Abstract
Purpose
Human infections caused by invasive non-typhoidal Salmonella (iNTS) are highly prevalent worldwide. However, data for such infections in China are scarce. This study reports the epidemiology of iNTS in China.
Methods
INTS isolates were recovered from blood and other clinical specimens collected during 2007–2016 across five provinces (Shanghai, Xinjiang, Fujian, Guangxi, and Chongqing) in China. Antimicrobial susceptibility was performed using the agar dilution method and molecular epidemiology was performed using standard microbiological techniques.
Results
A total of 178 iNTS isolates were recovered from approximately 9700 patient specimens during 2007–2016. The predominant serovars were Salmonella Enteritidis (57/178, 32%), Salmonella Choleraesuis (47/178, 26.4%), and Salmonella Typhimurium (24/178, 13.5%). Up to 50 isolates (28.1%) were from patients who were ≤1 year of age, while 28 (15.7%) were from patients who were ≥60 years. Among these isolates, high rates of resistance to nalidixic acid (114/178, 64%), sulfisoxazole (59%), ciprofloxacin (15.2%), and cefotaxime (8.4%) were found. Moreover, 53.4% (95/178) exhibited multidrug resistance, and 3.9% (7/178) showed co-resistance to third-generation cephalosporins and ciprofloxacin. Steadily increasing numbers of nalidixic acid, cefotaxime, and ciprofloxacin-resistant isolates, but decreasing numbers of multidrug resistance isolates were detected during the study period. Detection of quinolone genes in 114 nalidixic acid-resistant isolates showed that 58.3% (67/114) harbored plasmid-mediated quinolone resistance (PMQR) genes [aac(6´)-Ib-cr, qnrA, qnrB, oqxAB, qepA, qnrS, and qnrD] and 98.2% (112/114) exhibited mutations in quinolone resistance determining regions [gyrA, parC, and parE]. Furthermore, we detected beta-lactamases genes in the ceftriaxone-resistant isolates. The most common were blaTEM-1 (93.3%), followed by blaCTX-M-55 (40%), blaCMY-2 (33.3%), and blaOXA-1 (33.3%). Finally, a range of pulsed-field gel electrophoresis patterns were detected among the Salmonella Enteritidis and Salmonella Typhimurium isolates.
Conclusion
High rates of multidrug resistance and steadily increasing cefotaxime and ciprofloxacin-resistant iNTS could pose a significant challenge for the effective treatment of salmonellosis in China.
Keywords: invasive non-typhoidal Salmonella, fluoroquinolones, multidrug resistant, beta-lactamases, pulsed field gel electrophoresis, China
Plain language summary
Infections caused by invasive non-typhoidal Salmonella (iNTS) bacteria occur when non-typhoidal Salmonella, which normally cause diarrhea, enter the bloodstream and spread through the body. INTS infections have become a common cause of infection and death in children and elderly patients, accompanied with other diseases, in sub-Saharan Africa. However, data for such infections is unknown outside of sub-Saharan Africa. In particular, related data for China, with a large population, is scarce. This study reports on the epidemiology of iNTS in China, in which 178 iNTS isolates were isolated and Salmonella Enteritidis, Salmonella Choleraesuis, and Salmonella Typhimurium were the main serovars. Over half (53.4%) of the isolates exhibited multidrug resistance and 3.9% showed co-resistant to third-generation cephalosporins and ciprofloxacin. Steadily increasing nalidixic acid, cefotaxime, and ciprofloxacin-resistance, but decreasing multidrug resistance, were detected during the study period. The occurrence and diversity of mutations in QRDR and the PMQR genes, conferring fluoroquinolone resistance, in our research were much higher than those reported for iNTS isolates in other regions. A high proportion of cefotaxime-resistant isolates exhibited at least two beta-lactamase genes, which is seldom reported in iNTS isolates. In conclusion, high rates of multidrug resistance and the steadily increasing cefotaxime- and ciprofloxacin-resistant iNTS could pose a significant challenge for the effective treatment of salmonellosis in China.
Introduction
Invasive bacterial infections caused by invasive non-typhoidal Salmonella (iNTS) are a significant public health threat worldwide. An estimated 3.4 million cases of infection and over 680,000 deaths occur annually globally.1,2 In developed countries, such as the United States, the incidence of iNTS is low, and NTS infections that spread through contaminated food result in a self-limiting diarrheal disease, with invasive disease being rarely recorded.3 However, in Africa, iNTS ranks as the primary cause of community-acquired invasive bacterial disease and is responsible for nearly 17% of bacterial bloodstream cases.2,4,5 Infections with iNTS in low-income areas often result in meningitis and septicemia, and these severe infections more frequently occur in immunocompromised hosts and children who are malnourished and are co-infected with malaria.6,7 China is a big country with a population of about 1.4 billion, and 70–80% of bacterial food poisoning are caused by Salmonella.8 Despite the severe infections caused by iNTS worldwide, little data is available on iNTS infections in China.
Antimicrobial agents are critical to treat patients with complex iNTS infections to reduce the mortality rate. The massive use of antimicrobials to treat patients has caused the resistance rate to increase and the widespread occurrence of iNTS isolates with multidrug resistance (MDR) against traditional antimicrobial agents in Asia and Africa, which often results in death, representing an important public health problem.9–11 The extended-spectrum cephalosporins and fluoroquinolones have become the first-line drugs to treat iNTS cases; however, this conversion has resulted in the emergence of iNTS isolates resistant to these drugs.9
Therefore, we carried out a study to investigate iNTS infections in China from 2007 to 2016 to determine the molecular epidemiology and antimicrobial susceptibility characteristics of iNTS. The information provided will be useful to develop a comprehensive national surveillance program to help guide clinicians to choose the appropriate treatment for this disease.
Materials and methods
Ethics statement
Ethical approval for this study was provided by Shanghai Municipal Center for Disease Control and Prevention (Shanghai, China). This study was performed retrospectively, and individual patient identification was not accessed and informed consent was not required.
Sample collection and isolate identification
Approximately 9700 clinical samples, including blood, peritoneal fluid, joint fluid, bone fluid, cerebrospinal fluid (CSF), and aspirates were collected from patients presenting with fever at five Government General Hospitals and five Provincial Center for Disease Control and Prevention (CDC) laboratories in Shanghai, Xinjiang, Guangxi, Chongqing, and Fujian Provinces in China from January 2007 to December 2016. The samples were processed following standard blood culture procedures. Briefly, the clinical samples were inoculated into the bottles of aerobic blood culture and incubated in a BACTEC 9050 automated blood culture machine (Becton Dickinson, Franklin Lakes, NJ, USA) according to previously published methods.12 Commercial antisera (Statens Serum Institut, Copenhagen, Denmark) were used to detect the Salmonella serovars. Rainfall information was retrieved from http://www.cma.gov.cn/using the combined rainfall data for the five provinces. To avoid confounding effects, patients with multiple positive blood cultures for the same NTS serovar and antimicrobial susceptibility profile were considered as a single case. Cases in which the responsible microbial agent could not be identified were excluded from the analysis of categorical variables to prevent statistical bias.
