. Basophil tryptase mMCP-11 plays a crucial role in IgE-mediated, delayed-onset allergic inflammation in mice. Blood 128:2909–2918. doi: 10.1182/blood-2016-07-729392. [DOI] [PubMed] [Google Scholar]
- 135.Bagher M, Larsson-Callerfelt AK, Rosmark O, Hallgren O, Bjermer L, Westergren-Thorsson G. 2018. Mast cells and mast cell tryptase enhance migration of human lung fibroblasts through protease-activated receptor 2. Cell Commun Signal 16:59. doi: 10.1186/s12964-018-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chirumbolo S, Bjørklund G, Vella A. 2018. Mast cell activation test versus basophil activation test and related competing issues. J Allergy Clin Immunol 142:1018–1019. doi: 10.1016/j.jaci.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 137.Xie G, Wang F, Peng X, Liang Y, Yang H, Li L. 2018. Modulation of mast cell Toll-like receptor 3 expression and cytokines release by histamine. Cell Physiol Biochem 46:2401–2411. doi: 10.1159/000489646. [DOI] [PubMed] [Google Scholar]
- 138.Choi JE, Di Nardo A. 2018. Skin neurogenic inflammation. Semin Immunopathol 40:249–259. doi: 10.1007/s00281-018-0675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Van Linthout S, Miteva K, Tschöpe C. 2014. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 102:258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 140.Lin L, Hwang BJ, Culton DA, Li N, Burette S, Koller BH, Messingham KA, Fairley JA, Lee JJ, Hall RP, An L, Diaz LA, Liu Z. 2017. Eosinophils mediate tissue injury in autoimmune skin disease bullous pemphigoid. J Invest Dermatol 12:S0022-202X(17)33250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Freire PC, Muñoz CH, Stingl G. 2017. IgE autoreactivity in bullous pemphigoid: eosinophils and mast cells as major targets of pathogenic immune reactants. Br J Dermatol 177:1644–1653. doi: 10.1111/bjd.15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ohtsu H, Seike M. 2017. Histamine and histamine receptors in allergic dermatitis. Handb Exp Pharmacol 241:333–345. doi: 10.1007/164_2016_9. [DOI] [PubMed] [Google Scholar]
- 143.Venturelli N, Lexmond WS, Ohsaki A, Nurko S, Karasuyama H, Fiebiger E, Oyoshi MK. 2016. Allergic skin sensitization promotes eosinophilic esophagitis through the IL-33-basophil axis in mice. J Allergy Clin Immunol 138:1367–1380. doi: 10.1016/j.jaci.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 144.Graauw E, Beltraminelli H, Simon HU, Simon D. 2015. Eosinophilia in dermatologic disorders. Immunol Allergy Clin North Am 35:545–560. doi: 10.1016/j.iac.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 145.Long H, Zhang G, Wang L, Lu Q. 2016. Eosinophilic skin diseases: a comprehensive review. Clin Rev Allergy Immunol 50:189–213. doi: 10.1007/s12016-015-8485-8. [DOI] [PubMed] [Google Scholar]
- 146.Cook PC, Aynsley SA, Turner JD, Jenkins GR, Van Rooijen N, Leeto M, Brombacher F, Mountford AP. 2011. Multiple helminth infection of the skin causes lymphocyte hypo-responsiveness mediated by Th2 conditioning of dermal myeloid cells. PLoS Pathog 7:e1001323. doi: 10.1371/journal.ppat.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pearlman E. 1997. Immunopathology of onchocerciasis: a role for eosinophils in onchocercal dermatitis and keratitis. Chem Immunol 66:26–40. doi: 10.1159/000058664. [DOI] [PubMed] [Google Scholar]
- 148.Prendergast CT, Sanin DE, Mountford AP. 2016. CD4 T-cell hyporesponsiveness induced by schistosome larvae is not dependent upon eosinophils but may involve connective tissue mast cells. Parasite Immunol 38:81–92. doi: 10.1111/pim.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumar S. 2018. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 154:383–393. doi: 10.1111/imm.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kurioka A, Klenerman P, Willberg C. 2018. Innate-like CD8+ T cells and NK cells: converging functions and phenotypes. Immunology 14. doi: 10.1111/imm.12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar A, Suryadevara N, Hill TM, Bezbradica JS, Van Kaer L, Joyce S. 2017. Natural killer T cell an ecological evolutionary developmental biology perspective. Front Immunol 8:1858. doi: 10.3389/fimmu.2017.01858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peng H, Tian Z. 2017. Natural killer cell memory: progress and implications. Front Immunol 8:1143. doi: 10.3389/fimmu.2017.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rusek P, Wala M, Druszczyńska M, Fol M. 2018. Infectious agents as stimuli of trained innate immunity. Int J Mol Sci 19:456. doi: 10.3390/ijms19020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. 2009. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol 39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 155.Yachie A, Kanegane H, Kasahara Y. 2003. Epstein-Barr virus-associated T-natural killer cell lymphoproliferative diseases. Semin Hematol 40:124–132. doi: 10.1053/shem.2003.50012. [DOI] [PubMed] [Google Scholar]
- 156.Haustein UF. 1989. Bacterial skin flora, host defense and skin infections. Dermatol Monatsschr 175:665–680. [PubMed] [Google Scholar]
- 157.Reinhold U, Bauer R. 1988. Clinico-immunological analysis of the significance of natural killer cells in skin diseases. Hautarzt 39:129–135. [PubMed] [Google Scholar]
- 158.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. 2009. Human fetal lymphoid tissue inducer cells are interleukin 17 producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol 10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 159.Teunissen MBM, Munneke JM, Bernink JH, Spuls PI, Res PCM, Te Velde A, Cheuk S, Brouwer MWD, Menting SP, Eidsmo L, Spits H, Hazenberg MD, Mjösberg J. 2014. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol 134:2351–2360. doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- 160.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12:1045–1054. doi: 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, Chapman A, Smith CH, Di Meglio P, Nestle FO. 2014. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol 134:984–991. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Laffleur B, Debeaupuis O, Dalloul Z, Cogné M. 2017. B cell intrinsic mechanisms constraining IgE memory. Front Immunol 8:1277. doi: 10.3389/fimmu.2017.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rodríguez-Pinto D. 2005. B cells as antigen presenting cells. Cell Immunol 238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 164.Nihal M, Mikkola D, Wood GS. 2000. Detection of clonally restricted immunoglobulin heavy chain gene rearrangements in normal and lesional skin: analysis of the B cell component of the skin-associated lymphoid tissue and implications for the molecular diagnosis of cutaneous B cell lymphomas. J Mol Diagn 2:5–10. doi: 10.1016/S1525-1578(10)60609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shimizu Y, Newman W, Tanaka Y, Shaw S. 1992. Lymphocyte interactions with endothelial cells. Immunol Today 13:106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 166.Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, Young AJ, Hay JB, Debes GF. 2012. The skin, a novel niche for recirculating B cells. J Immunol 188:6027–6035. doi: 10.4049/jimmunol.1102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lafyatis R, Kissin E, York M, Farina G, Viger K, Fritzler MJ, Merkel PA, Simms RW. 2009. B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum 60:578–583. doi: 10.1002/art.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Simon D, Hösli S, Kostylina G, Yawalkar N, Simon HU. 2008. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol 121:122–128. doi: 10.1016/j.jaci.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 169.Holtfreter S, Kolata J, Broker BM. 2010. Towards the immune proteome of Staphylococcus aureus—the anti-S. aureus antibody response. Int J Med Microbiol 300:176–192. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 170.Kobayashi SD, Deleo FR. 2011. A MRSA-terious enemy among us: boosting MRSA vaccines. Nat Med 17:168–169. doi: 10.1038/nm0211-168. [DOI] [PubMed] [Google Scholar]
