Background:
US health care systems face a growing demand to incorporate innovations that improve patient outcomes at a lower cost. Funding agencies increasingly must demonstrate the impact of research investments on public health. The Learning Health System promotes continuous institutional innovation, yet specific processes to develop innovations for further research and implementation into real-world health care settings to maximize health impacts have not been specified.
Objective:
We describe the Research Lifecycle and how it leverages institutional priorities to support the translation of research discoveries to clinical application, serving as a broader operational approach to enhance the Learning Health System.
Methods:
Developed by the US Department of Veterans Affairs Office of Research and Development Research-to-Real-World Workgroup, the Research Lifecycle incorporates frameworks from product development, translational science, and implementation science methods. The Lifecycle is based on Workgroup recommendations to overcome barriers to more direct translation of innovations to clinical application and support practice implementation and sustainability.
Results:
The Research Lifecycle posits 5 phases which support a seamless pathway from discovery to implementation: prioritization (leadership priority alignment), discovery (innovation development), validation (clinical, operational feasibility), scale-up and spread (implementation strategies, performance monitoring), and sustainability (business case, workforce training). An example of how the Research Lifecycle has been applied within a health system is provided.
Conclusions:
The Research Lifecycle aligns research and health system investments to maximize real-world practice impact via a feasible pathway, where priority-driven innovations are adapted for effective clinical use and supported through implementation strategies, leading to continuous improvement in real-world health care.
Key Words: translation, implementation, technology transfer, quality of care
It can take decades for clinical research discoveries to be translated to routine health care.1 Eighty percent of medical research dollars do not result in direct public health impact.2 This Research-to-Real-World (R2R) gap is attributed to several barriers including a lack of alignment of research investments with the priorities of health systems, providers, and patients. This gap also persists due to limited investment in the clinical validation or product development following the discovery of innovations. It also exists because of limited strategies to effectively implement effective interventions derived from innovations into real-world health care settings.
Closing the R2R gap requires an approach that iteratively aligns research activities with the changing needs of health systems, providers, and patients, to more rapidly translate innovations into practice. The National Academy of Medicine’s Learning Health System framework strives to reduce this gap by using health system data to identify research priorities and continuously monitor improvements in the health system that address those priorities.3 However, the Learning Health System lacks an operational approach to rapidly move research innovations to clinical application and their integration into routine care.4 Key reasons for this delay include lack of adequate research infrastructure in most health systems, or if research support exists, a lack of alignment of research and clinical priorities and functions.
This paper describes a Research Lifecycle that extends the impact of the Learning Health System to more rapidly move research discoveries into the clinical application so that they enhance the real-world impact of research. The Research Lifecycle is based on the experiences of the US Department of Veterans Affairs Veterans Health Administration (VHA) Office of Research and Development (ORD). ORD is VHA’s national research program embedded within a health system (VHA), which has identified the Learning Health System as its strategic priority. With a clinical enterprise of over 150 hospitals and over 1000 community-based outpatient clinics, VHA is supported by a workforce that has access to research training opportunities as well as a national electronic medical record system to promote a Learning Health System.
Although VHA is uniquely positioned to inform the Research Lifecycle, the ultimate audience for the Research Lifecycle is the broader community of funders and health systems. It addresses the need for an operational framework to better align research investments to solve the problems facing practitioners within health systems, to better measure the impact of research innovations on public health and policy, and to ensure their more rapid implementation as effective interventions in routine practice.5
METHODS
The Research Lifecycle was developed by ORD, which funds research through competitive, investigator-initiated intramural funding opportunities for over 2500 VHA-employed investigators across the United States.6,7 With a Congressional appropriation exceeding $750 million/year, ORD funds discovery/biomedical research, clinical trials, rehabilitation research, and health services and implementation science. ORD also manages cross-disciplinary programs that test innovations derived from discoveries in clinical trials (eg, Cooperative Studies Program)8 and rapidly implement clinical trial results into routine practice (eg, Quality Enhancement Research Initiative—QUERI).9
ORD collaborates side by side with other VHA organizations including the Office of Academic Affiliations and Innovation Ecosystem. The Office of Academic Affiliations oversees training opportunities in research and quality improvement for VHA employees.10 The Innovation Ecosystem is comprised of the VHA Innovators Network and Diffusion of Excellence Initiative11 which support the development of innovative practices (eg, clinical, technological, or administrative processes) cultivated by frontline employees, by promoting their further development, scale-up and spread in routine health care settings. Innovations developed within VHA also spread to other health systems, extending their impact to include patients outside the VHA system.
