Abstract
A recent DNA-based assessment of the psyllid fauna of New Zealand recorded high genetic variation between populations that were expected to belong to the same psyllid species. Among these, a number of populations of the kōwhai psyllid Psylla apicalis (Ferris & Klyver, 1932), from a kōwhai species, Sophora microphylla Aiton (Fabaceae), presented high genetic variability. This gave new endorsement of an 87-year-old observation made by the entomologists Ferris and Klyver who, when describing the kōwhai psyllid, from Sophora tetraptera J.S. Muell., suggested that morphological variations could support more than one species. Accordingly, the morphological assessment conducted here, together with the genetic information now available, resulted in the description of Psylla frodobagginsi sp. nov. as a second New Zealand endemic psyllid species hosted by S. microphylla.
Introduction
The superfamily Psylloidea (Hemiptera, Sternorrhyncha) comprises almost 4000 described species worldwide [1]. Yet, there are several indications that many more species remain undescribed (e.g. [2, 3]) which confounds a clear appreciation of the number of psyllid species, our ability to distinguish them and their distribution worldwide. Thus, there is a fundamental need to better understand their systematics and to confirm the most recent taxonomic classification [4] for the downstream applied benefits of that, such as studies on the insect/bacteria interactions and on their dispersal models (e.g. [5]). This can be facilitated by the continuous updating of psyllid taxonomic diversity and distribution. Good examples tackling Australasia and the Pacific, as a hot spot for biodiversity of the Psylloidea [1, 2], include the most recent works focusing on Australia [6, 7], New Zealand [3, 8, 9], Cook Islands [10] and Hawaii [11, 12]. Expediting this appraisal has been inclusion of molecular phylogenetic studies to enable a better understanding of this superfamily’s evolutionary history (e.g. [13, 14]. Equally this relies on a pre-existing and accurate understanding of psyllid diversity to ensure representative sampling across families and genera.
Within Australasia, the psyllid fauna of New Zealand can contribute useful information towards a global understanding of these insects [3, 8], with a less challenging number of species per geographic area to consider than Australia [1, 2]. Thus far, New Zealand is known to be home to 73 described psyllid species [8, 15, 16] and at least another 47 undescribed or hypothesised taxa [3, 17]; this compares with more than 500 species described to date in Australia [1, 2] and an unknown number of undescribed taxa present there [2]. Among these, the genus Psylla, comprised of 98 species worldwide and none in Australia [1], is to date represented in New Zealand by two described species, P. apicalis and P. carmichaeliae Tuthill, 1952, and a proposed additional five undescribed taxa [3].
The hypothesis of cryptic variation within taxa of the genus ‘Psylla’ in New Zealand dated well before the advent of molecular analysis, which was the key driver for developing the current new description [3]. Essentially, when ‘Psyllia (sic) apicalis’ was described as the first and only Sophora-feeding psyllid, the authors highlighted how this species showed such a variable morphology that it could as well be due to the presence of two separate taxa [18]. Of note was the different wing patterns and colours. Nonetheless, the authors assumed the presence of intergradation between the patterns and believed that the differences were merely variants of the same species [18].
More recently, molecular characters developed for the New Zealand Psylloidea, based on the cytochrome oxidase I (COI) barcode gene region [19], reported the presence of high genetic variability of 7–8% divergence between different populations of P. apicalis. This led to the hypothesis of the presence of up to five additional taxa belonging within this genus [3]. Since it is not the aim of this work to review the New Zealand psyllid species attributed to the genus Psylla per se, these additional taxa have been left in this genus. Rather, while awaiting a taxonomic review of Psylla to include a phylogenetic comparison of the New Zealand species and those elsewhere, this study focuses on an observation in the South Island that there was potentially more than one Psylla species associated with S. microphylla. In order to provide a useful and valid tool to distinguish specimens of a new species here as Psylla frodobagginsi and not P. apicalis, specimens of both species have been collected and a new morphological description for P. frodobagginsi plus re-description of P. apicalis is presented here which fully reflects the molecular divergence between them [3]. Measurements, figures and description are reported for both species, together with the information obtained from examination of the holotype of P. apicalis. This aims to contribute to a better understanding of the number of New Zealand endemic species belonging to this genus. In turn this will facilitate future work to reconsider more broadly the taxonomy, systematics and phylogenetics of the genus Psylla.
