Abstract
Alcohol abuse leads to great medical, social, and economic burdens throughout the world. It is believed that the rewarding actions of alcohol are mediated by alterations in the mesolimbic dopaminergic system leading to increased levels of dopamine in the nucleus accumbens (nAc). Little is known about the role that ligand gated ion channels (LGIC), such as glycine receptors (GlyR), have in regulating levels of ethanol intake and place preference. In this study, we used Knock-in (KI) mice that have ethanol insensitive α1 GlyRs (KK385/386AA) and a combination of electrophysiological and behavioral approaches to examine how expression of ethanol resistant α1 GlyRs in brain neurons might affect binge drinking and conditioned place preference. Data show that tonic α1 GlyR-mediated currents that modulate accumbal excitability were exclusively sensitive to ethanol only in WT mice. Behavioral studies showed that the KI mice have a higher intake of ethanol upon first exposure to drinking and greater conditioned place preference to ethanol, suggesting that α1 GlyRs in the brain have a protective role against abuse. This study suggests that non-synaptic α1 containing GlyRs have a role in motivational and early reinforcing effects of ethanol and opens a novel opportunity for pharmacotherapy development to treat alcohol use disorders.
Keywords: Alcohol & Alcoholism, Animal Models, Glycine Receptor, G-protein, Nucleus accumbens, Receptor Pharmacology
Introduction
It is well accepted that alcohol (ethanol), at pharmacological doses, activates the mesolimbic dopaminergic reward circuit and increases dopamine levels in the nucleus accumbens (nAc) (Di Chiara and Imperato, 1988; Molander and Soderpalm, 2005a; Soderpalm et al., 2009), similarly to other abused drugs (Hyman et al., 2006; Jonsson et al., 2014; Nestler, 2005). However, little is known about the molecular mechanism that is involved in this action. Glycine receptors (GlyRs) are one of the major mediators of inhibition in the CNS and are distributed principally in the spinal cord and brainstem (Aguayo et al., 2014; Aguayo et al., 1996; Bradaia et al., 2004; Eggers and Berger, 2004; Eggers et al., 2000; Mariqueo et al., 2014; Sebe et al., 2003). However, several studies have reported the presence of GlyRs in supraspinal brain regions, such as the prefrontal cortex (PFC) (Lu and Ye, 2011; Salling and Harrison, 2014), OFC (Badanich et al., 2013), raphe (Maguire et al., 2014), ventral tegmental area (VTA) (Li et al., 2012; Ye et al., 2001), dorsal striatum (McCracken et al., 2017b) and nAc (Forstera et al., 2017; Jiang et al., 2004; Jonsson et al., 2014; Martin and Siggins, 2002; Molander et al., 2005; Molander and Soderpalm, 2005b; Muñoz et al., 2018). There is a solid body of data from in vivo studies indicating that an increase in GlyR-mediated inhibition in nAc, by application of the agonist or a glycine transporter 1 (GlyT1) inhibitor (Org24598), increases dopamine release and lowers ethanol consumption. On the other hand, reducing GlyR function by strychnine (STN), a highly selective GlyR antagonist, led to a decrease in the dopamine level and an increase in ethanol consumption (Lido et al., 2011; Molander et al., 2005; Molander and Soderpalm, 2005a; Salling and Harrison, 2014). Thus, these pharmacological and behavioral results suggest that GlyRs might be involved in regulating reward network excitability and addictive behaviors. Considering that GlyRs can be formed by three principal subunits, α1, α2 and α3 (Lynch, 2009), and that a recent study showed that D1 medium spiny neurons (MSN) in nAc have ethanol-sensitive GlyRs (Forstera et al., 2017), it is now important to learn which subunits are involved, the stoichiometry, and/or specific residues that might be important for ethanol actions in the mesolimbic circuit.
We recently generated Knock In (KI) mice that have their lysine residues at amino acid positions 385 and 386 in the intracellular loop of the α1 GlyR replaced with alanine (KK385/386AA) (Aguayo et al., 2014), residues that are important for ethanol sensitivity via Gβγ (Yevenes et al., 2010; Yevenes et al., 2008). Unlike other genetically engineered animals with mutations in the GlyR (Findlay et al., 2005; Findlay et al., 2003), these mice present a normal phenotype (lack of hyperreflexia, startle reflex), but exhibit shorter sedation times with ethanol (Aguayo et al., 2014). The examination of the role that the α1 subunit might have in ethanol actions is of relevance because a recent report suggested that even though α2 and α3 subunits are not sensitive to ethanol (Sanchez et al., 2015; Yevenes et al., 2010), they might be important for some ethanol behaviors (Blednov et al., 2015).
In the present study, we examined neuronal mechanisms which are likely associated to the control of dopamine release from VTA neurons and influence ethanol addictive behaviors. We found that the KI mice showed a higher intake on first exposure to ethanol and elevated conditioned place preference together with reduced GlyR sensitivity to ethanol in MSNs.
Materials and Methods
Mice
Animal care and experimental protocols for this study were approved by the Institutional Animal Care and Use Committees at the Universities of Concepción and Pittsburgh and followed the guidelines for ethical protocols and care of experimental animals established by the National Institutes of Health (NIH, Maryland, USA).
Mice with the conditional α1KK385–386AA KI allele (Aguayo et al., 2014) are available from the Jackson Laboratory (Stock number 023516; Bar Harbor, ME) stock. Mice were individually housed for behavioral experiments and in groups of 2–4 mice for cellular experiments on a 12-h light/dark cycle and given food and water ad libitum. Mice used in the present study are a global KI from the seventh generation of a hybrid genetic background, with a near uniform C57BL/6J inbred genetic background that consisted of approximately 0.8% Strain 129X1/S1 and 99.2% C57BL/6J. In this report, mice are referred to as WT (without the mutation) and KI (with α1KK385–386AA allele). All the mice were genotyped using a genomic DNA extraction kit (Wizard Genomic DNA purification kit, Promega Co. USA) and conventional PCR with the primers: KI sense 5’-TTG TAG CAT GGG AAA GCC AGA- 3’ and Anti Sense 5’- ATG CTG AAA CAG ATG CAA GCC AGG- 3’. Genotypes of PCR products were observed by agarose electrophoretic gel (2%) that demonstrated homozygous KI with one band of 400 bp, homozygous WT mice with a band of 300 bp, and heterozygous mice with two bands of 400 and 300 bp, as previously reported (Aguayo et al., 2014).
Preparation of brain slices
WT and KI mice (PND 21–40) were decapitated as previously described (Jun et al., 2011). The brain was quickly excised, placed in cutting solution containing (in mM): sucrose 194, NaCl 30, KCl 4.5, MgCl2 1, NaHCO3 26, NaH2PO4 1.2, Glucose 10 (pH 7.4) saturated with 95% O2 and 5% CO2, glued to the chilled stage of a vibratome (Leica VT1200S, Germany), and sliced to a thickness of 300 μm. Slices were transferred to an aCSF solution containing (in mM): NaCl 124, KCl 4.5, MgCl2 1, NaHCO3 26, NaH2PO4 1.2, Glucose 10, CaCl2 2 (pH 7.4 and 310–320 mOsm) saturated with O2 at 30°C for 1hr. The slices were then transferred to the recording chamber with aCSF solution saturated with 95% O2 and 5% CO2 at RT. The slices were observed on a DIC-IR microscope using 10x (0.25 n.a.) and 40x (0.8 n.a.) objectives (Nikon Eclipse FN1, Japan).
Enzymatic dissociation of accumbal neurons
Coronal brain slices that contained the nAc were isolated and transferred to a 35-mm culture dish. For enzymatic dissociation, nAc slices were incubated for 20 min in normal aCSF (saturated with 95% O2 and 5% CO2) in the presence of 0.6 mg/ml pronase (Calbiochem/EMD Bioscience, Darmstadt, Germany) at 37°C. Slices were removed from aCSF for trituration. nAc were dissected from the slices and the tissue was then triturated through a series of pipette tips of decreasing size in a 35-mm diameter culture dish. After 20 min, isolated neurons attached to the bottom of the culture dish and were ready for electrophysiological experiments.
Electrophysiology
Dissociated accumbal neurons:
MSNs were identified by their size, membrane resistance, and capacitance, and whole-cell current recordings from dissociated accumbal neurons were performed using the voltage-clamp technique at RT. Glycine-activated currents were studied in MSNs using whole-cell recordings. Patch pipettes were prepared from filament-containing borosilicate micropipettes (World Precision Instruments) using a P-87 micropipette puller (Sutter Instruments). The resistance was 4–6 MΩ for whole-cell configuration and patch pipettes of 10–20 MΩ were used for out-side out single channel recordings. Recordings of glycine currents were done using an Axopatch 200B amplifier (Axon Instruments, Union City, CA) at a holding potential of −60 mV. We used an internal solution containing (in mM): 120 CsCl, 4.0 MgCl2, 10 BAPTA, 0.5 Na2-GTP and 2.0 Na2-ATP (pH 7.4, 290–310 mOsmol) and an external solution containing (in mM): 150 NaCl, 5.4 KCl, 2.0 CaCl2, 1.0 MgCl2, 10 D-glucose and 10 HEPES (pH 7.4, 300–330 mOsm). Currents were displayed and stored on a personal computer using a 1322A Digidata (Axon Instruments, Union City, CA) and analyzed with Clampfit 10.1 (Axon Instruments, Union City, CA). Series resistance was monitored and only cells with a stable series resistance (less than 25 MΩ and that did not change more than 15% during recording) were included for data analysis. Recordings were made 2–7 h after euthanasia. The experimenter was not blinded to the genotype of the mice.
Brain slice recordings:
Coronal brain slices (300 μm) containing the nAc region were prepared from adult WT and KI mice (PND 21–40) as described previously (Jun et al., 2011) and perfused (2 ml/min) with oxygenated (95% O2/5% CO2, RT) aCSF at 30–32°C. Whole-cell current recordings of accumbal neurons were performed using the voltage-clamp technique. Patch pipettes having a 3–6 MΩ resistance were used for whole cell recording. Recordings were done at a holding potential of −60 mV using an internal solution containing (in mM): 120 KCl, 4.0 MgCl2, 10 BAPTA, 0.5 Na2-GTP and 2.0 Na2-ATP (pH 7.4, 290–310 mOsmol) and an aCSF solution containing (in mM): NaCl 124, KCl 4.5, MgCl2 1, NaHCO3 26, NaH2PO4 1.2, D-Glucose 10, CaCl2 2 (pH 7.4 and 315–320 mOsm) saturated with O2/CO2. Series resistance was monitored and only cells with a stable series resistance were included for data analysis. The experimenter was not blinded to treatments administered to the mice.