Research Lifecycle Development
In 2018–2019, the ORD R2R Workgroup was commissioned by the ORD Director to develop the Research Lifecycle to specify more seamless processes to move research discoveries to direct application in routine care. R2R Workgroup participants were VHA national leaders with diverse backgrounds including clinical services, quality improvement, data science, technology transfer, rehabilitation, biomedical science, and public health. Participants were selected using purposeful sampling to ensure representation from the major organizations within VHA and were not compensated for their participation.
Three 90-minute virtual meetings were convened by phone, and a 3-hour in-person meeting was held after 6 months. Meetings were facilitated by the R2R Workgroup lead who had a background in implementation science, health care policy, and clinical research. Discussions during the meetings focused on identifying barriers to seamless translation of innovations to clinical application, and from clinical application to further implementation and sustainability. Participants also made several recommendations for steps that ORD could take to improve more direct, seamless transition of innovations to implementation, which was operationalized as phases in the Research Lifecycle.
The Research Lifecycle was further refined based on a critical review of the Learning Health System Knowledge to Action Framework.12 This Framework operationalizes a continuous quality improvement approach for health care systems. Health care system data are used to identify priorities (data to knowledge), which in turn inform quality improvement efforts (knowledge to performance), and lead to continuous monitoring of the health system for additional opportunities for improvement (performance to data). The Lifecycle also incorporates stages of the National Institutes of Health Translational Pipeline.13 The Translational Pipeline focuses on moving discoveries to clinical application, from biomedical research (T0) to the preclinical phase (T1) to clinical intervention validation (T2) to intervention implementation (T3), to ultimately improved population health (T4). Central to the application of the Translational Pipeline in the Lifecycle is the use of implementation science, which is the study of methods that promote the systematic uptake of discoveries and effective interventions into routine practice.14 An additional model that informed the Research Lifecycle was the Center for Translation of Rehabilitation Engineering Advances and Technology (TREAT) commercialization process.15 TREAT emphasizes the importance of product development by end-users to ensure discoveries are acceptable to patients and providers. Finally, the Research Lifecycle was informed by experiences of the Diffusion of Excellence Initiative,11 which created a process to ensure that innovations were technologically and administratively feasible for scale-up in routine clinical practice.
A summary of the R2R Workgroup recommendations, as well as a draft Research Lifecycle, were presented to ORD leadership and the VHA National Research Advisory Council in September 2018. The Workgroup consolidated their feedback into the updated Research Lifecycle, depicted in Figure 1.
FIGURE 1.
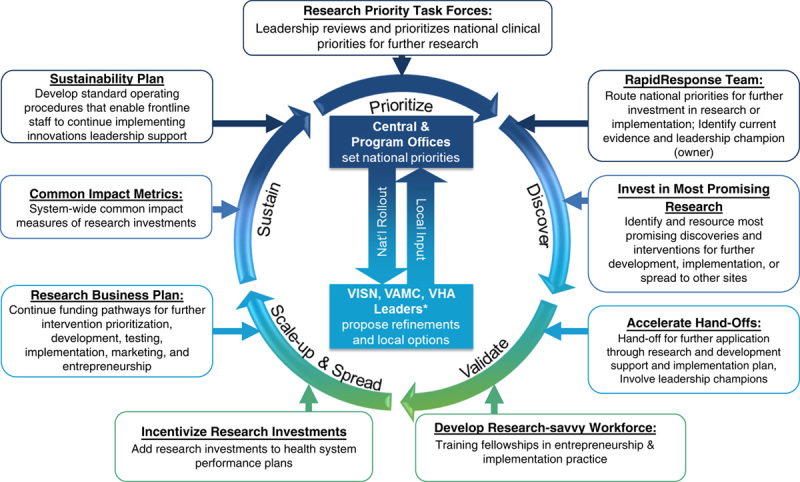
Research Lifecycle: moving high-priority innovations to application. VAMC indicates VA Medical Center; VHA, Veterans Health Administration; VISN, Veterans Integrated Service Network.