Materials and methods
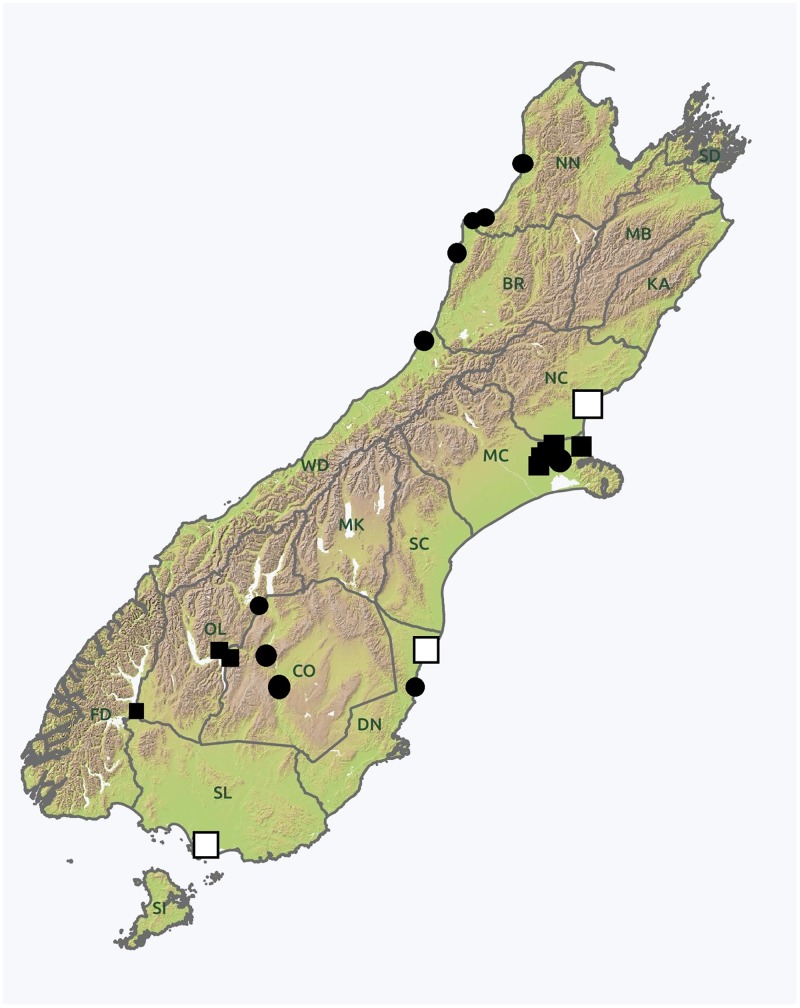
Psyllids were collected from kōwhai trees in 21 locations across 10 regions in the South Island of New Zealand, following Crosby’s regional delimitations [20] (Fig 1, Table 1). Collections were performed from public areas not requiring any permit. Samples were obtained by beating plant branches on a tray, then retrieving fallen insects using an entomological aspirator. Collected samples were stored, per tree, in high grade ethanol at -20°C.
Fig 1. Map of the South Island of New Zealand, showing the 21 locations from which psyllids could be collected.
Some of the locations included only P. apicalis (black squares), only P. frodobagginsi (black circles) or both the species (white squares). Regions delimitation follows Crosby et al. [20]. Reprinted from https://geographx.co.nz/ under a CC BY license, with permission from Geographx.
Table 1. Populations of psyllids analysed in this study.
The table provides information on the species, the population ID number, the collection date, the collectors, the GPS coordinates, the region within the South Island of New Zealand (based on Crosby’s regional boundaries [20]), the town location and the accession numbers for the DNA sequences previously available on GenBank for each population.
| Species | ID | Date | Collector | Coordinate | Region | Location | COI | 18S |
|---|---|---|---|---|---|---|---|---|
| P. apicalis | 7 | 07-Sep-14 | Martoni, F | 43°38'23.3"S 172°28'30.8"E | MC | Lincoln | MG132469-70 | MG195385 |
| 187 | 01-Sep-15 | Brown, SDJ | 43°31'22.44"S 172°35'7.44"E | MC | Christchurch | MG132465 | ||
| 200 | 02-Nov-15 | Martoni, F & Evans, H | 45°5'13.47"S 170°58'31.85"E | DN | Oamaru | |||
| 201 | 08-Nov-15 | Martoni, F & Evans, H | 46°24'09.7"S 168°21'38.2"E | SL | Invercargill | MG132466 | ||
| 204 | 08-Nov-15 | Martoni, F & Evans, H | 45°25'25.3"S 167°43'05.4"E | FD | Te Anau | MG132467 | ||
| 206 | 09-Nov-15 | Martoni, F & Evans, H | 45°02'12.8"S 168°39'38.3"E | OL | Queenstown | MG132468 | ||
| 208 | 09-Nov-15 | Martoni, F & Evans, H | 45°02‴S 168°39‴E | OL | Queenstown | |||
| 429 | 10-Sep-16 | Martoni, F | 43°09'23.4"S 172°43'50.5"E | NC | Amberley | |||
| 431 | 13-Oct-16 | Martoni, F | 43°38'31.4"S 172°28'15.1"E | MC | Lincoln | |||
| 433 | 13-Oct-16 | Martoni, F | 43°38'25.0"S 172°28'28.9"E | MC | Lincoln | |||
| 434 | 01-Oct-16 | Bowie, M | 43°38'14.0"S 172°29'09.0"E | MC | Lincoln | |||
| P. frodobagginsi | 55 | 30-Nov-14 | Martoni, F | 43°38‴S 172°28‴E | MC | Lincoln | MG132481 | MG195386 |
| 200 | 02-Nov-15 | Martoni, F & Evans, H | 45°5'13.47"S 170°58'31.85"E | DN | Oamaru | MG132471 | ||
| 201 | 08-Nov-15 | Martoni, F & Evans, H | 46°24'09.7"S 168°21'38.2"E | SL | Invercargill | |||
| 209 | 09-Nov-15 | Martoni, F & Evans, H | 45°02'09.7"S 169°11'33.8"E | CO | Cromwell | MG132472 | ||
| 215 | 09-Nov-15 | Martoni, F & Evans, H | 45°15'06.3"S 169°23'19.3"E | CO | Alexandra | MG132473 | ||
| 218 | 10-Nov-15 | Martoni, F & Evans, H | 44°41'37.9"S 169°08'08.5"E | OL | Wanaka | MG132474 | ||
| 226 | 11-Nov-15 | Martoni, F & Evans, H | 42°42'34.4"S 170°58'25.8"E | WD | Hokitika | MG132475 | ||
| 230 | 11-Nov-15 | Martoni, F & Evans, H | 42°27'25.8"S 171°12'18.7"E | BR | Greymouth | MG132476 | ||
| 234 | 12-Nov-15 | Martoni, F & Evans, H | 41°45'15.8"S 171°36'57.0"E | NN | Westport | MG132477 | ||
| 236 | 12-Nov-15 | Martoni, F & Evans, H | 41°45'07.1"S 171°37'14.6"E | NN | Westport | MG132478 | ||
| 238 | 12-Nov-15 | Martoni, F & Evans, H | 41°14'47.1"S 172°06'43.3"E | NN | Karamea | MG132479 | ||
| 244 | 04-Dec-15 | Bulman, SR | 45°21'57.5"S 170°50'51.6"E | DN | Moeraki | MG132480 | ||
| 429 | 10-Sep-16 | Martoni, F | 43°09'23.4"S 172°43'50.5"E | NC | Amberley |
Microscope slide preparation of specimens of both Psylla frodobagginsi and P. apicalis follows the work of Taylor et al. [6]. Morphology of adult characters follows the work of Rendón-Mera et al. [21]. Photographs were taken using a Nikon DS-Ri2 camera connected to a Nikon SMZ25 microscope. Pictures were the result of stacking images using the software Nikon NIS-Elements D v4.5. The magnification of each picture depended upon the dimensions of the insects and of the morphological character of interest. All the plates were then prepared using the software GIMP version 2.8.14. All psyllid specimens collected, together with the holotype and some of the paratypes, have been deposited in the Lincoln University Entomology Collection (LUNZ). Additional paratypes have been deposited at the New Zealand Arthropods Collection (NZAC). The holotype of P. apicalis was provided by the NZAC.
