KEY POINTS
Live attenuated vaccines are capable of causing symptomatic vaccine-derived infection, even weeks following vaccination.
Immunocompromised hosts, including those taking low-dose immunosuppressive medications, are at increased risk for infection with live vaccine strains.
Caution is required before using live attenuated vaccines in immunocompromised people; expert consultation may be required.
Severe and unusual adverse events following vaccination should be reported to local public health authorities for surveillance and investigative purposes.
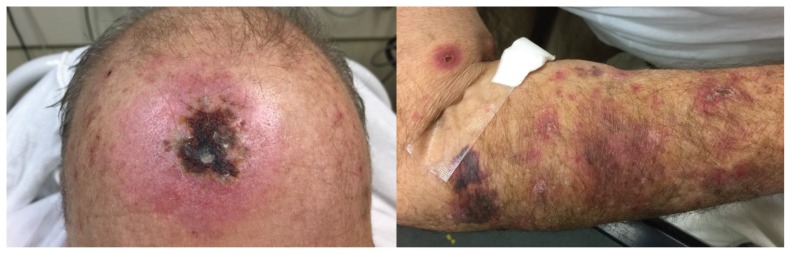
A 70-year-old man presented to the emergency department with a 2-week history of rash, which started as a localized eruption on his forehead and progressed to a vesicular rash involving his entire body (Figure 1). Over this same period, he noted increasing shortness of breath, tiredness, painful swallowing and chills. He did not report recent travel.
Figure 1:
Widespread vesicular and crusting lesions with surrounding erythema in a patient with disseminated vaccine strain zoster.
The patient had a past history of hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease and atrial flutter. Successful cardiac ablation had been performed 2 weeks before the onset of the rash. He also had rheumatoid arthritis, treated with methotrexate, 2.5 mg/d (6 d per week) for 3 years, hydroxychloroquine, 200 mg/d and prednisone, 10 mg/d. In the previous month, his prednisone dosage had been tapered from 10 mg/d. He was not receiving any biologic agents.
On examination, the patient’s blood pressure was 110/60 mm Hg, heart rate 86 beats/min and temperature 36.8°C. On examination, his cardiorespiratory and abdominal systems were initially within normal limits. Dermatologic assessment showed numerous widespread vesicles with erythematous bases across all aspects of his body. His oropharynx was erythematous, with associated lesions. Ocular examination was within normal limits.
On admission, the patient’s hemoglobin concentration was 120 (normal 140–180) g/L, leukocyte count 1.7 (normal 4.0–11.0) × 109/L, neutrophil count 1.3 (normal 2.0–7.5) × 109/L, lymphocyte count 0.3 (1.5–4.0) × 109/L and platelet count 84 (normal 150–400) × 109/L. During the course of evaluating this patient, a wide differential diagnosis was considered (Box 1). Given the multiple vesicular and crusted lesions, disseminated varicella zoster virus (VZV) infection was identified as the most likely cause. It was then ascertained from the patient and family that he had received a live attenuated herpes zoster vaccine (Zostavax II, Merck) about 1 month before the onset of symptoms. He also reported a history of chickenpox as a child.
Box 1:
Differential diagnosis for diffuse vesicular rash1
Infectious
Viral
Herpes simplex virus (disseminated herpes or eczema herpeticum)
Varicella zoster virus (chickenpox or disseminated zoster)
HIV
Measles
Parvovirus B19
Enteroviruses (including hand, foot and mouth disease)
Bacterial
Disseminated neisserial infections
Impetigo
Folliculitis
Rickettsial infections
Secondary syphilis
Ecthyma gangrenosum
Noninfectious
Severe idiosyncratic reactions (e.g., toxic epidermal necrolysis)
Delayed hypersensitivity (e.g., poison ivy)
Eczema vaccinatum
Erythema multiforme
With the clinical findings and vaccination history, intravenous treatment with acyclovir was started, 15 mg/kg every 8 h, and continued for the duration of the hospital stay. The patient was placed under airborne isolation. Shortly after admission, he was transferred to the intensive care unit for monitoring. Subsequently, leukopenia and thrombocytopenia developed, with evidence of progressive multiorgan failure with hypotension. Treatment with broad-spectrum antibiotics was started. The patient died on the fifth day after admission to the intensive care unit, following withdrawal of supportive measures and initiation of a palliative approach. Klebsiella spp. was subsequently cultured from 1 of 2 blood specimens.
Polymerase chain reaction testing of a lesional swab obtained at the time of initial presentation confirmed the presence of VZV on the first day after admission, and posthumous viral genotyping by the National Microbiology Laboratory confirmed the presence of the Oka (vaccine) strain. Autopsy findings included multiorgan failure from disseminated VZV infection, with pulmonary and colonic lesions and dilated cardiomyopathy. The case was referred to the local public health authority for reporting of an adverse event following vaccination and investigation. Close contacts and family members were contacted and evaluated to determine whether they were at high risk for complications of VZV infection.