RESULTS
R2R Workgroup members identified 5 phases of the Research Lifecycle that are designed to address the major barriers to allowing research to have more real-world impact. Lifecycle phases depicting the R2R translation process include prioritization, discovery, validation, scale-up and spread, and sustainment. Notable barriers to transition across these phases were also identified, including lack of systematic processes for aligning clinical priorities with research investments and inadequate funding and administrative mechanisms to support the translation of practices from discovery to validation, and ultimately, to clinical implementation. Without more seamless funding to further validate innovations for clinical use and their direct implementation in real-world practice, innovations face the “valley of death” which can leave many discoveries out of reach for patients.16,17
Research Lifecycle Phases
The 5 phases of the Research Lifecycle are described in Figure 1. Each phase is depicted by a circular arrow and each callout box represents specific processes recommended by the R2R Workgroup to overcome those barriers. Each phase offers researchers, health system leaders, and providers specific pathways to support innovation adoption. Together these phases represent how research priorities can be identified to foster innovations, how such innovations are further validated for their effectiveness in clinical environments. They also represent how innovations, if proven effective, can be further implemented (scaled) and sustained in the health system. At the core of the Lifecycle is the alignment of clinical priorities generated by national, regional, and local leaders and stakeholders, to help inform decisions to allocate investments in specific innovations and further implementation efforts throughout the research process.
Prioritization
Prioritization is defined as the selection of clinical issues that have the largest impact on the health system. Prioritization also involves a commitment of resources to support the development of interventions to address those issues. Health system leaders and regional/national policymakers are natural stakeholders, as key decision-makers in determining health care resources. However, frontline employees, patients, families, and policymakers are also crucial stakeholders in setting priorities. ORD uses multiple inputs for identifying national clinical priorities including policymakers (Congress), consumer groups (eg, Veterans Service Organizations), regional health system leaders, researchers, and frontline providers.18 In 2019, suicide prevention and mental health care access were top VHA clinical priorities.
Discovery
Discovery involves the identification of innovations that are most aligned with clinical priorities for their further development and implementation. ORD’s Evidence Synthesis Program (ESP)18 conducts reviews of high-priority topics to identify clinical needs and quality gaps to drive new research initiatives that address health system priorities. Through requests for applications and a scientific peer review process, ORD identifies innovations aligned with VHA priorities for further development, validation, or implementation. Discovery also involves the rapid deployment of research resources to address national priorities. For example, ORD investigators often consult VHA national leaders on the deployment of existing effective practices addressing clinical priorities. Central to this phase is identifying a national health system leader who is actively involved in further research and application addressing a clinical priority across the Lifecycle over time.
Validation
Validation is the process by which innovations identified in the discovery phase are developed and further tested as clinical interventions. Researchers use concepts such as “design for dissemination/implementation,”19 or user-centered design20,21 which incorporate input from end-users (eg, patients, frontline providers) to further validate innovations for feasibility, resource requirements, and safety. Validation also involves “derisking” of interventions prior to their application. Derisking17 is a process in which interventions are evaluated on whether they meet regulatory standards, and do not duplicate existing copyrights.