Antimicrobial susceptibility testing
Antimicrobial susceptibility to 12 drugs was performed for the iNTS isolates, using an agar dilution method according to the guidelines of Clinical and Laboratory Standards Institute (CLSI) standards.13 The tested antimicrobial agents comprised: Tetracycline (TET), polymyxin B (PB), imipenem (IPM), chloramphenicol (C), ciprofloxacin (CIP), ofloxacin (OFX), nalidixic acid (NA), sulfisoxazole (SUL), streptomycin (STR), cefepime (CFP) (fourth-generation cephalosporin), cefotaxime (CTX) (third-generation cephalosporin), and ampicillin (AMP). For ciprofloxacin, “ciprofloxacin susceptibility” was used to indicate a minimum inhibitory concentration (MIC) value ≤0.06 mg/L; the term “decreased ciprofloxacin susceptibility” was reserved for MIC values >0.06 mg/L and <1 mg/L; and the “resistance breakpoint” meant MIC values ≥1 mg/L.13 Escherichia coli isolates ATCC 25922 and ATCC 35218 served as quality control isolates. The results were interpreted on the basis of CLSI guidelines.13
Molecular analysis of quinolone and cephalosporin resistance
The analysis of quinolone resistance was conducted on 114 NA resistant iNTS isolates by amplification and sequencing of the quinolone resistance determining regions (QRDRs) of the DNA gyrase (gyrA and gyrB) and DNA topoisomerase IV (parC and parE) genes, and screening for the presence of the plasmid-mediated quinolone resistance (PMQR) genes, according to methods detailed in a previous study.14,15 PCR screening and sequencing were conducted on 15 iNTS isolates that were resistant to cefotaxime (MIC ≥4 mg/L) to confirm the presence of beta-lactamase genes blaTEM, blaSHV, blaOXA, blaCMY, and blaCTX-M using standard methods.15–18 The forward and reverse primers for the beta-lactamase genes are shown in Table S1. The PCR products were sent to Sangon Biotech Co., Ltd. (Shanghai, China) for sequencing. Sequence data were then analyzed using DNAstar (DNAstar Inc., Madison, WI, USA) and the sequences were aligned using GenBank online BLAST software (http://www.ncbi.nlm.nih.gov/BLAST/).
Pulse field gel electrophoresis
Pulsed-field gel electrophoresis (PFGE) was performed for S. Typhimurium and S. Enteritidis isolates using the restriction enzyme XbaI (Takara, Dalian, China) according to the PulseNet protocol, as described previously.8 Salmonella enterica serotype Braenderup H9812 served as standard control isolate. The PFGE results were analyzed using BioNumerics version 6.5 software (Applied Maths, Kortrijk, Belgium).
Statistical analysis
Comparison of frequencies was calculated using the Chi-squared test in SAS 9.2 (SAS Institute, Cary, NC, USA). A P-value <0.05 was considered to indicate statistical significance.
Results
Serovars and distribution of age
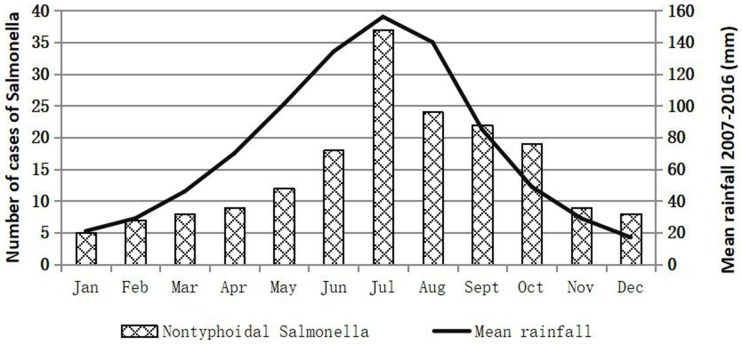
One hundred and seventy eight iNTS isolates were detected during the study period: 86 isolates from Shanghai, 33 isolates from Chongqing, 10 isolates from Fujian, 42 isolates from Guangxi, and 7 isolates in Xinjiang provinces were recovered from approximate 4900, 1400, 700, 2100 and 600 clinical samples, respectively (Table S2). One hundred and forty six (82%) were collected from blood, five (2.8%) from cerebrospinal fluid, and twenty-seven (15.2%) from joint fluid, peritoneal fluid, bone, and aspirates. Among the isolates, 57 (32%) were identified as S. Enteritidis, 47 (26.4%) as S. Choleraesuis, and 24 (13.5%) as S. Typhimurium, which were the top three serovars, followed by five (2.8%) S. London, four (2.2%) S. Derby, four (2.2%) S. Virehow, four (2.2%) S. Livingstone, four (2.2%) S. Give, three (1.7%) S. Weltevreden, two (1.1%) S. Indiana, two (1.1%) S. Meleagridis, two (1.1%) S. Thompson (1.1%), and seventeen other serovars (Table 1). The aggregate age distribution of infections is displayed in Table S3. Up to 28.1% (50/178, p<0.01) of the infections were in patients ≤1 year of age, while 15.7% (28/178, p<0.01) were in patients ≥60 years old. The male-to-female ratio was approximately 1.43 for iNTS infections. The numbers of iNTS isolations fluctuated over the months, and a seasonal trend was observed, in which an increase in iNTS infections appeared to occur during seasons of concentrated rainfall, mainly between May and October (Figure 1).
Table 1.