- 171.Berthelot JM, Jamin C, Amrouche K, Le Goff B, Maugars Y, Youinou P. 2013. Regulatory B cells play a key role in immune system balance. Joint Bone Spine 80:18–22. doi: 10.1016/j.jbspin.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 172.Aravena O, Ferrier A, Menon M, Mauri C, Aguillón JC, Soto L, Catalán D. 2017. TIM-1 defines a human regulatory B cell population that is altered in frequency and function in systemic sclerosis patients. Arthritis Res Ther 19:8. doi: 10.1186/s13075-016-1213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. 2006. The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 174.Andrew W, Andrew NV. 1949. Lymphocytes in the normal epidermis of the rat and of man. Anat Rec 104:217–241. doi: 10.1002/ar.1091040207. [DOI] [PubMed] [Google Scholar]
- 175.Bos JD, Kapsenberg ML. 1993. The skin immune system: progress in cutaneous biology. Immunol Today 14:75–78. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- 176.Foster CA, Yokozeki H, Rappersberger K, Koning F, Volc-Platzer B, Rieger A, Coligan JE, Wolff K, Stingl G. 1990. Human epidermal T cells predominantly belong to the lineage expressing alpha/beta T cell receptor. J Exp Med 171:997–1013. doi: 10.1084/jem.171.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bos JD, Zonneveld I, Das PK, Krieg SR, van der Loos CM, Kapsenberg ML. 1987. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J Invest Dermatol 88:569–573. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- 178.de Sousa JR, Quaresma JAS. 2018. The role of T helper 25 cells in the immune response to M. leprae. J Am Acad Dermatol 78:1009–1011. doi: 10.1016/j.jaad.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 179.Sousa JR, Pagliari C, de Almeida DSM, Barros LFL, Carneiro FRO, Dias LB Jr, de Souza Aarão TL, Quaresma J. 2017. Th9 cytokines response and its possible implications in the immunopathogenesis of leprosy. J Clin Pathol 70:521–527. doi: 10.1136/jclinpath-2016-204110. [DOI] [PubMed] [Google Scholar]
- 180.Silveira EL, Sousa JR, Aarão TLS, Fuzii HT, Dias Junior LB, Carneiro FR, Quaresma JA. 2015. New immunologic pathways in the pathogenesis of leprosy: role for Th22 cytokines in the polar forms of the disease. J Am Acad Dermatol 72:729–730. doi: 10.1016/j.jaad.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 181.Kutlu A, Bozkurt B, Ciftci F, Bozkanat E. 2007. Th1-Th2 interaction: is more complex than a see-saw? Scand J Immunol 65:393–395. doi: 10.1111/j.1365-3083.2007.01917.x. [DOI] [PubMed] [Google Scholar]
- 182.Lowes MA, Suárez-Fariñas M, Krueger JG. 2014. Immunology of psoriasis. Annu Rev Immunol 32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Di Cesare A, Di Meglio P, Nestle FO. 2009. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 184.Chien CH, Chiang BL. 2017. Regulatory T cells induced by B cells: a novel subpopulation of regulatory T cells. J Biomed Sci 24:86. doi: 10.1186/s12929-017-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Singh B, Krawetz MD, De Lima RM, Mukherjee R, Chaturvedi P, Lee-Chan E, Leiter EH, Summers KL. 2018. Role of TGF-β in self-peptide regulation of autoimmunity. Arch Immunol Ther Exp (Warsz) 66:11–19. doi: 10.1007/s00005-017-0482-6. [DOI] [PubMed] [Google Scholar]
- 186.Torres-Poveda K, Burguete-García AI, Bahena-Román M, Méndez-Martínez R, Zurita-Díaz MA, López-Estrada G, Delgado-Romero K, Peralta-Zaragoza O, Bermúdez-Morales VH, Cantú D, García-Carrancá A, Madrid-Marina V. 2016. Risk allelic load in Th2 and Th3 cytokines genes as biomarker of susceptibility to HPV-16 positive cervical cancer: a case control study. BMC Cancer 16:330. doi: 10.1186/s12885-016-2364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mihailova S, Ivanova-Genova E, Lukanov T, Stoyanova V, Milanova V, Naumova E. 2016. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J Neuroimmunol 293:123–128. doi: 10.1016/j.jneuroim.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 188.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 189.Mohr A, Malhotra R, Mayer G, Gorochov G, Miyara M. 2018. Human FOXP3(+) T regulatory cell heterogeneity. Clin Transl Immunology 7:e1005. doi: 10.1002/cti2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Farahani P, Halabian R, Vahedi E, Salimian J. 2017. Increased genes expression levels of cytokines related to Th17/Treg cells in peripheral blood mononuclear cell correlate with clinical severity in COPD and mustard gas-exposed patients. Iran J Allergy Asthma Immunol 16:396–403. [PubMed] [Google Scholar]
- 191.Komai T, Okamura T, Yamamoto K, Fujio K. 2016. The effects of TGF-βs on imune responses. Nihon Rinsho Meneki Gakkai Kaishi 39:51–58. (In Japanese.) doi: 10.2177/jsci.39.51. [DOI] [PubMed] [Google Scholar]
- 192.Weiner HL, da Cunha AP, Quintana F, Wu H. 2011. Oral tolerance. Immunol Rev 241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kitani A, Chua K, Nakamura K, Strober W. 2000. Activated self-MHC-reactive T cells have the cytokine phenotype of Th3/T regulatory cell 1 T cells. J Immunol 165:691–702. doi: 10.4049/jimmunol.165.2.691. [DOI] [PubMed] [Google Scholar]
- 194.Lewkowicz N, Kur B, Kurnatowska A, Tchorzewski H, Lewkowicz P. 2011. Expression of Th1/Th2/Th3/Th17-related genes in recurrent aphthous ulcers. Arch Immunol Ther Exp (Warsz) 59:399–406. doi: 10.1007/s00005-011-0134-1. [DOI] [PubMed] [Google Scholar]
- 195.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. 2007. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol 178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 196.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH, Anthony BA, Sverdrup FM, Krow-Lucal E, MacKenzie TC, Johnson DS, Meyer EH, Löhr A, Hsu A, Koo J, Liao W, Gupta R, Debbaneh MG, Butler D, Huynh M, Levin EC, Leon A, Hoffman WY, McGrath MH, Alvarado MD, Ludwig CH, Truong HA, Maurano MM, Gratz IK, Abbas AK, Rosenblum MD. 2014. Memory regulatory T cells reside in human skin. J Clin Invest 124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharma A, Rudra D. 2018. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol 9:883. doi: 10.3389/fimmu.2018.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li J, Tan J, Martino MM, Lui KO. 2018. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol 9:585. doi: 10.3389/fimmu.2018.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Owczarczyk-Saczonek A, Czerwińska J, Placek W. 2018. The role of regulatory T cells and anti-inflammatory cytokines in psoriasis. Acta Dermatovenerol Alp Pannonica Adriat 27:17–23. [PubMed] [Google Scholar]