Label data of holotypes and paratypes are reported using the conventions previously stated by Brown [22]: labels are delimited using quotes (‘…’), separate lines are indicated with a solidus (/) while all the metadata are reported in curly brackets ({…}). Reference to COI barcode molecular data gathered prior [3] is reported here for the specific specimens used in the descriptions below, except for the P. apicalis holotype (Table 1).
Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN) [23, 24], and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:346EDDD2-AA9C-4C18-8251-36A543EF941E.
The electronic edition of this work was published in a journal with an ISSN and has been archived and is available from the following digital repositories: PubMed Central and LOCKSS.
Results
From eight of the sampled locations, mostly west and south of the island, morphological examination confirmed that all the psyllids collected belonged to the same ‘large-sized’ morphospecies, later identified as Psylla apicalis (Fig 1, black squares) (see below). No other psyllid species was found on these plants. At ten other locations, mostly east and south of the island, all samples collected belonging to the ‘small-sized’ morphospecies were collected and later identified as Psylla frodobagginsi (Fig 1, black circles) (see below). Additionally, in three locations on the east and very south of the island (Fig 1, white squares), Psylla apicalis and P. frodobagginsi were collected from the same S. microphylla plant.
Taxonomy
Psylla frodobagginsi Martoni, 2019
urn:lsid:zoobank.org:act:EC986474-A6FF-4484-8E0D-11935DE60B5C (Fig 2).
Fig 2. Psylla frodobagginsi sp. nov.
Habitus dorsal view of female (a) and male (b); habitus lateral view of female (c) and male (d); wings of female (e) and male (f); head dorsal view of female (g) and male (h); lateral view of terminalia of female (i) and male (j), with particular of the aedeagus (k). Scale bar length = 500μm (a-f), 200μm (g-j) and 100μm (k).
Material examined
Holotype: ♂ deposited at the LUNZ. Entire specimen mounted on card triangle. Labels: ‘NEW ZEALAND, DN / Oamaru Botanic Gardens / 45°5’13.47”S 170°58’31.85”E / 2 Nov 2015 H. Evans, F. Martoni / On Sophora sp.’ {Printed on white card}. ‘ID 200—Martoni F. 2017 / PhD Thesis. Lincoln University, / Canterbury–New Zealand.’ {Printed on white card}. ‘HOLOTYPE ♂ / Psylla frodobagginsi / Martoni 2019’ {Printed on red card}
Paratypes: 1 ♀ deposited at the LUNZ. Entire specimen mounted on card triangle. Labels: ‘NEW ZEALAND, DN / Oamaru Botanic Gardens / 45°5’13.47”S 170°58’31.85”E / 2 Nov 2015 H. Evans, F. Martoni / On Sophora sp.’ {Printed on white card}. ‘ID 200—Martoni F. 2017 / PhD Thesis. Lincoln University, / Canterbury–New Zealand.’ {Printed on white card}. ‘PARATYPE ♀ / Psylla frodobagginsi / Martoni 2019’ {Printed on blue card}; 3 ♂, 3 ♀ deposited at LUNZ. Dissected specimens mounted on microscope slide. Labels: ‘PARATYPE / Psylla frodobagginsi / Martoni 2019’ {Hand-written on white card}. ‘NEW ZEALAND, OL Wanaka / Lake front parking. / 10 Nov 2015 –H. Evans, F. Martoni / On Sophora sp.’ {Hand-written on white card}; 3 ♂, 3 ♀ deposited at NZAC. Entire specimen mounted on card triangle. Labels: ‘PARATYPE / Psylla frodobagginsi / Martoni 2019’ {Printed on white card}. ‘NEW ZEALAND, DN Moeraki / 45°21’57.5”S 170°50’51.6”E / 04 Dec 2015 –S. Bulman / On Sophora sp.’ {Printed on white card}.
Other samples examined for this study: Countless specimens from the populations listed in Table 1 and Fig 1.