Validation also includes determining whether interventions are designed to be implemented once the research ends. For example, the ORD Cooperative Studies Program8 funds multisite clinical trials that require an implementation plan.9 The implementation plan requires that: (1) leaders and stakeholders are involved in the design of the intervention; (2) providers react to the intervention, including impacts on efficiency, satisfaction, and perceived barriers/facilitators; and (3) specific strategies are proposed that enable existing providers to implement the intervention (if proven effective) once research funding ends. The implementation plan ensures that leadership endorses the clinical intervention and that if proven effective, the intervention is feasible for implementation by existing providers who are not paid on the research trial. The implementation plan is based on the QUERI Implementation Roadmap22 and the hybrid effectiveness-implementation designs,23 which were developed to operationalize patient, provider, and leader involvement by incorporating implementation science methods and measures.14
Scale-Up and Spread
Scale-up and spread is the process by which interventions are implemented across different health settings. This phase involves research investments that refine interventions for marketing, technology transfer and further implementation in real-world practice. Many health systems including VHA’s ORD have embedded technology transfer programs which support their researchers with invention disclosures, commercialization, and royalty acquisitions of innovations. Another crucial component of scale-up and spread is the development and application of strategies to implement interventions across different practices. Described as the “how to” of implementation science, implementation strategies24 are specific methods derived from implementation science that is used to help providers adopt use interventions in real-world settings. They are the active ingredients that ensure effective interventions work at the clinic level, without research support. There is a growing body of effective implementation strategies such as audit and feedback (eg, monitoring and coaching providers on quality performance) and facilitation (eg, consulting providers on strategic thinking skills to garner support for the intervention within their practice).24 Implementation strategies are evaluated in separate studies for their effectiveness to continue and sustain the intervention once the research trial is over. For example, using a hybrid effectiveness-implementation design, one study could determine whether audit and feedback alone versus audit and feedback plus facilitation leads to more frontline providers using the clinical intervention with fidelity, and more patients receiving the intervention.14,24
Sustainment
Sustainment is the process of ensuring that interventions continue after the research studies are over. It involves demonstrating the impacts of the interventions on leadership to justify continued funding and support for intervention refinement and implementation. Sustainment also includes transitioning ownership of the intervention from investigators to local/national leaders and developing health system-wide policies that promote the use of the intervention.
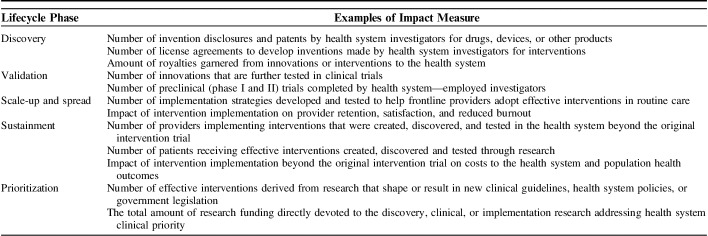
Optimizing sustainment requires measurement of the interventions’ impact beyond scientific criteria such as publications. Table 1 lists impact measures from the ORD 2020 budget that are derived from the National Academy of Sciences Degrees of Impact and the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) frameworks.25 These measures are intended as examples for assessing impacts beyond publications and grant funding, from the perspective of leaders of health systems who are interested in the value of the interventions generated from research. Examples of measures include innovation return-on-investment measures such as royalties, the number of patients receiving the intervention. They also include implementation return-on-investment such as the impact on the provider and patient satisfaction as well as intervention adoption and costs over time.
TABLE 1.
Research Lifecycle: Examples of Impact Measures For Health Systems
The sustainment phase also involves a business case analysis, which assesses the intervention’s value, or return-on-investment, from the health system’s perspective. A business case analysis is important to help leaders determine whether to resource implementation support over time once research funding ends. A key component of a business case analysis26 is determining the intervention’s impacts on overall health care costs, including costs maintaining implementation of the intervention over time. Business case analyses also entail the collection of outcomes pertinent to health system leaders, such as changes in provider turnover, and patient satisfaction.
Sustainment also includes prioritizing national budgets to support effective programs/policies derived from interventions. For example, the QUERI Partnered Evidence-based Policy Resource Center (PEPReC)27 informs national policies in the US Department of Veterans Affairs through rigorous evaluation of intervention sustainability. PEPReC is also helping VHA operationalize the Foundations for Evidence-based Policymaking Act,28 which requires all Cabinet-level agencies to have a plan to evaluate the level of evidence of their programs and policies, and use research to generate rigorous evidence to inform budget decisions.
Cross-cutting Research Lifecycle Components
The Research Lifecycle includes 2 cross-cutting components which affect all 5 phases: development of a research-savvy workforce and provision of clinical-level incentives for research investment.
To achieve the goals of the Lifecycle, health systems require an interdisciplinary workforce to produce innovations which address complex problems in health care. For example, VHA’s Office of Academic Affiliations Fellowship Programs supports postdoctoral training opportunities in health services research and quality improvement science. These fellowship programs incorporate Learning Health Care System components29,30 through curricula in implementation science, data science, and health systems engineering, and opportunities to apply these methods through projects with health system leaders. The VHA Office of Academic Affiliations and the Diffusion of Excellence Initiative provide fellowships in innovation and entrepreneurship, akin to the National Science Foundation I-Corps program.31 The goal of these fellowships is to create a cadre of frontline employees with the knowledge of managing innovation and increase health system capacity to better translate innovative ideas into direct patient impact.