- 200.Malhotra N, Leyva-Castillo JM, Jadhav U, Barreiro O, Kam C, O'Neill NK, Meylan F, Chambon P, von Andrian UH, Siegel RM, Wang EC, Shivdasani R, Geha RS. 2018. RORα-expressing T regulatory cells restrain allergic skin inflammation. Sci Immunol 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong H-A, Lowe MM, Sanchez Rodriguez R, Ali N, Laszik ZG, Sonnenburg JL, Millar SE, Rosenblum MD. 2017. Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe 21:467–477. doi: 10.1016/j.chom.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, Rosenblum MD. 2013. Cutting edge: memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol 190:4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chow Z, Mueller SN, Deane JA, Hickey MJ. 2013. Dermal regulatory T cells display distinct migratory behavior that is modulated during adaptive and innate inflammation. J Immunol 191:3049–3056. doi: 10.4049/jimmunol.1203205. [DOI] [PubMed] [Google Scholar]
- 204.Akagawa KS. 2012. Recent advances in tuberculosis immunity. Kekkaku 87:61–70. [PubMed] [Google Scholar]
- 205.McDonald DR. 2012. TH17 deficiency in human disease. J Allergy Clin Immunol 129:1429–1435. doi: 10.1016/j.jaci.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. 2009. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhong W, Jiang Y, Ma H, Wu J, Jiang Z, Zhao L. 2017. Elevated levels of CCR6+ T helper 22 cells correlate with skin and renal impairment in systemic lupus erythematosus. Sci Rep 7:12962. doi: 10.1038/s41598-017-13344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ting L, Yan L, Shuang Y, Congcong Y, Peng L, Jingjing Y, Daoxin M, Chunyan J. 2016. Increased frequency of circulating Th22 cells in patients with B-cell non-Hodgkin’s lymphoma. Oncotarget 7:56574–56583. doi: 10.18632/oncotarget.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Eyerich K, Eyerich S. 2015. Th22 cells in allergic disease. Allergo J Int 24:1–7. doi: 10.1007/s40629-015-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cheuk S, Wikén M, Blomqvist L, Nylén S, Talme T, Ståhle M, Eidsmo L. 2014. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 192:3111–3120. doi: 10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Michalak-Stoma A, Bartosińska J, Kowal M, Juszkiewicz-Borowiec M, Gerkowicz A, Chodorowska G. 2013. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers 35:625–631. doi: 10.1155/2013/856056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119:3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kaplan MH, Hufford MM, Olson MR. 2015. The development and in vivo function of T helper 9 cells. Nat Rev Immunol 15:295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li J, Chen S, Xiao X, Zhao Y, Ding W, Li XC. 2017. IL-9 and Th9 cells in health and diseases—from tolerance to immunopathology. Cytokine Growth Factor Rev 37:47–55. doi: 10.1016/j.cytogfr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rochfort KD, Cummins PM. 2015. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans 43:702–706. doi: 10.1042/BST20140319. [DOI] [PubMed] [Google Scholar]
- 216.Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, Campbell L, Yawalkar N, Kupper TS, Clark RA. 2014. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med 6:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Singh TP, Schön MP, Wallbrecht K, Gruber-Wackernagel A, Wang XJ, Wolf P. 2013. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PLoS One 8:e51752. doi: 10.1371/journal.pone.0051752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R. 2014. Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res 3:127. doi: 10.4103/2277-9175.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Caza T, Landas S. 2015. Functional and phenotypic plasticity of CD4(+) T cell subsets. Biomed Res Int 2015:521957. doi: 10.1155/2015/521957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Monin L, Gaffen SL. 2017. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb Perspect Biol 15:a028522. doi: 10.1101/cshperspect.a028522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Castro-Lopez N, Hung CY. 2017. Immune response to coccidioidomycosis and the development of a vaccine. Microorganisms 16:E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, Masson C, Toth B, Flatot J, Migaud M, Chrabieh M, Kochetkov T, Bolze A, Borghesi A, Toulon A, Hiller J, Eyerich S, Eyerich K, Gulácsy V, Chernyshova L, Chernyshov V, Bondarenko A, Grimaldo RM, Blancas-Galicia L, Beas IM, Roesler J, Magdorf K, Engelhard D, Thumerelle C, Burgel PR, Hoernes M, Drexel B, Seger R, Kusuma T, Jansson AF, Sawalle-Belohradsky J, Belohradsky B, Jouanguy E, Bustamante J, Bué M, Karin N, Wildbaum G, Bodemer C, Lortholary O, Fischer A, Blanche S, et al. . 2011. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nielsen MM, Witherden DA, Havran WL. 2017. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Born WK, Huang Y, Reinhardt RL, Huang H, Sun D, O’Brien RL. 2017. γδ T cells and B cells. Adv Immunol 134:1–45. doi: 10.1016/bs.ai.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 225.O’Brien RL, Born WK. 2015. Dermal γδ T cells—what have we learned? Cell Immunol 296:62–69. doi: 10.1016/j.cellimm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Macleod AS, Havran WL. 2011. Functions of skin-resident γδ T cells. Cell Mol Life Sci 68:2399–2408. doi: 10.1007/s00018-011-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Komori HK, Meehan TF, Havran WL. 2006. Epithelial and mucosal gamma delta T cells. Curr Opin Immunol 18:534–538. doi: 10.1016/j.coi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 228.Dambuza IM, He C, Choi JK, Yu CR, Wang R, Mattapallil MJ, Wingfield PT, Caspi RR, Egwuagu CE. 2017. IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat Commun 8:719. doi: 10.1038/s41467-017-00838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Campbell JJ, Ebsworth K, Ertl LS, McMahon JP, Newland D, Wang Y, Liu S, Miao Z, Dang T, Zhang P, Charo IF, Singh R, Schall TJ. 2017. IL-17-secreting γδ T cells are completely dependent upon CCR6 for homing to inflamed skin. J Immunol 199:3129–3136. doi: 10.4049/jimmunol.1700826. [DOI] [PubMed] [Google Scholar]
- 230.Floudas A, Saunders SP, Moran T, Schwartz C, Hams E, Fitzgerald DC, Johnston JA, Ogg GS, McKenzie AN, Walsh PT, Fallon PG. 2017. IL-17 receptor A maintains and protects the skin barrier to prevent allergic skin inflammation. J Immunol 199:707–717. doi: 10.4049/jimmunol.1602185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fay NS, Larson EC, Jameson JM. 2016. Chronic inflammation and γδ T cells. Front Immunol 7:210. doi: 10.3389/fimmu.2016.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Foppoli M, Ferreri AJ. 2015. Gamma-delta T-cell lymphomas. Eur J Haematol 94:206–218. doi: 10.1111/ejh.12439. [DOI] [PubMed] [Google Scholar]