Antimicrobial susceptibility among 178 invasive non-typhoidal Salmonella isolates from humans in China, during 2007 to 2016
| No. of strains resistant to indicated agent at the indicated breakpoint in mg/L (% resistance)a | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. enterica serotype | No. of strains | MDR phenotype | CIP | CTX | CFP ≥32 | PB ≥8 | OFX ≥2 | AMP ≥32 | SUL ≥512 | C ≥32 | TET ≥16 | NA ≥32 | STR ≥64 | ||
| ≥1 | >0.06and <1 | ≥4 | 0.25–2 | ||||||||||||
| Enteritidis | 57 | 24 (42.1) | 2 (3.5) | 8 (14) | 3 (5.3) | 10 (17.5) | 0 | 0 | 2 (3.5) | 5 (8.8) | 32 (56.1) | 0 | 6 (10.5) | 47 (82.5) | 32 (56.1) |
| Choleraesuis | 47 | 38 (80.9) | 15 (31.9) | 17 (36.2) | 2 (4.3) | 14 (29.8) | 1 (2.1) | 2 (4.3) | 16 (34) | 2 (4.3) | 35 (74.5) | 32 (68.1) | 30 (63.8) | 42 (89.4) | 29 (61.7) |
| Typhimurium | 24 | 17 (70.8) | 4 (16.7) | 6 (25) | 2 (8.3) | 4 (16.7) | 1 (4.2) | 0 | 5 (20.8) | 3 (12.5) | 13 (54.2) | 8 (33.3) | 18 (75) | 13 (54.2) | 12 (50) |
| Derby | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25) | 1 (25) | 0 | 0 |
| London | 5 | 2 (40) | 0 | 3 (60) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (60) | 2 (40) | 3 (60) | 0 | 0 |
| Give | 4 | 1 (25) | 0 | 2 (50) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (50) | 1 (25) | 1 (25) | 3 (75) | 1 (25) |
| Livingstone | 4 | 3 (75) | 0 | 3 (75) | 2 (50) | 2 (50) | 0 | 0 | 0 | 2 (50) | 3 (75) | 4 (100) | 4 (100) | 0 | 2 (50) |
| Virehow | 4 | 2 (50) | 2 (50) | 0 | 0 | 0 | 0 | 0 | 2 (50) | 0 | 2 (50) | 0 | 2 (50) | 2 (50) | 0 |
| Weltevreden | 3 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (66.7) | 0 | 0 | 0 | 0 |
| Indiana | 2 | 2 (100) | 2 (100) | 0 | 2 (100) | 0 | 0 | 0 | 2 (100) | 2 (100) | 2 (100) | 0 | 0 | 2 (100) | 0 |
| Meleagridis | 2 | 2 (100) | 0 | 1 (50) | 2 (100) | 0 | 1 (50) | 0 | 0 | 2 (100) | 2 (100) | 1 (50) | 1 (50) | 2 (100) | 1 (50) |
| Thompson | 2 | 2 (100) | 2 (100) | 0 | 2 (100) | 0 | 0 | 0 | 0 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| Others | 20 | 2 (10) | 0 | 1 (5) | 0 | 1 (5) | 0 | 0 | 0 | 0 | 7 (35) | 1 (5) | 3 (15) | 1 (5) | 2 (10) |
| Total | 178 | 95 (53.4) | 27 (15.2) | 42 (23.6) | 15 (8.4) | 31 (17.4) | 3 (1.7) | 2 (1.1) | 27 (15.2) | 18 (10.1) | 105 (59) | 52 (29.2) | 71 (39.9) | 114 (64) | 81 (45.5) |
Notes: aAll isolates were susceptible to Imipenem. The resistance breakpoint for ciprofloxacin is ≥1 mg/L and for cefotaxime is ≥4 mg/L.
Abbreviations: CIP, ciprofloxacin; CTX, cefotaxime; CFP, cefepime; PB, polymyxin B; OFX, ofloxacin; AMP, ampicillin; SUL, Sulfisoxazole; C, chloramphenicol; TET, tetracycline; NA, nalidixic acid; STR, streptomycin; MDR, multidrug resistant.
Figure 1.
Prevalence of invasive non-typhoidal Salmonella in the People’s Republic of China by rainfall.
Salmonella meningitis
Besides causing bloodstream infections, iNTS are also a cause of life-threatening meningitis in China. From 2007 to 2016, there were five cases of culture-confirmed iNTS meningitis China. The serovars responsible for the iNTS meningitis cases included S. Enteritidis (two), S. Typhimurium (one), S. Derby (one), and S. Indiana (one). The first case of Salmonella meningitis occurred in 2012. All meningitis occurred in children and three cases (60%) involved infants <1 year of age.
Antimicrobial resistance
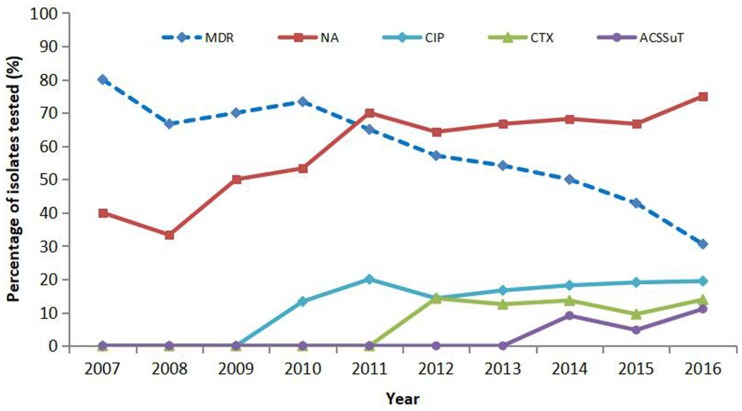
The results of antimicrobial resistance for the tested antibiotics for the 178 iNTS isolates are displayed in Table 1. Among these isolates, 114 (64%) were resistant to nalidixic acid, 105 (59%) to sulfisoxazole, 81 (45.5%) to streptomycin, 71 (39.9%) to tetracycline, 52 (29.2%) to chloramphenicol, 27 (15.2%) to ciprofloxacin, 27 (15.2%) to ofloxacin, 18 (10.1%) to ampicillin, 15 (8.4%) to cefotaxime, 3 (1.7%) to cefepime, and 2 (1.1%) to polymyxin B. Forty-two (61.8%) isolates showed decreased ciprofloxacin susceptibility. Overall, 95 (54.3%) serovars were MDR (MDR being defined as resistant to three or more antimicrobial classes). Furthermore, we detected seven (3.9%) isolates that displayed the ACSSuT pattern (ACSSuT is defined as resistant to tetracycline, sulfamethoxazole, streptomycin, chloramphenicol, and ampicillin) and seven (3.9%) isolates showed co-resistance to cefotaxime and ciprofloxacin. There was a gradual decline in the percentage of MDR isolates (from 80% in 2007 to 30.6% in 2016; P<0.05) during the study period, as well as a parallel increase in nalidixic acid-resistant (NAR) (from 40% in 2007 to 75% in 2016), cefotaxime resistance (from 0% in 2007 to 13.9% in 2016), ciprofloxacin resistance (from 0% in 2007 to 19.4% in 2016), and ACSSuT pattern isolates (from 0% in 2007 to 11.1% in 2016). All iNTS isolates exhibited susceptibility to imipenem (Table 2 and Figure 2).
Table 2.