Another cross-cutting component of the Lifecycle includes providing incentives to invest in research. Recently, VHA regional and medical center leadership performance criteria included assessments of investments in the scale-up and spread of innovations and effective interventions. VHA leaders receive an outstanding rating on a critical element of their performance plan if innovation or effective intervention is adopted across multiple facilities within a region.
Research Lifecycle Example
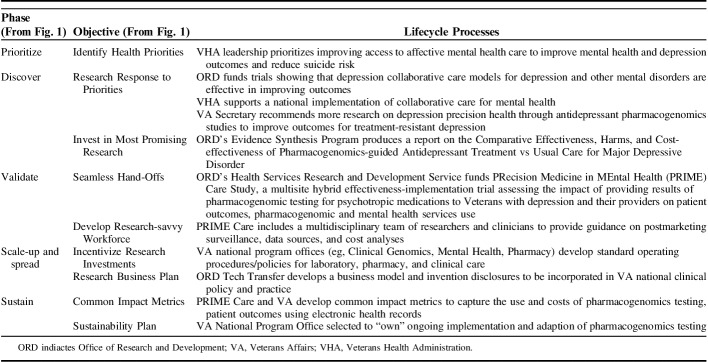
Table 2 provides an example of how the Research Lifecycle has been applied in VHA to improve access to innovative mental health treatments, particularly for depression. A common diagnosis among VHA patients, depression is associated with significant functional impairment and increased risk of mortality including from suicide.32 Over the past 2 decades, ORD invested in research in collaborative care models for depression care, which combine patient self-management support with care management and guideline-concordant pharmacotherapy informed by clinical information systems. Evidence suggests that collaborative care models are associated with improved patient health outcomes33 including reduced suicide risk.34 In 2006, VHA prioritized the implementation of collaborative care models for depression and other mental disorders to improve patient outcomes including suicide prevention.35,36
TABLE 2.
Research Lifecycle Example: Depression Treatment
In 2016, recognizing that some patients with depression were still not improving even with evidence-based pharmacotherapy, the US Department of Veterans Affairs Secretary asked ORD to support new research to optimize depression treatment through pharmacogenomic precision medicine. In response, ORD commissioned ESP to conduct a rapid review of research on pharmacogenomics testing strategies to inform the optimal antidepressant selection and patient outcomes.37 The resulting report highlighted gaps in evidence and informed the development of an ORD request for applications for funding studies on pharmacogenomic testing to improve the quality of depression treatment. In 2017, ORD funded PRIME Care38 as a multisite hybrid effectiveness-implementation trial to determine the impact of returning pharmacogenomic test results to providers on the choice of antidepressants and subsequent patient depression outcomes. PRIME Care involved multiple stakeholders including national leaders from Pharmacy, Genomics, and Mental Health program offices, as well as implementation researchers who applied principles from the CSP implementation plan to ensure study results could be implemented in routine VHA care. Once PRIME Care is completed, the implementation data collected on patient acceptance and provider feasibility will inform the further application of this intervention (if proven effective) in national clinical practice.
DISCUSSION
The Research Lifecycle proposes a systematic approach to reduce the gap between research innovation and public health impact, supporting the rapid translation of research discoveries to their application in real-world clinical settings. To date, no other framework has operationalized elements of product development, translational science, or implementation science to support the core functions of the Learning Health System to enhance the real-world impact of research. The Research Lifecycle promotes the further validation of discoveries aligned with priorities and ensures their clinical application in routine care to enhance the impact of research investments over time.
The VHA health care system is uniquely positioned to operationalize the Research Lifecycle because it has an embedded research program that can directly develop and test discoveries and then translate them into routine clinical practice. However, challenges remain in maximizing the full potential of the Research Lifecycle in VHA and elsewhere. First, as with many other funding agencies, VHA research currently lacks a formal infrastructure to administer the full range of funding mechanisms needed to promote further development and implementation of innovations. Central to the success of the Lifecycle involves the creation of funding opportunities that currently do not exist among federal research funders. Notably, researchers require developmental funding to further validate and replicate innovations for clinical application, and application funding to implement strategies to get effective interventions into the hands of providers and patients. To date, researchers have been incentivized to pursue funding opportunities that focus on innovation rather than validation, replication or implementation. The bolus of innovations without mechanisms to further develop and implement them is the likely reason in which few end up achieving a public health impact.