Diagnosis
Psylla frodobagginsi can be identified most easily by its small dimension, light colours, and uniformly spotted wings. Psylla frodobagginsi is smaller than P. apicalis, with males as small as 1.24mm, compared to the 1.65mm of P. apicalis, and females as small as 1.56mm, against the 2.03mm of P. apicalis. In addition, P. frodobagginsi is light coloured (Fig 2a–2d), with orange/light brown bands on pronotum, mesoprescutum, mesoscutum and abdomen, as opposed to dark brown/black bands in P. apicalis (Fig 3a–3d). Furthermore, P. apicalis presents a band of merged spots, usually black, crossing the width of the distal part of the wing and a second band, running parallel to the first one (Fig 3e and 3f), where no spots can be seen in the cells, as if the spots present here moved to compose the black band. While the colour of the dark band can vary a lot from black to light brown (as mentioned in [18]), the lack of spots in the area adjacent to the band (Fig 3e and 3f) is a clear diagnostic character that distinguish P. apicalis from P. frodobagginsi. Additionally, P. apicalis has a black spot in the cell cu1 (Fig 3e and 3f). Psylla frodobagginsi, on the contrary, presents the forewing uniformly covered in spots, without any band or darker region (Fig 2e and 2f).
Fig 3. Psylla apicalis.
Habitus dorsal view of female (a) and male (b); habitus lateral view of female (c) and male (d); wings of female (e) and male (f); head dorsal view of female (g) and male (h); lateral view of terminalia of female (i) and male (j), with particular of the aedeagus (k). Scale bar length = 500μm (a-f), 200μm (g-j) and 100μm (k).
Diagnosis is also supported by P. frodobagginsi presenting a number of characters that have a rounded shape, such as the tip of the female proctiger, the tip of the male parameres and the genal processes. In P. apicalis these are conical and pointy (Fig 3g and 3h) while in P. frodobagginsi these are rounded and more irregular, especially in the males (Fig 2g and 2h). The female proctiger of Psylla frodobagginsi ends in a rounded tip (Fig 2i), while that of P. apicalis appears to be slightly pointy (Fig 3i). Similarly, the apical part of the male parameres that are more rounded in P. frodobagginsi (Fig 2j and 2k), while they are pointy in P. apicalis (Fig 3j and 3k).
Colouration
Body light coloured, mostly yellowish/orange. Head yellow and pronotum of similar colour except for four darker spots (two at each extremity). Antennae yellow with apical part of segments 1–6 darker and segments 7 and 8 completely dark brown/black. Genal processes yellow and eyes just slightly darker. Mesothoracic prescutum of the same colour except for two triangular darker spots (orange/brown) in frontal position. Mesothoracic scutum yellowish orange with four dark bands running parallel to the head/terminalia axis. Abdomen yellowish/orange with darker bands on each segment. Wings are hyaline uniformly spotted. Both male and female terminalia are orange/brown.
Structure
Body very small, with females well under 2mm and males under 1.5mm. Head: vertex generally twice as wide as long. Genal processes short and quite rounded, more so in males than females. Antennae moderately long, more so in males (up to 1.3 times the width of the head) than in females (maximum 1.1 times). Thorax short and broad. Fore wing at least three times as long as width of head and between 2.2 and 2.4 times as long as wide. Veins Rs longer than M, and M1+2 on average half as long as vein M, but quite variable.
Male terminalia quite slender, with proctiger longer than parameres. Parameres thin and slender internally covered in setae that, in lateral view, can be seen protruding frontally and caudally. The tip of the parameres is rounded and pointing caudally. Female terminalia are straight and moderately long with proctiger longer than the subgenital plate (up to 1.36 times), wider in the proximal part and narrow to the point with a rounded bump separating the circum-anal ring from the narrower tip of the proctiger.
Measurements
Measurements are in mm (5 ♂, 5 ♀). Length of body (vertex to terminalia) ♂ 1.24–1.41, ♀ 1.56–1.72; length of body (vertex to apex of folded wings) ♂ 1.67–1.88, ♀ 1.98–2.11; width of head (HW) ♂ 0.52–0.54, ♀ 0.56–0.60; length of genal processes (GCL) ♂ 0.09–0.10, ♀ 0.10–0.11; length of vertex (VL) ♂ 0.16–0.18, ♀ 0.18–0.20; width of vertex (VW) ♂ 0.32–0.35, ♀ 0.36–0.41; length of antenna (AL) ♂ 0.64–0.72, ♀ 0.61–0.65; length of fore wing ♂ 1.62–1.66, ♀ 1.79–1.88; width of fore wing ♂ 0.69–0.74, ♀ 0.77–0.83; length of vein Rs ♂ 0.86–1.00, ♀ 1.05–1.09; length of vein M (M) ♂ 0.66–0.82, ♀ 0.64–0.88; length of vein M1+2 (M1) ♂ 0.34–0.39, ♀ 0.32–0.52; marginal width of cell m1 ♂ 0.18–0.24, ♀ 0.20–0.24; marginal width of cell cu1 ♂ 0.36–0.43, ♀ 0.40–0.44; length of vein Cu1b ♂ 0.20–0.26, ♀ 0.24–0.28; length (height) of proctiger (PL) ♂ 0.24–0.28; length of paramere ♂ 0.17–0.18; length of proximal aedeagal segment ♂ 0.30–0.34; length of distal aedeagal segment ♂ 0.11–0.16; length of proctiger (PL) ♀ 0.32–0.34; length of circumanal ring (CL) ♀ 0.16–0.17; length of subgenital plate (SL) ♀ 0.24–0.28.
Ratios
GCL:VL ♂ 0.50–0.59, ♀ 0.53–0.61; VL:VW ♂ 0.47–0.56, ♀ 0.47–0.53; VL:HW ♂ 0.30–0.35, ♀ 0.31–0.34; AL:HW ♂ 1.23–1.33, ♀ 1.05–1.12; PL:HW ♂ 0.46–0.52, ♀ 0.55–0.61; PL:CL ♀ 1.94–2.12; PL:SL ♀ 1.21–1.36; WL:HW ♂ 3.00–3.17, ♀ 3.08–3.24; WL:WW ♂ 2.24–2.39, ♀ 2.19–2.32; Rs:M ♂ 1.20–1.36, ♀ 1.20–1.70; M1:M ♂ 0.41–0.59, ♀ 0.36–0.81.