- 233.Siegers GM. 2018. Integral roles for integrins in γδ T cell function. Front Immunol 9:521. doi: 10.3389/fimmu.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. 2007. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Med 4:e176. doi: 10.1371/journal.pmed.0040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Imada K, Leonard WJ. 2000. The JAK-STAT pathway. Mol Immunol 37:1–11. doi: 10.1016/S0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 236.Imhof BA, Jemelin S, Emre Y. 2017. Toll-like receptors elicit different recruitment kinetics of monocytes and neutrophils in mouse acute inflammation. Eur J Immunol 47:1002–1008. doi: 10.1002/eji.201746983. [DOI] [PubMed] [Google Scholar]
- 237.Martin-Rodriguez S, Caballo C, Gutierrez G, Vera M, Cruzado JM, Cases A, Escolar G, Diaz-Ricart M. 2015. TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia. Eur J Clin Invest 45:160–169. doi: 10.1111/eci.12392. [DOI] [PubMed] [Google Scholar]
- 238.Majewska M, Szczepanik M. 2006. The role of Toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Postepy Hig Med Dosw (Online) 60:52–63. (In Polish.) [PubMed] [Google Scholar]
- 239.Oude Nijhuis CS, Vellenga E, Daenen SM, Kamps WA, De Bont ES. 2003. Endothelial cells are main producers of interleukin 8 through Toll-like receptor 2 and 4 signaling during bacterial infection in leukopenic cancer patients. Clin Diagn Lab Immunol 10:558–563. doi: 10.1128/cdli.10.4.558-563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. 2000. P-selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J Exp Med 192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Stocker CJ, Sugars KL, Harari OA, Landis RC, Morley BJ, Haskard DO. 2000. TNF-a, IL-4, and IFN-g regulate differential expression of pand E-selectin expression by porcine aortic endothelial cells. J Immunol 164:3309–3315. doi: 10.4049/jimmunol.164.6.3309. [DOI] [PubMed] [Google Scholar]
- 242.Kulidjian AA, Issekutz AC, Issekutz TB. 2002. Differential role of E-selectin and P-selectin in T lymphocyte migration to cutaneous inflammatory reactons induced by cytokines. Int Immunol 14:751–760. doi: 10.1093/intimm/dxf045. [DOI] [PubMed] [Google Scholar]
- 243.Schimmel L, Heemskerk N, van Buul JD. 2017. Leukocyte transendothelial migration: a local affair. Small GTPases 8:1–15. doi: 10.1080/21541248.2016.1197872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Silva M, Videira PA, Sackstein R. 2017. E-Selectin ligands in the human mononuclear phagocyte system: implications for infection, inflammation, and immunotherapy. Front Immunol 8:1878. doi: 10.3389/fimmu.2017.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang X, Chang Y, Wei W. 2016. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediators Inflamm 2016:6813016. doi: 10.1155/2016/6813016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Marcos-Ramiro B, García-Weber D, Millán J. 2014. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost 112:1088–1102. doi: 10.1160/TH14-04-0299. [DOI] [PubMed] [Google Scholar]
- 247.Chousterman BG, Swirski FK, Weber GF. 2017. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 248.Malavige GN, Ogg GS. 2017. Pathogenesis of vascular leak in dengue virus infection. Immunol 151:261–269. doi: 10.1111/imm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Winter V. 1965. Histochemistry of the skin. Cesk Dermatol 40:263–269. (In Czech.) [PubMed] [Google Scholar]
- 250.Loewenthal LJ, Hins SC. 1965. Anatomy and histochemistry of skin 1963. Dermatologica 131:465–470. doi: 10.1159/000254506. [DOI] [PubMed] [Google Scholar]
- 251.Loewenthal LJ. 1964. Anatomy and histochemistry of skin 1962. Dermatologica 29:507–515. doi: 10.1159/000254672. [DOI] [PubMed] [Google Scholar]
- 252.Loewenthal LJ. 1963. Anatomy and histochemistry of skin L961. Dermatologica 126:380–387. doi: 10.1159/000254939. [DOI] [PubMed] [Google Scholar]
- 253.Montagna W. 1967. Comparative anatomy and physiology of the skin. Arch Dermatol 96:357–363. doi: 10.1001/archderm.1967.01610040007003. [DOI] [PubMed] [Google Scholar]
- 254.Liu W, Krump NA, MacDonald M, You J. 2018. Merkel cell polyomavirus infection of animal dermal fibroblasts. J Virol 92:e01610-17. doi: 10.1128/JVI.01610-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Barbosa EA, Oliveira A, Plácido A, Socodato R, Portugal CC, Mafud AC, Ombredane AS, Moreira DC, Vale N, Bessa LJ, Joanitti GA, Alves C, Gomes P, Delerue-Matos C, Mascarenhas YP, Marani MM, Relvas JB, Pintado M, Leite J. 2018. Structure and function of a novel antioxidant peptide from the skin of tropical frogs. Free Radic Biol Med 115:68–79. doi: 10.1016/j.freeradbiomed.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 256.Krishnaswamy VR, Mintz D, Sagi I. 2017. Matrix metalloproteinases: the sculptors of chronic cutaneous wounds. Biochim Biophys Acta 1864:2220–2227. doi: 10.1016/j.bbamcr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 257.Choi Y, Bowman JW, Jung JU. 2018. Autophagy during viral infection—a double-edged sword. Nat Rev Microbiol 16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Karonitsch T, Kandasamy RK, Kartnig F, Herdy B, Dalwigk K, Niederreiter B, Holinka J, Sevelda F, Windhager R, Bilban M, Weichhart T, Säemann M, Pap T, Steiner G, Smolen JS, Kiener HP, Superti-Furga G. 2018. mTOR senses environmental cues to shape the fibroblast-like synoviocyte response to inflammation. Cell Rep 23:2157–2167. doi: 10.1016/j.celrep.2018.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Han X, Rodriguez D, Parker TL. 2017. Biological activities of frankincense essential oil in human dermal fibroblasts. Biochim Open 4:31–35. doi: 10.1016/j.biopen.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bautista-Hernández LA, Gómez-Olivares JL, Buentello-Volante B, Bautista-de Lucio VM. 2017. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. Eur J Microbiol Immunol 7:151–157. doi: 10.1556/1886.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Singh B, Fleury C, Jalalvand F, Riesbeck K. 2012. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev 36:1122–1180. doi: 10.1111/j.1574-6976.2012.00340.x. [DOI] [PubMed] [Google Scholar]
- 262.Huang Y, Deng X, Lang J, Liang X. 2018. Modulation of quantum dots and clearance of Helicobacter pylori with synergy of cell autophagy. Nanomedicine (Lond) 14:849–861. doi: 10.1016/j.nano.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 263.Hufbauer M, Akgül B. 2017. Molecular mechanisms of human papillomavirus induced skin carcinogenesis. Viruses 9:187. doi: 10.3390/v9070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Silva-Almeida M, Carvalho LO, Abreu-Silva AL, Souza CS, Hardoim DJ, Calabrese KS. 2012. Extracellular matrix alterations in experimental Leishmania amazonenses infection in susceptible and resistant mice. Vet Res 43:10. doi: 10.1186/1297-9716-43-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Xie J, Yao B, Han Y, Huang S, Fu X. 2016. Skin appendage-derived stem cells: cell biology and potential for wound repair. Burns Trauma 4:38. doi: 10.1186/s41038-016-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shi VY, Leo M, Hassoun L, Chahal DS, Maibach HI, Sivamani RK. 2015. Role of sebaceous glands in inflammatory dermatoses. J Am Acad Dermatol 73:856–863. doi: 10.1016/j.jaad.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 267.Zouboulis CC. 2004. Acne and sebaceous gland function. Clin Dermatol 22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 268.Zouboulis CC. 2010. The sebaceous gland. Hautarzt 61:467–468. doi: 10.1007/s00105-009-1894-y. [DOI] [PubMed] [Google Scholar]