Tonic Current Analysis:
To evaluate the tonic glycine current, the GlyT1 inhibitor (Org24598; 10 μM), EtOH (10–100 mM) and strychnine (STN; 1 μM) were applied in voltage-clamp configuration at a holding potential of –60 mV. Glycine currents were pharmacologically isolated via bath application of tetrodotoxin (TTX; 500 nM), NMDA receptor antagonist; D-2-amino-5-phosphonovalerate (D-APV; 40 μM), AMPA receptor antagonist; 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM) and the GABAA antagonist; bicuculline (10 μM). The maximal amplitude of the tonic current was estimated after 4 min of Org24598 and strychnine application and 2 min of ethanol exposure. The current shift was calculated as the mean holding current during a 30 s period of recording without mIPSC. The all-points holding current histograms were fit with a Gaussian curve. The difference between the peaks of these Gaussian curves in the presence and absence of drug was calculated to determine the change of holding current. Because these recordings comprised up to 20 minutes and included the application of STN, the effect of the high concentration of ethanol was studied in neurons not previously exposed to the modulator. For the lower concentrations, we cannot exclude the development of a small degree of tolerance.
Excitability Experiments:
To determine the effects of ethanol and strychnine on spike firing, current-clamp recordings were performed using a potassium gluconate internal pipette solution (in mM): 126 KGluc, 4 KCl, 10 HEPES, 10 BAPTA, 4 NaATP, 0.3 NaGTP, adjusted to 290 mOsm, pH = 7.2). Current injections (25–300 pA) were used to evoke action potential firing (APs) in nAc neurons and neuron excitability was evaluated before, during, and after EtOH and strychnine exposure. To examine the effects of EtOH and strychnine on glycine receptor function, EtOH (100 mM), with or without strychnine (1 μM), was added to the aCSF perfusion solution and bath applied throughout the entire recording session. We used a high concentration of ethanol to avoid any time-dependent change in the excitability of the neurons during the long term recording. Recordings were analyzed for resting membrane potential (mV), APs threshold (mV) and frequency (# spikes).
Immunocytochemistry
Dissociated accumbal neurons:
The acute dissociated neurons were fixed for 15 min with cold methanol (−20°C). After 3 washes with 1X PBS, neurons were blocked with normal horse serum (10%) for 30 min. Cells were incubated (overnight) with a combination of primary antibodies: α1GlyR (1:100, mouse monoclonal IgG, mAb4a clone; Cat. No. 146011, Synaptic System), synapsin 1 (1:200, goat polyclonal, A-158 clone, sc-55774, Santa Cruz Biotechnology) and Gβ (1:200, rabbit polyclonal, T-20 clone, sc-378, Santa Cruz Biotechnology). The specificity of the mAb4a clone was confirmed in immature and mature spinal neurons, with the latter expressing only α1 (Mariqueo et al., 2014). Subsequently, cells were washed with 1X PBS and incubated (2h) with a secondary anti-mouse, anti-goat or anti-rabbit antibody (Alexafluor 488, Cy3; and Alexafluor 647, Jackson Labs) diluted 1:200 for 2 hours. After 5 washes with 1X PBS, the preparations were mounted with Dako (DakoCytomation, USA) mounting solution. Confocal images (1024 × 1024 pixels, pixel size was 313 nm) of a single optical section were acquired with 40X /1.3 n.a objective in a LSM700 laser scanning microscope and ZEN software suit (Zeiss, Oberkochen, Germany) in the CMA core facility at the University of Concepcion. Dissociated accumbal neurons were chosen randomly from view-fields presenting multiple cells exhibiting different levels of fluorescence. Triple color immunofluorescent images were captured, processed, deconvoluted, rendered, stored and analyzed using the ZEN (Zeiss) ImageJ program (NIH)
Western blots
Tissue homogenates (100 μg; nAc, brainstem and hippocampus) after detergent treatment (10 mM Tris-HCl pH 7.4, 0.25 M Sucrose, 10 mM NEM, Protease inhibitor cocktail 1X) were subjected to electrophoresis on 10% SDS–PAGE gels. Proteins were blotted onto nitrocellulose membranes (Biorad) and blocked with 5% milk in 1X TBS, 0.1% Tween 20 for one hour with stirring. Subsequently, the membranes were incubated with primary α1GlyR antibodies (1:1000, mouse monoclonal IgG, mAb4a clone; Cat. No. 146011, Synaptic System) and anti α-tubulin (1:3000, mouse monoclonal, DM1A clone, Cat No. T9026, Sigma) for 1–2 hrs. After washes with 1X TBS and 0.1% Tween 20, membranes were incubated for 1 hr with anti-mouse secondary antibodies conjugated to HRP (1:5000, Santa Cruz). The immunoreactivity of the proteins was detected and visualized with ECL Plus Western Blotting Detection System (PerkinElmer, MA, USA). Levels of α-tubulin were used as a loading control. The Western blot was quantified using the “ImageJ” (NIH) program.
Dot Blot:
Tissues (nAc and brainstem) were lysed with a buffer containing 0.5 mM EDTA, 140 mM NaCl, 0.5%Triton X-100, and 100 mM DTT (1,4-Dithiothreitol). Five microliters of equal amounts of proteins were added to a nitrocellulose membrane and dried. Nonspecific sites were blocked with 5% evaporated milk and incubated with the primary GlyT1 antibody (1:200; goat polyclonal, N-20 clone, sc16701, Santa Cruz Biotechnology). Immunoreactive dots were detected with secondary antibodies conjugated with HRP (1:5000 dilution; Santa Cruz Biotechnology) and visualized with an ECL Plus Western Blotting Detection System (PerkinElmer, MA, USA)(Peters et al., 2013).
Behavioral Characterization
Mice (seventh generation) were used for all studies unless otherwise indicated. All mice were between 8 and 12 weeks of age at the time of testing.
Drinking in the dark (DID):
The protocol used has been previously described (Blednov et al., 2015). Briefly, two hours after the dark cycle began, three groups of WT and KI mice had limited access to either a 15% (v/v) ethanol solution or water (WT males=8; WT females=7; KI males=7; KI females=6). In independent experiments, we tested 5% sucrose (w/v) (WT males=3; WT females=3; KI males=4; KI females=5) and 0.1 mM quinine hydrochloride dihydrate solution (WT males=6 KI males=7) for 2 h (1–3 days and 1–11 days) and 4 h (4th day and 12th day). Mouse (male and female) weight measurements were taken immediately before and after the 2 h and 4 h access period. Quantities of ethanol, sucrose (g/kg body weight/2–4 hr) and quinine (mg/kg body weight/2–4 hr) consumption were statistically analyzed with two-way ANOVA, Bonferroni post hoc test.
Blood ethanol concentration (BEC):
Blood samples (20–100 μl) from WT and KI tails were collected after 20 min on day 1 and 4 of DID. Whole blood samples were spun-down in a centrifuge (10000 rpm × 1 min) and BEC was determined in serum using an Analox AM1 Alcohol Analyzer (Lunenburg, MA). BECs were statistically analyzed with Unpaired Student’s t test using Origin 6.0 software (Microcal, Inc. Northampton, MA).
Conditioned Place Preference (CPP):
This behavioral test is a Pavlovian conditioning paradigm primarily used to measure reward and motivation for substances or drugs of abuse. The apparatus consisted of 6 identical place-conditioning chambers (33×27×20 cm) enclosed in individual ventilated, light and sound attenuating enclosures and separated by a transparent wall. The amount of time spent in each side of the chamber was detected by infrared video recording. The conditioning chamber was placed over a floor made of two different textures. The positive stimulus floor (Cs+) consisted of a 0.6 cm grid. The negative stimulus floor (Cs-) was made with a 0.1 cm grid. The protocol used has been previously described (Cunningham et al., 2006). Briefly, male mice were handled and habituated to sham injection procedures at 8:00 am. The enclosure had a removable wall that allowed the mice to freely explore both chambers (day 1 per 10 min). 24 hours after habituation, the first conditioning session was initiated (1 or 2 injections per day). CPP conditioning trials were performed in the morning for 8 sessions or morning/afternoon for 16 sessions. In the conditioning trials (days 2–5 and 8–11 of the schedule), the conditioning boxes were separated by the wall and prepared with the appropriate floors (large or small grid depending on the group, floor and order assignment). Each male mouse was weighed and immediately injected i.p. with ethanol (2.0 g/kg) (if CS+ trial) or 0.9 % saline (if CS- trial) paired with a different floor and chamber. The mouse was placed in the center of the box and the sound-attenuating chamber was closed. The mouse activity during each trial was recorded for 5 min. Finally, twenty-four hours after the final conditioning session (day 12) the mouse was weighed, injected i.p. with 0.9 % saline and placed in the center of the box without the wall to record the test activity in a 30-min preference session. Then, the mouse was removed from the apparatus and returned to its home cage. Preference test video was analyzed using tracking video software Kinovea (mean time on Grid) and results were statistically analyzed with two-way ANOVA and Bonferroni post hoc test.
Reagents
Bicuculline, strychnine and quinine were obtained from Sigma-Aldrich (USA). Org24598, D-APV and CNQX were purchased from Tocris (Bristol, UK). TTX was purchased from Alomone labs (Jerusalem, Israel). Ethanol was purchased from Merck Millipore (USA).
Sample size
The target number of samples in each group for behavioral, biochemistry and electrophysiological experiments was determined on the basis of numbers reported in published studies (Aguayo et al., 2014; Mariqueo et al., 2014). Using those effect sizes and an alpha level set at 0.05 and at 80% power, we determined that 5—7 electrophysiological recordings from at least 2 mice was an appropriate sample size.
Replication
All sample sizes indicated in figures for behavioral and electrophysiological experiments represent biological replicates. The biochemistry experiments (Western blot, dot blot and immunocytochemistry) were successfully repeated at least three times.