Etymology
The name Psylla frodobagginsi refers to Frodo Baggins, one of the main characters of J. R. R. Tolkien’s literary trilogy commonly referred to as “The Lord of the Rings” [25, 26, 27]. In the books, Frodo Baggins is a hobbit, a member of a fictitious race similar to humans, but of smaller size. The name was chosen due to the smaller size of this psyllid species, together with the fact it is native to New Zealand. The cinematographic sets for P. Jackson’s movie trilogy “The Lord of the Rings” were for the majority placed in the South Island, reflecting the proportional distribution of the psyllid’s endemic host Sophora microphylla.
Distribution
Psylla frodobagginsi was recorded in the South Island of New Zealand from a total of 13 locations (Fig 1, Table 1). From three of these, this species was present at the same time on the same individual Sophora microphylla plant as P. apicalis. No collections were performed in the North Island. However, due to the presence of kōwhai there, P. frodobagginsi is hypothesised to be present, as well.
Host plant
All the populations examined in this study were collected from the plant Sophora microphylla, the small-leaved kōwhai, a plant endemic to New Zealand. Nonetheless, with previous records of P. apicalis collected also from S. tetraptera [17, 18] and S. prostrata Buchanan [17], these plants are to be considered possible host plants, too. However, probably due to the time of the year when collections were performed, no immature life stages were found. Such a lack of nymphs would suggest caution in the host plant assessment. Generally, in fact, a psyllid host plant is defined as ‘the plant on which psyllids can complete their life cycle, from nymphs to adults’ [28]. Nonetheless, considering the high number of adult individuals collected on the same plant species at a wide range of locations, and the absence of Psylla frodobagginsi on other plants previously examined [3], Sophora microphylla is hypothesised here as the host plant of P. frodobagginsi.
Psylla apicalis (Ferris and Klyver, 1932)
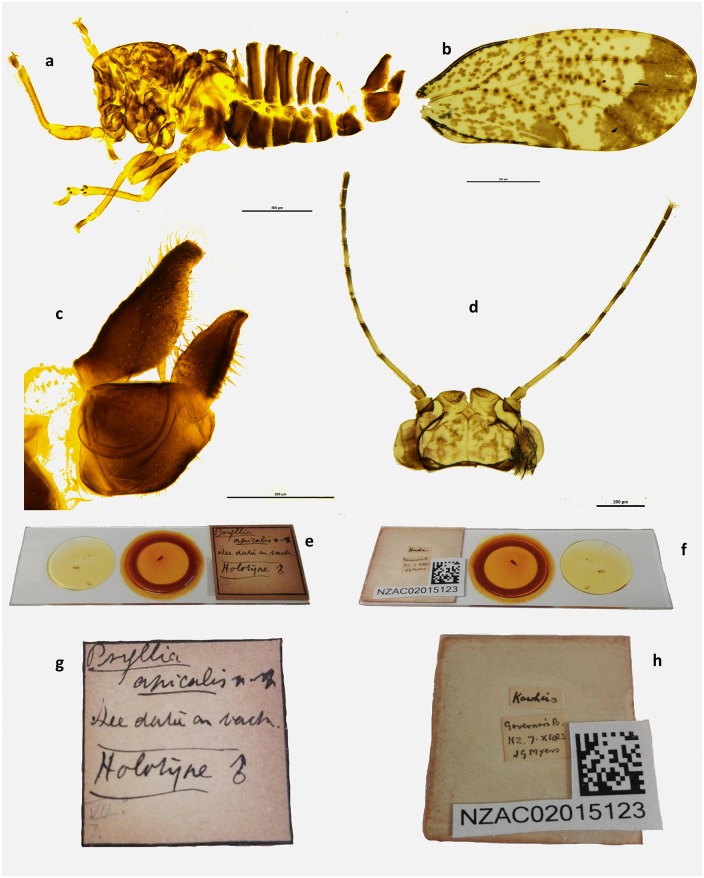
Fig 4. Psylla apicalis holotype (male).
Habitus lateral view (a); wing (b); terminalia lateral view (c); head dorsal view (d); slide front (e) and back (f) with frontal (g) and posterior (h) labels. Scale bar length = 500μm (a-b) and 200μm (c-d).
Material examined
Holotype: ♂ preserved at the NZAC. Dissected specimen mounted on microscope slide. Labels: ‘Psyllia (sic)/ apicalis n. sp. / see data on back / holotype ♂’ {hand written on white card, on front}. ‘Kōwhai / Governors’ B. / NZ. 7.X.1922 / JG Myers’ {hand written on white card, on back}. ‘NZAC02015123’ {typed on white card, on back} (Fig 4).
Samples measured for this study: 5 ♂, 5 ♀ deposited at LUNZ. Dissected specimens mounted on microscope slide. Labels: ‘NEW ZEALAND, MC, Christchurch / Canterbury University campus / 01 September 2015 / on Kōwhai, SDJ Brown’ {printed on white card}. (Fig 3).
Other samples examined for this study: Countless specimens from the populations listed in Table 1 and Fig 1.