Trends in resistance to quinolones, cefotaxime, cefepime, MDR and ACSSuT pattern among iNTS isolates during the study period
| Period of isolation | No.(%) susceptiblea | No. (%) MDRb | No. (%) CIPR | No. (%) NAR | No. (%) CTXR | No. (%) CFPR | No. (%) ACSSuT | Year isolation no. |
|---|---|---|---|---|---|---|---|---|
| 2007 | 0 | 8 (80) | 0 | 4 (40) | 0 | 0 | 0 | 10 |
| 2008 | 1 (16.7) | 4 (66.7) | 0 | 2 (33.3) | 0 | 0 | 0 | 6 |
| 2009 | 2 (20) | 7 (70) | 0 | 5 (50) | 0 | 0 | 0 | 10 |
| 2010 | 2 (13.3) | 11 (73.3) | 2 (13.3) | 8 (53.3) | 0 | 0 | 0 | 15 |
| 2011 | 2 (10) | 13 (65) | 4 (20) | 14 (70) | 0 | 0 | 0 | 20 |
| 2012 | 2 (14.3) | 8 (57.1) | 2 (14.3) | 9 (64.3) | 2 (14.3) | 0 | 0 | 14 |
| 2013 | 2 (8.3) | 13 (54.2) | 4 (16.7) | 16 (66.7) | 3 (12.5) | 0 | 0 | 24 |
| 2014 | 4 (18.2) | 11 (50) | 4 (18.2) | 15 (68.2) | 3 (13.6) | 0 | 2 (9.1) | 22 |
| 2015 | 2 (9.5) | 9 (42.9) | 4 (19) | 14 (66.7) | 2 (9.5) | 1 (4.8) | 1 (4.8) | 21 |
| 2016 | 4 (11.1) | 11 (30.6) | 7 (19.4) | 27 (75) | 5 (13.9) | 2 (5.6) | 4 (11.1) | 36 |
| Total | 21 (11.9) | 95 (53.4) | 27 (15.2) | 114 (64) | 15 (8.4) | 3 (1.7) | 7 (3.9) | 178 |
Abbreviations: susceptiblea, susceptible to all tested antibiotics; MDRb, resistance to three or more antimicrobial classes; CIPR, ciprofloxacin resistant; NAR, nalidixic acid resistant; CTXR, cefotaxime resistant; CFPR, cefepime resistant; ACSSuT, resistance to tetracycline, sulfamethoxazole, streptomycin, chloramphenicol and ampicillin.
Figure 2.
The trend of resistance to invasive non-typhoidal Salmonella (iNTS) isolates annually from 2007 to 2016. Numbers in brackets indicate the total number of iNTS isolates cultured in each year in China. Proportions that were nalidixic acid resistant (NAR) and multidrug resistant (MDR). Also shown are cefotaxime- (CTX) and ACSSuT resistant patterns among the iNTS isolates.
Among the iNTS serovars, S. Choleraesuis, S. Typhimurium, and S. Enteritidis comprised high proportions of MDR isolates: 38/47 [80.9%], 17/24 [70.8%], and 24/57 [43.1%], respectively. The proportions of MDR isolates of S. Choleraesuis and S. Typhimurium were significantly higher than that of S. Enteritidis (p<0.05). Fifteen (31.9%) S. Choleraesuis and four (16.7%) S. Typhimurium isolates exhibited resistance to ciprofloxacin. In addition, the uncommon serovars S. Livingstone, S. Meleagridis, S. Thompson, and S. Indiana showed resistance to cefotaxime, ciprofloxacin, and the ACSSuT pattern (Tables 1 and 3).
Table 3.
The resistance phenotype and bla genes of 15 cefotaxime-resistant isolates and 1 isolate with ACSSuT pattern without resistant to cefotaxime
| No. | Source/Geographic origin | Age | Date | Serovar | CTXa | CFPa | CIPa | Other resistance phenotype | Bla genes | PMQR genes |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Blood/SH | 21d | 12/1/6 | Indiana | 16 | 2 | 8 | OFX,SUL,NA | TEM-1, CTX-M-55, OXA-1 | qnrA, qnrB, Aac (6´)-Ib-cr |
| P2 | Blood/SH | 19y | 12/6/25 | Enteritidis | 32 | 2 | 0.06 | AMP, SUL,N A | TEM-1, OXA-1 | - |
| P3 | CSF/SH | 11m | 13/1/11 | Indiana | 16 | 2 | 8 | AMP, OFX, SUL, NA | TEM-1, CTX-M-55, OXA-1 | qnrS, Aac (6´)-Ib-cr |
| P4 | Blood/SH | 1y | 13/10/13 | Meleagridis | 4 | 0.5 | 0.06 | AMP, SUL, STR, NA | TEM-1, CTX-M-55, SHV-2 | - |
| P5 | Blood/GX | 52y | 13/4/5 | Choleraesuis | 128 | 2 | 1 | AMP,C,TET,STR,NA | TEM-1, CTX-M-55, OXA-1 | qnrB, qnrS, Aac (6´)-Ib-cr |
| P6 | Blood/FJ | 1y | 14/12/1 | Thompson | 8 | 0.125 | 4 | ACSSuT, NA | TEM-1, CMY-2 | - |
| P7 | Aspirates/CQ | 11m | 14/2/20 | Thompson | 8 | 0.125 | 2 | ACSSuT, NA | TEM-1 | - |
| P8 | Blood/GX | 64y | 14/11/8 | Typhimurium | 128 | 0.25 | 1 | AMP,C,TET, NA | TEM-1, CMY-2 | - |
| P9 | Blood/SH | 83y | 15/6/18 | Enteritidis | 128 | 4 | 0.5 | AMP, NA | TEM-1 | qnrD |
| P10 | Aspirates/GX | 36y | 15/11/18 | Meleagridis | 512 | 32 | 0.5 | AMP,SUL,C,TET,NA | TEM-1, CMY-2 | - |
| P11 | Blood/CQ | 3m | 16/6/10 | Enteritidis | 256 | 16 | 0.25 | AMP | TEM-1 | - |
| P12 | Blood/SH | 4m | 16/7/28 | Livingstone | 64 | 0.5 | 0.25 | ACSSuT | TEM-1, CMY-2 | - |
| P13 | Blood/SH | 51y | 16/7/5 | Livingstone | 64 | 0.5 | 0.25 | ACSSuT | TEM-1, CMY-2 | - |
| P14 | Blood/GX | 52y | 16/9/5 | Choleraesuis | 128 | 32 | 1 | ACSSuT, PB, NA | CTX- M-55, OXA-1 | Aac (6´)-Ib-cr |
| P15 | Blood/SH | 59y | 16/6/2 | Typhimurium | 256 | 32 | 0.5 | ACSSuT, NA | TEM-1,CTX-M-55 | qnrS |
| P16 | Aspirates/CQ | 9m | 15/9/15 | Typhimurium | 0.5 | 0.25 | 2 | ACSSuT, NA | None | None |
Notes: aIndicates units of related antibiotics is mg/L. Gray shading indicates the isolate with ACSSuT pattern without resistant to cefotaxime. None indicates that the relevant genes were not tested. - indicates that the relevant genes were tested but not detected.
Abbreviation: CSF, cerebrospinal fluid; d, day; m, month; y, year; SH, Shanghai; GX, Guangxi; CQ, Chongqing; FJ, Fujian.
