INTRODUCTION: THE NEED FOR ADDICTION CONSULT SERVICES IN THE GENERAL HOSPITAL SETTING
Substance use disorders (SUDs) affect 40 million Americans and place an enormous burden on society, with a cost of $740 billion annually.1,2 Hospitalization is an increasingly frequent and costly occurrence among individuals with an SUD, with almost one-quarter of hospitalized patients having an SUD, presenting a huge opportunity for intervention.3,4 Despite this, only a minority of patients receive any treatment of SUDs in a hospital setting.5
Previous research has demonstrated not only the feasibility of providing addiction treatment to individuals in an acute care setting but also its efficacy. A recent meta-analysis demonstrated brief intervention for unhealthy alcohol use in the hospital as associated with a significant reduction in weekly alcohol consumption 6 months later.6 Furthermore, hospitalized individuals with an alcohol use disorder have been shown more likely to participate in outpatient addiction treatment if they receive motivational interviewing and facilitated referral to treatment.7 Similarly, pharmacotherapy initiation for alcohol, tobacco, or opioid use disorder in acute care settings has been associated with several positive outcomes, including a reduction in substance use, improvement in treatment retention, and a decrease in hospital readmission rates.8–10 More specifically, 1 study of 302 individuals with an opioid use disorder initiated on methadone maintenance therapy in a hospital demonstrated an 82% follow-up rate to an outpatient methadone maintenance program.11 Given the high prevalence of SUDs and recurring need to access medical care, hospitalization offers a critical opportunity for effective intervention and provision of evidence-based addiction treatment to dramatically reduce morbidity and mortality.
To date, an important barrier identified in the delivery of evidence-based addiction treatment pertains to many health care providers overall feeling ill equipped to accurately screen for, diagnose, or treat SUDs.12,13 In an attempt to overcome this in hospital, 1 strategy has been the creation of an inpatient addiction consult service (ACS). Although varied in the nature and background of program clinical staff, patient recruitment strategies, and interventions offered, all ACSs share a common goal of reducing morbidity and mortality associated with SUDs by improving access to evidence-based addiction treatment while in a hospital and successfully transitioning individuals from acute to community settings. Hospital-based ACSs can further provide support, effective role modeling, and education about SUD interventions, which, in turn, may improve preparedness among health care providers in managing SUDs, reducing stigma associated with the condition, and ultimately improving clinical practice.14
CURRENT ADDICTION CONSULT SERVICE MODELS
To date, more than half a dozen hospital-based ACSs exist within North America. A range of model types, including different strategies of patient identification and team composition are described below.
Patient Identification
Although some hospitals use a universal screening approach for substance misuse or an SUD with validated tools, such as the Alcohol Use Disorders Identification Test, a majority of ACS referrals occur directly from the primary admitting medical or surgical team according to clinical judgment.
Team Composition
Although some pioneering consult services relied solely on a physician, current models often involve additional staffing, with compositions varying according to the needs and resources of each institution. A physician is essential to manage pharmacotherapy for withdrawal symptoms and long-term treatment. In addition, a nurse may assist with day-to-day clinical assessments and symptom management, whereas a social worker, nurse care manager, or resource specialist might assist with psychosocial interventions and community care transitions. A recovery coach or peer navigator may assist with motivational interviewing and patient navigation. Because many ACSs operate in an academic setting, often trainees are part of the team, including medical students, residents, and addiction fellows (medicine or psychiatry).
THE ADDICTION CONSULT
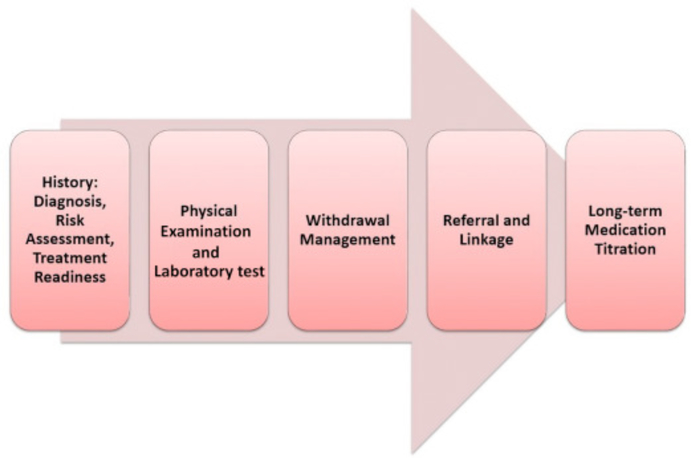
An individual who is referred for an addiction consult receives a comprehensive addiction history and physical examination (Fig. 1).
Figure 1.