Data analyses
Unless otherwise indicated, data were presented as mean ± SEM and the analyses were performed using the two-tailed unpaired Student’s t tests following an F test to confirm similar variances were used for figures 4b, c, d, f and h, S1c and d; S2c and Tables S1 and S2. Data with more than two groups or factors were analyzed by one-way ANOVA test followed by a Bonferroni post hoc test in figures 5, S3a and two-way ANOVA test followed by a Tukey or Bonferroni post hoc test for figures 1, 2, 3, 4a, e and g; S2d and S3b. Linear regression was performed in Figure 4a and these data were not tested for normality. Statistical analyses were performed with Origin 6.0 and 8.0 (Microcal, Inc. Northampton, MA). Values for *p<0.05 were considered statistically significant.
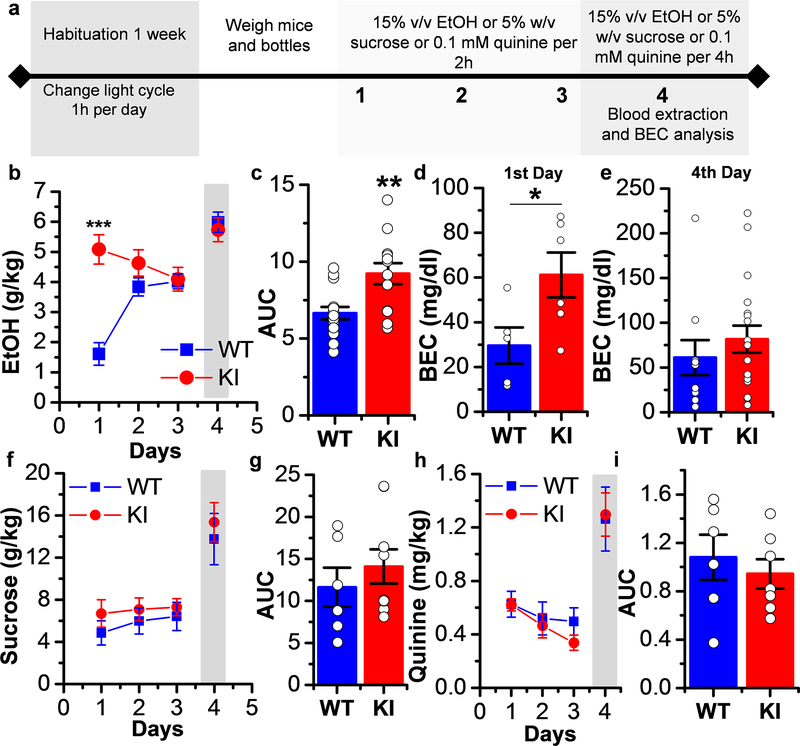
Figure 4. Increased ethanol first exposure consumption without change in sucrose and quinine intake in KI mice.
a) Experimental timeline of DID experiment for ethanol, sucrose or quinine. b) Graph summarizing the DID test in WT and KI mice. The gray shaded bar represents 4 hr sessions rather than the 2 hr sessions on the other days. WT mice had an escalated ethanol consumption, while ethanol consumption in KI mice was elevated for all the days tested (p=1.79281E−6, F3,55= 12.846, n=15 WT mice and n=13 KI mice, Two-way ANOVA, Bonferroni post hoc test). c) Summary graph of area under the curve (AUC) showing a higher area in KI mice (p=0.0026, t26=3.336, Unpaired Student’s t test, n=15 WT mice and n=13 KI mice). d) The graph shows that after the 1st day of consumption KI mice had higher BEC than WT mice (p=0.042, t9= 2.369, Unpaired Student’s t test, n=5 WT mice and n=6 KI mice). e) The graph shows that there were no differences in the blood ethanol concentration between WT and KI mice after the 4th day of consumption (p=0.417, t26= 0.825, Unpaired Student’s t test, n=10 WT mice and n=18 KI mice). f) The graph shows that no differences were found in sucrose consumption between WT and KI mice (n=6 WT mice and n=9 KI mice). The gray shaded bar represents 4 hr sessions rather than the 2 hr sessions on the other days. g) Summary graph of AUC showing no difference in the area between WT and KI mice (p=0.447, t13=0.784, Unpaired Student’s t test, n=6 WT mice and n=9 KI mice). h) The graph shows that no differences were found in quinine consumption between WT and KI mice (p=0.589, F1,47= 0.294, Two-way ANOVA, n=6 WT mice and n=7 KI mice). The gray shaded bar represents 4 hr sessions rather than the 2 hr sessions on the other days. i) Summary graph of AUC showing no difference in the area between WT and KI mice (p=0.539, t11= 0.6334, Unpaired Student’s t test, n=6 WT mice and n=7 KI mice). Data represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
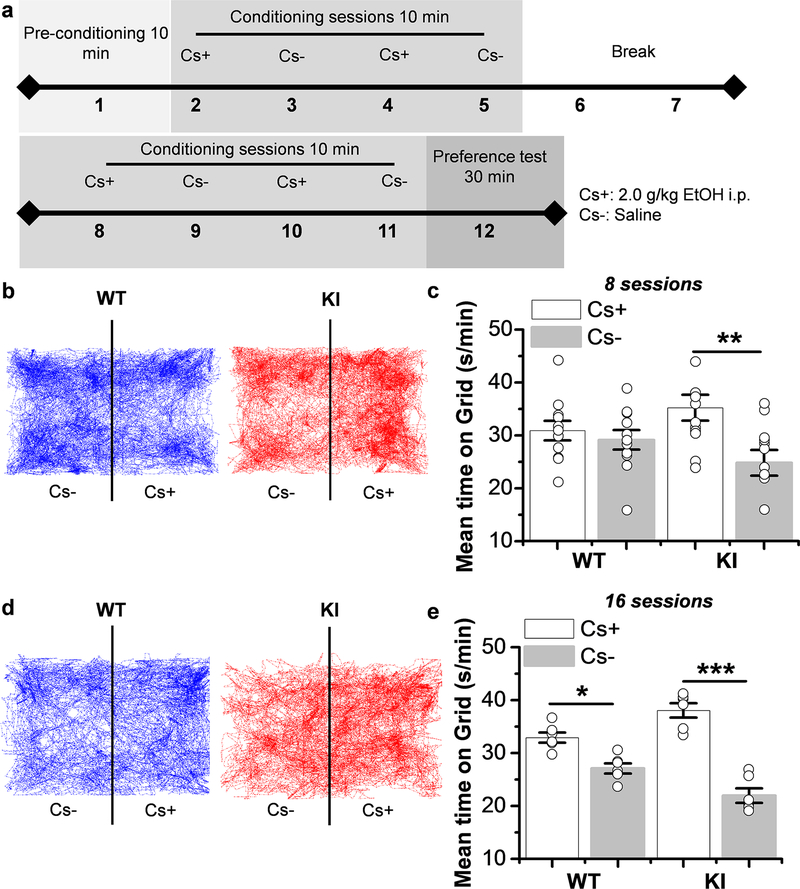
Figure 5. Increase in ethanol-conditioned place preference in KI mice.
a) Timeline design of CPP experiment. b) Representative trajectory traces of 30 min obtained from WT (left) and KI (right) mice during preference test after 8 conditioning sessions. c) The graph summarizes the Post conditioned place preference after 8 sessions in WT and KI mice and demonstrates that KI mice spent more time in the ethanol side (Cs+) than in the vehicle side (Cs-) (p=0.0062, F1,22= 9.183). WT mice did not show any preference for either side (p=0.508, F1,20= 0.454) (n=11 WT mice and n=12 KI mice).d) Representative trajectory traces of 30 min obtained from WT (left) and KI (right) mice during preference test after 16 conditioning sessions. e) The graph summarizes the Post conditioned place preference after 16 sessions in WT and KI mice and demonstrates that WT and KI mice spent more time in the ethanol side (Cs+) than in the vehicle side (Cs-) (WT: p=0.020, F1,10= 18.18 and KI: p=8.1E−6, F1,10= 69.77) (n=6 WT mice and n=6 KI mice). Data represent mean ± SEM, **p<0.01, ***p<0.001. One-way ANOVA, Bonferroni post hoc test.
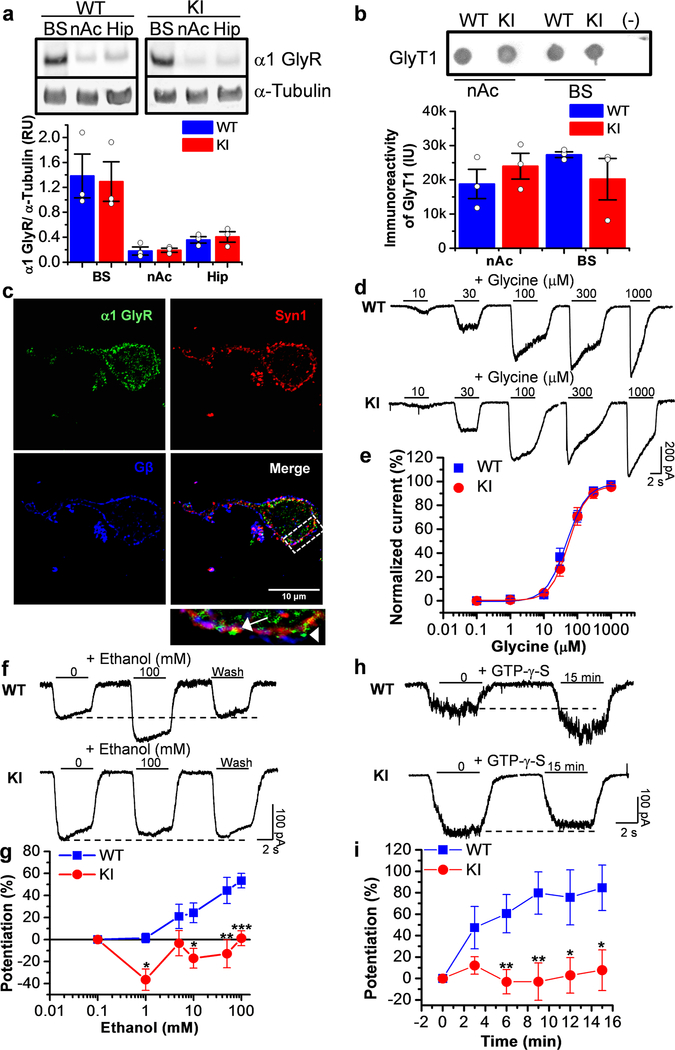
Figure 1. Presence of GlyRs sensitive to the effects of ethanol in nAc.