- 269.Ro BI, Dawson TL. 2005. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J Investig Dermatol Symp Proc 10:194–197. doi: 10.1111/j.1087-0024.2005.10104.x. [DOI] [PubMed] [Google Scholar]
- 270.Downing DT, Stewart ME, Wertz PW, Colton SW, Abraham W, Strauss JS. 1987. Skin lipids: an update. J Invest Dermatol 88:2s–6s. doi: 10.1111/1523-1747.ep12468850. [DOI] [PubMed] [Google Scholar]
- 271.Ekanayake-Mudiyanselage S, Thiele J. 2006. Sebaceous glands as transporters of vitamin E. Hautarzt 57:291–296. doi: 10.1007/s00105-005-1090-7. [DOI] [PubMed] [Google Scholar]
- 272.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. 2014. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Odoardi F, Neuhuber W, Flügel A. 2014. Pain-induced skin autoimmunity. Cell Res 24:1021–1022. doi: 10.1038/cr.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, et al. . 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Carmona-Gutierrez D, Bauer MA, Zimmermann A, Aguilera A, Austriaco N, Ayscough K, Balzan R, Bar-Nun S, Barrientos A, Belenky P, Blondel M, Braun RJ, Breitenbach M, Burhans WC, Büttner S, Cavalieri D, Chang M, Cooper KF, Côrte-Real M, Costa V, Cullin C, Dawes I, Dengjel J, Dickman MB, Eisenberg T, Fahrenkrog B, Fasel N, Fröhlich KU, Gargouri A, Giannattasio S, Goffrini P, Gourlay CW, Grant CM, Greenwood MT, Guaragnella N, Heger T, Heinisch J, Herker E, Herrmann JM, Hofer S, Jiménez-Ruiz A, Jungwirth H, Kainz K, Kontoyiannis DP, Ludovico P, Manon S, Martegani E, Mazzoni C, Megeney LA, Meisinger C, et al. . 2018. Guidelines and recommendations on yeast cell death nomenclature. Microb Cell 5:4–31. doi: 10.15698/mic2018.01.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Liu X, Lieberman J. 2017. A mechanistic understanding of pyroptosis: the fiery death triggered by invasive infection. Adv Immunol 135:81–117. doi: 10.1016/bs.ai.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chuang M, Chisholm AD. 2014. Insights into the functions of the death associated protein kinases from C. elegans and other invertebrates. Apoptosis 19:392–397. doi: 10.1007/s10495-013-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Penaloza-MacMaster P. 2017. CD8 T-cell regulation by T regulatory cells and the programmed cell death protein 1 pathway. Immunol 151:146–153. doi: 10.1111/imm.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kolb JP, Oguin TH III, Oberst A, Martinez J. 2017. Programmed cell death and inflammation: winter is coming. Trends Immunol 38:705–718. doi: 10.1016/j.it.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhou X, Jiang W, Liu Z, Liu S, Liang X. 2017. Virus infection and death receptor-mediated apoptosis. Viruses 27:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Morris G, Walker AJ, Berk M, Maes M, Puri BK. 2018. Cell death pathways: a novel therapeutic approach for neuroscientists. Mol Neurobiol 55:5767–5786. doi: 10.1007/s12035-017-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Callea M, Pedica F, Doglioni C. 2016. Programmed death 1 (PD-1) and its ligand (PD-L1) as a new frontier in cancer immunotherapy and challenges for the pathologist: state of the art. Pathologica 108:48–58. [PubMed] [Google Scholar]
- 283.Orzalli MH, Kagan JC. 2017. Apoptosis and necroptosis as host defense strategies to prevent viral infection. Trends Cell Biol 27:800–809. doi: 10.1016/j.tcb.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jorgensen I, Rayamajhi M, Miao EA. 2017. Programmed cell death as a defence against infection. Nat Rev Immunol 17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Galluzzi L, Kepp O, Chan FK, Kroemer G. 2017. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol 12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Annibali O, Crescenzi A, Tomarchio V, Pagano A, Bianchi A, Grifoni A, Avvisati G. 2018. PD-1/PD-L1 checkpoint in hematological malignancies. Leuk Res 67:45–55. doi: 10.1016/j.leukres.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 287.Wakao H, Sugimoto C, Kimura S, Wakao R. 2017. Mucosal-associated invariant T cells in regenerative medicine. Front Immunol 8:1711. doi: 10.3389/fimmu.2017.01711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhao C, Wang M, Cheng A, Yang Q, Wu Y, Zhu D, Chen S, Liu M, Zhao X, Jia R, Sun K, Chen X. 2018. Programmed cell death: the battlefield between the host and alpha-herpesviruses and a potential avenue for cancer treatment. Oncotarget 9:30704–30719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rothan HA, Fang S, Mahesh M, Byrareddy SN. 2019. Zika virus and the metabolism of neuronal cells. Mol Neurobiol 56:2551–2557. doi: 10.1007/s12035-018-1263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Solano-Gálvez SG, Abadi-Chiriti J, Gutiérrez-Velez L, Rodríguez-Puente E, Konstat-Korzenny E, Álvarez-Hernández DA, Franyuti-Kelly G, Gutiérrez-Kobeh L, Vázquez-López R. 2018. Apoptosis: activation and inhibition in health and disease. Med Sci 6:E54. doi: 10.3390/medsci6030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wang M, Yu F, Wu W, Wang Y, Ding H, Qian L. 2018. Epstein-Barr virus-encoded microRNAs as regulators in host immune responses. Int J Biol Sci 14:565–576. doi: 10.7150/ijbs.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Naderer T, Fulcher MC. 2018. Targeting apoptosis pathways in infections. J Leukoc Biol 103:275–285. doi: 10.1189/JLB.4MR0717-286R. [DOI] [PubMed] [Google Scholar]
- 293.McDougal CE, Sauer JD. 2018. Listeria monocytogenes: the impact of cell death on infection and immunity. Pathogens 7:8. doi: 10.3390/pathogens7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Fitzsimmons L, Kelly GL. 2017. EBV and apoptosis: the viral master regulator of cell fate? Viruses 9:339. doi: 10.3390/v9110339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Meyer B, Groseth A. 2018. Apoptosis during arenavirus infection: mechanisms and evasion strategies. Microbes Infect 20:65–80. doi: 10.1016/j.micinf.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 296.Zhou X, Jiang W, Liu Z, Liu S, Liang X. 2017. Virus infection and death receptor-mediated apoptosis. Viruses 9:316. doi: 10.3390/v9110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nainu F, Shiratsuchi A, Nakanishi Y. 2017. Induction of apoptosis and subsequent phagocytosis of virus-infected cells as an antiviral mechanism. Front Immunol 8:1220. doi: 10.3389/fimmu.2017.01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Cardoso CC, Pereira AC, de Sales Marques C, Moraes MO. 2011. Leprosy susceptibility: genetic variations regulate innate and adaptive immunity, and disease outcome. Future Microbiol 6:533–549. doi: 10.2217/fmb.11.39. [DOI] [PubMed] [Google Scholar]