Components of an addiction consult
History
A complete addiction history is important for confirmation of a substance use disorder, to assess risk for withdrawal, and to evaluate an individual’s readiness for addiction treatment. Patient medical comorbidities, number of active substance use disorders, and social circumstances have an impact on the ease and ability for providers to recommend and link patients to addiction treatment. An addiction history can be challenging to elicit, because it includes questions focused on stigmatized behaviors; accordingly, the establishment of a therapeutic relationship between patient and health care provider is of critical importance and can be aided by using nonjudgmental, stigma-free language.15 In addition, much of this history may be obtained when a patient is actively in withdrawal and distress; thus, it is also essential to focus the consult on the components that most directly have an impact on treatment decision making (see Table 1).
Table 1.
Questions to ask as part of the addiction consult history
| • What age was your first use? How much and how frequently were you using? How did you use initially (e.g. inhalation, insufflation, oral, injection etc.)? |
| • How much are you using currently? How frequently and by what route? |
| • Are you requiring more of the substance (e.g. amount or frequency) to achieve the same effect compared to your time of first use? |
| • Do you currently have cravings to use? |
| • When you stop using, how do you feel? |
| • Do you spend a lot of your day using or recovering from its effects? |
| • Have you experienced any negative consequences from your use? This may include: interactions with law enforcement, development of a medical complication, deterioration of personal or professional relationships, loss of employment, cessation of hobbies or other activities previously important to you. |
| • Have you ever engaged in dangerous behavior while using? This may include: driving, operating heavy machinery etc. |
| • Have you ever previously tried to cut down or quit your use? Were you successful? If so, how did you achieve this and what was your longest period of abstinence? What caused you to relapse? |
Substance use disorder—diagnosis and severity
A positive screen for unhealthy substance use requires assessment for a SUD using the 11 criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), including asking explicitly about cravings, tolerance, withdrawal, loss of control and consequences of use in the past 12 months.16 Patients who meet 2–3 criteria have a mild use disorder, 4–5 a moderate use disorder and 6 or more a severe disorder.16 Should an individual screen positive for more than one substance use disorder, the criteria should be assessed individually for each substance.
General risk assessment
A detailed assessment of associated risks should be undertaken as part of the addiction history. Specifically, patients should be asked about any previous or ongoing injection drug use, including inquiry about injection practices and risk for infectious diseases. Lastly, overdose risk and use of harm reduction services should be included (Table 2).
Table 2.
Risks associated with substance use and questions to ask as part of the addiction history
| Substance Use—Associated Risks | Questions to Ask in the Addiction History |
|---|---|
| Injection drug use | • Have you ever injected drugs? If so, do you currently inject? Approximately how frequently? |
| • Do you use clean injecting equipment? Have you ever previously shared injecting equipment? | |
| Infectious diseases (eg, HIV and hepatitis) | • Have you previously been tested for HIV or hepatitis? |
| • When was your last test and what was the result? | |
| • Do you ever exchange sex for drugs or money? | |
| Overdose risk | • Do you use alone? |
| • Have you previously overdosed? If so, approximately how many times? When was your last overdose? | |
| • Have you ever witnessed anyone else overdose? | |
| • Do you have a naloxone kit? | |
| • Do you ever take a test dose before using to assess your drug’s potency? | |
| Harm reduction services | • Do you access needle exchange programs or supervised consumption sites? |
Readiness for addiction treatment
Evaluating readiness for treatment is critical in developing an appropriate management plan. A good starting point is a patient’s previous treatment history, including treatment strategies (both pharmacologic and psychosocial) that were successful for a period of time and those that were unsuccessful, as an opportunity for discussion about a patient’s current goals and treatment options. Furthermore, having a patient identify previous periods of abstinence or reduced substance use offers the health care provider the opportunity to enact motivational interviewing techniques to instill confidence in patients of their ability to achieve such an outcome again (Table 3).
Table 3.
Readiness for addiction treatment and questions to ask in the addiction history
| Readiness for Addiction Treatment | Questions to Ask in the Addiction History |
|---|---|
| Previous addiction treatment | • Have you previously received any addiction treatment? This may include detox, medications, support groups, or recovery homes. |
| • If so, what types of treatment have you previously tried and approximately how many times? | |
| • How long ago was your last addiction treatment and what kind of treatment were you receiving? | |
| Previous abstinence or reduced substance use | • Have you ever previously been able to cut down your substance use or stop all together? |
| • If so, how many times? How did you achieve this and what was your longest period of abstinence or reduced substance use? | |
| • Was any or some of this time during a period of incarceration or other forced abstinence? | |
| Relapse history | • If you have had periods of abstinence or reduced substance use previously, what caused you to relapse? Can you identify any potential triggers? |
| Current goals for treatment | • What are your current treatment goals (eg, total abstinence vs reduction in substance use vs harm reduction)? |
Social history
A detailed social history, specifically understanding if an individual is housed, on income assistance, and has a strong network of support (eg, friends and family), can provide helpful collateral when formulating a management plan.