a) Western blot of brainstem (BS), nucleus accumbens (nAc) and hippocampus (Hip) from WT and KI animals for α1 GlyR and α−tubulin. Western blot analysis shows low levels of α1 GlyR in nAc and Hip and high levels in BS (n=3 animals). b) Dot blot from nAc and BS of WT and KI mice for GlyT1. The graph shows a quantitative analysis of immunoreactivity for GlyT1 in nAc and BS from WT and KI animals (n=3 mice). c) Confocal photomicrograph of dissociated neurons from nAc showing immunoreactivity to α1 GlyR (green), synapsin 1 (red) and Gβ (blue). The presence of colocalization of α1 GlyR with Syn1 represents a synaptic receptor (arrow); while α1 GlyR alone is non-synaptic (arrowhead). The scale bar represents 10 μm. d) Representative traces of glycine evoked currents (1–1000 μM) in dissociated neurons from WT and KI mice. e) The graph shows the glycine concentration-response curve in accumbal neurons from WT (blue squares) and KI mice (red circles). The EC50 was similar in both genotypes: 47 ± 6 μM WT and 54 ± 1 μM KI (WT n=15 neurons from 3 animals and KI n=12 neurons from 2 animals). f) Representative evoked current traces from WT and KI showing the effects of 100 mM ethanol measured with an EC10 of glycine (15 μM). g) The graph summarizes the effect of ethanol concentrations on accumbal neurons from WT (blue squares) and KI (red circles) animals. Data shows positive modulation only in WT neurons (n=12 neurons from 2 WT mice, n=22 neurons from 6 KI mice) (p=0.00675, F1,30= 8.4673). h) Representative evoked current traces from WT and KI showing the effects of G protein activation by intracellular dialysis of GTP-γ-S (0.2 mM) for 15 minutes. i) The time course graph summarizes the effects of G-protein activation by GTP-γ-S in MSNs from WT (blue squares) and KI (red circles) animals. An important potentiation was found only in WT neurons (n=5–8 neurons from 2 WT mice, n=9–11 neurons from 3 KI mice) (p=1.218E−7, F1,94= 32.802). Data are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Two-way ANOVA, Tukey test.
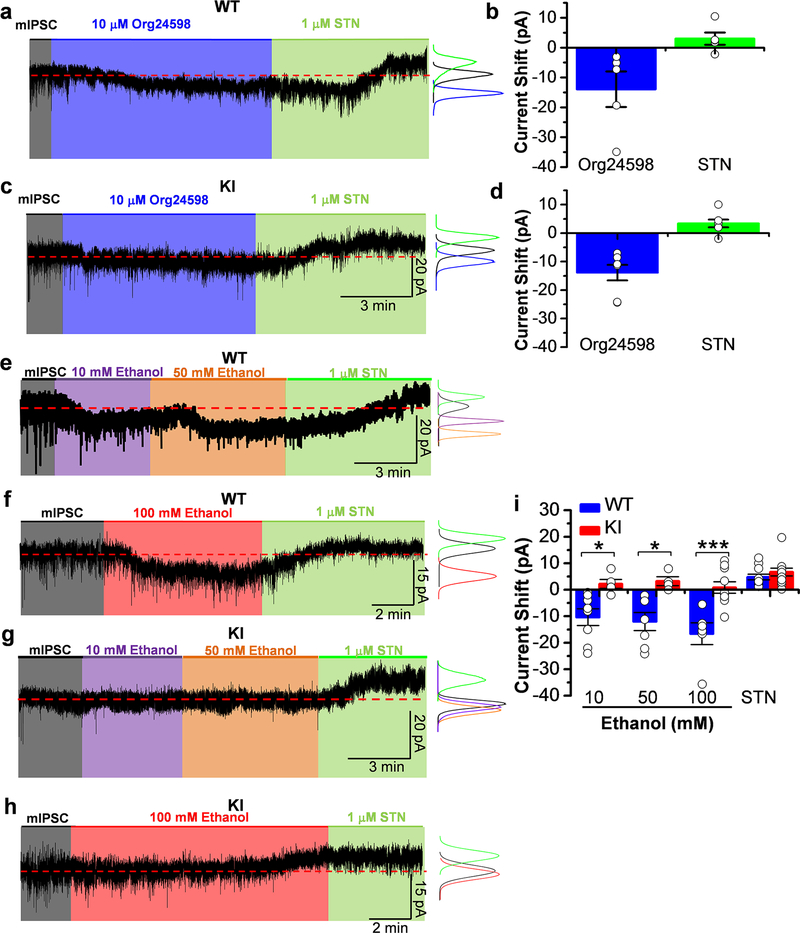
Figure 2. Presence of tonic inhibitory currents mediated by GlyRs that are sensitive to ethanol in WT nAc.
a and c) Representative electrophysiological trace from WT and KI neuron in the presence of 10 μM Org24598 (blue shaded area) and 1 μM STN (green shaded area). The red dotted line indicates the baseline. The histogram graph shows the analysis of the trace. Org24598 induced an inward current in nAc. STN abolished mIPSCs and produced a positive shift in the holding current in both genotypes. b and d) The graph summarizes the effects of Org24598 and STN on glycine tonic currents from WT and KI neurons. (n=5 neurons from 3 WT mice and n=7 neurons from 4 KI mice). e) Representative electrophysiological trace from a WT neuron in the presence of 10 (purple shaded area) and 50 mM ethanol (orange shaded area) and 1 μM STN (green shaded area). The red dotted line indicates the baseline. The histograms show the analysis of the trace. Low and high ethanol concentrations increased GlyR-mediated currents in the nAc. This effect was abolished by STN. f) Representative electrophysiological trace from a WT neuron in the presence of 100 mM ethanol (red shaded area) and 1 μM STN (green shaded area). The red dotted line indicates the baseline. The histogram graph shows the analysis of the trace. Ethanol increased the GlyR-mediated current in the nAc. This effect was abolished by STN. g) Representative electrophysiological trace from a KI neuron in the presence of 10 (purple shaded area) and 50 mM ethanol (orange shaded area) and 1 μM STN (green shaded area). The red dotted line indicates the baseline. The histogram graph shows the analysis of the trace. GlyR-mediated tonic currents in KI were not affected by low and high concentrations of ethanol. STN produced a positive shift in the holding current. h) Representative electrophysiological trace from a KI neuron in the presence of 100 mM ethanol (red shaded area) and 1 μM STN (green shaded area). The red dotted line indicates the baseline. The histogram graph shows the analysis of the trace. Ethanol did not affect the holding current; however STN abolished the mIPSCs and produced a positive shift in the holding current. Tonic currents in KI neurons were resistant to ethanol effects. i) The graph summarizes the effects of 10, 50 and 100 mM ethanol and STN on WT and KI glycine tonic currents, showing an increase in tonic current only in WT neurons (p=5.189E−7, F1,36=37.1487). Data are mean ± SEM. n=7 neurons from 3 WT mice and n=5 neurons from 2 KI mice for 10 and 50 mM ethanol and n=6 neurons from 3 WT mice and n=10 neurons from 4 KI mice for 100 mM ethanol. ns p>0.05, *p<0.05, ***p<0.001. Two-way ANOVA, Tukey test.
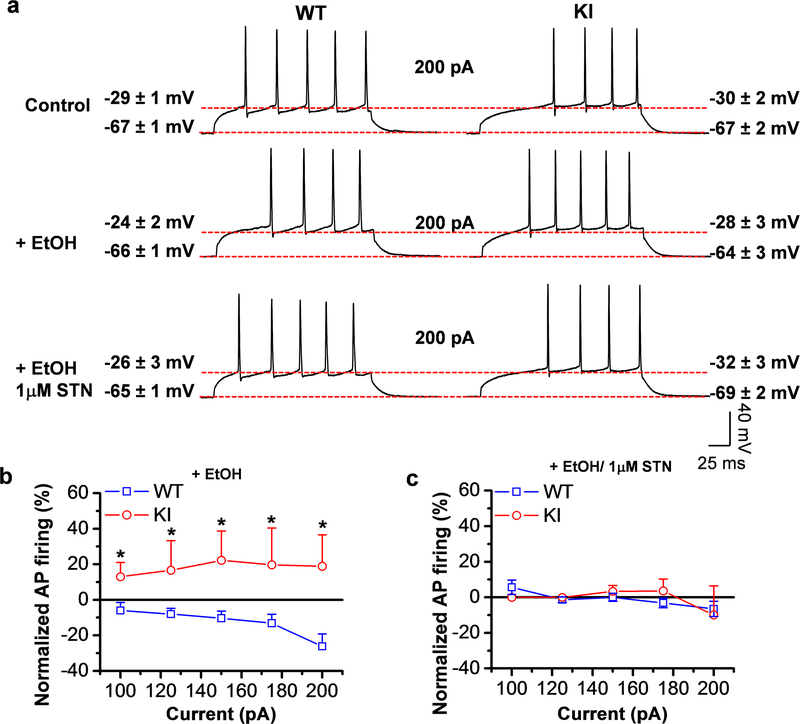
Figure 3. Ethanol decreased excitability in WT neurons.
a) Representative action potential traces from WT and KI neurons stimulated with 200 pA of current clamp in the absence and presence of 100 mM ethanol and co-application of 100 mM ethanol plus 1 μM STN. Similar resting (−67 ± 1 mV WT and −67 ± 2 mV KI) and threshold potentials (−29 ± 1 mV WT and −30 ± 2 mV KI) were found in control condition. Traces show a decrease in AP in WT neurons with 100 mM ethanol that was reverted by the co-application of ethanol plus STN. This effect was not present in KI neurons. b) Graph shows the decrease in AP in WT neurons with ethanol at 100–200 pA current clamp, while KI neurons were resistant to the effects of ethanol (p= 2.70E−6, F1,116= 24.3628, n=13–20 neurons from 3 WT mice and n=6–14 from 3 KI mice). c) Graph shows the recovery of APs in WT neurons by ethanol/STN at 100–200 pA, while KI neurons were resistant to the effects of ethanol. Data represent mean ± SEM.*p<0.05. Two-way ANOVA, Tukey test.
Results
GlyRs in accumbal neurons in the α1 KI mice are not potentiated by ethanol.