Second, additional incentives are needed within health systems to encourage research investment, application, and sustainability. A recent report on the future of health services research by the National Academy of Medicine5 concluded that research funding should be used to solve the operational-focused problems facing health systems such as improving clinical processes that lead to high-value care at a lower cost. A key barrier to achieving this recommendation is that researchers are more likely to be awarded for publications and grant productivity for innovations in specific disease areas rather than achieving systematic changes to quality or outcomes in patient care. It remains a challenge to measure the impact of research beyond publications, especially in showing whether innovations or the implementation of effective interventions achieve tangible impacts on quality of care or national practice/policy. For health systems without their own source of research resources, funding agencies should continue to invest in grant mechanisms that promote embedded research, where the health systems help set the study goals, to maximize the generalizability of research impacts. Health systems, in turn, will need to invest in a workforce with experience in research methods to fulfill the Learning Health System goal of continuous improvement through innovation, further validation, and implementation.
Overall, the Research Lifecycle is a guide for funders and health systems to more rapidly translate innovations into interventions that address clinical priorities that have a meaningful impact on population health. It should be further applied to health systems and refined based on the changing health care landscape and changing needs of patients. The Research Lifecycle provides a process for aligning research priorities with resources and talent, ensuring that discoveries lead to effective treatments to improve public health impact.
Footnotes
Supported by the US Department of Veterans Affairs, Veterans Health Administration, Health Services Research & Development Service. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs of the US Government.
The authors declare no conflict of interest.
REFERENCES
- 1.Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearb Med Inform. 2000;1:65–70. [PubMed] [Google Scholar]
- 2.Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374:86–89. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson TB., Jr The Institute of Medicine committee report “best care at lower cost: the path to continuously learning health care”. Circ Cardiovasc Qual Outcomes. 2012;5:e93–e94. [DOI] [PubMed] [Google Scholar]
- 4.Bindman AB, Pronovost PJ, Asch DA. Funding innovation in a learning health care system. JAMA. 2018;319:119–120. [DOI] [PubMed] [Google Scholar]
- 5.Whicher D, Rosengren K, Siddiqi S, Simpson L, The Future of Health Services Research: Advancing Health Systems Research and Practice in the United States. Washington, DC: National Academy of Medicine; 2018. [PubMed] [Google Scholar]
- 6.Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467–487. [DOI] [PubMed] [Google Scholar]
- 7.Kupersmith J. Introduction: Comparative effectiveness research-objectives, challenges, and contributions of the US Department of Veterans Affairs. Am J Med. 2010;123:e1–e2. [DOI] [PubMed] [Google Scholar]
- 8.Huang GD, Ferguson RE, Peduzzi PN, et al. Scientific and organizational collaboration in comparative effectiveness research: the VA cooperative studies program model. Am J Med. 2010;123:e24–e31. [DOI] [PubMed] [Google Scholar]
- 9.Kilbourne AM, Elwy AR, Sales AE, et al. Accelerating research impact in a learning health care system: VA’s quality enhancement research initiative in the choice act era. Med Care. 2017;55(suppl 7 suppl 1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilman SC, Chokshi DA, Bowen JL, et al. Connecting the dots: interprofessional health education and delivery system redesign at the Veterans Health Administration. Acad Med. 2014;89:1113–1116. [DOI] [PubMed] [Google Scholar]
- 11.Elnahal SM, Clancy CM, Shulkin DJ. A framework for disseminating clinical best practices in the VA Health System. JAMA. 2017;317:255–256. [DOI] [PubMed] [Google Scholar]
- 12.Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26:13–24. [DOI] [PubMed] [Google Scholar]
- 13.Fort DG, Herr TM, Shaw PL, et al. Mapping the evolving definitions of translational research. J Clin Transl Sci. 2017;1:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Technology and Market Assessment. Center for Translation of Rehabilitation Engineering Advances in Technology. 2019. Available at: www.treatcenter.org. Accessed April 8, 2019.