Molecular analysis of quinolone resistance
Detection of PMQR genes in the 114 NAR iNTS isolates showed that aac(6´)-Ib-cr (33.3%, 38/114) was the most common gene, 20 (17.5%) isolates harbored qnrA, 15 (13.2%) harbored qnrB, 8 (7%) harbored oqxABaac, 8 (7%) harbored qepA, 5 (4.4%) harbored qnrS, and 4 (3.5%) harbored qnrD. Sixty-seven (58.3%) NAR isolates were detected to harbor at least one PMQR gene. The co-existence of two or more PMQR genes in a single isolate was observed in 32 isolates.
Correlations among the ciprofloxacin MIC, NAR, and genomic mutations in the QRDR of 114 NAR isolates, are displayed in Table 4. All 47 NAR isolates with susceptibility to ciprofloxacin possessed a single point mutation in the gyrA gene (either at Ser83 or Asp87) only. Of the 40 NAR isolates with decreased ciprofloxacin susceptibility, 17 had a single point mutation in the gyrA or parC genes only, and 23 possessed a single gyrA mutation and at least one parC mutation. While for the 21 isolates with ciprofloxacin resistance (MIC 1–2 mg/L), 19 (90.5%) showed one mutation in gyrA and at least one parC mutation. All isolates with high ciprofloxacin MIC (≥4 mg/L) exhibited five mutations, double point mutations in the both genes gyrA and parC, and a single parE mutation (at positions 538 or 530 or 458). Overall, all the isolates that carried PMQR genes possessed mutations in the QRDRs.
Table 4.
Relationship between ciprofloxacin MIC, point mutations within the QRDRs of DNA gyrase and topoisomerase IV subunit genes and PMQR genes in 114 NAR iNTS isolates
| CIP MIC (mg/L) | No. of isolates | Amino acid change in GyrA | Amino acid change in ParC | Amino acid change in ParE | No. (%) Multiple mutations ratea | No. (%) Mutation rateb | No. (%) PMQR genes ratec |
|---|---|---|---|---|---|---|---|
| 0-0.125 | 47 | Asp87→Tyr [26], Asp87→Asn [12], Asp87→Gly [5], Ser83→Tyr [4] | 47 (100) | 25 (53.2) | |||
| 0.25–0.5 | 40 | Ser83→Tyr[15], Asp87→Gly [8], Asp87→Asn [6], Asp87→Tyr [5], Ser83→Phe [1] | Thr57→Ser [24], Ser80→Arg [8], Gly78→Cys [1] | 23 (57.5) | 40 (100) | 29 (72.5) | |
| 1–2 | 21 | Ser83→Tyr [14], Asp87→Gly [2], Asp87→Tyr [2], Asp87→Asn [1] | Thr57→Ser [19], Gly78→Cys [6], Ser80→Arg [1] | 19 (90.5) | 19 (90.5) | 10 (47.6) | |
| ≥4 | 6 | Ser83→Tyr [3], Ser83→Phe [3], Asp87→Asn [3], Asp87→Gly [3] | Thr57→Ser [6], Ser80→Arg [3], Ser80→Ile [3] | Asp530→Asn [2], Ala538→Thr [3], Ser458→Pro [1] | 6 (100) | 6 (100) | 3 (50) |
Notes: Alterations in the QRDRs of gyrB were not found in any of the isolates tested. The a indicates that isolates exhibit at least two mutations in the QRDRs. The b indicates that isolates exhibit one mutation in the QRDRs. The c indicates that isolates exhibit both mutations in the QRDRs and PMQR gene.
Abbreviations: CIP, ciprofloxacin; MIC, minimum inhibitory concentration.
Detection of beta-lactamases
Of the 15 isolates resistant to ceftriaxone, 14 (93.3%) were found to harbor blaTEM-1 genes. Six (40%) isolates were positive for blaCTX-M-55. blaCMY-2 and blaOXA-1 genes were observed in five isolates (33.3%). One (6.7%) isolate was positive for the blaSHV-2 gene. All isolates exhibited the detected beta-lactamase resistance genes and 12 (80%) isolates harbored at least two beta-lactamase resistance genes (Table 3).
Pulsed-field gel electrophoresis
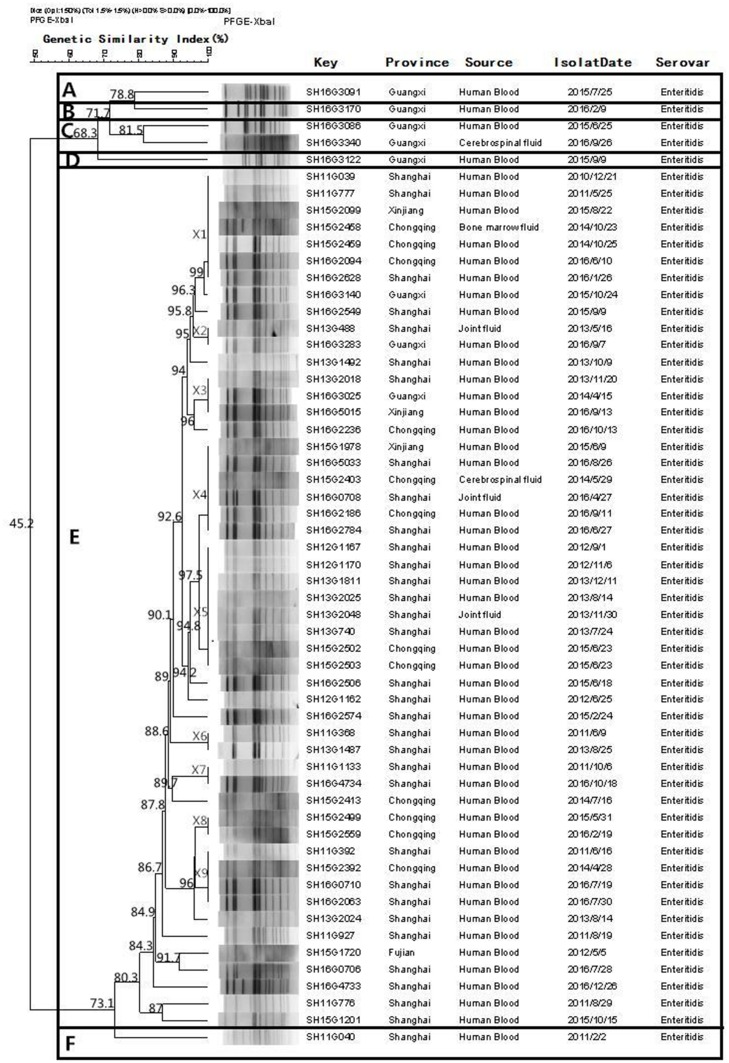
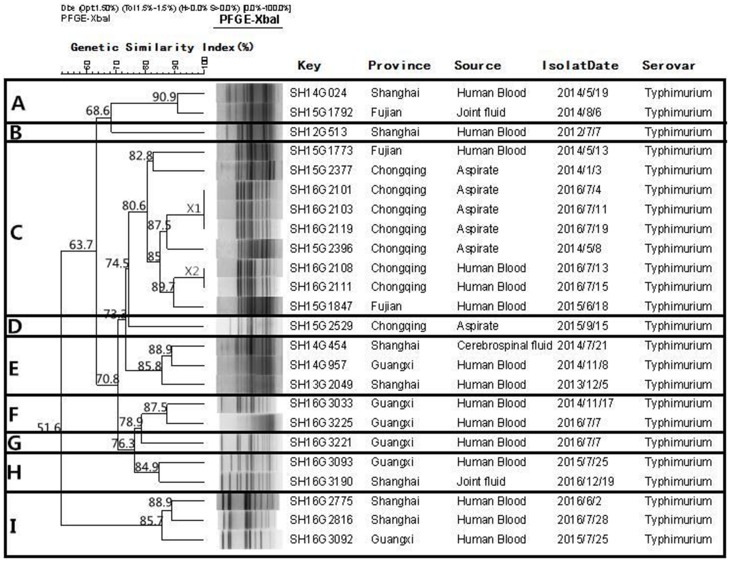
Fifty-seven S. Enteritidis and 24 S. Typhimurium isolates were subjected to PFGE genotyping. Figure 3 shows that 30 PFGE patterns (non-clonal) were observed from the 57 S. Enteritidis isolates, and these could be divided into six clusters at 80% similarity (A to F). Cluster E was the biggest cluster and included 51 (89.5%) isolates. Among the 24 S. Typhimurium isolates (Figure 4), 21 PFGE patterns (non-clonal) were identified, and these could be divided into nine clusters (A–I) at 80% similarity.