In agreement with previous studies in rat nAc and in mice (Molander and Soderpalm, 2005b) (Forstera et al., 2017), we found that both WT and KI mice expressed low levels of the α1 GlyR subunit in nAc when compared to brain stem (BS) (Fig. 1a). However, levels of GlyT1, a glial glycine transporter, in WT and KI mice nAc were similar to the levels found in BS (Fig. 1b). Western blot analysis also showed that there were no marked differences between the two genotypes (WT versus KI). Confocal microscopy in accumbal neurons showed the presence of α1 GlyR (green), with and without apposition to Synapsin1 (red), supporting the conclusion that while some GlyRs are synaptically found (arrow, Fig. 1c) most can be classified as non-synaptic locations (arrowhead, Fig. 1c) (Mariqueo et al., 2014). The high specificity of the α1 GlyR monoclonal antibody used was previously reported indicating that this fluorescent mark actually represents expression of this subunit (Forstera et al., 2017; Mariqueo et al., 2014).
Accumbal GlyR function was studied with patch clamp techniques in isolated neurons from WT and KI mice indicating no differences in several properties such as EC50 (Fig. 1d and e), current amplitude (Supporting Information Fig. S1a) and current density (Supporting Information Table S1). Interestingly, the EC50 values were similar to α1 GlyRs expressed in HEK293 cells (Yevenes et al., 2008) and D1 MSNs (Forstera et al., 2017), again supporting the presence of this subunit in nAc. We applied a range of low (1, 5 and 10 mM) and high (50 mM and 100 mM) concentrations of ethanol, and found that KI neurons were significantly less affected by the drug at most concentrations: 10 mM (WT: 24±9%, n=8 versus KI: −17±9%, n=19), 50 mM (WT: 45±11%, n=10 versus KI: −13±13%, n=21) and 100 mM ethanol (WT: 56±7%, n=18 versus KI: 1±6%, n=24, Fig. 1g). In WT neurons, ethanol (50 mM) potentiated 8 out of 10 WT cells (Supporting Information Fig. S1c) and the effect was reversible as shown in figure 1f. Interestingly, only 4 (19%) out of 21 KI neurons were potentiated by 50 mM ethanol (Supporting Information Fig. S1c).
The effect of 10 mM ethanol was also examined at the single channel level using outside-out single channel analysis, and a significant increase in open probability (nPo) in WT, but not in KI (Supporting Information Fig. S2a-e) was observed. The increase in nPo was 92±26% (n=10) in WT, however, in KI it was reduced (−26±17%, n=14). On the other hand, ethanol did not alter channel conductance in WT (40±3 pS in control vs 37±2 pS in ethanol) or KI neurons (43±4 pS in control; 37±4 pS in ethanol) (Supporting Information Fig. S2d). In agreement with data in HEK cells overexpressing α1 GlyRs, where the basic residues are important for ethanol sensitivity (Yevenes et al., 2010), intracellular dialysis with GTP-γ-S (non-hydrolyzable analog of GTP) only affected the current in WT neurons (85±21%, n=5) (Fig. 1h and i). No effect was observed in the neurons from KI animals because the mutation eliminates critical molecular characteristics for Gβγ modulation (KK385–386AA) (Yevenes et al., 2008). We found that 80% (4 out of 5) of the neurons were potentiated by GTP-γ-S in WT after 15 min (Supporting Information Fig. S1d). On the other hand, in KI neurons only 44% (4 out of 9) of neurons were potentiated by the GTP irreversible analog. Taken together, these data indicate that GlyRs in accumbal neurons are functional and sensitive to ethanol at low concentrations; additionally, the lack of potentiation in KI neurons suggests that GlyRs in the nAc are largely composed of α1 subunits. The presence of GlyRs sensitive to ethanol and GTP-γ-S in some KI neurons supports the idea that other α subunits are also expressed in nAc and this was recently confirmed using HA-tagged ribosomes from nAc of D1 and D2-Cre-RiboTag mice (Forstera et al., 2017). Specifically, the transcript for α1 was enriched in both MSN types. Transcript for α2 subunits, on the other hand, were enriched in D1 MSNs, but reduced in D2 MSNs (Forstera et al., 2017). Because synaptic GlyRs in accumbal neurons were found not to be affected by ethanol, it appears that non-synaptic complexes are the key targets for this allosteric modulator (Muñoz et al., 2018).
Wild type MSNs have an α1-mediated GlyR tonic current sensitive to low ethanol concentrations.
Confocal microscopy examination of nAc neurons showed the presence of non-synaptic GlyRs (see Fig. 1c) suggesting that these receptors might be involved with the generation of an inhibitory Cl− tonic current. The presence of such a current was confirmed using a selective glial glycine reuptake inhibitor for GlyT1 (Org24598) known to increase the extracellular basal glycine concentration in brain slices (Bradaia et al., 2004). We found that 10 μM Org24598 increased the holding current to a very similar extent in WT and KI neurons (−14±6 pA n=5 vs −14±3 pA n=7) and that these current shifts were inhibited by 1 μM strychnine in both genotypes (3±2 pA WT and 3±1 pA KI) (Fig. 2a-d). After establishing the existence of GlyR-mediated tonic currents in the nAc, we evaluated their sensitivity to ethanol. The tonic current in WT neurons was potentiated by a range of ethanol concentrations, starting at 10 mM (Fig. 2e, f and i). Figure 2e, for example, shows that the all-point histograms in presence of 10 (violet shaded area) and 50 mM (orange shaded area) are displaced towards higher values and that the effect was blocked by STN (green shaded area). We also examined the effect of 100 mM ethanol in WT (Fig. 2f, red shaded area) to be able to compare its effect on non-synaptic GlyRs with those in KI mice that were not affected by this concentration (Fig. 2h, red shaded area). Also, the current was blocked by strychnine confirming that it was mediated by the activation of GlyRs (Fig. 2e, f and i). On the other hand, the tonic current from the α1 KI mice was not affected by ethanol, even at a high concentration (1±2 pA, n=10), but still blocked by strychnine (6±2 pA, n=10) (Fig. 2g, h and i). Further analysis showed that the difference between WT and KI is significant at 10, 50 (*p<0.05) and 100 mM ethanol (Fig. 2i). Thus, these results demonstrate that mutations in intracellular sites of α1 are important for ethanol modulation of non-synaptic GlyRs and suggest that tonic inhibitory currents are mediated by GlyRs containing this subunit. Hence, this study is the first to associate the α1 subunit expressed in the nAc with the activation of tonic currents mediated by these inhibitory receptors.
Ethanol decreases excitability in WT accumbal neurons.
Based on the presence of a tonic glycinergic current, we hypothesized that α1 in the nAc may have a role in maintaining the excitatory and inhibitory balance. Thus, AP firing was monitored during current clamp recordings (25–300 pA) in the absence and presence of ethanol and STN. We found that ethanol reduced the number of APs in WT neurons indicating enhanced inhibition (Fig. 3a and b). For example, a 5–10 minutes superfusion of the brain slice with ethanol decreased AP firing to 26±7% of control with a 200 pA depolarizing current pulse (Fig. 3b). The reduction in excitability with ethanol was found in WT, but not in KI neurons, and the effect was blocked by application of strychnine, suggesting that the response was mediated by α1 containing GlyRs (Fig. 3b and c). Lower concentrations of ethanol (10–50 mM) also reduced AP firing (data not shown). Furthermore, no major differences were found in resting membrane potential, threshold potential, AP Half-Width, input resistance and AP peak amplitude in WT and KI neurons. Interestingly only an increase in AP threshold potential in WT MSNs neurons by STN was found (Supporting Information Table S2, see KI). Hence, we associated the decrease in AP firing by ethanol with α1 GlyR activation, in agreement with our previous work where we also show that strychnine increases AP firing in D1 MSNs (Forstera et al., 2017). Taken together, these findings suggest that α1 GlyRs are involved in the control of the excitatory-inhibitory drive in the nAc.
KI mice exhibited high first exposure ethanol consumption compared to WT mice.
Until now, there is no clear link between ethanol drinking behavior and changes in inhibitory LGIC sensitivity to ethanol in mutant receptors causing changes in the activation and gating properties. Recently published data showed that GlyR KI mice have reduced sedation when they are administered a sedative dose of ethanol (i.p. 3.5g/kg) (Aguayo et al., 2014). Those results demonstrated an important role in the sedative action of spinal α1 GlyRs. Similarly, the expression of GlyRs sensitive to ethanol in the nAc suggests that they might play a role in regulating the rewarding effects of ethanol. Therefore, we examined ethanol consumption using the limited access paradigm of drinking in the dark (DID) because mice are more active in the dark phase of the light cycle (Rhodes et al., 2005) (Fig. 4a). On day 1 of the study, we found a significantly higher ethanol consumption in the KI mice compared to controls (WT: 1.6±0.4 g/kg, n=15; KI: 5.1±0.5 g/kg, n=13) (Fig. 4b and c). The results in figure 4b show that KI mice consumed higher levels of ethanol than WT mice. Interestingly, the KI mice exhibited a sustained high level of ethanol consumption over the testing period. The higher drinking in KI mice at day 1 was corroborated by an increased blood ethanol concentration (BEC) (Fig. 4d), but not at day 4 (Fig. 4e). To characterize the ethanol consumption for an extended time, we examined limited access for 2 weeks. Similar results were found on day 1, the high consumption in KI mice was stable for the 2 weeks, while WT mice showed an increased level during week 1 and a slight decrease in consumption at week 2 (Supporting Information Fig. 3a). The KI mice, on the other hand, did not consume more sucrose (Fig. 4f and g) or quinine on the first exposure (Fig. 4h and i). Together, these results indicate that α1 GlyRs play a role in the initial levels of ethanol intake.
Increased ethanol conditioned place preference in the α1 KI mice.
Consequently, we evaluated conditioned place preference (CPP) in WT and KI mice to evaluate their motivation and preference for ethanol (Cunningham et al., 2006) (Fig. 5a). During preconditioning with 8 and 16 sessions, no place preference for WT and KI mice was detected (Supporting Information Fig. 3b). Interestingly, KI mice showed a significant increase in ethanol preference (Cs+) as compared to WT during post conditioning of 8 sessions (35±2 s/min versus 25±2 s/min, respectively, n=12, Fig. 5b and c) and a bigger increase after 16 sessions (38±1 s/min versus 22 ±1 s/min, respectively, n=6, Fig. 5d and e). On the other hand, no difference in ethanol-preference was found in WT mice at 8 sessions (31±2 s/min versus 29±2 s/min. n=11, p=0.973, Fig. 5b and c). However, at 16 sessions WT mice showed an increase in ethanol place preference (33±1 s/min versus 27±1 s/min. n=6, Fig. 5d and e). These results show that KI mice develop a faster preference to ethanol. All these behavioral data provide in vivo evidence to suggest that the motivational, rewarding, and reinforcing effects of ethanol are associated with the loss of ethanol sensitivity of α1 GlyRs.