Physical examination
All patients should be assessed for signs of withdrawal or intoxication as well as medical complications of use (Table 4).
Table 4.
Addiction-focused physical examination
| Vital signs | Pulse, blood pressure, respiratory rate, temperature, and oxygenation |
|---|---|
| Eye, ears, nose, and throat | Pupil size and scleral icterus |
| Cardiology | New or increased murmur |
| Pulmonary | Rales, wheeze, or decreased breath sounds |
| Abdominal | Hepatosplenomegaly, a nodular liver, and ascites |
| Genitourinary | Testicular size and gynecomastia |
| Lymph nodes | Lymphadenopathy |
| Skin | Diaphoresis, piloerection, abscesses, thrombophlebitis, Roth spots, Janeway lesions, jaundice, palmar erythema, and spider angioma |
| Neurologic/psychiatric | Mental status, delirium, hallucinations, restlessness, and anxiety |
Laboratory testing
Baseline laboratory assessment, including complete blood cell count, comprehensive metabolic panel, and coagulation studies, can help assess for safety of potential pharmacologic treatments (because some medications require dose adjustments or are contraindicated based on severity of liver or kidney disease) as well as sequelae of substance use.
Infectious disease testing, including HIV and hepatitis screening, should be offered.17–19 Full sexually transmitted disease screening and HIV pre-exposure prophylaxis also may be offered.20,21
A comprehensive urine drug test, including synthetic and semisynthetic opioids (eg, buprenorphine and fentanyl), can assist in confirming recent substance use as well as reveal substances that a patient has not reported. Urine drug testing may be helpful in harm reduction counseling, if, for example, a patient was not aware of using heroin contaminated with fentanyl.
Withdrawal risk and management
Assessing a patient’s risk for withdrawal and promptly treating the symptoms is a clinical priority. Suboptimal withdrawal symptom management has been shown a prominent reason for leaving a hospital against medical advice.22 Conversely, appropriate withdrawal treatment and subsequent linkage to outpatient treatment are important strategies to help support patients to comply with their medical care.23,24
Nicotine withdrawal
Nicotine cessation or a reduction in use may result in cravings and symptoms of the nicotine withdrawal syndrome (Table 5).16 Nicotine dependence from cigarette smoking can be quantified using the Fagerstrom Test for Nicotine Dependence.25 During hospitalization, a patient should be offered medications to treat nicotine withdrawal, including nicotine replacement therapy, with a starting dose chosen based on the Fagerstrom score and self-reported quantity of cigarettes used daily.
Table 5.
Nicotine withdrawal syndrome—symptoms to ask about as part of the addiction history and predicted onset of symptoms following smoking cessation
| Nicotine Withdrawal Syndrome Symptoms | Timing of Predicted Nicotine Withdrawal Syndrome |
|---|---|
| • Anxiety | After smoking cessation: |
| • Irritability | • 72 h—peak |
| • Restlessness | • 3–4 wk—subside |
| • Increased appetite and weight gain | |
| • Changes in mood (dysphoria or depression) | |
| • Insomnia | |
Alcohol withdrawal
Alcohol cessation or a reduction in use may result in cravings and symptoms of the alcohol withdrawal syndrome (AWS). AWS ranges from minor withdrawal symptoms to seizures and delirium tremens. Minor withdrawal can begin within 6 hours of the last drink, and includes anxiety, headache diaphoresis nausea insomnia and tachycardia.26,27 Severe AWS is associated with increased morbidity and mortality and may manifest as seizures, alcoholic hallucinosis and/or delirium tremens.