Figure 3.
Dendrogram of pulsed field gel electrophoresis (PFGE) patterns of 57 Salmonella Enteritidis isolates recovered from these five provinces. Six clusters (A–F) were identified.
Figure 4.
Dendrogram of pulsed field gel electrophoresis (PFGE) patterns of 24 Salmonella Typhimurium isolates recovered from these five provinces. Nine clusters (A–I) were identified.
Discussion
Infections caused by iNTS are a significant public health threat worldwide. Our study revealed the 178 iNTS isolates were identified from a number of specimen types, which was similar to earlier reports.13,19 A high percentage [30.3% (54/178) and 14.6% (26/178), respectively of iNTS infections were in patients ≤1 or ≥60 years of age. The isolation of iNTS isolates fluctuated over time, but increased significantly during the warm seasons and in those with concentrated rainfall. This confirmed a relationship between the pathogens and climatic variables, in which an organism might persist for longer in such reservoirs.13 These results suggested that improvements to drinking water infrastructure are required.
Our study found that S. Enteritidis and S. Typhimurium were the main serovars, causing approximately 45.5% of iNTS infections, which was similar to previous studies.20,21 Furthermore, one of the most significant discoveries in our study was that S. Choleraesuis was an important serovar, representing a high proportion of the isolates identified. We believe it may be explained as follows: Pork production in China ranked first in the world and pork is very popular in the Chinese diet. S. Choleraesuis is particularly prone to infecting pork meat. This is consistent with reports from Taiwan and Thailand, where S. Choleraesuis is an important invasive pathogen that causes severe infections.22–24 Although there is a lack of data pertaining to the prevalence of invasive NTS around the world, it would appear that there are regional discrepancies in the circulation of Salmonella serovars.25
Invasive Salmonella diseases are related to high case fatality rates (>20%) and prompt antimicrobial therapy is critical for this complex disease.1 The present study identified a high resistance rate to streptomycin, sulfonamides, and nalidixic acid, which were significantly higher than those in North America, where the resistance rates of iNTS isolates to streptomycin, sulfonamides, and nalidixic acid were 8.8, 8.4, and 11.3% from 2003–2013.6 Furthermore, we identified that a high proportion (53.4%) of the iNTS isolates displayed MDR; in particular, 80.9% of S. Choleraesuis and 70.8% of S. Typhimurium isolates exhibited MDR. These rates were higher compared with those in previous studies from United States and Kenya, in which the MDR rates were 9.0% and 4.0%, respectively.6,26 High levels of drug resistance and MDR constitute a significant public health threat and are strongly associated with higher morbidity and mortality.1
A gradual decline in the percentage of iNTS MDR isolates was detected during the study period. This was accompanied by a gradual increase in the number of NAR, ciprofloxacin, and cefotaxime-resistant isolates. This phenomenon is most probably explained by the reduction in the prescription of traditional antibiotics and an increased dependence on ciprofloxacin and cefotaxime as the first-line drugs to treat clinical iNTS infections in China. This is similar to reports from Kenya and United States, where resistance rates toward ceftriaxone (third-generation cephalosporin) increased during the study period.6,19,27 Indeed, in China the rates of resistance of iNTS isolates to ciprofloxacin, cefotaxime, and ACSSuT pattern were 15.2%, 8.4%, and 3.9%, respectively. In particular, the resistant rate emerged early in the study from 0, rising up to 19.4% for ciprofloxacin and 13.9% for cefotaxime in 2016, which is of great concern. These resistant rates were much higher than those previously reported in the United States and Africa, where such drug resistance has been identified, but remains uncommon.19,28–30 More importantly, co-resistance to ciprofloxacin, cefotaxime, ACSSuT pattern, and polymyxin B was identified in the iNTS isolates in the present study. These resistant phenotype isolates were detected among the uncommon Salmonella serovars, and most were found in infants that were ≤1 year of age, which makes the infections more life-threatening, reminding us that we should pay more concerns to these uncommon serovars.19,22,31 In our opinion, the emergence and high level resistance to ciprofloxacin, cefotaxime, and the ACSSuT pattern in iNTS are deeply worrying, because these drugs are considered critically important according to WHO criteria in human medicine and the ACSSuT pattern is difficult to treat,31,32 which greatly limits clinically usable treatment options and leaving only more expensive drugs, such as carbapenems and tigecycline, as possible treatment options.