- 299.Patnaik N, Agarwal S, Sharma S, Sharma S, Pandhi D. 2015. Evaluation of apoptosis in skin biopsies of patients of borderline leprosy and lepra type 1 reaction. Indian J Dermatol 60:60–65. doi: 10.4103/0019-5154.147795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Quaresma JA, Esteves PC, de Sousa Aarão TL, de Sousa JR, da Silva Pinto D, Fuzii HT. 2014. Apoptotic activity and Treg cells in tissue lesions of patients with leprosy. Microb Pathog 76:84–88. doi: 10.1016/j.micpath.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 301.Oliveira Fulco T, Andrade PR, de Mattos Barbosa MG, Pinto TG, Ferreira PF, Ferreira H, da Costa Nery JA, Real SC, Borges VM, Moraes MO, Sarno EN, Sampaio EP, Pinheiro RO. 2014. Effect of apoptotic cell recognition on macrophage polarization and mycobacterial persistence. Infect Immun 82:3968–3978. doi: 10.1128/IAI.02194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Adigun R, Bhimji SS. 2017. Necrosis, cell (liquefactive, coagulative, caseous, fat, fibrinoid, and gangrenous). StatPearls Publishing, Treasure Island, FL. [Google Scholar]
- 303.Man SM, Karki R, Kanneganti TD. 2017. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Brault M, Oberst A. 2017. Controlled detonation: evolution of necroptosis in pathogen defense. Immunol Cell Biol 95:131–136. doi: 10.1038/icb.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hanson B. 2016. Necroptosis: a new way of dying? Cancer Biol Ther 17:899–910. doi: 10.1080/15384047.2016.1210732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shlomovitz I, Zargarian S, Erlich Z, Edry-Botzer L, Gerlic M. 2018. Distinguishing necroptosis from apoptosis. Methods Mol Biol 1857:35–51. doi: 10.1007/978-1-4939-8754-2_4. [DOI] [PubMed] [Google Scholar]
- 307.Kitur K, Wachtel S, Brown A, Wickersham M, Paulino F, Peñaloza HF, Soong G, Bueno S, Parker D, Prince A. 2016. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep 16:2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ahn D, Prince A. 2017. Participation of necroptosis in the host response to acute bacterial pneumonia. J Innate Immun 9:262–270. doi: 10.1159/000455100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mutsafi Y, Altan-Bonnet N. 2018. Enterovirus transmission by secretory autophagy. Viruses 10:E139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.McEwan DG. 2017. Host-pathogen interactions and subversion of autophagy. Essays Biochem 61:687–697. doi: 10.1042/EBC20170058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Blázquez AB, Escribano-Romero E, Merino-Ramos T, Saiz JC, Martín-Acebes MA. 2014. Stress responses in flavivirus-infected cells: activation of unfolded protein response and autophagy. Front Microbiol 5:266. doi: 10.3389/fmicb.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang ZW, Li ZL, Yuan S. 2016. The role of secretory autophagy in Zika virus transfer through the placental barrier. Front Cell Infect Microbiol 6:206. doi: 10.3389/fcimb.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Desprès P, Amara A, Yssel H, Missé D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Carr AC, Maggini S. 2017. Vitamin C and immune function. Nutrients 9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Raposo SE, Fondell E, Ström P, Bälter O, Bonn SE, Nyrén O, Plymoth A, Bälter K. 2017. Intake of vitamin C, vitamin E, selenium, zinc and polyunsaturated fatty acids and upper respiratory tract infection—a prospective cohort study. Eur J Clin Nutr 71:450–457. doi: 10.1038/ejcn.2016.261. [DOI] [PubMed] [Google Scholar]
- 316.Sezikli M, Güzelbulut F, Akkan ÇZ. 2016. Influence of vitamin C and E supplementation on the eradication rates of triple and quadruple eradication regimens in Helicobacter pylori infection. Turk J Gastroenterol 27:290–291. [DOI] [PubMed] [Google Scholar]
- 317.Quraishi SA, Litonjua AA, Moromizato T, Gibbons FK, Camargo CA Jr, Giovannucci E, Christopher KB. 2015. Association between prehospital vitamin D status and hospital-acquired Clostridium difficile infections. JPEN J Parenter Enteral Nutr 39:47–55. doi: 10.1177/0148607113511991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Gostner J, Ciardi C, Becker K, Fuchs D, Sucher R. 2014. Immunoregulatory impact of food antioxidants. Curr Pharm Des 20:840–849. doi: 10.2174/13816128113199990047. [DOI] [PubMed] [Google Scholar]
- 319.Peelen E, Rijkers G, Meerveld-Eggink A, Meijvis S, Vogt M, Cohen Tervaert JW, Hupperts R, Damoiseaux J. 2013. Relatively high serum vitamin D levels do not impair the antibody response to encapsulated bacteria. Eur J Clin Microbiol Infect Dis 32:61–69. doi: 10.1007/s10096-012-1714-7. [DOI] [PubMed] [Google Scholar]
- 320.Hao S, Liang B, Huang Q, Dong S, Wu Z, He W, Shi M. 2018. Metabolic networks in ferroptosis. Oncol Lett 15:5405–5411. doi: 10.3892/ol.2018.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li L, Hao Y, Zhao Y, Wang H, Zhao X, Jiang Y, Gao F. 2018. Ferroptosis is associated with oxygen-glucose deprivation/reoxygenation-induced Sertoli cell death. Int J Mol Med 41:3051–3062. doi: 10.3892/ijmm.2018.3469. [DOI] [PubMed] [Google Scholar]
- 322.Wu C, Zhao W, Yu J, Li S, Lin L, Chen X. 2018. Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Sci Rep 8:574. doi: 10.1038/s41598-017-18935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Dhingra R, Ravandi A, Kirshenbaum LA. 2018. Ferroptosis: beating on death’s door. Am J Physiol Heart Circ Physiol 314:H772–H775. doi: 10.1152/ajpheart.00692.2017. [DOI] [PubMed] [Google Scholar]
- 324.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. 2017. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Latunde-Dada GO. 2017. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj 1861:1893–1900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 326.Müller T, Dewitz C, Schmitz J, Schröder AS, Bräsen JH, Stockwell BR, Murphy JM, Kunzendorf U, Krautwald S. 2017. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci 74:3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Chen D, Eyupoglu IY, Savaskan N. 2017. Ferroptosis and cell death analysis by flow cytometry. Methods Mol Biol 1601:71–77. doi: 10.1007/978-1-4939-6960-9_6. [DOI] [PubMed] [Google Scholar]
- 328.Cassat JE, Moore JL, Wilson KJ, Stark Z, Prentice BM, Van de Plas R, Perry WJ, Zhang Y, Virostko J, Colvin DC, Rose KL, Judd AM, Reyzer ML, Spraggins JM, Grunenwald CM, Gore JC, Caprioli RM, Skaar EP. 2018. Integrated molecular imaging reveals tissue heterogeneity driving host-pathogen interactions. Sci Transl Med 10:eaan6361. doi: 10.1126/scitranslmed.aan6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Díaz FE, Abarca K, Kalergis AM. 2018. An update on host-pathogen interplay and modulation of immune responses during Orientia tsutsugamushi infection. Clin Microbiol Rev 31:e00076-17. doi: 10.1128/CMR.00076-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Brinkworth JF. 2017. Infectious disease and the diversification of the human genome. Hum Biol 89:47–65. doi: 10.13110/humanbiology.89.1.03. [DOI] [PubMed] [Google Scholar]