Generalized tonic-clonic seizures can begin within 6 hours of the last drink. Alcohol hallucinosis presents after 12 hours from the last drink with visual, auditory and/or tactile hallucinations, but with intact orientation and normal vital signs.26 Delirium tremens, including disorientation, confusion, hypertension fever and agitated can begin after 48 hours from the last drink.26 Not all individuals with an alcohol use disorder experience severe symptoms of AWS. The Prediction of Alcohol Withdrawal Severity Scale is an instrument validated for use among hospitalized patients and may be used to assess the risk for developing severe, complicated withdrawal.28
The gold standard of treatment of individuals identified at high risk for severe, complicated alcohol withdrawal management is benzodiazepines to decrease the risk of delirium and seizures.26,29,30 Benzodiazepines can be administered as a front-loading regimen, fixed-dose taper, or by symptom-triggered scale. Front- loading regimens include administering diazepam, 20 mg every 2 hours, until withdrawal symptoms have abated.30 Fixed-dose tapers typically involve higher doses of benzodiazepines (eg, chlordiazepoxide, 100 mg, or diazepam, 20 mg) every 6 hours for at least 1 day, with scheduled decreasing doses over subsequent days and additional medication as needed. Symptom-triggered therapy can allow for shorter treatment duration and decreased amount of total medication required.26 The revised Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) is the most commonly used scale to assess withdrawal symptoms, with medication administered as frequently as every hour when the CIWA-Ar score is greater than or equal to 8.31 Not all patients, however, are medically appropriate for symptom-triggered monitoring. The evaluation of the CIWA-Ar scale does not include patients with a seizure history, thus these patients should receive at least 1 prophylactic benzodiazepine dose, even if asymptomatic.32 Furthermore, patients who are unable to communicate easily with nursing staff (eg, non–English-speaking or dementia patients) may not be able to answer subjective questions reliably and thus may not be appropriate for CIWA-Ar. Phenobarbital may be used either in addition or instead of benzodiazepines, especially in the intensive care setting.33,34
Benzodiazepine withdrawal
Benzodiazepine cessation after chronic use may cause a withdrawal syndrome with features similar to alcohol withdrawal.35 Symptoms may be more severe when withdrawing from higher-dose or short-acting benzodiazepine formulations.36 The window for benzodiazepine withdrawal can be highly variable, with onset occurring within 48 hours of discontinuing short-acting benzodiazepines and up to 10 days for long-acting benzodiazepines.35 The primary treatment of benzodiazepine dependence and withdrawal is a slow taper of benzodiazepines.35 Both fixed-dose and symptom triggered-tapering schedules are acceptable treatment modalities.37 Additionally, a fixed-dose phenobarbital protocol can be used safely and effectively in the inpatient setting.38
Opioid withdrawal
Opioid cessation, or a reduction in use, may result in cravings or symptoms of opioid withdrawal syndrome (OWS) (Table 6). The Clinical Opiate Withdrawal Scale (COWS) is a validated tool to assist with the diagnosis and severity of OWS.39,40 During OWS treatment, the COWS helps guide therapy and monitor for symptom improvement. A detailed review of medications dispensed through an established prescription monitoring program can provide helpful collateral information about patterns of use and risk for overdose.
Table 6.
Opioid withdrawal syndrome—symptoms to ask about as part of the addiction history and timing after opioid cessation
| Addiction History—Opioid Withdrawal Syndrome Symptoms | Timing of Predicted Opioid Withdrawal Syndrome |
|---|---|
| • Nausea | Onset of symptoms |
| • Vomiting | • 6–12 h after last dose of short-acting opioid a |
| • Diarrhea | • 24–48 h after last dose of long-acting opioid b |
| • Sweating | Peak symptoms |
| • Irritability | • 24–48 h after onset |
| • Restlessness | Symptoms subside |
| • Tremor | Days to weeks (short-acting vs long-acting, respectively) |
| •Yawning | |
Short-acting opioids: morphine, codeine, hydromorphone, oxycodone, hydrocodone, diacetylmorphine (heroin), meperidine, tramadol, and fentanyl.
Long-acting opioid: methadone.
Opioid detoxification alone is associated with high relapse rates and increased HIV incidence41 whereas maintenance opioid agonist therapy is associated with improved treatment retention, sustained abstinence, and a reduction in morbidity and mortality.42 Given this, maintenance opioid agonist treatment is recommended for the management of opioid withdrawal and the ACS should facilitate a seamless link to outpatient treatment programs post-hospital discharge. The efficacy of methadone or buprenorphine is comparable with low rates of adverse effects.43,44 An important consideration when prescribing buprenorphine is the potential for precipitated withdrawal, a syndrome of worsening withdrawal symptoms when buprenorphine, a partial opioid agonist with high affinity for the opioid receptor, is administered when a full opioid agonist is also systemically present. To avoid this, buprenorphine should not be administered until at least 12 hours after the last dose of a short-acting opioid and up to 48 hours after the last dose of a long-acting opioid.42 A COWS score of 8 to 12, representing mild opioid withdrawal, should be obtained prior to starting buprenorphine if there are concerns regarding precipitated withdrawal (Table 7).
Table 7.
Typical dosing schedule for methadone or buprenorphine for withdrawal
| Medication | Initial Dose | Next Doses in First 24 h | Day 2 |
|---|---|---|---|
| Methadone | 20 mg oral | Reassess every 2–3 h, giving additional 10 mg until COWS <5 (40 mg maximum in 24 h) | Continue on total dose from first 24 h; can increase to 40 mg if not already reached |
| Buprenorphine | 4 mg sublingual | Reassess every 2–3 h, giving additional 4 mg until COWS <5 (12 mg maximum in 24 h) | Continue on total dose from first 24 h; can increase up to 16 mg |
A common misconception in the United States is that hospitals or doctors need special licensing to provide methadone or buprenorphine in the inpatient setting; however, it is legal for both of these medications to be administered to manage withdrawal symptoms while patients complete their inpatient treatments.45
Adjuvant medications may be used for symptomatic management for patients who decline agonist treatment or while waiting to start buprenorphine. These medications may include a-agonists to treat anxiety or muscle spasms, antihistamines or benzodiazepines for anxiety, hypnotics for insomnia, anti-inflammatories for pain, and antidiarrheals or antispasmodics.