The main mechanisms of quinolone resistance in Salmonella are mediated by QRDR and PMQR genes.33 Most isolates (98.2%; 112/114) contained mutations in the QRDR and 58.8% (67/114) of the isolates carried PMQR genes. The occurrence and diversity of mutations in the QRDR and PMQR genes in the present study were much higher than those reported for iNTS in other Asian and African regions.13,22,24,34 Furthermore, in the present study, multipoint mutations in QRDR and/or two or more in PMQR genes co-existing in a single isolate were frequently detected, whereas this has seldom been observed among iNTS isolates from other regions.1 A single point mutation in QRDR will confer resistance to nalidixic acid and multipoint mutations in QRDR confer a high MIC value for ciprofloxacin, while mutations in the PMQR genes confer a modest decrease in susceptibility to ciprofloxacin.35 This might explain why these isolates have a high nalidixic acid-resistance rate and a high proportion of them had high a MIC value for ciprofloxacin. In addition, among the substitutions Asp530→Asn, Ala538→Thr, and Ser458→Pro, found in the QRDR of parE in our study, Ser458→Pro has been observed in a few parE sequences from S. Schwarzengrund that were related with ciprofloxacin resistance.36 Meanwhile, the substitutions Asp530→Asn and Ala538→Thr have not been previously reported, and were observed in the NAR and ciprofloxacin (MIC >4 mg/L) isolates; therefore, they are probably associated with fluoquinolone resistance in these isolates. The aac(6´)-Ib-cr gene was identified commonly in 33.3% (38/114) of isolates. Isolates carrying these PMQR genes will pose a potential threat because their resistance to ciprofloxacin will increase with the increase in chromosomal mutant selection.33,37
Similar to previous reports,20,22,34 the cefotaxime-resistant isolates in the present study contained the relevant beta-lactamases [TEM-1, CTX-M-55, OXA-1, SHV-2, and CMY-2] and high proportions (80%) of cefotaxime-resistant isolates were observed to harbor at least two bla genes, which may be interpreted as a high level of resistance of cefotaxime, which has rarely been documented in iNTS before.1,38
Interestingly, there was no correlation between the antimicrobial resistance and particular clonal genotypes of S. Enteritidis and S. Typhimurium. The PFGE analysis showed that the isolates of S. Enteritidis from different regions of the PFGE profiles (X1, X2, X3, X4, and X5) shared a distinctly high genetic similarity (>92%). This indicated the potential for cross-infections and horizontal transmission. The results of the PFGE study reveal that genotype diversity exists in S. Typhimurium in China, which suggested that there are multiple, independent clones in different regions of the world.
Conclusion
This study revealed that S. Enteritidis, S. Choleraesuis, and S. Typhimurium mainly cause iNTS infections; however, other serovars are also important. It is also a major threat to infant and elderly patients. An important alarm was the emergence and high level of resistance to cefotaxime, ciprofloxacin, and the ACSSuT pattern in iNTS isolates, which will pose new challenges for clinicians. To better manage and prevent the spread of antimicrobial resistance, especially in China with its large population, emphasis should be placed on the need for comprehensive surveillance to acquire accurate epidemiological information to help treat iNTS infections.
Acknowledgment
This work was supported by the National Key R&D Program of China (2017YFC1600101, 2017YFC1600104, 2018YFD0500500), a project supported by National Natural Science Foundation of China (31972762), a project supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2018), the Pearl River S&T Nova Program of Guangzhou (201806010183), the Province Science and Technology of Guangdong Research Project (2017A020208055), Walmart Foundation (SA1703162), and National Broiler Industry Technology System Project (cARS-41-G16).
Author contributions
Zhen Gu, Xiayidan Wufuer, Mingliu Wang, Meilian Huang, Jianhui Chen, Chunmei Jing and Xuebin Xu collected the clinical samples. Ming Liao, Jianmin Zhang and Mei Zeng designed the study. Zeqiang Zhan., Zhiying Xiong, Jianmin Zhang and Xuebin Xu carried out the experiment. Jianghong Meng, Zeqiang Zhan, Ming Liao, Jianmin Zhang and Mei Zeng analyzed the data. Zeqiang Zhan, Ming Liao, Jianmin Zhang and Mei Zeng and Xuebin Xu prepared the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine. 2015;33(Suppl 3):C21–C29. doi: 10.1016/j.vaccine.2015.03.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease. Emerg Infect Dis. 2015;21(6). doi: 10.3201/eid2101.140256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vugia DJ, Samuel M, Farley MM, et al. Invasive Salmonella infections in the United States, FoodNet, 1996-1999: incidence, serotype distribution, and outcome. Clin Infect Dis. 2004;38(Suppl 3):S149–S156. doi: 10.1086/381581 [DOI] [PubMed] [Google Scholar]
- 4.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis. 2012;12(6):480–487. doi: 10.1016/S1473-3099(12)70028-2 [DOI] [PubMed] [Google Scholar]
- 5.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(6):417–432. doi: 10.1016/S1473-3099(10)70072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelo KM, Reynolds J, Karp BE, Hoekstra RM, Scheel CM, Friedman C. Antimicrobial resistance among nontyphoidal salmonella isolated from blood in the United States, 2003–2013. J Infect Dis. 2015;214(10):1565–1570. doi: 10.1093/infdis/jiw415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon MA. Invasive nontyphoidal Salmonella disease: epidemiology, pathogenesis and diagnosis. Curr Opin Infect Dis. 2011;24(5):484–489. doi: 10.1097/QCO.0b013e32834a9980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan Z, Kuang D, Liao M, et al. Antimicrobial susceptibility and molecular typing of salmonella senftenberg isolated from humans and other sources in Shanghai, China, 2005 to 2011. J Food Prot. 2017;80(1):146–150. doi: 10.4315/0362-028X.JFP-16-255 [DOI] [PubMed] [Google Scholar]
- 9.Muthumbi E, Morpeth SC, Ooko M, et al. Invasive Salmonellosis in Kilifi, Kenya. Clin Infect Dis. 2015;61(Suppl 4):S290–S301. doi: 10.1093/cid/civ737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phu Huong Lan N, Le Thi Phuong T, Nguyen Huu H, et al. Invasive non-typhoidal salmonella infections in Asia: clinical observations, disease outcome and dominant serovars from an infectious disease hospital in Vietnam. PLoS Negl Trop Dis. 2016;10(8):e0004857. doi: 10.1371/journal.pntd.0004446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev. 2015;28(4):901–937. doi: 10.1128/CMR.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panchalingam S, Antonio M, Hossain A, et al. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin Infect Dis. 2012;55(Suppl 4):S294–S302. doi: 10.1093/cid/cis754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalonji LM, Post A, Phoba MF, et al. Invasive salmonella infections at multiple surveillance sites in the Democratic Republic of the Congo, 2011-2014. Clin Infect Dis. 2015;61(Suppl 4):S346–S353. doi: 10.1093/cid/civ713 [DOI] [PubMed] [Google Scholar]