Discussion
Although it is widely recognized that ethanol can potentiate or inhibit several LGICs, namely GABAA, NMDA, nACh and 5-HT3 (Howard et al., 2011), it is still unknown how these actions of ethanol are able to influence specific animal behaviors. Studies with mice lacking receptors (KO) have major difficulties such as changes in network activity, unforeseen compensations, and pathological disorders (Avila et al., 2013; Badanich et al., 2011). Studies with KI animals, on the other hand, require that the mutations selectively affect ethanol allosteric modulation, without changing channel activation and gating to maintain normal brain function. A previous study using the α1 (S246Q) GlyR KI mice that had reduced sensitivity to ethanol showed a marked hyperekplexic phenotype (Findlay et al., 2003). This study indicated that a single mutation in a critical amino acid important for channel opening can lead to major compensations, affecting normal behavior (Findlay et al., 2005; Findlay et al., 2003). In this respect, the KI mutation used in the present study did not appear to affect GlyR function, and selectively blocked the potentiation caused by ethanol (Aguayo et al., 2014). Examination of response-concentration curves in accumbal neurons show that the half-maximal potentiation (EC50) was near 20 mM in WT while in KI even 100 mM was unable to induce a potentiating response. In addition, the KI animal did not present hyperekplexic or startle response behaviors characteristic of altered inhibitory functions (Chung et al., 2013; Findlay et al., 2005; Findlay et al., 2003; Lape et al., 2012). Thus, the effects of the mutations examined in the present study appear to be relatively specific for ethanol-related behaviors.
Cellular actions of α1 GlyRs in nAc neurons
Several reports have shown that GlyRs are involved in dopamine release in the nAc (Molander et al., 2005; Molander and Soderpalm, 2005a) and that glycinergic ligands can indeed affect ethanol consumption (Molander et al., 2007; Molander et al., 2005; Molander and Soderpalm, 2005b). The present results using KI mice with a point mutation helped us to define a new cellular function of α1 containing GlyRs in a critical reward region. With western blot and immunocytochemistry studies, we showed the presence of synaptic and non-synaptic α1 containing GlyRs in nAc, in agreement with studies in spinal cord neurons (Mariqueo et al., 2014), indicating that nAc has all the molecular requirements for the presence of glycinergic Cl− currents, both tonic (Forstera et al., 2017) and phasic (Muñoz et al., 2018).
The most conventional action of ethanol on the mesolimbic dopamine system considers GABAAR activation as the main inhibitory synaptic transmission in MSNs and interneurons (Nestler, 2005). Studies have shown that application of dopamine on nAc slices affected non-synaptic GABAARs, without changing those synaptically located (Liang et al., 2014). The present study found that these nAc neurons have functional GlyRs with properties very similar to previous reports for α1 GlyRs (see Table S1) (Aguayo et al., 2014; Mariqueo et al., 2014; Yevenes et al., 2008). Hence, neuronal inhibition in nAc does not depend only on GABAARs, but GlyRs can provide a tonic inhibition that may dampen incoming excitation of MSNs. Only WT neurons expressed GlyRs that were sensitive to ethanol and Gβγ activation. Neurons from the KI mice showed some heterogeneity in their responses indicating that about 20% of the MSN in nAc expressed other GlyR subunits, in addition to α1. Furthermore, the conductance value obtained with single channel recordings support the conclusion that GlyRs in nAc are heteropentameric receptors because homomeric receptors have higher conductances (Yevenes et al., 2010; Yevenes et al., 2008; Yevenes et al., 2003). Altogether, the heterogeneity in KI neurons with respect to ethanol effects suggest that not only α1 GlyR subunits are expressed in the different accumbal neurons important for addictive behaviors (Hyman et al., 2006), a conclusion that is in agreement with a previous study (Forstera et al., 2017). Thus, the present data suggest that α1 in the brain is important for some rewarding effects of ethanol, but other subunits cannot be ruled out.
Physiological relevance of tonic GlyR-mediated currents in nAc inhibition
An inhibitory tonic current component mediated by non-synaptic GlyRs has been reported in several regions of the CNS, such as mPFC (Salling and Harrison, 2014), spinal cord (Bradaia et al., 2004), raphe (Maguire et al., 2014), OFC (Badanich et al., 2013), dorsal striatum (McCracken et al., 2017a) and recently in nAc (Forstera et al., 2017; McCracken et al., 2017a). In the present study, the results obtained using confocal microscopy showed the presence of non-synaptic α1 GlyR clusters in nAc neurons, which are most likely responsible for the tonic current that was evident after blocking GlyT1 with Org24598 and by its sensitivity to strychnine. This tonic current was potentiated by low ethanol concentrations only in WT neurons, while KI neurons were mostly resistant. It appears that the tonic current in MSNs decreases AP firing during ethanol exposure, in agreement with a previous study (Forstera et al., 2017). Thus, it appears non-synaptic α1 GlyR in MSNs are responsible of this decrease in AP firing, because synaptic GlyR in nAc was not affected by ethanol (Muñoz et al., 2018). Although further studies are needed to understand how these ethanol insensitive receptors increase ethanol preference, we suggest that potentiation of GlyRs might inhibit MSN GABAergic pathways contributing to activation of the reward circuitry through disinhibition. Also, it is possible that α1 GlyRs are also expressed in other brain regions projecting to the mesolimbic circuit and regulating its output. Nevertheless, given that α1 GlyRs are expressed mainly in the spinal cord (Aguayo et al., 2014), a role for α1 in the mesolimbic dopaminergic system has not been well established up to now (Blednov et al., 2015; Forstera et al., 2017; McCracken et al., 2017a).
Role of accumbal α1 GlyRs on cellular responses and behaviors
A recent study using α2 and α3 KO mice suggested that the changes in ethanol phenotypes were associated with loss of these subunits (Blednov et al., 2015). The conclusion that behavioral changes in the presence of ethanol are due to these subunits is unlikely because the α2 and α3 subunits do not have the molecular properties to be modulated by ethanol (Sanchez et al., 2015; Yevenes et al., 2010). Thus, these results are probably not due to loss of direct ethanol effects on these receptors, but can be associated to compensations present in both KO mice and/or the role of β GlyR subunit.
Potentiation of α1 GlyRs in spinal and brain stem neurons is likely responsible for the loss of muscle control and sedation during ethanol intoxication which is in agreement with a previous study that showed that α1 KI mice were less sedated by a high ethanol concentration (Aguayo et al., 2014). However, it is much more difficult to understand the precise sites and targets responsible for the addictive behavior of ethanol because several brain regions are affected during this complex behavior. Various studies using pharmacological and intracerebral dialysis techniques have reported that GlyRs in nAc and VTA are important for addictive-mediated behaviors (Li et al., 2012; Molander et al., 2005). This notion is in line with the widely recognized understanding that reward-related learning (seeking, motivation) is associated with activation of the direct nAc-VTA pathway (Macpherson et al., 2014; Nakanishi et al., 2014). Indeed, the activation of D1 MSNs is related to high cocaine preference and D2 MSNs to aversion (Lenz and Lobo, 2013). More recent studies, however, have raised some questions regarding the differences in these pathways (Kupchik et al., 2015).
The present study provides support of a new role for α1 GlyRs in nAc, in addition to the sedative actions of ethanol (Schmid et al., 1991; Williams et al., 1995; Ye et al., 2009), specifically on addictive behaviors (Van den Oever et al., 2012). Here, we evaluated reward-based learning in KI mice. The DID studies showed high consumption in the KI mice beginning at day 1, suggesting that KI mice do not develop aversion to the first ethanol exposure and this effect was not related with the taste, since sucrose and quinine intake showed no differences. On other hand, the WT mice drank much less at day 1, displaying an increase in consumption that reached higher consumption levels with time. Interestingly, at day 4, both groups of mice exhibited high consumption as revealed by BEC measurements. Consistent with higher ethanol consumption, this study also showed that KI mice had stronger and faster-developing place preference to ethanol after only 8 conditioning sessions. A previous study reported place preference for C57BL/6J mice using 16 conditioning sessions (Hilbert et al., 2013), as we found in WT mice. These data support the hypothesis that the inhibitory pathway is not affected by ethanol in the α1 KI mice, disrupting first exposure consumption and place preference. Thus, in agreement with previous reports, the present study suggests that ethanol-seeking behavior and changes in excitability in nAc (Jonsson et al., 2014) might be caused by non-synaptic α1 GlyRs, but not synaptic α1 (Muñoz et al., 2018), α2 and α3 subunits present in D1 MSNs (Forstera et al., 2017). Therefore, the results suggest that α1 has a role in reward-related learning that only now is being understood.
The data indicate that the time course of these ethanol behaviors are different. The drinking in KI mice start high and is maintained over time similar to WT levels. In parallel, KI mice show a high preference to ethanol after the training period. Several explanations are possible to explain these features, but we believe that they are related to the administration method, while DID is voluntary and self-limiting, the CPP is done with high alcohol and no voluntary conditions causing distinct receptor pharmacodynamics.
Activation of GlyRs in the reward circuitry might result in complex effects. For instance, in a neuronal circuitry like the nAc-VTA, inhibition of MSN will produce an excitatory response in a downstream synapse, causing desinhibition in the VTA. Based on our current understanding of the neuronal circuit of the reward system (Nakanishi et al., 2014), we propose that α1 GlyRs antagonize the excitation of D1 MSNs. It is well accepted that drugs of abuse, including ethanol, stimulate the release of dopamine from VTA, activating the direct pathway in nAc (Di Chiara and Imperato, 1988). In the KI mice, the stimulatory effect of dopamine is no longer antagonized by GlyR potentiation leading to higher D1 MSN excitability. This effect disinhibits downstream basal ganglia targets, such as the thalamus and cortex, promoting ethanol-seeking behavior (Fig. 6). However, because the KI mice represent a global mutation we cannot exclude the role of GlyRs with the KI mutation that are present in other brain regions (VTA, striatum). For instance, we recently found that GlyRs in VTA are remarkably sensitive to ethanol and have a high expression of α1 subunits (not shown). Additionally, the role of other subunits (i.e. α2β), for example, cannot be ruled out at this time as important to drive the higher early intake in the KI mice.