Stimulant withdrawal
Cocaine, methamphetamine, or other stimulant intoxication may present with hypertension, agitation, and anxiety, which should be managed symptomatically. Withdrawal from stimulants includes symptoms, such as depressed mood and somnolence, and does not routinely require medication management.46
Post-withdrawal management
After acute withdrawal has subsided, providers should engage patients in brief interventions and motivational interviewing to assess and enhance patients’ readiness and motivation for long-term treatment of their SUDs.6,7,47 All patients who are interested should receive a referral and connection to outpatient counseling as well as potentially residential treatment. Pharmacologic interventions should be offered as appropriate based on the substance use disorder.
Nicotine use disorder
Despite approximately 70% of adult smokers in the United States expressing a desire to quit smoking,48 only approximately half report being asked about their smoking by a health care provider in the previous 12 months.49 Brief physician advice has been shown to have a significant effect on smoking cessation.50 Medications can assist with quit rates 10-fold.51 Drug therapies for nicotine use disorder include combination nicotine replacement therapy, varenicline, or bupropion. Behavioral interventions should be offered, because these provide significant benefit when combined with pharmacotherapy.52,53
Alcohol use disorder
Among risky drinkers, brief intervention has proved effective in reducing alcohol consumption.54,55 Patients should be offered pharmacologic treatment of alcohol use disorder with any of the 3 US Food and Drug Administration– approved medications: naltrexone, acamprosate, and disulfiram, based on a patient’s medical characteristics and alcohol consumption goals.56,57 Medications can be initiated in a hospital with patients and then linked to outpatient care.
Benzodiazepine use disorder
The mainstay of treatment of benzodiazepine use disorder is a long-term taper over 1 month to 3 months.35 In the inpatient setting, however, long-term taper is not always feasible. Inpatient teams should attempt to connect with an outpatient provider and assess the risks, benefits, and feasibility of continuing a taper beyond the inpatient period. If a taper must be completed while an inpatient, providers should complete the taper over the maximum number of days. There are no pharmacologic maintenance treatments of benzodiazepine use disorder. Counseling may provide additional benefit as well as treatment of underlying psychiatric disorders.58
Opioid use disorder
Administration of opioids in-hospital and the timing of this influence a patient’s management strategy (eg, choice of opioid agonist treatment and timing of induction with buprenorphine). Long-term opioid agonist treatment with either buprenorphine or methadone should be recommended (see Table 7). Furthermore, both medications should be titrated toward a therapeutic dose while a patient is in hospital. Buprenorphine can be increased by 2 mg to 4 mg daily until a patient achieves a therapeutic daily dose of approximately 16 mg. Methadone can be increased by 5 mg to 10 mg every 2 days to 3 days, with the goal of a dose that treats withdrawal symptoms, alleviates cravings, and minimizes or eliminates the euphoric effects of concurrent opioid use.42 Before aggressive titration begins, efforts should be taken to ensure a patient has appropriate linkage to an outpatient buprenorphine or methadone provider who can continue treatment directly after hospital discharge.
If a patient presents who is not currently in withdrawal due to recent abstinence, but given concerns about relapse is otherwise motivated to be on pharmacologic treatment, this patient may be offered either naltrexone, which is an opioid antagonist, or opioid agonist therapy. If the patient elects agonist therapy, then buprenorphine or methadone can be slowly titrated.
If an outpatient prescriber cannot be located, the patient declines maintenance treatment, or maintenance is not possible (eg, incarceration), then the patient should be offered an opioid taper. Methadone may be tapered by 5 mg to 10 mg per day and buprenorphine tapered by 2 mg to 4 mg per day until discharge. Additionally, patients should be informed of the increased risk that exists for overdose in the setting of tapering and should be provided with overdose prevention education and naloxone at the time of discharge.