- 14.Jiang HX, Song L, Liu J, et al. Multiple transmissible genes encoding fluoroquinolone and third-generation cephalosporin resistance co-located in non-typhoidal Salmonella isolated from food-producing animals in China. Int J Antimicrob Agents. 2014;43(3):242–247. doi: 10.1016/j.ijantimicag.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 15.Menezes GA, Harish BN, Khan MA, Goessens WH, Hays JP. Antimicrobial resistance trends in blood culture positive Salmonella Typhi isolates from Pondicherry, India, 2005-2009. Clin Microbiol Infect. 2012;18(3):239–245. doi: 10.1111/j.1469-0691.2011.03546.x [DOI] [PubMed] [Google Scholar]
- 16.Qiao J, Zhang Q, Alali WQ, et al. Characterization of extended-spectrum beta-lactamases (ESBLs)-producing Salmonella in retail raw chicken carcasses. Int J Food Microbiol. 2017;248:72–81. doi: 10.1016/j.ijfoodmicro.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DTA, Kanki M, Nguyen PD, et al. Prevalence, antibiotic resistance, and extended-spectrum and AmpC β-lactamase productivity of Salmonella isolates from raw meat and seafood samples in Ho Chi Minh City, Vietnam. Int J Food Microbiol. 2016;236:115–122. doi: 10.1016/j.ijfoodmicro.2016.07.017 [DOI] [PubMed] [Google Scholar]
- 18.Eguale T, Birungi J, Asrat D, et al. Genetic markers associated with resistance to beta-lactam and quinolone antimicrobials in non-typhoidal Salmonella isolates from humans and animals in central Ethiopia. Antimicrob Resist Infect Control. 2017;6:13. doi: 10.1186/s13756-017-0171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feasey NA, Masesa C, Jassi C, et al. Three epidemics of invasive multidrug-resistant salmonella bloodstream infection in Blantyre, Malawi, 1998-2014. Clin Infect Dis. 2015;61(Suppl 4):S363–S371. doi: 10.1093/cid/civ691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lunguya O, Lejon V, Phoba MF, et al. Antimicrobial resistance in invasive non-typhoid Salmonella from the Democratic Republic of the Congo: emergence of decreased fluoroquinolone susceptibility and extended-spectrum beta lactamases. PLoS Negl Trop Dis. 2013;7(3):e2103. doi: 10.1371/journal.pntd.0002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kariuki S, Onsare RS. Epidemiology and genomics of invasive nontyphoidal salmonella infections in Kenya. Clin Infect Dis. 2015;61(Suppl 4):S317–S324. doi: 10.1093/cid/civ711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko WC, Yan JJ, Yu WL, et al. A new therapeutic challenge for old pathogens: community-acquired invasive infections caused by ceftriaxone- and ciprofloxacin-resistant Salmonella enterica serotype choleraesuis. Clin Infect Dis. 2005;40(2):315–318. doi: 10.1086/426593 [DOI] [PubMed] [Google Scholar]
- 23.Jones TF, Ingram LA, Cieslak PR, et al. Salmonellosis outcomes differ substantially by serotype. J Infect Dis. 2008;198(1):109–114. doi: 10.1086/588823 [DOI] [PubMed] [Google Scholar]
- 24.Luk-In S, Chatsuwan T, Pulsrikarn C, Bangtrakulnonth A, Rirerm U, Kulwichit W. High prevalence of ceftriaxone resistance among invasive Salmonella enterica serotype Choleraesuis isolates in Thailand: the emergence and increase of CTX-M-55 in ciprofloxacin-resistant S. Choleraesuis isolates. Int J Med Microbiol. 2018;308(4):447–453. doi: 10.1016/j.ijmm.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 25.Galanis E, Lo Fo Wong DM, Patrick ME, et al. Web-based surveillance and global Salmonella distribution, 2000-2002. Emerg Infect Dis. 2006;12(3):381–388. doi: 10.3201/eid1205.050854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwambana-Adams B, Darboe S, Nabwera H, et al. Salmonella infections in The Gambia, 2005-2015. Clin Infect Dis. 2015;61(Suppl 4):S354–S362. doi: 10.1093/cid/civ781 [DOI] [PubMed] [Google Scholar]
- 27.Oneko M, Kariuki S, Muturi-Kioi V, et al. Emergence of community-acquired, multidrug-resistant invasive nontyphoidal salmonella disease in Rural Western Kenya, 2009-2013. Clin Infect Dis. 2015;61(Suppl 4):S310–S316. doi: 10.1093/cid/civ674 [DOI] [PubMed] [Google Scholar]
- 28.Helms M, Simonsen J, Molbak K. Quinolone resistance is associated with increased risk of invasive illness or death during infection with Salmonella serotype Typhimurium. J Infect Dis. 2004;190(9):1652–1654. doi: 10.1086/424570 [DOI] [PubMed] [Google Scholar]
- 29.Crump JA, Medalla FM, Joyce KW, et al. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob Agents Chemother. 2011;55(3):1148–1154. doi: 10.1128/AAC.01333-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlieghe ER, Phe T, De Smet B, et al. Azithromycin and ciprofloxacin resistance in Salmonella bloodstream infections in Cambodian adults. PLoS Negl Trop Dis. 2012;6(12):e1933. doi: 10.1371/journal.pntd.0001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenstemacher P. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32(3):331–351. doi: 10.1086/318514 [DOI] [PubMed] [Google Scholar]
- 32.Helms M, Ethelberg S, Mølbak K, Group TDS. International Salmonella Typhimurium DT104 infections, 1992-2001. Emerg Infect Dis. 2005;11(6):859–867. doi: 10.3201/eid1106.041017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrari R, Galiana A, Cremades R, et al. Plasmid-mediated quinolone resistance (PMQR) and mutations in the topoisomerase genes of Salmonella enterica strains from Brazil. Braz J Microbiol. 2013;44(2):651–656. doi: 10.1590/S1517-83822013000200046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mather AE, Phuong TLT, Gao Y, et al. New variant of multidrug-resistant salmonella enterica serovar typhimurium associated with invasive disease in immunocompromised patients in Vietnam. MBio. 2018;9(5). doi: 10.1128/mBio.01056-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22(8):438–445. doi: 10.1016/j.tim.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 36.Baucheron S, Chaslus-Dancla E, Cloeckaert A, Chiu CH, Butaye P. High-level resistance to fluoroquinolones linked to mutations in gyrA, parC, and parE in Salmonella enterica serovar Schwarzengrund isolates from humans in Taiwan. Antimicrob Agents Chemother. 2005;49(2):862–863. doi: 10.1128/AAC.49.2.862-863.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robicsek A, Strahilevitz J, Jacoby GA, et al. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med. 2006;12(1):83–88. doi: 10.1038/nm1347 [DOI] [PubMed] [Google Scholar]
- 38.Antunes NT, Frase H, Toth M, Mobashery S, Vakulenko SB. Resistance to the third-generation cephalosporin ceftazidime by a deacylation-deficient mutant of the TEM beta-lactamase by the uncommon covalent-trapping mechanism. Biochemistry. 2011;50(29):6387–6395. doi: 10.1021/bi200403e [DOI] [PubMed] [Google Scholar]