- 331.Dietert K, Gutbier B, Wienhold SM, Reppe K, Jiang X, Yao L, Chaput C, Naujoks J, Brack M, Kupke A, Peteranderl C, Becker S, von Lachner C, Baal N, Slevogt H, Hocke AC, Witzenrath M, Opitz B, Herold S, Hackstein H, Sander LE, Suttorp N, Gruber AD. 2017. Spectrum of pathogen- and model-specific histopathologies in mouse models of acute pneumonia. PLoS One 12:e0188251. doi: 10.1371/journal.pone.0188251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Scott NE, Hartland EL. 2017. Post-translational mechanisms of host subversion by bacterial effectors. Trends Mol Med 23:1088–1102. doi: 10.1016/j.molmed.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 333.Eyerich K, Eyerich S. 2018. Immune response patterns in non-communicable inflammatory skin diseases. J Eur Acad Dermatol Venereol 32:692–703. doi: 10.1111/jdv.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Prescott SL, Larcombe DL, Logan AC, West C, Burks W, Caraballo L, Levin M, Etten EV, Horwitz P, Kozyrskyj A, Campbell DE. 2017. The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic imune programming. World Allergy Organ J 10:29. doi: 10.1186/s40413-017-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Poulakou G, Giannitsioti E, Tsiodras S. 2017. What is new in the management of skin and soft tissue infections in 2016? Curr Opin Infect Dis 30:158–171. doi: 10.1097/QCO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 336.Sousa JR, Sotto MN, Simões Quaresma JA. 2017. Leprosy as a complex infection: breakdown of the Th1 and Th2 immune paradigm in the immunopathogenesis of the disease. Front Immunol 8:1635. doi: 10.3389/fimmu.2017.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Nogueira MR, Latini AC, Nogueira ME. 2016. The involvement of endothelial mediators in leprosy. Mem Inst Oswaldo Cruz 111:635–641. doi: 10.1590/0074-02760160122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sunderkötter C, Steinbrink K, Henseleit U, Bosse R, Schwarz A, Vestweber D, Sorg C. 1996. Activated T cells induce expression of E-selectin in vitro and in an antigen-dependent manner in vivo. Eur J Immunol 26:1571–1579. doi: 10.1002/eji.1830260725. [DOI] [PubMed] [Google Scholar]
- 339.Sousa JR, Sousa Aarão TL, Rodrigues de Sousa J, Hirai KE, Silva LM, Dias LB Jr, Oliveira Carneiro FR, Fuzii HT, Quaresma JA. 2017. Endothelium adhesion molecules ICAM-1, ICAM-2, VCAM-1 and VLA-4 expression in leprosy. Microb Pathog 104:116–124. doi: 10.1016/j.micpath.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 340.Hirai KE, Aarão TL, Silva LM, de Sousa JR, de Souza J, Dias LB Jr, Carneiro FR, Fuzii HT, Quaresma JA. 2016. Langerhans cells (CD1a and CD207), dermal dendrocytes (FXIIIa) and plasmacytoid dendritic cells (CD123) in skin lesions of leprosy patients. Microb Pathog 91:18–25. doi: 10.1016/j.micpath.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 341.Alvarenga Lira ML, Pagliari C, de Lima Silva AA, de Andrade HF Jr, Duarte MI. 2015. Dermal dendrocytes FXIIIa+ are essential antigen-presenting cells in indeterminate leprosy. Am J Dermatopathol 37:269–273. doi: 10.1097/DAD.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 342.Sotto MN, Halpern I, Kauffman MR, Pagliari C. 2010. Factor XIIIa+ dermal dendrocyte parasitism in American tegumentary leishmaniasis skin lesions. Am J Dermatopathol 32:15–18. doi: 10.1097/DAD.0b013e3181ab4695. [DOI] [PubMed] [Google Scholar]
- 343.Silva WL, Pagliari C, Duarte MI, Sotto MN. 2016. Paracoccidioides brasiliensis interacts with dermal dendritic cells and keratinocytes in human skin and oral mucosa lesions. Med Mycol 54:370–376. doi: 10.1093/mmy/myv112. [DOI] [PubMed] [Google Scholar]
- 344.Sinnis P, Zavala F. 2008. The skin stage of malaria infection: biology and relevance to the malaria vaccine effort. Future Microbiol 3:275–278. doi: 10.2217/17460913.3.3.275. [DOI] [PubMed] [Google Scholar]
- 345.Briant L, Desprès P, Choumet V, Missé D. 2014. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 464-465:26–32. doi: 10.1016/j.virol.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 346.Duangkhae P, Erdos G, Ryman KD, Watkins SC, Falo LD Jr, Marques ETA Jr, Barratt-Boyes SM. 2018. Interplay between keratinocytes and myeloid cells drives dengue virus spread in human skin. J Invest Dermatol 138:618–626. doi: 10.1016/j.jid.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 347.Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat Med 6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 348.Palucka AK. 2000. Dengue virus and dendritic cells. Nat Med 6:748–749. doi: 10.1038/77470. [DOI] [PubMed] [Google Scholar]
- 349.Vielle NJ, Zumkehr B, García-Nicolás O, Blank F, Stojanov M, Musso D, Baud D, Summerfield A, Alves MP. 2018. Silent infection of human dendritic cells by African and Asian strains of Zika virus. Sci Rep 8:5440. doi: 10.1038/s41598-018-23734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Mayr L, Su B, Moog C. 2017. Langerhans cells: the ‘Yin and Yang’ of HIV restriction and transmission. Trends Microbiol 25:170–172. doi: 10.1016/j.tim.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 351.Kuo YP, Tsai KN, Luo YC, Chung PJ, Su YW, Teng Y, Wu MS, Lin YF, Lai CY, Chuang TH, Dai SS, Tseng FC, Hsieh CH, Tsai DJ, Tsai WT, Chen CH, Yu GY. 2018. Establishment of a mouse model for the complete mosquito-mediated transmission cycle of Zika virus. PLoS Negl Trop Dis 12:e0006417. doi: 10.1371/journal.pntd.0006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Feijó D, Tibúrcio R, Ampuero M, Brodskyn C, Tavares N. 2016. Dendritic cells and Leishmania infection: adding layers of complexity to a complex disease. J Immunol Res 2016:3967436. doi: 10.1155/2016/3967436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sinnis P, Zavala F. 2012. The skin: where malaria infection and the host immune response begin. Semin Immunopathol 34:787–792. doi: 10.1007/s00281-012-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Leitner WW, Costero-Saint Denis A, Wali T. 2011. Immunological consequences of arthropod vector-derived salivary factors. Eur J Immunol 41:3396–3400. doi: 10.1002/eji.201190075. [DOI] [PubMed] [Google Scholar]