Stimulant use disorder
There are no Food and Drug Administration–approved medications for stimulant use disorder because overall pharmacologic interventions have had mixed or negative results.59–61 Counseling, especially contingency management, is the most evidence-based intervention.62
DEMONSTRATED BENEFITS OF ADDICTION CONSULT SERVICES: ONGOING RESEARCH AND EVALUATION
ACSs have been demonstrated as feasible across North America, are successful at engaging patients in care, provide evidence-based addiction treatment, and assist in transitioning patients from acute to community treatment settings.63–68 Recipients of hospital-based ACSs have also been found to have higher rates of medical insurance coverage, increased engagement with primary care and HIV services, and decreased homelessness.69 For example, at Johns Hopkins University, providers achieved a decrease in emergency department utilization and increase in ambulatory care visits for patients treated within an integrated addiction and medical treatment model in a day hospital setting.70 In addition, inpatient addiction services can decrease addiction severity and increase self-reported abstinence rates at 30-days.63
Of importance, a challenge in the United States regarding the success of ACSs has been the restricted access of outpatient methadone or buprenorphine maintenance programs, limiting the ability to initiate long-term opioid agonist treatment in acute care settings.69 In an attempt to overcome this, some ACSs have created a bridge clinic, where patients can obtain opioid agonist medications short-term while seeking a long-term placement. Inpatient addiction treatment with appropriate outpatient linkage has been shown beneficial to many health as well as care utilization outcomes. One study of an ACS, which offers linked services, demonstrated a self-reported reduction in substance-related hospitalizations and emergency room visits.63 In both Vancouver, Canada, and Portland, Oregon, hospitalized patients with an SUD who require long-term antibiotics can choose to access a post-discharge residential care facility that includes harm reduction services in addition to maintenance addiction treatment.64 Such a program can be cost-saving, allowing patients to leave the more expensive inpatient setting, while also facilitating completion of antibiotic therapy. An ACS model is estimated to yield significant savings, by reducing readmissions as well as length of stay, likely through linkage to appropriate outpatient services.64
In addition, an ACS can have an important educational role, increasing trainee knowledge of addiction medicine concepts71 as well as creating a more patient- centered harm reduction culture within the hospital setting.
FUTURE CONSIDERATIONS
Although several positive outcomes have been reported with hospital-based ACSs, future research should focus on its impact on health outcomes, health care utilization, and health systems costs. Given the high prevalence of SUDs among hospitalized patients and their frequent need to access medical care on discharge, hospitalization offers a critical opportunity for effective intervention and evidence-based addiction treatment. Inpatient services alone are not sufficient, however, because having robust and available outpatient services for linkage seem critical to the successes of current consult service models. ACSs offer a feasible solution to address the intensive needs of patients with an SUD and consequently have immense potential to dramatically reduce the morbidity and mortality experienced by individuals with an SUD.
KEY POINTS.
Hospitalized patients have a high prevalence of substance use; however, this problem often goes unaddressed.
Inpatient addiction consult services are an important intervention to use the reachable moment of hospitalization to engage patients and initiate addiction treatment.
Addiction consultation involves taking an addiction-specific history, motivational interviewing, withdrawal symptom management, and initiation of long-term pharmacotherapy.
Addiction consult services have the potential to decrease readmissions and utilization costs for medical systems, improve substance use outcomes for patients, and increase provider knowledge.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
REFERENCES
- 1.National Institute on Drug Abuse. Trends & statistics. 2017. Available at: https://www.drugabuse.gov/related-topics/trends-statistics. Accessed July 10, 2017.
- 2.The National Center on Addiction and Substance Abuse. Addiction medicine: closing the gap between science and practice. 2012. Available at: https://www.centeronaddiction.org/addiction-research/reports/addiction-medicine-closing-gap-between-science-and-practice. Accessed July 10, 2017.
- 3.Brown RL, Leonard T, Saunders LA, et al. The prevalence and detection of substance use disorders among inpatients ages 18 to 49: an opportunity for prevention. Prev Med 1998;27(1):101–10. [DOI] [PubMed] [Google Scholar]
- 4.Weiss AJ, Elixhauser A, Barrett ML, et al. Opioid-related inpatient stays and emergency department visits by state, 2009–2014: statistical brief #219 In: healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017. Available at: http://www.ncbi.nlm.nih.gov/books/NBK441648/. Accessed July 10, 2017. [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Reports and detailed tables from the 2015. National Survey on Drug Use and Health (NSDUH) Available at: https://www.samhsa.gov/samhsa-data-outcomes-quality/major-data-collections/reports-detailed-tables-2015-NSDUH. Accessed March 29, 2017. [Google Scholar]