Updated description
Colouration
Body dark orange/black, with head and thorax showing black striped on a dark orange background. Head orange uniformly covered in darker spots. Eyes dark orange/brown. Genal processes brown to black. Pronotum darkened by the presence of seven darker spots (three at each extremity and one in the centre). Antennae yellow with apical part of segments 1–6 darker and segments 7 and 8 completely dark brown/black. Mesothoracic prescutum presenting the same two triangular darker spots of P. frodobagginsi with the addition of a multitude of black spots (Fig 3a and 3b). Mesothoracic scutum orange/brown with four black bands running parallel to the head/terminalia axis and a thin black line in the middle. Abdomen yellowish/orange with black wide bands on each segment that almost completely cover the yellow colour. Wings are hyaline, with dark brown-black spots that are not uniformly distributed. Spots merge to form a black diagonal band crossing the wing from cell cu1 to r1 and a black spot in the cu2 cell. The colour of band and spot can be lighter at times, almost resembling the colouration of P. frodobagginsi, but in P. apicalis there is an additional contiguous band, parallel to the dark one, that shows an absence of spots except on the veins. This clearer band on the wing is not hyaline, but completely transparent. Both male and female terminalia are dark brown to black.
Structure
Body larger than P. frodobagginsi, with males of this species generally bigger than females of P. frodobagginsi. All measurements were generally greater than those of P. frodobagginsi, some with no overlap of the ranges and showing a clear gap; in particular the length of the body for both sexes. On the other hand, ratios appear similar to those of P. frodobagginsi except for the Rs:M in males where that for P. frodobagginsi were between 1.20mm–1.36mm while those of P. apicalis are between 1.43mm–1.53mm.
Measurements
Measurements are in mm (5 ♂, 5 ♀) and the measurements of the Holotype (♂, Fig 4) are reported in brackets in bold, when it was possible to make the measurement. Length of body (vertex to terminalia) ♂ 1.65–1.90, ♀ 2.03–2.24; length of body (vertex to apex of folded wings) ♂ 2.15–2.43, ♀ 2.59–2.74; width of head (HW) ♂ 0.60–0.67 (0.63), ♀ 0.66–0.71; length of genal processes (GCL) ♂ 0.12 (0.1), ♀ 0.13–0.14; length of vertex (VL) ♂ 0.17–0.21 (0.2), ♀ 0.22–0.23; width of vertex (VW) ♂ 0.38–0.40 (0.41), ♀ 0.40–0.51; length of antenna (AL) ♂ 0.93–1.02 (1.11), ♀ 0.89–0.91; length of fore wing ♂ 1.88–1.98 (1.92), ♀ 2.09–2.40; width of fore wing ♂ 0.79–0.86 (0.84), ♀ 0.92–1.05; length of vein Rs ♂ 1.10–1.23 (1.20), ♀ 1.28–1.39; length of vein M (M) ♂ 0.75–0.86 (0.83), ♀ 0.87–0.96; length of vein M1+2 (M1) ♂ 0.42–0.51 (0.47), ♀ 0.49–0.62; marginal width of cell m1 ♂ 0.23–0.27 (0.20), ♀ 0.24–0.32; marginal width of cell cu1 ♂ 0.40–0.51 (0.48), ♀ 0.50–0.55; length of vein Cu1b ♂ 0.24–0.28 (0.28), ♀ 0.30–0.37; length (height) of proctiger (PL) ♂ 0.31–0.33 (0.33); length of paramere ♂ 0.20–0.22 (0.22); length of proximal aedeagal segment ♂ 0.41–0.45 (0.44); length of distal aedeagal segment ♂ 0.18–0.21 (0.21); length of proctiger (PL) ♀ 0.38–0.42; length of circumanal ring (CL) ♀ 0.17–0.21; length of subgenital plate (SL) ♀ 0.30–0.35.
Ratios
GCL:VL ♂ 0.57–0.71 (0.50), ♀ 0.56–0.61; VL:VW ♂ 0.45–0.52 (0.51), ♀ 0.45–0.57; VL:HW ♂ 0.27–0.32 (0.33), ♀ 0.32–0.34; AL:HW ♂ 1.50–1.58 (1.33), ♀ 1.32–1.35; PL:HW ♂ 0.46–0.53 (0.48), ♀ 0.57–0.59; PL:CL ♀ 1.90–2.28; PL:SL ♀ 1.14–1.27; WL:HW ♂ 2.92–3.27 (3.05), ♀ 3.12–3.64; WL:WW ♂ 2.26–2.48 (2.29), ♀ 2.25–2.35; Rs:M ♂ 1.43–1.53 (1.45), ♀ 1.39–1.53; M1:M ♂ 0.49–0.65 (0.57), ♀ 0.53–0.66.
Discussion
The results presented here enable the morphology of Psylla frodobagginsi to be distinguished from that of P. apicalis. This is in agreement with recently presented molecular data [3] where a COI divergence of 7–8% suggested the presence of two separate taxa on Sophora. Furthermore, this work finally allows the doubts presented by Ferris and Klyver [18], when first describing P. apicalis 87 years ago, to be resolved and confirms that there are two psyllid species on kōwhai. With Psylla frodobagginsi, there are now 74 described psyllid species present in New Zealand of which 41 are endemic.