- 16.Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840–842. [DOI] [PubMed] [Google Scholar]
- 17.Kim ES, Omura PMC, Lo AW. Accelerating biomedical innovation: a case study of the SPARK program at Stanford University, School of Medicine. Drug Discov Today. 2017;22:1064–1068. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Veteran Affairs. VA evidence synthesis program reports. Washington, DC: Department of Veterans Affairs. 2019. Available at: www.hsrd.research.va.gov/publications/esp/. Accessed April 9, 2019. [Google Scholar]
- 19.Tan ASL, Mazor KM, McDonald D, et al. Designing shared decision-making interventions for dissemination and sustainment: can implementation science help translate shared decision making into routine practice? MDM Policy Pract. 2018;3:2381468318808503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzpatrick NE, Maier J, Yasko L, et al. The Pitt Innovation Challenge (PInCh): driving innovation in translational research through an incentive-based, problem-focused competition. Acad Med. 2017;92:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyon AR, Bruns EJ. User-centered redesign of evidence-based psychosocial interventions to enhance implementation-hospitable soil or better seeds? JAMA Psychiatry. 2018;76:3–4. [DOI] [PubMed] [Google Scholar]
- 22.Kilbourne AM, Goodrich DE, Miake-Lye I, et al. The VA quality enhancement research initiative implementation roadmap: towards sustainability of evidence-based practices in a learning health care system. Med Care. 2019. In press. [DOI] [PMC free article] [PubMed]
- 23.Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harden SM, Smith ML, Ory MG, et al. RE-AIM in clinical, community, and corporate settings: perspectives, strategies, and recommendations to enhance public health impact. Front Public Health. 2018;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leatherman S, Berwick D, Iles D, et al. The business case for quality: case studies and an analysis. Health Aff (Millwood). 2003;22:17–30. [DOI] [PubMed] [Google Scholar]
- 27.Frakt AB, Prentice JC, Pizer SD, et al. Overcoming challenges to evidence-based policy development in a large, integrated delivery system. Health Serv Res. 2018;53:4789–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Congress. Foundations for evidence-based policymaking act. 2019. Available at: www.congress.gov/bill/115th-congress/house-bill/4174/text?q=%7B%22search%22%3A%5B%22HR+4174%22%5D%7D&r=1&s=3. Accessed April 8, 2019.
- 29.Forrest CB, Chesley FD, Jr, Tregear ML, et al. Development of the learning health system researcher core competencies. Health Serv Res. 2017;53:2615–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins D. Are we growing the right health services research workforce of the future? Thoughts from a National Delivery System. Health Serv Res. 2018;53(suppl 2):4034–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirschtritt ME, Heaton PM, Insel TR. Preparing physician-scientists for an evolving research ecosystem. JAMA. 2018;320:31–32. [DOI] [PubMed] [Google Scholar]
- 32.Valenstein M, Kim HM, Ganoczy D, et al. Higher-risk periods for suicide among VA patients receiving depression treatment: prioritizing suicide prevention efforts. J Affect Disord. 2009;112:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169:790–804. [DOI] [PubMed] [Google Scholar]
- 34.Bruce ML, Ten Have TR, Reynolds CF, III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081–1091. [DOI] [PubMed] [Google Scholar]
- 35.Post EP, Metzger M, Dumas P, et al. Integrating mental health into primary care within the Veterans Health Administration. Fam Syst Health. 2010;28:83–90. [DOI] [PubMed] [Google Scholar]
- 36.Bauer MS, Miller CJ, Kim B, et al. Effectiveness of implementing a collaborative chronic care model for clinician teams on patient outcomes and health status in mental health: a randomized clinical trial. JAMA Netw Open. 2019;2:e190230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson K, Dieperink E, Anderson J, et al. Rapid evidence review of the comparative effectiveness, harms, and cost-effectiveness of pharmacogenomics-guided antidepressant treatment versus usual care for major depressive disorder. Psychopharmacology (Berl). 2017;234:1649–1661. [DOI] [PubMed] [Google Scholar]
- 38.Hull LE, Lynch KG, Oslin DW. VA Primary Care and Mental Health Providers’ Comfort with Genetic Testing: survey results from the PRIME Care Study. J Gen Intern Med. 2019;34:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]