- 6.McQueen J, Howe TE, Allan L, et al. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev 2011;(8):CD005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pecoraro A, Horton T, Ewen E, et al. Early data from project engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract 2012;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med 2014;174(8):1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and post-discharge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA 2014;312(7):719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Defries T, Lozada M, et al. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-Day readmissions and emergency department visits. J Gen Intern Med 2015;30(3):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanahan CW, Beers D, Alford DP, et al. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med 2010;25(8):803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: Results from a survey of general internists. Subst Abus 2016;37(4):635–41. [DOI] [PubMed] [Google Scholar]
- 13.Wakeman SE, Baggett MV, Pham-Kanter G, et al. Internal medicine residents’ training in substance use disorders: a survey of the quality of instruction and residents’ self-perceived preparedness to diagnose and treat addiction. Subst Abus 2013;34(4):363–70. [DOI] [PubMed] [Google Scholar]
- 14.Wakeman SE, Kanter GP, Donelan K. Institutional substance use disorder intervention improves general internist preparedness, attitudes, and clinical practice. J Addict Med 2017;11(4):308–14. [DOI] [PubMed] [Google Scholar]
- 15.Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med 2015;128(1):8–9. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition. Washington (DC): American Psychiatric Association Publishing Co; 2013. [Google Scholar]
- 17.Hutchinson AB, Farnham PG, Sansom SL, et al. Cost-effectiveness of frequent HIV testing of high-risk populations in the United States. J Acquir Immune Defic Syndr 1999 2016;71(3):323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J 2016;92(1088):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh N, Verster A, Rodolph M, et al. WHO guidance on the prevention of viral hepatitis B and C among people who inject drugs. Int J Drug Policy 2014; 25(3):363–71. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). Update to interim guidance for Preexposure Prophylaxis (PrEP) for the prevention of HIV infection: PrEP for injecting drug users. MMWR Morb Mortal Wkly Rep 2013;62(23):463–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Gu ¨nthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA panel. JAMA 2016;316(2):191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil R, Small W, Wood E, et al. Hospitals as a ‘risk environment’: An ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med 2014; 105:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanucchi L, Lofwall MR. Putting parity into practice — integrating opioid-use disorder treatment into the hospital setting. N Engl J Med 2016;375(9):811–3. [DOI] [PubMed] [Google Scholar]
- 24.Wakeman SE, Ghoshhajra BB, Dudzinski DM, et al. Case records of the Massachusetts General Hospital. Case 35–2014: a 31-year-old woman with fevers, chest pain, and a history of HCV infection and substance-use disorder. N Engl J Med 2014;371(20):1918–26. [DOI] [PubMed] [Google Scholar]
- 25.Heatherton TF, Kozlowski LT, Frecker RC, et al. The fagerstro ¨m test for nicotine dependence: a revision of the fagerstro ¨m tolerance questionnaire. Br J Addict 1991;86(9):1119–27. [DOI] [PubMed] [Google Scholar]
- 26.Bayard M, McIntyre J, Hill K, et al. Alcohol withdrawal syndrome. Am Fam Physician 2004;69(6):1443–50. [PubMed] [Google Scholar]
- 27.Gortney JS, Raub JN, Patel P, et al. Alcohol withdrawal syndrome in medical patients. Cleve Clin J Med 2016;83(1):67–79. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado JR, Sher Y, Ashouri JF, et al. The “Prediction of Alcohol Withdrawal Severity Scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol 2014;48(4):375–90. [DOI] [PubMed] [Google Scholar]
- 29.Mayo-Smith MF. Pharmacological management of alcohol withdrawal: a meta-analysis and evidence-based practice guideline. JAMA 1997;278(2):144–51. [DOI] [PubMed] [Google Scholar]
- 30.Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse: withdrawal and treatment. Med Clin North Am 1997;81(4):881–907. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989;84(11):1353–7. [DOI] [PubMed] [Google Scholar]
- 32.Saitz R, Mayo-Smith MF, Roberts MS, et al. Individualized treatment for alcohol withdrawal: a randomized double-blind controlled trial. JAMA 1994;272(7): 519–23. [PubMed] [Google Scholar]
- 33.Askgaard G, Hallas J, Fink-Jensen A, et al. Phenobarbital compared to benzodiazepines in alcohol withdrawal treatment: a register-based cohort study of subsequent benzodiazepine use, alcohol recidivism and mortality. Drug Alcohol Depend 2016;161:258–64. [DOI] [PubMed] [Google Scholar]
- 34.Mo Y, Thomas MC, Karras GE Jr. Barbiturates for the treatment of alcohol withdrawal syndrome: a systematic review of clinical trials. J Crit Care 2016;32:101–7. [DOI] [PubMed] [Google Scholar]
- 35.Soyka M Treatment of benzodiazepine dependence. N Engl J Med 2017; 376(24):2399–400. [DOI] [PubMed] [Google Scholar]
- 36.Petursson H The benzodiazepine withdrawal syndrome. Addiction 1994;89(11): 1455–9. [DOI] [PubMed] [Google Scholar]