Discussion
This is a case report of disseminated vaccine (Oka) strain VZV infection resulting in multiorgan failure and death, following receipt of the live attenuated herpes zoster virus vaccine.
Varicella zoster virus infection is acquired either naturally through exposure to wild-type virus or through vaccination with live attenuated virus. The virus typically remains dormant in the dorsal root ganglia and may reactivate later in life. Herpes zoster, commonly known as shingles, typically manifests as a maculopapular vesicular rash occurring along 1 or 2 dermatomes that does not cross the midline and is often associated with neuropathic pain. Disseminated zoster often involves multiple noncontiguous dermatomes and can result in central nervous system, pulmonary and hepatic involvement. It occurs more commonly in people with compromised immune systems.2
Given the history of a localized eruption followed by dissemination, the presentation in our patient could be considered as disseminated zoster, although this syndrome can sometimes be challenging to differentiate from primary varicella. Varicella serologic testing was not performed during the patient’s hospital stay and likely would not have been informative given that his symptoms began about 1 month following vaccination with a live virus vaccine. Regardless of the diagnostic classification, this patient had widespread dissemination of VZV infection with a vaccine strain. The Oka vaccine strain is considered susceptible to the antiviral acyclovir.
There are 2 licensed herpes zoster vaccines available in Canada for adults aged 50 years or more. The purpose of the vaccines is to prevent shingles and reduce the duration and severity of symptoms, most importantly postherpetic neuralgia. A live attenuated zoster vaccine (Zostavax II) has been authorized for use in Canada since 2008. In 2017, a recombinant subunit zoster vaccine (Shingrix, GlaxoSmithKline) was authorized for use in Canada. A comparison of these vaccines is presented in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190270/-/DC1).
The Canadian National Advisory Committee on Immunization (NACI) considers both vaccines to be safe and immunogenic.3 They both have been shown to reduce the incidence of herpes zoster and postherpetic neuralgia.3 In 2014, the Canadian Immunization Committee recommended that live zoster vaccine be offered to immunocompetent adults aged 60–65 years or more without contraindications on the “basis of the epidemiology of varicella zoster virus, zoster vaccine characteristics, disease modelling and economic analysis, as well as on the feasibility and acceptability of zoster immunization programs.”3 In 2016, Ontario was the first province to publicly fund the vaccine for adults 65–70 years of age.4
Live virus vaccines are typically contraindicated in immunocompromised people. However, in light of the high burden of reactivation and complications of zoster in immunocompromised hosts, before 2018, vaccination of immunocompromised people with live zoster vaccine could be considered under certain circumstances.5 Since there was insufficient evidence of safety and efficacy in certain groups, NACI did not recommend live zoster vaccine for those with “HIV infection (regardless of CD4 count or viral load), post-organ or hematopoietic stem cell transplantation (HSCT), or in those receiving high dose corticosteroids, chemotherapy or other immune suppressing medications.”5 High-dose corticosteroids were defined as 20 mg/d or more of prednisone or its equivalent for an adult for 14 days or more.5 However, at the time of the case in the present report, live zoster vaccine could be considered for those receiving low-dose immunosuppression therapy or tumour necrosis factor inhibitors on a case-by-case basis.5 Low-dose immunosuppressives were defined as low-dose prednisone (< 20 mg/d), methotrexate (≤ 0.4 mg/kg per week), azathioprine (≤ 3.0 mg/kg per day) and 6-mercaptopurine (≤ 1.5 mg/kg per day).5 There were no recommendations regarding patients who were receiving multiple low-dose immunosuppressive medications.
In addition, NACI does not recommend routine serologic testing before or after zoster vaccination. This practice is based largely on the fact that most Canadians have had varicella, even if they do not recall this, and zoster vaccination is likely safe in healthy adults who are susceptible to VZV.2
In 2018, NACI updated the herpes zoster vaccine recommendations and provided a preferential recommendation for the recombinant zoster vaccine, Shingrix, over the live attenuated vaccine, Zostavax II.3 However, a recommendation still remains that the live vaccine may be considered for immunocompetent populations in whom recombinant vaccine is contraindicated, unavailable or inaccessible.3 Recombinant zoster vaccine does not have a specific indication for use in immunocompromised people, as the results of studies in these populations are still pending. It is expected that it will be a safer alternative to the live vaccine since it does not contain whole virus particles capable of causing symptomatic disease. Guidance from NACI now states that the recombinant zoster vaccine (not the live zoster vaccine) may be considered in immunocompromised adults 50 years of age or more.3
There have been few previous reports of death from disseminated VZV infection related to the live attenuated zoster vaccine in immunocompromised people.6,7 The authors of a review of post-marketing safety of the live zoster vaccine concluded that the safety profile was good and concordant with what had been previously found in postlicensure studies and clinical trials.8 Another review of over 14 000 patients who received zoster vaccination while taking immunosuppressant drugs showed a small increased risk of herpes zoster for up to 42 days after vaccination but suggested that the increased risk was likely due to reactivation of latent zoster virus rather than vaccine-derived virus.9
In our case, however, disseminated vaccine-strain infection beginning about 4 weeks after vaccination and resulting in multiorgan failure and death was confirmed. To our knowledge, there have been no other reports of death associated with live attenuated zoster vaccine in Canada.10
Balancing the risks of vaccination with the increased risk of herpes zoster is crucial for each patient. Our case highlights the importance of inquiring about and recording a vaccination history in potentially relevant patient encounters and considering an adverse event following vaccination in the differential diagnosis. Such adverse events should be reported to the local public health authority in the patient’s province or territory.11 Local reporting forms are available, or the reporting form from the Public Health Agency of Canada can be used (www.canada.ca/content/dam/phac-aspc/documents/services/immunization/adverse-events-following-immunization-reporting-declaration-manifestations-cliniques-inhabituelles-suite-immunisation-eng.pdf). Not all adverse events following vaccination need to be reported. Well-known, expected events such as injection site pain or fever do not need to be reported unless they are more severe than expected.