In a taxonomical context, the description of P. frodobagginsi highlights the need of a review of the genus Psylla in New Zealand. Firstly, because the number of species is suspected to be higher than the three species described so far, possibly including a number of undescribed taxa found associated with Carmichaelia spp., another group of the Fabaceae native to New Zealand (as previously suggested [3]). Secondly, based on divergent morphological and molecular characters, these species probably do not belong to the European genus Psylla at all, and they will need to be moved under a new New Zealand endemic genus (as previously suggested [8]). This is consistent with early quandaries about attribution of the two New Zealand described species to the Psylla. While Tuthill, who described P. carmichaeliae in 1952 (Tuthill 1952) found the species congeneric to P. apicalis he also highlighted similarities between both New Zealand species and Euphalerus nidifex Schwarz [29]. Nonetheless, he did not consider this sufficient to move the New Zealand species to Euphalerus [29]. Later, in 1985, Dale re-examined P. apicalis and P. carmichaeliae and moved them to the existing genus Euphalerus (Macrocorsinae) [17]. However, Euphalerus was subsequently redefined to include only species from the New World [30], leaving the New Zealand species in a genus requiring revision. Thus, the original doubt of Tuthill [29] was confirmed, that the Australasian species considered Euphalerus were not congeneric with the type species E. nidifex from Central America. Tuthill’s uncertainty was mostly due to morphological variation in the last instar of the immature life stages of the two New Zealand species, P. apicalis and P. carmichaeliae. However, characters such as the eight-segmented antennae and the marginal setae on the caudal plate, would eventually contribute to placing these taxa in the subfamily Psyllinae, but outside Psylla [8]. This, together with the new information in this current description, therefore supports the accumulation of evidence that a broader, world-wide study is necessary to elucidate the taxonomy of the family Psyllidae, comprising Arytainilla, Heteropsylla, Baeopelma, Psylla sensu stricto, and the New Zealand ‘Psylla’.
The South Island-wide geographic distribution of the specimens collected, together with the presence of both Psylla apicalis and P. frodobagginsi on the same individual plant in multiple locations, suggests that both species are occupying the same ecological niche. It remains quite unusual for the same plant to host multiple psyllid species [31]). However, in New Zealand this has been observed for other species, such as the adventives Psyllopsis fraxini and P. fraxinicola, both hosted by Fraxinus excelsior [8]. On the other hand, polyphagy appear to be a more common trait in psyllid pest species such as Bactericera cockerelli, the tomato potato psyllid (TPP), and Diaphorina citri, the Asian citrus psyllid [1]. The overlapping distribution of the two species associated with kōwhai, together with the small size of psyllids making it difficult to appreciate their distinctiveness outside of dedicated taxonomic studies such as this, might be the main reason why they have been considered the same species until today. The misconception has likely been perpetuated by the common name “kōwhai psyllid” which has been used indiscriminately to refer to any psyllid found on S. microphylla. Without ongoing, targeted taxonomic studies, species such as P. frodobagginsi effectively ‘hide in plain sight’ contrary to a common notion that new species are only to be found in unchartered or non-urban environments. Here we propose to refer to P. frodobagginsi as the “hobbit kōwhai psyllid”, as compared to P. apicalis that could become the “greater kōwhai psyllid”. Finally, the focus of this study on South Island was largely a consequence of S. microphylla, which is the most common of the eight Sophora species, in the wild being largely restricted to the South Island. However, as New Zealand’s national plant it is a common ornamental throughout the country. Therefore, additional sampling to include the North Island would provide some interesting information not only about the true distribution of both of these kōwhai-hosted psyllids in New Zealand, but also about how well, or consistently, despite a large range in climatic conditions they share the same ecological niche.
Acknowledgments
The authors would like to thank Martin Steinbauer (La Trobe University, Melbourne) and an anonymous reviewer for comments and suggestions that helped improving a previous version of this manuscript. Thanks to Lincoln University for funding the publication of this manuscript. The basemap of distribution map (Fig 1) was provided by Roger Smith, courtesy of Geographx (https://geographx.co.nz). Thanks to Samuel DJ Brown and Simon R Bulman (The New Zealand Institute of Plant and Food Research), and Mike Bowie (Lincoln University) for providing specimens. Thanks to Hannah Evans for the help during field collections. Thanks to Professor Phil Lester (Victoria University, Wellington), for the encouragements in pursuing the idea of describing this species; and to John Marris (LUNZ), for the helpful comments and suggestions. Many thanks to Marie-Claude Lariviere (NZAC) for providing the holotype of Psylla apicalis. Part of the data published in this article was reported in FM’s PhD thesis [32].
Data Availability
All relevant data are within the manuscript.
Funding Statement
Thanks to Lincoln University for funding the publication of this manuscript. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ouvrard D (2019) Psyl’list—The World Psylloidea Database. http://www.hemiptera-databases.com/psyllist - searched on 31 March 2019
- 2.Hollis D (2004) Australian Psylloidea. Jumping plantlice and lerp insects. Australian Biological Resources Study, Camberra (Australia), 216 pp.