- 355.Ong EZ, Zhang SL, Tan HC, Gan ES, Chan KR, Ooi EE. 2017. Dengue virus compartmentalization during antibody-enhanced infection. Sci Rep 7:40923. doi: 10.1038/srep40923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Boonnak K, Slike BM, Burgess TH, Mason RM, Wu SJ, Sun P, Porter K, Rudiman IF, Yuwono D, Puthavathana P, Marovich MA. 2008. Role of dendritic cells in antibody-dependent enhancement of dengue virus infection. J Virol 82:3939–3951. doi: 10.1128/JVI.02484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Usme-Ciro JA, Méndez JA, Laiton KD, Páez A. 2014. The relevance of dengue vírus genotypes surveillance at country level before vaccine approval. Hum Vaccin Immunother 10:2674–2678. doi: 10.4161/hv.29563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Wick MR. 2017. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol 34:301–311. doi: 10.1053/j.semdp.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 359.Inkeles MS, Teles RM, Pouldar D, Andrade PR, Madigan CA, Lopez D, Ambrose M, Noursadeghi M, Sarno EN, Rea TH, Ochoa MT, Iruela-Arispe ML, Swindell WR, Ottenhoff TH, Geluk A, Bloom BR, Pellegrini M, Modlin RL. 2016. Cell-type deconvolution with immune pathways identifies gene networks of host defense and immunopathology in leprosy. JCI Insight 1:e88843. doi: 10.1172/jci.insight.88843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Gurung P, Karki R, Vogel P, Watanabe M, Bix M, Lamkanfi M, Kanneganti TD. 2015. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest 125:1329–1338. doi: 10.1172/JCI79526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Teng O, Ang CKE, Guan XL. 2017. Macrophage-bacteria interactions—a lipid-centric relationship. Front Immunol 8:1836. doi: 10.3389/fimmu.2017.01836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Murray PJ. 2017. Macrophage polarization. Annu Rev Physiol 79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 363.Fortes MR, Miot HA, Kurokawa CS, Marques ME, Marques SA. 2011. Immunology of paracoccidioidomycosis. An Bras Dermatol 86:516–524. doi: 10.1590/S0365-05962011000300014. [DOI] [PubMed] [Google Scholar]
- 364.Saini C, Tarique M, Rai R, Siddiqui A, Khanna N, Sharma A. 2017. T helper cells in leprosy: an update. Immunol Lett 184:61–66. doi: 10.1016/j.imlet.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 365.Xu S, Shinohara ML. 2017. Tissue-resident macrophages in fungal infections. Front Immunol 8:1798. doi: 10.3389/fimmu.2017.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Glennie ND, Scott P. 2016. Memory T cells in cutaneous leishmaniasis. Cell Immunol 309:50–54. doi: 10.1016/j.cellimm.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. 2011. Macrophages in skin injury and repair. Immunobiology 216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 368.Banerjee A, Bhattacharya P, Joshi AB, Ismail N, Dey R, Nakhasi HL. 2016. Role of pro-inflammatory cytokine IL-17 in Leishmania pathogenesis and in protective immunity by Leishmania vaccines. Cell Immunol 309:37–41. doi: 10.1016/j.cellimm.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 369.Boer MC, Joosten SA, Ottenhoff TH. 2015. Regulatory T-cells at the interface between human host and pathogens in infectious diseases and vaccination. Front Immunol 6:217. doi: 10.3389/fimmu.2015.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Souza J, Sousa JR, Hirai KE, Silva LM, Fuzii HT, Dias LB Jr, Carneiro FR, Aarão TL, Quaresma JA. 2015. E-selectin and P-selectin expression in endothelium of leprosy skin lesions. Acta Trop 149:227–231. doi: 10.1016/j.actatropica.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 371.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, Mckinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. 2010. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol 11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Rojas-Zuleta WG, Vásquez G. 2016. Th9 lymphocytes: a recent history from IL-9 to its potential role in rheumatic diseases. Autoimmun Rev 15:649–655. doi: 10.1016/j.autrev.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 373.Smith LM, May RC. 2013. Mechanisms of microbial escape from phagocyte killing. Biochem Soc Trans 41:475–490. doi: 10.1042/BST20130014. [DOI] [PubMed] [Google Scholar]
- 374.Pel MJ, Pieterse CM. 2013. Microbial recognition and evasion of host immunity. J Exp Bot 64:1237–1248. doi: 10.1093/jxb/ers262. [DOI] [PubMed] [Google Scholar]
- 375.Quaresma JA, Lima LW, Fuzii HT, Libonati RM, Pagliari C, Duarte MI. 2010. Immunohistochemical evaluation of macrophage activity and its relationship with apoptotic cell death in the polar forms of leprosy. Microb Pathog 49:135–140. doi: 10.1016/j.micpath.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 376.Quaresma JA, de Almeida FA, de Souza Aarao TL, de Miranda Araujo Soares LP, Nunes Magno IM, Fuzii HT, Feio Libonati RM, Xavier MB, Pagliari C, Seixas Duarte MI. 2012. Transforming growth factor β and apoptosis in leprosy skin lesions: possible relationship with the control of the tissue immune response in the Mycobacterium leprae infection. Microbes Infect 14:696–701. doi: 10.1016/j.micinf.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 377.Oliveira RB, Sampaio EP, Aarestrup F, Teles RM, Silva TP, Oliveira AL, Antas PR, Sarno EN. 2005. Cytokines and Mycobacterium leprae induce apoptosis in human Schwann cells. J Neuropathol Exp Neurol 64:882–890. doi: 10.1097/01.jnen.0000182982.09978.66. [DOI] [PubMed] [Google Scholar]
- 378.Aarão TLS, de Sousa JR, Falcão ASC, Falcão LFM, Quaresma J. 2018. Nerve growth factor and pathogenesis of leprosy: review and update. Front Immunol 9:939. doi: 10.3389/fimmu.2018.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Quaresma JA, de Oliveira E, Cardoso de Brito A. 2008. Is TGF-beta important for the evolution of subcutaneous chronic mycoses? Med Hypotheses 70:1182–1185. doi: 10.1016/j.mehy.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 380.Xavier MB, Libonati RM, Unger D, Oliveira C, Corbett CE, de Brito AC, Quaresma JA. 2008. Macrophage and TGF-beta immunohistochemical expression in Jorge Lobo’s disease. Hum Pathol 39:269–274. doi: 10.1016/j.humpath.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 381.Jafari M, Shirbazou S, Sadraie SH, Kaka G, Norozi M. 2015. The role of apoptosis in the cellular response of liver and spleen of BALB/c mice in cutaneous leishmaniasis. Iran J Med Sci 40:133–142. [PMC free article] [PubMed] [Google Scholar]
- 382.Rethi B, Eidsmo L. 2012. FasL and TRAIL signaling in the skin during cutaneous leishmaniasis - implications for tissue immunopathology and infectious control. Front Immunol 3:163. doi: 10.3389/fimmu.2012.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Getti GT, Cheke RA, Humber DP. 2008. Induction of apoptosis in host cells: a survival mechanism for Leishmania parasites? Parasitology 135:1391–1399. doi: 10.1017/S0031182008004915. [DOI] [PubMed] [Google Scholar]
- 384.van Zandbergen G, Solbach W, Laskay T. 2007. Apoptosis driven infection. Autoimmunity 40:349–352. doi: 10.1080/08916930701356960. [DOI] [PubMed] [Google Scholar]
- 385.Chiramel AI, Best SM. 2018. Role of autophagy in Zika virus infection and pathogenesis. Virus Res 254:34–40. doi: 10.1016/j.virusres.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Carneiro LA, Travassos LH. 2016. Autophagy and viral diseases transmitted by Aedes aegypti and Aedes albopictus. Microbes Infect 18:169–171. doi: 10.1016/j.micinf.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 387.Mattoscio D, Medda A, Chiocca S. 2018. Human papilloma virus and autophagy. Int J Mol Sci 19:1775. doi: 10.3390/ijms19061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Cyrino LT, Araújo AP, Joazeiro PP, Vicente CP, Giorgio S. 2012. In vivo and in vitro Leishmania amazonensis infection induces autophagy in macrophages. Tissue Cell 44:401–408. doi: 10.1016/j.tice.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 389.Soong G, Chun J, Parker D, Prince A. 2012. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J Infect Dis 205:1571–1579. doi: 10.1093/infdis/jis244. [DOI] [PMC free article] [PubMed] [Google Scholar]