Adverse events should be reported if there is a temporal association with the vaccine and there is no other clear cause for the event. However, a causal association between the vaccination and the adverse event is not required to report the event. Severe events that should be reported include life-threatening events, death, hospital admission, disability and congenital malformations. Adverse events that are unusual or unexpected should also be reported. Reporting adverse events following vaccination ensures the events can be investigated by public health and reported in Canada’s passive vaccine safety surveillance system.10
In conclusion, disseminated VZV infection is a rare but potentially lethal complication of vaccination with the live attenuated zoster vaccine. When considering live vaccine administration in immunocompromised patients, caution should be taken, and expert consultation may be required. Severe and unusual adverse events following immunization should be reported to local public health authorities for investigation and inclusion in Canada’s vaccine safety surveillance system.
Acknowledgements
The authors thank Janice Vandezande for completing the follow-up of the public health investigation, and Lindsay Melvin, Abirami Vijenthira and Wayne Gold for their thoughtful review of this case.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained consent from the patient’s next of kin.
Contributors: Both authors contributed to the conception and design of the work, drafted the manuscript, critically revised it for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Kang JH. Febrile illness with skin rashes. Infect Chemother 2015;47:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herpes zoster (shingles) vaccine: Canadian immunization guide. Canadian immunization guide for health professionals. Ottawa: Public Health Agency of Canada; modified 2018 Aug. 30. Available: www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-8-herpes-zoster-(shingles)-vaccine.html (accessed 2019 June 24). [Google Scholar]
- 3.An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): updated recommendations on the use of herpes zoster vaccines. Ottawa: Public Health Agency of Canada; 2018. Available: www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/updated-recommendations-use-herpes-zoster-vaccines-eng.pdf (accessed 2019 June 24). [Google Scholar]
- 4.Publicly funded shingles (herpes zoster) immunization program: information for health care providers. Toronto: Ontario Ministry of Health and Long-Term Care; 2019. Available: www.health.gov.on.ca/en/pro/programs/immunization/docs/shingles_hcp_qa_en.pdf (accessed 2019 June 24). [Google Scholar]
- 5.An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): Update on the use of herpes zoster vaccine. Ottawa: Public Health Agency of Canada; 2014. Available: canada.ca/en/public-health/services/publications/healthy-living/update-use-herpes-zoster-vaccine.html (accessed 2019 June 24). [Google Scholar]
- 6.Costa E, Buxton J, Brown J, et al. Fatal disseminated varicella zoster infection following zoster vaccination in an immunocompromised patient. BMJ Case Rep 2016;2016:bcr2015212688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander KE, Tong PL, Macartney K, et al. Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection. Vaccine 2018;36:3890–3. [DOI] [PubMed] [Google Scholar]
- 8.Willis ED, Woodward M, Brown E, et al. Herpes zoster vaccine live: a 10 year review of post-marketing safety experience. Vaccine 2017;35:7231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheetham TC, Marcy SM, Tseng HF, et al. Risk of herpes zoster and disseminated varicella zoster in patients taking immunosuppressant drugs at the time of zoster vaccination. Mayo Clin Proc 2015;90:865–73. [DOI] [PubMed] [Google Scholar]
- 10.Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). Ottawa: Public Health Agency of Canada; modified 2019 July 16. Available: www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html (accessed 2019 Sept. 3). [Google Scholar]
- 11.Reporting adverse events following immunization (AEFI) in Canada. Ottawa: Public Health Agency of Canada; modified 2013 Oct. 3. Available: www.canada.ca/en/public-health/services/immunization/reporting-adverse-events-following-immunization/form.html (accessed 2019 June 24). [Google Scholar]