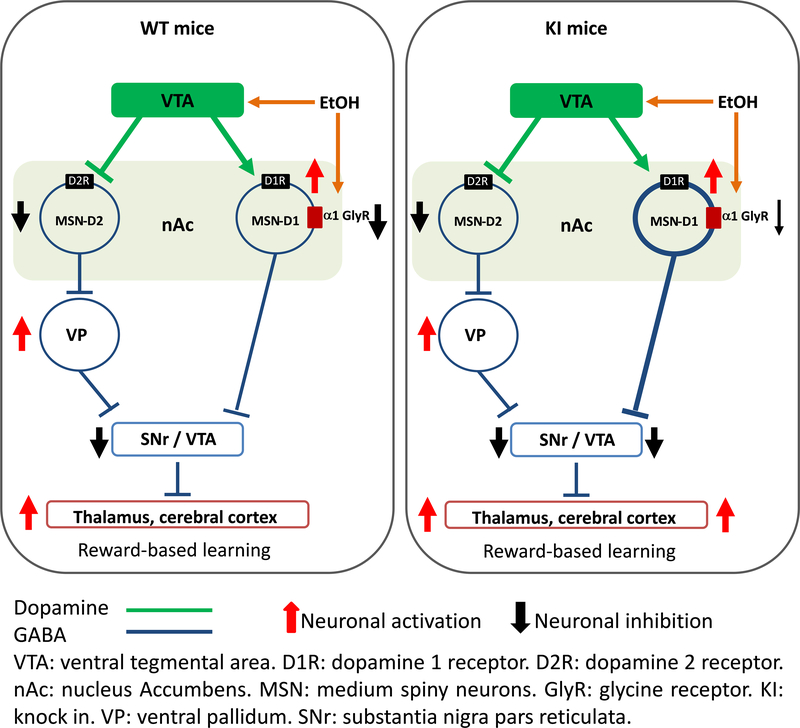
Figure 6. Glycine receptors containing α1 subunits affect preference to ethanol.
In the absence of ethanol, WT GlyRs activation inhibits (black arrow) D1 MSNs releasing the GABAergic inhibition of VTA by disinhibition (thalamus is excited, red arrow). In presence of ethanol, release of dopamine from VTA increase and further stimulate D1 MSN in the nAc (red arrow). In parallel, ethanol potentiates non-synaptic α1 GlyRs in D1 MSN decreasing membrane excitability, see figure 3 and (Forstera et al., 2017). On the other hand, mutated α1 GlyR in the nAc are insensitive to ethanol leading to a higher activation of MSNs (more GABA released, thicker blue line) and higher VTA inhibition (black arrow). This mechanism explains the enhanced behavior/learning results in this study that show high first exposure and preference to ethanol by these KI mice (see Fig. 4). (Modified from Nakanishi et al. (2014))
Our study supports the conclusion that α1 GlyRs play a crucial inhibitory role in the circuitry of the mesolimbic dopaminergic system, regulating the pathway and affecting first exposure to ethanol consumption and ethanol place preference. Also, it provides a new molecular and possible pharmacotherapeutic target for the prevention and treatment of alcohol abuse.
Supplementary Material
Figure S1. MSNs from WT are sensitive to the effects of ethanol.
a) Graph shows the amplitude of evoked currents of glycine concentration-response curves. (n=15 neurons from 3 WT mice and n=12 neurons from 2 KI mice). b) Graph shows similar current density for WT (7.5 ± 2 pA/pF) and KI (7 ± 2 pA/pF) neurons. Data are mean ± SEM (n=11 neurons from 3 WT mice and n=8 neurons from 2 KI mice). c) Scatter graph shows the effects of 50 mM ethanol in each recorded WT and KI neuron. (p=0.00852, t29=2.822, Unpaired Student’s t test, n=10 neurons from 2 WT mice, n=21 neurons from 6 KI mice). d) Scatter graph shows the effects of intracellular dialysis of GTP-γ-S in each recorded WT and KI neuron (p=0.0254, t12=2.552, Unpaired Student’s t test, n=5 neurons from 2 WT mice and n=9 neurons from 3 KI mice). Data are mean ± SEM. *p<0.05, **p<0.01.
Figure S2. Ethanol increased the nPo of GlyRs in dissociated neurons from WT nAc.
a-b). Single channel recordings from dissociated neurons from WT and KI before and after the application of 10 mM EtOH. c) The bar graph shows the percentage of change of open probability (nPo) after the application of 10 mM EtOH in WT and KI (p=0.0006, t22=3.99, Unpaired Student’s t test, n=10 neurons from 2 WT mice and n=14 neurons from 4 KI mice). d) The graph shows that the GlyR mean conductance was not affected by 10 mM EtOH (p=0.989, F1,67=0.0107, Two-way ANOVA, Tukey test, n=12-17 neurons from 2 WT mice and n=15-26 neurons from 4 KI mice). Data are mean ± SEM, ***p<0.001.
Figure S3. Increased ethanol consumption in KI mice through 2 weeks of limited access to ethanol and no preference at day one.
a) Graph summarizes the drinking in the dark (two weeks of ethanol-limited exposure) test in WT and KI mice. The gray shaded bars represent 4 hr sessions rather than the 2 hr sessions on the other days. WT mice had escalated ethanol consumption and remained stable until day 9, and then a decrease in intake was found, while ethanol consumption in KI mice was elevated and stable for all the days tested. Significant differences were found at day 1 (p=0.009, F1,11=9.981, One-way ANOVA, Bonferroni post hoc test) and day 2 (p=0.041, F1,11=5.306, One-way ANOVA, Bonferroni post hoc test, n=6 WT mice and n=7 KI mice). b) The graph shows the pre-conditioning session in WT and KI mice and reveals that WT and KI mice do not have place preference on day 1 (p=0.973, F1,43= 0.00114, Two-way ANOVA, Bonferroni post hoc test, n=11 WT mice and n=12 KI mice). Data represent mean ± SEM. *p<0.05, **p<0.01.
Table S1. Electrophysiological properties of glycine evoked currents in nAc.
Table S2. Electrophysiological properties of APs in nAc.
Acknowledgments
The authors would like to thank Lauren J. Aguayo and Carolina Benitez for expert technical assistance, and the Centro de Microscopía Avanzada (CMA Bio-Bio, ECM-12), University of Concepción. All reagents and materials used for this work are commercially available.
Funding and disclosure
This work was supported by NIH grant AA17875 (GEH and LGA), NIH AA025718 (LGA) and DPI 20140008 (LGA). The authors declare no conflict of interest.
Footnotes
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Aguayo LG, Castro P, Mariqueo T, Munoz B, Xiong W, Zhang L, Lovinger DM, Homanics GE (2014) Altered sedative effects of ethanol in mice with alpha1 glycine receptor subunits that are insensitive to Gbetagamma modulation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39:2538–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo LG, Tapia JC, Pancetti FC (1996) Potentiation of the glycine-activated Cl- current by ethanol in cultured mouse spinal neurons. The Journal of pharmacology and experimental therapeutics 279:1116–1122. [PubMed] [Google Scholar]
- Avila A, Vidal PM, Dear TN, Harvey RJ, Rigo JM, Nguyen L (2013) Glycine receptor alpha2 subunit activation promotes cortical interneuron migration. Cell Rep 4:738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Doremus-Fitzwater TL, Mulholland PJ, Randall PK, Delpire E, Becker HC (2011) NR2B-deficient mice are more sensitive to the locomotor stimulant and depressant effects of ethanol. Genes Brain Behav 10:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ (2013) Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38:1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Leiter CR, Osterndorff-Kahanek E, Harris RA (2015) Glycine receptors containing alpha2 or alpha3 subunits regulate specific ethanol-mediated behaviors. The Journal of pharmacology and experimental therapeutics 353:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradaia A, Schlichter R, Trouslard J (2004) Role of glial and neuronal glycine transporters in the control of glycinergic and glutamatergic synaptic transmission in lamina X of the rat spinal cord. The Journal of physiology 559:169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SK, Bode A, Cushion TD, Thomas RH, Hunt C, Wood SE, Pickrell WO, Drew CJ, Yamashita S, Shiang R, Leiz S, Longardt AC, Raile V, Weschke B, Puri RD, Verma IC, Harvey RJ, Ratnasinghe DD, Parker M, Rittey C, Masri A, Lingappa L, Howell OW, Vanbellinghen JF, Mullins JG, Lynch JW, Rees MI (2013) GLRB is the third major gene of effect in hyperekplexia. Hum Mol Genet 22:927–940. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA (2006) Drug-induced conditioned place preference and aversion in mice. Nature protocols 1:1662–1670. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Berger AJ (2004) Mechanisms for the modulation of native glycine receptor channels by ethanol. Journal of neurophysiology 91:2685–2695. [DOI] [PubMed] [Google Scholar]
- Eggers ED, O’Brien JA, Berger AJ (2000) Developmental changes in the modulation of synaptic glycine receptors by ethanol. Journal of neurophysiology 84:2409–2416. [DOI] [PubMed] [Google Scholar]
- Findlay GS, Harris RA, Blednov YA (2005) Male transgenic glycine receptor alpha1 (S267Q) mutant mice display a hyperekplexia-like increase in acoustic startle responses. Pharmacology, biochemistry, and behavior 82:215–222. [DOI] [PubMed] [Google Scholar]
- Findlay GS, Phelan R, Roberts MT, Homanics GE, Bergeson SE, Lopreato GF, Mihic SJ, Blednov YA, Harris RA (2003) Glycine receptor knock-in mice and hyperekplexia-like phenotypes: comparisons with the null mutant. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:8051–8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstera B, Munoz B, Lobo MK, Chandra R, Lovinger DM, Aguayo LG (2017) Presence of ethanol-sensitive glycine receptors in medium spiny neurons in the mouse nucleus accumbens. The Journal of physiology 595:5285–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert ML, May CE, Griffin WC 3rd (2013) Conditioned reinforcement and locomotor activating effects of caffeine and ethanol combinations in mice. Pharmacology, biochemistry, and behavior 110:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Murail S, Ondricek KE, Corringer PJ, Lindahl E, Trudell JR, Harris RA (2011) Structural basis for alcohol modulation of a pentameric ligand-gated ion channel. Proceedings of the National Academy of Sciences of the United States of America 108:12149–12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annual review of neuroscience 29:565–598. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Krnjevic K, Wang F, Ye JH (2004) Taurine activates strychnine-sensitive glycine receptors in neurons freshly isolated from nucleus accumbens of young rats. Journal of neurophysiology 91:248–257. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Adermark L, Ericson M, Soderpalm B (2014) The involvement of accumbal glycine receptors in the dopamine-elevating effects of addictive drugs. Neuropharmacology 82:69–75. [DOI] [PubMed] [Google Scholar]
- Jun SB, Cuzon Carlson V, Ikeda S, Lovinger D (2011) Vibrodissociation of neurons from rodent brain slices to study synaptic transmission and image presynaptic terminals. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW (2015) Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nature neuroscience 18:1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Plested AJ, Moroni M, Colquhoun D, Sivilotti LG (2012) The alpha1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:1336–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK (2013) Optogenetic insights into striatal function and behavior. Behavioural brain research 255:44–54. [DOI] [PubMed] [Google Scholar]
- Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH (2012) Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. The Journal of pharmacology and experimental therapeutics 341:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Marty VN, Mulpuri Y, Olsen RW, Spigelman I (2014) Selective modulation of GABAergic tonic current by dopamine in the nucleus accumbens of alcohol-dependent rats. Journal of neurophysiology 112:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lido HH, Ericson M, Marston H, Soderpalm B (2011) A role for accumbal glycine receptors in modulation of dopamine release by the glycine transporter-1 inhibitor org25935. Front Psychiatry 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye JH (2011) Glycine-activated chloride currents of neurons freshly isolated from the prefrontal cortex of young rats. Brain research 1393:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW (2009) Native glycine receptor subtypes and their physiological roles. Neuropharmacology 56:303–309. [DOI] [PubMed] [Google Scholar]
- Macpherson T, Morita M, Hikida T (2014) Striatal direct and indirect pathways control decision-making behavior. Frontiers in psychology 5:1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EP, Mitchell EA, Greig SJ, Corteen N, Balfour DJ, Swinny JD, Lambert JJ, Belelli D (2014) Extrasynaptic glycine receptors of rodent dorsal raphe serotonergic neurons: a sensitive target for ethanol. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariqueo TA, Agurto A, Munoz B, San Martin L, Coronado C, Fernandez-Perez EJ, Murath P, Sanchez A, Homanics GE, Aguayo LG (2014) Effects of ethanol on glycinergic synaptic currents in mouse spinal cord neurons. Journal of neurophysiology 111:1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Siggins GR (2002) Electrophysiological evidence for expression of glycine receptors in freshly isolated neurons from nucleus accumbens. The Journal of pharmacology and experimental therapeutics 302:1135–1145. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Lowes DC, Salling MC, Carreau-Vollmer C, Odean NN, Blednov YA, Betz H, Harris RA, Harrison NL (2017a) Glycine receptor alpha3 and alpha2 subunits mediate tonic and exogenous agonist-induced currents in forebrain. Proceedings of the National Academy of Sciences of the United States of America 114:E7179–E7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Lowes DC, Salling MC, Carreau-Vollmer C, Odean NN, Blednov YA, Betz H, Harris RA, Harrison NL (2017b) Glycine receptor α3 and α2 subunits mediate tonic and exogenous agonist-induced currents in forebrain. Proceedings of the National Academy of Sciences 114:E7179–E7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Lido HH, Lof E, Ericson M, Soderpalm B (2007) The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol and alcoholism (Oxford, Oxfordshire) 42:11–18. [DOI] [PubMed] [Google Scholar]
- Molander A, Lof E, Stomberg R, Ericson M, Soderpalm B (2005) Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcoholism, clinical and experimental research 29:38–45. [DOI] [PubMed] [Google Scholar]
- Molander A, Soderpalm B (2005a) Accumbal strychnine-sensitive glycine receptors: an access point for ethanol to the brain reward system. Alcoholism, clinical and experimental research 29:27–37. [DOI] [PubMed] [Google Scholar]
- Molander A, Soderpalm B (2005b) Glycine receptors regulate dopamine release in the rat nucleus accumbens. Alcoholism, clinical and experimental research 29:17–26. [DOI] [PubMed] [Google Scholar]
- Muñoz B, Yevenes GE, Förstera B, Lovinger DM, Aguayo LG (2018) Presence of Inhibitory Glycinergic Transmission in Medium Spiny Neurons in the Nucleus Accumbens. Frontiers in molecular neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Hikida T, Yawata S (2014) Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience 282:49–59. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nature neuroscience 8:1445–1449. [DOI] [PubMed] [Google Scholar]
- Peters C, Fernandez-Perez EJ, Burgos CF, Espinoza MP, Castillo C, Urrutia JC, Streltsov VA, Opazo C, Aguayo LG (2013) Inhibition of amyloid beta-induced synaptotoxicity by a pentapeptide derived from the glycine zipper region of the neurotoxic peptide. Neurobiology of aging 34:2805–2814. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & behavior 84:53–63. [DOI] [PubMed] [Google Scholar]
- Salling MC, Harrison NL (2014) Strychnine-sensitive glycine receptors on pyramidal neurons in layers II/III of the mouse prefrontal cortex are tonically activated. Journal of neurophysiology 112:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Yevenes GE, San Martin L, Burgos CF, Moraga-Cid G, Harvey RJ, Aguayo LG (2015) Control of ethanol sensitivity of the glycine receptor alpha3 subunit by transmembrane 2, the intracellular splice cassette and C-terminal domains. The Journal of pharmacology and experimental therapeutics 353:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Bohmer G, Gebauer K (1991) Glycine receptor-mediated fast synaptic inhibition in the brainstem respiratory system. Respir Physiol 84:351–361. [DOI] [PubMed] [Google Scholar]
- Sebe JY, Eggers ED, Berger AJ (2003) Differential effects of ethanol on GABA(A) and glycine receptor-mediated synaptic currents in brain stem motoneurons. Journal of neurophysiology 90:870–875. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Lof E, Ericson M (2009) Mechanistic studies of ethanol’s interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry 42 Suppl 1:S87–94. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB (2012) The synaptic pathology of drug addiction. Advances in experimental medicine and biology 970:469–491. [DOI] [PubMed] [Google Scholar]
- Williams KL, Ferko AP, Barbieri EJ, DiGregorio GJ (1995) Glycine enhances the central depressant properties of ethanol in mice. Pharmacology, biochemistry, and behavior 50:199–205. [DOI] [PubMed] [Google Scholar]
- Ye JH, Sokol KA, Bhavsar U (2009) Glycine receptors contribute to hypnosis induced by ethanol. Alcoholism, clinical and experimental research 33:1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, McArdle JJ (2001) Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. The Journal of pharmacology and experimental therapeutics 296:77–83. [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Avila A, Guzman L, Figueroa M, Peoples RW, Aguayo LG (2010) Molecular requirements for ethanol differential allosteric modulation of glycine receptors based on selective Gbetagamma modulation. The Journal of biological chemistry 285:30203–30213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG (2008) A selective G betagamma-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proceedings of the National Academy of Sciences of the United States of America 105:20523–20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG (2003) Modulation of glycine-activated ion channel function by G-protein betagamma subunits. Nature neuroscience 6:819–824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. MSNs from WT are sensitive to the effects of ethanol.
a) Graph shows the amplitude of evoked currents of glycine concentration-response curves. (n=15 neurons from 3 WT mice and n=12 neurons from 2 KI mice). b) Graph shows similar current density for WT (7.5 ± 2 pA/pF) and KI (7 ± 2 pA/pF) neurons. Data are mean ± SEM (n=11 neurons from 3 WT mice and n=8 neurons from 2 KI mice). c) Scatter graph shows the effects of 50 mM ethanol in each recorded WT and KI neuron. (p=0.00852, t29=2.822, Unpaired Student’s t test, n=10 neurons from 2 WT mice, n=21 neurons from 6 KI mice). d) Scatter graph shows the effects of intracellular dialysis of GTP-γ-S in each recorded WT and KI neuron (p=0.0254, t12=2.552, Unpaired Student’s t test, n=5 neurons from 2 WT mice and n=9 neurons from 3 KI mice). Data are mean ± SEM. *p<0.05, **p<0.01.
Figure S2. Ethanol increased the nPo of GlyRs in dissociated neurons from WT nAc.
a-b). Single channel recordings from dissociated neurons from WT and KI before and after the application of 10 mM EtOH. c) The bar graph shows the percentage of change of open probability (nPo) after the application of 10 mM EtOH in WT and KI (p=0.0006, t22=3.99, Unpaired Student’s t test, n=10 neurons from 2 WT mice and n=14 neurons from 4 KI mice). d) The graph shows that the GlyR mean conductance was not affected by 10 mM EtOH (p=0.989, F1,67=0.0107, Two-way ANOVA, Tukey test, n=12-17 neurons from 2 WT mice and n=15-26 neurons from 4 KI mice). Data are mean ± SEM, ***p<0.001.
Figure S3. Increased ethanol consumption in KI mice through 2 weeks of limited access to ethanol and no preference at day one.
a) Graph summarizes the drinking in the dark (two weeks of ethanol-limited exposure) test in WT and KI mice. The gray shaded bars represent 4 hr sessions rather than the 2 hr sessions on the other days. WT mice had escalated ethanol consumption and remained stable until day 9, and then a decrease in intake was found, while ethanol consumption in KI mice was elevated and stable for all the days tested. Significant differences were found at day 1 (p=0.009, F1,11=9.981, One-way ANOVA, Bonferroni post hoc test) and day 2 (p=0.041, F1,11=5.306, One-way ANOVA, Bonferroni post hoc test, n=6 WT mice and n=7 KI mice). b) The graph shows the pre-conditioning session in WT and KI mice and reveals that WT and KI mice do not have place preference on day 1 (p=0.973, F1,43= 0.00114, Two-way ANOVA, Bonferroni post hoc test, n=11 WT mice and n=12 KI mice). Data represent mean ± SEM. *p<0.05, **p<0.01.
Table S1. Electrophysiological properties of glycine evoked currents in nAc.
Table S2. Electrophysiological properties of APs in nAc.