- 3.Martoni F, Bulman SR, Pitman A, Taylor G, Armstrong K (2018) DNA barcoding highlights cryptic diversity in the New Zealand Psylloidea (Hemiptera: Sternorrhyncha). Diversity 50, 10 10.3390/d10030050. [DOI] [Google Scholar]
- 4.Burckhardt D, Ouvrard D (2012) A revised classification of the jumping plant-lice (Hemiptera: Psylloidea). Zootaxa 3509: 1–34. [Google Scholar]
- 5.Syfert MM, Serbina L, Burckhardt D, Knapp S, Percy DM (2017) Emerging New Crop Pests: Ecological Modelling and Analysis of the South American Potato Psyllid Russelliana solanicola (Hemiptera: Psylloidea) and Its Wild Relatives. PLOS ONE 12(1): e0167764 10.1371/journal.pone.0167764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor GS, Fagan-Jeffries EP, Austin AD (2016) A new genus and twenty new species of Australian jumping plant-lice (Psylloidea: Triozidae) from Eremophila and Myoporum (Scrophulariaceae: Myoporeae). Zootaxa 4073(1): 1–84 [DOI] [PubMed] [Google Scholar]
- 7.Taylor GS (2018) New species of Acizzia (Hemiptera: Psyllidae) from an Australian endemic Solanum (Solanaceae). Austral Entomology, 57: 297–302, 10.1111/aen.12278 [DOI] [Google Scholar]
- 8.Martoni F, Burckhardt D, Armstrong K (2016) An annotated checklist of the psyllids of New Zealand (Hemiptera: Psylloidea). Zootaxa 4144: 556–574 [DOI] [PubMed] [Google Scholar]
- 9.Martoni F, Bulman SR, Pitman A & Armstrong K. Elongation Factor-1α Accurately Reconstructs Relationships Amongst Psyllid Families (Hemiptera: Psylloidea), with Possible Diagnostic Implications. Journal of Economic Entomology 2017; 110: 2618–2622. 10.1093/jee/tox261 [DOI] [PubMed] [Google Scholar]
- 10.Martoni F, Brown SDJ (2018) An annotated checklist of the Cook Islands psyllids with keys to the species and two new records (Hemiptera, Psylloidea). ZooKeys 811: 91–108. 10.3897/zookeys.811.28829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Percy DM (2017) Making the most of your host: The Metrosideros-feeding psyllids (Hemiptera: Psylloidea) of the Hawaiian Islands. ZooKeys 649: 1–163. 10.3897/zookeys.649.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Percy DM (2018) Revision of the Hawaiian psyllid genus Swezeyana, with descriptions of seven new species (Hemiptera, Psylloidea, Triozidae). ZooKeys 2018, 758: 75–113. 10.3897/zookeys.758.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Percy DM, Crampton-Platt A, Sveinsson S, Lemmon AR, Moriarty Lemmon E, Ouvrard D et al. (2018) Resolving the psyllid tree of life: Phylogenomic analyses of the superfamily Psylloidea (Hemiptera). Systematic Entomology 43(4): 762–776. 10.1111/syen.12302 [DOI] [Google Scholar]
- 14.Cho G, Malenovský I, Lee S (2019) Higher-level molecular phylogeny of jumping plant lice (Hemiptera: Sternorrhyncha: Psylloidea). Systematic Entomology 10.1111/syen.12345 30988552 [DOI] [Google Scholar]
- 15.Henderson R, Teulon DAJ, Dale PJ, Eastop VF, Stufkens MAW (2010) Order Hemiptera, Suborder Sternorrhyncha In: Gordon D.P. (Ed.), New Zealand Inventory of Biodiversity. Volume two. Kingdom Animalia. Chaetognatha, Ecdysozoa, Ichnofossils; Canterbury University Press, Canterbury, New Zealand, pp. 400–406. [Google Scholar]
- 16.Martoni F, Armstrong KF (2019) Acizzia errabunda sp. nov. and Ctenarytaina insularis sp. nov.: descriptions of two new species of psyllids (Hemiptera: Psylloidea) discovered on exotic host plants in New Zealand. PLOS ONE 10.1371/journal.pone.0214220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale PJ (1985) A review of the Psylloidea (Insecta: Hemiptera) of the New Zealand subregion. Unpublished PhD thesis. The University of Auckland, 628 pp.
- 18.Ferris GF, Klyver FD (1932) Report upon a Collection of Chermidae (Homoptera) from New Zealand. Transactions of the New Zealand Institute 63: 34–61. [Google Scholar]
- 19.Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003) Biological identifications through DNA barcodes. Procedings of the Roayal Society B -Biological Sciences 270: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosby TK, Dugdale JS, Watt JC (1998) Area codes for recording specimen localities in the New Zealand subregion. New Zealand Journal of Zoology 25: 175–183. [Google Scholar]
- 21.Rendón-Mera DI, Serna F, Burckhardt D (2017) Generic synopsis of the jumping plant-lice (Hemiptera: Sternorrhyncha: Psylloidea) from Colombia. Zootaxa 4350 (3): 436–468. 10.11646/zootaxa.4350.3.2 [DOI] [PubMed] [Google Scholar]
- 22.Brown SDJ (2017) Austromonticola, a new genus of broad-nosed weevil (Coleoptera, Curculionidae, Entiminae) from montane areas of New Zealand. ZooKeys, 707: 73–130. 10.3897/zookeys.707.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ICZN (1999) International Code of Zoological Nomenclature. Fourth Edition The International Trust for Zoological Nomenclature, London, UK, 306 pp. [Google Scholar]
- 24.ICZN (2012) Amendment of Articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa 2012; 3450: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolkien JRR (1954a) The fellowship of the ring. George Allen and Unwin Publishers, United Kingdom: 423 pp. [Google Scholar]
- 26.Tolkien JRR (1954b) The two towers. George Allen and Unwin Publishers, United Kingdom: 352 pp [Google Scholar]
- 27.Tolkien JRR (1955) The return of the king. George Allen and Unwin Publishers, United Kingdom: 416 pp [Google Scholar]
- 28.Burckhardt D, Ouvrard D, Queiroz D, Percy D (2014) Psyllid host-plants (Hemiptera: Psylloidea): resolving a semantic problem. Florida Entomologist 97: 242–246. [Google Scholar]
- 29.Tuthill LD (1952) On the Psyllidae of New Zealand (Homoptera). Pacific Science 6, 83–12. [Google Scholar]
- 30.Hollis D, Martin JH (1997) Jumping plantlice (Insecta: Hemiptera) attacking Lonchocarpus species (Leguminosae), including “Black Cabbage Bark”, in Belize. Journal of Natural History 31, 237–267. [Google Scholar]
- 31.Ouvrard D, Chalise P & Percy DM. Host-plant leaps versus host-plant shuffle: a global survey reveals contrasting patterns in an oligophagous insect group (Hemiptera, Psylloidea). Systematics and Biodiversity 2015, 1–21. 10.1080/14772000.2015.1046969. [DOI] [Google Scholar]
- 32.Martoni F. Biodiversity, evolution and microbiome of the New Zealand Psylloidea (Hemiptera: Sternorrhyncha). Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2017, 279 pp. https://hdl.handle.net/10182/9340.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.