- 37.Mcgregor C, Machin A, White JM. In-patient benzodiazepine withdrawal: comparison of fixed and symptom-triggered taper methods. Drug Alcohol Rev 2003;22(2):175–80. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki SS, Jacapraro JS, Rastegar DA. Safety and effectiveness of a fixed-dose phenobarbital protocol for inpatient benzodiazepine detoxification. J Subst Abuse Treat 2012;43(3):331–4. [DOI] [PubMed] [Google Scholar]
- 39.Tompkins DA, Bigelow GE, Harrison JA, et al. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend 2009;105(1):154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs 2003;35(2):253–9. [DOI] [PubMed] [Google Scholar]
- 41.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012;345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuckit MA. Treatment of opioid-use disorders. N Engl J Med 2016;375(4): 357–68. [DOI] [PubMed] [Google Scholar]
- 43.Amato L, Davoli M, Minozzi S, et al. Methadone at tapered doses for the manage-ment of opioid withdrawal. Cochrane Database Syst Rev 2013;(2):CD003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gowing L, Ali R, White JM, et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev 2017;(5):CD002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noska A, Mohan A, Wakeman S, et al. Managing opioid use disorder during and after acute hospitalization: a case-based review clarifying methadone regulation for acute care settings. J Addict Behav Ther Rehabil 2015;4(2) [pii:1000138]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donroe JH, Tetrault JM. Substance use, intoxication, and withdrawal in the critical care setting. Crit Care Clin 2017;33(3):543–58. [DOI] [PubMed] [Google Scholar]
- 47.Velez CM, Nicolaidis C, Korthuis PT, et al. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med 2017;32(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babb S, Malarcher A, Schauer G, et al. Quitting smoking among adults — United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;65(52):1457–64. [DOI] [PubMed] [Google Scholar]
- 49.Nugent CN, Schoenborn CA, Vahratian A. Discussions between health care providers and their patients who smoke cigarettes: NCHS Data Brief No. 174. 2014. Available at: https://www.cdc.gov/nchs/data/databriefs/db174.pdf. Accessed July 10, 2017. [PubMed]
- 50.Stead LF, Buitrago D, Preciado N, et al. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013. 10.1002/14651858.CD000165.pub4. Accessed July 10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013;(5):CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stead LF, Koilpillai P, Fanshawe TR, et al. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016;(3):CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev 2015;(10):CD009670. [DOI] [PubMed] [Google Scholar]
- 54.Kaner EFS, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;(2):CD004148. [DOI] [PubMed] [Google Scholar]
- 55.Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract 2013;8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs 2013;27(4):287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedmann PD. Alcohol use in adults. N Engl J Med 2013;368(4):365–73. [DOI] [PubMed] [Google Scholar]
- 58.Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev 2015;(5):CD009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castells X, Cunill R, Perez-Mana C, et al. Psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev 2016;(9):CD007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pettinati HM, Kampman KM, Lynch KG, et al. A pilot trial of injectable, extended-release naltrexone for the treatment of co-occurring cocaine and alcohol dependence. Am J Addict 2014;23(6):591–7. [DOI] [PubMed] [Google Scholar]
- 61.Singh M, Keer D, Klimas J, et al. Topiramate for cocaine dependence: a systematic review and meta-analysis of randomized controlled trials. Addiction 2016; 111(8):1337–46. [DOI] [PubMed] [Google Scholar]
- 62.Minozzi S, Saulle R, De Crescenzo F, et al. Psychosocial interventions for psychostimulant misuse. Cochrane Database Syst Rev 2016;(9):CD011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakeman SE, Metlay JP, Chang Y, et al. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med 2017;32(8):909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med 2017;12(5):339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Toole DTP, Conde-Martel A, Young JH, et al. Managing acutely III substance-abusing patients in an integrated day hospital outpatient program. J Gen Intern Med 2006;21(6):570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy MK, Chabon B, Delgado A, et al. Development of a substance abuse consultation and referral service in an academic medical center: challenges, achievements and dissemination. J Clin Psychol Med Settings 2009;16(1):77–86. [DOI] [PubMed] [Google Scholar]
- 67.McDuff DR, Solounias BL, Beuger M, et al. A substance abuse consultation service. Am J Addict 1997;6(3):256–65. [DOI] [PubMed] [Google Scholar]
- 68.Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services – Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat 2017;79:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aszalos R, McDuff DR, Weintraub E, et al. Engaging hospitalized heroin- dependent patients into substance abuse treatment. J Subst Abuse Treat 1999;17(1–2):149–58. [DOI] [PubMed] [Google Scholar]
- 70.O’Toole TP, Pollini RA, Ford DE, et al. The effect of integrated medical-substance abuse treatment during an acute illness on subsequent health services utilization. Med Care 2007;45(11):1110–5. [DOI] [PubMed] [Google Scholar]
- 71.Klimas J, Ahamad K, Fairgrieve C, et al. Impact of a brief addiction medicine training experience on knowledge self-assessment among medical learners. Subst Abuse 2017;38(2):141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]